Highlights
-
•
Hypothalamus and amygdala functional connectivity is altered in adolescents with NT1.
-
•
The hypothalamus shows reduced connectivity with the hippocampus and parietal cortex.
-
•
The amygdala showed both reduced and increased functional connectivity.
-
•
Reduced connectivity was observed between amygdala, sensorimotor and visual network.
-
•
Increased functional connectivity was present between amygdala and salience network.
Keywords: Narcolepsy, Functional connectivity, Resting-state fMRI, Hypothalamus, Amygdala
Abstract
Introduction
functional and structural MRI studies suggest that the orexin (hypocretin) deficiency in the dorso-lateral hypothalamus of narcoleptic patients would influence both brain metabolism and perfusion and would cause reduction in cortical grey matter. Previous fMRI studies have mainly focused on cerebral functioning during emotional processing. The aim of the present study was to explore the hemodynamic behaviour of spontaneous BOLD fluctuation at rest in patients with Narcolepsy type 1 (NT1) close to disease onset.
Methods
Fifteen drug naïve children/adolescents with NT1 (9 males; mean age 11.7 ± 3 years) and fifteen healthy children/adolescents (9 males; mean age 12.4 ± 2.8 years) participated in an EEG-fMRI study in order to investigate the resting-state functional connectivity of hypothalamus and amygdala. Functional images were acquired on a 3 T system. Seed-based functional connectivity analyses were performed using SPM12. Regions of Interest were the lateral hypothalamus and the amygdala.
Results
compared to controls, NT1 patients showed decreased functional connectivity between the lateral hypothalamus and the left superior parietal lobule, the hippocampus and the parahippocampal gyrus. Decreased functional connectivity was detected between the amygdala and the post-central gyrus and several occipital regions, whereas it was increased between the amygdala and the inferior frontal gyrus, claustrum, insula, and putamen.
Conclusion
in NT1 patients the abnormal connectivity between the hypothalamus and brain regions involved in memory consolidation during sleep, such as the hippocampus, may be linked to the loss of orexin containing neurons in the dorsolateral hypothalamus. Moreover, also functional connectivity of the amygdala seems to be influenced by the loss of orexin-containing neurons. Therefore, we can hypothesize that dysfunctional interactions between regions subserving the maintenance of arousal, memory and emotional processing may contribute to the main symptom of narcolepsy.
1. Introduction
Narcolepsy type 1 (NT1) is a rare chronic sleep disorder, occurring in 0.025% to 0.050% of the population (Ohayon et al., 2002, Silber et al., 2002). Recent studies suggest that symptoms began during childhood in more than one-half of the patients (Dauvilliers et al., 2001, Rocca et al., 2015). The main symptoms of narcolepsy are excessive daytime sleepiness (EDS), sleep paralysis, hypnagogic hallucinations, and nocturnal sleep fragmentation (American Academy of Sleep Medicine, 2014). Furthermore, a pathognomonic symptom of NT1 is cataplexy, a sudden muscle tone loss that occurs during wakefulness and it is triggered by strong positive emotions such as laughing (Bassetti et al., 2019). However, NT1 disease burden includes a variety of non-sleep-related symptoms including depression, cognitive difficulties, fatigue, and poor school/work performances (Maski et al., 2017, Plazzi et al., 2018).
Low or absent orexin level (also known as hypocretin-1, Hcrt-1) has been reported in the cerebrospinal fluid (CSF) (Thannickal et al., 2003, Thannickal et al., 2000) of 90% of NT1 patients (Mignot et al., 2002). Orexin deficiency is known to be associated with the loss of hypothalamic orexin neurons (Peyron et al., 2000, Ritchie et al., 2010), which project to the amygdala (Bisetti et al., 2006, Sakurai et al., 2005, Yoshida et al., 2006) and modulate the sleep/wake cycle via widespread connections with other arousal-promoting nuclei (Mignot et al., 2002, Saper et al., 2005).
A close interaction between brain regions involved in emotion processing and NT1 has been well documented in both animals (Gulyani et al., 2002, Siegel et al., 1999) and humans (Hong et al., 2006, KHATAMI et al., 2007, Meletti et al., 2015, Vaudano et al., 2019). In particular, these previous evidences suggest the involvement of the amygdala, a limbic structure that mediates emotional information processing (LeDoux, 2000, Vuilleumier, 2005, Zald, 2003).
Neuroimaging studies have not demonstrated consistent structural brain alterations in NT1. Systematic structural neuroimaging techniques revealed gray matter loss in unilateral (Kim et al., 2009), or bilateral (Bušková et al., 2006, Draganski et al., 2002, Joo et al., 2009, Scherfler et al., 2012) hypothalamus, even though, no structural hypothalamic changes were reported in other works (Brenneis et al., 2005, Kaufmann et al., 2002, Overeem et al., 2003). Conflicting results were reported also about amygdala structural abnormalities. Two studies described decreased grey matter (Brabec et al., 2011, Kim et al., 2016), whereas other authors failed to detect any anomaly in the amygdala volume (Nemcova et al., 2015). Gray matter reduction was reported also in the thalamus, the nucleus accumbens and the fronto-temporal areas, which are projected by orexin neurons (Wada et al., 2019). Moreover, a recent study of our group showed increased volume of the hippocampus in children and adolescents with NT1 (Tondelli et al., 2018).
Functional MRI (fMRI) studies investigated BOLD activation during emotional processing (see for review: Engström et al., 2014, Wada et al., 2019), showing conflicting results with both increased and reduced hypothalamic response in narcoleptic patients compared to controls (Reiss et al., 2008, Schwartz et al., 2008, Vaudano et al., 2019). Significant participation in cataplexy triggered by emotions was observed within several emotional and reward system regions, such as the amygdala but also the insula, the nucleus accumbens, the anterior cingulate and the orbitofrontal cortices (Meletti et al., 2015, Vaudano et al., 2019). A number of these studies performed event-related fMRI with different aims and using different protocols, for instance to investigate neural correlates of humor processing in drug-free adult patients while viewing sequences of humorous pictures (Schwartz et al., 2008) or humorous cartoons during cataplectic attack (Reiss et al., 2008). Other studies focused on children and adolescents with NT1 investigating the neural correlates of spontaneous laughter (Vaudano et al., 2019) and of cataplexy (Meletti et al., 2015) while watching funny videos. Therefore, the resulting picture of the exploration of narcolepsy through the lens of functional MRI is complex and non-homogenous.
The hemodynamic behavior of spontaneous BOLD fluctuation at rest has not been extensively explored in NT1. Three studies examined brain functional connectivity in NT1 using graph theoretical analysis and/or independent component analysis (Drissi et al., 2016, Xiao et al., 2020, 2019). Xiao et al. (2019) reported altered functional connectivity within the executive (Seeley et al., 2007) and the salience network (Menon and Uddin, 2010), but also between the sensorimotor regions and the occipital and cingulate gyri in narcoleptic adult patients, whereas a disruption of both anterior and posterior components of the default mode network (Raichle et al., 2001, Gusnard and Raichle, 2001) was described by Drissi and colleagues (2016) in adolescents (14–20 years).
The aim of the present study was to investigate the resting state functional connectivity in drug-naïve children and adolescents with narcolepsy, focusing on the hypothalamus and the amygdala as seeds. We wanted to study functional connectivity close to disease onset in order to detect early neurodevelopmental features of NT1, before any structural, functional or behavioral adaptation and without drug-induced effects. We hypothesized that the loss of neurons containing orexin in NT1 may alter functional connectivity between the hypothalamus and brain regions involved in arousal and emotion processing.
2. Materials and methods
2.1. Participants
Twenty young normal-weight (body mass index, BMI: 22.19 ± 4.4 kg/m2) patients with NT1 (11 males; mean age 11.3 ± 2.9 years) were recruited between January 2014 and February 2015. Five patients were excluded from the study because of movements along the whole fMRI acquisition, incompleteness of functional session and/or lack of T1-weighted anatomical image. Therefore, 15 patients entered the group analyses. Detailed clinical features are described in our previous papers on the same population (Meletti et al., 2015, Vaudano et al., 2019) and summarized in Table 1. Briefly, patient inclusion criteria were the following: (a) drug-naive young patients from 7 to 18 years old, (b) a diagnosis of NT1, according to current criteria (American Academy of Sleep Medicine, 2014), (c) proven CSF orexin deficiency, and (d) video documentation of cataplectic attacks during a standardized video session (Pizza et al., 2013, Plazzi et al., 2011).
Table 1.
Clinical and Laboratory Data of Patients (included in the fMRI group analyses).
| Patient ID | Age (yr) | Gender | Disease duration (yr) | CSF Hcrt(pg/ml) | mESS score | Cataplexy frequency | BMI | MSLT-SL | MSLT-SOREMPs |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13 | M | 2.7 | 55.0 | n.r. | n.r. | 19.56 | 13.00 | 4 |
| 2 | 10 | F | 3.7 | 15.5 | 14 | >1d | 19.86 | 1.20 | 1 |
| 3 | 9 | M | 1.7 | 0.0 | 21 | >1d | 26.19 | 1.90 | 4 |
| 4 | 7 | F | 0.9 | 0.0 | 20 | >1d | 17.46 | 9.60 | 1 |
| 5 | 10 | M | 3.7 | 43.1 | 13 | 1y-1 m | 20.61 | 9.00 | 5 |
| 6 | 12 | F | 2.0 | 10.7 | 13 | 1w-1d | 23.7 | 3.30 | 5 |
| 7 | 15 | M | 1.0 | 0.0 | 17 | 1w-1d | 27.04 | 3.30 | 4 |
| 8 | 11 | F | 1.3 | 0.0 | 14 | 1w-1d | 23.9 | 1.00 | 5 |
| 9 | 14 | F | 3.2 | 0.0 | 12 | 1w-1d | 19.3 | 2.40 | 5 |
| 10 | 16 | M | 6.1 | 0.0 | 14 | 1w-1d | 21.03 | 2.40 | 5 |
| 11 | 11 | M | 3.3 | 25.0 | 14 | >1d | 16.53 | 9.80 | 4 |
| 12 | 14 | M | 2.8 | 39.8 | 18 | 1d | 33.9 | 5.50 | 5 |
| 13 | 7 | M | 0.3 | 0.0 | 10 | 1w-1d | 23.84 | 7.50 | 3 |
| 14 | 10 | M | 0.8 | 15.7 | 13 | >1d | 18.66 | 11.6 | 3 |
| 15 | 17 | F | 1.6 | 0.0 | 15 | 1w-1d | 21.3 | 2.6 | 5 |
Yr = years; F = female; M = male; min = minutes; hcrt = hypocretin-1 level in CSF (pathological levels < 110 pg/mL); ESS = Epworth Sleepiness Scale score (range 0–24, pathological score > 10); MSLT = multiple sleep latency test; SOREMPs = sleep-onset rapid eye movement periods.
For between-group comparison, 15 normal-weight healthy controls (HC, 9 males; mean age 12.4 ± 2.8 years) with no history of neurological diseases and normal sleep-wake habit took part in the fMRI study (for a detailed demographic description, see supplementary Table 1). The participants and their parents were interviewed by A.E.V., a certified sleep expert, to ascertain personal sleep habits, neurological status and to exclude sleep disorders . Part of the studied population of healthy children/adolescents has already been published in a recent study by Talami et al. (2020).
The local Ethics Committee has approved the experimental protocol (CE 268/15) and written informed consent was obtained from the parents of each subject, who assented to participate in the study.
2.2. Resting-state EEG-fMRI protocol
All MRI recordings were performed during the early afternoon 1–3 h after a meal and after a brief nap to avoid confounding effects of sleepiness. All subjects were instructed not to focus their thoughts on anything in particular, avoiding any structured mental activity such as counting, rehearsing, etc., and to keep their eyes closed during the resting state MR acquisition. At the end of the scanning session, the participants were asked whether they were awake and whether they were able to follow the instructions.
Scalp EEG was recorded by means of a 32 channels MRI-compatible EEG recording system (Micromed, Mogliano Veneto, Italy) in NT1 group only. Electrodes were placed according to conventional 10–20 locations and the reference was FCz. ECG was recorded from two chest electrodes. Surface electromyography (EMG) was recorded from sub chin electrodes. The impedance of the electrodes was lower than 10 kΩ. Before scanning, 10 min of out-of magnet good quality EEG data were collected. Foam pads were used to secure the EEG leads, minimize motion, and improve the comfort of the patient. The EEG data were transmitted via an optic fiber cable from the high-input impedance amplifier (1024 Hz sampling rate) to a computer located outside the scanner room. Both groups were also constantly observed and recorded by a small camcorder positioned on the head coil inside the scanner pointing to the participant’s face to obtain a simultaneous split-screen video-EEG documentation during the fMRI recording (Chaudhary et al., 2010, Ruggieri et al., 2015). The utility of the video is twofold. On one hand, it allows to monitor the subject’s face inside the scanner and to verify offline the presence of any physiological movement (head movements, swallowing, orobuccal movements, etc.), which has been modelled in the fMRI analyses according to previously published methods (Ruggieri et al., 2015). On the other, it was used to monitor any behavioral sign of sleep and specifically the occurrence of rapid eye movements (REM).
Functional data were acquired using a Philips Achieva system at 3 T and a gradient-echo echo-planar sequence from 30 axial contiguous slices (TR = 2000 ms; in-plane matrix = 80x80; voxel size: 3x3x4) over a 8 min session per participant (240 volumes) with continuous video-EEG recording. A TTL signal was sent every TR via a BNC trigger cable from the MRI console to the EEG computer allowing the synchronization between the acquisition of the functional volumes and the EEG data. A high-resolution T1-weighted anatomical image was acquired for each participant to allow anatomical localization. The volume consisted of 170 sagittal slices (TR = 9.9 ms; TE = 4.6 ms; in plane matrix = 256 × 256; voxel size = 1 × 1 × 1 mm).
2.3. EEG preprocessing and analysis
The correction of the gradient artifact on EEG acquired during fMRI was performed offline by means of the Brain Quick System Plus software (Micromed, Mogliano Veneto, Italy) (Allen et al., 2000). The EEG data were exported in .edf format and reviewed by means of the BrainVision Analyzer 2.0 software (Brain Products, Munich, Germany). A bandpass filter between 0.5 and 70 Hz was applied to the continuous recording and a cubic spline interpolation was used for channels showing high impedance or electrode displacement artifacts. Pulse related artefacts were removed using the EEG processing package of Brain Analyzer (Brain Products, Munich, Germany) (Allen et al., 1998). The preprocessed EEG was then submitted to an Independent Component Analysis (ICA) (Bell and Sejnowski, 1995, Makeig et al., 2004) in order to separate the generators of EEG activity thus maximizing the statistical independence among them. For each participant, the 30 EEG channels signal was decomposed in 30 components (F0 to F29). Each component was characterized by a time course describing the morphology of the component over time, and by a specific topography (i.e. an array containing the weights the specific component had in each channel). For all the patients two certified sleep experts (A.E.V. and F.P.) reviewed both the standard EEG recordings and the individual components of the ICA-processed recordings. The components carrying residual motion or gradient artifacts were marked as artifactual components. The residual cardiac artifact was subtracted by objective (CBC parameters, Vision Analyzer) ICA-based rejection of residual artifact-laden components. Then, the resulting cleaned EEG traces were further reviewed to identify the physiological brain rhythms, such as alpha rhythm, and any sign of sleep and hypnagogic sleep figures. To this end, a conventional scoring in 30 s epochs was performed according to the rules published by the American Academy of Sleep Medicine (AASM, 2007).
2.4. Resting-State fMRI data processing
fMRI data analysis was performed using MATLAB version R2013a (The MathWorks Inc, Natick, Mass) and SPM12 (Wellcome Department of Imaging Neuroscience, London, UK). Functional volumes of each participant were slice time corrected, realigned to the first volume acquired and normalized to the MNI (Montreal Neurologic Institute) template implemented in SPM12. A temporal filter (0.01–0.08 Hz) was applied using Resting-State fMRI Data Analysis Toolkit (REST) (Song et al., 2011) to reduce low frequency drifts and high frequency physiological noise. Finally, functional data were smoothed with a 6x6x8 mm FWHM Gaussian kernel.
2.4.1. Seed-based functional connectivity analyses: Lateral hypothalamus and amygdala
Functional connectivity maps were obtained using the voxel wise approach by computing functional connectivity between two regions of interest (ROI), the lateral hypothalamus and the amygdala, and each voxel within the brain.
The lateral hypothalamus was identified in each hemisphere. According to previous studies (Baroncini et al., 2012, Contreras-Rodríguez et al., 2017, Kullmann et al., 2014), the ROI were built as 2-mm radius spheres around the following coordinates: x = ± 6, y = − 10, z = − 10. The BOLD signal time course was extracted from the left and the right lateral hypothalamus of each participant by means of marsbar (http://marsbar.sourceforge.net/). First level (single subject) regression analyses were performed for each subject, using the BOLD signal time course of the seed regions as predictor of interest. The six head-motion parameters (translations and rotations) and the time courses representing mean signal fluctuations in gray matter, white matter and cerebrospinal fluid were entered as confounds. Single patient contrast images were generated by estimating the regression coefficient between each seed’s time series and the whole brain and were then included in a second-level full factorial model with a 2 × 2 design (group [patients vs healthy controls] × ROI [left and right lateral hypothalamus]). Age and sex were included as covariate.
The second seed was the amygdala. Since this limbic region contains dense orexin fibers and receptors and is also projected by orexin-containing neurons (Siegel et al., 1999), we exclusively considered the subregions which were functionally connected with the lateral hypothalamus. Therefore, the ROI was defined as the intersection between the healthy controls’ hypothalamic functional connectivity map and the amygdala belonging to the AAL Atlas (Tzourio-Mazoyer et al., 2002). The intersection was performed by means of marsbar using the function r1 & r2, which gives all the voxels included in ROI 1 (AAL amygdala) and in ROI 2 (hypothalamic connectivity map). The resulting volumes of the ROI were 608 mm3 (16.88 voxels) for the left amygdala and 96 mm3 (2.66 voxels) for the right amygdala. First and second-level resting-state analyses were performed as described above for the lateral hypothalamus.
A double statistical threshold (voxel-wise p < 0.001 and spatial extent) was adopted to achieve a combined significance, corrected for multiple comparisons, of α < 0.05, as computed by 3dClustSim AFNI routine, using the “-acf” option (https://afni.nimh.nih.gov/pub/dist/doc/program_help/3dClustSim.html).
Regression analyses were performed to examine correlation between hypothalamus and amygdala functional connectivity and disease duration (years), CSF orexin level, and mESS score. Gender, age, and BMI were considered as nuisance covariates.
3. Results
The simultaneously acquired EEG and video recordings allowed to ascertain that no patient slept during the fMRI acquisitions. Specifically, the sleep scoring procedure demonstrated the absence of any N1 or N2 epochs. No vertex spikes, K-complex or spindles were observed in any subject during the experimental recording; nor sleep onset rapid eye-movement (SOREM) periods were recorded. Similarly, the inspection of the video in controls did not show any sign of sleep during fMRI. Both patients and controls declared that they were awake during the experimental session.
3.1. Functional connectivity of lateral hypothalamus
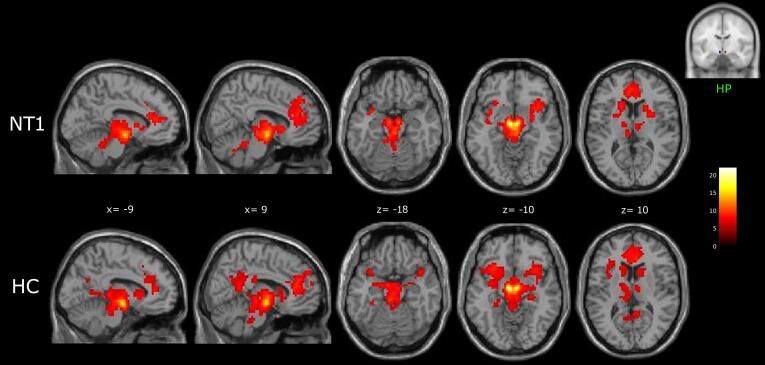
Seed-based analyses revealed that patients’ and control’s lateral hypothalamus was functionally connected with the thalamus, the basal ganglia (caudate nucleus, putamen, globus pallidus), the anterior insula (AI), the anterior cingulate cortex, the midbrain, and the cerebellum. In healthy participants, the lateral hypothalamus showed additional functional connection to the medial and inferior frontal gyrus (IFG), the precuneus/posterior cingulate cortex (PCC), the hippocampus, the parahippocampal gyrus and the amygdala (Fig. 1; supplementary Table 2).
Fig. 1.
Hypothalamus (HP) functional connectivity networks in NT1 patients and healthy controls (HC), shown in sagittal and axial slices (neurological convention) of a standard structural T1 weighted brain image (3dClustSim corrected: cluster size threshold k ≥ 31, corrected at α < 0.05). Color bar represents t-values.
3.1.1. Comparison of NT1 patients versus healthy controls
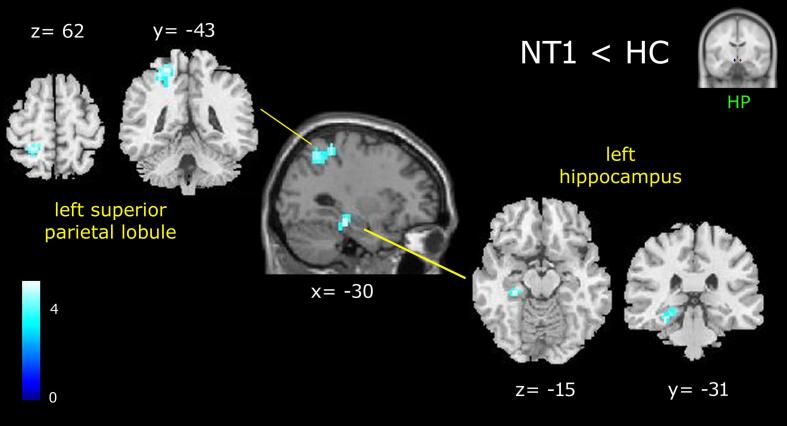
When compared to controls, NT1 showed a significant decrease in connectivity between the lateral hypothalamus and the superior parietal lobule, the hippocampus and the parahippocampal gyrus of the left hemisphere (Fig. 2; Table 2). No regions of increased connectivity in patients versus controls were detected.
Fig. 2.
Results of the NT1 < HC contrast, showing reduced functional connectivity of the patient’s lateral hypothalamus (cluster size threshold k ≥ 31, corrected at α < 0.05). Clusters are shown in a sagittal slice of a standard structural T1 weighted brain image and in coronal and axial slices of an anatomical template found in xjview toolbox (https://www.alivelearn.net/xjview). Color bar represents t-values.
Table 2.
Peak coordinates of reduced (NT1 < HC) functional connectivity of patient’s lateral hypothalamus.
| Cluster | Voxel level | MNI Coordinates | ||||
|---|---|---|---|---|---|---|
| K | Brain areas | BA | T | x | y | z |
| 96 | Superior parietal lobule | 5 | 5.02 | −24 | −43 | 62 |
| Precuneus | 7 | 4.76 | −27 | −58 | 50 | |
| 34 | Hippocampus | 4.85 | −30 | −28 | −14 | |
| Parahippocampal gyrus | 27 | 3.46 | −21 | −37 | −6 | |
BA, Brodmann area. Cluster size threshold k ≥ 31, corrected at α < 0.05.
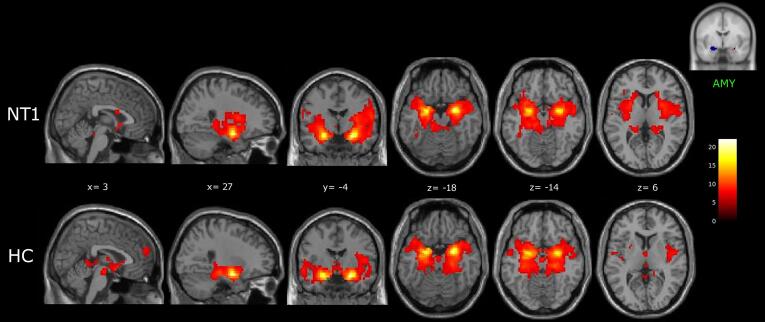
3.2. Functional connectivity of amygdala
The amygdala was functionally connected to several cortical and subcortical areas in both groups, including the hippocampus and the parahippocampal gyrus, the thalamus, the basal ganglia (putamen, globus pallidus, caudate nucleus), the anterior and posterior insula, the middle and superior temporal gyrus, and the cerebellum (Fig. 3; supplementary Table 3).
Fig. 3.
Amygdala (AMY) functional connectivity networks in NT1 patients and healthy controls (HC), shown in sagittal, coronal and axial slices (neurological convention) of a standard structural T1 weighted brain image (3dClustSim corrected: cluster size threshold k ≥ 25, corrected at α < 0.05). Color bar represents t-values.
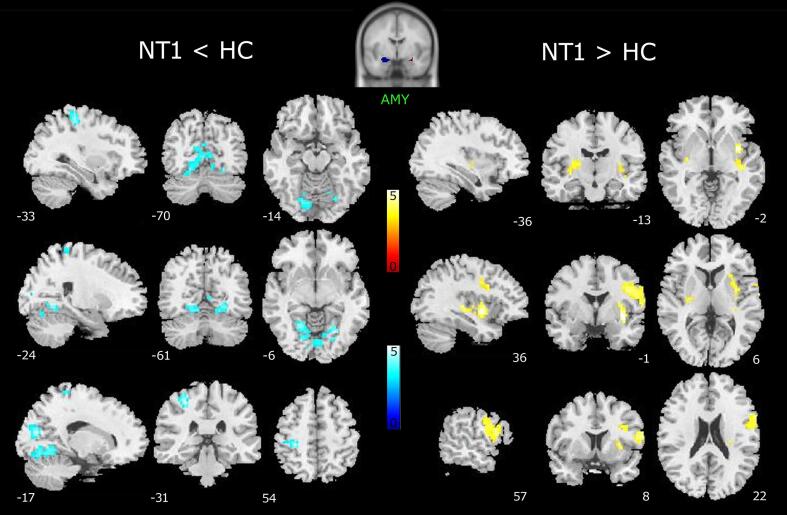
3.2.1. Comparisons between NT1 patients and healthy controls
Decreased functional connectivity was found between the patients’ amygdala and the post-central gyrus and several occipital regions (lingual gyrus and cuneus), whereas increased connectivity was detected between the seed and the inferior frontal gyrus (pars opercolaris), the anterior insula and the claustrum of the right hemisphere, the bilateral posterior insula, and the putamen (Fig. 4; Table 3).
Fig. 4.
Reduced (left) and increased (right) functional connectivity of patient’s amygdala (AMY; cluster size threshold k ≥ 25, corrected at α < 0.05). Results are superimposed on the ch2bet.nii template as provided by xjview toolbox. Color bars represent T-values.
Table 3.
Peak coordinates of reduced (NT1 < HC) and increased (NT1 > HC) functional connectivity of patient’s amygdala.
| Cluster |
Voxel level |
MNI Coordinates |
||||
|---|---|---|---|---|---|---|
| k | Brain areas | BA | T | x | y | z |
| NT1 < HC | ||||||
| 93 | Post-central gyrus | 3 | 5.54 | −30 | −31 | 54 |
| 409 | Lingual gyrus | 19 | 5.32 | 24 | −64 | −6 |
| Cuneus | 18 | 5.20 | −18 | −76 | 18 | |
| NT1 > HC | ||||||
| 99 | Anterior insula | 5.13 | 36 | −1 | −2 | |
| Claustrum | 4.60 | 33 | −7 | 10 | ||
| Putamen | 4.06 | 30 | 5 | 6 | ||
| 176 | Precentral gyrus | 4 | 4.87 | 45 | −4 | 30 |
| Inferior frontal gyrus (pars opercolaris) | 44 | 4.75 | 60 | 11 | 18 | |
| 27 | Putamen | 4.04 | –33 | −16 | 2 | |
| Posterior insula | 3.30 | −36 | −16 | −2 | ||
BA, Brodmann area. Cluster size threshold k ≥ 25, corrected at α < 0.05.
3.3. Correlation analyses
No significant correlation between functional connectivity of the seeds and measures of disease duration, CSF orexin level, mESS score was detected.
4. Discussion
As expected, we found alteration of both seeds functional connectivity during resting state in young patients with NT1. The possibility to study cerebral functioning in drug-naïve children and adolescents allowed to identify functional connectivity changes before any behavioral adaptation and possible drug-induced effects. When compared to healthy controls, NT1 patients demonstrated a complex change of functional connectivity mainly involving: (i) a decreased connectivity between the lateral hypothalamus and the superior and inferior parietal lobule, the hippocampus and the parahippocampal gyrus of the left hemisphere; (ii) decreased amygdala functional connectivity with the left post-central gyrus and the bilateral occipital areas, and (iii) increased amygdala connectivity with several regions of the right hemisphere (frontal operculum, claustrum), bilateral posterior insula and putamen.
4.1. Functional connectivity of hypothalamus
Since the loss of orexin-producing neurons has a central role in determining the main symptoms of NT1 (Liblau et al., 2015, Mignot et al., 2002), the lateral hypothalamus is one of the key brain regions of narcolepsy. Our results replicate previous seed-based studies in healthy volunteers (Kaufmann et al., 2006, Kullmann et al., 2014), which showed functional connectivity between the hypothalamus and several subcortical and cortical regions, such as the thalamus, the brainstem, the striatum, the orbitofrontal cortex, the cingulum, and temporal lobe regions.
The present work showed that in NT1 patients functional connectivity between the lateral hypothalamus and the hippocampus was reduced. Animal studies suggest that dense innervations is provided by hypothalamic orexin-containing neurons to the medial septum–diagonal band of Broca, which in turns sends cholinergic and GABAergic fibers to the hippocampus to controls the hippocampal theta rhythm and related learning and memory functions (Peyron et al., 1998, Wu et al., 2004, Wu et al., 2002). Moreover, orexin may enhance hippocampal neurogenesis improving spatial learning, memory abilities, and mood, whereas orexin deficiency results in learning and memory deficits, and depression (Arendt et al., 2013, Wayner et al., 2004, Yang et al., 2013, Zhao and Zhang, 2014).
Furthermore, the administration of modafinil, a wakefulness-promoting agent used for the management of daytime sleepiness in narcoleptic patients, brought to an increase of the regional metabolism in left hippocampus after two weeks of treatment (Kim et al., 2007), as well as increase theta rhythm in left temporal areas (Saletu et al., 2004). Therefore, we may infer that reduced functional connectivity between the lateral hypothalamus and the hippocampus may reflect a loss of orexinergic innervation. The central role of the hippocampus in narcolepsy is demonstrated also in previous neuroimaging studies showing hypoperfusion of brain regions involved in promoting wakefulness, including the hippocampus (Eun et al., 2005). Recently, our group demonstrated greater GM volume of the right hippocampus in NT1 adolescents when compared with controls using both VBM and shape analysis approach (Tondelli et al., 2018) suggesting a possible compensatory mechanism of the right hippocampus. On the other hand, structural neuroimaging data on NT1 adults patients are consistent with reduction of the hippocampal volume in narcolepsy (Joo et al., 2012, Kim et al., 2016, Nemcova et al., 2015) and showed a positive correlation with mean sleep and REM sleep latencies in MSLT (Joo et al., 2012).
Concerning the reduced connectivity between the hypothalamus and the left parietal cortex, it may reflect the attentional deficits previously reported in narcolepsy (Naumann et al., 2006). A recent study compared adult NT1 and NT2 patients showing specific alteration of the alerting network in orexin deficient patients (Filardi et al., 2017). The functioning of the alerting network is subserved by parietal and frontal areas and is modulated by the locus coeruleus noradrenergic system (Petersen and Posner, 2012), which is projected by the orexinergic system (Aston-Jones and Cohen, 2005). Our results are consistent with clinical observation that attention deficit/hyperactivity disorder (ADHD) is the most frequently described neurodevelopmental disorder in childhood narcolepsy (Lecendreux et al., 2015, Modestino and Winchester, 2013, Rocca et al., 2016). Unfortunately, we were not able to correlate our functional findings with attentive functioning since we did not measure attentive/cognitive abilities in our patients’ population.
4.2. Functional connectivity of amygdala
With regards to the functional connectivity of the amygdala, we found decreased connectivity with the post-central gyrus and occipital areas. Our results were consistent with recent resting-state studies, which showed altered topological properties of many brain nodes including the bilateral precentral and postcentral gyrus and right occipital regions in NT1 patients using Graph Theoretical Analysis (Xiao et al., 2020, 2019), and increased functional connectivity in the bilateral calcarine fissure within the visual network in adolescents with NT1 (Xiao et al., 2020). Changes in the excitability of intrinsic circuits in the motor cortex were found using transcranial magnetic stimulation study which demonstrated hypo-excitability of sensorimotor cortex during wakefulness in narcolepsy (Oliviero et al., 2005). Since the orexin-containing neurons project widely to the neocortex and excitatory orexin innervation of visual neurons has been described (Bayer et al., 2004), altered functional interaction between the amygdala and the sensorimotor and visual networks (Damoiseaux et al., 2006, De Luca et al., 2006) may be related to reduced orexin levels in narcolepsy.
On the contrary, increased functional connectivity was found with regions belonging to salience network (Menon and Uddin, 2010, Seeley et al., 2007), such as the right frontal operculum/anterior insula and the claustrum. Salience network refers to a cerebral circuit that directs attention towards the most behaviorally relevant stimuli (Uddin, 2015) and may be involved in the maintenance of tonic alertness (Sadaghiani et al., 2010). Alterations of this network were found also in previous rs-fMRI studies in both adults and adolescents with NT1 (Xiao et al., 2020, 2019). The interpretation of this increased functional connectivity between the amygdala and salience network regions is speculative. It could be either compensatory or due to orexin deficiency. Moreover, these alterations could also be relevant to explain some behavioral traits described in adolescents with NT1 such as hyperactivity.
Future studies may investigate the connectivity pattern of different amygdala subregions in narcolepsy, since a strong differentiation was found in both task-dependent and independent contexts in healthy subjects (Kerestes et al., 2017, Roy et al., 2009).
4.3. Limitations of the study
The present study has a main limitation: the absence of EEG recording during MRI in healthy volunteers, which is a critical tool to exclude sleep episodes. Nevertheless, in order to mitigate the possibility of falling asleep, all recordings were made after an afternoon nap. Additionally, video recordings were used to monitor any behavioral sign of sleep and, at the end of the recording session, the subjects were asked whether they slept during the recording. A further limit, which is intrinsic to all resting state protocols, is the impossibility to control the mental activity of the participants during the scanning session. It is recommended to give consistent instructions to mitigate this confounding effect, although its elimination is not possible (Duncan and Northoff, 2013).
5. Conclusions
The present seed-based fc-fMRI study suggests that the loss of orexin containing neurons in NT1 may cause abnormal connectivity between the hypothalamus and brain regions involved in sleep-wake regulation, such as the hippocampus. Moreover, functional connectivity of the amygdala, a limbic region which contains dense orexin fibers and receptors, seem to be influenced by the loss of dorsolateral hypothalamus orexin-containing neurons. Therefore, we can hypothesize that dysfunctional interactions between regions subserving the maintenance of wakefulness and emotional processing may contribute to the main symptom of narcolepsy characterized by orexin deficiency.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This study was supported by a grant “Dipartimenti di eccellenza 2018-2022”, MIUR, Italy, to the Department of Biomedical, Metabolic and Neural Sciences.
The funding source had no role in study design, data collection and analysis, interpretation of the findings or decision to publish.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102748.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Allen P.J., Josephs O., Turner R. A method for removing imaging artifact from continuous EEG recorded during functional MRI. Neuroimage. 2000;12(2):230–239. doi: 10.1006/nimg.2000.0599. [DOI] [PubMed] [Google Scholar]
- Allen P.J., Polizzi G., Krakow K., Fish D.R., Lemieux L. Identification of EEG events in the MR scanner: The problem of pulse artifact and a method for its subtraction. Neuroimage. 1998;8(3):229–239. doi: 10.1006/nimg.1998.0361. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . Darien, IL: American Academy of Sleep Medicine; 2014. International Classification of Sleep Disorders. 3rd ed. ICSD-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arendt D.H., Ronan P.J., Oliver K.D., Callahan L.B., Summers T.R., Summers C.H. Depressive behavior and activation of the orexin/hypocretin system. Behav. Neurosci. 2013;127:86–94. doi: 10.1037/a0031442. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G., Cohen J.D. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Annu. Rev. Neurosci. 2005;28(1):403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baroncini M., Jissendi P., Balland E., Besson P., Pruvo J.-P., Francke J.-P., Dewailly D., Blond S., Prevot V. MRI atlas of the human hypothalamus. Neuroimage. 2012;59(1):168–180. doi: 10.1016/j.neuroimage.2011.07.013. [DOI] [PubMed] [Google Scholar]
- Bassetti C.L.A., Adamantidis A., Burdakov D., Han F., Gay S., Kallweit U., Khatami R., Koning F., Kornum B.R., Lammers G.J., Liblau R.S., Luppi P.H., Mayer G., Pollmächer T., Sakurai T., Sallusto F., Scammell T.E., Tafti M., Dauvilliers Y. Narcolepsy — clinical spectrum, aetiopathophysiology, diagnosis and treatment. Nat. Rev. Neurol. 2019;15(9):519–539. doi: 10.1038/s41582-019-0226-9. [DOI] [PubMed] [Google Scholar]
- Bayer L., Serafin M., Eggermann E., Saint-Mleux B., Machard D., Jones B.E., Mühlethaler M. Exclusive postsynaptic action of hypocretin-orexin on sublayer 6b cortical neurons. J. Neurosci. 2004;24:6760–6764. doi: 10.1523/JNEUROSCI.1783-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell A.J., Sejnowski T.J. An Information-Maximization Approach to Blind Separation and Blind Deconvolution. Neural Comput. 1995;7(6):1129–1159. doi: 10.1162/neco.1995.7.6.1129. [DOI] [PubMed] [Google Scholar]
- Bisetti A., Cvetkovic V., Serafin M., Bayer L., Machard D., Jones B.E., Mühlethaler M. Excitatory action of hypocretin/orexin on neurons of the central medial amygdala. Neuroscience. 2006;142(4):999–1004. doi: 10.1016/j.neuroscience:2006.07.018. [DOI] [PubMed] [Google Scholar]
- Brabec J., Rulseh A., Hořínek D., Pala A., Guerreiro H., Bušková J., Petrovický P., Němcová V., Krásenský J., Seidl Z., Nimsky C., Šonka K. Volume of the amygdala is reduced in patients with narcolepsy - a structural MRI study. Act. Nerv. Super. Rediviva. 2011;53:177–181. [PubMed] [Google Scholar]
- Brenneis C., Brandauer E., Frauscher B., Schocke M., Trieb T., Poewe W., Hogl B. Voxel-based morphometry in narcolepsy. Sleep Med. 2005;6(6):531–536. doi: 10.1016/j.sleep.2005.03.015. [DOI] [PubMed] [Google Scholar]
- Bušková J., Vaneckova M., Sonka K., Seidl Z., Nevsimalova S. Reduced hypothalamic gray matter in narcolepsy with cataplexy. Neuroendocrinol. Lett. 2006;27:769–772. [PubMed] [Google Scholar]
- Chaudhary U.J., Kokkinos V., Carmichael D.W., Rodionov R., Gasston D., Duncan J.S., Lemieux L. Implementation and evaluation of simultaneous video-electroencephalography and functional magnetic resonance imaging. Magn. Reson. Imaging. 2010;28(8):1192–1199. doi: 10.1016/j.mri.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Contreras-Rodríguez O., Vilar-López R., Andrews Z.B., Navas J.F., Soriano-Mas C., Verdejo-García A. Altered cross-talk between the hypothalamus and non-homeostatic regions linked to obesity and difficulty to lose weight. Sci. Rep. 2017;7:1–9. doi: 10.1038/s41598-017-09874-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux J.S., Rombouts S.A.R.B., Barkhof F., Scheltens P., Stam C.J., Smith S.M., Beckmann C.F. Consistent resting-state networks. Proc. Natl. Acad. Sci. 2006;103:13848–13853. doi: 10.1073/pnas.0601417103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y., Montplaisir J., Molinari N., Carlander B., Ondze B., Besset A., Billiard M. Age at onset of narcolepsy in two large populations of patients in France and Quebec. Neurology. 2001;57(11):2029–2033. doi: 10.1212/wnl.57.11.2029. [DOI] [PubMed] [Google Scholar]
- De Luca M., Beckmann C.F., De Stefano N., Matthews P.M., Smith S.M. fMRI resting state networks define distinct modes of long-distance interactions in the human brain. Neuroimage. 2006;29(4):1359–1367. doi: 10.1016/j.neuroimage.2005.08.035. [DOI] [PubMed] [Google Scholar]
- Draganski B., Geisler P., Hajak G., Schuierer G., Bogdahn U., Winkler J., May A. Hypothalamic gray matter changes in narcoleptic patients. Nat. Med. 2002;8(11):1186–1188. doi: 10.1038/nm1102-1186. [DOI] [PubMed] [Google Scholar]
- Drissi N.M., Szakács A., Witt S.T., Wretman A., Ulander M., Ståhlbrandt H., Darin N., Hallböök T., Landtblom A.M., Engström M. Altered Brain Microstate Dynamics in Adolescents with Narcolepsy. Front. Hum. Neurosci. 2016;10:1–16. doi: 10.3389/fnhum.2016.00369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan N., Northoff G. Overview of potential procedural and participant-related confounds for neuroimaging of the resting state. J. Psychiatry Neurosci. 2013;38(2):84–96. doi: 10.1503/jpn10.1503/jpn.120059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström M., Hallböök T., Szakacs A., Karlsson T., Landtblom A.M. Functional magnetic resonance imaging in narcolepsy and the Kleine-Levin syndrome. Front. Neurol. 2014;5:1–6. doi: 10.3389/fneur.2014.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun Y.J., Hong S.B., Woo S.T., Jee H.K., Sun J.H., Yong W.C., Chang H.Y., Sung I.L, Mann H.L., Kyung H.L., Kim M.H., Byung T.K., Kim L. Cerebral perfusion abnormality in narcolepsy with cataplexy. Neuroimage. 2005;28:410–416. doi: 10.1016/j.neuroimage.2005.06.019. [DOI] [PubMed] [Google Scholar]
- Filardi M., Pizza F., Tonetti L., Antelmi E., Natale V., Plazzi G., Penzel T. Attention impairments and ADHD symptoms in adult narcoleptic patients with and without hypocretin deficiency. PLoS One. 2017;12(8):e0182085. doi: 10.1371/journal.pone.0182085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulyani S., Wu M.-F., Nienhuis R., John J., Siegel J.M. Cataplexy-related neurons in the amygdala of the narcoleptic dog. Neuroscience. 2002;112(2):355–365. doi: 10.1016/S0306-4522(02)00089-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong S.B., Tae W.S., Joo E.Y. Cerebral perfusion changes during cataplexy in narcolepsy patients. Neurology. 2006;66(11):1747–1749. doi: 10.1212/01.wnl.0000218205.72668.ab. [DOI] [PubMed] [Google Scholar]
- Joo E.Y., Kim S.H., Kim S.-T., Hong S.B. Hippocampal volume and memory in narcoleptics with cataplexy. Sleep Med. 2012;13(4):396–401. doi: 10.1016/j.sleep.2011.09.017. [DOI] [PubMed] [Google Scholar]
- Joo E.Y., Tae W.S., Kim S.T., Hong S.B. Gray matter concentration abnormality in brains of narcolepsy patients. Korean J. Radiol. 2009;10:552–558. doi: 10.3348/kjr.2009.10.6.552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann C., Schuld A., Pollmächer T., Auer D.P. Reduced cortical gray matter in narcolepsy: Preliminary findings with voxel-based morphometry. Neurology. 2002;58(12):1852–1855. doi: 10.1212/wnl.58.12.1852. [DOI] [PubMed] [Google Scholar]
- Kaufmann C., Wehrle R., Wetter T.C., Holsboer F., Auer D.P., Pollmächer T., Czisch M. Brain activation and hypothalamic functional connectivity during human non-rapid eye movement sleep: An EEG/fMRI study. Brain. 2006;129:655–667. doi: 10.1093/brain/awh686. [DOI] [PubMed] [Google Scholar]
- Kerestes R., Chase H.W., Phillips M.L., Ladouceur C.D., Eickhoff S.B. Multimodal evaluation of the amygdala’s functional connectivity. Neuroimage. 2017;148:219–229. doi: 10.1016/j.neuroimage.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KHATAMI RAMIN, BIRKMANN STEFFEN, BASSETTI CLAUDIO.L. Amygdala dysfunction in narcolepsy-cataplexy. J. Sleep Res. 2007;16(2):226–229. doi: 10.1111/jsr.2007.16.issue-210.1111/j.1365-2869.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- Kim H., Suh S., Joo E.Y., Hong S.B. Morphological alterations in amygdalo-hippocampal substructures in narcolepsy patients with cataplexy. Brain Imaging Behav. 2016;10(4):984–994. doi: 10.1007/s11682-015-9450-0. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Lyoo I.K., Lee Y.S., Lee J.Y., Yoon S.J., Kim J.E., Kim J.H., Hong S.J., Jeong D.U. Gray matter deficits in young adults with narcolepsy. Acta Neurol. Scand. 2009;119:61–67. doi: 10.1111/j.1600-0404.2008.01063.x. [DOI] [PubMed] [Google Scholar]
- Kim Y.K., Yoon I.-Y., Shin Y.-K., Cho S.S., Kim S.E. Modafinil-induced hippocampal activation in narcolepsy. Neurosci. Lett. 2007;422(2):91–96. doi: 10.1016/j.neulet.2007.04.085. [DOI] [PubMed] [Google Scholar]
- Kullmann S., Heni M., Linder K., Zipfel S., Häring H.-U., Veit R., Fritsche A., Preissl H. Resting-state functional connectivity of the human hypothalamus. Hum. Brain Mapp. 2014;35(12):6088–6096. doi: 10.1002/hbm.v35.1210.1002/hbm.22607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecendreux M., Lavault S., Lopez R., Inocente C.O., Konofal E., Cortese S., Franco P., Arnulf I., Dauvilliers Y. Attention-deficit/hyperactivity disorder (ADHD) symptoms in pediatric narcolepsy: A cross-sectional study. Sleep. 2015;38:1285–1295. doi: 10.5665/sleep.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J.E. Emotion Circuits in the Brain. Annu. Rev. Neurosci. 2000;23(1):155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Liblau R.S., Vassalli A., Seifinejad A., Tafti M. Hypocretin (orexin) biology and the pathophysiology of narcolepsy with cataplexy. Lancet Neurol. 2015;14(3):318–328. doi: 10.1016/S1474-4422(14)70218-2. [DOI] [PubMed] [Google Scholar]
- Makeig S., Debener S., Onton J., Delorme A. Mining event-related brain dynamics. Trends Cogn. Sci. 2004;8(5):204–210. doi: 10.1016/j.tics.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Maski K., Steinhart E., Williams D., Scammell T., Flygare J., McCleary K., Gow M. Listening to the patient voice in narcolepsy: Diagnostic delay, disease burden, and treatment efficacy. J. Clin. Sleep Med. 2017;13(03):419–425. doi: 10.5664/jcsm.6494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletti S., Vaudano A.E., Pizza F., Ruggieri A., Vandi S., Teggi A., Franceschini C., Benuzzi F., Nichelli P.F., Plazzi G. The Brain Correlates of Laugh and Cataplexy in Childhood Narcolepsy. J. Neurosci. 2015;35(33):11583–11594. doi: 10.1523/JNEUROSCI.0840-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V., Uddin L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010;214(5-6):655–667. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignot E., Lammers G.J., Ripley B., Okun M., Nevsimalova S., Overeem S., Vankova J., Black J., Harsh J., Bassetti C., Schrader H., Nishino S. The Role of Cerebrospinal Fluid Hypocretin Measurement in the Diagnosis of Narcolepsy and Other Hypersomnias Emmanuel Mignot. Arch. Neurol. 2002;59(10):1553. doi: 10.1001/archneur.59.10.1553. [DOI] [PubMed] [Google Scholar]
- Modestino E.J., Winchester J. Survey of Childhood ADHD Symptomatology Among Adult Narcoleptics. J. Atten. Disord. 2013;17:574–582. doi: 10.1177/1087054713480033. [DOI] [PubMed] [Google Scholar]
- Naumann A., Bellebaum C., Daum I. Cognitive deficits in narcolepsy. J. Sleep Res. 2006;15(3):329–338. doi: 10.1111/jsr.2006.15.issue-310.1111/j.1365-2869.2006.00533.x. [DOI] [PubMed] [Google Scholar]
- Nemcova V., Krásenský J., Kemlink D., Petrovicky P., Vaneckova M., Seidl Z., Rulseh A., Buskova J., Susta M., Sonka K. Hippocampal but not amygdalarvolume loss in narcolepsy with cataplexy. Neuro Endocrinol. Lett. 2015;36:682–688. [PubMed] [Google Scholar]
- Ohayon M.M., Priest R.G., Zulley J., Smirne S., Paiva T. Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology. 2002;58(12):1826–1833. doi: 10.1212/wnl.58.12.1826. [DOI] [PubMed] [Google Scholar]
- Oliviero A., Della Marca G., Tonali P.A., Pilato F., Saturno E., Dileone M., Versace V., Mennuni G., Di Lazzaro V. Functional involvement of cerebral cortex in human narcolepsy. J. Neurol. 2005;252:56–61. doi: 10.1007/s00415-005-0598-1. [DOI] [PubMed] [Google Scholar]
- Overeem S., Steens S.C.A., Good C.D., Ferrari M.D., Mignot E., Richard S.J. Voxel-Based Morphometry in Hypocretin-Deficient Narcolepsy. Sleep. 2003 doi: 10.1093/sleep/26.1.44. [DOI] [PubMed] [Google Scholar]
- Petersen S.E., Posner M.I. The attention system of the human brain: 20 years after. Annu. Rev. Neurosci. 2012;35(1):73–89. doi: 10.1146/annurev-neuro-062111-150525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron C., Faraco J., Rogers W., Ripley B., Overeem S., Charnay Y., Nevsimalova S., Aldrich M., Reynolds D., Albin R., Li R., Hungs M., Pedrazzoli M., Padigaru M., Kucherlapati M., Fan J., Maki R., Lammers G.J., Bouras C., Kucherlapati R., Nishino S., Mignot E. A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat. Med. 2000;6(9):991–997. doi: 10.1038/79690. [DOI] [PubMed] [Google Scholar]
- Peyron C., Tighe D.K., van den Pol A.N., de Lecea L., Heller H.C., Sutcliffe J.G., Kilduff T.S. Neurons Containing Hypocretin (Orexin) Project to Multip. Neuronal Syst. 1998;18(23):9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizza F., Franceschini C., Peltola H., Vandi S., Finotti E., Ingravallo F., Nobili L., Bruni O., Lin L., Edwards M.J., Partinen M., Dauvilliers Y., Mignot E., Bhatia K.P., Plazzi G. Clinical and polysomnographic course of childhood narcolepsy with cataplexy. Brain. 2013;136:3787–3795. doi: 10.1093/brain/awt277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzi G., Clawges H.M., Owens J.A. Clinical Characteristics and Burden of Illness in Pediatric Patients with Narcolepsy. Pediatr. Neurol. 2018;85:21–32. doi: 10.1016/j.pediatrneurol.2018.06.008. [DOI] [PubMed] [Google Scholar]
- Plazzi G., Pizza F., Palaia V., Franceschini C., Poli F., Moghadam K.K., Cortelli P., Nobili L., Bruni O., Dauvilliers Y., Lin L., Edwards M.J., Mignot E., Bhatia K.P. Complex movement disorders at disease onset in childhood narcolepsy with cataplexy. Brain. 2011;134(12):3480–3492. doi: 10.1093/brain/awr244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D.A., Raichle M.E. Searching for a baseline: Functional imaging and the resting human brain. Nat. Rev. Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Raichle M.E., MacLeod A.M., Snyder A.Z., Powers W.J., Gusnard D.A., Shulman G.L. A default mode of brain function. PNAS. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiss A.L., Hoeft F., Tenforde A.S., Chen W., Mobbs D., Mignot E.J., Greene E. Anomalous hypothalamic responses to humor in cataplexy. PLoS One. 2008;3(5):e2225. doi: 10.1371/journal.pone.0002225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie C., Okuro M., Kanbayashi T. Hypocretin Ligand Deficiency in Narcolepsy : Recent Basic and Clinical Insights. Curr. Neurol. Neurosci. Rep. 2010;10:180–189. doi: 10.1007/s11910-010-0100-z. [DOI] [PubMed] [Google Scholar]
- Rocca F.L., Finotti E., Pizza F., Ingravallo F., Gatta M., Bruni O., Plazzi G. Psychosocial profile and quality of life in children with type 1 narcolepsy: A case-control study. Sleep. 2016;39:1389–1398. doi: 10.5665/sleep.5970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocca F., Pizza F., Ricci E., Plazzi G. Narcolepsy during Childhood: An Update. Neuropediatrics. 2015;46(03):181–198. doi: 10.1055/s-0035-1550152. [DOI] [PubMed] [Google Scholar]
- Roy A.K., Shehzad Z., Margulies D.S., Kelly A.M.C., Uddin L.Q., Gotimer K., Biswal B.B., Castellanos F.X., Milham M.P. Functional connectivity of the human amygdala using resting state fMRI. Neuroimage. 2009;45(2):614–626. doi: 10.1016/j.neuroimage.2008.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggieri A., Vaudano A.E., Benuzzi F., Serafini M., Gessaroli G., Farinelli V., Nichelli P.F., Meletti S. Mapping (and modeling) physiological movements during EEG-fMRI recordings: The added value of the video acquired simultaneously. J. Neurosci. Methods. 2015;239:223–237. doi: 10.1016/j.jneumeth.2014.10.005. [DOI] [PubMed] [Google Scholar]
- Sadaghiani S., Scheeringa R., Lehongre K., Morillon B., Giraud A.-L., Kleinschmidt A. Intrinsic connectivity networks, alpha oscillations, and tonic alertness: A simultaneous electroencephalography/functional magnetic resonance imaging study. J. Neurosci. 2010;30(30):10243–10250. doi: 10.1523/JNEUROSCI.1004-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T., Nagata R., Yamanaka A., Kawamura H., Tsujino N., Muraki Y.o., Kageyama H., Kunita S., Takahashi S., Goto K., Koyama Y., Shioda S., Yanagisawa M. Input of orexin/hypocretin neurons revealed by a genetically encoded tracer in mice. Neuron. 2005;46(2):297–308. doi: 10.1016/j.neuron.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Saletu M., Anderer P., Saletu-Zyhlarz G.M., Mandl M., Arnold O., Zeitlhofer J., Saletu B. EEG-tomographic studies with LORETA on vigilance differences between narcolepsy patients and controls and subsequent double-blind, placebo-controlled studies with modafinil. J. Neurol. 2004;251(11):1354–1363. doi: 10.1007/s00415-004-0543-8. [DOI] [PubMed] [Google Scholar]
- Saper C.B., Scammell T.E., Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi: 10.1038/nature04284. [DOI] [PubMed] [Google Scholar]
- Scherfler C., Frauscher B., Schocke M., Nocker M., Gschliesser V., Ehrmann L., Niederreiter M., Esterhammer R., Seppi K., Brandauer E., Poewe W., Högl B. White and gray matter abnormalities in narcolepsy with cataplexy. Sleep. 2012;35:345–351. doi: 10.5665/sleep.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz S., Ponz A., Poryazova R., Werth E., Boesiger P., Khatami R., Bassetti C.L. Abnormal activity in hypothalamus and amygdala during humour processing in human narcolepsy with cataplexy. Brain. 2008;131(2):514–522. doi: 10.1093/brain/awm292. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27(9):2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel J.M., Nienhuis R., Gulyani S., Ouyang S., Wu M.F., Mignot E., Switzer R.C., McMurry G., Cornford M. Neuronal degeneration in canine narcolepsy. J. Neurosci. 1999;19(1):248–257. doi: 10.1523/JNEUROSCI.19-01-00248.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silber M.H., Krahn L.E., Olson E.J., Pankratz V.S. The epidemiology of narcolepsy in Olmsted County, Minnesota: A population-based study. Sleep. 2002;25:197–202. doi: 10.1093/sleep/25.2.197. [DOI] [PubMed] [Google Scholar]
- Song X.-W., Dong Z.-Y., Long X.-Y., Li S.-F., Zuo X.-N., Zhu C.-Z., He Y., Yan C.-G., Zang Y.-F., Harrison B.J. REST: A Toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6(9):e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talami F., Vaudano A.E., Meletti S. Motor and Limbic System Contribution to Emotional Laughter across the Lifespan. Cereb. Cortex. 2020;30:3381–3391. doi: 10.1093/cercor/bhz316. [DOI] [PubMed] [Google Scholar]
- Thannickal T.C., Moore R.Y., Nienhuis R., Ramanathan L., Gulyani S., Aldrich M., Cornford M., Siegel J.M., Arbor A. Reduced Number of Hypocretin Neurons in Human Narcolepsy. Neuron. 2000;27:469–474. doi: 10.1016/S0896-6273(00)00058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal T.C., Siegel J.M., Nienhuis R., Moore R.Y. Pattern of Hypocretin (Orexin) Soma and Axon Loss, and Gliosis, in Human Narcolepsy. Brain Pathol. 2003;13(3):340–351. doi: 10.1111/j.1750-3639.2003.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondelli, M., Pizza, F., Vaudano, A.E., Plazzi, G., Meletti, S., 2018. Cortical and Subcortical Brain Changes in Children and Adolescents With Narcolepsy Type 1 1–7. Doi: 10.1093/sleep/zsx192. [DOI] [PubMed]
- Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015;16(1):55–61. doi: 10.1038/nrn3857. [DOI] [PubMed] [Google Scholar]
- Vaudano A.E., Pizza F., Talami F., Plazzi G., Meletti S. The neuronal network of laughing in young patients with untreated narcolepsy. Neurology. 2019;92(5):e504–e515. doi: 10.1212/WNL.0000000000006853. [DOI] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: Neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wada M., Mimura M., Noda Y., Takasu S., Plitman E. Neuroimaging correlates of narcolepsy with cataplexy : A systematic review. Neurosci. Res. 2019;142:16–29. doi: 10.1016/j.neures.2018.03.005. [DOI] [PubMed] [Google Scholar]
- Wayner, M.J., Armstrong, D.L., Phelix, C.F., Oomura, Y., 2004. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 25, 991–996. Doi: 10.1016/j.peptides.2004.03.018. [DOI] [PubMed]
- Wu M., Zaborszky L., Hajszan T., Van Den Pol A.N., Alreja M. Hypocretin/Orexin Innervation and Excitation of Identified Septohippocampal Cholinergic Neurons. J. Neurosci. 2004;24:3527–3536. doi: 10.1523/JNEUROSCI.5364-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Zhang Z., Leranth C., Xu C., Van Den Pol A.N., Alreja M. Hypocretin Increases Impulse Flow in the Septohippocampal GABAergic Pathway : Implications for Arousal via a Mechanism of Hippocampal Disinhibition. J. Neurosci. 2002;22:7754–7765. doi: 10.1523/JNEUROSCI.22-17-07754.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao F., Karen S., Chao L., Dianjiang Z., Jun Z., Fang H. Resting-state brain network topological properties and the correlation with neuropsychological assessment in adolescent narcolepsy. Sleep. 2020;43:1–9. doi: 10.1093/sleep/zsaa018. [DOI] [PubMed] [Google Scholar]
- Xiao F., Lu C., Zhao D., Zou Q., Xu L., Li J., Zhang J., Han F. Independent Component Analysis and Graph Theoretical Analysis in Patients with Narcolepsy. Neurosci. Bull. 2019;35(4):743–755. doi: 10.1007/s12264-018-0307-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L., Zou B., Xiong X., Pascual C., Xie J., Malik A., Xie J., Sakurai T., Xie X. Hypocretin/orexin neurons contribute to hippocampus-dependent social memory and synaptic plasticity in mice. J. Neurosci. 2013;33(12):5275–5284. doi: 10.1523/JNEUROSCI.3200-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K., McCormack S., España R.A., Crocker A., Scammell T.E. Afferents to the orexin neurons of the rat brain. J. Comp. Neurol. 2006;494(5):845–861. doi: 10.1002/(ISSN)1096-9861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald D.H. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res. Rev. 2003;41(1):88–123. doi: 10.1016/S0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zhao, X., Zhang, R.x., Tang, S., Ren, Y.y., Yang, W.x., Liu, X.m., Tang, J.y. (2014). Orexin-A-induced ERK 1/2 activation reverses impaired spatial learning and memory in pentylenetetrazol-kindled rats via OX1R-mediated hippocampal neurogenesis. Peptides 54, 140–147. Doi: 10.1016/j.peptides. 2013.11.019. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.