Abstract
Background
The roll-out of COVID-19 vaccines is a multi-faceted challenge whose performance depends on pace of vaccination, vaccine characteristics and heterogeneities in individual risks.
Methods
We developed a mathematical model accounting for the risk of severe disease by age and comorbidity, and transmission dynamics. We compared vaccine prioritisation strategies in the early roll-out stage and quantified the extent to which measures could be relaxed as a function of the vaccine coverage achieved in France.
Findings
Prioritizing at-risk individuals reduces morbi-mortality the most if vaccines only reduce severity, but is of less importance if vaccines also substantially reduce infectivity or susceptibility. Age is the most important factor to consider for prioritization; additionally accounting for comorbidities increases the performance of the campaign in a context of scarce resources. Vaccinating 90% of ≥65 y.o. and 70% of 18–64 y.o. before autumn 2021 with a vaccine that reduces severity by 90% and susceptibility by 80%, we find that control measures reducing transmission rates by 15–27% should be maintained to remain below 1000 daily hospital admissions in France with a highly transmissible variant (basic reproduction number R0 = 4). Assuming 90% of ≥65 y.o. are vaccinated, full relaxation of control measures might be achieved with a vaccine coverage of 89–100% in 18–64 y.o or 60–69% of 0–64 y.o.
Interpretation
Age and comorbidity-based vaccine prioritization strategies could reduce the burden of the disease. Very high vaccination coverage may be required to completely relax control measures. Vaccination of children, if possible, could lower coverage targets necessary to achieve this objective.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Prioritisation, Relaxation of measures, Comorbidities
Research in context.
Evidence before this study
The impact of COVID-19 vaccination strategies as well as the extent to which control measures might be relaxed when a large proportion of the population will be vaccinated are affected by the complex interplay between vaccine characteristics (especially their efficacies and their potential impact on transmission), the groups that will receive the vaccines within the population, the epidemic dynamics and vaccine roll-out constraints. To identify analyses aiming at evaluating the impact of COVID-19 vaccination strategies, we conducted a search of the literature on March 23rd, 2021 using the query “ (COVID-19 OR SARS-CoV-2) AND vaccin* AND (priorit* OR optim* OR allocat* OR eval*) AND model* ” which returned 454 results on PubMed. Among these, we identified 26 analyses assessing the effect of vaccination campaigns against COVID-19, none of which accounted for the interaction between age and the presence of underlying medical conditions in prioritization strategies.
Added value of this study
We developed a modelling framework to investigate how heterogeneities in individual risks, vaccine characteristics and vaccine coverages affect the impact of the vaccination campaign and the future dynamics of the COVID-19 pandemic. From this framework, we are also able to derive estimates of the proportion of the population that should be vaccinated in both high-risk and low-risk groups to allow a return to a normal life, accounting for the proportion having naturally acquired immunity, the characteristics of the vaccines being rolled-out and the transmissibility of the dominant variant, providing crucial insights to guide medium-term healthcare planning.
Implications of all the available evidence
In a context of scarce resources, accounting for both age and comorbidities can increase the performance of the vaccination campaign, compared to a strategy solely based on age. With the spread of more transmissible variants such as B.1.1.7 in France, a complete relaxation of measures would require very high levels of vaccine coverage in both high and low risk groups, that are higher than vaccination intent currently measured in the French population. Vaccination of children, if possible, might facilitate return to a normal life.
Alt-text: Unlabelled box
1. Introduction
Over the last year, the COVID-19 pandemic has generated large numbers of hospitalisations and deaths. In addition, the drastic control measures implemented to contain disease spread have caused major social and economic disruptions. In most locations, immunity conferred by natural infection remains much lower than the one required for herd immunity [1]. In this context, the progressive roll-out of safe and effective COVID-19 vaccines provides a crucial pharmaceutical tool to exit the current crisis. It comes however with a number of challenges associated with availability, urgency and finally progressive phasing out of epidemic time.
It is important to further clarify how vaccines should be distributed when the number of vaccine doses is limited and one aims to minimize morbi-mortality and the stress on the healthcare system. This is crucial for countries that are still at an early stage of their campaign and for the many countries where vaccination has not started yet. For vaccines reducing the severity of the disease, modelling studies have shown that vaccination strategies prioritized towards older individuals may substantially reduce the number of COVID-19 deaths [2], [3], [4], [5] owing to the strong age dependence for severe infections [6,7]. It may also be relevant to consider comorbidities like obesity or diabetes in the prioritization scheme, as these are independent risk factors for mortality with an age-dependent effect [8,9]. As the extent of vaccine protection on the risk of infection is increasingly well characterized [10,11], a renewed examination of the various prioritisation strategies combining age and comorbidities will be required [12].
To guide medium term strategic planning, it is essential to anticipate how vaccination might impact the course of the pandemic in autumn 2021. In a context where healthcare systems have been on the brink of saturation several times and economies have been devastated by restrictive control measures, we argue that vaccination could be considered successful if it allowed relaxing control measures while keeping COVID-19 stress on the healthcare system at a manageable level. It is therefore important to determine what combination of control measures and vaccine coverage in different age groups might result in a small enough peak in COVID-19 hospital admissions after relaxation. Furthermore, examining how the vaccination of children might facilitate the control of the epidemic in autumn 2021 would be helpful in case vaccines were recommended in this age group.
Here, we developed a mathematical model to understand how vaccine characteristics, levels of vaccine coverage and heterogeneities in individual risks may affect the impact of vaccination in the short and medium term, using France as a case study. The model is used both to investigate the question of the relaxation of control measures in the autumn and that of prioritisation at the early stage of the campaign.
2. Methods
2.1. Data sources
We work with hospitalization data from the SI-VIC database, a national surveillance system describing the trajectories of COVID-19 patients in public and private French hospitals. The prevalence of comorbidities in different age groups is extracted from the Esteban survey [13], a cross-sectional national health study, carried out in France between 2014 and 2016, on a representative sample of the French adult population (see Supplement).
2.2. Epidemiological model and scenarios
We adapt an age-structured compartmental model [6] describing the spread of SARS-CoV-2 in the general population in metropolitan France (around 65 million inhabitants) [6] (see Supplement) to capture the impact of comorbidities on the age-stratified risk of developing severe COVID-19. It accounts for the interaction between age and comorbidity on the risk of hospitalisation, as estimated by the Centers for Disease Control and Prevention based on the COVID-NET surveillance network data [9] (see Supplement).
Let denote the event : ‘Having k comorbidities’, . Let denote the relative risk of hospitalisation given infection among individuals of age group a with k comorbidities. Let denote the mean probability of hospitalisation given infection among individuals of age group a and denote the prevalence of in age group a. We derive the probability of hospitalisation given infection among individuals of age group a by levels of comorbidity using the following expression:
The same type of adjustment is applied on the probabilities of ICU admission and death following hospitalisation using relative risks (that were not stratified by age) estimated from the same US-based surveillance network [14].
We assume that children aged 0–9 years old (y.o.) and those aged 10–17 y.o. are respectively 50% and 25% less susceptible to infection than adults [15,16].
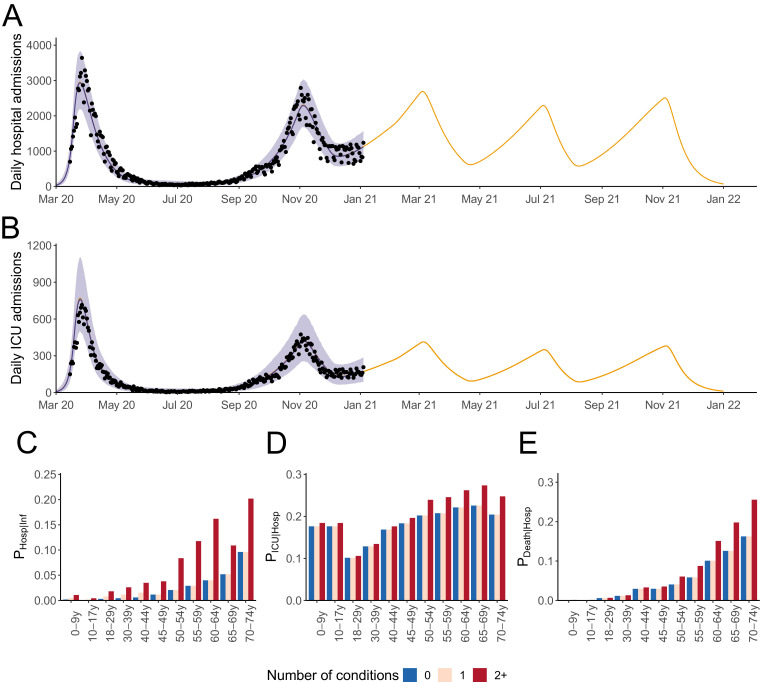
In our baseline scenario, we assume that we will observe in 2021 a series of epidemic waves with the same magnitude as the one in autumn 2020 (501,000 COVID-19 hospitalisations and 102,000 hospital deaths during 2021 in the absence of vaccination) (Fig. 1). In a sensitivity analysis, we assume that we will observe in 2021 a series of epidemic waves with a smaller magnitude than the one in autumn 2020 (330,000 hospitalisations and 66,000 hospital deaths during 2021 in the absence of vaccination) (Fig. S1). We do not explicitly account for seasonality but these epidemic waves are constructed to reflect the interplay between implemented measures and the impact of climate on transmission.
Fig. 1.
Daily (A) ICU and (B) hospital admissions in metropolitan France in our baseline epidemiological scenario in the absence of vaccination. (C) Probability of hospitalisation given infection, (D) probability of ICU admission given hospitalisation and (E) death given hospitalisation stratified by age group and number of conditions. The shaded areas in (A-B) correspond to 95% credible intervals.
2.3. Accounting for the roll-out of vaccines
2.3.1. Nature of vaccine protection
The first clinical trials suggested that vaccines were around 90% efficacious against severe outcomes (severity) [17], [18], [19]. However, their impact on the risk of transmission (infectivity) or of infection (susceptibility) remained uncertain for several months. Recent data from vaccine field studies suggest that vaccines could reduce susceptibility by around 80% [10,11,20,21]. To investigate how changes in the understanding of vaccine characteristics can impact the assessment of vaccine strategies, we explored three different scenarios regarding the efficacy of vaccines: (i) a vaccine reducing the severity by 90%, without any impact on infectivity or susceptibility, which was a conservative scenario in the absence of data about the effect of vaccines on transmission (vaccine Severity), (ii) a vaccine reducing the severity by 90% and the infectivity by 30%, that seemed a reasonable scenario in the absence of data regarding transmission (vaccine Transmission), (iii) a vaccine reducing the severity by 90% and the susceptibility by 80% (vaccine Susceptibility, see Supplement), the scenario that we now favor given latest data. This latter scenario implies a substantial impact on transmission as vaccinated individuals have a decreased risk of infection, and are thus less likely to transmit, even though their infectivity is the same as that of unvaccinated individuals. We assume that vaccine efficacy lasts until the end of the study period.
2.3.2. Vaccination campaign characteristics
We consider a two-doses distribution scheme, with vaccine efficacy acquired 15 days after the distribution of the first dose. We account for the constraints associated with the vaccine delivery schedule, the vaccination roll-out pace and the delay between doses. First doses are distributed when possible, always ensuring that a second dose will be available after a 21-day delay. We assume that the vaccination campaign starts on February 1st, 2021 under a roll-out pace of 200,000 doses per day, close to that in France throughout March 2021. The vaccine delivery schedule that we use is detailed in Table S1. As a sensitivity analysis, we also explore a scenario where vaccines are delivered under a roll-out pace of 450,000 doses per day, to account for the expected increase in roll-out pace as more doses will be available and operational capacities for doses distribution will expand from April.
2.3.3. Vaccine prioritisation strategies
We consider the following age and comorbidities groups: individuals (i) older than 75 y.o., (ii) aged 65–74 y.o. with 0, 1 or at least 2 conditions, (iii) aged 50–64 y.o. with 0, 1 or at least 2 conditions and (iv) aged 18–49 y.o. with 0, 1 or at least 2 underlying medical conditions. The size of these age groups in the French population is detailed in Table S2.
We first explore strategies targeted towards single age or comorbidity groups. We then explore prioritisation strategies, where a prioritisation order is defined. The vaccination starts within a group when 70% of vaccine coverages are reached in groups of higher priority. We consider 3 prioritisation strategies: (i) without prioritisation, where available doses are distributed at random in individuals older than 18 y.o. (At random 18y+), (ii) a prioritisation based on age (≥ 75 y.o. then 65–74 y.o. then 50–64 y.o. then 18–49 y.o.), (iii) a prioritisation based on age and comorbidities (≥ 75 y.o. then 65–74 y.o. with at least 2 conditions then with 1 condition then without any condition then 50–64 y.o. with at least 2 conditions and so on until reaching the 18–49 y.o. without any condition).
We assess the impact of each vaccination strategy on the proportion of deaths and hospital admissions averted during 2021.
2.4. Modelling the relaxation of control measures
We explore the extent to which control measures might be relaxed depending on vaccine coverage. We explore a range of scenarios where we relax measures from September 1st, 2021 by changing transmission intensity from the day of relaxation. For a range of vaccine coverages in individuals ≥65 y.o. and individuals aged 18–64 y.o., we derive the reductions in transmission rates in the general population that would remain necessary to ensure the peak in daily hospital admissions remains below 1000 (an arbitrary threshold that is about 3 times lower than the values observed during the first two pandemic waves in France) between September 1st, 2021 and April 1st, 2022. This is done for different values of the basic reproduction number that characterizes a situation with complete relaxation of measures and no immunity: (i) R0 of 2.5 and 3 (as estimated in several locations prior the implementation of control measures) (ii) R0 of 4 (to explore the potential impact of more transmissible variants [22], [23], [24]). Additional values (R0 = 3.5 and 4.5) are considered in the Supplement. This assessment is performed for different values of the proportions of the population ever infected in France on September 1st, 2021, when measures are relaxed (30%, range 25–35%) (see Supplement). We also consider a scenario where vaccines have been demonstrated to be safe for children, have the same efficacy in children as in adults and where children are vaccinated.
2.5. Role of the funding source
The Haute Autorité de Santé (HAS) is an independent public body of health technology assessment. The HAS was involved in defining the objectives of the study, in selecting parameters regarding vaccine comorbidities and vaccine deployment and critically commented on the manuscript. The corresponding author made the decision to submit the paper for publication.
3. Results
Our model can reproduce the dynamics of hospital admissions and admissions in intensive care units (ICU) observed since the beginning of the pandemic in metropolitan France (Fig. 1A and B). Accounting for the increased risk of developing a severe form of COVID-19 associated to identified comorbidities (Table S5), we derive estimates of the probability of hospitalisation given infection, the probability of ICU admission given hospitalisation and the probability of death given hospitalisation stratified by age groups and number of comorbidities (Fig. 1C–E). For instance, we estimate that individuals aged 70–74 y.o. have a probability of hospitalisation upon infection of 20.2% if they have at least 2 comorbidities and 9.6% if they have less than 2 comorbidities (Table S3).
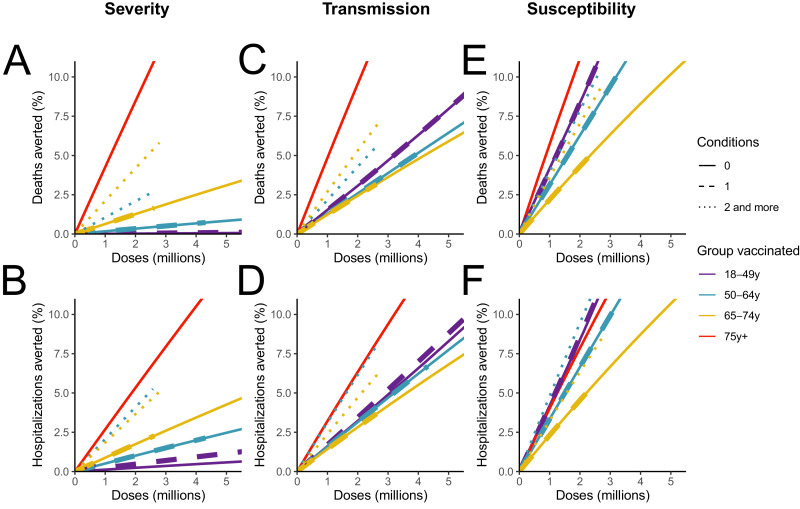
We first evaluate the impact of vaccination strategies targeted towards specific age and comorbidity groups (Fig. 2). When considering a vaccine that reduces the probability of severe outcomes among vaccinated individuals by 90% but has no impact on transmission and susceptibility (Vaccine Severity), the most efficient strategy to minimize hospitalisations and deaths is to allocate first doses to individuals older than 75 y.o. (8.5% reduction in deaths for the first 2 million doses, corresponding to the vaccination of 1 million individuals or a vaccine coverage of 16% in this group), followed by strategies targeting 65–74 y.o. (4.2% reduction) and 50–64 y.o. (2.1% reduction) with at least two comorbidities. Targeting individuals aged 18–49 y.o. has little impact (Fig. 2A-B). When considering a vaccine that also induces a moderate 30% reduction on transmission (Vaccine Transmission), we find that the vaccination of those older than 75 y.o. remains the most efficient strategy to minimize deaths. Vaccinating individuals aged 18–49 y.o. without comorbidities enables larger reductions in deaths (3.1% for 2 million doses) compared to a vaccine that does not impact transmission (<0.05% for 2 million doses). Finally, if the vaccine reduces severity by 90% and susceptibility by 80% (Vaccine Susceptibility), vaccinating individuals aged 18–49 y.o. without comorbidities can induce a reduction in deaths (8.3% for 2 million doses) that is relatively similar to that obtained when vaccinating individuals older than 75 y.o. (11.2% for 2 million doses) (Fig. 2E) and a reduction in hospitalisations even slightly higher (Fig. 2F). For such a vaccine the largest reductions in hospitalisations are obtained by targeting those aged 50–64 y.o. with at least two comorbidities and the benefits associated with the vaccination of young individuals (that contribute substantially to transmission) increase as the reproduction number gets closer to 1 (Fig. S2). Similar trends are observed when considering a vaccine with a lower efficacy (Fig. S3) or a vaccine rolled-out at a faster pace (Fig. S4).
Fig. 2.
Impact of vaccination strategies targeted at different age and comorbidity groups. (A) Deaths and (B) hospitalisations averted for the vaccine Severity that reduces severity by 90%. (C) Deaths and (D) hospitalisations averted for the vaccine Transmission that reduces severity by 90% and infectivity by 30%. (E) Deaths and (F) hospitalisations averted for the vaccine Susceptibility that reduces severity by 90% and susceptibility by 90%. To increase readability, results are reported for less than 5 million doses administered and less than 10% of deaths or hospitalisations averted.
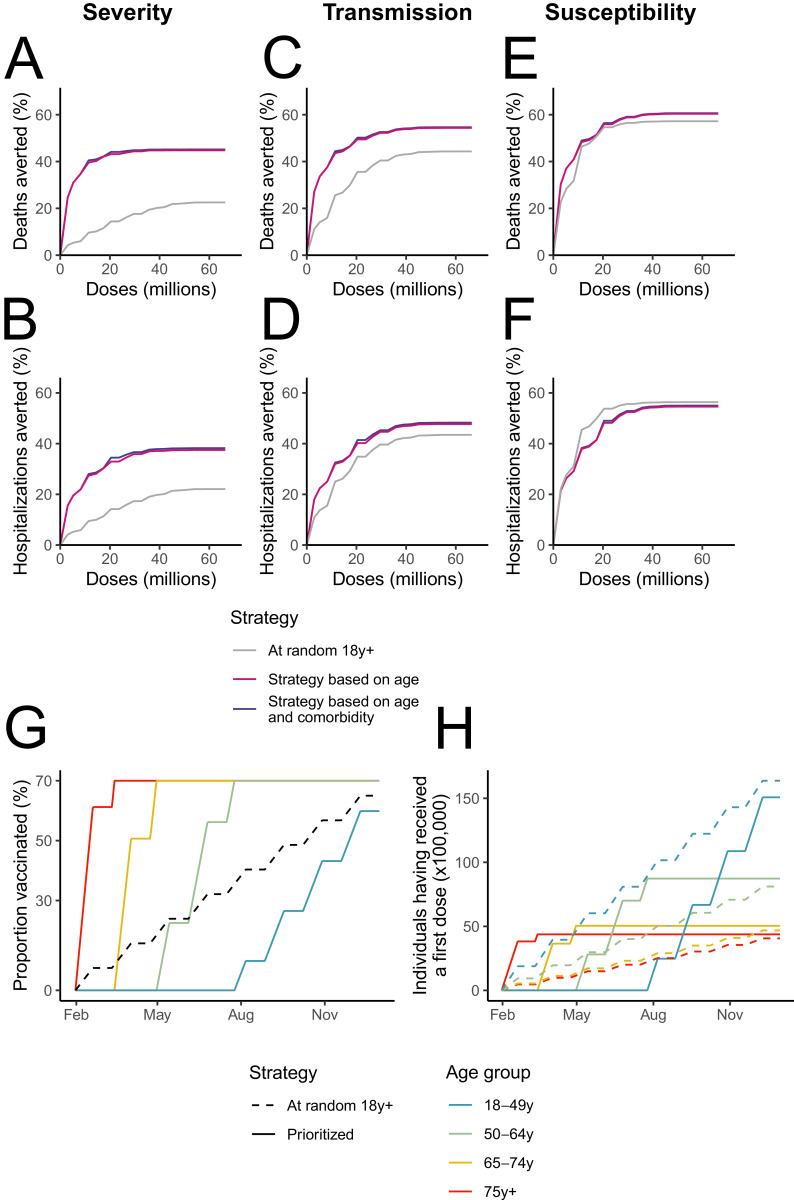
We then evaluate several prioritisation strategies (Fig. 3). For the vaccine Severity, prioritisation based on age or on age and comorbidities substantially outperforms distribution at random (Fig. 3A and B). For example, assuming 9.4 million vaccinated individuals (i.e. the number of individuals who will have received a first dose by May 1st, 2021), 42.1–42.2% deaths would be averted under the prioritized strategies, whereas only 11.6% deaths would be averted in the unprioritized strategy. Similar conclusions are drawn for a vaccine that also has a moderate impact on transmission, though the difference between the strategies shrinks (Fig. 3C and D). For a vaccine that also substantially reduces susceptibility (Fig. 3E and F), we find that the three strategies lead to similar reductions in deaths (51.4–51.4% for prioritized strategies and 50.8% for random distribution with 9.4 million vaccinated individuals). Unprioritized strategies are even slightly more efficient to reduce hospitalizations than prioritized strategies, which can be explained by the younger age distribution of hospitalizations compared to deaths. For the vaccine Susceptibility, the unprioritized strategy can outperform the prioritized ones if the reproduction number is closer to 1 (Fig. S5). The rankings between the strategy remain unchanged if vaccines are distributed at a faster pace (Fig. S6). Prioritization accounting for age and comorbidities is slightly better than a strategy solely based on age, with a gain that decreases as more doses are being distributed (Fig. S7). Finally, these conclusions remain unchanged when considering vaccine coverages that vary across age groups in line with vaccination intent currently measured in France (Fig. S8).
Fig. 3.
Impact of different vaccine prioritisation strategies. (A) Deaths and (B) hospitalisations averted (A) for the vaccine Severity that reduces severity by 90%. (C) Deaths and (D) hospitalisations averted for the vaccine Transmission that reduces severity by 90% and infectivity by 30%. (E) Deaths and (F) hospitalisations averted for the vaccine Susceptibility that reduces severity by 90% and susceptibility by 90%. (G) Proportion of the population and (H) number of individuals having received a first dose throughout 2021 in the different age groups by prioritisation strategy.
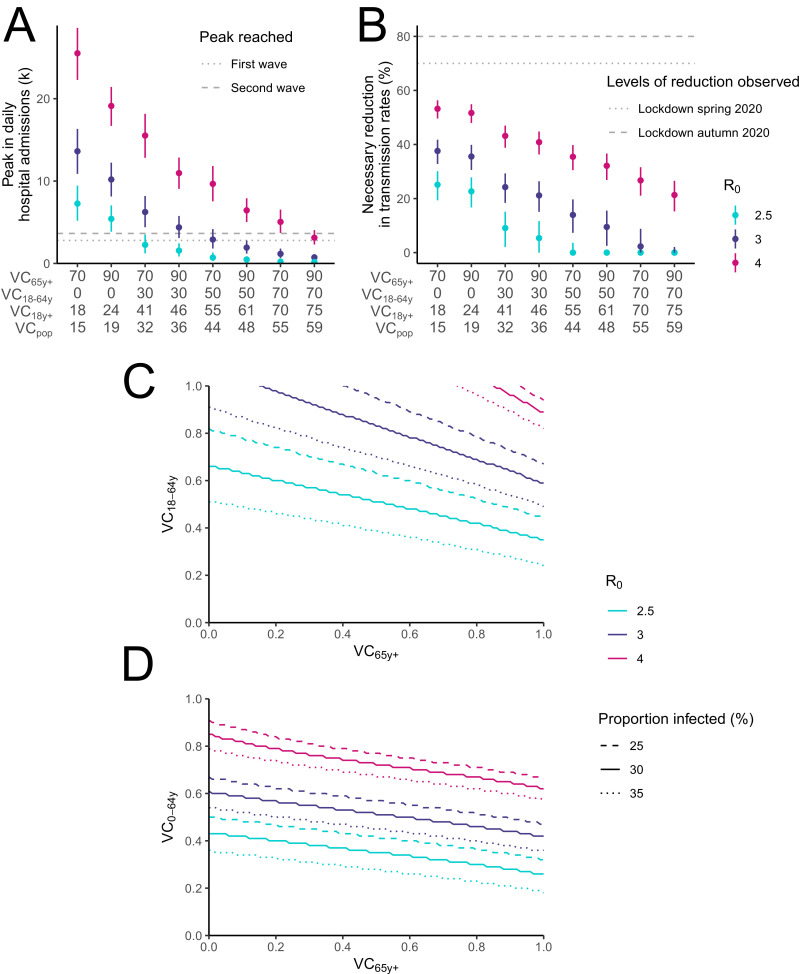
In Fig. 4A, we show the expected peak in daily hospital admissions in autumn 2021 if control measures were to be completely relaxed on September 1st 2021, as a function of the vaccine coverage reached in those aged ≥65 y.o. and 18–64 y.o. This is done under the assumption that 25–35% of the population will have been infected by SARS-CoV-2 by September 1st 2021 and considering the vaccine Susceptibility. If the basic reproduction number R0 of the dominant variant in autumn is similar to that measured in spring 2020 in a number of european countries (R0 around 3.0 [6,25]), a vaccine coverage of 90% in ≥65 y.o. and 70% in 18–64 y.o. (59% of the French population once we account for unvaccinated children) would result in a peak of 420–1100 daily hospital admissions. If the circulation of more transmissible variants such as B.1.1.7 increased R0 to 4, this would increase to 2300–4000 daily admissions at the peak, in between the peak values of the first (3642) and second (2791) waves in metropolitan Franc (Fig. 4A).
Fig. 4.
Manageable relaxation of measures by levels of vaccine coverage. (A) Peak in daily hospital admissions for different combinations of vaccine coverages in 18–64 y.o. (VC18–64y) and ≥65 y.o. (VC65y+). (B) Reduction in transmission rates necessary to avoid reaching 1000 daily hospital admissions. (C) Combinations of vaccine coverages in 18–64 y.o. and ≥65 y.o. and in (D) 0–64 y.o. and ≥65 y.o. necessary to avoid reaching 1000 daily hospital admissions. Different values of the basic reproduction number R0 assuming complete relaxation are explored. The reductions computed in (A-B) assume a proportion ever infected in France of 30% (range 25%−35% corresponding to the vertical bars) upon relaxation on September 1st 2021. Results are reported for the vaccine Susceptibility that reduces severity by 90% and susceptibility by 80%. For each combination of vaccine coverage in 18–64 y.o. and ≥65 y.o., we report the corresponding vaccine coverage in those older than 18 y.o. (VC18y+) and in the general population (VCpop). In (C-D), different values for the proportion of people ever infected in France at the date of relaxation of measures are explored.
To avoid reaching a peak of 1000 daily admissions for a vaccine coverage of 90% in ≥65 y.o. and 70% in 18–64 y.o., control measures would need to reduce transmission rates in the general population by 0–2% for R0 = 3.0 and 15–27% for R0 = 4.0. The required effort would increase to 3–16% (R0 = 3.0) and 27–37% (R0 = 4.0) if we only managed to vaccinate 50% of 18–64 y.o. (Fig. 4B). To put these reductions into context, control measures during the French strict lockdown in Spring 2020 and the softer lockdown in November 2020 reduced transmission rates by around 80% and 70%, respectively (Table S6).
We then explore the combination of vaccine coverages in ≥65 y.o. and 18–64 y.o. that would ensure the peak in daily hospital admissions remains below 1000 (Fig. 4C). Assuming R0 = 3.0, the vaccine coverage in 18–64 y.o. would need to be 62–84% and 54–73% for a vaccine coverage of 70% and 90% in ≥65 y.o, respectively. For R0 = 4.0, complete relaxation would not be achievable for a vaccine coverage of 70% in ≥65 y.o.; if 90% of ≥65 y.o. were vaccinated, it would require a vaccination coverage of ≥89% in 18–64 y.o. If children were included in the campaign, complete relaxation of control measures might be possible with the vaccination of 60–69% of 0–64 y.o. if 90% of ≥65 y.o. were vaccinated (Fig. 4D and S9). Vaccine coverages would need to be higher for vaccines that have a lesser impact on infectivity or susceptibility (Figs. S9 and S10) or that have lower efficacies (Fig. S11). In contrast, considering vaccines additionally reduce the infectivity of vaccinated individuals by 50% would require lower coverages (Fig. S12). For example, complete relaxation of measures might be possible with the vaccination of 81–93% of the 18–64 y.o. or 52–60% of the 0–64 y.o. if 90% of ≥65 y.o. were vaccinated, for R0 = 4.0. Lower vaccine coverages would be required if higher thresholds for the peak in daily hospital admissions were considered (Fig. S13). Higher values of R0 corresponding to a higher transmissibility of circulating strains would also make the situation harder to control (Fig. S14).
4. Discussion
We developed a mathematical model to investigate how vaccine characteristics, levels of vaccine coverage and heterogeneities in individual risks may affect the impact of SARS-CoV-2 vaccination strategies, both early on when prioritization may be necessary and at a later stage when relaxation of control measures may be considered.
We found that the impact of the vaccination campaign is strongly dependent on the nature of protection conferred by the vaccine, with important implications for campaign design. If the vaccine is protective against severe disease only, vaccination of those aged 18–49 y.o. is expected to have only limited impact on morbi-mortality as infections are mostly mild in this group. In this scenario, vaccination does not lead to a build up of herd immunity because vaccinated individuals can still get infected and transmit the virus. As a result, high levels of viral circulation may be observed even if vaccine coverage is high. In contrast, if the vaccine has an impact on infectivity or susceptibility, the vaccination of younger individuals that play a key role in transmission can substantially reduce viral circulation and indirectly prevent the occurrence of severe forms of COVID-19. These results have important implications for the prioritisation of vaccines in the context of limited resources, as this was the case in France throughout spring 2021. If vaccines only reduce disease severity with no impact on infectivity/susceptibility (direct effect only), prioritizing available doses to at-risk individuals largely outperforms strategies where vaccines are distributed at random (Fig. 3A,B,G, H). As the indirect effect of vaccination becomes larger (i.e. the vaccine also reduces infectivity and/or susceptibility), the gains achieved with age prioritisation decline (Fig. 3C–F), with similar levels of reductions reached in the absence of prioritisation when vaccines substantially reduce susceptibility (Fig. 3E). In contrast, the benefits of prioritization might be amplified by increased levels of viral circulation due to pandemic fatigue or the emergence of more transmissible strains (Fig. 2, S2).
Of the three possible effects of vaccines (i.e. reduction of severity, infectivity or susceptibility), the reduction in severity was the only one documented in early assessments of their impact [17,19,26]. In this context, prioritisation by age group and comorbidities was the most conservative approach to optimize allocation of first doses. Vaccine efficacy to reduce infectivity remains poorly characterized but there is increasing evidence that vaccines also substantially reduce susceptibility, at levels close to those considered in our vaccine Susceptibility scenario [10,20,21]. This information is crucial for the next stages of the vaccination campaign: while the vaccination of at-risk individuals needs to be maintained so that they benefit from the direct protection conferred by vaccines, it is also very important to achieve high vaccine coverages in younger age groups to benefit from the indirect effects of herd immunity. This is the only way to obtain an important relaxation of social distancing measures in the autumn.
Whether we can achieve full relaxation of control measures in the autumn will also depend on the transmission potential of the circulating viruses (usually characterized by the basic reproduction number R0) at that time. If R0 in autumn 2021 was equal to 3 like in spring 2020 [6], we expect that a vaccine coverage of 90% in ≥65 y.o and 70% in 18–64 y.o. would be sufficient to maintain the peak in daily hospital admissions below 1000. However, the variant B.1.1.7 that is now dominant in France is substantially more transmissible than historical lineages [22], [23], [24]. For R0=4, and assuming a vaccine coverage of 90% in ≥65 y.o., vaccine coverage would need to increase to ≥89% in those aged 18–64 y.o. These levels are substantially higher than current vaccination intent in the French population (from 36% in 18–24 y.o. to 58% in 50–64 y.o. according to a survey performed in March 2021[27]). If such vaccine coverages cannot be achieved, some control of viral circulation may have to be maintained, potentially through Test-Trace-Isolate, protective measures (e.g. masks) or a certain level of social distancing. We would nonetheless expect these measures to be substantially less strict than those that have been necessary so far in the absence of vaccines (Fig. 4) and that were associated with a significant economic, societal and health impact (e.g. treatment delays and mental health). If vaccination is restricted to adults, high levels of viral circulation may be expected among children, contributing to the infection of unprotected parents and grandparents. If it is demonstrated that vaccines are safe in 0–17 y.o. and if they effectively reduce infectivity or susceptibility in this age group, full relaxation of control measures could be considered with a vaccine coverage of 60–69% in those aged 0–64 y.o. and 90% in ≥65 y.o. To illustrate the impact of the vaccination of children, we explored scenarios where all age groups below 18 y.o. were eligible for vaccination; but strategies restricted to older children might also be considered. Heterogeneities in the proportion of the population already infected by SARS-CoV-2 also imply that the vaccine coverages required to go back to normal may differ across locations. Finally, the situation could be harder to control than anticipated here as we do not account for the increased severity reported for B.1.1.7 [28], the circulation of variants such as B.1.351 that may partly escape protection conferred by vaccines [29] or the emergence of new variants even more transmissible than B.1.1.7 (such as the B.1.617.2 variant [30]).
Compared to previous assessments of vaccination strategies, we explicitly accounted for how the probability to develop a severe form of COVID-19 increased with the number of comorbidities and for the interaction between the number of comorbidities and age. We could not consider the effect of comorbidities in those older than 75 y.o. due to insufficient data for this age group. However, our results show that the vaccination of individuals older than 75 y.o. regardless of their number of comorbidities results in larger reductions in the number of deaths than the vaccination of younger age-groups (e.g. 65–74 y.o.) with at least 2 conditions. While prioritizing according to age and comorbidities optimally reduces the number of deaths and hospitalisations at the beginning of the program, accounting for comorbidities becomes less important when more doses are available. In this later context, the slightly higher benefit obtained with prioritization accounting for age and comorbidities might be offset by logistical challenges associated with the targeting of a smaller group of individuals. A limitation of our study is that the list of comorbidities we consider does not perfectly match the one used to characterize the association with severe outcome [9]. Nevertheless, the impact on our results should be limited, especially since we are considering relative risks associated to the number of conditions and not to specific pathologies individually. We evaluated the impact of vaccination on deaths and hospitalisations, which does not capture the burden associated with long COVID [31].
Prioritization of vaccination strategies during a pandemic must adhere to ethical principles common to the allocation of scarce resources, including consideration of age, prognosis, burden, and instrumental value [32]. However, vaccine strategies are unique in that optimality may arise from the indirect effect of vaccination, i.e. the overall reduction in viral circulation in a vaccinated population, rather than from the direct protection provided by the vaccine. The extent of indirect effects is however more challenging to anticipate, as they arise from reduction in susceptibility and transmissibility as well as on vaccine coverage. Protecting those with the poorest outcomes does not conflict with relying on indirect effects, as long as it is an effective way to deliver interventions [33].
Our modelling framework has been developed to describe the spread of SARS-CoV-2 in the community and is therefore not suited to describe epidemic dynamics in healthcare settings or elderly homes. As such, we do not account for the increased risks observed among healthcare workers and elderly homes’ staff and residents. We may thus underestimate the impact of strategies prioritised towards the population older than 75 y.o., which implicitly takes into account the population of elderly homes. We also do not account for the gradual increase in vaccine induced-immunity between the two doses. This should have a limited impact on the ranking of vaccine prioritisation strategies and on the extent of measures relaxation assuming all vaccinated individuals have reached full protection. In a context of high uncertainty, this modelling analysis is not aimed at precisely forecasting the future course of the pandemic. Instead, by exploring a range of scenarios characterized by well-defined assumptions, it can help appreciate how different factors including vaccine coverage and the distribution of individual risks might impact the pandemic.
Our modelling results highlight how understanding of vaccine characteristics, individual risks and vaccine coverages across groups is essential to optimize the design of the vaccination campaign and determine the level of relaxation of control measures that may be expected in the autumn. These results provide valuable insights for the implementation of vaccination programs in many European countries with similar demographics, vaccine doses availability and vaccine coverages as France.
Funding
We acknowledge financial support from Haute Autorité de Santé, the Investissement d'Avenir program, the Laboratoire d'Excellence Integrative Biology of Emerging Infectious Diseases program (grant ANR-10-LABX-62-IBEID), Santé Publique France, the INCEPTION project (PIA/ANR-16-CONV-0005), AXA, Groupama and the European Union's Horizon 2020 research and innovation program under grants 101003589 (RECOVER) and 874735 (VEO).
Contributors
CTK and SC designed and planned the study. CTK, CM, PB, AG, VO and PC performed analyses. DLB, CP, VC, AF, LZ, PYB, SC critically commented on assumptions and model structure. CTK and SC wrote the first draft. All authors contributed to revisions of the manuscript. CTK accessed the hospitalization data used for the inference. AG and VO accessed data from the Esteban survey. The corresponding author made the decision to submit the manuscript for publication.
Data sharing
The data and the codes used for this study are available at the address https://gitlab.pasteur.fr/mmmi-pasteur/covidvaccinationstrategies-france.
Declaration of Competing Interest
PC reports consulting fees from Sanofi Pasteur for projects outside of the submitted work and unrelated to COVID-19. The other authors declare no competing interests.
Acknowledgments
We are much grateful to the COVID-NET team from the US CDC for having kindly provided us with complementary analyses from the COVID-NET database.
Footnotes
Funding: HAS.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.eclinm.2021.101001.
Appendix. Supplementary materials
References
- 1.O'Driscoll M., Ribeiro Dos Santos G., Wang L. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590:140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 2.Bubar K.M., Reinholt K., Kissler S.M. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science. 2021 doi: 10.1126/science.abe6959. published online Jan 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matrajt L., Eaton J., Leung T., Brown E.R. Vaccine optimization for COVID-19: who to vaccinate first? Sci Adv. 2020;7 doi: 10.1126/sciadv.abf1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moore S., Hill E.H., Tildesley M.J., Dyson L., Keeling M.J. Vaccination and non-pharmaceutical interventions for COVID-19: a mathematical modelling study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00143-2. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandmann F.G., Davies N.G., Vassall A., Edmunds W.J., Jit M. The potential health and economic value of SARS-CoV-2 vaccination alongside physical distancing in the UK: a transmission model-based future scenario analysis and economic evaluation. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00079-7. published online March 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salje H., Tran Kiem C., Lefrancq N. Estimating the burden of SARS-CoV-2 in France. Science. 2020;369:208–211. doi: 10.1126/science.abc3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verity R., Okell L.C., Dorigatti I. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reilev M., Kristensen K.B., Pottegård A. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: a nationwide cohort. Int J Epidemiol. 2020 doi: 10.1093/ije/dyaa140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko J.Y., Danielson M.L., Town M. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1419. published online Sept 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall V.J., Foulkes S., Saei A. Multicentre prospective cohort study (the SIREN Study) 2021. Effectiveness of BNT162b2 mRNA vaccine against infection and COVID-19 vaccine coverage in healthcare workers in England. published online Feb 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson M.G., Burgess J.L., Naleway A.L. Interim estimates of vaccine effectiveness of BNT162b2 and mRNA-1273 COVID-19 vaccines in preventing SARS-CoV-2 infection among health care personnel, first responders, and other essential and frontline workers - eight U.S. locations, December 2020-March 2021. MMWR Morb Mortal Wkly Rep. 2021;70:495–500. doi: 10.15585/mmwr.mm7013e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jentsch P.C., Anand M., Bauch C.T. Prioritising COVID-19 vaccination in changing social and epidemiological landscapes: a mathematical modelling study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00057-8. published online March 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balicco A., Oleko A., Szego E. Protocole Esteban : une Étude transversale de santé sur l’environnement, la biosurveillance, l’activité physique et la nutrition (2014–2016) Toxicol Anal Clin. 2017;29:517–537. [Google Scholar]
- 14.Kim L., Garg S., O'Halloran A. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the U.S. coronavirus disease 2019 (COVID-19)-associated hospitalization surveillance network (COVID-NET) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1012. published online July 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Viner R.M., Mytton O.T., Bonell C. Susceptibility to SARS-CoV-2 infection among children and adolescents compared with adults: a systematic review and meta-analysis. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.4573. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davies N.G., Klepac P. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. CMMID COVID-19 working group. [DOI] [PubMed] [Google Scholar]
- 17.Polack F.P., Thomas S.J., Kitchin N. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Voysey M., Costa Clemens S.A., Madhi S.A. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials. Lancet. 2021;397:881–891. doi: 10.1016/S0140-6736(21)00432-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baden L.R., El Sahly H.M., Essink B. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tande A.J., Pollock B.D., Shah N.D. Impact of the COVID-19 vaccine on asymptomatic infection among patients undergoing pre-procedural COVID-19 molecular screening. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab229. published online March 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gaymard A., Bosetti P., Feri A. Early assessment of diffusion and possible expansion of SARS-CoV-2 Lineage 20I/501Y.V1 (B.1.1.7, variant of concern 202012/01) in France, January to March 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.9.2100133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies N.G., Abbott S., Barnard R.C. Estimated transmissibility and impact of SARS-CoV-2 lineage B.1.1.7 in England. Science. 2021 doi: 10.1126/science.abg3055. published online March 3. DOI:10.1126/science.abg3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Volz E., Mishra S., Chand M. Assessing transmissibility of SARS-CoV-2 lineage B.1.1.7 in England. Nature. 2021:1–17. doi: 10.1038/s41586-021-03470-x. [DOI] [PubMed] [Google Scholar]
- 25.Flaxman S., Mishra S., Gandy A. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. 2020;584:257–261. doi: 10.1038/s41586-020-2405-7. [DOI] [PubMed] [Google Scholar]
- 26.Voysey M., Clemens S.A.C., Madhi S.A. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Santé Publique France. COVID-19 : point épidémiologique du 25 mars 2021. 2021 https://www.santepubliquefrance.fr/maladies-et-traumatismes/maladies-et-infections-respiratoires/infection-a-coronavirus/documents/bulletin-national/covid-19-point-epidemiologique-du-25-mars-2021 (Accessed March 27, 2021).
- 28.Davies N.G., Jarvis C.I. Increased mortality in community-tested cases of SARS-CoV-2 lineage B.1.1.7. Nature. 2021 doi: 10.1038/s41586-021-03426-1. published online March 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou D., Dejnirattisai W., Supasa P. Evidence of escape of SARS-CoV-2 variant B.1.351 from natural and vaccine-induced sera. Cell. 2021 doi: 10.1016/j.cell.2021.02.037. published online Feb 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finlay C., Brett A., Henry L.S. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.24.2100509. pii=2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ayoubkhani D., Khunti K., Nafilyan V. Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Persad G., Wertheimer A., Emanuel E.J. Principles for allocation of scarce medical interventions. Lancet. 2009;373:423–431. doi: 10.1016/S0140-6736(09)60137-9. [DOI] [PubMed] [Google Scholar]
- 33.Williams J., Degeling C., McVernon J., Dawson A. How should we conduct pandemic vaccination? Vaccine. 2021;39:994–999. doi: 10.1016/j.vaccine.2020.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.