Abstract
Background
Scar and vulnerability models assert that increased psychopathology may predict subsequent executive functioning (EF) deficits (and vice versa) over protracted timescales, yet most prior work on this topic has been cross-sectional. Thus, we tested the within- and between-person relations between EF, depression, and anxiety.
Methods
Older adult participants (n = 856) were assessed across four waves, approximately 2 years apart. Performance-based EF and caregiver-rated symptom measures were administered. Bivariate latent change score and random-intercept cross-lagged panel models were conducted.
Results
Within persons, random-intercept cross-lagged panel models revealed that prior greater depression forecasted lower subsequent EF, and vice versa (d = −0.292 vs. −0.292). Bivariate dual latent change score models showed that within-person rise in depression predicted EF decreases, and vice versa (d = −0.245 vs. −0.245). No within-person, cross-lagged, EF-anxiety relations emerged. Further, significant negative between-person EF-symptom relations were observed (d = −0.264 to −0.395).
Conclusion
Prospective, within-person findings offer some evidence for developmental scar and vulnerability models.
Keywords: anxiety, depression, executive functioning, latent change, random-intercept cross-lagged panel models
In daily life, most of us depend on our global executive functioning (EF) capacity to effectively accomplish tasks, communicate, handle emotions, make choices, prioritize goals, and solve problems [1,2]. Global EF is defined as a group of multidomain cognitive control systems entwined with attention, information processing, and other cognitive abilities [3,4]. Our global EF systems comprise facets of inhibition (capacity to abstain from autopilot actions), working memory (WM; ability to alter cognitive representations with incoming data in real-time), shifting (adeptness to flexibly switch from one mental set to another) [5], and verbal fluency [6]. Relatedly, evidence has shown consistently that language-based, temporal lobe-mediated verbal fluency ability (marked by scores on diverse time-limited word generation on animal- and phonemic-cued tests) had strong and unique relations with common EF variance (i.e., global EF capacity) in diverse youth and adult samples [7–10]. Given its importance, EF problems have been linked to issues with career, social relationships, diet, nutrition, and health [11,12]. Executive dysfunction-related health problems include cardiorespiratory, metabolic, neuroendocrine, and psychiatric disorders [13,14]. Thus, understanding the risk factors and consequences of EF decrements is essential.
Scar theories propose that increases in psychiatric symptoms can precede and predict future EF decline. Specifically, scar models posit that chronic increased depression and anxiety may build up oxidative and inflammatory-stress, thereby adversely impacting EF-related brain regions over protracted durations [15–17]. Relatedly, scar models such as the vascular- [18] and executive dysfunction syndrome-depression [19] hypotheses assert that increased depression and anxiety could impair future EF via buildup of tissue injury (e.g., lacunes, microinfarcts, and white matter hyperintensities) in cardiovascular systems, cognitive control-, and reward processing-related brain regions, over long timescales [20]. These brain areas might include frontal–striatal pathways (e.g., dorsolateral prefrontal cortex, basal ganglia, thalamus, and anterior cingulate cortex) [21,22].
Thus far, 47 longitudinal studies have offered support for scar models. For instance, higher depression severity during adolescence was associated with lower vocabulary score in early adulthood 8 years later [23]; however, whether such pattern applied to various stages in adulthood could not be inferred from that study. Other studies suggested such a possibility. Swedish and American adults with (vs. without) major depression displayed worsened episodic memory, EF, or verbal fluency after 6 months to 5 years despite symptom remission [24,25]. Likewise, among mid-life and older community adults, increased anxiety was related to reduced immediate and delayed auditory memory abilities following 12 years [26]. Similarly, 2 meta-analyses of 43 studies showed that heightened anxiety and depression dovetailed with larger EF decline and incidence of major neurocognitive disorders in diverse community and clinical samples across 1–17 years [27,28].
Simultaneously, vulnerability models argue that EF decline can function as a precursor of later heightened depression and anxiety. Vulnerability models assert that poorer EF may forecast future anxiety and depression across prolonged periods due to chronic problems with disengaging from negative self-referential perseverative thinking (e.g., worry and rumination) [29,30]. Likewise, EF deficits can make it perpetually hard to detach from threats, leading to excessive focus on anxiety-inducing factors in one’s surroundings and risk for increased anxiety [31,32]. Moreover, it has been thought that poorer EF, especially WM, can predict increased depression and anxiety across long durations, in part due to difficulties with adjusting to various changing emotion-eliciting contexts in versatile and optimal ways [33]. In sum, vulnerability theories argue that worse EF may forecast increased depression and anxiety over long durations.
To date, 31 prospective investigations have empirically supported vulnerability theories. For example, an earlier study demonstrated that poorer WM was related to future chronic course of increased depression [34]. Likewise, reduced inhibition, WM, shifting, verbal fluency, and other cognitive functioning indices were connected with pathological worry dimensionally and categorically 9 years later in community adults [35]. More recently, meta-analytic data on 29 studies (n = 121,749) showed that cognitive deficits were associated with increased major depression severity following several months to 45 years in diverse clinical and community-dwelling samples [36].
However, the mostly two-time-point, between-person, regression studies testing the prospective relations between mental health symptoms and global EF to date introduce shortcomings to clinical science. Such methods do not account for the nesting of repeated assessments within persons to capture change-to-future change trajectories across time [37]. Mounting global pressures related to neuropsychiatric illnesses, increasing life expectancy, and aging [38–40] make it crucial to explore whether change in global EF over long durations may be related to future change in mental illness during adulthood development. Further, between-person differences across time may be due to stable variations observed across the lifespan [41], or to individual differences in aging-associated rate of EF decrements [42–45]. The latter possibility can only be captured by using within-person methods that also capture change. Moreover, the foregoing scar and vulnerability theories posit that EF-symptom relations unfold within persons across long durations [46–51]. Awareness of within-person prolonged trajectories of increased depression or anxiety, EF decrements, and their covariation may guide the design of personalized prevention, diagnostic, and treatment efforts that rely on idiographic (or within-person) more than between-person data, as part of precision psychiatry [52–54]. It is also important to note that observations of between- and within-person differences in EF and psychopathological symptoms do not always align with each other [55–57]. To broaden and deepen comprehension of EF and mental health in mid-life and older adulthood, within-person (co)variations and change must be considered. Tethering within-person data analytic approaches with longitudinal study designs is thus important to comprehend the bidirectional within-person changes in EF and subsequent changes in symptoms (and vice versa).
Two cutting-edge techniques that attain these aims are random-intercept cross-lagged panel models (RI-CLPM) [58] and bivariate dual latent change score models (BLCS) [59]; two forms of longitudinal structural equation modeling (SEM). These longitudinal SEM approaches benefit researchers by accounting for prior lagged relations and regression to the mean, minimizing measurement unreliability, and using all available values instead of listwise deletion [57]. Further, by adjusting for temporally stable between-person differences and autoregressive effects, these models can test if change in one variable across a previous time-period or time-lag is associated with change in another variable at the next time-period or time-lag within persons. Accordingly, by evaluating lead–lag change-to-future change connections, RI-CLPM and BLCS models move us toward the ability to draw causal inferences [60]; inquiries essential to clinical science.
Thus far, three studies of adult participants have tested the longitudinal, dynamic, within-person relations between EF and anxiety, depression, or pertinent concepts with BLCS. Using BLCS, increase in anxiety was related to cognitive functioning decline in older adults [57]; despite that, the two-wave study prevented understanding of how symptom change predicted subsequent EF change (and conversely). Relatedly, BLCS models across three waves showed that 9-year growth in excessive worry dovetailed with future 9-year decline in global and unique EF facets [49]; however, whether change in EF forecasted later change in worry was not examined. Another five time-point study demonstrated that rise in trait neuroticism at one time-lag preceded and linked to reductions in spatial processing, WM, and processing speed at the next time-lag [57]; nonetheless, one-item assessments of cognitive functioning were used in the study. To our knowledge, no studies have tested EF-psychological symptom relations in older adults with RI-CLPM. However, a recent study in youths that utilized RI-CLPM [61] suggested the possibility of EF problems serving as risk factors for later increased depression and anxiety.
Building on this literature, this study aimed to examine the within-person associations between a global EF composite (formed via a latent composite of five measures) and depression or anxiety severity using RI-CLPM and BLCS in older adults. Based on scar theories, we hypothesized that within persons, higher anxiety or depression severity would reliably precede and relate to greater future EF decline at the next time-point and time-lag. Moreover, based on vulnerability models, we hypothesized that within persons, lower EF would forecast subsequent rise or increased depression or anxiety severity at the next time-point or time-lag. Last, using a SEM-based model comparison approach [62], we aimed to directly juxtapose the effect sizes indicating the scar (vs. vulnerability) hypothesis to determine if any differences emerged.
Method
Participants
The present study was a secondary analysis of the Aging, Demographics, and Memory Study (ADAMS) publicly available and restricted-use datasets [63]. Ethical approval was provided by the University of Michigan and Duke University Medical Center, and all participants voluntarily consented to enroll. Participants (n = 856) averaged 81.59 years of age (SD = 7.10, range = 70–110), 58.53% were female, and 76.87% identified as White, compared to the other 23.13% who identified African American or other ethnicities. In addition, 28.62% (n = 245) of the participants needed support for dressing, feeding, or bathing based on caregiver report, or were diagnosed with Diagnostic and Statistical Manual-Fourth Edition-Text Revised (DSM-IV-TR) [64,65]—defined dementia, major depressive disorder, stroke, or other neurological condition. These dementia syndromes included probable and possible Alzheimer’s disease, cardiovascular, and other causes. All DSM-IV-TR diagnoses were attained through expert consensus with a multidisciplinary team of neurologists, psychiatrists, geriatric psychologists, and other healthcare professionals [66]. The online supplementary material (OSM) offers more details on the sample characteristics.
Procedures
Participants completed performance-based EF measures and had a significant other caregiver (e.g., spouse and children) who could reliably report on their behavioral symptoms across multiple time-points. Data were collected across four waves in 2004 (Time 1; T1), 2006 (Time 2; T2), 2008 (Time 3; T3), and 2010 (Time 4; T4) [66]. The following caregiver-rated symptom assessment and EF tests were administered.
Measures
Mental health symptoms
The widely used caregiver-rated Neuropsychiatric Inventory (NPI) [67]—depression and anxiety domains were utilized to assess past-month depression and anxiety severity in the form of a structured interview. Caregiver ratings were used in this study as self-reported mental health symptom severity data was only available at T1 and T2 [63,68,69], and due to the reliable nature of caregiver-reported data that tends to align with self-rated symptom measures [70]. To measure depression, caregivers were asked about the presence and duration of depression symptoms (e.g., sadness, irritability, feels worthless, and suicidal thoughts) the participant may have exhibited. To assess for anxiety, caregivers were inquired on the presence of any anxiety symptoms (e.g., excessive worry, breathlessness, and behavioral avoidance). Also, for each participant’s symptom domain, the informants reported on the degree of the following four facets: severity (3-point scale; 1 = mild to 3 = marked); change from past typical behaviors (3-point scale; 0 = no; 1 = yes; 2 = exaggeration of previous problems); distress (6-point scale; 0 = not at all to 5 = very severely or extremely). Supplementary Tables S1 and S2 in the OSM show that these four manifest indicators for the depression and anxiety scales had excellent model fit using a series of confirmatory factor analysis (CFA) at distinct time-points. Further, the NPI has reliably shown strong internal consistency, as well as convergent and discriminant validity [67,71]. In this study, internal consistencies were high for the depression severity (Cronbach’s αs = 0.93, 0.94, 0.92, and 0.93 at T1, T2, T3, and T4, respectively) and anxiety severity scales (αs = 0.93, 0.96, 0.93, and 0.90 at T1, T2, T3, and T4, respectively).
Executive functioning
The following five measures of EF were used to create a composite latent global EF composite: (a) controlled oral word association (a verbal fluency assessment that captures unplanned generation of words within a time limit that start with some assigned letter) [72]; (b) animal fluency (another time-limited verbal fluency test based on the animal category) [73]; (c) serial 7 subtraction (extent of accuracy of counting down from 100 by 7 within a time limit) [74]; (d) backward digit span (degree of accuracy of recall in reverse order of integer strings of increasing length) [75]; and (e) symbol digit modality test (level of accuracy of replacing a single-digit integer for randomized displays of geometric patterns) [76]. These EF assessments have been shown to have good internal consistency, strong convergent, and discriminant validity [77–79]. In this study, the αs for the global EF composite were strong across all time-points (αs = 0.92, 0.83, 0.87, and 0.88 at T1, T2, T3, and T4, respectively). Moreover, at each time-point, a composite global EF index was created by standardizing each EF measure and averaging the scores across EF measures. Further, exploratory factor analysis and a series of CFA demonstrated that a one-factor latent global EF composite had good model fit across waves of assessment (refer to page 4 and Supplementary Table S3 of the OSM). In addition, these global EF scores have been normed based on age and education, and appropriate adjustments were made for participants with hearing impairments [80–83]. Also, Supplementary Table S4 in the OSM shows the descriptive statistics of the study variables based on SEM analyses.
Data analyses
All longitudinal SEM analyses were performed with the lavaan package [84] in R Version 3.6.3. Model fit was assessed utilizing practical fit indices and heuristic cut-offs: confirmatory fit index (CFI; CFI ≥ 0.90) [85] and root mean square error of approximation (RMSEA; RMSEA ≤ 0.09) [86]. To maximize all available data points, we used full information maximum likelihood, the gold standard [87], to manage missing data. In total, 16.29% of the data were missing. Further, the data were missing completely at random (χ2[df = 113] = 134.32, p = 0.084).
Next, we established longitudinal measurement invariance; a prerequisite for longitudinal SEM [88]. We progressively evaluated for configural invariance (equivalence of factor structure), metric invariance (equal factor structure and item loadings [λs], freely estimated item intercepts [τs], and item error variances [εs] across the time-points), scalar invariance (equal factor structure, λs, and τs, but freely estimated εs across time-points), and strict invariance (equal factor structure, λs, τs, and εs, across time-points) [62]. To test for measurement invariance, we conducted a Δχ2 difference test. A statistically significant Δχ2 meant that the more (vs. less) restricted model had poorer fit [89]. However, as Δχ2 is affected by sample size despite negligible misfit changes, the following change in practical fit indices, ΔCFI ≤ −0.01 or ΔRMSEA < +0.015 [90], from the less restricted to more restricted models signaled measurement nonequivalence.
The RI-CLPM was used to manage interdependent repeated assessments nested within persons, and to distinguish between within-person (dynamic state) variance and between-person (trait) variance [91]. RI-CLPM procedures permitted us to test these within-person reciprocal cross-lagged relations (γs) accounting for within-person autoregressive effects (βs; level of one variable forecasting its subsequent level), trait variances (αs), and regression to the mean [92,93]. Of primary interest were the within-person cross-lagged associations between level of EF at a prior time-point (T − 1) and level of depression or anxiety symptom severity (SYM) at the next adjacent time-point (T) following about 2 years (γs) (and vice versa), as shown in Equations (1) and (2).
| (1) |
| (2) |
Concurrently, BLCS approaches were utilized to test if within-person change in depression or anxiety symptom severity at a previous time-lag (ΔT − 1) would be related to change in EF at the next successive time-lag (ΔT) (and vice versa). BLCS is a cutting-edge method that empowers researchers to test within-person change-to-future change associations (coupling effects; δs) after accounting for trait-level initial status, trait-level constant change parameters (αs), and within-person autoregressive paths (proportional effects; change in a variable predicting subsequent change in itself; βs) [59]. The BLCS models relevant to our research question can be denoted in Equations (3) and (4) as follows.
| (3) |
| (4) |
As recommended, the within-person cross-lagged associations (γs) in the RI-CLPM and within-person coupling effects in the BLCS (δs) were constrained to be equal across waves of assessments to reduce SEs in parameter estimates (refer to Supplementary Figure S1 shows a BLCS model in [94]). Also, baseline psychopathology and EF were controlled for in all models.
As we aimed to directly compare the scar and vulnerability hypotheses, we contrasted a model that freely estimated the cross-lagged or coupling effects (EF predicting future SYM and conversely) to a model that constrained the cross-lagged or coupling effects to equality. A statistically significant change (Δ) in χ2 value in comparing these two models would indicate notable differences in the strength of effect sizes for one hypothesis versus the other [62]. If the Δχ2 test was not significant, the more parsimonious model with equality constraints on cross-lagged or coupling effects was chosen as the final model. Effect sizes were calculated using the formula, Cohen’s 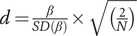 [95], where β is the unstandardized regression estimate, SD(β) its standard deviation, and N is the sample size. Cohen’s d values of 0.2, 0.5, and 0.8 indicated small, moderate, and large effects, respectively.
[95], where β is the unstandardized regression estimate, SD(β) its standard deviation, and N is the sample size. Cohen’s d values of 0.2, 0.5, and 0.8 indicated small, moderate, and large effects, respectively.
Power analysis
Following best practices [96], an a priori Monte Carlo power analysis based on a conservative effect size of d = 0.20 for the cross-lagged effects in the RI-CLPM and coupling effects (bidirectional change-to-future change EF-symptom relations) in the BLCS was performed using the RAMpath R package [97]. After 1,000 replications per condition, we observed 90.6–100.0% power to detect significant within-person cross-lagged or coupling effects. Further, there was 90.0–100.0% power to identify other significant parameter estimates.
Results
Longitudinal measurement invariance
Supplementary Table S5 in the OSM shows the longitudinal measurement invariance analyses for the constructs of interest. Overall, strict level of equivalence (equal λs, τs, εs) was observed for the constructs of depression severity, anxiety severity, and EF. Therefore, conducting analyses using RI-CLPM and BLCS approaches were appropriate.
Lagged relations between depression severity and executive function
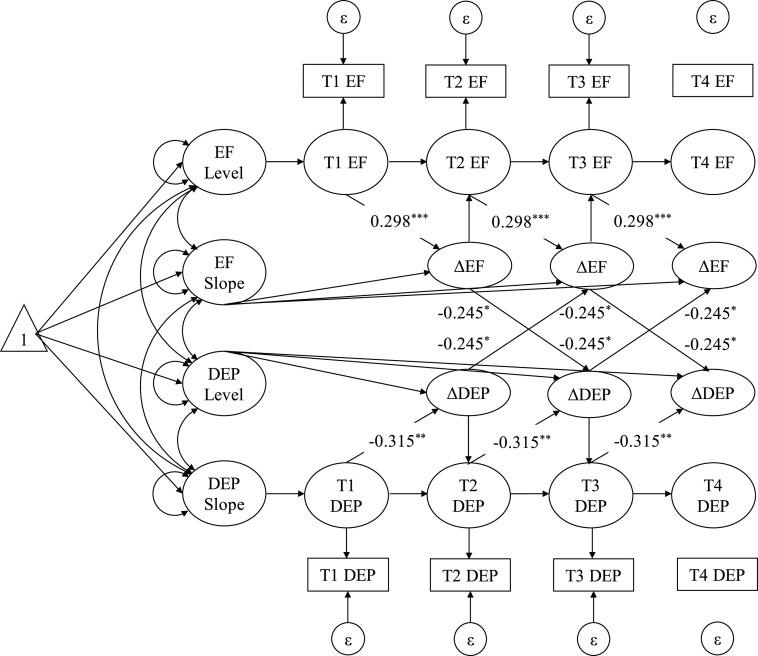
Table 1 displays all of the parameter estimates for the RI-CLPM testing the cross-lagged relations between depression severity and EF. The model with equality constraints on the cross-lagged effects did not significantly differ from the model that freely estimated those parameters (Δχ2[df = 1] = 0.017, p = 0.895). The parsimonious model with equality constraints showed good model fit (χ2[df = 24] = 45.160, p = 0.006, CFI = 0.984, RMSEA = 0.032). Within persons, higher prior depression severity substantially predicted lower EF at the subsequent time-point (β = −0.073, 95% CI [−0.119, −0.026], d = −0.292). Likewise, lower previous EF significantly predicted greater depression severity at the next time-point within persons (β = −0.073, 95% CI [−0.119, −0.026], d = −0.292). Also, between persons, higher random intercept depression severity was significantly correlated with lower random intercept EF (β = −0.055, 95% CI [−0.094, −0.016], d = −0.264).
Table 1.
Random-intercepts cross-lagged panel model of DEP and EF across four time-points.
| Estimate | 95% CI | Cohen’s d | |
|---|---|---|---|
| Within-person cross-lagged effects | |||
| (DEP)[T-1] ➔ (EF)[T] | −0.073** | [−0.119, −0.026] | −0.292 |
| (EF)[T-1] ➔ (DEP)[T] | −0.073** | [−0.119, −0.026] | −0.292 |
| Within-person autoregressive effects | |||
| (DEP)[T-1] ➔ (DEP)[T] | 0.248*** | [0.157, 0.338] | 0.518 |
| (EF)[T-1] ➔ (EF)[T] | 0.289** | [0.074, 0.504] | 0.252 |
| Between-person covariances | |||
| (EF)[T] ↔ (DEP)[T] | −0.027*** | [−0.042, −0.011] | −0.324 |
| RI(EF)[T] ↔ RI(DEP)[T] | −0.055** | [−0.094, −0.016] | −0.264 |
| RI(DEP)[T] ↔ (DEP)[T1] | 0.006 | [−0.067, 0.078] | 0.016 |
| RI(EF)[T] ↔ (EF)[T1] | −0.121*** | [−0.176, −0.066] | −0.415 |
| Between-person intercepts | |||
| Mean of (DEP)[T1] | 0.371*** | [0.316, 0.426] | 1.272 |
| Mean of (DEP)[T2] | 0.256*** | [0.176, 0.336] | 0.599 |
| Mean of (DEP)[T3] | 0.290*** | [0.212, 0.368] | 0.696 |
| Mean of (DEP)[T4] | 0.266*** | [0.173, 0.360] | 0.532 |
| Mean of (EF)[T1] | −0.000 | [−0.058, 0.058] | 0.000 |
| Mean of (EF)[T2] | −0.094* | [−0.168, −0.020] | −0.237 |
| Mean of (EF)[T3] | −0.655*** | [−0.735, −0.575] | −1.534 |
| Mean of (EF)[T4] | −0.785*** | [−0.872, −0.698] | −1.713 |
| Residual variances | |||
| Variance of RI(DEP)[T1-T4] | 0.000 | – | – |
| Variance of RI(EF)[T1-T4] | 0.904*** | [0.783, 1.025] | 1.400 |
| Variance of (DEP)[T1] | 0.670*** | [0.513, 0.827] | 0.804 |
| Variance of (EF)[T1] | 0.089*** | [0.059, 0.120] | 0.534 |
| Variance of (DEP)[T2-T4] | 0.412*** | [0.371, 0.453] | 1.883 |
| Variance of (EF)[T2-T4] | 0.088*** | [0.067, 0.109] | 0.768 |
Note: Model fit indices: χ2(df = 24) = 45.160, p = 0.006, CFI = 0.984, RMSEA = 0.032, 95% CI [0.017, 0.046]. Within-person cross-lagged effects refer to level in DEP at a prior time-point (T-1) predicting (➔) future Δ in EF at the next adjacent time-point (T) (and vice versa). Within-person coupling effects and proportional effects, residual covariances between DEP and EF, as well as variances of DEP and EF were each uniquely fixed to be equal across all three time-lags.
Abbreviations: CI, confidence interval; DEP, depression severity; EF, executive functioning; RI, random intercept.
p < 0.05.
p < 0.01.
p < 0.001.
Table 2 presents the parameter estimates for the BLCS models examining the change-to-future change associations between depression severity and EF. The freely estimated (vs. constrained) models were not significantly different from each other (Δχ2[df = 1] = 0.235, p = 0.628). The final model with equality constraints on the coupling effects showed acceptable model fit (χ2[df = 25] = 47.000, p = 0.005, CFI = 0.974, RMSEA = 0.039, 95% CI [0.021, 0.057]). Within persons, greater growth in depression severity at a prior time-lag significantly predicted EF decrement at the next time-lag (β = −0.540, 95% CI [−0.955, −0.124], d = −0.245). Likewise, within persons, EF decline at a previous time-lag was significantly associated with larger increase in depression severity at the subsequent time-lag (β = −0.540, 95% CI [−0.955, −0.124], d = −0.245). Figures 1 and 2 summarize the analyses of the lagged relations between depression severity and EF.
Table 2.
Bivariate dual latent change score model of DEP and EF across four time-points.
| Estimate | 95% CI | Cohen’s d | |
|---|---|---|---|
| Within-person coupling effects | |||
| (DEP)[ΔT-1] ➔ Δ(EF)[ΔT] | −0.540* | [−0.955, −0.124] | −0.245 |
| (EF)[ΔT-1] ➔ Δ(DEP)[ΔT] | −0.540* | [−0.955, −0.124] | −0.245 |
| Within-person proportional effects | |||
| (DEP)[ΔT-1] ➔ Δ(DEP)[ΔT] | −0.462*** | [−0.738, −0.185] | 0.315 |
| (EF)[ΔT-1] ➔ Δ(EF)[ΔT] | 0.093** | [0.033, 0.152] | −0.298 |
| Between-person covariances | |||
| (DEP)[T-1] ↔ Δ(DEP)[ΔT] | 0.010 | [−0.038, 0.059] | 0.038 |
| (EF)[T-1] ↔ Δ(EF)[ΔT] | −0.021 | [−0.058, −0.017] | −0.106 |
| (EF)[T-1] ↔ Δ(DEP)[T-1] | −0.092*** | [−0.139, −0.045] | −0.368 |
| (EF)[ΔT] ↔ Δ(DEP)[ΔT] | −0.010* | [−0.018, 0.001] | −0.192 |
| (DEP)[T-1] ↔ Δ(EF)[ΔT] | −0.014 | [−0.032, 0.005] | −0.149 |
| (EF)[T-1] ↔ Δ(DEP)[ΔT] | 0.002 | [−0.031, 0.035] | −0.011 |
| (EF)[T] ↔ Δ(DEP)[T] | 0.003 | [−0.016, 0.022] | −0.029 |
| Between-person intercepts | |||
| Mean of (DEP)[T1] | 0.367*** | [0.313, 0.422] | 1.258 |
| Mean of Δ(DEP)[T] | 0.079 | [−0.000, 0.159] | 0.185 |
| Mean of (EF)[T1] | 0.003 | [−0.055, −0.061] | 0.010 |
| Mean of Δ(EF)[T] | −0.103*** | [−0.137, −0.068] | −0.549 |
| Variances | |||
| Variance of (DEP)[T1] | 0.278*** | [0.152, 0.405] | 0.411 |
| Residuals of Δ(DEP)[T] | 0.395*** | [0.315, 0.475] | 0.925 |
| Variance of Δ(DEP)[T] | 0.000 | – | – |
| Variance of (EF)[T1] | 0.696*** | [0.646, 0.746] | 2.673 |
| Residuals of Δ(EF)[T] | 0.067*** | [0.055, 0.079] | 1.072 |
| Variance of Δ(EF)[T] | 0.000 | – | – |
Note: Model fit indices: χ 2(df = 25) = 47.000, p = 0.005, CFI = 0.974, RMSEA = 0.039, 95% CI [0.021, 0.057]. Within-person coupling effects refer to change (Δ) in DEP at a prior time-lag (ΔT-1) predicting (➔) future Δ in EF at the next adjacent time-lag (ΔT; and vice versa). Within-person coupling effects and proportional effects, residual covariances between DEP and EF, as well as variances of DEP and EF were each uniquely fixed to be equal across all three time-lags.
Abbreviations: CI, confidence interval; DEP, depression severity; EF, executive functioning.
p < 0.05;
p < 0.01;
p < 0.001.
Figure 1.
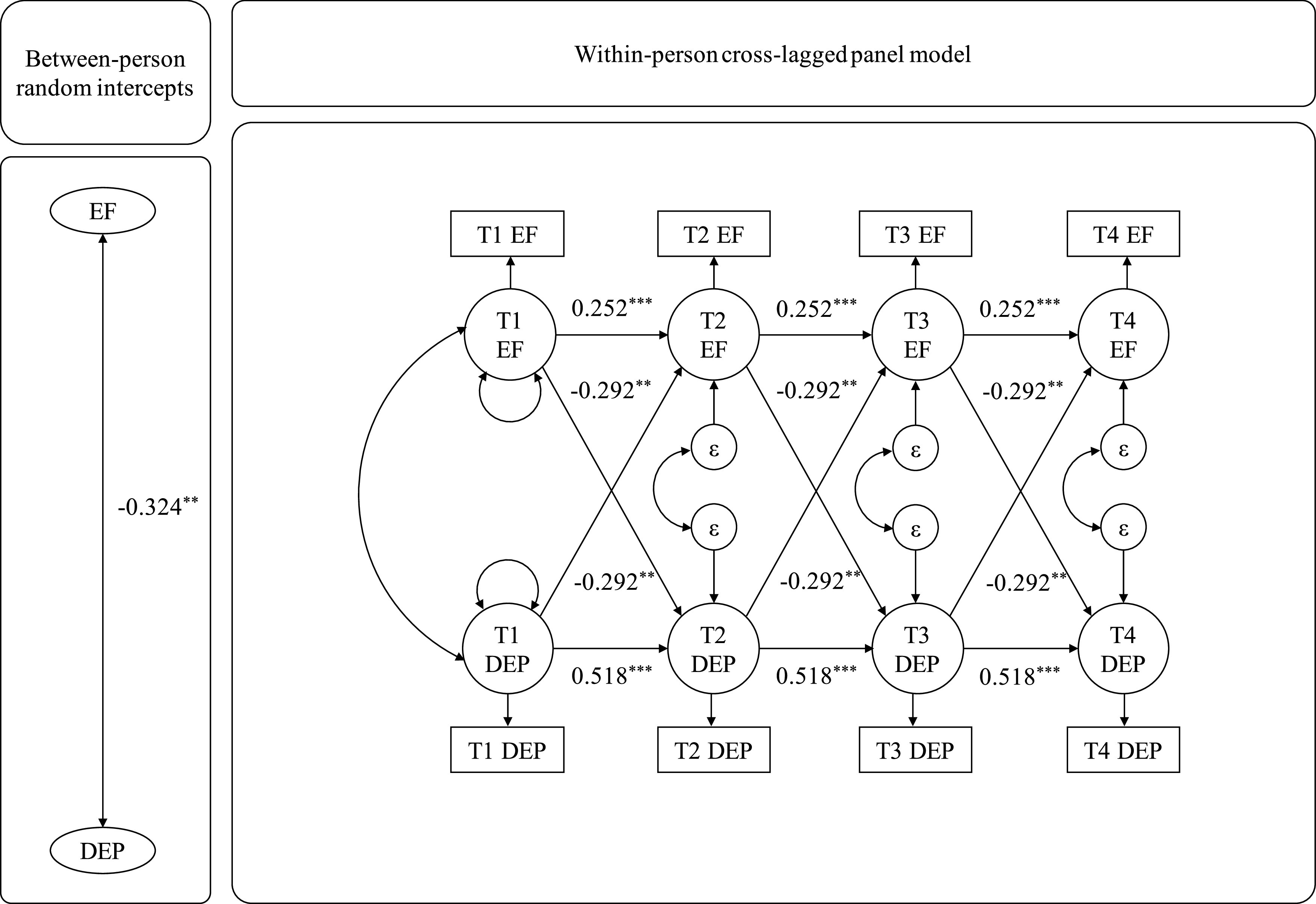
Random-Intercept Cross-Lagged Panel Models Between EF and Depression Severity.
Note. **p < .01; ***p < .001. Δ = within-person change in construct from a time-lag to the next adjacent time-lag; DEP = depression severity; EF = executive functioning.
Figure 2.
Bivariate Dual Latent Change Score Models Between EF and Depression Severity.
Note. **p < .01; ***p < .001. Δ = within-person change in construct from a time-lag to the next adjacent time-lag; DEP = depression severity; EF = executive functioning.
Lagged relations between anxiety severity and executive function
Table 3 shows the model parameter estimates for the RI-CLPM evaluating the cross-lagged relations between anxiety severity and EF. The freely estimated model was not significantly different from the constrained model (Δχ2[df = 1] = 0.069, p = 0.792). The final model with equality constraints on the cross-lagged effects demonstrated good model fit (χ2[df = 23] = 86.84, p < 0.001, CFI = 0.952, RMSEA = 0.057). Within persons, no cross-lagged relations were observed between prior anxiety severity and EF at the subsequent time-point (β = −0.025, 95% CI [−0.101, 0.051], d = −0.062). Likewise, no within-person cross-lagged relations were found between previous EF and anxiety severity at the next time-point (β = −0.025, 95% CI [−0.101, 0.051], d = −0.062). However, between persons, higher random intercept anxiety severity was significantly related to lower random intercept EF (β = −0.070, 95% CI [−0.104, −0.036], d = −0.395).
Table 3.
Random-intercepts cross-lagged panel model of ANX and EF across four time-points.
| Estimate | 95% CI | Cohen’s d | |
|---|---|---|---|
| Within-person cross-lagged effects | |||
| (ANX)[T-1] ➔ (EF)[T] | −0.025 | [−0.101, 0.051] | −0.062 |
| (EF)[T-1] ➔ (ANX)[T] | −0.025 | [−0.101, 0.051] | −0.062 |
| Within-person autoregressive effects | |||
| (ANX)[T-1] ➔ (ANX)[T] | 0.120* | [0.026, 0.213] | 0.245 |
| (EF)[T-1] ➔ (EF)[T] | 0.287 | [0.070, 0.643] | 0.151 |
| Between-person covariances | |||
| (EF)[T] ↔ (ANX)[T] | −0.008 | [−0.020, 0.003] | −0.128 |
| RI(EF)[T] ↔ RI(ANX)[T] | −0.070*** | [−0.104, −0.036] | −0.395 |
| RI(ANX)[T] ↔ (ANX)[T1] | 0.104* | [0.006, 0.201] | 0.200 |
| RI(EF)[T] ↔ (EF)[T1] | −0.125*** | [−0.175, −0.074] | −0.462 |
| Between-person intercepts | |||
| Mean of (ANX)[T1] | 0.229*** | [0.182, 0.277] | 0.916 |
| Mean of (ANX)[T2] | 0.141*** | [0.064, 0.217] | 0.347 |
| Mean of (ANX)[T3] | 0.176*** | [0.109, 0.243] | 0.497 |
| Mean of (ANX)[T4] | 0.149*** | [0.76, 0.222] | 0.387 |
| Mean of (EF)[T1] | −0.000 | [−0.058, 0.058] | 0.000 |
| Mean of (EF)[T2] | −0.096* | [−0.171, −0.021] | −0.243 |
| Mean of (EF)[T3] | −0.657*** | [−0.733, −0.582] | −1.617 |
| Mean of (EF)[T4] | −0.789*** | [−0.875, −0.704] | −1.762 |
| Variances | |||
| Variance of RI(ANX)[T1-T4] | 0.000 | – | – |
| Variance of RI(EF)[T1-T4] | 0.912*** | [0.816, 1.009] | 1.786 |
| Variance of (ANX)[T1] | 0.297** | [0.107, 0.486] | 1.584 |
| Variance of (EF)[T1] | 0.088*** | [0.052, 0.123] | 0.338 |
| Variance of (ANX)[T2-T4] | 0.262*** | [0.151, 0.373] | 0.441 |
| Variance of (EF)[T2-T4] | 0.090*** | [0.058, 0.122] | 0.540 |
Note: Model fit indices: χ2(df = 24) = 33.102, p = 0.102, CFI = 0.982, RMSEA = 0.021, 95% CI [0.008, 0.031]. Within-person cross-lagged effects refer to level in ANX at a prior time-point (T-1) predicting (➔) future Δ in EF at the next adjacent time-point (T) (and vice versa). Within-person coupling effects and proportional effects, residual covariances between ANX and EF, as well as variances of ANX and EF were each uniquely fixed to be equal across all three time-lags.
Abbreviations: ANX, anxiety severity; CI, confidence interval; EF, executive functioning; RI, random intercept.
p < 0.05.
p < 0.01.
p < 0.001.
Table 4 shows the parameter estimates for the BLCS models testing the change-to-future change relations between anxiety severity and EF. The constrained (vs. freely estimated) models were not significantly different (Δχ2[df = 1] = 0.005, p = 0.943). The final model with equality constraints on the coupling parameters showed acceptable model fit (χ2[df = 25] = 46.996, p < 0.001, CFI = 0.966, RMSEA = 0.057). Within persons, prior change in anxiety severity at a previous time-lag was not significantly associated with change in EF at the subsequent time-lag (β = −0.254, 95% CI [−0.951, 0.444], d = −0.068) and vice versa (β = −0.254, 95% CI [−0.951, 0.444], d = −0.068). 1
Table 4.
Bivariate dual latent change score model of ANX and EF across four time-points.
| Estimate | 95% CI | Cohen’s d | |
|---|---|---|---|
| Within-person coupling effects | |||
| (ANX)[ΔT-1] ➔ Δ(EF)[ΔT] | −0.254 | [−0.951, 0.444] | −0.068 |
| (EF)[ΔT-1] ➔ Δ(ANX)[ΔT] | −0.254 | [−0.951, 0.444] | −0.068 |
| Within-person proportional effects | |||
| (ANX)[ΔT-1] ➔ Δ(ANX)[ΔT] | 0.006 | [−0.597, 0.608] | 0.002 |
| (EF)[ΔT-1] ➔ Δ(EF)[ΔT] | 0.080** | [0.025, 0.135] | 0.274 |
| Between-person covariances | |||
| (ANX)[T-1] ↔ Δ(ANX)[ΔT] | −0.049*** | [−0.077, −0.021] | −0.336 |
| (EF)[T-1] ↔ Δ(EF)[ΔT] | −0.014 | [−0.044, 0.016] | −0.090 |
| (EF)[T-1] ↔ Δ(ANX)[T-1] | −0.101*** | [−0.144, −0.058] | −0.441 |
| (EF)[ΔT] ↔ Δ(ANX)[ΔT] | −0.007 | [−0.017, 0.002] | −0.134 |
| (ANX)[T-1] ↔ Δ(EF)[ΔT] | 0.007 | [−0.016, 0.030] | 0.056 |
| (EF)[T-1] ↔ Δ(ANX)[ΔT] | 0.031 | [−0.016, 0.078] | 0.124 |
| (EF)[T] ↔ Δ(ANX)[T] | 0.004 | [−0.009, 0.018] | 0.055 |
| Between-person intercepts | |||
| Mean of (ANX)[T1] | 0.224*** | [0.178, 0.270] | 0.935 |
| Mean of Δ(ANX)[T] | −0.052 | [−0.155, 0.051] | −0.096 |
| Mean of (EF)[T1] | 0.003 | [−0.055, 0.061] | 0.010 |
| Mean of Δ(EF)[T] | −0.102*** | [−0.136, −0.068] | −0.576 |
| Variances | |||
| Variance of (ANX)[T1] | 0.231* | [0.056, 0.407] | 0.246 |
| Residuals of Δ(ANX)[T] | 0.256*** | [0.138, 0.374] | 0.410 |
| Variance of Δ(ANX)[T] | 0.000 | – | – |
| Variance of (EF)[T1] | 0.692*** | [0.642, 0.742] | 2.657 |
| Residuals of Δ(EF)[T] | 0.071*** | [0.058, 0.084] | 0.974 |
| Variance of Δ(EF)[T] | 0.000 | – | – |
Note: Model fit indices: χ2(df = 25) = 46.996, p < 0.001, CFI = 0.966, RMSEA = 0.057, 95% CI [0.044, 0.071]. Within-person coupling effects refer to change (Δ) in ANX at a prior time-lag (ΔT-1) predicting (➔) future Δ in EF at the next adjacent time-lag (ΔT; and vice versa). Within-person coupling effects and proportional effects, residual covariances between ANX and EF, as well as variances of ANX and EF were each uniquely fixed to be equal across all three time-lags.
Abbreviations: ANX, anxiety severity; CI, confidence interval; EF, executive functioning.
p < 0.05.
p < 0.01.
p < 0.001.
Discussion
Partially supporting scar and vulnerability hypotheses, robust RI-CLPM and BLCS methods showed that within persons, higher prior level and change in depression (but not anxiety) severity predicted greater reduced EF at the next time-point and subsequent time-lag, and conversely. Simultaneously, these models demonstrated stronger between-person, cross-sectional magnitude between EF and anxiety compared to EF and depression severity. Overall, findings concurred with up-to-date, cross-sectional, between-person evidence from recent meta-analytic data (e.g., [36]). Results also extended an early seminal cross-sectional study [98] which observed that whereas patients with (vs. without) depression performed poorly on auditory and visual WM tasks, patients with anxiety disorders attained scores comparable to healthy controls. Findings also built on hierarchical linear modeling results that whereas inverse EF-depression relations tended to predominate within persons, negative EF-anxiety associations tended to be larger between persons [99]. The divergence between within- and between-person findings for anxiety is likely due to the fact that between-person analyses do not account for individual differences in person-specific changes across time. Whereas between-person differences across time could be due to group differences in stable variations observed across the lifespan, they may not be capturing individual differences in aging-associated rate of EF or mental health deterioration. In fact, whereas prior between-person findings were interpreted to suggest that moderate levels of anxiety (but not depression) could facilitate performance on EF tests up to a certain point, this relation has not held up when examined at the within-person level [100–102]. Another potential explanation pertains to the fact that anxiety (vs. depression) severity tends to be more stable across the lifespan, as illustrated by prospective [103] and gene–environment studies [104]. Accordingly, higher stability and lower variability in anxiety severity across the lifespan could translate to stronger predominance of between-person, as opposed to within-person, effects on EF over long durations. Clearly, more longitudinal work is needed to test these notions.
Why did rise in depression severity consistently predict future EF decline at the next time-point and time-lag within persons? Overall, our findings offered support for scar theories. Conceivably, recurrent depression episodes might be a factor in cognitive functioning decline and diseased neurological aging processes (e.g., shrinkage in learning- and EF-linked brain regions and white matter hyperintensities) over the years [105,106]. Biologically, elevated depression might have this adverse effect on EF across prolonged durations via chronic wear-and-tear of the hypothalamic–pituitary–adrenal axis function, such as buildup of glucocorticoids and proinflammatory cytokines (e.g., C-reactive protein) [107,108]. On that note, elevated depression might precede or speed up the onset of dementia, possibly via the accumulation of neurofibrillary plaques and tangles in emotion modulation-, EF-, and learning-related brain areas [109–111]. Equally tenable are scar models centering on behavioral, environmental, and lifestyle factors observed for extended durations in depression (e.g., decreased physical exercise, suboptimal sleep, diet, and nutrition), that could impact proinflammatory and cardiovascular processes [112,113]. Future longitudinal studies using RI-CLPM and BLCS models can further examine the “neurotoxic” scar effect of increased depression.
Findings suggested that reduced EF functioned as a risk factor for subsequent heightened depression (but not anxiety) within persons. This could be because poorer EF may have compromised abilities to harness “top-down” cognitive control over depressed mood (but not necessarily anxiety symptoms), and to refocus thoughts and actions to create and sustain more positive emotions (e.g., via engaging in mood-lifting activities or searching for suitable social support). However, the result that change in EF deficits did not forecast change in future anxiety within persons was inconsistent with prior longitudinal, between-person studies that found evidence supporting the vulnerability hypothesis. For instance, two studies showed that EF deficits were risk factors for generalized anxiety disorder symptoms across time (e.g., [35,114]). Also, using BLCS, two studies observed that within-person rise in anxiety or trait neuroticism at a time-lag predicted worsened cognitive functioning at the next time-lag [57,115] in community-dwelling Swedish adults. Similarly, another recent BLCS study found that within persons, worse cognitive functioning forecasted increased anxiety and depression across 4 years in patients with Parkinson’s disease [116]. Differences in data analysis (e.g., linear regression vs. SEM), sample characteristics (e.g., age and data collection site), anxiety measures (e.g., worry vs. anxiety symptoms), and study design (e.g., time-lags) might account for such variability in findings.
In addition, between persons, cross-sectional relations between lower EF and greater depression or anxiety severity were reliably observed. Observations at the between-person level are concordant with several community-based studies. For example, poorer EF facets (e.g., shifting and inhibition) or global cognition have been shown to consistently forecast increased worry, anxiety, and depression at a later time in children [117], adolescents [118], mid-life adults [35], and older adults [114], across 3–12 years. Our study extended those findings by bolstering arguments that the strength and sign of magnitudes between within- and between-person associations might not coincide [56]. The field can benefit from using prospective designs (e.g., cross-panel and ecological momentary assessment) and SEM to clarify the between- and within-person relations among EF, depression, and anxiety severity across years and smaller timescales (e.g., within-day and day-to-day fluctuations) [48,119].
Relatedly, the cross-sectional, between-person negative associations between anxiety or depression and EF in this study may be accounted for by the attentional control theory [120] and attentional scope model of rumination [121]. Note that these theories are inappropriate for explaining the within-person, cross-lagged, and long-term change-to-future change relations between EF and depression severity found herein as they argue that symptom-EF perturbation relations occur across brief durations or at one time-point [49,122]. Further, these models assert that elevated symptoms could deplete finite EF resources for task-pertinent processing and increased anxiety and depression would be reliably linked to greater cognitive rigidity (i.e., difficulty disengaging from threat or distractions) at a single time-point. Such mechanisms may unfold through excessive repetitive negative thinking, such as worry, brooding, and obsessions, as consistently evidenced by cross-sectional or experimental meta-analytic data [123–125].
Findings must be interpreted in light of study strengths and limitations. Unmeasured third variables (e.g., genetics) [126] may have contributed to observed outcomes. Additionally, although other studies have observed within- or between-person relations between depression and EF domains of shifting and inhibition [49], consistent with theory and neuroanatomical evidence [127], these EF facets were not measured herein. Also, as no structured diagnostic interviews were included, future studies that include such diagnostic instruments could determine if the results would be similar. In addition, given the predominantly White sample, subsequent investigations can determine if outcomes extend to culturally diverse populations by conducting multiple-group SEM (e.g., [128]). Limitations notwithstanding, study strengths included the large and well-powered sample size, administration of behavioral EF and caregiver-rated symptom assessments, four-wave cross-panel longitudinal dataset, and use of potent SEM approaches.
If the pattern of results herein was replicated, some clinical implications deserve consideration. Offering preventive interventions at early signals of increased depression might assist with remediating depression, but would also probably benefit EF capacities. Moreover, the field could benefit from continuing to test EF indices as reliable predictors or markers of treatment response for depression and anxiety, as suggested by various studies (e.g., [129,130]). Relatedly, based on recent evidence, such efforts might be augmented by investigating if cognitive-behavioral therapies (CBTs) (e.g., behavioral activation, cognitive remediation, problem-solving therapy, personalized, environment-focused, and technology-facilitated CBTs) [131–136], mindfulness-based interventions [137], EF training [138], and pharmacological treatments [139], could simultaneously alleviate depression and enhance EF capacities.
Footnotes
As part of a sensitivity analysis, we determined that the results were similar when analyses were restricted to a sample without baseline major depression and cognitive or physical disabilities (n = 611), as shown on page 9 of the OSM.
Financial Support
The ADAMS study has been funded by the following grants and agencies: the National Institute on Aging (grant numbers U01 AG09740 and K08 AG19180); the New Investigator Research Grant from the Alzheimer’s Association; the Paul Beeson Physician Faculty Scholars in Aging Research award; National Institutes of Health (grant number K12 HD01438); and the National Institute of Mental Health (grant number K24 70027). The original investigators and funding agency are not responsible for the analyses or interpretations presented here.
Conflict of Interest
The authors declare that there are no conflicts of interest.
Data Availability Statement
The data that support the findings of this study are available from Health and Retirement Study (HRS)—ADAMS—website. Data are available at https://hrs.isr.umich.edu/publications/biblio/5761 with the permission of the study team principal investigators and team members, G. H. Steven, G. F. Gwenith, D. H. Michael, M. L. Kenneth, O. Mary Beth, L. P. Brenda, R. W. David, and colleagues.
Authorship Contributions
The research team, Michelle G. Newman, and Nur Hani Zainal took full responsibility for the data, the accuracy of analyses and interpretation, as well as conduct of the research. All authors have made substantial contributions to analysis and interpretation of the study and its findings; drafted and revised the article for intellectual content; gave their final approval of the version to be submitted; and read and approved the manuscript.
Ethical Statement
This study was conducted in compliance with the American Psychological Association (APA) and Declaration of Helsinki ethical standards in the treatment of human participants and approved by the institutional review board (IRB). Informed consent was obtained from participants as per IRB requirements at the University of Michigan at Ann Arbor and Duke University. Since this study used a publicly available dataset, it was exempt from additional IRB approval.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2021.2217.
click here to view supplementary material
References
- [1].Nigg JT. On the relations among self-regulation, self-control, executive functioning, effortful control, cognitive control, impulsivity, risk-taking, and inhibition for developmental psychopathology. J Child Psychol Psychiatry. 2017;58:361–83. doi: 10.1111/jcpp.12675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].McClelland MM, Cameron CE. Developing together: the role of executive function and motor skills in children’s early academic lives. Early Child Res Q. 2019;46:142–51. doi: 10.1016/j.ecresq.2018.03.014. [DOI] [Google Scholar]
- [3].Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, Garcia-Barrera MA. The unity and diversity of executive functions: a systematic review and re-analysis of latent variable studies. Psychol Bull. 2018;144:1147–85. doi: 10.1037/bul0000160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Baggetta P, Alexander PA. Conceptualization and operationalization of executive function. Mind Brain Educ. 2016;10:10–33. doi: 10.1111/mbe.12100. [DOI] [Google Scholar]
- [5].Friedman NP, Miyake A, Young SE, DeFries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008;137:201–25. doi: 10.1037/0096-3445.137.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aita SL, Beach JD, Taylor SE, Borgogna NC, Harrell MN, Hill BD. Executive, language, or both? An examination of the construct validity of verbal fluency measures. Appl Neuropsychol Adult. 2019;26:441–51. doi: 10.1080/23279095.2018.1439830. [DOI] [PubMed] [Google Scholar]
- [7].Gustavson DE, Panizzon MS, Franz CE, Reynolds CA, Corley RP, Hewitt JK, et al. Integrating verbal fluency with executive functions: evidence from twin studies in adolescence and middle age. J Exp Psychol Gen. 2019;148:2104–19. doi: 10.1037/xge0000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Delis DC, Kaplan E, Kramer JH. The Delis–Kaplan executive function system: examiner’s manual. San Antonio, NM: The Psychological Corporation; 2001. [Google Scholar]
- [9].Friesen DC, Edwards K, Lamoureux C. Predictors of verbal fluency performance in monolingual and bilingual children: the interactive role of English receptive vocabulary and fluid intelligence. J Commun Disord. 2021;89:106074 doi: 10.1016/j.jcomdis.2020.106074. [DOI] [PubMed] [Google Scholar]
- [10].Gustavson DE, Elman JA, Panizzon MS, Franz CE, Zuber J, Sanderson-Cimino M, et al. Association of baseline semantic fluency and progression to mild cognitive impairment in middle-aged men. Neurology. 2020;95:e973–83. doi: 10.1212/wnl.0000000000010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Favieri F, Forte G, Casagrande M. The executive functions in overweight and obesity: a systematic review of neuropsychological cross-sectional and longitudinal studies. Front Psychol. 2019;10:2126 doi: 10.3389/fpsyg.2019.02126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Pineda-Alhucema W, Aristizabal E, Escudero-Cabarcas J, Acosta-López JE, Vélez JI. Executive function and theory of mind in children with ADHD: a systematic review. Neuropsychol Rev. 2018;28:341–58. doi: 10.1007/s11065-018-9381-9. [DOI] [PubMed] [Google Scholar]
- [13].Berryman C, Stanton TR, Bowering KJ, Tabor A, McFarlane A, Moseley GL. Do people with chronic pain have impaired executive function? A meta-analytical review. Clin Psychol Rev. 2014;34:563–79. doi: 10.1016/j.cpr.2014.08.003. [DOI] [PubMed] [Google Scholar]
- [14].Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174–80. doi: 10.1016/j.tics.2012.01.006. [DOI] [PubMed] [Google Scholar]
- [15].Gałecki P, Talarowska M, Anderson G, Berk M, Maes M. Mechanisms underlying neurocognitive dysfunctions in recurrent major depression. Med Sci Monit. 2015;21:1535–47. doi: 10.12659/MSM.893176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Juruena MF, Bocharova M, Agustini B, Young AH. Atypical depression and non-atypical depression: Is HPA axis function a biomarker? A systematic review. J Affect Disord. 2018;233:45–67. doi: 10.1016/j.jad.2017.09.052. [DOI] [PubMed] [Google Scholar]
- [17].Copeland WE, Shanahan L, Worthman C, Angold A, Costello EJ. Generalized anxiety and C-reactive protein levels: a prospective, longitudinal analysis. Psychol Med. 2012;42:2641–50. doi: 10.1017/S0033291712000554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Alexopoulos GS, Meyers BS, Young RC, Campbell S, Silbersweig D, Charlson M. ‘Vascular depression’ hypothesis. Arch Gen Psychiatry. 1997;54:915–22. doi: 10.1001/archpsyc.1997.01830220033006. [DOI] [PubMed] [Google Scholar]
- [19].Alexopoulos GS. Mechanisms and treatment of late-life depression. Transl Psychiatry. 2019;9:188–8. doi: 10.1038/s41398-019-0514-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Alexopoulos GS. Depression and cerebrovascular disease: What is to be done? Am J Geriatr Psychiatry. 2017;25:129–30. doi: 10.1016/j.jagp.2016.11.013. [DOI] [PubMed] [Google Scholar]
- [21].Alexopoulos GS, Hoptman MJ, Yuen G, Kanellopoulos D, Seirup JK, Lim KO, et al. Functional connectivity in apathy of late-life depression: a preliminary study. J Affect Disord. 2013;149:398–405. doi: 10.1016/j.jad.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Alexopoulos GS, Hoptman MJ, Kanellopoulos D, Murphy CF, Lim KO, Gunning FM. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J Affect Disord. 2012;139:56–65. doi: 10.1016/j.jad.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Beaujean AA, Parker S, Qiu X. The relationship between cognitive ability and depression: a longitudinal data analysis. Soc Psychiatry Psychiatr Epidemiol. 2013;48:1983–92. doi: 10.1007/s00127-013-0668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hammar Å, Sørensen L, Årdal G, Oedegaard KJ, Kroken R, Roness A, et al. Enduring cognitive dysfunction in unipolar major depression: a test–retest study using the Stroop paradigm. Scand J Psychol. 2010;51:304–8. doi: 10.1111/j.1467-9450.2009.00765.x. [DOI] [PubMed] [Google Scholar]
- [25].Wilson RS, Mendes de Leon CF, Bennett DA, Bienias JL, Evans DA. Depressive symptoms and cognitive decline in a community population of older persons. J Neurol Neurosurg Psychiatry. 2004;75:126. [PMC free article] [PubMed] [Google Scholar]
- [26].Gulpers BJA, Oude Voshaar RC, van Boxtel MPJ, Verhey FRJ, Köhler S. Anxiety as a risk factor for cognitive decline: a 12-year follow-up cohort study. Am J Geriatr Psychiatry. 2019;27:42–52. doi: 10.1016/j.jagp.2018.09.006. [DOI] [PubMed] [Google Scholar]
- [27].Gulpers B, Ramakers I, Hamel R, Köhler S, Oude Voshaar R, Verhey F. Anxiety as a predictor for cognitive decline and dementia: a systematic review and meta-analysis. Am J Geriatr Psychiatry. 2016;24:823–42. doi: 10.1016/j.jagp.2016.05.015. [DOI] [PubMed] [Google Scholar]
- [28].Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry. 2013;202:329–35. doi: 10.1192/bjp.bp.112.118307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Koster EHW, De Lissnyder E, Derakshan N, De Raedt R. Understanding depressive rumination from a cognitive science perspective: the impaired disengagement hypothesis. Clin Psychol Rev. 2011;31:138–45. doi: 10.1016/j.cpr.2010.08.005. [DOI] [PubMed] [Google Scholar]
- [30].Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: a meta-analytic review. Clin Psychol Rev. 2010;30:217–37. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- [31].Cabrera I, Brugos D, Montorio I. Attentional biases in older adults with generalized anxiety disorder. J Anxiety Disord. 2020;71:102207. doi: 10.1016/j.janxdis.2020.102207. [DOI] [PubMed] [Google Scholar]
- [32].Barry TJ, Vervliet B, Hermans D. An integrative review of attention biases and their contribution to treatment for anxiety disorders. Front Psychol. 2015;6:968. doi: 10.3389/fpsyg.2015.00968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Hantke NC, Gyurak A, Van Moorleghem K, Waring JD, Adamson MM, O’Hara R, et al. Disentangling cognition and emotion in older adults: the role of cognitive control and mental health in emotional conflict adaptation. Int J Geriatr Psychiatry. 2017;32:840–8. doi: 10.1002/gps.4535. [DOI] [PubMed] [Google Scholar]
- [34].Harvey PO, Le Bastard G, Pochon JB, Levy R, Allilaire JF, Dubois B, et al. Executive functions and updating of the contents of working memory in unipolar depression. J Psychiatr Res. 2004;38:567–76. doi: 10.1016/j.jpsychires.2004.03.003. [DOI] [PubMed] [Google Scholar]
- [35].Zainal NH, Newman MG. Executive function and other cognitive deficits are distal risk factors of generalized anxiety disorder 9 years later. Psychol Med. 2018;48:2045–53. doi: 10.1017/S0033291717003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Scult MA, Paulli AR, Mazure ES, Moffitt TE, Hariri AR, Strauman TJ. The association between cognitive function and subsequent depression: a systematic review and meta-analysis. Psychol Med. 2017;47:1–17. doi: 10.1017/S0033291716002075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Molenaar PCM, Huizenga HM, Nesselroade JR. The relationship between the structure of interindividual and intraindividual variability: a theoretical and empirical vindication of developmental systems theory. In: Staudinger UM, Lindenberger U, editors. Understanding human development: dialogues with lifespan psychology. Boston, MA: Springer US; 2003, p. 339–60. [Google Scholar]
- [38].Lang L, Clifford A, Wei L, Zhang D, Leung D, Augustine G, et al. Prevalence and determinants of undetected dementia in the community: a systematic literature review and a meta-analysis. BMJ Open. 2017;7:e011146 doi: 10.1136/bmjopen-2016-011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Wu Y-T, Brayne C, Matthews FE. Prevalence of dementia in East Asia: a synthetic review of time trends. Int J Geriatr Psychiatry. 2015;30:793–801. doi: 10.1002/gps.4297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cohen BE, Edmondson D, Kronish IM. State of the art review: depression, stress, anxiety, and cardiovascular disease. Am J Hypertens. 2015;28:1295–302. doi: 10.1093/ajh/hpv047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Gustavson DE, Panizzon MS, Elman JA, Franz CE, Reynolds CA, Jacobson KC, et al. Stability of genetic and environmental influences on executive functions in midlife. Psychol Aging. 2018;33:219–31. doi: 10.1037/pag0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Braun T, Schmukle SC, Kunzmann U. Stability and change in subjective well-being: the role of performance-based and self-rated cognition. Psychol Aging. 2017;32:105–17. doi: 10.1037/pag0000153. [DOI] [PubMed] [Google Scholar]
- [43].Johnson JK, Gross AL, Pa J, McLaren DG, Park LQ, Manly JJ, et al. Longitudinal change in neuropsychological performance using latent growth models: a study of mild cognitive impairment. Brain Imaging Behav. 2012;6:540–50. doi: 10.1007/s11682-012-9161-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Small BJ, Dixon RA, McArdle JJ. Tracking cognition–health changes from 55 to 95 years of age. J Gerontol B. 2011;66B:i153–61. doi: 10.1093/geronb/gbq093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Yu D, Yang PJ, Geldhof GJ, Tyler CP, Gansert PK, Chase PA, et al. Exploring idiographic approaches to children’s executive function performance: an intensive longitudinal study. J Pers Oriented Res. 2020;6:73–87. doi: 10.17505/jpor.2020.22401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Hülür G, Ram N, Willis SL, Schaie KW, Gerstorf D. Cognitive aging in the Seattle longitudinal study: within-person associations of primary mental abilities with psychomotor speed and cognitive flexibility. J Intelligence. 2016;4:12. doi: 10.3390/jintelligence4030012. [DOI] [Google Scholar]
- [47].Kievit RA, Brandmaier AM, Ziegler G, van Harmelen A-L, de Mooij SMM, Moutoussis M, et al. Developmental cognitive neuroscience using latent change score models: a tutorial and applications. Dev Cogn Neurosci. 2018;33:99–117. doi: 10.1016/j.dcn.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sliwinski MJ, Mogle JA, Hyun J, Munoz E, Smyth JM, Lipton RB. Reliability and validity of ambulatory cognitive assessments. Assessment. 2018;25:14–30. doi: 10.1177/1073191116643164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Zainal NH, Newman MG. Within-person increase in pathological worry predicts future depletion of unique executive functioning domains. Psychol Med. 2020;1–11. doi: 10.1017/S0033291720000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Brose A, Schmiedek F, Lövdén M, Lindenberger U. Daily variability in working memory is coupled with negative affect: the role of attention and motivation. Emotion. 2012;12:605–17. doi: 10.1037/a0024436. [DOI] [PubMed] [Google Scholar]
- [51].Allerhand M, Gale CR, Deary IJ. The dynamic relationship between cognitive function and positive well-being in older people: a prospective study using the English longitudinal study of aging. Psychol Aging. 2014;29:306–18. doi: 10.1037/a0036551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Lenze EJ, Nicol GE, Barbour DL, Kannampallil T, Wong AWK, Piccirillo J, et al. Precision clinical trials: a framework for getting to precision medicine for neurobehavioural disorders. J Psychiatry Neurosci. 2021;46:e97–e110. doi: 10.1503/jpn.200042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Marzano L, Bardill A, Fields B, Herd K, Veale D, Grey N, et al. The application of mHealth to mental health: opportunities and challenges. Lancet Psychiatry. 2015;2:942–8. doi: 10.1016/S2215-0366(15)00268-0. [DOI] [PubMed] [Google Scholar]
- [54].Zuidersma M, Riese H, Snippe E, Booij SH, Wichers M, Bos EH. Single-subject research in psychiatry: facts and fictions. Front Psych. 2020;11:539777 doi: 10.3389/fpsyt.2020.539777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Moore RC, Campbell LM, Delgadillo JD, Paolillo EW, Sundermann EE, Holden J, et al. Smartphone-based measurement of executive function in older adults with and without HIV. Arch Clin Neuropsychol. 2020;35:347–57. doi: 10.1093/arclin/acz084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Fisher AJ, Medaglia JD, Jeronimus BF. Lack of group-to-individual generalizability is a threat to human subjects research. Proc Natl Acad Sci. 2018;115:E6106–15. doi: 10.1073/pnas.1711978115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Zainal NH, Newman MG. Larger increase in trait negative affect is associated with greater future cognitive decline and vice versa across 23 years. Depress Anxiety. 2021;38:146–60. doi: 10.1002/da.23093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Hamaker EL, Kuiper RM, Grasman RPPP. A critique of the cross-lagged panel model. Psychol Methods. 2015;20:102–16. doi: 10.1037/a0038889. [DOI] [PubMed] [Google Scholar]
- [59].Grimm KJ, Ram N. Latent growth and dynamic structural equation models. Annu Rev Clin Psychol. 2018;14:55–89. doi: 10.1146/annurev-clinpsy-050817-084840. [DOI] [PubMed] [Google Scholar]
- [60].Mund M, Nestler S. Beyond the cross-lagged panel model: next-generation statistical tools for analyzing interdependencies across the life course. Adv Life Course Res. 2019;41:100249. doi: 10.1016/j.alcr.2018.10.002. [DOI] [PubMed] [Google Scholar]
- [61].Oh Y, Greenberg MT, Willoughby MT, Vernon-Feagans L, Greenberg MT, Blair CB, et al. Examining longitudinal associations between externalizing and internalizing behavior problems at within- and between-child levels. J Abnorm Child Psychol. 2020;48:467–80. doi: 10.1007/s10802-019-00614-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Widaman KF, Ferrer E, Conger RD. Factorial invariance within longitudinal structural equation models: measuring the same construct across time. Child Dev Perspect. 2010;4:10–8. doi: 10.1111/j.1750-8606.2009.00110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Steven GH, Gwenith GF, Michael DH, Kenneth ML, Mary Beth O, Brenda LP, et al. Aging, demographics and memory study (ADAMS): sample design, weighting and analysis for ADAMS. Ann Arbor, MI: Institute for Social Research, University of Michigan; 2009. [Google Scholar]
- [64].American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-IV). Washington, DC: American Psychiatry Press; 1994. [Google Scholar]
- [65].American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- [66].Langa KM, Plassman BL, Wallace RB, Herzog AR, Heeringa SG, Ofstedal MB, et al. The aging, demographics, and memory study (ADAMS): study design and methods. Neuroepidemiology. 2005;25:181–91. doi: 10.1159/000087448. [DOI] [PubMed] [Google Scholar]
- [67].Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- [68].Mehta KM, Stewart AL, Langa KM, Yaffe K, Moody-Ayers S, Williams BA, et al. “Below average” self-assessed school performance and Alzheimer’s disease in the aging, demographics, and memory study. Alzheimers Dement. 2009;5:380–7. doi: 10.1016/j.jalz.2009.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Fisher GG, Franks MM, Plassman BL, Brown SL, Potter GG, Llewellyn D, et al. Caring for individuals with dementia and cognitive impairment, not dementia: findings from the aging, demographics, and memory study. J Am Geriatr Soc. 2011;59:488–94. doi: 10.1111/j.1532-5415.2010.03304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Steffens DC, Fisher GG, Langa KM, Potter GG, Plassman BL. Prevalence of depression among older Americans: the aging, demographics and memory study. Int Psychogeriatr. 2009;21:879–88. doi: 10.1017/S1041610209990044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].de Medeiros K, Robert P, Gauthier S, Stella F, Politis A, Leoutsakos J, et al. The neuropsychiatric inventory-clinician rating scale (NPI-C): reliability and validity of a revised assessment of neuropsychiatric symptoms in dementia. Int Psychogeriatr. 2010;22:984–94. doi: 10.1017/S1041610210000876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Borkowski JG, Benton AL, Spreen O. Word fluency and brain damage. Neuropsychologia. 1967;5:135–40. doi: 10.1016/0028-3932(67)90015-2. [DOI] [Google Scholar]
- [73].Ardila A, Ostrosky‐Solís F, Bernal B. Cognitive testing toward the future: the example of semantic verbal fluency (ANIMALS). Int J Psychol. 2006;41:324–32. doi: 10.1080/00207590500345542. [DOI] [Google Scholar]
- [74].Manning RT. The serial sevens test. Arch Intern Med. 1982;142:1192. doi: 10.1001/archinte.1982.00340190148022. [DOI] [PubMed] [Google Scholar]
- [75].Wechsler D. Wechsler adult intelligence scale-III (WAIS- III) manual. New York: The Psychological Corporation; 1997. [Google Scholar]
- [76].Smith A. Symbol digit modalities test. Los Angeles, CA: Western Psychological Services; 1982. [Google Scholar]
- [77].Oliveira RM, Mograbi DC, Gabrig IA, Charchat-Fichman H. Normative data and evidence of validity for the Rey auditory verbal learning test, verbal fluency test, and Stroop test with Brazilian children. Psychol Neurosci. 2016;9:54–67. doi: 10.1037/pne0000041. [DOI] [Google Scholar]
- [78].Jaeggi SM, Buschkuehl M, Perrig WJ, Meier B. The concurrent validity of the N-back task as a working memory measure. Memory. 2010;18:394–412. doi: 10.1080/09658211003702171. [DOI] [PubMed] [Google Scholar]
- [79].Hinton-Bayre A, Geffen G. Comparability, reliability, and practice effects on alternate forms of the digit symbol substitution and symbol digit modalities tests. Psychol Assess. 2005;17:237–41. doi: 10.1037/1040-3590.17.2.237. [DOI] [PubMed] [Google Scholar]
- [80].Fong TG, Fearing MA, Jones RN, Shi P, Marcantonio ER, Rudolph JL, et al. Telephone interview for cognitive status: creating a crosswalk with the mini-mental state examination. Alzheimers Dement. 2009;5:492–7. doi: 10.1016/j.jalz.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Herzog AR, Wallace RB. Measures of cognitive functioning in the AHEAD study. J Gerontol B Psychol Sci Soc Sci. 1997;52B:37–48. doi: 10.1093/geronb/52B.Special_Issue.37. [DOI] [PubMed] [Google Scholar]
- [82].Plassman BL, Newman TT, Welsh KA, Helms M, JCS B. Properties of the telephone interview for cognitive status: application in epidemiological and longitudinal studies. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:235–41. [Google Scholar]
- [83].Welsh KA, Breitner JC, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1993;6:103–10. [Google Scholar]
- [84].Rosseel Y. Lavaan: an R package for structural equation modeling. J Stat Softw. 2012;48:1–36. doi: 10.18637/jss.v048.i02. [DOI] [Google Scholar]
- [85].Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–46. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- [86].Steiger JH. Structural model evaluation and modification: an interval estimation approach. Multivar Behav Res. 1990;25:173–80. doi: 10.1207/s15327906mbr2502_4. [DOI] [PubMed] [Google Scholar]
- [87].Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–76. doi: 10.1146/annurev.psych.58.110405.085530. [DOI] [PubMed] [Google Scholar]
- [88].McArdle JJ. Longitudinal dynamic analyses of cognition in the health and retirement study panel. Adv Stat Anal. 2011;95:453–80. doi: 10.1007/s10182-011-0168-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Cheung GW, Rensvold RB. Evaluating goodness-of-fit indexes for testing measurement invariance. Struct Equ Modeling. 2002;9:233–55. doi: 10.1207/S15328007SEM0902_5. [DOI] [Google Scholar]
- [90].Chen FF. Sensitivity of goodness of fit indexes to lack of measurement invariance. Struct Equ Modeling. 2007;14:464–504. doi: 10.1080/10705510701301834. [DOI] [Google Scholar]
- [91].Mulder JD, Hamaker EL. Three extensions of the random intercept cross-lagged panel model. Struct Equ Modeling. 2020;1–11. doi: 10.1080/10705511.2020.1784738. [DOI] [Google Scholar]
- [92].Falkenström F, Finkel S, Sandell R, Rubel JA, Holmqvist R. Dynamic models of individual change in psychotherapy process research. J Consult Clin Psychol. 2017;85:537–49. doi: 10.1037/ccp0000203. [DOI] [PubMed] [Google Scholar]
- [93].Usami S. On the differences between general cross-lagged panel model and random-intercept cross-lagged panel model: interpretation of cross-lagged parameters and model choice. Struct Equ Modeling. 2021;28:331–44. doi: 10.1080/10705511.2020.1821690. [DOI] [Google Scholar]
- [94].McArdle JJ. Latent variable modeling of differences and changes with longitudinal data. Annu Rev Psychol. 2009;60:577–605. doi: 10.1146/annurev.psych.60.110707.163612. [DOI] [PubMed] [Google Scholar]
- [95].Dunlap WP, Cortina JM, Vaslow JB, Burke MJ. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods. 1996;1:170–7. doi: 10.1037/1082-989x.1.2.170. [DOI] [Google Scholar]
- [96].Arend MG, Schafer T. Statistical power in two-level models: a tutorial based on Monte Carlo simulation. Psychol Methods. 2019;24:1–19. doi: 10.1037/met0000195. [DOI] [PubMed] [Google Scholar]
- [97].Zhang Z, Hamagami F, Grimm KJ, McArdle JJ. Using R package RAMpath for tracing SEM path diagrams and conducting complex longitudinal data analysis. Struct Equ Modeling. 2015;22:132–47. doi: 10.1080/10705511.2014.935257. [DOI] [Google Scholar]
- [98].Christopher G, MacDonald J. The impact of clinical depression on working memory. Cogn Neuropsychiatry. 2005;10:379–99. doi: 10.1080/13546800444000128. [DOI] [PubMed] [Google Scholar]
- [99].Laukka EJ, Dykiert D, Allerhand M, Starr JM, Deary IJ. Effects of between-person differences and within-person changes in symptoms of anxiety and depression on older age cognitive performance. Psychol Med. 2018;48:1350–8. doi: 10.1017/S0033291717002896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bierman EJM, Comijs HC, Jonker C, Beekman ATF. Effects of anxiety versus depression on cognition in later life. Am J Geriatr Psychiatry. 2005;13:686–93. doi: 10.1097/00019442-200508000-00007. [DOI] [PubMed] [Google Scholar]
- [101].Bierman EJ, Comijs HC, Rijmen F, Jonker C, Beekman AT. Anxiety symptoms and cognitive performance in later life: results from the longitudinal aging study Amsterdam. Aging Ment Health. 2008;12:517–23. doi: 10.1080/13607860802224276. [DOI] [PubMed] [Google Scholar]
- [102].Sari BA, Koster EHW, Derakshan N. The effects of active worrying on working memory capacity. Cogn Emot. 2017;31:995–1003. doi: 10.1080/02699931.2016.1170668. [DOI] [PubMed] [Google Scholar]
- [103].Wetherell JL, Gatz M, Pedersen NL. A longitudinal analysis of anxiety and depressive symptoms. Psychol Aging. 2001;16:187–95. doi: 10.1037/0882-7974.16.2.187. [DOI] [PubMed] [Google Scholar]
- [104].Lee LO, Gatz M, Pedersen NL, Prescott CA. Anxiety trajectories in the second half of life: genetic and environmental contributions over age. Psychol Aging. 2016;31:101–13. doi: 10.1037/pag0000063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Taylor WD, McQuoid DR, Payne ME, Zannas AS, MacFall JR, Steffens DC. Hippocampus atrophy and the longitudinal course of late-life depression. Am J Geriatr Psychiatry. 2014;22:1504–12. doi: 10.1016/j.jagp.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Elbejjani M, Fuhrer R, Abrahamowicz M, Mazoyer B, Crivello F, Tzourio C, et al. Depression, depressive symptoms, and rate of hippocampal atrophy in a longitudinal cohort of older men and women. Psychol Med. 2015;45:1931–44. doi: 10.1017/S0033291714003055. [DOI] [PubMed] [Google Scholar]
- [107].Zainal NH, Newman MG. Depression and worry symptoms predict future executive functioning impairment via inflammation. Psychol Med. 2021;1–11. doi: 10.1017/s0033291721000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lebedeva A, Sundström A, Lindgren L, Stomby A, Aarsland D, Westman E, et al. Longitudinal relationships among depressive symptoms, cortisol, and brain atrophy in the neocortex and the hippocampus. Acta Psychiatr Scand. 2018;137:491–502. doi: 10.1111/acps.12860. [DOI] [PubMed] [Google Scholar]
- [109].Butters MA, Klunk WE, Mathis CA, Price JC, Ziolko SK, Hoge JA, et al. Imaging Alzheimer pathology in late-life depression with PET and Pittsburgh compound-B. Alzheimer Dis Assoc Dis. 2008;22:261–8. doi: 10.1097/WAD.0b013e31816c92bf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Butters MA, Whyte EM, Nebes RD, Begley AE, Dew MA, Mulsant BH, et al. The nature and determinants of neuropsychological functioning in late-life depression. Arch Gen Psychiatry. 2004;61:587–95. doi: 10.1001/archpsyc.61.6.587. [DOI] [PubMed] [Google Scholar]
- [111].Butters MA, Sweet RA, Mulsant BH, Ilyas Kamboh M, Pollock BG, Begley AE, et al. APOE is associated with age-of-onset, but not cognitive functioning, in late-life depression. Int J Geriatr Psychiatry. 2003;18:1075–81. doi: 10.1002/gps.1006. [DOI] [PubMed] [Google Scholar]
- [112].Wang J, Zhou Y, Chen K, Jing Y, He J, Sun H, et al. Dietary inflammatory index and depression: a meta-analysis. Public Health Nutr. 2019;22:654–60. doi: 10.1017/S1368980018002628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Alvaro PK, Roberts RM, Harris JK. A systematic review assessing bidirectionality between sleep disturbances, anxiety, and depression. Sleep. 2013;36:1059–68. doi: 10.5665/sleep.2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Zhang X, Norton J, Carriere I, Ritchie K, Chaudieu I, Ancelin ML. Risk factors for late-onset generalized anxiety disorder: results from a 12-year prospective cohort (the ESPRIT study). Transl Psychiatry. 2015;5:e536. doi: 10.1038/tp.2015.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Petkus AJ, Reynolds CA, Wetherell JL, Kremen WS, Gatz M. Temporal dynamics of cognitive performance and anxiety across older adulthood. Psychol Aging. 2017;32:278–92. doi: 10.1037/pag0000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Petkus AJ, Filoteo JV, Schiehser DM, Gomez ME, Petzinger G. Worse cognitive performance predicts increased anxiety and depressive symptoms in patients with Parkinson’s disease: a bidirectional analysis. Neuropsychology. 2019;33:35–46. doi: 10.1037/neu0000498. [DOI] [PubMed] [Google Scholar]
- [117].Nelson TD, Kidwell KM, Nelson JM, Tomaso CC, Hankey M, Espy KA. Preschool executive control and internalizing symptoms in elementary school. J Abnorm Child Psychol. 2018;46:1509–20. doi: 10.1007/s10802-017-0395-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kertz SJ, Belden AC, Tillman R, Luby J. Cognitive control deficits in shifting and inhibition in preschool age children are associated with increased depression and anxiety over 7.5 years of development. J Abnorm Child Psychol. 2016;44:1185–96. doi: 10.1007/s10802-015-0101-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].McNeish D, Hamaker EL. A primer on two-level dynamic structural equation models for intensive longitudinal data in Mplus. Psychol Methods. 2020;25:610–35. doi: 10.1037/met0000250. [DOI] [PubMed] [Google Scholar]
- [120].Eysenck MW, Derakshan N. New perspectives in attentional control theory. Pers Individ Dif. 2011;50:955–60. doi: 10.1016/j.paid.2010.08.019. [DOI] [Google Scholar]
- [121].Whitmer AJ, Gotlib IH. An attentional scope model of rumination. Psychol Bull. 2013;139:1036–61. doi: 10.1037/a0030923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Beckwé M, Deroost N, Koster EHW, De Lissnyder E, De Raedt R. Worrying and rumination are both associated with reduced cognitive control. Psychol Res. 2014;78:651–60. doi: 10.1007/s00426-013-0517-5. [DOI] [PubMed] [Google Scholar]
- [123].Yang Y, Cao S, Shields GS, Teng Z, Liu Y. The relationships between rumination and core executive functions: a meta-analysis. Depress Anxiety. 2017;34:37–50. doi: 10.1002/da.22539. [DOI] [PubMed] [Google Scholar]
- [124].Moran TP. Anxiety and working memory capacity: a meta-analysis and narrative review. Psychol Bull. 2016;142:831–64. doi: 10.1037/bul0000051. [DOI] [PubMed] [Google Scholar]
- [125].Snyder HR, Kaiser RH, Warren SL, Heller W. Obsessive-compulsive disorder is associated with broad impairments in executive function: a meta-analysis. Clin Psychol Sci. 2015;3:301–30. doi: 10.1177/2167702614534210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Friedman NP, Miyake A, Altamirano LJ, Corley RP, Young SE, Rhea SA, et al. Stability and change in executive function abilities from late adolescence to early adulthood: a longitudinal twin study. Dev Psychol. 2016;52:326–40. doi: 10.1037/dev0000075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Xi C, Liu Z, Zhong M, Yang H, Peng W, Yi J. Impaired set-shifting in drug-naïve patients with borderline personality disorder: an event-related potentials study. J Affect Disord. 2021;280:64–71. doi: 10.1016/j.jad.2020.10.074. [DOI] [PubMed] [Google Scholar]
- [128].Zainal NH, Newman MG, Hong RY. Cross-cultural and gender invariance of transdiagnostic processes in the United States and Singapore. Assessment. 2021;28:485–502. doi: 10.1177/1073191119869832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Douglas KM, Porter RJ. Longitudinal assessment of neuropsychological function in major depression. Aust N Z J Psychiatry. 2009;43:1105–17. doi: 10.3109/00048670903279887. [DOI] [PubMed] [Google Scholar]
- [130].Mohlman J. Does executive dysfunction affect treatment outcome in late-life mood and anxiety disorders? J Geriatr Psychiatry Neurol. 2005;18:97–108. doi: 10.1177/0891988705276061. [DOI] [PubMed] [Google Scholar]
- [131].Chen F-T, Etnier JL, Chan K-H, Chiu P-K, Hung T-M, Chang Y-K. Effects of exercise training interventions on executive function in older adults: a systematic review and meta-analysis. Sports Med. 2020;50:1451–67. doi: 10.1007/s40279-020-01292-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Alexopoulos GS, Raue PJ, Banerjee S, Marino P, Renn BN, Solomonov N, et al. Comparing the streamlined psychotherapy “Engage” with problem-solving therapy in late-life major depression. A randomized clinical trial. Mol Psychiatry. 2020;1–10. doi: 10.1038/s41380-020-0832-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Alexopoulos GS. Digital mental health for older adults. Am J Geriatr Psychiatry. 2020;28:191–3. doi: 10.1016/j.jagp.2019.06.013. [DOI] [PubMed] [Google Scholar]
- [134].Alexopoulos GS, Raue PJ, Gunning F, Kiosses DN, Kanellopoulos D, Pollari C, et al. “Engage” therapy: behavioral activation and improvement of late-life major depression. Am J Geriatr Psychiatry. 2016;24:320–6. doi: 10.1016/j.jagp.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Alexopoulos GS, Wilkins VM, Marino P, Kanellopoulos D, Reding M, Sirey JA, et al. Ecosystem focused therapy in poststroke depression: a preliminary study. Int J Geriatr Psychiatry. 2012;27:1053–60. doi: 10.1002/gps.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Alexopoulos GS, Raue PJ, Kiosses DN, Mackin RS, Kanellopoulos D, McCulloch C, et al. Problem-solving therapy and supportive therapy in older adults with major depression and executive dysfunction: effect on disability. Arch Gen Psychiatry. 2011;68:33–41. doi: 10.1001/archgenpsychiatry.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Zainal NH, Newman MG. Mindfulness enhances cognitive functioning: a meta-analysis of 100 randomized controlled trials. 2021; 1–165. doi: 10.31234/osf.io/vzxw7. [DOI] [PMC free article] [PubMed]
- [138].Karbach J, Verhaeghen P. Making working memory work: a meta-analysis of executive-control and working memory training in older adults. Psychol Sci. 2014;25:2027–37. doi: 10.1177/0956797614548725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Bastos AG, Pinto Guimarães LS, Trentini CM. Neurocognitive changes in depressed patients in psychodynamic psychotherapy, therapy with fluoxetine and combination therapy. J Affect Disord. 2013;151:1066–75. doi: 10.1016/j.jad.2013.08.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2021.2217.
click here to view supplementary material
Data Availability Statement
The data that support the findings of this study are available from Health and Retirement Study (HRS)—ADAMS—website. Data are available at https://hrs.isr.umich.edu/publications/biblio/5761 with the permission of the study team principal investigators and team members, G. H. Steven, G. F. Gwenith, D. H. Michael, M. L. Kenneth, O. Mary Beth, L. P. Brenda, R. W. David, and colleagues.