Key Points
Question
In adults with painful midportion Achilles tendinopathy lasting longer than 3 months, does a single injection of platelet-rich plasma result in better function when compared with a sham injection 6 months after treatment?
Findings
This randomized clinical trial included 240 participants with pain at the midportion of the Achilles tendon. Treatment with a single injection of intratendinous platelet-rich plasma vs a subcutaneous dry needle resulted in a mean Victorian Institute of Sport Assessment-Achilles score at 6 months of 54.4 vs 53.4 (range, 0 [worst symptoms] to 100 [no symptoms]); this difference was not statistically significant.
Meaning
A single injection of platelet-rich plasma compared with a sham injection did not significantly reduce Achilles tendon dysfunction.
Abstract
Importance
Platelet-rich plasma injections are used as a treatment for chronic midportion Achilles tendinopathy, but evidence for this treatment is limited.
Objective
In adults with midportion Achilles tendinopathy, to assess the effects of a single platelet-rich plasma injection, compared with sham injection, on the outcome of the Victorian Institute of Sport Assessment-Achilles (VISA-A) score (a single composite measure of Achilles tendinopathy severity).
Design, Setting, and Participants
A participant-blinded, multicenter randomized clinical trial that included 240 people from 24 sites assigned to either a platelet-rich plasma injection or a sham injection between April 2016 and February 2020. Final follow-up was July 2020. Participants were older than 18 years with midportion Achilles tendon pain for more than 3 months as confirmed by ultrasound, magnetic resonance imaging, or both.
Interventions
A single intratendinous platelet-rich plasma injection (n = 121) or a single sham injection (insertion of a subcutaneous dry needle not entering the tendon) (n = 119).
Main Outcomes and Measures
The primary outcome was the VISA-A score, measured 6 months after treatment allocation. The VISA-A score contains 8 questions that cover 3 domains of pain, function, and activity, analyzed as a composite score (range, 0 [worst symptoms] to 100 [no symptoms]; minimal clinically important difference in score, 12 points). The primary analysis was adjusted for laterality, age, sex, and baseline VISA-A score.
Results
Among 240 patients assigned to a platelet-rich plasma or sham injection (mean age, 52 years; 138 [58%] women), 221 (92%) completed the trial. At 6-month follow-up, mean VISA-A score values in the plasma-rich plasma group vs the sham injection group were 54.4 vs 53.4 (adjusted mean difference, −2.7 [95% CI, −8.8 to 3.3]). The most common adverse events compared between patients in the platelet-rich plasma group vs the sham group were injection site discomfort (97 vs 73 patients), swelling (56 vs 52 patients) and bruising (48 vs 49 patients).
Conclusions and Relevance
Among patients with chronic midportion Achilles tendinopathy, treatment with a single injection of intratendinous platelet-rich plasma, compared with insertion of a subcutaneous dry needle, did not reduce Achilles tendon dysfunction at 6 months. These findings do not support the use of this treatment for chronic midportion Achilles tendinopathy.
Trial Registration
isrctn.org Identifier: ISRCTN13254422
This randomized clinical trial compares the efficacy of a single injection of intratendinous platelet-rich plasma vs injection with a subcutaneous dry needle (sham) from baseline until 6-month follow-up in adult patients with chronic midportion Achilles tendinopathy.
Introduction
Chronic midportion Achilles tendinopathy is defined as degeneration of the midportion of the Achilles tendon and is characterized by swelling and pain over the midportion of the tendon, resulting in activity limition.1,2,3,4 In 2016 data from Dutch general practice registers, a prevalence of 5.2 per 1000 people per year was reported.3 In data collected between 2007 and 2011 (United Healthcare Orthopaedic dataset from the PearlDiver patient record database), 36 per 100 000 patients diagnosed with tendinopathy sustained a subsequent Achilles tendon tear.2
Current therapies for chronic midportion tendinopathy consist of exercise, orthotics, electrotherapy, and injections. Injection therapies include prolotherapy (injection of an irritant solution), high-volume (injection of solution into the space between the tendon and tendon sheath) platelet-rich plasma (PRP), and corticosteroids.5 Of these, therapeutic injections with PRP, in which the plasma fraction of the patient’s blood is injected into the Achilles tendon, has gained interest from multiple organizations nationally including the International Olympic Committee and the National Institute for Health and Care Excellence.6,7,8,9 PRP injections are thought to promote tendon repair by introducing a high concentration of growth factors (produced from whole blood) directly at the site of degeneration to enhance regeneration.10,11 However, randomized trial evidence for PRP to treat midportion Achilles tendinopathy is limited.8 To date, randomized trials have had small sample sizes (<60 participants) and were not multicenter.
Therefore, a single blind multicenter randomized clinical trial was conducted to compare the effects of a single PRP injection, compared with sham injection, on pain function and quality of life in patients with chronic midportion Achilles tendinopathy.
Methods
Study Conduct and Oversight
This randomized multicenter clinical trial was conducted at 24 UK hospital trusts in the National Health Service (NHS). The National Research Ethics Committee approved this study at each site on October 30, 2015 (15/WM/0359). Participants provided written informed consent. Supplement 1 reports the trial protocol,12 and the statistical analysis plan is in Supplement 2.
Participants
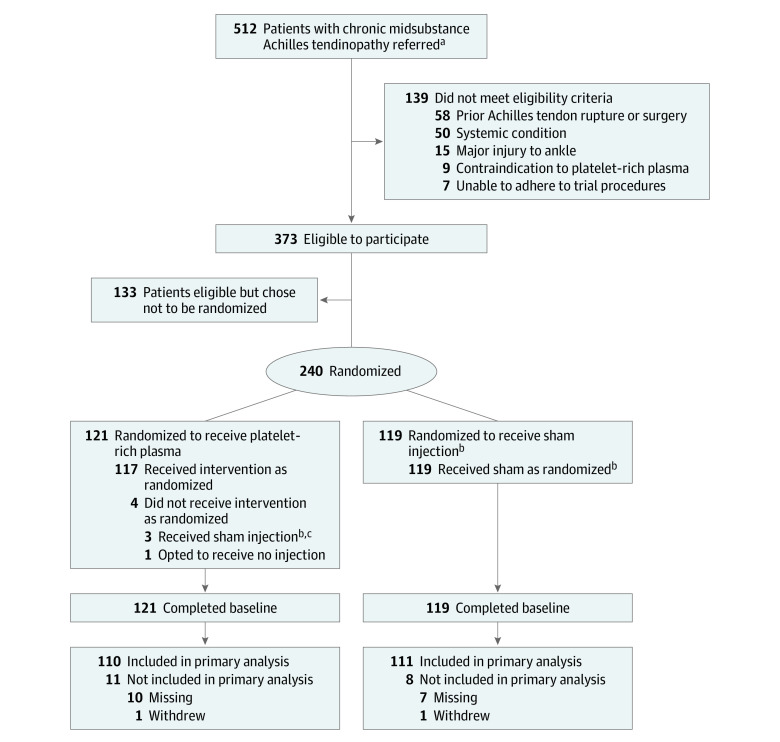
Research teams screened adults from orthopedic foot and ankle clinics (Figure 1). Eligible patients were referred for a surgical opinion and had received other previous treatments. Potential participants were provided with verbal and written information (eFigure 1 in Supplement 3) prior to written informed consent.
Figure 1. Recruitment, Randomization and Follow-up in the Achilles Tendinopathy Trial.
aReferred patients were screened from orthopedic foot and ankle clinics.
bIndicates subcutaneous dry needle injection.
cSwitched to sham injection due to equipment failure with platelet-rich plasma injection.
Eligible candidates were aged 18 years or older with pain at the midportion of the Achilles tendon for longer than 3 months (chronic presentation) with tendinopathy confirmed by ultrasound, magnetic resonance imaging, or both. Exclusion criteria included systemic conditions associated with tendinopathy (eg, diabetes or rheumatoid arthritis), inability to adhere to trial procedures, pregnancy, prior Achilles tendon surgery or rupture on the index side, previous major tendon or ankle injury, deformity to either lower leg, fracture of a long bone in either lower limb (tibia, fibula, femur, or any 2 of these bones) in the previous 6 months (expected duration of recovery), previous randomization in the study, previous receipt of PRP treatment into a tendon, or contraindication to receiving PRP (eg, hemodynamic instability, platelet dysfunction syndrome, active cancer, septicemia, anticoagulation therapy).
Allocation to Treatment
All baseline data were collected prior to allocation to treatment. Patients were randomized in an approximate 1:1 ratio to receive a PRP injection or a sham injection using a standard minimization algorithm with stratification by recruitment center and laterality (1 or both Achilles tendons affected) (Figure 1).13,14 Participants with bilateral Achilles tendinopathy were treated as 1 experimental unit (ie, the person was allocated rather than the tendon). For these participants, an index tendon was identified (most painful). The treatment allocation process was triggered by a telephone call from the research nurse to a secure, centralized, telephone-based service at Warwick Clinical Trials Unit. The minimization algorithm was implemented by a trained staff member, and treatment allocation was communicated verbally to the research nurse and confirmed by email. Only members of the independent team had access to the minimization algorithm and ability to allocate participants. Treatment allocation was not predictable within the trial team due to the independent secure allocation system.
Intervention and Masking
Each research team received training from the chief investigator (R.S.K.) for preparation and delivery of the trial interventions, with attendance recorded on a trial delegation log. Only those individuals listed on the delegation log were permitted to prepare and deliver the trial interventions.
The participants were blinded to their treatment allocation. Clinicians involved in preparing or delivering the intervention could not be blinded but had no role in collection or assessment of follow-up data.
All participants had approximately 9 mL of whole blood withdrawn from the antecubital fossa. This blood was mixed with 1 mL of sodium citrate anticoagulant. Following this procedure, a research nurse moved to a separate clinical area. Those randomized to receive PRP had the injection prepared immediately; there was no storage of whole blood. These participants waited 30 minutes after blood was withdrawn (the time required to prepare the PRP injection) before receiving the PRP injection. For participants randomized to receive the sham injection, their whole blood was discarded, and they also waited 30 minutes after blood withdrawal to simulate the approximate time required to prepare a PRP injection.
The PRP preparation involved whole-blood centrifugation using the study-specific Glo PRP system, which produces a leukocyte-rich preparation (Glofinn). A new study-specific centrifuge that used a 2-stage centrifugation process was issued to each recruitment center. In stage 1, the whole blood was centrifuged for 5 minutes at 1200 RCF (relative centrifugal force; 1200g), after which red blood cells were collected in an attachment and discarded. In stage 2, the remaining sample was centrifuged for 10 minutes at 1200 RCF, after which the PRP was transferred using an extraction syringe to produce approximately 3 mL of PRP. The PRP was administered immediately in its liquid form and was not stored. In the event that the project-specific centrifuge system failed once blood had been drawn for PRP, recruitment centers were advised to give the participant the sham intervention to maintain participant blinding to treatment allocation. Participants remained in the allocated group regardless of intervention received. The preparation procedures remained consistent across the trial duration.
For both interventions, participants were in the prone position when the intervention was administered and were unable to see the syringe. Additionally, all syringes were masked with black tape to ensure that the contents could not be seen. For both interventions, 5mL of 2% lidocaine was injected into the skin overlying the tendon.
Participants randomized to the PRP group received 1 injection into the Achilles tendon through a single skin portal and 5 penetrations of the tendon. Participants randomized to the sham injection group received 1 dry injection, inserted under the skin but not into the tendon, for 10 seconds to simulate the conventional time taken to inject PRP.
The trial team chose a comparator that would avoid any potential therapeutic value. Saline injections and dry needling have been associated with possible therapeutic values due to the local trauma caused within the tendon by the needle, which may facilitate a healing response (absent in this pathology) and possible treatment effects associated with pressure-volume changes within the tendon. Consequently, it was decided that all patients would have a needle inserted under the skin, but not into the tendon.
All participants were asked to avoid additional treatments during the 6-month follow-up period, and they received the same advice (verbal and written; eFigure 2 in Supplement 3) after receiving the PRP or sham injection. This postinjection advice informed participants that they may have increased pain for 24 to 48 hours and recommended that simple analgesia (eg, paracetamol) could be taken for increased pain, while anti-inflammatory medications (eg, ibuprofen) should be avoided for up to 4 weeks. They were also informed of potential adverse events and about what to do if they occurred. All participants were advised to return to normal activities when they felt able, while avoiding significant activity (eg, running or weight training) for 1 week.
At each recruitment site, quality assurance checks were planned to assess adherence using an intervention preparation and delivery checklist. In addition, 14 healthy volunteers, independent of the study sample, provided two 10-mL blood samples at 4 of the recruitment centers during the period of recruitment. Sample one was kept as a whole-blood control and sample two was used to produce PRP for analysis, for quality control. Samples were anonymized and transported to an independent test laboratory the same day (Institute of Inflammation and Aging, University of Birmingham, Birmingham, UK). Instrument performance was checked by the test laboratory each day and externally on a monthly basis (UKNEQAS, Watford, UK). Red blood cell, platelet, and white blood cell counts were recorded in a database, and all samples were destroyed after analysis.
Outcomes
Primary Outcome
The primary outcome was the Victorian Institute of Sport Assessment-Achilles score (VISA-A),15 a composite measure of severity of Achilles tendinopathy that contains 8 questions, and was mailed to participants for self-administration. This is the only validated Achilles tendinopathy–specific patient-reported outcome measure available. Questions 1 to 3 are related to pain in the Achilles region, questions 4 to 6 are related to function, and questions 7 and 8 are related to activity. Each of the first 7 questions are scored on a 0- to 10-point visual analog scale to report the magnitude of symptoms, and question 8 is scored on a 0- to 30-point categorical rating scale (overall score range, 0 [most-severe symptoms] to 100 [no symptoms]). The 6-month follow-up time point was selected for ethical reasons; if patients were still experiencing debilitating symptoms at this time point, investigators did not want to withhold access to other treatment.
Secondary Outcomes
Secondary outcomes were the VISA-A score at 3-month follow-up, health-related quality of life assessed by the 5-level Euroqol questionnaire (EQ-5D-5L; range, usually 0 to 1 [1 indicates full health], but negative values are possible and indicate worse than death)16 at 3- and 6-month follow-up, and pain assessed using a visual analog scale (range indicated on a 10-cm line: 0 cm [no pain] to 10 cm [worst imaginable pain]) at 2-week, 3-month, and 6-month follow-up periods.17 Expected events related to the study treatments were predefined and recorded as adverse events. All unexpected events were evaluated for relatedness to the study treatments.
Baseline data were collected by the recruiting center research teams on paper data collection forms. Baseline data included patient-reported ethnicity, collected using predefined fixed categories to characterize the study population and to assess generalizability of results. At 2-week, 3-month, and 6-month follow-up time points, data were collected centrally by the research team based at Warwick Clinical Trials Unit, who were blinded to treatment allocation throughout. Data were collected via mail or telephone. The primary method was by mailed forms for the primary end point. When participants did not return a mailed questionnaire, they were telephoned to collect the data. All participants received a paper copy of the questionnaire prior to the telephone call.
Statistical Analysis
There is no consensus on the minimal clinically important difference for the VISA-A score. Previous trials evaluating PRP injections have used between 10 and 12 points, consistent with other comparable musculoskeletal studies that report minimal clinically important difference values that represented between 10% and 15% of the scale.5,18
From a pilot study publication,4 VISA-A scores were observed to be approximately normally distributed with a standard deviation of 26. At the 5% significance level, 100 patients in each group (200 in total) were required for 90% power to observe a 12-point difference in VISA-A.19,20 Allowing for a 15% loss to follow-up, 240 participants were required. The sample-size calculations did not take into account planned control in the analysis for baseline VISA-A score and other covariates.
The primary comparison was the between-group VISA-A score 6 months after treatment allocation. For the primary analysis, patients were analyzed according to their allocated treatment. Mixed-effects linear regression analysis was used to estimate the treatment effect, including (fixed-effects) terms to adjust for laterality, age, sex and baseline VISA-A score. Recruitment center was included in the mixed-effects model as a random effect to allow for possible heterogeneity in patient outcomes due to other unknown center-related effects. Recruitment center was treated as a fixed effect when the random effect was inestimable. As a secondary analysis, the VISA-A was analyzed in a similar manner on a per-protocol, as received–basis (in contrast to on an as allocated).
Multivariable imputation by chained equations method was used to impute missing data for the sensitivity analysis for the primary outcome. The trial statistician was blinded to the treatment allocation throughout.
Prespecified subgroup analysis included laterality (single vs bilateral) and duration of symptom (≤median vs > median duration). The median duration of symptoms was determined by the baseline data. The subgroup analyses followed the methods described for the primary analysis. We performed an omnibus (likelihood ratio χ2) test for treatment-subgroup interaction in a model for the primary outcome. A 2-sided P value of less than .05 was considered statistically significant. All analyses were conducted using SAS version 9.4 (SAS Institute Inc). Because of the potential for type 1 error due to multiple comparisons, secondary analyses should be considered exploratory.
Results
Between April 27, 2016, and February 21, 2020, a total of 512 adults with chronic midportion Achilles tendinopathy were screened. Of these, 139 were ineligible, 133 declined participation, and 240 were randomized to receive PRP injection (n = 121) or sham injection (n = 119). Two hundred twenty-one participants (92%) completed the primary VISA-A score at the 6-month primary end point (Figure 1).
Three participants in the PRP group (n = 121) did not receive the allocated treatment due to equipment failure and received a sham injection. One participant in the PRP group withdrew from receiving any intervention after consent and treatment allocation and subsequently withdrew from all trial procedures. All participants in the sham injection group (n = 119) received the allocated treatment. All recorded quality assurance checks demonstrated adherence to the trial protocol, and external quality assurance of PRP samples using the same protocol were satisfactory (eTable 1 and eTable 2 in Supplement 3). The rate of successful blinding was balanced across the groups (eTable 3 in Supplement 3).
Thirty-seven participants in the PRP (n = 121) group and 40 in the sham group (n = 119) received additional treatments. These treatments were similar and balanced in the 2 groups (eTable 4 in Supplement 3).
The trial population had a mean (SD) age of 52.2 (10.5) years and 58% (138/230) were women. The most common pretrial treatment was physical therapy, received by 207 participants (86%). The median symptom duration in both groups was 24 months (interquartile range, 14-36) The groups were well balanced across baseline characteristics (Table 1).
Table 1. Baseline Characteristics of Study Participants Randomized to the Platelet-Rich Plasma or Sham Treatment Group.
| No. (%)a | ||
|---|---|---|
| Platelet-rich plasma (n=121) | Sham (n=119)b | |
| Men | 48 (39.7) | 54 (45.4) |
| Women | 73 (60.3) | 65 (54.6) |
| Race/ancestryc | ||
| White | 119 (98.3) | 111 (93.3) |
| Asian/Asian British | 1 (0.8) | 1 (0.8) |
| Multiple ethnic groups | 0 | 3 (2.5) |
| Other | 1 (0.8) | 4 (3.4) |
| Age, mean (SD) | 52.4 (11.1) | 52.0 (9.8) |
| Body mass index, mean (SD)d | 30.8 (5.8) | 31.0 (5.4) |
| Employmente | ||
| Full-time employed | 64 (52.9) | 66 (55.5) |
| Retired/inactive | 22 (18.2) | 19 (16.0) |
| Part-time employed | 16 (13.2) | 13 (10.9) |
| Self-employed | 9 (7.4) | 13 (10.9) |
| Unemployed | 4 (3.3) | 5 (4.2) |
| Unpaid work | 4 (3.3) | 1 (0.8) |
| Caregiver | 2 (1.7) | 2 (1.7) |
| Bilateral tendon involvement | 20 (16.5) | 19 (16.0) |
| Regular smoker | 10 (8.3) | 12 (10.1) |
| Symptom duration, median (IQR), mo | 24 (14-36) | 24 (14-36) |
| VISA-A score, mean (SD)f | 37.6 (19.3) | 33.2 (18.1) |
| Pain VAS, mean (SD)g | 4.2 (2.3) | 4.6 (2.4) |
| EQ-5D-5L utility score, mean (SD)h | 0.60 (0.18) | 0.53 (0.21) |
Abbreviations: IQR, interquartile range; VAS, Visual Analog Scale; VISA-A, Victorian Institute of Sport Assessment-Achilles score.
Numeric values indicate No. (%) unless otherwise indicated. Categorical percentages may not total 100 due to rounding.
Sham indicates subcutaneous injection of a dry needle.
This study included a race/ancestry category for “Black/African/Caribbean/Black British,” but it is not shown as no participants self-identified as such. Participants who self-identified as “Other” for race/ancestry did not provide more specific information.
Calculated as weight in kilograms divided by height in meters squared.
An employment status category of “Full-time student” was included but is not shown as no participants self-identified as such.
The score is a composite measure of severity of Achilles tendinopathy (range, 0 [worst] to 100 [best] with a lower score indicating more symptoms and a larger limitation of physical activity). A score of 35 is indicative of one-third of best.
Score range: 0 (no pain) to 10 (worst pain) with a score of 4 to 5 indicating moderate pain.
The score indicates a composite measure of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels (range, usually 0 to 1 [best health-related quality of life]), but negative values are possible and indicate worse than death.
Primary Outcome
There was no significant difference in VISA-A scores between the PRP group and the sham group at 6 months following treatment allocation (mean difference, −2.7 [95% CI, −8.8 to 3.3]) (Figure 2).
Figure 2. VISA-A Scores at Baseline and 6 Months for Each Participant by Group.
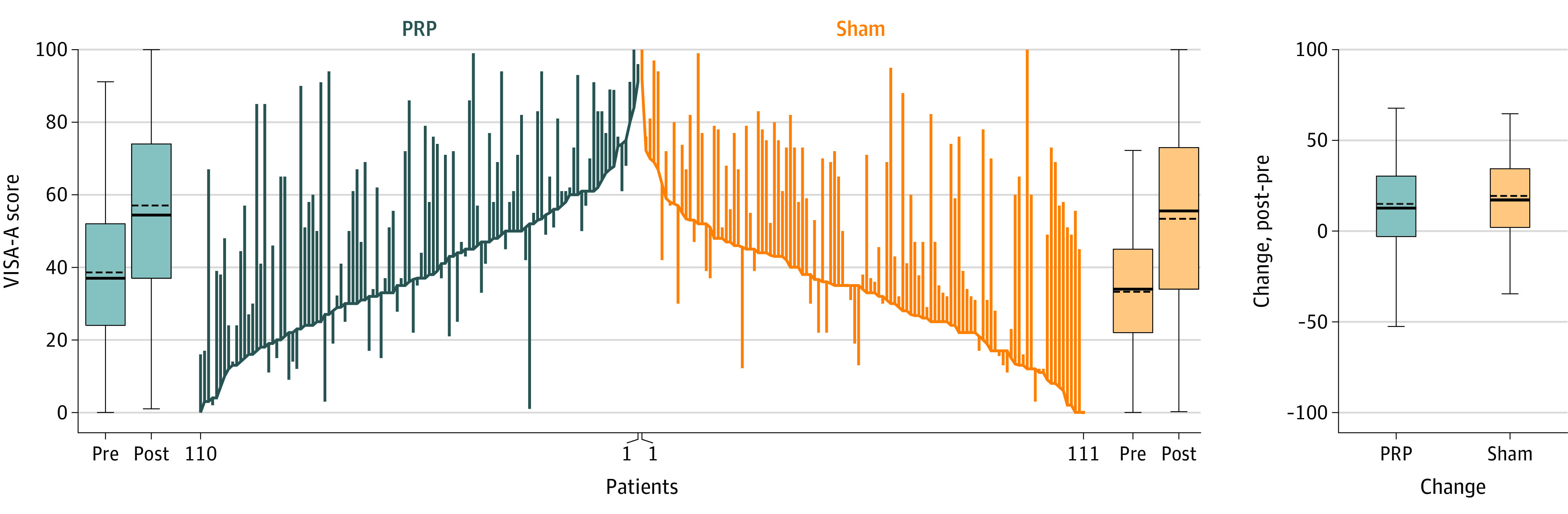
The baseline Victorian Institute of Sport Assessment-Achilles (VISA-A) scores for individual participants are connected using blue lines for the platelet-rich plasma (PRP) group and orange lines for the sham (subcutaneous dry needle injection) group. Changes from baseline (indicated by the pre label) to 6 months (post label) are represented by the vertical lines with upward lines indicating improvement of Achilles tendinopathy and downward lines indicating deterioration. Box plots show the summary of baseline, 6 months, and changes by group. Boxes from bottom to top show the 25th, 50th, and 75th percentiles; horizontal dashed lines indicate the mean; horizontal solid lines indicate the median; whiskers with caps indicate the range from the lower to upper adjacent values (25th percentile−1.5 × the interquartile range [IQR]; 75th percentile + 1.5 × the IQR).
Secondary Outcomes
There was no significant difference in the VISA-A score between the PRP group and the sham group at the 3-month time point (mean difference, −0.4 [95% CI, −5.1 to 4.3]; Table 2). There were no significant differences in quality of life (EQ-5D-5L utility and VAS score) or pain (VAS) at 2 weeks, 3 months, or 6-month time points (Table 3).
Table 2. VISA-A Scores by Treatment Group and Timea.
| Platelet-rich plasma | Shama | Between-group difference (95% CI)b | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | VISA-A, mean (SD)c | No. | VISA-A, mean (SD)c | Unadjusted | P value | Adjustedd | P value | |
| Primary end point (6 mo) | 110 | 54.4 (25.7) | 111 | 53.4 (24.2) | 1.0 (−5.6 to 7.6) | .76 | −2.7 (−8.8 to 3.3)e | .36 |
| Secondary end point (3 mo) | 116 | 47.0 (22.3) | 111 | 44.2 (20.5) | 2.8 (−2.8 to 8.4) | .33 | −0.4 (−5.1 to 4.3) | .88 |
Abbreviation: VISA-A, Victorian Institute of Sport Assessment-Achilles score.
Sham indicates subcutaneous injection of a dry needle.
Group difference is calculated as platelet-rich plasma injection group minus sham injection group.
The score is a composite measure of severity of Achilles tendinopathy (range, 0 [worst] to 100 [best] with a lower score indicating more symptoms and a larger limitation of physical activity). A score of 35 is indicative of one-third of best.
The model has been adjusted for age, sex, laterality, and baseline VISA-A score, with site included as a random effect in the 6-month analysis and as a fixed effect in the 3-month analysis due to inestimable random effect.
Indicates the primary outcome.
Table 3. Secondary Outcomes by Treatment Group and Timea.
| Platelet-rich plasma | Shamb | Between-group difference (95% CI) | ||||||
|---|---|---|---|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | Unadjusted | P value | Adjustedc | P value | |
| Pain VASc | ||||||||
| 2 wk | 113 | 3.7 (2.5) | 116 | 4.1 (2.7) | −0.3 (−1.0 to 0.3) | .33 | −0.1 (−0.7 to 0.6) | .85 |
| 3 mo | 116 | 3.6 (2.6) | 111 | 3.6 (2.6) | 0.0 (−0.7 to 0.7) | .95 | 0.2 (−0.4 to −0.9) | .47 |
| 6 mo | 110 | 2.7 (2.6) | 111 | 2.6 (2.7) | 0.2 (−0.5 to 0.9) | .64 | −0.4 (−0.3 to 1.1) | .22 |
| EQ-5D-5L VASd | ||||||||
| 3 mo | 116 | 67.6 (19.7) | 111 | 69.0 (19.3) | −1.4 (−6.5 to 3.7) | .59 | −2.6 (−7.9 to 2.7) | .33 |
| 6 mo | 110 | 72.8 (18.7) | 111 | 72.7 (19.7) | 0.2 (−4.9 to 5.2) | .95 | −0.6 (−5.8 to 4.7) | .81 |
| EQ-5D-5L utility scoree | ||||||||
| 3 mo | 116 | 0.654 (0.198) | 111 | 0.639 (0.211) | 0.014 (−0.039 to 0.068) | .59 | −0.005 (−0.057 to 0.047) | .85 |
| 6 mo | 110 | 0.690 (0.214) | 111 | 0.674 (0.217) | 0.016 (−0.041 to 0.073) | .58 | −0.002 (−0.059 to 0.055) | .94 |
Abbreviations: EQ-5D-5L, 5-level Euroqol questionnaire; VAS, Visual Analog Scale; VISA-A, Victorian Institute of Sport Assessment-Achilles score.
Analysis was adjusted for age, sex, laterality, and baseline VISA-A score, with site included as a random effect. A lower pain VAS score indicates less pain. A higher EQ-5D-5L index/VAS score indicates better health-related quality of life.
Sham is subcutaneous injection of a dry needle.
Score range: 0 (no pain) to 10 (worst pain) with a score of 4 to 5 indicating moderate pain.
Score range: 0 (indicates worst health) to 100 (best health).
The score indicates a composite measure of mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension has 5 levels (range, usually 0 to 1 [best health-related quality of life]), but negative values are possible and indicate worse than death.
Secondary per-protocol analyses and imputed analysis accounting for missingness were not substantially different (eTable 5 and eTable 6 in Supplement 3). Predefined subgroup analyses showed the differences between treatment groups in VISA-A scores at 6 months were not statistically significantly different between subgroups on either factor (eTable 7 in Supplement 3).
Adverse Events
The most common adverse event was mild discomfort at the injection site at 2 weeks after the injection (97 (82%) in the PRP group vs 73 (61%) in the sham group). At 6 months after the injection, mild discomfort at the injection site was reduced (9 in PRP group vs 1 in sham group). Swelling was the second most common adverse event observed at 2-week follow-up (56 [47%] in the PRP group vs 52 [44%] in the sham injection group), followed by bruising (48 [40%] in the PRP group vs 49 [41%] in the sham injection group) (Table 4). There was 1 related serious adverse event in which a patient in the PRP injection group developed severe pain following the injection. This participant subsequently had a tendon debridement and symptoms resolved.
Table 4. Analysis of Secondary Outcome Complications From Baseline to 6 Months by Allocated Treatment Group.
| Characteristics by follow-up period | No. (%)a | |
|---|---|---|
| Platelet-rich plasma | Sham | |
| 2 wk | ||
| No. | 119 | 119 |
| Bleeding at injection site | 24 (20.2) | 36 (30.3) |
| Bruising at injection site | 48 (40.3) | 49 (41.2) |
| Fainting | 0 | 0 |
| Infection at injection site | 0 | 0 |
| Mild discomfort at injection site | 97 (81.5) | 73 (61.3) |
| Swelling at injection site | 56 (47.1) | 52 (43.7) |
| Skin discoloration at injection site | 24 (20.2) | 22 (18.5) |
| Allergic reaction | 0 | 1 (0.8) |
| Otherb | 8 (6.7) | 6 (5.0) |
| 3 mo | ||
| No. | 116 | 110 |
| Bleeding at injection site | 0 | 0 |
| Bruising at injection site | 4 (3.4) | 7 (6.4) |
| Fainting | 0 | 0 |
| Infection at injection site | 0 | 0 |
| Mild discomfort at injection site | 20 (17.2) | 15 (13.6) |
| Swelling at injection site | 11 (9.5) | 11 (10.0) |
| Skin discoloration at injection site | 4 (3.4) | 0 |
| Allergic reaction | 0 | 0 |
| Otherb | 4 (3.4) | 3 (2.7) |
| 6 mo | ||
| No. | 110 | 111 |
| Bleeding at injection site | 0 | 0 |
| Bruising at injection site | 0 | 1 (0.9) |
| Fainting | 0 | 0 |
| Infection at injection site | 0 | 0 |
| Mild discomfort at injection site | 9 (8.2) | 1 (0.9) |
| Swelling at injection site | 3 (2.7) | 5 (4.5) |
| Skin discoloration at injection site | 0 | 0 |
| Allergic reaction | 0 | 0 |
| Otherb | 1 (0.9) | 2 (1.8) |
Numeric values indicate complications reported at least once per participant; percentages exclude unknown answers at each follow-up.
Category included episodes of cramping, ankle stiffness, numbness, nausea, temporary visual disturbance, and swelling behind the knee.
Discussion
In this clinical trial of patients with chronic midportion Achilles tendinopathy, a single PRP injection, compared with sham, injection did not result in a statistically significant difference in the VISA-A score at 6-month follow-up. The upper limit of the 95% CI for the mean difference excluded a clinically meaningful effect. There was no significant effect of the PRP injection on secondary outcomes of change in VISA-A score at 3-month follow-up or the EQ-5D-5L and pain at 2 weeks, 3 months, and 6 months. A single injection of PRP resulted in mild discomfort more frequently than a sham injection, and in some patients, mild discomfort persisted to the 6-month follow-up.
A 2020 systematic review identified 5 randomized clinical trials (201 participants) of PRP for Achilles tendinopathy.4,8,19,20,21,22 Only 1 small study (60 participants) had a significant mean change in VISA-A scores when compared with placebo (19.6 vs 8.8).20 Preclinical evidence demonstrated that PRP promoted tendon healing, due to the high concentration of growth factors delivered by injection to the site of degeneration, that enhanced tendon regeneration.23 However, these effects have not been demonstrated in humans.23
Limitations
This study has several limitations. First, the injections were not ultrasound guided, so it is hypothetically possible that the PRP was not injected directly into the tendinopathic area of the tendon; however, the Achilles tendon is superficial, and the symptomatic area can be easily identified. Therefore, this phenomenon is unlikely. Second, only 1 PRP injection was administered. It is possible that multiple PRP injections might have had a different effect.24 Third, 77 participants sought additional treatment during the 6-month follow-up, which may have influenced results. However, these therapies appeared balanced across the 2 groups. Fourth, the quality of every PRP sample that was injected was not independently assessed. However, a detailed quality assurance method was implemented.
Conclusions
Among patients with chronic midportion Achilles tendinopathy, treatment with a single injection of intratendinous platelet-rich plasma, compared with insertion of a subcutaneous dry needle, did not reduce Achilles tendon dysfunction at 6 months. These findings do not support the use of this treatment for chronic midportion Achilles tendinopathy.
Trial Protocol
Statistical Analysis Plan
eFigure 1. Patient Information Sheet
eFigure 2. Postinjection Care Sheet (Front and Back Pages)
eTable 1. List of Site Quality Assurance (QA) Assessments
eTable 2. PRP Quality Assurance (QA) Assessments
eTable 3. Blinding Success Report
eTable 4. Additional Treatments Received
eTable 5. Adjusted Treatment Difference of VISA-A Score at 6-Months Follow-up With 95% CI (Per Protocol Using Observed Dataset)
eTable 6. Adjusted Treatment Difference of VISA-A Score at 6-Months Follow-up With 95% CI by Treatment Group (Imputed Dataset)
eTable 7. Adjusted Treatment Difference of VISA-A Score at 6 Months According to Subgroup Characteristics
Nonauthor Collaborators
Data Sharing Statement
References
- 1.de Jonge S, van den Berg C, de Vos RJ, et al. Incidence of midportion Achilles tendinopathy in the general population. Br J Sports Med. 2011;45(13):1026-1028. doi: 10.1136/bjsports-2011-090342 [DOI] [PubMed] [Google Scholar]
- 2.Yasui Y, Tonogai I, Rosenbaum AJ, Shimozono Y, Kawano H, Kennedy JG. The risk of Achilles tendon rupture in the patients with Achilles tendinopathy: healthcare database analysis in the United States. Biomed Res Int. 2017;2017:7021862. doi: 10.1155/2017/7021862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riel H, Lindstrøm CF, Rathleff MS, Jensen MB, Olesen JL. Prevalence and incidence rate of lower-extremity tendinopathies in a Danish general practice: a registry-based study. BMC Musculoskelet Disord. 2019;20(1):239. doi: 10.1186/s12891-019-2629-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kearney RS, Parsons N, Costa ML. Achilles tendinopathy management: a pilot randomised controlled trial comparing platelet-richplasma injection with an eccentric loading programme. Bone Joint Res. 2013;2(10):227-232. doi: 10.1302/2046-3758.210.2000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kearney RS, Parsons N, Metcalfe D, Costa ML. Injection therapies for Achilles tendinopathy. Cochrane Database Syst Rev. 2015;5(5):CD010960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Engebretsen L, Steffen K, Alsousou J, et al. IOC consensus paper on the use of platelet-rich plasma in sports medicine. Br J Sports Med. 2010;44(15):1072-1081. doi: 10.1136/bjsm.2010.079822 [DOI] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence . Autologous blood injection for tendinopathy interventional procedures guidance (IPG438). Published January 23, 2013. Accessed June, 17, 2021. https://www.nice.org.uk/guidance/ipg438
- 8.Madhi MI, Yausep OE, Khamdan K, Trigkilidas D. The use of PRP in treatment of Achilles tendinopathy: a systematic review of literature. Ann Med Surg (Lond). 2020;55:320-326. doi: 10.1016/j.amsu.2020.04.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu CJ, Yu KL, Bai JB, Tian DH, Liu GL. Platelet-rich plasma injection for the treatment of chronic Achilles tendinopathy: a meta-analysis. Medicine (Baltimore). 2019;98(16):e15278. doi: 10.1097/MD.0000000000015278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. Cochrane Database Syst Rev. 2013;(12):CD010071. doi: 10.1002/14651858.CD010071.pub2 [DOI] [PubMed] [Google Scholar]
- 11.Alsousou J, Keene DJ, Hulley PA, et al. Platelet rich Plasma in Achilles Tendon Healing 2 (PATH-2) trial: protocol for a multicentre, participant and assessor-blinded, parallel-group randomised clinical trial comparing platelet-rich plasma (PRP) injection versus placebo injection for Achilles tendon rupture. BMJ Open. 2017;7(11):e018135. doi: 10.1136/bmjopen-2017-018135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kearney RS, Parsons N, Ji C, et al. Platelet rich plasma versus placebo for the management of Achilles tendinopathy: protocol for the UK study of Achilles tendinopathy management (ATM) multi-centre randomised trial. BMJ Open. 2020;10(2):e034076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Altman DG, Bland JM. Treatment allocation by minimisation. BMJ. 2005;330(7495):843. doi: 10.1136/bmj.330.7495.843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31(1):103-115. doi: 10.2307/2529712 [DOI] [PubMed] [Google Scholar]
- 15.Robinson JM, Cook JL, Purdam C, et al. ; Victorian Institute Of Sport Tendon Study Group . The VISA-A questionnaire: a valid and reliable index of the clinical severity of Achilles tendinopathy. Br J Sports Med. 2001;35(5):335-341. doi: 10.1136/bjsm.35.5.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooks R. EuroQol: the current state of play. Health Policy. 1996;37(1):53-72. doi: 10.1016/0168-8510(96)00822-6 [DOI] [PubMed] [Google Scholar]
- 17.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care Res (Hoboken). 2011;63(11)(suppl 11):S240-S252. doi: 10.1002/acr.20543 [DOI] [PubMed] [Google Scholar]
- 18.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94. doi: 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 19.de Vos RJ, Weir A, van Schie HT, et al. Platelet-rich plasma injection for chronic Achilles tendinopathy: a randomized controlled trial. JAMA. 2010;303(2):144-149. doi: 10.1001/jama.2009.1986 [DOI] [PubMed] [Google Scholar]
- 20.Boesen AP, Hansen R, Boesen MI, Malliaras P, Langberg H. Effect of high-volume injection, platelet-rich plasma, and sham treatment in chronic midportion Achilles tendinopathy: a randomized double-blinded prospective study. Am J Sports Med. 2017;45(9):2034-2043. doi: 10.1177/0363546517702862 [DOI] [PubMed] [Google Scholar]
- 21.Albano D, Messina C, Usuelli FG, et al. Magnetic resonance and ultrasound in achilles tendinopathy: predictive role and response assessment to platelet-rich plasma and adipose-derived stromal vascular fraction injection. Eur J Radiol. 2017;95:130-135. doi: 10.1016/j.ejrad.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 22.Krogh TP, Ellingsen T, Christensen R, Jensen P, Fredberg U. Ultrasound-guided injection therapy of Achilles tendinopathy with platelet-rich plasma or saline: a randomized, blinded, placebo-controlled trial. Am J Sports Med. 2016;44(8):1990-1997. doi: 10.1177/0363546516647958 [DOI] [PubMed] [Google Scholar]
- 23.Kaux JF, Drion P, Croisier JL, Crielaard JM. Tendinopathies and platelet-rich plasma (PRP): from pre-clinical experiments to therapeutic use. J Stem Cells Regen Med. 2015;11(1):7-17. doi: 10.46582/jsrm.1101003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vilchez-Cavazos F, Millán-Alanís JM, Blázquez-Saldaña J, et al. Comparison of the clinical effectiveness of single versus multiple injections of platelet-rich plasma in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Orthop J Sports Med. 2019;7(12):2325967119887116. doi: 10.1177/2325967119887116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
eFigure 1. Patient Information Sheet
eFigure 2. Postinjection Care Sheet (Front and Back Pages)
eTable 1. List of Site Quality Assurance (QA) Assessments
eTable 2. PRP Quality Assurance (QA) Assessments
eTable 3. Blinding Success Report
eTable 4. Additional Treatments Received
eTable 5. Adjusted Treatment Difference of VISA-A Score at 6-Months Follow-up With 95% CI (Per Protocol Using Observed Dataset)
eTable 6. Adjusted Treatment Difference of VISA-A Score at 6-Months Follow-up With 95% CI by Treatment Group (Imputed Dataset)
eTable 7. Adjusted Treatment Difference of VISA-A Score at 6 Months According to Subgroup Characteristics
Nonauthor Collaborators
Data Sharing Statement