Abstract
Hospital-onset COVID-19 infections (HOCIs) are associated with excess morbidity and mortality in patients and healthcare workers. The aim of this review was to explore and describe the current literature in HOCI surveillance. Medline, EMBASE, the Cochrane Database of Systematic Reviews, the Cochrane Register of Controlled Trials, and MedRxiv were searched up to 30 November 2020 using broad search criteria. Articles of HOCI surveillance systems were included. Data describing HOCI definitions, HOCI incidence, types of HOCI identification surveillance systems, and level of system implementation were extracted. A total of 292 citations were identified. Nine studies on HOCI surveillance were included. Six studies reported on the proportion of HOCI among hospitalized COVID-19 patients, which ranged from 0 to 15.2%. Six studies provided HOCI case definitions. Standardized national definitions provided by the UK and US governments were identified. Four studies included healthcare workers in the surveillance. One study articulated a multimodal strategy of infection prevention and control practices including HOCI surveillance. All identified HOCI surveillance systems were implemented at institutional level, with eight studies focusing on all hospital inpatients and one study focusing on patients in the emergency department. Multiple types of surveillance were identified. Four studies reported automated surveillance, of which one included real-time analysis, and one included genomic data. Overall, the study quality was limited by the observational nature with short follow-up periods. In conclusion, HOCI case definitions and surveillance methods were developed pragmatically. Whilst standardized case definitions and surveillance systems are ideal for integration with existing routine surveillance activities and adoption in different settings, we acknowledged the difficulties in establishing such standards in the short-term.
Keywords: SARS-CoV-2, COVID-19, Hospital-onset, Surveillance
Background
Nosocomially acquired coronavirus disease 2019 (COVID-19) reflects a failure in healthcare systems to prevent transmission and acquisition. Hospital-onset COVID-19 infections (HOCIs) are fuelling the global pandemic and are associated with excess morbidity and mortality in both patients and healthcare workers (HCWs). Hospitals are facing challenges in preventing cross-transmission, particularly in settings with extensive community transmission prior to implementation of stringent social distancing measures [1]. Factors such as continuation of clinical services, surge in intensive care, uncertainty in transmission dynamics, staff-to-staff transmission, and shortages and inadequate use of personal protective equipment (PPE) increased the difficulty in managing SARS-CoV-2 transmission in healthcare facilities. Understanding nosocomial acquisition, outbreaks, and transmission chains in real time are fundamental to ensuring infection-prevention measures are effective in controlling SARS-CoV-2 in healthcare. Tackling HOCI is vital to ensure patient safety, maintain public confidence and protect the health and wellbeing of healthcare professionals [2].
Surveillance systems reduce the incidence of nosocomial infection [3]. National surveillance systems of HOCI have been developed in the UK to support understanding national trends, emerging issues and geographical areas of concern [4,5]. However, they are limited by lack of resolution to clarify the finer details of transmission and acquisition events in a real-time manner to support infection prevention and control (IPC) interventions on the ground. Accurate, real-time recognition of HOCIs has the potential to improve patient outcomes, optimize IPC measures, and impact on the trajectory of the pandemic. In addition, surveillance will play a key part in healthcare recovery. To accomplish this, there is a need for standardized definitions to categorize HOCI cases, as well as surveillance systems which can be adopted globally and integrated with existing routine surveillance activities for other healthcare-associated infections (HCAIs). HCAIs are frequently defined as development of disease more than 48 h after admission. However, such a definition is not suitable for COVID-19 considering that the incubation period between infection of SARS-CoV-2 and symptoms onset is up to 14 days with a median of 5 days [6]. At present, multiple definitions of COVID-19 infections within healthcare have been developed pragmatically, along with ad hoc approaches to deploy systems for monitoring of HOCI. To inform and enable the development of a standardized yet translatable approach, we aimed to review the current literature in surveillance systems that are in place to identify and monitor HOCI.
Methods
Study eligibility
We conducted a systematic review of published literature to identify and compare existing surveillance frameworks for HOCI implemented in different countries in a range of clinical settings, and highlight any gaps in HOCI reporting. This review protocol was registered on the International Prospective Register of Systematic Reviews (PROSPERO) and is available in full on the PROSPERO website (CRD42021235412). In addition, we also identified the standardized HOCI definitions published by governmental health authorities (grey literature).
Search strategy and information sources
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [7]. A completed PRISMA checklist is included in the Supplementary data. A Patient, Intervention, Comparator, Outcome (PICO) framework was developed to determine key components and develop a search strategy to interrogate relevant electronic resources [8], including Medline, EMBASE, the Cochrane Database of Systematic Reviews, the Cochrane Register of Controlled Trials), and MedRxiv pre-print repository for a comprehensive list of articles. The PICO framework comprises: Population: any patient of any age in any healthcare setting with any respiratory sample yielding SARS-CoV-2 48 h from hospital admission will be included. Intervention: all studies of surveillance systems applied to HOCI will be evaluated. Comparison: as no gold standard surveillance systems are available for comparison, we compared with routine practice (spontaneous identification). Outcome: we will assess outcomes in four ways: (1) HOCI definitions, (2) HOCI incidence, (3) types of HOCI identification surveillance systems, and (4) level of implementation (single site, regional, national, international). The search criteria included any full-text article in English, Chinese, French, Spanish, Portuguese, Italian, German language published since 1st January 2020. A combination of Medical Subject Headings and equivalent terms were used (see Supplementary data). The literature search was performed up to 30th November 2020.
Study selection and data extraction
Abstracts were initially screened against eligibility criteria and duplicates removed. All studies involving HCW surveillance were excluded. Following this, full-text articles were assessed. Abstract screening and full-text article assessment was performed by two independent researchers (M.A., J.R.P.) using a web-based tool for systematic review (Rayyan QCRI) [9]. Data was extracted from eligible studies by two independent researchers (M.A., N.J.Z.).
Quality assessment
Study design-specific critical appraisal tools were used to evaluate the scientific rigour of all included papers. One reviewer (N.Z.) quality appraised the studies using: Effective Public Health Practice Project (EPHPP) tool for interventional or observational study designs [10]; Newcastle–Ottawa scale for cohort studies [11]; Joanna Briggs Institute checklist for case series [12]; Scale for the Assessment of Narrative Review Articles (SANRA) scale for narrative reviews [13]; AMSTAR 2 critical appraisal tool for systematic reviews [14]; and an adapted version of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist for modelling studies (with questions 1, 6, 8–14 and 19–21 omitted, as these were not relevant to non-economic modelling studies) [15]. In the absence of an appropriate standardized tool for appraisal of descriptive case studies, we documented key factors that were likely to influence study quality [16,17].
Ethics
Not applicable.
Results
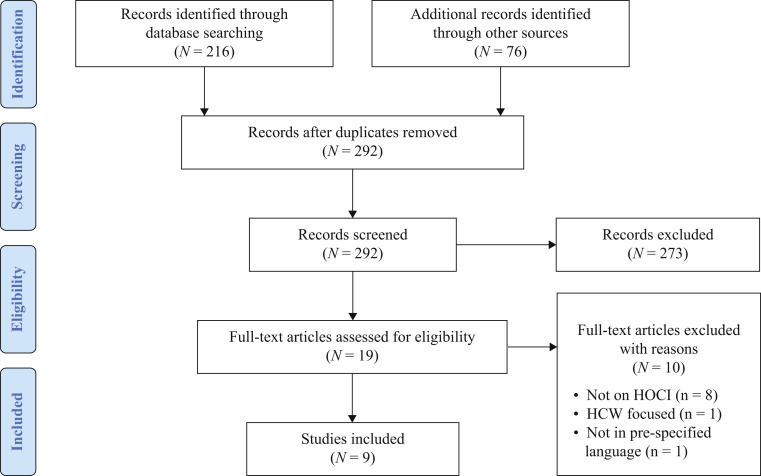
The literature search yielded a total of 292 non-duplicate citations. A flow chart of assessed studies is presented in Figure 1 . We identified 208 citations from Medline and EMBASE, eight citations from the Cochrane Database of Systematic Reviews, and 76 citations from Medrxiv. 273 citations were excluded at the abstract screening stage; most were excluded because the study did not evaluate HOCI surveillance (N = 139). Whilst one publication provided a protocol of an international surveillance system, at the time of review no data were available and hence it was not included [18]. Of the 19 citations that were reviewed at the full-text screening stage (full-texts available for all), nine were included in this review [2,[19], [20], [21], [22], [23], [24], [25], [26]]; the remaining 10 were excluded as they did not address HOCI surveillance. Of the nine studies included in this review, four were from the UK [2,23,25,26], two were from the USA [22,24], and one each were from Hong Kong [20], Korea [21] and Taiwan [19]. All of the studies were from the so-called ‘first wave’ of the pandemic. Surveillance was retrospective in four studies [22,[24], [25], [26]]. The quality of the included studies was appraised using assessment tools for interventional, observational, and case study designs. The quality grading of individual studies is included in the Supplementary data. Overall, the quality of studies was limited by their observational nature with short follow-up periods, and lack of prespecified protocols. Two observational studies [21,22] were deemed to have strong quality considering the aims of reporting incidence of COVID-19 infections. Two prospective surveillance studies [2,23] were deemed to have moderate quality when the comparison of efficacy of case detection was not compared against conventional approaches. One interventional study [20] was deemed to be of low quality (described escalating infection control responses) due to the absence of definition of outcome(s) of interest and providing pre-/postintervention comparison. The quality of two cohort studies [24,25] was limited due to the lack of a comparable non-COVID cohort.
Figure 1.
Study flow chart describing the identification, screening, eligibility and inclusion of literature at falls under the scope of this systematic review. HCW, healthcare worker; HOCI, hospital-onset COVID-19 infection.
HOCI definitions
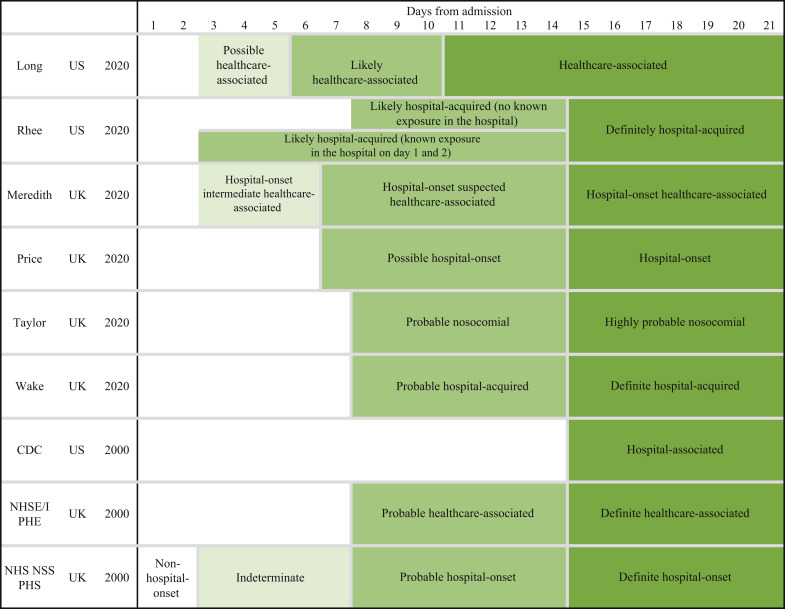
HOCI definitions were provided in six [2,[22], [23], [24], [25], [26]] of the nine included studies. Figure 2 is a schematic of the different definitions used in the studies (full description of the proposed definitions is included in the Supplementary data). One UK study [23] developed HOCI definitions before standardized national government definitions were published, while the other three UK studies [2,25,26] used the national definitions [27]. In addition, we identified national standardized definitions from National Health Service England and National Health Service Improvement (NHSE/I) and Public Health England (PHE) (UK) [28], National Health Service National Service Scotland (NHS NSS) and Public Health Scotland (PHS) (UK) [29], and Centers for Disease Control and Prevention (USA) [30] (Figure 2; Supplementary data).
Figure 2.
Visual representation of definitions of hospital-onset COVID-19 infection. CDC, Centers for Disease Control and Prevention; NHSE/I, National Health Service England and National Health Service Improvement; NHS NSS, National Health Service National Service Scotland; PHE, Public Health England; PHS, Public Health Scotland.
Table I summarizes the current evidence of surveillance of hospital-onset COVID-19 infection.
Table I.
Summary of studies in surveillance of hospital-onset COVID-19 infection
| Study | Country | Proportion of HOCI cases | Type of surveillance | Automated surveillance | Population | Inclusion of HCWs |
|---|---|---|---|---|---|---|
| [19] | Taiwan | Not reported | Real-time | Yes | General hospital inpatients | Unclear |
| [20] | China (HK) | 0.0% (0/42) nosocomial | Real-time | Yes | General hospital inpatients | Yes |
| [21] | Korea | Not reported | Retrospective | No | Emergency department inpatients | Yes |
| [22] | US | 12.5% (1/8) possibly healthcare-associated; 0.0% (0/8) definitely healthcare-associated | Retrospective | No | General hospital inpatients | No |
| [2] | UK | 15.2% (57/374) suspected or highly likely to be hospital-acquired | Prospective genomic | Yes | General hospital inpatients | Yes |
| [23] | UK | 9.9% (90/907) hospital-onset; 3.1% (28/907) possible hospital-onset | Real-time | Yes | General hospital inpatients | No |
| [24] | US | 0.3% (2/697) hospital-acquired | Retrospective | No | General hospital inpatients | No |
| [25] | UK | 8.3% (42/505) highly probable nosocomial; 3.0% (15/505) probable nosocomial | Retrospective | No | General hospital inpatients | Yes |
| [26] | UK | 1.2% (8/662) likely hospital-acquired; 5.9% (39/662) highly likely hospital-acquired | Retrospective | No | General hospital population inpatients | No |
HCW, healthcare worker; HOCI, hospital-onset COVID-19 infection.
HOCI incidence
Seven studies [2,20,[22], [23], [24], [25], [26]] reported a proportion of HOCI cases among all hospitalized confirmed COVID-19 patients which ranged from 0 to 15.2%.
Types of surveillance systems
The majority of studies (N = 6) [2,21,22,[24], [25], [26]] did not undertake surveillance as part of a multimodal approach. Of the three studies [19,20,23] that reported adoption of a multimodal strategy, only one study [19] articulated how such strategy had been applied to IPC activities, including screening, hospital zone partition, patient flow re-arrangement, education targeting both staff and patients, and evaluation and feedback.
Only one study [21] focused on a particular patient population (emergency room patient exposure). Four studies [2,20,21,25] included HCWs in the surveillance, and in one study the inclusion of HCWs was unclear [19].
Automated surveillance systems were reported in four studies [2,19,20,23]; two studies [2,23] reported automated utilization of administrative data (e.g., admission dates, ward location, etc.) as well as clinical and laboratory data to support their surveillance system; in two studies [19,20] it is unclear which data were automatically extracted. One study [2] involved genomic surveillance to investigate HOCI, incorporating large-scale sequencing of SARS-CoV-2 isolates from patients and HCWs. Of the four studies with automated surveillance, three [19,20,23] reported real-time analysis of incidence data. One study [2] performed epidemiological and cluster analysis, and another [23] performed network analysis. The recipients of the reports were hospital management, and IPC professionals in two studies [2,23] with front-line clinicians being additional recipients in one of these [2].
Interventions reported to have been implemented to prevent SARS-CoV-2 transmission within the healthcare institutions of each study site were examined, including environmental and hand hygiene practices, use of PPE, and antimicrobial stewardship activities. All sites segregated COVID-19 patients, and four sites [21,22,24,26] screened all admitted patients for SARS-CoV-2 (by reverse transcription polymerase chain reaction (RT-PCR) or other). It was unclear whether inpatient admission screening was performed in four sites [2,19,23,25].
Two studies [2,23] detected and reported nosocomial clusters, while this was unclear in three studies [19,25,26]. Eight studies [2,[19], [20], [21], [22], [23], [24], [25]] had developed the surveillance system for nosocomial outbreak detection; this was unclear in one study [26]. One study [25] captured the symptoms of the confirmed COVID-19 cases [26].
Level of implementation
All nine included studies reported single-centre, institution-wide implementation of HOCI surveillance [2,[19], [20], [21], [22], [23], [24], [25], [26]].
Discussion
Surveillance of healthcare-associated infections is key to effective infection prevention and control programmes and will form a core element in the recovery from the COVID-19 pandemic. Following an exhaustive systematic review of all available literature, at the time of writing there are only nine published studies that describe surveillance systems for HOCI. We interrogated these studies and identified heterogeneity in (1) definitions of HOCI; (2) reported incidence of HOCI; (3) surveillance methods. The level of HOCI surveillance implementation remained at institutional level.
Definitions
There was wide heterogeneity in HOCI definitions employed by the studies, with most using custom in-house definitions. Although this highlights the difficulties in ascertaining whether hospital-onset COVID-19 cases are truly healthcare-associated, it does complicate comparisons between institutions or surveillance systems in terms of disease burden.
Of the four UK studies, three utilized national definitions [2,25,26]. Although in line with the eventual definitions provided by the UK government, an additional UK study developed their own definition as their study was undertaken prior to the governmental publication [23].
Incidence
There are many examples of nosocomial COVID-19 outbreaks with variable but often high attack rates up to 60% with high mortality [31]. Unfortunately, as in community-acquired COVID-19, it is the elderly and frail population and those with underlying conditions that bear the highest burden of disease.
Surveillance methods
It is essential to share and disseminate knowledge on surveillance systems to guide hospitals or other healthcare institutions who have not yet developed a surveillance system or who seek to improve an existing one.
Level of implementation
Surveillance should ideally be accompanied with other preventive measures and hospitals should be prepared not only for the large influx of community-acquired COVID-19 cases, but also to protect those patients requiring urgent or semi-urgent care, as well as HCWs [32,33]. At the time when the review was conducted, none of the identified HOCI surveillance systems matched the surveillance definition developed by the Centers for Disease Control and Prevention (CDC) in that the results of the surveillance were not provided by a central network [34]. The UK later introduced a centralized system, through a research consortium, to investigate HOCIs across providers [5].
HCWs are at the interface between the community and the healthcare environment and can thus play an important role in initiating or amplifying nosocomial outbreaks of viral respiratory diseases, including COVID-19. Whole genome sequencing data has been used to demonstrated cross-transmission of SARS-CoV-2 between patients and HCWs [35,36]. Therefore, it is important to consider assessing HCW infection that is epidemiologically associated with patients who develop HOCIs. In turn this will rely on robust systems, especially genomics-based surveillance in place for testing symptomatic and asymptomatic staff and collaborating with occupational health will be essential. As COVID-19 vaccination programmes expand globally, these surveillance systems may provide a robust method to assess the impact of HCW vaccination. In turn, vaccine uptake may form part of these surveillance systems.
This study has several limitations. First, local HOCI surveillance has been likely to rely on already established systems. Therefore, some characteristics of surveillance systems may not have been adequately captured by this systematic review of published literature. We acknowledge that many institutes without established surveillance systems are likely to be under-resourced during the pandemic to develop new systems. Second, our search strategy was not designed to focus on outbreak reports, which are likely to have been detected by some form of surveillance system. However, we believe that it is justified to exclude these reports as, presumably, their focus would be more on outbreak management strategies than on the surveillance system per se.
In conclusion, HOCI surveillance is going to be an essential component in the pandemic recovery. Proactive, real-time surveillance will provide accurate and rapid data to support healthcare recovery. Establishing robust HOCI surveillance will enable assessment and effective IPC interventions. We acknowledge that this will not be easily applicable to all healthcare settings and in turn baseline standards need to be determined. Whilst the development of evidence-based standards is ideal, the variability of settings and resources coupled with need for urgent action, suggests that in the short term these will rely on expert consensus.
Acknowledgements
M.A. is supported by a grant from Geneva University Hospitals.
Author contributions
M.A., A.H., J.R.P. developed the study conception and design. NZ, M.A., J.R.P. undertook data curation. M.A., NZ, J.R.P. performed data analysis and interpretation. M.A., N.Z., A.H., J.R.P. drafted manuscript. N.Z., M.A., S.M., F.B., J.A.O., A.H., J.R.P. conducted critical revision of the manuscript for important intellectual content. N.Z., M.A., J.R.P. provided administrative, technical or material support. A.H. supported funding acquisition. J.R.P. is the guarantor of the study. The corresponding author attests that all listed authors meet the ICMJE criteria for authorship and that no others meeting the criteria have been omitted.
Conflict of interest statement
All other authors report no potential conflicts.
Funding sources
The underlying investigation received financial support from the World Health Organization (WHO). This research was also funded by National Institute for Health Research (NIHR) Health Protection Research Unit (HPRU) in Healthcare Associated Infections and Antimicrobial Resistance at Imperial College London in partnership with Public Health England (PHE), in collaboration with Imperial Healthcare Partners, University of Cambridge and University of Warwick, and Department for Health and Social Care (UK), who funded Centre for Antimicrobial Optimisation (CAMO) at Imperial College London. A.H. is an NIHR Senior Investigator. The views expressed in this publication are those of the authors and not necessarily those of the NHS, NIHR, the Department of Health and Social Care, the NHS or the WHO.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhin.2021.05.016.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ran L., Chen X., Wang Y., Wu W., Zhang L., Tan X. Risk factors of healthcare workers with coronavirus disease 2019: a retrospective cohort study in a designated hospital of Wuhan in China. Clin Infect Dis. 2020;71:2218–2221. doi: 10.1093/cid/ciaa287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Meredith L.W., Hamilton W.L., Warne B., Houldcroft C.J., Hosmillo M., Jahun A.S., et al. Rapid implementation of SARS-CoV-2 sequencing to investigate cases of health-care associated COVID-19: a prospective genomic surveillance study. Lancet Infect Dis. 2020;20:1263–1271. doi: 10.1016/S1473-3099(20)30562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gastmeier P., Geffers C., Brandt C., Zuschneid I., Sohr D., Schwab F., et al. Effectiveness of a nationwide nosocomial infection surveillance system for reducing nosocomial infections. J Hosp Infect. 2006;64:16–22. doi: 10.1016/j.jhin.2006.04.017. [DOI] [PubMed] [Google Scholar]
- 4.Scottish Government . 2020. COVID-19 Nosocomial Review Group.https://www.gov.scot/groups/covid-19-nosocomial-review-group/ Available at: [last accessed Feburary 2020] [Google Scholar]
- 5.COVID-19 Genomics UK Consortium . 2020. The COG-UK Project Hospital-Onset COVID-19 Infections (HOCI) Study.https://www.cogconsortium.uk/news_item/explainer-the-cog-uk-project-hospital-onset-covid-19-infections-hoci-study/ Available at: [last accessed Feburary 2020] [Google Scholar]
- 6.Men K., Wang X., Li Y., Zhang G., Hu J., Gao Y., et al. Estimate the incubation period of coronavirus 2019 (COVID-19) medRxiv. 2020 doi: 10.1101/2020.02.24.20027474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group T.P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 [PMC free article] [PubMed] [Google Scholar]
- 8.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak. 2007:7–16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ouzzami M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan QCRI. Qatar Comput Res Inst. 2016 [Google Scholar]
- 10.Effective Public Health Practice Project . 2003. Quality assessment tool for quantitative studies. [Google Scholar]
- 11.Wells G., Shea B., O’Connell D., Peterson J. Ottawa Hosp. Res. Inst.; Ottawa: 2000. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. [Google Scholar]
- 12.The Joanna Briggs Institute Critical Appraisal tools for use in JBI systematic reviews checklist for case series. Available at: http://joannabriggs.org/research/critical-appraisal-tools.html.
- 13.Baethge C., Goldbeck-Wood S., Mertens S. SANRA—a scale for the quality assessment of narrative review articles. Res Integr Peer Rev. 2019;26(4):5. doi: 10.1186/s41073-019-0064-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shea B.J., Reeves B.C., Wells G., Thuku M., Hamel C., Moran J., et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. doi: 10.1136/bmj.j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Husereau D., Drummond M., Petrou S., Carswell C., Moher D., Greenberg D., et al. Consolidated health economic evaluation reporting standards (CHEERS)-explanation and elaboration: A report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. 2013;16(2):231–250. doi: 10.1016/j.jval.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Braithwaite I., Callender T., Bullock M., Aldridge R.W. Automated and partly automated contact tracing: a systematic review to inform the control of COVID-19. Lancet Digit Health. 2020;2:e607–e621. doi: 10.1016/S2589-7500(20)30184-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S., Li A., Eshaghpour A., Ivanisevic S., Salopek A., Eikelboom J., Crowther M. Quality of early evidence on the pathogenesis, diagnosis, prognosis and treatment of COVID-19. BMJ Evid Based Med. 2020 doi: 10.1136/bmjebm-2020-111499. bmjebm-2020-111499. [DOI] [PubMed] [Google Scholar]
- 18.Saadatian-Elahi M., Picot V., Hénaff L., Pradel F.K., Escuret V., Dananché C., et al. Protocol for a prospective, observational, hospital-based multicentre study of nosocomial SARS-CoV-2 transmission: NOSO-COR Project. BMJ Open. 2020;10 doi: 10.1136/bmjopen-2020-039088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang Y.T., Lin C.Y., Tsai M.J., Hung C.T., Hsu C.W., Lu P.L., et al. Infection control measures of a Taiwanese hospital to confront the COVID-19 pandemic. Kaohsiung J Med Sci Kaohsiung J Med Sci. 2020;36:296–304. doi: 10.1002/kjm2.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng V.C.C., Wong S.C., Chen J.H.K., Yip C.C.Y., Chuang V.W.M., Tsang O.T.Y., et al. Escalating infection control response to the rapidly evolving epidemiology of the coronavirus disease 2019 (COVID-19) due to SARS-CoV-2 in Hong Kong. Infect Control Hosp Epidemiol. 2020;41:493–498. doi: 10.1017/ice.2020.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y.J., Choe J.Y., Kwon K.T., Hwang S., Choi G.S., Sohn J.H., et al. How to keep patients and staff safe from accidental SARS-CoV-2 exposure in the emergency room: Lessons from South Korea’s explosive COVID-19 outbreak. Infect Control Hosp Epidemiol. 2020;42:18–24. doi: 10.1017/ice.2020.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Long D.R., O’Reilly-Shah V., Rustagi A.S., Bryson-Cahn C., Jerome K.R., Weiss N.S., Sunshine J.E. Incidence of health care–associated COVID-19 during universal testing of medical and surgical admissions in a large US health system. Open Forum Infect Dis. 2020;7:ofaa435. doi: 10.1093/ofid/ofaa435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price J.R., Mookerjee S., Dyakova E., Myall A., Leung W., Weiße A.Y., et al. Development and delivery of a real-time hospital-onset COVID-19 surveillance system using network analysis. Clin Infect Dis. 2021;72:82–88. doi: 10.1093/cid/ciaa892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rhee C., Baker M., Vaidya V., Tucker R., Resnick A., Morris C.A., Klompas M. Incidence of nosocomial COVID-19 in patients hospitalized at a large US academic medical center. JAMA Netw Open. 2020;3:e2020498. doi: 10.1001/jamanetworkopen.2020.20498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taylor J., Rangaiah J., Narasimhan S., Clark J., Alexander Z., Manuel R., Balasegaram S. Nosocomial COVID-19: experience from a large acute NHS Trust in South-West London. J Hosp Infect. 2020;106:621–625. doi: 10.1016/j.jhin.2020.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wake R.M., Morgan M., Choi J., Winn S. Reducing nosocomial transmission of COVID-19: Implementation of a COVID-19 triage system. Clin Med (Lond) 2020;20:e141–e145. doi: 10.7861/clinmed.2020-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS England and NHS Improvement . 2020. Operating framework for urgent and planned services within hospitals.https://covidlawlab.org/wp-content/uploads/2020/06/Operating-framework-for-urgent-and-plannedservices-within-hospitals.pdf [last accessed February 2020] [Google Scholar]
- 28.NHS England and NHS Improvement . 2020. COVID-19: epidemiological definitions of outbreaks and clusters in particular settings.https://covidlawlab.org/wp-content/uploads/2020/06/Operating-framework-for-urgent-and-plannedservices-within-hospitals.pdf Available at: [last accessed February 2020] [Google Scholar]
- 29.National Service Scotland . 2021. Hospital onset COVID-19 cases in Scotland.https://beta.isdscotland.org/find-publications-and-data/population-health/covid-19/hospital-onset-covid-19-cases-in-scotland/ Available at: [last accessed February 2021] [Google Scholar]
- 30.Centers for Disease Control and Prevention . 2020. Responding to SARS-CoV-2 Infections in Acute Care Facilities.https://www.cdc.gov/coronavirus/2019-ncov/hcp/responding-acute-care-facilities.html Available at: [last accessed December 2020] [Google Scholar]
- 31.Abbas M., Robalo Nunes T., Martischang R., Zingg W., Iten A., Pittet D., Harbarth S. Nosocomial transmission and outbreaks of coronavirus disease 2019: the need to protect both patients and healthcare workers. Antimicrob Resist Infect Control. 2021;10:7. doi: 10.1186/s13756-020-00875-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mastan S., Cash T., Malik R.A., Charalambous C.P., Abdulla S., Collins T., et al. Limited implementation of measures to reduce nosocomial spread of COVID-19 in hip-fracture patients in the North West of England. J Hosp Infect. 2021;108:90–93. doi: 10.1016/j.jhin.2020.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noh J.Y., Song J.Y., Yoon J.G., Seong H., Cheong H.J., Kim W.J. Safe hospital preparedness in the era of COVID-19: The Swiss cheese model. Int J Infect Dis. 2020;98:294–296. doi: 10.1016/j.ijid.2020.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horan T.C., Andrus M., Dudeck M.A. CDC/NHSN surveillance definition of health care–associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309–332. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Lucey M., Macori G., Mullane N., Sutton-Fitzpatrick U., Gonzalez G., Coughlan S., et al. Whole-genome Sequencing to Track Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Transmission in Nosocomial Outbreaks. Clin Infect Dis. 2021;72:e727–e735. doi: 10.1093/cid/ciaa1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klompas M., Baker M.A., Rhee C., Tucker R., Fiumara K., Griesbach D., et al. A SARS-CoV-2 Cluster in an Acute Care Hospital. Ann Intern Med. 2021:M20-7567. doi: 10.7326/M20-7567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.