Abstract
Introduction:
Dental anomalies in Osteogenesis imperfecta (OI), such as tooth discoloration, pulp obliteration (calcified dental pulp space), and taurodontism (enlarged dental pulp space) vary between and within patients. To better understand the associations and variations in these anomalies, a cross-sectional study was designed to analyze the dental phenotype in OI patients at the individual tooth type.
Method:
A cohort of 171 individuals with OI type I, III and IV, aged 3–55 years, were recruited and evaluated for tooth discoloration, pulp obliteration, and taurodontism at the individual tooth level, using intraoral photographs and panoramic radiographs.
Results:
Genetic variants were identified in 154 of the participants. Patients with Helical α1 and α2 glycine substitutions presented the highest prevalence of tooth discoloration, while those with α1 Haploinsufficiency had the lowest (<10%). C-propeptide variants did not cause discoloration but resulted in the highest pulp obliteration prevalence (μ%20). The prevalence of tooth discoloration and pulp obliteration was higher in OI types III and IV and increased with age. Tooth discoloration was mainly observed in teeth known to have thinner enamel (i.e. lower anterior), while pulp obliteration was most prevalent in the first molars. A significant association was observed between pulp obliteration and tooth discoloration, and both were associated with a lack of occlusal contact. Taurodontism was only found in permanent teeth and affected mostly first molars, and its prevalence decreased with age.
Conclusion:
The dental phenotype evaluation at the tooth level revealed that different genetic variants and associated clinical phenotypes affect each tooth type differently, and genetic variants are better predictors of the dental phenotype than the type of OI. Our results also suggest that tooth discoloration is most likely an optical phenomenon inversely proportional to enamel thickness, and highly associated with pulp obliteration. In turn, pulp obliteration is proportional to patient age, it is associated with malocclusion and likely related to immature progressive dentin deposition. Taurodontism is an isolated phenomenon that is probably associated with delayed pulpal maturation.
Keywords: Dentinogenesis, Tooth abnormalities, Dentin, Oral medicine, Connective tissue, Osteogenesis Imperfecta
Introduction
Osteogenesis imperfecta (OI) is a heterogeneous group of inherited bone dysplasias, with an incidence of 1 per 10,000 individuals [1]. OI was classified based on the severity of bone fragility and the clinical/radiological features [1], into four main types: OI type I with mild bone fragility and blue sclerae; type II is pre- or perinatally lethal; type III is the most severe type associated with survival of the perinatal period, and type IV is of moderate severity [2]. The primary clinical manifestations of OI involve the skeleton, with high susceptibility to fractures and substantial growth deficiency[3]. However, OI is a generalized connective tissue disorder associated with extra-skeletal manifestations like blue sclera, hyperlaxity of ligaments, and hearing impairment [4]. Craniofacial and dental manifestations were also reported including a triangular face [5], severe cranial growth impairment [6], malocclusion [7], dentinogenesis imperfecta (DI), missing and/or unerupted teeth [8, 9].
Clinically, teeth affected by DI present tooth discoloration, ranging from grey-brown to opalescent blue [10], and radiographically, include altered root morphology, bulbous crowns, premature pulp obliteration (PO), and taurodontism [10, 11].
DI is common in OI patients, with a prevalence varies between 8% and 100% [8, 9, 12, 13] and was associated with a high prevalence of dental caries [14]. Interestingly, not all teeth are affected by OI; certain types of teeth seem to be more affected by discoloration [8]. For example, deciduous teeth are generally more discolored than permanent teeth, and first permanent molars are more discolored than the upper anterior teeth, also; not all the discolored teeth will present pulp obliteration or Taurodontism [8, 9, 15]. For this reason, this study focused on a systematic evaluation of tooth discoloration, pulp obliteration, and taurodontism at the single tooth level, in a large cohort of patients affected with OI types I, III, and IV and associated variants.
Materials and Methods
A total of 171 individuals diagnosed with OI were evaluated in the context of the Brittle Bone Disease study. This longitudinal observational study collects information from a consortium of specialized OI centers in North America. The consortium is part of the Rare Disease Clinical Research Network funded by the US National Institutes of Health [14, 16, 17].
All study participants were diagnosed with OI at the Montreal Shriners hospital, Canada. Patients were subsequently grouped according to the diagnosis into OI type I, III, and IV; and according to the variant type into haploinsufficiency variants, helical α1, and helical α2 glycine substitution, splice site variant, and C-propeptide variant.
Dental evaluation was given to participants three years of age and older at the Faculty of Dentistry of McGill University (Montreal, Canada) between 2016–2017. Patients that agreed to participate in the dental descriptive study were included, with the only exclusion criteria being the patient’s refusal to participate or individuals younger than 3 years old. The study obtained ethics approval from McGill ethics committee, number A09-M47–15B, and all participants or their legal guardians provided informed consent. This study followed the STROBE guidelines for cross sectional studies.
Dental Evaluations
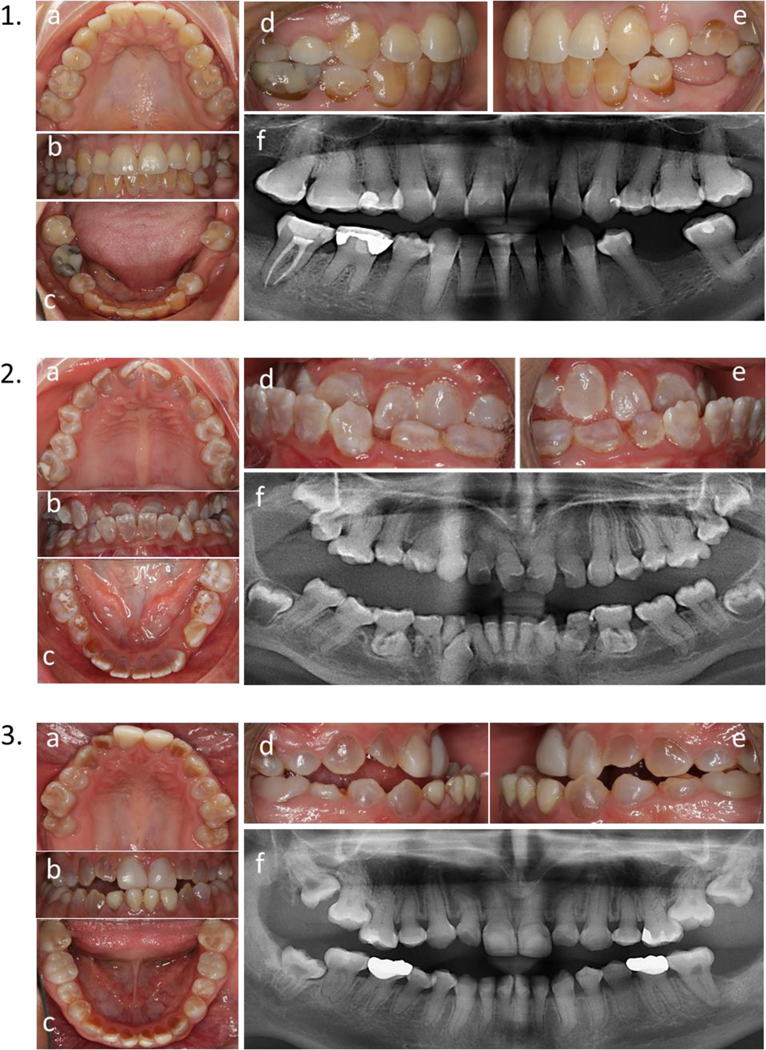
Calibrated intraoral photographs were taken with a Canon D70 camera equipped with a 100 mm Canon lens (1:3 magnification), and a ring flash (Canon model Macro Ring Lite MAR 14 Ex II) on TTL mode. For every individual, five photographs were taken from five different angles (facial view, lateral right view, lateral left view, upper occlusal view, and lower occlusal view) in a standardized setting by one photographer and were used to evaluate tooth discoloration. Figure 1. Panoramic radiographs were obtained for individuals five years and older and used to assess the presence or absence of PO and Taurodontism.
Figure 1.
1) 31 years old female with OI Type I. 2) 16 years old female with OI type III. 3) 17 years old male with OI type IV. a) upper occlusal view, b) frontal view, c) lower occlusal view. c) right side, d) left side, f) panoramic x-ray.
Evaluation criteria
Every tooth was evaluated individually for tooth discoloration. While for PO and taurodontism, only posterior teeth were assessed due to the panoramic x-ray limitations in anterior teeth imaging [18].
Tooth discoloration was defined as gray-blue or yellow-brown discoloration [13, 15]. PO was defined as pulp chambers and root canals that are opaque or not discernible [19]. Taurodontism was defined as a large pulp chamber where the distance from the trifurcation or bifurcation of the root to the cementoenamel junction is greater than the occluso-cervical distance [20, 21].
In order to predict the affect of oral environment on the dental anomalies, teeth with or without contact with an opposite one, were recorded using intraoral photographs. After that an association between the presence of pulp obliteration or tooth discoloration within teeth in contact was assessed.
Evaluations Reliability
The photographs and x-rays were evaluated by two examiner D.T. and H.M, who were blinded to the OI type, and a third Party, JM.R., was consulted in disagreement. Six months after the first evaluation, D.T. randomly repeated the evaluation of 70 patients for inter-examiner validity. The agreement proportion for the inter and intraexaminer reliability was > 0.8.
Statistical analysis
A descriptive analysis was used to summarize the data. Frequency scores were presented as percentages. Chi-square test and odds ratio were used to verify the association between the binomial variables and between the cofounders (age, gender). The Kruskal- Wallis test was used to compare the non-parametric variables. All the above statistical analyses were performed using SPSS (IBM SPSS statistics for windows, version 22.0. Armonk, NY). Generalized linear model analysis was used for the binary distribution to compare the dental findings in every tooth type using SAS 9.4 (SAS Institute Inc., Cary, NC, USA). A p-value< 0.05 was considered statistically significant after Bonferroni’s adjustment for multiple comparisons.
Results
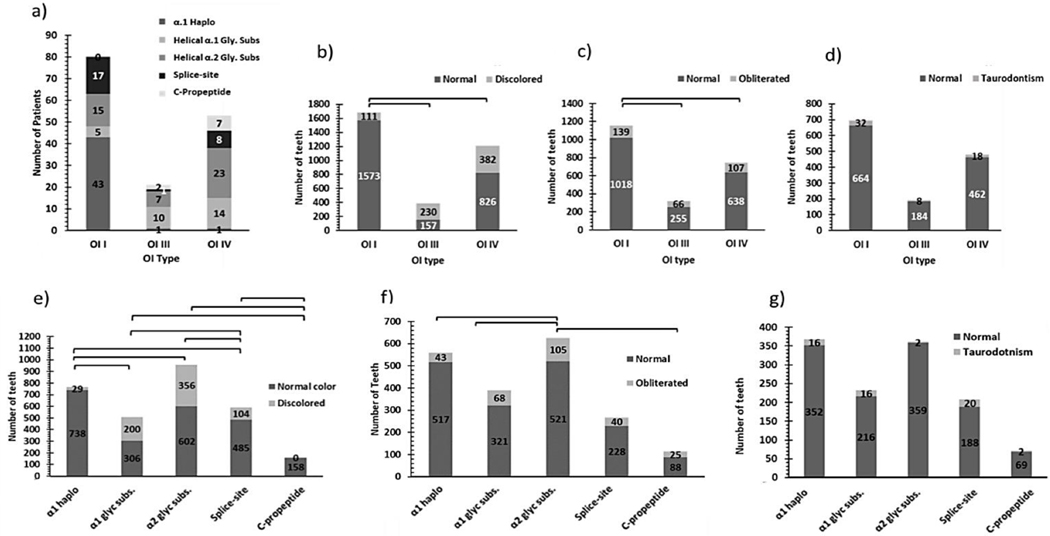
Results collected from a cohort of 171 patients diagnosed with OI are described in supplementary table 1. Genetic variants were identified in 154 individuals and were associated with different clinical and dental phenotypes (Figure 2). Tooth level analysis revealed significant differences in terms of tooth discoloration and PO between patients with different types of variants and OI; however, no such differences were observed regarding taurodontism. (Table 1.). Older individuals (>16 y.o.) consistently showed a higher prevalence of tooth discoloration and PO regardless of OI type, while the contrary was true for taurodontism. Dental manifestations were not affected by different sex (sup. table 14,15).
Figure 2.
The prevalence of the clinical and dental phenotype of OI patients as a function of their genetic variants a) The number of patients associated with different types of variants as a function of their OI type. b) The number of discolored teeth in different OI types. c) The number of obliterated teeth in different OI types. d) The number of teeth with taurodontism in different OI types. e) The number of discolored teeth associated with the different types of variants. f) The number of obliterated teeth associated with the different types of variants. g) The number of teeth with taurodontism associated with different variant types.
Table 1.
The descriptive statistics for the prevalence of tooth discoloration, pulp obliteration, and taurodontism in different Osteogenesis imperfecta types.
| OI Type | Min-Max | Median | Mean±SD | 95% CI | Total No. of teeth evaluated | % affected teeth |
|---|---|---|---|---|---|---|
| Tooth discoloration | ||||||
| Permanent Teeth | ||||||
| OI I | 0–28 | 0 | 1.46±5.2 | .27 – 2.65* | 1684 | 7 |
| OI III | 0–28 | 11 | 10.38±9.9 | 5.9 – 14.8 | 387 | 59 |
| OI IV | 0–28 | 0 | 6.82±9.8 | 4.19 – 9.46 | 1208 | 32 |
| Deciduous Teeth | ||||||
| OI I | 0–14 | 0 | .61±2.6 | 0.0 – 1.21* | 312 | 15 |
| OI III | 0–20 | 0 | 3.95±7.01 | −.05 – 4.52 | 96 | 49 |
| OI IV | 0–20 | 0 | 1.73±4.3 | .57 – 2.89 | 13 | 54 |
| Pulp Obliteration (PO) | ||||||
| Permanent teeth | ||||||
| OI I | 0–16 | 0 | .782±2.8 | .15–1.41* | 1095 | 6 |
| OI III | 0–16 | 0 | 2.95±4.7 | .81–5.09 | 265 | 23 |
| OI IV | 0–16 | 0 | 3.03±5.3 | 1.66–4.40 | 821 | 23 |
| Deciduous teeth | ||||||
| OI I | 0–8 | 0 | .231±1.2 | 0.0 – .52 | 208 | 9 |
| OI III | 0–8 | 0 | 1.0±2.1 | .06 – 1.93 | 55 | 40 |
| OI IV | 0–8 | 0 | .44±1.6 | 0.02 – .85 | 95 | 28 |
| Taurodontism | ||||||
| Permanent Teeth | ||||||
| OI I | 0–6 | 0 | .410±1.2 | .125–.695 | 664 | 5 |
| OI III | 0–2 | 0 | .364±.79 | .014–.714 | 184 | 4 |
| OI IV | 0–4 | 0 | .295±.88 | .069–.521 | 462 | 4 |
The Minimum(Min), Maximum(Max), Mean and standard deviation (SD), and the confidence interval (CI), were calculated based on the number of affected teeth/ patient.
The % of patients (Pt) having at least one affected tooth.
Indicates the significant different between OI type I and both OI type III and IV.
Tooth discoloration:
The descriptive statistics for tooth discoloration in different OI types and variants were summarized in Table 1, Figure 2., and Sup. Table 1. Tooth discoloration was more prevalent in severe forms of OI compared to milder one (70% in OI type III, and 48 % in OI type IV, 11 in OI type I) (Table 1. & Figure 2), and it was more prevalent in older individuals (>16 y.o.) (Sup table 15.).
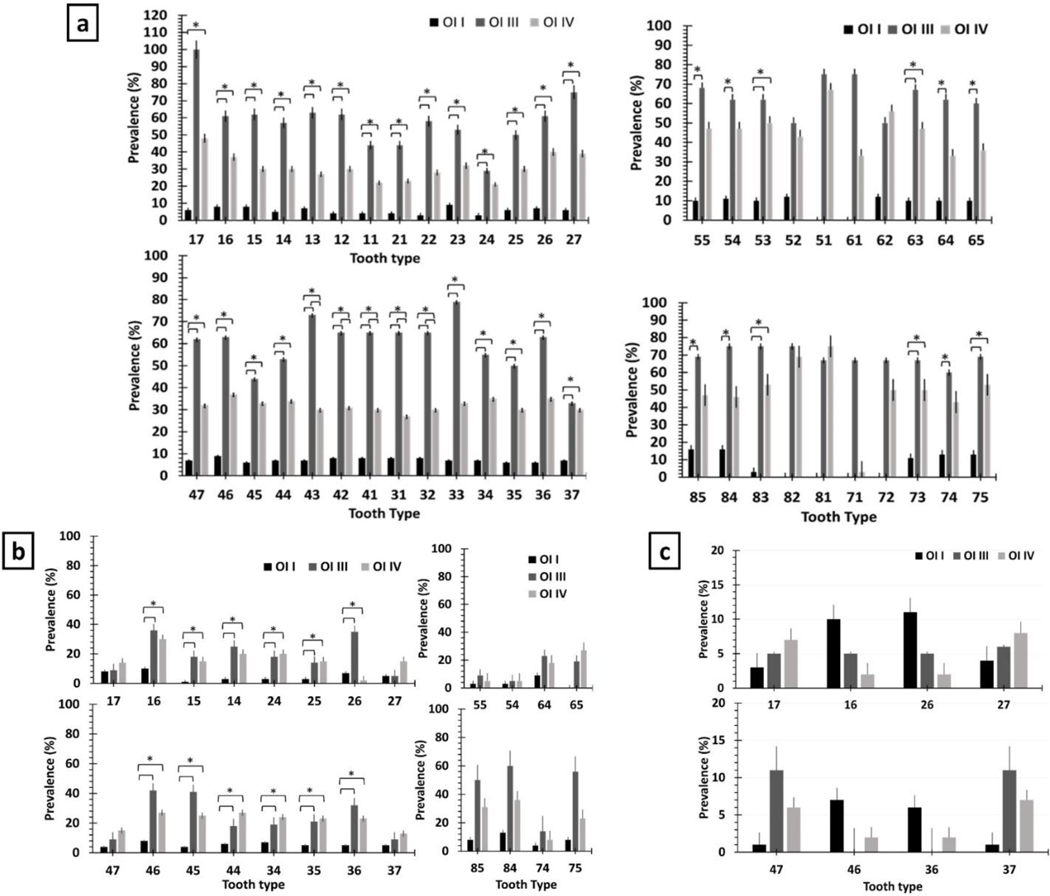
The permanent teeth with the highest prevalence of discoloration were tooth 17,27,33, and 43 (>75%) in patients with OI type III, the molars in those with type IV (≥40%), and lower incisors as well as tooth 15, 16, 23, and 46 in patients with OI type I (>8%) (Figure 3. & sup table 16,17,18.). Deciduous canines as well as all permanent teeth except 17 an 37,were more often affected with discoloration in OI III and IV than in OI I. Deciduous molars were likely to present discoloration in OI III compared to OI IV (Figure 3.).
Figure 3.
The prevalence of dentinogenesis imperfecta in permanent and deciduous teeth. b) The prevalence of pulp obliteration in different OI types in each tooth type. c) The prevalence of Taurodontism in permanent teeth. * indicates a significant difference.
Tooth discoloration was significantly more prevalent in patients with helical α1 and α2 glycine substitution variants (39%−37%, respectively) than in those with splice-site variants (18%), and α1 haploinsufficiency (4%). In contrast, patients with C-propeptide variants showed no tooth discoloration (Figure 2.). We also observed that patients with helical α2 glycine substitutions presented extensive tooth discoloration regardless of the OI type, while helical α1 glycine variants caused extensive tooth discoloration in patients with the moderate-severe OI. Also, α1 haploinsufficiency did not affect second molars, and α2 glycine substitution was significantly associated with discolored first molars than splice-site variants (Figure 2.).
Pulp Obliteration (PO):
PO was observed in both the permanent and deciduous dentitions (Table 1 and sup table 1) and more often affected individuals with the moderate-severe forms of the disease (OI types III & IV) than those with the milder form (OI type I)(p= .001, 0.003, respectively) (Table 1.). It also affected older individuals (16> y.o.) more often than younger ones, especially in OI type IV (OR= 1.55, 95%CI =1.1–2.4, p=.032). (Sup table 15).
Permanent first molars showed the highest prevalence of PO, while second molars showed the lowest prevalence in patients with OI types III and IV (Figure 2&3.). Also, deciduous lower teeth were affected more often than upper teeth, especially in patients with OI type III, and tooth 75 showed a significantly higher prevalence of PO in OI type III than in OI type I (p = 0.035). (Table 1. & Figure 3.)
Patients with α1 haploinsufficiency presented the lowest prevalence of PO than other variants, while the highest was seen in c-propeptide variants. (Figure 2&4.). In patients with helical α1 and α2 glycine substitutions, PO was most often observed in first molars, while in patients with splice-site variants, it was more common in first premolars. None of the patients with α1 haploinsufficiency or c-propeptide variants presented PO in teeth 14 and 15. (Figure 4.).
Figure 4.
Tooth level analysis of OI patients as a function of their type of variant. a) The prevalence of tooth discoloration in each tooth type as a function of type of variant. b) the prevalence of obliterated in each tooth type as a function of type of variant. c) The prevalence of taurodontism in each tooth type as a function of type of variant.
Taurodontism:
Taurodontism was less prevalent than the other dental features of OI (Tables 1 and Sup. Table 1), and only affected permanent teeth. Taurodontism was statistically more significant in younger individuals with OI type I (≤16 yo) (OR 8.42, 95% CI =1.10 – 64.2, p-value = .013). In OI type I, taurodontism affected first molars (10%−11%) the most, while in patients with OI types III & IV, the lower second molars were affected the most (6%−11%) (Figure 3.).
The prevalence of taurodontism in patients with splice-site variants was the highest, while it was significantly lower in patients with helical α2 glycine substitution than those with helical α1 glycine substitutions or C-propeptide variants. (Sup table 13.). Helical α2 glycine substitutions only caused taurodontism in lower second molars, while c-propeptide variants only caused taurodontism in upper second molars
The correlation between tooth discoloration, pulp obliteration, taurodontism, and teeth in contact:
There was a significant association between tooth discoloration and PO (OR 2.3, 95% CI 1.78–2.97; p-value <0.05). This association was significant among patients with helical α1 and splice-site variants associated with OI type IV (p-value <0.05); and more specifically for tooth 14, 15, and 16 with more than 75% of discolored teeth were obliterated (p-value <0.05) (Sup table 11.).
An inversive correlation was also observed between taurodontism and tooth discoloration (OR 0.33, 95% CI = 0.12-.92, p-value =.02) as only 9% of teeth with taurodontism were discolored. This 9% was only found in patients with sever OI associated with helical α1 glycine substitution variant, but the association was not statistically significant (OR=.45, 95% CI 0.96–1.19). Sup table 12
The risk of developing pulp obliteration or tooth discoloration when teeth are out of contact with the opposing tooth (malocclusion) was significantly higher than those in contact, p value >0.05. Table 3.
Table 3.
The risk of teeth out of contact to be obliterated or discolored
| No Contact | contact | X2 | P value | OR | CI | |
|---|---|---|---|---|---|---|
| Pulp Obliteration | ||||||
| (n) Normal pulp | 935 | 951 | 6.06 | 0.014 | - | - |
| (n) Obliterated Pulp | 176 | 132 | 1.153 | 1.036–1.283 | ||
| Tooth Discoloration | ||||||
| (n) Normal color | 794 | 1762 | 173 | 0.000 | - | - |
| (n) Discolored | 418 | 305 | 1.861 | 1.710–2.026 | ||
OR is the Odds ratio of teeth out of contact to be obliterated/discolored compared to those without contact
Discussion:
By assessing the dental phenotype of patients with OI from a tooth type perspective, this study showed that the dental phenotype varies according to the tooth type, variant, OI type, and patient’s age. Genetic variants seemed to be better predictors of the dental phenotype than the OI type itself. Regardless of the severity of the clinical phenotype, normal tooth color is not always an indicator for normal dentin [9, 22].
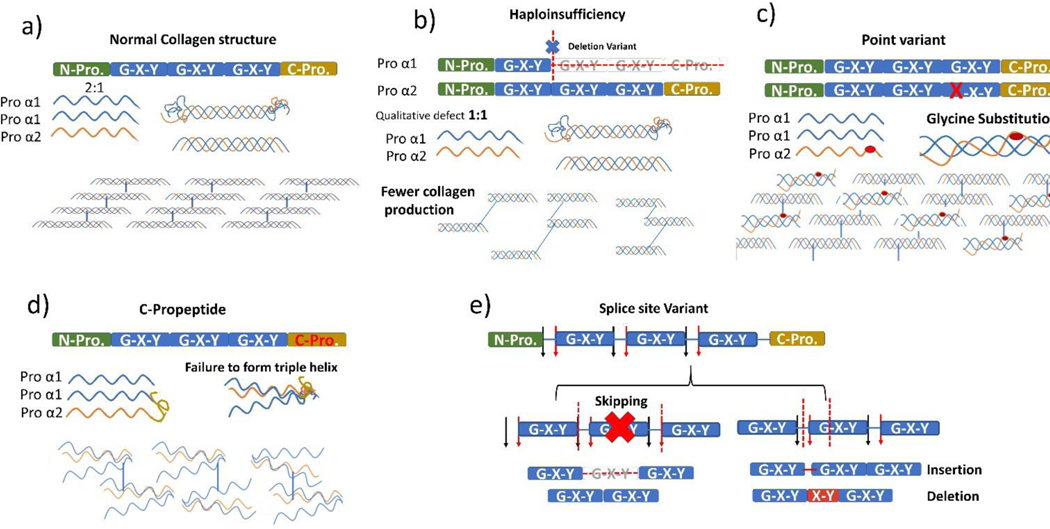
This was confirmed in our study by the fact that certain variants that didn’t cause tooth discoloration such as c-propeptide, were associated with a high prevalence of pulp obliteration and found only in patients with moderate-severe clinical phenotypes (OI types III & IV). The variants which occur within the domains that encode the carboxyl-terminal propeptide may alter the ability of collagen α-chains to aggregate into molecules because carboxyl-terminal propeptide is essential for correct selection and association of the three proα chains and the formation of the triple helix structure [23]. Figure 5.
Figure 5.
Variants in Col 1A1 and Col1A2. a) Normal collagen formation: 2 Pro α1 and 1 pro α2 are essential for the normal formation for the triple helix; change in the 2:1 ratio will result in a reduction of collagen production as in b). b) Haploinsufficiency variant affecting Pro α1 or Pro α2 would result in less collagen and a mild clinical phenotype (OI type I). c) Point variant, especially the Glycine substitution, would result in abnormal collagen structure. The severity will be dependent on the affected gene (Pro α1 or Pro α2). d)c-propeptide is the start point for the formation of the triple helix, and a mutation at this site will result in a loose bundle of collagen fibres and is associated with moderate to severe OI clinical phenotypes (OI types IV and III). e) Splice site variant is associated with a wide range of clinical phenotypes, where the site and type of variant will determine the severity. Skipping mutation is usually associated with a more severe form of OI, while insertions or deletion are associated with a mild form.
We also showed that helical α1 and helical α2 glycine substitutions caused discoloration more often than other variants as well as higher pulp obliteration than haploinsufficiency variants. Glycine substitutions delay helical folding, which in turns prolongs the access time for modifying enzymes and ends up disrupting the formation of the triple helix[1]. Glycine substitution variant is associated with more severe phenotypes when involving the α1 chains, whereas variants in the α2 chains tend to be less severe [1, 24]. Haploinsufficiency variants were associated with mild dental abnormalities, which is in agreement with previous literature [8, 9]. This could be explained by the nature of this variant that leads to decreased collagen type I production, while structurally collagen is not affected.
However, Splice site variants were associated with mild discoloration and pulp obliteration, but also with the highest prevalence of taurodontism. In this variant, clinical severity depends on the splice donor and acceptor site variants that could lead to exon skipping, and/or insertions/deletions. Exon skipping variants cause moderate to lethal OI phenotypes, while insertions/deletions usually cause mild forms of OI [24].
Tooth Discoloration
Tooth discoloration is a common dental findings in patients with OI, and was always associated with the severity of the OI phenotype. In our study helical α1 and helical α2 glycine substitutions caused discoloration more often than other mutations in all OI types regardless of the severity and mainly affected first molars and lower anteriors. This tooth type specification could be related to the timeline of dental development, which is known to affect the expression of pro-α1(I) and pro-α2(I) [25].
However, in our study, not all variants predicted the dental phenotypes, which is in agreement with previous observations [9, 26], indicating that the phenotype is influenced by additional factors such as variant position and the substituting amino acid [26]. Also, variations within the same patient indicated that other factors could be playing a role. For example, deciduous teeth were more affected than permanent teeth, which could be related to the significant production of collagen during the rapid dental development in the embryonic stage [27, 28]. However, we observed that lower incisors were discolored in many patients, while upper incisors which develop at the same stage, were rarely affected. Thus, the onset of tooth development does not seem to explain the variation in phenotype across the different tooth types.
Another possible explanation could be the speed of tooth development. Deciduous teeth and first molars, which were often discolored, develop much faster than the rest of the dentition (2 to 3 years). However, canines, which are the slowest teeth to develop (6–7 years), were severely affected by discoloration, which disproves this hypothesis.
A more plausible explanation for phenotype variations across tooth types could be the variation in enamel thickness. At the histological level, the dentin of patients with OI usually shows abnormalities regardless of the clinical tooth color [29, 30]. Tooth color is mainly determined by the shade of the underlying dentin that reflects yellow light [31], while tooth whiteness results from the light scattered off the enamel layer [32, 33]. Since enamel thickness varies across tooth types and surfaces [33, 34], these variations could explain why discoloration affects tooth types differently. In our cohort, tooth discoloration was always more apparent in the cervical area of the tooth, where the enamel is the thinnest. Also, we observed that teeth with thicker enamel, such as upper anteriors, were less likely to present discoloration than teeth with thinner enamel, such as lower anteriors. Likewise, deciduous teeth, which have thinner enamel than permanent teeth [35], were consistently associated with a higher prevalence of tooth discoloration. All these observations confirm the role of enamel thickness in tooth discoloration.
Pulp Obliteration:
After tooth eruption, dentin thickness may increase physiologically by secondary dentin formation or pathologically via reactionary and/or reparative dentin in response to external stimuli [36]. This is why in the general population, PO is usually observed in older individuals and in teeth exposed to irritants [37].
In our study, PO was associated with age, and it affected first molars the most, which are the first permanent teeth to erupt and get exposed the longest to the oral environment. These observations seem to indicate that PO in patients with OI could be the result of continuous dentin deposition, which is accelerated due to a higher sensitivity to irritants and exposure to the oral environment. Interestingly, C-propeptide, which caused no discoloration, was associated with the highest prevalence of PO, which might indicate that PO is not directly associated with tooth discoloration, at least in this type of variant. These findings help in understanding the nature of pulp obliteration in OI and should also be taken into consideration upon planning dental procedures, especially endodontic treatment, because obliterated tooth canals are hard to locate, debride and properly cleaned, which could result in tooth perforation and damage [38].
Taurodontism:
Taurodontism was found more often in younger individuals, and it affected the second molars more often than the first molars in moderate-sever OI. These findings could indicate that taurodontism represents a delay in the normal development and mineralization of the dentin during early childhood, which tends to resolve over time through the continuous deposition of secondary dentin.
Interestingly, even though patients with OI type I have milder dental features, they presented a similar prevalence of taurodontism to those of more severe types and affected first molars the most. This could indicate that unlike severe – moderate OI, dentin deposition in OI I might be relatively slower, and thus taurodontism becomes more apparent than expected.
Our results indicated that the severity of the clinical manifestations is not an indicator of the taurodontism, but specific variant like splice site will increase the risk of taurodontism. Regardless of the low risk of taurodontism in patients with OI, it still higher than in the normal population, and careful clinical and radiographical examination of the teeth will result in more accurate and risk-free dental plans.
The association between Tooth discoloration, Pulp obliteration, and taurodontism:
Generally, taurodontism and pulp obliteration were considered to be radiographic features of dentinogenesis imperfecta. However, our tooth level analysis revealed that the risk of taurodontism is significantly reduced when the tooth is discolored. Moreover, among patients with OI type I and IV, taurodontism was only found in normal colored teeth. Thus, it seems that rather than being a radiographic feature of dentinogenesis imperfecta, taurodontism is an independent phenomenon probably associated with delayed pulpal maturation.
In contrary, our results confirm the association between tooth discoloration and pulp obliteration similar to many previous studies [8, 9], but the tooth type analysis revealed for the first time that this relation is more of a tooth type-dependent, since it was stronger in tooth 14,15 and 16 and only in OI type IV. This was confirmed by the type of variant, where the helical α1 glycine substitution and the splice site variant showed a significant association between pulp obliteration and tooth discoloration, and both were more prominent in patients with OI type IV. This strong association will serve as clinical guidance to direct the treatment plan for patients with OI. On the other hand, the risk of teeth to be obliterated increases when the teeth are out of occlusion and so is discoloration. This might indicate that lacking occlusal forces is stimulating the odontoblasts to secrete more dentin resulting in pulp obliteration, as well as confirming the association between pulp obliteration and tooth discoloration. Interestingly, It seems that oral environment plays a role in Dentinogenesis imperfecta and may indicate that odontoblast has some mechanical receptors that responds to mechanical stresses.
Limitations, strengths, and future directions
The unbalanced distribution of patients with different OI types in our cohort could have contributed to the lack of statistical significance in some analyses. Lacking a control group is a clear limitation of the study, but in the normal population, tooth discoloration, taurodontism, and pulp obliteration are extremely rare. [39]
One of the major strengths of this research is the large cohort that reduced the risk of selection bias, with a 100% inclusion of patients with OI types I, III, and IV who were ≥ 3 and evaluated at the Shriners hospital, Montreal, Canada, between 2016–2017. Additionally, the fact that the investigators were blinded to the type of OI during the data collection minimized bias and maximized the validity of the results.
Further research is recommended to investigate the correlation between the enamel thickness and the tooth color in patients with OI.
Conclusion
Regardless of the limitation of this cross-sectional study, we shed the light on the importance of studying the effect of genetic variant at the tooth level, which allowed us to study the associations between dental anomalies themselves and the effect of the oral environment.
In OI, dental anomalies vary according to the type of tooth, variant, OI, and patient’s age. Where the type of variant serves as a better tool to predict dental phenotype. Interestingly, mutations causing OI do not always result in dental phenotypes that resemble the severity of the clinical phenotype; for example, C-propeptide variant was only found in patients with OI types III or IV, and was associated with normal tooth color, while α2 Gly. Substitution variant was associated with a high probability of tooth discoloration in all OI types evaluated.
Also, tooth discoloration seems to be associated with enamel thickness and pulp obliteration but not with taurodontism. And finally, pulp obliteration and tooth discoloration are affected by the oral environment that seems to play a significant role in the severity of the dental anomaly.
Clinical implications
The uniqueness of the dental phenotypes we observed for each OI type, and variant imply that dentists could play an essential role in the early diagnosis of OI, especially the milder forms of the disease that don’t present strong medical phenotypes. We also observed that certain variants could cause more dental complications than others, which could affect some teeth more than others. Thus, our findings could help personalize dental treatments according to the patients’ specific OI variant and help anticipate and prevent dental complications. Early diagnosis and intervention are essential for successful dental management to maintain adequate aesthetics, encourage favourable mandibular and maxillary development and establish favourable conditions for permanent dentition eruption. For example, patients with variants that are more prone to cause pulp obliteration could be considered for preemptive root canal treatments. Also, the associations between the clinical and radiographic findings we observed could help better plan radiographic examinations of OI patients.
Supplementary Material
Table 2.
The descriptive statistics for the prevalence of tooth discoloration, pulp obliteration, and taurodontism in different variant types.
| Variant | Median | Mean±SD | 95% CI |
|---|---|---|---|
| Tooth discoloration | |||
| α1 haploinsufficiency | 0 | 0.019±0.13 | −0.0 – 0.04 |
| Helical α.1.Gly. substitution | 0 | 0.48±0.48 | 0.39 – 0.59 |
| Helical α.2.Gly. substitution | 0 | 0.39±0.49 | 0.33– 0.45 |
| Splice-site | 0 | 0.69±0.38 | 0.02 – 0.11 |
| C-propeptide | 0 | 0 | 0 |
| Pulp obliteration | |||
| α1 haploinsufficiency | 0 | 0.08±0.26 | 0.02 – 0.10 |
| Helical α.1.Gly. substitution | 0 | 0.18±0.39 | 0.11 – 0.25 |
| Helical α.2.Gly. substitution | 0 | 0.18±0.38 | 0.13 – 0.23 |
| Splice-site | 0 | 0.13±0.33 | 0.07 – 0.18 |
| C-propeptide | 0 | 0.22±0.41 | .06 – 1.93 |
| Taurodontism | |||
| α1 haploinsufficiency | 0 | 0.04±0.20 | 0.01 – 0.06 |
| Helical α.1.Gly. substitution | 0 | 0.08±0.28 | 0.03 – 0.13 |
| Helical α.2.Gly. substitution | 0 | 0.01±0.08 | −0.00 – 0.02 |
| Splice-site | 0 | 0.96±0.32 | 0.06 – 0.18 |
| C-propeptide | 0 | 0.04±0.21 | −0.02 – 0.11 |
Mean and standard deviation (SD), and the confidence interval (CI), were calculated based on the number of affected teeth/patient.
Highlights.
Tooth level analysis revealed that different genetic variants and associated clinical phenotypes affect each tooth type differently.
Genetic variants are better predictors of the dental phenotype than the type of OI.
Tooth discoloration in OI seems to be an optical phenomenon associated with pulp obliteration and thin enamel.
Pulp obliteration and tooth discoloration are associated phenomena, and both are affected by the oral environment, while taurodontism is a separate feature.
The oral environment seems to play a role in the dental phenotype in patients with OI.
Acknowledgments
“The Brittle Bone Disease Consortium [1U54AR068069-0] is a part of the National Center for Advancing Translational Sciences (NCATS) Rare Diseases Clinical Research Network (RDCRN) and is funded through a collaboration between the Office of Rare Diseases Research (ORDR), NCATS, the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS), the National Institute of Dental and Craniofacial Research (NIDCR), and the Eunice Kennedy Shriver National Institutes of Child Health and Development (NICHD). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.”.
Abbreviations:
- OI
Osteogenesis Imperfecta
- DI
Dentinogenesis Imperfecta
- Gly
Glycine
- PO
pulp Obliterarion
Footnotes
We declare no conflict of interest.
The “Members of the BBDC” include Brendan Lee, V. Reid Sutton, Sandesh CS Nagamani, Francis Glorieux, Janice Lee, Paul Esposito, Maegen Wallace, Michael Bober, David Eyre, Danielle Gomez, Gerald Harris, Tracy Hart, Mahim Jain, Deborah Krakow, Jeffrey Krischer, Eric Orwoll, Lindsey Nicol, Cathleen Raggio, Peter Smith, Laura Tosi.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].Marini JC, Forlino A, Bachinger HP, Bishop NJ, Byers PH, Paepe A, et al. Osteogenesis imperfecta. Nature Reviews Disease Primers. 2017;3:17052. [DOI] [PubMed] [Google Scholar]
- [2].Sillence DO, Rimoin DL. Classification of osteogenesis imperfect. Lancet. 1978;1(8072):1041–2. [DOI] [PubMed] [Google Scholar]
- [3].Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int. 2016:1–11. [DOI] [PubMed] [Google Scholar]
- [4].Rauch F, Glorieux FH. Osteogenesis imperfecta. The Lancet. 2004;363(9418):1377–85. [DOI] [PubMed] [Google Scholar]
- [5].Maioli M, Gnoli M, Boarini M, Tremosini M, Zambrano A, Pedrini E, et al. Genotype–phenotype correlation study in 364 osteogenesis imperfecta Italian patients. Eur J Hum Genet. 2019:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Chang P-C, Lin S- Y, Hsu K- H. The craniofacial characteristics of osteogenesis imperfecta patients. Eur J Orthod. 2007;29(3):232–7. [DOI] [PubMed] [Google Scholar]
- [7].Rizkallah J, Schwartz S, Rauch F, Glorieux F, Vu D-D, Muller K, et al. Evaluation of the severity of malocclusions in children affected by osteogenesis imperfecta with the peer assessment rating and discrepancy indexes. Am J Orthod Dentofacial Orthop. 2013;143(3):336–41. [DOI] [PubMed] [Google Scholar]
- [8].O’Connell AC, Marini JC. Evaluation of oral problems in an osteogenesis imperfecta population. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(2):189–96. [DOI] [PubMed] [Google Scholar]
- [9].Andersson K, Dahllöf G, Lindahl K, Kindmark A, Grigelioniene G, Åström E, et al. Mutations in COL1A1 and COL1A2 and dental aberrations in children and adolescents with osteogenesis imperfecta–A retrospective cohort study. PLoS ONE. 2017;12(5):e0176466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Barron MJ, McDonnell ST, Mackie I, Dixon MJ. Hereditary dentine disorders: dentinogenesis imperfecta and dentine dysplasia. Orphanet J Rare Dis. 2008;3:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Kim J-W, Simmer J. Hereditary dentin defects. J Dent Res. 2007;86(5):392–9. [DOI] [PubMed] [Google Scholar]
- [12].Schwartz S, Tsipouras P. Oral findings in osteogenesis imperfecta. Oral Surg Oral Med Oral Pathol. 1984;57(2):161–7. [DOI] [PubMed] [Google Scholar]
- [13].Majorana A, Bardellini E, Brunelli PC, Lacaita M, Cazzolla AP, Favia G. Dentinogenesis imperfecta in children with osteogenesis imperfecta: a clinical and ultrastructural study. Int J Paediatr Denty. 2010;20(2):112–8. [DOI] [PubMed] [Google Scholar]
- [14].Ma MS, Najirad M, Taqi D, Retrouvey JM, Tamimi F, Dagdeviren D, et al. Caries prevalence and experience in individuals with osteogenesis imperfecta: A cross‐sectional multicenter study. Spec Care Dent. 2019;39(2):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Malmgren B, Norgren S. Dental aberrations in children and adolescents with osteogenesis imperfecta. Acta Odontol Scand. 2002;60(2):65–71. [DOI] [PubMed] [Google Scholar]
- [16].Najirad M, Ma MS, Rauch F, Sutton VR, Lee B, Retrouvey J-M, et al. Oral health-related quality of life in children and adolescents with osteogenesis imperfecta: cross-sectional study. Orphanet J Rare Dis. 2018;13(1):187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Retrouvey J-M, Taqi D, Tamimi F, Dagdeviren D, Glorieux FH, Lee B, et al. Oro-dental and craniofacial characteristics of osteogenesis imperfecta type V. Eur J Med Genet. 2019;62(12):103606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Peretz B, Gotler M, Kaffe I. Common errors in digital panoramic radiographs of patients with mixed dentition and patients with permanent dentition. Int J Dent. 2012;2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jacobsen I, Kerekes K. Long‐term prognosis of traumatized permanent anterior teeth showing calcifying processes in the pulp cavity. Scand J Dent Res. 1977;85(7):588–98. [DOI] [PubMed] [Google Scholar]
- [20].Kirzioglu Z, Ceyhan D, Gok Coban B. An assessment of the association of taurodontism with various dental anomalies, syndromes, systemic diseases and/or genetic diseases, and its role in identification. Aust J Forensic Sci. 2018;50(5):482–92. [Google Scholar]
- [21].Baranwal AK. Taurodontism: An anatomical challenge to clinical endodontics. Ann Prosthodont Restor Dent. 2016;2(4):105–9. [Google Scholar]
- [22].Levin LS, Young RJ, Pyeritz RE, Opitz JM, Reynolds JF. Osteogenesis imperfecta type I with unusual dental abnormalities. Am J Med Genet. 1988;31(4):921–32. [DOI] [PubMed] [Google Scholar]
- [23].Marini JC, Forlino A, Cabral WA, Barnes AM, San Antonio JD, Milgrom S, et al. Consortium for osteogenesis imperfecta mutations in the helical domain of type I collagen: regions rich in lethal mutations align with collagen binding sites for integrins and proteoglycans. Hum Mutat. 2007;28(3):209–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Forlino A, Marini JC. Osteogenesis imperfecta. The Lancet. 2016;387(10028):1657–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Andujar MB, Couble P, Couble M, Magloire H. Differential expression of type I and type III collagen genes during tooth development. Development. 1991;111(3):691–8. [DOI] [PubMed] [Google Scholar]
- [26].Rauch F, Lalic L, Roughley P, Glorieux FH. Genotype-phenotype correlations in nonlethal osteogenesis imperfecta caused by mutations in the helical domain of collagen type I. Eur J Hum Genet. 2010;18(6):642–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lund AM, Jensen BL, Nielsen LA, Skovby F. Dental manifestations of osteogenesis imperfecta and abnormalities of collagen I metabolism. J Craniofac Genet Dev Biol. 1998;18(1):30–7. [PubMed] [Google Scholar]
- [28].Luder H, Van Waes H, Raghunath M, Steinmann B. Mild dental findings associated with severe osteogenesis imperfecta due to a point mutation in the alpha 2 (I) collagen gene demonstrate different expression of the genetic defect in bone and teeth. J Craniofac Genet Dev Biol. 1996;16(3):156–63. [PubMed] [Google Scholar]
- [29].Waltimo J, Ojanotko‐Harri A, Lukinmaa PL. Mild forms of dentinogenesis imperfecta in association with osteogenesis imperfecta as characterized by light and transmission electron microscopy. J Oral Pathol Med. 1996;25(5):256–64. [DOI] [PubMed] [Google Scholar]
- [30].Lygidakis N, Smith R, Oulis C. Scanning electron microscopy of teeth in osteogenesis imperfecta type I. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;81(5):567–72. [DOI] [PubMed] [Google Scholar]
- [31].en osch JJ, Coops JC. ooth Color and eflectance as elated to Light Scattering and namel Hardness. J Dent Res. 1995;74(1):374–80. [DOI] [PubMed] [Google Scholar]
- [32].Vaarkamp J, ten Bosch JJ, Verdonschot EH. Propagation of Light through Human Dental Enamel and Dentine. Caries Res. 1995;29(1):8–13. [DOI] [PubMed] [Google Scholar]
- [33].Oguro, akajima M, Seki, Sadr A, agami J, Sumi. he role of enamel thickness and refractive index on human tooth colour. J Dent. 2016;51:36–44. [DOI] [PubMed] [Google Scholar]
- [34].Pahlevan A, Mirzaee M, Yassine E, Ranjbar Omrany L, Hasani Tabatabaee M, Kermanshah H, et al. Enamel thickness after preparation of tooth for porcelain laminate. J Dent (Tehran). 2014;11(4):428–32. [PMC free article] [PubMed] [Google Scholar]
- [35].De Menezes Oliveira MA, Torres CP, Gomes-Silva JM, Chinelatti MA, De Menezes FC, Palma-Dibb RG, et al. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc Res Tech. 2010;73(5):572–7. [DOI] [PubMed] [Google Scholar]
- [36].Arana–Chavez VE, Nanci A. High-resolution immunocytochemistry of noncollagenous matrix proteins in rat mandibles processed with microwave irradiation. J Histochem Cytochem. 2001;49(9):1099–109. [DOI] [PubMed] [Google Scholar]
- [37].Hamasha AA-H, Darwazeh A. Prevalence of pulp stones in Jordanian adults. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;86(6):730–2. [DOI] [PubMed] [Google Scholar]
- [38].Kim S, Kratchman S, Guess G. Contemporary endodontic microsurgery: procedural advancements and treatment planning considerations. Endodontics: Colleagues for Excellence. 2010:1–7. [Google Scholar]
- [39].Bauss O, Neter D, Rahman A. Prevalence of pulp calcifications in patients with Marfan syndrome. Oral Surg Oral Med O. 2008;106(6):e56–e61. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.