Abstract
This study focuses on synthesis of polyaniline composites and application as electrode material in the fabrication of hybrid supercapacitors. Hybrid supercapacitors were fabricated using aluminum foil as current collector, and conductive Polyaniline (PANI) composites as electrode materials. Cobalt oxide (Co2O3), ammonium peroxydisulfate (APS) were used along with PANI in the preparation of electrodes of the supercapacitor. Polyaniline and its various composites were used in the cathode and activated carbon in the anode (positive electrodes) of the asymmetric hybrid supercapacitor. Electrochemical performance of the supercapacitors has been evaluated on the basis of capacitive properties (i.e. capacitance, energy density and power density). Area of the supercapacitor, applied voltage for charging and composition of cathode materials have been optimized in this study. The optimum area of the supercapacitor was found 30 cm2 at optimum voltage 5 V. The result showed that supercapacitor fabricated using polyaniline (PANI) as cathode material with Al foil as current collector provided highest capacitance of 249 F/g, Energy density of 31 Wh/kg and Power density of 18 W/kg. Among various PANI composites (Ni-PANI, Cu-PANI, CNF-PANI) synthesized in this study, Ni-PANI composite showed highest capacitance of 336 F/g, energy density of 42 Wh/kg, and power density of 31 W/kg. The result indicates that Ni-PANI composite has high potential as cathode materials for the hybrid supercapacitor.
Keywords: Polyaniline, Composite, Hybrid supercapacitor, Capacitance, Energy density, Power density
Polyaniline, Composite, Hybrid supercapacitor, Capacitance, Energy density, Power density.
1. Introduction
With the rapid development of society and the increasing demand of green energy, energy storage devices have been receiving special attention [1]. Now a days, supercapacitor or other electrochemical capacitors have been receiving special attention for advanced energy storage devices and they have been used widely in the field of automotive systems, back-up power systems etc. [2]. Supercapacitors (SC) are also known as power devices that can be fully charged or discharged in seconds, consequently, it has higher power delivery or uptake and it can be achieved in few seconds [3]. Due to its high power density and energy density, supercapacitors are expected to overcome limitations of conventional energy storage devices (e.g. lithium ion batteries) [4].
Conducting polymer composites demonstrate remarkable energy storage capacity due to its greater electrical conductivity, higher charge storage ability, mechanical properties, comparatively cheaper and eco-friendliness [5, 6, 7, 8, 9, 10]. Electrodes prepared with conducting polymer can provide higher capacitance through rapid and reversible redox reaction [11, 12, 13, 14]. Carbon materials can be composited with conductive polymer composite (CPC) to achieve superior capacitive properties of the electrode. Therefore, novel conductive polymer composites (CPC) stand today as potential electrode material in assembling high performance supercapacitor. In order to fabricate an efficient supercapacitor using CPC as electrode material, it is crucial to consider specific properties of the material including electrical conductivity, stability to particular ions and recyclability. Conductive polymers that consist of conjugated structures (PANI, PPY, PEDOT etc.) and CPC with different inorganic materials (CNT, metal oxide, graphene) have been intensively studied as electrode materials in SC in the previous studies.
Jang et al. prepared PANI coated carbon nanofiber (CNF) composite, which showed highest sp. capacitance of 264 F/g [15]. Zhoua et al. developed align carbon nanotube (A-CNT) electrodes for supercapacitor, which exhibited higher operation voltage of 4 V with energy density of 82.8 Wh/kg and power density of 130.6 kW/L [16]. Li et al. synthesized GNR/PANI composites, and found a high sp. capacitance of 340 F/g and capacitance retention of 90% after 4200 cycles [17].
Xu et al. prepared PANI/GO (graphene oxide) composites, which possessed a capacitance of 555 F/g and it showed capacity retention of 92% after 2000 cycles [18]. Zhou et al. fabricated rGO (reduced graphene oxide) wrapped PANI nanofiber composites, the composite showed capacitance of 250 F/g with 74%of the capacitance retention after 1000 cycles [19]. Kumar et al. synthesized PANI grafted rGO composites, and found that the composite materials showed electrical conductivity of 8.66 S/cm, sp. capacitance of 250 F/g and a good cycling stability [20].
Zhang et al. prepared graphene/CNT/PANI composite which exhibited a sp. capacitance of 432 F/g with retention of 96% after 600 cycles [5]. Liu et al. prepared RuO2/PEDOT nanotubes which demonstrated maximum sp. capacitance of 1217 F/g and power density of 20 kW/kg [21]. Sharma et al. prepared MnO2 embedded MnO2/PPy composite, which exhibited an outstanding sp. capacitance of 620 F/g in comparison with MnO2 (225 F/g) or PPy (250 F/g) [22]. However, Deshmukh et al. prepared the PANI–RuO2 composite which demonstrated a high capacitance of 830 F/g [23]. Yang et al. synthesized a MoS2/PANI composite, which showed capacitance of 678 F/g with retention of 80% after 10000 cycles [24].
Although extensive researches have been published in the literature on fabrication of supercapacitor, however, performances of the current supercapacitors is still not satisfactory for many applications due to their lower energy density. In addition, most of the supercapacitors reported in the literature require high level of complexity and incurs with higher cost. Still, scientists are looking for new polymer based electrode and electrolyte for efficient supercapacitors with high electrochemical capacitance and rapid charge/discharge characteristics.
This study focuses on the synthesis of polyaniline, and preparation of its composite with combination of metal oxide, metal salt and organic acid with view to use as electrode material in the fabrication of supercapacitor. A customized facile synthesis procedure was followed to prepare PANI composite, and low cost flexible Al foil paper was used as current collector for the SC. A set of hybrid supercapacitors were prepared, and performance of the supercapacitor was evaluated on the basis of capacitive properties (capacitance, energy density and Power density). Voltage and area of the supercapacitor and composition of the electrode materials were optimized in this study. Suitable current collectors were selected on the basis of the performance of the supercapacitor.
2. Experimental
2.1. Materials
C6H5NH2 and (NH4)2S2O8 (APS) were collected from Merck, Germany and used in polyaniline synthesis. ZnCl2, CuCl2, Hydrochloric acid (37% HCl), C2H5OH, Acetone, and 25% ammonia solution were collected from Merck, Germany.
In this study Polyaniline, Oxalic Acid (C2H2O2), Sodium Sulphate (Na2SO4), Activated Carbon, Cobalt Oxide (Co3O4) were used to fabricate the supercapacitors. Polyaniline was synthesized in our laboratory, and other chemicals were collected from Merck limited. Aluminum and copper foil paper were used as current collector, and polypropylene sheet was used as separator material in the fabrication of supercapacitor.
2.2. Synthesis of polyaniline composite
Polyaniline was synthesized following chemical oxidative polymerization method in acidic medium. Similar procedure was also followed by previous studies [25, 26]. The initial aniline to peroxydisulfate mole ratio was maintained at 1.0 in the Polyaniline synthesis.
In case of preparation of Ni/PANI composite, aniline (18.26 ml) was dissolved in 600 ml of 2 M HCl. As the reaction is exothermic, the mixture was kept under ice bath. NiCl2 was added in the aniline solution. Then calculated amount of APS was dissolved in 2 M aqueous HCl solution, and added drop wise to the NiCl2 mixed aniline solution under continuous stirring for well mixing and continued for 45 min to prepare Ni/PANI composite. Finally the polymerization was completed, and the PANI composite was washed and dried following the procedure described in our previous studies [27]. Similarly, Cu/PANI, Mg/PANI, Carbon nanofiber (CNF)/PANI, and Benzene sulphonic acid/PANI composites were prepared in this study.
2.3. Fabrication of supercapacitor
Hybrid supercapacitor was fabricated with two electrode sections separated with a microporous separator. Electrode materials along with separator were compressed between current collectors, which act as terminals during charging and discharging. For making hybrid type supercapacitor two current collectors were used. The current collectors provide transportation of current from current source to the electrode, it also disperses heat generated within the cell [28].
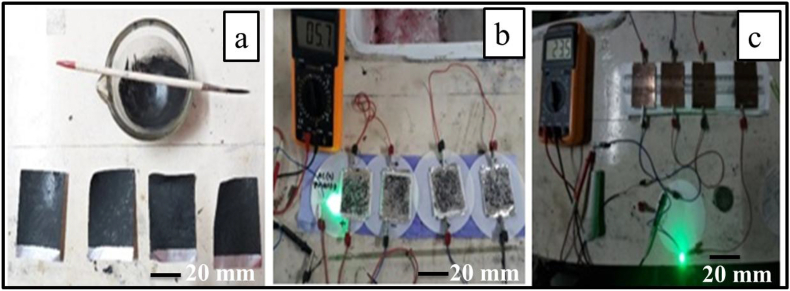
In this study 30 cm2 (5 cm × 6 cm) aluminum foil was used as current collector. Polyaniline or its composite was used as cathode material and activated charcoal was used as anode material. Polyaniline was mixed with cobalt oxide, APS and 0.8 M oxalic acid, and mixed composition was pasted onto the current collector by brush. On the other hand, activated charcoal was mixed with electrolyte (0.8 M Sodium sulfate) was paste onto another current collector by brush (Figure 1 a). Thereafter the electrode was left to dry properly. Then the separator was dipped into the Na2SO4 (electrolytic solution) and properly placed onto the anode electrode, and cathode electrodes were placed on the separator. Similarly, four cells were prepared and connected in series for one setup. Activated charcoal was used in positive electrodes and PANI electrode was used in negative electrodes. After that the whole setup was charged with a different voltage (3–7.5 V), and discharges it with 5 mm led bulb (Forward current of 20 mA, voltage of 2 V and power 0.04 W) (Figure 1b, c).
Figure 1.
Preparation of supercapacitor cell using synthesized PANI composite (a), Experimental setup for the evaluation of capacitive properties (b), and a 5 mm LED bulb lighted by PANI based supercapacitor (c).
2.4. Methods
Capacitance, energy density and power density have been calculated following Eqs. (1), (2), and (3), respectively. Michell et al. [29] and Ramya et al. [30] evaluated capacitance, energy density and power density using the equations.
| (1) |
Where C denotes capacitance in F/g, I represents current, t indicates discharge time in second, ΔV denotes voltage difference, m is the weight of materials.
| (2) |
Where E stands for the energy density (Wh/kg), and V stands for voltage. Power density (W/kg) is calculated following Eq. (3).
| (3) |
3. Result and discussion
3.1. Structural analysis
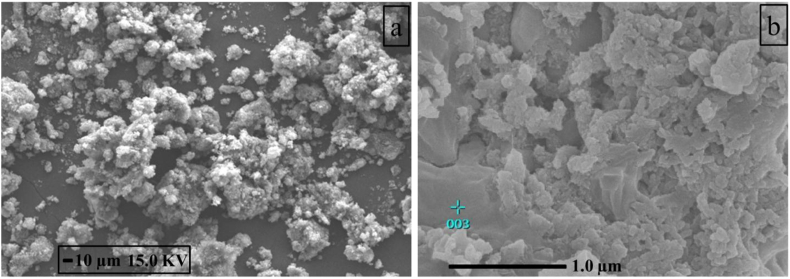
Polyaniline and its composites were synthesized in the laboratory and characterized. Scanning electron microscope (SEM) images of the synthesized PANI and Ni-PANI composite are presented in Figure 2 (a, b). SEM of PANI demonstrates the growth and interlocking of PANI particles (Figure 2 (a)). The surface of PANI is found uneven and irregular, similar phenomena have also been reported in the literature [27]. As seen in Figure 2(b), the surface area of Ni-PANI composite is larger than PANI.
Figure 2.
SEM of synthesized PANI (a), and Ni-PANI composite (b).
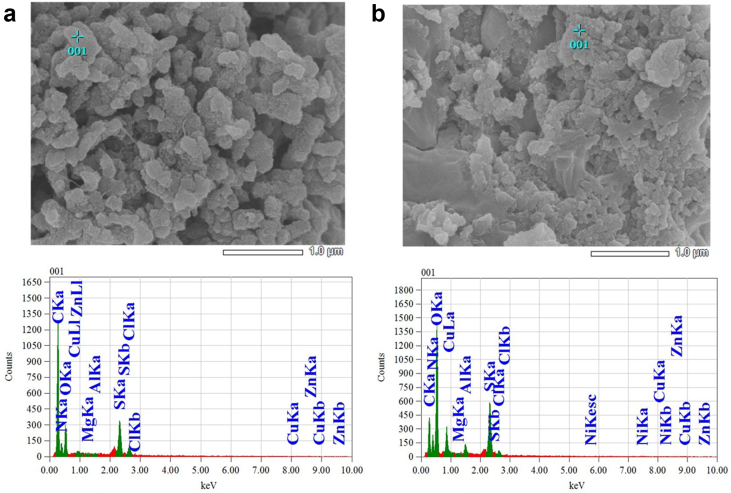
Energy Dispersive X-ray (EDX) spectra of the synthesized PANI and Ni-PANI composite are presented in Figure 3 and the corresponding elemental compositions are presented in Table 1. EDX spectrum of Ni-PANI composite shows the presence of Ni along with the other elemental species present in PANI. We identified the atomic and mass % of Ni in the composite sample is 3.78 and 12.99, respectively that proves the presence of Ni in the composite sample. Similar EDX measurements were performed in different places of same composite sample and were observed the presence of Ni in every case.
Figure 3.
EDX analysis of a) PANI, and b) Ni-PANI composites.
Table 1.
Elemental composition of PANI and Ni-PANI composite.
| Elements | Percent contribution, % |
|
|---|---|---|
| PANI | Ni-PANI composite | |
| C | 58.98 | 22.81 |
| N | 35.45 | 13.36 |
| O | - | 40.03 |
| Cl | 5.57 | 1.04 |
| S | - | 9.76 |
| Ni | - | 12.99 |
3.2. Electrochemical analysis
In the fabrication of supercapacitor, PANI, Cobalt oxide and APS were used as cathode material and activated charcoal as anode material. Al foil paper was used as current collector. In this study, voltage, surface area, and cathode material composition were optimized for individual cell. Performance of the supercapacitor was evaluated on the basis capacitive properties (capacitance, power density and energy density).
3.3. Optimization of applied voltage
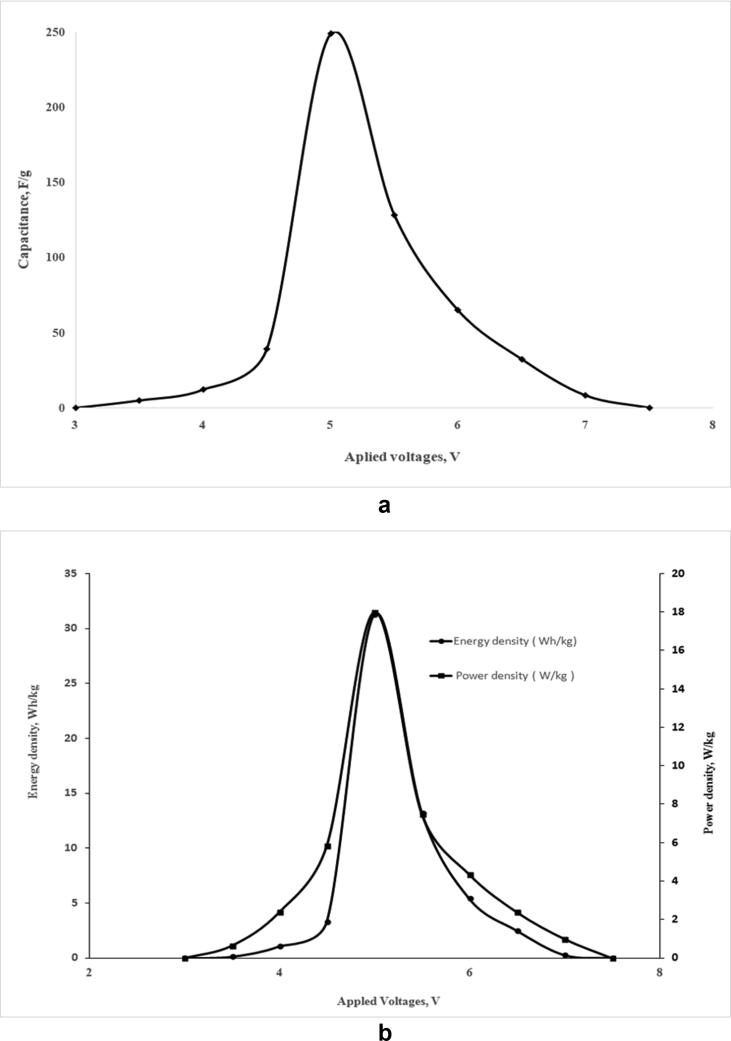
In this study, the fabricated supercapacitor was charged at different applied voltage (3–7.5 V) using a DC voltage supplier. Various electrical parameters such as capacitance, power density and energy density of the supercapacitor were evaluated at different applied voltage and presented in Figure 4. As shown in Figure 4(a), the capacitance increases with the increase of applied voltage up to 5 V at which the capacitance was recorded as 249 F/g. Further increase in applied voltage caused a gradual decrease in capacitance. Initially the capacitance was increased with increasing applied voltage from 3 V to 5 V, because faradaic charge transfer was increased with the application of voltage up to 5 V that resulted higher electrosorption on the surface of electrode, and pseudocapacitance became significant at that voltage. Then, the leakage current and degradation rate was increased with the application of higher voltage (more than 5 V) that resulted reduction in capacitance. Similar to this study, Wang et al. also reported that incorporation of Ni, Co, Mn, Cr in the composition of PANI composite improved operating voltage (>4.6 V) of PANI composite electrode materials [31].
Figure 4.
Effect of applied voltage on the capacitance (a), and the energy density and the power density (b) of the supercapacitor (composition of cathode materials: 0.2 gm PANI, 0.3 gm Cobalt oxide and 0.2 gm APS; anode material: activated charcoal; current collector: Al foil; area of the cell: 30 cm2).
Figure 4(b) demonstrates the variation of energy density and power density of the supercapacitor with various applied voltage. As shown in Figure 4(b), the energy density and the power density initially were increased with increasing the applied voltage up to 5 V. Thereafter, the energy density and the power density were decreased gradually with increasing applied voltage. The maximum values of energy density and power density were found 31 Wh/kg and 18 W/kg, respectively under the applied voltage of 5 V. The performance of the supercapacitor in terms of capacitance, energy density and power density was found maximum at the applied voltage of 5 V. Thus, the optimum voltage of the fabricated hybrid supercapacitor is considered as 5 V. The reason of increment in energy density and power density up to 5 V can be explained by the similar phenomena of pseudocapacitive action, and reduction of the properties at higher than 5 V can be explained by the leakage current. Optimization of voltage is important for any energy storage device; it indicates which purpose the device can be used in. In addition, energy storing capacity increases with increasing applied voltage, and higher applied voltage is advantageous [32]. However, stability of a device at higher voltage depends on electrode material, electrolyte and assembling of the device.
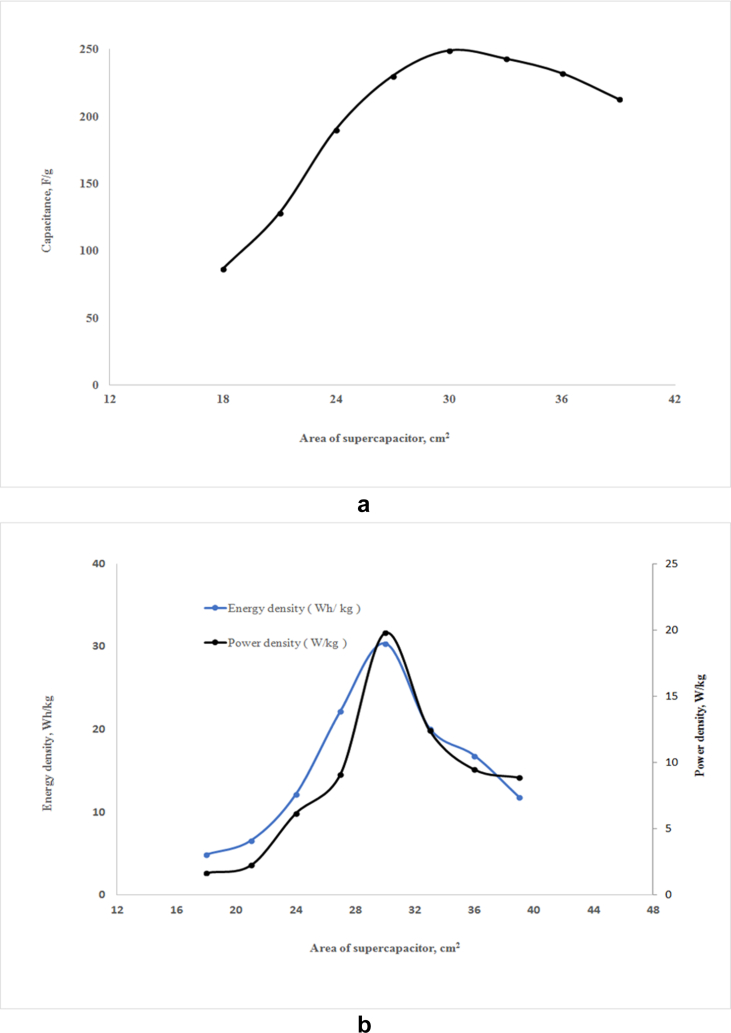
3.4. Optimization of area of the supercapacitor
Effective area is an important parameter of a supercapacitor. The supercapacitors with different areas were fabricated, and their performances were evaluated under an applied voltage of 5 V. As shown in Figure 5(a), the capacitance of the supercapacitor was varied with the variation of area. The figure shows that the supercapacitor with the area of 30 cm2 demonstrates the maximum capacitance of 249 F/g. Further increase in area decreases the capacitance of the supercapacitor.
Figure 5.
Effect of area on the capacitance (a), and the energy density and the power density (b) of the supercapacitor (Compositions of anode and cathode materials, current collector are identical with those discussed in Figure 5, and applied voltage 5 V).
The influence of the area of the supercapacitor on the energy density and the power density is shown in Figure 5(b). As observed from Figure 5(b), both the energy density and power density increase with increasing area up to 30 cm2. Further increase in area gradually decreases the energy density and power density. Maximum energy density and power density were found 31 Wh/kg and 18 W/kg, respectively at 30 cm2, which shows the optimum area for the fabricated supercapacitor is 30 cm2. This result indicates that increase in area of current collector reduces current transportation that results reduction in the capacitive properties of the supercapacitor cell. The effective area of a cell also depends on the type of material and applied voltage.
3.5. Effect of composition of cathode materials
A series of supercapacitor cell were prepared with PANI composition as cathode, activated charcoal as anode and Al foil paper as current collector, and the effects of addition of APS and/or Co3O4 with PANI as cathode materials were evaluated on the basis of capacitive properties of the supercapacitor. The performances of the supercapacitors as a function of variation in composition of cathode materials are presented in Table 2. As shown in Table 2, the supercapacitor where pure PANI was used as cathode material showed comparatively lower performance. In contrast, the supercapacitor where the cathode was composed of PANI, APS and cobalt oxide demonstrates the maximum capacitance of 249 F/g, energy density of 31 Wh/kg and Power density of 18 W/kg. The improvement in electrical properties can be explained by the synergetic behavior of PANI and Co3O4, APS acts as oxidant and the Co3O4 provides transport properties that results improvement in electrical properties and energy storage capacity [33, 34]. Co3O4 undergoes redox reactions. In first case, Co2+ ↔ Co3+ transformation takes place that involves a reversible intercalation of OH− to form CoOOH that results to an improvement of charge storage capacity of the electrode materials. Secondly, Co3+ ↔ Co4+ transformation occurs through the adsorption of OH− ions on the near-surface to form CoO2 that improve capacitance through irreversible non-faradaic process [35, 36]. On the other hand, APS improves capacitive properties of the electrode materials by increasing hydrophilicity of carbon materials and reaction between electroactive group and protons from electrolyte. Kacper Kopczyński et al also reported similar effect of APS on capacitance of carbon electrode materials [37].
Table 2.
Capacitance, energy density and power density as a function of different combination of cathode materials (cathode composition: PANI, Cobalt oxide and APS; anode material: activated charcoal; current collector Al foil; Voltage 5 V, area of the cell 30 cm2).
| Composition of cathode Materials | PANI:APS:Co3O4 | Capacitance (F/g) | Energy density (Wh/kg) | Power density (W/kg) |
|---|---|---|---|---|
| PANI | 1:0:0 | 16.7 | 0.38 | 0.31 |
| PANI + APS | 1:1:0 | 23.35 | 0.59 | 0.29 |
| PANI + Co3O4 | 1:0:1.5 | 80.8 | 4.89 | 2.31 |
| PANI + Co3O4 + APS | 1:1:1.5 | 249 | 31 | 18 |
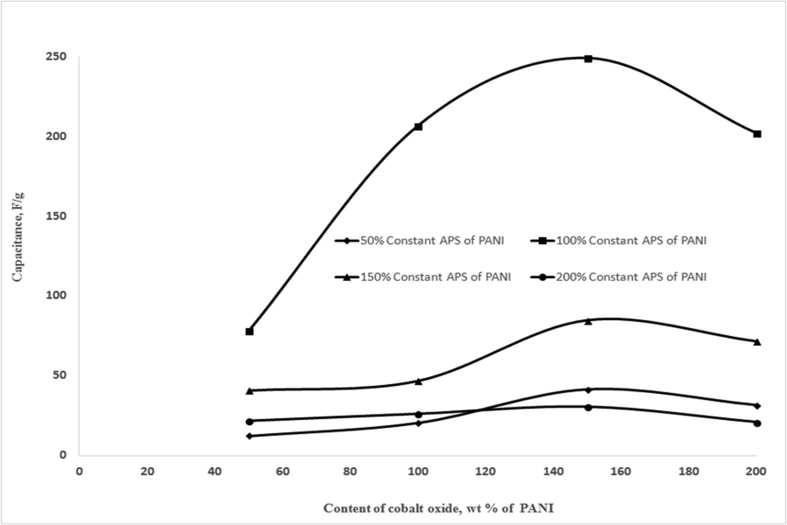
Compositions of cathode materials (PANI, APS, and Co3O4) also have been optimized in this study. A number of cathodes were prepared with fixed amount of PANI (0.2 g) with varying the amount of APS (50, 100, 150, 200, wt. % of PANI) and Co3O4 (50, 100, 150, 200, wt. % of PANI). The effects of APS and Co3O4 content along with PANI in the cathode of the supercapacitor were studied and presented in Figure 6 and Figure 7. The Figure 6 shows the variation of capacitance as a function of Co3O4 content at different percentages of APS. It was observed from Figure 6 that the capacitance was greatly influenced by the composition of APS and Co3O4 in cathode of the supercapacitor. Incorporation of Co2+ in the PANI matrix lowers the internal resistances and also causes localized charge transfer along the polymer chain that results improvement in electrical properties [38]. The supercapacitor with the cathode composition of PANI, APS (100 wt% of PANI) and Co3O4 (150 wt% of PANI) showed maximum capacitance of 249 F/g.
Figure 6.
Variation of capacitance as a function Co3O4 and APS content along with fixed amount of PANI in the cathode of the supercapacitor (Composition of cathode materials: 0.2 gm of PANI, varying amount of Cobalt oxide and APS; anode material: activated charcoal; current collector Al foil; Voltage 5 V, area of the cell 30 cm2).
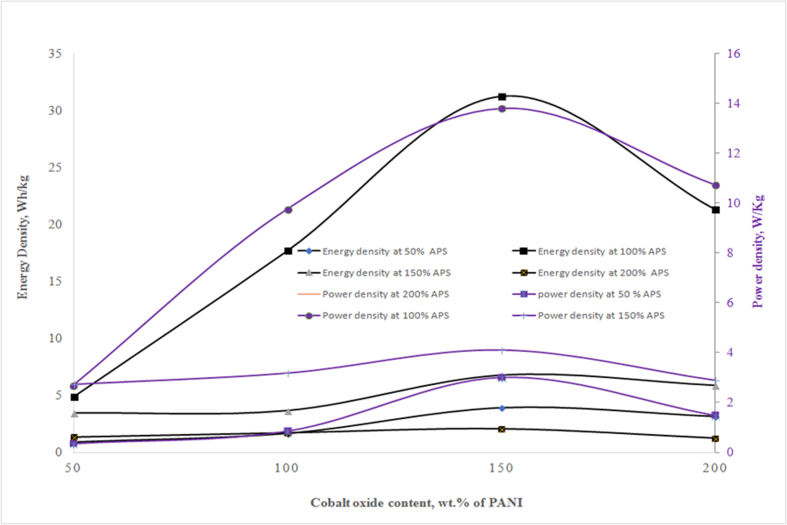
Figure 7.
Variation of energy density and power density as a function Co3O4 and APS content with fixed amount of PANI in the cathode of the supercapacitor (Composition of anode and cathode materials, current collector, Voltage and area of the cell are identical with those discussed in Figure 6).
The change of energy density and power density as a function of Co3O4 and APS content at fixed dose of PANI in cathode of the supercapacitor are presented in Figure 7. The figures reveal that cathode composed of PANI along with APS (100 wt% of PANI) and Co3O4 (150 wt% of PANI) demonstrates the best performance with maximum energy density of 31 Wh/kg and power density of 18 W/kg. Therefore the optimum composition of cathode was found PANI (0.2 g), APS (100 wt% of PANI) and Co3O4 (150 wt% of PANI). Incorporation of Co in the PANI composite provide fast electronic transmission path that results higher power capacity.
3.6. Effect of various PANI composites on the performance of supercapacitor
A set of supercapacitor was prepared using various PANI composite as electrode material along with optimum content of Co3O4 (150 wt% of PANI) and APS (100 wt% of PANI) in the composition. Al foil was used as current collector. Table 3 shows that supercapacitor with Ni-PANI composite as electrode material exhibited highest capacitance compared to the supercapacitor with other composite. Ni-PANI composite exhibited maximum capacitance of 336 F/g, energy density of 42 Wh/kg and power density of 31 W/kg. However, Zhang et al. reported capacitance of 160 F/g for Ni-PANI composite [39]. Better performance of Ni-PANI composite electrode material containing Co3O4 originated from the synergetic effect of PANI, Ni and Co3O4. Nickel and Cobalt can prevent electrode polarization of the PANI and improve the power capacity of the composite materials [40].
Table 3.
Electrical properties of SC prepared with various electrode materials.
| Cathode materials along with Co3O4 and APS | Capacitance (F/g) | Energy density (Wh/kg) | Power density (W/kg) |
|---|---|---|---|
| PANI | 249 | 31 | 18 |
| Ni-PANI composite | 336 | 42 | 31 |
| Cu-PANI composite | 266 | 19 | 18 |
| Carbon nanofiber-PANI composite | 108 | 10 | 6 |
| Benzene sulphonic acid-PANI composite | 23 | 9.8 | 5.5 |
In PANI, nitrogen has one lone pair of electrons and when metal such as Ni or Cu is doped with PANI, a bond is formed between nitrogen and metal. This bond formation may increase the cross linking among the PANI chains and enhance the electron transport rate or electrical conductivity [41]. In addition, the doping of Ni or Cu into the PANI may form more number of active centers which favors the high catalytic activity and high electrochemical surface area. In case of Ni-PANI, both PANI and Ni undergo the redox reactions, which synergistically improve the electrochemical charge storage capability of the electrode [42]. Therefore, elevated capacitance was observed in case of PANI doped with Ni (Ni-PANI) or Cu (Cu-PANI).
In case of addition of carbon nano-fiber (CNF) during the PANI synthesis, it was found that CNF was not well dispersed in the aqueous media that leads to unsatisfactory electrochemical performance of the PANI composite. On the other hand, incorporation of Benzene sulphonic acid into the PANI matrix was not found effective to improve electrochemical properties of PANI composite. Because capacitive properties depends on acid based dopant of anion [43].
4. Conclusion
In this study, Polyaniline and its composites were synthesized for the fabrication of supercapacitor, and the electrochemical performance of the supercapacitor cell was evaluated. Asymmetric hybrid supercapacitor was fabricated by using polyaniline and its various composites as cathode material and activated carbon as anode material. In the fabrication of the supercapacitor, aluminum foil was used as current collector. Area of the supercapacitor, applied voltage for charging, and composition of cathode material have been optimized in this study. Experimental result showed that supercapacitor of the optimum area of 30 cm2 fabricated with optimum cathode composition (PANI along with 100% APS and 150% cobalt oxide) and charged at optimum applied voltage of 5 V showed highest capacitance of 249 F/g. Among various PANI composites, Ni-PANI composite showed highest capacitance of 336 F/g, energy density of 42 Wh/kg, and power density of 31 W/kg. Ni-PANI composite synthesized is found a promising material for energy storage devices.
Declarations
Author contribution statement
Md. Mostafizur Rahman: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Prova Mehedi Joy, Md. Nasir Uddin: Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
M. Zobayer Bin Mukhlish, Mohammad Mizanur Rahman Khan: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by SUST research center (Research grant 2020-2021, Project code: AS/2020/1/19).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Yang J., Liu Y., Liu S., Li L., Zhang C., Liu T. Conducting polymer composites: material synthesis and applications in electrochemical capacitive energy storage. Mater. Chem. Front. 2017;1:251–268. [Google Scholar]
- 2.Miller J.R., Simon P. Materials science: electrochemical capacitors for energy management. Science (80-. ) 2008;321:651–652. doi: 10.1126/science.1158736. [DOI] [PubMed] [Google Scholar]
- 3.Conway B.E. Kluwer Academic/Plenum Publishers; New York, N.Y.: 1999. Electrochemical Supercapacitors : Scientific Fundamentals and Technological Applications. [Google Scholar]
- 4.Frackowiak E. Carbon materials for supercapacitor application. Phys. Chem. Chem. Phys. 2007;9:1774–1785. doi: 10.1039/b618139m. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C., Tjiu W.W., Liu T. All-carbon composite paper as a flexible conducting substrate for the direct growth of polyaniline particles and its applications in supercapacitors. Polym. Chem. 2013;4:5785–5792. [Google Scholar]
- 6.Eris S., Daşdelen Z., Sen F. Enhanced electrocatalytic activity and stability of monodisperse Pt nanocomposites for direct methanol fuel cells. J. Colloid Interface Sci. 2018;513:767–773. doi: 10.1016/j.jcis.2017.11.085. [DOI] [PubMed] [Google Scholar]
- 7.Tapas Das B.V. High performance ternary polyaniline-acetylene black-cobalt ferrite hybrid system for supercapacitor electrodes. Synth. Met. 2019;51:65–74. [Google Scholar]
- 8.Tapas Das B.V. Synthesis of polymer composite based on polyaniline-acetylene black copper ferrite for super capacitor electrodes. Polymer (Guildf) 2019;168:61–69. [Google Scholar]
- 9.Tapas Das B.V. Polyaniline-Acetylene Black-Copper Cobaltite based ternary hybrid material with enhanced electrochemical properties and its use in supercapacitor electrodes. Int. J. Energy Res. 2019:1–16. [Google Scholar]
- 10.Tapas Das B.V. Polyaniline based ternary composite with enhanced electrochemical properties and its use as supercapacitor electrodes. J. Energy Storage. 2019;26:100975. [Google Scholar]
- 11.Choi H., Yoon H. Nanostructured electrode materials for electrochemical capacitor applications. Nanomaterials. 2015;5:906–936. doi: 10.3390/nano5020906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramachandran R., Chen S.M., Gnana kumar G. An overview of electrochemical energy storage devices of various electrodes and morphological studies of supercapacitors. Int. J. Electrochem. Sci. 2015;10:10355–10388. [Google Scholar]
- 13.Yoon W., Lee S. Polypyrrole nanotubes conjugated with human olfactory receptors: high-Performance transducers for FET-Type bioelectronic noses. Angew. Chem. Int. Ed. 2009;48:2755–2758. doi: 10.1002/anie.200805171. [DOI] [PubMed] [Google Scholar]
- 14.Wang J., Mao Q. Methodology based on the PVT behavior of polymer for injection molding. Adv. Polym. Technol. 2012;32:474–485. [Google Scholar]
- 15.Jang J., Bae J., Choi M., Yoon S.H. Fabrication and characterization of polyaniline coated carbon nanofiber for supercapacitor. Carbon N. Y. 2005;43:2730–2736. [Google Scholar]
- 16.Zhoua Y., Xu H., Lachmanc N., Ghaffarid M., Wua S., Liua Y., Ugure A., Gleasone K.K., Wardlec Brian L., Zhang Q.M. Advanced asymmetric supercapacitor based on conducting polymer and aligned carbon nanotubes with controlled nanomorphology. Nanomater. Energy. 2014;9:176–185. [Google Scholar]
- 17.Li L., Raji A.R.O., Fei H., Yang Y., Samuel E.L.G., Tour J.M. Nanocomposite of polyaniline nanorods grown on graphene nanoribbons for highly capacitive pseudocapacitors. ACS Appl. Mater. Interfaces. 2013;5:6622–6627. doi: 10.1021/am4013165. [DOI] [PubMed] [Google Scholar]
- 18.Xu J., Wang K., Zu S., Han B., Wei Z. 17号 Nn1006539.Pdf. 2010;4:5019–5026. doi: 10.1021/nn1006539. [DOI] [PubMed] [Google Scholar]
- 19.Zhou S., Zhang H., Zhao Q., Wang X., Li J., Wang F. Graphene-wrapped polyaniline nanofibers as electrode materials for organic supercapacitors. Carbon N. Y. 2013;52:440–450. [Google Scholar]
- 20.Kumar N.A., Choi H.J., Shin Y.R., Chang D.W., Dai L., Baek J.B. Polyaniline-grafted reduced graphene oxide for efficient electrochemical supercapacitors. ACS Nano. 2012;6:1715–1723. doi: 10.1021/nn204688c. [DOI] [PubMed] [Google Scholar]
- 21.Liu R., Lee S. Synthesis and characterization of RuO2/poly(3,4-ethylenedioxythiophene) composite nanotubes for supercapacitors. Phys. Chem. Chem. Phys. 2010;12:4273–4274. doi: 10.1039/b918589p. [DOI] [PubMed] [Google Scholar]
- 22.Sharma R.K., Rastogi A.C., Desu S.B. Manganese oxide embedded polypyrrole nanocomposites for electrochemical supercapacitor. Electrochim. Acta. 2008;53:7690–7695. [Google Scholar]
- 23.Deshmukh P.R., Bulakhe R.N., Pusawale S.N., Sartale S.D., Lokhande C.D. Polyaniline-RuO2 composite for high performance supercapacitors: chemical synthesis and properties. RSC Adv. 2015;5:28687–28695. [Google Scholar]
- 24.Yang C., Chen Z., Shakir I., Xu Y., Lu H. Rational synthesis of carbon shell coated polyaniline/MoS2 monolayer composites for high-performance supercapacitors. Nano. Res. 2016;9:951–962. [Google Scholar]
- 25.Zulfequar M.D.C., Kumari K., Ali V., Kumar A., Kumar S. Conductivity and spectroscopic studies of polyaniline doped with binary dopant ZrOCl2/AgI. Bull. Mater. Sci. 2011;34:1237–1243. [Google Scholar]
- 26.F.M., Khasim S., Raghavendra S., Revanasiddappa M., Sajjan K., Lakshmi M. Synthesis, characterization and magnetic properties of polyaniline/γ-Fe2O3 composites. Bull. Mater. Sci. 2011;34:1557–1561. [Google Scholar]
- 27.Rahman M., Mahtab T., Bin M.Z. Enhancement of electrical properties of metal doped polyaniline synthesized by different doping techniques. Polym. Bull. 2020 [Google Scholar]
- 28.Lei Z., Christov N., Zhao X.S. Intercalation of mesoporous carbon spheres between reduced graphene oxide sheets for preparing high-rate supercapacitor electrodes. Energy Environ. Sci. 2011;4:1866–1873. [Google Scholar]
- 29.Mitchell E., Candler J., De Souza F., Gupta R.K., Gupta B.K., Dong L.F. High performance supercapacitor based on multilayer of polyaniline and graphene oxide. Synth. Met. 2015;199:214–218. [Google Scholar]
- 30.Ramya R., Sivasubramanian R., Sangaranarayanan M.V. Conducting polymers-based electrochemical supercapacitors - progress and prospects. Electrochim. Acta. 2013;101:109–129. [Google Scholar]
- 31.Wang H., Lin J., Xiang Z. Journal of Science: advanced Materials and Devices Polyaniline ( PANi ) based electrode materials for energy storage and conversion. J. Sci. Adv. Mater. Dev. 2016;1:225–255. [Google Scholar]
- 32.Lehtimäki S., Railanmaa A., Keskinen J., Kujala M., Tuukkanen S., Lupo D. Performance, stability and operation voltage optimization of screen-printed aqueous supercapacitors. Sci. Rep. 2017;7:1–9. doi: 10.1038/srep46001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takada S., Fujii M., Kohiki S. Intraparticle magnetic properties of Co3O4 nanocrystals. Nano Lett. 2001;1:379–382. [Google Scholar]
- 34.Ahmed S.R., Ogale S.B., Papaefthymiou G.C., Ramesh R., Kofinas P. Magnetic properties of CoFe2O4 nanoparticles synthesized through a block copolymer nanoreactor route. Appl. Phys. Lett. 2002;80:1616–1618. [Google Scholar]
- 35.Samal R., Dash B., Sarangi C.K., Sanjay K., Subbaiah T., Senanayake G., Minakshi M. Influence of synthesis temperature on the growth and surface morphology of Co3O4 nanocubes for supercapacitor applications. Nanomaterials. 2017;7:356. doi: 10.3390/nano7110356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Premlatha S., Chandrasekaran M., Bapu G.N.K.R. Preparation of cobalt-RuO2 nanocomposite modified electrode for highly sensitive and selective determination of hydroxylamine. Sens. Actuators, B. 2017;252:375–384. [Google Scholar]
- 37.Kopczyński K., Pęziak-Kowalska D., Lota K., Buchwald T., Parus A., Lota G. Persulfate treatment as a method of modifying carbon electrode material for aqueous electrochemical capacitors. J. Solid State Electrochem. 2017;21:1079–1088. [Google Scholar]
- 38.K.H., H.Z., Yu Shu Ping, Chang Xiao Cong, Wang Zhong Ming. Preparation and performance of Co3O4/polyaniline nano-composite for supercapacitor. Adv. Mater. Res. 2011;239:2042–2045. [Google Scholar]
- 39.Zhang H., Zhao Q., Zhou S., Liu N., Wang X., Li J., Wang F. Aqueous dispersed conducting polyaniline nanofibers: promising high specific capacity electrode materials for supercapacitor. J. Power Sources. 2011;196:10484–10489. [Google Scholar]
- 40.Li Y., Zhang Z., Chen Y., Chen H., Fan Y., Li Y., Cui D., Xue C. Facile synthesis of a Ni-based NiCo 2 O 4 -PANI composite for ultrahigh specific capacitance. Appl. Surf. Sci. 2019:144646. [Google Scholar]
- 41.N.S.S., K.S., Jain N., Patidar D. Temperature dependence of dc conductivity in polyaniline-metal halide composite. Indian J. Pure Appl. Phys. 2006;44:767–770. [Google Scholar]
- 42.Im H., Inamdar Akbar I., Chavan Harish S., Kim Hyungsang. Mesoporous Ni-PANI composite electrode for electrochromic energy storage applications. Sol. Energy Mater. Sol. Cells. 2019;201:110121. [Google Scholar]
- 43.Liu P., Yan J., Guang Z., Huang Y., Li X., Huang W. Recent advancements of polyaniline-based nanocomposites for supercapacitors. J. Power Sources. 2019;424:108–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.