Abstract
Background
Tumor-Treating Fields (TTFields) is an emerging treatment modality for glioblastoma (GBM). Studies have shown a good safety profile alongside improved efficacy in newly diagnosed GBM (ndGBM), while a less clear effect was shown for recurrent GBM (rGBM). Despite regulatory support, sectors of the neuro-oncology community have been reluctant to accept it as part of the standard treatment protocol. To establish an objective understanding of TTFields’ mechanism of action, safety, efficacy, and economical implications, we conducted a systematic literature review and meta-analysis.
Methods
A systematic search was conducted in PubMed, Scopus, and Cochrane databases. Twenty studies met the pre-defined inclusion criteria, incorporating 1636 patients (542 ndGBM and 1094 rGBM), and 11 558 patients (6403 ndGBM and 5155 rGBM) analyzed for the clinical outcomes and safety endpoints, respectively.
Results
This study demonstrated improved clinical efficacy and a good safety profile of TTFields. For ndGBM, pooled median overall survival (OS) and progression-free survival (PFS) were 21.7 (95%CI = 19.6-23.8) and 7.2 (95%CI = 6.1-8.2) months, respectively. For rGBM, pooled median OS and PFS were 10.3 (95%CI = 8.3-12.8) and 5.7 (95%CI = 2.8-10) months, respectively. Compliance of ≥75% was associated with an improved OS and the predominant adverse events were dermatologic, with a pooled prevalence of 38.4% (95%CI = 32.3-44.9). Preclinical studies demonstrated TTFields’ diverse molecular mechanism of action, its potential synergistic efficacy, and suggest possible benefits for certain populations.
Conclusions
This study supports the use of TTFields for GBM, alongside the standard-of-care treatment protocol, and provides a practical summary, discussing the current clinical and preclinical aspects of the treatment and their implication on the disease course.
Keywords: alternating electric fields, glioblastoma, meta-analysis, systematic review, Tumor-Treating Fields
Glioblastoma (GBM) is the most common primary malignant brain tumor among adults, accounting for 46% of malignant CNS tumors, with an annual incidence rate of ~3.2 per 100 000.1,2 In the last two decades, there has been some progress in understanding the pathophysiology and molecular background of the disease, however, modest advances were made in GBM treatment, and it remains an incurable and aggressive disease with a bleak prognosis. Current standard-of-care protocols for GBM combine three primary treatment modalities: resection, chemotherapy, and radiation. Patients with newly diagnosed GBM (ndGBM) are treated with maximal safe resection followed by radiotherapy (RT) plus concomitant and maintenance of temozolomide (TMZ) based on the results of a phase III EORTC study.3 The extent of tumor resection has been shown to affect prognosis significantly,4 and the introduction of tumor fluorescence derived from 5-ALA (5-aminolevulinic acid) that enabled better resection, has led to further improvement in progression-free survival (PFS).5 Chemotherapy regime includes TMZ as the first-line treatment and either bevacizumab or nitrosoureas, which serve as second-line therapy.6,7 Since the establishment of the EORTC protocol, little progress has been made, and different trials have shown median overall survival (OS) and median PFS from diagnosis varying from 14.6-16.7 to 5.5-7.3 months, respectively.8 Following disease recurrence, treatment options for GBM patients are limited. The OS from recurrence is commonly short and without effective therapy rarely exceeds 3-5 months, while with active treatment, the median survival is ~7 months (range 5-9.2 months) with 6-month PFS rates <20%.9
Besides the standard of care for GBM described above (surgery, radiation, and chemotherapy), a fourth treatment modality, Tumor-Treating Fields (TTFields; “Optune®”), was developed by Novocure Ltd., over the past two decades. Following preclinical studies in vitro and in vivo,10–12 two large phase III multicenter clinical trials, EF-119 and EF-1413 were conducted, showing a good safety profile, equivalence to chemotherapy in recurrent GBM (rGBM), and improved OS among ndGBM when added to standard protocol. Following these results, the FDA approved TTFields treatment for recurrent or refractory GBM in 2011,14 and as adjuvant treatment for ndGBM patients after completing standard-of-care surgery and chemoradiation in 2015.15 TTFields has also been recognized by the American Society of Clinical Oncology (ASCO),16 and the National Comprehensive Cancer Network (NCCN) guidelines granted a “category 1” recommendation for ndGBM patients with good performance status, and a “category 2B” recommendation for rGBM.17
Nevertheless, skepticism and criticism have been directed at the studies’ results. The studies were scrutinized for their unblinded design and lack of a sham device. Critics also noted that controls received less adjuvant chemotherapy than treatment group and noted the problematic time lag between diagnosis and randomization. Criticism was also directed at the unclear device mechanism of action, its high costs, and difficulty in use.18–24 Finally, as with any proprietary treatment, TTFields literature may be influenced by financial ties, demonstrating a strong association between favorable views of TTFields and financial conflicts of interest with the device manufacturer.23
To address the controversy and establish an objective understanding of the device’s mechanism of action and its efficacy for treating GBM, we conducted a systematic literature review and meta-analysis using accepted evidence-based techniques. The following systematic review summarizes the existing body of evidence concerning TTFields and uses quantitative meta-analytic methods to assess the treatment’s clinical efficacy and safety profile. We also review the preclinical literature, presenting TTFields’ molecular mechanism of action and discuss potential therapeutic clinical implications of combining it with other treatment methods to highlight the GBM patients’ who may gain the most benefit from its’ use, in addition to the current standard treatment paradigm. Finally, we aim to summarize the current data in an accessible manner for those who treat GBM patients regularly.
Materials and Methods
The meta-analysis presented below was conducted according to the PRISMA guidelines.25 The PRISMA checklist for this meta-analysis is given in the supplementary materials section of this study.
Literature Search
A thorough literature search was performed in three main medical database engines: PubMed (Medline), Scopus (ELSEVIER), and Cochrane Central Register of Controlled Studies (CENTRAL). The search algorithm was generated as follows: (glioblastoma OR GBM) AND (“tumor*treating field*” OR “tumor-treating field*” OR “alternating electric field*” OR “alternating-electric field*” OR ttfield* OR “novocure” OR optune OR NovoTTF-100A OR NovoTTF200A OR EF-14 OR EF-11).
The most recent search was conducted on October 9, 2020.
Studies Selection
Studies meeting the following criteria were included in the quantitative analysis: (1) written in English; (2) original study (randomized controlled trials [RCT], cohort studies, observational studies, or case series); (3) patients treated for GBM; (4) patients ≥18; (5) report either clinical efficacy, daily compliance, or adverse events (AEs). When multiple studies analyzed the same population, we chose the study with a larger sample or most extended follow-up. The studies were categorized by patients’ diagnosis: ndGBM or rGBM.
Data Extraction
A standardized electronic tool named State of the Art through Systematic Review (StArt) was used to systematize the data extraction and examination.26 Studies were screened by title and abstract; relevant studies were then screened by full text for final inclusion. For each eligible study, we extracted the following data: authors, year of publication, study type, intervention, GBM status, number of patients, gender, age, Karnofsky Performance Status (KPS), treatment compliance, and number of recurrences. Also, we extracted clinical endpoints: median OS, median PFS, PFS at 6 months, survival at 1, 2, and 3 years, AEs, and Kaplan-Meier (KM) curves.
Assessment of Methodological Quality and Risk of Bias
To evaluate the quality and risk of bias in the included studies’ methodological design, we used the Oxford Centre for Evidence-Based Medicine guidelines,27 the Newcastle-Ottawa Quality Assessment tool for non-RCT studies,28 and the RoB 2 for RCTs29 (Table 1 and Supplementary Tables 1 and 2). A quantitative analysis of publication bias was not conducted due to the small number of comparative studies.
Table 1.
Summary of Clinical and Socio-Demographic Characteristics of Studies
| Source | Study Design | Quality of Evidencea | Risk of Biasb | Intervention | Patients, No. | Age, y Median (Range) | KPS, Median (Range) | Male, n (%) | Recurrence Number | Daily Compliance, %(range) |
|---|---|---|---|---|---|---|---|---|---|---|
| Newly diagnosed GBM | ||||||||||
| Kirson et al. 2009 | P | 3 | 6 Stars | TTF + TMZ | 10 | 50 (32-70) | 90 (80-100) | 8 (80) | ||
| TMZ | 32c | |||||||||
| Stupp et al. 2017 (EF-14) | RCT | 2 | Some Concern | TTF + TMZ | 466 | 56 (19-83) | 90 (60-100) | 316 (68) | 75% of pt. ≥18 h/d | |
| TMZ | 229 | 57 (19-80) | 90 (70-100) | 157 (69) | ||||||
| Lu et al. 2019 | R | 3 | 7 Stars | TTF + TBI | 8 | |||||
| TTF + BBC | 12 | |||||||||
| Lazaridis et al. 2020 | R | 3 | TTF + CCNU + TMZ | 16 | 50 (27-70) | 90 (60-100) | 9 (66) | 83 (45-99) | ||
| Song et al. 2020 | P | 3 | TTF + TMZ + RT | 10 | 61 (49-73) | 90 (70-90) | 8 (80) | Concurrent phase 83.5 (49-93) Maintenance phase 77 (15-94) | ||
| Bokstein et al. 2020 | P | 3 | TTF + TMZ + RT | 10 | 60.2 (42-72) | 90 (80-100) | 8 (80) | RT: 79.3 (SD 8.36) First 3 months: 77.0 (SD 10.56) | ||
| Shi et al. 2020 | R | 3 | 6 Stars | TTFd | 5887 | <18: 0.3% 18-64: 69% ≥65: 30.7% | 3849 (65.4) | |||
| Recurrent GBM | ||||||||||
| Kirson et al. 2007 | P | 3 | 6 Stars | TTF | 10 | 53 (28-68) | 90 (70-100) | 7 (70) | Mean 18h/d | |
| Stupp et al. 2012 (EF-11) | RCT | 2 | Some Concern | TTF | 120 | 54 (24-80) | 80 (50-100) | 92 (77) | 1st: 9% 2nd: 48% ≥3rd: 43% | 86 (41-98) |
| BSC | 117 | 54 (29-74) | 80 (50-100) | 73 (62) | 1st: 15% 2nd: 46% ≥3rd: 39% | |||||
| Mrugala et al. 2014 (PRiDe) | R | 3 | 6 Stars | TTFd | 457 | 55 (18-86) | 80 (10-100) | 310 (67.6) | 1st: 33.3% 2nd: 26.9% ≥3rd: 27.4% Unknown: 12.5% | 70 (12-99) |
| BSC | 117 | 54 (29-74) | 80 (50-100) | 73 (62) | 1st: 15% 2nd: 46% ≥3rd: 39% | |||||
| Wong et al. 2015 | R | 3 | 6 Stars | TTF + BEV | 34 | 57 (30-77) | 70 (50-90) | 21 (62) | 1st: 17.6% 2nd: 26.5% ≥3rd: 55.9% | 83.5 |
| TTF + BEV + TCCC | 3 | 56 (51-56) | 70 (60-70) | 2 (66) | 1st: 0% 2nd: 66.6% ≥3rd: 33.3% | 66.7 | ||||
| Mahadevan et al. 2015 | R | 3 | 6 Stars | TTF ± SRS | 40 | 57 (47-79) | 24 (60) | |||
| Ansstas et al. 2016 | R | 4 | TTF | 8 | 51 (35-62) | 5 (62.5) | 3rd: 87.5% 4th: 12.5% | 74.2 (48.2-92.9) | ||
| Kesari et al. 2017 (EF-14) | RCT-PH | 2 | Some Concern | TTF + TMZ | 144 | 57 (29-83) | 90 (60-100) | 108 (75) | 1st: 100% | |
| TMZ | 60 | 58 (22-75) | 90 (70-100) | 45 (75) | 1st: 100% | |||||
| Lu et al. 2019 | R | 3 | 7 Stars | TTF + TBI | 18 | 57.8 (11.6)e | 12 (66.7) | |||
| TTF + BBC | 30 | 52.3 (9.9)e | 19 (63.3) | |||||||
| Korshoej et al. 2019 | P | 3 | TTF + SR Surgery | 15 | 57 (39-67) | ≥70 | 11 (73) | 1st: 100% | 90 (48-98) | |
| Onken et al. 2019 | O | 3 | 6 Stars | TTF + BSC | 30 | 52 (36-64) | 20 (67) | ndGBM:47% 1st: 53% | 1st month: 83 (40-97) 2nd month: 85 (56-97) | |
| BSC | 27 | 47e | 19 (70) | ndGBM: 100% | ||||||
| Zhu et al. 2020 (EF-19) | P | 3 | 6 Stars | TTF | 192 | 80 (9.83)e | 55.9 (12.2)e | 125 (65) | ||
| BSC | 117 | 80 (11.01)e | 53 (10.77)e | 73 (62) | 1st: 15% 2nd: 46% ≥3rd: 39% | |||||
| Fallah et al. 2020 | P | 3 | 7 Stars | TTF + BEV | 23 | 60 (17-78) | 18 (78) | |||
| Shi et al. 2020 | R | 3 | 6 Stars | TTFd | 4345 | <18: 0.5% 18-64: 76.8% ≥65: 22.7% | 2921 (67.2) |
Abbreviations: P, prospective; R, retrospective; RCT, randomized controlled trial; RCT-PH, post hoc analysis of RCT; O, observational study; TTF, Tumor-Treating Fields; TMZ, temozolomide; BSC, best standard of care; BEV, bevacizumab; TCCC, 6-thioguanine + lomustine + capecitabine + celecoxib; BBC, bevacizumab ± irinotecan or lomustine; TBI, temozolomide + bevacizumab + irinotecan; RT, radiotherapy; SR Surgery, skull-remodeling surgery; SRS, stereotactic radiosurgery.
aLevel of evidence according to Oxford Centre for Evidence-Based Medicine guidelines.
bRCTs: according to RoB 2 scale; non-RCTs: according to Newcastle-Ottawa Quality Assessment tool.
cHistorical control, matched on KPS and age.
dPatients may have received combination therapy in addition to TTFields.
eMean (SD).
Statistical Analysis
To generate a pooled distribution-free KM survival curves, we used the method proposed by Combescure et al.30 Published survival curves were digitalized using DigitizeIt software, and survival probabilities were extracted at fixed intervals. Corresponding numbers of at-risk patients in each interval were collected when available or estimated using the method proposed by Tierney et al.31,32 Pooled KM curve, median OS, median PFS, and survival rates with 95%CI were calculated using the R MetaSurv package.30 Also, as a sensitivity analysis, for all studies reporting median OS and PFS, we estimated pooled median OS and PFS using the R MetaMedian package33 (Supplementary Figure 1). The pooled prevalence of AEs was calculated using the MetaProp package in R.
The Cochrane Q chi-square test and I2 statistic were used to examine the heterogeneity across studies. The fixed-effects model was used for pooled results with low heterogeneity (I2 ≤ 50%); otherwise, the random-effects model was used for analysis. All analyses were performed using the R software.
Results
Studies Identification and Selection
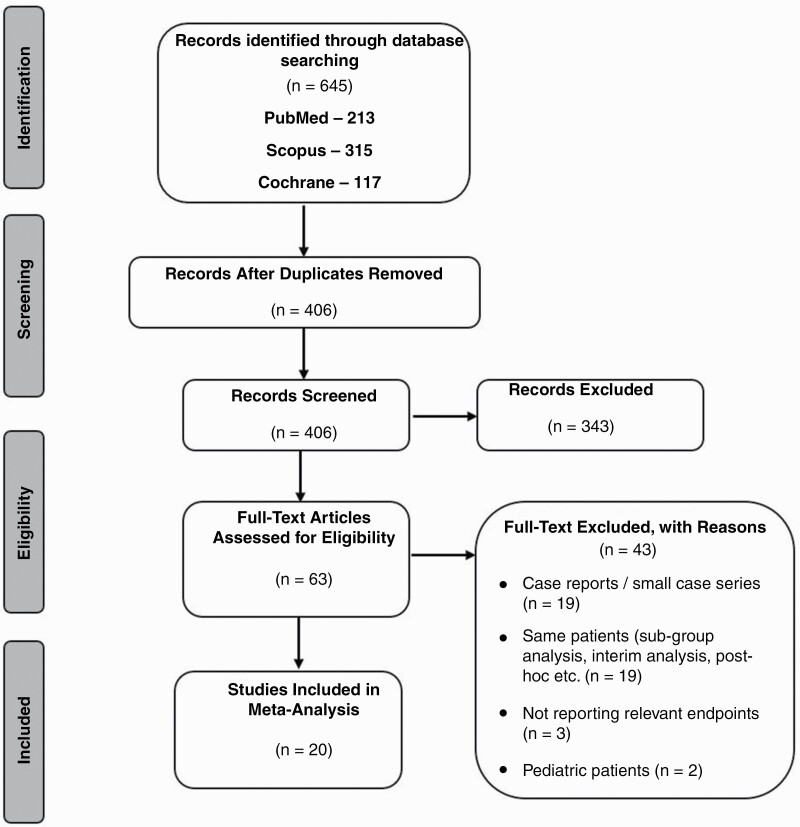
Of the initial 645 papers identified; 20 studies met the pre-defined inclusion criteria (Figure 1(. The studies consisted of: 2 RCTs (EF-119 and EF-1434), 5 prospective single-arm clinical trials,10,12,35–37 1 prospective observational study,38 2 registry-based studies (PRiDe on US patients,39 and a global post-marketing registry40), 3 retrospective studies,41–43 1 case series,44 and 3 post hoc analyses (2 of EF-1445,46 and 1 of EF-1147).45–47 Also, we included 3 conference presentations that have not yet been published in peer-reviewed journals due to the importance of their findings.48–50 Seven studies included ndGBM, 13 included rGBM, and 2 included both. The studies include 1636 (542 ndGBM and 1094 rGBM) patients analyzed for the clinical outcomes’ endpoints, and 11 558 (6403 ndGBM and 5155 rGBM) patients analyzed for the safety endpoints (Tables 1–4).
Figure 1.
Flowchart of studies included in this study.
Table 2.
Clinical Efficacy of TTFields
| Source | Intervention | Patients, No. | Median OS (95% CI) | Median PFS (95% CI) | PFS 6 Months,% | OS 1 Year,% | OS 2 Years,% | OS 3 Years,% |
|---|---|---|---|---|---|---|---|---|
| Newly diagnosed GBM | ||||||||
| Kirson et al. 2009 | TTF + TMZ | 10 | >39 | 38.8 | ||||
| TMZ | 32 | 14.7 | 7.8 | |||||
| Stupp et al. 2017 (EF-14) | TTF + TMZ | 466 | 20.9 (19.3-22.7) | 6.7 (6.1-8.1) | 56% | 73% | 43% | 26% |
| TMZ | 229 | 16.0 (14.0-18.4) | 4.0 (3.8-4.4) | 37% | 65% | 31% | 16% | |
| Lu et al. 2019 | TTF + TBI | 8 | 32.5 (17.0-49.0) | 6.6 (3.7-9.2) | ||||
| TTF + BBC | 12 | 17.8 (13.3-19.9) | 5.1 (3.3-6.1) | |||||
| Lazaridis et al. 2020 | TTF + CCNU + TMZ | 16 | 20a | |||||
| Song et al. 2020 | TTF + TMZ + RT | 10 | 6.9 | |||||
| Bokstein et al. 2020 | TTF + TMZ + RT | 10 | 8.9 (2.1-12.9) | 58.30% | ||||
| Recurrent GBM | ||||||||
| Kirson et al. 2007 | TTF | 10 | 15.6 (5.1-31) | 6.5 (0.75-31) | 50% | 67.50% | ||
| Stupp et al. 2012 (EF-11) | TTF | 120 | 6.6 | 2.2 | 21.40% | 20% | 8% | 5% |
| BSC | 117 | 6 | 2.1 | 15.10% | 20% | 4% | 1% | |
| Mrugala et al. 2014 (PRiDe) | TTF | 457 | 9.6 | 44% | 30% | |||
| BSC | 117 | 6 | 20% | 7% | ||||
| Wong et al. 2015 | TTF + BEV | 34 | 4.1 (0.3-22.7) | 2.8 (0.1-20.7) | ||||
| TTF + BEV + TCCC | 3 | 10.3 (7.7-13.6) | 8.1 (6.4-13.2) | |||||
| Mahadevan et al. 2015 | TTF ± SRS | 40 | 8 (12 SRS vs 4 no SRS) | |||||
| Ansstas et al. 2016 | TTF | 8 | 7.9 | 2.7 | ||||
| Kesari et al. 2017 (EF-14 post hoc) | TTF + TMZ | 144 | 11.8 | |||||
| TMZ | 60 | 9.2 | ||||||
| Lu et al. 2019 | TTF + TBI | 18 | 18.9 (10.7-25.3) | 10.7 (6.7-20.8) | ||||
| TTF + BBC | 30 | 11.8 (8.6-15.8) | 4.7 (3.6-6.3) | |||||
| Korshoej et al. 2019 | TTF + SR Surgery | 15 | 15 (9.6-16.2) | 8.8 (6.2-13.2) | 64% | 64% | ||
| Zhu et al. 2020 (EF-19) | TTF | 192 | 7.4 (PP 8.1) | 42% | ||||
| BSC | 117 | 6.4 | 23.40% | |||||
| Fallah et al. 2020 | TTF + BEV | 23 | 10.5 (8.2-14.9) Male: 9.9 Female: 16 | 4.1 (3.6-9.5) Male: 3.9 Female: 13 | 33% | 46% |
Abbreviations: P, prospective; R, retrospective; RCT, randomized controlled trial; RCT-PH, post hoc analysis of RCT; TTF, Tumor-Treating Fields; TMZ, temozolomide; BSC, best standard of care; BEV, bevacizumab; TCCC, 6-thioguanine + lomustine + capecitabine + celecoxib; BBC, bevacizumab ± irinotecan or lomustine; TBI, temozolomide + bevacizumab + irinotecan; RT, radiotherapy; SR Surgery, skull-remodeling surgery; SRS, stereotactic radiosurgery; PP, per-protocol population.
aMedian PFS from diagnosis. TTFields was initiated after a median interval of 19 weeks (range 12-33 weeks).
Table 3.
Effect of Compliance on Efficacy of TTFields Treatment
| Source | Diagnosis | Compliance | Patients, No. | Median OS |
|---|---|---|---|---|
| Mrugala et al. 2014 (PRiDe) | rGBM | ≥75% | 127 | 13.5 |
| <75% | 160 | 4 | ||
| Kanner et al. 2014 (EF-11) | rGBM | ≥75% | 92 | 7.7 |
| <75% | 28 | 4.5 | ||
| <60% | 10 | 5.8 | ||
| 60-79% | 33 | 6 | ||
| ≥80% | 77 | 7.7 | ||
| Zhu et al. 2020 (EF-19) | rGBM | ≥75% | 82 | 9.83 |
| <75% | 102 | 6.67 | ||
| Toms et al. 2019 (EF-14) | ndGBM | >90% | 43 | 24.9 |
| 80-90% | 166 | 21.5 | ||
| 70-80% | 91 | 21.7 | ||
| 60-70% | 46 | 19.9 | ||
| 50-60% | 42 | 18 | ||
| 30-50% | 40 | 17.9 | ||
| <30% | 22 | 18.2 |
Abbreviations: rGBM, recurrent glioblastoma; ndGBM, newly diagnosed glioblastoma.
Table 4.
TTFields-Related Local Adverse Events
| Source | Diagnosis | Patients, No. | Mild-Moderate dAEs | Severe dAEs | Headache | Heat Sensation | Electric Sensation |
|---|---|---|---|---|---|---|---|
| Kirson et al. 2007 | rGBM | 10 | 90% | ||||
| Kirson et al. 2009 | ndGBM | 10 | 100% | 20% | |||
| Stupp et al. 2012 (EF-11) | rGBM | 120 | 16% | ||||
| Mrugala et al. 2014 (PRiDe) | rGBM | 457 | 24.30% | 5.70% | 11.30% | 7.70% | |
| Stupp et al. 2017 (EF-14) | ndGBM | 456 | 52% | 2% | |||
| Onken et al. 2019 | ndGBM + rGBM | 30 | 40% | 3% | |||
| Korshoej et al. 2019 | rGBM | 15 | 47% | 60% | |||
| Lazaridis et al. 2020 | ndGBM | 16 | 37% | ||||
| Song et al. 2020 | ndGBM | 10 | 80% | 30% | |||
| Bokstein et al. 2020 | ndGBM | 10 | 80% | ||||
| Zhu et al. 2020 (EF-19) | rGBM | 192 | 36% | ||||
| Shi et al. 2020 | ndGBM | 5887 | 40% | <1% | 8% | 11% | 11% |
| rGBM | 4345 | 31% | 7% | 10% | 9% |
Abbreviations: dAEs, dermatologic adverse events; rGBM, recurrent glioblastoma; ndGBM, newly diagnosed glioblastoma.
Clinical Efficacy
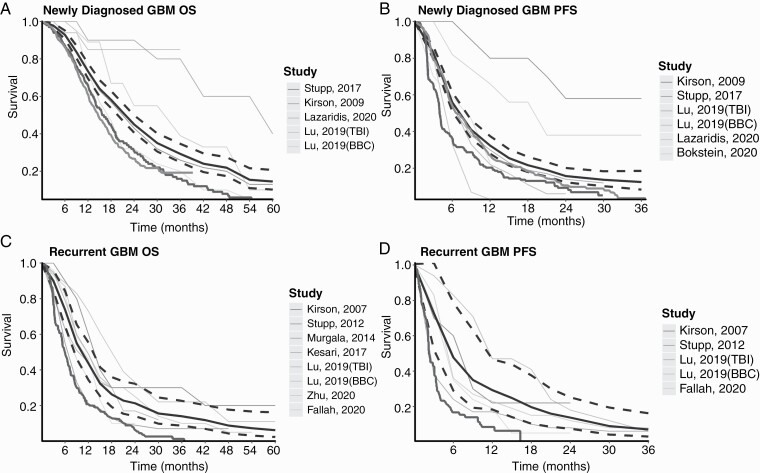
In ndGBM, the EF-14 RCT demonstrated significantly prolonged survival in patients treated with TTFields compared to controls, with a median OS of 20.9 vs 16.0 months, and a median PFS of 6.7 vs 4.0 months, respectively. Several smaller studies have investigated the efficacy of combined therapy in addition to TTFields + TMZ and the use of TTFields during the RT stage to increase its efficacy (Tables 1 and 2). To better assess the efficacy of TTFields, we constructed pooled KM survival plots. Four studies had OS KM curves12,34,41,42 and five had PFS KM curves,12,34,36,41,42 summing to 512 and 522 patients, respectively. The pooled OS and PFS survival curves with 95%CI compared to EF-14 and historical controls are shown in Figure 2A, B. The pooled median OS was 21.7 months (95%CI = 19.6-23.8). The 1-, 2-, and 3-year pooled OS rates were 73.5% (95%CI = 69.5-77.6), 45.1% (95%CI = 40.6-50), and 29.3% (95%CI = 24.8-34.7), respectively. The pooled median PFS was 7.2 months (95%CI = 6.1-8.2). The 6-, 12-, and 18-month pooled PFS rates were 55.9% (95%CI = 50.9-61.4), 32.4% (95%CI = 27.9-37.5), and 21.7% (95%CI = 17.9-26.2), respectively.
Figure 2.
Pooled Kaplan-Meier (KM) survival curves of patients treated with TTFields for GBM. Pooled KM survival curves of OS (A and C) and PFS (B and D). The gray-scale thin lines represent the survival in each individual study. The thick black line represents the summarized survival curve with the 95%CI (dashed black lines). The thick dark gray line represents the original survival curve of controls according to EF11 (rGBM: best standard-of-care treatment) and EF14 (ndGBM: RT + TMZ). The thick bright gray line represents the original survival curve of ndGBM patients treated with RT + TMZ in the historic study by Stupp et al.3 which established the current standard of care for ndGBM (EORTC protocol). (A) OS survival curve for ndGBM (512 patients), (B) PFS survival curve for ndGBM (522 patients), (C) OS survival curve for rGBM (984 patients), (D) PFS survival curve for rGBM (201 patients). Abbreviations: GBM, glioblastoma; ndGBM, newly diagnosed GBM; OS, overall survival; PFS, progression-free survival; rGBM, recurrent GBM; RT + TMZ, radiotherapy + temozolomide; TTFields, Tumor-Treating Fields.
In rGBM, TTFields efficacy remained vague, with contradicting results between EF-11 and later non-RCT studies. EF-11 reported median OS was not significantly superior in the TTFields arm than controls (6.6 vs 6 months, respectively). However, patients in the control group had a slightly better recurrence rate, which may have affected clinical outcomes.9 Several follow-up studies re-evaluated the efficacy of TTFields for rGBM patients, showing significantly improved outcomes compared to EF-11 controls. The Patient Registry Dataset (PriDe), a large post-marketing registry, reported a median OS of 9.6 months among TTFields patients, significantly higher than the 6 months among EF-11 controls.39 However, here too, the improved survival was suggested to be due to an earlier stage of disease course follow-up as compared to the EF-11 controls.39 Preliminary results from the EF-19, a new prospective study, demonstrated a median OS of 7.4 and 8.1 months in the intention-to-treat and per-protocol patients, respectively.50 A post hoc analysis of EF-14 RCT found that TTFields + TMZ vs TMZ alone after first recurrence significantly prolonged median OS (11.8 vs 9.2 months).45 Several smaller studies investigated the efficacy of combined therapy in addition to TTFields, and one suggested a skull-remodeling surgery to increase TTFields dose in the tumor (Tables 1 and 2).
To better assess the efficacy of TTFields among rGBM, and to clarify the contradicting results from the different studies concerning TTFields efficacy among rGBM patients, we constructed pooled KM survival plots of the relevant studies. Seven studies had OS KM curves,9,10,39,41,45,49,50 and four had PFS KM curves,9,10,41,49 summing to 984 and 201 patients, respectively (Tables 1 and 2). The pooled OS and PFS survival curves with 95%CI compared to EF-11 controls are shown in Figure 2C, D. The pooled median OS was 10.3 months (95%CI = 8.3-12.8). The 1-, 2-, and 3-year pooled OS rates were 43.7% (95%CI = 34.4-55.4), 21.3% (95%CI = 14-32.3), and 14% (95%CI = 8.7-22.6), respectively. The pooled median PFS was 5.7 months (95%CI = 2.8-10). The 6-, 12-, and 18-month pooled PFS rates were 47.8% (95%CI = 29-78.7), 29.3% (95%CI = 18.4-46.7), and 19.7% (95%CI = 10.3-37.6), respectively.
Compliance as a Prognostic Factor
Given its mechanism of action, and in contrast to systemic therapies, TTFields has no half-life; therefore, continuous application and optimal device compliance are required for therapeutic efficacy. It has been shown that a minimum of 4 weeks of continuous treatment is necessary for tumor stasis or shrinkage.51 Several studies examined the effect of compliance on patients’ prognosis, both in rGBM patients39,47,50,51 and ndGBM.46,52 The studies showed a significant increase in median OS and PFS, and in 1-year survival rate for patients with ≥75% daily compliance rate, as well as a stepwise increase in prognosis as the compliance rate increased (Table 3). Moreover, the studies showed compliance to be associated with improved outcomes independently of other prognostic factors such as performance status, age, and methylguanine-DNA-methyltransferase (MGMT) methylation status.46
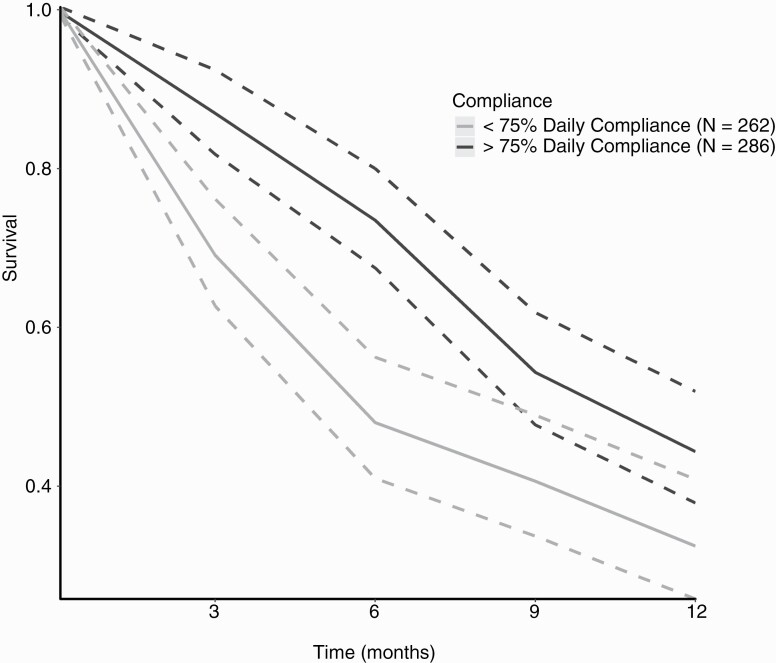
We examined the pooled effect of compliance on rGBM patients’ survival who used TTFields ≥75% of the day vs those who used the device for <75% of the day, summing to 262 and 286 patients, respectively. The pooled OS survival curves with 95%CI for both patient groups are shown in Figure 3. For patients with daily compliance of ≥75%, the pooled median OS was 10.3 months (95%CI = 8.6-12.3). The 6-, 9-, and 12-month pooled OS rates were 73.5% (95%CI = 67.5-80), 54.3% (95%CI = 47.7-61.9), and 44.4% (95%CI = 37.9-51.9), respectively. In contrast, for patients with daily compliance of <75%, the pooled median OS was 5.7 months (95%CI = 4.8-8.1). The 6-, 9-, and 12-month pooled OS rates were 48% (95%CI = 41-56.2), 40.6% (95%CI = 33.7-49), and 32.5% (95%CI = 25.8-40.9), respectively.
Figure 3.
Pooled Kaplan-Meier (KM) survival curves of OS according to daily compliance for rGBM patients. Pooled KM survival curves of OS for rGBM patients. The dark gray lines represent the pooled survival curve (solid line) with the 95%CI (dashed lines) of patients who used TTFields for ≥75% of the day (>18 h/d). The bright gray lines represent the pooled survival curve (solid line) with the 95%CI (dashed lines) of patients who used TTFields <75% of the day (<18 h/d). Abbreviations: OS, overall survival; rGBM, recurrent GBM; TTFields, Tumor-Treating Fields.
Safety
Twelve studies reported the frequency of AEs among patients treated with TTFields, summing 11 558 patients (Table 4). Overall, TTFields have a good safety profile with no known systemic toxicity, a finding attributed to the device’s localized mechanism of action. Moreover, studies showed significantly fewer severe AEs and either a lower or same overall incidence of AEs in patients treated with TTFields alone as compared to controls.9,34,40,50 Across all studies, the most predominant AEs associated with the use of TTFields are array-associated dermatologic AEs (dAEs), which include allergic and irritant dermatitis, mechanical lesions, ulcers, and skin infection.53 Most dAEs were mild to moderate, with a pooled prevalence of 38.4% (95%CI = 32.3-44.9) among TTFields patients (Supplementary Figure 2). Only two studies reported severe dAEs (≥ grade 3 AE); in EF-14, 2% of TTFields patients reported a grade 3 AE,34 and in the global post-marketing safety surveillance, less than 1% of patients experienced severe device-related dAEs (Table 4).40 The occurrence of these dAEs is most probably related to chronic skin exposure to irritants in transducer arrays, which are applied noninvasively to deliver TTFields through the skin to the tumor bed.53,54 Prophylactic strategies for dAEs include proper shaving and cleansing of the scalp, array relocation, and topical therapies.53 A recent preclinical study investigated the safety profile of topical treatments use with TTFields in vivo, reporting that all petroleum-based ointments tested led to an increase in electrical impedance, which lead to temperature increase beneath the arrays, and thus, are not recommended.55
During their disease course, GBM patients may require shunt placement. Since TTFields may interfere with ventricular shunts’ adjustable valves as well as with patients’ cardiac pacemakers or defibrillators, it is important to confirm the safety of concomitant use of TTFields with these implanted devices. A post-marketing surveillance study reported 104 patients treated with TTFields with a concurrent implanted device (79 non-adjustable shunts, 11 with adjustable shunts, and 14 with pacemakers/defibrillators). The study reported no unexpected safety issues on the concomitant use of TTFields with implanted devices.56 Case reports of patients treated with TTFields with either shunts or cardiac pacemakers, also reported no unexpected safety issues.57–59
Quality of Life
Due to the impact of GBM on patients’ progressive decline in neurologic function and health-related quality of life (HRQoL), it is crucial to address the effect of TTFields not only on prolongation of life but also on the patient’s well-being.
In EF-11, HRQoL was assessed using the EORTC QLQ-C30 questioner at baseline and every 3 months. Longitudinal data were available only for 27% of the patients. Assessing the change between baseline and 3 months, the study reported no meaningful differences between TTFields and controls in global health and social functioning domains. Cognitive, emotional, and role functioning favored TTFields, while physical functioning was slightly worse with TTFields.9
In EF-14, HRQoL was assessed using both EORTC QLQ-C30 and QLQ-BN20 questioners. The number of patients filling the HRQoL questioners decreased from 91.9% at baseline to 65.8% at 3 months and 41.7% at 12 months. HRQoL was maintained from baseline in 8 of 9 scales, except for itchy skin, which worsened in TTFields patients. TTFields cases had significantly better deterioration-free survival for physical and emotional functioning, global health, pain, and leg weakness, likely due to improved PFS. Social, role, and physical functioning were not affected.60 Finally, the study showed a significantly longer time to sustained 6- and 10-point decline in the Mini-Mental State Examination and KPS, respectively.34
A small two-center study examined 30 high-grade glioma patients treated with TTFields and 27 controls.38 The study assessed HRQoL using a device-specific questionnaire (DSQ), several modules of the EORTC questioner (QLQ-30, QLQ-BN20, QLQ-FA13), and the SSUK-8 (social support) questioner. Two months after treatment initiation, the surveys were completed by 91% of enrolled patients. The study showed that TTFields patients had significantly better emotional function, and reported significantly lower incidence of insomnia, pain, dyspnea, loss of appetite, nausea, and vomiting. Also, TTFields users had better emotional, physical, and cognitive functions than social and role functioning. The study showed TTFields frequently affected multiple aspects of the patients’ daily lives; nevertheless, 70% would recommend TTFields to others, and 67% would reuse the device.
Discussion
The study presented here provides a practical summary of TTFields modality for clinicians who treat GBM patients. It has some advantages over previous systematic TTFields reviews.61,62 It includes more clinical studies and uses both qualitative and quantitative analysis, thus, allowing for an extensive examination of the literature. The study also used several innovative statistical tools, creating pooled survival curves and allowing assessment of single-arm studies that are currently the majority of trials in TTFields literature. Lastly, it reviews the preclinical alongside clinical literature, promoting future research and therapeutic channels for the device.
Clinical Findings
The findings presented in this study support TTFields’ clinical efficacy for ndGBM and suggest it for rGBM. In ndGBM patients, TTFields use alongside the EORTC protocol, as established by both EF-14 RCT and later clinical studies, enhanced GBM patients’ survival to 21.7 months. In rGBM patients, TTFields use improved survival to 10.3 months, and therefore, it may be considered either as a single treatment or alongside other salvage therapies. Nevertheless, more studies are needed to better establish the clinical efficacy in rGBM cases.
This study also demonstrated that despite its restrictive effect on daily life, TTFields had an overall good impact on patients’ HRQoL. Particularly, patients’ cognitive and emotional well-being, as well as functional status, were favorably affected by TTFields. Subsequently, 70% of patients would recommend TTFields to others, and 67% would reuse it.38 However, these findings should be further assessed in larger cohorts.
TTFields treatment’s safety was also evaluated and showed no known systemic toxicity so far; a finding attributed to the device’s localized effect, which main AE was mild to moderate dermatitis in 38.4% of the patients. Also, no unexpected safety issues on the concomitant use of ventricular shunts or implanted cardiac devices were shown, although this trend should be cautiously considered until further validation of existing evidence is performed.
Treatment compliance of ≥75% of the day has been shown to significantly affect GBM patient’s survival, a finding that should be emphasized to both patients and their caregivers. It should be considered, though, that patient’s compliance may be affected by both social and clinical factors and may pose certain difficulties on them and their caregivers. For example, some patients may be reluctant to comply with the head-shaving required at every array change, and some may feel self-conscious due to wearing the arrays on a shaved head, calling attention to their condition.63 Also, since TTFields are administered in the home and outpatient setting, it places the burden of compliance on the patient and their caregivers. Thus, a good home support system is critical when considering therapy for a GBM patient.46,63
Cost-Effectiveness
The estimated monthly cost for TTFields in the United States is $21 000, leading to concerns regarding its cost-effectiveness.21,64,65 Several studies examined the incremental cost-effectiveness ratio (ICER) for ndGBM patients based on EF-14, reporting contradicting results. Two studies reported an ICER ranging at €510 273-549 909 per life-year gained (LYG), claiming TTFields is not cost-effective.66,67 A third study reported an ICER of $150 452/LYG, suggesting treatment can be considered cost-effective within the reported range of willingness-to-pay thresholds in the United States.68 Due to their post hoc design, these studies had limited access to potential cost factors such as hospitalizations which are major sources of health care expenditures.65 Also, the cost-effectiveness of TTFields vs salvage therapy has not been examined among rGBM. Notably, in Israel, 2020, TTFields became part of the standard-of-care treatment for GBM patients, given following the EORTC protocol and as maintenance therapy. It is, thus, subsidized by the Ministry of Health of Israel (The Ministry of Health of Israel website, in Hebrew). In summary, data are still scarce concerning the cost-effectiveness of TTFields in general and also regarding its use in specific subpopulations which may benefit most from it.64 Future prospective trials are needed concerning both ndGBM and rGBM and may provide a more accurate estimate of the real-world cost-effectiveness.65
Mechanism of Action and Combinational Treatment Potential
Examination of both preclinical and clinical studies demonstrates TTFields’ diverse mechanism of action and emphasizes its clinical potential to support and enhance treatment efficacy when combined with current treatment protocols and novel treatments, which may lead to improved patient outcomes. It disrupts DNA repair, cell mitosis10,11,69–71 and angiogenesis72; suppress migration and invasion71,72; induces apoptosis,69 autophagy,71,73 and immunogenic cell death71,74–76; causes temporary BBB (blood-brain barrier) disruption and increase cell membrane permeability.77,78
MGMT status among ndGBM patients is known to influence the efficacy of TMZ. The EF-14 study demonstrated improved clinical efficacy in patients treated with TMZ + TTFields regardless of MGMT status.34 A preclinical study investigating TTFields combination with TMZ, focusing on MGMT status and TMZ resistance reinforced these findings, demonstrating that TTFields efficacy was not dependent on MGMT status nor diminished in TMZ-resistant cell lines.71 The effect of TTFields on angiogenesis72 suggests possible synergistic potential with anti-angiogenetic agents such as bevacizumab. This combined treatment was examined in several preliminary studies, suggesting improved clinical efficacy with no additive side effects.41,43,45,49 TTFields, therefore, may be attractive to ndGBM patients who are unlikely to benefit from TMZ due to unmethylated MGMT promoter, and in addition to second-line treatment with bevacizumab.
Preclinical studies also demonstrated TTFields’ utility in reducing DNA repair mechanisms’ effectiveness within GBM cells following RT. Studies showed a synergistic effect of TTFields and RT when given either before or following RT, inducing DNA damage, inhibiting DNA repair and mitotic activity, and suppressing cell migration and invasion.79–82 Therefore, ndGBM patients may benefit not just from adjuvant treatment as conducted in EF-14, but also concurrent TTFields alongside RT. This notion was recently examined by two phase I studies, demonstrating preliminary efficacy results with no significant safety issues.35,36
GBM microenvironment is known to be highly immunosuppressive,83 making TTFields immunogenic effect of interest. Preclinical studies showed TTFields induces apoptosis,69 increases autophagy,71,73 promotes tumor cell immunogenicity and immunogenic cell death, drives increased immune recognition and tumor rejection,74–76 and improves the infiltrative capacity of CD4 and CD8 T cells in animal models.84 Moreover, early during TTFields treatment, a transient stage of increased peritumoral edema is often observed, followed by an objective radiographic response, suggesting that a major component of therapeutic efficacy by TTFields may be due to an immune-mediated process.76 This highlights the potential of combining TTFields with different immunotherapies.76 One example is anti-PD-1, which showed enhanced antitumor immunity when combined with TTFields in preclinical studies alongside improved clinical outcomes in a preliminary clinical study.85 Also, TTFields potential influence on T cells84 suggests potential synergistic effect with novel immunotherapies such as dendritic cell vaccines.75,86
In this context, dexamethasone used to control symptomatic cerebral edema, can induce immunosuppression and interfere with the patient’s antitumor immunity, impairing TTFields activity.43,87 Preliminary examination of dexamethasone use among TTFields patients revealed patients who used doses <4.1 mg per day had a significant increase in OS.87 Moreover, patients who showed tumor response to treatment had a significantly lower dexamethasone burden.88 Yet, this association should be further assessed since it may be affected by the fact that more severe GBM patients receive higher doses of dexamethasone and have a worse prognosis. The use of bevacizumab can aid in controlling vasogenic cerebral edema, thus partially replacing dexamethasone, which may increase TTFields efficacy.43,87,88
Preclinical studies demonstrated a dose-dependent induction of cell death in vitro,10,11,71 while computational models showed field intensity depends on tumor position alongside the surrounding tissue conductivity.89 Post hoc analyses of EF-14 showed higher dose density was associated with significantly longer OS and PFS and improved quality of life.90 Also, tumor progression has been shown to occur more frequently outside the field, where electric intensity is lower, requiring the device’s repositioning to control progression.91,92 A skull-remodeling surgery was proposed recently to reduce the skull’s high resistivity, which leads to a reduction of current intensity. The surgery comprises either skull-thinning, formation of burr holes, or craniotomy over the tumor, allowing direct TTFields current to the tumor and a minimum increase of 25% of TTFields intensity.93 A preliminary phase I trial validated the surgery safety and feasibility, also reporting prolongation in OS.37
TTFields’ effect on GBM membrane permeability and BBB disruption77,78 suggests a potential new application, assisting in delivering non-permeable pharmacological agents through the BBB, enhancing drug accessibility to GBM cells and potentially, improving the extent of tumor resection. For example, increased 5-ALA uptake was shown in GBM cells in preclinical studies.78
TTFields’ diverse mechanism of action and lack of significant side effects emphasizes its synergistic potential when combined with other treatments, leading to improved patient outcomes. It may improve the extent of tumor resection, enhance RT efficacy, provide benefit to specific populations such as patients with unmethylated MGMT, enhance immunotherapies, and support second-line treatment with bevacizumab or nitrosourea.
Limitations
Limitations of the studies included in the meta-analysis
The literature concerning TTFields has several limitations. Both EF-11 and EF-14 were criticized for their unblinded design and lack of a placebo or sham device. It was suggested that the survival benefit might reflect a strong placebo effect, an adherence bias, or perhaps be due to higher palliative care received from TTFields supporting team.18,20,24 It was also noted that in the EF-14 trial, the control patients group received less adjuvant chemotherapy than treatment group,24 and noted the problematic time lag between diagnosis and randomization, which impacts the generalizability of the present data.21 Nevertheless, it was claimed that a placebo effect would not be associated with a dose-response and cannot entirely explain the magnitude of the effect,18,20,94 and that using a sham device, requesting patients to shave their hair and spend most of their day carrying a heavy device, may be unethical.18 In addition to concerns regarding the studies’ design, physicians note the uncertain exact mechanism of action, the device’s high cost, and difficulty in use.20,21,64,94 A limitation in both EF-11 and EF-14 studies, common in other cancer clinical trials, is the high rates of missing longitudinal HRQoL data, which may influence the results reported in both studies since patients with better prognostic factors and good treatment response will be overrepresented at later stages. However, it should be noted that the EF-14 addressed this limitation using sensitivity analyses, which confirmed their findings.60
Limitations of the meta-analysis itself
Notably, this study has its own limitations. Given the characteristics of the studies published to date, which included only two RCTs alongside single-arm studies, we could not conduct a comparative meta-analysis but only to investigate the efficacy and safety of TTFields treatment by itself. The findings concerning TTFields efficacy in rGBM should be treated with caution since except for the EF-11 study and a post hoc analysis of EF-14, all other studies concerning rGBM were single-arm studies that may be affected by selection bias. Finally, and as in any meta-analysis, the presented results should be treated with caution due to differences in study methodology and quality, the difference in sample size, and the relatively small number of studies published to date. These limitations reflect the current literature limitations concerning TTFields and underlie the importance of further clinical research, ideally blinded RCTs.
Conclusions and Future Directions
According to this study results, further research is needed to establish the different aspects of TTFields’ influence on GBM disease. For example, it will be essential to determine which GBM patients may benefit the most from the therapy, according to their specific prognostic factors classification (ie, their hemoglobin level,95 IDH1/2 mutation, and MGMT methylation status, other molecular and genetic tumor features).
Also, examining TTFields effect when added to common treatment modalities, ie, a phase II RCT study examining RT with concurrent and maintenance TMZ + TTFields (experimental arm) vs RT with concurrent TMZ alone followed by maintenance TMZ + TTFields (control arm) is closing accrual; or limitation of dexamethasone use to maximize TTFields immunogenic efficacy, are essential.96 As noted above, combined TTFields treatment and bevacizumab was examined in several preliminary studies, suggesting improved clinical efficacy with no additive side effects, though further research is again, needed.41,43,45,49 Future studies should also consider using TTFields before 5-ALA administration, possibly resulting in better demonstration of the infiltrative tumor margin, thus, improving the extent of resection4 and creating an additive effect on patients’ survival.
These preliminary findings and investigation suggestions based on them, emphasize the necessity for further in-depth research, both in the lab and clinical settings, enabling the realization of the potential inherent in TTFields.
To conclude, this study systematically assessed the current literature concerning TTFields, allowing a better, unbiased, and practical understanding of it, and its findings further support the clinical benefit, safety, and potential therapeutic synergism of its use by GBM patients.
Supplementary Material
Acknowledgments
The authors thank Dr. Andrew A. Kanner, MD, of the Department of Neurosurgery, Rabin Medical Center, Petah Tikva and Dr. Yonatan Elbaz, PhD, of the Physics Department, Nuclear Research Center - Negev, Be’er-Sheva, for their critical reviewing of the manuscript.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement. D.T.B. received speaker honoraria from Novocure. The rest of the authors declare no conflict of interest.
References
- 1. Ostrom QT, Gittleman H, Fulop J, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 2015;17(suppl 4):iv1–iv62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23(10):1985–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stupp R, Mason WP, van den Bent MJ, et al. ; European Organisation for Research and Treatment of Cancer Brain Tumor and Radiotherapy Groups; National Cancer Institute of Canada Clinical Trials Group . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. [DOI] [PubMed] [Google Scholar]
- 4. Brown TJ, Brennan MC, Li M, et al. Association of the extent of resection with survival in glioblastoma. JAMA Oncol. 2016;2(11):1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Stummer W, Pichlmeier U, Meinel T, et al. ; ALA-Glioma Study Group . Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol. 2006;7(5):392–401. [DOI] [PubMed] [Google Scholar]
- 6. Anton K, Baehring JM, Mayer T. Glioblastoma multiforme: overview of current treatment and future perspectives. Hematol Oncol Clin North Am. 2012;26(4):825–853. [DOI] [PubMed] [Google Scholar]
- 7. Weller M, Cloughesy T, Perry JR, Wick W. Standards of care for treatment of recurrent glioblastoma—are we there yet? Neuro Oncol. 2013;15(1):4–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lukas RV, Mrugala MM. Pivotal therapeutic trials for infiltrating gliomas and how they affect clinical practice. Neurooncol Pract. 2017;4(4):209–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Stupp R, Wong ET, Kanner AA, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48(14):2192–2202. [DOI] [PubMed] [Google Scholar]
- 10. Kirson ED, Dbalý V, Tovarys F, et al. Alternating electric fields arrest cell proliferation in animal tumor models and human brain tumors. Proc Natl Acad Sci USA. 2007;104(24):10152–10157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kirson ED, Gurvich Z, Schneiderman R, et al. Disruption of cancer cell replication by alternating electric fields. Cancer Res. 2004;64(9):3288–3295. [DOI] [PubMed] [Google Scholar]
- 12. Kirson ED, Schneiderman RS, Dbalý V, et al. Chemotherapeutic treatment efficacy and sensitivity are increased by adjuvant alternating electric fields (TTFields). BMC Med Phys. 2009;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stupp R, Taillibert S, Kanner AA, et al. Maintenance therapy with tumor-treating fields plus temozolomide vs temozolomide alone for glioblastoma: a randomized clinical trial. JAMA. 2015;314(23):2535–2543. [DOI] [PubMed] [Google Scholar]
- 14. FDA Premarket Approval (PMA) - NovoTTF-100A. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034. Published 2011. Accessed May 21, 2020.
- 15. Premarket Approval (PMA) - U.S. Food and Drug Administration. 2015 OPTUNE (formerly the NovoTTF-100A system) - expanded indication approval P100034S013.https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpma/pma.cfm?id=P100034S013. Published 2015. Accessed May 22, 2020.
- 16. Dizon DS, Krilov L, Cohen E, et al. Clinical cancer advances 2016: annual report on progress against cancer from the American Society of Clinical Oncology. J Clin Oncol. 2016;34(9):987–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. NCCN Clinical Practice Guidelines in Oncology Central Nervous System Cancers. 2020. https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed June 2020.
- 18. Weller M. Tumor-treating fields: time for demystification. Ann Oncol. 2018;29(8):1628–1630. [DOI] [PubMed] [Google Scholar]
- 19. Cloughesy TF, Lassman AB. NovoTTF: where to go from here? Neuro Oncol. 2017;19(5):605–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Stupp R, Toms SA, Kesari S. Treatment for patients with newly diagnosed glioblastoma – reply. JAMA. 2016;315(21):2348–2349. [DOI] [PubMed] [Google Scholar]
- 21. Wick W. TTFields: where does all the skepticism come from? Neuro Oncol. 2016;18(3):303–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Printz C. Electrical device for patients with glioblastoma met with support, skepticism: some question the device’s efficacy, others tout it as a new standard of care. Cancer. 2015;121(7):969–970. [DOI] [PubMed] [Google Scholar]
- 23. Hayes MJ, Prasad V. Association between conflict of interest and published position on tumor-treating fields for the treatment of glioblastoma. J Cancer Policy. 2019;21:100189. [Google Scholar]
- 24. Sampson JH. Alternating electric fields for the treatment of glioblastoma. JAMA. 2015;314(23):2511–2513. [DOI] [PubMed] [Google Scholar]
- 25. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fabbri S, Silva C, Hernandes E, Octaviano F, Di Thommazo A, Belgamo A. Improvements in the StArt tool to better support the systematic review process. Paper presented at: Proceedings of the 20th International Conference on Evaluation and Assessment in Software Engineering; June 1–3, 2016; Limerick, Ireland. New York, NY: ACM Press; 2016:1–5. [Google Scholar]
- 27. Howick J, Chalmers I, Glasziou P, et al. The Oxford Levels of Evidence 2 OCEBM. https://www.cebm.net/2016/05/ocebm-levels-of-evidence/. Accessed July 11, 2020.
- 28. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. 2012. http//www.ohri.ca/programs/clinical_epidemiology/oxford.asp). Accessed July 2020.
- 29. Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. [DOI] [PubMed] [Google Scholar]
- 30. Combescure C, Foucher Y, Jackson D. Meta-analysis of single-arm survival studies: a distribution-free approach for estimating summary survival curves with random effects. Stat Med. 2014;33(15):2521–2537. [DOI] [PubMed] [Google Scholar]
- 31. Tierney JF, Stewart LA, Ghersi D, et al. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoyle MW, Henley W. Improved curve fits to summary survival data: application to economic evaluation of health technologies. BMC Med Res Methodol. 2011;11:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGrath S, Zhao X, Qin ZZ, et al. One-sample aggregate data meta-analysis of medians. Stat Med. 2019;38(6):969–984. [DOI] [PubMed] [Google Scholar]
- 34. Stupp R, Taillibert S, Kanner A, et al. Effect of tumor-treating fields plus maintenance temozolomide vs maintenance temozolomide alone on survival in patients with glioblastoma: a randomized clinical trial. JAMA. 2017;318(23):2306–2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Song A, Bar-Ad V, Martinez N, et al. Initial experience with scalp sparing radiation with concurrent temozolomide and tumor treatment fields (SPARE) for patients with newly diagnosed glioblastoma. J Neurooncol. 2020;147(3):653–661. [DOI] [PubMed] [Google Scholar]
- 36. Bokstein F, Blumenthal D, Limon D, et al. Concurrent Tumor Treating Fields (TTFields) and radiation therapy for newly diagnosed glioblastoma: a prospective safety and feasibility study. Front Oncol. 2020;10:411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Korshoej AR, Mikic N, Hansen FL, et al. Enhancing tumor treating fields therapy with skull-remodeling surgery. The role of finite element methods in surgery planning. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:6995–6997. [DOI] [PubMed] [Google Scholar]
- 38. Onken J, Goerling U, Heinrich M, et al. Patient Reported Outcome (PRO) among high-grade glioma patients receiving TTFields treatment: a two center observational study. Front Neurol. 2019;10:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mrugala MM, Engelhard HH, Dinh Tran D, et al. Clinical practice experience with NovoTTF-100A™ system for glioblastoma: the Patient Registry Dataset (PRiDe). Semin Oncol. 2014;41(Suppl 6):S4–S13. [DOI] [PubMed] [Google Scholar]
- 40. Shi W, Blumenthal DT, Oberheim Bush NA, et al. Global post-marketing safety surveillance of Tumor Treating Fields (TTFields) in patients with high-grade glioma in clinical practice. J Neurooncol. 2020;148(3):489–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lu G, Rao M, Zhu P, et al. Triple-drug therapy with bevacizumab, irinotecan, and temozolomide plus tumor treating fields for recurrent glioblastoma: a retrospective study. Front Neurol. 2019;10:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lazaridis L, Schäfer N, Teuber-Hanselmann S, et al. Tumour Treating Fields (TTFields) in combination with lomustine and temozolomide in patients with newly diagnosed glioblastoma. J Cancer Res Clin Oncol. 2020;146(3):787–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wong ET, Lok E, Swanson KD. Clinical benefit in recurrent glioblastoma from adjuvant NovoTTF-100A and TCCC after temozolomide and bevacizumab failure: a preliminary observation. Cancer Med. 2015;4(3):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ansstas G, Tran DD. Treatment with tumor-treating fields therapy and pulse dose bevacizumab in patients with bevacizumab-refractory recurrent glioblastoma: a case series. Case Rep Neurol. 2016;8(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kesari S, Ram Z; EF-14 Trial Investigators . Tumor-treating fields plus chemotherapy versus chemotherapy alone for glioblastoma at first recurrence: a post hoc analysis of the EF-14 trial. CNS Oncol. 2017;6(3):185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Toms SA, Kim CY, Nicholas G, et al. Increased compliance with tumor treating fields therapy is prognostic for improved survival in the treatment of glioblastoma: a subgroup analysis of the EF-14 phase III trial. J Neurooncol. 2019;141(2):467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kanner AA, Wong ET, Villano JL, et al. ; EF-11 Investigators . Post hoc analyses of intention-to-treat population in phase III comparison of NovoTTF-100A™ system versus best physician’s choice chemotherapy. Semin Oncol. 2014;41(Suppl 6):S25–S34. [DOI] [PubMed] [Google Scholar]
- 48. Mahadevan A, Barron L, Floyd SR, Kasper E, Wong ET. Survival benefit of tumor treating fields plus stereotactic radiosurgery for recurrent malignant gliomas. J Clin Oncol. 2015;33(15_suppl):e13036. [Google Scholar]
- 49. Fallah J, Chaudhary RT, Rogers LR, et al. Clinical outcomes of the combination of bevacizumab and TTFields in patients with recurrent glioblastoma: results of a phase II clinical trial. J Clin Oncol. 2020;38(15_suppl):2537. [Google Scholar]
- 50. Zhu J-J, O’Donnell R, Ram Z. EF-19, a post-approval registry study of tumor treating fields (TTFields) in recurrent glioblastoma (rGBM). J Clin Oncol. 2020;38(15_suppl):e14536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Vymazal J, Wong ET. Response patterns of recurrent glioblastomas treated with tumor-treating fields. Semin Oncol. 2014;41(Suppl 6):S14–S24. [DOI] [PubMed] [Google Scholar]
- 52. Qualls KW, Pandey M, Michael LM, Sorenson J, Baughman B, Ballo MT. Single institutional experience with tumor-treating field compliance and overall survival in patients with primary glioblastoma. Int J Radiat Oncol. 2019;105(1):E106–E107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lacouture ME, Davis ME, Elzinga G, et al. Characterization and management of dermatologic adverse events with the NovoTTF-100A System, a novel anti-mitotic electric field device for the treatment of recurrent glioblastoma. Semin Oncol. 2014;41(Suppl 4):S1–S14. [DOI] [PubMed] [Google Scholar]
- 54. Lukas RV, Ratermann KL, Wong ET, et al. Skin toxicities associated with tumor treating fields: case based review. J Neurooncol. 2017;135(3):593–599. [DOI] [PubMed] [Google Scholar]
- 55. Giladi M, Lacouture ME, Weinberg U, Bomzon Z, Palti Y. Compatibility of topical agents with tumor treating fields (TTFields) for treatment of associated skin events in glioblastoma (GBM). J Clin Oncol. 2020;38(15_suppl):e24126. [Google Scholar]
- 56. Kew Y, Bush NAO. INNV-24. Safety of tumor treating fields in glioblastoma patients with implanted non-programmable and programmable shunts, and pacemakers/defibrillators: 6.5-year updated retrospective analysis. Neuro Oncol. 2018;20(suppl_6):vi143. [Google Scholar]
- 57. Chan AK, Birk HS, Winkler EA, et al. Stability of programmable shunt valve settings with simultaneous use of the Optune transducer array: a case report. Cureus. 2016;8(7):e675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Mrugala MM, Graham CA, Rockhill JK, Silbergeld DL. P17.60 * Novo-TTF 100A system used successfully in a patient with a ventriculo-peritoneal shunt. Neuro Oncol. 2014;16(suppl 2):ii101. [Google Scholar]
- 59. McClelland S 3rd, Henrikson CA, Ciporen JN, et al. Tumor treating fields utilization in a glioblastoma patient with a preexisting cardiac pacemaker: the first reported case. World Neurosurg. 2018;119:58–60. [DOI] [PubMed] [Google Scholar]
- 60. Taphoorn MJB, Dirven L, Kanner AA, et al. Influence of treatment with tumor-treating fields on health-related quality of life of patients with newly diagnosed glioblastoma: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(4):495–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Magouliotis DE, Asprodini EK, Svokos KA, et al. Tumor-treating fields as a fourth treating modality for glioblastoma: a meta-analysis. Acta Neurochir (Wien). 2018;160(6):1167–1174. [DOI] [PubMed] [Google Scholar]
- 62. Shah PP, White T, Khalafallah AM, Romo CG, Price C, Mukherjee D. A systematic review of tumor treating fields therapy for high-grade gliomas. J Neurooncol. 2020;1(3: 433–443. [DOI] [PubMed] [Google Scholar]
- 63. Murphy J, Bowers ME, Barron L. Optune®: practical nursing applications. Clin J Oncol Nurs. 2016;20(5 Suppl):S14–S19. [DOI] [PubMed] [Google Scholar]
- 64. Zhang I, Knisely JPS. Tumor treating fields-effective, but at what cost? Transl Cancer Res. 2016;5:S1349–S1353. [Google Scholar]
- 65. Schiff D, Schrag D. Living in a material world: tumor-treating fields at the top of the charts. Neuro Oncol. 2016;18:1033–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bernard-Arnoux F, Lamure M, Ducray F, et al. The cost-effectiveness of tumor-treating fields therapy in patients with newly diagnosed glioblastoma. Neuro Oncol. 2016;18(8):1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Connock M, Auguste P, Dussart C, et al. Cost-effectiveness of tumor-treating fields added to maintenance temozolomide in patients with glioblastoma: an updated evaluation using a partitioned survival model. J Neurooncol. 2019;143(3):605–611. [DOI] [PubMed] [Google Scholar]
- 68. Guzauskas GF, Pollom EL, Stieber VW, et al. Tumor treating fields and maintenance temozolomide for newly-diagnosed glioblastoma: a cost-effectiveness study. J Med Econ. 2019;22(10):1006–1013. [DOI] [PubMed] [Google Scholar]
- 69. Gera N, Yang A, Holtzman TS, et al. Tumor treating fields perturb the localization of septins and cause aberrant mitotic exit. PLoS One. 2015;10(5):e0125269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Giladi M, Schneiderman RS, Voloshin T, et al. Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci Rep. 2016;5(1):18046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Silginer M, Weller M, Stupp R, et al. Biological activity of tumor-treating fields in preclinical glioma models. Cell Death Dis. 2017;8(4):e2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kim EH, Song HS, Yoo SH, Yoon M. Tumor treating fields inhibit glioblastoma cell migration, invasion and angiogenesis. Oncotarget. 2016;7(40):65125–65136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Shteingauz A, Porat Y, Voloshin T, et al. AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields). Cell Death Dis. 2018;9(11):1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wong ET, Timmons J, Swanson KD. Abstract 1707: tumor treating fields exert cellular and immunologic effects. Cancer Res. 2018;78(13 Suppl):1707. [Google Scholar]
- 75. Voloshin T, Kaynan N, Davidi S, et al. Tumor-treating fields (TTFields) induce immunogenic cell death resulting in enhanced antitumor efficacy when combined with anti-PD-1 therapy. Cancer Immunol Immunother. 2020;69(7):1191–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen D, Thomas N, Ren J, et al. IMMU-06. TTFields induces immunogenic cell death and sting pathway activation through cytoplasmic double-stranded DNA in glioblastoma cells. Neuro Oncol. 2019;21(Supplement_6):vi120. [Google Scholar]
- 77. Kessler AF, Salvador E, Domröse D, et al. Blood brain barrier (BBB) integrity is affected by tumor treating fields (TTFields) in vitro and in vivo. Int J Radiat Oncol. 2019;105(1):S162–S163. [Google Scholar]
- 78. Chang E, Patel CB, Pohling C, et al. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov. 2018;4:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kim EH, Kim YJ, Song HS, et al. Biological effect of an alternating electric field on cell proliferation and synergistic antimitotic effect in combination with ionizing radiation. Oncotarget. 2016;7(38):62267–62279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Giladi M, Munster M, Schneiderman RS, et al. Tumor treating fields (TTFields) delay DNA damage repair following radiation treatment of glioma cells. Radiat Oncol. 2017;12(1):206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Giladi M, Zielinska-Chomej K, Tichon A, et al. The effect of alternating electric fields (TTFields) on inhibition of repair of DNA damage induced by ionizing radiation and sensitization of glioma and non-small cell lung cancer cells to radiation. J Clin Oncol. 2014;32(15_suppl):e22239. [Google Scholar]
- 82. Giladi M, Munster M, Schneiderman RS, et al. Tumor Treating Fields (TTFields) sensitize glioma tumor cells to radiation therapy by delaying DNA damage repair through homologous recombination. Int J Radiat Oncol. 2015;93(3):E524–E525. [Google Scholar]
- 83. Nduom EK, Weller M, Heimberger AB. Immunosuppressive mechanisms in glioblastoma. Neuro Oncol. 2015;17(suppl 7):vii9–vii14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Kirson ED, Giladi M, Gurvich Z, et al. Alternating electric fields (TTFields) inhibit metastatic spread of solid tumors to the lungs. Clin Exp Metastasis. 2009;26(7):633–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Schulte J, Odia Y, Donovan L, Iwamoto F. RTHP-10. Impact of combination immunotherapy with tumor treating fields therapy in a glioma cohort. Neuro Oncol. 2017;19(suppl_6):vi221. [Google Scholar]
- 86. Yang L, Guo G, Niu XY, et al. Dendritic cell-based immunotherapy treatment for glioblastoma multiforme. Biomed Res Int. 2015;2015:717530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wong ET, Lok E, Gautam S, Swanson KD. Dexamethasone exerts profound immunologic interference on treatment efficacy for recurrent glioblastoma. Br J Cancer. 2015;113(2):232–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wong ET, Lok E, Swanson KD, et al. Response assessment of NovoTTF-100A versus best physician’s choice chemotherapy in recurrent glioblastoma. Cancer Med. 2014;3(3):592–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Korshoej AR, Hansen FL, Thielscher A, et al. Impact of tumor position, conductivity distribution and tissue homogeneity on the distribution of tumor treating fields in a human brain: a computer modeling study. PLoS One. 2017;12(6):e0179214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ballo MT, Urman N, Lavy-Shahaf G, et al. Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: a large-scale numerical simulation-based analysis of data from the phase 3 EF-14 randomized trial. Int J Radiat Oncol Biol Phys. 2019;104(5):1106–1113. [DOI] [PubMed] [Google Scholar]
- 91. Turner SG, Gergel T, Wu H, et al. The effect of field strength on glioblastoma multiforme response in patients treated with the NovoTTF™-100A system. World J Surg Oncol. 2014;12:162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Jeyapalan S, Toms SA, Hottinger A, et al. Abstract CT205: Tumor Treating Fields alters progression patterns in glioblastoma: an imaging analysis of the EF-14 Phase III trial. Cancer Res. 2019;79(13 Suppl):CT205. [Google Scholar]
- 93. Mikic N, Korshoej AR. Improving tumor-treating fields with skull remodeling surgery, surgery planning, and treatment evaluation with finite element methods. In: Makarov SN, Noetscher GM, and Nummenmaa A, eds. Brain and Human Body Modeling 2020. Cham: Springer International Publishing; 2021:63–77. [PubMed] [Google Scholar]
- 94. Thomas AA, Rauschkolb PK. Tumor treating fields for glioblastoma: should it or will it ever be adopted? Curr Opin Neurol. 2019;32(6):857–863. [DOI] [PubMed] [Google Scholar]
- 95. Kaisman-Elbaz T, Elbaz Y, Merkin V, et al. Hemoglobin levels and red blood cells distribution width highlights glioblastoma patients subgroup with improved median overall survival. Front Oncol. 2020;10:432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Grossman R, Limon D, Bokstein F, Ben HC, Blumenthal D, Ram Z. RTID-12. Phase 2 trial of Tumor Treating Fields (TTFields) plus radiation therapy (RT) plus temozolamide (TMZ) compared to rt plus temozolomide in newly diagnosed glioblastoma (NDGBM). Neuro Oncol. 2020;22(Supplement_2):ii196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.