Abstract
Background
Primary central nervous system (CNS) tumors are often associated with high symptom burden and a poor prognosis from the time of diagnosis. The purpose of this study is to describe patient-reported outcomes (PRO) data from long-term survivors (LTS; ≥5-year survival post-diagnosis).
Methods
Clinical/treatment/molecular characteristics and PROs (symptom burden/interference (MDASI-BT/SP), perceived cognition (Neuro-QoL), anxiety/depression (PROMIS), and general health status (EQ-5D-3L)) were collected on 248 adult LTS between 9/2016 and 8/2019. Descriptive statistics and regression analysis were used to report results.
Results
Participants had a median age of 47 years (19-82) and were primarily White (83%) males (51%) with high-grade tumors (59%) and few mutations. Forty-two percent of the 222 brain tumor LTS reported no moderate-to-severe symptoms, whereas 45% reported three or more; most common symptoms were fatigue (40%), difficulty remembering (29%), and drowsiness (28%). Among spine tumor LTS (n = 42), nearly half reported moderate-to-severe weakness, pain, fatigue, and numbness/tingling, with 72% experiencing activity-related interference. Severe anxiety, depression, and cognitive symptoms were reported in up to 23% of the sample. Brain tumor LTS at higher risk for severe symptoms were more likely to be young, unemployed, and have poor KPS (Karnofsky Performance Status), whereas high symptom-risk spinal cord tumor LTS had poor KPS and received any tumor treatment.
Conclusions
Findings indicate LTS fall into distinct cohorts with no significant symptoms or very high symptom burden, regardless of tumor grade or mutational profile. These LTS data demonstrate the need for survivorship care programs and future studies to explore the symptom trajectory of all CNS tumor patients for prevention and early interventions.
Keywords: CNS tumor, long-term survivor, patient-reported outcomes, survivorship, symptom burden
Primary tumors of the central nervous system (CNS) are exceedingly rare, representing less than 2% of all cancers.1 Many CNS tumor types are associated with high symptom burden2 and, far too often, a dismal prognosis.3 The majority of studies that have considered CNS tumor patients who live for long periods of time, a clearly unique patient population worthy of rigorous investigation, have neglected individuals with low-grade tumors4,5 in favor of those with high-grade tumors, such as glioblastoma (GBM),6–14 an extremely aggressive neoplasm with a poor prognosis.15 The diagnosis of any CNS tumor typically follows the development of neurologic signs and symptoms,16 some of which may be permanent. In addition, the impact of tumor therapies and concomitant medications on both symptoms and function have been described17 and may further impact the person’s ability to work, care for themselves, and participate in usual activities, regardless of tumor type and grade.
In the literature, CNS tumor “long-term survivors” (LTS) have been defined by different lengths of survival from diagnosis. Some researchers have used a 2-year time-point to define LTS6,18 whereas others have used 3-7–9,19,20 and 5-year time-points to define their LTS group,11 mostly determined by the tumor type under consideration and frequently constrained by small, homogenous patient cohorts. Further, other investigators have identified patients with GBM who have survived greater than 5 years after diagnosis as “extreme survivors.” 12 Both “long-term” and “extreme” are relative terms dependent on current survival rates for the tumor type. Additionally, past groups have often used “long-term” as synonymous with “extended” or “prolonged,” referencing abnormally lengthy survival. Regardless of how one defines a LTS, research involving CNS tumor patients who live for long periods of time with their disease has predominantly focused on seeking to identify biomarkers that might predict such lengthy survival. These studies, which most commonly focus on the 5%-13% of patients with GBM who survive greater than 5-year post-diagnosis10,21–25 and consider the classic genetic prognostic factors of O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation and isocitrate dehydrogenase (IDH) mutation,7,13,26 have found no universal molecular characteristic that predicts long-term survivorship.14
The phrase “cancer survivorship” intends to describe the broad range of experiences that patients, as well as their caregivers and families, may have throughout the cancer diagnosis and treatment continuum. The aforementioned studies of LTS have failed to consider the survivorship experience of both high-grade and low-grade CNS tumor patients. While we have identified one study that considered the survivorship experience among LTS, its patient cohort was composed of only those with primary malignant brain tumors.27 Importantly, to our knowledge, no studies have yet characterized in-depth, across multiple domains of symptoms, the survivorship experience of adult LTS living with a wide array of neuraxis tumors of high- or low-grade. Understanding this lived experience, including the patient’s perception of their symptom burden and functional limitations, is important to develop survivorship care that is integrated into standard neuro-oncology care. Thus, the objective of this report is to describe a cross-section of systematically collected patient-reported outcomes (PRO) data from a cohort of LTS diagnosed with a primary CNS tumor, by WHO 2016 criteria, at least 5 years prior to data capture to begin to describe what life is like for those living long term with a primary CNS tumor and explore characteristics associated with worse outcomes. Importantly, unlike past groups and because of the heterogeneity of our cohort, here we use “long-term” in reference to a survival time of interest that, while abnormal for some tumor types (eg, GBM), is expected for others (eg, pilocytic astrocytoma).
Methods
Cohort Assembly
LTS enrolled in the Neuro-Oncology Branch’s Natural History Study (NHS; NCI 16-C-0151) at the National Institutes of Health (Bethesda, MD, USA) were defined as those patients alive at least 5 years (≥60 months) from their initial diagnosis of a primary CNS tumor. The NHS is an IRB-approved observational protocol that follows patients diagnosed with primary CNS tumors longitudinally throughout their disease course. Informed consent was obtained from all patients enrolled in the study. For analyses, we used the most recently completed assessment that was ≥5 years from the time of initial diagnosis.
Clinical and Demographic Information
Clinical and demographic data were collected using standardized forms by the nurse practitioner evaluating the patient. Updates on clinical information were collected at each clinic visit, with a required yearly follow-up as stipulated by the NHS protocol. For patients with more than one clinical evaluation, the most recent assessment was used for analyses. Study staff collected demographic, clinical, and treatment information from September 2016 through August 2019.
Tumor Tissue Analysis
Tumor tissue from the initial diagnosis was submitted on study entry, with central review of the histologic diagnosis and molecular characterization performed by experienced neuropathologists (M.M.Q. and K.A.). Ultimately, an integrated diagnosis was reported, incorporating all available data to characterize the tumor based on current neuropathologic criteria.
General Molecular Testing
A retrospective analysis of the somatic genetic profile of the LTS cohort’s tumor tissue from diagnosis was completed using data from a custom next-generation sequencing (NGS) panel of 56 genes (DNA-based) and 25 gene fusions (RNA-based) commonly known to be altered in CNS tumors. Only formalin-fixed, paraffin-embedded (FFPE) tissue samples were analyzed to characterize the tumors at the time of diagnosis. Descriptive analysis was completed on the most common diagnoses in this cohort of lower-grade astrocytoma (WHO grades II and III), ependymoma (all WHO grades), and oligodendroglioma (all WHO grades) as well as GBM (WHO grade IV). At the time of data analysis, the designation of GBM had not been restricted to IDH-wildtype as outlined by cIMPACT-NOW.28,29 Therefore, our cohort includes both IDH statuses (n = 8, wildtype and n = 8, mutant) as well as both primary and secondary GBM.
Patient-Reported Outcomes
PROs, including measures of symptom burden and interference, emotional distress, patient-perceived cognitive functioning, and general health status, were collected at each clinical evaluation via an electronic data capture system. PRO evaluations may have been completed up to a week prior to a patient’s clinical evaluation.
Symptom Burden and Interference Severity
The MD Anderson Symptom Inventory—Brain Tumor (MDASI-BT)30 and -Spine Tumor (MDASI-SP)31 modules are self-report measures of symptom burden and interference that capture the occurrence of a set of symptoms within the past 24 hours.30–32 The MDASI-BT and MDASI-SP scales were completed by those with tumors located in the brain and spine, respectively. Scores were provided for both overall symptom severity and interference (activity-related and mood-related interference) for both the MDASI-BT and MDASI-SP. Scores were also reported for the symptom factor subgroupings as previously outlined in the scale validation (see Supplementary Table 1; MDASI-BT: affective symptoms, cognitive symptoms, neurologic symptoms, treatment-related symptoms, general disease symptoms, and gastrointestinal disease symptoms; MDASI-SP: disease-related symptoms, autonomic function, constitutional symptoms, and emotional symptoms). Subsequently, mean scores were calculated for each factor.
Emotional Distress, Patient-Perceived Cognitive Functioning, and General Health Status
Patient-Reported Outcomes Measurement Information System (PROMIS) self-reported measures were used, including the PROMIS Item Bank v1.0-Emotional Distress-Anxiety Short Form 8a33 and the v1.0-Emotional Distress-Depression Short Form 8a,33 to assess anxiety and depressive symptoms. The Quality of Life in Neurological Disorders (Neuro-QoL) Item Bank v2.0-Cognitive Function-Short Form34 was used to assess patient-perceived cognitive symptoms. Finally, the EQ-5D-3L was used to assess general health status along five dimensions (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression).35 For analyses, the five dimensions were dichotomized into (1) “Some problems,” which incorporated “Confined to bed,” “Unable,” and “Extreme problems” and (2) “No problems.”
Statistical Analyses
Descriptive statistics characterized the patient sample and provided PRO summary scores. Karnofsky Performance Status (KPS) was dichotomized as either “Good” (≥90) or “Poor” (≤80) based on previous work by members of our group identifying that a KPS of ≤80 is associated with higher symptom burden, increased interference with daily activities, and disease recurrence.30 For the purpose of molecular analysis, tumor diagnosis type was divided into three groups representing the most common diagnoses in our cohort: astrocytoma, ependymoma, and oligodendroglioma, which included a variety of subtypes and grades. An additional group was considered “other” which included atypical teratoid rhabdoid tumor, atypical choroid plexus papilloma, central neurocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma, glioneuronal tumor, rosette-forming glioneuronal tumor, anaplastic glioneuronal tumor, high-grade glioma, high-grade neuroepithelial tumor, medulloblastoma, meningioma, atypical meningioma, anaplastic meningioma, rhabdoid meningioma, oligoastrocytoma, pineoblastoma, pituitary carcinoma, pleomorphic xanthoastrocytoma, anaplastic pleomorphic xanthoastrocytoma, and undifferentiated pleomorphic sarcoma.
PRO scores were described as significant based on established cutoffs for moderate-severe reporting. A MDASI-BT/-SP symptom rated ≥536,37 or an interference score of ≥2 was considered moderate-to-severe.38 Results from the MDASI-BT/-SP were analyzed separately based on tumor location. For both the PROMIS-Anxiety and -Depression scores, 1 standard deviation (SD) above the mean (T-score >60) was considered moderate-to-severe anxiety or depressive symptoms.39 For the Neuro-QoL Cognitive Function, scores 1 SD below the mean (T-score <40) were considered moderate-to-severe cognitive deficits.39 EQ-5D-3L index scores were calculated using the Shaw et al. scoring algorithm and ranged from −0.11 to 1.0, with a score of 1.0 representing perfect health.40
Associations between and group differences among clinical factors (including sex, race, education level, current work status, KPS, levetiracetam use, dexamethasone use, current treatment phase, tumor location, tumor diagnosis, tumor grade, prior diagnosis change, radiation treatment, treatments other than radiation, disease recurrence, current age, and years from diagnosis) and PROs (symptom burden and interference, emotional distress, patient-perceived cognitive functioning, and general health status) were explored through chi-square tests, Fisher exact tests, and independent samples t tests. Adjustments to significance level for multiple comparisons were applied as appropriate based on Bonferroni’s method. All statistical analyses were performed using IBM SPSS Version 25.0.41
Results
Demographic and Clinical Characteristics
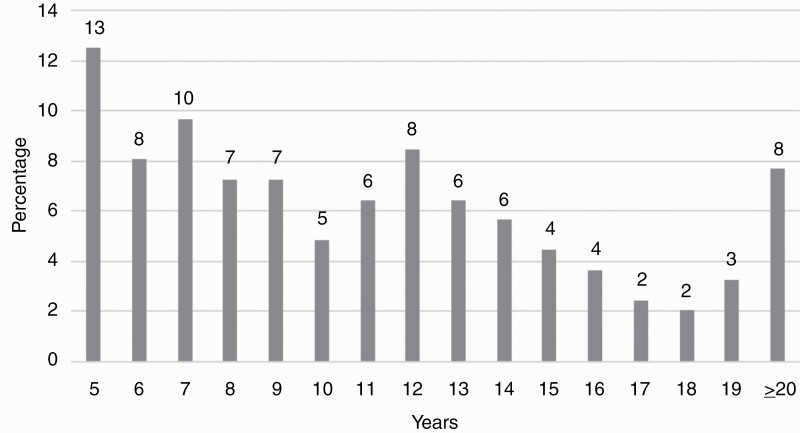
The study cohort consisted of 248 LTS between 5 and 29 years (median: 11 years) post-diagnosis of an oligodendroglioma, astrocytoma, GBM, ependymoma, or “other” CNS tumor (see Figure 1). Patients were predominantly White (83%) males (51%) ranging in age from 19 to 82 (= 47, SD = 13). The majority of patients had brain tumors (82%; 59% high-grade tumors) and underwent ≥2 surgeries (57%). Approximately one-third (34%) of LTS had an initial gross total resection, 56% of patients received radiation, and 69% experienced a tumor recurrence prior to the study time-point. Additional demographic and clinical characteristics of the sample are listed in Table 1.
Figure 1.
Distribution of survivorship years, n = 248.
Table 1.
Patient Demographics and Clinical Characteristics, n = 248
| n | % | ||
|---|---|---|---|
| Age | Median (Range)Mean (SD) | 4747 | (19-82) (13.3) |
| Sex | Male | 127 | 51 |
| Race | Asian | 10 | 4 |
| Black/African American | 16 | 6 | |
| White | 205 | 83 | |
| Other | 3 | 1 | |
| Ethnicity | Hispanic or Latino | 15 | 6 |
| Highest education level | High school or below | 32 | 13 |
| Some college/bachelor’s degree | 120 | 48 | |
| Graduate/professional degree | 87 | 35 | |
| Work status | Employed | 131 | 53 |
| Unemployeda | 40 | 16 | |
| Retired | 42 | 17 | |
| Disabled/FMLA | 19 | 8 | |
| Other | 8 | 3 | |
| Vital status | Deceased | 35 | 14 |
| Current tumor location | Brain | 203 | 82 |
| Spine | 20 | 8 | |
| Brain and spine | 23 | 9 | |
| Other | 2 | 1 | |
| Current diagnosis | Astrocytoma | 64 | 26 |
| Ependymoma | 47 | 19 | |
| Glioblastoma | 38 | 15 | |
| Oligodendroglioma | 47 | 19 | |
| Otherb | 45 | 18 | |
| Current tumor grade | Grade I | 30 | 12 |
| Grade II | 60 | 24 | |
| Grade III | 97 | 39 | |
| Grade IV | 50 | 20 | |
| No grade assigned | 4 | 2 | |
| No tissue diagnosis | 7 | 3 | |
| Prior diagnosis changec | Yes | 79 | 32 |
| KPS | ≥90 | 145 | 58 |
| ≤80 (30-80) | 102 | 41 | |
| Current treatment phase | Treatment | 69 | 28 |
| Surveillance | 179 | 72 | |
| Current progression status | No progression | 198 | 80 |
| Progression | 50 | 20 | |
| Years from diagnosis | Median (range) | 11 | (5-29) |
| Age at diagnosis | Median (range) | 35 | (2-73) |
| Number of surgeries | 0 | 7 | 3 |
| 1 | 99 | 40 | |
| 2 | 60 | 24 | |
| ≥3 | 82 | 33 | |
| Original surgery extent | No surgery | 7 | 3 |
| Biopsy | 44 | 18 | |
| Subtotal resection | 68 | 27 | |
| Gross total resection | 85 | 34 | |
| Resection NOS | 44 | 18 | |
| Number of radiation treatments | 0 | 40 | 16 |
| 1 | 139 | 56 | |
| ≥2 | 69 | 28 | |
| Number of treatmentsd | 0 | 64 | 26 |
| 1 | 79 | 32 | |
| 2 | 43 | 17 | |
| ≥3 | 62 | 25 | |
| Number of recurrences | 0 | 78 | 32 |
| 1 | 51 | 21 | |
| 2 | 50 | 20 | |
| ≥3 | 69 | 28 |
Abbreviations: FMLA, family and medical leave; KPS, Karnofsky Performance Status; NOS, not otherwise specified.
Missing data ranged from n = 1 to n = 14.
a11% of patients reported being unemployed due to their diagnosis.
bOther tumor types included: atypical teratoid rhabdoid tumor, atypical choroid plexus papilloma, central neurocytoma, dysembryoplastic neuroepithelial tumor, ganglioglioma, glioneuronal tumor, rosette-forming glioneuronal tumor, anaplastic glioneuronal tumor, high-grade glioma, high-grade neuroepithelial tumor, medulloblastoma, meningioma, atypical meningioma, anaplastic meningioma, rhabdoid meningioma, oligoastrocytoma, pineoblastoma, pituitary carcinoma, pleomorphic xanthoastrocytoma, anaplastic pleomorphic xanthoastrocytoma, and undifferentiated pleomorphic sarcoma.
cDue to transformation, pathology review, or further molecular testing.
dIncludes chemotherapy, vaccines, immunotherapy, Optune devices, hyperbaric oxygen treatments, and microdialysis chips.
Tumor Tissue Analysis and General Molecular Characteristics
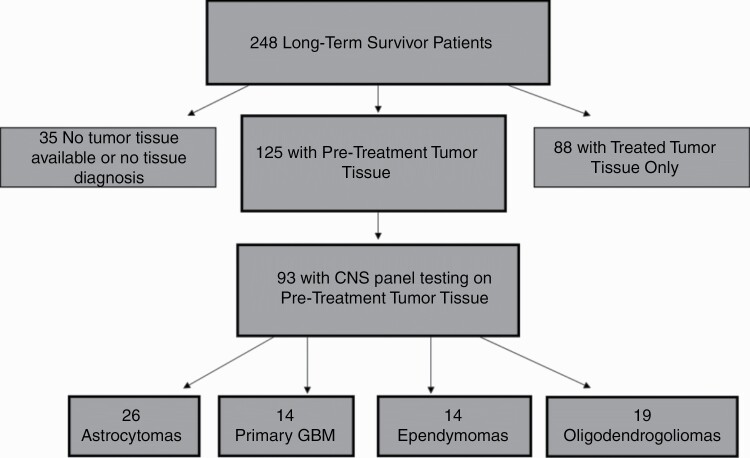
Of the 248 LTS in the study cohort, 213 (86%; pre-treatment tissue: n = 125 and post-treatment tissue: n = 88) had tumor tissue available for central review and analysis (see Figure 2). Of the 35 LTS with no tissue available, tissue was depleted for 29, and 6 were diagnosed based solely on neuroimaging. Of the 213 patients with tumor samples available, 173 (81%) had molecular testing done in addition to histopathologic review. Diagnosis was confirmed in 181 (85%) cases, with a change in diagnosis in 32 patients; 25 (78%) patients had a diagnosis change due to histological review, whereas 7 (22%) had a diagnosis change due to histological review and molecular testing. Of the 32 total LTS with a diagnosis change, 9 patients had their tumor type changed from one diagnosis to another, 20 patients had a change in glioma subtype, with the majority changed as a result of 1p/19q codeletion analysis, and 3 patients had a change in their ependymoma diagnosis subtype (either to higher grade or lower grade; see Supplementary Table 2).
Figure 2.
Schematic of the LTS cohort tumor tissue and CNS panel result availability. Abbreviations: CNS, central nervous system; LTS, long-term survivors.
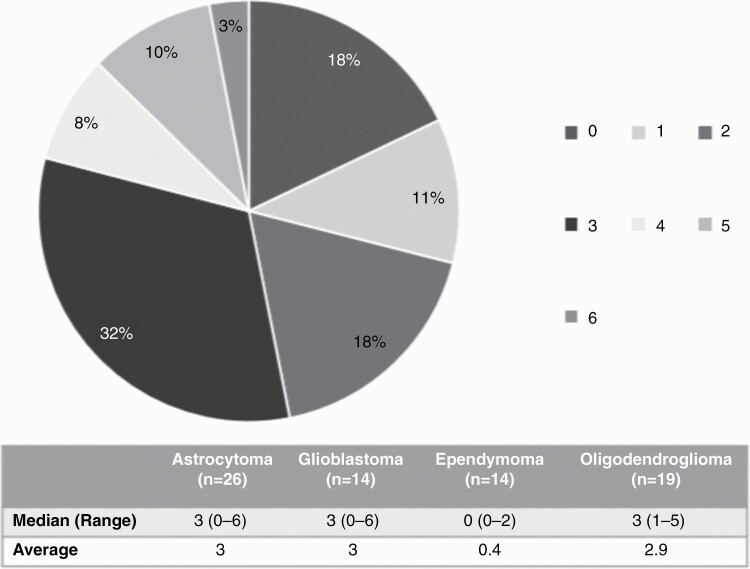
Across the various diagnoses in our cohort, the mutation rate was low (maximum number of 6; see Table 2), with 18% of tumors showing no detectable mutations. Sixteen patients within our cohort were diagnosed with GBM, of which 50% had an IDH mutation, and 63% had MGMT promoter methylation. Overall, only 19% showed both of these characteristics consistent with a better prognosis. GBM, astrocytoma, and oligodendroglioma tumor samples each had 3 mutations on average (see Figure 3), with mutations in the most commonly altered genes (IDH1, TP53, and ATRX) present. More specifically, tumor tissue of LTS with astrocytoma and GBM had high percentages of TP53 mutations with 67% and 75%, respectively (see Supplementary Figure 1B).
Table 2.
Description of Alterations Found by Tumor Diagnosis
| Number of Alterations | Total N | Diagnosis | n | Alterations |
|---|---|---|---|---|
| 0 | 13 | Astrocytoma | 2 | |
| Glioblastoma | 1 | |||
| Ependymoma | 10 | |||
| 1 | 8 | Astrocytoma | 1 | IDH1 |
| Glioblastoma | 2 | IDH1 EGFR amplification | ||
| Ependymoma | 2 | NF2 TERT | ||
| Oligodendroglioma | 3 | IDH1 | ||
| 2 | 13 | Astrocytoma | 4 | ATRX, IDH1 IDH1, TP53 IDH2, TP53 |
| Glioblastoma | 3 | TP53, PTEN TP53, TP53 ATRX, IDH1 | ||
| Ependymoma | 2 | BRAF, ATRX TERT, SETD2 | ||
| Oligodendroglioma | 4 | CIC, IDH1 IDH1, FUBP1 IDH2, MSH6 | ||
| 3 | 23 | Astrocytoma | 12 | IDH1, ATRX, ATRX IDH1, ATRX, TP53 MSH6, CIC, IDH1 IDH1, TP53, TP53 IDH1, TP53, CDK4 amplification |
| Glioblastoma | 4 | ATRX, TP53, IDH1 PTEN, RB1, TP53 TP53, CIC, BRAF TP53, IDH1, PIK3CA | ||
| Oligodendroglioma | 7 | TERT, TP53, IDH1 CIC, IDH1, FUBP1 IDH1, CIC, HIST1H3C TERT, IDH1, NRAS MET, TERT, IDH1 TERT, IDH1, CDKN2A loss | ||
| 4 | 6 | Astrocytoma | 1 | PTCH1, TERT, CIC, IDH1 |
| Glioblastoma | 3 | TP53, RB1, PTCH1, PTEN TP53, IDH1, PIK3CA, ATRX ATRX, IDH1, TP53, MYCN amplification | ||
| Oligodendroglioma | 2 | TP53, ATRX, IDH1, SETD2 TERT, IDH1, CIC, CIC | ||
| 5 | 8 | Astrocytoma | 3 | TP53, ATRX, NOTCH1, IDH1, CDKN2A ATRX, RB1, IDH1, TP53, TP53 TP53, TP53, ATRX, IDH1, IGF1R |
| Glioblastoma | 2 | CIC, TP53, TP53, TP53, IDH1 TERT, RB1, TP53, PTEN, RB1 | ||
| Oligodendroglioma | 3 | IDH1, RB1, CIC, CIC, NOTCH1 IGF1R, ATRX, IDH1, SMARCA4, SETD2 FUBP1, TERT, FGFR1, IDH1, PTEN | ||
| 6 | 2 | Astrocytoma | 1 | ATRX, NF1, IDH1, TP53, TP53, SMARCA4 |
| Glioblastoma | 1 | ATRX, ATRX, ATRX, TP53, TP53, IDH1 |
Figure 3.
Number of mutations in pre-treatment tissue.
Patient-Reported Outcomes
Table 3 summarizes all associations between PROs and clinical/treatment factors used in our analysis. For each factor considered, the table includes the mean symptom burden and interference score as well as the percentage of patients with none-mild or moderate-to-severe symptoms (emotional distress and patient-perceived cognitive functioning) and no problems or some problems/extreme problems with mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (general health status). Correlation coefficients (current age and years from diagnosis with MDASI-BT/SP) and mean age/years for each PRO are also presented for current age and years from diagnosis. Again, symptom reports are based on established criteria for none-to-mild vs moderate-to-severe. Below, results for each PRO scale (with symptom burden and interference presented separately based on the different MDASI scales: MDASI-BT and MDASI-SP for those with brain and spine tumors, respectively) are described in terms of descriptive severity data and a report of associations with both demographic and clinical characteristics is presented.
Table 3.
Associations Between Patient-Reported Outcomes and Clinical/Treatment Factors
| Symptom Burden and Interference | Emotional Distress | Patient-Perceived Cognitive Functioning | General Health Status | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MDASI-BT | MDASI-SP | PROMIS Anxiety | PROMIS Depression | Neuro-QoL Cognitive Function | EQ-5D-3L | |||||||||||||
| Mobility | Self-care | Usual Activities | Pain/Discomfort | Anxiety/Depression | ||||||||||||||
| NM | MS | NM | MS | NM | MS | NP | SP/EP | NP | SP/EP | NP | SP/EP | NP | SP/EP | NP | SP/EP | |||
| x– | x– | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | % | |
| Sex: Female | 1.8 | 2.3 | 85 | 15 | 88 | 12 | 75 | 25 | 57 | 43 | 79 | 21 | 55 | 45 | 61 | 39 | 55 | 45 |
| Sex: Male | 1.8 | 3.2 | 82 | 18 | 87 | 13 | 85 | 15 | 60 | 40 | 78 | 22 | 52 | 48 | 61 | 39 | 59 | 41 |
| Race: White | 1.8 | 2.8 | 83 | 17 | 87 | 13 | 80 | 20 | 57 | 43 | 77 | 23 | 52 | 48 | 61 | 39 | 58 | 42 |
| Race: Non-White | 2.2 | 2.1 | 83 | 17 | 90 | 10 | 89 | 11 | 65 | 35 | 86 | 14 | 55 | 45 | 62 | 38 | 62 | 38 |
| Education level: High school or below | 2.0 | 2.8 | 85 | 15 | 91 | 9 | 96 | 4 | 68 | 32 | 91 | 9 | 56 | 44 | 53 | 47 | 59 | 41 |
| Education level: Some college/Bachelor’s degree | 1.9 | 2.9 | 81 | 19 | 86 | 14 | 77 | 23 | 59 | 41 | 77 | 23 | 53 | 47 | 62 | 38 | 58 | 42 |
| Education level: Graduate/Professional degree | 1.7 | 2.3 | 85 | 15 | 87 | 13 | 79 | 21 | 58 | 42 | 81 | 19 | 53 | 47 | 64 | 36 | 56 | 44 |
| Current work status: Employed | 1.6 | 2.5 | 86 | 14 | 93 | 7* | 84 | 16 | 73 | 27* | 86 | 14* | 66 | 34* | 68 | 32 | 62 | 38 |
| Current work status: Not employed | 2.2 | 3.1 | 79 | 21 | 79 | 21* | 75 | 25 | 42 | 58* | 70 | 30* | 36 | 64* | 50 | 50 | 50 | 50 |
| KPS: ≥90 (Good) | 1.3* | 1.8 | 87 | 13 | 95 | 5* | 86 | 14 | 85 | 15* | 97 | 3* | 78 | 22* | 71 | 29* | 64 | 36 |
| KPS: ≤80 (Poor) | 2.7* | 3.3 | 78 | 22 | 76 | 24* | 70 | 30 | 21 | 79* | 51 | 49* | 16 | 84* | 46 | 54* | 48 | 52 |
| Levetiracetam use: Yes | 2.0 | 4.1 | 87 | 13 | 89 | 11 | 73 | 27 | 54 | 46 | 74 | 26 | 50 | 50 | 67 | 33 | 56 | 44 |
| Levetiracetam use: No | 1.7 | 2.7 | 81 | 19 | 86 | 14 | 86 | 14 | 62 | 38 | 81 | 19 | 55 | 45 | 57 | 43 | 59 | 41 |
| Dexamethasone use: Yes | 2.7 | 3.3 | 79 | 21 | 75 | 25 | 82 | 18 | 25 | 75* | 50 | 50* | 25 | 75* | 46 | 54 | 54 | 46 |
| Dexamethasone use: No | 1.7 | 2.7 | 84 | 16 | 89 | 11 | 81 | 19 | 63 | 37* | 82 | 18* | 57 | 43* | 63 | 37 | 58 | 42 |
| Current treatment phase: Treatment | 2.2 | 3.2 | 86 | 14 | 88 | 12 | 83 | 17 | 39 | 61* | 62 | 38* | 33 | 67* | 51 | 49 | 62 | 38 |
| Current treatment phase: Surveillance | 1.7 | 2.3 | 82 | 18 | 87 | 13 | 80 | 20 | 66 | 34* | 84 | 16* | 60 | 40* | 65 | 35 | 56 | 44 |
| Progression status: Progression | 2.6* | 2.8 | 79 | 21 | 82 | 18 | 71 | 29 | 42 | 58 | 73 | 27 | 35 | 65 | 48 | 52 | 58 | 42 |
| Progression status: No progression | 1.7* | 2.7 | 84 | 16 | 89 | 11 | 82 | 18 | 63 | 37 | 80 | 20 | 57 | 43 | 64 | 36 | 57 | 43 |
| Tumor location: Brain | 1.8 | – | 84 | 16 | 88 | 12 | 78 | 22 | 64 | 36* | 82 | 18 | 58 | 42* | 69 | 31* | 58 | 42 |
| Tumor location: Spine | – | 2.8 | 86 | 14 | 90 | 10 | 93 | 7 | 43 | 57* | 67 | 33 | 33 | 67* | 14 | 86* | 57 | 43 |
| Tumor location: Brain and Spine | 2.6 | 2.7 | 77 | 23 | 82 | 18 | 92 | 8 | 27 | 73* | 59 | 41 | 23 | 77* | 32 | 68* | 54 | 46 |
| Tumor diagnosis: Astrocytoma | 1.8 | 3.1 | 79 | 21 | 84 | 16 | 71 | 29 | 65 | 35* | 82 | 18 | 55 | 45 | 67 | 33 | 52 | 48 |
| Tumor diagnosis: Ependymoma | 2.3 | 2.7 | 85 | 15 | 93 | 7 | 96 | 4 | 35 | 65* | 61 | 39 | 37 | 63 | 41 | 59 | 56 | 44 |
| Tumor diagnosis: Oligodendroglioma | 1.2 | – | 94 | 6 | 89 | 11 | 86 | 14 | 72 | 28* | 79 | 21 | 68 | 32 | 70 | 30 | 68 | 32 |
| Tumor diagnosis: Other | 2.1 | 2.7 | 81 | 19 | 86 | 14 | 81 | 19 | 56 | 44* | 86 | 14 | 51 | 49 | 60 | 40 | 60 | 40 |
| Tumor grade: Grade I | 1.3 | 2.9 | 90 | 10 | 93 | 7 | 87 | 13 | 63 | 37 | 83 | 17 | 57 | 43 | 43 | 57 | 63 | 37 |
| Tumor grade: Grade II | 1.6 | 2.5 | 75 | 25 | 86 | 14 | 84 | 16 | 64 | 36 | 81 | 19 | 61 | 39 | 69 | 31 | 52 | 48 |
| Tumor grade: Grade III | 1.9 | 2.9 | 86 | 14 | 88 | 12 | 80 | 20 | 59 | 41 | 74 | 26 | 53 | 47 | 62 | 38 | 58 | 42 |
| Tumor grade: Grade IV | 2.1 | 1.1 | 87 | 13 | 86 | 14 | 67 | 33 | 48 | 52 | 77 | 23 | 44 | 56 | 62 | 38 | 60 | 40 |
| Tumor grade: No grade assigned | 1.0 | 5.3 | 100 | 0 | 100 | 0 | 100 | 0 | 50 | 50 | 100 | 0 | 25 | 75 | 50 | 50 | 50 | 50 |
| Tumor grade: No tissue diagnosis | 2.3 | 1.8 | 57 | 43 | 71 | 29 | 100 | 0 | 71 | 29 | 86 | 14 | 57 | 43 | 57 | 43 | 57 | 43 |
| Prior diagnosis change: Yes | 2.0 | 2.4 | 87 | 13 | 87 | 13 | 82 | 18 | 58 | 42 | 76 | 24 | 47 | 53 | 67 | 33 | 60 | 40 |
| Prior diagnosis change: No | 1.8 | 2.8 | 81 | 19 | 87 | 13 | 80 | 20 | 59 | 41 | 80 | 20 | 56 | 44 | 59 | 41 | 56 | 44 |
| Radiation treatments: Yes | 2.0* | 2.9 | 85 | 15 | 87 | 13 | 78 | 22 | 53 | 47* | 75 | 25 | 49 | 51* | 60 | 40 | 58 | 42 |
| Radiation treatments: No | 1.0* | 1.0 | 75 | 25 | 87 | 13 | 92 | 8 | 87 | 13* | 95 | 5 | 75 | 25* | 67 | 33 | 55 | 45 |
| Treatments (other than radiationa): Yes | 1.9 | 3.3 | 84 | 16 | 87 | 13 | 79 | 21 | 54 | 46 | 75 | 25 | 47 | 53 | 62 | 38 | 60 | 40 |
| Treatments (other than radiationa): No | 1.6 | 1.8 | 81 | 19 | 87 | 13 | 85 | 15 | 72 | 28 | 87 | 13 | 69 | 31 | 59 | 41 | 52 | 48 |
| Recurrence: Yes | 2.0 | 2.8 | 85 | 15 | 86 | 14 | 79 | 21 | 48 | 52* | 71 | 29* | 43 | 57* | 59 | 41 | 59 | 41 |
| Recurrence: No | 1.4 | 2.4 | 79 | 21 | 90 | 10 | 85 | 15 | 82 | 18* | 94 | 6* | 74 | 26* | 65 | 35 | 55 | 45 |
| r | r | |||||||||||||||||
| Current age | −0.073 | 0.088 | 48 | 45 | 47 | 47 | 47 | 49 | 46 | 49 | 46 | 50 | 46 | 48 | 48 | 46 | 48 | 46 |
| Years from diagnosis | 0.001 | 0.113 | 12 | 9 | 11 | 10 | 12 | 11 | 11 | 12 | 11 | 12 | 11 | 12 | 11 | 12 | 12 | 11 |
Abbreviations: , mean; KPS, Karnofsky Performance Status; MDASI-BT/SP, MD Anderson Symptom Inventory-Brain Tumor/Spine Tumor modules; MS, moderate-to-severe; NM, none-mild; NP, no problems; PROMIS, Patient-Reported Outcomes Measurement Information System; r, correlation coefficient; SP/EP, some problems/extreme problems.
aIncludes chemotherapy, vaccines, immunotherapy, Optune devices, hyperbaric oxygen treatments, and microdialysis chips.
*P < .0027, after adjustment for multiple comparisons.
Symptom Burden and Interference
MDASI-BT Symptom Burden and Interference Severity
Patients with brain tumors (n = 222) reported 0-21 moderate-to-severe symptoms (= 4, SD = 5). While 42% of brain tumor LTS reported no moderate-to-severe symptoms, 45% reported three or more, with the three most prevalent moderate-to-severe symptoms being fatigue (40%), difficulty remembering (29%), and drowsiness (28%). When queried on how their disease has interfered with their lives, over 40% of brain tumor LTS reported moderate-to-severe overall, activity-related, and mood-related interference.
After considering associations with demographic and clinical factors, it was determined that patients whose imaging showed disease progression at the time of PRO reporting had greater overall symptom burden (difference = 0.9, Hedges’ g = 0.52, 95% CI [0.3, 1.5]), neurologic symptom factor (difference = 1.3, Hedges’ g = 0.68, 95% CI [0.7, 2.0]), and activity-related interference (difference = 1.5, Hedges’ g = 0.49, 95% CI [0.5, 2.6]) means than patients whose imaging showed stable disease. In terms of tumor location, patients who had tumor involving both their brain and spine had greater overall (difference = 1.8, Hedges’ g = 0.65, 95% CI [0.5, 3.1]) and activity-related (difference = 2.7, Hedges’ g = 0.87, 95% CI [1.3, 4.1]) interference means than patients with tumors localized only to the brain.
Current age, current work status, KPS, progression on latest MRI, tumor location, tumor diagnosis type, radiation treatment, and past tumor recurrence yielded statistically significant results upon multiple comparisons. Younger age was correlated with a higher neurologic factor mean (r = −0.14, P = .038). Unemployed patients had greater overall interference (difference = 1.3, Hedges’ g = 0.46, 95% CI [0.5, 2.1]) and activity-related (difference = 1.6, Hedges’ g = 0.51, 95% CI [0.7, 2.4]) interference means than employed patients. Patients with poor KPS had a greater overall symptom mean (difference = 1.3, Hedges’ g = 0.80, 95% CI [0.9, 1.8]) as well as greater affective (difference = 1.2, Hedges’ g = 0.49, 95% CI [0.6, 1.9]), cognitive (difference = 1.6, Hedges’ g = 0.68, 95% CI [0.9, 2.2]), neurologic (difference = 1.7, Hedges’ g = 0.92, 95% CI [1.2, 2.2]), treatment-related (difference = 1.6, Hedges’ g = 0.82, 95% CI [1.0, 2.1]), and general disease (difference = 1.2, Hedges’ g = 0.73, 95% CI [0.7, 1.6]) symptom factor means than patients with good KPS. These patients also had a greater overall interference mean (difference = 3.0, Hedges’ g = 1.25, 95% CI [2.3, 3.7]) as well as greater activity (difference = 3.8, Hedges’ g = 1.50, 95% CI [3.1, 4.6]) and mood-related (difference = 2.2, Hedges’ g = 0.88, 95% CI [1.5, 2.9]) interference means than patients with good KPS.
When evaluated by tumor diagnosis, there were group differences for the general disease factor mean among the four diagnosis groups of astrocytoma, ependymoma, oligodendroglioma, and “other” (F(3, 218) = 6.22, P < .001). Patients with ependymoma or “other” CNS tumor types had a greater general disease factor mean than patients with oligodendroglioma. In terms of treatment history, patients who received radiation treatment had greater overall symptom (difference = 1.0, Hedges’ g = 0.57, 95% CI [0.5, 1.5]), cognitive factor (difference = 1.4, Hedges’ g = 0.58, 95% CI [0.7, 2.1]), neurologic factor (difference = 1.1, Hedges’ g = 0.55, 95% CI [0.6, 1.6]), treatment-related factor (difference = 1.1, Hedges’ g = 0.54, 95% CI [0.5, 1.7]), general disease factor (difference = 0.9, Hedges’ g = 0.56, 95% CI [0.5, 1.4]), overall interference (difference = 1.5, Hedges’ g = 0.55, 95% CI [0.7, 2.3]), and activity-related interference (difference = 2.0, Hedges’ g = 0.64, 95% CI [1.2, 2.8]) means than patients who did not receive radiation. Finally, those patients who had previous disease recurrence had greater neurologic (difference = 0.8, Hedges’ g = 0.42, 95% CI [0.3, 1.4]), treatment-related (difference = 0.8, Hedges’ g = 0.42, 95% CI [0.3, 1.4]), and general disease (difference = 0.6, Hedges’ g = 0.34, 95% CI [0.1, 1.0]) factor means as well as overall (difference = 1.3, Hedges’ g = 0.45, 95% CI [0.5, 2.0]) and activity-related (difference = 1.7, Hedges’ g = 0.56, 95% CI [1.0, 2.5]) interference means than patients who did not have a recurrence.
In summary, among brain tumor LTS, those with poor KPS and those who were unemployed had high symptom burden and interference. Additionally, receiving radiation and disease recurrence on MRI at the time of the report was associated with more severe disease-specific symptom burden and interference.
MDASI-SP Symptom Burden and Interference Severity
Patients with spine tumors (n = 42) reported 0-18 moderate-to-severe symptoms (= 5, SD = 4). Approximately two-thirds (67%) of spine tumor LTS reported ≥3 moderate-to-severe symptoms, with the four most prevalent being weakness in arms/legs/trunk (51%), pain (49%), fatigue (49%), and numbness/tingling (49%). Additionally, among spine tumor LTS, 72% reported moderate-to-severe activity-related interference, and 46% reported moderate-to-severe mood-related interference.
KPS, radiation treatment, and a history of any tumor treatment other than radiation, but neither tumor grade nor disease recurrence, yielded statistically significant results upon multiple comparisons. Spine tumor LTS with poor KPS had greater overall symptom (difference = 1.5, Hedges’ g = 0.79, 95% CI [0.5, 2.6]), autonomic function factor (difference = 2.3, Hedges’ g = 0.87, 95% CI [0.8, 3.8]), and activity-related interference (difference = 3.0, Hedges’ g = 1.09, 95% CI [1.2, 4.7]) means than patients with good KPS. Additionally, patients who received radiation treatment had greater autonomic function (difference = 2.4, Hedges’ g = 0.87, 95% CI 1.2, 3.6]) and constitutional/treatment factor (difference = 1.7, Hedges’ g = 0.76, 95% CI [0.7, 4.0]) means than patients who did not receive radiation. Finally, patients who received tumor treatment other than radiation had a greater autonomic function factor mean than patients who did not receive such treatment (difference = 2.6, Hedges’ g = 1.01, 95% CI [1.3, 3.9]). Thus, poorly functioning spinal cord tumor LTS with a history of any tumor treatment experience significant symptom burden and interference, regardless of tumor grade.
Emotional Distress: Anxiety and Depressive Symptoms
Moderate-to-severe anxiety symptoms were reported by 17% of LTS (PROMIS range: 37.1-78), with no difference in incidence between those with brain (16%) vs spinal cord (14%) tumors and the highest prevalence in those with disease in both the brain and spine (23%). There was a significant association between patients experiencing moderate-to-severe anxiety symptoms and the time since diagnosis, with those with moderate-to-severe anxiety 9.4 years from diagnosis and those with none-mild anxiety 11.6 years from diagnosis (Hedges’ g = 0.45, 95% CI [0.5, 3.9]). Additionally, higher anxiety scores were reported by females (2.9 points higher than males; Hedges’ g = 0.30, 95% CI [0.5, 5.2]), unemployed patients (3.2 points higher than employed patients; Hedges’ g = 0.33, 95% CI [0.7, 5.6]), and those with poor KPS (2.8 points higher than those with good KPS; Hedges’ g = 0.29, 95% CI [0.4, 5.2]).
Moderate-to-severe depressive symptoms were reported by 13% of LTS (PROMIS range: 37.7-81.1), with, as previously described for anxiety, the highest prevalence in patients with disease in both locations (brain and spine; 18%), compared to disease in the brain (12%) or spine (10%) alone where similar prevalence rates were observed. Patients who were unemployed were approximately four times more likely to report moderate-to-severe depressive symptoms (X2(1) = 11.28, P = .001), and scored, on average, 3.5 points higher on the PROMIS-Depression scale than their employed counterparts (Hedges’ g = 0.40, 95% CI [1.3, 5.8]). Patients with poor KPS were over six times more likely to report moderate-to-severe depressive symptoms as well (X2(1) = 19.19, P < .001). These individuals scored, on average, 5.3 points higher on the PROMIS-Depression scale than those with good KPS (Hedges’ g = 0.62, 95% CI [3.1, 7.5]). Notably, tumor grade, tumor type, and number of disease recurrences were not associated with depressive symptoms in this LTS cohort, whereas KPS and employment status were.
Patient-Perceived Cognitive Functioning
In this cohort, 19% of LTS reported moderate-to-severe cognitive dysfunction with an overall average Neuro-QoL Cognitive Function score of 48.9 (SD = 10.2; range: 24.4-64.2). The majority of demographic and clinical characteristics did not show any statistically significant association with moderate-to-severe cognitive dysfunction upon multiple comparisons, including tumor grade, tumor type, and disease recurrence. Neither levetiracetam nor dexamethasone use was found to have a statistically significant association with cognitive dysfunction. Approximately one-third (30%) of patients with poor KPS reported moderate-to-severe cognitive dysfunction compared to only 14% of patients with good KPS (X2(1) = 5.60, P = .018, OR = 2.70). Additionally, patients with poor KPS scored, on average, 6.9 points lower on the Neuro-QoL Cognitive Function scale than patients with good KPS (Hedges’ g = 0.72, 95% CI [3.6, 10.3]). Mean differences in T-scores were found for current work status and prior radiation treatment. On average, unemployed patients scored 4.4 points lower than employed patients (Hedges’ g = 0.43, 95% CI [1.0, 7.8]), and patients who had received radiation scored 5.3 points lower than patients who had not received such treatment (Hedges’ g = 0.52, 95% CI [0.9, 9.7]). Thus, the only disease or treatment characteristic associated with worse patient-perceived cognitive functioning was treatment with radiation therapy, with patients more likely to have a poor KPS and be unemployed.
General Health Status
Issues related to usual activities (47%), anxiety/depression (42%), mobility (41%), pain/discomfort (39%), and self-care (22%) were commonly reported by LTS. Current work status, current treatment phase, KPS, dexamethasone use, radiation treatment, and past tumor recurrence were all associated with problems with self-care, usual activities, and mobility, with older age also associated with mobility problems. Tumor location and diagnosis were associated with all EQ-5D-3L dimensions except self-care. Having received treatments other than radiation was only associated with the ability to perform usual activities. Current work status, KPS, current disease progression status, and tumor location and diagnosis were all statistically significantly associated with pain/discomfort. Finally, only KPS was statistically significantly associated with anxiety/depression. Thus, KPS was unique in displaying significant associations with each EQ-5D-3L dimension, key components of patients’ general health status.
Discussion
The objective of this comprehensive report of primary CNS tumor LTS was to describe a cross-section of systematically collected PRO data in domains of symptom burden and interference, emotional distress, patient-perceived cognitive functioning, and general health status to characterize the illness burden contributing to survivorship issues of those living longer than 5 years with a CNS tumor. The tumors of LTS were found to have few mutations and, similar to other LTS molecular biomarker studies, no common mutational characteristic. Findings from the analysis of PRO data highlight the symptom experience of LTS with CNS tumors and suggest that there are distinct patient cohorts based on symptom burden, with both low- and high-grade tumor patients at risk for moderate-severe symptoms.
Feasibility and Importance of General Molecular Characterization of LTS Tumor Tissue
Targeted molecular analysis of the LTS cohort supported previous findings that these tumors have a low mutation rate for genes commonly altered in CNS tumors. Due to the use of a targeted panel, comparison to large datasets, such as The Cancer Genome Atlas (TCGA), was not possible. As would be expected, ependymomas had the lowest median and average number of genetic alterations suggesting that methylation studies might be more informative in these tumors. Nevertheless, performing small and specific panels is informative in the clinical space for identifying alterations that can be targeted using current treatment methods. Due to the retrospective nature of the study, genetic material for sequencing was taken from archived FFPE tissue, creating issues with quality that is better assessed through targeted methods. As a supplement to this panel, these tumors are being further investigated using DNA methylome profiling, expanded targeted sequencing through a pan-cancer panel, and FFPE specific RNA-sequencing to better understand the underlying molecular changes.
Eight (of the 16) patients in our GBM subset had IDH-mutant tumors, and 63% were MGMT promoter methylated. This demonstrates that there is a group of MGMT unmethylated, IDH-wildtype GBM patients who, despite conventional thinking, have long-term survival post-diagnosis. Interestingly, our cohort had a high percentage of tumors with mutations in TP53.21,42 Mutant TP53 is believed to increase MGMT expression, reducing the response to temozolomide and conferring a worse survival; therefore, the high percentage of TP53 mutations in our LTS cohort was unexpected.43 According to TCGA data, 90%, 86%, and 79% of primary GBM tumors sampled had a mutation in either the RTK/PI3K, TP53, or RB pathway, respectively, with most having at least one mutation in each. In our cohort, 81% showed an alteration in either the RTK/PI3K or TP53 pathways alone, no LTS showed an alteration with RB1 alone, and 38% had a mutation in all three pathways.
The Potential Existence of Distinct Symptom Cohorts
While almost half of brain tumor LTS reported no moderate-to-severe symptoms, the other approximately half reported having three or more, suggesting a dichotomy with distinct cohorts of patients that have either no significant symptoms or considerable symptom burden and interference. Moderate-to-severe fatigue, difficulty remembering, and drowsiness were commonly reported in our brain tumor LTS cohort, consistent with the debilitating symptoms primary brain tumor patients have reported in other studies.2 Similarly, moderate-to-severe extremity weakness, pain, fatigue, and numbness/tingling are significant symptoms that negatively impact spine tumor LTS. Additionally, interference was especially prevalent among spine tumor LTS, with 72% reporting moderate-to-severe activity-related interference, and 46% reporting moderate-to-severe mood-related interference. These results underscore the importance of better understanding symptoms and interference and initiating symptom management programs for those LTS who are highly symptomatic, including those with tumors involving the spine, those with poor KPS, and those experiencing symptoms precluding the ability to work.
Anxiety and Depression Among LTS
To our knowledge, this is the first study reporting anxiety and depressive symptoms in a large cohort of primary CNS tumor LTS. Nearly 1 in 5 patients reported moderate-to-severe anxiety symptoms, which was significantly associated with unemployment, poor KPS, female gender, and closer proximity to the time of diagnosis. Depressive symptoms were somewhat less common, with 13% of patients reporting moderate-to-severe symptoms, but similar significant associations related to unemployment status and poor KPS were found. The impact of these psychological symptoms on quality of life was reflected on the EQ-5D-3L; 45% of LTS reported moderate-to-extreme impairment related to anxiety/depression. Additionally, the most impacted symptom domain on the MDASI-BT was the affective domain, further supporting the saliency of psychological symptoms for LTS.
Although a paucity of psychological symptom data exists on the brain tumor LTS population, the prevalence of anxiety and depressive symptoms reported in this study is comparable to what has been reported in other solid tumor LTS populations.44,45 While most LTS in our cohort are not undergoing active treatment, they still live with the uncertainty of disease recurrence, which can create significant psychological distress, particularly at the time of diagnostic neuroimaging when “scanxiety” has been documented.46 Indeed, 10%-23% of LTS reported moderate-to-severe emotional distress, which may not reach the threshold of a clinical psychiatric disorder, but warrants attention from neuro-oncology providers.
Mental health concerns, including anxiety and depression, are commonly identified as an unmet need among survivors,47 but often oncology providers lack either the necessary skills to recognize psychological conditions and/or the time to spend with patients to address these issues.48 The etiology of psychological symptoms in patients with CNS tumors is multifactorial and varies across individuals, with key contributing factors including preexisting psychiatric disorders, adverse effects from tumor-directed treatments, and the ability to cope with a life-threatening disease.49 There is a need to better understand the prevalence and impact of psychological symptoms among primary CNS tumor LTS to allow for the development of targeted psycho-oncology therapies (eg, cognitive-behavioral therapy). Coping and relaxation interventions at the time of diagnostic neuroimaging and clinical evaluation could also potentially mitigate adverse psychological symptoms in primary CNS tumor LTS.
Cognitive Dysfunction Among LTS
Approximately 1 in 5 LTS reported experiencing cognitive dysfunction, with patients who either had a poor KPS, were unemployed, or had a history of radiation therapy reporting worse cognitive function. The detrimental impact of this cognitive dysfunction on quality of life is reflected in the inability to work outside the home and a reduced performance status. The prevalence of psychological symptoms described previously may also play a role in subjective cognitive complaints. Given that 56% of our LTS cohort received radiation, the high rate of reported cognitive dysfunction may also reflect a significant association with long-term sequelae of CNS radiation and higher symptom burden due to their tumor treatment. A previous study that analyzed neuroimaging data and neuropsychological assessments reported that the ApoE4 allele might play a role in the decline of cognitive function in brain tumor patients.50 In a randomized controlled trial of patients with solid tumors metastatic to the brain treated with cranial radiation, neuropsychological assessments indicated that those who received radiosurgery and whole-brain radiation therapy showed greater decline in executive function than patients who received only radiosurgery.51 Future research on cognitive functioning in this patient population should include other factors such as genomic predispositions, characterization of radiation therapy received, and the use of both self-report and objective measures. Furthermore, understanding the impact of cognitive symptom burden in primary CNS tumor LTS may allow for more targeted and personalized treatment methods.
General Health Status of LTS
KPS was the strongest predictor of limitations in general health status for LTS, displaying significant associations with each EQ-5D-3L dimension and highlighting the unique clinical care needs of those with poor KPS. Therefore, efforts should be made to maintain and potentially improve patient functioning. Providers should encourage LTS to continue practicing their daily activities and build new healthy routines. Providers may also refer such patients to physical, occupational, and speech therapies as warranted, and exercise interventions should be encouraged as they have been associated with improved outcomes on measures of fatigue, sleep, mood, cognitive symptoms, and health-related quality of life in stable glioma patients.52
The Role of Prehabilitation and Survivorship Care Plans (SCPs) for CNS Tumor LTS
Although maintaining a high functional status throughout an extensive treatment course is challenging, prehabilitation as commonly used before orthopedic and, increasingly, other procedures and in populations including those with other systemic cancers, has promise in caring for LTS. Cancer prehabilitation is designed to improve physical and mental health in newly diagnosed cancer patients and can help prevent or decrease the severity of anticipated treatment that potentially could lead to disability.53,54 In our analyses, tumor diagnosis and tumor location were predictors in all categories except anxiety and depression. Therefore, implementation of a prehabilitation course may stem limitations later.
An SCP may be considered as well. SCPs highlight the importance of the evolution from active treatment to the post-treatment phase and may help both LTS and their caregivers.55 The provision of survivorship care aims to optimize the quality of life for patients and their families across the illness trajectory.56 SCPs guide the requisite discussion between patients and their providers about needs across domains of symptom management, health promotion, psychosocial support, socioeconomic impacts of cancer and its treatment, surveillance planning for cancer recurrence, and detection and management of late consequences of cancer and its treatment, thereby creating individualized treatment plans, surveillance schedules, and referrals to services and resources, such as palliative and supportive care.57 The variability of symptom burden and interference self-reports across the LTS cohort reflect both the existence of differential illness trajectories and the necessity of individualized care.
Limitations
The present study has a number of limitations. First, our subset of spine tumor LTS was small (8%), which may have limited our ability to identify statistically significant relationships with demographic and clinical factors. Next, because this study utilized cross-sectional secondary data, patient psychological symptoms were analyzed at a single time-point and did not take into account changes in mood over time. These psychological symptoms are likely to have been affected by a wide variety of variables that are external to disease trajectory and thus should be interpreted cautiously in terms of causality. Following LTS longitudinally would allow for a more precise evaluation of mood fluctuations over time and would help determine factors that might contribute to periods of severe psychological distress. The same limitation of using cross-sectional data also applies to other outcomes, including patient-perceived cognitive functioning and general health status. Finally, this sample only reflects the experience of patients treated at a large quaternary cancer center, which may have resulted in a degree of sampling bias.
Conclusions
LTS are an important group of patients in any disease process. From a patient care perspective, LTS provide a challenge as they are at risk for late and often chronic complications of both their disease and associated treatment, paradoxically because of their lengthy survival. As clearly demonstrated from the PRO data in this study, most patients, across many different CNS tumor types and grades (both high- and low-grade), have been adversely impacted by their disease and/or treatment. Our pathologically heterogeneous patients cohort exemplifies that it is not only high-grade tumor patients who face tremendous symptom burden in the long term. Importantly, our results underscore the need to incorporate longitudinal analyses into clinical trials, particularly natural history studies that afford opportunities to study illness and its impact over the entire trajectory of the disease.
From a biologic perspective, LTS may offer clues regarding disease characteristics that portend a better outcome either with previously unrecognized positive prognostic factors or markers that indicate an unusually good and prolonged response to treatment. These factors or markers may help optimize treatments for future patients and provide clues of disease susceptibility that may help find better treatments for all patients with the disease. Our LTS cohort provides us an opportunity for in-depth analysis to try to uncover these critical molecular and other biologic prognostic markers. Unfortunately, our targeted gene panel was unable to identify such a marker associated with long-term survival. However, we did note one critical molecular finding; contrary to conventional clinical wisdom, it is not only IDH-mutant GBM patients that survive long-term, as our cohort also included IDH-wildtype GBM patients. Ultimately, LTS are a unique patient population, both clinically and biologically, that must be further investigated.
Funding
The Natural History Study project is supported by Intramural Project 1ZIABC011768-03 (T.S.A.).
Supplementary Material
Acknowledgments
Our research team would like to thank Mr Adam Hayden, a long-term survivor of a CNS tumor and a patient advocate, for providing his perspective on the topic of long-term survivorship.
Conflict of interest statement. All authors declare no conflicts of interest.
References
- 1. National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) Program. Bethesda, MD: DCCPS, National Cancer Institute; 2018. [Google Scholar]
- 2. Armstrong TS, Vera-Bolanos E, Acquaye AA, et al. . The symptom burden of primary brain tumors: evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol. 2016;18(2):252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aldape K, Brindle KM, Chesler L, et al. . Challenges to curing primary brain tumours. Nat Rev Clin Oncol. 2019;16(8):509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mucha-Małecka A, Gliński B, Hetnał M, et al. . Long-term follow-up in adult patients with low-grade glioma (WHO II) postoperatively irradiated. Analysis of prognostic factors. Rep Pract Oncol Radiother. 2012;17(3):141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schomas DA, Laack NN, Rao RD, et al. . Intracranial low-grade gliomas in adults: 30-year experience with long-term follow-up at Mayo Clinic. Neuro Oncol. 2009;11(4):437–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marko NF, Toms SA, Barnett GH, et al. . Genomic expression patterns distinguish long-term from short-term glioblastoma survivors: a preliminary feasibility study. Genomics. 2008;91(5):395–406. [DOI] [PubMed] [Google Scholar]
- 7. Reifenberger G, Weber RG, Riehmer V, et al. . Molecular characterization of long-term survivors of glioblastoma using genome-and transcriptome-wide profiling. Int J Cancer. 2014;135(8):1822–1831. [DOI] [PubMed] [Google Scholar]
- 8. Shinawi T, Hill VK, Krex D, et al. . DNA methylation profiles of long- and short-term glioblastoma survivors. Epigenetics. 2013;8(2):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barbus S, Tews B, Karra D, et al. . Differential retinoic acid signaling in tumors of long-and short-term glioblastoma survivors. J Natl Cancer Inst. 2011;103(7):598–601. [DOI] [PubMed] [Google Scholar]
- 10. Poon MT, Sudlow CL, Figueroa JD, et al. . Longer-term (≥2 years) survival in patients with glioblastoma in population-based studies pre- and post-2005: a systematic review and meta-analysis. Sci Rep. 2020;10(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim J-Y, Jackman JG, Woodring S, et al. . Second primary cancers in long-term survivors of glioblastoma. Neurooncol Pract. 2019;6(5):386–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnston SK, Whitmire P, Massey SC, et al. . ENvironmental dynamics underlying responsive extreme survivors (ENDURES) of glioblastoma: a multidisciplinary team-based, multifactorial analytical approach. Am J Clin Oncol. 2019;42(8):655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Geisenberger C, Mock A, Warta R, et al. . Molecular profiling of long-term survivors identifies a subgroup of glioblastoma characterized by chromosome 19/20 co-gain. Acta Neuropathol. 2015;130(3):419–434. [DOI] [PubMed] [Google Scholar]
- 14. Park C-K, Bae JM, Park S-H. Long-term survivors of glioblastoma are a unique group of patients lacking universal characteristic features. Neurooncol Adv. 2019;2(1):1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marton E, Giordan E, Siddi F, et al. . Over ten years overall survival in glioblastoma: a different disease? J Neurol Sci. 2020;408:116518. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong T. See brain cancer as more than just the sum of biology. Nature. 2018;561(7724):S45. [DOI] [PubMed] [Google Scholar]
- 17. Helfer JL, Wen PY, Blakeley J, et al. . Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials clinical outcome assessment endpoints workshop (October 15, 2014, Bethesda MD). Neuro Oncol. 2016;18(suppl_2):ii26–ii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lieberman AN, Foo SH, Ransohoff J, et al. . Long term survival among patients with malignant brain tumors. Neurosurgery. 1982;10(4):450–453. [DOI] [PubMed] [Google Scholar]
- 19. Donson AM, Birks DK, Schittone SA, et al. . Increased immune gene expression and immune cell infiltration in high-grade astrocytoma distinguish long-term from short-term survivors. J Immunol. 2012;189(4):1920–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Malay S, Somasundaram E, Patil N, et al. . Treatment and surgical factors associated with longer-term glioblastoma survival: a National Cancer Database study. Neurooncol Adv. 2020;2(Suppl 1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Burgenske DM, Yang J, Decker PA, et al. . Molecular profiling of long-term IDH-wildtype glioblastoma survivors. Neuro Oncol. 2019;21(11):1458–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ostrom QT, Gittleman H, Truitt G, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2011–2015. Neuro Oncol. 2018;20(suppl_4):iv1–iv86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Grossman SA, Ye X, Piantadosi S, et al. . Survival of patients with newly diagnosed glioblastoma treated with radiation and temozolomide in research studies in the United States. Clin Cancer Res. 2010;16(8):2443–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hou LC, Veeravagu A, Hsu AR, et al. . Recurrent glioblastoma multiforme: a review of natural history and management options. Neurosurg Focus. 2006;20(4):E5. [DOI] [PubMed] [Google Scholar]
- 25. Ostrom QT, Cioffi G, Gittleman H, et al. . CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2012–2016. Neuro Oncol. 2019;21(Supplement_5):v1–v100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerber NK, Goenka A, Turcan S, et al. . Transcriptional diversity of long-term glioblastoma survivors. Neuro Oncol. 2014;16(9):1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lovely MP, Stewart-Amidei C, Page M, et al. . A new reality: long-term survivorship with a malignant brain tumor. Oncol Nurs Forum. 2013;40(3):267–274. [DOI] [PubMed] [Google Scholar]
- 28. Brat DJ, Aldape K, Colman H, et al. . cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. 2018;136(5):805–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Brat DJ, Aldape K, Colman H, et al. . cIMPACT-NOW update 5: recommended grading criteria and terminologies for IDH-mutant astrocytomas. Acta Neuropathol. 2020;139(3):603–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Armstrong TS, Mendoza T, Gring I, et al. . Validation of the M.D. Anderson Symptom Inventory Brain Tumor Module (MDASI-BT). J Neuro Oncol. 2006;80(1):27–35. [DOI] [PubMed] [Google Scholar]
- 31. Armstrong TS, Gning I, Mendoza TR, et al. . Reliability and validity of the MD Anderson Symptom Inventory–spine tumor module. J Neurosurg Spine. 2010;12(4):421–430. [DOI] [PubMed] [Google Scholar]
- 32. Cleeland CS, Nakamura Y, Mendoza TR, et al. . Dimensions of the impact of cancer pain in a four country sample: new information from multidimensional scaling. Pain. 1996;67(2–3):267–273. [DOI] [PubMed] [Google Scholar]
- 33. Pilkonis PA, Choi SW, Reise SP, et al. . Item banks for measuring emotional distress from the Patient-Reported Outcomes Measurement Information System (PROMIS®): depression, anxiety, and anger. Assessment. 2011;18(3):263–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cella D, Lai JS, Nowinski CJ, et al. . Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. The EuroQol Group. EuroQoL – a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199–208. [DOI] [PubMed] [Google Scholar]
- 36. Serlin RC, Mendoza TR, Nakamura Y, et al. . When is cancer pain mild, moderate or severe? Grading pain severity by its interference with function. Pain. 1995;61(2):277–284. [DOI] [PubMed] [Google Scholar]
- 37. Mendoza TR, Wang XS, Cleeland CS, et al. . The rapid assessment of fatigue severity in cancer patients: use of the Brief Fatigue Inventory. Cancer. 1999;85(5):1186–1196. [DOI] [PubMed] [Google Scholar]
- 38. Shi Q, Mendoza TR, Dueck AC, et al. . Determination of mild, moderate, and severe pain interference in patients with cancer. Pain. 2017;158(6):1108–1112. [DOI] [PubMed] [Google Scholar]
- 39. Health Measures. https://www.healthmeasures.net/index.php. Accessed August 2019.
- 40. Shaw JW, Johnson JA, Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43(3):203–220. [DOI] [PubMed] [Google Scholar]
- 41. IBM Corp. Released. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 42. Louis DN, Perry A, Reifenberger G, et al. . The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820. [DOI] [PubMed] [Google Scholar]
- 43. Kim HS, Kwon MJ, Song JH, et al. . Clinical implications of TERT promoter mutation on IDH mutation and MGMT promoter methylation in diffuse gliomas. Pathol Res Pract. 2018;214(6):881–888. [DOI] [PubMed] [Google Scholar]
- 44. Acquaye A, Vera-Bolanos E, Armstrong T, et al. . Mood disturbance in glioma patients. J Neuro Oncol. 2013;113(3):505–512. [DOI] [PubMed] [Google Scholar]
- 45. Brandenbarg D, Maass SW, Geerse OP, et al. . A systematic review on the prevalence of symptoms of depression, anxiety and distress in long‐term cancer survivors: implications for primary care. Eur J Cancer Care. 2019;28(3):e13086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bauml JM, Troxel A, Epperson CN, et al. . Scan-associated distress in lung cancer: quantifying the impact of “scanxiety”. Lung Cancer. 2016;100:110–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yi JC, Syrjala KL. Anxiety and depression in cancer survivors. Med Clin North Am. 2017;101(6):1099–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Krebber AM, Buffart LM, Kleijn G, et al. . Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psycho Oncol. 2014;23(2):121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Richter A, Woernle CM, Krayenbühl N, et al. . Affective symptoms and white matter changes in brain tumor patients. World Neurosurg. 2015;84(4):927–932. [DOI] [PubMed] [Google Scholar]
- 50. Correa DD, Satagopan J, Baser RE, et al. . APOE polymorphisms and cognitive functions in patients with brain tumors. Neurology. 2014;83(4):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chang EL, Wefel JS, Hess KR, et al. . Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10(11):1037–1044. [DOI] [PubMed] [Google Scholar]
- 52. Gehring K, Stuiver MM, Visser E, et al. . A pilot randomized controlled trial of exercise to improve cognitive performance in patients with stable glioma: a proof of concept. Neuro Oncol. 2020;22(1):103–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silver JK. Cancer prehabilitation and its role in improving health outcomes and reducing health care costs. Semin Oncol Nurs. 2015;31(1):13–30. [DOI] [PubMed] [Google Scholar]
- 54. Treanor C, Kyaw T, Donnelly M. An international review and meta-analysis of prehabilitation compared to usual care for cancer patients. J Cancer Surviv. 2018;12(1):64–73. [DOI] [PubMed] [Google Scholar]
- 55. Leeper HE, Acquaye AA, Bell S, et al. . Survivorship care planning in neuro-oncology. Neuro Oncol Pract. 2018;5(1):3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Institute of Medicine, National Research Council. From Cancer Patient to Cancer Survivor: Lost in Transition. Washington, DC: The National Academies Press; 2006. [Google Scholar]
- 57. Philip J, Collins A, Brand C, et al. . A proposed framework of supportive and palliative care for people with high-grade glioma. Neuro Oncol. 2018;20(3):391–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.