Abstract
Background
The Response Assessment in Neuro-Oncology Patient-Reported Outcome (RANO-PRO) working group aims to provide guidance on the use of PROs in brain tumor patients. PRO measures should be of high quality, both in terms of relevance and other measurement properties. This systematic review aimed to identify PRO measures that have been used in brain tumor studies to date.
Methods
A systematic literature search for articles published up to June 25, 2020 was conducted in several electronic databases. Pre-specified inclusion criteria were used to identify studies using PRO measures assessing symptoms, (instrumental) activities of daily living [(I)ADL] or health-related quality of life (HRQoL) in adult patients with glioma, meningioma, primary central nervous system lymphoma, or brain metastasis.
Results
A total of 215 different PRO measures were identified in 571 published and 194 unpublished studies. The identified PRO measures include brain tumor-specific, cancer-specific, and generic instruments, as well as instruments designed for other indications or multi- or single-item study-specific questionnaires. The most frequently used instruments were the EORTC QLQ-C30 and QLQ-BN20 (n = 286 and n = 247), and the FACT-Br (n = 167), however, the majority of the instruments were used only once or twice (150/215).
Conclusion
Many different PRO measures assessing symptoms, (I)ADL or HRQoL have been used in brain tumor studies to date. Future research should clarify whether these instruments or their scales/items exhibit good content validity and other measurement properties for use in brain tumor patients.
Keywords: activities of daily living, brain tumor, health-related quality of life, patient-reported outcome, symptoms
Traditional outcomes to assess the impact of a treatment in adult brain tumor patients are progression-free or overall survival and radiological response on imaging. With an increasing focus on patient-centered care and the need for therapies to show improvement in symptom burden, patient function, and health-related quality of life (HRQoL), the emphasis on the use of patient-reported outcome (PRO) measures is growing.1–3 PRO measures reflect the patient’s health status based on the patient’s self-report, without amendment or interpretation by a clinician or anyone else.4,5 PRO measures are important to quantify the symptom burden, patient function, or overall HRQoL, but might also cover topics such as needs and coping.
Findings from PROs together with outcomes such as survival and tumor response are essential to inform the research community and policy makers on the net clinical benefit of a tumor-directed treatment, and to assist physicians and patients in clinical decision-making. It is therefore important that PRO measures are of high quality in terms of relevance (content validity) and other measurement properties, to create high-quality evidence.4 The content that is considered relevant depends on the setting and the research question. Regulators aim to accurately capture the patient perspective during clinical trials to inform the regulatory decision-making process.6 Concepts of interest to the regulators are disease symptoms, symptomatic adverse events, and physical function.3,7 When considering patient-centered assessments in evaluation of therapeutic trials, clinical investigators may be interested in concepts that are a surrogate to primary survival or response outcomes such as disease-related symptoms or functions that reflect therapeutic tolerability, but also aspects such as activities in daily life and participation in society, as described by the World Health Organization International Classification of Functioning, Disability and Health (WHO ICF).8 In the WHO ICF framework, disability and functioning are seen as an interaction between the disease and contextual factors, such as environmental factors (eg, social structures) and personal factors (eg, coping styles and character). Although it is understandable from the regulator’s point of view to only assess those outcomes that directly reflect a treatment effect, for researchers and clinicians it may be important to capture information on the entire spectrum of the patients’ functioning and well-being, including contextual factors. This may be particularly useful in clinical practice, where the obtained information could be tailored to the specific needs of the individual patient. This is also emphasized by results of the Jumpstarting Brain Tumor Drug Development (JSBTDD) Coalition survey in glioma patients, showing that patients particularly would like treatments to result in maintenance of physical functioning (including walking), cognitive abilities, as well as reduction or relief of symptoms including pain, weakness, seizures, aphasia, alterations and mood, and perceived cognitive symptoms.9
As advocated by the FDA, developing appropriate instruments to measure clinical outcome assessments (COAs) in neuro-oncology, including PROs, should include identifying areas requiring new tools and reviewing existing tools that may be suitable or adapted for use in clinical trials.7 The JSBTDD initiative identified priority signs and symptoms, reviewed properties of existing COAs and considerations for trial design including COAs, and serves as a starting point for further guidelines on outcome assessment in neuro-oncology.9 PRO measures being used in brain tumor studies may not have been developed and/or validated for use in this condition, and with the development of new treatment strategies associated with different toxicity profiles, currently available PRO measures may also no longer be sufficiently comprehensive to address toxicities associated with new therapeutic approaches. In addition, it is also important that the instruments exhibit good other measurement properties, such as sensitivity to known group comparisons, reliability, and responsiveness.
The Response Assessment in Neuro-Oncology Patient-Reported Outcome (RANO-PRO) initiative builds on the JSBTDD initiative and aims to provide guidance on the use of PRO measures, with respect to their measurement properties and content, in research studies and daily practice for adult brain tumor patients.10 In addition, suggestions for the revision of existing PRO measures and the development of new PRO measures or measurement strategies for use in neuro-oncology will be provided if warranted. For this multistep process, PRO measures that have been used in studies of brain tumor patients first need to be identified and subsequently reviewed for content validity and other psychometric properties. This systematic review aimed to identify studies in which a PRO measure was used to assess symptoms and signs, (instrumental) activities of daily living [(I)ADL] or HRQoL in patients with glioma, meningioma, primary central nervous system lymphoma (PCNSL), or brain metastases. The results serve as a foundation for further evaluation.
Methods
Search Strategy
An extensive literature search for articles published up to June 25, 2020 (there was no specific start date) was conducted in the following electronic databases: PubMed/Medline, Embase, Web of Science, Cochrane Library, PsycINFO, Emcare, and Academic Search Premier. The search string consisted of a combination of terms related to “PRO measures” and “brain tumors” (see Supplementary File 1 for the complete search string in PubMed). The search string was adapted for the other electronic databases. In addition, clinicaltrials.gov was consulted for additional eligible studies initiated in the last decade but may not yet have been published; ie, with a study start between January 1, 2010 and June 25, 2020 (see Supplementary File 2 for the complete search string).
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed for screening procedures.11
Selection Criteria
All retrieved titles and abstracts were independently screened by two reviewers (M.E.V. and L.D.). Articles had to report the use of a PRO measure to assess aspect(s) of functioning, including symptoms and signs, (I)ADL or HRQoL in patients with glioma, meningioma, PCNSL, or brain metastases. All study designs (eg, randomized controlled trials and observational cohort studies) were deemed eligible. To align with the work of the RANO-PRO, (1) ≥75% of the study population had to concern patients with glioma, meningioma, PCNSL, and/or metastatic brain tumor. Other inclusion criteria were (2) at least one PRO measure is used and specified, (3) the PRO measure used addresses symptoms and signs, (I)ADL or HRQoL, (4) adult patients (≥18 years), (5) a sample of ≥10 patients, (6) original articles (eg, no systematic review articles, conference papers, or study protocols), (7) articles in English, (8) full-text availability, and (9) peer-reviewed articles. The first five inclusion criteria were also used for the assessment of additional eligible studies on clinicaltrials.gov. After screening titles and abstracts, full-texts of potentially relevant articles were examined for eligibility by the same two reviewers, applying the same criteria. Disagreements between the reviewers were discussed and resolved by consensus.
Data Extraction
The following data were extracted from the eligible articles and studies on clinicaltrials.gov to gain insight into the type of brain tumor studies that used PRO measures: tumor type, (estimated) sample size, study design, study ID number (if available), PRO measure used and whether the PRO measure was used as primary or secondary endpoint, and for studies on clinicaltrials.gov only the study status. The actual outcomes (eg, number and severity of specific symptoms) as assessed with the PRO measures were not reported, as this review was focused on identifying the instruments only. In addition, information about the PRO measures was retrieved from the articles, cited publications, or web resources to gain insight into the intended use and structure of the identified PRO measures, including the number of items, population of intended use, and subscales (reflecting the concepts of interest). Information about the population of intended use was based on our interpretation if official information was not available. Identified PRO measures were excluded from further analyses if they (1) did not assess symptoms and signs, (I)ADL or HRQoL (eg, coping styles or satisfaction with care), (2) were not intended to be used as PRO measure (eg, Lawton Instrumental Activities of Daily Living Scale or Hamilton Depression Rating Scale, which are completed by observers such as proxies), (3) were not available in English, or (4) could not be retrieved or otherwise judged for eligibility. If at least one subscale in a PRO measure was relevant, the PRO measure was considered eligible. Some PRO measures have been revised over the years, and therefore, different versions were identified. These versions may have (slightly) different items or response scales. For this review, the different revised questionnaire versions were not considered as separate instruments. The original versions and the short form versions of questionnaires were reported as separate instruments (eg, Medical Outcomes Study 36-item Short Form Health Survey [SF-36] and Medical Outcomes Study 12-item Short Form Health Survey [SF-12]). Data extraction was performed by one researcher (M.E.V.), however, ambiguities were discussed and agreed upon with another researcher (L.D.).
Results
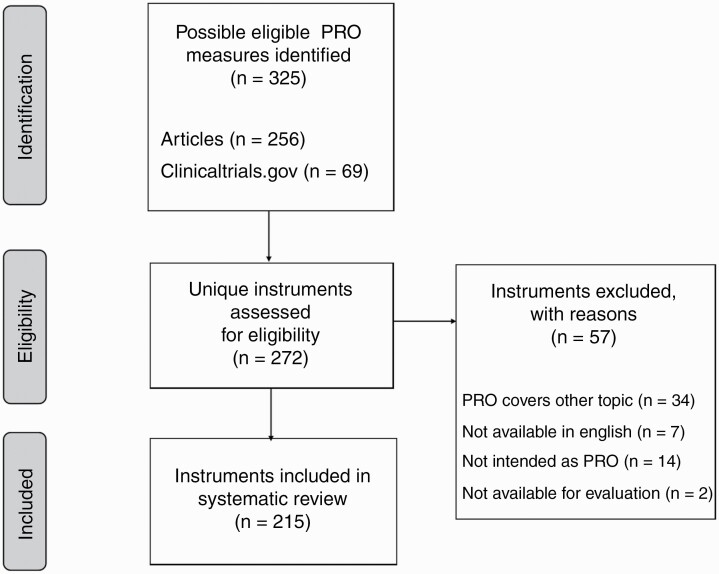
The literature search yielded 5715 unique records. Screening of titles and abstracts resulted in 997 potentially eligible articles. These articles were read full-text, and 621 met the inclusion criteria. Based on the study number and names reported in the 621 articles, 571 different studies were identified. The search on clinicaltrials.gov yielded 543 unique studies, which were screened for eligibility. A total of 210 studies met the inclusion criteria, of which 16 were already reported in one of the identified articles, resulting in 194 unpublished studies. In total, 765 unique published and unpublished studies were identified that used a PRO measure to report on symptoms and signs, (I)ADL and/or HRQoL in brain tumor patients. The flow diagram of the selection processes is shown in Supplementary Figure 1. The reference list of all articles and NCT (National Clinical Trial) numbers (ClinicalTrials.gov registry number) are shown in Supplementary Files 3 and 4.
Main Characteristics of Included Studies
The main characteristics of the included published (n = 571) and unpublished (n = 194) studies are presented in Table 1. The majority of the published (n = 401, 70%) and unpublished (n = 106, 55%) studies included glioma patients. Populations of mixed brain tumor types were included in 178 of the published studies (31%) and 10 of the unpublished studies (5%). The median sample size of brain tumor patients was 63 (IQR = 33-116) in the published studies and 60 (IQR = 37-136) in the unpublished studies.
Table 1.
Main Characteristics of the Unique Published (Described in Articles) and Unpublished (Registered on clinicaltrials.gov) Studies
| Characteristics | Published Studies (n = 571) | Unpublished Studies (n = 194) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Year of publication | ||||
| 1990-2000 | 35 | 6 | - | |
| 2001-2010 | 125 | 22 | - | |
| 2011-2020 | 411 | 72 | - | |
| Type of brain tumor (multiple tumor types possible) | ||||
| Glioma | 401 | 70 | 106 | 55 |
| Meningioma | 116 | 20 | 7 | 4 |
| PCNSL | 31 | 5 | 6 | 3 |
| Brain metastases | 152 | 27 | 78 | 40 |
| Unspecified/other brain tumors | 91 | 16 | 3 | 2 |
| Study design | ||||
| Interventional | ||||
| RCT | 106 | 19 | 78 | 40 |
| Non-RCT | 13 | 2 | 20 | 10 |
| Single arm | 92 | 16 | 81 | 42 |
| Observational | ||||
| Cross-sectional | 141 | 25 | 0 | 0 |
| Cohort | 192 | 34 | 15 | 8 |
| Case-control | 20 | 3 | 0 | 0 |
| Other | 7 | 1 | 0 | 0 |
| Endpoint of PRO measure | ||||
| Primary | 182 | 32 | 6 | 3 |
| Secondary | 257 | 45 | 173 | 89 |
| Co-primary | 107 | 19 | 6 | 3 |
| Primary and secondary | 25 | 4 | 9 | 5 |
| Follow-up of PRO measure | ||||
| Cross-sectional | 208 | 36 | 2 | 1 |
| Longitudinal | 363 | 64 | 192 | 99 |
| Study status | ||||
| Recruiting | - | 114 | 59 | |
| Not yet recruiting | - | 11 | 6 | |
| Active, not recruiting | - | 30 | 15 | |
| Completed | - | 28 | 14 | |
| Enrolling by invitation | - | 0 | 0 | |
| Suspended | - | 1 | 1 | |
| Terminated | - | 10 | 5 | |
PCNSL, primary central nervous system lymphoma; PRO, patient-reported outcome; RCT, randomized controlled trial.
The majority of the published studies were observational studies (n = 353, 62%) and included PRO measures most frequently as primary or co-primary outcome measures (n = 314). On the other hand, the unpublished studies were mostly interventional studies (n = 179, 92%) and included PRO measures almost always as secondary outcome measure (n = 173).
Identified PRO Measures
In total, 215 unique PRO measures that met our inclusion criteria were identified (Figure 1). These were both multi-item and single-item questionnaires. Information about the top 20 instruments (those that were used in at least 10 studies), including the number of items, population of intended use, and subscales are displayed in Table 2 (see Supplementary Table 1 for an overview of all PRO measures). The population of intended use was classified as brain tumor-specific, cancer-specific, central nervous system (CNS) disorders, and generic or for other indications. The concepts of interest were mixed in the studies, focusing on one aspect only (eg, symptom(s)) or covering multiple aspects (eg, symptoms and (I)ADL).
Figure 1.
Flow diagram (PRISMA) of the selection process of PRO measures. Abbreviations: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PRO, patient-reported outcome.
Table 2.
Characteristics of the 20 Most Frequently Identified Eligible PRO Measures
| Abbreviation | Full Name | # Used in Studies Published | # Used in Unpublished Studies | Population of Intended Use | # of Items | Subscales | Study Type in Which Used |
|---|---|---|---|---|---|---|---|
| EORTC QLQ-C30(+3) | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30(+3) | 186 | 100 | Cancer-specific | 30(+3) | 5 functional scales (physical; role; emotional; cognitive; social); 3 symptom scales (fatigue; nausea and vomiting; pain); 7 single items (dyspnea; insomnia; appetite loss, constipation; diarrhea; financial difficulties) and GHS/QoL scale | Interventional; observational |
| EORTC QLQ-BN20(+2)a | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Brain Cancer 20(+2) | 164 | 83 | Brain tumor-specific | 20(+2) | 4 (future uncertainty; visual disorder; motor dysfunction; communication deficit); 7 single items (headache; seizures; drowsiness; hair loss; itchy skin; weakness of legs; bladder control) | Interventional; observational |
| FACT-Br | Functional Assessment of Cancer Therapy-Brain | 121 | 46 | Brain tumor-specific | 50 | 5 (physical-; social/family-; emotional-; functional well-being; disease-specific concerns) | Interventional; observational |
| HADS | The Hospital Anxiety and Depression Scale | 62 | 8 | Generic | 14 | 2 (anxiety symptoms; depression symptoms) | Interventional; observational |
| SF-36 | Short Form 36 Health Survey/RAND-36/Medical Outcomes Study | 47 | 5 | Generic | 36 | 8 (physical functioning; physical role; pain; general health; vitality; social function; emotional role; mental health) | Interventional; observational |
| MDASI-BT | MD Anderson Symptom Inventory Brain Tumor | 25 | 24 | Brain tumor-specific | 28 | 6 factors (affective, cognitive, neurologic, constitutional, gastrointestinal, and interference) | Interventional; observational |
| BDI(-II) | Beck Depression Inventory (-II) | 38 | 9 | Generic | 21 | BDI: 3 (negative attitude toward self; performance impairment; somatic symptoms); BDI-II: 2 (affective; somatic) | Interventional; observational |
| EQ-5D | EuroQoL 5 dimensions (3 or 5 levels) | 36 | 24 | Generic | 6 | 5 (mobility; self-care; usual activities; pain/discomfort; anxiety/depression) | Interventional; observational |
| DT(+PL) | NCCN Distress Thermometer (and Problem List) | 28 | 2 | Cancer-specific | 1(+39) | 6 (overall distress; spiritual/religious concerns; practical-; family-; emotional-; physical problems) | Interventional; observational |
| FACIT-F | Functional Assessment of Chronic Illness Therapy-Fatigue | 19 | 5 | Other indications | 13 | 1 (disease-specific concerns) | Interventional; observational |
| STAI-Y | State-Trait Anxiety Inventory Form Y | 19 | 1 | Generic | 40 | 2 (trait anxiety; state anxiety) | Interventional; observational |
| EORTC QLQ-C15-PAL | European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Core 15 - Palliative Care | 15 | 5 | Cancer-specific | 15 | 10 (GHS; physical functioning; emotional functioning; fatigue; nausea/vomiting; pain; dyspnea; insomnia; appetite loss and constipation) | Interventional; observational |
| MOS CFS | Medical Outcomes Study Cognitive Functioning Scale | 15 | Generic | 6 | 1 (cognitive function) | Interventional; observational | |
| BFI | Brief Fatigue Inventory | 7 | 6 | Cancer-specific | 9 | 2 (severity of fatigue; impact on daily function) | Interventional; observational |
| FACT-cog | Functional Assessment of Cancer Therapy-Cognitive Function | 3 | 9 | Cancer-specific | 37 | 4 (perceived cognitive impairments; comments from others; perceived cognitive abilities; impact on QoL) | Interventional; observational |
| FACT-G | Functional Assessment of Cancer Therapy-General | 9 | 2 | Cancer-specific | 27 | 4 (physical-; social/family-; emotional-; functional well-being) | Interventional; observational |
| ZSDS | Zung Self-Rating Depression Scale | 10 | 1 | Other indications | 20 | 1 (depressive symptoms) | Interventional; observational |
| BAI | Beck Anxiety Inventory | 6 | 4 | Generic | 21 | 1 (anxiety symptoms) | Interventional; observational |
| QOLIE-31-P | Patient Weighted Quality of Life in Epilepsy | 10 | Other indications | 39 | 7 (emotional well-being; social function; energy/fatigue; cognitive functioning; seizure worry; medication effects; overall QoL) and health status | Interventional; observational | |
| SQLI | Spitzer Quality of Life Index | 10 | Cancer-specific | 5 | 5 (activity; daily living; health; support; outlook) | Interventional; observational |
aOut of the 247 times, the EORTC QLQ-BN20 was used in published and unpublished studies, 216 times (87%) this was in conjunction with the EORTC QLQ-C30.
The 215 identified PRO measures represent 40 (19%) brain tumor-specific instruments, 37 (17%) cancer-specific instruments, 13 (6%) CNS disorder instruments, 79 (37%) generic instruments, and 46 (21%) instruments intended for other indications.
Frequency of Use of PRO Measures
Although many different PRO measures were identified, only a few have been frequently used in the identified brain tumor studies. In both the published and unpublished studies, a mean of two PRO measures assessing symptoms and signs, (I)ADL and/or HRQoL were included. Of the 215 identified PRO measures, 20 instruments (9%) were used in 10 studies or more. The top 10 most frequently used instruments were used in 24-286 studies, and include brain tumor-specific instruments (n = 3) as well as cancer-specific instruments (n = 2), generic instruments (n = 4), and instruments for other indications (n = 1).
The most frequently used instrument is the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Core 30 (EORTC QLQ-C30) in n = 286 studies, often combined with the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire - Brain Cancer 20 (EORTC QLQ-BN20) module (used in n = 247 studies), but also used as standalone questionnaires. Other PRO measures in the top 10 of frequently used instruments measuring HRQoL are the brain tumor-specific Functional Assessment of Cancer Therapy-Brain (FACT-Br; n = 167), the generic EuroQoL five-dimensional instruments (EQ-5D; n = 60), the generic Medical Outcomes Study SF-36 (n = 52), the cancer-specific NCCN (National Comprehensive Cancer Network) distress thermometer and problem list (DT + PL; n = 30), and the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F; n = 24) questionnaire. Further, the MD Anderson Symptom Inventory-Brain Tumor (MDASI-BT) was frequently used to assess symptom burden and (I)ADL specific to brain tumor patients (n = 49). Lastly, the generic Hospital Anxiety and Depression Scale (HADS; n = 70) was frequently used to assess symptoms of anxiety and depression, and the generic Beck Depression Inventory (BDI/BDI-II; n = 47) to assess symptoms of depression only.
An important finding was that the majority of the identified PRO measures were only used once (121/215; 56%) or twice (29/215; 13%). The large majority (84%) of these instruments was used in observational studies and may reflect the evaluation of constructs in this population using multi- or single-item scales previously developed for other populations related to universal nature of the study outcome (ie, fatigue or depression), psychometric evaluation of these scales, or scale development.
Use of PRO Measures per Tumor Type and Study Type
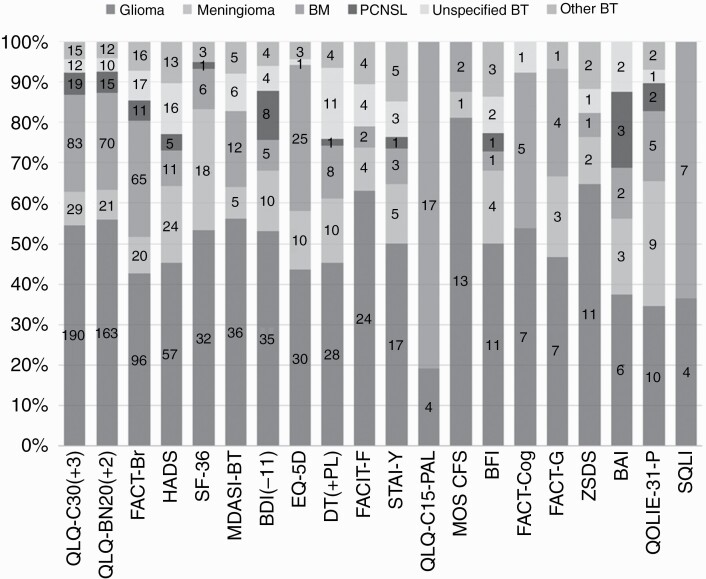
To assess how often the identified PRO measures were used in the different tumor types, we evaluated the 20 most frequently used PRO measures. Figure 2 shows that the majority of instruments were used in all brain tumor types.
Figure 2.
Percentage and number of times the 20 most frequently used PRO measures have been used in different types of brain tumor. Abbreviation: BM, brain metastases; BT, brain tumor; PCNSL, primary central nervous system lymphoma; PRO, patient-reported outcome.
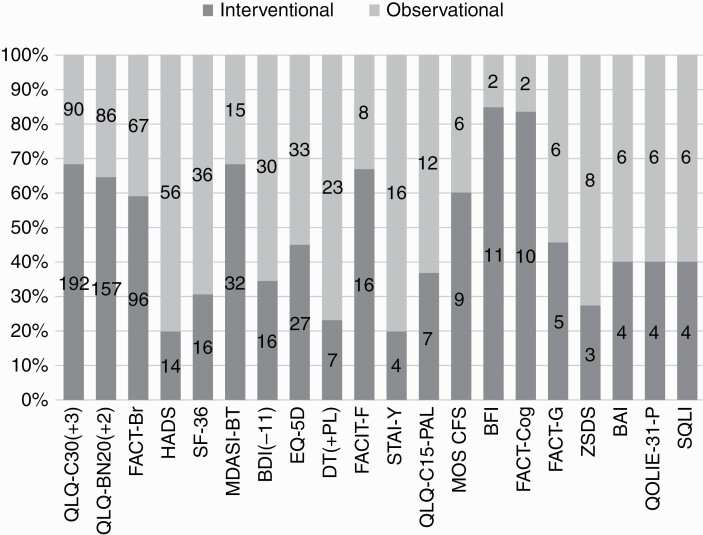
In addition, Figure 3 shows that the type of instrument is somewhat related to the study design. The most commonly used brain tumor-specific instruments (EORTC QLQ-BN20, FACT-Br, and MDASI-BT) were most often used in interventional studies, while generic PRO measures such as HADS, SF-36, EQ-5D, and BDI were most often used in observational studies.
Figure 3.
Percentage and number of times the 20 most frequently used PRO measures have been used in observational and interventional studies. Abbreviation: PRO, patient-reported outcome.
Discussion
In this systematic review, we have identified 215 different multi-item or single-item PRO measures that have been used in brain tumor studies to assess symptoms and signs, (I)ADL or HRQoL. The majority of the instruments (70%) were only used in one or two studies. The EORTC QLQ-C30, often used in conjunction with the EORTC QLQ-BN20, and FACT-Br were the most commonly used instruments in both interventional and observational studies. Of note, in European clinical trials, the EORTC instruments are more commonly used, whereas the FACT instruments are more often used in the United States. The identified PRO measures not only include instruments specifically designed for brain tumor patients, but also cancer-specific instruments, generic instruments, and instruments designed for other indications.
Although certain instruments were often used in brain tumor studies, they are not all designed and/or validated for use in (specific types of) brain tumor patients. For example, the EORTC QLQ-BN20 was developed for and validated in glioma patients, but is also frequently used in other types of brain tumor patients. Whether this questionnaire exhibits good content validity for these other types of patients remains to be investigated. A small study in WHO grade I meningioma did show that patients considered only 35% of the items of the EORTC QLQ-BN20 as relevant, while health care professionals considered all items as relevant.12 In addition, many cancer-specific instruments and generic instruments have not been validated in brain tumor patients, nor have instruments designed for other indications. Also, it may be possible that the questionnaires that have been used only once or twice are actually very relevant for brain tumor patients. Therefore, the content validity of the identified PRO measures, regardless of frequency of use in the various brain tumor populations, warrants clarification and will be further investigated as part of the RANO-PRO working plan. To this end, the content validity of scales/items in the available PRO measures used in brain tumor studies will be established by means of an international survey among patients, their caregivers, and health care professionals in the field of neuro-oncology. Participants will be asked to identify those aspects that are most relevant to brain tumor patients. Besides good content validity, future research should also clarify if the identified PRO measures, or separate multi- or single-item scales, exhibit other good measurement properties, such as reliability and responsiveness. These steps will identify those PRO measures or scales that measure relevant aspects, but still need to be optimized in terms of other measurement properties.
Given new developments in the treatment for brain tumor patients, with distinct toxicity profiles, and the changing needs of patients during the disease course, a new approach of measuring PROs is required. The current static questionnaires, consisting of a fixed set of items, may not meet the current demands of the regulators and academic researchers. A more flexible approach, in which a standard set of items could be complemented with validated scales, could be a solution. This means that only those scale(s) should be selected that are relevant to a specific setting or research objective, which may possibly also reduce the response burden for patients as only those items are administered that are relevant for that situation. Item libraries, which are large databases with multi-item and single-item scales, could be used to create short item lists that can be added to a core set of questions.13–15 This allows to measure adverse effects that are specific for a certain treatment, which may have been missed when using static questionnaires in which these are not covered. A difficulty with this approach is that many different (but overlapping) questionnaires will arise, hampering comparability between studies. The Fast Track COA Group, including representatives of RANO, the RANO-PRO working group, regulators, and patient representatives, recently established a core set of symptom and functional constructs that should at least be included in all clinical trials for high-grade glioma patients, enhancing comparability.16 These symptoms include pain, difficulty communicating, perceived cognition, seizures, and symptomatic adverse events (ie, depending on the treatment under investigation), and the functions include physical functioning and role and social functioning. The next step is to select items/scales from existing instruments that are suitable to assess these concepts, particularly in terms of content validity.
This systematic review has some limitations. Although a comprehensive search strategy was used, it is possible that relevant articles and PRO measures have been missed due to the search strategy and selection criteria that were applied. For example, if it was unclear which tumor types were evaluated, the study was excluded from further analyses. Also, it may be possible that there are existing questionnaires that are relevant for brain tumor patients, which have not been identified in this review as we only focused on studies in brain tumor patients. Another limitation is that there may be articles included describing the same study or dataset. Numbers or names for studies or datasets are not always reported in articles, which makes it difficult, if not impossible, to trace the use of the same study or dataset. Furthermore, the classification of PRO measures in terms of population of intended use (brain tumor-specific, cancer-specific, CNS disorders, and generic or other indications) was suboptimal, as the classification process is partly based on the judgment of the reviewers, as not all information could be extracted from the literature. Lastly, although we evaluated in which tumor types the instruments were used, based on the currently available data we cannot recommend which instrument would be most suitable for each tumor type. This also applied for evaluating specific treatments. First, more information on the content validity and other psychometric properties is warranted.
In conclusion, 215 multi and single-item PRO measures have been used in adult brain tumor studies to assess symptoms and signs, (I)ADL and HRQoL. The majority of these instruments are only used in one or two studies, while few are frequently being used. Future research should clarify whether the identified instruments or scales exhibit good content validity and other measurement properties for use in brain tumor patients.
Supplementary Material
Acknowledgments
J.W. Schoones from the Leiden University Medical Center in the Netherlands helped with the search strategy.
Funding
This work was supported by research grant GN-000616 from The Brain Tumour Charity, Fleet, UK.
Conflict of interest statement. E.L.R. has received honoraria for lectures or advisory board participation from Tocagen, AbbVie, and Daiichi Sankyo. M.W. has received research grants from AbbVie, Adastra, Merck, Sharp & Dohme (MSD), Merck (EMD), and Novocure, and honoraria for lectures or advisory board participation or consulting from AbbVie, Basilea, Bristol Myers Squibb (BMS), Celgene, Medac, Merck, Sharp & Dohme (MSD), Merck (EMD), Nerviano Medical Sciences, Orbus, Philogen, Roche, and Tocagen. P.D.B. received personal fees from UpToDate outside the submitted work. All other authors declare no conflict of interest.
References
- 1. Basch E. Patient-reported outcomes: an essential component of oncology drug development and regulatory review. Lancet Oncol. 2018;19(5):595–597. [DOI] [PubMed] [Google Scholar]
- 2. Dirven L, Armstrong TS, Taphoorn MJ. Health-related quality of life and other clinical outcome assessments in brain tumor patients: challenges in the design, conduct and interpretation of clinical trials. Neurooncol Pract. 2015;2(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kluetz PG, Slagle A, Papadopoulos EJ, et al. Focusing on core patient-reported outcomes in cancer clinical trials: symptomatic adverse events, physical function, and disease-related symptoms. Clin Cancer Res. 2016;22(7):1553–1558. [DOI] [PubMed] [Google Scholar]
- 4. European Medicines Agency. Appendix 2 to the guideline on the evaluation of anticancer medicinal products in man: the use of patient-reported outcome (PRO) measures in oncology studies.2016. http://www.ema.europa.eu/docs/en_GB/document_library/Other/2016/04/WC500205159.pdf. Accessed October 29, 2020.
- 5. US Food and Drug Administration. Patient-reported outcome measures: use in medical product development to support labeling claims.2009. https://www.fda.gov/media/77832/download. Accessed October 29, 2020.
- 6. Kluetz PG, O’Connor DJ, Soltys K. Incorporating the patient experience into regulatory decision making in the USA, Europe, and Canada. Lancet Oncol. 2018;19(5):e267–e274. [DOI] [PubMed] [Google Scholar]
- 7. Sul J, Kluetz PG, Papadopoulos EJ, et al. Clinical outcome assessments in neuro-oncology: a regulatory perspective. Neurooncol Pract. 2016;3(1):4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. World Health Organization. The International Classification of Functioning, Disability and Health.https://www.who.int/classifications/icf/en/. Accessed October 29, 2020.
- 9. Helfer JL, Wen PY, Blakeley J, Gilbert MR, Armstrong TS. Report of the Jumpstarting Brain Tumor Drug Development Coalition and FDA clinical trials clinical outcome assessment endpoints workshop (October 15, 2014, Bethesda MD). Neuro Oncol. 2016;18(Suppl 2):ii26–ii36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Dirven L, Armstrong TS, Blakeley JO, et al. Working plan for the use of patient-reported outcome measures in adults with brain tumours: a Response Assessment in Neuro-Oncology (RANO) initiative. Lancet Oncol. 2018;19(3):e173–e180. [DOI] [PubMed] [Google Scholar]
- 11. Moher D, Liberati A, Tetzlaff J, et al. ; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zamanipoor Najafabadi AH, Peeters MCM, Lobatto DJ, et al. Health-related quality of life of cranial WHO grade I meningioma patients: are current questionnaires relevant? Acta Neurochir (Wien). 2017;159(11):2149–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. MD Anderson. MDASI Symptom Library.https://www.mdanderson.org/content/dam/mdanderson/documents/Departments-and-Divisions/Symptom-Research/MDASI%20symptom%20library.pdf. Accessed October 29, 2020.
- 14. European Organisation for Research and Treatment of Cancer. Item Library.https://qol.eortc.org/item-library/. Accessed October 29, 2020.
- 15. National Cancer Institute. PRO-CTCAE Item Library.https://healthcaredelivery.cancer.gov/pro-ctcae/instrument.html. Accessed October 29, 2020.
- 16. Armstrong TS, Dirven L, Arons D, et al. Glioma patient-reported outcome assessment in clinical care and research: a response assessment in neuro-oncology collaborative report. Lancet Oncol. 2020;21(2):e97–e103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.