Abstract
Background
Large vestibular schwannomas (VS) pose a treatment challenge for both microsurgery (MS) and stereotactic radiosurgery (SRS). Technical developments have allowed for safer irradiation of large tumors. It remains unclear if SRS can achieve appropriate tumor control and acceptable cranial nerve toxicities. In this study, we assess outcomes of irradiation for large VS.
Methods
PubMed MEDLINE, EMBASE, Web of Science, and Cochrane were searched for all the studies assessing SRS outcome in large VS. Primary endpoints included clinical and radiographic tumor control, need for salvage surgery, serviceable hearing, cranial nerve V and VII impairment, presence of hydrocephalus requiring shunting, and presence of vertigo/dizziness.
Results
Twenty-two studies were identified that met selection criteria for analysis from an initial pool of 1272 reports. They were evaluated according to treatment protocol: 1) single-dose SRS (13 studies, 483 patients), 2) combination of MS and SRS (7 studies, 182 patients), and 3) fractionated SRS (3 studies, 82 patients). Tumor control was achieved in 89%, 94%, and 91% of patients, respectively. Odds ratios (ORs) of post- over pretreatment serviceable hearing were 0.42 (P < .01), 0.47 (P = .05), and 0.60 (P = .22); for facial nerve impairment, these ORs were 1.08 (P = .69), 3.45 (P = .28), and 0.87 (P = .71), respectively.
Conclusions
The management of large VS remains challenging. All treatment modalities resulted in high tumor control rates and worsening of pretreatment hearing. None, however, caused significant facial nerve impairment, suggesting that management strategies incorporating focal irradiation can be successful.
Keywords: acoustic neuroma, Koos IV, large vestibular schwannoma, radiosurgery, stereotactic radiosurgery
Vestibular schwannomas (VS) are benign tumors arising from the eighth cranial nerve (CN VIII).1 They represent the most common tumor of the cerebellopontine angle (CPA).2 Patients present with decreased hearing, tinnitus, and vestibular symptoms.3 Tumor Koos grade is key in predicting symptomatology, as grade III (tumor in the CPA without cerebellopontine trunk displacement) and grade IV (cerebellopontine trunk displacement) tend to cause hydrocephalus and symptoms of brainstem compression and vasogenic edema.4 Large tumors can also cause deficits of other CNs including facial numbness, weakness, and swallowing difficulties.5
Concerns about iatrogenic morbidity are heightened in the case of large VS. Classic treatments that work well for smaller tumors are more challenging, and with higher morbidities. Both microsurgery (MS) and radiation therapy (XRT) are able to reduce tumor burden; MS can be curative in cases of complete resection.6,7 Surgical complication rates are, however, higher for larger tumors.8,9 XRT can be given either as a single high dose in the form of stereotactic radiosurgery (SRS) or under fractionation regimens, either via fractionated SRS (fSRS), for example, 6–7 Gy × 3, or via fractionated stereotactic radiotherapy (fSRT), for example, 25 fractions of 2 Gy each.10 Neurosurgical complications are most commonly acute, while radiation complications may not be evident until years later.11
To maintain excellent tumor control and reduce CN impairment, some authors have advocated for a combination of subtotal resection and SRS, wherein the surgery is performed primarily to reduce the tumor size to a safe SRS target. Such a “nerve-centered” approach has reported excellent outcomes, with 93% tumor control and preservation of facial nerve function in 96% of patients, as found by a recent meta-analysis.12–14
An important question is whether large VS can successfully be treated with SRS as a single therapy. Contemporary radiosurgery platforms utilizes sophisticated planning software and high-resolution stereotactic MRI and CT which may facilitate safe and effective treatment of tumors with a diameter greater than 30 mm—historically considered the highest suitable dimension for SRS, given the risk of postradiation edema requiring surgical decompression.8,15 Recent studies have, in fact, shown promise of this approach, with high tumor control rates and acceptable comorbidities.16–18 The exact likelihood of tumor control and rate of CN toxicities remains unclear, given the lack of randomized controlled trials and significant variability in radiation regimens.11,16,19–22
In this study, we performed a systematic literature review to assess studies that utilize SRS on large VS. Given the high variability in the literature in defining “large” tumors, we included all studies where the authors claimed to be treating “large tumors,” providing their cutoff measures. We focus our attention on the radiation parameters, tumor control, the need for other interventions, and CN toxicities. By performing a classical meta-analysis, we endeavored to characterize clinically relevant outcomes for differing radiotherapy regimens on large VS.
Methods
Research Protocol and Search Question
Systematic literature searches were conducted (March 30, 2020) in 4 databases for any publication types and reports of human studies written in English, with no filters on publication date or other search limits applied. The databases searched were: 1) MEDLINE (via PubMed), 2) Embase (via OVID), 3) The Cochrane Library (via Wiley), and 4) Web of Science (via Clarivate Analytics). Detailed key words are reported in the Supplementary Material. Search results were combined in a bibliographic management tool (EndNote) and duplicates were removed both electronically and through manual review. Search results were then imported into the systematic review support tool, Covidence, for further management and review which included title/abstract screening and full-text screening phases. A detailed search strategy is provided in the Supplementary Material.
In accordance with current guidelines, this meta-analysis followed the PRISMA Checklist (Supplementary Material) and has been registered in PROSPERO (https://www.crd.york.ac.uk/prospero/)—protocol #CRD42020187373.
Eligibility Criteria and Primary Outcomes
Following preliminary searches, 3 broad categories of studies were identified: 1) those where single-dose SRS was used, 2) those where SRS was used in conjunction with tumor removal (always done before SRS), and 3) those where SRS was given via fractionation. For a more comprehensive analysis, we included all peer-reviewed publications that met the following criteria: 1) studies were in English; 2) outcome was not limited to quality of life assessment but included, at least, either a functional (CN status) outcome or tumor control; 3) at least one of the primary outcomes of interest was reported in the population of interest; and 4) the authors specifically discussed “large” VS (either in the entire paper or in a subcohort). Studies where normal fractionation (fSRT) (eg, 2 Gy/fraction in 20 fractions) was utilized were excluded.
Primary outcomes assessed included rate of tumor control (defined as no need for further intervention or lack of symptom progression, as specified in each manuscript), need for salvage surgery, radiographic control (defined as tumors either remaining within 10% of their original size or decreasing in size23,24), trigeminal nerve impairment, facial nerve impairment, serviceable hearing, presence of vertigo and/or dizziness, and presence of hydrocephalus requiring a ventriculoperitoneal shunt (VPS).
Data Collection
Abstract and full-text review was carried out independently and blindly by 2 authors (A1 and A2). Conflicts were resolved with discussion. Data were then extracted manually from the included articles and stored electronically. Data fields extracted included study characteristics, patient biographical characteristics, tumor characteristics, treatment characteristics, tumor response, before- and after-treatment rate of serviceable hearing (either grade 1 or 2 on the Gardner–Robertson scale, or grade A or B on the American Academy Otolaryngology-Head and Neck Surgery [AAO-HNS] scale), trigeminal symptoms, facial nerve symptoms, and presence of hydrocephalus requiring VPS (Supplementary Material). Odds ratios (ORs) were calculated for each variable. Tumor control was broadly defined as no need for further intervention and no symptom progression; radiographic tumor control was defined as tumors either remaining within 10% of their original size or decreasing in size at the time of the report made by the original authors.23,24
Statistical Analysis
Meta-analyses for the complication proportions were conducted for studies using fSRS, single-dose SRS, and MS and single-dose SRS. Statistical heterogeneity was tested through the Cochrane Q test, and a P-value ≤.20 was used to indicate the presence of heterogeneity (ie, a more conservative approach using a random-effects meta-analysis). Statistical heterogeneity was also assessed by the inconsistency statistic (I2). However, regardless of the heterogeneity test P-value or I2 statistic percentage, a random-effects analysis was used to calculate the pooled proportions.
For each meta-analysis of a specific complication type, the presence of publication bias was evaluated through a funnel plot (Supplementary Material). Egger’s test and the Begg–Mazumdar rank-correlation test were used to statistically assess the presence of publication bias. All analyses were conducted with the use of R (version 3.6.3, R Foundation for Statistical Computing, Vienna, Austria), packages meta, metaphor, and dmetar.
Results
Study Selection
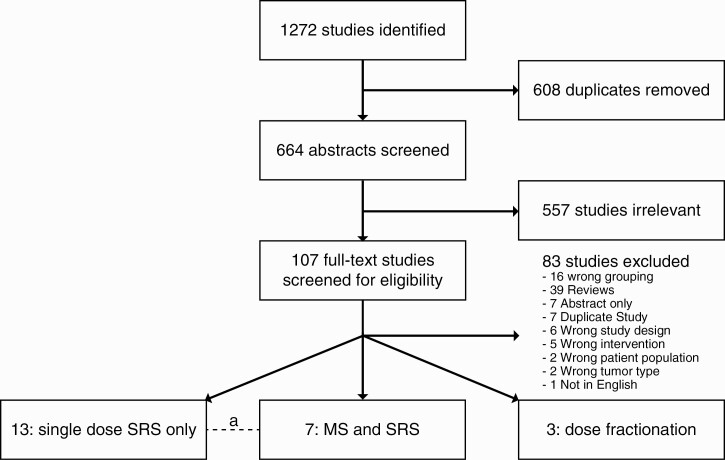
A total of 1272 studies were identified. After de-duplication and initial screening, 664 full-text studies were assessed for eligibility, resulting in 22 studies included here. Of these, 13 studies assessed single-dose SRS,15–18,23,25–32 7 a combination of MS followed by SRS in all patients,12,25,33–37 and 3 relied on fSRS.38–40 One study had 2 subcohorts (Figure 1).25 Of note, studies varied in how they defined “large VS,” as shown in Tables 1–3.
Figure 1.
Scheme of search results and assessment of eligibility. a: one study in common with 2 arms.
Table 1.
Summary of Studies Utilizing Single-Dose SRS for Large VS
| Author, Yeara | “Large VS” Definition | Patients/Tumors (% NF2) | Mean Age (range) | Prior Surgery (%) | Mean Follow-up (range) (mo) | Mean Marginal Dose (range) (Gy) | Mean Max. Dose (range) (Gy) | Mean Isodose (%) | Mean Isocenters (range) | Mean Tumor Volume (range) (mL) | Mean Tumor Diameter (range) (mm) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Bailo,20 2016 | >2.5 cm | 59 (0) | 63.8 (24–85) | 0 | 79 (36–164) | 13 (11–15) | 26 (23.8–32.6) | 50 | 20 (11–41) | 5.98 (2.5–14.9) | 28.5 (25.5–34.0) |
| Chung,28 2010 | >3 cm | 21 (N/A) | 49.5 (27–77) | 66.7 | 66 (12–155) | 11.9 (11–14) | 21.2 (19.2–28) | 57 | 18 (10–27) | 17.3 (12.7–25.2) | N/A |
| Huang,14 2018 | >3 cm and >10 mL | 35 (0) | 49.7 (21–74) | 25.7 | 48 (6–156) | 11 (10–12) | 22 (18.2–28.6) | 50 | 17 (10–36) | 14.8 (10.3–24.5) | N/A |
| Inoue,30 2004 | >4 cm | 5b (20) | 58.8 (44–67) | 66.7 | 106 (60–144) | 11.5 (10–12) | 25.67 (24–30) | 50 | N/A | 21.0 (17.3–24.9) | N/A |
| Inoue,31 2005 | >3 cm | 18c (11.1) | N/A (33–81) | 55 | N/A (72–156) | 11.5 (10–12) | N/A | N/A | N/A | 15.2 (5.3–28.5) | N/A |
| Iorio-Morin,32 2016 | Koos IV and >4 cm | 68 (0) | 58 (18–65) | 19.1 | 47 (6–125) | 12 (11–13) | N/A | 50 | N/A | 7.4 (4–19) | 27.0 (20.0–40.0) |
| Lefranc,33 2018 | Koos IV | 86 (N/A) | 54.6 (23–84) | 16.3 | 74.4 (36–192) | 10.94 (8–13) | N/A | 49.2 | N/A | 4.5 (1.4–8.7) | 25.8 (16.3–35.0) |
| Milligan,21 2012 | >2.5 cm | 22 (4.5) | 61 (N/A) | 4.5 | 28 (25–38) | 12 (12–14) | N/A | N/A | 13 (8–22) | 9.4 (5.3–19.1) | 28 (25–38) |
| van de Langenberg,34 2011 | >6 mL, indenting brainstem | 33 (0) | 54.8 (30–83) | 0 | 30 (12–72) | 11.6 (10.3–13) | 20.79 (18.1–25.5) | 90 | 9 (3–23) | 8.8 (6.1–17.7) | 30.0 (23.0–40.0) |
| Watanabe,35 2019 | >8 mL | 19 (0) | 71 (29–91) | 47.4 | 98 (49–204) | 11.9 (10–12) | 20.3 (20–24.8) | 60 | N/A | 11.5 (8.0–30.6) | N/A |
| Williams,36 2013 | >3 cm | 24 (0) | 61 (32–87) | 37.5 | 82.5 (7–222) | 11 (8–20) | 30 (20–47) | N/A | 8 (2–25) | 9.5 (3.1–24.7) | N/A |
| Yang,22 2011 | 3–4 cm extracanalicular | 65 (0) | 51 (19–89) | 26.2 | 36 (1–146) | 12 (11–15) | N/A | 50 | N/A | 9.0 (5.0–22.0) | N/A |
| Zeiler,37 2013 | 3–4 cm | 28 (7) | 56 (26–85) | 42.9 | 34.5 (6–99) | N/A | 25 (24–26) | 50 | N/A | N/A | 32.8 (30.0–40.0) |
| Author, Yeara | % R- CTRL, % C- CTRL (% re-op) | CN V Impairment (%) | CN VII Impairment (%) | Serviceable Hearing (%) | Hydrocephalus Requiring VPS (%) | Vertigo/ Dizziness (%) | |||||
| Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | ||
| Bailo,20 2016 | 88.6, 98.3 (1.7) | 27.1 | 20.3 | 15.3 | 8.5 | 35.6 | 8.5 | 8.5 | 25.4 | 10.2 | 6.8 |
| Chung,28 2010 | 85.7, 76.2 (9.5) | 38.1 | 38.1 | 57.1 | 57.1 | 4.8 | 0 | 0 | 9.5 | N/A | N/A |
| Huang,14 2018 | 85.7, 85.7 (11.4) | 34.30% | 11.40% | N/A | N/A | 8.6 | 2.9 | 0 | 5.7 | 25.7 | N/A |
| Inoue,30 2004 | 100, 100 (0) | N/A | N/A | 80 | 80 | 16.7 | 16.7 | N/A | N/A | N/A | N/A |
| Inoue,31 2005 | 93.3, 93.3 (5.6) | N/A | N/A | 55 | 55 | 25 | 20 | N/A | N/A | N/A | N/A |
| Iorio-Morin,32 2016 | 94.1, 94.1 (4.4) | 51.5 | 57.4 | 8.8 | 8.8 | 58.8 | 29.4 | 7.4 | 11.8 | 11.8 | 11.8 |
| Lefranc,33 2018 | 100, 90.7 (8.1) | 1.2 | 1.2 | 0 | 0 | 44.2 | 29.1 | 18.6 | 19.8 | 7 | 15.1 |
| Milligan,21 2012 | 90.9, 81.8 (9.1) | 59.1 | 72.7 | 4.5 | 18.2 | 45.5 | 13.6 | 4.5 | 18.2 | N/A | N/A |
| van de Langenberg,34 2011 | 87.9, 78.8 (15.2) | 36.4 | 12.1 | 3 | 9.1 | 36.4 | 21.2 | 6.1 | 12.1 | 51.5 | 54.5 |
| Watanabe,35 2019 | 83.3, 84.2 (15.8) | 15.8 | 26.3 | 21.1 | 21.1 | 36.8 | 0 | 15.8 | 31.6 | N/A | N/A |
| Williams,36 2013 | 88.9, 75.0 (12.5) | 45.8 | 29.2 | 33.3 | 54.2 | 25 | N/A | 4.2 | 12.5 | 20.8 | N/A |
| Yang,22 2011 | 89.2, 89.2 (10.8) | 40 | 46.2 | 23.1 | 24.6 | 33.8 | 27.7 | 7.7 | 13.8 | N/A | N/A |
| Zeiler,37 2013 | 92.0, 92.0 (4) | 48 | 24 | 36 | 28 | 16 | 16 | 0 | 12 | 20 | 0 |
Abbreviations: C-CTRL, clinical control; CN, cranial nerve; N/A, not applicable; NF2, neurofibromatosis 2; R-CTRL, radiographic control; SRS, stereotactic radiosurgery; VS, vestibular schwannomas.
aAll studies are retrospective.
bFive patients, 6 tumors.
cEighteen patients, 20 tumors.
Single-Dose SRS
Of the studies identified, 13 focused solely on single-dose SRS for a total of 483 patients (Table 1). Combining these studies, mean age was 56.6 (range: 18–91) years, mean tumor volume was 8.9 (range: 1.4–30.6) mL, mean tumor diameter was 27.9 (range: 20–40) mm, and mean follow-up was 59 (range: 1–222) months. Treatment was carried out with a mean marginal dose of 11.1 (range: 10–15) Gy and a mean maximal dose of 24.0 (range: 18.2–47) Gy; the mean isodose was at 53.8% (range: 49.2%–90%); an average of 15.1 (range: 2–41) isocenters was used. About 23.4% of patients had undergone prior surgery before SRS (range: 0%–80%).
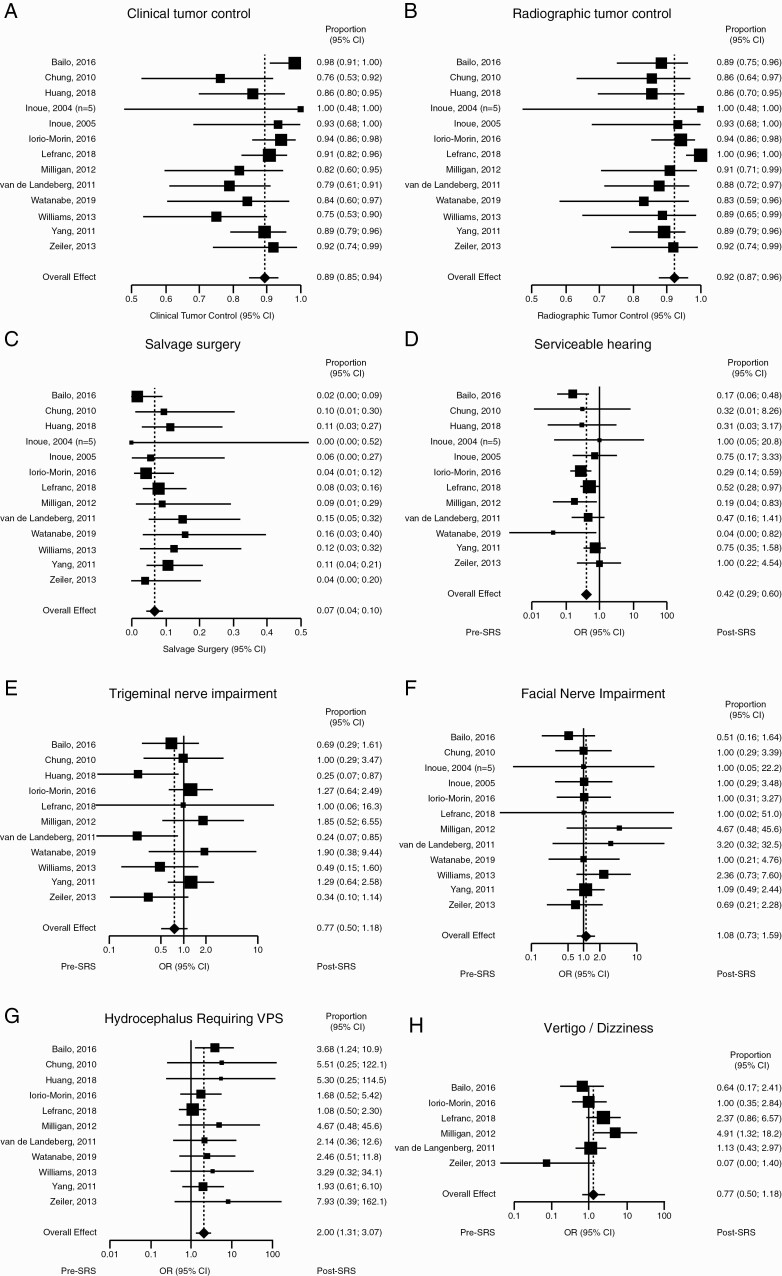
Clinical control was achieved in 89% (95% CI: 85%, 94%) of patients with moderate heterogeneity (I2 = 47%) (Figure 2A), and radiographic control in 92% (95% CI: 87%, 96%) with moderate heterogeneity (I2 = 57%) (Figure 2B). Salvage surgery was required in 7% (95% CI: 4%, 10%) of patients because of recurrent tumor growth, with minimal heterogeneity (I2 = 0%) (Figure 2C).
Figure 2.
Single-dose stereotactic radiosurgery (SRS). Forest plots showing (A) clinical tumor control, (B) radiographic tumor control, and (C) rate of salvage surgery. Forest plots summarizing odds ratio (OR) of (D) serviceable hearing, (E) trigeminal nerve impairment, (F) facial nerve impairment, (G) hydrocephalus requiring ventriculoperitoneal shunt (VPS), and (H) vertigo/dizziness.
Significant morbidities were observed before and after treatment. For all primary endpoint morbidities, ORs were calculated as post- over pretreatment symptom incidence. With respect to serviceable hearing, 12 studies (459 patients) were included. Overall, low heterogeneity was observed (I2 = 11%) for a combined OR of 0.42 (95% CI: 0.29, 0.60, P < .01; Figure 2D). Trigeminal nerve impairment was assessed in 11 studies (457 patients) for a combined OR of 0.77 (95% CI: 0.50, 1.18, P = .23; Figure 2E). Heterogeneity was moderate (I2 = 38%). Facial nerve impairment was assessed in 12 studies (447 patients) for a combined OR of 1.08 (95% CI: 0.73, 1.59, P = .69; Figure 2F). Heterogeneity was minimal (I2 = 0%). The presence of hydrocephalus requiring VPS was assessed in 11 studies (457 patients) for a combined OR of 2.00 (95% CI: 1.31, 3.07, P < .01; Figure 2G). Heterogeneity was minimal (I2 = 0%). The presence of vertigo or dizziness was assessed in 6 studies (293 patients) for a combined OR of 1.29 (95% CI: 0.62, 2.70, P = .50; Figure 2H). Heterogeneity was moderate (I2 = 52%). Overall, these results show that single-dose SRS results in a decrease in serviceable hearing and an increase in incidence of hydrocephalus requiring VPS. Funnel plots summarizing heterogeneity are shown in Supplementary Figure 1.
MS and SRS
Of the studies identified, 7 assessed the efficacy of MS followed by SRS, for a total of 182 patients in whom this combined approach was used (Table 2). Combining these studies, mean age was 53.0 (range: 18–85) years, mean tumor volumes before and after MS were 14.9 (range: 1.5–36.1) mL and 3.9 (range: 0.2–28.5) mL, respectively (ie, before SRS), mean tumor diameters before and after MS were 28.0 (range: 20–58) mm and 18.6 (range: 9–36.1) mm, respectively, and mean follow-up was 43.5 (range: 4–156) months. The average delay between MS and SRS was 5.8 (range: 1–24) months. Treatment was carried out with a mean marginal dose of 11.7 (range: 9.4–14.1) Gy and a mean maximal dose of 21.6 (range: 18–26) Gy; the mean prescription isodose was 66.7% (range: 50–90%); an average of 21.7 (range: 7–44) isocenters was used.
Table 2.
Summary of Studies Utilizing MS Followed by SRS
| Author, Year | “Large VS” Definition | N (% NF2)a | Mean Age (range) | Surgical Approach | Mean Follow-up (range) (mo) | Mean Marginal Dose (range) (Gy) | Mean Max. Dose (range) (Gy) | Mean Isodose (%) | Mean Isocenters (range) | MS-SRS Delay (range) (mo) | Mean Tumor Volume (range) (mL) | Mean Tumor Diameter (range) (mm) | % R- CTRL, % C- CTRL (% re-op) | CN V Impairment (%) | CN VII Impairment (%) | Serviceable Hearing (%) | Hydrocephalus Requiring VPS (%) | Vertigo/ Dizziness (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre- MS | Pre-SRS | Pre- MS | Pre-SRS | Pre- MS | Post- MS | Post-SRS | Pre- MS | Post- MS | Post-SRS | Pre- MS | Post- MS | Post-SRS | Pre- MS | Post-SRS | Pre- MS | Post-SRS | ||||||||||||
| Daniel,17 2017b | Koos IV | 32 (0) | 51.7 (32–85) | RS | 29 (4–78) | 12 (11–12) | N/A | 50 | 20 (7–33) | 6.3 (3.8–13.9) | 12.5 (1.5–34.9) | 3.5 (0.5–12.8) | 33.2 (20–45) | 90.6, 90.6 (9.4) | 6.3 | 0 | 0 | 3.1 | 0 | 0 | 40.6 | 37.5 | 37.5 | N/A | N/A | 12.5 | N/A | |
| Fuentes,38 2008b | >3.5 cm | 8 (N/A) | 53 (24–78) | RS | 46 (12–73) | 11.8 (11–13) | 23.75 (22–26) | N/A | N/A | 9 (6–12) | N/A | 1.16 (0.3–2.2) | 40 (35–45) | 18 (9–20) | 100, 100 (0) | 25 | N/A | N/A | N/A | 25 | 25 | 12.5 | N/A | N/A | N/A | N/A | N/A | N/A |
| Inoue,30 2004c | >4 cm | 3 (0) | 51.3 (41–66) | N/A, ICD | 34 (12–72) | 12 (12-12) | 23.3 (22–24) | 50 | N/A | 3.67 (2–5) | N/A | 17.1 (2.2–28.5) | N/A | N/A | 100, 100 (0) | N/A | N/A | N/A | N/A | N/A | N/A | 33.3 | 33.3 | 33.3 | N/A | N/A | N/A | N/A |
| Iwai,42 2003b | >3 cm | 14 (14.3) | 47 (18–64) | RS | 32 (12–72) | 12.1 (10–14.1) | N/A | N/A | N/A | 2.9 (1–6) | N/A | N/A | 42 (30–58) | 18.9 (9.8–36.1) | 78.6, 92.9 (7.1) | 50 | N/A | N/A | 0 | 28.6 | 28.6 | 21.4 | 7.1 | 7.1 | N/A | N/A | N/A | N/A |
| Iwai,39 2015b | >2.5 cm | 40 (N/A) | 60.5 (33–82) | RS | 65 (18–156) | 12 (10–12) | N/A | N/A | N/A | 3 (1–12) | N/A | N/A | 32.5 (25–52) | 18.6 (9.1–27.1) | 90, 90 (10) | 75 | N/A | N/A | 32.5 | 22.5 | 7.5 | 35 | 27.5 | 17.5 | 0 | 0 | N/A | N/A |
| Pan,40 2012b | >3 cm | 18 (N/A) | 50 (N/A) | RS, ICD | 57.7 (N/A) | 12 (12-12) | N/A | 50 | N/A | 3.6 | 17.5 (N/A) | 9.35 (N/A) | N/A | N/A | 100, 94 (N/A) | N/A | N/A | N/A | 5.6 | 11.1 | 11.1 | 61.1 | 61.1 | 61.1 | N/A | 5.6 | N/A | N/A |
| 17 (N/A) | 49 (N/A) | RS, ECD | 52.7 (N/A) | 12 (12-12) | N/A | 50 | N/A | 7 | 16.4 (N/A) | 1.1 (N/A) | N/A | N/A | 100, 94 (N/A) | N/A | N/A | N/A | 0 | 88.2 | 88.2 | 64.7 | 0 | 0 | N/A | 5.9 | N/A | N/A | ||
| van de Langenberg,41 2011c | Koos III or IV | 50 (0) | 52 (21–84) | TL (25) or RS (25) | 33.8 (12–84) | 11 (9.4–11.9) | 21.1 (18–26) | 90 | 22.8 (7–44) | 8.5 (2–24) | 14.9 (4.1–36.1) | 3.34 (0.2–11.8) | 35 (26–54) | N/A | 90, 92, 2 | 28 | 30 | 30 | 2 | 40 | 18 | 8 | 2 | 2 | 4 | 8 | 48 | 48 |
Abbreviations: CN, cranial nerve; ECD, extracapsular decompression; ICD, intracapsular decompression; MS, microsurgery; N/A, not applicable; NF2, neurofibromatosis 2; RS, retrosigmoid; SRS, stereotactic radiosurgery; TL, translabyrinthine; VPS, ventriculoperitoneal shunt; VS, vestibular schwannomas.
aAll studies had patients with 1 VS per patient.
bA prospective study.
cA retrospective study.
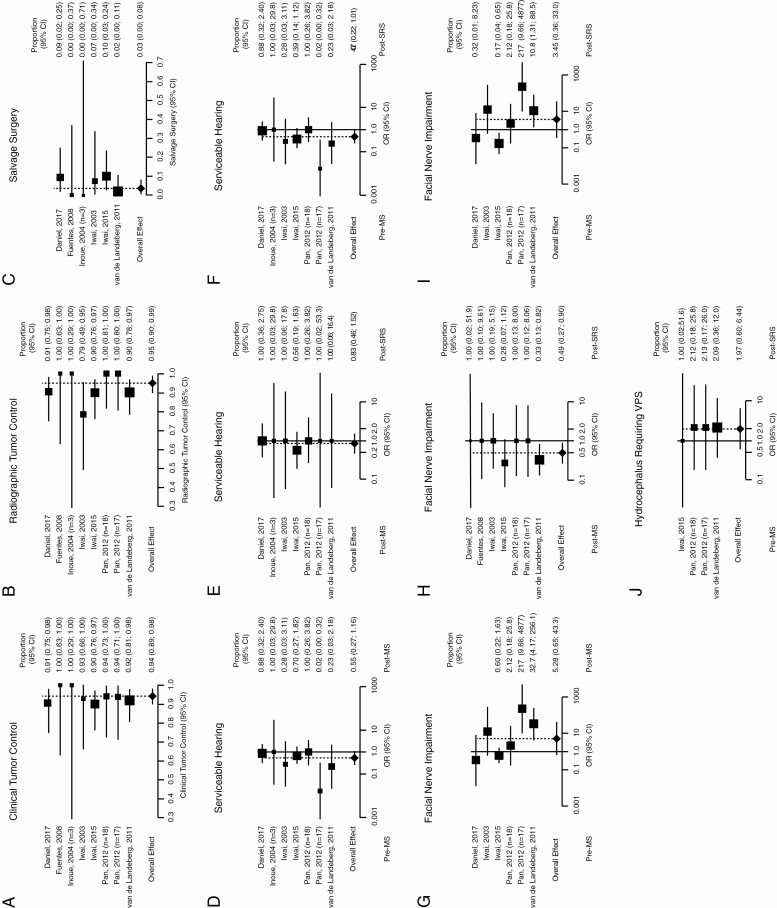
Overall clinical control (ie, after both procedures) was achieved in 94% (95% CI: 89%, 98%) of patients with minimal heterogeneity (I2 = 0%) (Figure 3A), and radiographic control in 95% (95% CI: 90%, 99%) with low heterogeneity (I2 = 16%) (Figure 3B). Salvage surgery was required in 3% (95% CI: 0%, 8%) of patients because of tumor recurrence with minimal heterogeneity (I2 = 0%) (Figure 3C).
Figure 3.
Combination of microsurgery (MS) and stereotactic radiosurgery (SRS). Forest plots showing combined post-MS and post-SRS (A) clinical tumor control, (B) radiographic tumor control, and (C) rate of salvage surgery. Forest plots summarizing odds ratio (OR) of serviceable hearing (D), pre-MS and post-MS, (E) post-MS and post-SRS, and (F) pre-MS and post-SRS. Forest plots summarizing OR of facial nerve impairment (G) pre- and post-MS, (H) post-MS and post-SRS, and (I) pre-MS and post-SRS. (J) Forest plot summarizing OR of pre-MS and post-SRS hydrocephalus requiring ventriculoperitoneal shunt (VPS).
To assess whether either treatment alone (MS or SRS) or their combinations resulted in significant worsening of serviceable hearing and facial nerve impairment (the only 2 metrics reliably assessed across studies), ORs were calculated as post- over pretreatment symptom incidence for the following pairs: pre-MS and post-MS, post-MS and post-SRS, and pre-MS and post-SRS. With respect to serviceable hearing, 6 studies were included, of which 1 had 2 arms (174 patients). Comparing pre-MS with post-MS, low heterogeneity was observed (I2 = 30%) for a combined OR of 0.55 (95% CI: 0.27, 1.16, P = .12; Figure 3D). Comparing post-MS with post-SRS, minimal heterogeneity was observed (I2 = 0%) for a combined OR of 0.83 (95% CI: 0.46, 1.52, P = .55; Figure 3E). Comparing pre-MS with post-SRS, moderate heterogeneity was observed (I2 = 31%) for a combined OR of 0.47 (95% CI: 0.22, 1.01, P = .05; Figure 3F).
With respect to facial nerve impairment, 6 studies were included, of which 1 had 2 arms (179 patients). Comparing pre-MS with post-MS, high heterogeneity was observed (I2 = 81%) for a combined OR of 5.28 (95% CI: 0.65, 43.25, P = .12; Figure 3G). Comparing post-MS with post-SRS, minimal heterogeneity was observed (I2 = 0%) for a combined OR of 0.49 (95% CI: 0.27, 0.90, P = .02; Figure 3H). Comparing pre-MS with post-SRS, high heterogeneity was observed (I2 = 81%) for a combined OR of 3.45 (95% CI: 0.36, 32.96, P = .28; Figure 3I). Overall, this shows that the combination of MS and SRS does not result in facial nerve impairment; rather, the use of SRS following MS is associated with some recovery of function.
The presence of hydrocephalus requiring VPS was assessed in 3 studies (125 patients), for a combined OR of 1.97 (95% CI: 0.60, 6.44, P = .26; Figure 3J). Heterogeneity was minimal (I2 = 0%). Funnel plots summarizing heterogeneity are shown in Supplementary Figure 2. The prevalence of trigeminal nerve impairment was assessed in only 2 studies.12,36 The presence of vertigo/dizziness was assessed in only 1 study.36
Fractionated SRS
Of the studies identified, 3 utilized fSRS, for a total of 82 patients. Studies that relied on fSRT were excluded. These studies are summarized in Table 3. Combining these studies, mean age was 57.2 (range: 17–85) years, mean tumor diameter was 33.8 (range: 23–50) mm, and mean follow-up was 67.3 (range: 7–175) months. The mean prescription isodose was 80.2% (range: 70%–95%). About 28.0% of patients had undergone prior treatment before fSRS (range: 15.2%–36.8%).
Table 3.
Summary of Studies Utilizing Fractionated Radiation
| Author, Yeara | “Large VS” Definition | N (% NF2) | Mean Age (range) | Prior Surgery (%) | Mean Follow-up (range) (mo) | Fractionation Schedule | Mean Isodose (range) (%) | Mean Tumor Volume (range) (mL) | Mean Tumor Diameter (range) (mm) | % R- CTRL, % C- CTRL (% re-op) | CN V Impairment (%) | CN VII Impairment (%) | Serviceable Hearing (%) | Hydrocephalus Requiring VPS (%) | Vertigo/Dizziness (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | Pre-SRS | Post-SRS | |||||||||||
| Casentini,43 2015 | >8 mL | 33 (6.1) | 63 (28–85) | 15.2 | 48 (12–111) | 2–5 fr, total 14–19.5 Gy | 77.6 (70–82) | 11.0 (8.0–24.) | N/A | 57.6, 87.9 (6.1) | 21.2 | 24.2 | 27.3 | 27.3 | 24.2 | 21.2 | 15.2 | 21.2 | 12.1 | 9.1 |
| Kalapurakal,45 1999 | PP > 1 cm, MPTD > 2 cm | 19 (N/A) | 68 (34–84) | 36.8 | 54 (34–65) | 6 fr × 5 or 6 Gy each | 85 (80–95) | N/A | 35 (23–49) | 100, 100 (0) | 21.1 | 21.1 | 15.8 | 15.8 | 47.4 | 47.4 | N/A | N/A | N/A | N/A |
| Teo,44 2016 | Koos IV, >3 cm | 30 (20) | 44 (17–82) | 36.7 | 97 (7–175) | 3 fr × 6 Gy each | 79.9 (71–90) | N/A | 33.0 (25.0–50.0) | 80.0, 80.0 (6.7) | N/A | N/A | 83.3 | 76.7 | 43.3 | 16.7 | 0 | 10 | N/A | N/A |
Abbreviations: C-CTRL, clinical control; CN, cranial nerve; fr, fraction; MPTD, mid-porous transverse diameter; N/A, not applicable; NF2, neurofibromatosis 2; PP, pons-petrous distance; R-CTRL, radiographic control; Re-op, re-operation; SRS, stereotactic radiosurgery; VPS, ventriculoperitoneal shunt; VS, vestibular schwannomas.
aAll studies are retrospective.
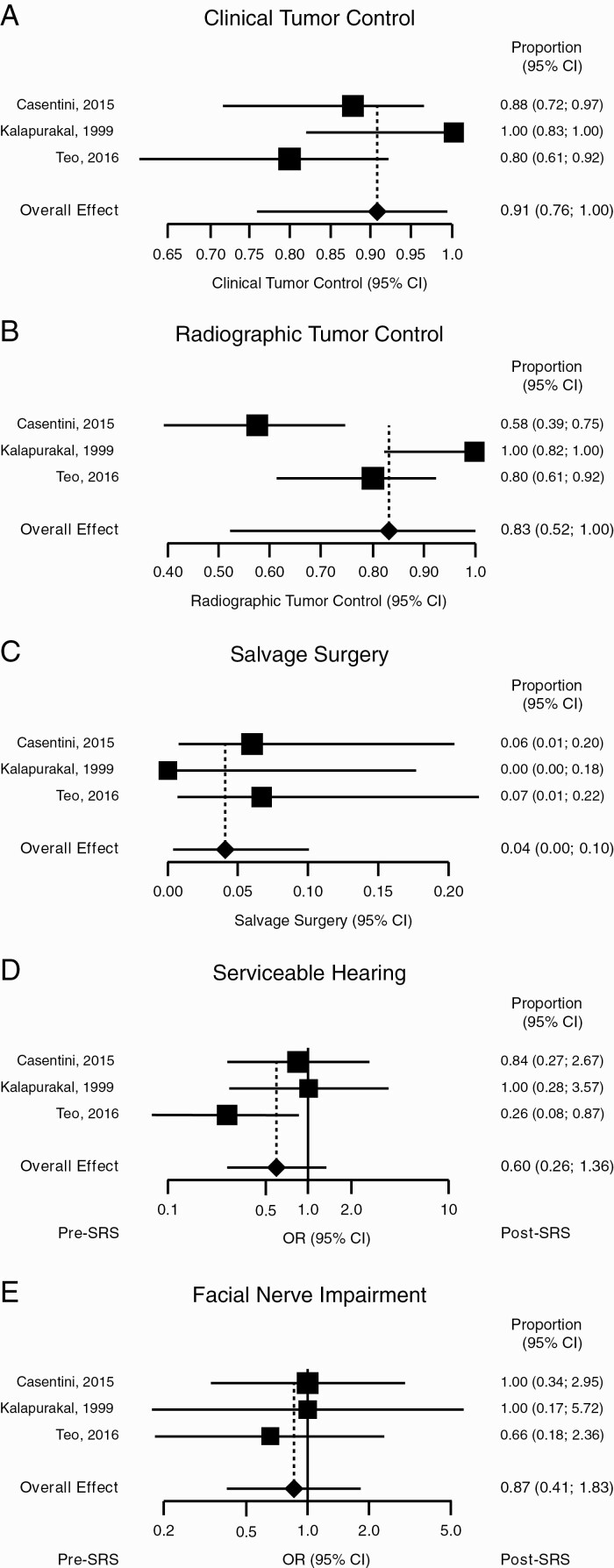
Clinical control was achieved in 91% (95% CI: 76%, 100%) of patients, with moderate heterogeneity (I2 = 69%) (Figure 4A), and radiographic control in 83% (95% CI: 52%, 100%) with high heterogeneity (I2 = 89%) (Figure 4B). Salvage surgery was required for 4% (95% CI: 0%, 10%) of patients, with minimal heterogeneity (I2 = 0%) (Figure 4C). With respect to serviceable hearing, moderate heterogeneity was observed (I2 = 28%) for a combined OR of 0.60 (95% CI: 0.26, 1.36, P = .22; Figure 4D). With respect to facial nerve impairment, heterogeneity was minimal (I2 = 0%) for a combined OR of 0.87 (95% CI: 0.41, 1.83, P = .71; Figure 4E). Funnel plots summarizing heterogeneity are shown in Supplementary Figure 3.
Figure 4.
Fractionated stereotactic radiosurgery (SRS). Forest plots showing (A) clinical tumor control, (B) radiographic tumor control, (C) rate of salvage surgery, (D) odds ratio (OR) of serviceable hearing, and (E) OR of facial nerve impairment.
Trigeminal nerve impairment was assessed in only 2 studies.38,40 Vertigo/dizziness was assessed in only 1 study.38 Hydrocephalus requiring VPS was assessed in only 2 studies.38,39
Discussion
The management of large VS remains a challenge, as bulky size increases the morbidity of MS and has historically hindered the delivery of XRT. In this study, we analyze the use of SRS in treating large VS and show how single-dose SRS, the combination of MS and SRS, and fractionated SRS result in excellent clinical (89%, 94%, and 91%, respectively) and radiographic (92%, 95%, and 83%, respectively) tumor control, with low incidence of salvage surgery (7%, 3%, and 4%, respectively). Both single-dose SRS and the combination of MS with SRS resulted in significant worsening of pretreatment serviceable hearing; this finding was not seen for fractionated SRS, possibly because of small sample size. Trigeminal nerve impairment was assessed only by single-dose studies, which did not result in significant post-treatment impairment. Facial nerve impairment was not increased following any of these approaches. The incidence of hydrocephalus requiring VPS was increased following single-dose SRS but not after the combination of MS and SRS; however, in the latter case, only 3 studies assessed such a complication. Unfortunately, studies did not clearly associate toxicities with dose given, partly because of the wide range of treatment regimens.
It is important to note how the definition of “large VS” differed across studies: some authors used a maximal diameter cutoff, others used a volume cutoff; others yet used both metrics, with 5 studies relying on Koos grade. Such a wide-ranging definition of large tumors indicates different levels of comfort with irradiating “borderline” tumors and can explain, at least in part, some of the variability here observed.
In recent years, XRT protocols have been applied to larger tumors, going beyond the historical limit of 2.5–3.0 cm in diameter.19 A promising approach centered on the combination of MS with SRS—MS aimed at reducing the bulk of the tumor followed by SRS to target the remnant. As shown here, this approach achieves good tumor control despite the inherent difficulty in treating large VS without any significant worsening of pre-existing CN impairment, at the cost of reducing serviceable hearing. Our findings are consistent with another meta-analysis that solely focused on such a “nerve-centered approach.” 14 Patient selection will have to be carefully carried out: one of the main reasons why SRS is chosen in the first place is to avoid surgery. On the other hand, surgical intervention in experienced centers can be associated with low morbidity and minimal need for any future treatments.35,41 Noticeably, in this series, the use of MS and SRS did not result in a significant increase in hydrocephalus requiring VPS, thus indicating how decompression may prevent future hydrocephalus. Since such a measure was assessed in only 3 studies, more data are needed to determine if, in fact, this is the case.
In recent years, fSRS has been utilized for the treatment of VS and other tumors in the hope of reducing toxicities associated with high radiation doses without compromising tumor control. In this study, we show how a careful use of this treatment modality (ie, performed by an experienced practitioner with close postprocedural monitoring and assessment of acute complication development) is safe even for large VS.42
Currently, no definitive randomized clinical trial has shown the superiority of the treatment modalities here discussed over the others. The problem is further compounded by the wide range in treatment doses. Initially, VS were irradiated with marginal doses of 16 Gy and above, which achieved excellent tumor control (>95%) with significant cranial nuropathies.11 Now, rarely do authors go above 14 Gy; however, such a regimen has not completely fallen out of practice.11,16,18,31,43–49 Here, we have shown how different approaches can successfully achieve tumor control; further structured studies will be needed to better define a treatment paradigm in treatment of large VS.
Numerous limitations of the current study exist. The studies identified represent retrospective case series with no randomized clinical trials; data are further reported in a highly heterogenous manner. Furthermore, separating studies in subgroups based on treatment, albeit necessary to compare similar interventions, resulted in a limited sample size of each subgroup, with some with a limited number of studies and cases. Despite the limited size, statistical analysis was still possible and carried out, conscious of the fact that our conclusions will be strengthened by further studies with larger sample size. To understand how each treatment modality fares compared to the others, further structured studies are necessary.
In conclusion, large VS pose a therapeutic challenge given the high likelihood of compromise of local structures, the tendency to continue growing in size, and the difficulties associated with both MS and SRS. SRS, either as a single dose, in conjunction with MS, or fractionated, is a valid treatment alternative, as it achieves good tumor control with acceptable CN morbidity.
Conclusions
The radiosurgical treatment of large VS remains a technically challenging endeavor. However, thanks to novel low-dose radiation regimens (either single dose, fractionated, or in combination with surgical debulking) and accurate patient selection, it can provide excellent tumor control and low level of CN toxicities. Further structured studies, however, are urgently needed to determine the relative success of each of these different approaches and to reach a higher level of evidence.
Funding
A.A. and P.J.C. were partially supported by the National Center for Advancing Translational Science of the National Institute of Health under award number UL1TR002384.
Conflict of interest statement. None declared.
Supplementary Material
References
- 1. Gupta VK, Thakker A, Gupta KK. Vestibular schwannoma: what we know and where we are heading. Head Neck Pathol. 2020;14(4):1058–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Propp JM, McCarthy BJ, Davis FG, et al. Descriptive epidemiology of vestibular schwannomas. Neuro Oncol. 2006;8(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Miller LE, Brant JA, Naples JG, et al. Quality of life in vestibular schwannoma patients: a longitudinal study. Otol Neurotol. 2020;41(2):e256–e261. [DOI] [PubMed] [Google Scholar]
- 4. Erickson NJ, Schmalz PGR, Agee BS, et al. Koos classification of vestibular schwannomas: a reliability study. Neurosurgery. 2019;85(3):409–414. [DOI] [PubMed] [Google Scholar]
- 5. Lees KA, Tombers NM, Link MJ, et al. Natural history of sporadic vestibular schwannoma: a volumetric study of tumor growth. Otolaryngol Head Neck Surg. 2018;159(3):535–542. [DOI] [PubMed] [Google Scholar]
- 6. Fabbris C, Gazzini L, Paltrinieri D, et al. Exclusive surgical treatment for vestibular schwannoma regrowth or recurrence: a meta-analysis of the literature. Clin Neurol Neurosurg. 2020;193:105769. [DOI] [PubMed] [Google Scholar]
- 7. Mahboubi H, Sahyouni R, Moshtaghi O, et al. CyberKnife for treatment of vestibular schwannoma: a meta-analysis. Otolaryngol Head Neck Surg. 2017;157(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bambakidis NC, Lo SS, Selman WR. Large vestibular schwannomas. J Neurosurg. 2011;115(5):894–895. [DOI] [PubMed] [Google Scholar]
- 9. Troude L, Boucekine M, Montava M, et al. Predictive factors of early postoperative and long-term facial nerve function after large vestibular schwannoma surgery. World Neurosurg. 2019;127:e599–e608. [DOI] [PubMed] [Google Scholar]
- 10. Jian BJ, Kaur G, Sayegh ET, et al. Fractionated radiation therapy for vestibular schwannoma. J Clin Neurosci. 2014;21(7):1083–1088. [DOI] [PubMed] [Google Scholar]
- 11. Kondziolka D, Lunsford LD, McLaughlin MR, et al. Long-term outcomes after radiosurgery for acoustic neuromas. N Engl J Med. 1998;339(20):1426–1433. [DOI] [PubMed] [Google Scholar]
- 12. Daniel RT, Tuleasca C, George M, et al. Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by Gamma Knife radiosurgery. Acta Neurochir (Wien). 2017;159(7):1197–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Radwan H, Eisenberg MB, Sandberg Knisely JP, et al. Outcomes in patients with vestibular schwannoma after subtotal resection and adjuvant radiosurgery. Stereotact Funct Neurosurg. 2016;94(4):216–224. [DOI] [PubMed] [Google Scholar]
- 14. Starnoni D, Daniel RT, Tuleasca C, et al. Systematic review and meta-analysis of the technique of subtotal resection and stereotactic radiosurgery for large vestibular schwannomas: a “nerve-centered” approach. Neurosurg Focus. 2018;44(3):E4. [DOI] [PubMed] [Google Scholar]
- 15. Huang CW, Tu HT, Chuang CY, et al. Gamma Knife radiosurgery for large vestibular schwannomas greater than 3 cm in diameter. J Neurosurg. 2018;128(5):1380–1387. [DOI] [PubMed] [Google Scholar]
- 16. Bailo M, Boari N, Franzin A, et al. Gamma Knife radiosurgery as primary treatment for large vestibular schwannomas: clinical results at long-term follow-up in a series of 59 patients. World Neurosurg. 2016;95:487–501. [DOI] [PubMed] [Google Scholar]
- 17. Milligan BD, Pollock BE, Foote RL, et al. Long-term tumor control and cranial nerve outcomes following γ knife surgery for larger-volume vestibular schwannomas. J Neurosurg. 2012;116(3):598–604. [DOI] [PubMed] [Google Scholar]
- 18. Yang HC, Kano H, Awan NR, et al. Gamma Knife radiosurgery for larger-volume vestibular schwannomas. Clinical article. J Neurosurg. 2011;114(3):801–807. [DOI] [PubMed] [Google Scholar]
- 19. Cusimano MD. Defining the optimal management for patients with large vestibular schwannomas. Can J Neurol Sci. 2013;40(3):280–281. [DOI] [PubMed] [Google Scholar]
- 20. Boari N, Bailo M, Gagliardi F, et al. Gamma Knife radiosurgery for vestibular schwannoma: clinical results at long-term follow-up in a series of 379 patients. J Neurosurg. 2014;121(suppl.):123–142. [DOI] [PubMed] [Google Scholar]
- 21. Patel KS, Ng E, Kaur T, et al. Increased cochlear radiation dose predicts delayed hearing loss following both stereotactic radiosurgery and fractionated stereotactic radiotherapy for vestibular schwannoma. J Neurooncol. 2019;145(2):329–337. [DOI] [PubMed] [Google Scholar]
- 22. Udawatta M, Kwan I, Preet K, et al. Hearing preservation for vestibular schwannomas treated with stereotactic radiosurgery or fractionated stereotactic radiotherapy. World Neurosurg. 2019;129:e303–e310. [DOI] [PubMed] [Google Scholar]
- 23. Chung WY, Pan DH, Lee CC, et al. Large vestibular schwannomas treated by Gamma Knife surgery: long-term outcomes. J Neurosurg. 2010;113(suppl.):112–121. [DOI] [PubMed] [Google Scholar]
- 24. Chung WY, Liu KD, Shiau CY, et al. Gamma Knife surgery for vestibular schwannoma: 10-year experience of 195 cases. J Neurosurg. 2005;102(suppl.):87–96. [PubMed] [Google Scholar]
- 25. Inoue H, Nishi H, Shibazaki T, Ono N. Hearing preservation after radiosurgery combined with or without microsurgery for large vestibular schwannomas: preliminary results. Radiosurgery. 2004;5:107–114. [Google Scholar]
- 26. Inoue HK. Low-dose radiosurgery for large vestibular schwannomas: long-term results of functional preservation. J Neurosurg. 2005;102(suppl.):111–113. [PubMed] [Google Scholar]
- 27. Iorio-Morin C, AlSubaie F, Mathieu D. Safety and efficacy of Gamma Knife radiosurgery for the management of Koos grade 4 vestibular schwannomas. Neurosurgery. 2016;78(4):521–530. [DOI] [PubMed] [Google Scholar]
- 28. Lefranc M, Da Roz LM, Balossier A, et al. Place of Gamma Knife stereotactic radiosurgery in grade 4 vestibular schwannoma based on case series of 86 patients with long-term follow-up. World Neurosurg. 2018;114:e1192–e1198. [DOI] [PubMed] [Google Scholar]
- 29. van de Langenberg R, Hanssens PE, Verheul JB, et al. Management of large vestibular schwannoma. Part II. Primary Gamma Knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115(5):885–893. [DOI] [PubMed] [Google Scholar]
- 30. Watanabe S, Yamamoto M, Kawabe T, et al. Long-term follow-up results of stereotactic radiosurgery for vestibular schwannomas larger than 8 cc. Acta Neurochir (Wien). 2019;161(7):1457–1465. [DOI] [PubMed] [Google Scholar]
- 31. Williams BJ, Xu Z, Salvetti DJ, et al. Gamma Knife surgery for large vestibular schwannomas: a single-center retrospective case-matched comparison assessing the effect of lesion size. J Neurosurg. 2013;119(2):463–471. [DOI] [PubMed] [Google Scholar]
- 32. Zeiler FA, Bigder M, Kaufmann A, et al. Gamma knife radiosurgery for large vestibular schwannomas: a Canadian experience. Can J Neurol Sci. 2013;40(3):342–347. [DOI] [PubMed] [Google Scholar]
- 33. Fuentes S, Arkha Y, Pech-Gourg G, et al. Management of large vestibular schwannomas by combined surgical resection and Gamma Knife radiosurgery. Prog Neurol Surg. 2008;21:79–82. [DOI] [PubMed] [Google Scholar]
- 34. Iwai Y, Ishibashi K, Watanabe Y, et al. Functional preservation after planned partial resection followed by Gamma Knife radiosurgery for large vestibular schwannomas. World Neurosurg. 2015;84(2):292–300. [DOI] [PubMed] [Google Scholar]
- 35. Pan HC, Sheehan J, Sheu ML, et al. Intracapsular decompression or radical resection followed by Gamma Knife surgery for patients harboring a large vestibular schwannoma. J Neurosurg. 2012;117(suppl.):69–77. [DOI] [PubMed] [Google Scholar]
- 36. van de Langenberg R, Hanssens PE, van Overbeeke JJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by Gamma Knife surgery: radiological and clinical aspects. J Neurosurg. 2011;115(5):875–884. [DOI] [PubMed] [Google Scholar]
- 37. Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003;59(4):283–289; discussion 289. [DOI] [PubMed] [Google Scholar]
- 38. Casentini L, Fornezza U, Perini Z, et al. Multisession stereotactic radiosurgery for large vestibular schwannomas. J Neurosurg. 2015;122(4):818–824. [DOI] [PubMed] [Google Scholar]
- 39. Teo M, Zhang M, Li A, et al. The outcome of hypofractionated stereotactic radiosurgery for large vestibular schwannomas. World Neurosurg. 2016;93:398–409. [DOI] [PubMed] [Google Scholar]
- 40. Kalapurakal JA, Silverman CL, Akhtar N, et al. Improved trigeminal and facial nerve tolerance following fractionated stereotactic radiotherapy for large acoustic neuromas. Br J Radiol. 1999;72(864):1202–1207. [DOI] [PubMed] [Google Scholar]
- 41. Goldbrunner R, Weller M, Regis J, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro Oncol. 2020;22(1):31–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kirkpatrick JP, Soltys SG, Lo SS, Beal K, Shrieve DC, Brown PD. The radiosurgery fractionation quandary: single fraction or hypofractionation? Neuro Oncol. 2017;19(suppl. 2):ii38–ii49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Murphy ES, Suh JH. Radiotherapy for vestibular schwannomas: a critical review. Int J Radiat Oncol Biol Phys. 2011;79(4):985–997. [DOI] [PubMed] [Google Scholar]
- 44. Berkowitz O, Han YY, Talbott EO, et al. Gamma Knife radiosurgery for vestibular schwannomas and quality of life evaluation. Stereotact Funct Neurosurg. 2017;95(3):166–173. [DOI] [PubMed] [Google Scholar]
- 45. Kondziolka D. Role of radiosurgery for larger vestibular schwannomas. J Neurosurg. 2011;115(5):896–897. [DOI] [PubMed] [Google Scholar]
- 46. Niranjan A, Mathieu D, Flickinger JC, Kondziolka D, Lunsford LD. Hearing preservation after intracanalicular vestibular schwannoma radiosurgery. Neurosurgery. 2008;63(6):1054–1062; discussion 1062–1053. [DOI] [PubMed] [Google Scholar]
- 47. Niranjan A, Mathieu D, Kondziolka D, et al. Radiosurgery for intracanalicular vestibular schwannomas. Prog Neurol Surg. 2008;21:192–199. [DOI] [PubMed] [Google Scholar]
- 48. Johnson S, Kano H, Faramand A, Niranjan A, Flickinger JC, Lunsford LD. Predicting hearing outcomes before primary radiosurgery for vestibular schwannomas. J Neurosurg. 133(4):947–1284. [DOI] [PubMed] [Google Scholar]
- 49. Pollock BE, Lunsford LD, Norén G. Vestibular schwannoma management in the next century: a radiosurgical perspective. Neurosurgery. 1998;43(3):475–481; discussion 481–473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.