Abstract
Background:
Around half of anterior cruciate ligament (ACL) injuries are treated through reconstruction, but the literature lacks mechanical investigation of reconstructions in a dynamic athletic task and rupture environment. The current objective was to ascertain the feasibility of investigating ACL reconstructions in a rupture environment during simulated landing tasks in a validated mechanical impact simulator.
Methods:
Four cadaveric lower extremities were subjected to simulated landing in a mechanical impact simulator. External joint loads that mimicked magnitudes recorded from an in vivo population were applied to each joint in a stepwise manner. Simulations were repeated until ACL failure was achieved. Repeated measures design was used to test each specimen in the native ACL and hamstrings, quadriceps, and patellar tendon reconstructed states.
Findings:
ACL injuries were generated in 100% of specimens. Graft substance damage occurred in 58% of ACLRs, and in 75% of bone tendon bone grafts. Bone tendon bone and quadriceps grafts survived greater simulated loading than hamstrings grafts, but smaller simulated loading than the native ACL. Median peak strain prior to failure was 20.3% (11.6, 24.5) for the native ACL and 17.4% (9.5, 23.3) across all graft types.
Interpretation:
The simulator was a viable construct for mechanical examination of ACLR grafts in rupture environments. Post-surgery, ACL reconstruction complexes are weaker than the native ACL when subjected to equivalent loading. Bone tendon bone grafts most closely resembled the native ligament and provided the most consistently relevant rupture results. This model advocated reconstruction graft capacity to sustain forces generated from immediate gait and weightbearing during rehabilitation from an ACL injury.
Key Terms: ACL, ACL reconstruction, knee biomechanics, simulated landing, sports medicine
INTRODUCTION
Of the estimated 250,000 annual anterior cruciate ligament (ACL) injuries in the United States,34 127,000 will opt for ACL reconstruction (ACLR).36 ACLR is considered the surgical standard of care as it restores knee stability, particularly related to anterior tibial shear (ATS), and will allow between 53–84% of athletes to return to sport following this traumatic injury.3,38,66,67,69 However, ACLR grafts fail to precisely emulate the native ACL across multiple variables, including fiber orientation,45 graft stiffness,27 and kinetic and kinematic joint restraint outside of the ATS degree of freedom.6
Robotic manipulators have been utilized extensively to conduct repeated measures investigations that examine the mechanical differences between the native ACL and various ACLR graft techniques.6,71,72,75 These studies typically involve the repeated application of automated clinical tests (Lachman’s Exam, anterior drawer test, pivot-shift exam) to cadaveric specimens across multiple ACLR states. While the outcomes deliver important kinetic and kinematic information pertaining to the functional mechanics of the native ACL and ACLR grafts, these investigations fail to mimic physiologic conditions of athletic motion. Further, robotic simulations have yet to generate physiologically relevant ACL rupture environments. In contrast, impact simulators that mimic drop landing tasks have more recently accomplished this objective.12,14,39 The mechanical impact simulator has generated ruptures in native ACLs in 88% of tested specimens with an injury distribution that matches clinical presentation.13 However, testing of ACLR constructs has yet to be performed in the dynamic loading and rupture environments of these landing simulators. The introduction of a methodology that can viably study ACLR constructs in a simulated rupture environment will allow both clinicians and investigators to directly examine graft mechanics in a realistic environment and quantify their differences relative to the native ACL structure. Enhanced understanding of graft mechanical function will allow for improvements in the selection of ACLR graft type, which should lead to more efficacious outcomes after patients return to activity.
The objective of this pilot study was to ascertain the feasibility of investigating ACLR mechanics in a rupture environment during simulated landing tasks in a validated mechanical impact simulator. While ACLR fixation strength4,29,60,62,73 is lower than ACLR graft tensile strength,40 the natural ACL inclination angle (49.5°)33 combined with the initial contact (IC) angle of our mechanical impact simulator (25°)12 nearly align the ACLR graft substance in parallel with the line of action for ATS force. As the ACL restrains up to 85% of ATS force in the knee,21 it was hypothesized that the mechanical impact simulator model would generate non-fixation failures in the ACLR grafts. Though ACLR grafts have greater tensile strength than the native ACL,40 review literature also indicates that grafts exhibit a significantly greater change in graft force per unit of displacement42 as well as greater tibial displacement in response to ATS than the native ACL.74 As such, this is likely to lead to higher strain and more rapid failure in graft constructs. It was further hypothesized that ACLR grafts would achieve higher peak strain prior to failure than the native ACL, but that the failures would occur with reduced external loading compared to the native ACL.
METHODS
The mechanical impact simulator is a proven and validated tool that has been utilized to induce clinically-representative ACL injuries on cadaveric lower extremities.12,13 Through the application of physiologic factors that include knee abduction moment, anterior shear force, internal tibial rotation moment, and vertical ground reaction forces, this device has been used to investigate a multitude of variables pertaining to intra-articular mechanics during jump landing tasks and ACL injury events.9–13,44,46,55–57 Following a modified version of a previously established testing paradigm,12 four cadaveric specimens purchased from an anatomical donations program (Anatomy Gifts Registry, Hanover, MD, USA) were subjected to simulations in the mechanical impact simulator (Figure 1). Briefly, specimens were inverted and suspended in an aluminum frame at a 25° knee flexion angle with the femoral shaft aligned with the vertical axis of a 6-axis load cell (Omega160 IP65/IP68, ATI Industrial Automation, Inc., Apex, NC, USA). In this position, a vertical load of 0.5 * bodyweight was suspended 31 cm above each specimen. When initiated by an electronic trigger, this load was released to gravity and was delivered to heel of the specimen, which created an impulse force representative of the ground reaction force generated during landing. The quadriceps and hamstrings tendons were placed under a constant 1:1 force ratio of 400 N each throughout each simulation, as has been specified in previous literature.9–13 This magnitude of loading was justified as quadriceps tension of 400 N has been documented to increase ACL strain by 3–5% within a 0–40° range of knee flexion;51,61 whereas, greater quadriceps tension applied between 15–25° of knee flexion either decreased or did not change ACL loading.32,41
Figure 1:

(A) Meta-view of custom designed mechanical impact simulator for creation of ACL ruptures,12 (B) cable pulley system used to deliver pneumatically actuated loads to the quadriceps and hamstrings tendons, (C) external fixation frame attached to the tibia and used to deliver pneumatically actuated KAM, ATS, and ITR loads to each specimen. This figure has been reproduced from Bates, et al. 2018 Am J Sports Med.13,35
A custom clamp was secured through the tibia of each specimen and provided attachment sites for frame-mounted pneumatic cylinders to externally deliver specified knee abduction moment (KAM), anterior tibial shear (ATS), and internal tibial rotation (ITR) loads. These loads were based off of three dimensional kinetics that were calculated from an in vivo cohort of 44 athletes (age = 23.3 (4.1) years; mass = 72.6 (13.9) kg; height = 172 (10) cm) who previously performed drop vertical jump tasks in a motion capture laboratory.9–13 Load magnitudes for each external input matched based on the percentile of the in vivo cohort and trials were selected to represent the 0th, 17th, 33rd, 50th, 67th, 83rd, and 100th percentiles (Table 1). These loads were initiated immediately prior to release of the weight sled, such that the external loads were enacted on the knee at the time of impulse delivery. Trials were executed in a step-wise fashion and ramped from the 0th – 100th percentile. After the 100th percentile, trials were continued with 20% increases in magnitude until the damage was reported to the ACL or bony structure of the knee. This non-randomized order was necessary to preserve the maximum amount of bone stock for subsequent ACLRs. This behavior demonstrates the viability of each specimen to be utilized in the examination of multiple reconstruction techniques. Repeated measures testing to assess multiple ACLRs in a single specimen is an established principle of robotics models.6,71,72,75 However, a distinct difference between robotics simulations models and the mechanical impact simulator is that robotic models are not designed to investigate failure events.7 Further investigations would be required to determine whether isolated examination or a randomized test order would influence the survivability of BTB and QT grafts.
Table 1:
Magnitudes of external loads applied to the specimen knee joints.
| Percentile of in vivo Population Cohort | KAM Load (Nm) | ATS Load (N) | ITR Load (Nm) | Test Sequence |
|---|---|---|---|---|
| 0% | 1.7 | 47 | 1.0 | 1st |
| 17% | 9.4 | 56 | 5.5 | 2nd |
| 33% | 13.5 | 63 | 9.6 | 3rd |
| 50% | 17.4 | 73 | 14.6 | 4th |
| 67% | 26.8 | 80 | 18.6 | 5th |
| 84% | 36.5 | 93 | 23.1 | 6th |
| 100% | 57.3 | 196 | 53.7 | 7th |
| 120% | 68.8 | 235 | 64.4 | 8th |
| 140% | 80.2 | 274 | 75.2 | 9th |
| 160% | 91.7 | 314 | 85.9 | 10th |
| 180% | 103.1 | 353 | 96.7 | 11th |
| 200% | 114.6 | 392 | 107.4 | 12th |
Following the failure of the native ACL, a dual fellowship-trained orthopedic sports medicine surgeon (RKM) performed ACL reconstruction surgery with a hamstrings tendon allograft (HT) and step-wise testing was repeated until failure. Failure of the HT was subsequently followed by ACLR with a quadriceps tendon allograft (QT). The construct was again tested until failure of the QT allograft which was followed by ACLR with a bone-patellar-tendon-bone allograft (BTB). This final ACLR construct was then subjected to a final round of step-wise external load trials until failure occurred. Thus, each specimen was exposed to the impactor simulation protocol in a total of four ACL states. All ACLR grafts were prepared with allograft material from separate donor specimens with physically active lifestyles and no history of knee trauma or bone cancer. All activities were performed at Mayo Clinic and were approved as “not human subjects research” under IRB 15–005819.
Hamstrings Graft
The HT was prepared as a quadruple bundle graft using a previously described technique.28 Graft diameter was specified to be a minimum of 9.0 mm on both the femoral and tibial ends. ACL remnant from the native ligament was removed, then an ACLR was performed following the GraftLink (Arthrex, Inc., Naples, FL, USA) technique. The graft was secured with adjustable loop fixation on the femur (Tightrope, Arthrex, Inc., Naples, FL, USA) and tied over a button on the tibia (Tightrope Button, Arthrex, Inc., Naples, FL, USA), followed by tensioning of the graft construct in extension. Anterior drawer tests were performed to confirm graft integrity.
Quadriceps Graft
The QT was harvested with a patella bone block in the standard fashion. The bone plug end of the QT was prepared to a diameter of no less than 9.0 mm and the tibial side was prepared with a FiberLoop (Arthrex, Inc., Naples, FL, USA) whipstitch on the distal end. Graft diameter was specified to be a minimum of 9.0 mm on both the femoral and tibial ends. Fixation and graft material remaining from the HT ACLR were removed and the same anatomic tunnels utilized for the HT ACLR were reused for the QT ACLR. If necessary, the femoral tunnel was enlarged to match the size of the QT bone block. The tibial socket was reamed in a retrograde fashion with a FlipCutter (Arthrex, Inc., Naples, FL, USA) to create a full tibial tunnel. The QT was inserted through the tibial tunnel into the femoral tunnel and secured with a metal interference screw (Arthrex, Inc., Naples, FL, USA) with a minimum diameter of 9.0 mm. The specimen was then fully extended and the QT was pulled taught to the maximum possible manual tension provided by the surgeon. In this tensioned state, the QT was secured on the tibial side. Two types of fixation were utilized on separate specimens for the tibial side. Specimens 1 & 2 were tied over a button on the tibia (Tightrope Button, Arthrex, Inc., Naples, FL, USA), while specimens 3 & 4 were secured with a PEEK interference screw (Arthrex, Inc., Naples, FL, USA). Anterior drawer tests were performed to confirm graft integrity.
Bone-Patellar-Tendon-Bone Graft
The BTB bone plugs were prepared with a diameter of no less than 9.0 mm on either end. Fixation and graft tissue remaining from the QT ACLR were removed, and the same anatomic tunnels utilized for the QT ACLR were reused for the BTB ACLR. If necessary, the femoral and/or tibial tunnels were enlarged to match the size of the BTB bone blocks. The BTB was inserted through the tibial tunnel such that the proximal and distal bone plugs were fully enveloped within the femoral and tibial tunnels, respectively. Once the BTB graft was in position, the bone plug was secured in the femoral tunnel with a metal interference screw (Arthrex, Inc., Naples, FL, USA) with a minimum diameter of 10 mm. The specimen was then fully extended and the BTB was pulled taught to the maximum possible manual tension provided by the surgeon. In this tensioned state, the remaining bone plug was secured inside the tibial tunnel with a PEEK interference screw (Arthrex, Inc., Naples, FL, USA). Anterior drawer tests were performed to confirm graft integrity.
Data Analysis
Each specimen was affixed to a 6-axis load cell such that the knee joint center point was located 200 mm superior to the face of the load cell and the femoral shaft was aligned with the vertical axis. A transformation matrix was used to translate forces from the 6-axis load cell to the joint center and to rotate them relative to the tibia. In particular, the ATS force was recorded as the ACL has been shown to restrain up to 85% of the ATS force in the knee.20,21 In addition, custom barbed 3mm microminiature differential variance reluctance transducers (DVRT, LORD MicroStrain, Willingston, VT, USA) were implanted onto the native ACL and each of the ACLR grafts prior to mounting the specimen on the mechanical impact simulator. Once mounted, anterior and posterior drawer tests were performed on each specimen in each ACL state to identify the neutral strain length (slack length) of each ligament as has been previously described in the literature.9–13,44,54,57 Displacement recorded by the DVRT throughout each simulation was compared relative to the ligament slack length to continuously calculate ligament strain (Eq 1):
All data were sampled at 10 kHz and subsequently filtered through a fourth-order, zero lag low-pass Butterworth filter with a cutoff frequency of 50 Hz. Data sampling, filtering, and analysis were performed with customized LabVIEW (version 2019, National Instruments Co., Austin, TX, USA) and MATLAB (version 2019a, The MathWorks, Inc., Natick, MA, USA) software.
Due to the limited sample size of this pilot investigation (N = 4), values were reported as medians (25%, 75% quartile range). Nonparametric Kruskal-Wallis Tests were performed to determine the significance of ACL state relative to each dependent variable (ACL strain, ATS force, number of trials prior to rupture). Wilcoxon Each Pair tests were then performed post-hoc to assess peak strain, strain at IC, and ATS force differences individually between each pair of ACL states. Significance was set a priori at α < 0.05. Statistical analyses of ACL strain and ATS force were performed separately on the entire specimen cohort as well as on the sub-cohort of specimens that suffered substance failures of their respective ACL state.
RESULTS
All four specimens (Table 2) completed mechanical impact simulations for each of the four ACL states (native, HT, QT, and BTB). Grafts diameters were consistent between specimen and graft type (Table 3). There were no documented cases of graft instability or loosening following reconstruction, and reconstruction resolved anterior instability induced by prior simulations in all cases (Table 4). This was true across all specimens and graft types.
Table 2:
Specimen demographics
| Specimen | Age (years) | Height (cm) | Mass (kg) | BMI | Sex | Race | Side | Dominant |
|---|---|---|---|---|---|---|---|---|
| S1 | 34 | 178 | 94.3 | 30 | M | Caucasian | Left | Right |
| S2 | 46 | 185.5 | 78.9 | 23 | M | African | Left | Right |
| S3 | 36 | 162.5 | 80.3 | 30 | F | Caucasian | Right | Undisclosed |
| S4 | 47 | 157.5 | 74.8 | 30 | F | Caucasian | Right | Left |
| Median | 41 (34.5, 46.8) | 170.3 (158.8, 183.6) | 79.6 (75.8, 90.8) | 30 (24.8, 30) | --- | --- | --- | --- |
Table 3:
ACLR graft demographics for each respective specimen.
| Specimen | Graft | Femoral Diameter (mm) | Tibial Diameter (mm) | Total Length (mm) | Femoral Screw | Tibial Screw | Donor Age (years) | Donor Mass (kg) | Donor BMI | Donor Sex | Point of Failure |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | BTB | 10 | 10 | 110 | metal | PEEK | 40 | 117.9 | 37 | M | Both |
| S2 | BTB | 10 | 10 | 94 | metal | PEEK | 39 | 88.5 | 26 | M | Graft |
| S3 | BTB | 10 | 10 | 103 | metal | PEEK | 45 | 102.1 | 36 | F | Fixation |
| S4 | BTB | 10 | 10 | --- | metal | PEEK | 42 | 90.7 | 28 | M | Graft |
| S1 | Hamstring | 10.5 | 10 | 75 | --- | --- | 40 | 117.9 | 37 | M | Fixation |
| S2 | Hamstring | 9 | 10 | 70 | --- | --- | 39 | 88.5 | 26 | M | Fixation |
| S3 | Hamstring | 9 | 9 | 83 | --- | --- | 40 | 117.9 | 37 | M | Graft |
| S4 | Hamstring | 9 | 9 | 80 | --- | --- | 42 | 90.7 | 28 | M | Graft |
| S1 | Quadriceps | 10 | 9 | 65 | metal | --- | 40 | 117.9 | 37 | M | Fixation |
| S2 | Quadriceps | 10 | 10 | 80 | metal | --- | 39 | 88.5 | 26 | M | Graft |
| S3 | Quadriceps | 10 | 10 | 110 | metal | PEEK | 40 | 117.9 | 37 | M | Graft |
| S4 | Quadriceps | 10 | 10 | --- | metal | PEEK | 42 | 90.7 | 28 | M | Fixation |
Table 4:
Pre- and post-testing clinical evaluation scores for each ACL state. Clinical exams were conducted by a fellowship trained orthopedic surgeon (RKM).
| Specimen | ACL State | Anterior Drawer | Lachman | Posterior Drawer | Medial Stability | Lateral Stability | Other Notes | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PRE | POST | PRE | POST | PRE | POST | PRE | POST | PRE | POST | |||
| S1 | Native | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S1 | HT | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 3 | 0 | 0 | |
| S1 | QT | 0 | 2 | 0 | 2 | 0 | 0 | 3 | 3 | 0 | 0 | |
| S1 | BTB | 0 | 2 | 0 | 2 | 0 | 0 | 3 | 3 | 0 | 0 | |
| S2 | Native | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S2 | HT | 0 | 3 | 0 | 3 | 0 | 1 | 0 | 0 | 0 | 0 | |
| S2 | QT | 0 | 3 | 0 | 3 | 1 | 1 | 0 | 3 | 0 | 0 | MM post. Horn disruption |
| S2 | BTB | 0 | 3 | 0 | 3 | 1 | 1 | 3 | 3 | 0 | 0 | |
| S3 | Native | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | HT | 0 | 2 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | QT | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S3 | BTB | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | Native | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | HT | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | QT | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| S4 | BTB | 0 | 3 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
Ligament failure was documented in 100% of specimens tested in the native ACL state (Table 4 & 5). For the reconstructed states, graft failure was achieved most commonly in the BTB state (75%), followed by the HT and QT states (50% each; Table 3). ACL state was significant to the number of simulation trials survived by a specimen (P = 0.05). On average, rupture for the native ACL occurred during the ninth trial (140th loading percentile), while the BTB and QT grafts occurred during the fifth trial (67th loading percentile), and the HT grafts occurred during the second trial (17th loading percentile). For reconstructed states, graft disruption was the observed in 58% of cases, while fixation failure was observed in 50% of cases. For the BTB and QT states, fixation failures occurred at a 1:1 ratio between the tibial and femoral attachments; whereas, all HT fixation failures occurred at the tibial attachment. Fixation failures included suture disruption (x2), displacement of the tibial or femoral bone plug (x3), and TightRope Button fracture (x1). Concomitant structural damage was noted on the first reconstruction of S1, as the MCL was disrupted. Concomitant structural damage was also noted on the second reconstruction of S2 as the MCL and posterior horn of the medial meniscus were disrupted.
Table 5:
Failure outcomes for each respective ACL state.
| Ligament Type | Specimens Tested | Ligament Ruptures | Specimens | Complete | Partial | Non-compliant | Fixation Failures | Specimens | Femoral | Tibial | Fixation Failure Description | Number of Trials Until Failure | P-value vs. Native | P-value vs. BTB | P-value vs. HT | P-value vs. QT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native ACL | 4 | 4 | S1,S2,S3,S4 | 1 | 3 | 0 | --- | --- | --- | --- | --- | 10.0 (4.3, 13.5) | --- | 0.19 | 0.04* | 0.19 | |
| BTB | 4 | 3 | S1,S2,S4 | 0 | 3 | 0 | 2 | S1,S3 | 1 | 1 | Femoral plug loosening; Tibial plug pulled out | 5.5 (2.8, 6.8) | 0.19 | --- | 0.10 | 0.88 | |
| Hamstrings | 4 | 2 | S3,S4 | 0 | 1 | 1 | 2 | S1,S2 | 0 | 2 | Loosening of button suture; Button fracture | 2.0 (1.3, 2.8) | 0.04* | 0.10 | --- | 0.10 | |
| Quadriceps | 4 | 2 | S2,S3 | 0 | 1 | 1 | 2 | S1,S4 | 1 | 1 | Femoral plug pulled out; Tibial suture failure | 5.5 (2.8, 6.0) | 0.19 | 0.88 | 0.10 | --- |
Indicates statistically significant difference (P ≤ 0.05).
ACL state was not significant relative to median peak strain prior to failure (P = 0.07) or median strain at IC (P = 0.28). However, QT grafts did exhibit greater median peak strain prior to failure than HT grafts (Table 6). For failures that occurred in the ligament substance, ACL state was not significant relative to median peak strain prior to failure (P = 0.24) or at IC (P = 0.33; Table 7). ACL state was not significant to median peak strain prior to failure (P = 0.12) or median strain at IC (P = 0.18) relative to any individual magnitude of external loading applied at the knee (Figure 2). No HT grafts survived past the 17th percentile knee loading, QT grafts past the 67th percentile knee loading, or BTB grafts past the 100th percentile knee loading. ACL state was not significant to the median ATS force immediately prior to failure (P = 0.54) or ATS at IC (P = 0.27; Table 7).
Table 6:
Ligament strains and ATS force for all specimens (n = 16) with pairwise Wilcoxon P-values between each ligament state.
| Ligament Type | Number of Specimens | Peak ACL Strain (%) | P-value vs. Native ACL | P-value vs. BTB | P-value vs. HT | P-value vs. QT | ACL Strain @ IC (%) | P-value vs. Native ACL | P-value vs. BTB | P-value vs. HT | P-value vs. QT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native ACL | 4 | 20.3 (11.6, 24.5) | --- | 0.31 | 0.22 | 0.31 | 15.6 (4.6, 16.8) | --- | 0.19 | 0.22 | 0.47 | |
| BTB | 4 | 13.5 (4.2, 18.7) | 0.31 | --- | 0.86 | 0.06 | 4.1 (0.7, 11.8) | 0.19 | --- | 0.86 | 0.31 | |
| HT | 4 | 11.9 (6.2, 17.0) | 0.22 | 0.86 | --- | 0.05* | 5.3 (0.0, 7.7) | 0.22 | 0.86 | --- | 0.38 | |
| QT | 4 | 24.8 (19.6, 31.3) | 0.31 | 0.06 | 0.05* | --- | 8.3 (3.3, 14.2) | 0.47 | 0.31 | 0.38 | --- | |
| Ligament Type | Number of Specimens | Peak ATS (N) | P-value vs. Native | P-value vs. BTB | P-value vs. HT | P-value vs. QT | Peak ATS @ IC (N) | P-value vs. Native | P-value vs. BTB | P-value vs. HT | P-value vs. QT | |
| Native ACL | 4 | 768 (518, 1213) | --- | 0.67 | 0.38 | 0.47 | 75 (39, 424) | --- | 0.47 | 0.06 | 0.11 | |
| BTB | 4 | 634 (570, 744) | 0.67 | --- | 0.38 | 0.89 | 25 (−7, 106) | 0.47 | --- | 0.99 | 0.99 | |
| HT | 4 | 532 (255, 701) | 0.38 | 0.38 | --- | 0.38 | 25 (22, 29) | 0.06 | 0.99 | --- | 0.86 | |
| QT | 4 | 693 (495, 761) | 0.47 | 0.89 | 0.38 | --- | 23 (5, 84) | 0.11 | 0.99 | 0.86 | --- |
Indicates statistically significant difference (P ≤ 0.05).
Table 7:
Ligament strains and ATS force for only those specimens that experienced graft substance failures (n = 11) with pairwise Wilcoxon P-values between each ligament state. Specimens with isolated fixation failure were not included in this analysis.
| Ligament Type | Number of Specimens | Graft Failures Peak ACL Strain (%) | P-value vs. Native ACL | P-value vs. BTB | P-value vs. HT | P-value vs. QT | Graft Failures ACL Strain @ IC (%) | P-value vs. Native ACL | P-value vs. BTB | P-value vs. HT | P-value vs. QT | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Native ACL | 4 | 20.3 (11.6, 24.5) | --- | 0.38 | 0.25 | 0.25 | 15.6 (4.6, 16.8) | --- | 0.22 | 0.49 | 0.82 | |
| BTB | 3 | 9.5 (2.5, 19.1) | 0.38 | --- | 0.99 | 0.15 | 1.2 (0.6, 13.3) | 0.22 | --- | 0.77 | 0.39 | |
| HT | 2 | 11.6 (6.2, 17.0) | 0.25 | 0.99 | --- | 0.25 | 6.5 (5.3, 7.7) | 0.49 | 0.77 | --- | 0.25 | |
| QT | 2 | 28.1 (23.3, 33.0) | 0.25 | 0.15 | 0.25 | --- | 12.6 (9.3, 15.9) | 0.82 | 0.39 | 0.25 | --- | |
| Ligament Type | Number of Specimens | Graft Failures Peak ATS (N) | P-value vs. Native | P-value vs. BTB | P-value vs. HT | P-value vs. QT | Graft Failures Peak ATS @ IC (N) | P-value vs. Native | P-value vs. BTB | P-value vs. HT | P-value vs. QT | |
| Native ACL | 4 | 768 (518, 1213) | --- | 0.86 | 0.49 | 0.99 | 75 (39, 424) | --- | 0.86 | 0.11 | 0.49 | |
| BTB | 3 | 672 (562, 769) | 0.86 | --- | 0.78 | 0.39 | 47 (2, 126) | 0.86 | --- | 0.77 | 0.99 | |
| HT | 2 | 478 (255, 701) | 0.49 | 0.78 | --- | 0.25 | 27 (25, 29) | 0.11 | 0.77 | --- | 0.99 | |
| QT | 2 | 754 (740, 769) | 0.99 | 0.39 | 0.25 | --- | 61 (19, 103) | 0.49 | 0.99 | 0.99 | --- |
Indicates statistically significant difference (P ≤ 0.05).
Figure 2:
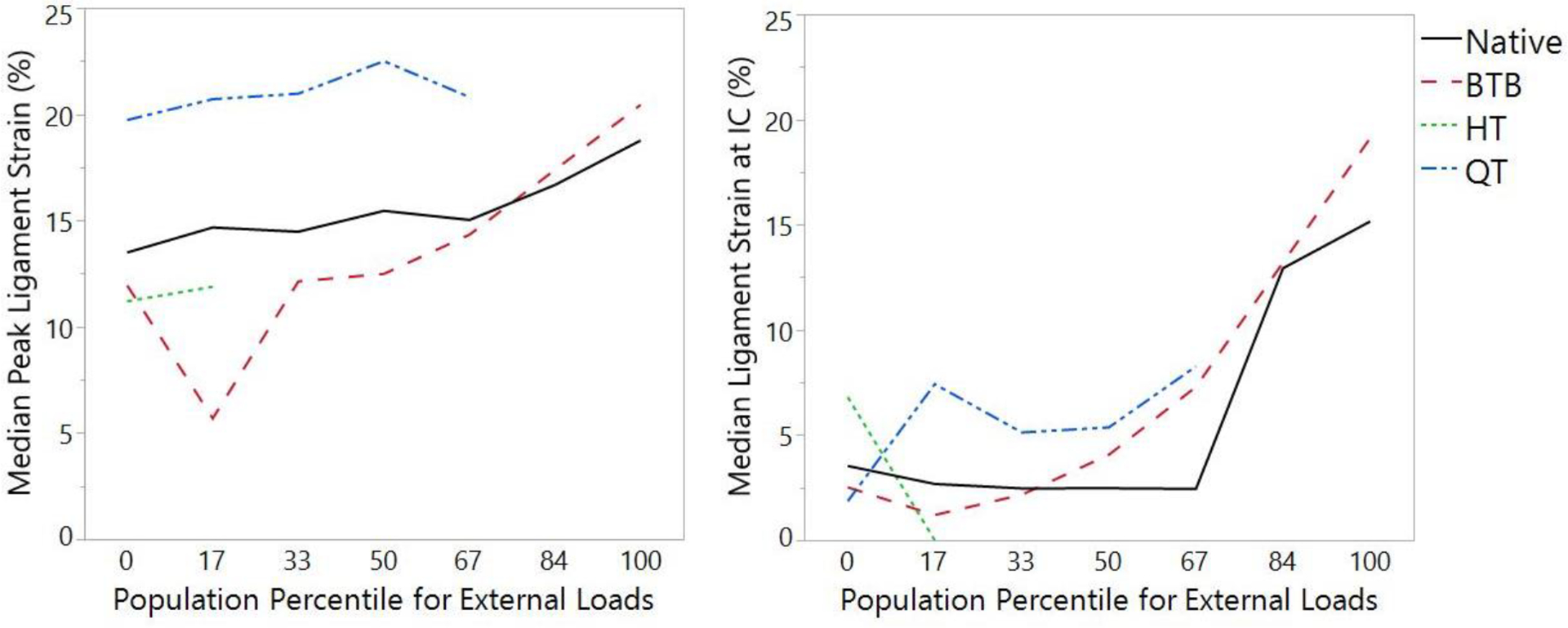
Median peak ligament strain for each ACL state at each magnitude of external loading (0th to 100th percentile of the in vivo cohort) applied to the specimen’s knee. Waveforms that do not span across all external loading indicate that no specimens survived impact simulation at the indicated loading for that ACL state.
DISCUSSION
Our mechanical impact simulator model partially satisfied the primary hypothesis as 58% of the ACLRs studied experienced non-fixation failures in the graft substance during injury simulation. Accordingly, the mechanical impact simulator demonstrated utility for the study of intra-articular ACLR graft mechanics following reconstruction. However, potential remains to improve this ACLR model as the simulator has documented successful creation of clinically-representative ruptures in 88% of native ACLs.13 Indeed, ligament rupture was realized in 100% of native ACL simulated for the present study. Further, utility of the impact simulator to investigate ACLR mechanics and rupture is limited by the magnitude of external loading that can be sustained by surgical graft constructs. Compared to native ACLs, ACLR grafts failed in half as many trials (or fewer). Only in S1 did an ACLR graft equal the loading of the native ACL as both the native ACL and BTB graft failed at the 33rd percentile trial. This outcome of native tissues performing superior to ACLR grafts was largely expected and mechanically justifies the activity restrictions that are recommended during rehabilitation after ACLR.1,16,30
The present simulations provided additional utility in that the data supports rehabilitation protocols that encourage immediate weight-bearing and gait following ACLR.2,22,64 Mechanically, only one graft on one specimen (HT, S1) was unable to survive impact during the baseline landing simulation. Previous study has indicated that landing from a 31 cm drop generates mean ground reaction force impulse of 4.1 * bodyweight, which is quadruple the 1.25 * bodyweight generated during gait.5,23,70 Therefore, the survival of all grafts and 96% of the graft fixation sites tested in the present investigation through at least one landing simulation indicated that time point zero grafts exhibit a sufficient safety factor to sustain gait. Further investigation is needed to make additional recommendations of mechanically-justified time points for patient rehabilitation from ACLR to initiate specific athletic tasks. As investigations of this nature would require graft healing, the current paradigm would need to be adjusted for animal models as has been done in other modalities of ACL investigation, although this would provide other limitations for relevancy to humans.17–19,24,25,47
The secondary hypothesis was partially supported as ACLR grafts did not express greater peak strain to failure than the native ACLs, but did fail with lower external loading applied to the joint than native ACLs. Of the various graft types, BTB and QT grafts survived the highest number of trials and, consequently, the highest magnitudes of loading during simulated landings. This is likely due to the use of fixation screws in all BTB grafts and half of the QT grafts. Literature has demonstrated that fixation screws offer stronger ultimate strength at the time of surgery than suspensory fixation.29,37,60 As such, it remains unsurprising that grafts secured with fixation screws proved to exhibit survivability under greater external loading in the current impact model. Both BTB and HT grafts exhibit ultimate tensile strength that is significantly greater than the native ligament (2977 N vs. 4140 N vs. 2160 N, respectively); thus, it is unlikely that HT grafts failed in fewer trials due to structural properties of the graft.26,27,31,73 In fact, despite similarities in tensile strength, a greater proportion of BTB ACLRs failed in the graft substance than the HT ACLRs. BTB and QT grafts expressed greater survivability than HT grafts despite being performed in a secondary and tertiary manner on each specimen, respectively. Likewise, QT graft tensile strength (2353 N)27,63 is lower than both HT and BTB grafts; yet, in the current study, QT grafts successfully sustained as many impacts as BTB grafts (Table 5). Accordingly, it is further unlikely that fixation failures were a byproduct of graft structural integrity.
The relevance of ACLR graft tensioning remains a contentious topic in the literature. In the current model, ACLR grafts were tensioned to the maximal ability of the operating surgeon. Potential discrepancies in mechanical behavior between ACLR constructs and the native ACL may be related to nearly one-third of athletes who suffer second ACL injury following return to sport.48,49,65,68 Accordingly, future investigations should quantify tension placed on ACLR grafts at the time of surgery and assess whether this variable associates with graft failure in a dynamic environment.
The obvious limitation of ACLR in the mechanical impact simulator is the lack of biologic healing available in a cadaveric model. Reduced survivability during simulated injury events in the present work explicitly confirmed that ACLRs, irrespective of graft type, were weaker mechanical constructs than the native ACL complex. While this may relate to the lack of healing in the current model, literature has also shown that it is faulty to assume that athletes return to sport only after biologic healing has resolved. Following ACLR, resolution of bony healing requires 12 months, graft remodeling requires 6–24 months (dependent on graft type), graft revascularization requires 24 months, return of neuromuscular deficits to baseline requires 24 months, and muscle strength deficits remain at 24 months.45 Further, ACLR grafts experience slight tissue degeneration for 3–10 months post-surgery which slowly dissipates for 1–3 years, cell proliferation occurs between 3–10 months post-surgery, and type III collagen returns to the native ACL rate around 3 years.53 Despite this, athletes are commonly cleared for return to sport 6–9 months after surgical intervention which, in some cases, makes the return to sport closer to minimal healing than to healing completion. Therefore, so long as the damage induced by the mechanical impact simulator model occurred within the ACLR graft complex and not the mechanical hardware used for fixation, the clinical relevance of the reported results is maintained relative to the incomplete or minimal healing throughout rehabilitation and at the return to sport. In all cases, from a mechanical and health standpoint, it may be wise to advocate delay of a full return to sport until the biologic healing has completely resolved. Lack of healing in the present model can be exacerbated when concomitant injuries occur during ACL rupture. As indicated in published literature, the mechanical impact simulator model generates concomitant MCL rupture in approximately 30% of ACL injuries in accordance with clinical presentation.13 For the present model, this meant that three ACLRs were examined under conditions where the MCL was compromised (Table 4). In future investigations, methods of MCL repair should be explored to restore medial knee stability following MCL damage.
Regardless of the healing limitation, 7 of 12 ACLR cases exhibited failure in the graft substance, which was directly indicative of graft integrity in a dynamic environment. In 6 of 7 cases, the ACLR graft failed earlier than the native ligament and the seventh case matched the native ACL. As literature indicates that both the native ACL and ACLRs are the primary restraint to ATS in the knee,20,21,47 each structure should have been subjected to similar loads from the matched, repeated-measures trials. Thus, the present failure outcomes demonstrated that ACLR graft complexes, at the time of reconstruction, offer reduced knee stability compared to the native ligament as grafts failed earlier in the stepwise simulations than the native ACLs. Unfortunately, graft substance failures only comprised 58% of ACLRs tested in the current model. This rate of return would make the mechanical impact simulator (as presented) both a cost and time-intensive model to study ACLRs. However, 75% of BTB grafts tested failed midsubstance, which indicated the mechanical impact simulator may be a sustainable model for that specific ACLR construct. As such the impact simulator model may offer the greatest viability with respect to BTB ACLR grafts that are secured with interference screws. Superior performance of the BTB graft in this impact simulation model is unsurprising as BTB grafts clinical exhibit more favorable performance than HT grafts in KT laxity and Pivot Shift tests,76 as well as failure rate outcomes.43,50,52,58,59 As previously noted, this investigative model can withstand multiple ACLRs performed on a single specimen. Further study is needed to assess whether multiple iterations of the same technique are viable with this model, as this would increase the probability of relevant graft failures and sustainability/utility of the model. Additional animal model study is also needed to determine whether the graft substance increases in ultimate failure strength or remains constant during biological healing.
An additional limitation of the current investigation, and most pilot studies, is the sample size. Significant statistical results were very limited in the present investigation due to the high biologic variability in ACL strain measurements that is documented in previous literature8,13,15 and examination of only four specimens. Prior mechanical impact simulator investigations analyzed between 19–40 specimens.9–13,44,46,55–57 Even with a repeated-measures test design for separate grafts in the same specimens, a repeated-measures within-between interactions power analysis based on the current data (lowest effect size 0.865, across 4 groups, with a median of 5 repeated measures per group, and a correlation between measures of 0.622) indicated that a minimum of eight specimens would be required. As such, the current pilot study is underpowered and the limited statistical significance observed between ACL groups should be considered accordingly.
CONCLUSION
The mechanical impact simulator provided a construct for the examination of ACLR mechanics in a rupture environment. Clinical relevance of the outcomes reported is dependent on the mechanism of failure demonstrated by each individual graft as lack of biologic healing can lead to non-representative failures in this model. As such, the integration of an animal surrogate into the mechanical impact simulator model would be ideal. ACLR graft ruptures in the current model indicated that ACLR complexes at the time of surgery are weaker than the native ligament when subjected to equivalent loading. Of the three common graft constructs, BTB most closely resembled the native ligament and provided the most consistently relevant rupture results.
HIGHLIGHTS.
Validated apparatus simulated physiologic landing forces on cadaveric limbs.
Each specimen was tested in native and three reconstructed conditions.
Ligament injuries were generated in all specimens and conditions.
Reconstruction complexes withstood less loading than the native ligament.
Bone tendon bone reconstructions best resembled the native ligament mechanics.
ACKNOWLEDGEMENTS
We acknowledge funding provided by NIH grants from the National Institute of Arthritis and Musculoskeletal and Skin Diseases R01-AR056259, R01-AR055563, L30-AR070273 and the National Institute of Children and Human Development K12-HD065987.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was performed at Mayo Clinic in Rochester, MN, USA
CONFLICT OF INTEREST STATEMENT
There were no potential conflicts of interest in the preparation of this manuscript. Specifically, there are no financial relationships with any manufacturers, including, but not limited to grants, honoraria, consulting fees, royalty fees, ownership, or support in preparation of the manuscript.
REFERENCES
- 1.Adams D, Logerstedt DS, Hunter-Giordano A, Axe MJ, Snyder-Mackler L. Current Concepts for Anterior Cruciate Ligament Reconstruction: A Criterion-Based Rehabilitation Progression. J Orthop Sports Phys Ther. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrade R, Pereira R, van Cingel R, Staal JB, Espregueira-Mendes J. How should clinicians rehabilitate patients after ACL reconstruction? A systematic review of clinical practice guidelines (CPGs) with a focus on quality appraisal (AGREE II). Br J Sports Med. 2019. [DOI] [PubMed] [Google Scholar]
- 3.Ardern CL, Taylor NF, Feller JA, Webster KE. Fifty-five per cent return to competitive sport following anterior cruciate ligament reconstruction surgery: an updated systematic review and meta-analysis including aspects of physical functioning and contextual factors. Br J Sports Med. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Arnold MP, Burger LD, Wirz D, Goepfert B, Hirschmann MT. The biomechanical strength of a hardware-free femoral press-fit method for ACL bone-tendon-bone graft fixation. Knee Surg Sports Traumatol Arthrosc. 2017;25(4):1234–1240. [DOI] [PubMed] [Google Scholar]
- 5.Bates NA, Ford KR, Myer GD, Hewett TE. Impact differences in ground reaction force and center of mass between the first and second landing phases of a drop vertical jump and their implications for injury risk assessment. J Biomech. 2013;46(7):1237–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bates NA, Myer GD, Shearn JT, Hewett TE. Anterior cruciate ligament biomechanics during robotic and mechanical simulations of physiologic and clinical motion tasks: a systematic review and meta-analysis. Clin Biomech. 2015;30(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. A Novel Methodology for the Simulation of Athletic Tasks on Cadaveric Knee Joints with Respect to In Vivo Kinematics. Ann Biomed Eng. 2015;43(10):2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bates NA, Nesbitt RJ, Shearn JT, Myer GD, Hewett TE. Relative strain in the anterior cruciate ligament and medial collateral ligament during simulated jump landing and sidestep cutting tasks: implications for injury risk. Am J Sports Med. 2015;43(9):2259–2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bates NA, Schilaty ND, Krych AJ, Hewett TE. Influence of relative injury risk profiles on ACL and MCL strain during simulated landing leading to a noncontact ACL injury event. Clin Biomech. 2019;69:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bates NA, Schilaty ND, Krych AJ, Hewett TE. Variation in ACL and MCL strain prior to initial contact is dependent on injury risk-level during simulated landings. Orthopedic Journal of Sports Medicine. 2019;7(11):2325967119884906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Multiplanar loading of the knee and its influence on ACL and MCL strain during simulated landings and noncontact tears. Am J Sport Med. 2019;47(8):1844–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Novel mechanical impact simulator designed to generate clinically relevant anterior cruciate ligament ruptures. Clin Biomech. 2017;44:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bates NA, Schilaty ND, Nagelli CV, Krych AJ, Hewett TE. Validation of Non-Contact Anterior Cruciate Ligament Tears Produced by a Mechanical Impact Simulator Against the Clinical Presentation of Injury. Am J Sport Med. 2018;46(9):2113–2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beaulieu ML, Wojtys EM, Ashton-Miller JA. Risk of anterior cruciate ligament fatigue failure is increased by limited internal femoral rotation during in vitro repeated pivot landings. Am J Sports Med. 2015;43(9):2233–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beynnon B, Howe JG, Pope MH, Johnson RJ, Fleming BC. The measurement of anterior cruciate ligament strain in vivo. Int Orthop. 1992;16(1):1–12. [DOI] [PubMed] [Google Scholar]
- 16.Beynnon BD, Johnson RJ, Naud S, et al. Accelerated versus nonaccelerated rehabilitation after anterior cruciate ligament reconstruction: a prospective, randomized, double-blind investigation evaluating knee joint laxity using roentgen stereophotogrammetric analysis. Am J Sports Med. 2011;39(12):2536–2548. [DOI] [PubMed] [Google Scholar]
- 17.Boguszewski DV, Shearn JT, Wagner CT, Butler DL. Investigating the effects of anterior tibial translation on anterior knee force in the porcine model: is the porcine knee ACL dependent? J Orthop Res. 2011;29(5):641–646. [DOI] [PubMed] [Google Scholar]
- 18.Boguszewski DV, Wagner CT, Butler DL, Shearn JT. Effect of ACL graft material on anterior knee force during simulated in vivo ovine motion applied to the porcine knee: An in vitro examination of force during 2000 cycles. J Orthop Res. 2015;33(12):1789–1795. [DOI] [PubMed] [Google Scholar]
- 19.Boguszewski DV, Wagner CT, Butler DL, Shearn JT. Effect of ACL graft material on joint forces during a simulated in vivo motion in the porcine knee: examining force during the initial cycles. J Orthop Res. 2014;32(11):1458–1463. [DOI] [PubMed] [Google Scholar]
- 20.Butler DL, Guan Y, Kay MD, et al. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech. 1992;25(5):511–518. [DOI] [PubMed] [Google Scholar]
- 21.Butler DL, Noyes FR, Grood ES. Ligamentous restraints to anterior-posterior drawer in the human knee. A biomechanical study. J Bone Joint Surg Am. 1980;62(2):259–270. [PubMed] [Google Scholar]
- 22.Cavanaugh JT, Powers M. ACL Rehabilitation Progression: Where Are We Now? Curr Rev Musculoskelet Med. 2017;10(3):289–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chao EY, Laughman RK, Schneider E, Stauffer RN. Normative data of knee joint motion and ground reaction forces in adult level walking. J Biomech. 1983;16(3):219–233. [DOI] [PubMed] [Google Scholar]
- 24.Cook JL, Smith P, Stannard JP, et al. A canine arthorscopic anterior cruciate ligament reconstruction model for study of synthetic augmentation of tendon allografts. J Knee Surg. 2017;30(7):704–711. [DOI] [PubMed] [Google Scholar]
- 25.Cook JL, Smith PA, Stannard JP, et al. A canine hybrid double-bundle model for study of arthroscopic ACL reconstruction. J Orthop Res. 2015;33(8):1171–1179. [DOI] [PubMed] [Google Scholar]
- 26.Cooper DE, Xianghua D, Burstein AL, Warren RF. The strength of the central third patellar tendon graft: A biomechanical study. Am J Sport Med. 1993;21(6):818–824. [DOI] [PubMed] [Google Scholar]
- 27.Dargel J, Gotter M, Mader K, et al. Biomechanics of the anterior cruciate ligament and implications for surgical reconstruction. Strategies Trauma Limb Reconstr. 2007;2(1):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Desai VS, Anderson GR, Wu IT, et al. Anterior Cruciate Ligament Reconstruction With Hamstring Autograft: A Matched Cohort Comparison of the All-Inside and Complete Tibial Tunnel Techniques. Orthopaedic journal of sports medicine. 2019;7(1):2325967118820297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eguchi A, Ochi M, Adachi N, et al. Mechanical properties of suspensory fixation devices for anterior cruciate ligament reconstruction: comparison of the fixed-length loop device versus the adjustable-length loop device. Knee. 2014;21(3):743–748. [DOI] [PubMed] [Google Scholar]
- 30.Escamilla RF, Macleod TD, Wilk KE, Paulos L, Andrews JR. Anterior cruciate ligament strain and tensile forces for weight-bearing and non-weight-bearing exercises: a guide to exercise selection. J Orthop Sports Phys Ther. 2012;42(3):208–220. [DOI] [PubMed] [Google Scholar]
- 31.Hamner DL, Brown CH, Steiner ME, Hecker AT, Hayes WC. Hamstring Tendon Grafts for Reconstruction of the Anterior Cruciate Ligament: Biomechanical Evaluation of the Use of Multiple Strands and Tensioning Techniques. The Journal of Bone & Joint Surgery. 1999;81-A(4):549–557. [DOI] [PubMed] [Google Scholar]
- 32.Hashemi J, Breighner R, Jang TH, et al. Increasing pre-activation of the quadriceps muscle protects the anterior cruciate ligament during the landing phase of a jump: an in vitro simulation. Knee. 2010;17(3):235–241. [DOI] [PubMed] [Google Scholar]
- 33.Illingworth KD, Hensler D, Working ZM, et al. A simple evaluation of anterior cruciate ligament femoral tunnel position: the inclination angle and femoral tunnel angle. Am J Sports Med. 2011;39(12):2611–2618. [DOI] [PubMed] [Google Scholar]
- 34.Johnson DL, Warner JJP. Diagnosis for anterior cruciate ligament surgery. Clin Sports Med. 1993;12(4):671–684. [PubMed] [Google Scholar]
- 35.Kiapour AM, Demetropoulos CK, Kiapour A, et al. Strain Response of the Anterior Cruciate Ligament to Uniplanar and Multiplanar Loads During Simulated Landings: Implications for Injury Mechanism. Am J Sports Med. 2016;44(8):2087–2096. [DOI] [PubMed] [Google Scholar]
- 36.Kim S, Bosque J, Meehan JP, Jamali A, Marder R. Increase in outpatient knee arthroscopy in the United States: a comparison of National Surveys of Ambulatory Surgery, 1996 and 2006. J Bone Joint Surg Am. 2011;93(11):994–1000. [DOI] [PubMed] [Google Scholar]
- 37.Kousa P, Jarvinen TL, Vihavainen M, Kannus P, Jarvinen M. The fixation strength of six hamstring tendon graft fixation devices in anterior cruciate ligament reconstruction. Part II: tibial site. Am J Sports Med. 2003;31(2):182–188. [DOI] [PubMed] [Google Scholar]
- 38.Lai CC, Ardern CL, Feller JA, Webster KE. Eighty-three per cent of elite athletes return to preinjury sport after anterior cruciate ligament reconstruction: a systematic review with meta-analysis of return to sport rates, graft rupture rates and performance outcomes. Br J Sports Med. 2017. [DOI] [PubMed] [Google Scholar]
- 39.Levine JW, Kiapour AM, Quatman CE, et al. Clinically relevant injury patterns after an anterior cruciate ligament injury provide insight into injury mechanisms. Am J Sports Med. 2013;41(2):385–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin KM, Boyle C, Marom N, Marx RG. Graft selection in anterior cruciate ligament reconstruction. Sports Med Arthrosc Rev. 2020;28(2):41–48. [DOI] [PubMed] [Google Scholar]
- 41.Lipps DB, Oh YK, Ashton-Miller JA, Wojtys EM. Effect of increased quadriceps tensile stiffness on peak anterior cruciate ligametn strain during a simulated pivot landing. J Orthop Res. 2014;32(3):423–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Appl Bionics Biomech. 2018;2018:4657824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McMurray NS, Bates NA, Fischer S, Schilaty ND, Hewett TE. Investigation of second anterior cruciate ligament tears and associated risk factors from 2011 to 2016 using a geographic database. Int J Sports Phys Ther. 2020;Accepted: in-press. [PMC free article] [PubMed] [Google Scholar]
- 44.McPherson AL, Bates NA, Schilaty ND, et al. Ligament strain response between lower extremity contralateral pairs during in vitro landing simulation. Orthopaedic journal of sports medicine. 2018;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nagelli CV, Hewett TE. Should Return to Sport be Delayed Until 2 Years After Anterior Cruciate Ligament Reconstruction? Biological and Functional Considerations. Sports Med. 2017;47(2):221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Navacchia A, Bates NA, Schilaty ND, Krych AJ, Hewett TE. Knee Abduction and Internal Rotation Moments Increase ACL Force During Landing Through the Posterior Slope of the Tibia. J Orthop Res. 2019;37(8):1730–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nesbitt RJ, Herfat ST, Boguszewski DV, et al. Primary and secondary restraints of human and ovine knees for simulated in vivo gait kinematics. J Biomech. 2014;47(9):2022–2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paterno MV, Rauh MJ, Schmitt LC, Ford KR, Hewett TE. Incidence of Second ACL Injuries 2 Years After Primary ACL Reconstruction and Return to Sport. Am J Sports Med. 2014;42(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinczewski L, Morgan M, Salmon LJ, et al. 15 year survival of endoscopic anterior cruciate ligament reconstruction in patients aged 18 years and under. Orthopaedic journal of sports medicine. 2015;3(3):Suppl 1. [DOI] [PubMed] [Google Scholar]
- 50.Reinhardt KR, Hetsroni I, Marx RG. Graft selection for anterior cruciate ligament reconstruction: a level I systematic review comparing failure rates and functional outcomes. Orthop Clin North Am. 2010;41(2):249–262. [DOI] [PubMed] [Google Scholar]
- 51.Renstrom P, Arms SW, Stanwyck TS, Johnson RJ, Pope MH. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am J Sports Med. 1986;14(1):83–87. [DOI] [PubMed] [Google Scholar]
- 52.Samuelsen BT, Webster KE, Johnson NR, Hewett TE, Krych AJ. Hamstring Autograft versus Patellar Tendon Autograft for ACL Reconstruction: Is There a Difference in Graft Failure Rate? A Meta-analysis of 47,613 Patients. Clin Orthop Relat Res. 2017;475(10):2459–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scheffler SU, Unterhauser FN, Weiler A. Graft remodeling and ligamentization after cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 2008;16(9):834–842. [DOI] [PubMed] [Google Scholar]
- 54.Schilaty ND, Bates NA, Hewett TE. Effect of Sagittal Plane Mechanics on ACL Strain During Jump Landing. J Orthop Res. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schilaty ND, Bates NA, Krych AJ, Hewett TE. Frontal Plane Loading Characteristics of Medial Collateral Ligament Strain Concurrent With Anterior Cruciate Ligament Failure. Am J Sport Med. 2019;47(9):2143–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schilaty ND, Bates NA, Nagelli C, Krych AJ, Hewett TE. Sex differences of medial collateral and anterior cruciate ligament strains with cadaveric impact simulations. Orthopaedic journal of sports medicine. 2018;6(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schilaty ND, Bates NA, Nagelli CV, Krych AJ, Hewett TE. Sex Differences of Knee Kinetics that Occur with Anterior Cruciate Ligament Strain on Cadaveric Impact Simulations. Orthopedic Journal of Sports Medicine. 2018;6(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schilaty ND, Bates NA, Sanders TL, et al. Incidence of Second Anterior Cruciate Ligament Tears (1990–2000) and Associated Factors in a Specific Geographic Locale. Am J Sports Med. 2017;45(7):1567–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schilaty ND, Nagelli C, Bates NA, et al. Incidence of Second Anterior Cruciate Ligament Tears and Identification of Associated Risk Factors from 2001 – 2010 using a Geographic Database. Orthopaedic journal of sports medicine. 2017;5(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Seil R, Rupp S, Krauss PW, Benz A, Kohn DM. Comparison of initial fixation strength between biodegradable and metallic interference screws and a press-fit fixation technique in a porcine model. Am J Sport Med. 1998;26(6):815–819. [DOI] [PubMed] [Google Scholar]
- 61.Shimokochi Y, Shultz SJ. Mechanisms of noncontact anterior cruciate ligament injury. J Athl Train. 2008;43(4):396–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith PA, DeBerardino TM. Tibial fixation properties of a continuous-loop ACL hamstring graft construct with suspensory fixation in porcine bone. J Knee Surg. 2015;28(6):506–512. [DOI] [PubMed] [Google Scholar]
- 63.Staubli HU, Schatzmann L, Brunner P, Rincon L, Nolte LP. Quadriceps tendon and patellar ligament: cryosectional anatomy and structural properties in young adults. Knee Surg Sports Traumatol Arthrosc. 1996;4(2):100–110. [DOI] [PubMed] [Google Scholar]
- 64.Tyler TF, McHugh MP, Gleim GW, Nicholas SJ. The effect of immediate weightbearing after anterior cruciate ligament reconstruction. Clin Orthop Relat Res. 1998;357:141–148. [DOI] [PubMed] [Google Scholar]
- 65.Webster KE, Feller JA. Exploring the high reinjury rate in younger patients undergoing anterior cruciate ligament reconstruction. Am J Sport Med. 2016;44(11):2827–2832. [DOI] [PubMed] [Google Scholar]
- 66.Webster KE, Feller JA. Return to Level I Sports After Anterior Cruciate Ligament Reconstruction: Evaluation of Age, Sex, and Readiness to Return Criteria. Orthopaedic journal of sports medicine. 2018;6(8):2325967118788045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Webster KE, Feller JA, Kimp AJ, Whitehead TS. Low Rates of Return to Preinjury Sport After Bilateral Anterior Cruciate Ligament Reconstruction. Am J Sports Med. 2019;47(2):334–338. [DOI] [PubMed] [Google Scholar]
- 68.Webster KE, Feller JA, Leigh WB, Richmond AK. Younger patients are at increased risk for graft rupture and contralateral injury after anterior cruciate ligament reconstruction. Am J Sports Med. 2014;42(3):641–647. [DOI] [PubMed] [Google Scholar]
- 69.Webster KE, Feller JA, Whitehead TS, Myer GD, Merory PB. Return to Sport in the Younger Patient With Anterior Cruciate Ligament Reconstruction. Orthopaedic journal of sports medicine. 2017;5(4):2325967117703399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Winter DA. Biomechanics and Motor Control of Human Movement. 3rd ed. ed. New York: John Wiley & Sons, Inc.; 2005. [Google Scholar]
- 71.Woo SL, Abramowitch SD, Kilger R, Liang R. Biomechanics of knee ligaments: injury, healing, and repair. J Biomech. 2006;39(1):1–20. [DOI] [PubMed] [Google Scholar]
- 72.Woo SL, Debski RE, Vangura AJ, et al. Use of robotic technology to study the biomechanics of ligaments and their replacements. Operative Techniques in Orthopaedics. 2000;10(1):87–91. [Google Scholar]
- 73.Woo SL, Hollis JM, Adams DJ, Lyon RM, Takai S. Tensile properties of the human femur-anterior cruciate ligament-tibia complex: the effects of specimen age and orientation. Am J Sport Med. 1991;19(3):217–227. [DOI] [PubMed] [Google Scholar]
- 74.Woo SL, Wu C, Dede O, Vercillo F, Noorani S. Biomechanics and anterior cruciate ligament reconstruction. J Orthop Surg Res. 2006;1:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Woo SLY. Evaluation of Knee Stability with Use of a Robotic System. The Journal of Bone and Joint Surgery (American). 2009;91(Supplement_1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xie X, Liu X, Chen Z, et al. A meta-analysis of bone-patellar tendon-bone autograft versus four-strand hamstring tendon autograft for anterior cruciate ligament reconstruction. Knee. 2015;22(2):100–110. [DOI] [PubMed] [Google Scholar]


