Highlights
-
•
CBT-based intervention has a positive effect on health outcomes in patients with hypertension.
-
•
CBT-based intervention might be more effective for blood pressure management in hypertension patients when it is group-based, long term, and cognitive therapy based.
Keywords: Hypertension, Cognitive behavioral therapy, Physiological indicators, Psychological indicators, Quality of sleep
Abstract
Recently, the benefits of cognitive behavioral therapy (CBT)-based interventions for patients with hypertension have been recognized, but there has been no systematic review that has comprehensively analyzed the efficacy of CBT on health outcomes in this population. We aimed to explore the therapeutic effect of CBT-based interventions on hypertension patients through a meta-analysis.
Relevant randomized controlled trials (RCTs) were obtained by searching electronic databases. The primary outcomes were physiological indicators (blood pressure, blood lipid profile). Secondary outcomes were psychological indicators (anxiety, depression), and the quality of sleep. Stata version 15.0 software was used to analyze the results.
A total of 15 RCTs were included. The main analysis revealed that CBT-based interventions reduced systolic pressure: −8.67 (95% CI: −10.67 to −6.67, P = 0.000); diastolic pressure: −5.82 (95% CI: −7.82 to −3.81, P = 0.000); total cholesterol levels: −0.43 (95% CI: −0.76 to −0.10, P = 0.010); depressive symptoms: −3.13 (95% CI: −4.02 to −2.24, P = 0.000); anxiety symptoms: −3.63 (95% CI: −4.40 to −2.87, P = 0.000); and improved quality of sleep: −2.93 (95% CI: −4.40 to −1.47, P = 0.000). Additionally, the results of subgroup analysis indicated that long-term group-based CBT-based interventions were particularly beneficial for blood pressure management in hypertension patients.
CBT-based interventions are effective in reducing systolic pressure, diastolic pressure, total cholesterol levels, anxiety symptoms, depressive symptoms, and improving quality of sleep in hypertension patients.
1. Introduction
Hypertension is a chronic disease characterized by continuously elevated arterial blood pressure. It is an important cause of, and a risk factor for, cardiovascular and cerebrovascular diseases (Mills et al., 2016), affecting the structure and function of the heart, brain, kidneys, and other important organs (Ndanuko et al., 2016). It causes myocardial infarction, heart failure, chronic kidney disease, and other complications, including high fatality and disability rates (Biswas et al., 2003). The latest data indicates that the number of adults with hypertension will reach 1.5 billion by 2025, which is about 30% of the world’s population (Hu et al., 2015, Li et al., 2015). It is now a global problem, and is deleterious for human physical and mental health (Liu et al., 2017b) and imposes a heavy burden on the patient, their family, and society. Therefore, effectively preventing and treating hypertension is of particular importance.
Currently, drug therapy is the main treatment for high blood pressure (Mann, 2011), and lifestyle changes are also highly recommended (Williams et al., 2018). However, due to the long course of the disease and duration of the need for medication, patients are prone to negative emotions such as anxiety and depression during treatment (Kretchy et al., 2014). Furthermore, these psychological problems have become an important factor affecting the occurrence and development of hypertension (Jonas et al., 1997, Rutledge and Hogan, 2002). Therefore, in the treatment of hypertension, timely adoption of psychological interventions may be conducive to the treatment and prognosis of the disease. Cognitive behavioral therapy (CBT) is a group of short-term psychological therapies that aim to change unreasonable cognitions and thereby eliminate dysfunctional behaviors (Creswell et al., 2010). CBT can effectively solve general psychological problems and is often used to treat depression, anxiety, sleep disorders, and chronic pain (McMain et al., 2015). In recent years, an increasing number of studies (Abgrall-Barbry and Consoli, 2006, Liu et al., 2017a, Xue et al., 2008) have applied CBT as an intervention for hypertension. Abgrall-Barbry and colleagues (Abgrall-Barbry and Consoli, 2006) compared the therapeutic effects of CBT, relaxation, meditation, and biofeedback therapy on hypertension, showing that these methods had an anti-hypertensive effect, with CBT being the most efficacious. Xue and colleagues (Xue et al., 2008) conducted a five-week group cognitive behavioral self-management project for patients with mild-to-moderate essential hypertension to evaluate its benefits for blood pressure management and found that patients’ blood pressure decreased significantly. Similarly, Lei Liu and colleagues (Liu et al., 2017a) conducted a cohort study on hypertensive patients in the Chinese working population and found that a psychological intervention based on CBT plus medication was more effective in improving blood pressure compared to usual medication alone. However, Nolan and colleagues (Nolan et al., 2018) conducted a remote intervention based on CBT for hypertension patients and found the difference in systolic blood pressure reduction between the intervention group and the control group was statistically significant, whereas the change in diastolic blood pressure was not.
The results of the above studies of CBT-based interventions for hypertension are inconsistent, and there are few relevant meta-analyses. To address this gap in the research we undertook a systematic review of the literature to evaluate whether comprehensive CBT-based interventions have a positive effect on physiological and psychological indicators and the quality of sleep in hypertension patients. In doing so, we aimed to provide a scientific basis for CBT intervention therapy in patients with hypertension and to provide references for how to design appropriate CBT-based interventions efficiently.
2. Methods
This systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Moher et al., 2009). The review protocol was registered at the PROSPERO International Prospective Register of Systematic Reviews (Registration ID: CRD42020213587 PROSPERO 2020 website: https://www.crd.york.ac.uk/prospero/#recordDetails). Ethical approval and patient consent were not required as this was a systematic review and meta-analysis of previously published studies.
2.1. Search strategy
Databases searched included PubMed, Embase, Cochrane Central Register of Controlled Trials, Scopus, Proquest, Web of Science, CINAHL and Chinese databases (WanFang, China National Knowledge Infrastructure). Key search words were “hypertension” and “cognitive behavioral therapy”. We searched using the form of subject words + free words with Boolean operators AND/OR in the abstract, key words, or title, with a language limitation of English and Chinese. In the process of retrieval, the search terms were modified according to the search rules for the different databases. We also searched the reference lists of the original papers to find additional relevant articles. Our retrieval time was from inception to October 2020. Articles collected were managed by Endnote X8 Software (Clarivate Analytics, PA, USA). Two researchers conducted literature reviews separately. In case of disagreement, a third researcher was consulted, and consensus reached.
2.2. Study selection
Inclusion criteria were developed using the population, intervention, control, outcomes, study type (PICOS) approach:
-
(1)
P: The target population was adults (≥18) with essential hypertension regardless of disease stage and severity, including grade I hypertension, grade II hypertension, grade III hypertension, and isolated systolic hypertension. Participants in this review were diagnosed with hypertension according to established definitions or guidelines. Trials that reported the recruitment of subjects with definite hypertension but without specific diagnostic criteria were also included.
-
(2)
I: Interventions were described as CBT or based on CBT principles. The strategies had to be under the umbrella of CBT including cognitive therapy and behavioral therapy, and common CBT techniques such as problem-solving, relaxation, goal-setting, behavioral experiments, and cognitive restructuring. The interventions could be CBT alone or CBT combined with other methods, delivered face-to-face or remotely (e.g., via telephone and internet) and used in individual or a group form.
-
(3)
C: The control conditions included non-CBT interventions (e.g., medication, education), or usual care or wait list. If there were multiple comparison groups, we chose the usual care group.
-
(4)
O: The primary outcomes were physiological indicators (blood pressure, blood lipid profile: low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), triglycerides (TG), total cholesterol). Secondary outcomes included psychological indicators (anxiety, depression), and the quality of sleep. We used the Pittsburgh Sleep Quality Index (PSQI) score to represent the quality of sleep. Articles reporting one or more of the above outcomes were included.
-
(5)
Studies had to involve a randomized controlled trial (RCT) design, with no restrictions on the length of follow-up.
-
(6)
Articles written in English or Chinese.
-
(7)
Accessibility of full-text publication.
The study exclusion criteria were:
-
(1)
Participants with cognitive impairment or substance abuse.
-
(2)
Solely cognitive or behavioral interventions as opposed to a comprehensive, integrated CBT approach;
-
(3)
Lacking quantitative analysis;
-
(4)
Literature reviews or protocols, incomplete in terms of data used or inconsistent statistical methods;
-
(5)
Duplicate publications;
-
(6)
Judged to be of low quality on the PEDro tool (Verhagen et al., 1998);
-
(7)
Not peer-reviewed journal articles.
2.3. Data extraction
The following information was extracted: (a) Basic information, including first author, year of publication, country; (b) Study design, including information on participants (number, age, gender, diseases), drop-out rates, frequency/length of follow-up, intervention method, comparison group, outcomes. Data were extracted independently by two researchers. Where data was incomplete the corresponding author was contacted to obtain the data. The primary outcome variables were physiological indicators (blood pressure, blood lipid profile: LDL-C, HDL-C, TG, total cholesterol). Secondary outcomes included psychological indicators (anxiety, depression), and the quality of sleep.
2.4. Quality assessment
Two reviewers independently read the full texts of the included articles and assessed their methodological quality using the PEDro tool (Verhagen et al., 1998). PEDro includes ten items: random allocation of subjects into groups, concealed randomization, similarity of baseline information between groups, blinding to subjects, blinding to assessors and researchers, attrition rate, use of “intention to treat” analysis, use of variability measures, and use of between-group comparison methods. Based on these ten items, PEDro categorizes the quality of studies into three levels: high quality (8 or more points), moderate quality (4–7 points), and lower quality (3 points or less). If there were disagreements in rating the quality of the included studies, they were resolved through consultation with a third researcher.
2.5. Statistical analysis
Stata version 15.0 software (Harris et al., 2008) was used for analysis, and a p-value <0.05 was considered to be statistically significant. A separate meta-analysis was performed for each outcome variable. The pooled mean difference (MD), with a 95% confidence interval (CI) was used for continuous outcome variables. Standardized mean difference (SMD) and 95% CI were used to measure the effect size of continuous outcome variables. When the SMD was between 0.2 and 0.5, the effect size was small; between 0.5 and 0.7, this was medium; and more than 0.7, this was large. The significance level was set as 0.05 (two-sided).
The Chi-square test and I2 were used for heterogeneity testing among the included articles. If I2 < 50% or P>0.05, the level of homogeneity was considered good and if I2 > 50%, it was considered to be heterogeneous. The random-effect model was adopted no matter the heterogeneity. We conducted sensitivity analysis by removing each individual study at a time from the meta-analysis to evaluate the stability of the pooled results and investigate the potential source of the heterogeneity if it was significant. To explore the heterogeneity, we performed subgroup analysis based on the country (developed country vs. developing country); intervention type (CBT combined with other interventions vs. CBT alone); treatment form (group vs. individual); treatment course (≥12 weeks vs. <12 weeks); duration of session (≥50 min vs. <50 min); number of session (≥10 vs. <10); mode of delivery (remote vs. face-to-face); use of a hypertension-specific manual (yes vs. no); drop-out rate (≥20% vs. <20%); patients with comorbid mood symptoms (yes vs. no); and treatment used specific components of CBT (yes vs. no). The definition of using components for CBT was based on the Comprehensive Psycho-therapeutic Intervention Rating Scale and previous studies (Koelen et al., 2014, Liu et al., 2019, Trijsburg et al., 2002, Yang et al., 2020). The following components of CBT were included in the subgroup analysis: psychoeducation, behavioral strategies, cognitive strategies, affective strategies, interpersonal strategies, exposure, body-directed strategies, behavior experiments, mindfulness and attention, homework assignments, goal-setting and planning, problem-solving, stress management, dietary interventions, and physical activity. These components were identified as “yes” (mentioned as an important technique), or “no” (not mentioned and not a core technique).
Publication bias was evaluated using the Egger test. A p-value of less than 0.05 represented statistically significant publication bias. If the number of meta-analysis studies was 10 or above, a funnel plot was used to analyze whether there was a publication bias.
3. Results
3.1. Literature search
A total of 1781 articles were included, including 1780 articles from literature retrieval and one article from references of a relevant review and meta-analysis. After removing duplicate articles, 1376 articles were screened for titles and abstracts. From these, 1304 publications were identified and discarded, including those that clearly did not fulfill the inclusion criteria. Finally, 72 articles were retrieved for full-text screening. During this assessment, two researchers read the full text of the article independently, screened and excluded all articles strictly according to the inclusion rules, and carefully recorded the reasons for the exclusion. In the case of any disagreement, a third researcher was invited to review the article until consensus was reached. Through full-text screening, 57 articles were excluded for the following reasons: protocol or review; non-English or Chinese; unrelated subjects; non-CBT-based intervention; no control group; non-RCT; PEDro ≤ 3; no access to the full article, or insufficient data. The specific process of identifying relevant articles for inclusion in the systematic review and meta-analysis is described in Fig. 1. This resulted in 15 studies being included in the meta-analysis.
Fig. 1.
PRISMA flow diagram.
3.2. Study characteristics
Full details of the included studies are displayed in Table 1. A total of 2195 participants were included in the 15 RCTs. Among these, 1102 participants were in intervention groups and 1093 in control groups. The mean age of those in the intervention groups was 55.40 and 55.23 in control groups. The mean proportions of females were 48.92% and 47.38% in the intervention and control groups, respectively. Twelve studies were undertaken in developing countries (Birashk et al., 2018, Hualei et al., 2013, Jing, 2020, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yu et al., 2018, Yuanyuan, 2017, Yurong et al., 2012) and three in developed countries (Clemow et al., 2018, Mensorio et al., 2019, Sung et al., 2012).
Table 1.
Characteristics of randomized controlled trials included in this meta-analysis.
| Author | Country | Setting | Sample size (intervention/control) | Gender: female, n(%) | Age (Mean ± SD) | Hypertension diagnosis | Intervention | Format | Manual | Control group | Outcome measure | Dropout rate (%) | Quality of article |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Birashk et al. (2018) (Birashk et al., 2018) | Iran | health center and hospital | 60 (20/20/20)1 | NR | NR | essential hypertension | CBT + drug therapy | group | yes | MBSR; drug therapy | ① | I: 25.00 C1: 25.00 C2: 20.00 | 4(moderate) |
| Chao et al. (2019) (Qing et al., 2019) | China | hospital | 400 (200/200) | I: 92(46.00) C: 93(46.50) | I: 50.94 ± 6.84C: 51.48 ± 6.83 | WHO diagnostic criteria for hypertension | CBT + TAU | individual | no | TAU | ②③ | NR | 5(moderate) |
| Clemow et al. (2018) (Clemow et al., 2018) | USA | urban medical center | 92 (46/46) | I: 38(83.00) C: 33(72.00) | I: 48.40 ± 8.40C: 48.70 ± 9.00 | SBP: 140–180 mm Hg DBP: 90–110 mm Hg | CBT | group | yes | TAU | ①② | I: 13.04C: 10.87 | 7(moderate) |
| Fu (2010) (Qingmei, 2010) | China | hospital | 80 (40/40) | I: 19(47.50) C: 17(42.50) | I: 43.12 ± 6.45C: 43.28 ± 6.25 | WHO diagnostic criteria for hypertension | CBT + drug therapy | NR | no | drug therapy | ①② | NR | 5(moderate) |
| Huang et al.(2013) (Hualei et al., 2013) | China | community health service center | 599 (302/297) | I: 187(61.92) C: 181(60.94) | I: 47.41 ± 8.09C: 47.96 ± 7.94 | mild hypertension SBP: 140–159 mm Hg DBP: 90–99 mm Hg | CBT + drug therapy | group | no | drug therapy | ①② | NR | 5(moderate) |
| Li (2017) (Mingming, 2017) | China | hospital | 120 (60/60) | I: 28(46.67) C: 27(45.00) | I: 60.83 ± 10.66C: 59.05 ± 11.12 | Chinese guidelines for hypertension prevention and treatment | CBT + drug therapy | NR | no | drug therapy | ①② | NR | 5(moderate) |
| Liu (2017) (Yuanyuan, 2017) | China | nursing home | 80 (40/40) | I: 15(46.90) C: 17(51.50) | I: 70.94 ± 3.62C: 70.24 ± 3.58 | WHO diagnostic criteria | CBT | group | yes | wait-list | ② | I: 20.00C: 17.50 | 5(moderate) |
| Liu et al. (2018) (Yu et al., 2018) | China | community health service center | 184 (102/82) | I: 39(38.24) C: 43(52.44) | I: 70.98 ± 4.13C: 72.16 ± 4.36 | Chinese guidelines for hypertension prevention and treatment 2010 | CBT + TAU | NR | no | TAU | ①④ | NR | 4(moderate) |
| Mao (2013) (Youyou, 2013) | China | hospital | 80 (40/40) | I: 20(50.00) C: 20(50.00) | I: 55.35 ± 6.37C: 54.80 ± 5.98 | WHO diagnostic criteria | CBT + TAU | NR | no | TAU | ①② | NR | 5(moderate) |
| Mensorio et al. (2019) (Mensorio et al., 2019) | Spain | Public hospital | 106 (55/51) | 47 (44.34) | 53.00 ± 8.90 | NR | SII based on CBT + UMC | individual | no | UMC | ①②③ | I: 21.82C: 5.88 | 7(moderate) |
| Shen et al. (2012) (Yurong et al., 2012) | China | hospital | 80 (40/40) | 32 (40.00) | 50.00 ± 3.70 | essential hypertension | CBT + drug therapy | individual | no | drug therapy | ①② | NR | 4(moderate) |
| Su (2020) (Jing, 2020) | China | hospital | 100 (50/50) | I: 23(46.00) C: 22(44.00) | I: 59.86 ± 2.75C: 59.82 ± 2.71 | essential hypertension | CBT + TAU | NR | no | TAU | ① | NR | 5(moderate) |
| Sung et al. (2012) (Sung et al., 2012) | Korea | local health center | 56 (28/28) | I: 20(72.00) C: 14(50.00) | I: 66.00 ± 7.00C: 63.00 ± 11.00 | SBP: 140–159 mm Hg DBP: 90–99 mm Hg | Forest Therapy based on CBT | group | no | self-monitoring | ①③ | I: 0.00 C: 0.00 | 5(moderate) |
| Tian et al. (2015) (Weiwei et al., 2015) | China | hospital | 52 (26/26) | I: 0(0.00) C: 0(0.00) | I: 42.60 ± 9.60C: 43.20 ± 9.80 | Chinese guidelines for hypertension prevention and treatment 2010 | CBT + TAU | NR | no | TAU | ①② | NR | 5(moderate) |
| Yang et al. (2017) (Xinju et al., 2017) | China | hospital | 106 (53/53) | I: 33(62.26) C: 34(64.15) | I: 56.16 ± 9.70C: 56.48 ± 11.16 | JNC-8 Diagnostic criteria for hypertension | iCBT + TAU | individual | no | TAU | ①②④ | I: 7.55 C: 5.66 | 6(moderate) |
1: I = CBT + drug therapy; C1 = drug therapy; C2 = MBSR; MBSR = mindfulness - based stress reduction; TAU = treat as usual; UMC = usual medical care; SII = self-administered Internet-based intervention.
Outcome measure: ① physiological indicator = blood pressure, blood glucose, blood lipid profile (LDL - C, HDL - C, TG, total cholesterol), heart rate, BMI, waist, hip perimeter, oxidative stress, interleukin 6; ② psychological indicators = depression, anxiety stress; ③ QOL; ④ quality of sleep.
Regarding interventions, a single CBT method was used in three studies (Clemow et al., 2018, Sung et al., 2012, Yuanyuan, 2017), and CBT combined with drug therapy or treatment as usual was used in the remaining 12 studies (Birashk et al., 2018, Hualei et al., 2013, Jing, 2020, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yu et al., 2018, Yurong et al., 2012). Additionally, control groups that adopted drug therapy or usual interventions were described in 14 articles (Birashk et al., 2018, Clemow et al., 2018, Hualei et al., 2013, Jing, 2020, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Sung et al., 2012, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yu et al., 2018, Yurong et al., 2012). A wait list control group was used in only one study (Yuanyuan, 2017). Two studies used remote interventions, including interventions over the internet (Mensorio et al., 2019, Xinju et al., 2017), and 13 studies used traditional face-to-face interventions (Birashk et al., 2018, Clemow et al., 2018, Hualei et al., 2013, Jing, 2020, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Sung et al., 2012, Weiwei et al., 2015, Youyou, 2013, Yu et al., 2018, Yuanyuan, 2017, Yurong et al., 2012). Four studies used an individual CBT intervention (Mensorio et al., 2019, Qing et al., 2019, Xinju et al., 2017, Yurong et al., 2012), five studies used a group-based CBT intervention (Birashk et al., 2018, Clemow et al., 2018, Hualei et al., 2013, Sung et al., 2012, Yuanyuan, 2017), while six did not report the treatment form (Jing, 2020, Mingming, 2017, Qingmei, 2010, Weiwei et al., 2015, Youyou, 2013, Yu et al., 2018). Only three studies reported using an intervention manual (Birashk et al., 2018, Clemow et al., 2018, Yuanyuan, 2017). The specific settings of the interventions were as follows: the mean number of sessions was 9.91, the mean duration of sessions was 64.75 min, and the mean duration of treatment was 10.04 weeks. Four types of outcome measures were included in this analysis: physiological indicators, psychological indicators, quality of life, and quality of sleep. Thirteen studies reported physiological indicators (Birashk et al., 2018, Clemow et al., 2018, Hualei et al., 2013, Jing, 2020, Mensorio et al., 2019, Mingming, 2017, Qingmei, 2010, Sung et al., 2012, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yu et al., 2018, Yurong et al., 2012), 11 psychological indicators (Clemow et al., 2018, Hualei et al., 2013, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yuanyuan, 2017, Yurong et al., 2012), three quality of life (Mensorio et al., 2019, Qing et al., 2019, Sung et al., 2012), and two reported quality of sleep (Jing, 2020, Mensorio et al., 2019, Xinju et al., 2017, Yu et al., 2018). The mean drop-out rates were 14.57% and 12.13% in the intervention and control groups. respectively. Detailed characteristics of the intervention methods and control group activities are in Table 1.
3.3. Pre to post-treatment effects of CBT-based interventions
3.3.1. Effects on physiological indicators
Nine studies (Hualei et al., 2013, Jing, 2020, Mingming, 2017, Sung et al., 2012, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yu et al., 2018, Yurong et al., 2012), with a total sample of 1377 participants, analyzed the effect of CBT-based interventions on blood pressure. The number of people in the intervention and control groups were 701 and 676, respectively. CBT-based interventions were more beneficial in reducing systolic pressure compared to the control conditions, with a mean reduction of systolic pressure of −8.67 (95% CI: −10.67 to −6.67, P = 0.000), and a large effect size (SMD −0.87 (95% CI: −1.18 to −0.55, P = 0.000)). The heterogeneity was statistically significant (I2 = 58.50%, P = 0.013) (Table 2). The forest plot of the effect is presented in Fig. 2A.
Table 2.
Total effect of CBT - based interventions on blood pressure, total cholesterol, triglyceride, LDL - C, depressive symptom, anxiety symptom, and the quality of sleep.
| Index | Outcomes: post-to pre - treatment effect |
|||||||
|---|---|---|---|---|---|---|---|---|
| Studies,n | Participants | I2% (P) | Q-test | MD (95%,CI) | P | SMD (95%,CI) | P | |
| Systolic pressure | 9 | 1377 | 58.50 (0.013) | 19.27 | −8.67 (−10.67, −6.67)*** | 0.000 | −0.87 (−1.18, −0.55)*** | 0.000 |
| Diastolic pressure | 9 | 1377 | 80.20 (0.000) | 40.45 | −5.82 (−7.82, −3.81)*** | 0.000 | −0.77 (−1.07, −0.47)*** | 0.000 |
| Total cholesterol | 2 | 679 | 74.60 (0.047) | 3.94 | −0.43 (−0.76, −0.10)* | 0.010 | −0.49 (−0.64, −0.33)*** | 0.000 |
| Triglyceride | 2 | 679 | 0.00 (0.419) | 0.65 | 0.00 (−0.07, 0.07) | 0.978 | 0.05 (−0.10, 0.20) | 0.502 |
| LDL | 2 | 679 | 0.00 (0.401) | 0.71 | 0.10 (−0.15, 0.34) | 0.441 | −0.00 (−0.21, 0.20) | 0.971 |
| Depression | 9 | 1620 | 99.10 (0.000) | 870.24 | −3.13 (−4.02, −2.24)*** | 0.000 | −1.07 (−1.82, −0.31)** | 0.005 |
| Anxiety | 10 | 1673 | 98.50 (0.000) | 600.01 | −3.63 (−4.40, −2.87)*** | 0.000 | −1.27 (−1.68, −0.86)*** | 0.000 |
| The quality of sleep | 2 | 290 | 73.20 (0.050) | 3.74 | −2.93 (−4.40, −1.47)*** | 0.000 | −0.94 (−1.29, −0.59)*** | 0.000 |
*P < 0.05, **P < 0.01, ***P < 0.001.
MD = Mean difference; SMD = Standard mean difference.
Fig. 2.
Forest plots of the effects of CBT - based interventions on blood pressure. A: Systolic blood pressure; B: Diastolic Blood pressure.
Subgroup analysis was performed to examine the effect of CBT-based interventions with different characteristics on improving systolic pressure. The results demonstrated that CBT-based interventions with the following characteristics had a better effect on systolic pressure: when they involved group treatment, patients did not have comorbid mood symptoms (Table 3).
Table 3.
Subgroup analysis on the effect of CBT - based interventions on systolic pressure.
| Subgroups | Systolic pressure: post- to pre-treatment effect |
||||||
|---|---|---|---|---|---|---|---|
| Studies(n) | Participants(n) | I2% (P) | Q-test | MD (95%,CI) | SMD (95%,CI) | P (between) | |
| Behavioral strategies | 0.210 | ||||||
| Important | 2 | 136 | 44.90 (0.178) | 1.81 | −12.54 (−19.15, −5.93)*** | −0.86 (−1.38, −0.35)** | |
| Not important | 7 | 1241 | 57.20 (0.030) | 14.01 | −8.09 (−10.07, −6.12)*** | −0.87 (−1.25, −0.49)*** | |
| Cognitive strategies | 0.940 | ||||||
| Important | 5 | 917 | 75.20 (0.003) | 16.11 | −8.59 (−12.09, −5.10)*** | −0.81 (−1.23, −0.40)*** | |
| Not important | 4 | 460 | 0.00 (0.405) | 2.91 | −8.73 (−10.25, −7.21)*** | −0.93 (−1.51, −0.35)** | |
| Body directed strategies | 0.730 | ||||||
| Important | 6 | 993 | 67.40 (0.009) | 15.36 | −8.93 (−11.45, −6.41)*** | −1.03 (−1.55, −0.50)*** | |
| Not important | 3 | 384 | 42.60 (0.175) | 3.48 | −8.10 (−12.13, −4.07)*** | −0.63 (−0.83, −0.42)*** | |
| Mindfulness and attention | 0.090 | ||||||
| Important | 2 | 162 | 15.90 (0.275) | 1.19 | −5.11 (−9.39, −0.83)* | −0.43 (−0.74, −0.11)** | |
| Not important | 7 | 1215 | 57.60 (0.028) | 14.15 | −9.27 (−11.34, −7.20)*** | −0.99 (−1.38, −0.59)*** | |
| Homework assignment strategies | 0.650 | ||||||
| Important | 2 | 719 | 69.30 (0.071) | 3.26 | −11.33 (−22.50, −0.16)* | −0.48 (−0.63, −0.33)*** | |
| Not important | 7 | 658 | 60.60 (0.019) | 15.22 | −8.72 (−11.04, −6.41)*** | −1.00 (−1.44, −0.56)*** | |
| Dietary intervention intervention | 0.480 | ||||||
| Important | 3 | 364 | 0.00 (0.510) | 1.35 | −8.33 (−9.81, −6.84)*** | −1.06(−1.82, −0.29)** | |
| Not important | 6 | 1013 | 72.00 (0.003) | 17.85 | −9.77 (−13.48, −6.06) *** | −0.76 (−1.10, −0.41)*** | |
| Physical activity | 0.720 | ||||||
| Important | 4 | 484 | 25.30 (0.260) | 4.02 | −8.35 (−10.38, −6.32)*** | −0.92 (−1.48, −0.36)** | |
| Not important | 5 | 893 | 73.80 (0.004) | 15.26 | −9.11 (−12.81, −5.42)*** | −0.82 (−1.25, −0.39)*** | |
| Treatment form | 0.710 | ||||||
| Group | 2 | 655 | 0.00 (0.697) | 0.15 | −7.22 (−9.54, −4.91)*** | −0.48 (−0.63, −0.32)*** | |
| Individual | 2 | 186 | 89.60 (0.002) | 9.57 | −9.42 (−20.90, 2.06) | −0.71 (−1.47, 0.04) | |
| Number of session | 0.340 | ||||||
| ≥10 | 2 | 679 | 0.00 (0.715) | 0.13 | −6.87 (−9.02, −4.72)*** | −0.48 (−0.63, −0.33)*** | |
| <10 | 3 | 238 | 84.40 (0.002) | 12.82 | −10.21 (−16.71, −3.72)** | −1.09 (−1.94, −0.24)* | |
| Treatment course | 0.150 | ||||||
| ≥12w | 2 | 679 | 0.00 (0.715) | 0.13 | −6.87 (−9.02, −4.72)*** | −0.48 (−0.63, −0.33)*** | |
| <12w | 5 | 414 | 73.10 (0.005) | 14.87 | −10.82 (−15.82, −5.82)*** | −0.85 (−1.32, −0.39)*** | |
| Patients with comorbid mood symptoms | 0.700 | ||||||
| Yes | 2 | 200 | 71.30 (0.062) | 3.48 | −11.13 (−23.43, 1.18) | −0.55 (−0.83, −0.27)*** | |
| No | 7 | 1177 | 61.60 (0.016) | 15.64 | −8.69 (−10.79, −6.59)*** | −0.97 (−1.38, −0.56)*** | |
*P < 0.05, **P < 0.01, ***P < 0.001.
MD = Mean difference; SMD = Standard mean difference.
Similarly, CBT-based interventions significantly reduced diastolic pressure, with a reduced pooled mean across these studies of −5.82 (95% CI: −7.82 to −3.81, P = 0.000) with a large effect size (SMD −0.77 (−1.07 to −0.47, P = 0.000)). Statistically significant heterogeneity was observed (I2 = 80.20%, P = 0.000). The forest plot of the effect is presented in Fig. 2B.
As shown in Table 4, CBT-based interventions statistically reduced diastolic pressure when the CBT intervention format involved a group-based intervention, and when more than 10 sessions were given.
Table 4.
Subgroup analysis on the effect of CBT - based interventions on diastolic pressure.
| Subgroups | Diastolic pressure: post- to pre - treatment effect |
||||||
|---|---|---|---|---|---|---|---|
| Studies(n) | Participants(n) | I2% (P) | Q-test | MD (95%,CI) | SMD (95%,CI) | P (between) | |
| Behavioral strategies | 0.920 | ||||||
| Important | 2 | 136 | 85.10 (0.010) | 6.69 | −6.08 (−14.58, 2.43) | −0.74 (−1.93, 0.45) | |
| Not important | 7 | 1241 | 80.90 (0.000) | 31.37 | −5.63 (−7.73, −3.52)*** | −0.77 (−1.09, −0.46)*** | |
| Cognitive strategies | 0.300 | ||||||
| Important | 5 | 917 | 84.50 (0.000) | 25.80 | −5.05 (−7.95, −2.16)** | −0.71 (−1.15, −0.28)** | |
| Not important | 4 | 460 | 60.40 (0.056) | 7.57 | −7.07 (−9.52, −4.61)*** | −0.86 (−1.26, −0.45)*** | |
| Body directed strategies | 0.360 | ||||||
| Important | 6 | 993 | 82.10 (0.000) | 27.99 | −5.17 (−7.65, −2.68)*** | −0.75 (−1.18, −0.32)** | |
| Not important | 3 | 384 | 73.80 (0.022) | 7.64 | −7.15 (−10.64, −3.66)*** | −0.84 (−1.19, −0.48)*** | |
| Mindfulness and attention | <0.001 | ||||||
| Important | 2 | 162 | 0.00 (0.615) | 0.25 | −0.08 (−2.57, 2.41) | −0.02 (−0.33, 0.29) | |
| Not important | 7 | 1215 | 65.80 (0.007) | 17.55 | −6.97 (−8.59, −5.35)*** | −0.96 (−1.24, −0.68)*** | |
| Homework assignment strategies | 0.710 | ||||||
| Important | 2 | 719 | 66.30 (0.085) | 2.97 | −6.35 (−9.57, −3.13)*** | −0.66 (−0.89, −0.42)*** | |
| Not important | 7 | 658 | 83.90 (0.000) | 37.24 | −5.55 (−8.30, −2.79)*** | −0.80 (−1.24, −0.36)*** | |
| Dietary intervention | 0.590 | ||||||
| Important | 3 | 364 | 73.60 (0.023) | 7.57 | −6.54 (−9.41, −3.67)*** | −0.95 (−1.37, −0.53)*** | |
| Not important | 6 | 1013 | 83.30 (0.000) | 29.90 | −5.43 (−8.27, −2.58)*** | −0.68 (−1.07, −0.30)*** | |
| Physical activity | 0.290 | ||||||
| Important | 4 | 484 | 63.80 (0.040) | 8.28 | −6.95 (−9.25, −4.66)*** | −0.93 (−1.23, −0.63)*** | |
| Not important | 5 | 893 | 85.00 (0.000) | 26.59 | −4.85 (−8.03, −1.66)** | −0.66 (−1.13, −0.19)** | |
| Treatment form | 0.910 | ||||||
| Group | 2 | 655 | 33.10 (0.221) | 1.49 | −4.32 (−7.22, −1.42)** | −0.42 (−0.84, −0.01)* | |
| Individual | 2 | 186 | 95.50 (0.000) | 22.18 | −4.90 (−15.02, 5.23) | −0.65 (−1.20, 0.70) | |
| Number of session | 0.810 | ||||||
| ≥10 | 2 | 679 | 0.00 (0.406) | 0.69 | −4.83 (−6.13, −3.54)*** | −0.56 (−0.72, −0.41)*** | |
| <10 | 3 | 238 | 92.00 (0.000) | 25.01 | −5.58 (−11.52, 0.36) | −0.89 (−1.90, 0.12) | |
| Treatment course | 0.770 | ||||||
| ≥12w | 2 | 679 | 0.00 (0.406) | 0.69 | −4.83 (−6.13, −3.54)*** | −0.56 (−0.72, −0.41)*** | |
| <12w | 5 | 414 | 86.60 (0.000) | 29.76 | −5.47 (−9.60, −1.34)** | −0.72 (−1.30, −0.15)* | |
| Patients with comorbid mood symptoms | 0.950 | ||||||
| Yes | 2 | 200 | 74.40 (0.048) | 3.90 | −5.95 (−10.76, −1.14)* | −0.68 (−1.04, −0.32)*** | |
| No | 7 | 1177 | 83.60 (0.000) | 36.55 | −5.77 (−8.13, −3.41)*** | −0.81 (−1.18, −0.43)*** | |
*P < 0.05, **P < 0.01, ***P < 0.001.
MD = Mean difference; SMD = Standard mean difference.
Two studies (Hualei et al., 2013, Youyou, 2013) with a total sample of 679 participants analyzed the effect of CBT-based interventions on total cholesterol, TG, and LDL-C. The numbers of people in the intervention and control groups were 342 and 337, respectively. Meta-analysis showed a significant reduction in total cholesterol, with mean reduction of −0.43 (95% CI: −0.76 to −0.10, P = 0.010), and a medium effect size of SMD −0.49 (95% CI: −0.64 to −0.33, P = 0.000). The heterogeneity was statistically significant (I2 = 74.60%, P = 0.047) (Table 2). The forest plot of the effect is presented in Fig. 3A. The meta-analysis did not show a significant reduction in either TG (0.00, 95% CI: −0.07 to 0.07, P = 0.978) or LDL-C (0.10, 95% CI: −0.15 to 0.34, P = 0.441). The heterogeneity was not statistically significant for TG (I2 = 0.00%, P = 0.419) or LDL-C (I2 = 0.00%, P = 0.401) (Table 2). The forest plot of the effect is presented in Fig. 3B and 3C.
Fig. 3.
Forest plots of the effects of CBT - based interventions on: A: Total cholesterol; B: Triglyceride; C: LDL - C. D: Quality of sleep.
3.3.2. Effects on psychological indicators
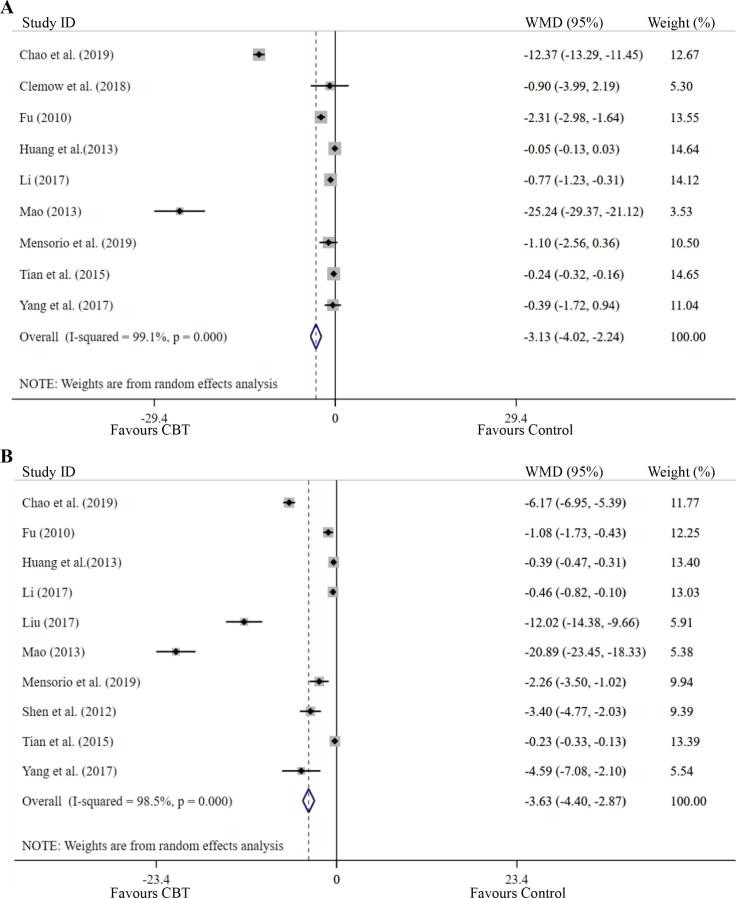
The effect on depressive symptoms was analyzed in nine studies (Clemow et al., 2018, Hualei et al., 2013, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013) with a total sample of 1620 participants. The number of people in the intervention and control groups was 810 and 810, respectively. CBT-based interventions were more beneficial for treating depressive symptoms than the control condition, with a mean reduction of depression of −3.13 (95% CI: −4.02 to −2.24, P = 0.000) and a large effect size (SMD −1.07 (95% CI: −1.82 to −0.31, P = 0.005)). The heterogeneity was statistically significant (I2 = 99.10%, P = 0.000) (Table 2). The forest plot of the effect is presented in Fig. 4A.
Fig. 4.
Forest plots of the effects of CBT - based interventions on: A: Depression symptom; B: Anxiety symptom.
Subgroup analysis was performed to examine the effect of CBT-based interventions with different characteristics on improving depressive symptoms. As shown in Table 5, the results demonstrated that CBT-based interventions with the following characteristics had a better effect on depressive symptoms: face-to-face treatment delivery, greater than 10 sessions, and in participants with comorbid mood symptoms. The subgroup analysis also examined the effects of CBT-based interventions with different components on improving depressive symptoms. CBT-based interventions showed a better effect when they used physical activity as the core technique, and when they did not use behavioral strategies, homework assignment strategies, or problem-solving strategies as core techniques.
Table 5.
Subgroup analysis on the effect of CBT - based interventions on depressive symptom.
| Subgroups | Depressive symptom: post- to pre - treatment effect |
||||||
|---|---|---|---|---|---|---|---|
| Studies(n) | Participants(n) | I2% (P) | Q-test | MD (95%,CI) | SMD (95%,CI) | P (between) | |
| Behavioral strategies | 0.003 | ||||||
| Important | 2 | 183 | 0.00 (0.909) | 0.01 | −1.06 (−2.39, 0.26) | −0.21 (−0.50, 0.08) | |
| Not important | 7 | 1437 | 99.30 (0.000) | 868.70 | −3.53 (−4.50, −2.56)*** | −1.31 (−2.25, −0.37)** | |
| Cognitive strategies | <0.001 | ||||||
| Important | 7 | 1409 | 99.30 (0.000) | 863.37 | −3.88 (−4.93, −2.84)*** | −1.24 (−2.21, −0.28)* | |
| Not important | 2 | 211 | 0.00 (0.673) | 0.18 | −0.80 (−1.24, −0.36)*** | −0.47 (−0.76, −0.19)** | |
| Body directed strategies | 0.110 | ||||||
| Important | 6 | 1329 | 99.30 (0.000) | 721.97 | −2.80 (−3.79, −1.81)*** | −1.01 (−2.03, 0.01) | |
| Not important | 3 | 291 | 98.50 (0.000) | 133.60 | −8.46 (−15.37, −1.55)* | −1.18 (−2.38, 0.03) | |
| Homework assignment strategies | 0.005 | ||||||
| Important | 3 | 810 | 82.10 (0.004) | 11.14 | −0.48 (−1.13, 0.18) | −0.31 (−0.63, 0.02) | |
| Not important | 6 | 810 | 99.40 (0.000) | 833.99 | −6.56 (−10.76, −2.35)** | −1.44 (−2.48,-0.40)** | |
| Problem solving | 0.003 | ||||||
| Important | 2 | 183 | 0.00 (0.909) | 0.01 | −1.06 (−2.39, 0.26) | −0.21 (−0.50, 0.08) | |
| Not important | 7 | 1437 | 99.30 (0.000) | 868.70 | −3.53 (−4.50, −2.56)*** | −1.31 (−2.25, −0.37)** | |
| Dietary intervention | 0.006 | ||||||
| Important | 4 | 651 | 99.30 (0.000) | 424.61 | −9.96 (−16.87, −3.06)** | −1.78 (−2.96, −0.61)** | |
| Not important | 5 | 969 | 77.10 (0.002) | 17.46 | −0.24 (−0.44, −0.04)* | −0.44 (−0.84, −0.04)* | |
| Physical activity | 0.002 | ||||||
| Important | 5 | 771 | 99.30 (0.000) | 611.22 | −7.93 (−12.77, −3.10)** | −1.55 (−2.59, −0.50)** | |
| Not important | 4 | 849 | 71.30 (0.015) | 10.47 | −0.15 (−0.32, 0.02) | −0.41 (−0.88, 0.07) | |
| Country | 0.003 | ||||||
| Developed country | 2 | 183 | 0.00 (0.909) | 0.01 | −1.06 (−2.39, 0.26) | −0.21 (−0.50, 0.08) | |
| Developing country | 7 | 1437 | 99.30 (0.000) | 868.70 | −3.53 (−4.50, −2.56)*** | −1.31 (−2.25, −0.37)** | |
| Treatment form | 0.290 | ||||||
| Group | 2 | 691 | 0.00 (0.589) | 0.29 | −0.05 (−0.14, 0.03) | −0.10 (−0.25, 0.05) | |
| Individual | 3 | 597 | 99.30 (0.000) | 287.73 | −4.63 (−13.10, 3.83) | −1.02 (−2.78, 0.74) | |
| Treatment delivery way | <0.001 | ||||||
| Remote | 2 | 197 | 0.00 (0.482) | 0.50 | −0.71 (−1.70, 0.27) | −0.20 (−0.48, 0.08) | |
| Face to face | 7 | 1423 | 99.30 (0.000) | 868.82 | −3.79 (−4.80, −2.79)*** | −1.31 (−2.26, −0.37)** | |
| Number of session | 0.510 | ||||||
| ≥10 | 4 | 851 | 98.40 (0.000) | 186.67 | −5.90 (−9.23, −2.58)** | −1.08 (−2.11, −0.04)* | |
| <10 | 4 | 649 | 99.50 (0.000) | 661.83 | −3.53 (−9.69, 2.63) | −1.17 (−2.56, 0.23) | |
| Treatment course | 0.040 | ||||||
| ≥12w | 4 | 1170 | 99.60 (0.000) | 824.84 | −9.45 (−17.33, −1.57)* | −1.42 (−2.93, 0.09) | |
| <12w | 5 | 450 | 90.30 (0.000) | 41.25 | −0.93 (−1.77, −0.10)* | −0.76 (−1.34, −0.18)* | |
| Patients with comorbid mood symptoms | 0.001 | ||||||
| Yes | 4 | 680 | 99.50 (0.000) | 605.03 | −9.72 (−15.45, −3.99)** | −1.85 (−2.94, −0.77)** | |
| No | 5 | 940 | 66.90 (0.017) | 12.09 | −0.17 (−0.34, 0.01) | −0.37 (−0.75, 0.00) | |
*P < 0.05, **P < 0.01, ***P < 0.001.
MD = Mean difference; SMD = Standard mean difference.
The effect on anxiety symptoms was analyzed in 10 studies (Hualei et al., 2013, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yuanyuan, 2017, Yurong et al., 2012) with a total sample of 1673 participants. The number of people in the intervention and control groups was 836 and 837, respectively. CBT-based interventions were more beneficial for anxiety symptoms than the control interventions, with a mean reduction of anxiety of −3.63 (95% CI: −4.40 to −2.87, P = 0.000), and a large effect size, with SMD −1.27 (95% CI: −1.68 to −0.86, P = 0.000). The heterogeneity was statistically significant (I2 = 98.50%, P = 0.000) (Table 2). The forest plot of the effect is presented in Fig. 4B.
Subgroup analysis was performed to examine the effect of CBT-based interventions with different characteristics on improving anxiety symptoms. As shown in Table 6, CBT-based interventions statistically reduced anxiety symptoms and were more effective as an individual treatment and when it emphasized cognitive strategies as the core technique (Fig. 5).
Table 6.
Subgroup analysis on the effect of CBT - based interventions on anxiety symptom.
| Subgroups | Anxiety symptom: post- to pre - treatment effect |
||||||
|---|---|---|---|---|---|---|---|
| Studies(n) | Participants(n) | I2% (P) | Q-test | MD (95%,CI) | SMD (95%,CI) | P (between) | |
| Behavioral strategies | 0.250 | ||||||
| Important | 3 | 236 | 96.20 (0.000) | 52.80 | −5.77 (−10.42, −1.13)* | −1.40 (−2.31, −0.49)** | |
| Not important | 7 | 1437 | 98.70 (0.000) | 479.63 | −3.01 (−3.82, −2.20)*** | −1.22 (−1.72, −0.73)*** | |
| Cognitive strategies | 0.002 | ||||||
| Important | 8 | 1462 | 98.80 (0.000) | 591.31 | −4.43 (−5.33, −3.53)*** | −1.45 (−1.94, −0.97)*** | |
| Not important | 2 | 211 | 86.70 (0.006) | 7.49 | −1.26 (−3.01, 0.49) | −0.58 (−0.85, −0.30)*** | |
| Body directed strategies | 0.200 | ||||||
| Important | 7 | 1382 | 98.30 (0.000) | 344.85 | −2.85 (−3.60, −2.10)*** | −1.18 (−1.57, −0.79)*** | |
| Not important | 3 | 291 | 99.20 (0.000) | 244.65 | −7.72 (−15.17, −0.27)* | −1.56 (−3.06, −0.05)* | |
| Homework assignment strategies | 0.070 | ||||||
| Important | 4 | 875 | 97.10 (0.000) | 102.06 | −2.72 (−4.09, −1.35)*** | −1.04 (−1.59, −0.48)*** | |
| Not important | 6 | 798 | 99.00 (0.000) | 497.94 | −5.82 (−8.85, −2.80)*** | −1.43 (−2.03, −0.83)*** | |
| Dietary intervention | 0.010 | ||||||
| Important | 4 | 651 | 98.90 (0.000) | 279.55 | −7.41 (−12.27, −2.55)** | −1.60 (−2.48, −0.72)*** | |
| Not important | 6 | 1022 | 96.20 (0.000) | 130.71 | −1.32 (−1.85, −0.79)*** | −1.05 (−1.44, −0.65)*** | |
| Physical activity | 0.020 | ||||||
| Important | 5 | 771 | 99.00 (0.000) | 389.78 | −5.88 (−9.24, −2.52)** | −1.36 (−2.12, −0.60)*** | |
| Not important | 5 | 902 | 96.90 (0.000) | 130.35 | −1.69 (−2.33, −1.05)*** | −1.18 (−1.66, −0.71)*** | |
| Treatment form | 0.730 | ||||||
| Group | 2 | 664 | 98.90 (0.000) | 93.37 | −6.14 (−17.54, 5.25) | −1.59 (−3.25, 0.07) | |
| Individual | 4 | 677 | 90.60 (0.000) | 31.90 | −4.11 (−6.24, −1.99)*** | −1.04 (−1.50, −0.57)*** | |
| Treatment delivery way | 0.650 | ||||||
| Remote | 2 | 197 | 62.90 (0.101) | 2.69 | −3.16 (−5.39, −0.94)** | −0.72 (−1.01, −0.43)*** | |
| Face to face | 8 | 1476 | 98.80 (0.000) | 580.57 | −3.71 (−4.54, −2.89)*** | −1.42 (−1.92, −0.93)*** | |
| Number of session | 0.400 | ||||||
| ≥10 | 3 | 759 | 99.20 (0.000) | 250.48 | −6.93 (−11.08, −2.78)** | −1.65 (−2.92, −0.38)* | |
| <10 | 6 | 794 | 98.60 (0.000) | 349.26 | −4.68 (−7.80, −1.57)** | −1.26 (−1.70, −0.82)*** | |
| Duration of session | 0.340 | ||||||
| ≥50 min | 5 | 876 | 98.90 (0.000) | 352.67 | −3.45 (−4.36, −2.54)*** | −1.71 (−2.60, −0.82)*** | |
| <50 min | 2 | 480 | 91.50 (0.001) | 11.83 | −4.84 (−7.56, −2.13)*** | −1.36 (−1.80, −0.93)*** | |
| Treatment course | 0.080 | ||||||
| ≥12w | 4 | 1170 | 99.30 (0.000) | 459.21 | −7.23 (−12.16, −2.30)** | −1.58 (−2.33, −0.83)*** | |
| <12w | 6 | 503 | 96.30 (0.000) | 134.03 | −2.77 (−3.94, −1.60)*** | −1.06 (−1.54, −0.59)*** | |
| Patients with comorbid mood symptoms | 0.000 | ||||||
| Yes | 5 | 745 | 99.10 (0.000) | 462.18 | −7.86 (−11.71, −4.01)*** | −1.71 (−2.56, −0.86)*** | |
| No | 5 | 928 | 91.20 (0.000) | 45.36 | −0.77 (−1.16, −0.38)*** | −0.81 (−0.95, −0.68)*** | |
*P < 0.05, **P < 0.01, ***P < 0.001.
MD = Mean difference; SMD = Standard mean difference.
Fig. 5.
Funnel plots for: A: Systolic blood pressure; B: Diastolic blood pressure; C: Depression symptom; D: Anxiety symptom.
3.3.3. Effects on quality of sleep
Two studies (Xinju et al., 2017, Yu et al., 2018), with a total sample of 290 participants, analyzed the effect of CBT-based interventions on sleep quality. The number of people in the intervention group and the control group was 155 and 135, respectively. CBT-based interventions were more beneficial in improving the quality of sleep than the control condition, with a mean reduction of the PSIQ score of −2.93 (95% CI: −4.40 to −1.47, P = 0.000), and large effect size of SMD −0.94 (95% CI: −1.29 to −0.59, P = 0.000). The heterogeneity was statistically significant (I2 = 73.20%, P = 0.050) (Table 2). The forest plot of the effect is presented in Fig. 3D.
3.3.4. Effects on health-related behaviors
One study (Jing, 2020), with 100 participants, analyzed the effect of the CBT-based intervention on health-related behaviors and found that the intervention group's health behavior scores, including medication compliance, quitting smoking and drinking, reasonable diet, and exercise, were relatively higher than the control group.
3.4. Risk of bias and quality assessment
We used the PEDro tool to assess the quality of the included studies. All were of medium quality and the score was 5.13 on average. Specifically, three studies scored 4 (Birashk et al., 2018, Yu et al., 2018, Yurong et al., 2012), nine studies scored 5 (Hualei et al., 2013, Jing, 2020, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Sung et al., 2012, Weiwei et al., 2015, Youyou, 2013, Yuanyuan, 2017), one study scored 6 (Xinju et al., 2017), and two studies (Clemow et al., 2018, Mensorio et al., 2019) scored 7.
3.5. Publication bias
Since only two studies were included to analyze the effect of CBT-based interventions on total cholesterol, HDL-C and the quality of sleep, no T or p-value of the Egger analysis was available for these variables. As can be seen in Table 7, we found minimal publication bias on the following outcome variables: systolic pressure (P = 0.487), diastolic pressure (P = 0.958) and depressive symptoms (P = 0.076). However, there was significant publication bias for anxiety symptoms (P = 0.008). The one-study-removed method was used to assess sensitivity, and it was found that removing one study at a time did not change the overall results for all outcome variables.
Table 7.
Egger's regression analysis on publication bias.
| Variables | T | P | 95%, CI |
|---|---|---|---|
| Systolic pressure | −0.73 | 0.487 | (−3.55, 1.87) |
| Diastolic pressure | −0.05 | 0.958 | (−5.47, 5.22) |
| Total cholesterol | — | — | — |
| Triglycerides | — | — | — |
| LDL – L | — | — | — |
| Depression | −2.08 | 0.076 | (−16.15, 1.01) |
| Anxiety | −3.54 | 0.008 | (−12.44, −2.62) |
| The quality of sleep | — | — | — |
4. Discussion
In this paper we have presented the results of a meta-analysis of the efficacy of CBT-based interventions for hypertension patients. The results indicated that CBT-based interventions were superior to control interventions, significantly reducing systolic pressure, diastolic pressure, total cholesterol levels, depressive symptoms and anxiety symptoms, as well as improving the quality of sleep.
4.1. Pre to post-treatment effects of CBT-based interventions
4.1.1. Effects on physiological indicators
Consistent with previous research (Clemow et al., 2018, Shapiro et al., 1997), we found that CBT-based interventions significantly reduced systolic and diastolic blood pressure in patients with hypertension. It has been reported that high blood pressure control using the recommended guidelines (Hypertension, 2013, James et al., 2014) is the most effective way to reduce cardiovascular mortality in hypertension patients (Burnier, 2017). However, studies have shown that 50% of people with hypertension receiving “usual treatment” had uncontrolled blood pressure (Conn et al., 2015), primarily due to inadequate medication adherence (Burnier, 2014, De Geest et al., 2014). In this context CBT-based interventions could improve medication compliance by correcting patients’ misconceptions about medication usage, thereby reducing blood pressure. Another possible explanation for the effectiveness of CBT is that patients’ health-related behaviors improved. Previous research indicates that CBT interventions can result in increased physical activity (Xue et al., 2008), modifications to unhealthy eating patterns (Mensorio et al., 2019), and promote quitting smoking and drinking alcohol (Jing, 2020) in hypertension patients, thereby improving their blood pressure control.
Further subgroup analysis showed that the use of a CBT group-based approach and an intervention lasting longer than 10 sessions is more effective in reducing systolic blood pressure and diastolic blood pressure compared with individual treatment and interventions of less than 10 sessions. This may be because group-based interventions facilitate social support among patients, reinforcing the effects of the intervention (Wolgensinger, 2015), and longer intervention times are required to change the maladaptive cognitions and behavior of patients with hypertension.
We found that the total cholesterol levels of patients in the intervention groups was reduced by a greater amount compared to that of patients in the control groups. Patients with high blood pressure are more prone to negative emotions, such as depression and anxiety, causing increased sympathetic nervous activity, which results in a series of physiological and pathological changes, including excessive secretion of catecholamines, disordered lipid metabolism, and increased heart rate (Chen and Huang, 2006, Lehto et al., 2008). Through the CBT intervention, negative emotions can be alleviated, and the sympathetic excitability of the patients reduced, thereby promoting stability in lipid metabolism. Similarly, studies by Mao (Youyou, 2013) found that CBT-based interventions significantly reduced total cholesterol in patients with hypertension. As only two studies reported TG and low-density lipoprotein results, we did not find a significant reduction in these. We should therefore be cautious in drawing conclusions in this area and need to include more studies to confirm our findings.
4.1.2. Effects on psychological indicators
We found that CBT-based interventions had a larger effect on depressive and anxiety symptoms in hypertension patients compared to the control interventions. This study is the first meta-analysis on the effect of comprehensive CBT-based intervention on negative emotions in patients with hypertension, and the tentative conclusion is that such interventions have a significant impact on anxiety and depressive symptoms in these patients.
Subgroup analysis on depression and anxiety found that face-to-face and individualized, rather than group-based, treatment had a more significant effect on the improvement of depressive and anxiety symptoms in hypertension patients, in agreement with Liu and colleagues’ previous study (Liu et al., 2019). We also found hypertension patients benefited more in relation to their depression and anxiety when the intervention emphasized a cognitive strategy as the core technique. Previous studies have also suggested that CBT-based intervention using this strategy are more effective in reducing depression and anxiety symptoms in hypertension patients (Qing et al., 2019, Qingmei, 2010). In addition, the number of sessions offered during the intervention was important. Interventions involving greater than 10 sessions were more effective in improving depressive symptoms, possibly because cognitive reconstruction of dysfunctional thoughts takes time (Liu et al., 2019).
4.1.3. Effects on quality of sleep
Two studies reporting sleep quality were included in our meta-analysis, and the results showed that CBT-based interventions were able to significantly reduce the PSQI score and improve sleep quality in patients with hypertension. Similarly, a review by Takaesu and colleagues (Takaesu and Inoue, 2012) found that CBT-based interventions can relieve symptoms of insomnia in patients with metabolic syndrome comorbidities, while also preventing the recurrence of insomnia. The proposed mechanism for this is that offering sleep hygiene education, stimulation control and relaxation therapy helps patients to gradually establish an improved sleep-wake biological rhythm, thus improving their sleep quality.
4.2. Strengths and limitations of the study
We undertook a systematic review and meta-analysis on the efficacy of CBT-based interventions for patients with hypertension using a reasonable number of RCTs with a moderate quality study design and minimal publication bias. Despite the findings of this systematic review, there are several limitations that need to be acknowledged. Firstly, this meta-analysis showed high heterogeneity. The possible reason is that as yet there is no standardized procedure for CBT-based interventions for hypertension, so there have been notable differences in study design, treatment form, duration of treatment, number of sessions, duration of sessions, intervention composition, and the professional background of therapists, including nurses, general practitioners or psychologists. Further, the studies were from different types of institution, including 10 from hospitals (Birashk et al., 2018, Jing, 2020, Mensorio et al., 2019, Mingming, 2017, Qing et al., 2019, Qingmei, 2010, Weiwei et al., 2015, Xinju et al., 2017, Youyou, 2013, Yurong et al., 2012), two from medical centers (Clemow et al., 2018, Sung et al., 2012), two from community health service centers (Hualei et al., 2013, Yu et al., 2018), and one from a nursing home (Yuanyuan, 2017). Secondly, only the results before and after the intervention were compared and analyzed, and long-term follow-up results were not discussed due to insufficient data. Therefore, the long-term effect of CBT-based interventions on patients with hypertension was unclear. Thirdly, only two studies (Clemow et al., 2018, Mensorio et al., 2019) used concealed randomization and one (Mensorio et al., 2019) had assessors who were blind to participants’ group allocation, while none of the others achieved the corresponding blinding methods, leading to the overall quality of evidence being relatively low due to a high risk of bias. Fourth, two articles (Sung et al., 2012, Yu et al., 2018) did not fully realize randomized grouping. We conducted a strict quality evaluation on these two papers and after finding that they met the remaining inclusion conditions, we decided to include them in the analysis. Last but not the least, medication (Ferdinand and Nasser, 2017), psychological factors (Hamer et al., 2010, Liu et al., 2017b), including stress, distress, as well as lifestyle factors (Beilin et al., 1999, Huntgeburth et al., 2005, Omboni, 2020, Samadian et al., 2016), including smoking, alcohol, have not been included in the meta-analysis due to insufficient data, but also due to the fact that they have an influence on blood pressure management. More research is needed to explore these relationships.
4.3. Implications
An increasing number of studies have applied CBT-based interventions in the management of chronic pain, diabetes, coronary heart disease, and other chronic diseases, and found a positive effect. At present, relatively few RCTs have applied CBT interventions in patients with hypertension. However, this meta-analysis found a positive effect of CBT-based interventions on blood pressure management. Given other researchers (Shapiro et al., 1997) have found that CBT offered as an adjunctive treatment was twice as effective as the control treatment in reducing drug requirements, future studies could examine its impact in terms of decreasing the costs and side effects of antihypertensive medications. This will have a profound impact on the prevention and management of hypertension.
4.4. Conclusion
The findings of this systematic review and meta-analysis suggest that CBT-based interventions are efficacious in reducing systolic pressure, diastolic pressure, total cholesterol level, anxiety symptoms, depressive symptoms, and improving quality of sleep in patients with hypertension. In addition, CBT maybe more effective for blood pressure management in these patients when it is offered long term and in group-based settings.
Funding
The first author was sponsored by Griffith University via Griffith University International Postgraduate Research Scholarship (GUIPRS).
6. Clinical trial registration
N/A.
CRediT authorship contribution statement
Yanni Li: Wrote original draft, data collection and analysis. Nicholas Buys: Critically reviewed and editted original draft. Zhanjiang Li: Critically reviewed paper. Li Li: Critically reviewed paper. Qifa Song: Critically reviewed paper. Jing Sun: Contributed to conceptulization, data curation, formal analysis, methodology, supervision, edited the original draft, edited and revised the manuscript for final submission.
Declaration of Competing Interest
There are no known conflict of interests.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pmedr.2021.101477.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Abgrall-Barbry G., Consoli S.M. Psychological approaches in hypertension management. Presse Med. 2006;35(6 Pt 2):1088–1094. doi: 10.1016/s0755-4982(06)74753-4. [DOI] [PubMed] [Google Scholar]
- Beilin, L.J., Puddey, I.B., Burke, V., 1999. Lifestyle and hypertension. Am J Hypertens. 12(9 Pt 1), 934-945. https://doi.org/ 10.1016/s0895-7061(99)00057-6. [DOI] [PubMed]
- Birashk, B., Sheybani, F., Gharraee, B., Pirmoradi, M., Hajsadeghi, S., 2018. Comparison effectiveness of MBSR and CBT on interleukin 6 and oxidative stress in hypertensive patients. Int. J. Life Sci. Pharma Res. 8(3), L39-L45. https://doi.org/10.22376/ijpbs/lpr.2018.8.3.L39-45.
- Biswas S., Dastidar D.G., Roy K.S., Pal S.K., Biswas T.K., Ganguly S.B. Complications of hypertension as encountered by primary care physician. J. Indian Med. Assoc. 2003;101(4):257–259. [PubMed] [Google Scholar]
- Burnier M. Managing 'resistance': is adherence a target for treatment? Curr. Opin. Nephrol. Hypertens. 2014;23(5):439–443. doi: 10.1097/MNH.0000000000000045. [DOI] [PubMed] [Google Scholar]
- Burnier M. Drug adherence in hypertension. Pharmacol. Res. 2017;125(Pt B):142–149. doi: 10.1016/j.phrs.2017.08.015. [DOI] [PubMed] [Google Scholar]
- Chen C.C., Huang T.L. Association of serum lipid profiles with depressive and anxiety disorders in menopausal women. Chang Gung Med J. 2006;29(3):325–330. [PubMed] [Google Scholar]
- Clemow L.P., Pickering T.G., Davidson K.W., Schwartz J.E., Williams V.P., Shaffer J.A., Williams R.B., Gerin W. Stress management in the workplace for employees with hypertension: a randomized controlled trial. Transl. Behav. Med. 2018;8(5):761–770. doi: 10.1093/tbm/iby018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn V.S., Ruppar T.M., Chase J.A., Enriquez M., Cooper P.S. Interventions to Improve Medication Adherence in Hypertensive Patients: Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2015;17(12):94. doi: 10.1007/s11906-015-0606-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creswell C., Hentges F., Parkinson M., Sheffield P., Willetts L., Cooper P. Feasibility of guided cognitive behaviour therapy (CBT) self-help for childhood anxiety disorders in primary care. Ment. Health Fam. Med. 2010;7(1):49–57. [PMC free article] [PubMed] [Google Scholar]
- De Geest S., Ruppar T., Berben L., Schönfeld S., Hill M.N. Medication non-adherence as a critical factor in the management of presumed resistant hypertension: a narrative review. EuroIntervention. 2014;9(9):1102–1109. doi: 10.4244/EIJV9I9A185. [DOI] [PubMed] [Google Scholar]
- Ferdinand K.C., Nasser S.A. Management of essential hypertension. Cardiol. Clin. 2017;35(2):231–246. doi: 10.1016/j.ccl.2016.12.005. [DOI] [PubMed] [Google Scholar]
- Hamer M., Batty G.D., Stamatakis E., Kivimaki M. Hypertension awareness and psychological distress. Hypertension. 2010;56(3):547–550. doi: 10.1161/hypertensionaha.110.153775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R.J., Deeks J.J., Altman D.G., Bradburn M.J., Harbord R.M., Sterne J.A.C. Metan: fixed- and random-effects meta-analysis. Stata J. 2008;8(1):3–28. [Google Scholar]
- Hu B.o., Liu X., Yin S., Fan H., Feng F., Yuan J., Li Y. Effects of psychological stress on hypertension in middle-aged Chinese: a cross-sectional study. PLoS ONE. 2015;10(6):e0129163. doi: 10.1371/journal.pone.0129163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hualei H., Ying Z., Hongwei W., Yuanpeng R., Yong L., Haoyao S., Aiqin W. Effects of cognitive behavior therapy on psychological and physiological status of hypertension patients in community. Chin J. Behav. Med. Brain Sci. 2013;22(4):335–337. [Google Scholar]
- Huntgeburth M., Ten Freyhaus H., Rosenkranz S. Alcohol consumption and hypertension. Curr. Hypertens. Rep. 2005;7(3):180–185. doi: 10.1007/s11906-005-0007-2. [DOI] [PubMed] [Google Scholar]
- Hypertension, E.E.T.F.f.t.M.o.A., 2013. 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens. 31(10), 1925-1938. https://doi.org/10.1097/HJH.0b013e328364ca4c. [DOI] [PubMed]
- James P.A., Oparil S., Carter B.L., Cushman W.C., Dennison-Himmelfarb C., Handler J., Lackland D.T., LeFevre M.L., MacKenzie T.D., Ogedegbe O., Smith S.C., Jr., Svetkey L.P., Taler S.J., Townsend R.R., Wright J.T., Jr., Narva A.S., Ortiz E. 2014 evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA. 2014;311(5):507–520. doi: 10.1001/jama.2013.284427. [DOI] [PubMed] [Google Scholar]
- Jing S. Effects of cognitive behavioral therapy on health behavior in patients with essential hypertension. Nurs. Garden. 2020;7(8):175–176. (Chinese) [Google Scholar]
- Jonas B.S., Franks P., Ingram D.D. Are symptoms of anxiety and depression risk factors for hypertension? Longitudinal evidence from the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Arch. Fam. Med. 1997;6(1):43–49. doi: 10.1001/archfami.6.1.43. [DOI] [PubMed] [Google Scholar]
- Koelen J.A., Houtveen J.H., Abbass A., Luyten P., Eurelings-Bontekoe E.H., Van Broeckhuysen-Kloth S.A., Bühring M.E., Geenen R. Effectiveness of psychotherapy for severe somatoform disorder: meta-analysis. Br. J. Psychiatry. 2014;204(1):12–19. doi: 10.1192/bjp.bp.112.121830. [DOI] [PubMed] [Google Scholar]
- Kretchy I.A., Owusu-Daaku F.T., Danquah S.A. Mental health in hypertension: assessing symptoms of anxiety, depression and stress on anti-hypertensive medication adherence. Int. J. Ment. Health Syst. 2014;8:25. doi: 10.1186/1752-4458-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehto S.M., Hintikka J., Niskanen L., Tolmunen T., Koivumaa-Honkanen H., Honkalampi K., Viinamäki H. Low HDL cholesterol associates with major depression in a sample with a 7-year history of depressive symptoms. Prog. Neuro-Psychopharmacol. Biol. Psychiatry. 2008;32(6):1557–1561. doi: 10.1016/j.pnpbp.2008.05.021. [DOI] [PubMed] [Google Scholar]
- Li Z., Li Y., Chen L., Chen P., Hu Y. Prevalence of depression in patients with hypertension: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94(31) doi: 10.1097/MD.0000000000001317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Gill N.S., Teodorczuk A., Li Z.J., Sun J. The efficacy of cognitive behavioural therapy in somatoform disorders and medically unexplained physical symptoms: A meta-analysis of randomized controlled trials. J. Affect. Disord. 2019;245:98–112. doi: 10.1016/j.jad.2018.10.114. [DOI] [PubMed] [Google Scholar]
- Liu L., Li M., Song S., Shi A., Cheng S., Dang X., Chen H., Zhang H., Ziguli A., Cao L., Wang P., Luan H., Ma Y., Zhang S., Wang Z., Wang X., Gao R., Tian G. Effects of long-term psychological intervention on blood pressure and health-related quality of life in patients with hypertension among the Chinese working population. Hypertens. Res. 2017;40(12):999–1007. doi: 10.1038/hr.2017.80. [DOI] [PubMed] [Google Scholar]
- Liu M.Y., Li N., Li W.A., Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol. Res. 2017;39(6):573–580. doi: 10.1080/01616412.2017.1317904. [DOI] [PubMed] [Google Scholar]
- Mann S.J. Drug therapy for resistant hypertension: simplifying the approach. J. Clin. Hypertens. (Greenwich) 2011;13(2):120–130. doi: 10.1111/j.1751-7176.2010.00387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMain S., Newman M.G., Segal Z.V., DeRubeis R.J. Cognitive behavioral therapy: current status and future research directions. Psychother. Res. 2015;25(3):321–329. doi: 10.1080/10503307.2014.1002440. [DOI] [PubMed] [Google Scholar]
- Mensorio M.S., Cebolla-Martí A., Rodilla E., Palomar G., Lisón J.F., Botella C., Fernández-Aranda F., Jimenez-Murcia S., Baños R.M. Analysis of the efficacy of an internet-based self-administered intervention (“Living Better”) to promote healthy habits in a population with obesity and hypertension: An exploratory randomized controlled trial. Int. J. Med. Inform. 2019;124:13–23. doi: 10.1016/j.ijmedinf.2018.12.007. [DOI] [PubMed] [Google Scholar]
- Mills K.T., Bundy J.D., Kelly T.N., Reed J.E., Kearney P.M., Reynolds K., Chen J., He J. Global disparities of hypertension prevalence and control: a systematic analysis of population-based studies from 90 countries. Circulation. 2016;134(6):441–450. doi: 10.1161/CIRCULATIONAHA.115.018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingming L. The influence of psychological intervention on patients with hypertension accompanied by psychological disorder. Henan Med. Res. 2017;26(8):1531–1532. [Google Scholar]
- Moher D., Liberati A., Tetzlaff J., Altman D.G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndanuko R.N., Tapsell L.C., Charlton K.E., Neale E.P., Batterham M.J. Dietary patterns and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2016;7(1):76–89. doi: 10.3945/an.115.009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan R.P., Feldman R., Dawes M., Kaczorowski J., Lynn H., Barr S.I., MacPhail C., Thomas S., Goodman J., Eysenbach G., Liu S., Tanaka R., Surikova J. Randomized controlled trial of E-counseling for hypertension: REACH. Circ. Cardiovasc. Qual. Outcomes. 2018;11(7) doi: 10.1161/circoutcomes. [DOI] [PubMed] [Google Scholar]
- Omboni S. Smoking and hypertension: what is behind the mask? J. Hypertens. 2020;38(6):1029–1030. doi: 10.1097/hjh.0000000000002423. [DOI] [PubMed] [Google Scholar]
- Qing, C., Jianhong, L., Hua, H., Hui, G., Xiaoxia, Z., Wanxia, Y., 2019. Interventional study of cognitive behavioral therapy on anxiety and depression in patients with hypertension. J. Nurs. Adv. 34(22), 2029-2031+2040. https://doi.org/10.16821/j.cnki.hsjx.22.003. (Chinese).
- Qingmei, F., 2010. The clinical effect of cognitive behavioral therapy on patients with essential hypertension accompanied by mood disorder. Chin. J. Pract. Rural Doctors. 17(11), 51-52. https://doi.org/j.issn.1672-7185.2010.11.030. (Chinese).
- Rutledge T., Hogan B.E. A quantitative review of prospective evidence linking psychological factors with hypertension development. Psychosom. Med. 2002;64(5):758–766. doi: 10.1097/01.psy.0000031578.42041.1c. [DOI] [PubMed] [Google Scholar]
- Samadian F., Dalili N., Jamalian A. Lifestyle modifications to prevent and control hypertension. Iran J Kidney Dis. 2016;10(5):237–263. [PubMed] [Google Scholar]
- Shapiro D., Hui K.K., Oakley M.E., Pasic J., Jamner L.D. Reduction in drug requirements for hypertension by means of a cognitive-behavioral intervention. Am. J. Hypertens. 1997;10(1):9–17. doi: 10.1016/s0895-7061(96)00258-0. [DOI] [PubMed] [Google Scholar]
- Sung J., Woo J.M., Kim W., Lim S.K., Chung E.J. The effect of cognitive behavior therapy-based “forest therapy” program on blood pressure, salivary cortisol level, and quality of life in elderly hypertensive patients. Clin. Exp. Hypertens. 2012;34(1):1–7. doi: 10.3109/10641963.2011.618195. [DOI] [PubMed] [Google Scholar]
- Takaesu Y., Inoue Y. Treatment strategy of insomnia for the patients with metabolic syndrome. Nihon rinsho. 2012;70(7):1216–1221. [PubMed] [Google Scholar]
- Trijsburg R.W., Frederiks G.C.F.J., Gorlee M., Klouwer E., den Hollander A.M., Duivenvoorden H.J. Development of the comprehensive psychotherapeutic interventions rating scale (CPIRS) Psychother. Res. 2002;12(3):287–317. doi: 10.1093/ptr/12.3.287. [DOI] [Google Scholar]
- Verhagen A.P., de Vet H.C., de Bie R.A., Kessels A.G., Boers M., Bouter L.M., Knipschild P.G. The Delphi list: a criteria list for quality assessment of randomized clinical trials for conducting systematic reviews developed by Delphi consensus. J. Clin. Epidemiol. 1998;51(12):1235–1241. doi: 10.1016/s0895-4356(98)00131-0. [DOI] [PubMed] [Google Scholar]
- Weiwei T., Wen D., Yan Z., Yu X., Xiaoxi K., Yaxin T., Zhang J. Clinical observation of cognitive behavioral therapy for patients of flight crew with hypertension. Mental Health Sichuan. 2015;28(6):507–510. [Google Scholar]
- Williams, B., Mancia, G., Spiering, W., Agabiti Rosei, E., Azizi, M., Burnier, M., Clement, D.L., Coca, A., de Simone, G., Dominiczak, A., Kahan, T., Mahfoud, F., Redon, J., Ruilope, L., Zanchetti, A., Kerins, M., Kjeldsen, S.E., Kreutz, R., Laurent, S., Lip, G.Y.H., McManus, R., Narkiewicz, K., Ruschitzka, F., Schmieder, R.E., Shlyakhto, E., Tsioufis, C., Aboyans, V., Desormais, I., 2018. 2018 ESC/ESH Guidelines for the management of arterial hypertension. Eur. Heart J. 39(33), 3021-3104. https://doi.org/10.1093/eurheartj/ehy339.
- Wolgensinger, L., 2015. Cognitive behavioral group therapy for anxiety: recent developments. Dialogues Clin. Neurosci. 17(3), 347-351. https://doi.org/10.31887/DCNS.2015.17.3/lwolgensinger. [DOI] [PMC free article] [PubMed]
- Xinju, Y., Yuanfeng, Z., Juan, L., Yazhen, L., Ying, L., Yanjiang, W., Xiaojiang, J., 2017. The efficacy of internet -based cognitive behaviour therapy on blood pressure for comorbid hypertension and insomnia. Med. J. Chin. PLA. 42(4), 331-335. https://doi.org/10.11855/j.issn.0577-7402.2017.04.11. (Chinese).
- Xue F., Yao W., Lewin R.J. A randomised trial of a 5 week, manual based, self-management programme for hypertension delivered in a cardiac patient club in Shanghai. BMC Cardiovasc. Disord. 2008;8:10. doi: 10.1186/1471-2261-8-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Li Z., Sun J. Effects of cognitive behavioral therapy-based intervention on improving glycaemic, psychological, and physiological outcomes in adult patients with diabetes mellitus: A meta-analysis of randomized controlled trials. Front. Psychiatry. 2020;11:711. doi: 10.3389/fpsyt.2020.00711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youyou, M., 2013. Clinical Application of Psycho-cardiology in Hypertension. Fujian Medical University. (Chinese Master Dissertation).
- Yu, L., Xin, B., Jiahong, W., 2018. Effect evaluation of sleep intervention on elderly patients with hypertension. Prev. Med. 30(8), 776-779. https://doi.org/10.19485/j.cnki.issn2096-5087.2018.08.005. (Chinese).
- Yuanyuan, L., 2017. Effect of Cognitive - Behavioral Group Counseling on Anxiety of Elderly Patients with Hypertension - Taking Two Nursing homes in Beitun as an Example. Shihezi University. (Chinese Master Dissertation).
- Yurong S., Mengqi K., Danhua Z., Yuanhua C., Weide L. A study on the effect of psychological intervention for hypertension. Med. J. Chin. People’s Health. 2012;24:2910–2912. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.