Abstract
Background
There is a need of well-powered randomized clinical trials in fibromyalgia. However, challenges for recruitment are presented. This study aims to describe and assess the perception of barriers and facilitators and the associated factors for the participation of underrepresented and non-underrepresented fibromyalgia patients.
Methods
We performed an online survey through REDCap (Research Electronic Data Capture) targeting fibromyalgia patients from April 7 to July 3, 2020 during the COVID-19 stay home mandate and it was restricted to the United States of America. We described and compared the survey characteristics between underrepresented and non-underrepresented participants, and we performed logistic regression models to assess the associated factors with clinical trial participation.
Results
In total, 481 completed the survey including 168 underrepresented fibromyalgia patients. Only (1) 11.09 % reported previous participation in clinical trials and the significant perceived barriers were investigator-related (lack of friendliness of research staff and the opportunity to receive the results) and center-related (privacy and confidentiality policies, and the institution's reputation); (2) the participation rate and perceived barriers and facilitators were similar between underrepresented and non-underrepresented patients; and was positively associated with low income, higher age, and clinical trial awareness from their physician; and negatively associated with the perception of investigator-related barriers; and (4) for the underrepresented population, the presence of emotional support.
Conclusion
Our findings suggest low rates of participation, regardless of underrepresented population status. Strategies as involving their physician as liaison to increase the awareness of clinical trials, as well as improving patient-researcher communication should be considered in this population.
Keywords: Fibromyalgia, Underrepresented population, Clinical trials participation, Recruitment
Fibromyalgia, Underrepresented population, Clinical trials participation, Recruitment
1. Introduction
Participant recruitment for randomized clinical trials (RCT) has always been a great challenge for clinical research. A study demonstrated that less than one-third of trials accomplished recruiting the established number of subjects in time [1]. Additionally, studies showed that 40% out of 253 trials were terminated prematurely, and around half of the trials had to prolong recruitment time due to ineffective recruitment strategies [2]. Evidence has also shown potential participation barriers such as certain social, economic, cultural traits, lack of clinical trial awareness, mistrust and lack of disease education, particularly in the underrepresented population [3, 4].
Previous studies have shown less participation of underrepresented population in Randomized Clinical Trials (RCTs) [5]. Underrepresented population includes race, ethnicity and other factors such as gender, sex, and low educational and socioeconomic status [6, 7]. This lack of participation reduces opportunities for discovering effects related to underrepresented populations [8]. It contributes thus to inequitable distribution of benefits and risks of trial participation [9]. The influence of the underrepresented population in RCT recruitment varies across diseases based on their clinical and epidemiological characteristics; thus disease-specific studies to understand barriers and facilitators for their participation are needed [10, 11].
Fibromyalgia is a condition where 90% of enrolled subjects are women [12] and has a higher prevalence in minority populations [13]. Moreover, racial/ethnic minorities report significantly greater levels of symptoms [14]. Fibromyalgia is a debilitating, poorly understood, and complex chronic pain condition [15], where most patients are highly impacted by the disease, lacking access to healthcare delivery and suffering significant comorbidities, such as functional and psychiatry disorders [16, 17]. Besides, given the perception and intensity of pain vary among subjects and across time, it may represent a barrier to accomplish the schedule and visits of a clinical trial [18]. Also, the definite diagnosis could extend from months to years; thus, patients do not know about it early enough to participate in a trial [15]. Currently, the response to available treatments is variable and the risk of adverse events is high [19, 20]. Other conditions as breast cancer has reported that despite the high prevalence in women, and even higher risk of breast cancer-specific mortality in race/ethnicity minorities, they have a lower representation in clinical trials [21]. Therefore, given there is a need to improve the recruitment of women and minorities in clinical trials, fibromyalgia patients represent an example of the challenges on recruitment and adherence of an underrepresented population.
Understanding and overcoming these barriers for fibromyalgia patients is essential to implement well-powered RCTs for new treatments and to reduce the negative impacts of poor recruitment, that not only affects RCTs overall costs [22] but also and most importantly impacts the generalizability of the results [10, 11]. Moreover, engender participation opportunities to underrepresented populations into fibromyalgia clinical trials may also clarify clinical outcomes as well as provide a deep understanding of different treatment response profiles. However, there is a lack of exploring and understanding of potential factors influencing the recruitment and participation for underrepresented and non-underrepresented fibromyalgia patients.
Therefore, given the high prevalence of fibromyalgia in women and minorities, it is critical to determine the clinical trial participation barriers and facilitators for fibromyalgia patients to improve the recruitment strategies of current and future clinical trials. This study aims to describe the clinical trial participation of underrepresented and non-underrepresented fibromyalgia patients and to assess their perception of barriers and facilitators for recruitment and the associated factors to their participation to propose further strategies enhancing recruitment.
2. Methods
2.1. Study design
We performed a cross-sectional study in which a self-administered anonymous survey was offered on the internet from April 7 to July 3, 2020. The survey period coincided with COVID-19 stay-at-home mandates in most US states and thus facilitated participation through the internet. The survey was approved by the Institutional Review Board of MassGeneral Brigham's ethics committee under the protocol number 2017P002524. It was performed and managed using REDCap (Research Electronic Data Capture) electronic data capture tools hosted at Partners HealthCare Research Computing, Enterprise Research Infrastructure & Services (ERIS) group [23]. Prior to starting the survey, participants were asked for their preferred language (English or Spanish) and their consent to participate after a description of the survey, including the aim to understand the participation of fibromyalgia patients in clinical trials and their anonymity participation (Supplementary Material 1). Before the Google Ads release, the survey was tested in a small sample (n = 21) of participants during March 2020. We then made minor changes about the wording of the questions and answers and eligibility criteria according to the initial experience. Based on our pre-established settings on Google ads, potential participants who fit our search criteria received an advertisement and an encrypted link from our survey [23].
2.2. Study population
Out target population for the survey was fibromyalgia patients. Eligibility screening assessed the completion of self-reported fibromyalgia characteristics (diagnosis of fibromyalgia, pain score more than zero over the last six months measured by a numeric scale 0–10 and time since diagnosis more than six months) and at least one criteria of underrepresented or non-underrepresented characteristic. Underrepresented was defined by NIH with the objective to enhance diversity in clinical trials [7]. This includes race (Black or African American, American Indian or Alaska Native, Asian, Native Hawaiian or other Pacific Islander or two or more races), ethnicity (Hispanic or Latino or Spanish Origin), low income according to poverty threshold (<$15 000 household income for one or two people, < $20 000 for three or four people, < $30 000 for four, five or six people) [24], low education referring to people without high school diploma or equivalent or higher (No school, home school, some school).
2.3. Survey recruitment
We used Google Ads with a spending of 40 dollars per day, a bid of 0.20/click, to maximize impression and clicks on google search. This tool was used previously for recruitment [25, 26, 27, 28] and was consistent with platform best practices during the study [29]. Google Trends gives live information about what is being searched on a determined area or at a certain time or under a specific subtopic [30, 31]. Therefore, you can delineate the profile of the users and infer the characteristics of a determined group of individuals [31, 32, 33]. This have been used in the literature to discuss a few main health topics and how they are affecting the population. Studies has shown that an adequate use of keywords and the understanding of Google analytics metrics, Google Ads can be an useful recruitment tool [34, 35, 36, 37]; also it has shown to be effective to target specific populations [36]. Moreover, it has shown a good performance recruiting underrepresented populations [38]. See the ad preview in supplementary table 1. Since our target population was subjects with fibromyalgia with iterative fibromyalgia-related information searches through Google, we focalized the scope of the Google ads by subject using five keywords (fibromyalgia, fibromyalgia syndrome, fibromyalgia pain, fibromyalgia symptoms, and fibromyalgia pain relief) and restricted its distribution geographically only to the United States (achieving impressions and clicks from all states). No restriction, neither by sex nor age was established. The survey was available for anyone.
2.4. Survey instrument
We created an ad hoc bilingual (English/Spanish) survey for the study consisting of 101 items and subdivided into four main sections: (1) fibromyalgia characteristics, (2) sociodemographic variables, (3) clinical awareness and experience in clinical trials and (4) perceived factors that might influence clinical trial participation, the latter were an adaptation (through a consensus process among the recruitment team of current a NIH-funded fibromyalgia trial [39] and lead by an expert epidemiologist) of a previous survey used to measure participation in clinical trials [40]. Responses options varied by the type of questions and ranged from numerical, categorical, ordinal (Likert scale) to open-ended comments.
Fibromyalgia variables included confirmation of diagnosis, pain score average over the last six months (0–10 numeric scale), time since diagnosis in years. Sociodemographic variables included, age, sex, ethnicity (Hispanic or Latino or Spanish Origin, not Hispanic), race (American Indian or Alaska Native, Asian, Black or African American, Native Hawaiian or other Pacific Islander, white, Two or more races, other), education (Home-school, No school, Some school, GED or equal, High school graduated, Associate or Bachelor's, Master's or higher), household income (<15 000 USD, 15 000–20 000 USD, 21 000–30 000 USD, 31 000–45 000 USD, 46 000–60 000 USD, 61 000–120 000 USD, >120 000 USD), number of people per household. Other variables included: religion, civil status, income, government support, employment, housework hours, emotional support and feeling down, depressed, or hopeless in the last two weeks. Clinical awareness was measured by previous knowledge of clinical trials by their physicians and whether they were asked previously to participate in a clinical study and the likelihood to participated in clinical trials measured by a 5-point Likert scale. Previous participation was measured by a dichotomic question asking, “In your lifetime, in how many trials have you taken part? – I have never participated in a clinical trial, or I have participated in ____,” and the number of previous clinical trial participation. Perceived factors that might influence participation were measured by a four point-Likert scale. A copy of the survey as fielded to participants is supplied in supplementary table S1.
2.5. Statistical analysis
For the descriptive analysis of categorical variables, absolute and relative frequencies were used. For the sociodemographic quantitative variables, means and their respective standard deviations were reported. We compared the survey answers (participant characteristics, trial awareness, and perception of barriers and facilitators) between underrepresented and non-underrepresented participants (using the full criteria, and also among subclassification of underrepresented population, by only socioeconomic criteria – low income and low education – or only by race/ethnicity criteria – non-white – as sensitivity analysis) by unpaired t test or Fisher's exact test, for quantitative or categorical data, respectively.
We performed univariate logistic regression models to test the association of clinical trial participation (no = 0, yes = 1) with participant characteristics, trial awareness, and perception of barriers and facilitators to participating. We determined the effects of confounders in these models by adding independent variables (participant characteristics, trial awareness, and perception of barriers and facilitators) in subsequent multivariate logistic regression models. Variables were considered as confounders if they changed the β coefficient of the clinical trial participation variable by more than 10 % and if the p-value was smaller than 0.10. The variable that was not considered a confounder was kept in the model if the p-value <0.05 and if it does not inflate the standard error of the clinical trial participation variable substantially, in order to avoid collinear terms. The selection of independent variables was based on the “purposeful selection method” [41]. We reported the logistic regression results using odds ratios (ORs), a relative measure of association between exposure and outcome. The OR represents the odds that an outcome (clinical trial participation) will occur given a particular exposure (participant characteristics, trial awareness, and perception of barriers and facilitators to participating). OR higher than 1 indicates increased occurrence of the outcome, and OR lower than 1 indicate the opposite [42].
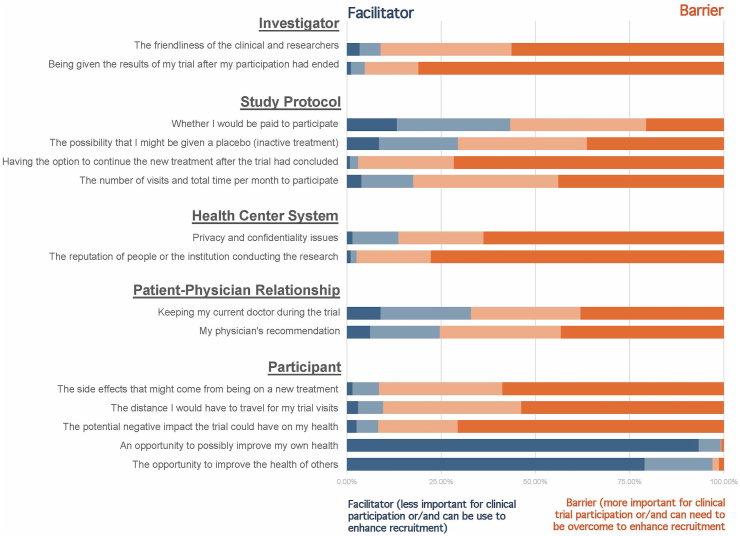
Also, we performed a Kruskal Wallis test to evaluate the differences between the perceived importance across domains from the Likert scale, as was done in a previous study [43]. To do so, we coded the four possible answers: 4 = very important, 3 = somewhat important, 2 = not very important, and 1 = not at all important. Using these values, we estimated a median score for each domain (investigator, trial protocol, center, patient, and physician-patient) by adding all the values obtained for each subdomain (See Figure 1 and Table 3). We used the Dunn test as a post hoc analysis to perform a pairwise comparison with multiple comparison adjustments using the Bonferroni method.
Figure 1.
Perception of facilitators and barriers for clinical trial participation among overall fibromyalgia patients.
Table 3.
Associated factors with clinical participation of overall and underrepresented fibromyalgia patients.
| Factors | Model 1: Overall sample (n = 337)∗ |
Factors | Model 2: Underrepresented sample (n = 128)∗∗ |
||
|---|---|---|---|---|---|
| OR | (95% IC) | OR | (95% IC) | ||
| Low-income | 2.20 | (1.04–4.61) | Low-income | 11.81 | (1.09–127.67) |
| Age | 1.04 | (1.00–1.06) | Age | 1.07 | (1.00–1.14) |
| Trial awareness by Physician | 4.20 | (1.85–9.57) | Presence of emotional support | 10.33 | (1.13–94.22) |
| Perception of investigator-related barriers | 0.66 | (0.51–0.87) | Clinical trial awareness | 10.89 | (1.27–92.85) |
| Caucasian/white | 0.73 | (0.16–3.50) | Married | 2.14 | (0.33–15.37) |
| Low education | 0.55 | (0.06–4.86) | Pain score | 0.60 | (0.00–0.16) |
Dependent variable: clinical trial participation (yes = 1, no = 0).
Pseudo R2: 10% (overall sample), adjusted by race/ethnicity, employment, and low education status, Degrees of freedoms: 332.
Pseudo R2: 29% (underrepresented sample), adjusted by marital status and pain score. Degrees of freedoms: 122.
Given the small proportion of missing data (0.62% for the questions that aimed to assess clinical trial participation), we decided that, when data were not available for a certain participant, we would not include it in the analysis. That said, we did not perform any imputation when data was not available for certain questions. All tests were two-sided with an alpha level of 0.05. Analyses were performed using Stata software v15.0.
3. Results
3.1. Sample characteristics
The survey was answered by 481 fibromyalgia patients, with a predominance of female responders (n = 465, 96.67 %) compared to males (n = 16, 3.3%). The mean age was 55.93 (SD 12.47), the average pain intensity in the past six months was 7.84 (SD 1.57), and the average of disease duration was 12.72 years (SD 10.35). Most of them were white (86.33%) and non-Hispanic (92.23%). The majority of responders were classified as middle or high-income (77.33%) and reported an education level of at least high-school completed (96.03%). The detailed characteristics description is shown in Table 1.
Table 1.
Responders characteristics of overall and sociodemographic variable of overall and underrepresented fibromyalgia patients.
| Overall 1 481 (100%) |
Non-Underrepresented 1 313 (65.07%) |
Underrepresented 1 168 (34.93%) |
p -value2 | |
|---|---|---|---|---|
|
Fibromyalgia | ||||
| Time since diagnosis (Mean) | 12.72 (10.35 SD) | 13.14 (10.70 SD) | 11.95 (9.64 SD) | 0.4017 |
| Pain score | 7.84 (1.57 SD) | 7.63 (1.59 SD) | 8.26 (1.44 SD) | <0.0001 |
|
Age (mean) |
55.93 (12.72 SD) |
57.3 (12.62 SD) |
53.36 (12.54 SD) |
0.0012 |
|
Gender | ||||
| Men | 16 (3.33%) | 9 (2.88%) | 7 (4.17%) | 0.248 |
| Women |
465 (96.67%) |
304 (97.12%) |
161 (95.83%) |
|
|
Race | ||||
| Black or African American | 30 (6.51%) | 0 (0%) | 30 (18.99%) | <0.0001 |
| Other |
431 (93.49%) |
303 (100%) |
128 (81.01%) |
|
|
Ethnicity | ||||
| Hispanic | 29 (7.77%) | 0 (0%) | 29 (24.58%) | <0.0001 |
| Not Hispanic |
344 (92.23%) |
255 (100%) |
89 (75.42%) |
|
|
Income | ||||
| Low income | 102 (22.67%) | 0 (0%) | 102 (63.35%) | <0.0001 |
| Middle or High income |
348 (77.33%) |
289 (100%) |
59 (36.65%) |
|
|
Education | ||||
| Low education | 19 (3.97%) | 0 (0%) | 19 (11.38%) | <0.0001 |
| High School complete or higher |
459 (96.03%) |
311 (100%) |
148 (88.62%) |
|
|
Civil status | ||||
| Married/Cohabited | 255 (53.57%) | 199 (64.40%) | 56 (33.53%) | <0.0001 |
| Other |
221 (46.43%) |
110 (35.60%) |
111 (66.47%) |
|
|
Religion | ||||
| Yes | 393 (87.72%) | 262 (89.42%) | 131 (84.52%) | 0.132 |
| No |
55 (12.28%) |
31 (10.58%) |
24 (15.48%) |
|
|
Income | ||||
| Self-Income | 197 (41.13%) | 135 (43.41%) | 62 (36.90%) | 0.167 |
| Other |
282 (58.87%) |
176 (56.59%) |
106 (63.10%) |
|
|
Government support (Monthly) | ||||
| Yes | 199 (43.07%) | 113 (37.17%) | 86 (54.43%) | <0.0001 |
| No |
263 (56.93%) |
191 (62.83%) |
72 (45.57%) |
|
|
Employment | ||||
| Employed | 120 (25.47%) | 81 (24.20%) | 39 (23.92%) | |
| Other |
351 (74.52%) |
227 (73.70%) |
124 (76.07%) |
|
|
Housework daily | ||||
| Less than 3 h | 250 (55.07%) | 169 (56.90%) | 81 (51.59%) | 0.279 |
| 3 or more hours |
204 (44.93%) |
128 (43.10%) |
76 (48.41%) |
|
|
Over the last 2 weeks, how often have you been bother by: Feeling down, depressed, or hopeless | ||||
| Not at all or several days | 251 (52.84%) | 169 (54.17%) | 82 (50.31%) | 0.424 |
| Nearly every day or more than half the days |
224 (47.16%) |
143 (45.83%) |
81 (49.49%) |
|
|
How often you have someone you can count on to listen to you when you need to talk about yourself? | ||||
| A little or none of the time | 166 (34.95%) | 92 (29.49%) | 74 (45.40%) | 0.001 |
| Some/most of the time or all of the time | 309 (65.05%) | 220 (70.51%) | 89 (54.60%) | |
Mean (SD) or Frequency (%).
Fisher's exact test; or t-test.
We identified 168 (34.93%) underrepresented fibromyalgia patients, mostly due to socioeconomic reasons (low income or low education). These patients were significantly younger than non-underrepresented (53.46 vs. 57.49, p < 0.001), with higher pain scores (8.26 vs. 7.63, p < 0.001), were not married (Fisher's exact p < 0.001), were receiving government support (Fisher's exact p = 0.001), and had less emotional support (Fisher's exact p = 0.004) (Table 1).
3.2. Clinical trial participation
Only 53 (11.09%) responders reported a previous participation in a clinical trial. The participation rate was similar for underrepresented (n = 18, 11.22%) or non-underrepresented population (n = 35, 11.04%, Fisher's exact p = 0.99); consistently, we found no differences in our sensitivity analysis among subclassification of underrepresented population, by only socioeconomic criteria (low income and low education) or only by race/ethnicity criteria (non-white). From the patients who had previously participated in a clinical trial, the median for study participation was 1 (IQR = 1 to 2). These patients were recruited from invitation by their doctor/healthcare provider (41.86%), from the hospital website or online/internet advertisements (30.23%), media (TV and Radio) (11.62%), social Network (Facebook, Instagram, others) (9.30%), flyers (2.33%), or others/not sure (20.93%) (Table 2).
Table 2.
Fibromyalgia patients' responses of clinical trial awareness and participation in RCTs.
| Overall 1 481 (100%) |
Non-Underrepresented 1 313 (65.07%) |
Underrepresented 1 168 (34.93%) |
p -value2 | |
|---|---|---|---|---|
| Clinical trial awareness | ||||
|
Has your physician talked to you about clinical trials? | ||||
| Yes | 47 (10.71%) | 33 (11.26%) | 14 (9.59%) | 0.593 |
| No |
392 (89.29%) |
260 (88.74%) |
132 (90.41%) |
|
|
Have you ever been asked to participate in clinical trials? | ||||
| Yes | 65 (13.63%) | 42 (13.46%) | 23 (13.94%) | 0.885 |
| No |
412 (86.37%) |
270 (86.54%) |
142 (86.06%) |
|
|
How likely would you be to participate in RCTs? | ||||
| Likely or very likely | 410 (85.77%) | 277 (88.78%) | 133 (80.12%) | 0.01 |
| Not sure, not likely or would not participate |
68 (14.23%) |
35 (11.22%) |
33 (19.88%) |
|
|
Participation in Clinical trials | ||||
| Yes | 53 (11.09%) | 35 (11.22%) | 18 (10.84%) | 0.99 |
| No |
425 (88.91%) |
277 (88.78%) |
148 (89.16%) |
|
|
Trial experience | ||||
| I took part and am still in the trial | 5 (4.03%) | 3 (4.05%) | 2 (4.0%) | 0.971 |
| I took part and completed the trial | 39 (31.45%) | 25 (33.78%) | 14 (28.0%) | |
| I took part but withdrew before the end | 2 (1.61%) | 1 (1.35%) | 1 (2.0%) | |
| I wanted to take part but was not eligible | 45 (36.29%) | 26 (35.14%) | 21 (38.0%) | |
| I declined to take part in the trial | 33 (26.61%) | 19 (25.68%) | 14 (28.0%) | |
Mean (SD) or Frequency (%).
Fisher's exact test; or t-test.
We performed univariate logistic models to assess associated factors with clinical trial participation in the total sample, and in the underrepresented patients; the full description of the models is reported in supplementary table S2. From the multivariate model (n = 377, Table 3), we found the following associated factors with clinical trial participation of overall fibromyalgia patients: 1) low income (OR = 2.2, 95% CI: 1.04 to 4.62); 2) age (OR = 1.04, 95% CI: 1.01 to 1.06) – older participant has higher odds for clinical trial participation; 3) clinical trial awareness from their physician (OR = 4.2, 95% CI: 1.85 to 9.57); and 4) the perception of barriers related to the investigator (lack of friendliness and to receive the results at the end of the trial) were negative associate with clinical trial participation (OR = 0.66, 95% CI: 0.51 to 0.87) – the participant who perceived the investigator-related factors as barriers has less odds for clinical trial participation. The model was adjusted by race, ethnicity, employment, and educational level.
Regarding underrepresented patients, we found in the multivariate model (n = 128, Table 3) that low income (OR = 11.81, 95 % CI: 1.09 to 127.66), age (OR = 1.07, 95% CI: 1.00 to 1.14), the presence of emotional support (OR = 10.33, 95% CI: 1.13 to 94.22), and awareness of clinical trials (OR = 10.89, 95% CI: 1.28 to 92.85) were positively associated to clinical trial participation. The multivariate model was adjusted by pain score and marital status. These characteristics were significantly different between underrepresented categories (Table 1).
3.3. Clinical trial retention
Seventy four percent (n = 39) of the subjects who previously participated in a clinical trial reported trial completion and six considered withdrawing from the study. Only two participants reported withdrawal before the end of the study. The rest of the participants did not complete the retention section of the survey.
3.4. Perception of participation barriers and facilitators
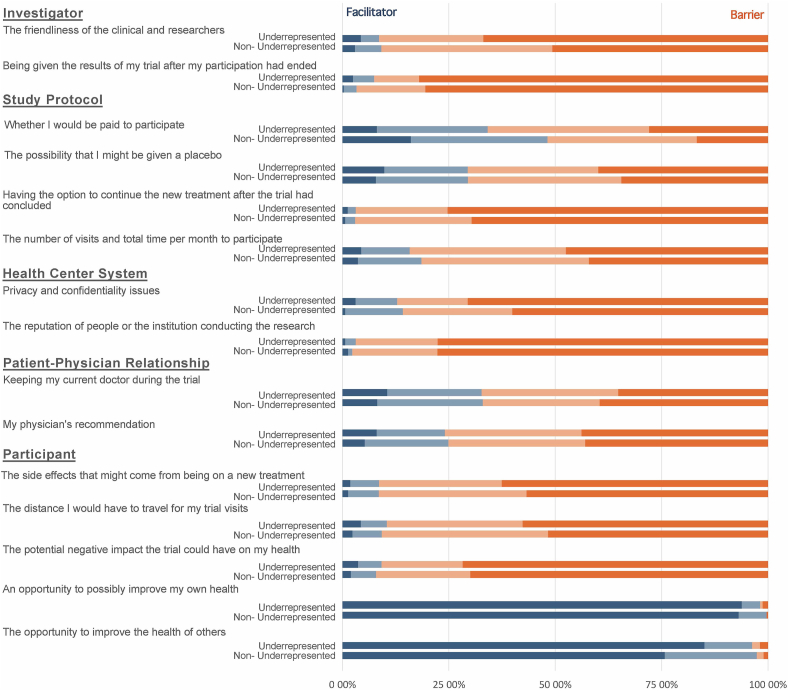
The summary of the answers is shown in Figures 1 and 2. The most important perceived barriers were factors related to the investigator (lack of friendliness of research staff and the opportunity to receive the results after the clinical trial participation) (ten points, from 0 to 10), and to the research center (privacy and confidentiality policies, and the institution reputation) (ten points, from 0 to 10). The less important perceived barriers were factors related to the participant (potential side effects or negative impact on health, the distance they need to travel for the visits, and the opportunity to improve their own health or the health of others). This perception was not different between underrepresented and non-underrepresented by domains or specific questions (Table 4).
Figure 2.
Perception of facilitators and barriers for clinical trial participation among underrepresented and non-underrepresented fibromyalgia patients.
Table 4.
Perception on clinical trial participation barriers.
| Not important or not at all important 1 | Important or Very important 1 | p-value2 | ||
|---|---|---|---|---|
| Investigator |
The friendliness of the clinical and researchers | 42 (8.96%) | 427 (91.04%) | |
| Non-underrepresented | 28 (9.15%) | 278 (90.85%) | 0.839 | |
| Underrepresented | 14 (8.59%) | 149 (91.41%) | ||
| Being given the results of my trial after my participation had ended | 22 (4.74%) | 442 (95.26%) | ||
| Non-underrepresented | 10 (3.3%) | 293 (96.7%) | 0.045 | |
| Underrepresented |
12 (7.45%) |
149 (92.55%) |
||
| Study Protocol |
Whether I would be paid to participate | 202 (43.35%) | 264 (56.65%) | |
| Non-underrepresented | 147 (48.2%) | 158 (51.8%) | 0.004 | |
| Underrepresented | 55 (34.16%) | 106 (65.84%) | ||
| The possibility that I might be given a placebo (inactive treatment) | 138 (29.49%) | 330 (70.51%) | ||
| Non-underrepresented | 90 (29.51%) | 215 (70.49%) | 0.989 | |
| Underrepresented | 48 (29.45%) | 115 (70.55%) | ||
| Having the option to continue the new treatment after the trial had concluded | 14 (3.01%) | 451 (96.99%) | ||
| Non-underrepresented | 9 (2.97%) | 294 (97.03%) | 0.945 | |
| Underrepresented | 5 (3.09%) | 157 (96.91%) | ||
| The number of visits and total time per month to participate | 81 (17.61%) | 379 (82.39%) | ||
| Non-underrepresented | 56 (18.54%) | 246 (81.46%) | 0.467 | |
| Underrepresented |
25 (15.82%) |
133 (84.18%) |
||
| Health Center System |
Privacy and confidentiality issues | 64 (13.73%) | 402 (86.27%) | |
| Non-underrepresented | 43 (14.19%) | 260 (85.81%) | 0.696 | |
| Underrepresented | 21 (12.88%) | 142 (87.12%) | ||
| The reputation of people or the institution conducting the research | 12 (2.58%) | 454 (97.42%) | ||
| Non-underrepresented | 7 (2.3%) | 298 (97.7%) | 0.599 | |
| Underrepresented |
5 (3.11%) |
156 (96.89%) |
||
| Patient-Physician Relationship |
Keeping my current doctor during the trial | 154 (32.91%) | 314 (67.09%) | |
| Non-underrepresented | 101 (33.01%) | 205 (66.99%) | 0.949 | |
| Underrepresented | 53 (32.72%) | 109 (67.28%) | ||
| My physician's recommendation | 115 (24.63%) | 352 (75.37%) | ||
| Non-underrepresented | 76 (24.92%) | 229 (75.08%) | 0.84 | |
| Underrepresented |
39 (24.07%) |
123 (75.93%) |
||
| Participant | The side effects that might come from being on a new treatment | 40 (8.55%) | 428 (91.45%) | |
| Non-underrepresented | 26 (8.52%) | 279 (91.48%) | 0.981 | |
| Underrepresented | 14 (8.59%) | 149 (91.41%) | ||
| The distance I would have to travel for my trial visits | 45 (9.64%) | 422 (90.36%) | ||
| Non-underrepresented | 28 (9.21%) | 276 (90.79%) | 0.67 | |
| Underrepresented | 17 (10.43%) | 146 (89.57%) | ||
| The potential negative impact the trial could have on my health | 39 (8.37%) | 427 (91.63%) | ||
| Non-underrepresented | 24 (7.92%) | 279 (92.08%) | 0.634 | |
| Underrepresented | 15 (9.2%) | 148 (90.8%) | ||
| An opportunity to possibly improve my own health | 4 (0.85%) | 464 (99.15%) | ||
| Non-underrepresented | 1 (0.33%) | 304 (99.67%) | 0.09 | |
| Underrepresented | 3 (1.84%) | 160 (98.16%) | ||
| The opportunity to improve the health of others | 14 (3%) | 452 (97%) | ||
| Non-underrepresented | 8 (2.62%) | 297 (97.38%) | 0.507 | |
| Underrepresented | 6 (3.73%) | 155 (96.27%) |
Frequency (%).
Fisher's exact test.
When comparing all the domains using the Kruskal Wallis test, we found a significant difference between them (H(4) = 1662.15, p < 0.0001). After the pairwise analysis corrected for multiple comparisons, we found significant differences between investigator-related and center-related factors domains compared to the rest of the domains (p < 0.0001 for all the comparisons), but not between the two of them (p = 1.00).
4. Discussion
Our findings suggest low rates of self-reported clinical trial participation among fibromyalgia patients (11.09%), regardless of their status as underrepresented. However, the low-income category is the component of the underrepresented population definition which most influences participation. We found the significant perceived barriers were investigator-related (lack of friendliness of research staff and the opportunity to receive the results after the trial) and center-related (privacy and confidentiality policies, and the institution reputation) with similar perception for both underrepresented and non-underrepresented populations. Moreover, in the overall sample, the participation was positively associated with patients with low income, higher age, and clinical trial awareness from their physician; and negatively related to the perception of investigator-related barriers; and for the underrepresented population (in addition to low income, age, and awareness), the presence of emotional support was also a positively associated factor with the participation.
Previous systematic reviews on fibromyalgia described the lack of well-powered RCTs, with most of the studies having less than 400 patients, making it difficult to extrapolate treatments to the general population [44, 45, 46]. Moreover, minorities including race, ethnicity and low income populations are not well represented in chronic pain studies [47]. The low rate of self-reported clinical trial participation is aligned with a previous online survey [40], which reported 11% of participation of fibromyalgia patients and significantly lower than other included diseases such as amyotrophic lateral sclerosis (36%), Parkinson's disease (36%), and multiple sclerosis (20%). To our knowledge, this is the first exploration of clinical trial participation of underrepresented fibromyalgia patients. However, contrary to studies on cancer clinical trials [10], which reported a lower participation rate among underrepresented individuals, we found no differential participation rate in this subgroup. This suggests that the barriers to participating are homogeneously distributed in the overall fibromyalgia population and could be associated with individual characteristics (education, income, race, ethnicity, etc.) rather than the full definition of underrepresented population.
Our survey aimed to help identify barriers or facilitators in clinical trial participation among patients with fibromyalgia. Our findings are consistent with recruitment surveys done in the general population [48]. In these surveys, receiving information about clinical trials from their healthcare provider was significantly associated with participation. Similarly, another study showed that 50–80% of eligible patients preferred not to participate in clinical trials as their physician's decision was not to offer the trial [49]. One explanation of this result can be related to trust in the medical and scientific community that is one of the major barriers, especially for the underrepresented population [50]. Physician referrals may affect patients' decision making by building better trust. These results support the engagement of physicians in clinical trials to enhance the participation of minorities. Thus, developing strategies targeting the potential barriers of minority-serving physicians' participation in clinical trials such as lack of time, lack of resources, communication difficulties, lack of training, and lack of rewards and recognition for physicians may be beneficial [51].
Our study found association between low-income and a higher participation in clinical trials, despite a previous oncology study did not considered low-income as a barrier [52], suggesting the effect of this variable might depend on the underlying condition. The fact that the association of these variables with clinical trial participation remained significant when the results were stratified for being underrepresented helps us refine recruiting strategies for this population. The low-income variable remained significantly related to clinical trial participation, even when controlled for employment status. Thus, we can speculate that low-income patients were not attracted to participate in the studies because they were unemployed and with more free time, but because of other factors, such as pursuing alternative treatments for their conditions or financial compensation.
Age also affected the trial participation in the overall and underrepresented fibromyalgia population. We found that older patients were significantly more likely to participate. The literature suggests that the elderly population is vastly underrepresented in clinical trials restricting the generalizability of the efficacy and safety of interventions [53]. Our study indicates that particularly older patients with fibromyalgia are prone to a higher participation rate. Therefore, they should be targeted in the recruitment strategies. Possible explanations for this finding include time-commitment availability and seeking alternative experimental treatment options after multiple unsuccessful treatments in the past. Targeting this population can thus help to improve underrepresented population participation.
Moreover, investigator-related barriers were considered important for participation, such as the staff's lack of friendliness and giving more information (e.g., results) related to their participation in the study. In different populations, recruiters considered the effective communication and presentation of the trial information simply and clearly as a key role for recruitment [54, 55, 56]. Previous studies with vulnerable populations such as women with HIV have reported that researchers' characteristics such as respectfulness, flexibility, being empathic, building a strong rapport, and good communication skills are important for participation [57]. Also, patients with poor perceptions of health and quality of life as fibromyalgia patients have reported a decreasing trust in physicians [58], consistent with several medical appointments with different specialist as the diagnosis is made by exclusion. Therefore, promoting a trustable and safe environment by the investigators at the research center might play an important role for this population, increasing recruitment and adherence to their participation.
Regarding underrepresented fibromyalgia patients, the distinctive associated factor was the presence of emotional support. It is well known the frequent functional and psychiatry comorbidities in fibromyalgia that can negatively affect the patients' mood [16, 17]. Additionally, they are a particular population with low access to healthcare and constant debilitating pain and fatigue decreasing their quality of life [59, 60]. Therefore, the emotional support in these patients could increase the resilience and adaptation to the disease and facilitate the seek for novel therapies.
Early planning for a recruitment strategy for large clinical trials is particularly important, given the substantial costs involved and the large expected enrollment goals. From our sample, we found specific characteristics associated with previous involvement in research. We believe that the participation of underrepresented and non-underrepresented populations can be enhanced considering these critical characteristics. First, we suggest an in-depth training of the recruitment and consent team improving their communication skills, standardizing the way they explain procedures and answer concerns, and adding a post-study procedure that includes a summary report of the trial results or publications. Second, we found two potential factors that could improve the strategies to target potential participants: low-income population and older age. Collaboration with non-profit or governmental organizations that provide assistance to patients and the use of digital databases to identify specific profiles using IRB-approved methods (such as online marketing services – Google ads or social media – or electronic medical records) are options to consider in the recruitment plan. Third, we found the importance of increasing RCTs awareness and the role of health care providers as liaisons for recruitment. Thus, we recommend creating an early partnership with primary care physicians, rheumatologists, and pain medicine specialists to develop targeted strategies at clinics with information to be forwarded to their patients instead of broad-based alternatives. Finally, we found that emotional support is also important, especially in the underrepresented population. Therefore, identifying and targeting support groups of fibromyalgia patients or implementing institutional support groups that could help as a long-term partnership for referral of fibromyalgia patients, especially for large and long RCTs.
Although we suggest potential strategies to increase the participation rate, it is important to highlight that recruitment should not be restricted to these subpopulations to avoid affecting the study's external validity. On the contrary, to be added to classic and broader strategies.
There are some limitations to our survey. The first one is the unknown number of non-response. Thus, the generalization of these findings is limited by our convenience sample. However, our participants' characteristics (Table 1) are similar to previous epidemiological studies on fibromyalgia [61, 62], suggesting that our sample could be representative of these patients. Furthermore, the identification of underrepresented populations by an internet-based survey could be argued as a potential biased method. Nevertheless, recent studies have shown the high availability of internet in the USA [63], and the access is similar for underrepresented and non-underrepresented populations [64]. Also, our survey was relatively short, thus did not inhibit participation once started. Finally, we surveyed subjects during the COVID-19 stay-at-home mandate in most of the US states. It is possible that participation and generalization increased during this time due to more internet use and home stay. The second factor is that self-reported data increases the risk of recall bias or the probability of having incomplete information in the survey regarding participation in past clinical studies. The final limitation is that we cannot request confirmation of diagnosis as the data collected is based on non-identifiable information; therefore, we rely on the individual confirmation of the diagnosis of fibromyalgia that could be uncertain. However, it needs to be underscored that subjects had no secondary gain to participate in the trial (they did not receive any monetary compensation). Thus, it is less likely subjects who do not have fibromyalgia would be imprecise with this information. Also, as online tools for targeting this population, Google ads were shown in previous survey studies as a reliable method to reach patients based on their internet search patterns [65, 66, 67, 68].
In summary, our findings suggest low rates of clinical trial participation of fibromyalgia patients, regardless of their status as underrepresented. However, the low-income category is the component of the underrepresented population definition that most influences their participation. Strategies to enhance recruitment should consider targeting support groups and low-income populations, involving their physician as liaison to increase the awareness of clinical trials and improve patient-researcher communication.
Declarations
Author contribution statement
Alejandra Cardenas-Rojas: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Kevin Pacheco-Barrios and Felipe Fregni: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Luis Castelo-Branco, Stefano Giannoni-Luza, Ana Balbuena-Pareja, Maria Alejandra Luna-Cuadros, Luna Vasconcelos Felippe, Elif Uygur-Kucukseymen, Paola Gonzalez-Mego, Muhammed Enes Gunduz, Emad Salman Shaikh and Anna Carolyna Lepesteur Gianlorenco: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This work was supported by National Center for Complementary and Alternative Medicine (R01 AT009491-01A1).
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Google ads preview and survey.
Univariate logistic regression models.
References
- 1.Isaksson E., Wester P., Laska A.C., Näsman P., Lundström E. Identifying important barriers to recruitment of patients in randomised clinical studies using a questionnaire for study personnel. Trials. 2019 Oct;20(1):618. doi: 10.1186/s13063-019-3737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kakumanu S., Manns B.J., Tran S., Saunders-Smith T., Hemmelgarn B.R., Tonelli M. Cost analysis and efficacy of recruitment strategies used in a large pragmatic community-based clinical trial targeting low-income seniors: a comparative descriptive analysis. Trials. 2019;20(1):577. doi: 10.1186/s13063-019-3652-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Otado J., Kwagyan J., Edwards D., Ukaegbu A., Rockcliffe F., Osafo N. Culturally competent strategies for recruitment and retention of african American populations into clinical trials. Clin. Trans. Sci. 2015 Oct;8(5):460–466. doi: 10.1111/cts.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heller C., Balls-Berry J.E., Nery J.D., Erwin P.J., Littleton D., Kim M. Strategies addressing barriers to clinical trial enrollment of underrepresented populations: a systematic review. Contemp. Clin. Trials. 2014 Nov;39(2):169–182. doi: 10.1016/j.cct.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sateren W.B., Trimble E.L., Abrams J., Brawley O., Breen N., Ford L. How sociodemographics, presence of oncology specialists, and hospital cancer programs affect accrual to cancer treatment trials. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 2002 Apr;20(8):2109–2117. doi: 10.1200/JCO.2002.08.056. [DOI] [PubMed] [Google Scholar]
- 6.Killien M., Bigby J.A., Champion V., Fernandez-Repollet E., Jackson R.D., Kagawa-Singer M. Involving minority and underrepresented women in clinical trials: the National Centers of Excellence in Women’s Health. J. Wom. Health Gend. Base Med. 2000 Dec;9(10):1061–1070. doi: 10.1089/152460900445974. [DOI] [PubMed] [Google Scholar]
- 7.NIH Policy and Guidelines on The Inclusion of Women and Minorities as Subjects in Clinical Research. grants.nih.gov. https://grants.nih.gov/policy/inclusion/women-and-minorities/guidelines.htm. (Accessed 7 July 2021).
- 8.Moser M., Lunn J. Responses to captopril and hydrochlorothiazide in black patients with hypertension. Clin. Pharmacol. Ther. 1982 Sep;32(3):307–312. doi: 10.1038/clpt.1982.165. [DOI] [PubMed] [Google Scholar]
- 9.Emanuel E., Wendler D., Grady C. What makes clinical research ethical? J. Am. Med. Assoc. 2000;283:2701–2711. doi: 10.1001/jama.283.20.2701. [DOI] [PubMed] [Google Scholar]
- 10.Ford J.G., Howerton M.W., Lai G.Y., Gary T.L., Bolen S., Gibbons M.C. Barriers to recruiting underrepresented populations to cancer clinical trials: a systematic review. Cancer. 2008 Jan;112(2):228–242. doi: 10.1002/cncr.23157. [DOI] [PubMed] [Google Scholar]
- 11.Otado J., Kwagyan J., Edwards D., Ukaegbu A., Rockcliffe F., Osafo N. Culturally competent strategies for recruitment and retention of african American populations into clinical trials. Clin. Trans. Sci. 2015 Oct;8(5):460–466. doi: 10.1111/cts.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marques A.P., de do Santo A.S.E., Berssaneti A.A., Matsutani L.A., Yuan S.L.K. Prevalence of fibromyalgia: literature review update. Rev. Bras. Reumatol. 2017 Jul 1;57(4):356–363. doi: 10.1016/j.rbre.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Gansky S.A., Plesh O. Widespread pain and fibromyalgia in a biracial cohort of young women. J. Rheumatol. 2007;34(4) [PubMed] [Google Scholar]
- 14.Marr N.C., van Liew C., Carovich T.F., Cecchini G.A., McKinley L.E., Cronan T.A. The effects of racial/ethnic minority status on sleep, mood disturbance, and depression in people with fibromyalgia. Psychol. Res. Behav. Manag. 2020;13:343–353. doi: 10.2147/PRBM.S242699. http://www.pmc/articles/PMC7174195/ [Internet] [cited 2021 Apr 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezende M.C., Paiva E.S., Helfenstein M.J., Ranzolin A., Martinez J.E., Provenza J.R. EpiFibro--a nationwide databank for fibromyalgia syndrome: the initial analysis of 500 women. Rev. Bras. Reumatol. 2013;53(5):382–387. [PubMed] [Google Scholar]
- 16.Lichtenstein A., Tiosano S., Amital H. The complexities of fibromyalgia and its comorbidities. Curr. Opin. Rheumatol. 2018 Jan;30(1):94–100. doi: 10.1097/BOR.0000000000000464. [DOI] [PubMed] [Google Scholar]
- 17.Weir P.T., Harlan G.A., Nkoy F.L., Jones S.S., Hegmann K.T., Gren L.H. The incidence of fibromyalgia and its associated comorbidities: a population-based retrospective cohort study based on International Classification of Diseases, 9th Revision codes. J. Clin. Rheumatol.: Pract. Rep. Rheum. Musculoskel. Dis. 2006 Jun;12(3):124–128. doi: 10.1097/01.rhu.0000221817.46231.18. [DOI] [PubMed] [Google Scholar]
- 18.Wolfe F., Walitt B., Perrot S., Rasker J.J., Häuser W. Fibromyalgia diagnosis and biased assessment: sex, prevalence and bias. PloS One. 2018;13(9) doi: 10.1371/journal.pone.0203755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ablin J., Fitzcharles M., Buskila D., Shir Y., Sommer C., Hauser W. Treatment of fibromyalgia syndrome: recommendations of recent evidence-based interdisciplinary guidelines with special emphasis on complementary and alternative therapies. Evid. base Compl. Alternative Med. 2015;2013 doi: 10.1155/2013/485272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kia S., Choy E. Update on treatment guideline in fibromyalgia syndrome with focus on pharmacology. Biomedicines. 2017;5(20) doi: 10.3390/biomedicines5020020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zavala V.A., Bracci P.M., Carethers J.M., Carvajal-Carmona L., Coggins N.B., Cruz-Correa M.R. Cancer health disparities in racial/ethnic minorities in the United States. Br. J. Canc. 2021;124:315–332. doi: 10.1038/s41416-020-01038-6. [Internet] Springer Nature [cited 2021 Jun 17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Isaksson E., Wester P., Laska A.C., Näsman P., Lundström E. Identifying important barriers to recruitment of patients in randomised clinical studies using a questionnaire for study personnel. Trials. 2019 Oct;20(1):618. doi: 10.1186/s13063-019-3737-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009 Apr;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. https://pubmed.ncbi.nlm.nih.gov/18929686/ [Internet] [cited 2021 Mar 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Poverty Thresholds, United States Census Bureau. https://www.census.gov/data/tables/time-series/demo/income-poverty/historical-poverty-thresholds.html. (Accessed July 7 2021).
- 25.Buller D.B., Meenan R., Severson H., Halperin A., Edwards E., Magnusson B. Comparison of 4 recruiting strategies in a smoking cessation trial. Am. J. Health Behav. 2012 Sep;36(5):577–588. doi: 10.5993/AJHB.36.5.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan A.J., Jorm A.F., Mackinnon A.J. Internet-based recruitment to a depression prevention intervention: lessons from the Mood Memos study. J. Med. Internet Res. 2013 Feb;15(2):e31. doi: 10.2196/jmir.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O’Dwyer S.T., Moyle W. Using Google Adwords to recruit family carers of people with dementia. Australas. J. Ageing. 2014 Jun;33(2):128–131. doi: 10.1111/ajag.12100. [DOI] [PubMed] [Google Scholar]
- 28.Temple E. A comparison of internet-based participant recruitment methods: engaging the hidden population of cannabis users in research. J. Res. Pract. 2011;7(2) [Google Scholar]
- 29.Google Google Ads policies. https://support.google.com/adspolicy/answer/600894
- 30.Carneiro H.A., Mylonakis E. Google trends: a web-based tool for real-time surveillance of disease outbreaks. Clin. Infect. Dis. 2009 Nov;49(10):1557–1564. doi: 10.1086/630200. [DOI] [PubMed] [Google Scholar]
- 31.Mavragani A., Ochoa G. Google trends in infodemiology and infoveillance: methodology framework. JMIR Publ. Health Surv. 2019 May 29;5(2) doi: 10.2196/13439. https://pubmed.ncbi.nlm.nih.gov/31144671/ [Internet] [cited 2021 Feb 22] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervellin G., Comelli I., Lippi G. Is Google Trends a reliable tool for digital epidemiology? Insights from different clinical settings. J. Epidemiol. Glob. Health. 2017 Sep 1;7(3):185–189. doi: 10.1016/j.jegh.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mavragani A., Ochoa G., Tsagarakis K.P. Assessing the methods, tools, and statistical approaches in Google trends research: systematic review. J. Med. Internet Res. 2018 Nov 1;20(11) doi: 10.2196/jmir.9366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liang J., Yang H., Gao J., Yue C., Ge S., Qu B. MOPSO-based CNN for keyword selection on google ads. IEEE Access. 2019;7:125387–125400. [Google Scholar]
- 35.Barrera A.Z., Kelman A.R., Muñoz R.F. Keywords to recruit Spanish- and English-speaking participants: evidence from an online postpartum depression randomized controlled trial. J. Med. Internet Res. 2014 Jan 9;16(1):e6. doi: 10.2196/jmir.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Johnson K.J., Mueller N.L., Williams K., Gutmann D.H. Evaluation of participant recruitment methods to a rare disease online registry. Am. J. Med. Genet. 2014 Jul 1;164(7):1686–1694. doi: 10.1002/ajmg.a.36530. [DOI] [PubMed] [Google Scholar]
- 37.About audience targeting - google ads help. https://support.google.com/google-ads/answer/2497941?hl=en [Internet]. [cited 2021 Apr 22]. Available from:
- 38.Carlini B.H., Safioti L., Rue T.C., Miles L. Using internet to recruit immigrants with language and culture barriers for tobacco and alcohol use screening: a study among Brazilians. J. Immigr. Minority Health. 2015 Mar 22;17(2):553–560. doi: 10.1007/s10903-013-9934-1. [DOI] [PubMed] [Google Scholar]
- 39.Castelo-Branco L., Uygur Kucukseymen E., Duarte D., El-Hagrassy M.M., Bonin Pinto C., Gunduz M.E. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia-targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ open. 2019 Oct;9(10) doi: 10.1136/bmjopen-2019-032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.DasMahapatra P., Raja P., Gilbert J., Wicks P. Clinical trials from the patient perspective: survey in an online patient community. BMC Health Serv. Res. 2017 Feb;17(1):166. doi: 10.1186/s12913-017-2090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bursac Z., Gauss C.H., Williams D.K., Hosmer D.W. Purposeful selection of variables in logistic regression. Source Code Biol. Med. 2008 Dec 16;3 doi: 10.1186/1751-0473-3-17. https://pubmed.ncbi.nlm.nih.gov/19087314/ [Internet] [cited 2021 Apr 26] Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kraemer H.C. Reconsidering the odds ratio as a measure of 2×2 association in a population. Stat. Med. 2004 Jan 30;23(2):257–270. doi: 10.1002/sim.1714. [Internet] [cited 2021 Apr 26] [DOI] [PubMed] [Google Scholar]
- 43.Zafra-Tanaka J.H., Pacheco-Barrios K., Inga-Berrospi F., Taype-Rondan A. Self-perceived competencies in the diagnosis and treatment of mental health disorders among general practitioners in Lima, Peru. BMC Med. Educ. 2019;19(1):464. doi: 10.1186/s12909-019-1900-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim S.Y., Busch A.J., Overend T.J., Schachter C.L., van der Spuy I., Boden C. Flexibility exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019 Sep 2;2019(9) doi: 10.1002/14651858.CD013419. [Internet] [cited 2021 Apr 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bidonde J., Busch A.J., Schachter C.L., Webber S.C., Musselman K.E., Overend T.J. Mixed exercise training for adults with fibromyalgia. Cochrane Database Syst. Rev. 2019 May 24;2019(5) doi: 10.1002/14651858.CD013340. [Internet] [cited 2021 Apr 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thorpe J., Shum B., Moore R.A., Wiffen P.J., Gilron I. Combination pharmacotherapy for the treatment of fibromyalgia in adults. Cochrane Database Syst. Rev. 2018 Feb 19;20(3):297–300. doi: 10.1002/14651858.CD010585.pub2. [Internet] [cited 2021 Apr 26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Campbell L.C., Robinson K., Meghani S.H., Vallerand A., Schatman M., Sonty N. Challenges and opportunities in pain management disparities research: implications for clinical practice, advocacy, and policy. J. Pain. 2012;13:611–619. doi: 10.1016/j.jpain.2012.02.004. Churchill Livingstone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Baquet C.R., Commiskey P., Daniel Mullins C., Mishra S.I. Recruitment and participation in clinical trials: socio-demographic, rural/urban, and health care access predictors. Canc. Detect. Prev. 2006;30(1):24–33. doi: 10.1016/j.cdp.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benson A.B., 3rd, Pregler J.P., Bean J.A., Rademaker A.W., Eshler B., Anderson K. Oncologists’ reluctance to accrue patients onto clinical trials: an Illinois Cancer Center study. J. Clin. Oncol.: Off. J. Am. Soc. Clin. Oncol. 1991 Nov;9(11):2067–2075. doi: 10.1200/JCO.1991.9.11.2067. [DOI] [PubMed] [Google Scholar]
- 50.Shavers-Hornaday V.L., Lynch C.F., Burmeister L.F., Torner J.C. Why are African Americans under-represented in medical research studies? Impediments to participation. Ethn. Health. 1997;2(1–2):31–45. doi: 10.1080/13557858.1997.9961813. [DOI] [PubMed] [Google Scholar]
- 51.Rahman S., Majumder M.A.A., Shaban S.F., Rahman N., Ahmed M., Abdulrahman K Bin. Physician participation in clinical research and trials: issues and approaches. Adv. Med. Educ. Pract. 2011;2:85–93. doi: 10.2147/AMEP.S14103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicholson L.M., Schwirian P.M., Klein E.G., Skybo T., Murray-Johnson L., Eneli I. Recruitment and retention strategies in longitudinal clinical studies with low-income populations. Contemp. Clin. Trials. 2011 May;32(3):353–362. doi: 10.1016/j.cct.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shenoy P., Harugeri A. Elderly patients’ participation in clinical trials. Perspet. Clin. Res. 2015;6(4):184–189. doi: 10.4103/2229-3485.167099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Picillo M., Kou N., Barone P., Fasano A. Recruitment strategies and patient selection in clinical trials for Parkinson’s disease: going viral and keeping science and ethics at the highest standards. Park. Relat. Disord. 2015 Sep;21(9):1041–1048. doi: 10.1016/j.parkreldis.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Kaur G., Smyth R.L., Powell C.V.E., Williamson P. A survey of facilitators and barriers to recruitment to the MAGNETIC trial. Trials. 2016 Dec;17(1):607. doi: 10.1186/s13063-016-1724-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Duncan M., Korszun A., White P., Eva G., investigators S. Qualitative analysis of feasibility of recruitment and retention in a planned randomised controlled trial of a psychosocial cancer intervention within the NHS. Trials. 2018 Jun;19(1):327. doi: 10.1186/s13063-018-2728-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loutfy M.R., V L.K., Mohammed S., Wu W., Muchenje M., Masinde K. Recruitment of HIV-positive women in research: discussing barriers, facilitators, and research personnel’s knowledge. Open AIDS J. 2014 Dec;8:58–65. doi: 10.2174/1874613601408010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Freburger J.K., Callahan L.F., Currey S.S., Anderson L.A. Use of the Trust in Physician Scale in patients with rheumatic disease: psychometric properties and correlates of trust in the rheumatologist. Arthritis Rheum. 2003 Feb;49(1):51–58. doi: 10.1002/art.10925. [DOI] [PubMed] [Google Scholar]
- 59.Choy E., Perrot S., Leon T., Kaplan J., Petersel D., Ginovker A. A patient survey of the impact of fibromyalgia and the journey to diagnosis. BMC Health Serv. Res. 2010 Apr;10:102. doi: 10.1186/1472-6963-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Häuser W., Fitzcharles M.-A. Facts and myths pertaining to fibromyalgia. Dialogues Clin. Neurosci. 2018 Mar;20(1):53–62. doi: 10.31887/DCNS.2018.20.1/whauser. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gore M., Tai K.-S., Chandran A., Zlateva G., Leslie D. Clinical characteristics, pharmacotherapy, and healthcare resource use among patients with fibromyalgia newly prescribed pregabalin or tricyclic antidepressants. J. Med. Econ. 2012;15(1):32–44. doi: 10.3111/13696998.2011.629263. [DOI] [PubMed] [Google Scholar]
- 62.Neumann L., Buskila D. Epidemiology of fibromyalgia. Curr. Pain Headache Rep. 2003 Oct;7(5):362–368. doi: 10.1007/s11916-003-0035-z. [DOI] [PubMed] [Google Scholar]
- 63.Bennett G.G., Glasgow R.E. The delivery of public health interventions via the Internet: actualizing their potential. Annu. Rev. Publ. Health. 2009;30:273–292. doi: 10.1146/annurev.publhealth.031308.100235. [DOI] [PubMed] [Google Scholar]
- 64.Hudnut-Beumler J., Po’e E., Barkin S. The use of social media for health promotion in hispanic populations: a scoping systematic review. JMIR Publ. Health Surv. 2016 Jul;2(2):e32. doi: 10.2196/publichealth.5579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villanti A.C., Jacobs M.A., Zawistowski G., Brookover J., Stanton C.A., Graham A.L. Impact of baseline assessment modality on enrollment and retention in a facebook smoking cessation study. J. Med. Internet Res. 2015 Jul;17(7):e179. doi: 10.2196/jmir.4341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lane T.S., Armin J., Gordon J.S. Online recruitment methods for web-based and mobile health studies: a review of the literature. J. Med. Internet Res. 2015 Jul;17(7):e183. doi: 10.2196/jmir.4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Upadhyay U.D., Jovel I.J., McCuaig K.D., Cartwright A.F. Using Google Ads to recruit and retain a cohort considering abortion in the United States. Contraception X. 2020;2:100017. doi: 10.1016/j.conx.2019.100017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Watson N.L., Mull K.E., Heffner J.L., McClure J.B., Bricker J.B. Participant recruitment and retention in remote eHealth intervention trials: methods and lessons learned from a large randomized controlled trial of two web-based smoking interventions. J. Med. Internet Res. 2018 Aug;20(8) doi: 10.2196/10351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Google ads preview and survey.
Univariate logistic regression models.
Data Availability Statement
Data included in article/supplementary material/referenced in article.