Abstract
Background
Erectile dysfunction is one of the common complications of testicular cancer with a prevalence of 11.3%–84%. It has devastating effects on men and their partner's quality of life, sexual satisfaction, and sexual experience. The findings of the previous studies on this matter were uneven and inconsistent. Therefore, this systematic review and meta-analysis is conducted to acquire a more recent and comprehensive result.
Methods and materials
PubMed, Scopus, Goggle scholar, Science Direct, African Index Medicus, African Journal online, EMBASE, and Cochrane Library databases were searched. All necessary data were extracted using a standardized data extraction format. Data were analyzed using STATA 14 statistical software. A heterogeneity of studies was assessed using the I2 statistics. Publication bias was checked by using a funnel plot and Egger's regression test. A random-effects model was computed to estimate the pooled prevalence of erectile dysfunction.
Result
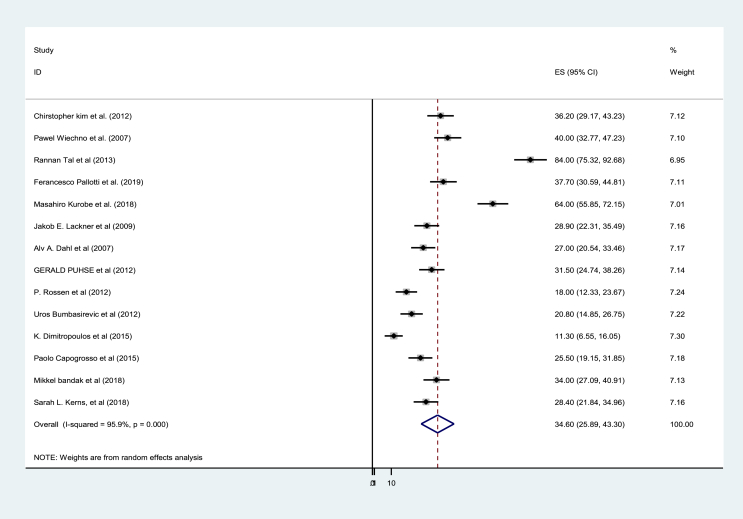
Fourteen full-text studies were included in this systematic review and meta-analysis. The pooled prevalence of erectile dysfunction among testicular cancer survivors was found to be 34.60% (95% CI: 25.89, 43.30 [I2 = 95.9% p = 0.000]). Study design subgroup analysis indicated that the pooled prevalence of erectile dysfunction was 50.02% (95% CI: 22.78, 77.28% [I2 = 96.1 p = 0.000]), and 27.36% (95% CI: 19.23, 34.48% [I2 = 91.6, P = 0.000]) in the case-control and cohort studies, respectively. Likewise, the level of erectile dysfunction was varied based on ED erectile dysfunction measuring tools and testicular cancer treatment modalities.
Conclusion
In this study erectile dysfunction was found to be a highly prevalent complication in testicular cancer survivors. It had also causes of heterogeneity in terms of treatment modalities, study designs, and measuring tools. Therefore prevention of this complication should be the concern of the responsible bodies.
Keywords: Testicular cancer, Erectile dysfunction, Pooled prevalence, Clinical research, Chronic diseases
Testicular cancer, erectile dysfunction, pooled prevalence, clinical research, chronic diseases
1. Introduction
Testicular cancer (TC) is a malignant tumor of the male sex organ that mainly affects reproductive age groups [1]. The global incidence of TC showed a 1.80 doubling increase from 37,231 in 1990 to 66,833 new cases in 2016 [2].
TC survivors are at a greater risk of reduced sexual interest, sexual activity, sexual enjoyment, erectile dysfunction (ED), ejaculatory problems, increased sexual discomfort, and changes in body image than the healthy male population [3]. It has a paramount and persistent impact on a patient's sexuality due to its location and treatments [4].
ED is defined as the inability to obtain or maintain an erection firm enough for sexual intercourse [5]. It may be provoked by the adverse effects of cancer treatment, such as fatigue, pain, or anxiety about cancer therapy, and depressed moods about having cancer [5].
ED can be in the organic and/or psychogenic form [3]. Organic ED results from the effects of radiotherapy and chemotherapy, whereas psychogenic ED is associated with changes in body image, loss of sense of manliness after orchiectomy, reduced spirits of well-being, and other psychosocial fluctuations associated with cancer [3].
ED is one of the common complications of TC with a prevalence of 11.3%–84%, according to different studies [3, 6]. It has devastating effects on men and their partner's quality of life, sexual satisfaction, and sexual experience [7, 8]. Its impact is not only related to the sexual life of the survivors, it rather comprises psychological, biological, relational, and cultural elements of life [9].
Treatment modalities, psychological emotion, relationship, body image, types of testicular cancer, patient age, and degree of ED before starting cancer treatment are some of the determinants that affect the erectile function of TC survivors [1, 5, 10, 11, 12, 13, 14, 15].
Nowadays, the prevalence of ED is increased due to the increment of TC survivors and decrement of the mortality rate of patients with TC [2]. The progressively increasing number of survivors and succeeding accomplishment of primary cancer treatments, cause specific complications that continue to affect cancer survivors negatively [4, 16].
However, the number of TC survivors is steadily increasing with decreasing mortality rate and with the increment of complications, including ED in the last two decades [2, 16]. The findings of the previous studies on this matter were uneven and inconsistent. Hence, designed and applied rationalized intervention for ED that currently exists in TC survivors by using those fragmented study findings as evidence is not acceptable.
Therefore, we decided to conduct a systematic review and meta-analysis of the existing data to acquire a more recent and comprehensive result. This evidence will give a new information for policy makers, which enables them to design scientific directives to decrease the magnitude of ED among TC survivors.
2. Methods
2.1. Protocol and registration
The findings of this review were reported according to the preferred reporting item on the systematic review and meta-analysis statement [17]. It is not registered in the Prospero database.
2.2. Eligibility criteria
The inclusion criteria were: 1. Any primary studies that clearly reported the prevalence of ED among TC survivors, 2. Studies conducted between 2001 and 2020, 3. Studies published in English, and 4. Studies available at the electronic source before July 2020. On the other hand, qualitative studies, citations without complete abstract and/or full text, anonymous reports, editorials, conference presentations, letters, expert opinions, case reports, and duplications were excluded.
2.3. Information source
PubMed, Scopus, Goggle scholar, Science Direct, African Index Medicus, African Journal online, EMBASE, and Cochrane Library up to July 2020. Furthermore, the reference lists of related papers were also plaid to identify additional studies. In addition, articles with incomplete data were accessed by communicating with the corresponding author.
2.4. Searching strategy
The main search terms and phrases were “prevalence,” “magnitude,” “epidemiology,” “proportion,” “erectile dysfunction,” “sexual dysfunction,” “impotence,” “sexual disorder,” “testicular cancer,” and “testicular tumor,” testicular neoplasm,” survivors,” patients.” “OR” and “AND” were used discretely and together as Boolean operators.
2.5. Study selection
Saved studies were exported to reference manager software, Endnote version 7, and to remove duplicate studies. Five independent reviewers screened the title and abstract. The disagreement was handled based on established article selection criteria. Five independent authors reviewed the abstract and full text of the articles.
2.6. Data extraction
Data was extracted by adopting the Joanna Briggs Institute (JBI) data extraction format [18]. Five authors (SK, YW, AS, EA, and MM) independently extracted all necessary data using this format. The data extraction format included primary author, publication year, country, region, measuring tool, study design, response rate, sample size, and prevalence.
2.7. Outcome measurement
The outcome variable of study was ED in testicular cancer, which is the inability to obtain or maintain an erection firm enough for sexual intercourse, which was measured by different tools [5]. The pooled prevalence was calculated by dividing the total number of ED in all review studies to the total number of involved TC survivors in the study and multiplying by 100 [19]. Erectile dysfunction = (Number of erectile dysfunction/number of participants) ∗100.
2.8. Quality assessment
The Newcastle-Ottawa Quality Assessment tool was used to check the quality of studies in this review [20]. The assessment tool contains 1) representativeness of the sample, 2) sample size, 3) non-respondents and 4) ascertainment of the exposure, 5) independent blind assessment, and 6) statistical test. Finally, based on this tool, an article with a scale of 6 out of 10 was considered as good quality.
Each original study was evaluated by five authors independently using this tool. If there were disagreements between those five authors, the consensus was reached by taking the mean score of the five authors.
2.9. Statistical analysis
Publication bias was checked by a funnel plot and more objectively through Begg's and Egger's regression test [21]. Heterogeneity of studies was quantified using the I-Squared Statistic, in which 25%, 50%, and 75% represented low, moderate, and high heterogeneity, respectively [22, 23]. Pooled analysis was conducted using a weighted inverse variance random-effects model [24]. Subgroup analysis was done by treatment modalities and ED measuring tools. Sensitivity analysis was employed to see the effect of a single study on the overall estimation. STATA version 14 statistical software was used for meta-analysis.
3. Results
3.1. Characteristics of reviewed studies
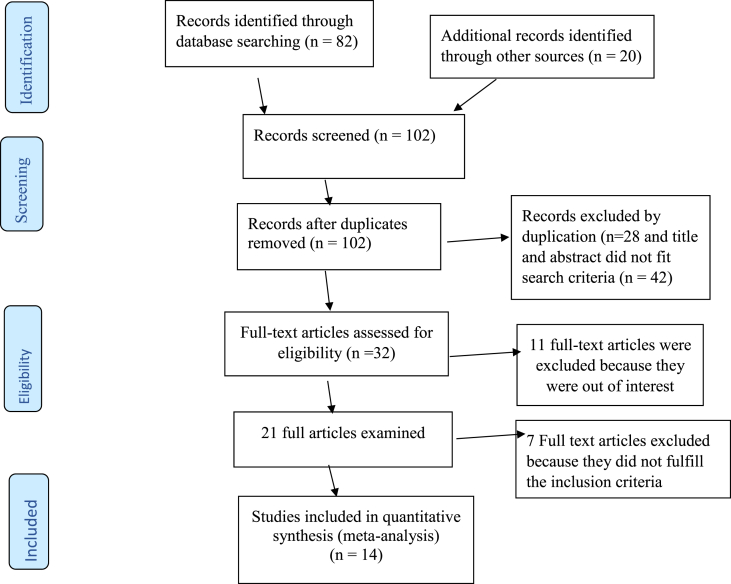
Originally, 82 records were collected in relation to ED in TC survivors PubMed, Google scholar, Africa Index Medicus, Africa Journal Online, EMBASE, and Science Direct databases. Twenty studies were investigated from other sources. From these, 70 were not considered for further evaluation as a result of duplication and title and abstract did not appropriate search criteria. Of the 32 articles that remained, 11 were excluded because they were out of scope. Therefore, 21 full-text articles were retrieved and evaluated for eligibility based on the inclusion criteria. Seven articles were excluded as a result of not fulfilling our inclusion criteria [1, 3, 4, 6, 10, 12, 14, 15, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37]. Finally, 14 studies that fulfilled the eligibility criteria were included in the systematic review and meta-analysis (Figure 1).
Figure 1.
Flowchart of the selection of studies for a systematic review and meta-analysis of the prevalence of erectile dysfunction, 2020.
Data for the 14 eligible studies were extracted and analyzed in this study. The pooled prevalence of ED was estimated by using 7043 TC survivors. Moreover, from 14 eligible studies, 2 were case control studies, 7 were cohort studies and 5 were conducted by cross-sectional study designs (Table 1).
Table 1.
Descriptive summary of 14 studies included in the meta-analysis of the prevalence of erectile dysfunction among testicular cancer survivors.
| Authors name | Publication Year | Country | Criterion tool | Treatment modalities | Study design | Response rate (%) | Sample size | Total N outcome | Prevalence (%) |
|---|---|---|---|---|---|---|---|---|---|
| Chirstopher kim et al. | 2012 | US | BMSFI | radiation, surgery and chemotherapy | case control | 100 | 246 | 89 | 36.2 |
| Pawel Wiechno et al. | 2007 | Poland | IIEF | radiation, surgery and chemotherapy | Cohort | 100 | 326 | 130 | 40 |
| Rannan Tal et al | 2013 | US | IIEF | radiation, surgery and chemotherapy | Cross-sectional | 100 | 76 | 64 | 84 |
| Ferancesco Pallotti et al. | 2019 | Italy | IIEF | surgery and chemotherapy | Cohort | 100 | 241 | 91 | 37.7 |
| Masahiro Kurobe et al. | 2018 | Japan | IIEF | radiation, surgery and chemotherapy | case control | 100 | 50 | 32 | 64 |
| Jakob E. Lackner et al | 2009 | Austria | IIEF | chemotherapy | Cohort | 100 | 83 | 24 | 28.9 |
| Alv A. Dahl et al | 2007 | Norway | BMSFI | chemotherapy and radiation | Cross-sectional | 84 | 1084 | 162 | 27 |
| Gerald Puhse et al | 2012 | Germany | EFBFI | radiation, surgery and chemotherapy | Cohort | 56 | 238 | 75 | 31.5 |
| P. Rossen et al | 2012 | Denmark | QLQ-PR25 | radiation, surgery and chemotherapy | Cohort | 66 | 611 | 401 | 18 |
| Uros Bumbasirevic et al | 2012 | Serbian | Nine-item generic questionnaire | surgery and chemotherapy | Cross-sectional | 96 | 202 | 42 | 20.8 |
| K. Dimitropoulos et al | 2015 | Greece | IIEF | surgery | Cohort | 100 | 53 | 6 | 11.3 |
| Paolo Capogrosso et al | 2015 | Italy | IIEF | surgery | Cohort | 100 | 143 | 35 | 25.5 |
| Mikkel bandak et al | 2018 | Copenhagen | IIEF | surgery and radiation | Cross-sectional | 100 | 2479 | 209 | 34 |
| Sarah L. Kerns, et al | 2018 | USA | AHO | chemotherapy | Cross-sectional | 100 | 1214 | 345 | 28.4 |
3.1.1. Quality appraisal
The Newcastle-Ottawa Scale quality appraisal criteria were used. The studies included in this systematic review and meta-analysis had no low quality. Therefore, all 14 studies were included (Table 2).
Table 2.
Scoring of the quality of articles by authors using The Newcastle-Ottawa Quality Assessment tool.
| Study | Selection |
Comparability |
Outcome |
Total Score | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Quality assessor | Representativeness of sample (∗, ∗) | Sample size (∗) | Non respondents (∗) | Ascertainment (∗∗) | Study controls for most important factor (∗) | The study control for any additional factor (∗) | Assessment of the outcome (∗) | Statistical test (∗) | ||
| Chirstopher kim et al. | SK | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 0 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | ||
| EA | 1 | 2 | 1 | 1 | 0 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Pawel Wiechno et al. | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Rannan Tal et al | SK | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| YW | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 2 | 1 | 1 | 1 | 1 | 1 | ||
| Ferancesco Pallotti et al. | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Masahiro Kurobe et al. | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 7 |
| YW | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Jakob E. Lackner et al | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Alv A. Dahl et al | SK | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 6 |
| YW | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | ||
| Gerald Puhse et al | SK | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 6 |
| YW | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | ||
| EA | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ||
| P. Rossen et al | SK | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| YW | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | ||
| AS | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Uros Bumbasirevic et al | SK | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 7 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| K. Dimitropoulos et al | SK | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 7 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| Paolo Capogrosso et al | SK | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 6 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | ||
| AS | 1 | 0 | 1 | 1 | 1 | 1 | 0 | 1 | ||
| EA | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 0 | ||
| Mikkel bandak et al | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| Sarah L. Kerns, et al | SK | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 8 |
| YW | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| AS | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| EA | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ||
| MM | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||
3.2. Meta-analysis
3.2.1. Prevalence of erectile dysfunction
In this study, the pooled prevalence of ED among TC survivors was found to be 34.60% (95% CI: 25. 89, 43.30%). Severe heterogeneity was detected across the studies (I2 = 95.9% and p = 0.000) (Figure 2).
Figure 2.
Pooled prevalence of erectile dysfunction among testicular cancer survivors, 2020 (n = 14).
3.2.2. Subgroup analysis
As a result of substantial heterogeneity, subgroup analysis was performed based on the study design, treatment, and measuring tools. In this respect, the prevalence was higher in the case control study, 50.02% (95% CI: 22.78, 77.28 [I2 = 96.1 and p = 0.000]) than that of the cohort study, 27.36% (95% CI: 19.23, 34.48 [I2 = 91.6 and P = 0.000]). The prevalence of erectile dysfunction, which was measured by IIEF was higher than the prevalence, which was measured by others. The prevalence of ED among those who were treated with three treatment modalities (radiation, surgery, and chemotherapy) was also higher than those who were treated with less than three treatment modalities (surgery and chemotherapy, chemotherapy and radiation, surgery and radiation, surgery only, and chemotherapy only) (Table3).
Table 3.
Subgroup analysis of prevalence of erectile dysfunction among testicular cancer survivors, 2020.
| Variables | Characteristics | Estimates (95% CI) | I2 tests with p-value |
|---|---|---|---|
| Study designs | Case-control | 50.02% (22.78%, 77.26%) | 96.1%, P = 0.000 |
| Cohort | 27.36% (19.23%, 35.48%) | 91.6%, P = 0.000 | |
| Cross-sectional | 38.66% (19.82%, 57.51%) | 97.4%, P = 0.000 | |
| Treatment modalities | Three treatments | 45.46% (27.23%, 63.70%) | 97.5%, P = 0.000 |
| Less three treatments | 26.5% (20.29%, 32. 71%) | 87.3%, P = 0.000 | |
| Measuring tools | IIEF | 40.50% (25.42, 55.58) | 97.5%, P = 0.000 |
| BMSFI | 31.49% (22.48,40.50) | 71.9%, P = 0.06 | |
| Others | 24.48% (18.30, 30.66) | 74.7%, P = 0.008 |
Others = International Index of Erectile Function and Brief Sexual Function Inventory, European Organization for Research and Treatment of Cancer (EORTC QLQ-PR25), nine-item generic questionnaire, and adverse health outcomes.
A random-effects model was employed to estimate the pooled prevalence of erectile dysfunction. Different factors associated with the heterogeneity such as a study design, publication year, sample size, and response rate were inspected using multivariate meta-regression models. From these variables, none of them were statistically significant (Table4).
Table 4.
Related factors with heterogeneity of erectile dysfunction prevalence among testicular cancer survivors in the current meta-analysis, 2020.
| Variables | Coefficient | P-value |
|---|---|---|
| Study design | -18.72 (-57.72, 20.27) | 0.300 |
| Publication year | .533 (-2.94, 4.01) | 0.733 |
| Sample size | -.011 (-.035, .013) | 0.340 |
| Response rate | .092 (-.879, 1.063) | 0.833 |
3.2.2.1. Publication bias
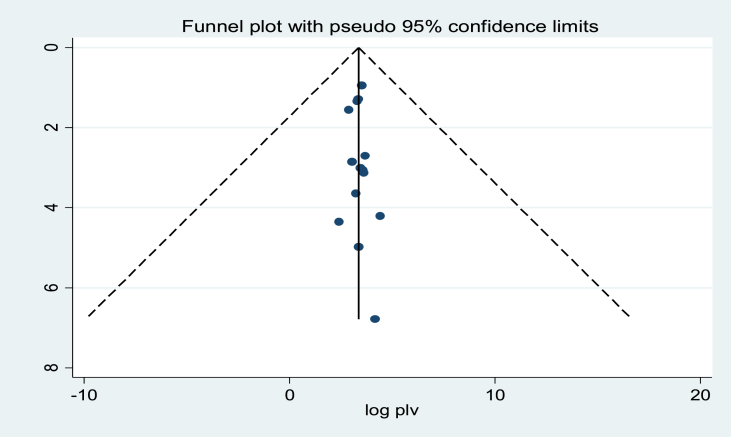
A funnel plot showed asymmetrical distribution (Figure 3). The result of the Egger test was also statistically significant with Bo = 3.33 and p = 0.000. To see publication bias further, trim fill analysis was done and unpublished studies were not found.
Figure 3.
Funnel plot for publication bias, logprop, or lnp (log of proportion) represented in the X-Axis and standard error of log proportion in the Y-Axis.
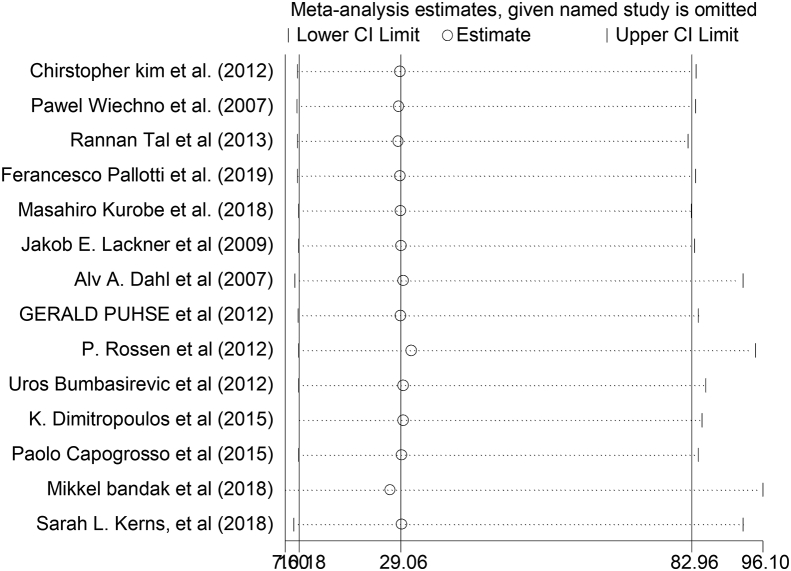
3.2.2.2. Sensitivity analysis
Among all 14 reviewed studies in the current analysis, no study had shown an impact on the overall estimation (Figure 4).
Figure 4.
The sensitivity analysis showed the pooled prevalence when the studies were omitted step by step.
4. Discussion
This meta-analysis showed that the pooled prevalence of ED among TC survivors was 34.6%. This pooled prevalence of ED among TC survivors is higher than the studies conducted in London (8%) [35], China (16.9%) [38], and Netherlands (11.5%) [39].
This discrepancy may be due to the age difference of study participants, the time of study, study design, types of TC (unilateral or bilateral), and treatment modalities. A study conducted in London included only young men (16–39 years old). But the current study includes all age groups and older age increases the prevalence of ED [5, 40]. In the case of a study conducted in China, the study included only case-control studies and patients experienced unilateral orchiectomy. Nevertheless, this systematic review and meta-analysis study includes any primary studies that clearly reported the prevalence of ED among TC survivors and patients underwent both unilateral orchiectomy and/or bilateral orchiectomy. Evidence supported that bilateral orchiectomy associated with the prevalence of ED in TC survivors due to complete cessation or very low production of testosterone [31]. Moreover, the discrepancy between a study conducted in Netherlands and this study may be due to the time variation and the study design that was included in the systematic review and meta-analysis. A study done in Netherlands was conducted before two decades and included only cohort studies conducted from 1975 to 2000. But the current study includes all primary studies conducted from 2001 to 2020. In the last two decades, the survival rate of TC is highly increased, this in turn increases the prevalence of ED in TC survivors due to long effects of TC complications [30].
A subgroup analysis reveals that the prevalence of ED was 50.02% in studies conducted in the control studies and the prevalence of ED was 27.36% in the cohort study. This showed that the significant heterogeneity of the prevalence of ED between study designs.
The inconsistency might be due to that unlike cohort study, in case control study already confirmed case are identified and selected to compare the level of exposure status with controls. Therefore, the prevalence of cases is expected to be high. Cases are purposely included in the sample so that to be comparable with controls in their exposure status. However, in cohort study, the researcher follows healthy individuals to compare the number of case in exposed and unexposed group. Here cases are those individuals who develop diseases during follow up period. Therefore, it is expected cases in cohort study to be smaller than in case control studies.
The heterogeneity of ED prevalence is also appreciated across treatment modalities. The prevalence of ED was 45.46% among those who were treated with three treatment modalities and the prevalence of ED was 26.5% among those TC survivors treated with less than three treatment modalities. This variance might be due to the synergistic effect of three treatment modalities on erectile function. Evidence showed that chemotherapy, surgery, and radiation can all cause a sexual antagonistic effect by causing neuropathy, nerve disconnected, and interruption of normal blood supply to maintain erection and vasculogenicity, respectively [3, 5]. The combination of these treatments has much more negative effect on erection than treatments less than this combination.
The heterogeneity of ED prevalence is also seen across different measurement tools. The prevalence of ED in the studies used IIEF as a criterion tool, which was higher than other studies that used BMSFI and other criterion tools. This might be due to the different cut points to determine ED among tools. This finding is supported by a study conducted in China [38].
In this study ED was found to be a highly prevalent complication in testicular cancer survivors. It had also causes of heterogeneity in terms of treatment modalities, study designs, and measuring tools. Therefore prevention of this complication should be the concern of the responsible bodies.
There were numerous limitations in our study. First, only English articles or reports were included to carry out the analysis. Second, the severity of ED was not described because data were unobtainable in most of the studies. Third, the subgroup analysis based on age was not done due to the lack of data in the included studies. Cofounders like stage are not taken into account. Finally, all the studies were conducted in Europe or America; the results may not be inferred to patients in Asia or Africa.
Declarations
Author contribution statement
Sitotaw Kerie Bogale and Ayele Semachew Kasa: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Yinager Workineh, Emiru Ayalew and Melak Menberu: Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
The authors do not have permission to share data.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Kim C., McGlynn K.A., McCorkle R., Li Y., Erickson R.L., Ma S., Niebuhr D.W., Zhang G., Zhang Y., Bai Y. Sexual functioning among testicular cancer survivors: a case–control study in the US. J. Psychosom. Res. 2012;73(1):68–73. doi: 10.1016/j.jpsychores.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pishgar F., Haj-Mirzaian A., Ebrahimi H., Saeedi Moghaddam S., Mohajer B., Nowroozi M.R., Ayati M., Farzadfar F., Fitzmaurice C., Amini E. Global, regional and national burden of testicular cancer, 1990–2016: results from the Global Burden of Disease Study 2016. BJU Int. 2019;124(3):386–394. doi: 10.1111/bju.14771. [DOI] [PubMed] [Google Scholar]
- 3.Tal R., Stember D.S., Logmanieh N., Narus J., Mulhall J.P. Erectile dysfunction in men treated for testicular cancer. BJU Int. 2014;113(6):907–910. doi: 10.1111/bju.12331. [DOI] [PubMed] [Google Scholar]
- 4.Pühse G., Wachsmuth J.U., Kemper S., Husstedt I.W., Evers S., Kliesch S. Chronic pain has a negative impact on sexuality in testis cancer survivors. J. Androl. 2012;33(5):886–893. doi: 10.2164/jandrol.110.012500. [DOI] [PubMed] [Google Scholar]
- 5.Voznesensky M., Annam K., Kreder K.J. Understanding and managing erectile dysfunction in patients treated for cancer. J. Oncol. Pract. 2016;12(4):297–304. doi: 10.1200/JOP.2016.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dimitropoulos K., Karatzas A., Papandreou C., Daliani D., Zachos I., Pisters L., Tzortzis V. Sexual dysfunction in testicular cancer patients subjected to post-chemotherapy retroperitoneal lymph node dissection: a focus beyond ejaculation disorders. Andrologia. 2016;48(4):425–430. doi: 10.1111/and.12462. [DOI] [PubMed] [Google Scholar]
- 7.Yafi F.A., Jenkins L., Albersen M., Corona G., Isidori A.M., Goldfarb S., Maggi M., Nelson C.J., Parish S., Salonia A. Erectile dysfunction. Nat. Rev. Dis. Prim. 2016;2(1):1–20. doi: 10.1038/nrdp.2016.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tuinman M.A., Fleer J., Sleijfer D.T., Hoekstra H.J., Hoekstra-Weebers J.E. Marital and sexual satisfaction in testicular cancer survivors and their spouses. Support. Care Cancer. 2005;13(7):540–548. doi: 10.1007/s00520-004-0758-3. [DOI] [PubMed] [Google Scholar]
- 9.Varela V.S., Zhou E.S., Bober S.L. Management of sexual problems in cancer patients and survivors. Curr. Probl. Cancer. 2013;37(6):319–352. doi: 10.1016/j.currproblcancer.2013.10.009. [DOI] [PubMed] [Google Scholar]
- 10.Pallotti F., Petrozzi A., Cargnelutti F., Radicioni A.F., Lenzi A., Paoli D., Lombardo F. Long-term follow up of the erectile function of testicular cancer survivors. Front. Endocrinol. 2019;10:196. doi: 10.3389/fendo.2019.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jankowska M. Sexual functioning of testicular cancer survivors and their partners–A review of literature. Rep. Practical Oncol. Radiother. 2012;17(1):54–62. doi: 10.1016/j.rpor.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossen P., Pedersen A.F., Zachariae R., von der Maase H. Sexuality and body image in long-term survivors of testicular cancer. Eur. J. Cancer. 2012;48(4):571–578. doi: 10.1016/j.ejca.2011.11.029. [DOI] [PubMed] [Google Scholar]
- 13.Chovanec M., Vasilkova L., Petrikova L., Obertova J., Palacka P., Rejlekova K., Sycova-Mila Z., Kalavska K., Svetlovska D., Mladosievicova B. Long-term sexual functioning in germ-cell tumor survivors. BMC Cancer. 2020;20(1):1–10. doi: 10.1186/s12885-020-07301-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eberhard J., Ståhl O., Cohn-Cedermark G., Cavallin-Ståhl E., Giwercman Y., Rylander L., Eberhard-Gran M., Kvist U., Fugl-Meyer K.S., Giwercman A. Sexual function in men treated for testicular cancer. J. Sex. Med. 2009;6(7):1979–1989. doi: 10.1111/j.1743-6109.2009.01298.x. [DOI] [PubMed] [Google Scholar]
- 15.Bandak M., Lauritsen J., Johansen C., Kreiberg M., Skøtt J.W., Agerbaek M., Holm N.V., Daugaard G. Sexual function in a nationwide cohort of 2,260 survivors of testicular cancer after 17 years of followup. J. Urol. 2018;200(4):794–800. doi: 10.1016/j.juro.2018.04.077. [DOI] [PubMed] [Google Scholar]
- 16.Schepisi G., De Padova S., De Lisi D., Casadei C., Meggiolaro E., Ruffilli F., Rosti G., Lolli C., Ravaglia G., Conteduca V. Psychosocial issues in long-term survivors of testicular cancer. Front. Endocrinol. 2019;10:113. doi: 10.3389/fendo.2019.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jordan Z., Lockwood C., Munn Z., Aromataris E. The updated Joanna Briggs Institute model of evidence-based healthcare. Int. J. Evid. Base. Healthc. 2019;17(1):58–71. doi: 10.1097/XEB.0000000000000155. [DOI] [PubMed] [Google Scholar]
- 19.Noordzij M., Dekker F.W., Zoccali C., Jager K.J. Measures of disease frequency: prevalence and incidence. Nephron Clin. Pract. 2010;115(1):c17–c20. doi: 10.1159/000286345. [DOI] [PubMed] [Google Scholar]
- 20.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 21.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ioannidis J.P. Interpretation of tests of heterogeneity and bias in meta-analysis. J. Eval. Clin. Pract. 2008;14(5):951–957. doi: 10.1111/j.1365-2753.2008.00986.x. [DOI] [PubMed] [Google Scholar]
- 23.Higgins J.P., Thompson S.G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 24.Borenstein M., Hedges L.V., Higgins J.P., Rothstein H.R. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res. Synth. Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12. [DOI] [PubMed] [Google Scholar]
- 25.Wiechno P., Demkow T., Kubiak K., Sadowska M., Kamińska J. The quality of life and hormonal disturbances in testicular cancer survivors in Cisplatin era. Eur. Urol. 2007;52(5):1448–1455. doi: 10.1016/j.eururo.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 26.Kurobe M., Kawai K., Suetomi T., Iwamoto T., Waku N., Kawahara T., Kojima T., Joraku A., Miyazaki J., Nishiyama H. High prevalence of hypogonadism determined by serum free testosterone level in Japanese testicular cancer survivors. Int. J. Urol. 2018;25(5):457–462. doi: 10.1111/iju.13537. [DOI] [PubMed] [Google Scholar]
- 27.Lackner J.E., Koller A., Schatzl G., Marberger M., Kratzik C. Androgen deficiency symptoms in testicular cancer survivors are associated with sexual problems but not with serum testosterone or therapy. Urology. 2009;74(4):825–829. doi: 10.1016/j.urology.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Dahl A.A., Bremnes R., Dahl O., Klepp O., Wist E., Fosså S.D. Is the sexual function compromised in long-term testicular cancer survivors? Eur. Urol. 2007;52(5):1438–1447. doi: 10.1016/j.eururo.2007.02.046. [DOI] [PubMed] [Google Scholar]
- 29.Bumbasirevic U., Bojanic N., Pekmezovic T., Janjic A., Janicic A., Milojevic B., Tulic C. Health-related quality of life, depression, and sexual function in testicular cancer survivors in a developing country: a Serbian experience. Support. Care Cancer. 2013;21(3):757–763. doi: 10.1007/s00520-012-1577-6. [DOI] [PubMed] [Google Scholar]
- 30.Capogrosso P., Boeri L., Ferrari M., Ventimiglia E., La Croce G., Capitanio U., Briganti A., Damiano R., Montorsi F., Salonia A. Long-term recovery of normal sexual function in testicular cancer survivors. Asian J. Androl. 2016;18(1):85. doi: 10.4103/1008-682X.149180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bandak M., Lauritsen J., Johansen C., Kreiberg M., Skøtt J.W., Agerbaek M., Holm N.V., Daugaard G. Sexual function and quality of life in a national cohort of survivors of bilateral testicular cancer. Eur. Urol. Foc. 2018 doi: 10.1016/j.euf.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Lackner J., Schatzl G., Koller A., Mazal P., Waldhoer T., Marberger M., Kratzik C. Treatment of testicular cancer: influence on pituitary-gonadal axis and sexual function. Urology. 2005;66(2):402–406. doi: 10.1016/j.urology.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 33.Kerns S.L., Fung C., Monahan P.O., Ardeshir-Rouhani-Fard S., Zaid M.I.A., Williams A.M., Stump T.E., Sesso H.D., Feldman D.R., Hamilton R.J. Cumulative burden of morbidity among testicular cancer survivors after standard cisplatin-based chemotherapy: a multi-institutional study. J. Clin. Oncol. 2018;36(15):1505. doi: 10.1200/JCO.2017.77.0735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartmann J., Albrecht C., Schmoll H., Kuczyk M., Kollmannsberger C., Bokemeyer C. Long-term effects on sexual function and fertility after treatment of testicular cancer. Br. J. Cancer. 1999;80(5):801–807. doi: 10.1038/sj.bjc.6690424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ljungman L., Eriksson L., Flynn K., Gorman J., Ståhl O., Weinfurt K., Wiklander M., Lampic C., Wettergren L. Sexual dysfunction and reproductive concerns in young men diagnosed with testicular cancer: an observational study. J. Sex. Med. 2019;16(7):1049–1059. doi: 10.1016/j.jsxm.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 36.Agrawal V., Dinh P.C., Jr., Fung C., Monahan P.O., Althouse S.K., Norton K., Cary C., Einhorn L., Fossa S.D., Adra N. Adverse health outcomes among US testicular cancer survivors after cisplatin-based chemotherapy vs surgical management. JNCI Cancer Spectr. 2020;4(2):pkz079. doi: 10.1093/jncics/pkz079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Iersel L., Li Z., Chemaitilly W., Schover L.R., Ness K.K., Hudson M.M., Klosky J.L. Erectile dysfunction in male survivors of childhood cancer. JAMA Oncol. 2018;4(11):1613–1616. doi: 10.1001/jamaoncol.2018.4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong J, Zhang J, Cai Z, Ma C, Li H: Erectile dysfunction in testicular cancer survivors: a meta-analysis of case-control studies. Arch. Med. Sci., 16(1). [DOI] [PMC free article] [PubMed]
- 39.Jonker-Pool G., Van de Wiel H.B., Hoekstra H.J., Sleijfer D.T., Van Driel M.F., Van Basten J.P., Koops H.S. Sexual functioning after treatment for testicular cancer—review and meta-analysis of 36 empirical studies between 1975–2000. Arch. Sex. Behav. 2001;30(1):55–74. doi: 10.1023/a:1026468707362. [DOI] [PubMed] [Google Scholar]
- 40.Gareri P., Castagna A., Francomano D., Cerminara G., De Fazio P. Erectile dysfunction in the elderly: an old widespread issue with novel treatment perspectives. Int. J. Endocrinol. 2014;2014 doi: 10.1155/2014/878670. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.