Abstract
Background
Laminectomy produces trauma in spinal cord injury (SCI) animal models resulting in impinging artefacts and welfare issues. Mechanizing laminectomy using a dental burr assisted (DBA) technique to reduce the impact of conventionally performed laminectomy on animal welfare without any alterations in the outcome of the model was previously demonstrated. However, further validation was necessary to establish it as an alternative in developing SCI rats as a model of chronic pain and memory loss.
Novel method
DBA technique was employed to perform laminectomy at T10-T11 vertebrae in rats undergoing contusion SCI as a model of chronic pain and memory loss. In a 56-day study, 24 female Wistar rats (Crl: WI) were assigned randomly to four equal groups: conventionally laminectomised, DBA laminectomised, conventionally laminectomised with SCI and DBA laminectomised with SCI.
Results
The study revealed DBA technique to cause less surgical bleeding (p = 0.001), lower Rat Grimace Scale (p = 0.0006); resulted in better body weight changes (p = 0.0002 on Day 7 and p = 0.0108 on Day 28) and dark phase activity (p = .0.0014 on Day 1; p = 0.0422 on Day 56). Different techniques did not differ in Basso Beattie Bresnahan score, novel object recognition, mechanical allodynia, number of surviving neurons and the area of vacuolation- indicating that the new method doesn’t affect the validity of the model.
Comparison with existing method(s)
In comparison with the conventional technique, motorised laminectomy can be a valid tool that evokes lesser pain and ensures higher well-being in rats modelled for chronic pain and memory loss.
Conclusions
The intended outcome from the model is not influenced by techniques whereas the DBA-technique is a refined alternative to the conventional method in achieving better welfare in SCI studies.
Keywords: Dental burr assisted laminectomy, Refinement, Spinal cord injury, BBB score, Rat model, Chronic pain, Memory loss
Dental burr assisted laminectomy, Refinement, Spinal cord injury, BBB score, Rat model, Chronic pain, Memory loss.
1. Introduction
Traumatic spinal cord injury (SCI) is a debilitating condition that deteriorates the quality of life by producing pain and loss of function and could lead to mortality in patients. According to the WHO, globally 250,000–500,000 individuals suffer from SCI every year [1], making SCI research highly relevant. In animal modelling of the disease, rats figured in 2209 studies published during 2017, contributing to 72.4% of all species used [2] with thoracic spinal region in 81% of the animals. The majority of studies used contusion (41%) as type of injury [2]. Majority of studies use females [3, 4], to avoid urinary infections and hematuria considering the ease of urine evacuation [5]. Besides, women when compared to men had a higher prevalence of nociceptive pain post SCI and use opiates and NSAIDs is higher proportions [6]and these reasons play a major role in selection of the animal model and methodology in SCI studies. Apart from the direct impact, other major effects of SCI like chronic severe pain [7, 8, 9] and memory loss [10] are also studied using rats.
A majority of the studies elaborating SCI model development does not detail the intricacies of laminectomy [11, 12, 13, 14, 15]. Since its first report in 1948 [16], laminectomy associated epidural fibrosis is considered as one among the most common post-operative complications in spinal surgeries and has no effective treatment [17]. A condition known as arachnoiditis is associated with laminectomies that could not be prevented in rat model with tenoxicam and it was concluded that effective hemostasis and minimizing tissue damage during laminectomies is the effective way out to prevent the condition [18]. Long-term housing of animal models with spinal cord injury requires special care [19]. However, most of the chronic studies focus on motor ability assessment tests like BBB scores [13, 20], thigh perimeter and cold spray test [20], combined behavioural score with several reflex tests, swim test and testing hyperalgesia using hot plate [13] thereby overlooking welfare parameters.
Recently, a motorised dental burr assisted (DBA) laminectomy to impart added welfare was proven successful [21] with its benefits demonstrated in a 28-day study during a 28-day period, including improved weight gain, reduced pain and better activity levels in rats. However, data was lacking on effects of DBA technique on parameters like chronic hyperalgesia and cognition. Ascertaining the capability of DBA technique to impart the previously demonstrated positive effects on welfare without altering the expected outcome in chronic studies is essential for researchers to adopt the newly introduced technique to create laminectomy.
The present study was designed to validate the DBA laminectomy in applying the SCI contusion rat model as a model of chronic pain and memory loss and to probe its impact on welfare. Parameters studied were bleeding during surgery; Basso Beattie and Bresnahan (BBB) score; body weight changes; Rat Grimace Scale (RGS); open field activity and rearing in light phase; home cage activity and rearing during dark phase; sucrose preference as an indicator for pleasure seeking behaviour; novel object recognition test (NOR) to assess memory loss during acute and chronic stages of the study; mechanical allodynia at chronic stage; and terminal histopathology to compare vacuole area and functional neurons.
It was hypothesized that animals subjected to SCI would differ in BBB scores, histopathology, mechanical allodynia and NOR compared to animals undergoing only laminectomy (LAM) without SCI, whereas no difference will be observed between two different techniques – conventional (CONV) and dental burr assisted (DBA). Regarding body weights, RGS, open field activity in light phase, home cage activity during dark phase and sucrose preference, it was hypothesized that both SCI and LAM groups as well as the techniques employed with CONV and DBA would exhibit significant difference between each other. Body weights score, rearing and activity and motivation to find sweetened water as an indicator of general health, lower pain sensation promoting natural behavioural repertoire, were expected to be higher whereas RGS score was predicted to be lower with the less traumatic DBA technique, as per data obtained from the previous acute study [21].
2. Materials & methods
2.1. Ethical approval, animals, housing and care
The study (SCT/IAEC/220/March/2017/91) was sanctioned by the Institutional Animal Ethics Committee of Sree Chitra Tirunal Institute for Medical Sciences and Technology (SCTIMST). The internal review committee of SCTIMST (IRC/XIII/2016-approval dated 10/07/2016) gave technical approval to conduct this study. Confirmation and compliance to the ARRIVE reporting guidelines [22] was maintained for the study. Female Wistar rats from Charles River Laboratories (Crl: WI), 9–12 weeks of age at the start of the study with body weight of 240–280 g (supplied by Hylasco Biotechnology, India) were used for the study. The animals were housed in groups of three per cage in polysulfone (800 cm2 floor area and 18.5 cm height) IVC cages at 22 ± 2 °C, around 55% relative humidity [23] and 12–15 fresh air changes per hour and was provided with sterile corn cob as bedding material. Pelleted rodent diet (Safe rodent diet, D131, Augy, France) and potable drinking water ad libitum, about 75 g of autoclaved nesting material (Enviro Dri, Shepherd, Cleveland, Ohio, USA) that was replaced with fresh material during each cage change once a week was supplied. FELASA guidelines [24] was followed to ascertain animal health. Automated 12:12 light: dark cycle not exceeding 325 Lux at one-meter height from the floor illuminated the animal room. The animals were separated and housed individually to acclimatize and handling and gentling was done twice daily for a week. Post-procedurally, animals were housed individually until their wound healed, to facilitate tracking of their activity and behaviour. The laminectomised animals also were individually housed for the same period of time for a fair comparison between groups. Single animal was considered as the experimental unit for all the experiments. All the procedures were performed between 9.00 am and 2.00 pm by trained and experienced investigators. Home cage activity during the dark phase was performed between 7.00 pm and 11.00 pm.
2.2. Study design
The group size of six animals per group was based on our previous study [21] and estimated using the resource equation method [25]. Twenty-four rats were assigned equally to four groups after randomization using an online research randomizer (https://www.randomizer.org/). Laminectomy by conventional technique formed the group ‘CONV-LAM’ and motorised laminectomy formed ‘DBA-LAM’. Conventional laminectomy followed by SCI formed ‘CONV-SCI’ and the group ‘DBA-SCI’ consisted of animals that underwent motorised laminectomy followed by SCI.
Unique identification codes were used to designate rats from different groups and the investigators (including the surgeon) were blinded during the observations. Histopathologists were blinded regarding groups and coded samples were sent and analyzed. Unblinding was done after the statistical analysis of data. Intra-operative bleeding score could not be blinded since the operator was aware of the procedure, but was scored by a trained and independent observer.
2.3. Anesthesia, surgery and post-operative care
Anesthesia was induced with xylazine at 5 mg/kg and ketamine at 80 mg/kg body weight, mixed together as a single intraperitoneal injection of about 0.5 ml total volume. Upon loss of righting reflexes, the dorsum of the animals was clipped clean and the 10th thoracic vertebra (T10) was anatomically located and confirmed using radiography. Under a deep surgical plane of anesthesia in a designated procedure room under strict aseptic conditions the surgical site was prepared with povidone iodine and draped using sterile window drapes. Anesthesia was maintained using a precision gas anesthesia delivery system (E-Z system corporation, Palmer, PA), which delivered isofluorane gas at 2% (Forane, Abott India Limited, Mumbai, India) in oxygen (0.5–1 l/min) during the entire length of the procedure with the help of a face mask.
For performing laminectomy using conventional technique and motorised dental burr assisted technique, the procedure described by previously by Harikrishnan et al, 2019 [21] was followed. In short, after a skin incision of 2.5 cm at T10-T11 vertebrae, and a blunt dissection of paraspinous muscles, the spinous process was removed with a pair of micro-rongeurs. In conventional technique, the dorsal laminae were dissected using micro rongeurs and fine mosquito forceps with manual-shearing force. In contrast to this manual procedure, the laminae in the DBA laminectomy were longitudinally drilled bi-laterally using a dental burr (Carbide burrs, SSWHP-559, NJ, USA) controlled using a pedal-switch of a micro-motor (Marathon-4, max RPM-35000, SDE-H37LI, Saeyang Microtech, Korea). Care was taken not to injure the underlying spinal cord while the drilling was done. Once free bone movement was noticed the laminae on both the sides were totally removed, using a fine jeweler’s forceps, to clearly visualize the intact spinal cord. Bleeding, if any was stopped by application of sterile cotton swabs.
Spinal cord injury was induced as previously described [21] using a custom manufactured spring-loaded 2.5 impounder tip, designed to deliver a force of 200 kdyn to the spinal tissue at the T10 level and the surgical wound was closed [21]. A nervous twitch of the hindquarter of the animal at the moment of impact and the formation of a sub-dural hematoma at the point of impact in spinal tissue confirmed the SCI. Immediately after the surgery during the post-operative recovery phase when the rats were still under the influence of anesthesia, 5ml of sterile isotonic saline was subcutaneously injected using a sterile 23G needle at the scruff of the neck region as a post-operative measure to hydrate the animals.
The animals were returned to home cages after regaining consciousness. Multimodal analgesia was given using buprenorphine (Bupregesic, Neon Labs Ltd., Thane, India) at a dose of 0.05 mg/kg body weight twice daily and meloxicam (Melonex, Intas Pharmaceuticals Ltd., Ahmedabad, India) at a dose of 1 mg/kg body weight once daily subcutaneously for five post-operative days. Antibiotic was given for five days with cephtriaxone (Finecef, Abott Healthcare Pvt. Ltd., Thane, India) at a dose of 15 mg/kg once daily as subcutaneous injection.
Retention of urine was observed in all the animals that underwent SCI after conventional as well as DBA laminectomy. Manual evacuation by applying gentle pressure on the abdomen was done twice daily to relieve urine retention for two weeks. To promote food consumption, daily two wheat biscuits (Britannia Tiger Glucose Cookie, 5/1A Hungerford Street, Kolkata, India) was supplied to all the animals until the end of the study.
A drop of over 20% of the baseline body weight, self-mutilation, recurrent urinary infections, absence of regaining active appearance and self-grooming one-week post-surgery were the pre-determined humane end-points. The rats were handled and examined twice daily during the entire study.
2.4. Bleeding score
Criteria to grade intra-operative bleeding was established prior to the surgery and the personnel was familiarized with the protocol. The level of bleeding was categorized as shown in Table 1 and the scores of groups were compared.
Table 1.
Intra-operative bleeding score – A novel technique to categorize the level of Intra-operative bleeding.
| Scores | Class Description | Definition attributed to the assessment |
|---|---|---|
| 1 | Low | Scanty, stopping on a few dabs, doesn’t obscure the view or procedure. |
| 2 | Mild | Scanty, does not stop on a few dabs and continue to ooze, but doesn’t obscure view or procedure. |
| 3 | Moderate | Bleeding that does not stop on a few dabs and obscures the view, but is not obscuring the procedure. |
| 4 | Severe < 2 min | Obscures the site and stops the procedure, but for less than two min |
| 5 | Severe >2 min | Obscures the site and stops the procedure for over two min |
The level of bleeding was categorized into 5 grades as per tabulated here and was scored during the surgery.
2.5. Basso Beattie Bresnahan (BBB) score
Starting the first post-operative day (Day 1), BBB scores was recorded on weekly intervals on Days 7, 14, 21, 28 and 56 as previously described by Basso et al [26] and compared between the groups. In short, a score of zero indicated no hind limb movement whereas a score of 21, a normally mobile rat. A BBB score of 0 on the first post-operative day was set as a criterion to be qualified for inclusion in the study.
2.6. Body weights measurement
Animals were weighed on Days 1, 7, 14, 21, 28 and 56 and the difference in body weights with respect to the baseline body weight as on Day 0 before surgery was compared between the groups.
2.7. RGS score
The RGS set up and the 5 min video recordings were exactly the same as previously reported [21]. The RGS scores on Days 1, 7, 14, 21, 28 and 56 and the difference with the baseline recorded on Day 0 before surgery was calculated as previously described [27]. In short, the RGS consists of four action units: orbital tightening, nose/cheek flattening, ear changes and whisker changes, which each are graded into not present (scored 0), moderately present (scored 1) and obviously present (scored 2). The RGS is presented as mean of all action units with a maximum score of two.
2.8. Light phase activity in open field
Using a video camera recorder (Sony HDR-CX405, Tokyo, Japan) mounted on a tripod, the animals were subjected to open field activity recording for 5 min on Days 1, 7, 14, 21, 28 and 56. Total activity time and rearing counts (partial rearings and full rearings combined) were compared between groups. The open field cage had dimensions of 65 × 50 × 20 cm. The animals were left at the centre of the field and personnel left the room immediately during the recording. The field was cleaned between animals using 70% ethanol wipes.
2.9. Home cage activity during dark phase
Using a night vision camera (Eurovigil IVIEW HD300 2MP, Parel, Mumbai, India), the dark phase activity was recorded for 5 min on Days 1, 7, 14, 21, 28 and 56. Total activity time and rearing counts (partial rearings and full rearings combined) were compared between groups. The nesting materials present in the cages were removed just for the night of recording for a clear view of the activity.
2.10. Sucrose preference test
Cages were supplied with two similar drinking bottles– one with water and the other-sweetened water with 1.5% sucrose. Weight of the bottles prior to supply and after 24 h were recorded. After a habituation of 24 h, baseline value was taken before the surgery (Day 0) and on Days 7 and 56 post-surgery. On the days of testing, the cage filter top was removed, leaving only the top grill with clamps to accommodate two bottles simultaneously in the IVC racks. The solution consumed was calculated for each bottle by the formula sucrose intake in grams/total intake in grams X 100, where the total intake was the total consumption of sucrose and water.
2.11. Novel object recognition test
The test set up was a modification of the methodology described previously [28]. A poly-sulfone cage measuring 800 cm2 of floor area was used for the test with top grill fixed with objects diagonally at both ends. During the familiarization phase, animals were exposed to two similar objects (Plastic cubes, sides 5 cm long). As the novel object, a plastic spherical toy of 8 cm in diameter was used. Exploration was defined as facing the object closely, biting, touching using either paws of snout, licking, rearing and sniffing. The test was done 5 min and 24 h after familiarization with familiar object and each phase lasted for 5 min with video recorded. After each test, the objects were wiped using 70% ethanol wipes. The tests were repeated on Days 5–7 for assessing acute phase memory and on Days 56–58 for chronic phase memory.
As a measure of memory performance, the Discrimination Index (DI) was measured (DI = TN-TF/TN + TF, where TN = total time spent with the novel object and TF = total time spent with the familiar object). The tests were video recorded and the time spent with each object was registered.
2.12. Von Frey test
Plantar von Frey testing was applied to the CONV-LAM and DBA-LAM groups and dorsal von Frey testing was applied as the hind limbs were paralyzed in the CONV-SCI and the DBA-SCI groups on days 58–60 as previously described [29]. Briefly, the plantar test for both hindlimbs started by applying the 5.18 von Frey filament (VFF) tip to the plantar aspect of the right hind paw until the filament started to bend and for a duration of 1 s. A rapid removal of the paw from the VFF marked a positive response and the test was continued by applying a thinner VFF. In case of a negative response, a thicker VFF (not exceeding 6.1 VFF) was tried until 10 applications were completed to observe a positive response. VFF that produced a positive response in more than 50% of the applications (thinnest used VFF with positive results in 2 out of 3 trials) in a series of 10 tests was considered as the paw withdrawal threshold.
The dorsal VFF test for both hindlimbs started with perpendicular application of the 4.56 VFF tip to the dorsal surface between the first and second metatarsal, approximately 1 cm proximal to the joint until the filament slightly bended with three repetitions. A response in a minimum of two applications out of three was considered as positive. In case of a negative response, the next thicker VFF were applied until the lowest VFF that would evoke a positive withdrawal response (not exceeding 5.88 VFF).
2.13. Histopathology
The animals were euthanized 60–62 days after initiation of LAM or SCI. Animals were first anaesthetized with ketamine and xylazine in the doses as previously described for anesthesia. Thereafter, 30 mg total dose of thiopentone sodium (Thiosol, Neon Laboratories Limited, Mumbai, India) was injected intraperitoneally in a volume of 2–3 ml to observe instantaneous apnea and blanching of both the eyes followed by other signs of death. Immediately after euthanasia, the area of laminectomy was identified and finely dissected. The tissue was then immersed in 10% neutrally buffered formalin (NBF) and retained for three days for fixation and then washed in running ap water. Decalcification was done by immersing in 14% EDTA (pH- 7.3) with continuous shaking for four days, where after the tissues were washed continuously in running tap water for 3 h. The area of laminectomy was selectively grossed transversely with a microtome blade to obtain a cross sectional view, and transferred into cassettes. The lesion was selected based on examination of serial sections with lesions from the epicenter of impact and the region with maximum damage was selected for the study by the histopathologist. The tissue was put back into 10% NBF and after embedding in paraffin, cross sections of 8μm thickness were taken from the lesion epicenter and staining was done using hematoxylin, eosin and Cresyl violet (Nissl’s) on a duplicate set of sections. Photographs of stained sections were taken with a camera (Nikon E600, Nikon, Japan) attached to the microscope. The lesions were analyzed by ImageJ software (U. S. National Institutes of Health, Bethesda, Maryland, USA), by assessing the percentage of damage observed. Nissl’s positive cell count was done at the ventral and dorsal horns of the right and left sides and the centre of the spinal cord, and the total number of live neuronal cells was calculated by adding up the cells counted from each segment by a pathologist.
2.14. Statistical analysis
All the statistical tests were performed using GraphPad Prism 8.4.2 (679) for Windows (GraphPad Software, San Diego, CA, USA) except for two-way ANOVA, which was done using SPSS (IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA). Data is expressed as mean with standard deviation. Normality was checked with Kolmogorov-Smirnov test and for the data that followed Gaussian distribution, ANOVA was applied to compare between groups. Tukey’s multiple comparison test was done for further post-hoc comparison. Data that was not normally distributed were analyzed using Kruskal-Wallis test and Dunn’s multiple comparison test was performed for post-hoc comparisons. Scatter plots with single dots each representing an individual animal and whiskers representing the standard deviation are presented in the graphs. To determine whether the type of procedure interacts with ventral or dorsal horns for the dependent variable of live neurons in histopathology data, normality assessed and log transformed (log10) data was used to perform a two-way ANOVA. Correlation between surviving neuronal count and BBB scores on days 1 and 56 was calculated within each technique. CONV-induced LAM and SCI groups where correlated, and the same analysis was performed in the DBA-induced LAM and SCI groups.
Since the major objective of the study was to compare two techniques (CONV and DBA), only the statistical differences between CONV-LAM and DBA-LAM and between CONV-SCI and DBA-SCI respectively are presented in the graphs. P < 0.05 was considered as statistically significant.
3. Results
All the animals completed the study period uneventfully and no animals were excluded. The mean surgery time did not differ significantly between groups. The mean surgery time in minutes were 21.3 ± 2.14 for CONV-LAM, 20.5 ± 1.6 for DBA-LAM, 22 ± 2 for CONV-SCI and 21.3 ± 2.1 for DBA-SCI. Area of laminectomy were 12.83 ± 2.03 for CONV-LAM, 12.33 ± 2.9 for DBA-LAM, 13.7 ± 2.4 for CONV-SCI and 12.6 ± 3.6 for DBA-SCI in mm2 and did not differ significantly between the groups.
3.1. Bleeding score
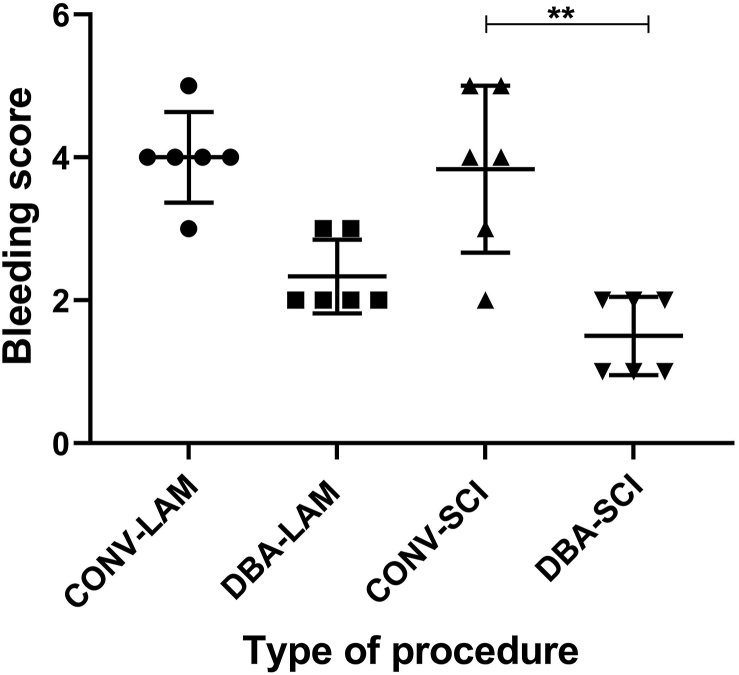
Conventional approach often led to obscuring of the site with blood, whereas DBA produced scanty dribbling of blood. There were significant differences between the bleeding scores of groups (Kruskal-Wallis statistic = 16.26, P = 0.001) (Figure 1). Multiple comparisons revealed that the severity of bleeding in DBA-SCI group was significantly less in comparison to CONV-SCI group (P = 0.0089) and to the CONV-LAM group (P = 0.0033).
Figure 1.
Intra-operative bleeding scores comparison. Bleeding scores comparison of rats (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorised dental burr assisted (DBA-LAM) technique during the surgery. Scores differed between groups as determined by Kruskal-Wallis test with Dunn’s multiple comparisons. Data are presented as scatter plot with each dot representing one animal, the horizontal line the mean and the whiskers the standard deviation. ∗∗ = P < 0.01.
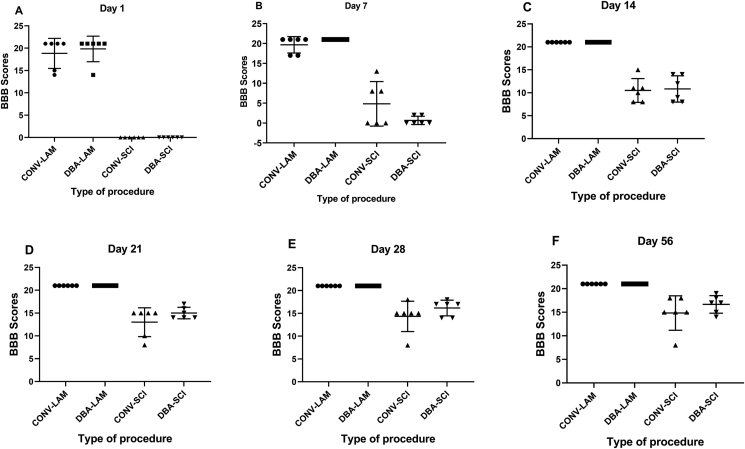
3.2. BBB score
There were no differences between different techniques within the respective LAM and SCI groups at any of the time points studied. Owing to the severe contusion effected at the T10-T11 level, on Day 1, all the animals in both the-SCI groups had a BBB score of 0, whereas the BBB scores were 18.83 ± 3.2 and 19.83 ± 2.7 in CONV-LAM and DBA-LAM respectively. Pairwise comparisons are presented in detail in Figure 2A-F.
Figure 2.
Post-operative BBB score comparison. BBB scores of rats (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorized dental burr assisted (DBA-LAM) technique at, or subjected to spinal cord injury-induced using conventional (CONV-SCI) or dental burr assisted (DBA-SCI) technique during the study period. Scores differed between groups on Day 1 (A), Day 7 (B), Day 14 (C), Day 21 (D), Day 28 (E) and Day 56 (F) postoperatively as determined by Kruskal-Wallis test with Dunn’s multiple comparisons. Data are presented as scatter plot with each dot representing one individual animal, the horizontal line the mean and the whiskers the standard deviation. ∗∗ = P < 0.01.
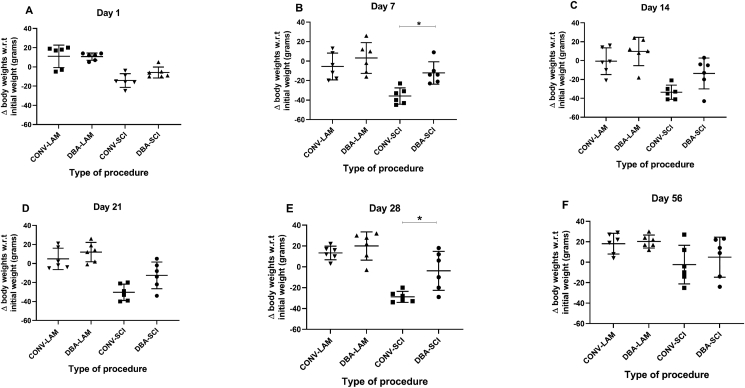
3.3. Body weights
The groups differed in the change in body weights with respect to the baseline body weights (body weight changes) on Day 1 (Kruskal- Wallis statistic = 16.48, P = 0.0009), Day 7 (F(3,20) = 10.55, P = 0.0002) (Figure 3B), Day 14 (F(3,20) = 11.06, P = 0.0002) (Figure 3C), Day 21 (F(3,20) = 17.06, P < 0.0001) (Figure 3D) and on Day 28 (F(3,20) = 18.76, P < 0.0001) (Figure 3E). The bodyweight changes did not differ between groups on Day 56 (Figure 3F). The bodyweight changes differed between techniques in SCI group on Day 7 (P = 0.0192) and 28 (P = 0.0108) where the DBA animals showed less body weight loss. Pairwise comparisons are presented in detail in Figure 3A-F.
Figure 3.
Post-operative body weights comparison. Postoperative weight fluctuation (compared to the initial body weights) in rats (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorized dental burr assisted (DBA-LAM) technique, or subjected to spinal cord injury induced using conventional (CONV-SCI) or dental burr assisted (DBA-SCI) technique during the study period on Day 1 (A), Day 7 (B), Day 14 (C), Day 21 (D), Day 28 (E) and Day 56 (F) postoperatively. Differences were analysed by Kruskal-Wallis test with Dunn’s multiple comparisons on Day 1 and by ANOVA with Tukey’s multiple comparisons on Days 7, 14, 21, 28 and 56. Data are presented as scatter plot with each dot representing one individual animal, the horizontal line the mean and the whiskers the standard deviation. ∗ = P < 0.05.
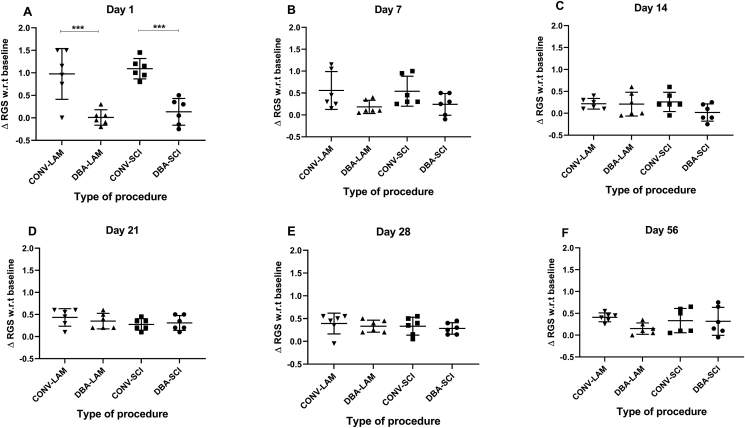
3.4. RGS score
The baseline RGS scores did not differ between the groups. On Day 1, the RGS scores showed significant differences between the groups (F (3, 20) = 15.48, P = 0.0006) (Figure 4A). On all the other time points, RGS scores showed no significant differences (Figure 4B-F). Pairwise comparisons are given in detail for all the time points in Figure 4A-F. On Day 1, multiple comparisons revealed that RGS scores of CONV-SCI and CONV-LAM groups were significantly higher than those of DBA-SCI (P = 0.0021) and DBA-LAM (P = 0.0025) respectively (Figure 4A).
Figure 4.
Post-operative RGS scores comparison. Change of RGS scores from baseline values of rats (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorized dental burr assisted (DBA-LAM) technique at, or subjected to spinal cord injury induced using conventional (CONV-SCI) or dental burr assisted (DBA-SCI) technique during the study period. RGS scores differed significantly on Day 1 (A) post-operatively between groups, as determined by Kruskal-Wallis test with Dunn’s multiple comparisons. No differences between groups were observed Day 7(B), 14 (C), 21 (D), Day 28 (E) or Day 56 (F). Data are presented as a scatter plot with each dot representing one individual animal, the horizontal line the mean and the whiskers the standard deviation. ∗∗∗ = P < 0.001.
3.5. Light phase activity in open field
No differences were observed in nether LAM nor SCI groups at any of the time points studied in open field activity or rearing (data not shown).
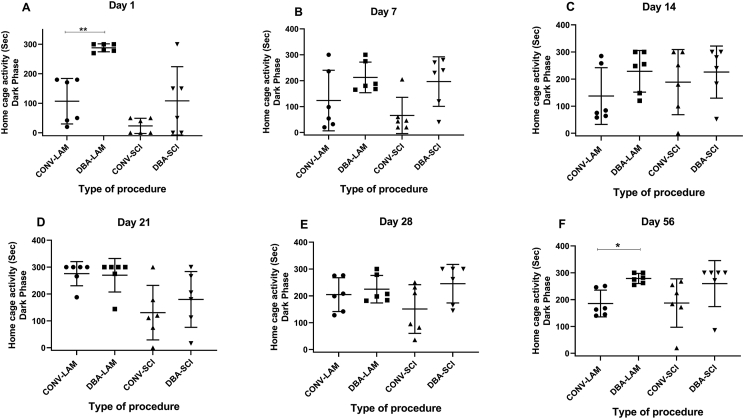
3.6. Home cage activity during dark phase
The groups showed difference in dark phase-home cage activity levels on Day 1 (F (3, 20) = 14.76, P < 0.0001) (Figure 5A) and on Day 56 (Kruskal Wallis statistic = 11.9, P = 0.0077) (Figure 5F) and on both these days, post-hoc comparisons using Tukey’s test revealed that, CONV-LAM animals showed less activity in comparison to DBA-LAM group (P = 0.0014 and P = 0.0422 respectively). Pairwise comparisons of dark phase-home cage activity are presented in detail for all the time points in Figure 5A-F.
Figure 5.
Home cage activity in the dark phase. Home cage activity time in dark phase of rats (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorized dental burr assisted (DBA-LAM) technique, or subjected to spinal cord injury induced using conventional (CONV-SCI) or dental burr assisted (DBA-SCI) technique during the study period. Time of activity differed significantly between groups on Day 1 (A) as determined by ANOVA with Tukey’s multiple comparisons and on Day (F) 56 as determined by Kruskal-Wallis test with Dunn’s multiple comparisons. Differences were analysed by ANOVA with Tukey’s multiple comparisons on Day 1 and by Kruskal-Wallis test with Dunn’s multiple comparisons on Days 7, 14, 21, 28 and Day 56. No differences between groups were observed Day 7(B), 14 (C), 21 (D) and Day 28 (E). Data are presented as a scatter plot with each dot representing one individual animal, the horizontal line the mean and the whiskers the standard deviation. ∗ = P < 0.05, ∗∗ = P < 0.01.
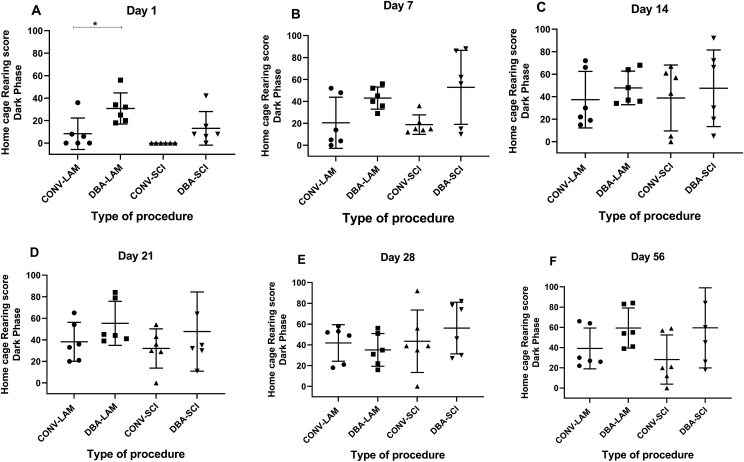
Number of rearings also showed differences between groups on Day 1 (F (3, 20) = 6.67, P = 0.0027) (Figure 6A) where DBA-LAM group showed significantly higher rearings in comparison to CONV-LAM group (P = 0.0237). No differences were observed between the two techniques in SCI induced animals in the rest of the time points. Pairwise comparisons of home cage-dark phase activity are presented in detail for all the time points in Figure 6A-F.
Figure 6.
Home cage rearing score in dark phase. Rearing scores of rats in their home cage during the dark phase (n = 6 per group) subjected to laminectomy using conventional (CONV-LAM) or motorized dental burr assisted (DBA-LAM) technique, or subjected to spinal cord injury induced using conventional (CONV-SCI) or dental burr assisted (DBA-SCI) technique during the study period. Time of activity differed significantly between groups on Day 1 (A) as determined by ANOVA with Tukey’s multiple comparisons. Differences were analysed by ANOVA with Tukey’s multiple comparisons on Day 1(A) and 14(C) and by Kruskal-Wallis test with Dunn’s multiple comparisons on Days 7 (B), 21(D), 28(E) and 56(F). Rearing score in home cage during dark phase differed significantly on Day 1 (A) whereas no differences between groups were observed Days 7(B), 14 (C), 21 (D), Day 28 (E) or Day 56 (F). ∗ = P < 0.05.
3.7. Sucrose preference test
Neither the sucrose preferential intake nor the total water intake differed between the groups at any of the time points studied (data not shown).
3.8. Novel object recognition test
Discrimination Index (DI) between groups did not differ in the NOR test of 5-minute and 24-hour delay on Day 7. DI scores measured 0.7 ± 0.15 for CONV-LAM, 0.8 ± 0.16 for DBA- LAM and 0.9 ± 0.2 for CONV-SCI and 0.7 ± 0.2 for DBA-SCI groups at 5 min delay on Day 7. On Day 7, at 24-hour delay, DI scores for CONV-LAM, DBA-LAM, CONV-SCI and DBA-SCI measured 0.7 ± 0.1, 0.7 ± 0.2, 0.8 ± 0.2 and 0.8 ± 0.1 respectively. On Day 56, the groups differed significantly in the 5-minute delayed NOR test (Kruskal Wallis statistic = 17.17, P = 0.0007). DI scores of CONV-LAM, DBA-LAM, CONV-SCI and DBA-SCI measured 0.57 ± 0.23, 0.7 ± 0.14, 0.003 ± 0.15 and -0.12 ± 0.3, respectively on Day 56 in 5 min NOR test. However, different techniques did not differ in DI scores neither within LAM nor in SCI groups. On Day 56, 24-hour NOR test showed no differences between groups of which, the DI measurements were 0.3 ± 0.4, 0.4 ± 0.4, -0.05 ± 0.15 and 0.09 ± 0.3 respectively (data not shown).
3.9. Von Frey test
The paw withdrawal threshold (PWT) for the right leg in SCI animals were 93.33 ± 15.5 g, 86.7 ± 19.7 g, 21 ± 18.5 g and 11.16 ± 8 g for CONV- LAM, DBA- LAM, CONV- SCI and DBA- SCI respectively whereas for the left leg, PWT were 86.66 ± 19.7 g, 86.66 ± 19.69, 11.33 ± 5.4 g and 13.16 ± 10.31 g, respectively. Plantar test was employed for the LAM animals whereas dorsal von Frey test was employed for all SCI animals. No differences could be observed between groups that underwent similar surgeries using different techniques. The groups differed significantly (Kruskal- Wallis statistic- 18.35, P = 0.0004) in PWT scores of the right and (Kruskal- Wallis statistic- 18.36, P = 0.0004) the left hind paws (data not shown).
3.10. Histopathology
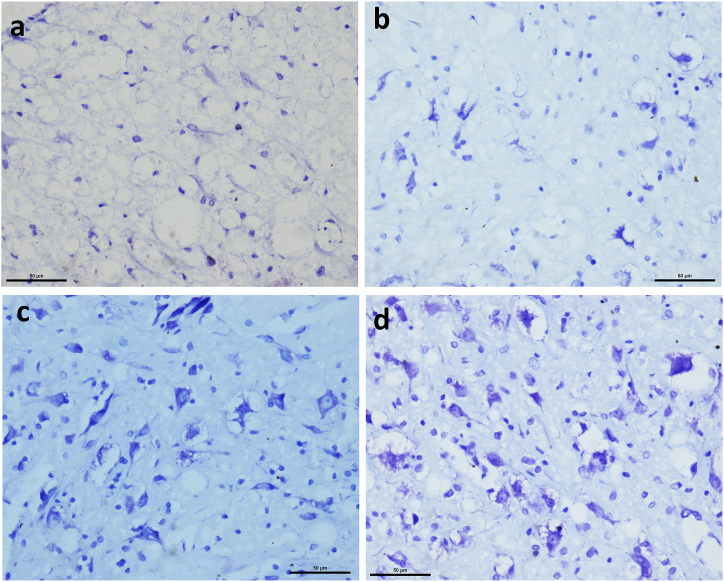
There were no differences in the number of live neurons between spinal cords that underwent CONV and DBA technique for laminectomy, within the respective LAM and SCI groups. A Two-Way ANOVA revealed that no interaction existed between the CONV or DBA procedures in neither the LAM nor the SCI groups with ventral or dorsal horn neurons (F (3, 40) = 1.055, p = 0.379). There was a positive correlation in animals that underwent DBA technique between surviving number of neurons and BBB scores on day 1 (r = 0.8952, p < 0.0001) and day 56 (r = 0.8049, p = 0.0016). A similar positive correlation was also observed in the animals which underwent CONV technique on Day 1 (r = 0.9441, p < 0.0001) and Day 56 (r = 0.7761, p = 0.0030).
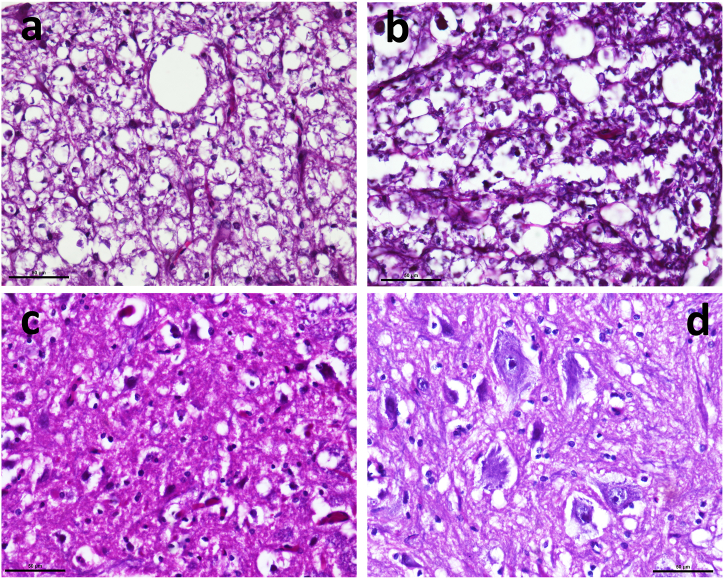
The vacuolization in H and E staining revealed that a difference between groups existed (KW Statistic = 17.41, P = 0.0006) and the both the SCI groups had more vacuoles than both the LAM groups (data not shown). However, there were no difference in vacuole percentage between the two different techniques employed to create either LAM or SCI. Representative figures of H and E and Nissl’s staining for all the groups are given as Figures 7 and 8.
Figure 7.
Representative slides from H and E stained (40X) sections. Representative slides from H and E stained (40X) sections. (a) CONV-LAM (b) DBA-LAM (c) CONV-SCI and (d) DBA-SCI.
Figure 8.
Representative slides from Cresyl Violet (Nissl’s) stained (40X) sections. (A) CONV-LAM (b) DBA-LAM (c) CONV-SCI, and (d) DBA-SCI.
4. Discussion
SCI models are of high severity presenting variable lesions from hind quarter paralysis and urine retention to even respiratory issues based on the level of injury. This is due to deep destructive changes in the grey and white matter of the spinal cord, inducing pathological shifts, death of neurons and glial cells, degeneration of nerve fibers, demyelination, and activation of microglia and macrophages. Given that this model currently is necessary in the path to find a cure to spinal damage, the high severity calls for increased efforts to refine the model for ethical reasons.
It is known that rat models mimic paraspinous muscle spasms, behavioural pain, tactile allodynia and nerve root scarring and adhesion that characterize human chronic post-laminectomy low back pain and sciatica [30]. It is established that the smallest possible laminectomy should be created to avoid mechanical deformity of spinal tissue and prevent confounding pain related behaviour [31]. It has also been established that laminectomy causes scoliosis in experimental animals [32]. Recent reports on occurrence of fibrosis due to the use of electrocautery for laminectomy in rat model [33] points to the fact that procedural bleeding and complications can arise in animal models owing to laminectomy.
However, laminectomy as a procedure and its potential complications in animal models remain sparsely documented. The lack of precise descriptive protocols in developing laminectomies in experimental rat pain models has been previously reported [31]. Studies [26,34, 35, 36, 37] aiming to establish SCI protocols using SCI models for preclinical tests usually overlook to present the details of procedural part of laminectomy and its complications.
Our study confirms the findings from the previous report [21] that there is no practical over-burden in terms of time consumed nor laminectomy dimensions for the procedure per animal while DBA technique is adopted. The bleeding scores are indicative of the potential trauma and blood loss that can occur during conventional laminectomy and reinforces the hypothesis of inflicting less trauma by mechanization of the technique.
The present study of 56 days did not show any differences in the BBB scores between the two techniques at any time point, in neither LAM nor in SCI groups reckoning the finding that the outcome in terms of BBB scores is comparable while using both the techniques.
It was earlier demonstrated that body weight loss and acute phase RGS scores in SCI animals that underwent procedures with the DBA technique [21] were lower. The present study confirms these findings indicative of the impact of DBA technique on the overall welfare of animals. Employing body weight changes and RGS scores in various groups in preclinical SCI studies shall be useful to investigators.
In the previous study [21], meaningful conclusions could not be made on activity and rearing in day time open field test and so it was decided to incorporate dark phase home cage activity. Contradictory findings have been reported in light phase versus dark phase testing in rodents. Pronounced behavioural inhibition has been demonstrated in DBA mice in the light phase [38] whereas other studies demonstrate usefulness of light phase trials in rodents [39]. It is well known that the behaviour is fundamentally modulated by light/dark cycle [38] and its rhythm profoundly affects activity [40]. Different results were obtained in time needed to perform the trial where DBA mice required much less time to perform a task during their active (dark) phase [38]. Sprague Dawley rats exhibited better acquisition and performance of an operant task requiring attentional efforts when the training originally occurred during the dark phase; the animals showed better remote memory in Morris Water Maze when retested after 2 weeks after the last day of training [41]. In our present study, there were no significant differences in the open field activity or rearing behaviour during the light phase at any time points whereas significant differences were observed between techniques on Day 1 and on Day 56 for both the parameters and on Day 1 for rearing score in the dark phase. The outcome of these tests brings out the relevance of employing activity tests not only in the light phase when studying post-procedural behaviour, even though this may have to be done in less convenient working hours. Further, it shall be noted that the animals showed better species-specific behavioural repertoire and activity when the DBA technique is employed.
Despite its popularity being easy to perform and less time consuming, sucrose preference data is often presented with high variability [42] and the present study confirms this variability in data obtained. As previously devised for mice [43], automation of the dual bottle preference set up in rats should be pursued and investigated whether significant advantage can be achieved.
The NOR test was modified to suit the paraplegic animals with poor mobility owing to SCI in this study for the first time. Placing objects firmly hung on the top grill worked well where the SCI animals from both the groups exhibited significantly lower short-term memory at chronic phase. The results from this study is in agreement with the documented observations that tactile allodynia is well established in rats with SCI [44] and the abnormalities of the dorsal horn neurons plays an important part in chronic pain in relation to SCI [45]. It is also elucidated that DBA technique exerts no effect of its own on this parameter.
It is conventionally considered that in comparison to group or pair housing of animals after surgery, single housing can prevent mutilation of surgical wounds. There is data suggesting that group housing can be of benefit during postoperative recovery in mice [46]. It will be of benefit in future to assess the recovery patterns in pair-housed SCI rats post-procedurally and to compare with the same in individually housed rats.
Neurodegeneration and damage to oligodendrocytes, activation of microglia by blood components and the iron released due to the lysis of blood polarizing the pro-inflammatory M1 phenotype of microglia are all contributed by bleeding at the site of SCI. These factors can aggravate SCI and delay healing and so, preventing bleeding is beneficial to the recovery process [36] in clinical applications. The present study is affirmative of these findings and for a better welfare of the model, a technique with better bleeding control is being put forth. Neuronal injury assessed using Nissl’s-stained functional neuronal assessment [47, 48] is used widely to assess functionality of spinal cord in rat spinal cord injury models and conclusive results could be obtained from the present study as well. Pathological evaluation based on neuronal count was affirmative of the fact that the outcome owing to different techniques were not different whereas differences in neuronal activity could be identified between the LAM and SCI procedures. Vacuolation was noticed in both the LAM and SCI groups, which was also in agreement with previous reports on vacuolization observed 24 h and six weeks post-laminectomy [49] even though vacuolization in the present study was observed at eight weeks post-laminectomy. The correlation study revealed a stronger correlation of surviving neuronal number to the BBB scores on Day 1 compared to Day 56 in both CONV and DBA techniques. As the animals regained motor coordination in due course as time progressed, the correlation became weaker. It is possible that the lower number of functionally active neurons are responsible for lower BBB scores on Day 1, thereby a stronger correlation. On Day 56, better BBB scores due to the remaining active neuronal compensatory reorganization [50], without the change in the total number of active neurons, resulted in a comparatively weaker correlation. This study focused on chronic effects of the DBA technique on the animal model and so histopathologic data from acute and subacute stages could not be processed. It will be definitely of high interest to probe into this aspect. More quantitative measures like electrophysiology and immunohistochemistry to draw information on neuro-inflammatory markers shall be performed to explore the DBA technique further to ensure the effectiveness of the technique in future.
5. Conclusion
Effect of local anesthetics in favour of laminectomy was not attempted in this study. This would be a point of interest in future to further refine and describe the model in broader terms.
In conclusion, the present study confirmed the findings from previous study demonstrating the advantage of DBA technique over the conventional technique to create laminectomy in rat thoracic vertebra for developing SCI. The applicability of the so created SCI model as a model of chronic pain and memory loss associated with the SCI lesion is successfully verified. SCI model is considered as a model of high severity, and employing DBA technique to perform laminectomy is recommended based on the evidence that it has less impact on animal welfare when compared with the conventional technique yet without compromising the validity and utility of the model. A major chunk of studies relies upon the sole semi-quantitative parameter of that of BBB scores to interpret results from SCI models. Demonstration of using a combination of quantitative as well as qualitative tests is of high utility in bringing out underlying facts from studies is being established by this study. Quantitative tests like von Frey test, body weight measurements, live neuron count and vacuole percentage in pathology sections along with qualitative tests like bleeding score, RGS, activity during photo and scotophase, and NOR can fortify and produce more meaningful data in comparison to using BBB scores as the sole parameter assessed. Data from this study demonstrate that the motorised DBA technique to develop laminectomy can be employed to develop chronic SCI in rats.
Declarations
Author contribution statement
Harikrishnan V. S: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Hamza Palekkodan, Ansar Fasaludeen: Analyzed and interpreted the data.
Lissy K. Krishnan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Klas S. P. Abelson: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data associated with this study has been deposited at Figshare under the URL: https://doi.org/10.6084/m9.figshare.13058915.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors hereby acknowledge the Director, SCTIMST and the Head, Biomedical Technology Wing for the support, and permission to use facilities given. The animal handlers of Division of Laboratory Animal Science, Manoj M, Sunil Kumar and Pradeep Kumar B have supported immensely with their handling, gentling and assistance of procedures. Sarath Kumar RS, technical assistant of animal laboratory has helped in the setting up of night time videography and management of supplies for the study whereas Dr. Naresh Kasoju of tissue culture facility has helped with image analysis using software. All the support received are hereby acknowledged.
References
- 1.Bickenbach J., Officer A., Shakespeare T., von Groote P., Organization W.H. World Health Organization; 2013. International Perspectives on Spinal Cord Injury. [Google Scholar]
- 2.Sharif-Alhoseini M., Khormali M., Rezaei M., Safdarian M., Hajighadery A., Khalatbari M., Meknatkhah S., Rezvan M., Chalangari M., Derakhshan P. Animal models of spinal cord injury: a systematic review. Spinal Cord. 2017;55(8):714–721. doi: 10.1038/sc.2016.187. [DOI] [PubMed] [Google Scholar]
- 3.Hosier H., Peterson D., Tsymbalyuk O., Keledjian K., Smith B.R., Ivanova S., Gerzanich V., Popovich P.G., Simard J.M. A direct comparison of three clinically relevant treatments in a rat model of cervical spinal cord injury. J. Neurotrauma. 2015;32(21):1633–1644. doi: 10.1089/neu.2015.3892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chiu C., Cheng H., Hsieh S. Contusion spinal cord injury rat model. Bio-protocol. 2017;7(12) doi: 10.21769/BioProtoc.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onifer S.M., Rabchevsky A.G., Scheff S.W. Rat models of traumatic spinal cord injury to assess motor recovery. ILAR J. 2007;48(4):385–395. doi: 10.1093/ilar.48.4.385. [DOI] [PubMed] [Google Scholar]
- 6.Budh C.N., Lund I., Hultling C., Levi R., Werhagen L., Ertzgaard P., Lundeberg T. Gender related differences in pain in spinal cord injured individuals. Spinal Cord. 2003;41(2):122–128. doi: 10.1038/sj.sc.3101407. [DOI] [PubMed] [Google Scholar]
- 7.Sharp K., Boroujerdi A., Steward O., Luo Z.D. A rat chronic pain model of spinal cord contusion injury. Methods Mol. Biol. 2012;851:195–203. doi: 10.1007/978-1-61779-561-9_14. [DOI] [PubMed] [Google Scholar]
- 8.Yu D., Thakor D.K., Han I., Ropper A.E., Haragopal H., Sidman R.L., Zafonte R., Schachter S.C., Teng Y.D. Alleviation of chronic pain following rat spinal cord compression injury with multimodal actions of huperzine a. Proc. Natl. Acad. Sci. U. S. A. 2013;110(8):E746–755. doi: 10.1073/pnas.1300083110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siddall P.J., Taylor D.A., McClelland J.M., Rutkowski S.B., Cousins M.J. Pain report and the relationship of pain to physical factors in the first 6 months following spinal cord injury. Pain. 1999;81(1-2):187–197. doi: 10.1016/s0304-3959(99)00023-8. [DOI] [PubMed] [Google Scholar]
- 10.Wu J., Stoica B.A., Luo T., Sabirzhanov B., Zhao Z., Guanciale K., Nayar S.K., Foss C.A., Pomper M.G., Faden A.I. Isolated spinal cord contusion in rats induces chronic brain neuroinflammation, neurodegeneration, and cognitive impairment. Involvement of cell cycle activation. Cell Cycle. 2014;13(15):2446–2458. doi: 10.4161/cc.29420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang F., Huang S.-L., He X.-J., Li X.-H. Determination of the ideal rat model for spinal cord injury by diffusion tensor imaging. Neuroreport. 2014;25(17):1386–1392. doi: 10.1097/WNR.0000000000000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen N.-F., Sung C.-S., Wen Z.-H., Chen C.-H., Feng C.-W., Hung H.-C., Yang S.-N., Tsui K.-H., Chen W.-F. Therapeutic effect of platelet-rich plasma in rat spinal cord injuries. Front. Neurosci. 2018;12(252) doi: 10.3389/fnins.2018.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer F.R., Peduzzi J.D. Functional recovery in rats with chronic spinal cord injuries after exposure to an enriched environment. J. Spinal Cord Med. 2007;30(2):147–155. doi: 10.1080/10790268.2007.11753926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao J., Li B., Duan H-q, Zhao C-x, Zhang Y., Sun C., Pan B., Liu C., Kong X-h, Yao X., Feng S-q. Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Reg. Res. 2017;12(6):959–968. doi: 10.4103/1673-5374.208591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun Y., Gong F., Yin J., Wang X., Wang X., Sun Q., Zhu Z., Su X., Zheng J., Liu L., Li Y., Hu X., Li J. Therapeutic effect of apocynin through antioxidant activity and suppression of apoptosis and inflammation after spinal cord injury. Exp. Ther. Med. 2017;13(3):952–960. doi: 10.3892/etm.2017.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Key J.A., Ford L.T. Experimental intervertebral-disc lesions. J. Bone Joint Surg. Am. 1948;30a(3):621–630. [PubMed] [Google Scholar]
- 17.Gürer B., Kahveci R., Gökçe E.C., Ozevren H., Turkoglu E., Gökçe A. Evaluation of topical application and systemic administration of rosuvastatin in preventing epidural fibrosis in rats. Spine J. 2015;15(3):522–529. doi: 10.1016/j.spinee.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Cemil B., Kurt G., Aydın C., Akyurek N., Erdogan B., Ceviker N. Evaluation of tenoxicam on prevention of arachnoiditis in rat laminectomy model. Eur. Spine J.: Off. Publ. Eur. Spine Soci. Eur. Spin. Deform. Soci. Eur. Sec. Cervic. Spine Res. Soci. 2011;20(8):1255–1258. doi: 10.1007/s00586-011-1706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sedý J., Urdzíková L., Jendelová P., Syková E. Methods for behavioral testing of spinal cord injured rats. Neurosci. Biobehav. Rev. 2008;32(3):550–580. doi: 10.1016/j.neubiorev.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Vaquero J., Zurita M., Oya S., Santos M. Cell therapy using bone marrow stromal cells in chronic paraplegic rats: systemic or local administration? Neurosci. Lett. 2006;398(1-2):129–134. doi: 10.1016/j.neulet.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 21.S H V., Krishnan L.K., Abelson K.S.P. A novel technique to develop thoracic spinal laminectomy and a methodology to assess the functionality and welfare of the contusion spinal cord injury (sci) rat model. PloS One. 2019;14(7) doi: 10.1371/journal.pone.0219001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the arrive guidelines for reporting animal research. PLoS Biol. 2010;8(6) doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Council N.R. 2010. Guide for the Care and Use of Laboratory Animals. [Google Scholar]
- 24.Mähler Convenor M., Berard M., Feinstein R., Gallagher A., Illgen-Wilcke B., Pritchett-Corning K., Raspa M. Felasa recommendations for the health monitoring of mouse, rat, hamster, Guinea pig and rabbit colonies in breeding and experimental units. Lab. Anim. 2014;48(3):178–192. doi: 10.1177/0023677213516312. [DOI] [PubMed] [Google Scholar]
- 25.M R. 1988. The Design of Experiments; p. 620. New York. [Google Scholar]
- 26.Basso D.M., Beattie M.S., Bresnahan J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma. 1995;12(1):1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 27.Sotocinal S.G., Sorge R.E., Zaloum A., Tuttle A.H., Martin L.J., Wieskopf J.S., Mapplebeck J.C.S., Wei P., Zhan S., Zhang S., McDougall J.J., King O.D., Mogil J.S. The rat grimace scale: a partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain. 2011;7:55. doi: 10.1186/1744-8069-7-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 29.Au - Detloff M.R., Au - Fisher L.C., Au - Deibert R.J., Au - Basso D.M. Acute and chronic tactile sensory testing after spinal cord injury in rats. JoVE. 2012;62 doi: 10.3791/3247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massie J.B., Huang B., Malkmus S., Yaksh T.L., Kim C.W., Garfin S.R., Akeson W.H. A preclinical post laminectomy rat model mimics the human post laminectomy syndrome. J. Neurosci. Methods. 2004;137(2):283–289. doi: 10.1016/j.jneumeth.2004.02.036. [DOI] [PubMed] [Google Scholar]
- 31.Košta V., Kojundžić S.L., Sapunar L.C., Sapunar D. The extent of laminectomy affects pain-related behavior in a rat model of neuropathic pain. Eur. J. Pain. 2009;13(3):243–248. doi: 10.1016/j.ejpain.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Liszka O. Spinal cord mechanisms leading to scoliosis in animal experiments. Acta Med. Pol. 1961;2:45–63. [PubMed] [Google Scholar]
- 33.Köksal V., Mercantepe T., Tumkaya L., Oktem I. Less use of bipolar cautery can prevent post-laminectomy epidural fibrosis: an experimental study in rats. Turkish Neurosurg. 2019;30 doi: 10.5137/1019-5149.JTN.27544-19.2. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed R.U., Alam M., Zheng Y.-P. Experimental spinal cord injury and behavioral tests in laboratory rats. Heliyon. 2019;5(3):e01324. doi: 10.1016/j.heliyon.2019.e01324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moon L.D., Leasure J.L., Gage F.H., Bunge M.B. Motor enrichment sustains hindlimb movement recovered after spinal cord injury and glial transplantation. Restor. Neurol. Neurosci. 2006;24(3):147–161. [PubMed] [Google Scholar]
- 36.Fan H., Chen K., Duan L., Wang Y.Z., Ju G. Beneficial effects of early hemostasis on spinal cord injury in the rat. Spinal Cord. 2016;54(11):1058. doi: 10.1038/sc.2016.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barros Filho T.E., Molina A.E. Analysis of the sensitivity and reproducibility of the basso, beattie, bresnahan (bbb) scale in wistar rats. Clinics. 2008;63(1):103–108. doi: 10.1590/s1807-59322008000100018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roedel A., Storch C., Holsboer F., Ohl F. Effects of light or dark phase testing on behavioural and cognitive performance in dba mice. Lab. Anim. 2006;40(4):371–381. doi: 10.1258/002367706778476343. [DOI] [PubMed] [Google Scholar]
- 39.Yang M., Weber M., Crawley J. Light phase testing of social behaviors: not a problem. Front. Neurosci. 2008;2(29) doi: 10.3389/neuro.01.029.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weinert D., Sturm J., Waterhouse J. Different behavior of the circadian rhythms of activity and body temperature during resynchronization following an advance of the ld cycle. Biol. Rhythm Res. 2002;33:187–198. [Google Scholar]
- 41.Gritton H.J., Kantorowski A., Sarter M., Lee T.M. Bidirectional interactions between circadian entrainment and cognitive performance. Learn. Mem. 2012;19(3):126–141. doi: 10.1101/lm.023499.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scheggi S., De Montis M.G., Gambarana C. Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol. 2018;21(11):1049–1065. doi: 10.1093/ijnp/pyy083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu M.Y., Yin C.Y., Zhu L.J., Zhu X.H., Xu C., Luo C.X., Chen H., Zhu D.Y., Zhou Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018;13(7):1686–1698. doi: 10.1038/s41596-018-0011-z. [DOI] [PubMed] [Google Scholar]
- 44.Detloff M.R., Quiros-Molina D., Javia A.S., Daggubati L., Nehlsen A.D., Naqvi A., Ninan V., Vannix K.N., McMullen M.K., Amin S., Ganzer P.D., Houlé J.D. Delayed exercise is ineffective at reversing aberrant nociceptive afferent plasticity or neuropathic pain after spinal cord injury in rats. Neurorehabilit. Neural Rep. 2016;30(7):685–700. doi: 10.1177/1545968315619698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hao J.-X., Kupers R.C., Xu X.-J. Response characteristics of spinal cord dorsal horn neurons in chronic allodynic rats after spinal cord injury. J. Neurophysiol. 2004;92(3):1391–1399. doi: 10.1152/jn.00121.2004. [DOI] [PubMed] [Google Scholar]
- 46.Van Loo P.L., Kuin N., Sommer R., Avsaroglu H., Pham T., Baumans V. Impact of 'living apart together' on postoperative recovery of mice compared with social and individual housing. Lab. Anim. 2007;41(4):441–455. doi: 10.1258/002367707782314328. [DOI] [PubMed] [Google Scholar]
- 47.He Z., Zhou Y., Huang Y., Wang Q., Zheng B., Zhang H., Li J., Liu Y., Wu F., Zhang X., Tong S., Wang M., Wang Z., He H., Xu H., Xiao J. Dl-3-n-butylphthalide improves functional recovery in rats with spinal cord injury by inhibiting endoplasmic reticulum stress-induced apoptosis. Am J Transl Res. 2017;9(3):1075–1087. [PMC free article] [PubMed] [Google Scholar]
- 48.Fang H., Liu C., Yang M., Li H., Zhang F., Zhang W., Zhang J. Neurotrophic factor and trk signaling mechanisms underlying the promotion of motor recovery after acute spinal cord injury in rats. Exp Ther Med. 2017;14(1):652–656. doi: 10.3892/etm.2017.4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Z., Tu J. The roads to mitochondrial dysfunction in a rat model of posttraumatic syringomyelia. BioMed Res. Int. 2015:831490. doi: 10.1155/2015/831490. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Filipp M., Travis B., Henry S., Idzikowski E., Magnuson S., Loh M., Hellenbrand D., Hanna A. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regener. Res. 2019;14(1):7–19. doi: 10.4103/1673-5374.243694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at Figshare under the URL: https://doi.org/10.6084/m9.figshare.13058915.