Abstract
Background
Codeine, a common drug of abuse, has been reported to induce organ damage; however, there are scanty available data on the effects of codeine on the brain.
Objective
Thus, we tested the hypothesis that redox dysregulation and inflammation of the brain induced by codeine exposure is 8-OHdG and/or caspase 3-dependent.
Methods
New Zealand White rabbits (Oryctolagus cuniculus) received vehicle (control; n = 7), low-dose codeine (4 mg/kg/day p.o; n = 6), or high-dose codeine (10 mg/kg/day p.o; n = 6) for six weeks. Body weight was checked before and after the study.
Results
Findings showed that codeine exposure resulted in redox dysregulation (evident by elevated MDA and H2O2 accompanied by reduced enzymatic antioxidant activities), elevated MPO activity, and distorted cytoarchitecture of the brain tissue. The observed codeine-induced redox imbalance and brain inflammation was accompanied by depletion of neuronal and purkinje cells, reduced AchE activity, and elevated 8-OHdG levels and caspase 3 activity.
Conclusions
The current study demonstrates that chronic codeine use induces oxido-inflammatory response and apoptosis of the brain tissue that is associated with neuronal and purkinje cells injury, and impaired AchE activity through 8-OHdG and/or caspase 3-dependent pathway.
Keywords: Codeine, Drug abuse, Brain, Oxidative stress, Genotoxicity, Apoptosis
Highlights
-
•
Codeine led to redox dysregulation and inflammation of the brain.
-
•
This was accompanied by distorted cytoarchitecture of the brain.
-
•
Codeine also caused depletion of neuronal and purkinje cells with reduced acetylcholinesterase activity.
-
•
Codeine-induced brain injury was mediated by upregulation of 8-OHdG/caspase 3 signaling.
Codeine, Drug abuse, Brain, Oxidative stress, Genotoxicity, Apoptosis.
1. Introduction
Drug use disorder is a menace of global worry [1]. It is the inappropriate use of drug either for medical or nonmedical reasons to the detriment of one's health [1]. It differs from the abuse and misuse of a drug [2]. Drug abuse is the excessive, maladaptive and addictive nonmedical use of a particular drug with an attendant adverse effect on health [3, 4]. In contrast, the misuse of a drug is a deliberate or accidental use of prescribed medication in a way that it was not intended with possible personal, professional, or social problems [2]. The pattern of drug use disorder varies from one country to another. In the UK, it was observed that about 51% of the patients abused cannabis, 43% alcohol, and 38% are involved with polysubstance [5]. In Australia, about 38% of the population aged at least fourteen years has used an illicit drug at some stage in their lives, and 17% have recent use of an illicit drug [6]. In Israel, a lifetime prevalence of drug abuse was documented to be 24% [7]. In Nigeria, the prevalence of drug abuse is estimated to be about 14.4% [8]. Common drugs of abuse include cannabis, cocaine, heroin, methamphetamine, and opioids, such as tramadol and codeine [1].
Codeine, also known as 3-methylmorphine, is one of the most commonly taken opioid medications at the centre of opioid addiction problems in most countries, including the United States [9] and Nigeria [10]. It is naturally found in Papaver somniferum. It is a phenanthrene derivative extracted from opium or synthetically produced by the methylation of morphine [11]. The global demand for codeine rose to about 27% [12], with a peak in consumption in 2011 and UK being the highest producer (22%), followed closely by France (21%), US (17%), and Australia (8%) [11]. Although its therapeutic values for pain, cough, Restless Leg Syndrome, and persistence cannot be undermined, its addictive potential puts it at risk of abuse. Though previous studies have documented its potential to enhance copulatory locomotion [10], it reduces fertility indices [10], causes testicular toxicity [13, 14], and low sperm quality [14, 15] via stimulation of oxidative stress, inflammation, and apoptosis. Further studies demonstrated that codeine induced hepatic [16] and cardiorenal damage [17] via caspase 3-dependent apoptosis.
The brain is a complex organ that coordinates most of the physiological functions. The cerebral cortex is the largest part of the brain and fulfils many functions such as reasoning and motor skills through processing, sensation, and memory. The cerebellum, also called the little brain, controls balance and movement. It also plays a crucial role in cognitive functions. It possesses one of the largest neurons in the brain [18, 19]. The hippocampus is a part of the limbic system, and it is located within the cerebrum. Its primary role is the coordination of the learning system. The brain is vulnerable to oxidative injury because of its high content of polyunsaturated fatty acids, the presence of redox-active metals, and its high utilization of oxygen [20]. Thus, oxidative stress has been implicated in neurodegenerative diseases [21].
The brain is very susceptible to the neurotoxic effects of drugs. Toxicity may be secondary to endocrine disruption [22], oxidative stress following impairment of the antioxidant scavenging capacity in the brain [23], DNA and apoptosis [24, 25], and epigenetic modification [26]. Despite the widespread abuse of codeine and its potential to exert oxidative damage to body tissues, insufficient data are reporting its effects on the brain. With this background in mind, this study was designed to evaluate the impact of codeine, a common drug of abuse, on the brain and the likely role of oxidative stress and apoptosis.
2. Materials and methods
2.1. Animals
This study was carried out according to the National Institutes of Health Guide for Care and Use of Laboratory Animals and was approved by the Ethics Review Committee of the Ministry of Health, Oyo State (Approval number: AD13/479/1396). A conscious effort to minimize the number of animals used for the study and their suffering was made. The study utilized 10 weeks old male inbred New Zealand White rabbits (Oryctolagus cuniculus) of similar weights. The rabbits were housed in a well-ventilated room maintained at natural conditions. The animals had unrestricted access to standard animal chow and water. They were acclimatized for two weeks. Twenty-one (21) rabbits were randomly assigned to control, low-dose codeine-treated, and high-dose codeine-treated groups (n = 7/group).
2.2. Treatment
The control group received distilled water (vehicle; p.o) daily. The low-dose codeine-treated group was administered (po) 4 mg/kg/day codeine, while the high-dose codeine-treated group had 10 mg/kg/day codeine po for six weeks [10, 13, 14, 15, 16, 17]. Treatments were discontinued at least 24 h before the termination of the study. Besides the treatment, body weights were assessed throughout the study period and just before the animals were culled. The brain organosomatic index (OSI) was determined as the percentage of the ratio of the weight of the brain to the final body weight. The gross and relative brain weight (OSI) were used as indices of toxicity.
2.3. Sample preparation
At the end of the experiment, animals were anaesthetized with ketamine (40 mg/kg)/xylazine (4 mg/kg) intraperitoneally after 12 h overnight fast. The brain was harvested and weighed. After weighing, the brain was carefully divided into two equal hemispheres. One hemisphere was homogenized in phosphate buffer with a glass homogenizer and the other hemisphere was used for histopathological analysis. The homogenate was used to determine acetylcholinesterase (AchE) activity, oxidative stress markers [malondialdehyde (MDA), H2O2, glutathione (GSH), and advanced glycation end products (AGE)], neutrophil infiltration (myeloperoxidase, MPO, activity), antioxidant buffering system [superoxide dismutase (SOD), catalase, glutathione S-transferase (GST), and glutathione peroxidase (GPx)], and markers of genotoxicity (8-hydroxy-2-deoxyguanosine, 8-OHdG), and apoptosis (caspase 3).
2.4. Biochemical assay
Brain AchE activity was determined by colorimetric method using standard reagents (Elabscience). The assay is based on Ellman's method in which AchE produces thiocholine that in turn reacts with DTNB 5,5-dithiobis (2-nitrobenzoic acid) to form a colorimetric product at 412 nm. The generated colorimetric product is proportional to the AchE activity present.
Markers of oxidative stress, neutrophil inflammation, and enzymatic antioxidants were assayed using standardized enzymatic colorimetric method with assay kit as previously documented in our studies [13, 27, 28, 29, 30]. Briefly, MDA, the marker of lipid peroxidation, was measured as the amount of thiobarbituric acid reactive substance, TBARS, generated during lipid peroxidation. This method is based on the reaction between 2- thiobarbituric acid (TBA) and malondialdehyde, an end product of lipid peroxidation. On heating in acidic pH, a pink chromogen complex ([TBS] 2-malondialdehyde adduct) is formed and measured by its absorbance at 532 [13,30].
The concentration of hydrogen peroxide (H2O2), a marker of reactive oxygen species (ROS) generation, was determined by the method of Wolff [13]. This method utilizes a colour reagent that contains xylenol orange dye in an acidic solution with sorbitol and ferrous ammonium sulphate that reacts to produce a purple colour in proportion to the concentration of H2O2.
The colorimetric method for the determination of GSH concentration (a marker of oxidative stress) is based on the formation of a relatively stable (yellow) colour when 5′, 5′-dithiobis-(2-nitrobenzoic acid) (Ellman's reagent) is added to sulfhydryl compounds. The chromophoric product resulting from the reaction of Ellman's reagent with the reduced glutathione, 2- nitro-5-thiobenzoic acid possesses a molar absorption at 412 nm which was read at 412 nm in a spectrophotometer [13].
The activity of MPO (a marker of neutrophil infiltration) was determined by colorimetric assay. This assay is based on hydrogen peroxide-dependent oxidation of guaiacol [13, 30].
The assay of SOD activity was based on the auto-oxidation of epinephrine at pH 10.2. Superoxide (O2−) radical generated by the xanthine oxidase reaction causes the oxidation of epinephrine to adrenochrome and the yield of adrenochrome produced per O2− introduced increases with increasing pH and also increases with increasing concentration of epinephrine. These results led to the proposal that auto-oxidation of epinephrine proceeds by at least two distinct pathways, only one of which is a free radical reaction involving superoxide (O2−) radical and can hence be inhibited by superoxide dismutase.
Determination of catalase activity was based on the oxidation of TMB (3,5,3′,5′-tetramethylbenzidine) to generate a blue coloured-, cation free- radical with a peak absorbance at 653 nm.
GST was determined as previously described [13]. The method is based on the relatively high activity of GST with 1-chloro-2,4,-dinitrobenzene as the second substrate. The conventional assay for GST activity utilizes 1-chloro-2,4,- dinitrobenzene as substrate. The absorption increases at 340 nm provides a direct measurement of enzymatic reaction.
The assay of GPx is based on the oxidation of GSH to produce GSSG as part of the reaction in which it reduces cumene hydroperoxide. Glutathione reductase (GR) then reduces the GSSG to produce GSH, and in the same reaction consumes NADPH. The decrease of NADPH (measured at OD = 340 nm) is proportional to GPx activity.
AGE (a marker of oxidative protein denaturation), 8OHdG (a marker of oxidative DNA damage, genotoxicity), and caspase 3 activity (a marker of apoptosis) were determined using ELISA kits (Elabscience) per the manufacturer's guidelines.
2.5. Histological studies
The brain from each rabbit was fixed in 10% Formol-saline and processed for paraffin wax embedment. The paraffin-embedded samples were sectioned at 3 μm and stained with Haematoxylin and eosin (H & E) stains. Slides were examined using a light microscope for histological examinations. Photomicrographs of the hippocampus, cortex, and cerebellum were taken at 100× and 400× magnifications.
2.6. Statistical analysis
Statistical analysis was performed using GraphPad Prism (Versions 5). Data are expressed as mean ± SEM of 7 replicates per group. Analysis of variance (ANOVA) was used to compare the mean values across groups and Tukey's posthoc test was used for pairwise comparison. P values <0.05 were considered significant.
3. Results
3.1. Effect of codeine exposure on body weight, brain weight, and brain OSI
The body weights of the animals were comparable across the groups at the onset of the study (Figure 1A). Although the final body weights and weight gain were slightly reduced in the codeine-exposed groups, the observed differences were not significant (Figure 1 (B, C)). The absolute brain weight was similar between the control and low-dose codeine-treated animals but was significantly reduced by 15% in the high-dose codeine-exposed rabbits when compared with the control group (Figure 1D). The brain weight related to final body weight was used to indicate the brain weight index (brain OSI). Codeine-exposed male rabbits had comparable brain OSI with the control group (Figure 1E).
Figure 1.
Effect of codeine on initial body weight (A), final body weight (B), body weight gain (C), brain weight (D), and brain organosomatic index, OSI (E). LDC: Low-dose codeine; HDC: High-dose codeine. Data were analyzed by ANOVA followed by Tukey's post hoc test. Values are expressed as mean ± SEM of 7 rats per group ∗p < 0.05 vs. control, #p < 0.05 vs LDC.
3.2. Histopathological examinations
The histopathological findings are summarized in Table 1. Histopathological examinations revealed hippocampus with normal neuronal cells and preserved structural organization of the CA1, CA2 AND CA3 in the control and low-dose codeine-treated groups (Figure 2 (A, B, G, H)), while the hippocampus of high-dose codeine-exposed animals exhibited moderate distortion of structural organization with moderate depletion of CA2 (Figure 2 (L, M)). The cortex of the vehicle-treated control and low-dose codeine-treated groups showed normal laminae, neuronal cells capillaries, and stroma (Figure 2 (C, D, I, J)), while the cortex of the high-dose codeine-exposed group showed normal laminae, neuronal cells, and stroma, but the capillaries appeared moderately dilated (Figure 2 (O, P)). The control and low-dose codeine-treated animals' cerebellar cortex showed normal folia, molecular cell layer, with normal Purkinje cells in the Purkinje cells layer. The granular layer appeared normal with normal white matter (Figure 2 (E, F, L, K)). The cerebellar cortex of the high-dose codeine-treated animals showed normal folia, molecular cell layer, and granular layer, but the Purkinje cells layer showed mild depletion of Purkinje cells (Figure 2 (Q, R)).
Table 1.
Scoring of the histopathological changes of the brain tissues.
| Groups | Histopathological score |
|||
|---|---|---|---|---|
| Distorted structural organization | C2 depletion | Capillary dilatation | Depletion of Purkinje cells | |
| Control | − | − | − | − |
| LDC | + | ++ | ++ | − |
| HDC | + | − | ++ | + |
Score represent values from sections of 7 rabbits per group and 5 fields per section. Score level (−) was considered as no significant alterations. Scores (+, ++, and +++) were considered as mild, moderate, and severe levels, exhibiting <25, 50, and 75% histopathological change of the total field examined respectively. LDC: low-dose codeine; HDC: high-dose codeine.
Figure 2.
Photomicrographs of the H and E stain of the brain. LDC: Low-dose codeine; HDC: High-dose codeine. The control animals show normal hippocampus with normal neuronal cells (blue arrow). The structural organization of the CA1, CA2 and CA3 appear normal (white arrow) (A, D). The low dose codeine-treated animals show normal hippocampus with normal neuronal cells (blue arrow). The structural organization of the CA1, CA2 and CA3 appear normal (white arrow) (B, E). The high dose codeine-treated animals show normal hippocampus with moderately normal structural organization. However, the CA2 seen show moderate depletion black arrow, while other CA2, 3 and 4 appear normal (white arrow) (C, F). The control animals had cortex with normal laminae (spanned) and the neuronal cells appear normal. The capillaries seen are normal and the stroma also appear normal (slender arrow) (G, J). The low dose codeine-treated animals had cortex with normal laminae (spanned) and the neuronal cells appear normal. The capillaries seen are normal and the stroma also appear normal (slender arrow) (H, K). The animals that received high dose codeine had cortex with normal laminae (spanned) and the neuronal cells appear normal. The capillaries seen are moderately dilated and the stroma also appear normal (slender arrow) (I, L). The control animals had cerebellar cortex with normal folia. The molecular cell layer appear normal (white arrow), the purkinje cells layer show normal purkinje cells (black arrow), and granular layer appear normal (red arrow). The white matter appears normal (blue arrow). No pathological lesion was observed (M, P). The low dose codeine-treated animals had cerebellar cortex with normal folia. The molecular cell layer appear normal (white arrow), the purkinje cells layer show normal purkinje cells (black arrow), and granular layer appear normal (red arrow). The white matter also appears normal (blue arrow). No pathological lesion was seen (N, Q). The animals that received high dose codeine had cerebellar cortex with normal folia. The molecular cell layer appear normal (white arrow), the purkinje cells layer show mild depletion of purkinje cells (black arrow), and granular layer appear normal (red arrow) (O, R).
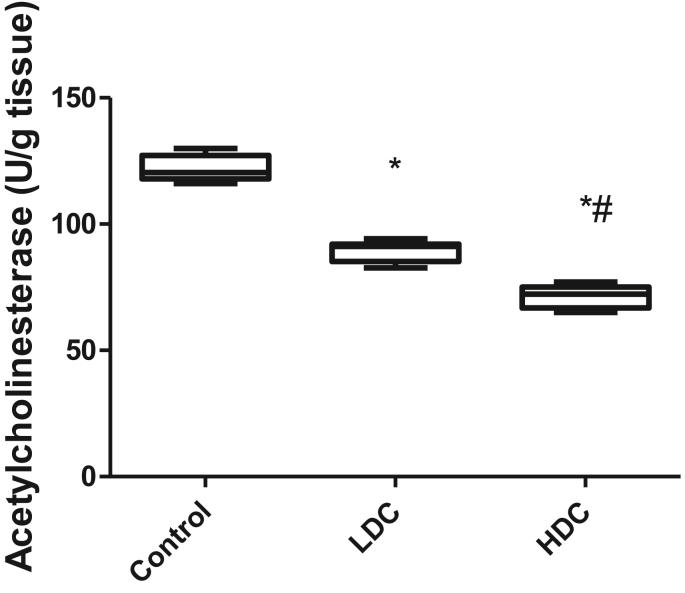
3.3. Effect of codeine exposure on acetylcholinesterase activity in brain tissue
Codeine exposure in male rabbits led to a significant decrease in acetylcholinesterase activity (Figure 3). Low-dose-codeine treatment significantly reduced acetylcholinesterase activity when compared to the vehicle-treated control group by 25.28%. High-dose codeine treatment caused a marked decline in acetylcholinesterase activity when compared with the control (by 38.72%) and low-dose codeine-treated groups (by 17.99%).
Figure 3.
Effect of codeine on acetylcholinesterase activity. LDC: Low-dose codeine; HDC: High-dose codeine. Data were analyzed by ANOVA followed by Tukey's post hoc test. Values are expressed as mean ± SEM of 7 rats per group ∗p < 0.05 vs. control, #p < 0.05 vs LDC.
3.4. Effect of codeine exposure on oxidative stress and pro-inflammatory biomarkers in brain tissue
Codeine exposure in male experimental animals led to a significant increase in MDA concentration in the brain tissue homogenate of the codeine-treated groups when compared to the control. When compared with the vehicle-treated control and low-dose codeine-treated animals, the high-dose codeine-treated animals had a significantly raised concentration of MDA by 25.22% and 33% respectively (Figure 2A). Also, there was a significant increase in the generation of H2O2 in a dose-dependent manner in the codeine-treated groups when compared to the control (Figure 2B). Significantly reduced GSH concentrations were observed in codeine-exposed rats; the high-dose codeine-treated group showed marked reduction in GSH levels (43.35%) than the low-dose codeine-treated group (23.64%) when compared with the control (Figure 2C). AGE concentrations was observed to be elevated in a dose-dependent manner in the codeine-treated groups compared to the control (9.97% and 14.74% higher in the low dose and high dose codeine-treated animals respectively compared with the control) (Figure 4D). In addition, there was a significant rise in MPO activity (26.59% in the low dose codeine-treated animals and 32.91% in the high dose codeine-treated animals) compared to the control group (Figure 4E). In addition, codeine treatment caused significant decrease in the activities of enzymatic antioxidants, SOD, catalase, GST, and GPx, when compared with the vehicle-treated control group. The reduction observed in the high-dose codeine-treated group was significantly more (16.03%, 27.7%, 27.06%, and 30.99% respectively for SOD, catalase, GST, and GPx compared to the control) than that observed in the low-dose codeine-treated group (5.34%, 12.22%, 15.1%, and 15.15% respectively for SOD, catalase, GST, and GPx compared to the control) (Figure 5 (A, B, C, D)).
Figure 4.
Effect of codeine on MDA (A), H2O2 (B), GSH (C), AGE (D), and MPO activity (E). LDC: Low-dose codeine; HDC: High-dose codeine. ∗ Data were analyzed by ANOVA followed by Tukey's post hoc test. Values are expressed as mean ± SEM of 7 rats per group ∗p < 0.05 vs. control, #p < 0.05 vs LDC.
Figure 5.
Effect of codeine on the activities of SOD (A), catalase (B), GST (C), and GPx (D). LDC: Low-dose codeine; HDC: High-dose codeine. Data were analyzed by ANOVA followed by Tukey's post hoc test. Values are expressed as mean ± SEM of 7 rats per group ∗p < 0.05 vs. control, #p < 0.05 vs LDC.
3.5. Effect of codeine exposure on genotoxicity and apoptosis in brain tissue
Codeine exposure resulted in elevated level of 8-OHdG when compared with the control group (Figure 6A) and a significant rise in caspase 3 activity (Figure 6B). The observed increases in 8-OHdG concentration and caspase 3 activity were more prominent in the high-dose codeine-treated group (40.95% and 84.08% for 8-OHdG and caspase 3 activity respectively compared with the control) than the low-dose codeine-treated group (26.87% and 75.95% for 8-OHdG and caspase 3 activity respectively compared with the control).
Figure 6.
Effect of codeine on 8OHdG (A), and caspase 3 activity (B). LDC: Low-dose codeine; HDC: High-dose codeine. Data were analyzed by ANOVA followed by Tukey's post hoc test. Values are expressed as mean ± SEM of 7 rats per group ∗p < 0.05 vs. control, #p < 0.05 vs LDC.
4. Discussion
In the present study, we tested the hypothesis that redox dysregulation and inflammation of the brain induced by codeine use is 8-OHdG and/or caspase 3-dependent. Our data demonstrate that codeine treatment in an animal model led to redox dysregulation (elevated MDA and H2O2 accompanied by reduced enzymatic antioxidant activities), elevated inflammation evident by a significant rise in MPO activity, and distorted cytoarchitecture of the brain tissue. The observed codeine-induced redox imbalance and brain inflammation was accompanied by reduced acetylcholinesterase activity, and elevated 8-OHdG levels and caspase 3 activity.
Our findings that codeine exposure caused alteration in the cytoarchitecture of the brain and degeneration of neuronal cells are consistent with previous findings on codeine [11] and morphine [31], the metabolite of codeine. However, since codeine has been reported to cause distortion of the histoarchitectural integrity of the brain and neuronal cell degeneration despite its global use as a substance of abuse and its therapeutic values in the management of cough, pain, and diarrhoeal disease, it may thus be a public health challenge. In addition, findings that codeine-induced distortion of the brain's cytoarchitecture are accompanied by depletion of purkinje cells, impaired acetylcholinesterase activity, genotoxicity, and caspase 3-mediated apoptosis of the neuronal cells are novel and noteworthy hence provide indications and scientific justification of increased risk cerebral and cerebellar dysfunctions. Also, it was observed that codeine use resulted in reduced absolute brain weight, although this was only significant in the high-dose codeine-treated group. However, body weight gain and brain OSI were not affected. This may infer that the neurotoxicity induced by codeine is not associated with alteration in visceral fat accumulation [17] but suppression of the cellular metabolism of the brain [32].
Redox imbalance in the brain has been reported as an independent risk factor that is strongly associated with neurodegenerative diseases [21, 23]. Although earlier studies documented that opioids such as morphine and tramadol induce oxidative brain damage [31, 33], the current study is perhaps the first to report that codeine exposure significantly increased H2O2 generation and lipid peroxidation as well as protein denaturation of the brain tissue. This was accompanied by reductions in the activities of enzymatic antioxidants. Oxidative stress is an imbalance in ROS generation and antioxidant buffering capacity leading to the accumulation of ROS and other pro-oxidant with resultant damage to cellular macromolecules [34]. Initially, under oxidative stress, antioxidant enzyme concentrations increase in an attempt to compensate for the increased ROS production [35]. SOD forms the first defense line. It scavenges superoxide radicals and converts them to H2O2 [13] thus dampening the impacts of the radicals, while catalase detoxifies the generated H2O2 [13, 20]. GST and GPx also play a key role in H2O2 metabolism. They are primarily responsible for H2O2 elimination [13]. When ROS and other pro-oxidant generation become robust, the antioxidant defense system is overwhelmed and their concentrations and activities decline. The marked increase in MDA, H2O2, and AGE observed following codeine administration and the significant fall in the enzymatic antioxidant activities suggest that the 6-week codeine exposure caused exaggerated ROS generation and consumption of antioxidants, thus permitting deleterious effects on the brain.
Inflammation of the brain tissue is a well-established risk factor that has been implicated in neurodegenerative conditions. The finding from this study that codeine administration led to increased MPO activity in the brain infers that codeine triggered neutrophil infiltration in the brain [17] and thus inflammation. Since oxidative stress can stimulate inflammation and vice versa, the observed raised MPO activity may be a result of codeine-induced oxidative stress or the other way round. Evidently, codeine-driven oxido-inflammatory response explains the rise in 8-OHdG observed. Excess ROS generation does not allow an attack on the lipid membrane and cytosolic protein; it also results in an attack of the DNA. Oxidative DNA damage causes mutations of the mitochondrial DNA [36]. Similar to the brain, nuclear and mitochondrial DNA are highly susceptible to oxidative stress via ROS production. ROS can trigger damage to the mitochondrial DNA [37, 38], with consequent accumulation of 8-OHdG [13]. Hence, 8-OHdG is an established biomarker of oxidative DNA damage (genotoxicity). The observed increase in 8OHdG in the brain following codeine administration corroborates our previous findings that demonstrated the potential of codeine to stimulate oxidative DNA damage of the testis [13, 14], sperm [15], liver [16], heart [17], and kidney [17].
Our study also shows that codeine-induced redox dysregulation and inflammatory response were associated with elevated caspase 3 activity. Possibly, codeine-induced ROS generation led to an increase in the mitochondrial membrane permeability with resultant mitochondrial failure [39]. This likely culminates in the release of cytochrome c from within the inner mitochondrial membrane into the cytosol in the cytoplasm. It binds to Apaf-1 to form a complex that activates caspase 9 and ultimately caspase 3 [40]. It is also likely that the activated caspase 3 cleaved inhibitor of caspase-activated DNAse I [41, 42] which degrades nuclear chromosomal DNA and causes apoptosis, chromatin degradation, and DNA fragmentation [43].
Codeine-induced oxidative stress and inflammation, as well as caspase-3-mediated apoptosis and DNA damage in the brain explains, at least partly, the observed distorted cytoarchitecture of the brain and depleted hippocampal neuronal cells and Purkinje fibers. The Purkinje cells are focal neurons of the cerebellar cortex and perhaps some of the most conspicuous neurons in the vertebrate central nervous system. All afferent pathways converge on the purkinje cells, while the axons serve as the primary exit of the cerebellar cortex after synapsing on the deep cerebellar nuclei [44]. The projections of the Purkinje cells connect different cerebellar zones. Most of the Purkinje cells release gamma-aminobutyric acid (GABA). Hence, codeine-induced depletion of Purkinje cells impairs the GABAergic terminals with reduced GABA release and increased tonic firing and excitation of the brain-stem that it innervates [45]. Our previous finding that codeine enhances sexual locomotor activity [10] may be due to the inhibitory activity of codeine on GABAergic neurons.
On the other hand, the hippocampus has been established as a brain structure for the formation of long-term memories. Also, the CA2 neurons of the hippocampus have been implicated in the pathogenesis of schizophrenia [46] and neurodegenerative diseases such as Lewy body dementia, Parkinson's disease, Alzheimer's disease, and transmissible spongiform encephalopathies [47]. Observation in the current study that codeine depleted CA2 neurons explains opioid-induced cerebellar dysfunction reported in previous studies [31, 48, 49] and could be attributed to the ability of codeine to induce oxidative stress, inflammation, and apoptosis in the brain tissue.
AchE is a carboxylic ester hydrolase that is an integral part of the cholinergic signaling. It has diverse unrelated biological functions involved in embryogenesis, neuro-modulation and stress response [50]. It is found in neuromuscular junctions and cholinergic synapses where it activates acetylcholine receptors and breaks down acetylcholine to choline and acetate [51], thus terminating synaptic transmission and inhibiting nerve firing at nerve endings. Since it is key for optimal functioning of the central and peripheral nervous system, it is employed as a biomarker of brain injury [51, 52, 53, 54]. Interestingly, our finding that codeine induced neuronal injury, which is accompanied by reduced AchE activity, expands the existing literature that confirms the use of AchE as a biomarker in brain injury. It could also be inferred that the impaired AchE activity following codeine exposure with possible dis-inhibition of synaptic transmission and nerve firing at nerve endings compliments the inhibitory activity of codeine on GABAergic neurons accounts for codeine-induced increased copulatory locomotor activity earlier reported [10]. Thus, it is credible to infer that codeine induces oxido-inflammatory response and apoptosis in the brain tissue and results in brain injury evident by elevated AchE, distorted structural organization of the brain, C2 depletion, capillary dilatation and depletion of Purkinje cells.
5. Conclusion
Summing up, the findings of the current study clearly indicate that codeine exposure could lead to oxido-inflammatory response and apoptosis of the brain tissue associated with neuronal and purkinje cells injury and impaired AchE activity through 8-OHdG and/or caspase 3-dependent pathway. These events are accompanied by reduced brain weight. Hence, the relevance of codeine exposure, particularly as a substance of abuse, should be given more attention.
Declarations
Author contribution statement
Ajayi A.F.: Author name as it appears on the manuscript: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Akhigbe R.E.: Author name as it appears on the manuscript: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ajayi A.F., Akhigbe R.E. The physiology of male reproduction: impact of drugs and their abuse on male fertility. Andrologia. 2020 doi: 10.1111/and.13672. [DOI] [PubMed] [Google Scholar]
- 2.Preuss C.V., Kalava A., King K.C.. Prescription of Controlled Substances: Benefits and Risks. https://www.statpearls.com/ArticleLibrary/viewarticle/40661. [PubMed]
- 3.Manbe D.A. Expert Committee on Drug Dependence; 2008. Crime and Drug Abuse Among Nigerian Youths: A Critical Examination in World Health Organization (WHO) 28th Report (unpublished) [Google Scholar]
- 4.Fareo D.O. Drug abuse among Nigerian adolescents: strategies for counselling. J. Int. Soc. Res. 2012;5(20):341–347. [Google Scholar]
- 5.Barnett J.H., Werners U., Secher S.M. Substance use in a population-based clinic sample of people with first episode psychosis. Br. J. Psychiatr. 2007;190:518–520. doi: 10.1192/bjp.bp.106.024448. [DOI] [PubMed] [Google Scholar]
- 6.Gary A.W., Chan C.K., Quek L., Connor J.P., Saunders J.B., Baker P., Brackenridge C., Kelly A.B. The topography of multiple drug use among adolescent Australians: Findings from the National Drug Strategy Household Survey. Addictive Behaviors. 2013;38(4):2068–2073. doi: 10.1016/j.addbeh.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 7.Katz G., Durst R., Shufman E. Substance abuse in hospitalized psychiatric patients. Isr. Med. Assoc. J. 2008;10(10):672–675. [PubMed] [Google Scholar]
- 8.United Nations Office on Drugs and Crime (UNODC) 2018. Drug Use in Nigeria.www.unodc.org Retrieved from: [Google Scholar]
- 9.Dydyk A.M., Sizemore D.C., Patel B.C., Ronquillo Y, Porter B.R.. Utah Controlled Substance Prescribing. https://www.ncbi.nlm.nih.gov/books/NBK567778/. [PubMed]
- 10.Ajayi A.F., Akhigbe R.E. Assessment of sexual behaviour and fertility indices in male rabbits following chronic use. Andrology. 2020;8:509–515. doi: 10.1111/andr.12717. [DOI] [PubMed] [Google Scholar]
- 11.Achukwu P.U., Omorodion N.T., Tosan E., Aloh H.E., Charles E., Okoyeocha O.M.E. Codeine and its histopathological effect on brain of Albino rats: an experimental study. Acta Sci. Nutr. Health. 2019;3(2):125–133. [Google Scholar]
- 12.INCB . 2012. Comments on the Reported Statistics on Narcotic Drugs Austria; pp. 73–93. [Google Scholar]
- 13.Akhigbe R.E., Ajayi A.F. Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO and caspase 3. PloS One. 2020;15(3) doi: 10.1371/journal.pone.0224052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ajayi A.F., Akhigbe R.E. In vivo exposure to codeine induces reproductive toxicity: role of HER 2 and p53/Bcl-2 signaling pathway. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e05589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ajayi A.F., Akhigbe R.E. Codeine induced sperm DNA damage is mediated predominantly by oxidative stress rather than apoptosis. Redox Rep. 2020;25(1):33–40. doi: 10.1080/13510002.2020.1752003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akhigbe R.E., Ajayi L.O., Adelakun A.A., Olorunnisola O.S., Ajayi A.F. Codeine-induced hepatic injury is via oxido-inflammatory damage and caspase-3-mediated apoptosis. Mol. Biol. Rep. 2020;47(12):9521–9530. doi: 10.1007/s11033-020-05983-6. [DOI] [PubMed] [Google Scholar]
- 17.Akhigbe R.E., Ajayi L.O., Ajayi A.F. Codeine exerts cardiorenal injury via upregulation of adenine deaminase/xanthine oxidase and caspase 3 signaling. Life Sci. 2018;273:118717. doi: 10.1016/j.lfs.2020.118717. [DOI] [PubMed] [Google Scholar]
- 18.Afifi A.K., Bergman R.A. second ed. McGraw-Hill; New York: 2005. Functional Neuroanatomy: Text and Atlas; pp. 201–222. [Google Scholar]
- 19.Eroschenko V.P. 10th ed. Lippincott Williams & Wilkins; Philadelphia, Pennsylvania, USA: 2005. DiFiore's atlas of histology with functional correlations; pp. 1–448. [Google Scholar]
- 20.Ebokaiwe A.P., Adedara I.A., Owoeye O., Farombi E.O. Neurotoxicity of Nigerian bonny light crude oil in rats. Drugs Chem. Toxicol. 2013;36(2):187–195. doi: 10.3109/01480545.2012.710619. [DOI] [PubMed] [Google Scholar]
- 21.Valko M., Leibfritz D., Moncola J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 22.Takeda K., Tsukue N., Yoshida S. Endocrine-disrupting activity of chemicals in diesel exhaust and diesel exhaust particles. Environ. Sci. 2004;11:33–45. [PubMed] [Google Scholar]
- 23.Archibong A.E., Inyang F., Ramesh A., Greenwood M., Nayyar T., Kopsombut P. Alteration of pregnancy related hormones and fetal survival in F-344 rats exposed by inhalation to benzo(a)pyrene. Reprod Toxicol. 2002;16:801–808. doi: 10.1016/s0890-6238(02)00058-8. [DOI] [PubMed] [Google Scholar]
- 24.Meyn M.S. Ataxia-telangiectasia and cellular responses to DNA damage. Cancer Res. 1995;55:5991–6001. [PubMed] [Google Scholar]
- 25.Nicol C.J., Harrison M.L., Laposa R.R., Gimelshtein I.L., Wells P.G. A teratologic suppressor role for p53 in benzo[a] pyrene-treated transgenic p53-deficient mice. Nat. Genet. 1995;10:181–187. doi: 10.1038/ng0695-181. [DOI] [PubMed] [Google Scholar]
- 26.Wilson V.L., Jones P.A. Inhibition of DNA methylation by chemical carcinogens in vitro. Cell. 1983;32:239–246. doi: 10.1016/0092-8674(83)90514-7. [DOI] [PubMed] [Google Scholar]
- 27.Ajayi A.F., Akhigbe R.E. Antispermatogenic mechanism of Trona is associated with lipid peroxidation but not testosterone suppression. J. Hum. Reprod. Sci. 2017;10:124–127. doi: 10.4103/jhrs.JHRS_104_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ajayi A.F., Akhigbe R.E., Ajayi L.O., Adeleye G.S., Adebayo-Gege G.I. Serum and gastric tissue electrolyte levels in carbimazole-treated and levothyroxine-treated male New Zealand white rabbits. World J. Pharm. Pharmaceut. Sci. 2018;7(10):142–155. [Google Scholar]
- 29.Azeez O.M., Akhigbe R.E., Anigbogu C.N. Exposure to petroleum hydrocarbon: implications in lung lipid peroxidation and antioxidant defense system in rat. Toxicol. Int. 2012;19:306–309. doi: 10.4103/0971-6580.103678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hamed M.A., Aremu G.O., Akhigbe R.E. Concomitant administration of HAART aggravates anti-Koch-induced oxidative hepatorenal damage via dysregulation of glutathione and elevation of uric acid production. Biomed. Pharmacother. 2021;137:111309. doi: 10.1016/j.biopha.2021.111309. [DOI] [PubMed] [Google Scholar]
- 31.Atici S. Opioid neurotoxicity: comparison of morphine and tramadol in an experimental rat model. Int. J. Neurosci. 2004;114:1001–1011. doi: 10.1080/00207450490461314. [DOI] [PubMed] [Google Scholar]
- 32.Akhigbe R.E. Discordant results in plant toxicity studies in Africa: attempt of standardization. In: Kuete V., editor. Toxicological Survey of African Medicinal Plants. Elsevier; Amsterdam: 2014. pp. 53–59. Chapter 4. [Google Scholar]
- 33.Juliana F., Joana B., Sandra L., Lu´ıs Pedro A., Jõao L., Roxana M., Od´ılia Q., F´elix C., Oliveira D., Jorge R. Effective analgesic doses of tramadol or Tapentadol induce brain, lung and heart toxicity in wistar Rats. Toxicology. 2017;385:38–47. doi: 10.1016/j.tox.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 34.Akhigbe R., Ajayi A. The impact of reactive oxygen species in the development of cardiometabolic disorders: a review. Lipids Health Dis. 2021;20:23. doi: 10.1186/s12944-021-01435-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gutteridge J.M.C., Halliwell B. Antioxidants in Nutrition, Health, and Disease. Oxford University Press; New York: 1994. Antioxidants: elixirs of life or media hype? pp. 40–62. [Google Scholar]
- 36.Li J.M., Shah A.M. Endothelial cell superoxide generation: regulation and relevance for cardiovascular pathophysiology. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;287(5):R1014–R1030. doi: 10.1152/ajpregu.00124.2004. [DOI] [PubMed] [Google Scholar]
- 37.Cooke M.S., Evans M.D., Dizdaroglu M., Lunec J. Oxidative DNA damage: mechanisms, mutation and disease. Faseb. J. 2003;17(10):1195–1214. doi: 10.1096/fj.02-0752rev. [DOI] [PubMed] [Google Scholar]
- 38.Ratan R.R., Murphy T.H., Baraban J.M. Oxidative stress induces apoptosis in embryonic cortical neurons. J. Neurochem. 1994;62(1):376–379. doi: 10.1046/j.1471-4159.1994.62010376.x. [DOI] [PubMed] [Google Scholar]
- 39.Huang S.H., Lin C.M., Chiang B.H. Protective effects of Angelica sinensis extract on amyloid beta-peptide-induced neurotoxicity. Phytomedicine. 2008;15(9):710–721. doi: 10.1016/j.phymed.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 40.Ghribi O., Herman M.M., Savory J. The endoplasmic reticulum is the main site for caspase-3 activation following aluminum-induced neurotoxicity in rabbit hippocampus. Neurosci. Lett. 2002;324(3):217–221. doi: 10.1016/s0304-3940(02)00147-7. 2002. [DOI] [PubMed] [Google Scholar]
- 41.Sakahira H., Enari M., Nagata S. Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature. 1998;391:96–99. doi: 10.1038/34214. [DOI] [PubMed] [Google Scholar]
- 42.Elmore S. Apoptosis: a review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Porter A.G., Janicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 44.Ellis H. Blackwell; Oxford, UK: 2006. Clinical Anatomy: Applied Anatomy for Students and Junior Doctors; pp. 93–98. [Google Scholar]
- 45.Welsh J.P., Yuen G., Placantonakis D.G., Vu T.Q., Haiss F., O'Hearn E., Molliver M.E., Aicher S.A. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv. Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- 46.Penn D.L. Social cognition in schizophrenia: an overview. Schizophr. Bull. 2008;34:408–411. doi: 10.1093/schbul/sbn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dickson D.W. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology. 1991;41:1402–1409. doi: 10.1212/wnl.41.9.1402. [DOI] [PubMed] [Google Scholar]
- 48.Dai Y.-H., Ou K.-L., Chu P.-W. Cerebellar and oculomotor dysfunction induced by rapid infusion of pethidine. BMJ Case Rep. 2014 doi: 10.1136/bcr-2014-203868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones E., Umansankar U., Mallu H., Hampton T., Kulendran A., Patel M. Lesson of the month: oxycodone-induced leukoencephalopathy: a rare diagnosis. Clin. Med. 2020;20(6):600–602. doi: 10.7861/clinmed.2020-06500-0-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soreq H., Seidman S. Acetylcholinesterase—new roles for an old actor. Nat. Rev. Neurosci. 2001;2:294–302. doi: 10.1038/35067589. [DOI] [PubMed] [Google Scholar]
- 51.Lionetto M.G., Caricato R., Calisi A., Giordano M.E., Schettino T. Acetylcholinesterase as a biomarker in environmental and occupational medicine: new insights and future perspectives. BioMed Res. Int. 2013 doi: 10.1155/2013/321213. Article ID 321213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Donat C.K., Schuhmann M.U., Voigt C., Nieber K., Schliebs R., Brust P. Alterations of acetylcholinesterase activity after traumatic brain injury in rats. Brain Inj. 2007;21(10):1031–1037. doi: 10.1080/02699050701630359. [DOI] [PubMed] [Google Scholar]
- 53.Khan R.A., Khan M.R., Sahreen S. Brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats: efficiency of Sonchus asper. Behav. Brain Funct. 2012;8:21. doi: 10.1186/1744-9081-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Q.-H., Li A.-M., He S.-L., Yao X.-D., Zhu J., Zhang Z.-W. Serum total cholinesterase activity on admission is associated with disease severity and outcome in patients with traumatic brain injury. PloS One. 2015;10(6) doi: 10.1371/journal.pone.0129082. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.