Abstract
Axillary Impella devices are increasingly employed for long-term support of patients with systolic heart failure and shock. Axillary access allows for awake support and ambulation, which carries an inherent risk of disconnection or malposition. We report a series of two cases where device replacement due to dysfunction and malposition can be completed safely through the original axillary graft using axillary graft thrombectomy, given that the clot burden could be a major source of morbidity to the patient.
Keywords: Impella, axillary artery, graft thrombectomy, heart failure
Introduction
Over the last two decades, heart failure has increased in prevalence due to improved patient survival after myocardial infarction, and the aging population. 1 While inotropes are considered the first line of treatment for acute cardiogenic shock, in more severe cases, a form of temporary mechanical support (TMCS) is needed to provide a bridge to more durable ventricular assist devices (VADs) or transplantation. 2 The Impella® has gained popularity in recent years as a less invasive temporary VAD. The larger versions (Impella® 5.0 and 5.5) can be used in cardiogenic shock for up to several weeks, especially with the axillary approach. 3 As the device is used for a longer duration, the risk of device dysfunction or malposition increases. Such an event may require device replacement. We share our experience in a series of two cases of surgically implanted axillary Impella® where malfunction and malposition necessitate device exchange.
Case reports
Patient 1
A 63-year-old male with a past history of hypertension and obesity was transferred to our hospital in cardiogenic shock. He was admitted to an outside hospital with dyspnea, orthopnea, and confusion. Transthoracic echocardiogram showed four-chamber dilatation with global ventricular hypokinesis and a left ventricular (LV) ejection fraction of 15%. Cardiac catheterization demonstrated normal coronary arteries but highly elevated LV end diastolic pressure (30 mm Hg). He also has elevated serum creatinine (2 mg/dL). His initial management included inotropic support, diuresis, and insertion of an Impella® CP (Abiomed, Inc., Danvers, MA, USA) through the right femoral artery.
Over the following 5 days, the patient’s condition deteriorated with persistently low cardiac output, worsening renal function (creatinine = 5.5 mg/dL), hepatic function (aspartate aminotransferase (AST) = 13,189 U/L, alanine aminotransferase (ALT) = 4158 U/L), and persistent hemolysis. Patient was transferred to our institution for escalation of heart failure management. Given his rapid decline and evidence of cardio-metabolic shock, TMCS was switched to Impella® 5.0 through an axillary approach. In the hybrid operating room (OR), a right axillary cut down was performed and a 10-mm Dacron (Hemashield® Platinum; Boston Scientific, Natick, MA, USA) graft was anastomosed to the side of the axillary artery, and then tunneled for 3 cm below the lateral edge of the wound. An Impella® 5.0 left ventricular assist device (LVAD; Abiomed, Inc.) was inserted under fluoroscopy per the manufacturer instructions. The patient condition improved over the next days with normalization of liver function and correction of hemolysis; however, his renal failure persisted and required a renal replacement therapy. On post-operative day (POD) 8, while the patient was getting out of bed, a critical red alarm was noticed due to Impella® red plug being disconnected from the controller connector cable. Attempts to reconnect the plug resulted in bending of one of the pins in the socket leading to the inability to reconnect the device. Therefore, inotropic support was increased and the patient underwent urgent device exchange. Fortunately, we avoided placing the patient on veno-arterial extracorporeal membrane oxygenator (V-A ECMO), which is needed if the patient is unstable. Instead, we increased inotropic support and did the exchange in short period of time. In the OR, the axillary wound was re-explored, the Dacron graft was controlled, and the Impella® device was removed without difficulty. Because of minimal back bleeding from the Dacron graft, we preceded with graft thrombectomy. We passed a #5 Fogarty catheter via the graft into the axillary artery beyond the anastomosis and removed a large organized thrombus from the graft on the first pass. We repeated that several more times until no thrombus was retrieved. Subsequently, a new Impella® 5.0 LVAD was inserted through the tunneled graft in the usual manner. The patient had an excellent recovery with inpatient physical therapy and renal replacement. On POD 40, he received a combined heart–kidney transplant with excellent post-operative course and was discharged home 10 days after transplant. The patient continues to do well 3 months after his double organ transplant.
Patient 2
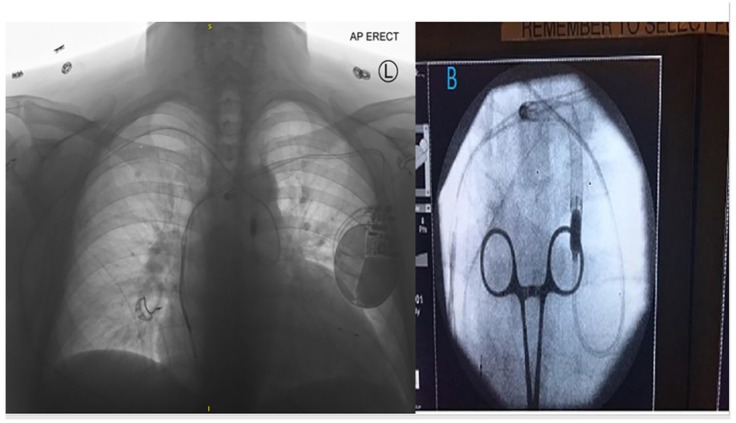
A 65-year-old male with dilated cardiomyopathy was admitted to the hospital as UNOS Status 3 awaiting heart transplantation on inotropic support. Due to secondary pulmonary hypertension and worsening cardiac hemodynamics, he underwent Impella® 5.5 (Abiomed, Inc.) implantation through the right axillary artery. In the OR, a 10-mm Hemashield® Dacron graft was sewn on to the side of the artery. The graft was tunneled for 2–3 cm below and lateral to the main incision. LV guide-wiring and Impella® advancement through the graft was completed per the manufacturer instruction. The patient had an uneventful post-operative course during which his hemodynamics improved. On POD 6, while participating in physical therapy exercises that involved squatting, repetitive movement of the upper extremities and torso, a displacement alarm was noted. The Smart Assist Monitor of the Impella® LVAD system alerted a loss of LV pressure signal. Urgent chest X-ray showed the cannula displaced into the aortic arch with the catheter looped retrograde in the descending aorta (Figure 1). The patient’s hemodynamics were maintained by increasing level of inotropic support, and have not used ECMO support. A trial to reposition the device at the bedside under fluoroscopy failed, and the patient was taken to the hybrid OR for device replacement under fluoroscopy and transesophageal echocardiography guidance. The hemostatic valve anchoring sutures were cut and the Hemashield® graft was pulled out through the skin exit site for approximately 3 cm without reopening the axillary incision. After removing the hemostatic plug and the Impella device, a #5 Fogarty catheter was used to clear the graft of thrombus; this was repeated until no clots were retrieved (Figure 2). Subsequently, a new introducer was inserted and the new Impella® 5.5 was passed through the graft. After that, the hemostatic plug was secured with sutures and the external catheter shaft was fixed at two points using catheter holders in a lazy S fashion. A segment of a Foley catheter was also wrapped and tied around the proximal 10 inches of the catheter shaft to fix it (Figure 3). He was supported with the Impella® 5.5 for a total of 29 days before undergoing a successful heart transplant. The patient continues to do well 2 months after his heart transplant.
Figure 1.
Chest X-ray showing the Impella cannula ejected into the aortic arch with the catheter shaft looped in the descending aorta.
Figure 2.
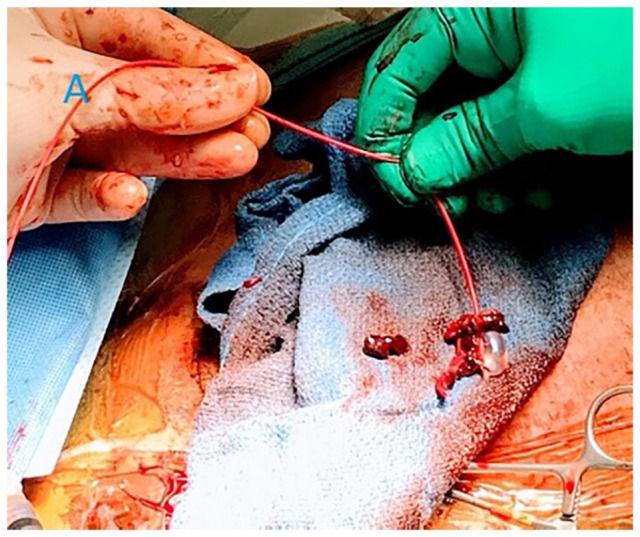
Intra-operative picture showing thrombus retrieved from the axillary graft.
Figure 3.
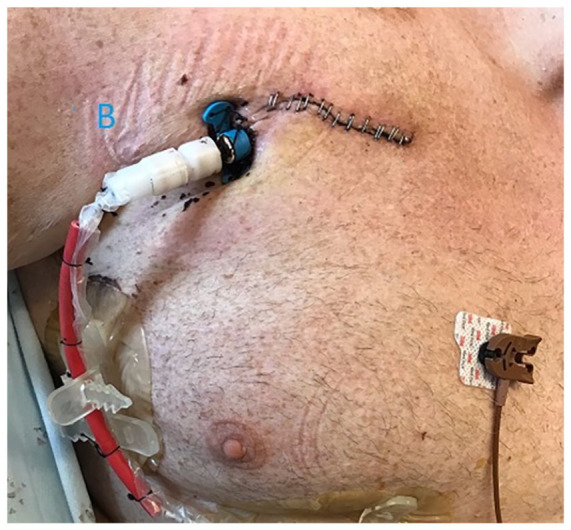
Post-op image showing the final lay out of the external catheter shaft.
None of the patients had stroke, distal arterial embolization, or limb ischemia throughout the duration of support with the Impella® device or afterwards, and the explanted Impella® cannula was free from any clots.
Discussion
Multiple approaches had been described for Impella placement, such as femoral arteries and axillary arteries, some centers use the left axillary approach especially in right-handed people; however, if there is a pacemaker placement in that side, the Impella will be inserted in the right side. Utilization of the right axillary artery for implantation of the Impella® 5.0 and 5.5 is considered the most optimal approach in our institution for the following reasons: the axillary artery is rarely calcified, is spared from arteriosclerosis, and is closely aligned with the LV axis. In addition, such an approach avoids crossing the aortic arch, allows the patient to ambulate and participate in physical therapy, can provide support for longer duration, and may be the only available site due to other invasive catheters and devices. 4 The longer the duration of Impella® LVAD support, the higher the chance of device malfunction, pump thrombosis, malposition, and infection. 5 Infection rates are reported to be around 7% for the axillary approach. 6 Tunneling the graft to exit at 1–2 inches below the main axillary wound may decrease the rate of infection; however, this needs to be investigated further.
Axillary access for TMCS allows patients to mobilize and participate in physical therapy, which has a significant impact on long-term survival. In such patients, modifications of physical therapy regimens may be needed to maintain proper device positioning. Such modifications include avoidance of repetitive upper extremity movements and squatting that may cause migration of the device in and out of the left ventricle, or disconnection of the shaft from the controller. In patient 1, when the catheter shaft was disconnected from the controller, attempts to reconnect the device with excessive force resulted in damage to the pins within the connectors. While in patient 2, the repetitive movements with squatting caused the cannula to be pushed in first, and then ejected to the aortic arch with a redundancy loop in the descending aorta.
When device repositioning is needed, it is important to note that the Impella® cannula and catheter shaft cannot be rewired. The surgeon has to rely on the cannula stiffness as support for repositioning it through the aortic valve and into the LV. If reinsertion fails, the device has to be replaced with a new one. During replacement, one has to be cognizant of the presence of thrombus within the axillary graft. Therefore, it is of utmost importance to perform proper thrombectomy until the graft is cleared of any clots before reinsertion of a new cannula to prevent proximal or distal thrombus embolization. If graft thrombectomy with balloon catheter deemed risky or unsatisfactory, a formal exploration of the axillary artery and graft is warranted. Also, dilating and pushing a transcatheter artery sheath through previous axillary graft would cause the clot burden to break and embolize distally. Even though the device can be reinserted through the sheath, this would not be our recommended approach. Some groups described blocking the artery with a balloon while clearing the graft to control bleeding and distal thermos dislodging. 7
A correctly positioned Impella® 5.5 or 5.0 in the LV is defined by the manufacturer as maintaining a distance of 4.5–5 cm between the cannula blood inlet and the aortic valve. Any shorter distance, even with good flow and acceptable LV pressure signal, predisposes the device to distal ejection. Furthermore, the external catheter shaft has to be firmly secured. We recommend using two catheter holders to maintain the chord in a lazy S configuration and wrapping the proximal 10 inches with a longitudinally cut Foley catheter (or other rubber tubings) and then tied it to the shaft at multiple points (Figure 3). The Impella cannula can be rewired. Especially in patient 2, the cannula could have been engaged with a standard guide-wire from a femoral approach. Alternatively, the Impella could be snared to bring it back to the LV, or a femoral buddy-wire could keep one leaflet open to facilitate LV engagement by the Impella. Both patients were on bivalirudin drip, which discontinued 1 h before surgery and switch to heparin drip with ACT 200 till they are stable, and then converted to bivalirudin.
Conclusion
We described in this report the technique of replacing an axillary Impella® catheter through the tunneled graft with and without re-exploring the main axillary incision. With careful attention to graft thrombectomy, we have shown that this technique is safe and reproducible. The approach of not re-exploring the axillary wound during placement is a valuable option that should be attempted with the new generation Impella® 5.5.
The Impella 5.5 is smaller but not more flexible as compared to the 5.0; instead the cannula is stiffer. Benefits of this approach include reduction in operative time, blood loss, post-operative pain, and infection risk. Appropriate final intra-ventricular pump position, external shaft configuration and fixation, and modification of physical therapy activities should further reduce the risk of malposition or malfunction events.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Our institution does not require ethical approval for reporting individual cases or case series.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Written informed consent was obtained from the patient(s) for their anonymized information to be published in this article.
ORCID iD: Samuel Jacob  https://orcid.org/0000-0001-5656-6474
https://orcid.org/0000-0001-5656-6474
References
- 1. Amat-Santos IJ, Varela-Falcón LH, Abraham WT. Current and future percutaneous strategies for the treatment of acute and chronic heart failure. Rev Esp Cardiol 2017; 70(5): 382–390. [DOI] [PubMed] [Google Scholar]
- 2. Castillo-Sang MA, Prasad SM, Singh J, et al. Thirty-five day Impella 5.0 support via right axillary side graft cannulation for acute cardiogenic shock. Innovations 2013; 8(4): 307–309. [DOI] [PubMed] [Google Scholar]
- 3. Sassard T, Scalabre A, Bonnefoy E, et al. The right axillary artery approach for the Impella Recover LP 5.0 microaxial pump. Ann Thorac Surg 2008; 85(4): 1468–1470. [DOI] [PubMed] [Google Scholar]
- 4. Boll G, Fischer A, Kapur NK, et al. Right axillary artery conduit is a safe and reliable access for implantation of Impella 5.0 microaxial pump. Ann Vasc Surg 2019; 54: 54–59. [DOI] [PubMed] [Google Scholar]
- 5. Khalid N, Rogers T, Shlofmitz E, et al. Adverse events and modes of failure related to the Impella percutaneous left ventricular assist devices: a retrospective analysis of the MAUDE database. EuroIntervention 2019; 15: 44–46. [DOI] [PubMed] [Google Scholar]
- 6. Mastroianni C, Bouabdallaoui N, Leprince P, et al. Short-term mechanical circulatory support with the Impella 5.0 device for cardiogenic shock at La Pitié-Salpêtrière. Eur Heart J Acute Cardiovasc Care 2017; 6(1): 87–92. [DOI] [PubMed] [Google Scholar]
- 7. Tongers J, Flierl U, Sieweke JT, et al. Safe exchange of a transfemoral Impella pump. Cardiovasc Revasc Med 2019; 20(9): 827–828. [DOI] [PubMed] [Google Scholar]