Abstract
Objective
We aimed to assess the relationship between major air pollutants and the natural history and mortality of idiopathic pulmonary fibrosis (IPF).
Methods
We conducted a retrospective cohort study from 2013 to 2019 among 52 patients with IPF from the pneumology department of a tertiary hospital. According to their geocoded residential address, each patient was assigned a mean concentration of carbon monoxide (CO), nitrogen dioxide, particulate matter 2.5 and 10, ozone, and sulfur dioxide, as measured at a single surveillance station in central Madrid, Spain. We analyzed forced vital capacity (FVC), CO diffusing capacity, 6-minute walking test, degree of dyspnea, radiologic pattern, and signs of pulmonary hypertension in all patients.
Results
Patients’ mean age was 66 ± 10 years, and 79% were men. The mean predicted FVC was 78.9 ± 0.5%. Forty-two patients met the criteria for severe disease, and 18 patients died. Mortality was significantly associated with increased CO exposure (for each 0.1 mg/m2 increase: odds ratio 2.45, 95% confidence interval 1.39–4.56). We observed no association between any of the other investigated contaminants and IPF mortality or severity.
Conclusions
Air pollution, specifically that caused by carbon monoxide, can increase mortality in patients with IPF.
Keywords: Idiopathic pulmonary fibrosis, air pollution, disease severity, mortality, carbon monoxide, urban center
Introduction
Interstitial lung disease (ILD) is a highly heterogeneous group of diseases that have common clinical, radiologic, and functional manifestations, which mainly involve alveolar interstitial structures. 1
Several known entities are grouped into ILD, although the causal agent can be identified in only approximately one-third of cases. According to the consensus of the American Thoracic Society (ATS) and the European Respiratory Society (ERS), 2 there are three differentiated groups of ILD. The first includes idiopathic (i.e., of unknown cause) interstitial pneumonia.3,4 The second comprises known-cause ILDs or associated-cause ILDs, including those associated with connective tissue disease; those induced by drugs, organic dust (extrinsic allergic alveolitis), or inorganic dust (pneumoconiosis); and those associated with inherited diseases. The third group includes a set of entities that, despite being idiopathic, have well-defined symptoms and histology (e.g., sarcoidosis, alveolar proteinosis, histiocytosis).5,6
Various pathological mechanisms have been described wherein atmospheric pollutants trigger effects in the respiratory system. Biomass and carbon combustion cause smoke that generates breathable particles and organic compounds such as benzopyrene, formaldehyde, and benzene, 7 which can alter lung defense mechanisms, such as mucociliary clearance and macrophage function.8,9 These particles cause direct bronchial irritation and inflammation and increase oxidative stress owing to DNA alterations.10–12 Similarly, thickening of the tunica intima in the pulmonary vessels has been reported to be greater in individuals exposed to biomass smoke than in smokers. 13 Carbon monoxide (CO) can also be released during combustion and can bind to hemoglobin, creating carboxyhemoglobin, with a consequent reduction in its oxygen-carrying capacity. 14 Nitrogen dioxide (NO2) causes changes in the airway caliber and viscoelastic properties of the lungs and also impairs gas exchange. 15 Particulate matter (PM) refers to particles such as ash, soot, pollen, or cement, with an aerodynamic diameter ranging from 2.5 microns (PM2.5) to 10 microns (PM10). PM affects both superficial and deep respiratory tissues and can even generate allergies, depending on the diameter. Decreased ozone (O3) levels have been associated with lung function disorders. 16 Sulfur dioxide (SO2) results from the combustion of coal and oil, which produces sulfuric acid that forms an aerosol acid that irritates the airways and results in the worsening of diseases such as asthma and chronic obstructive pulmonary disease (COPD). 17
Volatile organic compounds cause direct mucosal irritation, inflammation, and airway obstruction and can induce oxidative stress. These compounds can also enhance sensitization mechanisms through a synergistic effect such that an allergic reaction with less allergen content can occur and can decrease s-nitrosoglutathione, an endogenous bronchodilator. 18
Air pollution can produce numerous short- and long-term adverse effects on human health. The impact of urban air pollution has been associated with an increased incidence of COPD, 19 COPD exacerbations, poorly controlled asthma, and respiratory mortality. 20 It is estimated that for every 10 mg/m3 of PM10, mortality from respiratory disease and cardiovascular disease is increased by 3.4% and 1.4%, respectively. 21 A relationship between the degree of air pollution and COPD severity has been reported. Several papers have published evidence of a relationship between outdoor NO2 values and PM10 and a decreased forced vital capacity (FVC). 22 Long-term exposure to environmental pollution, even at low levels, has been associated with a higher prevalence of respiratory symptoms.
As for asthma, there is also evidence of increased symptoms and bronchodilator use in relation to increased levels of NO2, PM2.5, and PM10.23,24 A systematic review confirmed an increase in the prevalence of asthma among children of 3% per 10 μg/m3-increase in formaldehyde levels. 25
In terms of ILDs, we only found three articles that assessed the effects of pollution. The first was a retrospective study conducted in a South Korean cohort that analyzed the association between exposure to NO2 and O3 and acute exacerbation of idiopathic pulmonary fibrosis (IPF). 26 The second was a study from the United States that examined the impact of PM10 and PM2.5 on the progression of IPF according to the decline in FVC; a significant association with PM10 only was reported. 27 In the third study, a French group assessed the association between PM2.5, PM10, and O3 levels and the progression and exacerbation of IPF, with significant results. 28
The aim of our study was to analyze the relationship between major urban air pollutants (CO, NO2, PM2.5, PM10, O3, and SO2) and clinical, radiologic, and functional severity and mortality of IPF. The length of time needed for high concentrations of CO, NO2, PM2.5, PM10, O3, and SO2 to cause a decline in lung function has not been defined. To date, studies have only assessed the period 6 weeks prior to a relapse. We aimed to focus on pollution levels over an increased period of 12 weeks before an event because it has been demonstrated that measuring mean air pollution over longer periods often results in stronger associations with lung changes. 29 Our study was probably the first of its type to focus on respiratory disease conducted in Spain, specifically in the Community of Madrid.
Methods
We conducted a retrospective cohort study in patients diagnosed with IPF. Two consultants collected the data (a consultant for pulmonary fibrosis and a consultant for other ILDs) through the Department of Pneumology at La Paz University Hospital. The patients included in this study were diagnosed with IPF according to the consensus criteria of the ATS/ERS. The follow-up period was from 2013 to 2019, and the patients were evaluated every 4 months, which corresponded to routine patient visits during their follow-up in our unit. Clinical data were collected during the consultations, including administration of a questionnaire to assess dyspnea level as well as treatments received and diagnosis of other diseases. Patients who did not attend at least one visit or who lived more than 15 km from the surveillance station were excluded, as described in other studies.26,27
Lung function tests during each consultation (spirometry, plethysmography, diffusion capacity of carbon monoxide, and 6-minute walking test [6MWT]) were performed according to standardized ATS/ERS criteria. To this end, we used an integrated module in MasterLab-body 6.0 version equipment (Viasys, Würzburg, Germany).
Computed tomography was performed during both diagnosis and follow-up visits with a computed tomography scanner with 16 detectors (SOMATOM Emotion; Siemens Medical Solutions, Erlangen, Germany). All scans were performed by the same radiology staff. All echocardiograms were performed by the same members of the Department of Cardiology, using Philips IE33 and Philips EPIQ (Philips, Andover, MA, USA) ultrasound machines.
Two IPF severity criteria are necessary to define a severe episode and one of these must be related to worsening lung function. These criteria were chosen on the basis of standards used to refer patients with IPF for lung transplantation 30 :
Increased degree of dyspnea with respect to the previous visit (modified Medical Research Council dyspnea scale).
Absolute decrease in FVC ≥10% of the predicted value compared with the previous visit.
Absolute decrease in diffusion capacity of CO ≥15% of the predicted value compared with the previous visit.
Decrease of >50 m in 6MWT CO compared with the previous visit.
Worsening of the radiologic pattern in high-resolution computed tomography owing to the appearance of new ground glass opacities or increased signs of fibrosis (honeycombing, loss of volume, or traction bronchiectasis).
Signs of pulmonary hypertension on the echocardiogram.
Using air quality control stations, the Integral Air Quality System of Madrid provides hourly reports on the concentrations of CO, NO2, PM2.5, PM10, O3, and SO2 measured in g/m2 except for CO, which is reported in mg/m2. Each patient was assigned air pollutant levels according to those obtained from a single surveillance station in central Madrid. We did not used the inverse distance weighted method because we only used one station. 23 The distance between the surveillance station and each patient's residence was determined using the Google Maps Distance Calculator, after geocoding the patient’s address.
We examined patient variables including age at the time of inclusion, sex, smoking, comorbidities, and scheduled treatments. We compared the pollution data from the station with patients’ health conditions and according to the severity criteria indicated above. Patient mortality was determined by reviewing primary care medical records in which patient outcomes are recorded.
This study was conducted according to the guidelines of the Declaration of Helsinki and was approved by the Institutional Review Board of La Paz University Hospital (protocol code PI-3742, approval date: June 20, 2019). Owing to the retrospective nature of the study, informed consent was not required from patients.
Statistical analysis
Patients were followed from 2013 to death, pulmonary transplantation, or until December 2019. Patients who underwent transplantation were considered alive at the time of transplantation. We used a generalized linear model to assess the impact of air pollution on IPF severity. For each pollutant, the mean observed concentration was compared between each visit, except for the first visit, which was compared with the mean for the previous 90 days. The obtained odds ratio (OR) and 95% confidence interval (CI) was used to estimate the probability of presenting with a severe episode for each 5-unit increase in NO2, PM2.5, PM10, O3, and SO2 and for each 0.1-unit increase in CO. We also used a logistic regression model to estimate the impact of pollution on the likelihood of death. The model was adjusted for the presence of antifibrotic treatment.
All statistical tests were considered bilateral, and p-values <0.05 were considered significant. We analyzed the data using SAS 9.3 (SAS Institute, Cary, NC, USA).
Results
Two of the 55 patients included in the cohort were subsequently excluded because their place of residence was not specified, and one of them lived more than 15 km from the surveillance station. Table 1 lists patients’ baseline characteristics. Patients’ mean age was 66 ± 10 years, and 79% were men. Among the 52 enrolled patients with IPF, 42 experienced at least 1 severe episode during follow-up, and approximately one-third of the sample died. There were no significant differences between patients with severe episodes, those who did not have severe episodes, and those who died in terms of age at the time of inclusion, sex, smoking, comorbidities, or scheduled treatments (Tables 1, 2, and 3).
Table 1.
Patients’ baseline characteristics.
| Variable | |
|---|---|
| Age, years | 66 ± 10 |
| Men | 41 (79) |
| Smoking history | |
| Current | 6 (11.5) |
| Ex-smoker | 17 (32.7) |
| None | 29 (55.8) |
| Packs per year | 31 ± 3 |
| FVC, % pred. | 78.9 ± 10.5 |
| FEV1, % pred. | 84.05 ± 12.51 |
| FEV1/FVC | 81 ± 6.1 |
| Mean duration between each visit, days | 105.1 ± 100.5 |
| Nintedanib | 7 (13.7) |
| Pirfenidone | 40 (78.4) |
| Non-antifibrotic treatment | 4 (7.8) |
| Comorbidities | 24 (46) |
| Prednisone | 13 (25) |
| LTOT | 28 (53.8) |
| Antibiotics | 8 (15.3) |
| Inhaled therapy | 13 (25) |
| Anticoagulation | 9 (17.3) |
*Values are expressed as mean ± standard deviation or number (percentage).
*Any treatment was taken into account from the moment of inclusion.
FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; LTOT, long-term oxygen therapy; pred., predicted.
Table 2.
Patients’ baseline characteristics by severity group.
| Characteristics | Non-severe (n = 10) | Severe (n = 42) | p |
|---|---|---|---|
| Age, years | 72.5 ± 6.0 | 68.8 ± 10.2 | 0.30 |
| Men | 4 (9.8) | 37 (90.2) | 1.00 |
| Smoking history | |||
| Current | 0 (0) | 6 (100) | 0.14 |
| Ex-smoker | 3 (17.6) | 14 (82.4) | 0.34 |
| None | 2 (6.9) | 27 (93.1) | 0.15 |
| FVC, % pred | 72.3 ± 19.1 | 79 ± 12.2 | 0.48 |
| Antifibrotic treatment | 5 (50) | 42 (100) | 0.06 |
| Comorbidities | 2 (8.3) | 22 (91.7) | 0.82 |
| Prednisone | 1 (7.7) | 12 (92.3) | 1.00 |
| LTOT | 1 (3.6) | 27 (96.4) | 0.16 |
| Antibiotics | 0 (0) | 8 (100) | 1.00 |
| Inhaled therapy | 2 (15.4) | 11 (84.6) | 0.38 |
| Anticoagulation | 1 (11.1) | 8 (88.9) | 0.66 |
| Referral to transplantation | 1 (9.1) | 10 (90.9) | 0.69 |
*Values are expressed as mean ± standard deviation or number (percentage).
*Any treatment was taken into account from the moment of inclusion.
FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; LTOT, long-term oxygen therapy; pred., predicted.
Table 3.
Patients’ baseline characteristics by mortality.
| Characteristics | Alive (n = 34) | Died (n = 18) | p |
|---|---|---|---|
| Age, years | 68±9.8 | 71.3±9.9 | 0.30 |
| Men | 25 (61) | 16 (39) | 0.29 |
| Smoking history | |||
| Current | 4 (66.7) | 2 (33.3) | 0.10 |
| Ex-smoker | 8 (41.1) | 9 (52.9) | 0.14 |
| None | 22 (75.9) | 7 (24.1) | 0.13 |
| Comorbidities | 16 (66.7) | 8 (33.3) | 0.34 |
| FVC, % pred | 80.9±12.2 | 73.1±14.4 | 0.29 |
| Antifibrotic treatment | 33 (70) | 14 (30) | 0.059 |
| Prednisone | 9 (69.2) | 4 (30.8) | 1.00 |
| LTOT | 16 (57.1) | 12 (42.9) | 0.14 |
| Antibiotics | 4 (50) | 4 (50) | 0.42 |
| Inhaled therapy | 8 (61.5) | 5 (38.5) | 0.74 |
| Anticoagulation | 4 (44.4) | 5 (55.6) | 0.13 |
| Referral to transplantation | 9 (81.8) | 2 (18.2) | 0.28 |
*Values are expressed as mean±standard deviation or number (percentage).
*Any treatment was taken into account from the moment of inclusion.
FVC, forced vital capacity; FEV1, forced expiratory volume in the first second; LTOT, long-term oxygen therapy; pred., predicted.
Effects of pollution on the severity of idiopathic pulmonary fibrosis
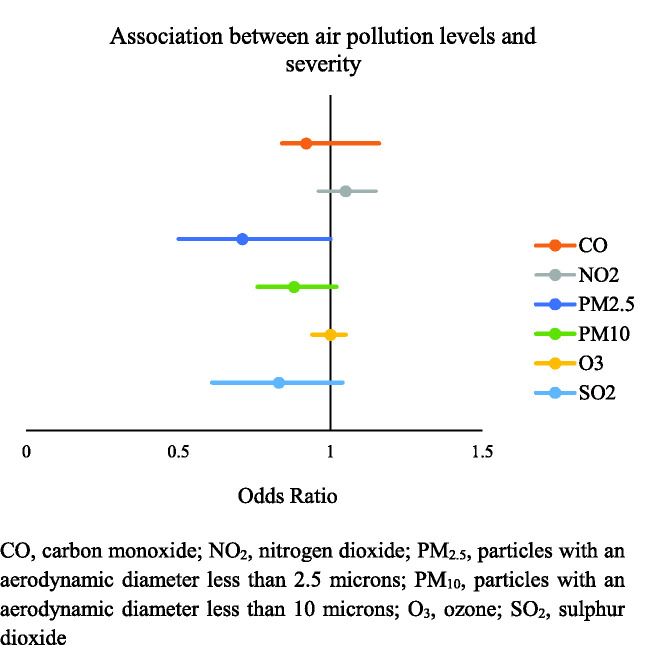
Mean levels of exposure to NO2, PM2,5, and PM10 were 58.18 ± 0.78 μg/m2, 11.21 ± 0.22 μg/m2, and 21.22 ± 0.49 μg/m2, respectively; these were all above the recommended level proposed by the World Health Organization (WHO). 31 Likewise, the presence of severe episodes was not associated with a higher mean concentration of these contaminants (Figure 1).
Figure 1.
Association between exposure to air pollution and disease severity.
CO, carbon monoxide; NO2, nitrogen dioxide; PM2.5, particles with an aerodynamic diameter less than 2.5 microns; PM10, particles with an aerodynamic diameter less than 10 microns; O3, ozone; SO2, sulfur dioxide.
Effects of pollution on idiopathic pulmonary fibrosis mortality
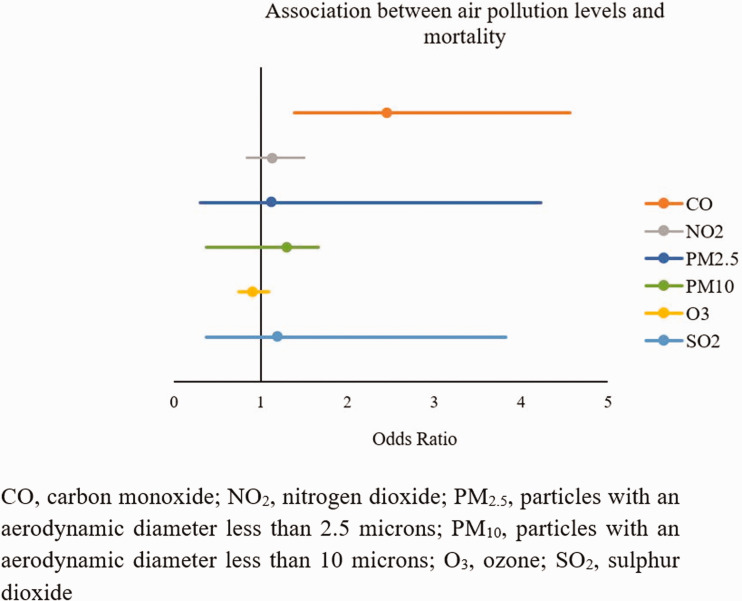
We used records of air pollution from the previous 12 weeks prior to death in the evaluation of mortality. We found that mortality was significantly associated with increased CO exposure (for each increase of 0.1 mg/m2: OR 2.45, 95% CI 1.39–4.56, p = 0.005). With respect to the other investigated contaminants, no association with mortality was detected: NO2: OR 1.13, 95% CI, 0.84–1.50; PM2.5: OR 1.12, 95% CI, 0.30–4.23; PM10: OR 0.78, 95% CI, 0.37–1.66; O3: OR 0.90, 95% CI, 0.75–1.09]; SO2: OR 1.19, 95% CI, 0.37–3.82 (Figure 2).
Figure 2.
Relationship between exposure to air pollution and disease mortality.
Discussion
Pollution is a potentially modifiable risk factor for many respiratory diseases through reducing exposure levels via preventive measures.10–14 Our results suggest that environmental pollutants can increase mortality in patients with IPF.
This study was the first to investigate the effect of air pollution on the severity and mortality of IPF in the Community of Madrid, Spain, taking into account some radiologic criteria and lung function parameters used in other studies26,27 as well as patients’ respiratory symptoms. The results confirmed that medium-term exposure to high CO levels is a risk factor for IPF mortality.
In the current study, our analysis of the impact of air pollution on the natural history of IPF could not be restricted to the same exposure period for all patients given that patients attended visits on different dates. To estimate disease severity, we considered the exposure to be the mean of contaminant levels between each visit, which were related to those patients with criteria for severe IPF at each visit. We found no significant association between pollution levels and IPF severity. These results are consistent with those reported by Sesé et al., 28 who also detected no significant association between pollution levels and IPF severity. This agreement might be related to the small sample sizes in the two studies as compared with the large populations that are typically included in air pollution studies. 20 To improve the quality of such studies, larger sample sizes are necessary, as well as unification of the severity criteria, such as with the introduction of clinical variables in standardized quality-of-life questionnaires.
We hypothesized that mortality could be associated with a high level of exposure to urban air pollutants. We therefore analyzed the exposure to these pollutants during the 12 weeks prior to death so as to account for a possible gap between pollutant exposure and the event. For this purpose, we used other similar studies as reference, such as that by Johannson et al., 26 who analyzed acute exacerbations over 6 weeks. It should be noted that the patients included in our study showed no significant differences in their baseline characteristics (age, sex, smoking, comorbidities, and antifibrotic treatment) (Tables 2 and 3).
The main result of our study was regarding the relationship between medium-term exposure to high CO levels and the risk of IPF mortality. The patients who died were exposed to higher CO concentrations, such that for every 0.1 mg/m2 increase in CO exposure, the risk of death increased 2.45-fold. This was the first study to measure and confirm the influence of CO on mortality in patients with IPF. Similar studies have yielded differing results. Johannson et al. 26 reported no significant association between the levels of various pollutants and mortality whereas Sesé et al. 28 reported this relationship with PM2.5 and PM10. However, those authors did not analyze CO and its possible relationship with mortality. Added to this is the possible difference in pollution levels between Seoul, France, and Madrid; the uneven distribution of surveillance stations in the cited cities and countries; and that the differences in pollutant concentrations in our study were on the basis of peak levels whereas those in the above studies were based on accumulated exposure.
CO is a toxic, odorless, and colorless gas that is produced by the incomplete combustion of hydrocarbons. Sixty percent of the CO detected in Madrid is the result of tobacco combustion and the burning of biomass and fossil fuels, whereas 40% occurs naturally. 32 Prolonged exposure to low CO levels can have adverse effects, especially cardiovascular, neurological, and respiratory effects. 32 We found no published studies that focused on increased CO levels and increased mortality in patients with IPF. Therefore, the results of this study can be applied to different areas, although the findings should be interpreted with caution. Our results can provide the basis for initiatives related to stricter CO control, which can be achieved by increasing the number of surveillance stations measuring this pollutant and introducing measures to reduce CO emissions.
This study has several limitations. First, the sample size was relatively small, which could have prevented the identification of other effects of air pollution. Second, the study was subject to errors in classification of the exposure. The varying distance between patients’ residence and the selected surveillance station could have resulted in misestimation of the actual exposure. Additionally, exposure to air pollution in the home or workplace was not taken into account, which could play an important role in such studies. No information on temperature or relative humidity was available, and no adjustments were made for seasonal periods. Last, the exclusion of patients living in rural areas, where air quality measurements were unavailable, ruled out cases with lower exposures.
In conclusion, the findings of this study suggested that air pollution, specifically that caused by CO, can increase mortality in patients with IPF. These results, which are in line with the WHO recommendations for reducing polluting emissions, require further research. This line of research must be continued using protocols that allow for more accurate quantification of ambient air quality, paying special attention to CO, which could open up new research avenues to analyze the impact of CO on other diffuse ILDs.
Acknowledgment
The authors would like to thank the IdiPaz Institute for their excellent technical assistance.
Footnotes
Author contributions: Conceptualization: P.M., L.G.C., C.P., and R.A.S.; Methodology: P.M., C.C., L.G.C., and R.A.S.; Software: P.M. and C.C.; Validation: C.C., M.I.T., P.M., M.F., and R.A.S.; Formal analysis: P.M. and C.C.; Investigation: L.G.C., C.C., R.A.S.; Resources: C.C., P.M., and R.A.S.; Data curation: P.M. and G.B.; Writing – Original Draft Preparation: P.M. and R.A.S.; Writing - Review and Editing: C.C., P.M., I.E., and R.A.S.; Visualization: C.C., P.M., C.P., and R.A.S.; Supervision: L.G.C., C.C., and R.A.S.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project received a grant for this research from The Spanish Society of Pneumology and Thoracic Surgery (SEPAR and Boehringer Ingelheim) and The Madrid Society of Pneumology and Thoracic Surgery (NEUMOMADRID).
ORCID iD: Pablo Mariscal Aguilar https://orcid.org/0000-0003-2459-3624
References
- 1.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. [DOI] [PubMed] [Google Scholar]
- 2.Xaubet A, Molina-Molina M, Acosta O, et al. Guidelines for the medical treatment of idiopathic pulmonary fibrosis. Arch Bronconeumol 2017; 53: 657–658. [DOI] [PubMed] [Google Scholar]
- 3.Lederer DJ andMartinez FJ.. Idiopathic Pulmonary Fibrosis. N Engl J Med 2018; 378: 1811–1823. [DOI] [PubMed] [Google Scholar]
- 4.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collard HR, Ryerson CJ, Corte TJ, et al. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. An International Working Group Report. Am J Respir Crit Care Med 2016; 194: 265–275. [DOI] [PubMed] [Google Scholar]
- 6.Naccache JM Cadranel J andNunes H.. Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Am J Respir Crit Care Med 2017; 195: 541–542. [DOI] [PubMed] [Google Scholar]
- 7.Samet JM, Avila-Tang E, Boffetta P, et al. Lung cancer in never smokers: clinical epidemiology and environmental risk factors. Clin Cancer Res 2009; 15: 5626–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perez-Padilla R Schilmann A andRiojas-Rodriguez H.. Respiratory health effects of indoor air pollution. Int J Tuberc Lung Dis 2010; 14: 1079–1086. [PubMed] [Google Scholar]
- 9.Smith KR, McCracken JP, Weber MW, et al. Effect of reduction in household air pollution on childhood pneumonia in Guatemala (RESPIRE): a randomised controlled trial. Lancet 2011; 378: 1717–1726. [DOI] [PubMed] [Google Scholar]
- 10.Turner MC, Krewski D, Pope CA, et al. Long-term ambient fine particulate matter air pollution and lung cancer in a large cohort of never-smokers. Am J Respir Crit Care Med 2011; 184: 1374–1381. [DOI] [PubMed] [Google Scholar]
- 11.Lopes FD, Pinto TS, Arantes-Costa FM, et al. Exposure to ambient levels of particles emitted by traffic worsens emphysema in mice. Environ Res 2009; 109: 544–551. [DOI] [PubMed] [Google Scholar]
- 12.Torres-Duque C, Maldonado D, Pérez-Padilla R, et al. Biomass fuels and respiratory diseases: a review of the evidence. Proc Am Thorac Soc 2008; 5: 577–590. [DOI] [PubMed] [Google Scholar]
- 13.Rivera RM, Cosio MG, Ghezzo H, et al . Comparison of lung morphology in COPD secondary to cigarette and biomass smoke. Int J Tuberc Lung Dis 2008; 12: 972–977. [PubMed] [Google Scholar]
- 14.Gillespie-Bennett J, Pierse N, Wickens K, et al. The respiratory health effects of nitrogen dioxide in children with asthma. Eur Respir J 2011; 38: 303–309. [DOI] [PubMed] [Google Scholar]
- 15.Mendell MJ, Mirer AG, Cheung K, et al. Respiratory and allergic health effects of dampness, mold, and dampness-related agents: a review of the epidemiologic evidence. Environ Health Perspect 2011; 119: 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurai J, Noma H, Sano H, et al. Association of short-term ozone exposure with pulmonary function and respiratory symptoms in schoolchildren: a panel study in a western Japanese city. J Med Invest 2018; 65: 236–241. [DOI] [PubMed] [Google Scholar]
- 17.Wigenstam E, Elfsmark L, Bucht A, et al. Inhaled sulfur dioxide causes pulmonary and systemic inflammation leading to fibrotic respiratory disease in a rat model of chemical-induced lung injury. Toxicology 2016; 368: 28–36. [DOI] [PubMed] [Google Scholar]
- 18.Billionnet C, Gay E, Kirchner S, et al. Quantitative assessments of indoor air pollution and respiratory health in a population-based sample of French dwellings. Environ Res 2011; 111: 425–434. [DOI] [PubMed] [Google Scholar]
- 19.Gauderman WJ, Gilliland GF, Vora H, et al. Association between air pollution and lung function growth in Southern California children: results from a second cohort. Am J Respir Crit Care Med 2002; 166: 76–84. [DOI] [PubMed] [Google Scholar]
- 20.Jerrett M, Burnett RT, Pope CA, et al. Long-term ozone exposure and mortality. N Engl J Med 2009; 360: 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu C, Chen R, Sera F, et al. Ambient Particulate Air Pollution and Daily Mortality in 652 Cities. N Engl J Med 2019; 381: 705–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zemp E, Elsasser S, Schindler C, et al. Long-term ambient air pollution and respiratory symptoms in adults (SAPALDIA study). The SAPALDIA Team. Am J Respir Crit Care Med 1999; 159: 1257–1266. [DOI] [PubMed] [Google Scholar]
- 23.Deng Q, Lu C, Li Y, et al. Exposure to outdoor air pollution during trimesters of pregnancy and childhood asthma, allergic rhinitis, and eczema. Environ Res 2016; 150: 119–127. [DOI] [PubMed] [Google Scholar]
- 24.Lu C, Norbäck D, Li Y, et al. Early-life exposure to air pollution and childhood allergic diseases: an update on the link and its implications. Expert Rev Clin Immunol 2020; 16: 813–827. [DOI] [PubMed] [Google Scholar]
- 25.McGwin G Lienert J andKennedy J.. Formaldehyde exposure and asthma in children: a systematic review. Environ Health Perspect 2010; 118: 313–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannson KA, Vittinghoff E, Lee K, et al. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J 2014; 43: 1124–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winterbottom CJ, Shah RJ, Patterson KC, et al. Exposure to ambient particulate matter is associated with accelerated functional decline in idiopathic pulmonary fibrosis. Chest 2018; 153: 1221–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sesé L, Nunes H, Cottin V, et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax 2018; 73: 145–115. [DOI] [PubMed] [Google Scholar]
- 29.Rider CF andCarlsten C.. Air pollution and DNA methylation: effects of exposure in humans. Clin Epigenetics 2019; 11: 131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weill D, Benden C, Corris PA, et al. A consensus document for the selection of lung transplant candidates: 2014–an update from the Pulmonary Transplantation Council of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2015; 34: 1–15. [DOI] [PubMed] [Google Scholar]
- 31.Akbar S, Ross Anderson H, Avaliani S, et al. Air quality guidelines. Global update 2005. Copenhagen, Denmark: World Health Organization Europe, 2006. Available from: https://www.euro.who.int/en/health-topics/environment-and-health/Housing-and-health/publications/pre-2009/air-quality-guidelines.-global-update-2005.-particulate-matter,-ozone,-nitrogen-dioxide-and-sulfur-dioxide. [Google Scholar]
- 32.Buchelli H, Fernández R, Rubinos G, et al. Elevated carboxyhemoglobin: sources of carbon monoxide exposure. Arch Bronconeumol 2014; 50: 465–468. [DOI] [PubMed] [Google Scholar]