Abstract
Mitochondrial autophagy (mitophagy) is the selective clearance of damaged or incomplete mitochondria by autophagy, which is critical for the functional integrity of the entire mitochondrial network and cell survival. Because dysfunction of mitophagy is closely related to many diseases, it is important to study the specific molecular mechanism and pathophysiological significance of mitophagy. FUN14 domain-containing 1 (FUNDC1) is a newly identified mitochondrial outer membrane protein that induces receptor-mediated mitophagy by its interaction with LC3 during hypoxia. The expression, phosphorylation, regulation and significance of FUNDC1 are reviewed in the context of a large number of pathophysiological conditions. Emerging evidence has demonstrated that levels and phosphorylation states of FUNDC1 are closely related to occurrence, progression and prognosis of various diseases including heart diseases and cancers, indicating that FUNDC1 may serve as a promising biomarker and potential therapeutic target.
Keywords: Autophagy, Biomarker, FUNDC1, Heart diseases, Hypoxia, Mitochondria, Mitochondrial ROS, Platelets
Introduction
Mitochondria are critical energy power houses and headquarters of cell apoptosis. Dysfunctional mitochondria lead to increased levels of reactive oxygen species (ROS) and subsequently cause oxidative damage to cellular DNA and proteins.1, 2, 3, 4 Therefore, dysfunctional or superfluous mitochondria must be eliminated to ensure mitochondrial quality and functions. Accumulation of dysfunctional mitochondria is detrimental to cells and organisms, causing a variety of ageing-related diseases, such as cancers, metabolic disorders, Parkinson's disease and cardiovascular diseases.5, 6, 7, 8, 9 Additionally, accumulation of damaged mitochondria is also one of the typical characteristics of the etiology of these diseases.
Autophagy is a fundamental catabolism process for degradation of large macromolecules and organelles through encapsulation by autophagosome with double-membrane structure. Mitochondrial autophagy (mitophagy) is a selective and efficient process of eliminating superfluous or damaged mitochondria by autophagy at the organelle level under stressful conditions such as hypoxia, inflammation, oxidative stress, and irradiation, and acts as a critical mitochondrial quality control mechanism to sustain mitochondrial homeostasis.10, 11, 12, 13, 14, 15, 16
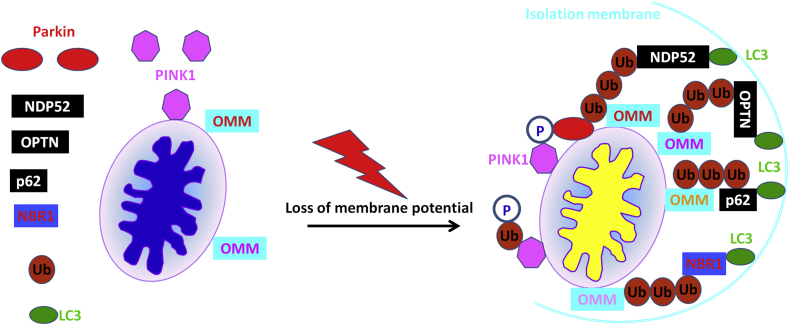
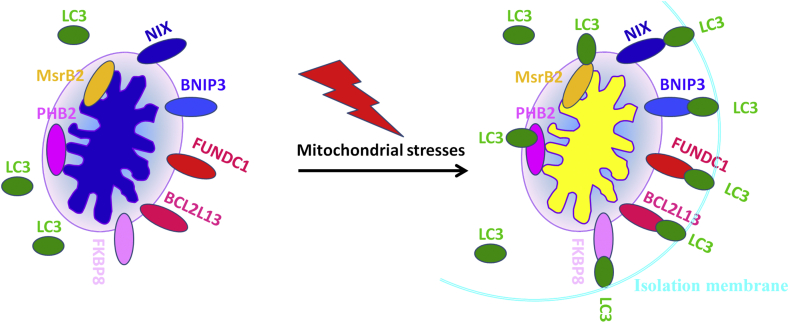
Cells possess different mitophagy mechanisms, and distinct stimuli can induce mitophagy by multiple signaling cascades under various cellular contexts. There are two categories of mitophagy pathways in yeast and mammalian cells: one is driven by Parkin/PTEN-induced putative protein kinase 1 (PINK1)-mediated receptor-independent mitochondrial clearance (Fig. 1) and the other is governed by receptor-dependent mitochondrial autophagy (Fig. 2).6,17, 18, 19, 20, 21, 22, 23 PINK1/Parkin-dependent mitophagy involves dysfunctional mitochondrial recognization by PINK1 and Parkin activation. This results in ubiquitination of outer mitochondrial membrane proteins, which are subsequently recognized by several autophagic receptors including optineurin (OPTN), nuclear dot protein 52 (NDP52), p62 and Neighbor of BRCA1 (NBR1). The autophagic receptors then bridge cargos of mitochondria with microtubule-associated protein 1 light chain (LC3) to induce selective autophagy (Fig. 1).17-25 In comparison, mitophagy receptors including NIX (a homolog of the E1B 19K/Bcl-2 binding and pro-apoptotic protein Nip3), BNIP3 (BCL2 interacting protein 3), BCL2L13 (BCL2 like 13), FKBP8 (FKBP prolyl isomerase 8), PHB2 (prohibitin 2), MsrB2 (methionine sulfoxide reductase B2) and FUN14 domain-containing 1 (FUNDC1) can interact with LC3 to activate selective autophagy of mitochondria (Fig. 2).6,17, 18, 19, 20, 21, 22, 23, 24 Therefore, deciphering the regulatory mechanism underlying mitophagy provides the foundation for us to better understand the pathogenesis of ageing-related diseases and pave new roads for fighting against these incurable diseases currently.
Figure 1.
Parkin/PTEN-induced putative protein kinase 1 (PINK1)-mediated receptor-independent mitochondrial clearance. PINK1 is a serine/threonine protein kinase which is encoded by the PINK1 gene. PINK1 can be activated on the outer mitochondrial membrane under conditions of decrease of mitochondrial membrane potential. The activated PINK1 can phosphorylate a potent E3 ligase, Parkin and ubiquitin simultaneously. The phosphorylated Parkin then induces ubiquitination of several outer mitochondrial membrane-associated proteins (OMM). At the same time, the activated PINK1 can also recruit various autophagy receptors to mediate mitophagy, in which optineurin (OPTN) and nuclear dot protein 52 (NDP52) play primary, yet redundant roles, and p62 and Neighbor of BRCA1 (NBR1) are dispensable for mitophagy mediated by Parkin. Importantly, PINK1-dependent ubiquitin phosphorylation recruits multiple receptors for mitophagy, whereas ubiquitination of mitochondrial protein substrates by Parkin significantly amplifies this signal. Specifically, PINK1 produces a novel and essential phospho-ubiquitin signature on mitochondria to recruit OPTN and NDP52 and induce mitophagy. Parkin enhances the mitophagy signal by producing more ubiquitin chains on mitochondria, which are phosphorylated by PINK1 subsequently. Finally, the phospho-ubiquitin chains engage multiple autophagy receptors to recruit ULK1, WIPI1, DFCP1 and LC3. Yellow and blue mitochondria represent unhealthy and healthy mitochondria, respectively.
Figure 2.
Receptor-mediated mitophagy. Mitophagy receptors including FUNDC1 (UN14 domain containing 1), NIX (a homolog of the E1B 19K/Bcl-2 binding and pro-apoptotic protein Nip3), BNIP3 (BCL2 interacting protein 3), BCL2L13 (BCL2 like 13), FKBP8 (FKBP prolyl isomerase 8), PHB2 (prohibitin 2) and MsrB2 (methionine sulfoxide reductase B2) have been confirmed to mediate mitophagy by their interaction with LC3 in various mammalian cells and yeast under stress conditions. Furthermore, FUNDC1 has been confirmed to mediate mitophagy in mice platelets in vivo. However, whether NIX, BNIP3, BCL2L13, FKBP8, PHB2 and MsrB2 can also play important roles in eliminating damaged or unwanted mitochondrion in platelets in vitro or in vivo remain to be investigated. Yellow and blue mitochondria represent unhealthy and healthy mitochondria, respectively.
FUNDC1 is a newly identified mitochondrial-localized protein with conserved sequences from Drosophila melanogaster to Homo sapiens.7, 8, 9, 10 FUNDC1-mediated mitophagy serves as a primary regulatory mechanism of the mitochondrial quality control that is fundamental for cellular homeostasis and function under (patho)physiological settings. It is necessary to point out that knockdown of FUNDC1 has no effect on starvation-induced LC3 conversion, but only partially prevents mitophagy induced by the uncoupler carbonylcyanide p-trifluoromethoxy-phenylhydrazone (FCCP),10 suggesting that selective mitophagy rather than general autophagy is induced by FUNDC1. Another pivotal issue is how FUNDC1-required mitophagy is initiated and regulated in pathophysiological settings.7, 8, 9, 10 Specifically, what are the mitochondrial sensors which can sense and initiate FUNDC1-mediated mitophagy in response to a number of mitochondrial stresses? Currently, it has been demonstrated that post-translational modifications including protein phosphorylation/dephosphorylation are implicated in the regulation of mitophagic processes. Additionally, phosphorylation/dephosphorylation of mitophagy receptors plays an important role in either inhibiting or enhancing mitophagy in a manner of context-dependent mode.7, 8, 9, 10 Therefore, it would be very interesting to uncover how FUNDC1-required mitophagy is initiated and activated in response to a variety of cellular and environmental cues, particularly in different diseases conditions.
The expression, phosphorylation, regulation and significance of FUNDC1, a key mitochondrial outer membrane protein that induces receptor-mediated mitophagy by its interaction with LC3 during hypoxia, is reviewed in the context of various pathophysiological conditions. Emerging evidence has demonstrated that levels and phosphorylation states of FUNDC1 are closely related to occurrence, progression and prognosis of various diseases particularly pan-cancer and heart diseases, indicating that FUNDC1 may serve as a promising biomarker and potential therapeutic target.
Identification and function of FUNDC1 in mitochondria
Mitochondrion is one of the important specialized organelles in eukaryotic cells for oxygen consumption and adenosine triphosphate (ATP) production through oxidative phosphorylation and electron transport chains.1, 2, 3, 4 As biosynthetic and bioenergetic organelles, mitochondria are pivotally involved in calcium signaling, redox homeostasis, inflammatory responses and cell apoptosis. They are also sources of ROS. Low levels of ROS may serve as signaling molecules involved in cell cycle, growth and proliferation, whereas excess ROS, as an inevitable by-product, may result in oxidative stress to organelles, cells and organisms. Mitophagy is an important mechanism for maintaining both the quality and quantity of mitochondria.1,7, 8, 9 Compelling evidence is emerging to support dysregulated mitophagy having multiple functions in aging-related diseases such as Parkinson's disease, Alzheimer's disease, diabetes mellitus, metabolic abnormality syndrome, and cardiovascular diseases (especially acute myocardial infarction).1,7, 8, 9 What is unknown is how individual mitochondrion is recognized for autophagic degradation and how this process is initiated and regulated under various disease contexts. Therefore, these unsolved problems of FUNDC1-dependent mitophagy direct our efforts to figure out a novel mechanistic insight into its pathological role in ageing-related diseases, which may also provide insights for designing new therapies to treat and/or prevent aging-induced disorder development and progression.
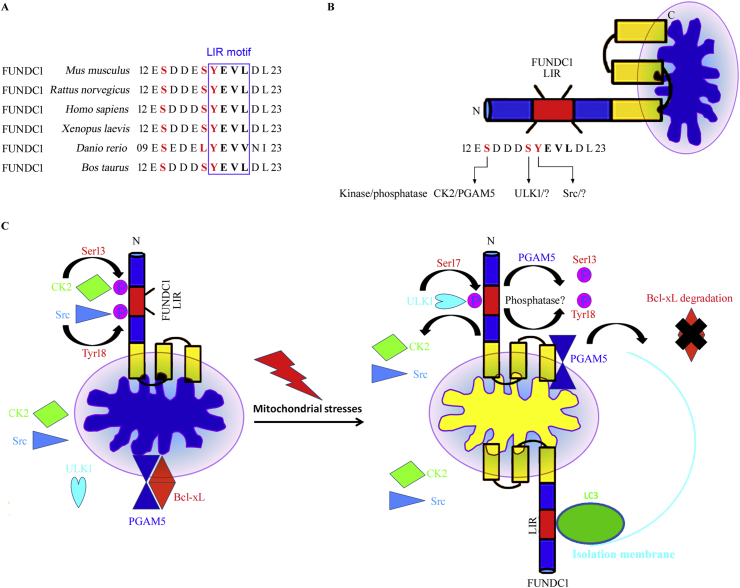
FUNDC1 localized on the mitochondrial outer membrane was first identified as a novel mitophagy receptor by our group in 2012.10 The FUNDC1 of human contains 155 amino acids encoding three conserved α-helical stretches with high hydropholicity, in which there are a C-terminal region, a N-terminal region exposed to the cytosol and an intermembrane space. A segment of LC3-interacting region (LIR) in the cytosol-exposed N-terminal region is responsible for the interaction with LC3 to induce mitophagy.10 Mutations or deletions of the conserved LIR-motifs of FUNDC1 inhibit its interaction with LC3 and the subsequent mitophagy. Although the LIR structures of different mitophagy receptors are different, they all conform to the general structural formula: A-XX-B, where A is an aromatic amino acid (W/F/Y), B is an aliphatic amino acid (L/I/V), and X can be any amino acid. Both the A and the B in LIR insert into the hydrophobic pockets deep on the LC3 surface. Specifically, the exact structure of LIR in FUNDC1 is Y18-E19-V20-L21. This LIR structure of FUNDC1 initiated mitophagy by docking with Y and L pocket of the LC3 protein through hydrophobic action, which could be eliminated by knockdown or mutation of the LIR motif. FUNDC1-mediated mitophagy is extremely dependent on the physical interaction between the typical LIR motif and LC3 (Fig. 3).5,8,9
Figure 3.
Regulatory mechanism of mitochondrial stresses (hypoxia, et al)-induced FUNDC1-dependent mitophagy in mammalian systems. (A) Conserved LIR domain sequences between different species are demonstrated. (B) Schematic representation of the mitochondrial location, structure and reversible phosphorylation of FUNDC1 at the illustrated critical amino acide residues in the mitochondrial outer membrane region. (C) Under physiological conditions, the protein kinases of casein kinase 2 (CK2) and Src phosphorylate FUNDC1 at Ser13 and Tyr18, respectively, therefore suppressing the interaction between FUNDC1 and LC3 and FUNDC1-mediated mitophagy. BCL-XL interacts with and blocks PGAM5 under normoxia conditions. Under mitochondrial stress conditions, such as hypoxia treatment or uncoupler carbonylcyanide p-trifluoromethoxy-phenylhydrazone (FCCP) stimulation, CK2 and Src cannot phosphorylate FUNDC1 at Ser13 and Tyr18, whereas phosphoglycerate mutase family member 5 (PGAM5) is released from BCL-XL, promoting the dephosphorylation of FUNDC1 at Ser13 to enhance FUNDC1-mediated mitophagy. Simultaneously, ULK1 can interact with and phosphorylate FUNDC1 at Ser17, thus promoting the FUNDC1–LC3 interaction and the subsequent autophagic degradation of mitochondria. However, the phosphatases responsible for the dephosphorylation of FUNDC1 at Ser13 and Tyr18 remain to be explored.
Mutation or knockdown this LIR domain inhibited the formation of autophagosome membrane. Knockdown of endogenous FUNDC1 inhibited hypoxia-triggered mitophagy, which was able to be reversed by the expression of wild-type FUNDC1, but not the LIR-deletion FUNDC1 mutant. Mitophagy induced by over-expression of FUNDC1 or hypoxia was significantly inhibited in Autophagy-Related Gene 5 (ATG5) knockdown cells, whereas Autophagy-Related Gene 6 (ATG 6)/BECLIN1 knockdown did not suppress FUNDC1-induced mitophagy, suggesting that FUNDC1-induced mitophagy is ATG-5-dependent but BECLIN1-independent.10,25,26 However, how various stresses are detected to initiate and activate FUNDC-mediated mitophagy remains to be defined.
Post-translational modification of FUNDC1 in mitophagy
Next, the question of how the interaction of FUNDC1 with LC3 is tightly and specifically regulated by its characteristic LIR motif in response to stress stimuli was addressed. Under normoxia conditions, FUNDC1 was stable in the mitochondrial outer membrane without occurrence of mitophagy.10 Therefore, beyond protein level regulation, cells must have other fine mechanisms to inhibit or activate FUNDC1 in the regulation of mitophagy.
Phosphorylation of FUNDC1
To further understand the interaction of FUNDC1 with LC3 and the mechanism of mitophagy regulation, numerous spectral analyses were conducted and the results revealed that there were both phosphorylation and dephosphorylation states of FUNDC1.10 Phosphorylation and dephosphorylation of proteins, which are dynamic and reversible processes mediated by protein kinases and phosphatases, respectively, play an important regulatory role in numerous biology events in eukaryotic cells. Therefore, the possible post-translational regulation of phosphorylation in FUNDC1 was explored.10,27 The analysis of mass spectra indicated that Tyrosine 18 (Tyr18) in the conserved LIR domain was a potential phosphorylation site of FUNDC1 and the phosphorylation level of FUNDC1 at Tyr18 was down-regulated in response to hypoxia treatment or depolarization of mitochondrial membrane potential. The Western blotting results employing an antibody specifically against the phosphorylated FUNDC1 at Tyr18 demonstrated that the endogenous FUNDC1 in HeLa cells exhibited a phosphorylated form under normoxia, whereas the expression level of the phosphorylated FUNDC1 was dramatically downregulated upon hypoxia treatment. To explore the phosphorylation regulation mechanism of FUNDC1 in mitophagy, several scholars have investigated FUNDC1-related kinases.10,27,28
Protein kinase Src and FUNDC1
Computational analysis and bioinformatics studies predicted that the Src kinase located at mitochondria might be responsible for the phosphorylation of FUNDC1 at Tyr18. Src kinase is a tyrosine kinase and a regulatory protein which is pivotally involved in cell growth, differentiation, proliferation and survival. The data uncovered that inactivation of Src kinase, evidenced by the dephosphorylation of Tyr416, was markedly related to the dephosphorylated FUNDC1 and the induction of mitophagy after hypoxia treatment.10 Further research revealed that it was the active form of Src, not the kinase-dead form of Src, that could directly phosphorylate FUNDC1 at Tyr18, but not at Ser17, Ser13 or Tyr11 of FUNDC1. The in vitro catalytic reaction with Src and FUNDC1 further showed that Src was the direct protein kinase of FUNDC1. Ectopic expression of wild type Src dramatically prevented the formation of the Cherry-LC3 punctum caused by wild type FUNDC1, but not the FUNDC1 mutant of Y18W. The mutant FUNDC1 with Y18W could not be phosphorylated even in the presence of wild type Src. Furthermore, Src suppressed mitophagy induced by FUNDC1 in response to hypoxia stimulation. In addition, LC3 preferentially interacted with the FUNDC1 in dephosphorylated form because phosphorylated FUNDC1 may conflict with the hydrophobic pocket of LC3 and eliminate its binding affinity to LC3.10,25 After exposure to hypoxia, FUNDC1 undergoes conformational modification via dephosphorylation, which may attenuate the steric hindrance for LC3 interaction,10,25,26 resulting in the co-localization between FUNDC1 and LC3-II. Collectively, phosphorylation of Tyr18 in the characteristic LIR motif of FUNDC1 by Src kinase under the physiological normoxia conditions inhibits FUNDC1-mediated mitophagy. In contrast, dephosphorylated FUNDC1 and the inactivated Src kinase under the hypoxia conditions lead to a dramatic increase of the interaction and co-localization between FUNDC1 and LC3-II, driving the selective incorporation of the damaged mitochondria into the LC3-bound isolation membrane for the autophagic degradation of mitochondria by autolysosomes with positive lysosomal associated membrane protein 1. These findings highlight the latest mechanistic insights into how the mitochondrial outer membrane protein acts as a mitophagy receptor to fine-tune the core autophagic machinery and how FUNDC1-required mitochondrial autophagy is controlled by Src. Notably, the dephosphorylation-dependent binding of the LIR of FUNDC1 with LC3 enhances hypoxia-induced mitophagy, which is different from that of other LIR-containing receptors because the dephosphorylation of autophagy receptors usually inhibits the binding affinities of LC3 and suppresses autophagy (such as BCL2/adenovirus E1B 19 kDa protein-interacting protein 3 and Parkin).6,10,17, 18, 19, 20, 21, 22, 23
Protein kinase CK2 and FUNDC1
Mass spectrometric analyses further revealed that Serine 13 (Ser13) of FUNDC1 was also phosphorylated in addition to Tyr18.27 Thus, the molecular details of FUNDC1-mediated mitophagy through the phosphorylation of FUNDC1 at Ser13 were focused on. It was found that either hypoxia treatment or FCCP administration induced a dramatic dephosphorylation of FUNDC1 at Ser13, an effect that was followed by an increase in LC3-II expression as well as a decrease in the levels of mitochondrial proteins (such as Tim23 and Tom20), similar to the actions induced by FUNDC1 dephosphorylation at Tyr18. The post-transcriptional dephosphorylation modification of FUNDC1 at Ser13 was reversible for that removal of FCCP restored mitochondrial potential accompanied with enhanced interaction between FUNDC1 and LC3. Next, the protein kinase responsible for the phosphorylation of FUNDC1 at Ser13 was investigated. The sequence alignment and analysis indicated that the acidic amino acids around Ser13 were highly conserved and conformed to the signature motif of casein kinase 2 (CK2), a constitutive protein serine/threonine kinase. The catalytic subunits of CK2 could phosphorylate FUNDC1 in vitro. Under physiological conditions, depletion of the two catalytic subunits α1 and α2 of CK2 significantly abolished its capacity to phosphorylate FUNDC1 at Ser13 and enhanced FUNDC1-mediated mitophagy, confirming that CK2 is the protein kinase responsible for the phosphorylation of FUNDC1 at Ser13.
The regulatory mechanism of FUNDC1-required mitophagy via phosphorylation of Ser 13 and Tyr18 is addressed.10,27 Both the phosphorylated Ser13 and the phosphorylated Tyr18 cooperated functionally to control mitophagy mediated by FUNDC1, although these two phosphorylation sites are regulated by different protein kinases and distinct mechanisms. Tyr18 phosphorylation of FUNDC1 may be a molecular switch for mitophagy mediated by FUNDC1. The suppression of either of the two kinases, CK2 and Src, was not able to activate FUNDC1-mediated mitophagy sufficiently, but the coordinated inhibition of these two kinases significantly induced mitophagy.
ULK1 and FUNDC1
Although unc-51 like autophagy activating kinase 1 (ULK1) is highly involved in the induction of autophagy, its mechanism and substrate(s) in mitophagy is still unknown. In addition, ULK1, a Ser/Thr kinase required for the formation of the early autophagosome,28 was also involved in the regulation of FUNDC1-required mitophagy.10,27, 28, 29 Both ULK1 and the newly identified mitophagy receptor FUNDC1 are needed in mitophagy upon hypoxia or FCCP. However, whether and how FUNDC1 is regulated by upstream signals of ULK1 to induce receptor-dependent mitophagy collaboratively also remains to be explored. Interestingly, the expression and activity of ULK1 increased and it translocated to dysfunctional fragmented mitochondria upon induction of pronounced mitophagy in response to either mitochondrial uncouplers or hypoxia. Cellular phosphoproteome analysis and mass spectrometric analysis indicated that Serine 17 (Ser17) of FUNDC1 is a potential phosphorylation site. Ectopic expression of ULK1 significantly enhanced the phosphorylation of Ser17 of FUNDC1, whereas knockout of ULK1 markedly abrogated Ser17 phosphorylation of FUNDC1.28 ULK1 could physically interact with FUNDC1 at mitochondria and phosphorylate FUNDC1 at Ser17 to promote the binding of FUNDC1 to LC3, resulting in enhanced autophagic degradation of mitochondrial proteins. A FUNDC1 mutant with ULK1-binding-deficiency inhibited the translocation of ULK1 to mitochondria and prevented mitophagy.28 Myc-ULK1 immunoprecipitated from cells could phosphorylate FUNDC1 at Ser17 in vitro. In addition, the expression level of the phosphorylated FUNDC1 at Ser17 was upregulated by ULK1 in a time-dependent manner in response to hypoxia or FCCP and there was a close correlation between the levels of the phosphorylated Ser17 of FUNDC1 and the expression of ULK1. ULK1 phosphorylated FUNDC1 at damaged mitochondria to promote mitophagy by the enhancement of the interaction of FUNDC1 with LC3, which is an important process for linking autophagosomes and fragmented mitochondria. It should be noted that the phosphorylation of FUNDC1 at Ser17 enhances mitophagy while the phosphorylation of FUNDC1 at both Tyr18 and Ser13 inhibits mitophagy under hypoxia and FCCP treatment conditions. Most importantly, both a phosphor-mimicking FUNDC1 mutant and a kinase-active ULK1 could rescue FUNDC1-dependent mitophagy in ULK1-null cells. Collectively, FUNDC1 can recruit ULK1 to the dysfunctional mitochondria, where ULK1 phosphorylates FUNDC1 at Ser17 to accelerate mitophagy.
Structural basis on FUNDC1
Although several functional investigations on the post-translational modification, particularly phosphorylation in FUNDC1-dependent mitophagy have been conducted extensively, the effect of phosphorylation on the interaction of FUNDC1 with LC3-II is not sufficiently explored from a point of view of structure.25,26
The structural basis of the phosphorylation of LIR in FUNDC1 was investigated by Kuang, et al by employing nuclear magnetic resonance (NMR).25 The solution structure of a peptide mimicking the LIR Y (18)EVL (21) of FUNDC1 in complex with LC3 has been solved to explore the exact molecular details of how the phosphorylation regulation is involved in FUNDC1-required mitophagy. The reversible phosphorylation modification of the amino acid Tyr18 in the LIR of FUNDC1 was revealed as a novel molecular switch of FUNDC1-mediated mitophagy in the functional and structural analyses. The dephosphorylated FUNDC1 in the cytosolic portion was both necessary and sufficient to induce mitophagy. The characteristic LIR domain of FUNDC1 was exposed to the cytosol and located within the 50 amino acid residues in the N-terminal. This cytosolic portion of FUNDC1 was confirmed to function as a degron for the initiation of mitophagy by the recruitment of LC3-positive phagophore membranes, and the reversible phosphorylation regulation was critical for the switching of FUNDC1-dependent mitophagy, in which the dephosphorylation of Tyr18 is the most important factor while the role of phosphorylation at Ser13 is limited during the initiation of mitophagy. The typical LIR domain of FUNDC1 bound to LC3 in a manner of noncanonical conformation. The solution structure of LC3 in complex with a synthesized peptide (Residues 10–26 of FUNDC1; F-pep) containing 17 amino acids and mimicking the LIR motif of FUNDC1 was determined by applying NMR spectroscopy, in which 34 H-bond restraints, 182 dihedral angle restraints and 5050 nuclear Overhauser effect (NOE) distance restraints were finally used in the complex structure. The Tyr18 of the hydroxyl group in the side-chain of FUNDC1 was located near the side-chain carboxyl group in the Asp19 of the LC3 HP1, whereas Ser13 of the LIR of FUNDC1 was positioned in the hydrophobic part between the side chain of Lys51 and Lys49 of FUNDC1. The phosphorylation states of the Tyr18 in the characteristic LIR domain of Y (18)EVL (21) played central roles in the regulation of the FUNDC1 binding affinity with LC3 and controlled the inducing activity of FUNDC1-mediated mitophagy. In comparison, the binding affinity of Ser13 in FUNDC1 did not change significantly, so Ser13 of FUNDC1 may be auxiliary in promoting FUNDC1-mediated mitophagy. Therefore, the phosphorylation of Tyr18 in LIR of FUNDC1 may serve as an important molecular switch in FUNDC1-mediated mitophagy, which may represent a new therapeutic target for fighting diseases.
The structural basis of the post-translational modification mechanism of FUNDC1 which affects the binding affinity of FUNDC1 for LC3-II was further investigated by using crystal structure analysis of X-ray crystallography.26 The crystal results of LC3-II in complex with a peptide encompassing phosphorylated Ser17 and mimicking the LIR domain of FUNDC1 demonstrated the critical amino acid residues of LC3-II in the recognition of the phosphorylated/dephosphorylated FUNDC1. The side chain of the LC3-II Lys49 significantly shifted and produced an important hydrogen bond and electro-static binding affinity with the phosphate group of the phosphorylated Ser17 in FUNDC1. Additionally, the structural and mutational analyses indicated that Lys49 in LC3-II was pivotal for sensing the phosphorylation status of Ser17 of FUNDC1 in FUNDC1-mediated selective mitophagy. These data collectively suggest that Lys49 of the LC3 not only controls its binding to LIR of FUNDC1 as a molecular switch, but also is responsible for the specific recognition of the phosphorylation modification of FUNDC1, which is critical for the formation of autophagosome and the subsequent removal of the damaged mitochondria by selective autophagy. Future studies should focus on whether and how the LC3 Lys49 is involved in the pathogenesis of distinct diseases. Importantly, both the phosphorylated Tyr18 and Ser13 of FUNDC1 markedly inhibited their bindings to the Arg10 and hydrophobic pocket of LC3-II. Thus the biochemical and structural findings support a model for the recognition of FUNDC1 by LC3-II specifically and indicate that the reversible phosphorylation and dephosphorylation regulation of the FUNDC1-mediated mitophagy may serve as a switch for the selective autophagic degradation of the damaged mitophagy by lysosome. It will be very interesting to know whether there is gene polymorphism of FUNDC1 in humans, and if so, whether the pathogenesis of patients is associated with mutation or deletion of LIR of FUNDC1.
In vivo analyses of the interaction between FUNDC1 and LC3 and in vitro structural studies are needed to elucidate a detailed regulatory mechanism of the exact recognition of FUNDC1 by LC3-II and provide useful information of how FUNDC1-mediated mitophagy employs the post-translational phosphorylation modification to sense environmental clues and elaborately regulate mitochondrial homestasis by mitophagy in a large number of disease settings. However, how FUNDC1 responds to different degrees and different forms of mitophagy needs to be further discussed when interaction with LC3.
Dephosphorylation of FUNDC1
PGAM5 and FUNDC1
Dephosphorylation states of FUNDC1 indicate that there is a phosphatase responsible for the dephosphorylation of FUNDC1. Numerous phosphatases related to hypoxic stress or other stress responses were screened and examined. It was identified that the phosphoglycerate mutase family member 5 (PGAM5), a serine/threonine phosphatase located at mitochondria, can interact with and then dephosphorylate LIR motif of FUNDC1 at Ser13 upon FCCP treatment or hypoxia stimulation.10,27, 28, 29, 30, 31 The dephosphorylated form of FUNDC1 at Ser13 catalyzed by PGAM5 promoted the interaction between FUNDC1 and LC3, which could be abrogated by the introduction of a cell-penetrating peptide encompassing unphosphorylated Ser13 of LIR in FUNDC1 or knockdown of PGAM5. Reciprocal immunoprecipitate experiments and mutants assays further supported the direct interaction of FUNDC1 with PGAM5 through dephosphorylation modification at Ser13.
The effect of PGAM5 in activation of FUNDC1-dependent mitophagy could be reversed by the phosphorylation of FUNDC1 by CK2. Therefore, a mechanistic and regulatory signaling loop comprising the phosphatase PGAM5 and CK2 links mitochondrial stress signals to the reversible phosphorylation/dephosphorylation of FUNDC1 to sense, initiate, induce and fine-tune mitophagy in response to mitochondrial stresses.
The remaining unanswered question is what signal specifically activates the FUNDC1/PGAM5 axis and how the cells sense the mitochondrial stress signal in initiation of mitophagy. Previous investigations have proved that Bcl-xL can interact with PGAM5 directly to regulate cell apoptosis. In-depth study further exhibited that both hypoxia and FCCP could attenuate the interaction of Bcl-xL/PGAM5, resulting in the release of PGAM5 to activate mitophagy. These mitochondrial stresses enhance the interaction between PGAM5 and FUNDC1, and promote the dissociation of the Src and CK2 kinases from FUNDC1. A seesaw model of the association between FUNDC1 and PGAM5 and with the Src and CK2 kinases was proposed. Therefore, hypoxia or FCCP treatment induces the translocation of the catalytic subunits of CK2 into the cell nuclei and accordingly reduces the phosphorylation of FUNDC1 by CK2 at the outer mitochondrial membrane. The relevant phosphatase responsible for the dephosphorylation of FUNDC1 at Tyr18 or at Ser17 has yet to be identified.
BCL2L1 and FUNDC1
One of the critical issues not addressed is how FUNDC1-mediated mitophagy is modulated in response to different cellular and environmental cues through Bcl-xL/PGAM5. Notably, activation of PGAM5 is strongly associated with an anti-apoptotic protein, Bcl2 Like 1 (BCL2L1). The Bcl2 family controls mitochondrial apoptosis, mitophagy, and mitochondrial homeostasis.30, 31, 32, 33, 34, 35 It is Bcl2L1, and not Bcl2, that interacts with PGAM5 to inhibit PGAM5 and prevent FUNDC1 from being phosphorylated at the Ser13 binding site through its BH3 domain, thereby inhibiting hypoxia-induced mitophagy. The phosphorylation of FUNDC1 is regulated by the binding of Bcl-xL to PGAM5 to suppress the phosphatase activity of PGAM5, preventing FUNDC1 from its interaction with LC3. Under normoxia conditions, Bcl2L1 interacts with PGAM5 through the BH3 domain and inhibits PGAM5 activation, which in turn prevents Ser13 dephosphorylation. Under hypoxia conditions, BCL2L1 degrades and PGAM5 is released, promoting Ser13 dephosphorylation, thereby initiating FUNDC1-mediated mitophagy. These results collectively show that the Bcl2L1-PGAM5-FUNDC1 axis is pivotal to responding to hypoxia-induced mitophagy. Future investigations will be very interesting to focus on how cells sense external stimuli to regulate dephosphorylation states of FUNDC1 under a variety of pathophysiological conditions. It also will be very exciting to see whether there is the polymorphism of PGAM5/BCL2L1 in healthy human and patients, and if so, whether the polymorphism is the pathogenesis of the diseases.
FUNDC1 in diseases
FUNDC1 is highly conserved between species especially in higher eukaryotic organisms and ubiquitously expressed in a variety of cell lines and different tissues of mammals,7, 8, 9, 10 suggesting that it must be implicated in cellular functions under various pathophysiological conditions. Thus, the focus centers on the role of FUNDC1 in a number of diseases.
Expression and phosphorylation states of FUNDC1 in hearts under ischemia/reperfusion (I/R)
Dysfunctional mitochondria are closely related to the occurrence and progression of various cardiovascular diseases, particularly cardiac I/R injury, cardiomyopathy and heart failure. Furthermore, cardiomyocytes contain large amounts of mitochondria, appropriately 30%–40% of the total volume, and the heart of an adult consumes about 6 kg ATP per day through mitochondrial oxidative phosphorylation system (OXPHOS) to support cardiac function.36, 37, 38, 39, 40 In addition, FUNDC1-mediated mitophagy is primarily activated in cardiomyocytes during acute I/R injury and chronic heart failure. Therefore, the expression and phosphorylation regulation of FUNDC1 in heart diseases were explored.
Zhou et al reported that the level of CK2α increased in a time-dependent manner following acute cardiac I/R in the infarction area, leading to mitochondrial damage and cardiac dysfunction.41, 42, 43 Functional analysis revealed that increased CK2α upregulated the levels of phosphorylated FUNDC1 at Ser13, a result that came along with mitophagy arrest and cardiomyocyte mitochondrial dysfunction. Knockout of CK2α restored FUNDC1-mediated mitophagy and thus sent a protective molecular mechanism to protect mitochondria and heart against I/R injury. Furthermore, there are three different regulatory mechanism for FUNDC1-related mitophagy in cardiomyocyte under normoxia (physiological), hypoxic (ischemic) and hypoxia-reoxygenation (ischemia-reperfusion) conditions. In normoxia (physiological) context, there were high levels of phosphorylated FUNDC1 at Tyr18 whereas only subtle phosphorylated FUNDC1 at Ser13 appeared, suggesting that phosphorylated FUNDC1 at Tyr18 primarily governed the baseline FUNDC1 activity. In contrast, in the period of hypoxia (ischemia), the level of the phosphorylated FUNDC1 at Tyr18 was significantly down-regulated while the level of the phosphorylated FUNDC1 at Ser13 remained relatively unchanged, indicating that hypoxia/ischemia-induced FUNDC1 activation is achieved through FUNDC1 dephosphorylation at Tyr18. Such a scenario is a good example of hypoxia-induced FUNDC1 mitophagy activation,7, 8, 9, 10,41, 42, 43 which could be potentially served as a novel cardioprotective mechanism with the aim to maintain cardiomyocyte mitochondrial integrity and function. Interestingly, at the stage of reperfusion after ischemia (reoxygenation following hypoxia), the level of the phosphorylated FUNDC1 at Tyr18 demonstrated little changes whereas the level of the phosphorylated FUNDC1 at Ser13 was upregulated progressively, indicating inactivation of FUNDC1 by Ser13 upon reperfusion exposure. It is necessary to point out that the total levels of the phosphorylated FUNDC1 at Tyr18 and Ser13 were down-regulated first at the ischemia stage and then upregulated gradually at the stage of reperfusion, negatively coinciding with the activation status of FUNDC1-dependent mitophagy. These data indicate that suppression of FUNDC1-mediated mitophagy is the pathogenesis of cardiac I/R injury and the level of the phosphorylated FUNDC1 at Ser13 and Tyr18 is closely related to the degree of cardiac injury, which might be a potential biomarker for the diagnosis and prognosis of acute myocardial infarction.
Interestingly, an unphosphorylated membrane-permeable peptide encompassing Ser13 mimicking the LIR and including the 9th-25th amino acids significantly prevented mitophagy mediated by the interaction between FUNDC1 and LC3 in cardiomyocytes in response to FCCP. This not only reinforces the importance of the phosphorylation status of FUNDC1 and specificity of the FUNDC1/LC3 interaction, but also suggests that this peptide strategy is an important tool for identifying mimetic peptides or screening natural compounds to manipulate FUNDC1-dependent mitophagy. These investigations reveal that mitophagy enhancement through CK2 inhibition, Src repression or PGAM5 activation is useful in recycling damaged mitochondria by degrading them in lysosome in disease settings. Given that mitochondrion is a major orchestrator of the cellular response to a broad array of stress types in cardiovascular illness by regulating apoptosis, oxidative stress, calcium homeostasis, and bioenergetics, the above findings not only decipher the sequence of events leading to FUNDC1-related mitophagy attenuation but also provide a promising therapeutic tool for the treatment of acute cardiac injury through many well-organized pre-clinical studies. However, most of the above investigations have been conducted employing cell lines in vitro and/or animal models in vivo. The next step is to verify these findings in clinical practice through appropriate human studies. Last but not the least, a credible real-time quantitative method for mitophagy detection, measurement, and evaluation in vivo is also required in order to address the predominant regulatory mechanism of various FUNDC1 phosphorylation sites as well as its involvement in mitochondrial quality control under distinct pathophysiological processes.
Phosphorylation regulation of FUNDC1 in platelets under I/R
Platelets in blood circulation are anucleated cells with a life span of about 7–10 days. Platelets were previously described as small pieces of cytoplasm removed from the megakaryocyte cytoplasm of the bone marrow, but later studies have revealed that they are critical in physiological hemostasis and pathological thrombosis.7, 8, 9 Increasing investigations have demonstrated that platelets are also involved in wound healing, inflammation, thrombosis, immunity, organ transplantation rejection, tumorigenesis and metastasis besides thrombosis and hemostasis. Interestingly, both autophagy and mitophagy have been detected in resting platelets, platelets from I/R mice models and platelets from patients with diabetes mellitus. Importantly, the molecular regulation of platelet mitophagy has been proposed as one of the major focuses in mitochondrial field and cardiology research.7, 8, 9 Since lots of excellent reviews have covered the molecular regulation of mitophagy in nucleated cells and the clinical significance in mice models and clinical patients, we mainly focus on the molecular regulation of FUNDC1-mediated mitophagy in anucleated platelets and propose targeting platelet FUNDC1-dependent mitophagy as a possible target for treating lots of cardiovascular diseases including I/R hearts.
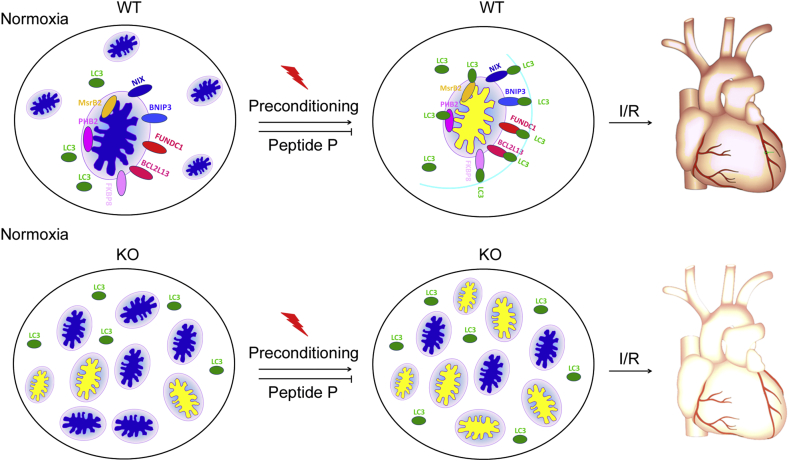
Because platelet activation is a process of consuming high levels of energy involving drastic structural and morphological changes, we therefore hypothesized that mitochondrial quality control mediated by FUNDC1-required mitophagy must play regulatory roles in platelet activation. The data revealed that FUNDC1 ablation in whole body knockout mice (F1KO), platelet FUNDC1 specific knockout mice and platelet ATG5 specific knockout mice significantly inhibited platelet mitophagy and reduced platelet activation in response to various stimuli. Interestingly, exposure of wild type mice to hypoxia dramatically inhibited platelet activation, whereas platelet activation of F1KO mice was not significantly reduced under hypoxic conditions compared with F1KO mice under normal conditions. Interestingly, the levels of both total FUNDC1 and phosphorylated FUNDC1 at Tyr18 decreased in platelets under hypoxia treatment and I/R in mice, suggesting that phosphorylated FUNDC1 at Tyr18 may be implicated in the pathogenesis of I/R.7, 8, 9 In addition, a membrane-penetrating peptide mimicking the unphosphorylated LIR domain of FUNDC1 competitively interacted with LC3 platelets, prevented FUNDC1-dependent mitophagy and enhanced I/R heart injury under I/R, whereas hypoxic preconditioning promoted FUNDC1-mediated platelet mitophagy, down-regulated phosphorylated FUNDC1 and alleviated I/R heart injury under I/R,7, 8, 9 indicating that the phosphorylation states of FUNDC1 in platelets are indicators of I/R biomarker and targeting platelet FUNDC1 may represent a novel strategy to fight heart diseases (Fig. 4).
Figure 4.
Preconditioning especially hypoxic preconditioning induces mitophagy in platelets and cardiomocytes, which decreases I/R heart injury and plays cardiac protection roles in mice. Preconditioning especially hypoxic preconditioning induces FUNDC1-dependent platelet mitophagy, which can significantly reduce I/R heart injury and restores cardiac functions especially ejection fraction and fractional shortening. However, whether other mitophagy receptors such as NIX, BNIP3, BCL2L13, FKBP8 and PHB2 and MsrB2 also have similar effects remains to be investigated.
Zhou et al recently also reported that the peroxisome proliferators-activated receptor γ (PPARγ)/FUNDC1/mitophagy pathways are involved in platelet activation in cardiac I/R mice models.41, 42, 43 Specifically, the level of PPARγ progressively decreased in both the patients with acute myocardial infarction receiving reperfusion and I/R mice models. Correlation analysis revealed that decreased level of PPARγ was significantly associated with reduced level of phosphorylated FUNDC1. I/R also activated platelet mitophagy in FUNDC1-dependent manner. Platelet mitophagy improved mitochondrial quality and platelet activations such as expression of P-selectin, platelet aggregation, platelet spreading and micro-thromboses. Importantly, a clinical drug melatonin significantly inhibited platelet activation, restored level of PPARγ and prevented FUNDC1-dependent mitophagy. The data collectively suggest that platelet mitophagy is highly involved in platelet activation and I/R injury, further supporting our findings.7, 8, 9 Our data along with the results of Hao Zhou et al40, 41, 42 indicate that manipulation of FUNDC1-mediated mitophagy by melatonin and membrane-permeable peptides may represent a new strategy for cardioprotection of cardiac I/R models (Fig. 4).
Mitochondria are cellular powerhouses that produce ATP at the expense of oxygen. They are also the major sources and the targets of ROS, thus highly sensitive to the changes of the oxygen conditions such as I/R. Response of mitochondria to ischemia is to activate mitophagy, a process that selectively removes the damaged organelles by autolysosomal degradation. Mitophagy was detected in both cardiomyocytes and platelets during I/R.1, 2, 3, 4,7, 8, 9 We propose a molecular regulation of mitophagy in response to hypoxia and highlight the role of mitophagy in both platelets and cardiomyocytes as a major mechanism for cardioprotection against I/R injury (Fig. 4).44,45 Mitophagy regulates both mitochondrial quantity and quality in vivo in response to hypoxia or hypoxic conditions especially I/R heart injury in both platelets and cardiomyocytes. It was further suggested that monitoring mitochondrial quality and mitochondrial functional status of platelets offered useful and convenient approach before the application of ischemic and pharmacological preconditioning, providing a new strategy to protect cardiac function and fight cardiovascular diseases. Because platelets are sensitive to fluctuated oxygen concentrations in blood circulation, and the phosphorylation and expression of FUNDC1 along with FUNDC1-mediated mitophagy in platelets can be detected effectively and non-invasively, it will represent a novel biomarker in disease diagnosis, treatment and prognosis.
Expression and significance of FUNDC1 in pan-cancer
Hypoxia is highly implicated in a number of human diseases, such as solid tumors and metabolic disorders. As one of the primary oxygen consumers, mitochondrion is extremely sensitive to hypoxia attack.46, 47, 48, 49, 50 It has been acknowledged that hypoxic environment can trigger mitophagy, but whether mitochondrial biogenesis is activated or inhibited by hypoxia is under dispute. Mitophagy theoretically has critical roles in tumor formation, metastasis and relapse although it remains unknown whether cancer growth is induced or blunted bifunctional or defective FUNDC1-mediated mitophagy, respectively (the former promotes mitochondrial renewal whereas the latter is associated with toxic mitochondria accumulation). It is hypothesized that FUNDC1-dependent mitophagy, possibly triggered by hypoxia in a manner critically depending on hypoxia inducible factor 1α, could be considered as a scavenger to neutralize ROS and thus normalize oxidative phosphorylation, which is vital for cancer development. In addition to the anti-oxidative property, FUNDC1-related mitophagy may sense and then transmit many stressful signals (such as inflammation, nutrient substrate conversion, or immunoregulation) both at the transcriptional and post-translational levels, ultimately affecting cancer metabolism, proliferation, mutation, mobilization and transdifferentiation.46, 47, 48, 49, 50 However, in light of the important role played by mitochondrial quality control in tumorigenesis, a description of these stress signaling regulatory mechanisms will provide useful information of how mitochondrial events are orchestrated and how mitochondrial activities are cooperated with tumor fate, which is needed in the clinical management of patients with cancer.
FUNDC1 anchors to the mitochondrial outer membrane and interacts with LC3 to form mitophagosomes.10 As an integral mitochondrial outer membrane protein, FUNDC1 is important in hypoxia-induced mitophagy, which is negatively regulated by the phosphorylation of FUNDC1, because the phosphorylation state of Tyr-18 in FUNDC1 LIR is able to weaken the interaction between FUNDC1 and LC3. Hypoxia, a common characteristic of cancers, induces FUNDC1-dependent mitophagy. In comparison, hypoxia also can trigger FUNDC1 ubiquitylation at its lysine 119 and degrade FUNDC1 by a mitochondrially localized E3 ubiquitin ligase, membrane-associated RING-CH protein 5 to fine-tune mitophagy.51 Long term hypoxia will result in the dephosphorylation of FUNDC1 and enhance mitophagic flux. Although FUNDC1-mediated mitophagy is implicated in various pathophysiological conditions especially tumors, its role remains to be explored. FUNDC1 is usually regarded to play protective roles in a variety of metabolic and cardiovascular diseases for the fact that FUNDC1-dependent mitophagy alleviates mitochondrial injury incurred by hypoxia and I/R and thus benefits human beings. In contrast, the roles of FUNDC1, which might be context dependent, vary significantly among different cancers.52, 53, 54, 55, 56, 57 While FUNDC1 was able to enhance the progression of cancer and represented poor prognosis in some tumors, it was also able to inhibit carcinogenesis through mitochondrial autophagy and quality control. The mechanism of FUNDC1-mediated mitophagy in various cancers remains to be studied. Therefore, the expression pattern, prognostic value and immunological role of FUNDC1 in pan-cancer were investigated across multiple databases.
The expression profiles of FUNDC1 mRNA over a cancer-wide range were examined in ONCOMINE. The expression levels of FUNDC1 mRNA were significantly higher in various tumor tissues, such as prostate cancers, pancreatic cancers, breast cancers, ovarian cancers, lung cancers, colorectal cancers and cervical cancers compared with those of the respective controls. The RNA sequencing results in TCGA revealed that the expression level of FUNDC1 mRNA was significantly lower in the tissues of uterine corpus endometrial carcinoma, prostate adenocarcinoma, lung squamous cell carcinoma, lung adenocarcinoma, liver hepatocellular carcinoma, kidney chromophobe, head and neck squamous carcinoma-HPV positive, colon adenocarcinoma, cholangiocarcinoma, bladder urothelial carcinoma and breast invasive carcinoma than that in the control adjacent normal tissues. The data suggest that FUNDC1 mRNA was implicated in the above cancers.52, 53, 54, 55, 56, 57
GEPIA was also utilized to examine the RNA sequencing data in TCGA. The effects of FUNDC1, as well as its overall effects on each type of cancer, were analyzed. FUNDC1 was generally a favorable prognostic factor in various cancers. Particularly, the high expression of FUNDC1 was significantly correlated with a better overall survival in kidney renal clear cell carcinoma, lung squamous cell carcinoma and disease-free survival in thyroid carcinoma compared with that of the low expression of FUNDC1. In comparison, the high level of FUNDC1 was significantly correlated with a poorer overall survival for the patients with brain lower grade glioma and hepatocellular carcinoma.52
The expression profiles of FUNDC1 protein were significantly correlated with eight cancers, including skin cancers, ovarian cancers, lung cancers, head and neck cancers, colorectal cancers, breast cancers, brain cancers and bladder cancers.52 Interestingly, FUNDC1 displayed protective roles in ovarian cancers, bladder cancers and lung cancers. In contrast, FUNDC1 exhibited detrimental effects on skin cancers, head and neck cancers, colorectal cancers, breast cancers and brain cancers. Furthermore, FUNDC1 was a newly identified detrimental prognostic factor in liver hepatocellular carcinoma, which may challenge the protective roles of FUNDC1 in hepatocellular carcinogenesis. The results on lung cancers were different from those employing PrognoScan in some degrees, because the high levels of FUNDC1 benefited only lung squamous cell carcinoma, but not lung adenocarcinoma. FUNDC1 expression demonstrated protective roles on the overall survival of the patients with bladder cancers, whereas the level of FUNDC1 worsened the relapse-free survival of the patients with bladder cancers. In contrast, the expression of FUNDC1 affected the overall survivals of the patients with head and neck squamous cell carcinoma and the patients with ovarian cancers, but the relapse-free survival rates were not affected. Interestingly, FUNDC1 exhibited protective roles on the relapse-free survival, but it did not display any significant effects on the overall survival of the patients with breast invasive carcinoma. Moreover, FUNDC1 played a protective role on relapse-free survival for rectum adenocarcinoma in the patients with colorectal cancers. The results indicate that the level of FUNDC1 protein varied in different cancers.52, 53, 54, 55, 56, 57
The possible relevance and the pathogenesis of FUNDC1 in cancers were explored further by integrating the pathological and clinical data in the Kaplan-Meier Plotter. Moreover, the relationship between the level of FUNDC1 and clinical characteristics of the patients with LIHC was investigated. The results showed that FUNDC1 displayed detrimental roles in the overall survival in the patients with liver hepatocellular carcinoma.52, 53, 54, 55, 56, 57
The data on the correlation of FUNDC1 expression level and immune infiltration for LIHC and lung squamous cell carcinoma are contradictory. Because it has been accepted that FUNDC1 plays a prognostic role in pan-cancer and tumor microenvironment affects the survival of the cancer patients, it will be very meaningful to investigate the relationship between FUNDC1 expression and immune infiltration in different types of cancers.52 The data suggest that the expression of FUNDC1 demonstrates significant correlations with tumor purity, and the infiltration degrees of dendritic cells, neutrophils, macrophages, B cells, CD8+ T cells and CD4+ T cells in most cancers.
The correlations between the level of FUNDC1 and immune infiltration in various cancers particularly lung squamous cell carcinoma and liver hepatocellular carcinoma were explored by employing the TIMER and GEPIA databases. The results uncovered that the expression levels of FUNDC1 in tumor tissues were significantly higher than that in control tissues generally. The up-regulated level of FUNDC1 was detrimental to the survival of the patients with LIHC, although FUNDC1 afforded a protection role in pan-cancer.52, 53, 54, 55, 56, 57 These data collectively indicate that the expression level of FUNDC1 may serve as a prognostic biomarker for the patients with pan-cancer.
It has been acknowledged that FUNDC1 is closely correlated with immunity in cancers. In addition, there is significant correlation between the expression level of FUNDC1 and cell proliferation in breast cancers and cervical cancers.52, 53, 54, 55, 56, 57 Therefore, it is not surprising that FUNDC1 affects the survival of the patients with various cancers through a mechanism involving cell proliferation and mitophagy. The effects of FUNDC1 on cell proliferation and metastasis vary significantly under different types of cancer and different stages of one particular cancer. For example, the mitophagy mediated by FUNDC1 inhibited hepatocarcinogenesis and hepatocyte specific deletion of FUNDC1 enhanced the initiation and progression of hepatocellular carcinomas induced by the chemical carcinogen diethylnitrosamine. On the contrary, transgenic FUNDC1 protected mice against the hepatocellular carcinomas development. However, it was also revealed in another study that FUNDC1 was an unfavorable prognosis factor for the patients with liver hepatocellular carcinoma. The reason might be that the models of mice and patients are different. Another reason might be that there are different patient characteristics of vascular invasion, tumor stage, tumor grade, hepatitis infection, alcohol consumption, race and gender. More precise techniques of RNA sequencing of single cell should be employed in future investigations to explore the relationships between FUNDC1 expression and cancers at the cellular and molecular levels.
The application of FUNDC1 as a prognostic factor in pan-cancer will provide insights from an immune-oncological perspective, which will aid in the development of immunotherapies and benefit in-depth mechanistic researches. Future studies should focus on the post-translational modification of FUNDC1, especially phosphorylation/dephosphorylation of FUNDC1 in pan-cancer. In summary, the expression of FUNDC1 is able to affect the prognosis of the patients with pan-cancer, suggesting that FUNDC1 might serve as an independent prognostic biomarker in the patients with pan-cancer.52, 53, 54, 55, 56, 57
Expression of FUNDC1 in breast cancer
Breast cancer is one of the most common cancers among female patients. Although early detection and individualized treatment are helpful in improving the survival of breast cancer patients, breast cancer is still one of the leading causes for deaths of young patients. Therefore, the facts highlight the significance of early diagnosis for breast cancer patients to advance precise diagnostics and development new prognostic biomarkers for the patents with breast cancer. Aberrant expression of mitochondrial protein may be closely related to the progression and metastasis of various cancers including breast cancer.52, 53, 54, 55, 56, 57 As a newly identified membrane of mitochondria-associated endoplasmic reticulum membranes, the expression pattern and functions of FUNDC1 in breast cancer remain largely unclear. Thus, the expression profile of FUNDC1 in breast cancer tissues was explored. The data uncovered that the expression of FUNDC1 in the tissues of breast cancer was significantly higher than that of normal breast epithelium. Mechanically, FUNDC1 enhanced proliferation, migration and invasion of the cells of breast cancer. The elevated mRNA level of FUNDC1 was significantly and negatively correlated with the survival of the patients with breast cancer. The expression levels of FUNDC1 protein in breast cancer tissues were positively correlated with tumor size, metastasis and death, respectively. The analysis results of Kaplan-Meier survival demonstrated the worse outcome for the breast cancer patients with high FUNDC1 levels. The data collectively suggest that FUNDC1 is implicated in the pathogenesis, progression and prognosis of breast cancer, and high expression of FUNDC1 predicts worse prognosis for the patients with breast cancer, and FUNDC1 may be a promising biomarker and target for breast cancer therapy.52, 53, 54, 55, 56, 57 Future studies should pinpoint how FUNDC1-mediated mitophagy are activated under the conditions of breast cancer. What's more, whether phosphorylation/dephosphorylation states of FUNDC1 participate in the pathogenesis, progression and prognosis of breast cancer is unclear.
Expression of FUNDC1 in cervical cancer
Cervical cancer is one of the important gynecological cancers accounting for the second highest incidence globally. The relationship between the expression level of FUNDC1 and the prognostic outcomes in cervical cancer remains to be investigated.52, 53, 54, 55, 56, 57 The levels of FUNDC1 mRNA in the tissues of cervical cancers were significantly higher than that in the adjacent normal cervical tissues. The expression levels of FUNDC1 in cervical cancer tissues were significantly higher than that in the corresponding adjacent noncancerous cervical tissues. Furthermore, the disease-free survival and the overall survival of the patients with cervical cancers were analyzed by using Kaplan-Meier, and the differences of the expression of FUNDC1 were compared by applying Log-rank test. The expression levels of FUNDC1 protein were negatively correlated with both the disease-free survival and the overall survival. In comparison, the expression level of FUNDC1 protein was an important prognostic factor for cervical cancer. The elevated levels of FUNDC1 were negatively correlated with the prognosis of the patients with cervical cancers, indicating that FUNDC1 might be employed as an independent prognostic factor for the prognosis and survival of the patients with cervical cancers. Because all the postoperative patients with cervical cancer had received radiotherapy and cisplatin-based adjuvant chemotherapy, the effects of FUNDC1 on the sensitivity of the cervical cancer cells to chemoradiotherapy were explored. The decreased levels of FUNDC1 enhanced the radiosensitivity in cervical cancers. Notably, FUNDC1 might be potential biomarker and may serve as a novel therapeutic target for the chemoradiotherapy for the patients with cervical cancers.52, 53, 54, 55, 56, 57 Future studies should focus on the roles of phosphorylation modifications of FUNDC1 in cervical cancers and the potential significance of the phosphorylated FUNDC1 in cervical cancers.
Expression of FUNDC1 in liver cancer
Hepatocellular carcinoma is one of the most common primary liver malignancies and cause of tumor-related mortality worldwide. Although it has been hypothesized that dysfunctional mitochondria may contribute to chronic inflammatory diseases, particularly hepatocellular carcinoma, the role of FUNDC1-mediated mitophagy in tumorigenesis remains to be explored.52, 53, 54, 55, 56, 57 Increased levels of FUNDC1 proteins were observed in most human hepatocellular carcinomas. Notably, FUNDC1 mRNA was also transcriptionally up-regulated in the tumor tissues of hepatocellular carcinoma compared with the corresponding controls. In addition, the roles of FUNDC1 and FUNDC1-required mitophagy in the initiation and progression of hepatocellular carcinomas were investigated by employing diethylnitrosamine (DEN)-induced mice models. The specific deletion of FUNDC1 in hepatocyte enhanced the initiation and progression of hepatocellular carcinomas induced by DEN, whereas ectopic expression of FUNDC1 in hepatocytes protected the mice models against the development of hepatocellular carcinomas. Specific deletion of FUNDC1 in hepatocytes enhanced the susceptibility to hepatocellular carcinomas induced by DEN and led to a higher death rate. These data suggest that increased levels of FUNDC1 in tumor might be related to the tumor development in DEN-treated mice models, and targeting FUNDC1 may represent as a novel strategy in fighting liver cancer.52, 53, 54, 55, 56, 57 However, the effects of phosphorylated FUNDC1 at sites of Tyr18 and Ser13 on hepatocellular carcinomas remain to be further explored.
Expression and phosphorylation regulation of FUNDC1 in sepsis
Sepsis is a systemic inflammatory response syndrome which causes oxidative injury to various organs. Because mitochondrial autophagy effectively eliminates damaged mitochondria and alleviates oxidative stress, and hydrogen gas serves as a selective antioxidant, it is hypothesized that hydrogen gas may play protective roles in sepsis through FUNDC1-mediated mitophagy.58 To test the hypothesis, cecal ligation and puncture (CLP) was employed in male C57BL6J mice in the presence of 2% hydrogen gas. Both the total expression level of FUNDC1 and the phosphorylated level of FUNDC1 at Tyr18 in the liver tissues of the septic mice group were significantly lower than that in the sham control group. Accordingly, the mitophagy of the liver tissues of the septic mice was markedly higher than that in the control group. Interestingly, hydrogen gas treatment down-regulated both the total expression level of FUNDC1 and the phosphorylated level of FUNDC1 at Tyr18 and promoted FUNDC1-dependent mitophagy, whereas a membrane-penetrating peptide mimicking the LIR domain of FUNDC1 demonstrated opposite effects. Notably, hydrogen gas treatment alleviated organ injury particularly liver and enhanced the survival time of the septic mice models. These results collectively demonstrated that FUNDC1-mediated mitophagy was involved in sepsis by phosphorylation modification regulation, and further suggested that the phosphorylated level of FUNDC1 at Tyr18 may be an important prognostic biomarker in septic mice. Notably, hydrogen gas inhalation may represent a novel strategy for fighting sepsis through a mechanism of FUNDC1-required mitophagy. However, the significance of the expression and phosphorylation of FUNDC1 in septic patients remains unknown.
Perspective for FUNDC1-mediated mitophagy research
Up till now, only three serine/threonine/tyrosine phosphorylation sites have been identified on the mitophagy receptor FUNDC1 with functional significance. Defining the functional regulation of these phosphorylation sites and their kinases/phosphatases under various pathophysiological conditions are needed. Since these three phosphorylation sites and their kinases are involved in the regulation of mitophagy receptor function, the changes of each phosphorylation site and the function of protein kinase mutual regulation in the occurrence and development of diseases deserve further exploration.59,60 At the same time, the pathogenesis, progression and prognosis of various diseases combined with phosphorylation of FUNDC1 will be studied, with a focus on further revealing the pathogenesis and discovering potential intervention targets by targeting FUNDC1.
Although there is emerging roles of FUNDC1-mediated mitophagy in a number of mitochondrial-related diseases, elucidating these complex pathophysiological processes and translating molecular information into personalized translational medicine still has a long way to go.61, 62, 63, 64, 65 Distinct phosphorylation states of FUNDC1 for selective elimination of damaged mitochondria in response to different stress signals possibly exists in different disease conditions. Thus, it is necessary to identify the specific upstream signaling molecules, particularly the protein kinases and phosphatases responsible for the pathogenesis of these diseases.66,67 In addition, whether other mitophagy receptors such as Nix is implicated in phosphorylation regulation and diseases also should be explored.68,69
Conclusions
In conclusion, mitochondria are the primary oxygen consumers and also produce ATP and mitochondrial ROS. Mitochondria can sense hypoxic signals to conduct appropriate responses. Hypoxia activates FUNDC1-dependent mitophagy by the reversible phosphorylation regulation of LIR of FUNDC1. Additionally, hypoxia enhances BCL-xL degradation, releasing PGAM5 and facilitating the dephosphorylation and activation of FUNDC1. The extent of FUNDC1-mediated mitophagy depends on both the duration and the acuteness of the hypoxic settings and different contexts. It needs further investigation on the exact mechanisms by which mitochondria can sense and integrate the different cellular or disease environmental cues to maintain the homeostasis of the whole mitochondrial network. Although FUNDC1-dependent mitophagy has been proposed to play protective roles in mitochondria-related diseases, the causal relationship between FUNDC1-mediated mitophagy and the pathogenesis of these diseases remains to be further explored to provide prevention and therapeutic strategies to the diseases.
Emerging evidence has demonstrated that levels of FUNDC1 are closely related to occurrence, progression and prognosis of various diseases including heart diseases and cancers, indicating that FUNDC1 may serve as a promising biomarker and therapeutic target.
Ethics approval and consent to participate
The manuscript is a review, so there is no ethics approve and consent to participate.
Authors contribution
Weilin Zhang has made substantial contributions to the conception and design of the work, the acquisition, analysis, interpretation of data, the creation of new software used in the work, has drafted the work, and has approved the submitted version, agrees to be personally accountable for the author's own contributions and for ensuring that questions related to the accuracy or integrity of any part of the work.
Conflict of Interests
No potential conflicts of interests were disclosed.
Funding
This research was supported by the Natural Science Foundation of China [grant number 31871392], the Beijing Natural Science Foundation [grant number 5192014] and China postdoctoral grant number [2013 M541041] to Weilin Zhang. Weilin Zhang is a recipient of the Innovation Foundation of Beijing University of Aeronautics and Astronautics (BUAA) and the Academic Innovation Award of Ministry of Education [grant number 401059].
Acknowledgements
I am very grateful to Prof. Quan Chen and all other laboratory members for their original experimental investigations on FUNDC1. Thank Dr. Hao Zhou and Dr. Seung Hee Lee for their expert comments and critical reading. I apologize for not citing many outstanding references because of space limitation.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Wallace D.C. Mitochondrial genetic medicine. Nat Genet. 2018;50:1642–1649. doi: 10.1038/s41588-018-0264-z. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Phil Soc. 1966;41:445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biochim Biophys Acta. 2011;1807:1507–1538. doi: 10.1016/j.bbabio.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 4.Pickles S., Vigié P., Youle R.J. Mitophagy and quality control mechanisms in mitochondrial maintenance. Curr Biol. 2018;28:R170–R185. doi: 10.1016/j.cub.2018.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gustafsson Å.B., Dorn G.W. 2nd. Evolving and expanding the roles of mitophagy as a homeostatic and pathogenic process. Physiol Rev. 2019;99:853–892. doi: 10.1152/physrev.00005.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ney P.A. Mitochondrial autophagy: Origins, significance, and role of BNIP3 and NIX. Biochim Biophys Acta. 2015;1853:2775–2783. doi: 10.1016/j.bbamcr.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W., Chen C., Wang J., Liu L., He Y., Chen Q. Mitophagy in cardiomyocytes and in platelets: a major mechanism of cardioprotection against ischemia/reperfusion injury. Physiology (Bethesda) 2018;33:86–98. doi: 10.1152/physiol.00030.2017. [DOI] [PubMed] [Google Scholar]
- 8.Zhang W., Ren H., Xu C. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. Elife. 2016;5 doi: 10.7554/eLife.21407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang W., Siraj S., Zhang R., Chen Q. Mitophagy receptor FUNDC1 regulates mitochondrial homeostasis and protects the heart from I/R injury. Autophagy. 2017;13:1080–1081. doi: 10.1080/15548627.2017.1300224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Feng D., Chen G. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol. 2012;14:177–185. doi: 10.1038/ncb2422. [DOI] [PubMed] [Google Scholar]
- 11.Gatica D., Lahiri V., Klionsky D.J. Cargo recognition and degradation by selective autophagy. Nat Cell Biol. 2018;20:233–242. doi: 10.1038/s41556-018-0037-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ohsumi Y. Historical landmarks of autophagy research. Cell Res. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lemasters J.J. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res. 2005;8:3–5. doi: 10.1089/rej.2005.8.3. [DOI] [PubMed] [Google Scholar]
- 14.Sarraf S.A., Youle R.J. Parkin mediates mitophagy during beige-to-white fat conversion. Sci Signal. 2018;11(527) doi: 10.1126/scisignal.aat1082. [DOI] [PubMed] [Google Scholar]
- 15.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shirihai O.S., Song M., Dorn G.W.I.I. How mitochondrial dynamism orchestrates mitophagy. Circ Res. 2015;116:1835–1849. doi: 10.1161/CIRCRESAHA.116.306374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schweers R.L., Zhang J., Randall M.S. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci U S A. 2007;104(49):19500–19505. doi: 10.1073/pnas.0708818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murakawa T., Yamaguchi O., Hashimoto A. Bcl-2-like protein 13 is a mammalian Atg32 homologue that mediates mitophagy and mitochondrial fragmentation. Nat Commun. 2015;6 doi: 10.1038/ncomms8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhujabal Z., Birgisdottir A.B., Sjøttem E. FKBP8 recruits LC3A to mediate Parkin-independent mitophagy. EMBO Rep. 2017;18:947–961. doi: 10.15252/embr.201643147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wei Y., Chiang W.C., Sumpter R., Jr., Mishra P., Levine B. Prohibitin 2 is an inner mitochondrial membrane mitophagy receptor. Cell. 2017;168(1-2):224–238. doi: 10.1016/j.cell.2016.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee S.H., Lee S., Du J. Mitochondrial MsrB2 serves as a switch and transducer for mitophagy. EMBO Mol Med. 2019;11 doi: 10.15252/emmm.201910409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Youle R.J., Narendra D.P. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi A.M., Ryter S.W., Levine B. Autophagy in human health and disease. N Engl J Med. 2013;368:651–662. doi: 10.1056/NEJMra1205406. [DOI] [PubMed] [Google Scholar]
- 24.Lazarou M., Sliter D.A., Kane L.A. The ubiquitin kinase PINK1 recruits autophagy receptors to induce mitophagy. Nature. 2015;524(7565):309–314. doi: 10.1038/nature14893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuang Y., Ma K., Zhou C. Structural basis for the phosphorylation of FUNDC1 LIR as a molecular switch of mitophagy. Autophagy. 2016;12:2363–2373. doi: 10.1080/15548627.2016.1238552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lv M., Wang C., Li F. Structural insights into the recognition of phosphorylated FUNDC1 by LC3B in mitophagy. Protein Cell. 2017;8:25–38. doi: 10.1007/s13238-016-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen G., Han Z., Feng D. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell. 2014;54:362–377. doi: 10.1016/j.molcel.2014.02.034. [DOI] [PubMed] [Google Scholar]
- 28.Wu W., Tian W., Hu Z. ULK1 translocates to mitochondria and phosphorylates FUNDC1 to regulate mitophagy. EMBO Rep. 2014;15:566–575. doi: 10.1002/embr.201438501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu H., Xue D., Chen G. The BCL2L1 and PGAM5 axis defines hypoxia-induced receptor-mediated mitophagy. Autophagy. 2014;10:1712–1725. doi: 10.4161/auto.29568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Jiang H., Chen S., Du F., Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 31.Lee K.H., Kang T.B. The molecular links between cell death and inflammasome. Cells. 2019;8(9) doi: 10.3390/cells8091057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anderson M.A., Huang D., Roberts A. Targeting BCL2 for the treatment of lymphoid malignancies. Semin Hematol. 2014;51:219–227. doi: 10.1053/j.seminhematol.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 33.Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- 34.McArthur K., Chappaz S., Kile B.T. Apoptosis in megakaryocytes and platelets: the life and death of a lineage. Blood. 2018;131:605–610. doi: 10.1182/blood-2017-11-742684. [DOI] [PubMed] [Google Scholar]
- 35.Strasser A., Vaux D.L. Viewing BCL2 and cell death control from an evolutionary perspective. Cell Death Differ. 2018;25:13–20. doi: 10.1038/cdd.2017.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu S., Zou M.H. Mitochondria-associated endoplasmic reticulum membranes in the heart. Arch Biochem Biophys. 2019;662:201–212. doi: 10.1016/j.abb.2018.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu S., Lu Q., Wang Q. Binding of FUN14 domain containing 1 with inositol 1,4,5-trisphosphate receptor in mitochondria-associated endoplasmic reticulum membranes maintains mitochondrial dynamics and function in hearts in vivo. Circulation. 2017;136:2248–2266. doi: 10.1161/CIRCULATIONAHA.117.030235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu S., Lu Q., Ding Y. Hyperglycemia-driven inhibition of AMP-activated protein kinase α2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139:1913–1936. doi: 10.1161/CIRCULATIONAHA.118.033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo R., Zong S., Wu M., Gu J., Yang M. Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell. 2017;170(6):1247–1257. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 40.Wang X., Zhang X., Wu D. Mitochondrial flashes regulate ATP homeostasis in the heart. Elife. 2017;6 doi: 10.7554/eLife.23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou H., Zhu P., Wang J., Zhu H., Ren J., Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2α-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25:1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou H., Zhu P., Guo J. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou H., Li D., Zhu P. Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARγ/FUNDC1/mitophagy pathways. J Pineal Res. 2017;63 doi: 10.1111/jpi.12438. [DOI] [PubMed] [Google Scholar]
- 44.Boengler K., Schlüter K.D., Schermuly R.T., Schulz R. Cardioprotection in right heart failure. Br J Pharmacol. 2020;177(23):5413–5431. doi: 10.1111/bph.14992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitchell M.B., Winter C.B., Banerjee A., Harken A.H. The relationship between ischemia-reperfusion injury, myocardial stunning and cardiac preconditioning. Surg Gynecol Obstet. 1993;177:97–114. [PubMed] [Google Scholar]
- 46.Ippolito L., Giannoni E., Chiarugi P., Parri M. Mitochondrial redox hubs as promising targets for anticancer therapy. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.00256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang T., Suo C., Zheng C., Zhang H. Hypoxia and metabolism in metastasis. Adv Exp Med Biol. 2019;1136:87–95. doi: 10.1007/978-3-030-12734-3_6. [DOI] [PubMed] [Google Scholar]
- 48.Ast T., Meisel J.D., Patra S. Hypoxia rescues frataxin loss by restoring iron sulfur cluster biogenesis. Cell. 2019;177(6):1507–1521. doi: 10.1016/j.cell.2019.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sprenger H.G., Langer T. The good and the bad of mitochondrial breakups. Trends Cell Biol. 2019;29:888–900. doi: 10.1016/j.tcb.2019.08.003. [DOI] [PubMed] [Google Scholar]
- 50.Lee P., Chandel N.S., Simon M.C. Cellular adaptation to hypoxia through hypoxia inducible factors and beyond. Nat Rev Mol Cell Biol. 2020;21(5):268–283. doi: 10.1038/s41580-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen Z., Liu L., Cheng Q. Mitochondrial E3 ligase MARCH5 regulates FUNDC1 to fine-tune hypoxic mitophagy. EMBO Rep. 2017;18:495–509. doi: 10.15252/embr.201643309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan Q., Sun N., Zheng J. Prognostic and immunological role of FUN14 domain containing 1 in pan-cancer: friend or foe? Front Oncol. 2020;9 doi: 10.3389/fonc.2019.01502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li W., Li Y., Siraj S. FUN14 domain-containing 1-mediated mitophagy suppresses hepatocarcinogenesis by inhibition of inflammasome activation in mice. Hepatology. 2019;69:604–621. doi: 10.1002/hep.30191. [DOI] [PubMed] [Google Scholar]
- 54.Wu L., Zhang D., Zhou L. FUN14 domain-containing 1 promotes breast cancer proliferation and migration by activating calcium-NFATC1-BMI1 axis. EBioMedicine. 2019;41:384–394. doi: 10.1016/j.ebiom.2019.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hou H., Er P., Cheng J. High expression of FUNDC1 predicts poor prognostic outcomes and is a promising target to improve chemoradiotherapy effects in patients with cervical cancer. Cancer Med. 2017;6:1871–1881. doi: 10.1002/cam4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hui L., Wu H., Wang T.W., Yang N., Guo X., Jang X.J. Hydrogen peroxide-induced mitophagy contributes to laryngeal cancer cells survival via the upregulation of FUNDC1. Clin Transl Oncol. 2019;21:596–606. doi: 10.1007/s12094-018-1958-5. [DOI] [PubMed] [Google Scholar]
- 57.Roperto S., Russo V., De Falco F., Rosati A., Catoi C., Roperto F. FUNDC1-mediated mitophagy in bovine papillomavirus-infected urothelial cells. Vet Microbiol. 2019;234:51–60. doi: 10.1016/j.vetmic.2019.05.017. [DOI] [PubMed] [Google Scholar]
- 58.Yan M., Yu Y., Mao X. Hydrogen gas inhalation attenuates sepsis-induced liver injury in a FUNDC1-dependent manner. Int Immunopharm. 2019;71:61–67. doi: 10.1016/j.intimp.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 59.Cai Q., Jeong Y.Y. Mitophagy in alzheimer's disease and other age-related neurodegenerative diseases. Cells. 2020;9:150. doi: 10.3390/cells9010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chan D.C. Mitochondrial dynamics and its involvement in disease. Annu Rev Pathol. 2020;15:235–259. doi: 10.1146/annurev-pathmechdis-012419-032711. [DOI] [PubMed] [Google Scholar]
- 61.Paik D.T., Cho S., Tian L., Chang H.Y., Wu J.C. Single-cell RNA sequencing in cardiovascular development, disease and medicine. Nat Rev Cardiol. 2020;17(8):457–473. doi: 10.1038/s41569-020-0359-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xie Q., Faust K., Van Ommeren R., Sheikh A., Djuric U., Diamandis P. Deep learning for image analysis: personalizing medicine closer to the point of care. Crit Rev Clin Lab Sci. 2019;56:61–73. doi: 10.1080/10408363.2018.1536111. [DOI] [PubMed] [Google Scholar]
- 63.Long K.B., Young R.M., Boesteanu A.C. CAR T cell therapy of non-hematopoietic malignancies: detours on the road to clinical success. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.02740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Horwitz R.I., Charlson M.E., Singer B.H. Medicine based evidence and personalized care of patients. Eur J Clin Invest. 2018;48 doi: 10.1111/eci.12945. [DOI] [PubMed] [Google Scholar]
- 65.Wang Q., Lv H., Lu L., Ren P., Li L.J. Neonatal hypoxic-ischemic encephalopathy: emerging therapeutic strategies based on pathophysiologic phases of the injury. Matern Fetal Neonatal Med. 2019;32:3685–3692. doi: 10.1080/14767058.2018.1468881. [DOI] [PubMed] [Google Scholar]
- 66.Sabbah H.N. Targeting the mitochondria in heart failure: a translational perspective. JACC Basic Transl Sci. 2020;5:88–106. doi: 10.1016/j.jacbts.2019.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chiarelli R., Martino C., Roccheri M.C. Cadmium stress effects indicating marine pollution in different species of sea urchin employed as environmental bioindicators. Cell Stress Chaperones. 2019;24:675–687. doi: 10.1007/s12192-019-01010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rogov V.V., Suzuki H., Marinković M. Phosphorylation of the mitochondrial autophagy receptor Nix enhances its interaction with LC3 proteins. Sci Rep. 2017;7(1) doi: 10.1038/s41598-017-01258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang W., Ma Q., Siraj S. Nix-mediated mitophagy regulates platelet activation and life span. Blood Adv. 2019;3(15):2342–2354. doi: 10.1182/bloodadvances.2019032334. [DOI] [PMC free article] [PubMed] [Google Scholar]