Abstract
Secreted frizzled-related protein 5 (SFRP5) plays a pivotal role in regulating the development of many tissues and organs, however, as an inhibitor of Wnt signaling, the role of SFRP5 in vitiligo remains unknown. Hence, we speculated that SFRP5 might be associated with melanogenesis in melanocytes by regulating Wnt signaling in vitiligo. In this study, we found that SFRP5 was overexpressed in the skin lesions of patients with vitiligo. Compared with that in normal epidermal melanocytes (PIG1), the expression of SFRP5 was increased in vitiligo melanocytes (PIG3V). To investigate the effect of SFRP5 on melanin synthesis, PIG1 cells were infected with recombinant SFRP5 adenovirus (AdSFRP5), and PIG3V cells were infected with recombinant siSFRP5 adenovirus (AdsiSFRP5). The results showed that SFRP5 overexpression inhibited melanin synthesis in PIG1 cells through downregulation of microphthalmia-associated transcription factor (MITF) and its target proteins via suppression of the Wnt/β-catenin signaling pathway. Accordingly, SFRP5 silencing increased melanin synthesis and activated the Wnt signaling pathway in PIG3V cells. Moreover, SFRP5 overexpression also downregulated the transcriptional activity of T cell factor/lymphoid enhancer factor (TCF/LEF) in PIG1 cells. Furthermore, this inhibitory effect of SFRP5 on melanin synthesis was reversed by treatment with the β-catenin agonist, SKL2001. The inhibitory action of SFRP5 in pigmentation was further confirmed in vivo using a nude mouse model. Hence, our results indicate that SFRP5 can inhibit melanogenesis in melanocytes. Additionally, our findings showed that SFRP5 plays a vital role in the development of vitiligo, and thus may serve as a potential therapeutic target for vitiligo.
Keywords: Melanin synthesis, Melanocytes, MITF, SFRP5, Vitiligo, Wnt signaling
Introduction
Vitiligo is an acquired chronic depigmentation disorder characterized by the destruction of melanocytes in the epidermis,1, 2, 3 which affects 0.5–1% individuals worldwide.4,5 Almost half of the patients have been shown to present symptoms before the age of 20 years.6,7 Moreover, vitiligo often affects the face and other visible areas of the body, resulting in a severe psychological burden and decreased quality of life in patients.8, 9, 10 Although several etiological theories have been proposed, which indicate that genetic predisposition,11 immune responses,12,13 melanocytorrhagy14 and metabolic abnormalities15 might be involved in the pathogenesis of vitiligo, the exact mechanism of loss of melanocytes in depigmented lesions is still unclear.
Wnt signaling is known to play a crucial role in melanocyte stem cell differentiation.16,17 Wnt1 and Wnt3a promote the differentiation of neural crest cells into melanocytes.18,19 Xingyu Mei et al20 showed that Wnt5a gene in the Wnt/β-catenin classical pathway promoted the differentiation and proliferation of melanocytes. Moreover, it has been shown that Wnt/β-catenin signaling plays a pivotal role in proliferation, migration, and differentiation in vitiligo pigmentation systems.21 Claire Regazzetti et al22 demonstrated that in lesional vitiligo, oxidative stress decreases Wnt expression/activation in melanocytes, which supports Wnt agonists to repigment vitiligo lesions. In addition, under certain conditions, the Wnt/β-catenin pathway has been shown to participate in the activation of microphthalmia-associated transcription factor (MITF) and its downstream melanogenic enzymes in vitiligo.23,24 These data suggest that stimulation of Wnt signaling may serve as an adjunct to current therapies for vitiligo.
Secreted frizzled-related protein 5 (SFRP5) is a member of the SFRP family of secreted proteins.25 SFRP5 is structurally very similar to the Fz receptor in the Wnt signaling pathway, and can inhibit the activity of Wnt signaling pathway by competitively inhibiting the Fz receptor.26 Hence, it is well known as an inhibitor of the Wnt signaling pathway.25,27 SFRP5 plays a crucial role in regulating the development of other tissues and organs but its effect on vitiligo remains unclear.
Our interest in SFRP5 involves its upregulation in the depigmentation lesions of vitiligo. In our previous study, we found that the mRNA and protein expression of SFRP5 was significantly increased in vitiligo melanocytes (PIG3V) than the expression in normal epidermal melanocytes (PIG1). Hence, we hypothesized that SFRP5 plays a vital role in the onset of vitiligo. In this study, we aim to investigate the effect of SFRP5 on melanogenesis in human melanocytes and elucidate the underlying mechanism in vitro and in vivo.
Materials and methods
Biopsy collection
Skin specimens for immunohistochemistry were taken from sixteen vitiligo patients and sixteen matched healthy volunteers. All the biopsy samples were obtained from patients diagnosed with vitiligo upon examination of their clinical and histologic manifestations at the Department of Dermatology, the First Affiliated Hospital of Chongqing Medical University. Skin biopsy samples were obtained from lesional and adjacent perilesional normal skin (usually within 1 cm from the lesion margin) for all patients. Written informed consent was obtained from each patient prior to skin biopsy. This study was approved by the Clinical Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 2020039) and conducted in accordance with the Declaration of Helsinki.
Immunohistochemistry
The biopsy samples were embedded in paraffin, sectioned (4 μm thick sections), and incubated with primary antibody at 4 °C overnight after antigen retrieval. Next, all sections were subjected to immunohistochemical analysis using immunohistochemistry SP-9000 Kit (Zhongshan Golden Bridge, Beijing, China).
Cell culture
Human normal melanocyte cell line (PIG1) and vitiligo melanocyte cell line (PIG3V) were kindly donated by Dr. Chunying Li (Xijing Hospital, Fourth Military Medical University, Xi'an, China). Cells were cultured in Medium 254 (Gibco, Grand Island, NY, USA) supplemented with Human Melanocyte Growth Supplement (Gibco), 5% fetal bovine serum (Gibco), and 1% penicillin-streptomycin antibiotic mix (Invitrogen, CAL, USA) at 37 °C with 5% CO2.28 The B16 melanoma cell lines were cultured in Dulbecco's modified Eagle's medium (HyClone Laboratories, UT, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 1% penicillin-streptomycin antibiotic mix at 37 °C in a 5% CO2 atmosphere.
Adenoviral transfection
Recombinant adenovirus SFRP5 (AdSFRP5) and recombinant adenovirus red fluorescent protein (AdRFP, as control) were kindly donated by Dr. T.C. He (Medical Center of University of Chicago, Chicago, IL, USA). Recombinant adenovirus siSFRP5 (AdsiSFRP5) and recombinant adenovirus green fluorescent protein (AdGFP, as control) were obtained from Hanheng Biotechnology Co., Ltd (Shanghai, China). They were transfected into PIG1 and PIG3V cells, respectively. After 8 h of cultivation, the medium was replaced with fresh medium.
Melanin content and tyrosinase activity assay
The melanin content of melanocytes was determined as previously described with slight modifications.29,30 Briefly, PIG1 or PIG3V cells were treated with different adenoviruses. When the fluorescence brightness reached 40–50%, the cells were digested, collected (1 × 107 cells), and lysed in 0.1 M phosphate buffer (pH 6.8). For melanin content determination, pellets were solubilized in 1 M NaOH. The absorbance at 490 nm was measured using a microplate reader. For the tyrosinase activity assay, each sample was incubated with 2 mM l-DOPA (Sigma–Aldrich, Shanghai, China) in 0.1 M phosphate buffer (pH 6.8) for 90 min at 37 °C. After incubation, the tyrosinase activity was measured at 490 nm. The melanin content and tyrosinase activity were normalized with that in the control group.
Immunocytochemical staining
Immunocytochemical staining was performed as previously described.31 After treatment with different processing factors, melanocytes were examined for α-melanocyte-stimulating hormone (α-MSH), SFRP5, and β-catenin expression. Rabbit polyclonal anti-α-MSH antibody (Bioss, Beijing, China), anti-β-catenin antibody (Cell Signaling Technology), and mouse monoclonal anti-SFRP5 antibody (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) were used as the primary antibodies. The secondary antibodies included Alexa Fluor 594 goat anti-mouse IgG (Zhongshan Golden Bridge, Beijing, China) and FITC goat anti-rabbit IgG (Zhongshan Golden Bridge, Beijing, China). 4′,6-Diamidino-2-phenylindole (DAPI; Beyotime, Beijing, China) was used as a nuclear counterstain. The fluorescence was detected and captured using a confocal microscope.
Transmission electron microscopy (TEM)
TEM was performed using a previously described method.2,32,33 In brief, after experimental treatments, cells were collected, washed twice with phosphate buffered saline (PBS), and fixed in ice-cold glutaraldehyde for 24 h at 4 °C. The cell pellet was then rinsed with Millonig's buffer and minced after fixing in 1.0% OsO4. The pellet was stained with 2.0% aqueous uranyl acetate, followed by ethanol dehydration and embedding in Spurr's plasticresin. Ultrathin sections were visualized using the Tecnai™ G2 Spirit BioTWIN (FEI, Hillsboro, OR, USA).
Real-time polymerase chain reaction (RT-PCR)
After experimental treatments, total RNA was isolated using TRIzol reagent (Thermo Fisher Scientific) in accordance with the RNA extraction protocol. Total RNA (1.5 μg) was used for cDNA synthesis by reverse transcriptase PCR. The cDNA was amplified on a RT-PCR system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) using SYBR-Green PCR Master Mix. β-actin was used as the endogenous control. The primers were designed and synthesized by Takara (Table 1). Each experiment was performed three times.
Table 1.
Primer sequences for RT-PCR.
| Genes | Sequence of primers 5′ to 3′ |
|---|---|
| β-actin | F:CACCACACCTTCTACAATGAGC R: GTGATCTCCTTCTGCATCCTGT |
| SFRP5 | F:TGCCCTTGCCCACAGTTAGA R:GAGGGAACAGGGATAGGAGAACA |
| MITF | F: CAACAACCTCGGAACTGGGACT R:TCCATGCTCATACTGCTCCTCC |
| TRP1 | F: CTGTGCTGAGCCAGGCTAAGTG R: GCCATCACCCCAGGAGACAA |
| TRP2 | F:GGAGTGGTCCCTACATCCTACG R:GAATGGATGTTCTGCCGAATC |
| TYR | F: GACAAATCCAGAACCCCAAGG R:GAGGCATCCGCTATCCCAGT |
F, forward; R, reverse.
Western blotting
Cells were washed three times with PBS and lysed in RIPA lysis buffer (Beyotime, Beijing, China) supplemented with a protease inhibitor, phenylmethanesulfonyl fluoride (Sigma–Aldrich). Lysates were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and blotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore, Billerica, MA, USA). The primary antibodies used were as follows: anti-SFRP5 (GTX114747) obtained from GeneTex (Irvine, CA, USA); anti-MITF (ab140606), anti-TYR (ab170905), anti-TRP1 (ab178676), and anti-TRP2 (ab221144) purchased from Abcam (Cambridge, MA, USA); anti-β-catenin (#8480), anti-β-cateninS675 (#4176), anti-GSK3β (#12456), anti- GSK3βS9 (#9322), and anti-β-actin (#3700) purchased from Cell Signaling Technology (Danvers, MA, USA). The PVDF membranes were incubated with primary antibody overnight at 4 °C, followed by incubation with horseradish peroxidase-conjugated anti-rabbit antibody (Zhongshan Golden Bridge, Beijing, China) for 1 h at 37 °C. Finally, the protein bands were visualized with the SuperSignal West Pico Chemiluminescent Substrate Kit (EMD Millipore, Billerica, MA, USA).
Promoter analysis
Promoter analysis was performed as previously described.34,35 Melanocytes were seeded in T-25 flasks and transfected with 4 μg of p-BGluc T cell factor/lymphoid enhancer factor (TCF/LEF) plasmids. After 24 h, the transfected melanocytes were seeded into 24-well plates and treated with AdSFRP5, AdRFP, AdGFP, and AdsiSFRP5 for 12 h. At the scheduled time points, cell supernatants were assayed using the luciferase assay kit, and the level of luciferase expression was detected by GloMax luminescence detector (Gairdner, Wuhan, China).
Xenograft mouse experiment
The in vivo experiments were approved by the Animal Experiment Administration Committee of Chongqing Medical University, Laboratory Animal Research. Approximately 5 × 106 AdRFP-, AdSFRP5-, AdGFP-, or AdsiSFRP5-infected B16 cells were briefly suspended in 100 μL of serum-free media and injected subcutaneously into nude mice. The changes in skin color of nude mice were observed every other day. The mice were sacrificed by cervical vertebra dislocation after 2 weeks, and the skin tissues around the injection site were collected and embedded in paraffin for immunohistochemical staining. All procedures related to animal handling, care, and treatment were performed in strict accordance with the recommendations in the guidelines for the management of experimental animals. Additionally, the experimental protocol was approved by the Animal Ethics Committee of the First Affiliated Hospital of Chongqing Medical University (Approval Number: 2020039).
Statistical analysis
All statistical analyses were performed with SPSS software (version 20.0; SPSS, Inc., Chicago, IL, USA). Student's t-test was used to evaluate differences between two groups. Data represent mean ± standard deviation (SD) for at least 3 independent experiments. A value of P < 0.05 was considered statistically significant.
Results
SFRP5 is upregulated in vitiligo lesions and vitiligo melanocytes
Immunohistochemical staining was performed to detect endogenous SFRP5 expression in the skin tissues of healthy control volunteers as well as lesional and perilesional normal skin of vitiligo patients. The results revealed that the protein expression of SFRP5 was higher in vitiligo lesions compared with that in perilesional and healthy control skin (Fig. 1A). The levels of SFRP5 expression in PIG1 and PIG3V cells were analyzed by RT-PCR and Western blotting. The results indicated that the mRNA and protein expression of SFRP5 was significantly increased in PIG3V cells compared with that in PIG1 cells (Fig. 1B and C).
Figure 1.
Relative expression of SFRP5 in vitiligo and normal skin. (A) Immunohistochemical staining of SFRP5 in vitiligo lesional (n = 16), perilesional normal skin (n = 16) and normal healthy control skin (n = 16). Scale bar = 100 μm (magnification: 400×). (B) The mRNA levels of SFRP5 in normal melanocytes (PIG1) and vitiligo melanocytes (PIG3V). (C) The protein levels of SFRP5 in PIG1 and PIG3V. Error bars are the means ± SD, n = 3. ∗∗P < 0.01 vs. control.
SFRP5 inhibits pigmentation of melanocytes
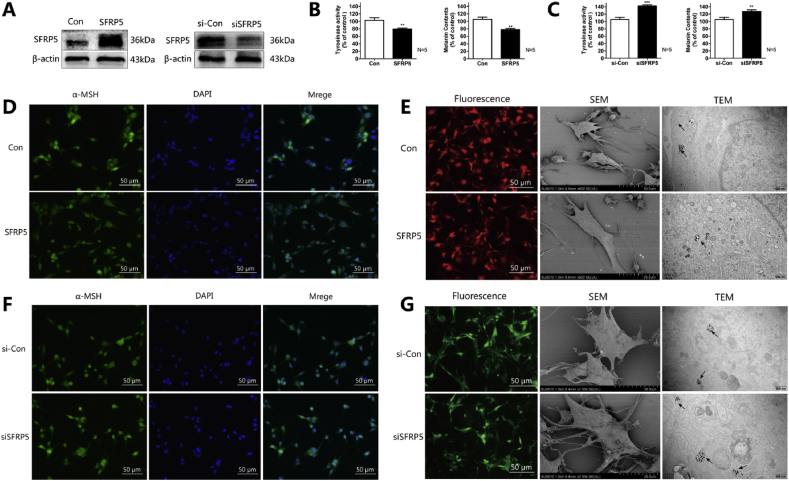
To investigate the role of SFRP5 in the regulation of melanogenesis, PIG1 cells were infected with AdSFRP5 and AdRFP (as control), separately. Western blot analysis revealed that AdSFRP5 was successfully transfected into PIG1 cells (Fig. 2A, left panel). SFRP5 overexpression did not affect PIG1 cell proliferation (Fig. S1 online); moreover, flow cytometry revealed that apoptosis and cell cycle were not altered by SFRP5 overexpression (Fig. S2, S3 online). However, SFRP5 overexpression significantly decreased the level of tyrosinase activity (Fig. 2B, left panel). Consistent with the changes in tyrosinase activity, there was a significant decrease in melanin content (Fig. 2B, right panel) and α-MSH expression (Fig. 2D). In addition, the number of dendrites was also detected by scanning electron microscopy, and the results indicated that SFRP5 overexpression remarkably decreased the number of dendrites, as well as the number of melanosomes, as assayed by TEM (Fig. 2E). Furthermore, the effect of SFRP5 downregulation on melanogenesis was also examined. As shown in Fig. 2A (right panel), siSFRP5 adenovirus transfection markedly reduced the expression of SFRP5 in PIG3V cells compared with the AdGFP group. In addition, SFRP5 knockdown significantly upregulated the tyrosinase activity (left panel) and melanin content (right panel) of PIG3V cells (Fig. 2C). Consistently, the expression of α-MSH was also remarkably increased (Fig. 2F). The numbers of dendrites and melanosomes were also increased by SFRP5 downregulation (Fig. 2G). Taken together, these results indicate that SFRP5 has an inhibitory effect on melanin synthesis.
Figure 2.
SFRP5 inhibits melanogenesis in normal human melanocytes. (A) Western blot analysis of the expression levels of SFRP5 in AdSFRP5-infected PIG1 cells (left panel) and AdsiSFRP5-infected PIG3V cells (right panel). (B) SFRP5 inhibits tyrosinase activity (left panel) and melanin content in PIG1 cells (right panel). (C) Tyrosinase activity (left panel) and melanin content were measured in SFRP5 knockdown PIG3V cells (right panel). (D) Immunocytochemical analysis the α-MSH expression in the PIG1 cells infected with AdSFRP5 or AdRFP. Nuclei were counterstained with DAPI (blue). (E) SFRP5 decreases the numbers of dendrites and melanosomes. (F) Immunocytochemical analysis the α-MSH expression in the PIG1 cells infected with AdsiSFRP5 or AdGFP. Nuclei were counterstained with DAPI (blue). (G) The numbers of dendrites and melanosomes in SFRP5 knockdown PIG1 cells. Error bars are the means ± SD, n = 3. ∗∗∗P < 0.001 or ∗∗P < 0.01 vs. control.
SFRP5 inhibits the expression levels of melanogenesis-associated proteins
To investigate whether SFRP5 affects the expression of melanogenesis-related proteins, we further examined the mRNA and protein expression of the melanogenesis-associated proteins, MITF, tyrosinase (TYR), tyrosine-related protein 1 (TRP1), and tyrosine-related protein 2 (TRP2) in PIG1 cells treated with AdSFRP5 and AdRFP. Results showed that the mRNA and protein levels of MITF, TYR, TRP1, and TRP2 were significantly decreased in AdSFRP5-treated cells compared with those in AdRFP-treated cells (Fig. 3A and B). Similarly, the effect of SFRP5 downregulation on PIG3V cells was examined. As shown in Fig. 3C and D, silencing of SFRP5 significantly increased the mRNA and protein levels of MITF, TYR, TRP1, and TRP2.
Figure 3.
SFRP5 down-regulates the melanogenesis-associated proteins. (A) The expressions of MITF, TYR, TRP1 and TRP2 were examined by means of RT-PCR in AdSFRP5-infected PIG1 cells. (B) Western blot analysis of the protein expression levels of MITF, TYR, TRP1 and TRP2 in AdSFRP5-infected PIG1 cells. (C) RT-PCR analyzed the MITF, TYR, TRP1 and TRP2 mRNA expression levels in AdsiSFRP5-infected PIG3V cells. (D) Western blot analysis of the protein expression levels of MITF, TYR, TRP1 and TRP2 in AdsiSFRP5-infected PIG3V cells. Error bars are the means ± SD, n = 3. ∗∗∗P < 0.001, ∗∗P < 0.01 or ∗P < 0.05 vs. control.
SFRP5 inhibits Wnt/β-catenin signaling in melanocytes
SFRP5 is a known antagonist of the Wnt signaling pathway. While Wnt signaling plays a significant role in regulating melanocyte function, it is unclear whether SFRP5 inhibits melanogenesis in melanocytes through this pathway. Hence, we examined the effect of SFRP5 on the Wnt/β-catenin signaling pathway in melanocytes. Results showed that SFRP5 downregulation decreased the phosphorylation level of glycogen synthase kinase 3β (GSK3β) at Ser9, resulting in a decrease in the phosphorylation of β-catenin at Ser675 and total β-catenin level (Fig. 4A). Consistently, SFRP5 knockdown significantly increased the phosphorylation of GSK3β at Ser9 and of β-catenin at Ser675 (Fig. 4B). Next, we performed TCF/LEF luciferase reporter assay to investigate whether SFRP5 inactivates β-catenin signaling. Treatment with AdSFRP5 inhibited luciferase activity and green fluorescence protein expression in PIG1 cells, while treatment with AdsiSFRP5 increased luciferase activity compared with control treatment (Fig. 4C). Finally, to further confirm the effect of β-catenin signaling on melanogenesis, double immunocytochemical staining was performed. Confocal microscopic examination showed a significantly reduced proportion of nuclear β-catenin in PIG1 cells overexpressing SFRP5 (Fig. 4D), but a significantly increased proportion of nuclear β-catenin in SFRP5-silenced cells (Fig. 4E).
Figure 4.
SFRP5 inhibits β-catenin signaling in melanocytes. (A) Western blot analysis of the protein expression levels of β-cateninS675, total β-catenin, GSK-3βS9 and GSK-3β in AdSFRP5-infected PIG1 cells. (B) Western blot analysis of the protein expression levels of β-cateninS675, total β-catenin, GSK-3βS9 and GSK-3β in AdsiSFRP5-infected PIG3V cells. (C) A TCF/LEF reporter luciferase assay was performed using PIG1 cells infected with AdSFRP5, AdRFP, AdsiSFRP5 or AdGFP. (D) The localization of β-catenin was analyzed using a confocal microscope in PIG1 cells that overexpress SFRP5. Nuclei were counterstained with DAPI (blue). (E) The localization of β-catenin was analyzed using a confocal microscope in PIG3V that knockdown SFRP5. Nuclei were counterstained with DAPI (blue). Error bars are the means ± SD, n = 3. ∗∗∗P < 0.001, ∗∗P < 0.01 or ∗P < 0.05 vs. control.
The inhibitory effect of SFRP5 on melanogenesis can be reversed by the β-catenin agonist SKL2001
To further confirm the role of Wnt/β-catenin signaling pathway in the inhibitory effects of SFRP5 on melanogenesis in PIG1 cells, SKL2001 was used to activate the Wnt/β-catenin signaling pathway. The results of concentration screening showed that SKL2001 had an optimal activation effect on Wnt signaling pathway at a concentration of 10 μM (Fig. 5A). Hence, this concentration was selected for subsequent experiments. As shown in Fig. 5B, after treatment with SKL2001, the expression levels of the melanogenesis-associated proteins, MITF, TYR, TRP1, and TRP2, were significantly increased. In addition, β-catenin activation markedly abrogated the SFRP5-induced downregulation of melanogenesis-associated proteins (Fig. 5B). Consistently, the effect of SFRP5 on the level of tyrosinase activity (Fig. 5C) and melanin content (Fig. 5D) was also reversed by treatment with SKL2001.
Figure 5.
SKL2001 reverses the inhibitory effect of SFRP5 on melanogenesis. (A) The optimal concentration of SKL2001 was detected by Western blot. (B) Western blot analysis of protein expression levels of SFRP5, MITF, TYR, TRP1 and TRP2 in PIG1 cells. (C) Results of tyrosinase activity measurement. (D) Results of melanin content measurement. Error bars are the means ± SD, n = 3. ∗∗∗P < 0.001, ∗∗P < 0.01 or ∗P < 0.05 vs. control.
SFRP5 suppresses melanogenesis of B16 cells in vivo
Since PIG1 cells are normal human-derived immortalized melanocytes,36 pigmentation changes are difficult to observe after transplanting them into nude mouse. Although B16 cells are mouse-derived melanoma cells, they were widely used for the research of melanin synthesis.37, 38, 39, 40 Hence, we chose B16 cells to establish a xenograft model in nude mice. The results showed that the skin was slightly lighter in the AdSFRP5 group than in the control group. Similarly, the AdsiSFRP5 group showed skin hyperpigmentation compared with the AdGFP group (Fig. 6A). Subsequently, ex vivo mouse skin was used for immunohistochemical staining, and the results showed that α-MSH expression was decreased in the AdSFRP5 group, but increased in the AdsiSFRP5 group (Fig. 6B).
Figure 6.
SFRP5 inhibits the melanogenesis of B16 cells in vivo. (A) The pigmentation on the back skin of nude mice. (B) Immunohistochemical staining for the expression of α-MSH. Scale bar = 100 μm (magnification: 400×).
Discussion
In the present study, we first revealed the overexpression of SFRP5 in vitiligo lesions and vitiligo melanocytes. In addition, we proved that SFRP5 overexpression suppressed melanogenesis by inhibiting the Wnt/β-catenin signaling pathway in normal melanocytes. Subsequently, we showed that treatment with the β-catenin agonist reversed the inhibitory effect of SFRP5 on melanogenesis in normal melanocytes. Our data further demonstrated that SFRP5 silencing contributed to repigmentation in vitiligo melanocytes through the Wnt/β-catenin signaling. Finally, we confirmed the inhibitory effects of SFRP5 on melanogenesis in a nude mouse model. Collectively, our study demonstrates that SFRP5 inhibits melanin synthesis in vitiligo melanocytes via the Wnt/β-catenin signaling pathway, and more importantly, specific knockdown of SFRP5 in melanocytes may be a promising therapeutic approach for vitiligo treatment (Fig. 7).
Figure 7.
Wnt/β-catenin signaling pathway regulates melanogenesis of melanocytes. The expression of SFRP5 is increased in vitiligo melanocytes compared with normal melanocytes. In normal melanocytes, overexpression of SFRP5 inhibits the Wnts and then reduces the accumulation of β-catenin in the cytoplasm, subsequently reduces β-catenin entering the nucleus. We use SKL2001 to activate β-catenin, increase its entry into the nucleus, and then proceed nuclear translocation to start the transcription and translation of downstream target genes. In vitiligo melanocytes, We knock down the SFRP5, then the Wnt signaling pathway is activated, β-catenin accumulates in the cytoplasm, subsequently enters the nucleus, nuclear translocation occurs, and transcription and translation of downstream target genes are initiated.
Vitiligo is an acquired disfiguring skin disorder, mainly characterized by progressive depigmentation due to the destruction of epidermal melanocytes.41,42 Although multiple mechanisms are involved in the pathogenesis of vitiligo, the most accepted hypothesis is that both genetic and non-genetic factors affect the function and survival of melanocytes, eventually leading to their immune-mediated destruction.42, 43, 44 As one of the SFRP family members, SFRP5 has been reported to be associated with several immune-related disorders, such as rheumatoid arthritis,45 psoriasis,46 and type 2 diabetes mellitus,27 which are close to vitiligo.7 In addition, Wnt signaling pathway activation is closely related to repigmentation in vitiligo.47, 48, 49, 50 However, as an antagonist of the Wnt signaling pathway, the link between SFRP5 and vitiligo remains indistinct. In our previous study, we found that SFRP5 had a lower expression in melanoma tissues than in normal skin tissues, which inhibited the migration and invasion of melanoma cells.51 In this study, we first demonstrated that the expression of SFRP5 in vitiligo skin lesions was increased. Furthermore, the mRNA and protein expression of SFRP5 was significantly increased in PIG3V cells compared with that in PIG1 cells. Emerging evidences highlight that vitiligo has an inverse relationship with malignant melanoma,52, 53, 54, 55, 56 which is consistent with our results.
The formation and maturation of melanosomes and the ability to synthesize melanin are key to vitiligo repigmentation.57,58 On the basis of its overexpression in vitiligo skin in vivo, we hypothesized that SFRP5 inhibits melanogenesis. Overexpression or silencing of SFRP5 expression was then achieved by recombinant or control adenoviruses, respectively, and the direct biological effects of SFRP5 on melanocytes were investigated. The results showed that SFRP5 overexpression reduced melanin content and tyrosinase activity and inhibited α-MSH immunoreactivity in PIG1 cells. Interestingly, SFRP5 overexpression was found to be associated with reduced numbers of dendrites and melanosomes. It is well known that melanin synthesis is an extremely complex process, which mainly involves three enzymes of the tyrosine gene family, namely, TYR, TRP1, and TRP2, of which TYR is the key enzyme for this process.59 MITF can regulate the expression of TYR, TRP1, and TRP2, thereby participating in the regulation of the whole melanin synthesis process.60 As the ability to synthesize melanin is the key to vitiligo repigmentation, we further investigated the effect of SFRP5 on the levels of melanogenesis-associated proteins. The results indicated that SFRP5 inhibited the mRNA and protein expression of the melanogenesis-associated proteins. These data revealed that SFRP5 can inhibit the biological function of melanocytes.
Next, we also provided evidence that SFRP5 behaves as a negative regulator of the canonical Wnt pathway in human melanocytes. Increasing evidence has highlighted the negative effect of SFRP5 as an antagonist of β-catenin.61,62 Moreover, previous findings have also revealed that activation of Wnt/β-catenin signaling results in accumulation of β-catenin, which forms a complex with LEF-1 to upregulate the gene expression of MITF.63 In addition, β-catenin directly interacts with the MITF protein and activates the MITF-specific target genes.50,64,65 In our study, SFRP5 overexpression in PIG1 cells resulted in decreased levels of the active form of β-catenin. Moreover, we found that SFRP5 overexpression induced the downregulation of GSK3βS9, β-cateninS675, and total β-catenin. Furthermore, SFRP5 overexpression significantly decreased the expression of MITF and LEF-1, which are known downstream target genes of β-catenin. More importantly, the inhibitory effect of SFRP5 on melanogenesis in melanocytes was reversed by treatment with the β-catenin agonist.
Finally, we assessed the efficacy of the SFRP5 overexpression on B16 cells in vivo. The results indicated that SFRP5 overexpression lightened the back skin of nude mice, while knockdown of SFRP5 promoted repigmentation of the back skin. Moreover, the most suitable animal model can be generated by using ultraviolet B (UVB) to induce pigmentation in brown guinea pigs66, 67, 68 or C57BL/6J mouse,69,70 and then performing various treatments and observing the color changes. However, because of our limited experimental conditions, UVB induction could not be performed. Moreover, the above-mentioned method is applicable to processing factors that can inhibit pigmentation in mouse skin, but our processing factors both increased and decreased pigmentation. Furthermore, there are several recent studies using nude mice for pigmentation research.71, 72, 73 Hence, we selected a normal nude mouse model.
In conclusion, our results showed that SFRP5 inhibits melanin synthesis in melanocytes through the Wnt/β-catenin signaling pathway in vitro and exhibits melanin-inhibiting activity in vivo. As for the possible mechanism of SFRP5 upregulation in vitiligo melanocytes and vitiligo skin lesions, we speculate that it may be regulated by epigenetics,74 inflammation,75 and immune-mediated factors.76 In recent years, epigenetic changes in SFPR5, such as SFRP5 gene methylation have been closely related to the occurrence and development of various tumors. Moreover, SFRP5 has been reported to act as a tumor suppressor gene in various cancers,77 and it is well known that transcriptional suppression of tumor suppressor genes leads to tumor development. For example, epigenetic inactivation of the SFRP5 gene in human breast cancer is associated with poor prognosis,78 hypermethylation of SFRP5 in multiple myeloma is associated with advanced disease stage,79 epigenetic silencing of SFRP5 promotes the metastasis and invasion of chondrosarcoma,80 and SFRP5 methylation is closely related to the incidence of colorectal cancer and poor prognosis of gastric cancer.81,82 Therefore, we speculate that in vitiligo skin lesions, SFRP5 expression may increase due to its demethylation. However, this needs to be confirmed by further experiments. An in-depth investigation of the impact of SFRP5 epigenetic changes on the biological function of melanocytes may contribute to the clinical development of targeted vitiligo drugs.
Availability of data and materials
Data sets used or analyzed during the current study can be obtained reasonably request of the corresponding authors.
Data statement
All data generated or analyzed during this study are authentic. Besides, the datasets during the current study are available from the corresponding author on reasonable request.
Authors contribution
JC and TWG planned the experiments; YMC and DPZ performed the experiments, AI and PTKN prepared the figures and analyzed the data; XHY and LZZ helped to perform experiments; YJZ collected the samples; DPZ composed the manuscript. All authors read and approved the final manuscript.
Conflict of Interests
The authors declare no conflict of interests.
Funding
This work was supported by the National Natural Science Foundation of China [grant number 81773307]; and a research grant from Chongqing Science and Technology Commission [grant number cstc2018jcyjAX0195].
Acknowledgements
We thank Dr. T.C. He (Medical Center of University of Chicago, Chicago IL, USA) for his kind provision of the recombinant adenoviruses, plasmid and Dr. Chunying Li (Xijing Hospital, Fourth Military Medical University, Xi'an, Shaanxi, China) for her kind provision of the human melanocyte cell lines PIG1 and PIG3V. We also thank the vitiligo patients and healthy donors for their participation in this study.
Footnotes
Peer review under responsibility of Chongqing Medical University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.gendis.2020.06.003.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
figs1.
figs2.
figs3.
References
- 1.Sahoo A., Lee B., Boniface K. MicroRNA-211 regulates oxidative phosphorylation and energy metabolism in human vitiligo. J Invest Dermatol. 2017;137(9):1965–1974. doi: 10.1016/j.jid.2017.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.He Y., Li S., Zhang W. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci Rep. 2017;7 doi: 10.1038/srep42394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shi Q., Zhang W., Guo S. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016;23(3):496–508. doi: 10.1038/cdd.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Su M., Yi H., He X. miR-9 regulates melanocytes adhesion and migration during vitiligo repigmentation induced by UVB treatment. Exp Cell Res. 2019;384(1) doi: 10.1016/j.yexcr.2019.111615. [DOI] [PubMed] [Google Scholar]
- 5.Speeckaert R., van Geel N. Vitiligo: an update on pathophysiology and treatment options. Am J Clin Dermatol. 2017;18(6):733–744. doi: 10.1007/s40257-017-0298-5. [DOI] [PubMed] [Google Scholar]
- 6.Sehgal V.N., Srivastava G. Vitiligo: compendium of clinico-epidemiological features. Indian J Dermatol Venereol Leprol. 2007;73(3):149–156. doi: 10.4103/0378-6323.32708. [DOI] [PubMed] [Google Scholar]
- 7.Ezzedine K., Eleftheriadou V., Whitton M. Vitiligo. Lancet. 2015;386(9988):74–84. doi: 10.1016/S0140-6736(14)60763-7. [DOI] [PubMed] [Google Scholar]
- 8.Sawant N.S., Vanjari N.A., Khopkar U. Gender differences in depression, coping, stigma, and quality of life in patients of vitiligo. Dermatol Res Pract. 2019;2019 doi: 10.1155/2019/6879412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodrigues M., Ezzedine K., Hamzavi I. New discoveries in the pathogenesis and classification of vitiligo. J Am Acad Dermatol. 2017;77(1):1–13. doi: 10.1016/j.jaad.2016.10.048. [DOI] [PubMed] [Google Scholar]
- 10.Silverberg J.I., Silverberg N.B. Quality of life impairment in children and adolescents with vitiligo. Pediatr Dermatol. 2014;31(3):309–318. doi: 10.1111/pde.12226. [DOI] [PubMed] [Google Scholar]
- 11.Jin Y., Birlea S.A., Fain P.R. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44(6):676–680. doi: 10.1038/ng.2272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sandoval-Cruz M., Garcia-Carrasco M., Sanchez-Porras R. Immunopathogenesis of vitiligo. Autoimmun Rev. 2011;10(12):762–765. doi: 10.1016/j.autrev.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Gey A., Diallo A., Seneschal J. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168(4):756–761. doi: 10.1111/bjd.12166. [DOI] [PubMed] [Google Scholar]
- 14.Jiang W., Li S., Chen X. Berberine protects immortalized line of human melanocytes from H2O2-induced oxidative stress via activation of Nrf2 and Mitf signaling pathway. J Dermatol Sci. 2019;94(1):236–243. doi: 10.1016/j.jdermsci.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Boniface K., Seneschal J., Picardo M. Vitiligo: focus on clinical aspects, immunopathogenesis, and therapy. Clin Rev Allergy Immunol. 2018;54(1):52–67. doi: 10.1007/s12016-017-8622-7. [DOI] [PubMed] [Google Scholar]
- 16.Yamada T., Akamatsu H., Hasegawa S. Melanocyte stem cells express receptors for canonical Wnt-signaling pathway on their surface. Biochem Biophys Res Commun. 2010;396(4):837–842. doi: 10.1016/j.bbrc.2010.04.167. [DOI] [PubMed] [Google Scholar]
- 17.Yamada T., Hasegawa S., Inoue Y. Wnt/beta-catenin and kit signaling sequentially regulate melanocyte stem cell differentiation in UVB-induced epidermal pigmentation. J Invest Dermatol. 2013;133(12):2753–2762. doi: 10.1038/jid.2013.235. [DOI] [PubMed] [Google Scholar]
- 18.Dorsky R.I., Moon R.T., Raible D.W. Control of neural crest cell fate by the Wnt signalling pathway. Nature. 1998;396(6709):370–373. doi: 10.1038/24620. [DOI] [PubMed] [Google Scholar]
- 19.Dunn K.J., Williams B.O., Li Y. Neural crest-directed gene transfer demonstrates Wnt1 role in melanocyte expansion and differentiation during mouse development. Proc Natl Acad Sci U S A. 2000;97(18):10050–10055. doi: 10.1073/pnas.97.18.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mei X., Wu Z., Huang J. Screening and analysis of differentially expressed genes of human melanocytes in skin cells mixed culture. Am J Transl Res. 2019;11(5):2657–2667. [PMC free article] [PubMed] [Google Scholar]
- 21.Birlea S.A., Costin G.E., Roop D.R. Trends in regenerative medicine: repigmentation in vitiligo through melanocyte stem cell mobilization. Med Res Rev. 2017;37(4):907–935. doi: 10.1002/med.21426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Regazzetti C., Joly F., Marty C. Transcriptional analysis of vitiligo skin reveals the alteration of Wnt pathway: a promising target for repigmenting vitiligo patients. J Invest Dermatol. 2015;135(12):3105–3114. doi: 10.1038/jid.2015.335. [DOI] [PubMed] [Google Scholar]
- 23.Heuberger J., Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harb Perspect Biol. 2010;2(2) doi: 10.1101/cshperspect.a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris J.E. Melanocyte regeneration in vitiligo requires WNT beneath their wings. J Invest Dermatol. 2015;135(12):2921–2923. doi: 10.1038/jid.2015.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bovolenta P., Esteve P., Ruiz J.M. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. J Cell Sci. 2008;121(Pt 6):737–746. doi: 10.1242/jcs.026096. [DOI] [PubMed] [Google Scholar]
- 26.Tong S., Ji Q., Du Y. Sfrp5/Wnt pathway: a protective regulatory system in atherosclerotic cardiovascular disease. J Interferon Cytokine Res. 2019;39(8):472–482. doi: 10.1089/jir.2018.0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu L.B., Chen X.D., Zhou X.Y., Zhu Q. The Wnt antagonist and secreted frizzled-related protein 5: implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Biosci Rep. 2018;38(4) doi: 10.1042/BSR20180011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jian Z., Li K., Song P. Impaired activation of the Nrf2-ARE signaling pathway undermines H2O2-induced oxidative stress response: a possible mechanism for melanocyte degeneration in vitiligo. J Invest Dermatol. 2014;134(8):2221–2230. doi: 10.1038/jid.2014.152. [DOI] [PubMed] [Google Scholar]
- 29.Xu P., Su S., Tan C., Lai R.S., Min Z.S. Effects of aqueous extracts of Ecliptae herba, Polygoni multiflori radix praeparata and Rehmanniae radix praeparata on melanogenesis and the migration of human melanocytes. J Ethnopharmacol. 2017;195:89–95. doi: 10.1016/j.jep.2016.11.045. [DOI] [PubMed] [Google Scholar]
- 30.Cui H., Guo S., He H. SASH1 promotes melanin synthesis and migration via suppression of TGF-β1 secretion in melanocytes resulting in pathologic hyperpigmentation. Int J Biol Sci. 2020;16(7):1264–1273. doi: 10.7150/ijbs.38415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baba Y., Funakoshi T., Mori M. Expression of monoacylglycerol lipase as a marker of tumour invasion and progression in malignant melanoma. J Eur Acad Dermatol Venereol. 2017;31(12):2038–2045. doi: 10.1111/jdv.14455. [DOI] [PubMed] [Google Scholar]
- 32.Pelkonen L., Reinisalo M., Morin-Picardat E. Isolation of intact and functional melanosomes from the retinal pigment epithelium. PloS One. 2016;11(8) doi: 10.1371/journal.pone.0160352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong X.X., Ding G.Z., Zhao W.E. Differences in the melanosome distribution within the epidermal melanin units and its association with the impairing background of leukoderma in vitiligo and halo nevi: a retrospective study. Arch Dermatol Res. 2017;309(5):323–333. doi: 10.1007/s00403-017-1730-7. [DOI] [PubMed] [Google Scholar]
- 34.Tan T., Chen J., Hu Y. Dihydrotanshinone I inhibits the growth of osteosarcoma through the Wnt/beta-catenin signaling pathway. OncoTargets Ther. 2019;12:5111–5122. doi: 10.2147/OTT.S204574. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Yamaguchi K., Zhu C., Ohsugi T. Bidirectional reporter assay using HAL promoter and TOPFLASH improves specificity in high-throughput screening of Wnt inhibitors. Biotechnol Bioeng. 2017;114(12):2868–2882. doi: 10.1002/bit.26394. [DOI] [PubMed] [Google Scholar]
- 36.Le Poole I.C., van den Berg F.M., van den Wijngaard R.M. Generation of a human melanocyte cell line by introduction of HPV16 E6 and E7 genes. In Vitro Cell Dev Biol Anim. 1997;33(1):42–49. doi: 10.1007/s11626-997-0021-6. [DOI] [PubMed] [Google Scholar]
- 37.Azumi J., Takeda T., Shimada Y. The organogermanium compound THGP suppresses melanin synthesis via complex formation with L-DOPA on mushroom tyrosinase and in B16 4A5 melanoma cells. Int J Mol Sci. 2019;20(19) doi: 10.3390/ijms20194785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jia Q., Hu S., Jiao D. Synaptotagmin-4 promotes dendrite extension and melanogenesis in alpaca melanocytes by regulating Ca(2+) influx via TRPM1 channels. Cell Biochem Funct. 2020;38(3):275–282. doi: 10.1002/cbf.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lv J., Fu Y., Gao R. Diazepam enhances melanogenesis, melanocyte dendricity and melanosome transport via the PBR/cAMP/PKA pathway. Int J Biochem Cell Biol. 2019;116 doi: 10.1016/j.biocel.2019.105620. [DOI] [PubMed] [Google Scholar]
- 40.Guo L., Yin Z., Wen L. Flower extracts from Paeonia decomposita and Paeonia ostii inhibit melanin synthesis via cAMP-CREB-associated melanogenesis signaling pathways in murine B16 melanoma cells. J Food Biochem. 2019;43(4) doi: 10.1111/jfbc.12777. [DOI] [PubMed] [Google Scholar]
- 41.Xie H., Zhou F., Liu L. Vitiligo: how do oxidative stress-induced autoantigens trigger autoimmunity? J Dermatol Sci. 2016;81(1):3–9. doi: 10.1016/j.jdermsci.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Yi X., Guo W., Shi Q. SIRT3-Dependent mitochondrial dynamics remodeling contributes to oxidative stress-induced melanocyte degeneration in vitiligo. Theranostics. 2019;9(6):1614–1633. doi: 10.7150/thno.30398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kingo K., Aunin E., Karelson M. Expressional changes in the intracellular melanogenesis pathways and their possible role in the pathogenesis of vitiligo. J Dermatol Sci. 2008;52(1):39–46. doi: 10.1016/j.jdermsci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Sastry K.S., Naeem H., Mokrab Y., Chouchane A.I. RNA-seq reveals dysregulation of novel melanocyte genes upon oxidative stress: implications in vitiligo pathogenesis. Oxid Med Cell Longev. 2019;2019 doi: 10.1155/2019/2841814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kwon Y.J., Lee S.W., Park Y.B. Secreted frizzled-related protein 5 suppresses inflammatory response in rheumatoid arthritis fibroblast-like synoviocytes through down-regulation of c-Jun N-terminal kinase. Rheumatology. 2014;53(9):1704–1711. doi: 10.1093/rheumatology/keu167. [DOI] [PubMed] [Google Scholar]
- 46.Gerdes S., Laudes M., Neumann K. Wnt5a: a potential factor linking psoriasis to metabolic complications. Exp Dermatol. 2014;23(6):438–440. doi: 10.1111/exd.12413. [DOI] [PubMed] [Google Scholar]
- 47.Li D., Wang X., Fu Y. Transcriptome analysis of the breast muscle of xichuan black-bone chickens under tyrosine supplementation revealed the mechanism of tyrosine-induced melanin deposition. Front Genet. 2019;10 doi: 10.3389/fgene.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwai-Takekoshi L., Balasubramanian R., Sitko A. Activation of Wnt signaling reduces ipsilaterally projecting retinal ganglion cells in pigmented retina. Development. 2018;145(21) doi: 10.1242/dev.163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang S., Liu B., Ji K. MicroRNA-5110 regulates pigmentation by cotargeting melanophilin and WNT family member 1. Faseb J. 2018;32(10):5405–5412. doi: 10.1096/fj.201800040R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang I., Park J.H., Park H.S. Neural stem cells inhibit melanin production by activation of Wnt inhibitors. J Dermatol Sci. 2013;72(3):274–283. doi: 10.1016/j.jdermsci.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Chen Y., Zou D., Wang N. SFRP5 inhibits the migration and invasion of melanoma cells through Wnt signaling pathway. OncoTargets Ther. 2018;11:8761–8772. doi: 10.2147/OTT.S181146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nardin C., Jeand’heur A., Bouiller K. Vitiligo under anti-PD1 therapy is associated with increased survival in melanoma patients. J Am Acad Dermatol. 2020;82(3):770–772. doi: 10.1016/j.jaad.2019.11.017. [DOI] [PubMed] [Google Scholar]
- 53.Teulings H.E., Overkamp M., Ceylan E. Decreased risk of melanoma and nonmelanoma skin cancer in patients with vitiligo: a survey among 1307 patients and their partners. Br J Dermatol. 2013;168(1):162–171. doi: 10.1111/bjd.12111. [DOI] [PubMed] [Google Scholar]
- 54.Schallreuter K.U., Tobin D.J., Panske A. Decreased photodamage and low incidence of non-melanoma skin cancer in 136 sun-exposed caucasian patients with vitiligo. Dermatology. 2002;204(3):194–201. doi: 10.1159/000057881. [DOI] [PubMed] [Google Scholar]
- 55.Paradisi A., Tabolli S., Didona B. Markedly reduced incidence of melanoma and nonmelanoma skin cancer in a nonconcurrent cohort of 10,040 patients with vitiligo. J Am Acad Dermatol. 2014;71(6):1110–1116. doi: 10.1016/j.jaad.2014.07.050. [DOI] [PubMed] [Google Scholar]
- 56.Czajkowski R., Mecinska-Jundzill K. Current aspects of vitiligo genetics. Postepy Dermatol Alergol. 2014;31(4):247–255. doi: 10.5114/pdia.2014.43497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zavala G., Sandoval C., Meza D. Differentiation of adipose-derived stem cells to functional CD105(neg) CD73(low) melanocyte precursors guided by defined culture condition. Stem Cell Res Ther. 2019;10(1) doi: 10.1186/s13287-019-1364-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vaish U., Kumar A.A., Varshney S. Micro RNAs upregulated in Vitiligo skin play an important role in its aetiopathogenesis by altering TRP1 expression and keratinocyte-melanocytes cross-talk. Sci Rep. 2019;9(1) doi: 10.1038/s41598-019-46529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pillaiyar T., Manickam M., Jung S.H. Downregulation of melanogenesis: drug discovery and therapeutic options. Drug Discov Today. 2017;22(2):282–298. doi: 10.1016/j.drudis.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Hwang Y.S., Kim Y.J., Kim M.O. Cannabidiol upregulates melanogenesis through CB1 dependent pathway by activating p38 MAPK and p42/44 MAPK. Chem Biol Interact. 2017;273:107–114. doi: 10.1016/j.cbi.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 61.Zhu J., Wang Y., Duan J. DNA Methylation status of Wnt antagonist SFRP5 can predict the response to the EGFR-tyrosine kinase inhibitor therapy in non-small cell lung cancer. J Exp Clin Canc Res. 2012;31(1) doi: 10.1186/1756-9966-31-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang H., Fan R., Wang X.Q. Methylation of Wnt antagonist genes: a useful prognostic marker for myelodysplastic syndrome. Ann Hematol. 2013;92(2):199–209. doi: 10.1007/s00277-012-1595-y. [DOI] [PubMed] [Google Scholar]
- 63.Yasumoto K., Takeda K., Saito H. Microphthalmia-associated transcription factor interacts with LEF-1, a mediator of Wnt signaling. EMBO J. 2002;21(11):2703–2714. doi: 10.1093/emboj/21.11.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho M., Ryu M., Jeong Y. Cardamonin suppresses melanogenesis by inhibition of Wnt/beta-catenin signaling. Biochem Biophys Res Commun. 2009;390(3):500–505. doi: 10.1016/j.bbrc.2009.09.124. [DOI] [PubMed] [Google Scholar]
- 65.Schepsky A., Bruser K., Gunnarsson G.J. The microphthalmia-associated transcription factor Mitf interacts with beta-catenin to determine target gene expression. Mol Cell Biol. 2006;26(23):8914–8927. doi: 10.1128/MCB.02299-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Feng L., Shi N., Cai S. De novo molecular design of a novel octapeptide that inhibits in vivo melanogenesis and has great transdermal ability. J Med Chem. 2018;61(15):6846–6857. doi: 10.1021/acs.jmedchem.8b00737. [DOI] [PubMed] [Google Scholar]
- 67.Ahn H.Y., Choo Y.M., Cho Y.S. Anti-pigmentation effects of eight phellinus linteus-fermented traditional crude herbal extracts on Brown Guinea pigs of ultraviolet B-induced hyperpigmentation. J Microbiol Biotechnol. 2018;28(3):375–380. doi: 10.4014/jmb.1711.11043. [DOI] [PubMed] [Google Scholar]
- 68.Park J.I., Lee H.Y., Lee J.E. Inhibitory effect of 2-methyl-naphtho[1,2,3-de]quinolin-8-one on melanosome transport and skin pigmentation. Sci Rep. 2016;6 doi: 10.1038/srep29189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.You Y.J., Wu P.Y., Liu Y.J. Sesamol inhibited ultraviolet radiation-induced hyperpigmentation and damage in C57BL/6 mouse skin. Antioxidants (Basel) 2019;8(7) doi: 10.3390/antiox8070207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Q., Wang Y., Pang S. Alcohol extract from Vernonia anthelmintica willd (L.) seed counteracts stress-induced murine hair follicle growth inhibition. BMC Compl Alternative Med. 2019;19(1) doi: 10.1186/s12906-019-2744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cario M., Pain C., Kaulanjan-Checkmodine P. Epidermal Keratin 5 expression and distribution Is under dermal influence. Pigment Cell Melanoma Res. 2020;33(3):435–445. doi: 10.1111/pcmr.12844. [DOI] [PubMed] [Google Scholar]
- 72.Kawakami T., Okano T., Takeuchi S. Approach for the derivation of melanocytes from induced pluripotent stem cells. J Invest Dermatol. 2018;138(1):150–158. doi: 10.1016/j.jid.2017.07.849. [DOI] [PubMed] [Google Scholar]
- 73.Lim W.S., Kim C.H., Kim J.Y. Adipose-derived stem cells improve efficacy of melanocyte transplantation in animal skin. Biomol Ther (Seoul) 2014;22(4):328–333. doi: 10.4062/biomolther.2014.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kim T.O., Han Y.K., Yi J.M. Hypermethylated promoters of tumor suppressor genes were identified in Crohn’s disease patients. Intest Res. 2020;18(3):297–305. doi: 10.5217/ir.2019.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang C. Obesity, inflammation, and lung injury (OILI): the good. Mediators Inflamm. 2014;2014 doi: 10.1155/2014/978463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Maekawa T., Kulwattanaporn P., Hosur K. Differential expression and roles of secreted frizzled-related protein 5 and the wingless homolog Wnt5a in periodontitis. J Dent Res. 2017;96(5):571–577. doi: 10.1177/0022034516687248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Surana R., Sikka S., Cai W. Secreted frizzled related proteins: implications in cancers. Biochim Biophys Acta. Jan 2014;1845(1):53–65. doi: 10.1016/j.bbcan.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 78.Veeck J., Geisler C., Noetzel E. Epigenetic inactivation of the secreted frizzled-related protein-5 (SFRP5) gene in human breast cancer is associated with unfavorable prognosis. Carcinogenesis. May 2008;29(5):991–998. doi: 10.1093/carcin/bgn076. [DOI] [PubMed] [Google Scholar]
- 79.van Andel H., Kocemba K.A., Spaargaren M. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options. Leukemia. May 2019;33(5):1063–1075. doi: 10.1038/s41375-019-0404-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheng W., Zhang Z.C., Shi D.Y. Epigenetic silencing of SFRP5 promotes the metastasis and invasion of chondrosarcoma by expression inhibition and Wnt signaling pathway activation. Chem Biol Interact. Dec 25 2018;296:1–8. doi: 10.1016/j.cbi.2018.08.020. [DOI] [PubMed] [Google Scholar]
- 81.Wang H., Duan X.L., Qi X.L. Concurrent hypermethylation of SFRP2 and DKK2 activates the Wnt/β-catenin pathway and is associated with poor prognosis in patients with gastric cancer. Mol Cell. Jan 2017;40(1):45–53. doi: 10.14348/molcells.2017.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Silva A.L., Dawson S.N., Arends M.J. Boosting Wnt activity during colorectal cancer progression through selective hypermethylation of Wnt signaling antagonists. BMC Cancer. 2014;14 doi: 10.1186/1471-2407-14-891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sets used or analyzed during the current study can be obtained reasonably request of the corresponding authors.