Abstract
Autism is a heterogeneous neurodevelopmental and neuropsychiatric disorder with no precise etiology. Deficits in cognitive functions uncover at early stages and are known to have an environmental and genetic basis. Since autism is multifaceted and also linked with other comorbidities associated with various organs, there is a possibility that there may be a fundamental cellular process responsible for this. These reasons place mitochondria at the point of interest as it is involved in multiple cellular processes predominantly involving metabolism. Mitochondria encoded genes were taken into consideration lately because it is inherited maternally, has its own genome and also functions the time of embryo development. Various researches have linked mitochondrial mishaps like oxidative stress, ROS production and mt-DNA copy number variations to autism. Despite dramatic advances in autism research worldwide, the studies focusing on mitochondrial dysfunction in autism is rather minimal, especially in India. India, owing to its rich diversity, may be able to contribute significantly to autism research. It is vital to urge more studies in this domain as it may help to completely understand the basics of the condition apart from a genetic standpoint. This review focuses on the worldwide and Indian scenario of autism research; mitochondrial abnormalities in autism and possible therapeutic approaches to combat it.
Keywords: Autism, Copy number variation (CNV), Mitochondria encoded genes, Neurodevelopmental disorder, World-wide scenario
Introduction
Autism is a common, highly heritable and heterogeneous neurodevelopmental disorder that has underlying cognitive features and commonly co-occurs with other conditions. The behaviors, strengths and challenges of people with autism have attracted the attention of scientists and clinicians for at least 500 years.1 The prevalence of autism was 4-2/1000 before four decades and has been continuously on the rise since then, currently, 1 in 59 children are diagnosed with autism.2 Autistic symptoms include hyperactivity, repetitive behavior, and late-onset in gross and fine motor development, sensory conflicts, language impairment, restricted interests, social communication and self-injury.3 The onset of autism is conclusively before 3 years of age.4 As autism is not a single clinical entity, it can be seen as a social sign of hundreds of genetic and genomic disorders. Although, numerous environmental factors and over 500 distinct genetic loci related to autism have been identified recently, its etiology remains undetermined.5 It is a possibility that autism is linked to fundamental cellular processes as it affects various organs, consists of many symptoms and is multidimensional. These reasons place mitochondria at the point of interest as it is involved in multiple cellular processes predominantly involving metabolism. Moreover, mitochondrial dysfunctions and genetic mutations have also been implicated in various neurodegenerative and physiological disorders,6,7 urging similar studies in autism as well. During embryonic development, the immature brain is vulnerable to reduced bio-energetic capacity and this can develop into neurodevelopmental disorders. Genes from the mitochondria are gaining importance due to their maternal inheritance, independent genome and also because they are functionally active during the development of the embryo. Further, various researches have linked mitochondrial mishaps like oxidative stress, ROS production and mt-DNA copy number variations to autism, while some investigations have rejected such an association.8 The observation of neurological disorders in mothers of children with autism further strengthens the allegation that inherited mitochondrial abnormalities may manifest in the form of autism. Although autism research is conducted across the globe, the work based on mitochondrial dysfunction is limited especially in India. The diversity in the population and heritage of India's may help to shine a light on the similarities and differences in the subpopulations with autism. Since different mt-DNA haplotypes seem to have varying autism risks.9 It is vital to urge more studies in this domain as it may help to completely understand the basics of the condition apart from a genetic standpoint. These studies will also help identify environmental factors that may cause mitochondrial damage and subsequently contribute to the development of autism. It is imperative to comprehend the fundamentals of the condition to enhance the usage of precise medication to ameliorate the symptoms of autism. This review focuses on the mitochondrial abnormalities associated with autism, the extent of research focusing on mitochondrial dysfunction in autism in India and worldwide and possible therapeutic approaches.
Autism – the worldwide scenario
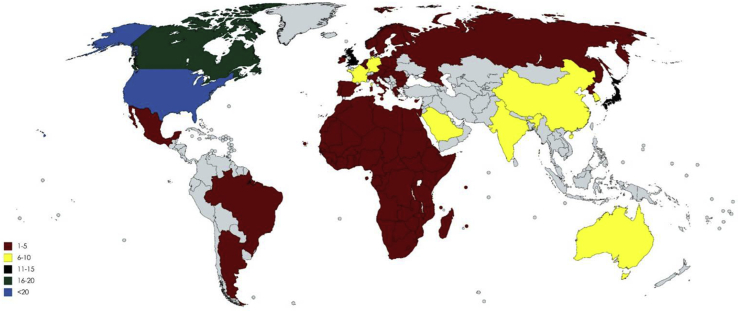
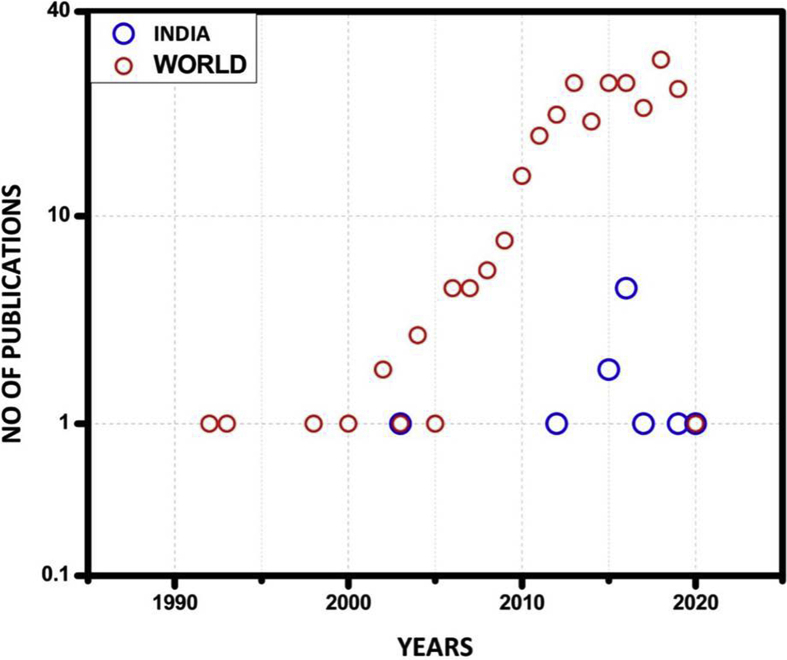
Although autism is predominant in many parts of the world, mitochondrial dysfunction studies in autism are rather limited. A total of 252 articles have been published in this regard. The majority of the articles have been published in the USA. The number of publications are USA (110), Canada (18), Mexico (02), Brazil (04), Africa (03), Europe (05), India (10), South Korea (06), North Korea (06), Japan (14), Saudi Arabia (10), Australia (10), UK (15), Norway (05), China (09), Israel (02), OHIO (06), Argentina (02), Russia (01), Germany (08), Denmark (02), Venezuela (02) and France (06) (Fig. 1). Furthermore, there is a dearth in these types of investigations in India (Fig. 2). It may be worthwhile to conduct relevant studies in India as its diverse population may help uncover essential variants in the mitochondrial genes in individuals with autism. Recently Geetha et al, had found associations between socioeconomic, environmental, pregnancy and newborn-related risk factors and autism. Further, many factors during fetal development were found to affect autism. A better understanding of the prenatal factors associated with autism may be important to further comprehend the inherent nature of autism. These evidences shine light on the genetic basis and other risk factors of autism.10 Whether mitochondrial changes occurring during embryonic neural developmental or inherited mitochondrial mutations have an impact on autism continues to remain enigmatic. More in-depth work is required around the world to accurately pinpoint the associations between mitochondrial dysfunctions and autism, these studies are essential to find innovative diagnostic tools and therapeutics and contribute significantly to the field of neurobiology. This may also help expecting mothers to safely avoid potential risk factors. Similarly, a deep understanding of the functions of the mitochondria in cells may be important to pinpoint specific causes of the condition.
Figure 1.
List of publications worldwide focusing on mitochondrial dysfunction in autism.
• 1–5 = Mexico (02), Brazil (04), Africa (03), Europe (05), North Korea (06), Israel (02), Argentina (02), Russia (01), Denmark (02), Venzuela (02) and Norway (05)
• 6–10 = India (10), South Korea (06), Saudi Arabia (10), Australia (10), China (09), Ohio (06), Germany (08) and France (06)
• 11–15 = Japan (14) and UK (15)
• 16–20 = Canada (18)
• <20 = USA (110).
Figure 2.
Graph depicting the number of studies over a period of 30 years in India and word-wide.
Mitochondrial bio-functions
Mitochondria were named “bio-blast” in the 19th century,11 the name was abandoned in favor of current etymology and named as mitochondria. It was initially found too challenging to isolate and characterize the underlying mechanism of mitochondria until Mitchell's postulated the chemiosmosis hypothesis and said that the energy needed for synthesis of ATP (Adenosine triphosphate), is stored in the form of an electrochemical proton gradient in the inner mitochondrial membrane.12 It is a bioenergetics circuit which also orchestrates the glycolysis and urea cycle in cytosol,13 beta-oxidation14 and TCA cycle in matrix, and ketogenesis14 and oxidative phosphorylation in inner mitochondrial membrane.15 Mitochondria are also involved in heme biosynthesis,16 steroidogenesis,17 biogenesis of iron-sulfur cluster,18 gluconeogenesis,14 calcium storage,19 ROS production and elimination, apoptosis, management of metabolic waste and maintenance of redox homeostasis20 and also stabilizes the protein in endoplasmic reticulum. A mitochondrion is the area of energy production and is named as a powerhouse of the cell.21 The ATP production is a chain process where it begins with glycolysis followed by the conversion of pyruvate to oxaloacetate in TCA cycle from which the proton gradient (NADH and FADH2) is maintained in the mitochondrial complexes (I–V) by two-electron carriers; ubiquinone and cytochrome c.22 This satisfies the high-energy demand in the brain, where neuronal cells take 4.7 billion ATP molecules per second.23 The high-energy demand of the neuronal cells and the brain may be the underlying reason that causes oxidative damage in the cells and contributes to autism.
Splice between autism and mitochondrial dysfunction
Mitochondrial dysfunction could be caused by primary dysfunction that is caused by a mutation in a gene which directly participates in ATP production and secondary dysfunction caused due to other genetic, metabolic and biochemical abnormalities that affect the ability of mitochondria to generate the ATP.21 TWAS (Transcriptome-wide association studies) has been used to recognize the patient risk genes of complex traits24 and this approach has been applied to autism as well as25 since biomarkers for autism remain inconsistent.26 An ongoing meta-examination demonstrated that mitochondrial disease is found in around 5% of children with autism, and 30%–50% of them have biomarkers for mitochondrial abnormality.27 Mitochondrial dysfunction has also been identified in the gastrointestinal tract of autism patients.28 Moreover, a higher rate of irregular electron transport chain (ETC) activity has been reported in lymphocytes, granulocytes29,30 and post-mortem cerebrum tissue31 of autistic individuals. Recent research has claimed that autism is associated with physiological disturbances alongside mitochondrial dysfunction.32 Also, 5%–8% of autistic children have mitochondrial dysfunctions that are rarer than the classic mitochondrial disease. This evidence suggests that autism may have a unique relationship with abnormal mitochondrial function.33 Further, many studies have also implicated different mitochondrial complexes in the pathogenesis of autism.
Complexes dysfunction of mitochondrion coded genes in autistic disorder
Mitochondrial complexes and autism
Mitochondrial dysfunctions highly influence autism. Abnormalities in the complexes may cause defects in the intracellular systems contributing to autism-like behavior. Biochemical markers for mitochondrial dysfunction comprise of lactate, 12 autistic children. Deletions were also observed pyruvate, lactate-to-pyruvate ratio, alanine, alanine-to-lysine ratio, ubiquinone, and acyl-carnitine and in addition, there are indirect markers, such as creatine kinase (CK), carnitine, aspartate aminotransferase (AST), ammonia, alanine aminotransferase (ALT), MRCC-I/caspase-7, GSH/GST, and MRCC-I/COQ10.34, 35, 36, 37 Abnormalities of lactate dehydrogenase, caspase 7, glutathione and glutathione S-transferase have been recorded in autistic individuals indicating mitochondrial dysfunction.38 Interestingly, complex- III, V, pyruvate and I dehydrogenase activity was found to be significantly reduced in the mitochondrial genes ND4 and cytochrome b (cytb). Further, the ratio of mtDNA was higher than nuclear DNA suggesting a variation in the copy number of the genes in affected individuals.39 A similar decrease in enzymatic activity (64%) of complex 1 was seen in 16 of 25 autism and the biochemical parameters such as blood pyruvate and lactate level was elevated in 19/25 (76%) and 9/17(53%) autistic children respectively.34 When 28 autistic children were investigated enzymatic defects were observed in complex I in 14/28(50%) individuals; complex I and III in 5/28 (17.9%) of them and combination of complex I, III, and IV in 5/28(17.9%) patients. Increased levels of pyruvate, lactate levels in the blood, urine, cerebrospinal fluid (46.4%, 13/28) were also observed in autistic individuals.40 When mutational analysis was conducted, homoplasmic mutations were observed in the cytb gene affecting CIII activity.41 A reduction in the mt-DNA content of the cytb gene was also observed along with mitochondrial hyperproliferation and respiratory chain block in CIII in 25 children with autism.42 Additionally, quantity of CI, CIII and CV proteins were higher in the cecum biopsies of 10 autistic children as compared to controls.28 Another study involving mutation screens found an association between the point mutations 1811A > G, 14569G > A, 16126T > C and autism risk in 24 autistic individuals.43 Similar substitutions and point mutations were found in the ATP6, COII and NC7 genes in 24 autism patients.44 Furthermore, hyperlactatemia in mitochondrial respiratory chain complex II–IV was also recorded in autism children.45 These studies display the effect of mutations in mitochondrial complexes in autism (Table 1). These mutations may result in excess ROS production resulting in oxidative stress and damage to the cells.
Table 1.
Mitochondrial complex disinfection influences on autism.
| Mitochondrial complexes | Mode of identification | References |
|---|---|---|
| Complex I II IV | Citrate synthase activities | 29 |
| Complex I III IV | High protein expression in cecum and rectum regions except complex II | 29 |
| Complex III | Mitochondrial hyperproliferation and most stingy partisan respiratory chain block | 43 |
| Complex II–IV | Hyperlactacidemia | 32 |
| Complex 1 | MT-ND5 and other variants of ATP6 and NDUFS4 genes disrupts the functions of Complex 1 | 46 |
ROS production
Production of ROS by mitochondria is a redox signal integrating mitochondria with that of the rest of the cell. Redox signaling can arise with the aid of mitochondria freeing hydrogen peroxide that modulates the action of target proteins via the reversible oxidation of crucial protein thiols, thus altering the function of enzymes, kinases, phosphatases and transcription factors in mitochondria, the cytosol or the nucleus. The prime targets of ROS and membrane phospholipids levels have been altered in autistic condition.44 When the ROS level was higher, the autism-A (abnormal mitochondria) lymphoblastic cell lines (LCLs) consistently showed depletion in Reserve Capacity (RC) of the mitochondria.28,32 ROS attacks polyunsaturated fatty acids, which are constitutive of cell membranes, resulting in secretion of lipid peroxidation (LPO) end products. Several reviews have indicated extended degrees of different LPO markers in autism, confirming an increase in oxidative stress in autism.47 In regular situations, a dynamic equilibrium exists between the production of reactive oxygen species (ROS) and the antioxidant capacity of the cell. The ROS inside the cells are neutralized with the aid of antioxidant protection mechanisms. Superoxide dismutase (SOD), catalase, and glutathione peroxidase (GSH-Px) are the primary enzymes involved in the direct elimination of ROS. Intriguingly, ROS levels were found up-regulated in autism patients, while the regulators of ROS were reduced.48 The elevated synthesis of ROS may bring about autistic pathology.49 SOD2 is the mitochondrial isoform of SOD which utilizes manganese to scavenge the superoxide radicals delivered by mitochondria. The suppressed response of SOD2 results in mitochondrial dysfunction in autism.50,51 Moreover, ROS can also result in inflammation in individuals with autism as the link between oxidative stress and inflammation is well documented. The accumulation of oxidized proteins, the consequence of high levels of ROS can result in neuroinflammation in autism.52 Protein oxidation can also result in the secretion of inflammatory signals that stimulates the macrophages into producing TNF.53 Moreover, reduced levels of GSH can also contribute to inflammation.54 ROS can also result in the increase of proinflammatory cytokines that can directly cause the inflammatory disorders in individuals with autism.53 ROS also has the ability to activate a wide range of signaling pathways that can result in a relatively high level of proinflammatory chemicals.55 In addition, NADPH oxidase derived ROS has been known to cause inflammation. Individuals with autism exhibit high levels of TLR-4 (Toll like receptor 4) in the T cells when compared to typically developing children.56 This result suggests that ROS may contribute to the increased neuroinflammation in children with ASD. Moreover, IL-17A/IL-17R signaling is also increased in children with autism. This contributes to increased ROS production further increasing the inflammatory states.57 ROS imbalance results in oxidative stress and this may have detrimental consequences in the cells.
Oxidative stress
Oxidative stress refers to a pathologic state raised from the imbalance between ROS and capacity of the cell to detoxify it, an obstruction in the process leads to severe harm to all macromolecules (protein, lipid, and DNA) and interfere with many communication pathways.58 The brain has a relatively large vulnerability to oxidative injury as compared to other organs, due to which oxidative stress has been concerned within the pathologic process of major psychiatric disorders.59 Several reports have indicated accrued levels of different LPO markers in autism, confirming a rise in oxidative stress in autism. A study focused on children with autism and their siblings reported higher LPO within the blood of kids with autism as compared to their developmentally traditional and non-autistic siblings. Likewise, increased generation of mitochondrial free radicals, higher concentrations of ROS and reactive nitrogen species was present in patients with autism as compared to controls.60 These reports provide an extra link between oxidative stress and genetic factors within the pathological process of autism. Furthermore, along with oxidative stress, another factor associated with autism is programmed cell death or apoptosis.
Apoptosis
As many cellular mechanisms participate and maintain normal development of the nervous system; activation of programmed cell death happens due to spatial, temporal errors in the stimuli and errors in the apoptosis pathway itself.58 Many active factors act upon fetal development in the brain, adult, and infants that cause abnormalities in apoptosis. Apoptosis can possibly be involved in the development of autism.61 The cyclic dipeptides (proline–phenylalanine) are the stimuli for the activation of apoptosis.62 It has been hypothesized that the exogenous cyclic dipeptides would interfere with the neural development at the early stage, which might result in psychiatric disorders such as schizophrenia and autism.63 Further, the apoptotic protein and expression of Caspase 3 and impaired anti-apoptotic pathway is found to be higher in autistic children.64 The quantification of GABA and Caspase 3, 9 levels shows the inversely proportionate ratio in autism cases. A higher frequency of Caspase 3 and 9 and lower GABA levels were recorded in people with autism.65 This shows a significant linkage between autism and apoptosis. Cathepsin D is a lysosomal protease that regulates and mediates cellular apoptosis by inducing tumor necrosis factor (TNF) and interferon (IFN-γ). Cathepsin D is increased in the cerebellum of autistic children, decreased levels of Bcl-2 anti-apoptotic protein and elevated activity of Caspase 3 is also found in autistic patients.64 Also, increased levels of pro-apoptotic p53 gene expression and decreased anti-apoptotic Bcl-2 was observed in the parietal cortex of the autistic brain.66 Similar results were also found in the cerebellum and frontal cortex of autistic individuals.67 This study suggests that apoptosis is also linked to the pathogenesis of autism. Besides these signaling pathways, variations in the mitochondrial genome may also cause discrepancies in the autism brain.
Copy number variation (CNV)
In 1985 Coleman and Blass marked evidence of mitochondrial DNA dysfunction and variation in copy number in autism patients with lactic acidosis.68 CNVs contribute to genetic heterogeneity; the rate of mutation is much higher if CNV is 100–1000 times. CNVs in specific regions on the chromosome (12, 19, 20, and 21) are associated with ASD,69,70 although no such variations are found in the mt D-Loop of autism patients.71 The ratios of mt-DNA for all three genes (ND1, CytB, and ND4) to nuclear DNA were increased in the frontal cortex of autistic subjects compared with the controls. Moreover, the ratio of the mitochondrial genes ND1, ND4 and CytB to pyruvate kinase was significantly higher in autistic individuals as compared to controls. This indicates that autistic subjects had a higher copy number than their normally developing counterparts. These changes in the copy number were observed in the brain tissue of people with autism.39 These reports indicate an association between mt-DNA copy number and autism. Aside from the mt-DNA, certain parts of the ETC like the aspartate/glutamate carrier have been concomitant with the pathogenesis of autism.
Aspartate/glutamate carrier in autism
The aspartate glutamate carrier (AGC) encoded by the solute carrier family 25, member 12, gene (SLC25A12) is essential for the exchange of aspartate from the mitochondria with glutamate from the cytosol. This is an important step for various biochemical processes like lactose metabolism, urea synthesis and glycolysis.30 Recent advances in autism research has implicated SNPs in SLC25A12 in the etiology of autism. When a meta-analysis was conducted with 2001 families with 735 individuals with autism and 632 typically developing individuals a significant association was found between polymorphism of rs2292813 and rs2056202 in autism.72 A similar association was confirmed in the Finnish populations.73 When linkage and association tests were carried out on these two SNPs in autistic individuals, a strong linkage and association was found.74 Interestingly, when the two SNPs were correlated the four traits of autism, it was found that the less frequent allele in rs2056206 was related with a reduced level of rituals and routines linked with autism. Moreover, C alleles of both rs2056202 and rs2292813 were also concomitant with autism in Irish affected child-parent trios.75 Similarly, the T alleles were associated with a reduced autism risk.76 Further, SLC25A12 knock-out mice displayed a delay in development and also died by week 3. They had smaller brains as compared to controls and harbored defects in myelination, axonal transport and displayed signs of neurodegeneration. The myelination deficit was also rescued by the introduction of pyruvate, displaying the importance of the gene in development.77 SLC25A12 was also found to be expressed at a higher level in the post-mortem brains of autistic individuals and was also present during neuronal development of areas of the brain associated with autism. Silencing or overexpressing this gene in mouse embryonic cortical neurons had an effect on dendrite length and the mobility of dendritic mitochondria, implicating it in the pathology of autism.78 Moreover, expression of AGC1 was found to be increased in autistic individuals along with excessive calcium levels, showing increased metabolism in the mitochondria, but in contrast to the other work, there was no association between the variants of the SLC25A12 gene and autism in this study.31 Finally, there was also no association between the gene and the distribution of lactate in 241 families with one affected individual.79 These evidences show a promising link between gene variants and autism but the data still remains inconclusive. More research is required to further understand the role of the SLC25A12 gene in pathophysiology of autism. In addition, the improper degradation of damaged cell organelles may also be responsible for the autism like symptoms.
Autophagy and autism
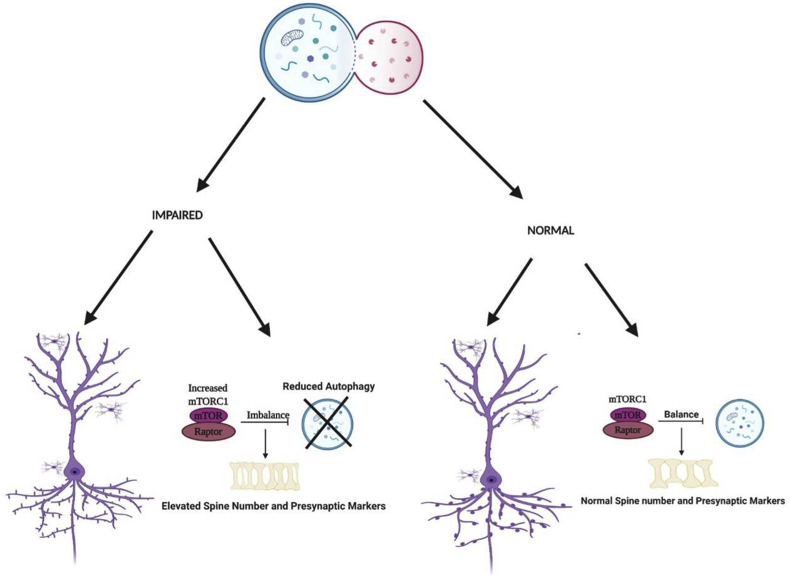
Autophagy is a process by which the intracellular organelles are regularly degraded to ensure the maintenance of the health of the cells. Mitophagy controls mitochondria number and health by a similar mechanism. Failure to do so can result in various disorders in the body. AMBRA1, a gene associated with autophagy, is associated with autism in a sexually dimorphic manner.57 Further, a heterozygous mutation in AMBRA1 exhibit autism-like behavior in females.80 Impaired autophagy and hyperactivated mTOR were also shown to contribute to reduced spine pruning and spinal deficits as well as autism-like behaviors, displaying the role of autophagy in autism (Fig. 3).81 Deficiency of tripartite motif protein 32 (TRIM32), a maintainer of mTOR, is known to cause elevated autophagy and an impaired generation of GABAergic neurons along with autistic behaviors.23 Valproic acid (VPA) is known to suppress autophagy and causes autistic characteristics in rats. Rapamycin and sulindac ameliorated these characteristics by targeting mTOR and WNT signaling.82 Similarly, Notch signaling has also been associated with autism by regulation of autophagy and spine growth.83 mTOR inhibition was also found to increase autophagy and rescue VPA-induced autism.84 A more in-depth analysis of autophagy may help develop new drugs for treating autism symptoms. Moreover, activity-dependent neuroprotective protein (ADNP) involved in the autophagy pathway, is vital for mammalian brain development and also results in autism-like conditions.85 Also, deletion of ATG7 vital for autophagy has been allied with an increase in dendritic spines, synaptic markers and autism-like repetitive behaviors.86,87 Ectopic expression of Foxp1 (R521X), a heterozygous mutation found in patients with autism and mental retardation, led to cytoplasmic aggregates and macroautophagy in neuroblastoma N2a cells. The cortical neurons expressing this also had delayed migration and abnormal dendritic morphology.88 LC3 and Beclin-1 mRNA associated with autophagy was found varied in mouse models of autism.68 Autophagy related proteins and genes may be a new target for the betterment of autism symptoms. A more precise understanding of the molecular basis of autophagy in autism is essential to develop novel therapeutics.86
Figure 3.
mTOR role in autophagy of Autism. Impaired autophagy due to increased mTOR, giving rise to reduced spine pruning resulting in elevated spine numbers as compared with normally functioning autophagy within the cell.
Therapeutic intervention: a future research
The mTOR signaling pathway performs a significant role in mitochondria, meanwhile, several regulatory genes such as p70S6K, eIF4B and eIF4E have shown assuring therapeutic advances in idiopathic autism subjects.89 The mTOR rapamycin signaling inhibitor treatment was found to improve behavioral abnormalities of autism.90 Hence, it becomes crucial to also consider the treatment of mitochondrial dysfunction in autism.
Treatment of mitochondrial dysfunction in autism
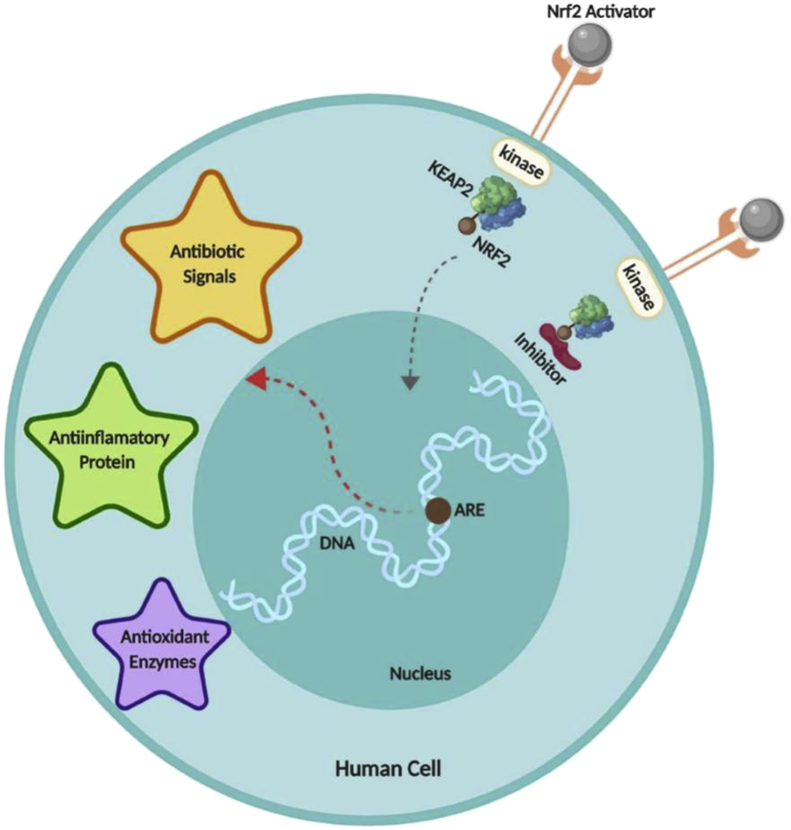
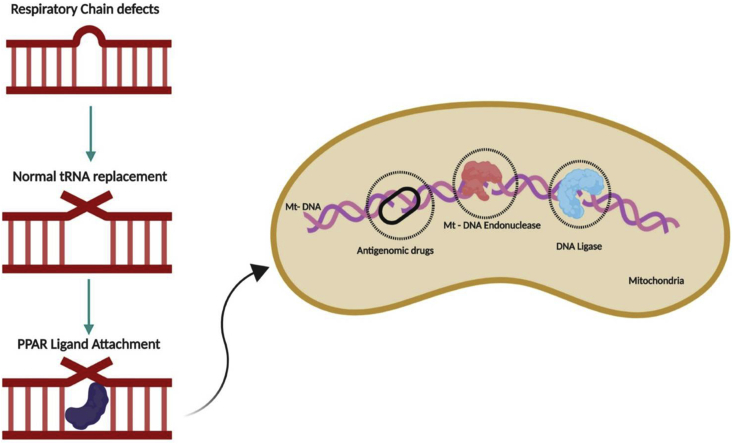
Mitochondrial dysfunction has no known cure. Hence, treatment for it mostly includes reduction in oxidative stress, removal of ROS and treatment of organs affected by it.91 Antioxidant supplementation in individuals with MD increases the GSH levels, involved in ROS removal.4,92 Methylcobalamin, and folinic acid have also been reported to increase the GSH concentrations in children with ASD reducing certain autistic behaviors.93 Likewise, mitochondrial dysfunction has been associated with cerebral folate deficiency and specific treatment with folinic acid along with milk-free diet has been reported to result in significant improvements in children with ASD with the symptoms of cerebral folate deficiency.94, 95, 96 Several other antioxidants, including vitamin B6 with magnesium in higher doses, have also been reported to significantly improve autistic behaviors and language.50,97,98 Carnitine is involved in mitochondrial energy production and is relatively deficient in children with autism.42,99 Some studies have reported improvements in the symptoms with the use of carnitine100 and carnitine may lower the toxicity of a potential Clostridial metabolite (a derivative of propionic acid) that has been recovered in the urine of some individuals with ASD.101 Correspondingly, Nrf-2, a known regulator of oxidative stress, has been considered as a target for the effective treatment of autism (Fig. 4).46 Under normal conditions, Nrf-2 binds to its repressor Kelch-like ECH-associated protein 1 (Keap1) and is inhibited. Upon activation, Nrf-2 translocates to the nucleus and binds to ARE genes which release antibiotic, anti-inflammatory and antioxidant enzymes. Hence, Nrf-2 has emerged as a probably candidate for targeting.102 Further, hyperlactatemia was recorded in mitochondrial respiratory chain complex in autistic children, reduction in lactic acid levels may help these better cope with their symptoms.45 Inflammatory response, glucose and lipid metabolism is regulated by the proliferator-activated receptors (PPAR) α, δ, and γ are ligand-activated receptors. FAO enzymes upregulated by PPAR activation leads to the defined PPAR-driven mitochondrial FAO flux. Regulation of FAO in autism by PPAR activators would also be an effective therapeutic intervention. Future therapies can be implemented by delivering tRNA molecules that can be replicated through the replication machinery instead of defective tRNA, the expression of these genes can be boosted by the PPAR ligands. In addition, antigenomic drugs can also be provided to inhibit the replication of defective mitochondrial genes. These methods are depicted in Fig. 5. Additionally, various mutations and CNVs in mitochondrial genes have been observed in autistic individuals. Gene therapy can be used to express proteins and help prevent the replication of mutant mtDNA.103 Gene knock-in and knock-out studies can be considered to develop better ways to treat mitochondrial dysfunction in autism. More deep-rooted evidence on mt-DNA is essential to developing new drugs to ensure a better quality of life for affected individuals.
Figure 4.
NRF-2 in autism treatment. Activators and inhibitors of NRF-2 are used to ameliorate the symptoms of autism by providing protection from oxidative stress. When NRF-2 is activated, it translocates to the nucleus, activating ARE genes which release anti-inflammatory, antibiotic and antioxidant enzymes that may help in alleviating the oxidative stress.
Figure 5.
Treatment of defective mitochondria. This figure represents the possible therapeutic techniques that can be used for the treatment of defective mitochondria. When defects in ETC is observed, correct tRNAs can be imported through gene technology into the mitochondria. These tRNAs will replicate using the cellular machinery providing correct proteins; PPAR ligands can be introduced to increase the replication of mitochondrial genes to enhance the availability of suitable genes; antigenomic drugs can also be administered to inhibit replication of defective genes in the mitochondria.
Conclusion
Autism is a complex neurological monogenic disorder which has a strong genetic influence for its occurrence.104 As the prevalence of autism continues to increase, it has become extremely imperative to understand the underlying basis of the condition.105 At the advent of the genomic era, various genetic factors have been linked to autism. Mt-DNA research has gained momentum recently, contributing to various studies associating it with autism. The current review supports the involvement of mitochondrial dysfunction in autism. The activity of mitochondria coded bio-energetic complexes I, III, IV, V is relatively lower in autistic children. This literature also links the role of mitochondria in ROS production, copy number variation, and apoptosis in autistic cases. Future studies can be focused on in-depth analysis of mt-DNA candidate genes that are closely associated with ATP synthesis. Copy number variation and ROS scavenging activity can be focused on, in relation to autism. Studies based on mitochondrial dysfunction may provide a breakthrough in the field of autism research. This will also help understand the genetics underlying this condition.
Authors contribution
Balachandar Vellingiri: Conceptualization, resources and supervision. Kamarajan Rajagopalan: Data analysis, illustrations and figures; Kaavya Jayaramayya: Data analysis, writing–original draft; Madesh Jeevanandam: Literature search; Mahalaxmi Iyer: writing–original draft, Literature search, data analysis.
Conflict of Interests
The authors delare no conflict of interest.
Funding
This work was supported by Science and Engineering Research Board (SERB) [grant number ECR/2016/001688].
Acknowledgements
The authors would like to thank Government of India, New Delhi for providing the fund to carry out this review article. Also, we thank Bharathiar University, Coimbatore, India for providing the necessary infrastructure facilities required for completing the article.
Footnotes
Peer review under responsibility of Chongqing Medical University.
References
- 1.Lord C., Brugha T.S., Charman T. Autism spectrum disorder. Nat Rev Dis Primers. 2020;6(1):1–23. doi: 10.1038/s41572-019-0138-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baio J., Wiggins L., Christensen D.L. Prevalence of autism spectrum disorder among children aged 8 years—autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ. 2018;67(6):1–23. doi: 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacFabe D.F., Cain N.E., Boon F., Ossenkopp K.-P., Cain D.P. Effects of the enteric bacterial metabolic product propionic acid on object-directed behavior, social behavior, cognition, and neuroinflammation in adolescent rats: relevance to autism spectrum disorder. Behav Brain Res. 2011;217(1):47–54. doi: 10.1016/j.bbr.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 4.James S.J., Cutler P., Melnyk S. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am J Clin Nutr. 2004;80(6):1611–1617. doi: 10.1093/ajcn/80.6.1611. [DOI] [PubMed] [Google Scholar]
- 5.Goldani A.A., Downs S.R., Widjaja F., Lawton B., Hendren R.L. Biomarkers in autism. Front Psychiatry. 2014;5 doi: 10.3389/fpsyt.2014.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohana D.S., Mahalaxmi I., Aswathy N., Dhivya V., Balachandar V. Does retina play a role in Parkinson’s disease? Acta Neurol Belg. 2020;120(2):257–265. doi: 10.1007/s13760-020-01274-w. [DOI] [PubMed] [Google Scholar]
- 7.Jayaramayya K., Iyer M., Venkatesan D. Unraveling correlative roles of dopamine transporter (DAT) and Parkin in Parkinson’s disease (PD) – a road to discovery? Brain Res Bull. 2020;157:169–179. doi: 10.1016/j.brainresbull.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Mousavizadeh K., Rsajabi P., Alaee M., Dadgar S., Houshmand M. Usage of mitochondrial D-loop variation to predict risk for Huntington disease. Mitochondrial DNA. 2015;26(4):579–582. doi: 10.3109/19401736.2013.878902. [DOI] [PubMed] [Google Scholar]
- 9.Chalkia D., Singh L.N., Leipzig J. Association between mitochondrial DNA haplogroup variation and autism spectrum disorders. JAMA Psychiatry. 2017;74(11):1161–1168. doi: 10.1001/jamapsychiatry.2017.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geetha B., Sukumar C., Dhivyadeepa E., Reddy J.K., Balachandar V. Autism in India: a case–control study to understand the association between socio-economic and environmental risk factors. Acta Neurol Belg. 2019;119(3):393–401. doi: 10.1007/s13760-018-01057-4. [DOI] [PubMed] [Google Scholar]
- 11.Altmann R. 1894. Die Elementarorganismen Und Ihre Beziehungen Zu Den Zellen. [Google Scholar]
- 12.Mitchell P. Coupling of phosphorylation to electron and hydrogen transfer by a chemi-osmotic type of mechanism. Nature. 1961;191(4784):144–148. doi: 10.1038/191144a0. [DOI] [PubMed] [Google Scholar]
- 13.Watford M. The urea cycle: a two-compartment system. Essays Biochem. 1991;26:49–58. [PubMed] [Google Scholar]
- 14.Michal G., Schomburg D. Wiley; New Jersey, NJ: 2012. Biochemical Pathways: An Atlas of Biochemistry and Molecular Biology. [Google Scholar]
- 15.Galluzzi L., Kepp O., Trojel-Hansen C., Kroemer G. Mitochondrial control of cellular life, stress, and death. Circ Res. 2012;111(9):1198–1207. doi: 10.1161/CIRCRESAHA.112.268946. [DOI] [PubMed] [Google Scholar]
- 16.Ajioka R.S., Phillips J.D., Kushner J.P. Biosynthesis of heme in mammals. Biochim Biophys Acta Mol Cell Res. 2006;1763(7):723–736. doi: 10.1016/j.bbamcr.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 17.Miller W.L. Vol. 20. 2011. Role of mitochondria in steroidogenesis; pp. 1–19. (Pediatric Adrenal Diseases). [Google Scholar]
- 18.Lill R., Mühlenhoff U. Iron–sulfur-protein biogenesis in eukaryotes. Trends Biochem Sci. 2005;30(3):133–141. doi: 10.1016/j.tibs.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Rizzuto R., Pozzan T. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol Rev. 2006;86(1):369–408. doi: 10.1152/physrev.00004.2005. [DOI] [PubMed] [Google Scholar]
- 20.Spinelli J.B., Haigis M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat Cell Biol. 2018;20(7):745–754. doi: 10.1038/s41556-018-0124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siddiqui M.F., Elwell C., Johnson M.H. Mitochondrial dysfunction in autism spectrum disorders. Autism Open Access. 2016;6(5) doi: 10.4172/2165-7890.1000190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rossignol D.A., Frye D.A. Mitochondrial dysfunction in autism spectrum disorders: a systematic review and meta-analysis. Mol Psychiatry. 2012;17(3):290–314. doi: 10.1038/mp.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu X.-H., Qiao H., Du F. Quantitative imaging of energy expenditure in human brain. Neuroimage. 2012;60(4):2107–2117. doi: 10.1016/j.neuroimage.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gusev A., Ko A., Shi H. Integrative approaches for large-scale transcriptome-wide association studies. Nat Genet. 2016;48(3):245–252. doi: 10.1038/ng.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang H., Liu X., Jin Y., Lee S., Wee C., Shen D. Enhancing the representation of functional connectivity networks by fusing multi-view information for autism spectrum disorder diagnosis. Hum Brain Mapp. 2019;40(3):833–854. doi: 10.1002/hbm.24415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shen L., Zhao Y., Zhang H. Reviews on Biomarker Studies in Psychiatric and Neurodegenerative Disorders. 2019. Advances in biomarker studies in autism spectrum disorders; pp. 207–233. [DOI] [PubMed] [Google Scholar]
- 27.Rossignol D.A., Frye R.E. A review of research trends in physiological abnormalities in autism spectrum disorders: immune dysregulation, inflammation, oxidative stress, mitochondrial dysfunction and environmental toxicant exposures. Mol Psychiatry. 2012;17(4):389–401. doi: 10.1038/mp.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rose S., Bennuri S.C., Murray K.F., Buie T., Winter H., Frye R.E. Mitochondrial dysfunction in the gastrointestinal mucosa of children with autism: a blinded case-control study. PLoS One. 2017;12(10) doi: 10.1371/journal.pone.0186377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giulivi C., Zhang Y.-F., Omanska-Klusek A. Mitochondrial dysfunction in autism. JAMA. 2010;304(21):2389–2396. doi: 10.1001/jama.2010.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Napoli E., Wong S., Hertz-Picciotto I., Giulivi C. Deficits in bioenergetics and impaired immune response in granulocytes from children with autism. Pediatrics. 2014;133(5):e1405–e1410. doi: 10.1542/peds.2013-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmieri L., Papaleo V., Porcelli V. Altered calcium homeostasis in autism-spectrum disorders: evidence from biochemical and genetic studies of the mitochondrial aspartate/glutamate carrier AGC1. Mol Psychiatry. 2010;15(1):38–52. doi: 10.1038/mp.2008.63. [DOI] [PubMed] [Google Scholar]
- 32.Rose S., Niyazov D.M., Rossignol D.A., Goldenthal M., Kahler S.G., Frye R.E. Clinical and molecular characteristics of mitochondrial dysfunction in autism spectrum disorder. Mol Diagn Ther. 2018;22(5):571–593. doi: 10.1007/s40291-018-0352-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castora F.J. Mitochondrial function and abnormalities implicated in the pathogenesis of ASD. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:83–108. doi: 10.1016/j.pnpbp.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 34.Weissman J.R., Kelley R.I., Bauman M.L. Mitochondrial disease in autism spectrum disorder patients: a cohort analysis. PLoS One. 2008;3(11) doi: 10.1371/journal.pone.0003815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haas R.H., Parikh S., Falk M.J. Mitochondrial disease: a practical approach for primary care physicians. Pediatrics. 2007;120(6):1326–1333. doi: 10.1542/peds.2007-0391. [DOI] [PubMed] [Google Scholar]
- 36.Morava E., van den Heuvel L., Hol F. Mitochondrial disease criteria: diagnostic applications in children. Neurology. 2006;67(10):1823–1826. doi: 10.1212/01.wnl.0000244435.27645.54. [DOI] [PubMed] [Google Scholar]
- 37.El-Ansary A., Bjørklund G., Khemakhem A.M., Al-Ayadhi L., Chirumbolo S., Bacha A.B. Metabolism-associated markers and Childhood Autism Rating Scales (CARS) as a measure of autism severity. J Mol Neurosci. 2018;65(3):265–276. doi: 10.1007/s12031-018-1091-5. [DOI] [PubMed] [Google Scholar]
- 38.Khemakhem A.M., Frye R.E., El-Ansary A., Al-Ayadhi L., Bacha A.B. Novel biomarkers of metabolic dysfunction is autism spectrum disorder: potential for biological diagnostic markers. Metab Brain Dis. 2017;32(6):1983–1997. doi: 10.1007/s11011-017-0085-2. [DOI] [PubMed] [Google Scholar]
- 39.Gu F., Chauhan V., Kaur K. Alterations in mitochondrial DNA copy number and the activities of electron transport chain complexes and pyruvate dehydrogenase in the frontal cortex from subjects with autism. Transl Psychiatry. 2013;3(9) doi: 10.1038/tp.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoffner J., Hyams L., Langley G.N. Fever plus mitochondrial disease could be risk factors for autistic regression. J Child Neurol. 2010;25(4):429–434. doi: 10.1177/0883073809342128. [DOI] [PubMed] [Google Scholar]
- 41.Iommarini L., Ghelli A., Leone G. Mild phenotypes and proper supercomplex assembly in human cells carrying the homoplasmic m. 15557G > A mutation in cytochrome b gene. Hum Mutat. 2018;39(1):92–102. doi: 10.1002/humu.23350. [DOI] [PubMed] [Google Scholar]
- 42.Filipek P.A., Juranek J., Smith M. Mitochondrial dysfunction in autistic patients with 15q inverted duplication. Ann Neurol. 2003;53(6):801–804. doi: 10.1002/ana.10596. [DOI] [PubMed] [Google Scholar]
- 43.Moosavizadeh K., Askari M., Arian H. Association of mtDNA mutation with autism in Iranian patients. J Res Med Sci. 2013;18(10) [PMC free article] [PubMed] [Google Scholar]
- 44.Piryaei F., Houshmand M., Aryani O., Dadgar S., Soheili Z.S. Investigation of the Mitochondrial ATPase 6/8 and tRNA(Lys) Genes Mutations in Autism. Cell J. 2012;14(2):98–101. [PMC free article] [PubMed] [Google Scholar]
- 45.Guevara-Campos J., González-Guevara L., Cauli O. Autism and intellectual disability associated with mitochondrial disease and hyperlactacidemia. Int J Mol Sci. 2015;16(2):3870–3884. doi: 10.3390/ijms16023870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durham L. 2019. Treatment of a Subtype of ASD. Published online May 9. [Google Scholar]
- 47.Essa M.M., Subash S., Braidy N. Role of NAD+, oxidative stress, and tryptophan metabolism in autism spectrum disorders. Int J Tryptophan Res. 2013;6(Suppl 1):S15–S28. doi: 10.4137/IJTR.S11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhou P., Xia D., Xia Y. Synergistic effect of vancomycin and l-homocarnosine alleviates Staphylococcus aureus-induced osteomyelitis in rats. Biomed Pharmacother. 2019;111:31–35. doi: 10.1016/j.biopha.2018.11.102. [DOI] [PubMed] [Google Scholar]
- 49.Al-Gadani Y., El-Ansary A., Attas O., Al-Ayadhi L. Metabolic biomarkers related to oxidative stress and antioxidant status in Saudi autistic children. Clin Biochem. 2009;42(10–11):1032–1040. doi: 10.1016/j.clinbiochem.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 50.Meguid N.A., Ghozlan S.A., Mohamed M.F. Expression of reactive oxygen species–related transcripts in Egyptian children with autism. Biomark Insights. 2017;12 doi: 10.1177/1177271917691035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Carrasco M., Salazar C., Tiznado W., Ruiz L.M. Alterations of mitochondrial biology in the oral mucosa of Chilean children with autism spectrum disorder (ASD) Cells. 2019;8(4) doi: 10.3390/cells8040367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Popa-Wagner A., Mitran S., Sivanesan S., Chang E., Buga A.M. ROS and brain diseases: the good, the bad, and the ugly. Oxid Med Cell Longev. 2013;2013 doi: 10.1155/2013/963520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Salzano S., Checconi P., Hanschmann E.-M. Linkage of inflammation and oxidative stress via release of glutathionylated peroxiredoxin-2, which acts as a danger signal. Proc Natl Acad Sci U S A. 2014;111(33):12157–12162. doi: 10.1073/pnas.1401712111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ghezzi P. Role of glutathione in immunity and inflammation in the lung. Int J Gen Med. 2011;4:105–113. doi: 10.2147/IJGM.S15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pangrazzi L., Balasco L., Bozzi Y. Oxidative stress and immune system dysfunction in autism spectrum disorders. Int J Mol Sci. 2020;21(9) doi: 10.3390/ijms21093293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nadeem A., Ahmad S.F., Bakheet S.A. Toll-like receptor 4 signaling is associated with upregulated NADPH oxidase expression in peripheral T cells of children with autism. Brain Behav Immun. 2017;61:146–154. doi: 10.1016/j.bbi.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 57.Nadeem A., Ahmad S.F., Attia S.M., Al-Ayadhi L.Y., Bakheet S.A., Al-Harbi N.O. Oxidative and inflammatory mediators are upregulated in neutrophils of autistic children: role of IL-17A receptor signaling. Prog Neuropsychopharmacol Biol Psychiatry. 2019;90:204–211. doi: 10.1016/j.pnpbp.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Wei H., Alberts I., Li X. The apoptotic perspective of autism. Int J Dev Neurosci. 2014;36:13–18. doi: 10.1016/j.ijdevneu.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 59.Hwang O. Role of oxidative stress in Parkinson's disease. Exp Neurobiol. 2013;22(1):11–17. doi: 10.5607/en.2013.22.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ji L., Chauhan A., Wegiel J., Essa M.M., Chauhan V. Gelsolin is proteolytically cleaved in the brains of individuals with Alzheimer's disease. J Alzheimers Dis. 2009;18(1):105–111. doi: 10.3233/JAD-2009-1127. [DOI] [PubMed] [Google Scholar]
- 61.Vargas D.L., Nascimbene C., Krishnan C., Zimmerman A.W., Pardo C.A. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57(1):67–81. doi: 10.1002/ana.20315. [DOI] [PubMed] [Google Scholar]
- 62.Brauns S.C., Milne P., Naudé R., Van de venter M. Selected cyclic dipeptides inhibit cancer cell growth and induce apoptosis in HT-29 colon cancer cells. Anticancer Res. 2004;24(3A):1713–1720. [PubMed] [Google Scholar]
- 63.Semon B.A. Dietary cyclic dipeptides, apoptosis and psychiatric disorders: a hypothesis. Med Hypotheses. 2014;82(6):740–743. doi: 10.1016/j.mehy.2014.03.016. [DOI] [PubMed] [Google Scholar]
- 64.Sheikh A., Li X., Wen G., Tauqeer Z., Brown W., Malik M. Cathepsin D and apoptosis related proteins are elevated in the brain of autistic subjects. Neuroscience. 2010;165(2):363–370. doi: 10.1016/j.neuroscience.2009.10.035. [DOI] [PubMed] [Google Scholar]
- 65.El-Ansary A., Zayed N., Al-Ayadhi L. GABA synaptopathy promotes the elevation of caspases 3 and 9 as pro-apoptotic markers in Egyptian patients with autism spectrum disorder. Acta Neurol Belg. 2021;121(2):489–501. doi: 10.1007/s13760-019-01226-z. [DOI] [PubMed] [Google Scholar]
- 66.Eftekhari A., Ahmadian E., Panahi-Azar V., Hosseini H., Tabibiazar M., Maleki Dizaj S. Hepatoprotective and free radical scavenging actions of quercetin nanoparticles on aflatoxin B1-induced liver damage: in vitro/in vivo studies. Artif Cells Nanomed Biotechnol. 2018;46(2):411–420. doi: 10.1080/21691401.2017.1315427. [DOI] [PubMed] [Google Scholar]
- 67.Li X., Chauhan A., Sheikh A.M. Elevated immune response in the brain of autistic patients. J Neuroimmunol. 2009;207(1–2):111–116. doi: 10.1016/j.jneuroim.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Coleman M., Blass J.P. Autism and lactic acidosis. J Autism Dev Disord. 1985;15(1):1–8. doi: 10.1007/BF01837894. [DOI] [PubMed] [Google Scholar]
- 69.Lou H., Li S., Yang Y. A map of copy number variations in Chinese populations. PLoS One. 2011;6(11) doi: 10.1371/journal.pone.0027341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wiśniowiecka-Kowalnik B., Nowakowska B.A. Genetics and epigenetics of autism spectrum disorder—current evidence in the field. J Appl Genet. 2019;60(1):37–47. doi: 10.1007/s13353-018-00480-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cruz A.C.P., Ferrasa A., Muotri A.R., Herai R.H. Frequency and association of mitochondrial genetic variants with neurological disorders. Mitochondrion. 2019;46:345–360. doi: 10.1016/j.mito.2018.09.005. [DOI] [PubMed] [Google Scholar]
- 72.Aoki Y., Cortese S. Mitochondrial aspartate/glutamate carrier SLC25A12 and autism spectrum disorder: a meta-analysis. Mol Neurobiol. 2016;53(3):1579–1588. doi: 10.1007/s12035-015-9116-3. [DOI] [PubMed] [Google Scholar]
- 73.Turunen J.A., Rehnström K., Kilpinen H., Kuokkanen M., Kempas E., Ylisaukko-oja T. Mitochondrial aspartate/glutamate carrier SLC25A12 gene is associated with autism. Autism Res. 2008;1(3):189–192. doi: 10.1002/aur.25. [DOI] [PubMed] [Google Scholar]
- 74.Ramoz N., Reichert J.G., Smith C.J. Linkage and association of the mitochondrial aspartate/glutamate carrier SLC25A12 gene with autism. Am J Psychiatry. 2004;161(4):662–669. doi: 10.1176/appi.ajp.161.4.662. [DOI] [PubMed] [Google Scholar]
- 75.Sebastiano V., Maeder M.L., Angstman J.F. In situ genetic correction of the sickle cell anemia mutation in human induced pluripotent stem cells using engineered zinc finger nucleases. Stem Cells. 2011;29(11):1717–1726. doi: 10.1002/stem.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu J., Yang A., Zhang Q. Association between genetic variants in SLC25A12 and risk of autism spectrum disorders: an integrated meta-analysis. Am J Med Genet B Neuropsychiatr Genet. 2015;168(4):236–246. doi: 10.1002/ajmg.b.32304. [DOI] [PubMed] [Google Scholar]
- 77.Sakurai T., Ramoz N., Barreto M. Slc25a12 disruption alters myelination and neurofilaments: a model for a hypomyelination syndrome and childhood neurodevelopmental disorders. Biol Psychiatry. 2010;67(9):887–894. doi: 10.1016/j.biopsych.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lepagnol-Bestel A.-M., Maussion G., Boda B. SLC25A12 expression is associated with neurite outgrowth and is upregulated in the prefrontal cortex of autistic subjects. Mol Psychiatry. 2008;13(4):385–397. doi: 10.1038/sj.mp.4002120. [DOI] [PubMed] [Google Scholar]
- 79.Correia C., Coutinho A.M., Diogo L. Brief report: high frequency of biochemical markers for mitochondrial dysfunction in autism: no association with the mitochondrial aspartate/glutamate carrier SLC25A12 gene. J Autism Dev Disord. 2006;36(8):1137–1140. doi: 10.1007/s10803-006-0138-6. [DOI] [PubMed] [Google Scholar]
- 80.Dere E., Dahm L., Lu D. Heterozygous ambra1 deficiency in mice: a genetic trait with autism-like behavior restricted to the female gender. Front Behav Neurosci. 2014;8 doi: 10.3389/fnbeh.2014.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tang G., Gudsnuk K., Kuo S.-H. Loss of mTOR-dependent macroautophagy causes autistic-like synaptic pruning deficits. Neuron. 2014;83(5):1131–1143. doi: 10.1016/j.neuron.2014.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Qin L., Dai X., Yin Y. Valproic acid exposure sequentially activates Wnt and mTOR pathways in rats. Mol Cell Neurosci. 2016;75:27–35. doi: 10.1016/j.mcn.2016.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Xiang Z., Jia Y., He X., Wang L., Cui W. The Notch signaling pathway inhibitor Dapt alleviates autism-like behavior, autophagy and dendritic spine density abnormalities in a valproic acid-induced animal model of autism. Prog Neuropsychopharmacol Biol Psychiatry. 2019;94 doi: 10.1016/j.pnpbp.2019.109644. [DOI] [PubMed] [Google Scholar]
- 84.Zhang J., Zhang J.-X., Zhang Q.-L. PI3K/AKT/mTOR-mediated autophagy in the development of autism spectrum disorder. Brain Res Bull. 2016;125:152–158. doi: 10.1016/j.brainresbull.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 85.Sragovich S., Merenlender-Wagner A., Gozes I. ADNP plays a key role in autophagy: from autism to schizophrenia and Alzheimer's disease. BioEssays. 2017;39(11) doi: 10.1002/bies.201700054. [DOI] [PubMed] [Google Scholar]
- 86.Kim H., Cho M., Shim W. Deficient autophagy in microglia impairs synaptic pruning and causes social behavioral defects. Mol Psychiatry. 2017;22(11):1576–1584. doi: 10.1038/mp.2016.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hui K.K., Tanaka M. Autophagy links MTOR and GABA signaling in the brain. Autophagy. 2019;15(10):1848–1849. doi: 10.1080/15548627.2019.1637643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li X., Han X., Tu X. An autism-related, nonsense Foxp1 mutant induces autophagy and delays radial migration of the cortical neurons. Cerebral Cortex. 2019;29(7):3193–3208. doi: 10.1093/cercor/bhy185. [DOI] [PubMed] [Google Scholar]
- 89.Ganesan H., Balasubramanian V., Iyer M. mTOR signalling pathway-A root cause for idiopathic autism? BMB Rep. 2019;52(7):424–433. doi: 10.5483/BMBRep.2019.52.7.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schiavone S., Trabace L. Pharmacological targeting of redox regulation systems as new therapeutic approach for psychiatric disorders: a literature overview. Pharmacol Res. 2016;107:195–204. doi: 10.1016/j.phrs.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 91.Edmonds J.L., Kirse D.J., Kearns D., Deutsch R., Spruijt L., Naviaux R.K. The otolaryngological manifestations of mitochondrial disease and the risk of neurodegeneration with infection. Arch Otolaryngol Head Neck Surg. 2002;128(4):355–362. doi: 10.1001/archotol.128.4.355. [DOI] [PubMed] [Google Scholar]
- 92.Atkuri K.R., Cowan T.M., Kwan T. Inherited disorders affecting mitochondrial function are associated with glutathione deficiency and hypocitrullinemia. Proc Natl Acad Sci U S A. 2009;106(10):3941–3945. doi: 10.1073/pnas.0813409106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mostafa G.A., Kitchener N. Serum anti-nuclear antibodies as a marker of autoimmunity in Egyptian autistic children. Pediatr Neurol. 2009;40(2):107–112. doi: 10.1016/j.pediatrneurol.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 94.Moretti P., Sahoo T., Hyland K. Cerebral folate deficiency with developmental delay, autism, and response to folinic acid. Neurology. 2005;64(6):1088–1090. doi: 10.1212/01.WNL.0000154641.08211.B7. [DOI] [PubMed] [Google Scholar]
- 95.Ramaekers V., Sequeira J.M., Quadros E.V. Clinical recognition and aspects of the cerebral folate deficiency syndromes. Clin Chem Lab Med. 2013;51(3):497–511. doi: 10.1515/cclm-2012-0543. [DOI] [PubMed] [Google Scholar]
- 96.Pacheva I., Ivanov I. Targeted biomedical treatment for autism spectrum disorders. Curr Pharmaceut Des. 2019;25(41):4430–4453. doi: 10.2174/1381612825666191205091312. [DOI] [PubMed] [Google Scholar]
- 97.Levy S.E., Hyman S.L. Use of complementary and alternative treatments for children with autistic spectrum disorders is increasing. Pediatr Ann. 2003;32(10):685–691. doi: 10.3928/0090-4481-20031001-10. [DOI] [PubMed] [Google Scholar]
- 98.Niederhofer H., Staffen W., Mair A. Galantamine may be effective in treating autistic disorder. BMJ. 2002;325(7377) doi: 10.1136/bmj.325.7377.1422/a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mostafa G.A., El-Gamal H.A., El-Wakkad A.S., El-Shorbagy O.E., Hamza M.M. Polyunsaturated fatty acids, carnitine and lactate as biological markers of brain energy in autistic children. Int J Child Neuropsychiatry. 2005;2(2):179–188. [Google Scholar]
- 100.Fahmy S.F., El-hamamsy M.H., Zaki O.K., Badary O.A. l-Carnitine supplementation improves the behavioral symptoms in autistic children. Res Autism Spectr Disord. 2013;7(1):159–166. [Google Scholar]
- 101.Shaw W. Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of Clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr Neurosci. 2010;13(3):135–143. doi: 10.1179/147683010X12611460763968. [DOI] [PubMed] [Google Scholar]
- 102.Ahmed S.M.U., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: pivotal roles in inflammation. Biochim Biophys Acta Mol Basis Dis. 2017;1863(2):585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 103.DiMauro S., Rustin P. A critical approach to the therapy of mitochondrial respiratory chain and oxidative phosphorylation diseases. Biochim Biophys Acta Mol Basis Dis. 2009;1792(12):1159–1167. doi: 10.1016/j.bbadis.2008.10.015. [DOI] [PubMed] [Google Scholar]
- 104.Venugopal A., Chandran M., Eruppakotte N., Kizhakkillach S., Breezevilla S.C., Vellingiri B. Monogenic diseases in India. Mutat Res. 2018;776:23–31. doi: 10.1016/j.mrrev.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 105.Ganesan H., Balasubramanian V., Iyer M. mTOR signalling pathway – a root cause for idiopathic autism? BMB Rep. 2019;52(7):424–433. doi: 10.5483/BMBRep.2019.52.7.137. [DOI] [PMC free article] [PubMed] [Google Scholar]