Abstract
Cirrhosis is characterized as the progress of regenerative nodules surrounded by fibrous bands in response to chronic hepatic injury and causes portal hypertension and end-stage hepatic disease. Following liver injury, liver progenitor cells (LPCs) can be activated and differentiate into hepatocytes in order to awaken liver regeneration and reach homeostasis. Recent research has uncovered some new sources of LPCs. Here, we update the mechanisms of LPCs-mediated liver regeneration in cirrhosis by introducing the origin of LPCs and LPCs’ niche with a discussion of the influence of LPC-related cells. This article analyzes the mechanism of regeneration and activation of LPCs in cirrhosis in recent years aiming to provide help for clinical application.
Keywords: Activation, Cytokines, Extracellular matrix, Hepatic stellate cells, Liver cirrhosis, Liver progenitor cells, Liver regeneration, Niche
Introduction
Liver is an important organ which detoxifies various metabolites, synthesizes proteins and produces biochemicals necessary for digestion and growth. Liver, moreover, has a strong regenerative and compensatory capacity. Hepatocytes are produced by the proliferation and division of hepatocytes and the differentiation of liver progenitor cells (LPCs). However, the ability of liver cells to divide and proliferate is weakened under pathological conditions such as cirrhosis, in which, thereupon, progenitor cells are activated to differentiate into hepatocytes and bile duct cells to maintain liver homeostasis. The proliferation of mature hepatocytes and the expansion of LPCs are two ways of liver regeneration. After partial hepatectomy or acute liver injury, liver regeneration occurs mainly through proliferation of liver cells. In severe or chronic liver injury, the proliferation of liver cells is impaired and LPCs are activated, which could differentiate toward hepatocytes and cholangiocytes, and repopulate the injured epithelial and promote the recovery of liver function.
Origin and activation of LPCs
In adult liver and biliary tree, there are two distinct populations of progenitor cell, namely Liver Progenitor Cells (LPCs), located in the smallest branches of the biliary tree (canals of Hering and bile ductules), and Biliary Tree Stem Cells (BTSCs), found in the peribiliary glands (PBGs) of large intrahepatic and extrahepatic bile ducts.1,2 In chronic and severe injury, liver progenitor cells (LPCs) are first activated and then differentiate into hepatocytes and bile duct cells to maintain liver homeostasis.
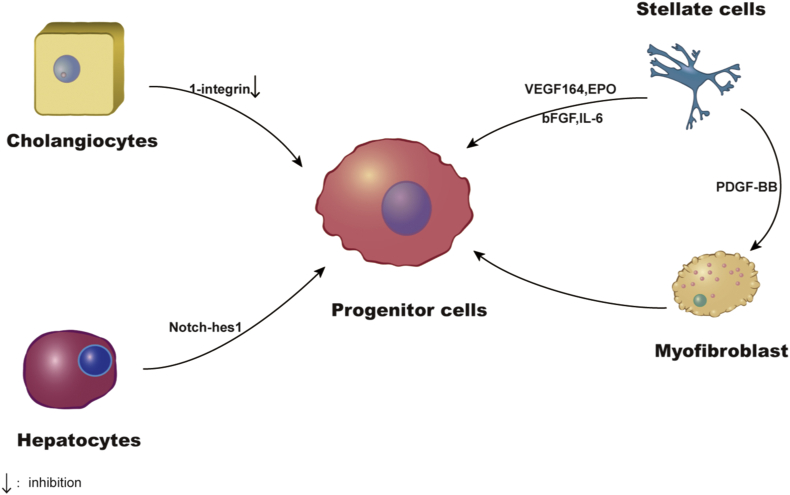
Actually all new hepatocytes do not come from facultative stem cells, also known as “oval cells” or “atypical ductal cells”. These conclusions were confirmed by studies based on genes and nucleoside analogues that marks and tracks the origin and contribution of various cell populations during liver regeneration.3 A study to determine the origin of cells using the method of genetic lineage tracing found that hepatocytes, rather than cholangiocytes, are the major source of cells for primitive ductules formed in response to chronic liver damage, and in which Notch-hes1 signal plays an important role in this process.4 In addition, other studies found that the loss of 1-integrin in hepatocytes induced liver injury triggers a ductular reactions (DRs) on the origin of cholangiocytes, about 25% of which are from non-hepatocyte origin. β1- integrin knockdown or overexpression of p21 could inhibit hepatocytes proliferation, which led to the proliferation of hepatocytes derived from cholangiocyte.5 This suggests that cholangiocytes are also a source of progenitor cells. HSC expresses the stem cell/progenitor marker CD133 and can differentiate into hepatocytes after dealing with a variety of cytokines such as VEGF164, bFGF, EPO and IL-6.6 After 7 days of culture of CD133+HSC on a medium containing PDGF-BB, they were transformed into myofibroblast like cells.6 In vivo lineage tracing studies which uses an injury mouse model induced by bile duct ligation and Choline-Deficient, Ethionine Supplemented (CDE) diet have shown that HSC is a source of myofibroblasts and progenitor cells.7 The stationary hepatic stellate cells transform into myofibroblast promoted by Hedgehog (Hh) pathway, and some of which become regenerative progenitor cells.8 Hence we could draw conclusion that multiple cell sources have the ability to convert into LPC (Fig. 1).
Figure 1.
The origin of liver progenitor cells. LPCs come from a variety of cells such as cholangiocytes, hepatocytes, HSCs and myofibroblasts.
Hepatocytes contributing to liver regeneration can be differentiated from LPCs activation following liver injury. Hepatocyte-mediated regeneration predominates as normal liver undergoes partial hepatectomy or acute injury, while in chronic and severe injury, however, ductular reactions (DRs) of activated biliary epithelial cells containing LPCs appear in the periportal regions.9 DRs refer to epithelial components and their related inflammatory niche. The proliferation of the hepatocytes can be inhibited by disrupting Ikk-NF-κB pathway, however this can also stimulate the production of Hh ligands which emits viability signals to progenitors and myofibroblasts resulting in the accumulation of these cell types and the cause of fibrogenesis.10 Investigation showed autophagy and the Wnt/β-catenin pathway are activated during the differentiation of LPCs. Autophagy was inhibited by down-regulation of Atg5 gene expression, which attenuated the differentiation of LPC and inhibited the Wnt/β-catenin pathway. In addition, the expression of β-catenin gene down-regulated without inhibiting autophagy also hindered the differentiation of LPCs.11 Autophagy regulates the signal of Wnt/β-catenin promoting LPC differentiation after bile duct ligation, the Notch signaling pathway increased, and DAPT (a Notch pathway inhibitor) treatment reduced the expression of CK19, OV6, Sox9, and EpCAM, blocking bile duct cell proliferation and cholestatic liver fibrosis (CLF).12 This study shows that in CLF, LPC differentiating into bile duct cells requires activation of Notch signals. Monocytes produce TNF-like weak inducer of apoptosis (TWEAK)13 and Wnt3a14 to stimulate oval cell proliferation.
LPC niche
Stem cells are maintained and controlled in their unique niches which enable their behavior to ensure tissue homeostasis and regeneration throughout life. LPCs niche is a special microenvironment which contains of different cell types. Extracellular matrix (ECM), growth factors, and cytokines released from niche cells help control the characteristics of LPCs and balance their expansion and differentiation. Liver progenitor cells, namely human hepatic progenitor cells and rodents oval cells, function when hepatocyte proliferation is prolongedly or severely impaired in liver injury. The oval cell, named for its morphological characteristics, is a dual-energy precursor cell that can be used to study the formation of bile ducts and regeneration of liver parenchyma. Both supportive and non-supportive stromal lines secrete extracellular vesicles (EVs), and EVs also play an important role in liver fibrosis, such as, connective tissue growth factor 2 (CCN2) is overexpressed in liver fibrosis and can contribute to promote fibrosis through HSCs.15 HSCs can transfer mir-214 to adjacent cells through exosomes and regulate expression of CCN2. During chronic liver injury, secretion of mir-214 is decreased, leading to the upregulation of CCN2 and promoting the development of liver fibrosis. In addition, secretion of exosome Twist1 was inhibited in liver fibrosis, which reduced the expression of mir-214 and promoted the process of liver fibrosis.16 Activated HSCs can transport exosome CCN2 to other HSCs, which may lead to the amplification of fibrogenic signaling.
Related cells
Hepatic stellate cells (HSCs)
HSCs are located in the Disse space between hepatic epithelial cells and sinusoidal endothelial cells. Producing extracellular matrix after chronic liver injury, HSCs are the main cells involved in liver fibrosis. It was found that LPCs were co-cultured in transwell plates with an immortalized human HSC line to induce LPCs to differentiate into hepatocytes.17 HSC releases many cytokines and growth factors that affect the survival, proliferation, migration, and differentiation of LPC as listed in Table 1.18, 19, 20, 21, 22 LPCs lead to the accumulation of HSCs through the TGF β signaling pathway in the ductular reaction, resulting in enhanced fibrosis and disease progression.23
Table 1.
The effect of related cells on LPCs.
| Cell | Cytokines/pathway | Function | References |
|---|---|---|---|
| HSC | Lymphotoxin-beta | LPCs proliferation | 18 |
| IL-6 | LPCs proliferation | 6 | |
| The fibroblast growth factor 7 | LPCs expansion | 15 | |
| Hedgehog (Hh) pathway | LPCs proliferation and differentiation | 5 | |
| Notch | LPCs differentiation | 14 | |
| Hepatocyte growth factor | LPCs proliferation and differentiation | 22 | |
| Macrophage | TWEAK | LPCs expansion | 13,27 |
| Wnt pathway | LPCs differentiation; | 14 | |
| promote the catheter response | |||
| Hepatocyte | Hedgehog (Hh) pathway | LPCs proliferation and differentiation | 10 |
Macrophage
Hepatic macrophages consist of two subgroups: tissue-resident Kupffer cells (KCs) and MDMs infiltrating peripheral blood. In damaged liver tissues, macrophages are clustered in areas where LPC response is evident, and also in tissues other than the response sites of precursor cells.24 In 2-AAF/PH-treated rats liposomes coated with clodronate reduced macrophages, inhibited LPCs expansion, and reduced residual liver regeneration.25 Resident and recruited macrophages play a key role in response to tissue damage. KC depletion reduced LPC proliferation in PBS liposome treated mice fed with CDE.26 These data suggest that KCs are necessary for early recruitment of monocytes to liver during CDE diet-induced injury and regeneration.26 Hence, KC may have effects on LPC proliferation by recruitment of monocyte.
Cytokine TNF-like weak apoptosis inducer (TWEAK), a member of the pro-inflammatory TNF family is produced by macrophages, monocytes and T lymphocytes. TWEAK can stimulates oval cell proliferation in mouse liver and cell culture models.13,27 TWEAK directly stimulates LPC mitosis in an Fn14-dependent and NFκB-dependent manner, and signals sent by this pathway mediate LPC response to CDE induced damage and regeneration.28 Notch and Wnt signaling directly promote the catheter response of LPCs through interactions with activated myofibroblasts or macrophages in human diseased liver and mouse models. Macrophage phagocytic hepatocyte fragment induces the expression of Wnt3a, which leads to the typical Wnt signal in the adjacent oval cells to express the Notch pathway inhibitor Numb, blocks the differentiation of LPCs into cholangiocytes, and turns into hepatocytes.14
Extracellular matrix
Recent studies have shown that ECM and LPCs interact in liver injury. LPC-ECM interaction mechanism consists of the following points29:
-
1.
Molecules on the LPC surface act as receptors for the matrix ligands, and the activation of the matrix ligands can initiate the downstream intracellular signaling pathway in the LPC.
-
2.
The matrix sequesters and presents growth factors, cytokines and chemokines, which are regulators of LPC behavior.
-
3.
LPC itself influences matrix composition and remodeling by producing matrix proteins or matrix degrading enzymes, so they can contribute to homeostasis.
-
4.
Intracellular signaling pathways are involved in LPC-ECM interactions.
Laminin can be detected around the LPCs and heavily correlates to LPC responses in many models of chronic liver injury and LPC activation in rodents and is closely related to LPC response, such as chronic fibrotic carbon tetrachloride (CCl4) injury in C57BL/6 Wt mice and collagen Iα1 (r/r) mice.30 In addition to the models mentioned above, other models include the pyrrolizidine alkaloid- and retrorsine-induced LPC proliferation model in transgenic mice with hepatitis B surface antigen gene, CDE diet-treated C57/B6 mice.24 Similarly, laminin in humans has been detected associated with LPCs in hepatitis B/C virus-related chronic hepatitis, and following fibrosing cholestatic hepatitis that occurred in a patient who received a liver transplant for hepatitis C.24 Study using the collagen Iα1 (r/r) mouse (r/r) to test the relationship between collagen degradation, laminin deposition and LPC response, found that r/r mice had a markedly attenuated LPC response compared to wild-type, which correlated with persistence of collagen type I and a failure to deposit laminin, both in the model of chronic CCl4 injury and CDE-induced LPC activation.30 This suggests that degradation of the collagen matrix is required to induce LPC response and that degradation of collagen matrix enables the formation of a laminin rich progenitor cell niche.
It was reported that zone 1-like matrix proteins, collagen types III and IV and laminin, enabled attachment and expansion of LPCs independent of feeders, whereas zone 3-like matrix proteins, collagen type I and fibronectin, elicited growth arrest and differentiation and inhibited attachment.31 Moreover, liver cells cultured on laminin for more than 1 month have a strong proliferation capacity, while the cells in type I collagen differentiate into liver cells and bile duct cells.32 Pluripotent progenitor cells cultured on a matrix containing a mixture of laminin and type IV collagen could differentiate into liver cells, while stem cells cultured on type I collagen were similar to biliary tract cells.33
Besides, α6β1 and α5β132 integrins as well as CD4434 on the surface of LPCs, and their related downstream signals, are important mediators of interactions between LPCs and the ECM.
Study suggests that matrix hardness has no major effect on hepatocyte differentiation, but may affect cell survival or adhesion during differentiation. In addition, the ECM components, especially type IV collagen, have a significant effect on hepatocyte differentiation of liver progenitor cells.35 Both the ECM protein and the stiffness properties affect differentiation of cholangiocyte. In particular, rigidity-mediated differentiation of cholangiocyte is observed in cells cultured on fibronectin, whereas type IV collagen-induced differentiation is independent of matrix stiffness.35 The related effect factors are listed in the Table 2.
Table 2.
The effect of microenvironment on LPCs.
| Factors | Source | Function | References |
|---|---|---|---|
| EVs | stromal lines | maintains the survival and clonogenic potential of HSPCs | 15 |
| Cytokines | Niche cells | LPCs activation, proliferation and differentiation | 16, 19,31 |
| Laminin | Extracellular matrix | LPCs proliferation and differentiation | 24 |
| collagen type I | Extracellular matrix | LPCs proliferation and differentiation | 23,30,31 |
| collagen types III | Extracellular matrix | LPCs proliferation | 31 |
| collagen types IV | Extracellular matrix | LPCs proliferation | 31 |
| 1-like matrix proteins | Extracellular matrix | LPCs proliferation | 31 |
| 3-like matrix proteins | Extracellular matrix | LPCs differentiation; | 31 |
| Matrix stiffness | Extracellular matrix | LPCs survival or adhesion | 35 |
Conclusion
LPC plays an important role in the process of liver cirrhosis repair. LPC has more than one source of cells, such as hepatocytes, cholangoicytes, HSCs, and fibroblasts. The niche has important influence on the proliferation and differentiation of LPC as well. Various components in ECM, in particular, matrix hardness, have an impact on the proliferation of LPC. Other cells in liver, such as macrophages, also function by secreting cytokines and various signaling pathways (Fig. 2).
Figure 2.
LPC-related cells in liver cirrhosis. The proliferation and activation of LPCs are regulated by niche cells secreting cytokines.
Although many advances have been made in the field of LPC-mediated regeneration, there are still many problems to be studied. It is still unknown that the proportion of various cell sources in disease conditions. Therefore, further research is needed to find the main source of LPC, so as to determine the following research direction. The relevant cellular and molecular mechanisms need to be further refined, so as to promote the transformation of LPC into hepatocytes to achieve liver function compensation from bench to bedside.
Conflict of Interests
The authors declare no conflict of interest.
Research involving human and/or animal rights
This Mini Review does not contain any studies with human or animal subjects.
Funding
This project is supported by the Project of Shanghai Municipal Health Commission [grant number 20204Y0012]; National Key R&D Program of China [grant number 2017YFC0908100]; Corhort Study of HCC and Liver Diseases, Double First-Class Fundation, Shanghai Jiao Tong University [grant number W410170015]; Overall Leverage Clinical Medicine Center, NHFPC Fundation [grant number 2017ZZ01018].
Footnotes
Peer review under responsibility of Chongqing Medical University.
Contributor Information
Kang He, Email: hekang929@163.com.
Qiang Xia, Email: xiaqiang@shsmu.edu.cn.
Abbreviations
- BTSCs
Biliary Tree Stem Cells
- CCN2
connective tissue growth factor 2
- CDE
Choline-Deficient, Ethionine
- CLF
cholestatic liver fibrosis
- DRs
ductular reactions
- ECM
Extracellular matrix
- EVs
Extracellular vesicles
- Hh
Hedgehog
- HSPCs
hematopoietic stem and progenitor cells
- HSCs
Hepatic stellate cells
- KO
knockout
- KCs
Kupffer cells
- LPCs
liver progenitor cells
- PBGs
peribiliary glands
- TWEAK
TNF-like weak inducer of apoptosis
References
- 1.Lanzoni G., Cardinale V., Carpino G. The hepatic, biliary, and pancreatic network of stem/progenitor cell niches in humans: a new reference frame for disease and regeneration. Hepatology (Baltimore, Md) 2016;64(1):277–286. doi: 10.1002/hep.28326. [DOI] [PubMed] [Google Scholar]
- 2.Alvaro D., Gaudio E. Liver capsule: biliary tree stem cell subpopulations. Hepatology. 2016;64(2) doi: 10.1002/hep.28546. [DOI] [PubMed] [Google Scholar]
- 3.Yanger K., Knigin D., Zong Y. Adult hepatocytes are generated by self-duplication rather than stem cell differentiation. Cell Stem Cell. 2014;15(3):340–349. doi: 10.1016/j.stem.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sekiya S., Suzuki A. Hepatocytes, rather than cholangiocytes, can be the major source of primitive ductules in the chronically injured mouse liver. Am J Pathol. 2014;184(5):1468–1478. doi: 10.1016/j.ajpath.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Raven A., Lu W.Y., Man T.Y. Cholangiocytes act as facultative liver stem cells during impaired hepatocyte regeneration. Nature. 2017;547(7663):350–354. doi: 10.1038/nature23015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kordes C., Sawitza I., Müller-Marbach A. CD133+ hepatic stellate cells are progenitor cells. Biochem Biophys Res Commun. 2007;352(2):410–417. doi: 10.1016/j.bbrc.2006.11.029. [DOI] [PubMed] [Google Scholar]
- 7.Michelotti G.A., Xie G., Swiderska M. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123(6):2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Swiderska-Syn M., Syn W.K., Xie G. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2014;63(8):1333–1344. doi: 10.1136/gutjnl-2013-305962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Williams M.J., Clouston A.D., Forbes S.J. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146(2):349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 10.Jung Y., Witek R.P., Syn W.-K. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59(5):655–665. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ma Z., Li F., Chen L. Autophagy promotes hepatic differentiation of hepatic progenitor cells by regulating the Wnt/beta-catenin signaling pathway. J Mol Histol. 2019;50(1):75–90. doi: 10.1007/s10735-018-9808-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X., Du G., Xu Y. Inhibition of notch signaling pathway prevents cholestatic liver fibrosis by decreasing the differentiation of hepatic progenitor cells into cholangiocytes. Lab Invest. 2016;96(3):350–360. doi: 10.1038/labinvest.2015.149. [DOI] [PubMed] [Google Scholar]
- 13.Jakubowski A., Ambrose C., Parr M. TWEAK induces liver progenitor cell proliferation. J Clin Invest. 2005;115(9):2330–2340. doi: 10.1172/JCI23486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boulter L., Govaere O., Bird T.G. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18(4):572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gressner O.A., Lahme B., Demirci I., Gressner A.M., Weiskirchen R. Differential effects of TGF -beta on connective tissue growth factor (CTGF/CCN2) expression in hepatic stellate cells and hepatocytes. J Hepatol. 2007;47(5):699–710. doi: 10.1016/j.jhep.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Chen L., Chen R., Kemper S., Charrier A., Brigstock D.R. Suppression of fibrogenic signaling in hepatic stellate cells by Twist1-dependent microRNA-214 expression: role of exosomes in horizontal transfer of Twist1. Am J Physiol Gastrointest Liver Physiol. 2015;309(6):G491–G499. doi: 10.1152/ajpgi.00140.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Yao H.-L., Cui C.-B. Paracrine signals from mesenchymal cell populations govern the expansion and differentiation of human hepatic stem cells to adult liver fates. Hepatology (Baltimore, Md) 2010;52(4):1443–1454. doi: 10.1002/hep.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruddell R.G., Knight B., Tirnitz-Parker J.E.E. Lymphotoxin-beta receptor signaling regulates hepatic stellate cell function and wound healing in a murine model of chronic liver injury. Hepatology (Baltimore, Md) 2009;49(1):227–239. doi: 10.1002/hep.22597. [DOI] [PubMed] [Google Scholar]
- 19.Streetz K.L., Tacke F., Leifeld L. Interleukin 6/gp130-dependent pathways are protective during chronic liver diseases. Hepatology (Baltimore, Md) 2003;38(1):218–229. doi: 10.1053/jhep.2003.50268. [DOI] [PubMed] [Google Scholar]
- 20.Takase H.M., Itoh T., Ino S. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27(2):169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lin N., Tang Z., Deng M. Hedgehog-mediated paracrine interaction between hepatic stellate cells and marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun. 2008;372(1):260–265. doi: 10.1016/j.bbrc.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 22.Ishikawa T., Factor V.M., JU Marquardt. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology (Baltimore, Md) 2012;55(4):1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chobert M.-N., Couchie D., Fourcot A. Liver precursor cells increase hepatic fibrosis induced by chronic carbon tetrachloride intoxication in rats. Lab Invest J Tech Meth Pathol. 2012;92(1):135–150. doi: 10.1038/labinvest.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lorenzini S., Bird T.G., Boulter L. Characterisation of a stereotypical cellular and extracellular adult liver progenitor cell niche in rodents and diseased human liver. Gut. 2010;59(5):645–654. doi: 10.1136/gut.2009.182345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiang S., Dong H.-H., Liang H.-F. Oval cell response is attenuated by depletion of liver resident macrophages in the 2-AAF/partial hepatectomy rat. PloS One. 2012;7(4) doi: 10.1371/journal.pone.0035180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elsegood C.L., Chan C.W., Degli-Esposti M.A. Kupffer cell-monocyte communication is essential for initiating murine liver progenitor cell-mediated liver regeneration. Hepatology (Baltimore, Md) 2015;62(4):1272–1284. doi: 10.1002/hep.27977. [DOI] [PubMed] [Google Scholar]
- 27.Bird T.G., Lu W.-Y., Boulter L. Bone marrow injection stimulates hepatic ductular reactions in the absence of injury via macrophage-mediated TWEAK signaling. Proc Natl Acad Sci USA. 2013;110(16):6542–6547. doi: 10.1073/pnas.1302168110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tirnitz-Parker J.E.E., Viebahn C.S., Jakubowski A. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology (Baltimore, Md) 2010;52(1):291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 29.Zhu C., Coombe D.R., Zheng M.H., Yeoh G.C.T., Li L. Liver progenitor cell interactions with the extracellular matrix. J Tiss Eng Regen Med. 2013;7(10):757–766. doi: 10.1002/term.1470. [DOI] [PubMed] [Google Scholar]
- 30.Kallis Y.N., Robson A.J., Fallowfield J.A. Remodelling of extracellular matrix is a requirement for the hepatic progenitor cell response. Gut. 2011;60(4):525–533. doi: 10.1136/gut.2010.224436. [DOI] [PubMed] [Google Scholar]
- 31.McClelland R., Wauthier E., Uronis J., Reid L. Gradients in the liver's extracellular matrix chemistry from periportal to pericentral zones: influence on human hepatic progenitors. Tissue Eng Part A. 2008;14(1):59–70. doi: 10.1089/ten.a.2007.0058. [DOI] [PubMed] [Google Scholar]
- 32.Tanimizu N., Saito H., Mostov K., Miyajima A. Long-term culture of hepatic progenitors derived from mouse Dlk+ hepatoblasts. J Cell Sci. 2004;117(Pt 26):6425–6434. doi: 10.1242/jcs.01572. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale V., Wang Y., Carpino G. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology (Baltimore, Md) 2011;54(6):2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 34.Chiu C.-C., Sheu J.-C., Chen C.-H. Global gene expression profiling reveals a key role of CD44 in hepatic oval-cell reaction after 2-AAF/CCl4 injury in rodents. Histochem Cell Biol. 2009;132(5):479–489. doi: 10.1007/s00418-009-0634-9. [DOI] [PubMed] [Google Scholar]
- 35.Kourouklis A.P., Kaylan K.B., Underhill G.H. Substrate stiffness and matrix composition coordinately control the differentiation of liver progenitor cells. Biomaterials. 2016;99:82–94. doi: 10.1016/j.biomaterials.2016.05.016. [DOI] [PubMed] [Google Scholar]