Abstract
The role of microglia in mediating age-related changes in cognition and hippocampal synaptic function was examined by microglial depletion and replenishment using PLX3397. We observed age-related differences in microglial number and morphology, as well as increased Iba-1 expression, indicating microglial activation. PLX3397 treatment decreased microglial number, with aged rats exhibiting the lowest density. Young rats exhibited increased expression of pro-inflammatory cytokines during depletion and repopulation and maintenance of Iba-1 levels despite reduced microglial number. For aged rats, several cytokines increased with depletion and recovered during repopulation; however, aged rats did not fully recover microglial cell number or Iba-1 expression during repopulation, with a recovery comparable to young control levels rather than aged controls. Hippocampal CA3–CA1 synaptic transmission was impaired with age, and microglial depletion was associated with decreased total synaptic transmission in young and aged rats. A robust decline in N-methyl-D-aspartate-receptor-mediated synaptic transmission arose in young depleted rats specifically. Microglial replenishment normalized depletion-induced synaptic function to control levels; however, recovery of aged animals did not mirror young. Microglial depletion was associated with decreased context-object discrimination memory in both age groups, which recovered with microglial repopulation. Aged rats displayed impaired contextual and cued fear memory, and microglial replenishment did not recover their memory to the level of young. The current study indicates that cognitive function and synaptic transmission benefit from the support of aged microglia and are hindered by removal of these cells. Replenishment of microglia in aging did not ameliorate age-related cognitive impairments or senescent synaptic function.
Keywords: aging, cognition, hippocampus, microglial depletion, microglial repopulation
1 |. INTRODUCTION
As the resident immune cells of the central nervous system (CNS), microglia actively survey the environment for pathogens and debris, responding via pro- and anti-inflammatory signaling and phagocytosis.
Microglia also play a critical role in shaping the neural terrain through synapse formation and elimination. Within this scope, microglia bidirectionally regulate synaptic strength (Akiyoshi et al., 2018; Li, Du, Liu, Wen, & Du, 2012; Stellwagen & Malenka, 2006) and impact synaptic density through phagocytosis (Maggi et al., 2011; C. Wang, Yue, et al., 2020; C. F. Wang, Zhao, et al., 2020). Microglia approach synaptic boutons and terminals in an activity-dependent manner (Akiyoshi et al., 2018); adenosine triphosphate and purines promote synaptic loss or stripping, and CX3CL1 and CD200 foster synaptic protection (Norden & Godbout, 2013). Microglia also support the formation of new synapses through brain-derived neurotrophic factor signaling (Parkhurst et al., 2013) and thus play an essential role in synaptic plasticity, learning and memory, and behavior (Torres et al., 2016; C. Wang, Yue, et al., 2020).
Aging, however, alters the communication between microglia and neurons. Reduced levels of anti-inflammatory cytokines, such as interleukin (IL)-4, transforming growth factor β, and IL-10, and blunted fractalkine and CD200/CD200R signaling are observed in aging and are associated with impaired long-term potentiation (LTP; Griffin et al., 2006; Yirmiya & Goshen, 2011). Aged microglia appear to be less responsive to negative feedback loops directed by these pathways, resulting in persistent M1 activated states (Norden & Godbout, 2013). When microglia are activated, synchronization and strengthening of synaptic activity through microglia-neuron contact is attenuated (Akiyoshi et al., 2018). With age, microglia shift to a senescent or dystrophic state (Streit, Sammons, Kuhns, & Sparks, 2004), leading to reduced phagocytosis, greater neuroinflammation, increased oxidative stress, and heightened neuronal insult under states of disease or injury (Angelova & Brown, 2019; Koellhoffer, McCullough, & Ritzel, 2017). Thus, decreased microglial malfunction, through en masse microglial depletion, repopulation, or removal of senescent microglia (Bussian et al., 2018), may serve as a potential therapeutic tool.
Data regarding the effects of microglial depletion (Elmore et al., 2014; Lehmann, Weigel, Poffenberger, & Herkenham, 2019; Lewen et al., 2020; Michels et al., 2019; Parkhurst et al., 2013; Rice et al., 2015; Soch et al., 2020; Torres et al., 2016; C. Wang, Yue, et al., 2020; C. F. Wang, Zhao, et al., 2020; Wang et al., 2016) and repopulation (Henry et al., 2020; Ikezu et al., 2020; Lehmann et al., 2019; Michels et al., 2019; Rice et al., 2017) on synaptic density and function, the inflammatory environment, and behavioral measures of cognition, motor capacity, and social interaction are highly mixed. Generally, the variable findings support the notion that microglia are beneficial or detrimental in a context-specific fashion (Colonna & Butovsky, 2017), with microglial depletion-associated impairments arising when depletion occurs during or shortly after an injury and benefits emerging with microglial repopulation following injury induction and disease states.
However, little work has examined the effects of microglial depletion and repopulation in the aging brain. Elmore et al. (2018) observed mild improvements in spatial memory and LTP in aged mice with replenished microglia, though inflammatory markers remained unchanged. Few data were presented regarding the impact of microglial depletion on hippocampal function in aging. Given the findings from Parkhurst et al. (2013), in which microglial depletion disrupted learning-induced synaptic formation in the motor cortex, we wished to investigate how microglia contribute to N-methyl-D-aspartate (NMDA) receptor-mediated synaptic function in the aged hippocampus. By utilizing the colony stimulating factor 1 receptor (CSF1R) inhibitor PLX3397, we hypothesized that microglia would be critical to optimal hippocampal function, with cognitive deficits and impaired synaptic transmission arising in microglial depleted aged rats. We expected that microglial repopulation would improve hippocampal capacity in aged rats toward a youthful phenotype. Interestingly, we observed that microglial depletion impaired both young and aged rats on hippocampal-dependent tasks and NMDA receptor-specific synaptic transmission in young rats. In addition, young and aged rats with repopulated microglia exhibited subtle cognitive and synaptic detriments, indicating a failure to properly reintegrate into the neural circuitry.
2 |. MATERIALS AND METHODS
2.1 |. Subjects
Young (4–6 months old) and aged (23–24 months old) male Fischer-344 rats were obtained from the National Institute on Aging (Bethesda, MD) and maintained at the University of Florida in a temperature- and humidity-controlled animal facility. Rats were pair housed and remained on a 12:12 light/dark cycle (lights on: 7 a.m.). After 1 week of acclimation to the facility, rats were handled and divided into treatment groups: control (normal rat chow; N = 8–10), microglial depleted (PLX3397-treated rat chow for 21 days; N = 7–8), and microglial replenished (PLX3397-treated rat chow for 21 days, followed by 14 days of normal chow; N = 8–12). A small cohort of young rats (N = 4/group) was euthanized for immunohistochemical analysis of brain tissue to verify efficacy of PLX3397 treatment in reducing the microglial population. After the full treatment period, the remaining rats were assessed cognitively, and half of them underwent electrophysiological recordings while the remaining had tissue collected for immunoblotting and cytokine analysis. All procedures involving animals were approved by the Institutional Animal Care and Use Committee at the University of Florida and were in agreement with guidelines recognized by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals.
2.2 |. Chemical reagent
PLX3397 (also known as Pexidartinib; MedChemExpress, NJ) inhibits the tyrosine protein kinase c-Kit, CSF1R, and FMS-related tyrosine kinase 3, resulting in substantial reductions in microglial population within the brain (Elmore et al., 2014; Rice et al., 2015; Spangenberg et al., 2016; Thompson et al., 2015). This compound was incorporated into standard rat chow at 1,200 mg/kg (TD.170153 formula, Envigo, Huntingdon, UK) to provide approximately 45 mg/kg of PLX3397 in the food. Prior to behavioral performance, the microglial depleted group consumed PLX3397-treated chow for 21 days, based on previous studies (Elmore et al., 2014; Jin et al., 2017; Li et al., 2017). Rats experiencing microglial replenishment underwent the same treatment dosage and duration, followed by 14 days on standard untreated rat chow (Elmore, Lee, West, & Green, 2015; Lehmann et al., 2019; O’Neil, Witcher, McKim, & Godbout, 2018). Drug treatment for these rats was initiated 2 weeks prior to the microglial depletion group to ensure that all groups underwent behavioral training at the same time. Control rats remained on standard rodent chow throughout the study. All rats were weighed throughout the experiment to examine effects of treatment on their overall body weight. The percent change in body weight was calculated as [(weight after treatment-weight prior to treatment)/weight prior to treatment] * 100, for two time periods depending on the treatment group: one 3 weeks after drug treatment (microglial depleted) and another 2 weeks after drug removal (microglial replenished).
2.3 |. Behavior
2.3.1 |. Novel context-object discrimination (COD)
As conducted previously (Czerniawski, Miyashita, Lewandowski, & Guzowski, 2015), rats explored two distinct contexts (A and B), each with their own extramaze cues and paired objects for 5 min, for two consecutive days of training. Context A was a small black pool whereas Context B consisted of a plexiglass box. Objects were approximately 5.25 in. tall × 3.25 in. wide (flower, in Context B) and 4 in. tall × 2.6 in. wide (fish, in Context A). The interval between exposure to the two contexts was approximately 2 hr. Context exposure order was counterbalanced between the 2 days of training. On the third day, rats were tested on their capacity to bind these objects with their designated contexts by placing them in Context B with a congruent object (from Context B) and an incongruent object (from Context A) for 5 min and measuring the amount of time they examined each object. During the training and testing phase, exploration time of the objects was recorded, and for the test phase a discrimination index (DI) score was calculated as (incongruent − congruent)/(incongruent + congruent). In addition, distance traveled and velocity (EthovisionXT 6, Noldus, Wageningen, the Netherlands) were recorded for evaluation of the impact of age and treatment on locomotion.
2.3.2 |. Fear conditioning and extinction
Apparatus
The chamber (PhenoTyper, Noldus) was composed of clear walls and metal bar flooring, through which the shock was delivered. EthoVision XT 6.0 (Noldus) on a Dell Precision T3400 delivered the tone and shock stimuli through a shock scrambler (Med Associates, VT) and USB-IO box (Noldus). During the cued testing and extinction trials, a triangular plexiglass insert and black plexiglass flooring, along with a vanilla scent, were applied to sufficiently modify the chamber to provide a different context.
Behavioral paradigm
Rats were shifted from the vivarium 45–60 min prior to task performance and habituated in an area adjacent to conditioning and testing. Rats underwent cued fear conditioning, including two tone-shock pairings (0.8 dB, 0.6 mA), where the tone (conditioned stimulus, CS; 15 s) co-extinguished with 2 s of the shock. Rats had 180 s to explore the context initially, prior to the first tone/shock pair, and 60 s after the final pairing before removal from the chamber. The interval between the two shocks was 180 s. The chamber walls had a distinct pattern on them to provide additional contextual cues for later testing. Rats were examined in 10 s bins for freezing behavior, defined as a lack of movement excluding respiration (Gould & Feiro, 2005).
Twenty-four hours after fear conditioning, rats were returned to the original context and examined for contextual fear memory (i.e., freezing) for 5 min. At least 2 hr after contextual fear testing, the chamber was modified (triangular insert, black plexiglass flooring, vanilla scent) and rats were placed in for 180 s (pre-CS period) prior to a 180 s-long tone presentation (CS period). Rats remained in the chamber for 60s after the tone presentation. They were evaluated for cued fear memory (i.e., freezing during CS presentation) and generalized fear responses (i.e., freezing prior to CS presentation). For cued fear extinction, rats were placed in this modified chamber and exposed to the tone under the same parameters as described above for five consecutive days.
2.4 |. Extracellular field potential recordings
Hippocampal slices were prepared from young and aged control, microglial depleted, and microglial replenished rats. Methods for hippocampal slice preparation and electrophysiological recording of total and NMDA receptor (NMDAR)-mediated synaptic responses have been published previously (Bean et al., 2015; Bodhinathan, Kumar, & Foster, 2010; Kumar & Foster, 2013, 2014, 2019; Kumar, Rani, et al., 2018; Kumar, Rani, Tchigranova, Lee, & Foster, 2012; Lee, Kumar, Rani, & Foster, 2014).
Rats were anesthetized with isoflurane (Halocarbon Laboratories, River Edge, NJ) and swiftly decapitated. The brains were rapidly removed and stored briefly in chilled, oxygenated (95% O2/5% CO2) artificial cerebrospinal fluid (aCSF) before hippocampi were dissected away. Hippocampal slices (~450–500 μm) were cut parallel to the alvear fibers using a tissue chopper. The slices were transferred to a standard interface recording chamber (Harvard Apparatus, Boston, MA) continuously perfused with standard oxygenated (95% O2, 5% CO2) aCSF at a flow rate of 2 ml/min. The pH and temperature were maintained at 7.4 and 30 ± 0.5°C, respectively. Humidified air (95% O2, 5% CO2) was continuously blown over the slices.
Extracellular synaptic field potentials (fEPSP) from CA3 to CA1 synaptic contacts were recorded with glass micropipettes (4–6 MΏ) filled with recording medium (aCSF). A concentric bipolar stimulating electrode (outer pole: stainless steel, 200 μm diameter; inner pole: Platinum/Iridium, 25 μm diameter, FHC, Bowdoinham, ME) was positioned approximately 1 mm from the recording electrode localized in the middle of the stratum radiatum. A single diphasic stimulus pulse of 100 μs was passed via stimulators (SD9 Stimulator, Grass Instrument Co, West Warwick, RI) to the Schaffer collateral commissural pathway, in order to evoke field potentials at 0.033 Hz. Field potentials were amplified 100×, bandpass-filtered between 1 Hz and 1 kHz by a differential AC amplifier (A-M Systems, Everett, WA), converted to digital units, and stored on a computer for off-line analysis (Data Wave Technologies, Longmont, CO). Two cursors were placed around the initial descending phase of the waveform, and the maximum slope (mV/ms) of the fEPSP was determined by a computer algorithm that found the maximum change across all sets of 10 consecutively recorded points (20 kHz sampling rate) between the two cursors. To measure the influence of treatment on basal synaptic transmission, input–output curves for the total fEPSP slope were constructed for a range of increasing stimulation intensities (4–40 V).
Following collection of input–output data of total fEPSP, the paired-pulse facilitation (PPF) ratio was determined, as described previously (Bodhinathan et al., 2010; Foster & Dumas, 2001; Foster & McNaughton, 1991; Kumar, 2010; Kumar & Foster, 2007). Briefly, the paired pulse was delivered through a single stimulating electrode at various interpulse intervals (IPI; 50, 100, 200, and 400 ms). The first pulse was set to elicit 50% of the maximal fEPSP, as determined by the input–output curve. Five paired responses were recorded for each IPI. The PPF ratio was calculated by dividing the slope of the second synaptic response by the slope of the first response.
The NMDAR-mediated component of synaptic transmission was obtained by incubating slices in aCSF containing low Mg2+ (0.5 mM), 6,7-dinitroquinoxaline-2,3-dione (Cayman Chemical, 30 μM), and picrotoxin (Tocris Bioscience, 10 μM), as described previously (Bodhinathan et al., 2010; Kumar & Foster, 2013; Kumar, Rani, et al., 2018; Kumar, Thinschmidt, & Foster, 2019; Lee et al., 2014). Input–output curves for the NMDAR-mediated slope were constructed for increasing stimulation intensities from slices obtained from control and treated animals.
2.5 |. Immunohistochemistry
2.5.1 |. Tissue collection
A small cohort of young rats (controls and microglial depleted) were utilized to evaluate the efficacy of PLX3397 in reducing microglial number, as well as to examine general microglial morphology within the brain. Rats were anesthetized with isoflurane and rapidly decapitated, with one hemisphere placed immediately in 4% paraformaldehyde (PFA). Brains remained in PFA at 4°C for 48 hr, were transferred to 30% sucrose, and upon sinking in sucrose were mounted in optimal cutting temperature (OCT) compound and frozen at −80°C for later processing. From the other hemisphere, the frontal cortex and hippocampus were collected on an ice-cold Petri dish and flash frozen in liquid nitrogen. Samples were stored at −80°C. Once the drug was shown to be effective, a group of microglial replenished rats were anesthetized with isoflurane and transcardially perfused, first with ice-cold 1X phosphate buffered saline (PBS) and then with 4% PFA. Brains were extracted and stored in 4% PFA at 4°C for 24 hr, transferred to 30% sucrose, and then mounted in OCT and stored at −80°C. In addition, hippocampal slices from rats undergoing electrophysiology were collected, fixed in 4% PFA and stained for confirmation of PLX3397 treatment at the site of the recording. To ascertain if the CSF1R inhibitor treatment impacted macrophages, as well as microglia, a lobe of the liver was collected from a subset of rats and frozen at −80°C for later processing.
2.5.2 |. Immunofluorescent procedure
Brains were sliced as 40 μm-thick coronal sections, with the following regions collected for further examination: prefrontal cortex (PFC), hippocampus, and cerebellum. Sections were maintained in cryoprotectant solution (30% ethylene glycol, 15% glucose, 0.04% sodium azide in 1x PBS) until further processing. As previously conducted (Yegla & Foster, 2019) four free-floating sections were rinsed in 1X PBS, blocked for 2 hr in 10% donkey serum and 0.3% TritonX-100, and incubated overnight in 1% bovine serum albumin (BSA), 0.3% TritonX100, and goat anti-Iba-1 (ionized calcium binding adaptor molecule-1; 1:500, Abcam, Cambridge, MA). Sections were then rinsed and incubated for 2 hr in 1% BSA and Alexa Fluor 488 donkey anti-goat (1:500; Life Technologies, Waltham, MA). Slices were rinsed in PBS, mounted onto slides and coverslipped with VectaShield containing DAPI (Vector, Burlingame, CA).
2.5.3 |. Image analysis
Four representative sections of the PFC (A/P range: +3.7 to +2.0), hippocampus (A/P range: −3.5 to −5.5), and cerebellum (A/P range: −10 to −12) from each rat were selected for immunohistochemical analysis. Sections were captured at 400x utilizing a Leica DM2500 microscope (Wetzlar, Germany), equipped with a Retiga 4000R camera (QImaging, Surrey, BC, Canada) with QCapture Pro7 software (QImaging). Utilizing NIH ImageJ, images were processed to reduce background and enhance the boundaries of microglia (Iba-1+ cells). The image was converted from pixels/in. to microns (56pixels/μm) and analyzed for microglial count and branching parameters, including branch length, branch density, and branch complexity, utilizing the Skeleton (2D/3D) and FracLac plugins. These plugins provide output measures, such as fractal dimension, lacunarity, circularity, density, and span ratio, all of which provide information on the complexity of the microglial cells evaluated. Briefly, fractal dimension assesses how many branches proceed from the cell’s center. Lacunarity is representative of how many gaps there are, incorporating the cell body and branches (more gaps = higher lacunarity). Circularity evaluates how similar to a circle the cell is (perfect circle = 1) and span ratio describes how oblong it is. Density integrates circularity and span ratio to describe the percentage of space the cell embodies of its surrounding area. A subset of Iba-1+ cells, specifically those from hippocampal sections of rats undergoing electrophysiological recordings, were also examined for additional parameters such as total area and average size.
2.6 |. Immunoblotting
After thawing on ice, prefrontal and hippocampal tissue were sonicated with lysis buffer (215 μl for hippocampus, 300 μl for PFC; 10X PBS, 1% ethylene diamine tetra acetic acid, 0.1% Tween, 1% phosphatase and proteinase inhibitors, Thermo Scientific, Waltham, MA; (Bean et al., 2015; Lee et al., 2014). Liver samples were maintained in liquid nitrogen, pulverized with a mortar and pestle and sonicated in lysis buffer (70–100 mg/ml). Samples remained on ice for 1 hr, with 15 min intervals of vortexing. Samples were centrifuged at 20,000g for 10 min at 4°C, and the supernatant was collected for immunoblotting. A bicinochoninic acid (BCA) protein assay (Thermo Fisher) was utilized to estimate protein concentration. After denaturing the protein for 10 min at 95–100°C, 30 μg of protein, mixed with Laemmli and 2-mercaptoethanol was loaded into a gel (BioRad, Hercules, CA) for electrophoresis at 100 V for 60 min followed by 120 V for 25 min. The protein was transferred to a polyvinylidene difluoride membrane at 20 V for 90 min. The membrane was rinsed in PBS, blocked in 5% BSA, and incubated in 1% BSA in PBS with goat anti-Iba-1 (1:500; abcam), mouse anti-PSD-95 (postsynaptic density-95; 1:2,000; Invitrogen, Carlsbad, CA), and rabbit anti-β-tubulin (1:1,000; Sigma) on a shaker overnight at 4°C. The membrane was rinsed and incubated in 1% BSA with PBS with 1:500 of secondary antibodies (AlexaFluor 594 donkey anti-mouse and anti-rabbit, AlexaFluor 488 donkey antigoat, Invitrogen, Carlsbad, CA). The membrane was imaged with ChemiDoc MP Imager (BioRad) and analyzed with NIH ImageJ for differences in integrated density.
2.7 |. Prefrontal and hippocampal cytokines
A portion of the prefrontal and hippocampal tissue homogenized in PBS buffer (see Section 2.6) was processed for pro- and anti-inflammatory cytokine levels. Utilizing the Milliplex Multiplex immunoassay kit (Millipore, Burlington, MA), the following cytokines were measured: IL-4, IL-1β, IL-6, IL-10, IL-12p70 IL-13, interferon-γ (IFNγ), and tumor necrosis factor-α (TNFα). The data were converted from pg/ml to pg/mg, using the total protein estimation acquired from the BCA output.
2.8 |. Statistical analysis
Behavioral, immunohistochemical, immunoblotting, and cytokine data were analyzed with a two-factor analysis of variance (ANOVA), including age and treatment type as between-subjects factors. Cued fear extinction was analyzed with a two-way repeated-measures ANOVA, with day (level = 5) as the within-subjects factor.
Immunohistochemical analyses evaluating regional differences also were conducted with a two-way repeated-measures ANOVA, with either PFC, hippocampus, and cerebellum as the within-subjects factors or CA1, CA3, and DG as the within-subjects factors. Electrophysiological measures were evaluated with a two-way repeated-measures ANOVA, with stimulation intensity or interpulse intervals as the within-subjects factor. For significant interactions, Fisher’s least significant difference post hoc test was applied to identify the source of the interaction. Under circumstances of non-normality, the nonparametric Mann–Whitney U or Kruskall–Wallis test was applied. Alternatively, for heteroscedasticity of variances, the Browns– Forsythe or Welch test was utilized. For violations of sphericity on repeated measures, the Greenhouse–Geisser statistic and degrees of freedom were reported. Relationships between variables, including behavior, electrophysiology, and immunohistochemistry, were evaluated via Pearson correlation (r). Data with p-values ≤.05 were interpreted as significant.
3 |. RESULTS
3.1 |. PLX3397 treatment did not produce overt adverse physiological effects
Treatment did not impact weight during the length of the study. However, body weight was influenced by age (F1,27 = 37.06, p <.001), with young rats increasing weight, and aged rats gradually decreasing weight as the study progressed (Figure S1). Thus, PLX3397 treatment was tolerated well by the rats, and the presence of the drug in rat chow did not exert a decline in food consumption or metabolism.
3.2 |. PLX3397 reduced microglial population, with greater effects in aged rats
To confirm the effects of PLX3397 treatment on microglial population, a small cohort of young rats (N = 4) were dosed and compared to controls (N = 4) for changes in microglial count and morphological complexity (Figure 1a). Rats treated with PLX3397 had significantly decreased microglial counts in the PFC (Kruskall–Wallis: 6.54, p = .01), hippocampus (F1,6.25 = 83.03, p <.001), and cerebellum (F1,8 = 16.17, p = .004), with an overall loss of about 61% compared to controls (Figure 1b). There was a marginal effect of treatment on branch length across the brain (F1,8 = 5.13, p = .05), in which PLX3397-treated rats displayed longer microglial branching (Figure 1c). Regional differences were noted in branch length (F2,16 = 4.11, p = .04), as well as the number of branches per cell (F2,16 = 3.75, p = .05; Figure 1d). The cerebellum drove these results, exhibiting more branches per cell (p = .04) but shorter branches (p = .005) compared to the PFC. Despite the substantial depletion of microglia, there were no significant differences between treated and control rats on lacunarity, fractal dimension, span ratio, circularity, or density (all p >.05). Thus, although microglial numbers were significantly reduced with PLX3397 treatment, the remaining microglia did not change in their morphological complexity.
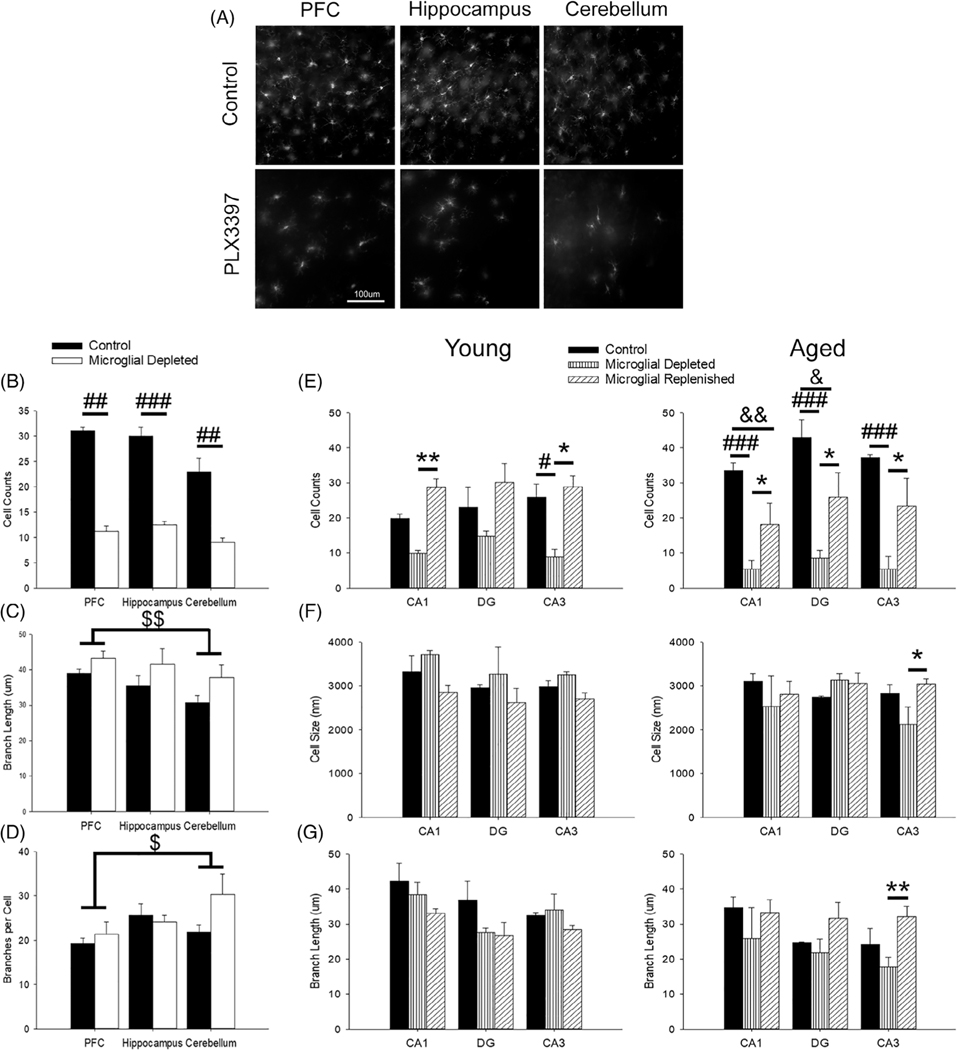
FIGURE 1.
Efficacy of PLX3397 treatment in depleting microglia. (a) Representative images of Iba-1-positive cells in PFC, hippocampus, and cerebellum between young controls and PLX3397-treated rats and their quantification for Iba-1-positive cell counts (b), branch length (c), and branches per cell (d) within the PFC, hippocampus, and cerebellum (4 slices/rat; 4 rats/group). PLX3397 treatment significantly reduced microglial cell counts throughout the brain (b; p <.01) and marginally elongated microglial branch length (c; p = .05). Regionally, the cerebellum displayed more branches per microglia (d; p = .04) but shorter branches overall compared to the PFC (c; p = .005). (e) From behaviorally trained rats, which included all age and treatment groups (three slices/rat; 2–3 rats/group), hippocampal microglia were significantly reduced with PLX3397 treatment (p ≤.01) and repopulated upon treatment removal. Within the CA1 and DG, however, aged rats with a replenished microglial population did not return to aged control levels (p ≤.03). Aged control rats exhibited greater microglial cell count in the CA1 compared to most groups (p ≤.03). Within the CA3 region, aged microglial depleted rats exhibited smaller cell bodies (f; p <.02) and shortened branches compared to most groups (g; p <.02). All data are represented as means ± SEM. # p ≤.05, ## p ≤.01, ### p ≤.001, control versus microglial depleted; & p ≤.05, && p ≤.01, control versus microglial replenished; * p ≤.05, ** p ≤.01, microglial depleted versus microglial replenished; $ p ≤.05, $ $ p ≤.01, PFC versus cerebellum
Once efficacy of PLX3397 treatment on microglial density was confirmed, rats undergoing behavioral training and electrophysiology were treated accordingly. These rats included all age and treatment groups, and microglial characterization was focused on hippocampal regions. In general, aged controls exhibited the greatest number of microglia. For region CA1, aged control rats exhibited a significantly greater number of Iba-1 immunoreactive cells relative to all other groups (p ≤.03), except young replenished. Treatment significantly decreased microglial number in area CA1 (F2,10 = 16.09, p = .001), CA3 (F2,10 = 13.66, p = .001), and DG (F2,9 = 8.52, p = .008; Figure 1e), with aged depleted rats exhibiting the smallest number of cells. Microglia increased in number during repopulation; however, age-related differences in repopulation were noted. While aged replenished rats increased their microglial population compared to aged depleted rats (p = .02), the number did not recover relative to age-matched controls (p = .01) and young replenished rats (p <.05). Post hoc analysis indicated a lower number of microglia in region CA1 and the DG of aged replenished relative to age-matched controls.
Examination of microglial cell size and branch length indicated that region CA3 of aged animals was particularly sensitive to treatment. The size of microglia within the CA3 exhibited an age by treatment interaction (F2,10 = 5.35, p = .03; Figure 1f), such that CA3 microglia of aged depleted rats had the smallest cell bodies relative to the aged replenished group. Examination of branch length across all regions revealed age differences (F1,8 = 5.81, p = .04), with aged rats displaying shorter microglial branch length. This age-related decline was magnified by treatment within the CA3 only (F2,9 = 7.00, p = .02), such that aged microglial depleted rats had shorter branching compared to young (control, depleted, and replenished) and aged replenished (p ≤.02; Figure 1g).
No significant differences in the morphological complexity of the microglia were observed between controls and rats treated with PLX3397, except for an age effect for lacunarity of microglia within the DG (F1,10 = 6.81, p = .03). Young microglia were more lacunar, suggesting that they had more or larger gaps between the branching of their microglia compared to aged rats and thus had greater morphological complexity. No additional morphological measures significantly varied between the groups. In conclusion, microglia exhibited a strong impact of aging, with greater microglial activation indicated as more microglia and shorter branches (Hopperton, Mohammad, Trépanier, Giuliano, & Bazinet, 2018; Shaerzadeh et al., 2020; Streit et al., 2004; Streit & Xue, 2013). PLX3397-treated young and aged animals exhibited microglial depletion, and aged depleted rats had the fewest microglia along with shorter branches. Age differences related to treatment included attenuated recovery of microglial number during repopulation, particularly in region CA1, and shorter microglial branches in CA3 relative to aged repopulated rats.
3.3 |. PLX3397 treatment induced off-site declines of Iba-1 protein expression in the liver
Brain and liver tissue were examined for treatment-induced shifts in microglial and synaptic markers, as well as off-target effects on macrophages within the liver (Figure 2a,b,e). Confirming immunohistochemical data, treatment significantly decreased brain (PFC and hippocampus) expression of the microglial activation marker Iba-1 (F2,23 = 10.13, p = .01, Figure 2c) for microglial depleted rats (p = .001) compared to controls. In contrast to microglial counts, microglial replenished rats displayed lower Iba-1 levels than controls (p = .001). These effects were driven primarily by changes in the hippocampus of aged rats. Post hoc ANOVAs within each region indicated an age by treatment interaction for the hippocampus (F2,27 = 9.93, p <.001) due to decreased Iba-1 expression in the depleted and repopulated hippocampus of aged rats relative to age-matched controls. The pattern is consistent with an increase in microglial activation and microglial number in aged control animals and a decrease in microglial cell numbers in aged depleted and repopulated animals. In contrast, the absence of a treatment effect on Iba-1 expression in young animals suggests a potential shift in microglial activation or function of remaining microglial cells (Ito et al., 1998; Norden, Trojanowski, Villanueva, Navarro, & Godbout, 2016; Sogn, Puchades, & Gundersen, 2013).
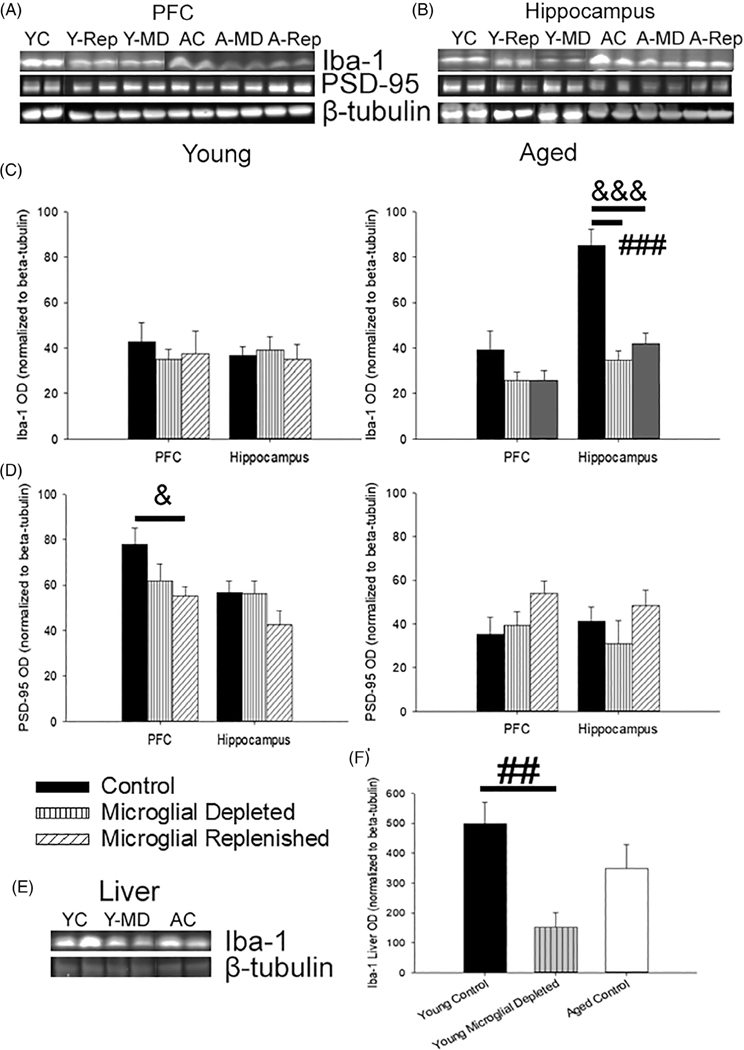
FIGURE 2.
PLX3397 treatment reduced Iba-1 expression in the brain and liver and shifted synaptic and astrocytic markers. (a,b) Representative images of immunoblots examining changes in microglial and synaptic protein markers in the PFC and hippocampus following microglial depletion and replenishment (5–9 young rats/treatment; 4–7 aged rats/treatment). (c) Levels of hippocampal Iba-1 decreased with microglial depletion and replenishment in aged animals. (d) Corresponding shifts in postsynaptic dynamics, measured by PSD-95, were examined. Age-related declines in PSD-95 were noted (p <.001); though within young rats, PLX3397 treatment tended to decrease prefrontal PSD-95 levels (p = .09), which failed to recover with microglial replenishment (p = .03). To examine off-site effects of PLX3397 treatment, liver Iba-1 levels were measured (e) and found to significantly decrease from microglial depletion within young (f), demonstrating an impact of PLX3397 treatment on both the brain and periphery. All data are represented as means ± SEM. ## p ≤.01, ### p ≤.001, control versus microglial depleted; & p ≤.05, &&& p ≤.001, control versus microglial replenished; ** p ≤.01, microglial depleted versus microglial replenished
Prefrontal and hippocampal expression of the postsynaptic marker, PSD-95, was significantly lower in aged rats (F1,23 = 17.18, p <.001; Figure 2d). Within the PFC, age interacted with treatment (F2,33 = 4.21, p = .02), but no main effect of treatment was observed (p >.05). Post hoc ANOVAs in each age group indicated that, compared to controls, microglial depletion (p = .09) and replenishment (p = .03) decreased prefrontal PSD-95 levels in young rats, which was not observed in the hippocampus. Thus, examination of protein expression confirmed a robust decrease in Iba-1 levels in aged animals, which did not recover to control levels during repopulation. In addition, young rats exhibited decreased expression of PSD-95 during repopulation.
To look for off-target effects of PLX3397, liver was collected from a subset of rats (young control, N = 9; young depleted, N = 4; aged control, N = 4) to examine Iba-1 expression as a marker for macrophages (Figure 2e). Although it did not differ by age, Iba-1 significantly varied within the liver with treatment (F1,15 = 7.82, p = .01; Figure 2f), declining with PLX3397 treatment in young (p = .007) compared to young controls, suggesting an impact of PLX3397 on peripheral macrophages.
3.4 |. Cytokines increased with microglial depletion and resolved during repopulation in an age-dependent manner
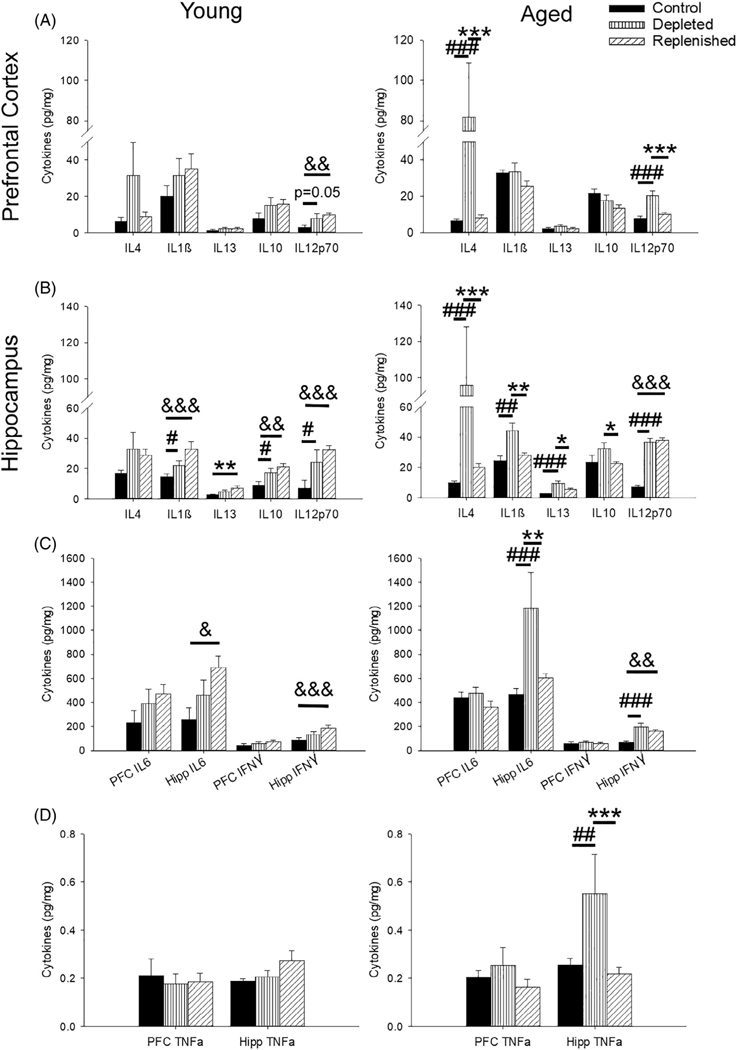
Prefrontal and hippocampal cytokines were measured to determine the impact of microglial depletion and replenishment on pro- and anti-inflammatory cytokines, which are known to interact with or be released by microglia (Colonna & Butovsky, 2017; Fumagalli, Lombardi, Gressens, & Verderio, 2018; Werneburg, Feinberg, Johnson, & Schafer, 2017). Age effects were observed across all treatment conditions with older animals exhibiting higher levels of prefrontal IL12p70 (F1,29 = 15.70, p <.001) and hippocampal IL-1β (F1,25 = 8.92, p = .006), IL-6 (F1,22 = 7.32, p = .01), IL-10 (F1,25 = 20.16, p <.001), and TNFα (F1,25 = 6.01, p = .02; Figure 3a–c). A main effect of treatment was observed for hippocampal IL-12p70 (F2,25 = 17.67, p <.001) and IFNγ (F2,25 = 12.87, p <.001), and post hoc tests within each age group indicated that hippocampal IL-12p70 and IFNγ increased in young and aged, depleted and replenished rats compared to control levels (p <.001; Figure 3b,c). Finally, age and treatment interactions were noted for IL-4 (PFC: F2,28 = 2.89, p = .07; hippocampus: F2,25 = 6.16, p = .007), IL-10 (PFC: F2,29 = 3.81, p = .03; hippocampus: F2,25 = 4.42, p = .02), prefrontal IL-12p70 (F2,29 = 5.29, p = .01), hippocampal IL-1β (F2,25 = 6.96, p = .004), hippocampal IL-6 (F2,25 = 5.42, p = .01), hippocampal IL-13 (F2,25 = 4.36, p = .02), and hippocampal TNFα (F2,25 = 6.26, p = .006; Figure 3a–d). Post hoc analyses demonstrated two distinct patterns, which were discernible by age. In young rats, treatment increased cytokine levels during depletion, which continued to increase during microglial replenishment. Specifically, young replenished animals exhibited a significant increase in prefrontal IL12p70 and IL-6 (p <.04) and hippocampal IL-1β, IL-6, IL-13, IL-10, IL12p70, and IFNγ (p <.02), relative to young controls. Aged rats, on the other hand, exhibited increased cytokine levels during microglial depletion, which recovered during repopulation. Thus, relative to aged controls, depletion was associated with a significant increase in expression of prefrontal and hippocampal IL-4, prefrontal and hippocampal IL-12p70, and hippocampal IL-1β, IL-6, IL-13, IFNγ, and TNFα (p <.005), which fully recovered upon microglial replenishment.
FIGURE 3.
PLX3397 increased both pro- and anti-inflammatory cytokines in the PFC and hippocampus. (a) Prefrontal levels of IL-4 and IL12p70 increased with PLX3397 treatment. Young depleted and replenished rats exhibited heightened levels of IL-12p70 compared to age-matched controls (vs. depleted: p = .05; vs. replenished: p = .01), whereas microglial depletion in aged rats resulted in a surge of IL-4 (p ≤.008) and IL-12p70 (p ≤.001) compared to all groups. (b–d) The hippocampus displayed a large number of changes in cytokine levels, especially for aged rats. Overall, increased levels were observed for both young depleted and replenished rats compared to controls, including IL-1β, IL-10, and IL-13 (b). In contrast, the greatest differences in cytokine levels in aged rats was with microglial depletion, which exhibited the largest increase for IL-4, IL1β, IL-13, IL-10, IL-6, and TNFα. IL-12p70 and IFNγ were the only cytokines in the hippocampus to display the same pattern of treatment-induced increases for both young and aged rats (b,c). (N = 4–6 rats/group) All data are represented as means ± SEM. # p ≤.05, ## p ≤.01, ### p ≤.001, control versus microglial depleted; & p ≤.05, && p ≤.01, &&& p ≤.001, control versus microglial replenished; * p ≤.05, ** p ≤.01, *** p ≤.001, microglial depleted versus microglial replenished
3.5 |. Microglial depletion impaired hippocampal-dependent memory on COD regardless of age
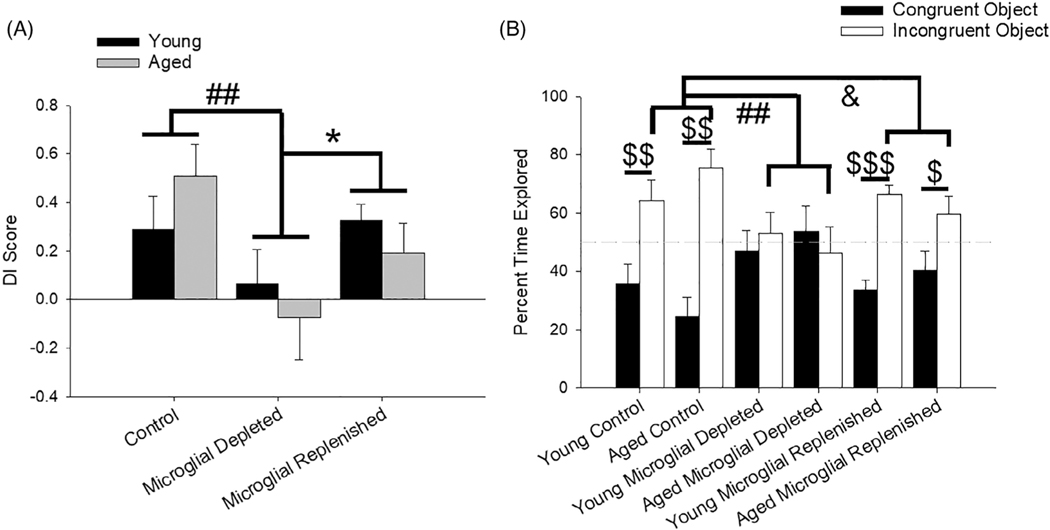
Due to the necessity of hippocampal engagement to accurately bind relational information, such as pairing a context and an object (Cooper & Ritchey, 2020; Howard, Kumaran, Ólafsdóttir, & Spiers, 2011; Libby, Hannula, & Ranganath, 2014), the COD task was utilized to investigate the impact of microglial depletion and replenishment on hippocampal function. Treatment effects were observed for the training and testing phases, in the absence of an effect of age or an age and treatment interaction. Microglial depleted and replenished rats increased their exploration from Context A to Context B (p ≤.003), and microglial depleted rats explored the objects in Context B more than controls (p = .01; Figure S2a). Overall, locomotion was also altered by treatment type for both distance traveled (F2,47 = 6.10, p <.01; Figure S2b) and velocity (F2,47 = 7.04, p <.01; Figure S2c) during the training phase, increasing specifically in microglial replenished rats compared to control and microglial depleted rats (p <.01 for both groups and measures).
During the test session, whereby rats explored a context-congruent and -incongruent object, microglial depletion impaired hippocampal memory, with a significant main effect of treatment on DI score (F2,37 = 4.86, p = .01; Figure 4a). Microglial depleted rats exhibited worse memory than controls (p <.01) and rats with replenished microglia (p <.05). Delving into the amount of time each group explored the objects during the test phase, all groups were comparable when exploring the congruent object (p >.05), but there was a significant treatment effect (F2,37 = 6.57, p <.01) for exploring the incongruent object (Figure 4b). Post hoc analyses indicated that controls explored the incongruent object more than microglial depleted (p <.01) and replenished rats (p = .02).
FIGURE 4.
Treatment effects on hippocampal-dependent memory on a novel COD task. (a) When tested between a context-congruent and -incongruent object, microglial depleted rats had worse memory than control (p <.01) and microglial replenished rats (p <.05). (b) More specifically, rats did not vary on the amount of time they explored the context-congruent object, but treatment impacted the percentage of time they explored the context-incongruent object, with control rats exploring it more than microglial depleted (p <.01) and replenished (p = .02) rats. Moreover, controls and microglial replenished rats exhibited a heightened preference for the context-incongruent object over the context-congruent object. All data are represented as means ± SEM (N = 7–10 young/treatment; N = 6–7 aged/treatment). ## p ≤.01, control versus microglial depleted; & p ≤.05, control versus microglial replenished; * p ≤.05, microglial depleted versus microglial replenished; $ p ≤.05, $ $ p ≤.01, $ $ $, p ≤.001, congruent versus incongruent object
3.6 |. Microglial depletion enhanced generalized fear responses in young
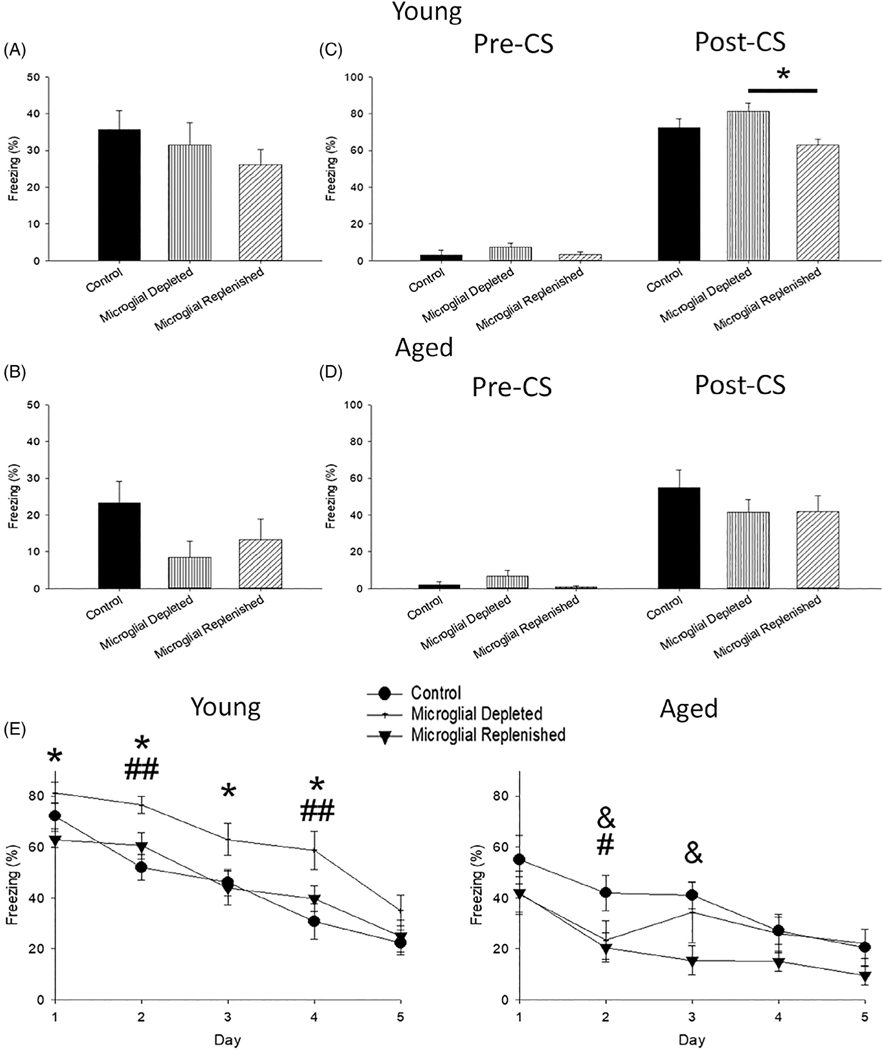
In addition to examining hippocampal function in the COD task, contextual and cued fear memory and cued extinction were conducted to investigate functional changes due to treatment and aging in the amygdala, hippocampus, and PFC, which are critically involved in these behavioral processes (Alvarez, Biggs, Chen, Pine, & Grillon, 2008; Bergstrom & Johnson, 2014; Hong, Song, Lee, Kim, & Choi, 2009; Klavir, Prigge, Sarel, Paz, & Yizhar, 2017; Marschner, Kalisch, Vervliet, Vansteenwegen, & Büchel, 2008; Phillips & LeDoux, 1992; Sehlmeyer et al., 2009). During the initial fear conditioning session, no significant differences in age (p >.05) or treatment (p >.05) on freezing behavior were observed in the pre-CS period. During the post-CS period, aged rats froze less than young (Mann–Whitney U = 224.0, p = .03). This age-specific decrease in freezing was consistently observed during contextual (U = 161.5, p = .001; Figure 5a,b) and cued fear memory testing (U = 135.5, p <.001; Figure 5c,d) and extinction (F2,46 = 37.13, p <.001; Figure 5e), consistent with previous findings (Kudo, Wati, Qiao, Arita, & Kanba, 2005; Villeda et al., 2011).
FIGURE 5.
Treatment was associated with increased freezing, specifically in young rats. Compared to young (a,c), aged rats exhibited less freezing during contextual (b) and cued (d) fear memory. (b) PLX3397 treatment slightly reduced freezing in aged microglial depleted rats compared to age-matched controls (p = .06). Interestingly microglial depleted rats, regardless of age, exhibited overgeneralization of the fear response, as seen with greater pre-CS freezing (c,d; p = .01). (c) Young microglial depleted rats also displayed greater post-CS freezing compared to young microglial replenished rats (p = .03). This pattern persisted during extinction training (e), with young microglial depleted rats freezing the most (p <.02) and aged rats, especially microglial replenished, freezing the least (p <.05). All data are represented as means ± SEM (N = 8–12 young/treatment; N = 7–8 aged/treatment). & p ≤.05, control versus microglial replenished; * p ≤.05, microglial depleted versus microglial replenished; # p ≤.05, ## p ≤.01, control versus microglial depleted
Microglial depletion increased freezing behavior, particularly for young animals. During the pre-CS period of the cued memory test (Figure 5c,d), microglial depleted rats exhibited significantly greater freezing than other groups (Kruskal–Wallis = 8.74, p = .01). A significant age and treatment interaction was noted during the cue presentation (i.e., CS) of cued fear memory testing (Kruskal–Wallis = 21.86, p = .001). Examination within each age group indicated that young microglial depleted rats froze significantly more than young microglial replenished rats (p = .03). Furthermore, this heightened freezing response of young depleted rats persisted for the first 4 days of extinction training, compared to young control and replenished rats (Figure 5e). Post hoc analysis of an age and treatment interaction (F2,46 = 4.86, p = .01) demonstrated that, in contrast, aged microglial replenished rats exhibited reduced freezing compared to age-matched controls (p = .02). Thus, microglial depletion was associated with a greater generalized fear response, particularly in young rats. This was evident by the increased freezing during the pre-CS period for both young and aged rats and during cued testing and extinction training for young depleted rats.
3.7 |. Microglial depletion was associated with decreased total and NMDAR-mediated synaptic transmission in the hippocampus, particularly in young rats
Hippocampal slices were prepared (Figure 6a), and the same slices utilized for all measures, including PPF, total fEPSP, and NMDAR-mediated fEPSP, for young and aged controls (5–6 slices/group; 3 rats/group), microglial depleted (4–10 slices/group; 3–5 rats/group), and microglial replenished rats (10 slices/group; 5 rats/group). The PPF ratio was examined as a measure of presynaptic function (representative traces: Figure 6b,d). As expected, there was a main effect of increasing IPI (F1.83,78.55 = 314.47, p <.001; Figure 6c,e), with a progressive decline in PPF ratio as intervals lengthened (p <.001 for all comparisons). PPF measures displayed a significant main effect of age (F1,43 = 4.82, p = .03), due to decreased facilitation for aged rats (Figure 6e), and an age and treatment interaction (F2,43 = 5.13, p = .01). Post hoc tests for each interval in young animals indicated a modest decrease in the PPF ratio following treatment, relative to controls (Figure 6c). In contrast, post hoc tests for aged animals indicated treatment increased the PPF ratio predominantly at 100 and 200 ms IPIs (Figure 6e).
FIGURE 6.
Treatment effects on presynaptic function and basal synaptic transmission. (a) Schematic illustration of a rat hippocampal slice demonstrating the placement of the recording and stimulating electrodes. The stimulating electrode was placed in the stratum radiatum near the CA3/CA1 synapse to stimulate the afferent Schaffer collateral pathway, and the recording pipette was placed in the stratum radiatum to record fEPSP. Representative fEPSP traces (first and second fEPSP) illustrating PPF from young (b) and aged (d) rats under control, microglial depleted, and microglial replenished conditions. (c,e) Bar diagram depicting mean PPF recorded at different interpulse intervals. A significant reduction in PPF was observed in young rats treated with PLX3397, predominantly for microglial depleted compared to young controls (p <.05; c). In contrast, PPF increased for aged microglial depleted (p = .04) and replenished rats (p = .08) relative to aged controls with interpulse intervals of 100 and 200 ms. Representative traces evoked by 16, 20, and 24 V highlighting total synaptic transmission between treatment groups for young (f) and aged (h) rats. Line diagrams illustrating input/output curve for total synaptic responses recorded from young (g) and aged (i) rats under different treatment conditions. Microglial depletion greatly reduced the fEPSP in young and aged rats (vs. controls, p = .07; vs. replenished, p = .001), while microglial replenishment normalized these to control levels (g,i). Representative NMDAR-mediated fEPSP traces evoked by 20, 24, and 28 V stimulation from young (j) and aged (l) rats. Input/output curve of NMDAR-mediated synaptic responses recorded for increasing stimulation intensities from young (k) and aged (m) rats. In contrast to total synaptic transmission, the NMDAR-mediated component was impaired in young microglial depleted rats specifically (vs. young controls, p = .001; vs. young replenished, p = .005; k), while all aged rats regardless of treatment exhibited reduced NMDAR responses relative to young (p <.05; m). All data are represented as means ± SEM (young: 6–7 slices/group, 3–4 rats/group; aged: 6–10 slices/group, 3–5 rats/group). # p ≤.05, ## p ≤.01, ### p ≤.001, control versus microglial depleted; & p ≤.05, control versus microglial replenished; * p ≤.05, ** p ≤.01, microglial depleted versus microglial replenished
Examination of input/output curves for the fast component of synaptic transmission, mediated by α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid (AMPA) receptors and measured as the total fEPSP slope, revealed a significant main effect of stimulation intensity (F2.04,89.67 = 119.03, p <.001), which increased with higher intensities. In addition, a main effect of age (F2,44 = 10.84, p = .002), with decreased synaptic responses for aged animals, and a main effect of treatment (F2,44 = 5.98, p = .005; Figure 6f–i) were noted. A decrease in the fEPSP was observed for microglial depleted rats relative to microglial replenished (p = .001), as well as a tendency for the response to be decreased in the microglial depleted condition relative to controls (p = .07). ANOVAs within each age group confirmed a treatment effect for young (F2,21 = 3.37, p = .05; Figure 6g) but not aged rats (p >.05; Figure 6i). Young microglial depleted rats exhibited a reduced response relative to young microglial replenished (p = .02). The absence of a treatment effect for the older group is likely due to a floor effect of the control condition.
The NMDAR-mediated component was then isolated to examine the effect of treatment on NMDAR function in region CA1 (representative traces: Figure 6j,l). Again, a significant main effect of stimulation intensity (F1.46,61.27 = 59.71, p <.001; Figure 6k,m) was observed with increasing responses with greater stimulation. Treatment significantly impacted the NMDAR-mediated synaptic response (F2,42 = 4.44, p = .02). Treatment interacted with age (F2,42 = 3.77, p = .03), whereby young rats drove the treatment effects. Young microglial depleted rats showed large decreases compared to young controls (p = .001) and young microglial replenished rats (p = .005; Figure 6k). The lack of a response to treatment within the aged rats is likely due to floor effects within aged controls (Figure 6m).
3.8 |. Relationship between microglial complexity, hippocampal synaptic function, and cognitive capacity
To determine the relationship between microglial markers or hippocampal neurotransmission with cognitive capacity, correlations were conducted. Collapsing across age groups, performance on the COD task was positively correlated with microglial branch length across hippocampal subregions (r = .68, p = .02; Figure S3a) and the number of branches per microglia within the DG (r = .69, p = .03; Figure S3b). Similarly, stronger cued fear memory was associated with longer microglial branch length throughout the hippocampus (r = .53, p = .04; Figure S3c).
In addition to microglial number and structural complexity, cognitive performance correlated with measures of synaptic function. A significant association between rats’ discriminative capacity on the COD task and the NMDAR component of basal transmission in the CA1 was observed at higher stimulation intensities (24, 28, 32, 36, and 40 mV; r >.45; p <.05), and this effect was predominantly driven by young rats (Figure S4). There were significant relationships between the PPF ratio for longer IPI and percentage freezing to the CS with all rats included (IPI200ms: r = .51; p = .01; IPI400ms: r = .36, p = .08; Figure S5a). This pattern was partially driven by rats with microglial replenishment (IPI400ms: r = .59, p = .08), which also displayed persistent freezing to the CS (Figure S5b).
4 |. DISCUSSION
Neuroinflammation associated with microglial activation may have toxic effects, impairing synaptic function (Block, Zecca, & Hong, 2007; Di Filippo, Sarchielli, Picconi, & Calabresi, 2008; Li, Lu, Tay, Moochhala, & He, 2007; Liu, Wu, Hayashi, & Nakanishi, 2012). Normal aging is associated with microglial senescence and sensitization, resulting in an intensified and protracted activation linked to cognitive deficits, and depressive-like behavior (Bussian et al., 2018; Norden & Godbout, 2013). Several studies indicate that minimizing microglial activation, through microglial depletion and repopulation, is beneficial during acute trauma or inflammation (Coleman, Zou, & Crews, 2020; Colonna & Butovsky, 2017; Henry et al., 2020; Sheppard, Coleman, & Durrant, 2019). One question is whether a reduction of microglial activation during aging, through depletion and/or repopulation of microglia, alleviates age-related cognitive and synaptic deficits (Streit & Xue, 2013). The goal of this study was to examine the importance of microglia to hippocampal synaptic function, specifically NMDAR-mediated contributions, and cognitive capacity in aging. Through the utilization of CSF1R inhibition, we found significant impairments in hippocampal synaptic transmission and discriminative memory in both young and aged rats associated with depletion of microglia.
Consistent with the literature, we observed age-related differences in microglia associated with increased microglial activation. For control animals, we confirmed an age-related increase in Iba-1 expression in the hippocampus, increased number of CA1 microglial cells, and a slight reduction in microglial branch length, consistent with microglial activation (Chan et al., 2018; Damani et al., 2011; Hefendehl et al., 2014; Perkins, Piazza, & Deak, 2018; Shaerzadeh et al., 2020; Tremblay, Zettel, Ison, Allen, & Majewska, 2012). Similarly, we confirmed an age-related decrease in CA1 synaptic transmission (Barnes, Rao, & Shen, 1997; Billard & Rouaud, 2007; Bodhinathan et al., 2010; Kumar & Foster, 2013), which may be linked to microglial activation (Barter et al., 2020; Kim et al., 2018).
Several age-related differences in the microglial response to PLX3397 treatment were noted. Young and aged animals exhibited decreased microglial numbers following PLX3397 treatment, which was particularly robust for aged animals, who exhibited the most microglia in the control condition and the lowest in the depleted condition. However, despite a decrease in microglial number, young rats did not display a decline in Iba-1 protein levels, suggesting a possible compensatory mechanism through activation of the remaining microglia. In contrast, aged repopulated animals did not fully recover Iba-1 protein expression or CA1 and DG microglial cell number to that observed in aged controls. Elmore et al. (2018) observed a similar attenuation in microglial repopulation in aged animals. The partial recovery in cell number and Iba-1 protein expression in older animals may indicate a delayed microglial response during recovery, due to cellular migration (Damani et al., 2011; Hefendehl et al., 2014), replicative senescence of microglia (Flanary, Sammons, Nguyen, Walker, & Streit, 2007), or repopulation with rejuvenated microglia of higher functionality, such that fewer microglia were required (Streit & Xue, 2010). The hippocampus of aged rats may respond differently to treatment due to elevated microglial activation under control conditions. In contrast, recovery in cytokine levels and the increase in branch length relative to the depleted state, which was particularly evident for region CA3, is consistent with a decrease in activated microglia during repopulation for aged animals.
The effect of treatment on cytokine expression differed across age groups. For young and aged rats, depletion was associated with an increase in cytokine expression. The source of these cytokines is unclear. It is notable that several cytokines continued to be elevated with microglial repopulation in young rats, suggesting an increase in neuroinflammation. In contrast, cytokine levels normalized in aged rats during repopulation, the exception being the proinflammatory cytokines IL12p70 and INFγ, which were elevated in young and aged animals during repopulation. The increase in INFγ in the hippocampus with PLX3397 treatment may arise from several different cell types including neurons (Monteiro, Roque, Marques, Correia-Neves, & Cerqueira, 2017), astrocytes (Lau & Yu, 2001; Xiao & Link, 1998), infiltrating T cells (Lynch, 2014; Mosser & Edwards, 2008), endothelial cells (Wei et al., 2000), and remaining microglia (Kawanokuchi et al., 2006; Mäkelä, Koivuniemi, Korhonen, & Lindholm, 2010; Wang & Suzuki, 2007). The increase in cytokines during depletion and repopulation is consistent with other reports suggesting altered neuroinflammatory signaling (Han et al., 2019; Michels et al., 2019; Yang et al., 2018), which may have an activating or compensatory influence on remaining microglia. This shift may be more marked or persistent in young rats since aging is already associated with increased neuroinflammation and microglial activation. For example, in young animals, some microglial markers exhibit little change or increased expression despite partial microglial depletion (Bennett et al., 2018).
Age-related differences in the electrophysiological response to treatment were also observed. Microglial depletion was associated with decreased and increased PPF in young and aged animals, respectively. The exact mechanism for altered presynaptic function is unclear; however, microglia can regulate presynaptic function through a variety of mechanisms (Marinelli, Basilico, Marrone, & Ragozzino, 2019; Tremblay et al., 2011; Vezzani & Viviani, 2015; Werneburg et al., 2017). In addition to direct effects of microglia, changes in presynaptic function may reflect neuroadaptive mechanisms that develop over time in response to changes in excitatory synaptic transmission (Zucker & Regehr, 2002). For example, Lewen et al. (2020) demonstrated that a few days of microglial depletion had no impact on cholinergic-elicited gamma oscillations in hippocampal slice cultures. In contrast, C. Wang, Yue, et al. (2020) found that sustained (30 days), but not acute (5 days), microglial depletion impaired synaptic transmission and presynaptic structure.
Treatment was associated with age differences in the NMDA receptor synaptic response. Microglial depletion was accompanied by a decrease in the NMDAR-mediated synaptic response specifically in young rats, and repopulation normalized this effect. The absence of a treatment effect in aged animals may be due to a floor effect for NMDA receptor transmission in this group. In contrast, the rapid component of synaptic transmission, mediated by AMPA receptors, decreased during microglial depletion and recovered with replenishment for both age groups, consistent with work indicating reductions in AMPA receptor-mediated miniature excitatory postsynaptic currents in the motor cortex following microglial depletion (Parkhurst et al., 2013). Although AMPA receptor-mediated synaptic transmission recovered during microglial replenishment for aged animals, the response did not recover to levels observed in young. Age-related differences in AMPA receptor-mediated synaptic transmission, during baseline and in response to PLX3397 treatment, may reflect differences in neuroinflammation and the balance of pro- and anti-inflammatory cytokines. Treatments that induce microglial activation result in an initial decrease in synaptic transmission in young animals, which increases during recovery (Barter et al., 2020; Kim et al., 2018). The pro-inflammatory cytokine, IL-1β, decreases basal synaptic transmission (Bellinger, Madamba, & Siggins, 1993; Lynch, 1998; Rothwell & Luheshi, 2000), and hippocampal IL-1β levels were elevated in young and aged depleted groups.
We observed that microglial depletion was associated with disruption in hippocampal-dependent memory in the COD task, regardless of age, and COD memory recovered during replenishment. The mechanism for cognitive impairment and recovery associated with depletion and repopulation is unknown. The impairing effect of microglial depletion on discriminative capacity cannot be due to confounds of initial learning, given that this group explored the objects more than controls and displayed normal locomotor function. Microglial replenished rats, on the other hand, exhibited increased locomotion, as observed previously (Elmore et al., 2018; Maggi et al., 2011), yet had intact discriminative memory.
The decline in AMPA and NMDA receptor function during aging is thought to underlie impaired memory (Foster, 2012). Object recognition and the association between objects and places depend on AMPA and NMDA receptor function in the hippocampus and associated brain regions (Warburton, Barker, & Brown, 2013; Winters & Bussey, 2005). Similarly, optimal performance on fear conditioning and extinction tasks examined in the current study depends upon NMDAR function within the amygdala, hippocampus, and PFC (Brim et al., 2013; Campeau, Miserendino, & Davis, 1992; Davis, 2011; Fanselow & Kim, 1994; Maren, Aharonov, Stote, & Fanselow, 1996; Miserendino, Sananes, Melia, & Davis, 1990; Vieira et al., 2015), possibly explaining the observed impairments in extinction for young depleted animals, which exhibited a robust decrease in NMDAR function. In turn, neuroinflammation associated with activated microglia may contribute to the decrease in hippocampal synaptic transmission through the release of cytokines, chemokines and microRNA, synapse elimination, or oxidative stress (Kumar, Yegla, et al., 2018; Vasek et al., 2016; Wolf, Boddeke, & Kettenmann, 2017). For example, INFγ activates microglia to increase IL-1β (Maher, Clarke, Kelly, Nally, & Lynch, 2006) and the production of reactive oxygen species (Müller, Fontana, Zbinden, & Gähwiler, 1993; Ta et al., 2019). As noted above, the increase in IL-1β may contribute to a decrease in basal synaptic transmission (Bellinger et al., 1993; Lynch, 1998; Rothwell & Luheshi, 2000), and oxidative stress decreases NMDA receptor function (Bodhinathan et al., 2010; Kumar & Foster, 2013; Kumar, Rani, et al., 2018; Kumar et al., 2019; Lee et al., 2014). Finally, long-term INFγ treatment decreases AMPA receptor function (Vikman, Owe-Larsson, Brask, Kristensson, & Hill, 2001). Correlation analyses indicated that shorter microglial branch length, a characteristic of activated microglia, and smaller NMDAR-mediated synaptic responses were associated with poorer cognitive performance, suggesting that activated microglia may have contributed to cognitive impairment either directly or through altered synaptic function.
Altered microglial function during aging, such as microglial priming, may contribute to cognitive decline (Barrientos, Frank, Watkins, & Maier, 2010; Elmore et al., 2018; Niraula, Sheridan, & Godbout, 2017; Wynne, Henry, & Godbout, 2009). In contrast to the beneficial effects of microglial depletion and repopulation observed for acute trauma or inflammation in young animals, the current study indicates that cognitive function in the presence of aged microglia (i.e., control condition) is better than the depleted microglia condition, and microglial repopulation did not improve cognition in aged animals. Thus, it appears that microglia of aged rats provide better support for cognition than the depleted state. In the case of replenished microglia, the renewed microglial population may be reintegrated in the presence of a pro-inflammatory CNS environment (Elmore et al., 2018; Lehmann et al., 2019; O’Neil et al., 2018), leading to the continuation of synaptic and behavioral impairments.
One potential confound of the PLX3397 treatment was a corresponding reduction in Iba-1 expression in the liver, demonstrating effects on peripheral macrophages. The treatment parameters had been based on studies that exhibited little to no effect of PLX3397 in the periphery (Elmore et al., 2014; Hilla, Diekmann, & Fischer, 2017; Szalay et al., 2016). Nonetheless, reductions in macrophages have been noted previously with PLX3397 (Abou-Khalil et al., 2014; Cannarile et al., 2017; DeNardo et al., 2011; Prada et al., 2013) and PLX5622 treatments (Cavnar et al., 2013; Dagher et al., 2015; Lee, Shi, Fan, West, & Zhang, 2018; Nissen, Thompson, West, & Tsirka, 2018). Modification of dosage and treatment duration may ameliorate these off-target effects. However, previous studies altering the concentration of PLX5622, resulting in partial and delayed microglial depletion, continued to decrease macrophage numbers (Dagher et al., 2015; Nissen et al., 2018). Thus, utilization of a pharmacological agent that more selectively targets microglia will be crucial in ascertaining the precise contributions that microglia have on hippocampal synaptic and cognitive function in aging.
Overall, this is the first study to examine age differences in synaptic function in the CA1 region during microglial deletion. PLX3397 treatment decreased the microglial population in both age groups and altered markers of microglial activation (e.g., Iba-1) and neuroinflammation (e.g., cytokine expression) in an age-dependent manner. Microglial depletion was associated with decreased NMDAR-mediated synaptic transmission for young rats and both age groups exhibited a decrease in the AMPA receptor-mediated component of synaptic transmission and impaired hippocampal-dependent memory. Microglial repopulation was associated with recovery of synaptic transmission and memory; however, repopulation did not rejuvenate synaptic transmission or cognitive function of aged animals to mirror a “younger” phenotype. This study emphasizes the critical contribution that microglia provide in modulating synaptic and cognitive function throughout the lifespan.
Supplementary Material
Acknowledgments
Funding information
Evelyn F. McKnight Brain Research Foundation; National Institute on Aging,Grant/Award Numbers: AG037984,AG052258, AG068205; University of Florida Claude D Pepper Older American Independence Center, Grant/Award Number: P30-AG028740
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
REFERENCES
- Abou-Khalil R, Yang F, Mortreux M, Lieu S, Yu YY, Wurmser M, … Colnot C. (2014). Delayed bone regeneration is linked to chronic inflammation in murine muscular dystrophy. Journal of Bone and Mineral Research, 29(2), 304–315. 10.1002/jbmr.2038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyoshi R, Wake H, Kato D, Horiuchi H, Ono R, Ikegami A, … Nabekura J. (2018). Microglia enhance synapse activity to promote local network synchronization. eNeuro, 5(5), ENEURO.0088–ENEU18.2018. 10.1523/ENEURO.0088-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez RP, Biggs A, Chen G, Pine DS, & Grillon C. (2008). Contextual fear conditioning in humans: Cortical-hippocampal and amygdala contributions. The Journal of Neuroscience, 28(24), 6211–6219. 10.1523/JNEUROSCI.1246-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova DM, & Brown DR (2019). Microglia and the aging brain: Are senescent microglia the key to neurodegeneration? Journal of Neurochemistry, 151(6), 676–688. 10.1111/jnc.14860 [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, & Shen J. (1997). Age-related decrease in the N-methyl-D-aspartateR-mediated excitatory postsynaptic potential in hippocampal region CA1. Neurobiology of Aging, 18(4), 445–452. 10.1016/s0197-4580(97)00044-4 [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, & Maier SF (2010). Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging and Disease, 1(3), 212–231. [PMC free article] [PubMed] [Google Scholar]
- Barter J, Kumar A, Rani A, Colon-Perez LM, Febo M, & Foster TC (2020). Differential effect of repeated lipopolysaccharide treatment and aging on hippocampal function and biomarkers of hippocampal senescence. Molecular Neurobiology, 57(10), 4045–4059. 10.1007/s12035-020-02008-y [DOI] [PubMed] [Google Scholar]
- Bean LA, Kumar A, Rani A, Guidi M, Rosario AM, Cruz PE, … Foster TC (2015). Re-opening the critical window for estrogen therapy. The Journal of Neuroscience, 35(49), 16077–16093. 10.1523/JNEUROSCI.1890-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger FP, Madamba S, & Siggins GR (1993). Interleukin 1 beta inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Research, 628(1–2), 227–234. 10.1016/0006-8993(93)90959-q [DOI] [PubMed] [Google Scholar]
- Bennett RE, Bryant A, Hu M, Robbins AB, Hopp SC, & Hyman BT (2018). Partial reduction of microglia does not affect tau pathology in aged mice. Journal of Neuroinflammation, 15(1), 311. 10.1186/s12974-018-1348-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrom HC, & Johnson LR (2014). An organization of visual and auditory fear conditioning in the lateral amygdala. Neurobiology of Learning and Memory, 116, 1–13. 10.1016/j.nlm.2014.07.008 [DOI] [PubMed] [Google Scholar]
- Billard JM, & Rouaud E. (2007). Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. The European Journal of Neuroscience, 25(8), 2260–2268. 10.1111/j.1460-9568.2007.05488.x [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, & Hong JS (2007). Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nature Reviews. Neuroscience, 8(1), 57–69. 10.1038/nrn2038 [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, & Foster TC (2010). Redox sensitive calcium stores underlie enhanced after hyperpolarization of aged neurons: Role for ryanodine receptor mediated calcium signaling. Journal of Neurophysiology, 104(5), 2586–2593. 10.1152/jn.00577.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brim BL, Haskell R, Awedikian R, Ellinwood NM, Jin L, Kumar A, … Magnusson KR (2013). Memory in aged mice is rescued by enhanced expression of the GluN2B subunit of the NMDA receptor. Behavioural Brain Research, 238, 211–226. 10.1016/j.bbr.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussian TJ, Aziz A, Meyer CF, Swenson BL, van Deursen JM, & Baker DJ (2018). Clearance of senescent glial cells prevents tau-dependent pathology and cognitive decline. Nature, 562(7728), 578–582. 10.1038/s41586-018-0543-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campeau S, Miserendino MJ, & Davis M. (1992). Intra-amygdala infusion of the N-methyl-D-aspartate receptor antagonist AP5 blocks acquisition but not expression of fear-potentiated startle to an auditory conditioned stimulus. Behavioral Neuroscience, 106(3), 569–574. 10.1037//0735-7044.106.3.569 [DOI] [PubMed] [Google Scholar]
- Cannarile MA, Weisser M, Jacob W, Jegg AM, Ries CH, & Rüttinger D. (2017). Colony-stimulating factor 1 receptor (CSF1R) inhibitors in cancer therapy. Journal for Immunotherapy of Cancer, 5(1), 53. 10.1186/s40425-017-0257-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavnar MJ, Zeng S, Kim TS, Sorenson EC, Ocuin LM, Balachandran VP, … DeMatteo RP (2013). KIT oncogene inhibition drives intratumoral macrophage M2 polarization. The Journal of Experimental Medicine, 210(13), 2873–2886. 10.1084/jem.20130875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TE, Grossman YS, Bloss EB, Janssen WG, Lou W, McEwen BS, … Morrison JH (2018). Cell-type specific changes in glial morphology and glucocorticoid expression during stress and aging in the medial prefrontal cortex. Frontiers in Aging Neuroscience, 10, 146. 10.3389/fnagi.2018.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Zou J, & Crews FT (2020). Microglial depletion and repopulation in brain slice culture normalizes sensitized proinflammatory signaling. Journal of Neuroinflammation, 17(1), 27. 10.1186/s12974-019-1678-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonna M, & Butovsky O. (2017). Microglia function in the central nervous system during health and Neurodegeneration. Annual Review of Immunology, 35, 441–468. 10.1146/annurevimmunol-051116-052358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper RA, & Ritchey M. (2020). Progression from feature-specific brain activity to hippocampal binding during episodic encoding. The Journal of Neuroscience, 40(8), 1701–1709. 10.1523/JNEUROSCI.1971-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czerniawski J, Miyashita T, Lewandowski G, & Guzowski JF (2015). Systemic lipopolysaccharide administration impairs retrieval of context-object discrimination, but not spatial, memory: Evidence for selective disruption of specific hippocampus-dependent memory functions during acute neuroinflammation. Brain, Behavior, and Immunity, 44, 159–166. 10.1016/j.bbi.2014.09.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dagher NN, Najafi AR, Kayala KM, Elmore MR, White TE, Medeiros R, … Green KN (2015). Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. Journal of Neuroinflammation, 12, 139. 10.1186/s12974-015-0366-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damani MR, Zhao L, Fontainhas AM, Amaral J, Fariss RN, & Wong WT (2011). Age-related alterations in the dynamic behavior of microglia. Aging Cell, 10(2), 263–276. 10.1111/j.1474-9726.2010.00660.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. (2011). NMDA receptors and fear extinction: Implications for cognitive behavioral therapy. Dialogues in Clinical Neuroscience, 13(4), 463–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeNardo DG, Brennan DJ, Rexhepaj E, Ruffell B, Shiao SL, Madden SF, … Coussens LM (2011). Leukocyte complexity predicts breast cancer survival and functionally regulates response to chemotherapy. Cancer Discovery, 1(1), 54–67. 10.1158/2159-8274.CD-10-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo M, Sarchielli P, Picconi B, & Calabresi P. (2008). Neuroinflammation and synaptic plasticity: Theoretical basis for a novel, immune-centred, therapeutic approach to neurological disorders. Trends in Pharmacological Sciences, 29(8), 402–412. 10.1016/j.tips.2008.06.005 [DOI] [PubMed] [Google Scholar]
- Elmore MR, Lee RJ, West BL, & Green KN (2015). Characterizing newly repopulated microglia in the adult mouse: Impacts on animal behavior, cell morphology, and neuroinflammation. PLoS One, 10(4), e0122912. 10.1371/journal.pone.0122912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MR, Najafi AR, Koike MA, Dagher NN, Spangenberg EE, Rice RA, … Green KN (2014). Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron, 82(2), 380–397. 10.1016/j.neuron.2014.02.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmore MRP, Hohsfield LA, Kramár EA, Soreq L, Lee RJ, Pham ST, … Green KN (2018). Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell, 17(6), e12832. 10.1111/acel.12832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, & Kim JJ (1994). Acquisition of contextual Pavlovian fear conditioning is blocked by application of an NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid to the basolateral amygdala. Behavioral Neuroscience, 108(1), 210–212. 10.1037//0735-7044.108.1.210 [DOI] [PubMed] [Google Scholar]
- Flanary BE, Sammons NW, Nguyen C, Walker D, & Streit WJ (2007). Evidence that aging and amyloid promote microglial cell senescence. Rejuvenation Research, 10(1), 61–74. 10.1089/rej.2006.9096 [DOI] [PubMed] [Google Scholar]
- Foster TC (2012). Dissecting the age-related decline on spatial learning and memory tasks in rodent models: N-methyl-D-aspartate receptors and voltage-dependent Ca2+ channels in senescent synaptic plasticity. Progress in Neurobiology, 96(3), 283–303. 10.1016/j.pneurobio.2012.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC, & Dumas TC (2001). Mechanism for increased hippocampal synaptic strength following differential experience. Journal of Neurophysiology, 85(4), 1377–1383. 10.1152/jn.2001.85.4.1377 [DOI] [PubMed] [Google Scholar]
- Foster TC, & McNaughton BL (1991). Long-term enhancement of CA1 synaptic transmission is due to increased quantal size, not quantal content. Hippocampus, 1(1), 79–91. 10.1002/hipo.450010108 [DOI] [PubMed] [Google Scholar]
- Fumagalli M, Lombardi M, Gressens P, & Verderio C. (2018). How to reprogram microglia toward beneficial functions. Glia, 66(12), 2531–2549. 10.1002/glia.23484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould TJ, & Feiro OR (2005). Age-related deficits in the retention of memories for cued fear conditioning are reversed by galantamine treatment. Behavioural Brain Research, 165(2), 160–171. 10.1016/j.bbr.2005.06.040 [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, & Lynch MA (2006). The age-related attenuation in long-term potentiation is associated with microglial activation. Journal of Neurochemistry, 99(4), 1263–1272. 10.1111/j.1471-4159.2006.04165.x [DOI] [PubMed] [Google Scholar]
- Han X, Li Q, Lan X, El-Mufti L, Ren H, & Wang J. (2019). Microglial depletion with Clodronate liposomes increases proinflammatory cytokine levels, induces astrocyte activation, and damages blood vessel integrity. Molecular Neurobiology, 56(9), 6184–6196. 10.1007/s12035-019-1502-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefendehl JK, Neher JJ, Sühs RB, Kohsaka S, Skodras A, & Jucker M. (2014). Homeostatic and injury-induced microglia behavior in the aging brain. Aging Cell, 13(1), 60–69. 10.1111/acel.12149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry RJ, Ritzel RM, Barrett JP, Doran SJ, Jiao Y, Leach JB, … Loane DJ (2020). Microglial depletion with CSF1R inhibitor during chronic phase of experimental traumatic brain injury reduces neurodegeneration and neurological deficits. The Journal of Neuroscience, 40(14), 2960–2974. 10.1523/JNEUROSCI.2402-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilla AM, Diekmann H, & Fischer D. (2017). Microglia are irrelevant for neuronal degeneration and axon regeneration after acute injury. The Journal of Neuroscience, 37(25), 6113–6124. 10.1523/JNEUROSCI.0584-17.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong I, Song B, Lee S, Kim J, & Choi S. (2009). Extinction of cued fear memory involves a distinct form of depotentiation at cortical input synapses onto the lateral amygdala. The European Journal of Neuroscience, 30(11), 2089–2099. 10.1111/j.1460-9568.2009.07004.x [DOI] [PubMed] [Google Scholar]
- Hopperton KE, Mohammad D, Trépanier MO, Giuliano V, & Bazinet RP (2018). Markers of microglia in post-mortem brain samples from patients with Alzheimer’s disease: A systematic review. Molecular Psychiatry, 23(2), 177–198. 10.1038/mp.2017.246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard LR, Kumaran D, Ólafsdóttir HF, & Spiers HJ (2011). Double dissociation between hippocampal and parahippocampal responses to object-background context and scene novelty. The Journal of Neuroscience, 31(14), 5253–5261. 10.1523/JNEUROSCI.6055-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikezu S, Yeh H, Delpech JC, Woodbury ME, Van Enoo AA, Ruan Z, … Ikezu T. (2020). Inhibition of colony stimulating factor 1 receptor corrects maternal inflammation-induced microglial and synaptic dysfunction and behavioral abnormalities. Molecular Psychiatry. 10.1038/s41380-020-0671-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, & Kohsaka S. (1998). Microglia-specific localisation of a novel calcium binding protein, Iba1. Molecular Brain Research, 57(1), 1–9. 10.1016/s0169-328x(98)00040-0 [DOI] [PubMed] [Google Scholar]
- Jin WN, Shi SX, Li Z, Li M, Wood K, Gonzales RJ, & Liu Q. (2017). Depletion of microglia exacerbates postischemic inflammation and brain injury. Journal of Cerebral Blood Flow and Metabolism, 37(6), 2224–2236. 10.1177/0271678X17694185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawanokuchi J, Mizuno T, Takeuchi H, Kato H, Wang J, Mitsuma N, & Suzumura A. (2006). Production of interferon-gamma by microglia. Multiple Sclerosis, 12(5), 558–564. 10.1177/1352458506070763 [DOI] [PubMed] [Google Scholar]
- Kim KM, Zamaleeva AI, Lee YW, Ahmed MR, Kim E, Lee HR, … Rajadas J. (2018). Characterization of brain dysfunction induced by bacterial lipopeptides that Alter neuronal activity and network in rodent brains. The Journal of Neuroscience, 38(50), 10672–10691. 10.1523/JNEUROSCI.0825-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Prigge M, Sarel A, Paz R, & Yizhar O. (2017). Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nature Neuroscience, 20(6), 836–844. 10.1038/nn.4523 [DOI] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD, & Ritzel RM (2017). Old maids: Aging and its impact on microglia function. International Journal of Molecular Sciences, 18(4), 769–794. 10.3390/ijms18040769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo K, Wati H, Qiao C, Arita J, & Kanba S. (2005). Age-related disturbance of memory and CREB phosphorylation in CA1 area of hippocampus of rats. Brain Research, 1054(1), 30–37. 10.1016/j.brainres.2005.06.045 [DOI] [PubMed] [Google Scholar]
- Kumar A. (2010). Carbachol-induced long-term synaptic depression is enhanced during senescence at hippocampal CA3-CA1 synapses. Journal of Neurophysiology, 104(2), 607–616. 10.1152/jn.00278.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, & Foster TC (2007). Shift in induction mechanisms underlies an age-dependent increase in DHPG-induced synaptic depression at CA3 CA1 synapses. Journal of Neurophysiology, 98(5), 2729–2736. 10.1152/jn.00514.2007 [DOI] [PubMed] [Google Scholar]
- Kumar A, & Foster TC (2013). Linking redox regulation of NMDAR synaptic function to cognitive decline during aging. The Journal of Neuroscience, 33(40), 15710–15715. 10.1523/JNEUROSCI.2176-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, & Foster TC (2014). Interaction of DHPG-LTD and synaptic-LTD at senescent CA3-CA1 hippocampal synapses. Hippocampus, 24 (4), 466–475. 10.1002/hipo.22240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, & Foster TC (2019). Alteration in NMDA receptor mediated Glutamatergic neurotransmission in the hippocampus during senescence. Neurochemical Research, 44(1), 38–48. 10.1007/s11064-018-2634-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Scheinert RB, Ormerod BK, & Foster TC (2018). Nonsteroidal anti-inflammatory drug, indomethacin improves spatial memory and NMDA receptor function in aged animals. Neurobiology of Aging, 70, 184–193. 10.1016/j.neurobiolaging.2018.06.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Rani A, Tchigranova O, Lee WH, & Foster TC (2012). Influence of late-life exposure to environmental enrichment or exercise on hippocampal function and CA1 senescent physiology. Neurobiology of Aging, 33(4), 828.e1–828.e17. 10.1016/j.neurobiolaging.2011.06.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, & Foster TC (2019). Subunit contribution to NMDA receptor hypofunction and redox sensitivity of hippocampal synaptic transmission during aging. Aging (Albany NY), 11(14), 5140–5157. 10.18632/aging.102108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yegla B, & Foster TC (2018). Redox signaling in neurotransmission and cognition during aging. Antioxidants & Redox Signaling, 28 (18), 1724–1745. 10.1089/ars.2017.7111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LT, & Yu AC (2001). Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor alpha and interferon-gamma following traumatic and metabolic injury. Journal of Neurotrauma, 18(3), 351–359. 10.1089/08977150151071035 [DOI] [PubMed] [Google Scholar]
- Lee S, Shi XQ, Fan A, West B, & Zhang J. (2018). Targeting macrophage and microglia activation with colony stimulating factor 1 receptor inhibitor is an effective strategy to treat injury-triggered neuropathic pain. Molecular Pain, 14, 1744806918764979. 10.1177/1744806918764979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WH, Kumar A, Rani A, & Foster TC (2014). Role of antioxidant enzymes in redox regulation of N-methyl-D-aspartate receptor function and memory in middle-aged rats. Neurobiology of Aging, 35(6), 1459–1468. 10.1016/j.neurobiolaging.2013.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann ML, Weigel TK, Poffenberger CN, & Herkenham M. (2019). The behavioral sequelae of social defeat require microglia and are driven by oxidative stress in mice. The Journal of Neuroscience, 39(28), 5594–5605. 10.1523/JNEUROSCI.0184-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewen A, Ta TT, Cesetti T, Hollnagel JO, Papageorgiou IE, Chausse B, & Kann O. (2020). Neuronal gamma oscillations and activity-dependent potassium transients remain regular after depletion of microglia in postnatal cortex tissue. Journal of Neuroscience Research, 98(10), 1953–1967. 10.1002/jnr.24689 [DOI] [PubMed] [Google Scholar]
- Li L, Lu J, Tay SS, Moochhala SM, & He BP (2007). The function of microglia, either neuroprotection or neurotoxicity, is determined by the equilibrium among factors released from activated microglia in vitro. Brain Research, 1159, 8–17. 10.1016/j.brainres.2007.04.066 [DOI] [PubMed] [Google Scholar]
- Li M, Li Z, Ren H, Jin WN, Wood K, Liu Q, … Shi FD (2017). Colony stimulating factor 1 receptor inhibition eliminates microglia and attenuates brain injury after intracerebral hemorrhage. Journal of Cerebral Blood Flow and Metabolism, 37(7), 2383–2395. 10.1177/0271678X16666551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Du XF, Liu CS, Wen ZL, & Du JL (2012). Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Developmental Cell, 23(6), 1189–1202. 10.1016/j.devcel.2012.10.027 [DOI] [PubMed] [Google Scholar]
- Libby LA, Hannula DE, & Ranganath C. (2014). Medial temporal lobe coding of item and spatial information during relational binding in working memory. The Journal of Neuroscience, 34(43), 14233–14242. 10.1523/JNEUROSCI.0655-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wu Z, Hayashi Y, & Nakanishi H. (2012). Age-dependent neuroinflammatory responses and deficits in long-term potentiation in the hippocampus during systemic inflammation. Neuroscience, 216, 133–142. 10.1016/j.neuroscience.2012.04.050 [DOI] [PubMed] [Google Scholar]
- Lynch MA (1998). Age-related impairment in long-term potentiation in hippocampus: A role for the cytokine, interleukin-1 beta? Progress in Neurobiology, 56(5), 571–589. 10.1016/s0301-0082(98)00054-9 [DOI] [PubMed] [Google Scholar]
- Lynch MA (2014). The impact of neuroimmune changes on development of amyloid pathology; relevance to Alzheimer’s disease. Immunology, 141(3), 292–301. 10.1111/imm.12156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maggi L, Scianni M, Branchi I, D’Andrea I, Lauro C, & Limatola C. (2011). CX(3)CR1 deficiency alters hippocampal-dependent plasticity phenomena blunting the effects of enriched environment. Frontiers in Cellular Neuroscience, 5, 22. 10.3389/fncel.2011.00022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher FO, Clarke RM, Kelly A, Nally RE, & Lynch MA (2006). Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. Journal of Neurochemistry, 96(6), 1560–1571. 10.1111/j.1471-4159.2006.03664.x [DOI] [PubMed] [Google Scholar]
- Mäkelä J, Koivuniemi R, Korhonen L, & Lindholm D. (2010). Interferon-gamma produced by microglia and the neuropeptide PACAP have opposite effects on the viability of neural progenitor cells. PLoS One, 5 (6), e11091. 10.1371/journal.pone.0011091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, & Fanselow MS (1996). N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behavioral Neuroscience, 110(6), 1365–1374. 10.1037//07357044.110.6.1365 [DOI] [PubMed] [Google Scholar]
- Marinelli S, Basilico B, Marrone MC, & Ragozzino D. (2019). Microglia-neuron crosstalk: Signaling mechanism and control of synaptic transmission. Seminars in Cell & Developmental Biology, 94, 138–151. 10.1016/j.semcdb.2019.05.017 [DOI] [PubMed] [Google Scholar]
- Marschner A, Kalisch R, Vervliet B, Vansteenwegen D, & Büchel C. (2008). Dissociable roles for the hippocampus and the amygdala in human cued versus context fear conditioning. The Journal of Neuroscience, 28(36), 9030–9036. 10.1523/JNEUROSCI.1651-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michels M, Avila P, Pescador B, Vieira A, Abatti M, Cucker L, … DalPizzol F. (2019). Microglial cells depletion increases inflammation and modifies microglial phenotypes in an animal model of severe sepsis. Molecular Neurobiology, 56(11), 7296–7304. 10.1007/s12035-019-1606-2 [DOI] [PubMed] [Google Scholar]
- Miserendino MJ, Sananes CB, Melia KR, & Davis M. (1990). Blocking of acquisition but not expression of conditioned fear-potentiated startle by NMDA antagonists in the amygdala. Nature, 345(6277), 716–718. 10.1038/345716a0 [DOI] [PubMed] [Google Scholar]
- Monteiro S, Roque S, Marques F, Correia-Neves M, & Cerqueira JJ (2017). Brain interference: Revisiting the role of IFNγ in the central nervous system. Progress in Neurobiology, 156, 149–163. 10.1016/j.pneurobio.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Mosser DM, & Edwards JP (2008). Exploring the full spectrum of macrophage activation. Nature Reviews. Immunology, 8(12), 958–969. 10.1038/nri2448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller M, Fontana A, Zbinden G, & Gähwiler BH (1993). Effects of interferons and hydrogen peroxide on CA3 pyramidal cells in rat hippocampal slice cultures. Brain Research, 619(1–2), 157–162. 10.1016/0006-8993(93)91607-t [DOI] [PubMed] [Google Scholar]
- Niraula A, Sheridan JF, & Godbout JP (2017). Microglia priming with aging and stress. Neuropsychopharmacology, 42(1), 318–333. 10.1038/npp.2016.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen JC, Thompson KK, West BL, & Tsirka SE (2018). Csf1R inhibition attenuates experimental autoimmune encephalomyelitis and promotes recovery. Experimental Neurology, 307, 24–36. 10.1016/j.expneurol.2018.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, & Godbout JP (2013). Review: Microglia of the aged brain: Primed to be activated and resistant to regulation. Neuropathology and Applied Neurobiology, 39(1), 19–34. 10.1111/j.1365-2990.2012.01306.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, & Godbout JP (2016). Sequential activation of microglia and astrocyte cytokine expression precedes increased Iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia, 64(2), 300–316. 10.1002/glia.22930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil SM, Witcher KG, McKim DB, & Godbout JP (2018). Forced turnover of aged microglia induces an intermediate phenotype but does not rebalance CNS environmental cues driving priming to immune challenge. Acta Neuropathologica Communications, 6(1), 129. 10.1186/s40478-018-0636-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, Lafaille JJ, … Gan WB (2013). Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell, 155(7), 1596–1609. 10.1016/j.cell.2013.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins AE, Piazza MK, & Deak T. (2018). Stereological analysis of microglia in aged male and female Fischer 344 rats in socially relevant brain regions. Neuroscience, 377, 40–52. 10.1016/j.neuroscience.2018.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, & LeDoux JE (1992). Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience, 106(2), 274–285. 10.1037//0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- Prada CE, Jousma E, Rizvi TA, Wu J, Dunn RS, Mayes DA, … Ratner N. (2013). Neurofibroma-associated macrophages play roles in tumor growth and response to pharmacological inhibition. Acta Neuropathologica, 125(1), 159–168. 10.1007/s00401012-1056-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RA, Pham J, Lee RJ, Najafi AR, West BL, & Green KN (2017). Microglial repopulation resolves inflammation and promotes brain recovery after injury. Glia, 65(6), 931–944. 10.1002/glia.23135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice RA, Spangenberg EE, Yamate-Morgan H, Lee RJ, Arora RP, Hernandez MX, … Green KN (2015). Elimination of microglia improves functional outcomes following extensive neuronal loss in the hippocampus. The Journal of Neuroscience, 35(27), 9977–9989. 10.1523/JNEUROSCI.0336-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, & Luheshi GN (2000). Interleukin 1 in the brain: Biology, pathology and therapeutic target. Trends in Neuroscience, 23(12), 618–625. 10.1016/s0166-2236(00)01661-1 [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schöning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, & Konrad C. (2009). Human fear conditioning and extinction in neuroimaging: A systematic review. PLoS One, 4(6), e5865. 10.1371/journal.pone.0005865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaerzadeh F, Phan L, Miller D, Dacquel M, Hachmeister W, Hansen C, … Khoshbouei H. (2020). Microglia senescence occurs in both substantia nigra and ventral tegmental area. Glia, 68(11), 2228–2245. 10.1002/glia.23834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard O, Coleman MP, & Durrant CS (2019). Lipopolysaccharide-induced neuroinflammation induces presynaptic disruption through a direct action on brain tissue involving microglia-derived interleukin 1 beta. Journal of Neuroinflammation, 16(1), 106. 10.1186/s12974-019-1490-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soch A, Sominsky L, Younesi S, De Luca SN, Gunasekara M, Bozinovski S, & Spencer SJ (2020). The role of microglia in the second and third postnatal weeks of life in rat hippocampal development and memory. Brain, Behavior, and Immunity, 88, 675–687. 10.1016/j.bbi.2020.04.082 [DOI] [PubMed] [Google Scholar]
- Sogn CJ, Puchades M, & Gundersen V. (2013). Rare contacts between synapses and microglial processes containing high levels of Iba1 and actin—A post-embedding immunogold study in the healthy rat brain. The European Journal of Neuroscience, 38(1), 2030–2040. 10.1111/ejn.12213 [DOI] [PubMed] [Google Scholar]
- Spangenberg EE, Lee RJ, Najafi AR, Rice RA, Elmore MR, Blurton-Jones M, West BL, & Green KN (2016). Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain, 139(Pt 4), 1265–1281. 10.1093/brain/aww016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellwagen D, & Malenka RC (2006). Synaptic scaling mediated by glial TNF-alpha. Nature, 440(7087), 1054–1059. 10.1038/nature04671 [DOI] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, & Sparks DL (2004). Dystrophic microglia in the aging human brain. Glia, 45(2), 208–212. 10.1002/glia.10319 [DOI] [PubMed] [Google Scholar]
- Streit WJ, & Xue QS (2010). The brain’s aging immune system. Aging and Disease, 1(3), 254–261. [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, & Xue QS (2013). Microglial senescence. CNS & Neurological Disorders Drug Targets, 12(6), 763–767. 10.2174/18715273113126660176 [DOI] [PubMed] [Google Scholar]
- Szalay G, Martinecz B, Lénárt N, Környei Z, Orsolits B, Judák L, … Dénes A. (2016). Microglia protect against brain injury and their selective elimination dysregulates neuronal network activity after stroke. Nature Communications, 7, 11499. 10.1038/ncomms11499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ta TT, Dikmen HO, Schilling S, Chausse B, Lewen A, Hollnagel JO, & Kann O. (2019). Priming of microglia with IFN-γ slows neuronal gamma oscillations in situ. Proceedings of the National Academy of Sciences of the United States of America, 116(10), 4637–4642. 10.1073/pnas.1813562116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson ML, Jimenez-Andrade JM, Chartier S, Tsai J, Burton EA, Habets G, … Mantyh PW (2015). Targeting cells of the myeloid lineage attenuates pain and disease progression in a prostate model of bone cancer. Pain, 156(9), 1692–1702. 10.1097/j.pain.0000000000000228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres L, Danver J, Ji K, Miyauchi JT, Chen D, Anderson ME, … Tsirka SE (2016). Dynamic microglial modulation of spatial learning and social behavior. Brain, Behavior, and Immunity, 55, 6–16. 10.1016/j.bbi.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Stevens B, Sierra A, Wake H, Bessis A, & Nimmerjahn A. (2011). The role of microglia in the healthy brain. The Journal of Neuroscience, 31(45), 16064–16069. 10.1523/JNEUROSCI.4158-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay M, Zettel ML, Ison JR, Allen PD, & Majewska AK (2012). Effects of aging and sensory loss on glial cells in mouse visual and auditory cortices. Glia, 60(4), 541–558. 10.1002/glia.22287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasek MJ, Garber C, Dorsey D, Durrant DM, Bollman B, Soung A, … Klein RS (2016). A complement-microglial axis drives synapse loss during virus-induced memory impairment. Nature, 534(7608), 538–543. 10.1038/nature18283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezzani A, & Viviani B. (2015). Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology, 96(Pt A), 70–82. 10.1016/j.neuropharm.2014.10.027 [DOI] [PubMed] [Google Scholar]
- Vieira PA, Corches A, Lovelace JW, Westbrook KB, Mendoza M, & Korzus E. (2015). Prefrontal NMDA receptors expressed in excitatory neurons control fear discrimination and fear extinction. Neurobiology of Learning and Memory, 119, 52–62. 10.1016/j.nlm.2014.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikman KS, Owe-Larsson B, Brask J, Kristensson KS, & Hill RH (2001). Interferon-gamma-induced changes in synaptic activity and AMPA receptor clustering in hippocampal cultures. Brain Research, 896(1–2), 18–29. 10.1016/s0006-8993(00)03238-8 [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, … WyssCoray T. (2011). The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature, 477(7362), 90–94. 10.1038/nature10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Yue H, Hu Z, Shen Y, Ma J, Li J, … Gu Y. (2020). Microglia mediate forgetting via complement-dependent synaptic elimination. Science, 367(6478), 688–694. 10.1126/science.aaz2288 [DOI] [PubMed] [Google Scholar]
- Wang CF, Zhao CC, Liu WL, Huang XJ, Deng YF, Jiang JY, & Li WP (2020). Depletion of microglia attenuates dendritic spine loss and neuronal apoptosis in the acute stage of moderate traumatic brain injury in mice. Journal of Neurotrauma, 37(1), 43–54. 10.1089/neu.2019.6460 [DOI] [PubMed] [Google Scholar]
- Wang X, & Suzuki Y. (2007). Microglia produce IFN-gamma independently from T cells during acute toxoplasmosis in the brain. Journal of Interferon & Cytokine Research, 27(7), 599–605. 10.1089/jir.2006.0157 [DOI] [PubMed] [Google Scholar]
- Wang X, Zhao L, Zhang J, Fariss RN, Ma W, Kretschmer F, … Wong WT (2016). Requirement for microglia for the maintenance of synaptic function and integrity in the mature retina. The Journal of Neuroscience, 36(9), 2827–2842. 10.1523/JNEUROSCI.3575-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburton EC, Barker GR, & Brown MW (2013). Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology, 74, 41–47. 10.1016/j.neuropharm.2013.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YP, Kita M, Shinmura K, Yan XQ, Fukuyama R, Fushiki S, & Imanishi J. (2000). Expression of IFN-gamma in cerebrovascular endothelial cells from aged mice. Journal of Interferon & Cytokine Research, 20(4), 403–409. 10.1089/107999000312342 [DOI] [PubMed] [Google Scholar]
- Werneburg S, Feinberg PA, Johnson KM, & Schafer DP (2017). A microglia-cytokine axis to modulate synaptic connectivity and function. Current Opinion in Neurobiology, 47, 138–145. 10.1016/j.conb.2017.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, & Bussey TJ (2005). Glutamate receptors in perirhinal cortex mediate encoding, retrieval, and consolidation of object recognition memory. The Journal of Neuroscience, 25(17), 4243–4251. 10.1523/JNEUROSCI.0480-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf SA, Boddeke HW, & Kettenmann H. (2017). Microglia in physiology and disease. Annual Review of Physiology, 79, 619–643. 10.1146/annurev-physiol-022516-034406 [DOI] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, & Godbout JP (2009). Immune and behavioral consequences of microglial reactivity in the aged brain. Integrative and Comparative Biology, 49(3), 254–266. 10.1093/icb/icp009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao BG, & Link H. (1998). IFN-gamma production of adult rat astrocytes triggered by TNF-alpha. Neuroreport, 9(7), 1487–1490. 10.1097/00001756-199805110-00044 [DOI] [PubMed] [Google Scholar]
- Yang X, Ren H, Wood K, Li M, Qiu S, Shi FD, … Liu Q. (2018). Depletion of microglia augments the dopaminergic neurotoxicity of MPTP. The FASEB Journal, 32(6), 3336–3345. 10.1096/fj.201700833RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yegla B, & Foster T. (2019). Effect of systemic inflammation on rat attentional function and neuroinflammation: Possible protective role for food restriction. Frontiers in Aging Neuroscience, 11, 296. 10.3389/fnagi.2019.00296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, & Goshen I. (2011). Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain, Behavior, and Immunity, 25 (2), 181–213. 10.1016/j.bbi.2010.10.015 [DOI] [PubMed] [Google Scholar]
- Zucker RS, & Regehr WG (2002). Short-term synaptic plasticity. Annual Review of Physiology, 64, 355–405. 10.1146/annurev.physiol.64.092501.114547 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.