Abstract
Background:
Glucocorticoid (GC) administration prior to exposure-based cognitive-behavioural therapy (CBT) has emerged as a promising approach to facilitate treatment outcome in anxiety disorders. Further components relevant for improved CBT efficacy include raised endogenous GCs and reductions in information-processing biases to threat.
Aims:
To investigate hydrocortisone as an adjunct to CBT for spider fear and the modulating role of threat bias change and endogenous short-term and long-term GCs for treatment response.
Methods:
Spider-fearful individuals were randomized to receiving either 20 mg of hydrocortisone (n = 17) or placebo (n = 16) one hour prior to single-session predominantly computerised exposure-based CBT. Spider fear was assessed using self-report and behavioural approach measures at baseline, 1-day and 1-month follow-up. Threat processing was assessed at baseline and 1-day follow-up. Cortisol and cortisone were analysed from hair and saliva samples at baseline.
Results/outcomes:
Self-report, behavioural and threat processing indices improved following CBT. Hydrocortisone augmentation resulted in greater improvement of self-report spider fear and stronger increase in speed when approaching a spider, but not on threat bias. Neither threat bias nor endogenous GCs predicted symptom change, and no interactive effects with hydrocortisone emerged. Preliminary evidence indicated higher hair cortisone as predictor of a stronger threat bias reduction.
Conclusions/interpretation:
Our data extend earlier findings by suggesting that GC administration boosts the success of exposure therapy for specific fear even with a low-level therapist involvement. Future studies corroborating our result of a predictive hair GC relationship with threat bias change in larger clinical samples are needed.
Keywords: Cognitive-behaviour therapy, glucocorticoids, threat processing, specific phobia, hydrocortisone
Introduction
Meta-analytical data suggest that exposure-based cognitive-behavioural therapy (CBT) is the most effective treatment for anxiety disorders (e.g., Carpenter et al., 2018; Hofmann and Smits, 2008). However, a substantial subgroup of patients still fail to achieve clinically significant symptom improvement, with high rates of non-response, dropout and relapse (e.g., Ali et al., 2017; Fernandez et al., 2015; Taylor et al., 2012). This fact has prompted a substantial effort to identify key mechanisms for an effective treatment outcome and to develop strategies to optimize the effects of exposure therapy.
One promising approach is the combination of psychological treatment strategies with the administration of glucocorticoids (GCs), which are assumed to influence key neural processes crucially involved in CBT learning (Bentz et al., 2010; De Quervain et al., 2017). Specifically, GCs are assumed to facilitate learning and memory by binding to GC and mineralocorticoid (MC) receptors located in limbic and frontal brain areas that underlie these information processes (De Quervain et al., 2017; Krugers et al., 2011). Previous evidence from animal and human studies suggests that GCs enhance the consolidation of emotional memories (e.g., Van Stegeren et al., 2007), while they impair the retrieval of aversive learning episodes (e.g., De Quervain et al., 1998). Hence, previous research has focused on whether GC administration has the potential to ameliorate clinical symptoms by inhibiting the retrieval of the fearful memory and/or enhancing the extinction process (De Quervain et al., 2017, 2019). In fact, growing evidence documents that coupling of exposure-based CBT with exogenous GC administration facilitates treatment outcome in anxiety disorder patients (Bentz et al., 2010; De Quervain et al., 2017). For instance, studies have indicated that GCs offer an effective augmentation of CBT in patients suffering from social phobia (Soravia et al., 2006), specific phobia (De Quervain et al., 2011; Soravia et al., 2006, 2014) and posttraumatic stress disorder (PTSD) (Yehuda et al., 2014).
In addition to these findings suggesting that exogenous GC administration enhances the effectiveness of exposure-based CBT, a growing body of literature indicates that endogenous GC functioning also affects CBT outcome. For example, spider phobia patients who received a single session of exposure therapy benefited more from the intervention when they were treated in the morning, when endogenous cortisol levels were increased due to circadian rhythmicity of cortisol output (Lass-Hennemann and Michael, 2014). In addition, higher plasma cortisol levels during exposure or a higher salivary cortisol awakening response on the day of exposure therapy were related to improved CBT outcome in panic disorder and agoraphobia patients (Meuret et al., 2015; Siegmund et al., 2011). Despite these promising findings, recent meta-analytical data did not support the notion of short-term salivary or plasma basal cortisol levels as a predictor of treatment response in anxiety disorders (Fischer and Cleare, 2017).
Part of the reason for mixed findings might be related to limitations in the assessment of long-term GC regulation. While anxiety disorders are assumed to exert long-term effects on GC secretion (Elnazer and Baldwin, 2014), traditional cortisol assessment strategies reflect short-term secretory activity over periods ranging from minutes (saliva, plasma) to hours (urine). Due to the fact that acute GC secretion is highly volatile and affected by various situational factors, these methods provide rather unreliable assessments of long-term GC output. An important advancement in this respect is hair steroid analysis, which represents a marker of long-term GC secretion integrated over periods of several months (Stalder et al., 2017). In fact, a recent study in this context observed higher hair cortisol concentrations as a predictor of improved therapy response in depression and anxiety disorder patients (Fischer et al., 2018). Hence, hair GC analysis may effectively complement research into endocrine predictors of clinical symptom change in response to exposure therapy. Another research gap in this context is to examine whether GC functioning may also have the potential to mediate the efficacy of exogenous GC treatment, as indicated by preliminary results in PTSD patients (Yehuda et al., 2014).
Besides the role of endocrine markers for CBT outcome, recent work has provided first evidence that one mechanistic driver of improvement in clinical symptoms might be changes in information-processing biases to threat (Reinecke and Harmer, 2015). In particular, studies on panic disorder patients have shown that automatic hypervigilance for threat information is reduced after only one session of exposure-based CBT (prior to clinical symptom change), and that the magnitude of this reduction predicts symptom improvement during the following 4 weeks (Reinecke et al., 2013b). Other findings also include changes in information-processing biases following treatment in specific phobia (Van den Hout et al., 1997; Reinecke et al., 2012), generalised anxiety disorder (e.g., Mogg et al., 1995; Reinecke et al., 2013a) and social anxiety disorder (Calamaras et al., 2012). Together, these results point toward the possibility that a normalization of threat bias causally relates to a reduction of clinical symptoms.
Interestingly, emerging evidence has indicated that pharmacologically elevated GCs might reduce working memory bias for fearful faces in healthy individuals (Putman et al., 2007) and, when applied before exposure-based CBT, acutely decrease threat bias in social phobia patients (Van Peer et al., 2010). In combination with the above evidence of a mechanistic role of threat bias change in clinical symptom improvement during CBT, it is conceivable that clinical augmentation effects of GCs are based on reductions in fear memory linked threat bias which, in turn, may allow a more pronounced consolidation of extinction memories (Reinecke and Harmer, 2015). However, a detailed investigation of this notion is still pending. In particular, no data are available on the predictive relationship between endogenous GC functioning before CBT and threat bias change.
In this double-blind experimental medicine study, 36 participants with high levels of spider fear were randomly allocated to receiving a single dose of 20 mg hydrocortisone versus placebo one hour before exposure-based CBT. We aimed to extend previous research by focusing on the role of GCs obtained from hair samples as a unique measure of long-term integrated GC secretion. Specifically, besides cortisol, concentrations of cortisone were determined in both hair and saliva to obtain a more robust GC index (e.g., Perogamvros et al., 2010; Stalder et al., 2013), and the ratio of cortisol and cortisone was implemented as a further GC estimate. Threat bias was measured before and on the day after CBT, and spider fear was assessed at baseline, on the day after treatment and 1 month later. We hypothesized that: i) higher basal endogenous GCs would predict better treatment response, ii) lower endogenous GCs would predict increased efficacy of GC-enhanced CBT and iii) participants in the hydrocortisone group would reveal a greater reduction in threat bias 1 day after the CBT session as compared to the control group, with greater bias change predicting greater decrease in spider fear during 1-month follow-up. Finally, explorative analyses were conducted to examine the possibility of an influence of higher endogenous GCs on a stronger reduction in threat bias.
Methods and materials
Participants
While previous research found a large effect size for self-report spider fear between a treatment and no-treatment control group at 1-month follow-up (Müller et al., 2011), we predicted a medium effect (f = 0.25) for the 1-month difference between hydrocortisone- versus placebo-augmented single-session CBT. G-power suggested a total sample size of 28 for a 2 group × 3 time ANOVA with an α-level of significance of 0.05 to achieve power of 1−ß = 0.80. To allow for dropouts at follow-up, 36 spider-fearful individuals were recruited through advertisements at local universities and on community websites. Inclusion criteria were age 18–60 years, non-smoker or smoking less than five cigarettes per day, no use of psychoactive medication in the previous six weeks, a body mass index (BMI) of between 18 and 30 kg/m2, a score of 14 or higher on the Spider Anxiety Screening (SAS) (Rinck et al., 2002) at baseline, and fulfilling DSM-5 criteria for specific spider phobia with the exception of the ‘impairment of functioning’ criterion, considering that avoiding confrontation with spiders is relatively easy in Western Europe. Exclusion criteria were pregnancy, lactation, GC-containing medication within the last three days (the last month for hair analyses), lifetime history of bipolar disorder, psychosis, alcohol, medication or drug abuse or dependence, or a current primary depressive disorder as assessed using the Structured Clinical Interview for DSM-V (SCID) (First et al., 2015). In addition, participants with a first-degree family member with a history of severe psychiatric disease were excluded, as well as participants with lifetime history of severe physical illness, previous exposure-based CBT for spider fear and inadequate English skills. This study received approval from the South Central Oxford ethics committee (REC 15/SC/0270), and all participants provided written consent for participation in the study.
General procedure
In a single-centre, double-blind parallel design, participants were randomised to receiving a single oral dose of 20 mg hydrocortisone (Auden Mckenzie) or placebo (microcrystalline cellulose, Rayonex GmbH) one hour before a single-session CBT. The researcher responsible for treatment, data collection and outcome evaluation remained naive to drug group allocation until completion of data analysis. Placebo and hydrocortisone tablets were encapsulated in identical lactose capsules. Generation of the randomisation sequence, treatment allocation and drug dispensing were executed by a researcher not in direct contact with study participants. The randomization sequence was generated using a random number generator (www.random.org) and was based on blocked randomization (blocks of four) while stratifying for gender. Of the total sample of 36 participants, 18 were randomized to hydrocortisone and 18 to placebo, of which 5 and 6 qualified for a full diagnosis of specific phobia, respectively.
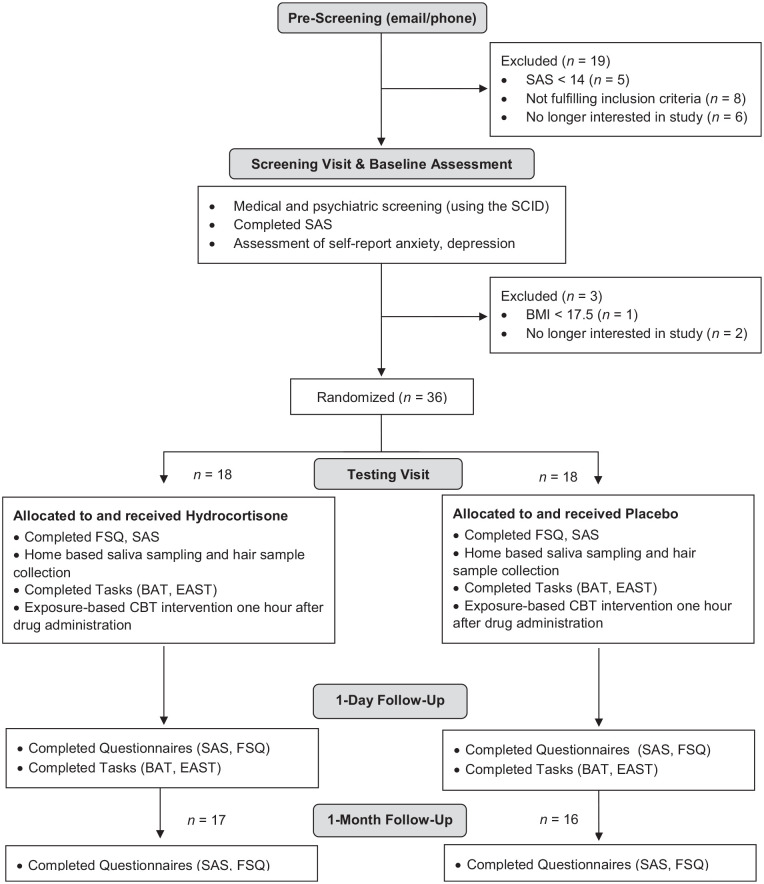
Figure 1 illustrates the flowchart of study procedures. During an initial eligibility screening visit, sociodemographic (age, gender, years of education) and clinical (specific phobia versus specific fear) data were assessed, and participants completed a battery of psychological questionnaires to characterise the sample. This included the Beck Depression Inventory (BDI-II) (Beck et al., 1996), the Perceived Stress Scale (PSS) (Cohen et al., 1983) and the State-Trait Anxiety Inventory (STAI) (Spielberger, 1989).
Figure 1.
Flowchart of experimental procedure.
BAT: Behavioural Approach Test; CBT: cognitive-behavioural therapy; EAST: Extrinsic Affective Simon Task; FSQ: Fear of Spiders Questionnaire; SAS: Spider Anxiety Screening; SCID: Structured Clinical Interview for DSM-V.
After successful screening, participants returned for three study visits, with baseline assessments and intervention (single-session CBT combined with single-dose hydrocortisone versus placebo) taking place on day 1, and outcome testing visits taking place 1 day and 1 month after intervention. Saliva and hair samples were taken on the intervention day. For the intervention visit, all participants were asked to fast for two hours prior to their appointment to ensure homogeneous effects of hydrocortisone across subjects. Considering elevated and more variable endogenous GC levels in the morning (Weitzman et al., 1971), capsule administration took place between 12:00 and 18:30. To capture any acute changes, blood pressure and heart rate were measured before and one hour after drug administration (expected peak level), and participants completed visual analogue scales (VAS) rating their mood and physiological symptoms. At the end of the intervention day, participants and experimenter also guessed whether the active capsule or placebo had been administered. Clinical symptoms of spider fear were assessed on all three visits. At baseline and on the day after intervention, we also assessed threat processing using a behavioural computer task. Of the initial study sample, n = 16 of the placebo group and n = 17 of the hydrocortisone group returned to the 1-month follow-up assessment (Table 1).
Table 1.
Sociodemographic, hair-related and clinical characteristics of participants in the placebo versus hydrocortisone group.
| Placebo (n = 16) | Hydrocortisone (n = 17) | p-values | |
|---|---|---|---|
| Sociodemographic data | |||
| Female (%) | 12 (75) | 14 (82.4) | 0.69 |
| Age (M, SD) | 24.25 (6.13) | 25.12 (7.98) | 0.73 |
| Years of education (M, SD) | 17.88 (2.80) | 17.06 (2.88) | 0.42 |
| Smoking, n (%) | 0 (0) | 1 (5.9) | 0.52 |
| Drinking (units/week) (M, SD) | 4.73 (4.73) | 6.12 (4.87) | 0.42 |
| Oral contraceptives (%) | 6 (37.5) | 6 (35.3) | 1.0 |
| BMI (M, SD) | 21.21 (1.37) | 22.02 (3.30) | 0.37 |
| Hair-related characteristics | |||
| Hair wash frequency (M, SD) | 3.47 (2.45) | 3.41 (1.54) | 0.93 |
| Hair treatment (%) | 3 (18.8) | 4 (25.0) | >0.99 |
| Curls/waves (%) | 5 (31.3) | 3 (18.8) | 0.69 |
| Clinical measures | |||
| STAIT (M, SD) | 38.13 (8.33) | 34.76 (7.13) | 0.22 |
| PSS (M, SD) | 22.5 (4.86) | 17.35 (6.48) | 0.02 |
| BDI-II (M, SD) | 4.19 (5.05) | 1.65 (2.34) | 0.07 |
| Specific spider phobia (%) | 6 (37.5) | 4 (23.5) | 0.47 |
| SAS (M, SD) | 18.5 (2.88) | 19.12 (2.06) | 0.48 |
| FSQ (M, SD) | 69.94 (14.79) | 65.47 (13.19) | 0.37 |
| BAT speed | 0.16 (0.12) | 0.18 (0.16) | 0.80 |
| BAT distance | 395.44 (143.95) | 443.53 (174.28) | 0.40 |
| Threat bias | |||
| EAST spider evaluation* (M, SD) | 21.93 (51.24) a | −0.84 (29.96) b | 0.14 |
| Endogenous glucocorticoid levels | |||
| Salivary cortisol + 0 (M, SD) | 10.10 (6.43) c | 8.45 (5.10) d | 0.60 |
| Salivary cortisol + 30 (M, SD) | 12.78 (5.02) c | 14.37 (7.82) d | 0.76 |
| Salivary cortisol + 45 (M, SD) | 12.61 (4.28) c | 14.28 (8.82) d | 0.64 |
| Salivary cortisone + 0 (M, SD) | 8.45 (3.23) c | 9.34 (3.18) d | 0.32 |
| Salivary cortisone + 30 (M, SD) | 10.74 (1.92) c | 14.76 (3.28) d | < 0.01 |
| Salivary cortisone + 45 (M, SD) | 11.28 (2.67) c | 15.41 (3.67) d | 0.03 |
| Hair cortisol (M, SD) | 7.68 (4.56) d | 7.83 (8.00) d | 0.65 |
| Hair cortisone (M, SD) | 17.91 (9.19) d | 16.31 (8.16) d | 0.64 |
BAT: Behavioural Approach Test; BDI-II: Beck Depression Inventory-II; BMI: Body Mass Index; EAST: Extrinsic Affective Simon Task; FSQ: Fear of Spiders Questionnaire; PSS: Perceived Stress Scale; SAS: Spider Anxiety Screening; STAIT: State-Trait Anxiety Inventory.
Indices reflect the difference in reaction time between fear-compatible (spider and positive valence) and fear-incompatible (spider and negative valence) stimuli pairings with negative values reflecting longer responses to fear-incompatible stimuli pairings.
Value refers to n = 14.
Value refers to n = 16.
Value refers to n = 12.
Value refers to n = 15.
Single-session exposure-based CBT
The 45-minute treatment involved a combination of the following components. i) Psychoeducation included written information about the anxiety response, the role of avoidance and escape behaviour in maintaining anxiety, and overwriting the fear association by exposure. ii) Computer-based exposure involved participants visually exploring nine large spider pictures that were presented for three minutes each. To facilitate visual attention towards the spider, participants had to click on star symbols superimposed on the images. This approach was validated in previous research and has been shown to lead to a significant decrease in self-report and behavioural measures of spider fear post-treatment and until 1-month follow-up when compared to non-treatment controls (Müller et al., 2011). iii) In vivo exposure involved participants viewing a medium-sized dead spider in a sealed, transparent petri-dish, guided by the therapist and with the goal of reducing fear levels to at least 50% of baseline fear. All participants chose to engage in all three parts of treatment. All diagnostic assessments and treatments were carried out by a trained researcher and supervised by an experienced clinical psychologist (AR).
Outcomes
Clinical symptom measures
Self-report spider fear
Spider fear was measured using the self-report four-item questionnaire Spider Anxiety Screening (Rinck et al., 2002) and the extensive self-report Fear of Spiders Questionnaire (FSQ) (Szymanski and O’Donohue, 1995).
Behavioural Approach Test (BAT)
To provide a behavioural measure of spider fear, participants were asked to approach a terrarium containing a tarantula carapace (which appeared to be a live spider) on a windowsill 6 m away from them, as quickly and closely as they felt able. The therapist did not provide any support or encouragement to the participant, other than detailing to them the initial task instructions. We measured distance (cm) covered and speed of approach as distance divided by time (m/s). This measure of spider fear has been used in previous studies and demonstrated good 1-week test–retest reliability (r = 0.84, p < 0.001; Reinecke et al., 2010, 2012).
Threat processing
Threat bias was assessed using the Extrinsic Affective Simon Task (EAST) which has been shown to be sensitive to brief treatment and demonstrated sufficient reliability and validity in previous work (Reinecke et al., 2012). Stimuli were 20 words of pleasant (e.g., happiness, pleasure) or unpleasant valence (e.g., fear, dangerous) and 10 spider or butterfly photographs (300 × 400 pixels) in original and mirrored version (the animal’s gaze points left versus right). The task was presented in seven blocks, with error feedback provided throughout. During a valence practice block (80 trials), valence words were presented four times in counterbalanced order, and participants categorised words based on valence with a left and a right response button, to associate either button with a specific valence. During a target practice block, participants categorised five pictures of dragonflies (twice in original and twice in mirrored form) based on their gaze direction as left or right. In the five experimental blocks, participants categorised 40 words and 40 pictures (pseudorandom presentation order) based on valence and gaze direction, respectively. This resulted in compatible trials in which butterflies were associated with positive valence and spiders with negative valence, and in incompatible trials in which spiders were paired with positive valence and butterflies with negative valence. For each participant and each test time, median reaction time (RT) to spider images with the unpleasant key (compatible reaction) versus with the pleasant key (incompatible response) were calculated (for correct trials only). Threat bias was computed as the difference in RTs between compatible and incompatible trials of spider pictures. Higher scores indicate more negative implicit associations with spider images. Owing to technical issues, two participants of the placebo group did not perform the EAST 1 day after treatment, resulting in a sample of n = 17 in the hydrocortisone and n = 14 in the placebo group for EAST analyses.
Glucocorticoid measurements
Saliva samples
Saliva samples were taken using salivettes (Sarstedt, Nümbrecht, Germany). Participants were required to gently chew on a cotton swab for 30–60 seconds. On the morning of the intervention visit, participants collected three saliva samples at home (immediately after waking up, 30 minutes and 45 minutes after waking) before breakfast, smoking or teeth brushing, and kept these in the fridge until their visit. In order to increase the likelihood of participants adhering to the protocol for these morning samples (Kudielka et al., 2003), salivettes were placed in mock MEMS 6 TrackCap containers (Aardex Ltd., Zug, Switzerland). Participants were under the impression that the mock containers were fully functional and able to track the times at which the containers were opened and the saliva samples were obtained.
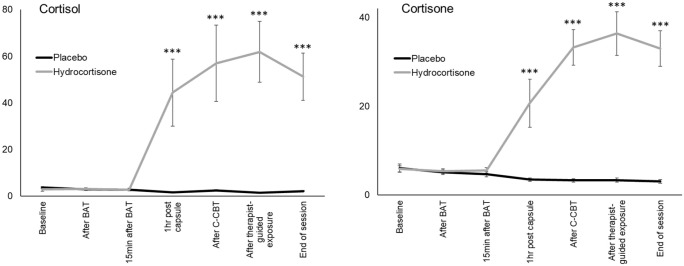
Morning saliva samples were returned by 18 participants in the placebo group and 18 in the hydrocortisone group. However, salivary data from two participants in the placebo group were excluded from further analysis due to non-compliance. Specifically, one participant missed noting down exact time points of sampling and another participant exceeded instructed sampling times by more than 15 minutes (Dockray et al., 2008; Okun et al., 2010). Additional saliva samples were taken during the intervention visit: at baseline, immediately after the BAT, 15 minutes after the BAT, at drug-peak level, after computer-based exposure (CBE), after therapist-guided exposure and at the end of the session (Figure 2). Saliva samples were stored at −20°C in the laboratory freezer until biochemical analysis. Salivary cortisol and cortisone concentrations were detected using liquid chromatography tandem mass spectrometry (LC–MS/MS) following a previously published protocol (Gao et al., 2015). Morning salivary data from four participants (hydrocortisone: n = 2, placebo n = 2) were excluded from analysis due to missing data on either the sample immediately after waking up or two out of the three sampling points, resulting in a sample with complete follow-up data of n = 12 for the placebo group and n = 15 for the hydrocortisone group. To reflect the cortisol and cortisone awakening response, respectively, the area under the curve with respect to increase (AUCI) was calculated (Pruessner et al., 2003).
Figure 2.
Saliva cortisol and cortisone levels measured at seven time points during the treatment session. The hydrocortisone group (n = 17) showed significantly higher cortisol and cortisone levels compared to the placebo group (n = 16) one hour after drug administration, for the remainder of the session. Error bars show standard error of the mean. Asterisks indicate significant group differences.
BAT: Behavioural Approach Test; C-CBT: computerised part of CBT session.
Hair samples
At the beginning of the intervention visit, a hair strand (~3 mm diameter) was cut as close as possible to the scalp from a posterior vertex position. Hair strands were obtained from 30 participants (placebo: n = 15, hydrocortisone: n = 15) at baseline and restricted to participants who had a hair length of at least 1-2 cm at the posterior vertex region of the scalp and showed no signs of hair loss or baldness. The scalp-near 1 cm hair segment was analysed. Based on an average hair growth rate of 1 cm per month (Wennig, 2000), hair GC concentrations in this segment are thought to reflect cumulative GC secretion over the previous 1-month period. Information on hair-specific characteristics (washes per week, waves or curls, hair treatments) were obtained using an in-house questionnaire. Cortisol and cortisone levels in hair were quantified using LC–MS/MS as described in Gao et al. (2013).
Statistical analysis
Statistical analyses were performed using SPSS for Windows, version 22 (IBM, Chicago, IL). Statistical tests were two-tailed and based on an alpha-level of significance of 0.05. Due to missing morning salivary data on one sampling point (45 minutes after awakening) for one participant and missing salivary data on up to three out of the seven sampling points during CBT in 11 participants, parameter estimation was carried out from data sets derived by a multiple imputation bootstrapping procedure (Schäfer, 1997). As expected, GC data lacked normality, and thus log-transformations were applied to minimize biased results (Miller and Plessow, 2013). For descriptive purposes, mean data in figures are presented in original units.
Group comparisons regarding sociodemographic, clinical-psychological and hair-related characteristics were conducted using t-tests for continuous variables and Fisher's exact tests for dichotomous variables. To demonstrate that hydrocortisone administration in fact resulted in an acute increase in salivary cortisol and cortisone, two-way repeated measures ANOVAs with measurement time [7] as within-subject factor and group [2] as between-subject factor were conducted. To establish differential changes in heart rate, blood pressure and VAS ratings from baseline to drug-peak level between the two groups, time × group (placebo, hydrocortisone) ANOVAs were run for each of these measures. Time (baseline, next day, 1-month follow-up) × group (hydrocortisone, placebo) mixed-model ANOVAs were run for subjective and behavioural measures of spider fear (SAS, FSQ, BAT) with significant interaction effects having been further explored by simple contrasts separately comparing next-day and 1-month follow-up scores to baseline scores.
For EAST analyses, one participant in the hydrocortisone group was excluded due to an RT outlier (> 3 SD above the mean) as measured before CBT treatment, resulting in a sample of n = 14 in the placebo and n = 16 in the hydrocortisone group. Indices of threat bias were entered into a time (baseline, next-day) × group (hydrocortisone, placebo) mixed-model ANOVA in order to examine the effect of hydrocortisone treatment on threat bias. Further, hierarchical multiple linear regression analyses were run to establish whether changes in threat bias from baseline to the day after treatment predicted symptom recovery during 1-month follow-up: next-day SAS scores were entered as predictor of no interest in a first step to control for its potential influence on the outcome at 1 month. Group, threat bias change and the group-threat bias interaction term were additionally entered as predictors of interest in a second step. The dependent variable was change on the SAS score between the day after treatment and 1-month follow-up. Similar regression analyses were run for 1-month change scores on the FSQ and in BAT speed and distance.
To investigate whether baseline GCs (i.e., cortisol and cortisone as measured in saliva and hair) predicted symptom recovery during 1-month follow-up, hierarchical multiple linear regressions were conducted. Baseline SAS scores were entered as predictor in a first step to control for its potential influence on the outcome at 1 month. Group, baseline endogeneous GCs and the group-GC interaction term were additionally entered as predictors of interest in a second step (separately for salivary and hair GCs). The dependent variables were change scores on the SAS, FSQ, BAT speed and distance between baseline and 1-month follow-up, respectively. Multiple linear regression analyses were further run to explore whether the baseline GCs predicted change in threat bias from baseline to 1-day follow-up. Baseline EAST scores were entered as a control variable in a first step and group, baseline GCs, and the group-GC interaction term in a second step. The dependent variable was change in threat bias from baseline to the day after treatment.
Results
Group matching, manipulation check and drug side effects
Groups (placebo: n = 16, hydrocortisone: n = 17) were well balanced on sociodemographic and clinical parameters, as well as subjective and behavioural measures of spider fear (p-values > 0.07), except for higher scores on the PSS in the placebo group (p = 0.02). Further, no group differences emerged with regard to threat processing (p = 0.14) as well as GC measures (p-values > 0.32), except for higher salivary cortisone levels in the hydrocortisone group as measured 30 and 45 minutes after awakening (both p < 0.05, Table 1).
Hydrocortisone versus placebo resulted in an acute increase in cortisol and cortisone (cortisol group × time: F6,186 = 39.99, p < 0.001; cortisone group × time: F6,186 = 38.11; p < 0.001), with groups differing from one hour after capsule intake until the end of the session (all p < 0.001, other p > 0.18, Figure 2).
The hydrocortisone versus placebo group were not significantly different in heart rate, blood pressure and VAS ratings at drug-peak level, but the placebo group gave higher anxiety ratings at baseline (F1,32 = 5.47, p = 0.03, Table 2). While there was a reduction in heart rate and systolic blood pressure in the drug group from baseline to drug-peak level, this was not seen in the placebo group (both F1,31 > 5.35, both p < 0.04). Nevertheless, neither the experimenter nor participants were able to correctly guess group allocation (hydrocortisone guesses: experimenter placebo 43.8%, hydrocortisone 41.2%, p > 0.99; participant placebo 18.8%, hydrocortisone 29.4%, p = 0.69), suggesting that double-blindness was maintained.
Table 2.
Heart rate, blood pressure and visual analogue scale ratings in the two groups before drug intake and at drug-peak-level. F-tests show the interaction of group (placebo, hydrocortisone) × time (baseline, drug-peak).
| Baseline |
Drug peak |
F-test | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Placebo (n = 16) | Hydrocortisone (n = 17) | Placebo (n = 16) | Hydrocortisone (n = 17) | p-value | |||||
| M | SD | M | SD | M | SD | M | SD | ||
| Physiological measures | |||||||||
| Heart rate | 72.6 | 12.8 | 74.8 | 10.8 | 69.5 | 10.9 | 64.1 | 7.4 | 0.04 |
| Systolic blood pressure | 118.6 | 13.8 | 124.6 | 14.5 | 119.1 | 9.4 | 116.0 | 9.6 | 0.03 |
| Diastolic blood pressure | 73.1 | 9.2 | 75.8 | 8.3 | 73.8 | 5.7 | 73.1 | 8.3 | 0.19 |
| Visual analogue ratings | |||||||||
| Anxious | 24.7 | 19.1 | 11.7 | 12.5 | 17.1 | 13.5 | 16.8 | 17.6 | 0.09 |
| Tearful | 3.7 | 6.1 | 2.8 | 7.4 | 4.7 | 6.8 | 2.5 | 5.4 | 0.66 |
| Hopeless | 5.2 | 7.5 | 0.7 | 2.1 | 4.4 | 6.2 | 0.9 | 2.4 | 0.42 |
| Sad | 6.9 | 8.1 | 2.0 | 4.1 | 5.3 | 7.2 | 1.2 | 2.8 | 0.62 |
| Depressed | 5.6 | 7.1 | 2.4 | 9.0 | 4.3 | 4.6 | 1.4 | 3.1 | 0.94 |
| Sleepy | 27.1 | 21.1 | 29.7 | 22.6 | 36.0 | 25.2 | 25.5 | 20.6 | 0.07 |
| Nauseous | 3.6 | 7.2 | 0.7 | 2.1 | 4.7 | 6.6 | 2.1 | 4.4 | 0.83 |
| Dizzy | 4.4 | 7.7 | 2.0 | 5.7 | 6.9 | 10.1 | 1.6 | 3.7 | 0.23 |
| Heart racing | 8.6 | 9.5 | 1.9 | 3.7 | 5.4 | 5.7 | 1.8 | 5.4 | 0.20 |
| Alert | 47.7 | 20.0 | 47.5 | 28.4 | 34.0 | 21.7 | 45.4 | 24.8 | 0.07 |
The effect of hydrocortisone treatment
Self-report spider anxiety
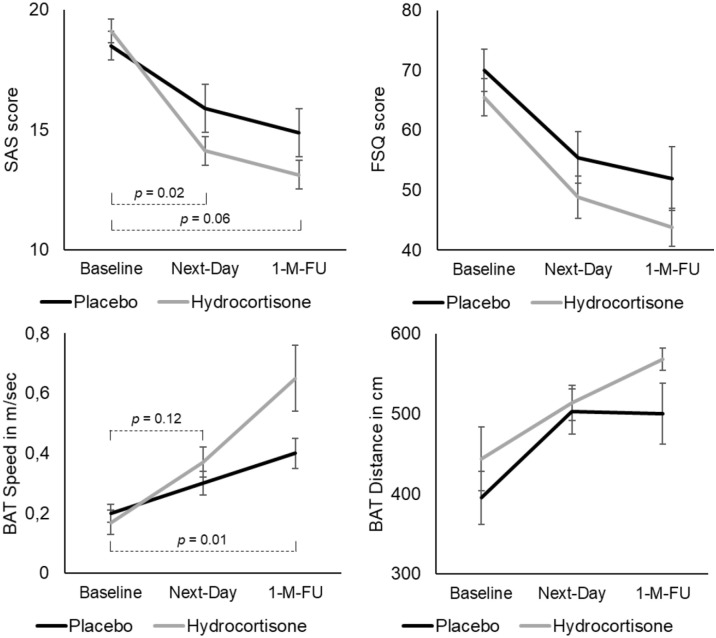
Both groups showed a significant decrease in self-report spider fear (SAS, FSQ) over time (both F2,62 > 38.12, both p < 0.001, Figure 3). While group differences in FSQ changes did not reach statistical significance (F2,62 = 0.28, p = 0.75), there was a significant interaction effect between the two groups and the three time points on the SAS (F2,62 = 3.83, p = 0.03). Follow-up comparisons revealed a significantly stronger decrease in SAS scores in the hydrocortisone compared to the placebo group from baseline to the day after treatment (F1,31 = 6.32, p = 0.02). The group comparison for baseline versus 1-month follow-up SAS scores failed to reach statistical significance (F1,31 = 3.98, p = 0.06).
Figure 3.
Clinical changes from baseline to the day and month after treatment in the two groups. Adding hydrocortisone (n = 17) versus placebo (n = 16) to single-session exposure-based CBT led to significantly stronger reductions in self-report spider fear within a day of treatment (SAS), and to significantly stronger increase in speed when approaching a spider over the month following treatment (BAT).
BAT: Behavioural Approach Test; FSQ: Fear of Spiders Questionnaire; SAS: Spider Anxiety Screening.
Behavioural Approach Test
Both groups showed significant increases over time in speed and covered distance when approaching a spider (both F2,62 > 15.94, both p < 0.001, Figure 3). While the reductions in covered distance were not different between groups (F2,62 = 0.92, p = 0.40), the group × time interaction reached significance for speed (F2,62 = 5.68, p = 0.01). Follow-up comparisons suggested a significantly stronger increase in speed from baseline to 1-month follow-up in the hydrocortisone versus placebo group (F1,31 = 6.87, p = 0.01), while this was not evident for baseline to the day after treatment (F1,31 = 2.63, p = 0.12).
Threat bias
While threat bias significantly reduced from baseline to the day after treatment (F1,28 = 6.41, p = 0.02), neither a group difference (baseline: placebo M = 21.93, SD = 51.24, hydrocortisone M = −0,84, SD = 29,96; next-day: placebo M = −4.04, SD = 21.06, hydrocortisone M = −8.78, SD = 33.69; F1,28 = 1.53, p = 0.23) nor a group × time interaction (F1,28 = 1.81, p = 0.19) was revealed.
Prediction of clinical recovery during 1-month follow-up
Threat bias change
Early change in threat bias (or the group interaction term) was not a significant predictor of 1-month follow-up change on SAS, FSQ, BAT speed and distance (all R2 < 0.34, all t < 0.87, all p > 0.40).
Baseline salivary awakening response
Neither baseline salivary cortisol AUCI nor the cortisol AUCI × group interaction was a significant predictor of 1-month follow-up change in SAS, FSQ, BAT speed or distance (all R2 < 0.60, all t < 1.73, all p > 0.10). The same pattern of results was revealed for salivary cortisone AUCI (all R2 < 0.61, all t < 1.99, all p > 0.06) or the AUCI cortisol/cortisone ratio (all R2 < 0.51, all t < 1.40, all p > 0.18).
Baseline hair
Baseline hair cortisol (or the group interaction term) was not a significant predictor of 1-month follow-up change on SAS, FSQ, BAT speed and distance (all R2 < 0.58, all t < 1.48, all p > 0.15). The same pattern of findings emerged for hair cortisone levels (all R2 < 0.58, all t < 1.60, all p > 0.09) and for the hair cortisol/cortisone ratio (all R2 < 0.59, all t < 1.08, all p > 0.29).
Prediction of change in threat bias by endogenous GCs
Baseline salivary awakening response
Neither baseline salivary cortisol AUCI nor the interaction term predicted change in threat bias at 1-day follow-up (R2 = 0.48, all t < 1.09, all p > 0.29). The same pattern of results was revealed for salivary cortisone AUCI (R2 = 0.49, all t < 1.10, all p > 0.29) or the AUCI cortisol/cortisone ratio (R2 = 0.47, all t < 0.74, all p > 0.47).
Baseline hair
Baseline hair cortisol (or the group interaction term) did not emerge as a significant predictor of change in threat bias at 1-day follow-up (R2 = 0.65, all t < 1.51, all p > 0.15). However, hair cortisone predicted change in threat bias at 1-day follow-up across groups bordering on statistical significance (R2 = 0.71, t = 2.07, p = 0.05), with higher baseline hair cortisone predicting a stronger reduction in threat bias. No significant effect for a hair cortisone × group interaction was revealed (R2 = 0.71, t = 1.89, p = 0.07). The hair cortisol/cortisone ratio (or the group interaction term) did not emerge as a significant predictor of change in threat bias at 1-day follow-up (R2 = 0.62, all t < 0.85, all p > 0.41).
Discussion
The current double-blind, placebo-controlled, randomised study replicates earlier findings of clinical augmentation effects with combined GC administration and exposure-based CBT for spider fear and extend these by showing such an effect for a low-level therapist involvement. Specifically, GC enhancing effects were revealed for self-report spider fear (SAS) within 1 day and 1 month (at trend level) after treatment and for a stronger increase in speed when approaching a spider (BAT) over the month following treatment. Different from our hypothesis, baseline endogenous GC levels (as evidenced by short-term and long-term GCs in saliva and hair, respectively) were not predictive of treatment response and did not modulate the efficacy of exogenous GC treatment. Further, changes in threat bias were not predictive of clinical symptom change and did not emerge as a function of GC administration. Explorative analyses revealed a tentative predictive relationship between hair cortisone levels and change in threat bias from baseline to 1-day follow-up.
The current findings replicate the effectiveness of a predominantly computerised, one-session CBT for ameliorating subjective and behavioural measures of spider fear until 1-month follow-up (Müller et al., 2011). Notably, our data support the notion that coupling of this innovative intervention with exogenous GC administration facilitates treatment response for spider fear. This is in line with previous studies suggesting efficacy of GC augmentation of exposure-based CBT in patients with anxiety disorders (De Quervain et al., 2011, 2017, 2019; Soravia et al., 2006, 2014). The current GC enhancing effects were observed for self-report spider fear (SAS) within 1 day and 1 month (at trend level) after treatment and for a stronger increase in speed when approaching a spider (BAT) over the month following treatment. However, no such clinical effects emerged for the other spider fear symptom measures (i.e., FSQ, BAT distance). The lack of efficacy of GC administration on these measures may be related to the fact that only 31% of the current sample qualified for a diagnosis of a specific phobia. This may have led to reduced room for improvement on subjective and behavioural spider fear indices which might have been sufficiently targeted by the effective one-session CBT protocol. Here, it is conceivable that the four-item questionnaire SAS might have been more powerful in detecting the clinical augmentation effect than the 18-item FSQ in the current small sample.
While the current data support the notion of exogenous GC administration to improve clinical symptom change in response to CBT, no such effect occurred for baseline endogenous GC levels. Specifically, long-term and short-term GCs were not predictive of treatment response and did not modulate the efficacy of hydrocortisone treatment. The current null findings with regard to the cortisol and cortisone awakening response are in line with meta-analytical data suggesting no effect of salivary cortisol as a predictor for treatment response (Fischer and Cleare, 2017). Despite the advantage of hair steroid analysis as an index of long-term integrated GCs, no predictive relationship was revealed, which is in contrast to one previous study on outpatients suffering from depression and anxiety disorders (e.g., PTSD, panic disorder, agoraphobia; Fischer et al., 2018). Again, the fact that the current study mainly focused on healthy spider-fearful individuals might have resulted in reduced variance in baseline long-term GC levels impeding the detection of meaningful relationships. Hence, future studies are needed to investigate the potential of pre-treatment endogenous GCs to distinguish individuals who are likely to benefit from CBT and/or adjunct hydrocortisone administration from those who are not, by focusing on larger clinical samples. While not predicting treatment response, there was a hint for hair cortisone levels to predict reductions in threat bias. Proceeding from findings suggesting a predictive value of early bias threat change for clinical symptom improvement in anxiety disorders (Reinecke et al., 2013a, 2013b), this calls for further research investigating whether pre-treatment GCs might serve as a predictor of this relationship in clinical samples.
However, against our hypothesis, hydrocortisone administration did not augment early bias reduction. Further, in contrast to previous studies (e.g., Reinecke et al., 2013b), our data did not provide evidence for the assumption that early bias change (or the group interaction effect) predicted 1-month follow-up change on subjective and behavioural spider fear. Besides differences in the applied paradigms (e.g., dot-probe task as a measure of threat bias), it is conceivable that this apparent discrepancy is again related to the current subclinical sample.
Despite the sample size being determined using an a priori power calculation, it may have been too small to detect effects in this population of participants, necessitating further studies examining particularly the interactive hypotheses in larger samples. Further, the sample mainly consisted of women with a high educational status, limiting the generalizability of findings. In addition, it is important to note that our exposure-based CBT approach deviated from Müller et al. (2011) in that we included an in vivo exposure to a dead spider and psychoeducation in addition to the CBE. Thus, we cannot conclude that hydrocortisone administration may be equally effective when solely applying the computer session. With regard to salivary GC analyses, it is important to note that we did not objectively verify compliance of the sampling protocol in the morning hours. While participants’ awareness of electronic monitoring of salivary sampling has been observed to result in improved compliance (Kudielka et al., 2003), we cannot exclude whether salivary GC data were confounded due to non-adherence to the fixed time sampling protocols. This should also be considered when interpreting the significant pre-treatment differences with regard to home-based salivary cortisone data. However, given that these group differences did not emerge with respect to salivary cortisol, this possibility seems rather unlikely. Nevertheless, further studies assessing the GC awakening response with improved correspondence to methodological guidelines (Stalder et al., 2016) are needed to exclude variability caused by non-compliance issues. Importantly, hair cortisol data, which are considered to be a more reliable marker of long-term cortisol secretion (i.e., not affected by non-adherence), showed a similar pattern of main findings. Another limitation is that we can draw no conclusion on the exact mechanism underlying the effect of hydrocortisone-enhanced CBT. Besides the assumption that GCs facilitate learning and memory by binding to GC and MC receptors located in limbic and frontal brain areas that underlie these information processes (De Quervain et al., 2017; Krugers et al., 2011), a possible interaction between CBT and GC receptors and/or MC receptors should also be considered.
To conclude, the current study replicates previous findings highlighting GC administration as an effective adjunct to exposure-based CBT even with a low-level therapist involvement. Despite the use of hair steroid analysis, which provides considerable advancement for the assessment of long-term integrated GC secretion, no predictive value of pre-treatment endogenous GCs for CBT response or the efficacy of hydrocortisone-enhanced CBT emerged. Interestingly, our explorative analyses revealed that higher baseline hair cortisone levels were tentatively predictive of a stronger reduction in threat bias. However, GC administration did not result in a greater reduction in threat bias and no predictive value of threat bias change for clinical symptom recovery emerged. Future studies integrating approaches from biopsychology and neuroscience (e.g., Nakataki et al., 2017) are needed to corroborate these findings in larger, clinical samples. The investigation of such underpinnings of successful treatment of anxiety disorders may help to develop more effective, economic and personalized treatment formats.
Acknowledgments
We thank Nils Kappelmann and Caroline Nettekoven for help with data collection, and Mike Browning for medical supervision.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by a research grant from the Oxfordshire Health Services Research Committee awarded to AR (OHSRC 1021), and it was supported by the NIHR Oxford Health Biomedical Research Centre. AR is funded by a fellowship from MQ: Transforming Mental Health. SSS was funded by a visiting fellowship from the German Research Foundation (STE 2399/1-1) and is currently funded by a habilitation fellowship for women from the Faculty of Medicine Carl Gustav Carus, Technische Universität Dresden. LC is funded by the NIHR Oxford Health Biomedical Research Centre. The funders had no role in the design of the study, data collection or interpretation of results. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health.
ORCID iDs: Susann Steudte-Schmiedgen  https://orcid.org/0000-0002-1171-7133
https://orcid.org/0000-0002-1171-7133
Emily Fay  https://orcid.org/0000-0002-0133-8420
https://orcid.org/0000-0002-0133-8420
Liliana Capitao  https://orcid.org/0000-0003-3117-5156
https://orcid.org/0000-0003-3117-5156
References
- Ali S, Rhodes L, Moreea O, et al. (2017) How durable is the effect of low intensity CBT for depression and anxiety? Remission and relapse in a longitudinal cohort study. Behav Res Ther 94: 1–8. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. (1996) Beck depression inventory-II. San Antonio 78: 490–498. [Google Scholar]
- Bentz D, Michael T, De Quervain DJ, et al. (2010) Enhancing exposure therapy for anxiety disorders with glucocorticoids: From basic mechanisms of emotional learning to clinical applications. J Anxiety Disord 24: 223–230. [DOI] [PubMed] [Google Scholar]
- Calamaras MR, Tone EB, Anderson PL. (2012) A pilot study of attention bias subtypes: Examining their relation to cognitive bias and their change following cognitive behavioral therapy. J Clin Psychol 68: 745–754. [DOI] [PubMed] [Google Scholar]
- Carpenter JK, Andrews LA, Witcraft SM, et al. (2018) Cognitive behavioral therapy for anxiety and related disorders: A meta-analysis of randomized placebo-controlled trials. Depress Anxiety 35: 502–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. (1983) A global measure of perceived stress. J Health Soc Behav 24: 385–396. [PubMed] [Google Scholar]
- De Quervain DJ, Bentz D, Michael T, et al. (2011) Glucocorticoids enhance extinction-based psychotherapy. Proc Natl Acad Sci U S A 108: 6621–6625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Quervain DJ, Roozendaal B, McGaugh JL. (1998) Stress and glucocorticoids impair retrieval of long-term spatial memory. Nature 394: 787–790. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ, Schwabe L, Roozendaal B. (2017) Stress, glucocorticoids and memory: Implications for treating fear-related disorders. Nat Rev Neurosci 18: 7–19. [DOI] [PubMed] [Google Scholar]
- De Quervain DJ, Wolf OT, Roozendaal B. (2019) Glucocorticoid-induced enhancement of extinction-from animal models to clinical trials. Psychopharmacology (Berl) 236: 183–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dockray S, Bhattacharyya MR, Molloy GJ, et al. (2008) The cortisol awakening response in relation to objective and subjective measures of waking in the morning. Psychoneuroendocrinology 33: 77–82. [DOI] [PubMed] [Google Scholar]
- Elnazer HY, Baldwin DS. (2014) Investigation of cortisol levels in patients with anxiety disorders: A structured review. Curr Top Behav Neurosci 18: 191–216. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Salem D, Swift JK, et al. (2015) Meta-analysis of dropout from cognitive behavioral therapy: Magnitude, timing, and moderators. J Consult Clin Psychol 83: 1108–1122. [DOI] [PubMed] [Google Scholar]
- First MB, Williams JBW, Karg RS, et al. (2015) Structured Clinical Interview for DSM-5—Research Version (SCID-5 for DSM-5, Research Version; SCID-5-RV). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Fischer S, Cleare AJ. (2017) Cortisol as a predictor of psychological therapy response in anxiety disorders – Systematic review and meta-analysis. J Anxiety Disord 47: 60–68. [DOI] [PubMed] [Google Scholar]
- Fischer S, King S, Papadopoulos A, et al. (2018) Hair cortisol and childhood trauma predict psychological therapy response in depression and anxiety disorders. Acta Psychiatr Scand 138: 526–535. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Kirschbaum C. (2015) Quantitative analysis of estradiol and six other steroid hormones in human saliva using a high throughput liquid chromatography-tandem mass spectrometry assay. Talanta 143: 353–358. [DOI] [PubMed] [Google Scholar]
- Gao W, Stalder T, Foley P, et al. (2013) Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J Chromatogr B 928: 1–8. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Smits JA. (2008) Cognitive-behavioral therapy for adult anxiety disorders: A meta-analysis of randomized placebo-controlled trials. J Clin Psychiatry 69: 621–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krugers HJ, Zhou M, Joels M, et al. (2011) Regulation of excitatory synapses and fearful memories by stress hormones. Front Behav Neurosci 5: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. (2003) Compliance with saliva sampling protocols: Electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med 65: 313–319. [DOI] [PubMed] [Google Scholar]
- Lass-Hennemann J, Michael T. (2014) Endogenous cortisol levels influence exposure therapy in spider phobia. Behav Res Ther 60: 39–45. [DOI] [PubMed] [Google Scholar]
- Meuret AE, Trueba AF, Abelson JL, et al. (2015) High cortisol awakening response and cortisol levels moderate exposure-based psychotherapy success. Psychoneuroendocrinology 51: 331–340. [DOI] [PubMed] [Google Scholar]
- Miller R, Plessow F. (2013) Transformation techniques for cross-sectional and longitudinal endocrine data: Application to salivary cortisol concentrations. Psychoneuroendocrinology 38: 941–946. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Millar N, et al. (1995) A follow-up study of cognitive bias in generalized anxiety disorder. Behav Res Ther 33: 927–935. [DOI] [PubMed] [Google Scholar]
- Müller BH, Kull S, Wilhelm FH, et al. (2011) One-session computer-based exposure treatment for spider-fearful individuals–efficacy of a minimal self-help intervention in a randomised controlled trial. J Behav Ther Exp Psychiatry 42: 179–184. [DOI] [PubMed] [Google Scholar]
- Nakataki M, Soravia LM, Schwab S, et al. (2017) Glucocorticoid administration improves aberrant fear-processing networks in spider phobia. Neuropsychopharmacology 42: 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okun ML, Krafty RT, Buysse DJ, et al. (2010) What constitutes too long of a delay? Determining the cortisol awakening response (CAR) using self-report and PSG-assessed wake time. Psychoneuroendocrinology 35: 460–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perogamvros I, Keevil BG, Ray DW, et al. (2010) Salivary cortisone is a potential biomarker for serum free cortisol. J Clin Endocrinol Metab 95: 4951–4958. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, et al. (2003) Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology 28: 916–931. [DOI] [PubMed] [Google Scholar]
- Putman P, Hermans EJ, Koppeschaar H, et al. (2007) A single administration of cortisol acutely reduces preconscious attention for fear in anxious young men. Psychoneuroendocrinology 32: 793–802. [DOI] [PubMed] [Google Scholar]
- Reinecke A, Harmer CJ. (2015) A cognitive-neuropsychological account of treatment action in anxiety: Can we augment clinical efficacy? Psychopathol Rev 3: 77–109. [Google Scholar]
- Reinecke A, Becker ES, Rinck M. (2010) Visual working memory and threat monitoring: Spider fearfuls show disorder-specific change detection. Behav Res Ther 48: 770–778. [DOI] [PubMed] [Google Scholar]
- Reinecke A, Rinck M, Becker ES, et al. (2013. a) Cognitive-behavior therapy resolves implicit fear associations in generalized anxiety disorder. Behav Res Ther 51: 15–23. [DOI] [PubMed] [Google Scholar]
- Reinecke A, Soltau C, Hoyer J, et al. (2012) Treatment sensitivity of implicit threat evaluation, avoidance tendency and visual working memory bias in specific phobia. J Anxiety Disord 26: 321–328. [DOI] [PubMed] [Google Scholar]
- Reinecke A, Waldenmaier L, Cooper MJ, et al. (2013. b) Changes in automatic threat processing precede and predict clinical changes with exposure-based cognitive-behavior therapy for panic disorder. Biol Psychiatry 73: 1064–1070. [DOI] [PubMed] [Google Scholar]
- Rinck M, Bundschuh S, Engler S, et al. (2002) Reliabilität und validität dreier instrumente zur messung von angst vor spinnen. Diagnostica 48: 141–149. [Google Scholar]
- Schäfer JL. (1997) Analysis of Incomplete Multivariate Data. New York: Chapman and Hall/CRC. [Google Scholar]
- Siegmund A, Koster L, Meves AM, et al. (2011) Stress hormones during flooding therapy and their relationship to therapy outcome in patients with panic disorder and agoraphobia. J Psychiatr Res 45: 339–346. [DOI] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Aerni A, et al. (2006) Glucocorticoids reduce phobic fear in humans. Proc Natl Acad Sci U S A 103: 5585–5590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soravia LM, Heinrichs M, Winzeler L, et al. (2014) Glucocorticoids enhance in vivo exposure-based therapy of spider phobia. Depress Anxiety 31: 429–435. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. (1989) State-Trait Anxiety Inventory: A Comprehensive Bibliography, 2nd edn. Palo Alto, CA: Consulting Psychologists Press. [Google Scholar]
- Stalder T, Kirschbaum C, Alexander N, et al. (2013) Cortisol in hair and the metabolic syndrome. J Clin Endocrinol Metab 98: 2573–2580. [DOI] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, et al. (2016) Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology 63: 414–432. [DOI] [PubMed] [Google Scholar]
- Stalder T, Steudte-Schmiedgen S, Alexander N, et al. (2017) Stress-related and basic determinants of hair cortisol in humans: A meta-analysis. Psychoneuroendocrinology 77: 261–274. [DOI] [PubMed] [Google Scholar]
- Szymanski J, O’Donohue W. (1995) Fear of spiders questionnaire. J Behav Ther Exp Psychiatry 26: 31–34. [DOI] [PubMed] [Google Scholar]
- Taylor S, Abramowitz JS, McKay D. (2012) Non-adherence and non-response in the treatment of anxiety disorders. J Anxiety Disord 26: 583–589. [DOI] [PubMed] [Google Scholar]
- Van den Hout M, Tenney N, Huygens K, et al. (1997) Preconscious processing bias in specific phobia. Behav Res Ther 35: 29–34. [DOI] [PubMed] [Google Scholar]
- Van Peer JM, Spinhoven P, Roelofs K. (2010) Psychophysiological evidence for cortisol-induced reduction in early bias for implicit social threat in social phobia. Psychoneuroendocrinology 35: 21–32. [DOI] [PubMed] [Google Scholar]
- Van Stegeren AH, Wolf OT, Everaerd W, et al. (2007) Endogenous cortisol level interacts with noradrenergic activation in the human amygdala. Neurobiol Learn Mem 87: 57–66. [DOI] [PubMed] [Google Scholar]
- Weitzman ED, Fukushima D, Nogeire C, et al. (1971) Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab 33: 14–22. [DOI] [PubMed] [Google Scholar]
- Wennig R. (2000) Potential problems with the interpretation of hair analysis results. Forensic Sci Int 107: 5–12. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Bierer LM, Pratchett LC, et al. (2014) Cortisol augmentation of a psychological treatment for warfighters with posttraumatic stress disorder: Randomized trial showing improved treatment retention and outcome. Psychoneuroendocrinology 51: 589–597. [DOI] [PubMed] [Google Scholar]