Abstract
Studies have shown that prenatal stress can negatively impact neurodevelopment, but little is known about its effect on early cognitive development. We assessed the impact of prenatal stress on cognition in 152 7.5-month-old infants using Cohen's Perceived Stress Scale (PSS), maternal telomere length (MTL), and a Stressful Life Events (SLE) Scale. A visual recognition memory task consisting of nine blocks, each with one familiarization trial (two identical stimuli) followed by two test trials (one familiar stimulus, one novel), was administered. Outcomes assessed included: average time looking at stimuli (measure: processing speed), time to reach looking time criterion (measure: attention), and the proportion of time looking at the novel stimulus (measure: recognition memory). We examined the association of each stress measure with each outcome adjusted for infant age and sex, which of the two stimuli in each set was novel, household income, and maternal age, education, and IQ. Higher prenatal stress was associated with shorter looking durations [PSS (β = −1.6, 95% CI: −2.5, −0.58); SLE (β = 0.58, 95% CI: −0.08, 1.24); MTL (β = 1.81, 95% CI: 0.18, 3.44)] and longer time to reach criterion [PSS (β = 9.1, 95% CI: 1.6, 16.6); SLE (β = 12.2, 95% CI: 1.9, 24.1); MTL (β = −23.1, 95% CI: −45.3, −0.9)], suggesting that higher prenatal stress is associated with decreased visual attention in infancy.
Keywords: birth cohort, cognition, neurodevelopment, prenatal maternal stress, telomere length, visual attention
1 |. INTRODUCTION
Stress is an environmental factor that can be measured in multiple ways—using objective measures (e.g., number or type of Stressful Life Events [SLE] in a certain period), subjective measures (a person's perception of their stress level), and biological measures (biomarkers of the stress response). Telomere length (TL) has been identified as a biomarker of longer-term chronic stress over the mother's lifetime, with shortening likely happening as a result of oxidative stress (Feiler et al., 2018; Mathur et al., 2016; Oliveira et al., 2016; Wojcicki et al., 2015).There is evidence to suggest that all these indicators of maternal stress are associated with neurodevelopment (Barrett et al., 2013, 2014; Dipietro, 2012; Kingston et al., 2015; Laplante et al., 2004; Vehmeijer et al., 2018; Zhu et al., 2014). Previous studies on stress have relied largely on global measures of cognition, like the Bayley Scales of Infant Development (BSID), rather than measures of specific cognitive domains. One concern with this approach is that the BSID has been shown to be a generally poor predictor of long-term cognitive outcomes (Anderson & Burnett, 2017; Anderson et al., 2010; McCall & Carriger, 1993). Research in developmental psychology has shown that infant looking behaviors can be used as reliable and stable measures of basic cognitive processes such as memory, visual attention, and information processing (Fagan et al., 2007; Marino & Gervain, 2019; McCall & Carriger, 1993; Pascalis & de Haan, 2003; Rose & Feldman, 1995; Rose et al., 2009). Importantly, these measures have been shown to be predictive of cognitive abilities later in childhood (Rose & Feldman, 1995, 1997; Rose et al., 2001) and tend to be stable within individuals across time (Aslin, 2007; Breeman et al., 2015; Linsell et al., 2018; Mangin et al., 2017). Thus, these measures may serve to identify adverse impacts on neurodevelopment.
Recently, our research group used eye-tracking technology to develop an automated visual recognition memory (VRM) task (Dzwilewski et al., 2020), based on Susan Rose's seminal work in 2001. The goal of this research was to characterize the association between multiple measures of prenatal maternal stress and multiple aspects of cognition including recognition memory, visual attention, and information processing speed as assessed by the VRM task.
2 |. METHODS
2.1 |. Study population
The mother–infant pairs included in this study are part of the Illinois Kids Development Study (IKIDS), an on-going prospective pregnancy and birth cohort study. Pregnant women were recruited for participation from two obstetric clinics in Urbana–Champaign, IL from December 2013 to January 2019. During their first prenatal visit, women received a brochure with information about the study, and they filled out a card to indicate whether they were interested in learning more about the study. Participant eligibility criteria and the enrollment process have been previously described (Dzwilewski et al., 2020; Merced-Nieves et al., 2020). Researchers met periodically with the women during pregnancy and after birth to collect key demographic, lifestyle, health, and diet information. Infants included were considered full-term if they were ≥37 weeks gestation. The infant’s cognitive assessment was completed at 7–8 months of age.
2.2 |. Prenatal stress
Three measures of prenatal maternal stress were used—a measure of perceived stress, a SLE scale, and TL in maternal blood. Pregnant women completed the 10-item Perceived Stress Scale (PSS; Cohen & Williamson, 1988) two times during pregnancy (8–14 and 34–37 weeks of gestation). The PSS is a well-validated scale that evaluates how stressful respondents believe their lives were during the previous month. As described previously (Merced-Nieves et al., 2020), very few women in the IKIDS cohort met the criteria of “high stress” under the PSS standard cut-offs. Hence, median scores at each time point for women included in this analysis were used to classify the women into three stress categories: low (scored below the median at both time points), medium (scored above the median at one of the two time points), and high (scored above the median at both time points) instead of using the published cut-offs. At birth, women also completed a brief SLE scale based on the one developed by Barrett et al. (2014) that asked about potential SLE experienced during pregnancy. The SLE Scale is a six-item questionnaire that asks whether each of six possible major SLE (i.e., loss of job, death in the family) has occurred during pregnancy. SLEs were relatively rare in this sample, thus women were dichotomized to those who reported no SLEs (0) and those who reported one or more SLEs (1+) during pregnancy.
Finally, TL was assessed in maternal blood collected at 16–18 weeks of pregnancy. Relative TL was measured by quantitative polymerase chain reaction (qPCR), expressed as the ratio of telomere to single-copy gene abundance (T/S ratio; Cawthon, 2002; Lin et al., 2010). Genomic DNA was extracted from whole blood stored at −80ºC using the QIAamp DNA Mini Kit (QIAGEN). DNA quantity and quality were assessed using a NanoDrop 2000c Spectrophotometer (Nanodrop Products). The DNA quality control criteria were an OD260/OD280 between 1.7 and 2.0 and concentrations greater than 10 ng/μl. All samples passed quality control. DNA was stored at −80ºC for batch TL analysis. Specifics of the qPCR condition are described in detail previously (Lin et al., 2010). All samples used the same lot of reagents for TL assay. All DNA samples from this study used the same lot of DNA extraction kits. The assays were run from DNA source plates of 96 samples per batch. To account for batch differences, we ran a combined batch with a subset of samples from each 96-well source plate and adjusted each 96-well plate, accordingly. The average inter-assay coefficient of variation for this study was 2.1%.
2.3 |. Visual recognition memory
Infants were assessed using a computerized paired-comparison VRM task based on the one developed by Rose et al. (1992). The testing methods and task were described in detail in a recent publication (Dzwilewski et al., 2020). Briefly, study infants were assessed at 224 to 259 days of age while seated on a parent’s lap in front of a large screen high definition television. Hereafter, this is referred to as the infant’s 7.5-month visit. The testing area was surrounded by black curtains to reduce distractions. In addition, to prevent their behavior or reactions from influencing the infant’s behavior, mothers were asked to remain as neutral as possible in their demeanor and avoid looking at the stimuli by looking downward during testing. Infant looking behaviors were tracked using an EyeLink 1000 Plus infrared eye tracker, and data from the eye-tracking system were reduced and extracted using DataViewer software (SR Research Ltd.). The eye tracker was calibrated at the start of each test session as previously described (Dzwilewski et al., 2020).
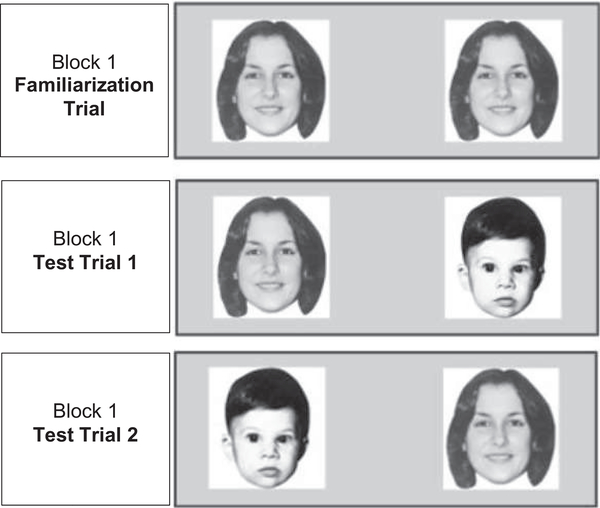
The task consisted of nine blocks of three trials each—one familiarization trial followed by two test trials (Figure 1). The first five blocks displayed black-and-white photographs of human faces and the last four blocks displayed colored two-dimensional images of geometrical shapes. During the familiarization trial, the infant was shown two identical stimuli side-by-side. The face stimuli remained until the infant had accumulated 20 s looking at them. The geometrical shape stimuli remained until the infant had accumulated 10 s of looking at them. During each test trial, the face or shape seen in the familiarization trial was paired with a novel stimulus of the same type. The novel stimulus was presented on one side during the first test trial and the opposite side during the second test trial. Novelty preference in the first and second test trials of each block was averaged for analysis to ensure that any looking preferences were stimulus-based rather than due to a side preference. During test trials, infants were required to accumulate 1 s looking at the stimuli, and then the stimuli remained for an additional 5 s regardless of infant looking.
FIGURE 1.
Example of stimulus displayed across block 1 of the visual recognition memory task. Re-printed with permission from Dzwilewski et al. (2020). Characterization of performance on an automated visual recognition memory task in 7.5-month-old infants
Face and shape data were analyzed separately. Infants were only included in the statistical analyses if they completed the full set of five blocks of human faces or the full set of four blocks of geometric shapes. For the shapes, the third block of trials was excluded from the analyses because previous work (Dzwilewski et al., 2020) showed that infants had a strong preference for one of the two stimuli (pink and blue stimulus; see Figure S1). Each infant was assessed in one of four conditions. In conditions A and B, the same stimuli were familiar, and these conditions were considered set 1; in conditions C and D, the other stimulus in each stimulus pair was the familiar stimulus, and these conditions were considered set 2. In conditions A and C, the novel stimulus was initially presented on the right side (test trial 1) and then on the left (test trial 2); whereas, in conditions B and D, the novel stimulus was presented on the left side first and then the right. Dzwilewski et al. (2020) showed that averaging across the two test trials for each block eliminates the need to account for which side the novel image appeared on first. However, which stimulus set was familiar impacted outcome measures assessed and thus does need to be included in statistical models.
For this study, we focused on three looking time outcomes. Average run duration during the familiarization trials, defined as the average time the infant spent looking at a stimulus before looking away, was used to measure information processing speed. The time to reach familiarization criterion, defined as the time the infant took to accumulate a set number of seconds looking at the familiarization stimuli, was used as a measure of visual attention, as off-task behavior prolonged time to reach criterion. The proportion of looking time the infant spent looking at a novel stimulus during the test trials, novelty preference, was used to measure recognition memory.
2.4 |. Covariates
Covariate data collected during a visit to the participant’s home (at 8–14 weeks of gestation) were used in this analysis and included information about key demographic, lifestyle, health, and diet variables collected via questionnaire and structured interviews. Maternal covariates included age, education, and household income. Maternal verbal IQ was assessed using the Peabody Picture Vocabulary Test, 4th edition (Dunn & Dunn, 2007), administered during a follow-up assessment during the child's first year of life. Additional covariates collected during study visits included maternal smoking and alcohol consumption; for the current analyses, the data available were for the first trimester of pregnancy. All infants included in the current analyses were full-term (≥37 weeks gestation). Both condition and infant sex, which was obtained at the time of birth, were considered as potential modifiers.
2.5 |. Statistical analysis
All statistical analyses were performed using SAS 9.4 software (SAS Institute Inc.). General linear models were used to examine the association of each prenatal maternal stress measure (PSS, SLE, TL) with performance on the VRM task (i.e., average run duration, time to reach familiarization, and novelty preference). Based on previous findings, all possible two-way and three-way interactions between infants’ sex, stress, and which set of stimuli was novel (set 1 vs. set 2) were assessed for each stress exposure-outcome model. For models where an interaction was found to have a p-value > .15, the interaction was removed from the model, sex and stimulus set were retained as covariates, and main effects of the stress measure on the outcome across both sexes and stimulus sets were assessed. For models where a three-way interaction was found to have a p-value < .15, models were stratified by sex and stimulus set. For models where a two-way interaction was found to have a p-value < .15, models were stratified by sex or stimulus set as appropriate.
A priori knowledge and a directed acyclic graph (Hernan et al., 2000) were used to select the covariates included in the models. Socioeconomic indicators (maternal education, household income, mother's age at birth) were identified as potential confounders for inclusion in models. Maternal education was dichotomized to college educated versus not college educated. Household income was dichotomized at less than $60,000 per year versus $60,000 per year or more. Infant’s age at exam and which set of stimuli was novel (set 1 vs. set 2) were also included in the models because they are known to be correlated with performance on the task. Regression diagnostics were performed to identify influential data points, defined as observations that had extreme Cook's Distance (D < 0.1), which were removed from the models.
A number of sensitivity analyses were performed to assess the robustness of any observed associations by considering adjustment for additional covariates. Among the women in our final analyses, there were few smokers and relatively few women reporting alcohol consumption. We assessed potential confounding by smoking or alcohol in secondary analyses removing women who reported any smoking or adjusting for first-trimester alcohol intake by including it in the models as a cofounder. Lastly, because of the correlation between pre- and postnatal perceived stress (rs = .57–.60), adjustment for postnatal stress was also done as a sensitivity analysis in PSS models.
3 |. RESULTS
3.1 |. Descriptive data
As of June 2019, there were available data on both cognitive outcomes and perceived stress for 152 mother–child dyads, SLE for 152 dyads, and maternal telomere length (MTL) for 132 dyads. Each adjusted model had a different number of observations due to incomplete covariate data (Table 1). At the time of enrollment, more than 80% of the mothers had at least a college degree and about 70% had an annual household income of $60,000 per year or greater. Maternal smoking and alcohol consumption data were only available for the first trimester. Only 4% of the subsample included in these analyses reported any smoking during the first trimester, and about 30% reported any alcohol consumption. However, alcohol consumption included recollections from weeks 0 to 14; thus, some of the drinking reported was before knowledge of the pregnancy. In addition, most women reported less than four drinks per week on average. Infants in these analyses had an average gestational age of 39 weeks, and about 20% were delivered via cesarean section. As shown in Table 1, the demographics of the mother–child dyads in these analyses are similar to those of the full cohort.
TABLE 1.
Demographic and lifestyle characteristics of IKIDS cohort and mother–infant pairs included in analyses
| IKIDS participants with infants enrolled (n = 476) |
Perceived Stress Scale analyses (n = 152) |
Stressful Life Events analyses (n = 152) |
Maternal blood TL analyses (n = 132) |
|||||
|---|---|---|---|---|---|---|---|---|
| N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | N (%) | Mean (SD) | |
| Maternal race | ||||||||
| White, non-Hispanic | 385 (80.9) | 132 (87) | 133 (87) | 115 (87) | ||||
| Othera | 90 (18.9) | 20 (13) | 19 (13) | 17 (13) | ||||
| Maternal age (years) | 30.3 (4.1) | 30.6 (3.8) | 30.7 (3.8) | 30.7 (3.8) | ||||
| Maternal education | ||||||||
| Some college or less | 88 (18.5) | 21 (14) | 21 (14) | 19 (14) | ||||
| College degree or higher | 388 (81.5) | 131 (86) | 131 (86) | 113 (86) | ||||
| Annual household income | ||||||||
| <$60,000 | 136 (29) | 42 (28) | 42 (28) | 39 (30) | ||||
| ≥$60,000 | 340 (71) | 110 (72) | 110 (72) | 93 (70) | ||||
| Maternal smoking (first trimester) | 24 (5) | 6 (4) | 6 (4) | 5 (4) | ||||
| Maternal drinking (first trimester) | 141 (30) | 47 (31) | 48 (32) | 38 (29) | ||||
| Cesarean mode of delivery | 122 (26) | 36 (24) | 37 (25) | 27 (21) | ||||
| Gestational age (weeks) | 39.3 (1.5) | 39.5 (1.2) | 39.5 (1.2) | 39.6 (1.2) | ||||
| Infant sex | ||||||||
| Female | 242 (50.8) | 79 (52) | 80 (53) | 67 (51) | ||||
| Male | 234 (49.2) | 73 (48) | 72 (47) | 65 (49) | ||||
Maternal race for the Illinois Kids Development Study (IKIDS) cohort had a missing observation.
3.2 |. Maternal stress
The median maternal PSS score at 8–14 weeks was 11 with a range from 0 to 26; the median at 34–37 weeks of gestation was 10 with a range from 0 to 32. Prenatal stress scores at the two time points were moderately correlated (rs = .60). The median postnatal PSS score (survey administered at the 7.5-month visit) was 9 with a range from 0 to 27. Prenatal stress scores at both time points were moderately correlated with the postnatal stress scores (rs = .57 and .60, respectively). A total of 54 (36%) of the women reported at least one SLE during pregnancy. The median number of SLEs reported was 0 with a range of 0 to 4. The PSS scores and SLE were significant but weakly correlated (rs = .18720; p-value = <.0001 and rs = .16243; p-value = .0007, respectively). The average maternal TL was 0.88 T/S with a range of 0.61 to 1.28 T/S. Maternal TL was not significantly correlated with either PSS or the SLE.
3.3 |. Associations of prenatal maternal stress with time to reach familiarization
3.3.1 |. Perceived Stress Scale
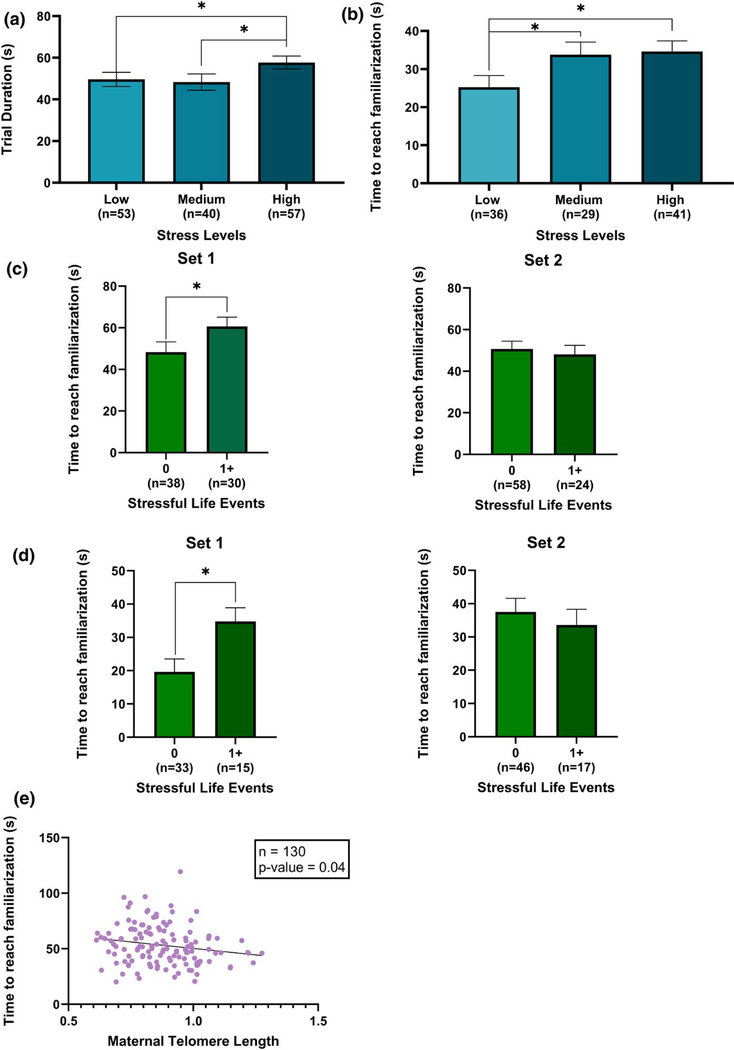
In models for time to reach familiarization, there was a main effect of perceived stress in both the face (p = .02) and shapes (p = .001) blocks. Infants whose mothers had high levels of perceived stress during pregnancy had longer time to reach familiarization than those whose mothers had low levels of perceived stress (β = 9.1; 95% CI: 1.6, 16.6 s; p-value = .02), whereas those whose mothers had medium levels of perceived stress did not differ from those with low stress (Table 2). Post hoc comparisons revealed that infants whose mothers had high levels of perceived stress during pregnancy also had longer time to reach familiarization than those whose mothers had medium levels of perceived stress (β = 9.3; 95% CI: 1.2, 17.5 s; p-value = .03) in the faces blocks. In the shapes blocks, infants whose mothers had high and medium levels of perceived stress had longer time to reach familiarization than those whose mothers had low levels of perceived stress (β = 9.4; 95% CI: 3.2, 15.7 s; p-value = .003 and β = 8.3; 95% CI: 1.6, 15.1 s; p-value = .02; Table 2). The covariate-adjusted mean ± SE time to reach familiarization for infants in the faces blocks was 48.6 ± 3.4 in the low stress group (n = 53), 48.3 ± 3.9 in the medium stress group (n = 40), and 57.7 ± 3.1 in the high stress group (n = 53; Figure 2a). The covariate-adjusted mean ± SE time to reach familiarization for infants in the shapes blocks was 25.2 ± 3.1 in the low stress group (n = 36), 33.6 ± 3.3 in the medium stress group (n = 29), and 34.6 ± 2.8 in the high stress group (n = 41; Figure 2b).
TABLE 2.
Findings for examining the association between stress measures and cognitive outcomes
| Main effect |
||||
|---|---|---|---|---|
| Outcome measure | Stress measure | p-Value | β estimate | 95% confidence interval |
| Perceived Stress Scale | ||||
| Trial duration (faces) | Low | Ref | ||
| Medium | .96 | −0.22 | −8.6, 8.1 | |
| High | .02 | 9.1 | 1.6, 16.6 | |
| Trial duration (shapes) | Low | Ref | ||
| Medium | .02 | 8.4 | 1.6, 15.1 | |
| High | .003 | 9.4 | 3.2, 15.7 | |
| Average run duration (faces) | Low | Ref | ||
| Medium | .23 | −0.53 | −1.4, 0.33 | |
| High | .04 | −0.83 | −1.6, −0.06 | |
| Average run duration (shapes) | Low | Ref | ||
| Medium | .32 | −0.39 | −1.1, 0.40 | |
| High | .19 | −0.47 | −1.2, 0.25 | |
| Novelty preferences (faces) | Low | Ref | ||
| Medium | .96 | −0.0007 | −0.03, 0.03 | |
| High | .6 | 0.007 | −0.02, 0.03 | |
| Novelty preferences (shapes) | Low | Ref | ||
| Medium | .58 | −0.02 | −0.07, 0.04 | |
| High | .29 | 0.03 | −0.02, 0.08 | |
| Stressful Life Events | ||||
| Trial duration (faces) | 0 | Ref | ||
| 1+ | .11 | 5.6 | −1.3, 12.5 | |
| Trial duration (shapes) | 0 | Ref | ||
| 1+ | .24 | 4 | −2.8, 10.8 | |
| Average run duration (faces) | 0 | Ref | ||
| 1+ | .14 | −0.51 | −1.2, 0.17 | |
| Average run duration (shapes) | 0 | Ref | ||
| 1+ | .08 | −0.58 | −1.2, 0.08 | |
| Novelty preferences (faces) | 0 | Ref | ||
| 1+ | .85 | −0.002 | −0.02, 0.02 | |
| Novelty preferences (shapes) | 0 | Ref | ||
| 1+ | .63 | 0.01 | −0.04, 0.06 | |
| Trial duration (faces) | .04 | −23.1 | −45.3, −0.86 | |
| Trial duration (shapes) | .5 | −7.66 | −30.1, 14.8 | |
| Average run duration (faces) | Maternal telomere length | .23 | 1.46 | −0.93, 3.8 |
| Average run duration (shapes) | .56 | 0.65 | −1.6, 1.9 | |
| Novelty preferences (faces) | .07 | −0.07 | −0.15, 0.01 | |
| Novelty preferences (shapes) | .15 | −0.16 | −0.38. 0.06 | |
Note: Bold were those that reached significance p < .05 and italic were those p < .10. Main effect results are presented here, stratified models are presented in text and graphs.
FIGURE 2.
(a) Association between maternal perceived stress and time to reach familiarization face blocks. Infants’ in the high stress group had longer time to reach familiarization than those in the low stress (*p-value = .02) and the medium stress (*p-value = .03) groups. (b) Association between maternal perceived stress and time to reach familiarization in shapes blocks. Infants’ in the high and medium stress groups had longer time to reach familiarization than those in the low stress group (*p-value = .003 and .02, respectively). (c) Association of stressful live events with time to reach familiarization in faces blocks. There was a significant difference the infants whose mothers didn’t experience stressful life events during pregnancy and those who did in set 1 (*p-value = .02), there wasn’t a significant difference in set 2. (d) Association of stressful live events with time to reach familiarization in shapes blocks. There was a significant difference the infants whose mothers didn’t experience stressful life events during pregnancy and those who did in set 1 (*p-value = .001), there wasn’t a significant difference in set 2. (d) Association of stressful live events with time to reach familiarization in shapes blocks. There was a significant difference the infants whose mothers didn’t experience stressful life events during pregnancy and those who did in set 1 (*p-value = .001), there wasn’t a significant difference in set 2. (e) Association of maternal telomere length with time to reach familiarization in face blocks. Shorter maternal telomere length was associated with longer time to reach familiarization
3.3.2 |. Stressful Life Events
In models for time to reach familiarization, the interaction between SLEs and condition in both the face blocks (p-value = .0575) and the shapes blocks (p-value = .021) met our criteria to run models stratified by condition. In models stratified by condition, there was a significant association of SLEs with time to reach familiarization for set 1, but not set 2 for both faces (β = 12.2; 95% CI: 1.9, 22.5; p-value = .02) and shapes (β = 15.2; 95% CI: 6.2, 24.1; p-value = .001), in which infants whose mothers reported SLEs during pregnancy had longer time to reach familiarization than those whose mothers did not experience any SLEs during pregnancy (Table 2). The covariate-adjusted mean ± SE time to reach familiarization for infants in set 1 (faces blocks) was 48.3 ± 4.9 in the no SLE group (n = 38) and 60.6 ± 4.5 in the at least one SLE group (n = 30; Figure 2c). The covariate-adjusted mean ± SE time to reach familiarization for infants who saw set 1 (shapes blocks) was 19.6 ± 3.9 in the no SLE group (n = 33) and 34.8 ± 4.1 in the at least one SLE group (n = 15; Figure 2d).
3.3.3 |. Maternal telomere length
In models for time to reach familiarization, there was a main effect of maternal TL on time to reach familiarization in the face blocks (β = −23.1; 95% CI: −45.3, −0.9 s; p-value = .04). Shorter maternal TL was associated with longer time to reach familiarization (Figure 2e). There was no significant association between maternal TL and time to reach familiarization in shapes blocks (Table 2).
3.4 |. Associations of prenatal maternal stress with average run duration
3.4.1 |. Perceived Stress Scale
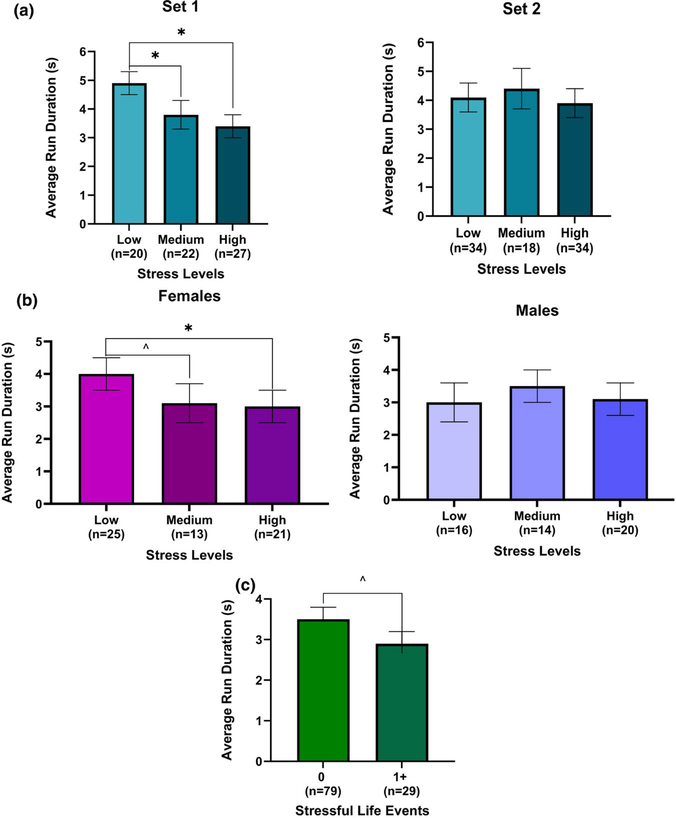
In models for average run duration (average time looking at stimuli before looking away) in the face blocks, the interaction between perceived stress and condition had a p-value of .126. In models stratified by condition, there was a significant association of perceived stress with average run duration in infants tested with set 1, but not those tested with set 2 (Figure 3a). Infants whose mothers had high and medium levels of perceived stress during pregnancy had significantly shorter average run durations than infants whose mothers had low levels of perceived stress (β = −1.6; 95% CI: −2.5, −0.58 s; p-value = .002 and β = −1.2; 95% CI: −2.2, −0.09 s; p-value = .03, respectively; Table 2). The covariate-adjusted mean ± SE average run duration in faces trials for set 1 was 4.9 ± 0.4 in the low stress group (n = 20), 3.8 ± 0.5 in the medium stress group (n = 22), and 3.4 ± 0.4 in the high stress group (n = 27; Figure 3a). In the shapes blocks the interaction between perceived stress and sex had a p-value of .12. In models stratified by sex, there was a significant association with perceived stress in females, but not males (Figure 3b). Females whose mothers had high levels of perceived stress during pregnancy had significantly shorter run durations than females whose mothers had low levels of perceived stress (β = −1.0; 95% CI: −1.9, −0.18 s; p-value = .02). There was also a trend for shorter run durations in females whose mothers had medium levels of perceived stress during pregnancy compared to females whose mothers had low levels of perceived stress (β = −.96; 95% CI: −1.9, 0.03 s; p-value = .06; Table 2). The covariate-adjusted mean ± SE run duration for females was 4.0 ± 0.5 in the low stress group (n = 25), 3.1 ± 0.6 in the medium stress group (n = 13), and 3.0 ± 0.5 in the high stress group (n = 21; Figure 3b).
FIGURE 3.
(a) Association of maternal perceived stress with average run duration in the face blocks in set 1 and 2. Infants in the high (*p-value = .002) and medium (*p-value = .03) stress group, in set 1, had significantly shorter run durations than those in the low stress group. (b) Association of maternal perceived stress with average run duration in shapes blocks in females and males. Females in the high stress group had significantly shorter run duration in the high stress group (*p-value = .02). There was a similar trend between females in the low stress group and the females in the medium stress group (^p-value = .06). (c) Association of stressful life events with average run duration in shapes blocks. Infants whose mothers had at least one stressful life events during pregnancy had shorter run duration than those who didn’t have any (^p-value = .08)
3.4.2 |. Stressful Life Events
In models for average run duration, there were no significant associations of SLEs with average run duration in the face blocks. There was an effect of SLEs on average run duration in the shapes blocks (Figure 3c). Infants whose mothers had one or more SLE during pregnancy had shorter average run duration than those whose mothers did not experience any SLEs during pregnancy (β = −0.58; 95% CI: −1.24, 0.08; p-value = .08; Table 2). The covariate-adjusted mean ± SE run duration for the shapes blocks was 3.5 ± 0.29 in the no SLE group (n = 79) and 2.9 ± 0.32 in the at least one SLE group (n = 32; Figure 3c).
3.4.3 |. Maternal telomere length
In models for average run duration, there was no significant association between maternal TL and run duration in either the faces or shapes blocks (Table 2).
3.5 |. Associations of prenatal maternal stress with novelty preference
3.5.1 |. Perceived Stress Scale
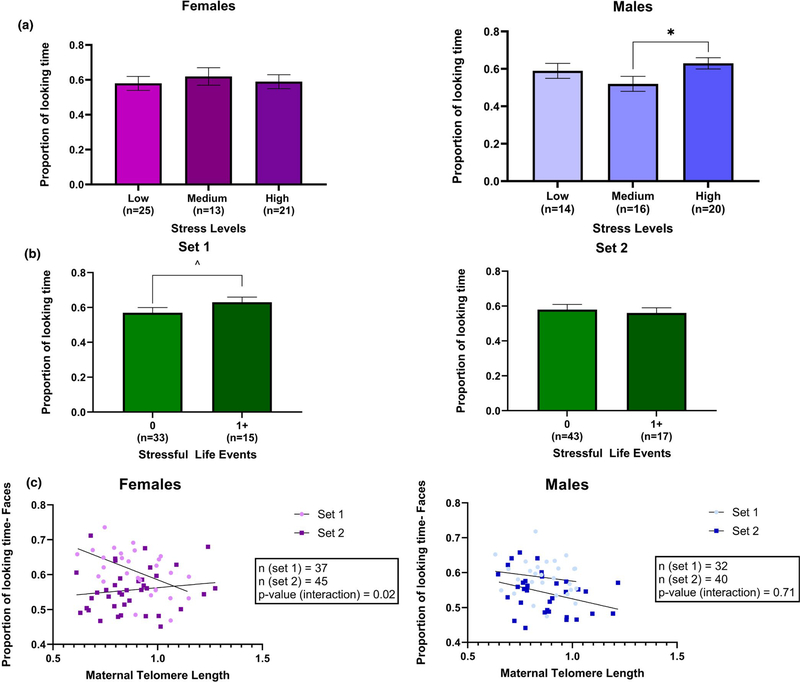
In models, there were no significant associations of perceived stress with novelty preference, the proportion of looking time at the novel versus familiar stimulus, in the face blocks. In the shapes blocks, the interaction between perceived stress and sex had a p-value of .07. In models stratified by sex, there was a significant association of perceived stress with novelty preference in males, but not females (Figure 4a). There was no statistically significant difference between males whose mothers had high levels of perceived stress and those whose mothers had low levels of perceived stress (Table 2). Post hoc comparisons revealed that males whose mothers had high levels of perceived stress during pregnancy had significantly greater proportion of looking time to the novel stimulus than males whose mothers had medium levels of perceived stress (β = 0.1; 95% CI: 0.02, 0.19; p-value = .001). The covariate-adjusted mean ± SE proportion of time looking at the novel stimulus for males was 0.59 ± 0.04 in the low stress group (n = 14), 0.52 ± 0.03 in the medium stress group (n = 16), and 0.63 ± 0.03 in the high stress group (n = 20; Figure 4a).
FIGURE 4.
(a) Association of maternal perceived stress with novelty preference in shapes blocks in females and males. Males in the high stress group had greater novelty preference than those in the medium stress group (*p-value = .001). (b) Association of stressful life event with novelty preference in shapes blocks in set 1 and 2. Infants whose mothers had at least one stressful life event had greater novelty preference than those who had none in set 1 (^p-value = .07). (c) Association of maternal telomere length with novelty preference in face blocks. Females, in set 1, whose mothers had shorter telomere length had greater novelty preference than those whose mothers had longer telomere length
3.5.2 |. Stressful Life Events
In models for novelty preference, there were no significant associations of SLEs in the face blocks. In the shapes blocks, there was an interaction between SLEs and condition (p-value = .12). In models stratified by condition, there was an association of novelty preference with SLEs for set 1, but not set 2 (Figure 4b). Infants whose mothers had one or more SLE during pregnancy had a greater proportion of looking time to the novel stimulus than those whose mothers did not experience any SLEs during pregnancy (β = 0.06; 95% CI: −0.004, 0.13; p-value = .07; Table 2). The covariate-adjusted mean ± SE proportion of looking time for infants in set 1 was 0.57 ± 0.03 in the no SLEs group (n = 33) and 0.63 ± 0.03 in the at least one SLE group (n = 15; Figure 4b).
3.5.3 |. Maternal telomere length
In models for novelty preference, there was a significant three-way interaction between maternal TL, sex, and condition in the face blocks (p = .04). When stratified by sex, there was a significant two-way interaction between maternal TL and condition in females, but not males (Figure 4c; β = −0.26; 95% CI: −0.48, −0.04; p-value = .02). When further stratified by condition, females in set 1 showed a significant decrease in novelty preference with longer maternal TL (β = −0.23; 95% CI: −0.38, −0.07; p-value = .006). There were no significant associations between maternal TL and novelty preference in shapes blocks (Table 2).
3.6 |. Sensitivity analyses
Findings were essentially unchanged when we excluded infants whose mothers reported smoking during the first trimester or when we adjusted models for maternal alcohol consumption during the first trimester. In sensitivity analyses substituting postnatal perceived stress for prenatal perceived stress, similar and significant associations to those for prenatal perceived stress were observed for time to reach familiarization, but not other outcomes. There was a non-significant association of higher postnatal perceived stress with longer time to reach familiarization in the shapes blocks (β = 5.3; 95% CI: −0.16, 10.8 s; p-value = .06).
4 |. DISCUSSION
4.1 |. Summary of results
Our study showed that prenatal maternal stress is associated with changes in performance on the VRM task at 7.5 months of age. Results showed all three maternal stress measures were associated with longer time to reach familiarization. As mentioned previously, the amount of time it takes an infant to reach looking time criterion reflects attention. This is because the more time spent looking away or off-task extends the amount of time to reach criterion (20 s of looking time in face trials and 10 s of looking time in shape trials). Thus, these findings suggest a potential adverse impact of maternal stress on visual attention.
Another consistent finding, although the pattern of results varied somewhat across stress measures, was that greater stress was associated with shorter average run durations. As mentioned previously, studies (e.g., Colombo et al., 1991) have interpreted shorter fixations to indicate faster information processing speed. Hence, together these results suggest a positive impact of prenatal stress on information processing. However, in this sample, there were significant negative correlations between average run duration and time to reach familiarization (rs = −.62 and −.54 [p-value < .0001]; face and shapes trials, respectively), with shorter average run durations associated with longer time to reach familiarization. One possible interpretation is that the infants having shorter average run durations (“faster processors”) are spending more time off task since they need less time to familiarize themselves with the stimuli and thus, are taking longer to reach the familiarization criterion. However, another possible interpretation is that shorter run durations may be indicative of attentional problems. That is, infants that are more easily distracted may look away more frequently resulting in shorter average run durations and longer times to reach the familiarization criterion.
4.2 |. Interactions with condition and sex
Interestingly, the associations between stress and the different outcomes assessed (time to reach familiarization, average run duration, and novelty preference) sometimes were only seen in infants who saw a certain condition (set 1 vs. set 2) and/or in infants of a specific sex. When the interactions with condition were analyzed further, there were significant associations in infants who viewed set 1, but not set 2. Previous work in the IKIDS cohort has shown that infants who viewed set 1 had longer time to reach familiarization, shorter average run durations, and greater novelty preferences than those who viewed set 2 (Dzwilewski et al., 2020). Clearly, there are differences in how infants respond to the two sets of stimuli. As reported by Dzwilewski et al. (2020), infants had a slight preference for the stimuli presented in set 2. Thus, it appears that the familiarization stimuli in set 1 were not as interesting to infants and, as a result, a subtle deficit in attention may be more evident when they are viewing these stimuli versus those in set 2 during the familiarization phase of the task.
A number of sex differences in the results were also observed. There was a significantly greater novelty preference in boys whose mothers were in the high maternal perceived stress group compared to those in the medium stress group, whereas shorter MTL was associated with greater novelty preference in girls that saw set 1 as the familiar stimuli. Also, in girls, higher maternal perceived stress was associated with shorter run duration. Previous literature suggests that males and females may be differentially sensitive to prenatal maternal stress (Barrett et al., 2013, 2014; Merced-Nieves et al., 2020). However, the results presented in this study did not show a clear pattern to suggest that one sex was more sensitive to stress than the other, and the associations were not consistent across different measures of stress. The lack of a consistent pattern of associations, together with the fact that the number of observations per group was quite small in analyses stratified by sex, suggests that these results indicating sex differences should be interpreted cautiously.
4.3 |. Stress measures
As mentioned previously, all three measures of maternal stress were associated with the cognitive outcomes assessed. In particular, all measures of maternal stress (PSS, SLE, and MTL) were associated with longer time to reach familiarization. This was despite the relatively low levels of both perceived stress and SLE in this population and despite a lack of significant correlations between the stress measures, with the exception of a weak correlation between perceived stress and SLE. There are multiple reasons that could explain the lack of significant correlations between stress measures. First, each of these measures taps into a different aspect of maternal stress. Another possible reason is the timing of each measure. Second, the weak correlation between perceived stress and SLE could be due to the fact that the PSS was administered during the first and third trimester and the questions asked about perceived stress during the previous month, whereas, the SLE scale asked about events anytime during the pregnancy. Finally, MTL was measured at 16–18 weeks of gestation, relatively early in pregnancy, and this measure has been identified as a measure of longer-term chronic stress over the mother's lifetime, with the shortening likely happening as a result of oxidative stress (Feiler et al., 2018; Mathur et al., 2016; Oliveira et al., 2016; Wojcicki et al., 2015).
4.4 |. Sensitivity analyses
Sensitivity analyses revealed that our findings were not altered when accounting for maternal smoking and drinking during the first trimester, which were very low in this cohort. Sensitivity analyses did show that the increase in time to reach familiarization with higher prenatal perceived stress could potentially be explained by postnatal perceived stress. Similar to prenatal stress models, higher postnatal stress levels were associated with longer time to reach familiarization. A similar relationship was also observed previously in the IKIDS cohort (Merced-Nieves et al., 2020), where pre- and postnatal perceived stress had similar associations with performance of 4.5-month-old infants on a physical reasoning task. However, as in Merced-Nieves et al. (2020), it is difficult to differentiate the impact of pre- and postnatal stress since the two are correlated (rs = .57–.60). To tease out the impact of preversus postnatal maternal stress, a larger sample size would be needed to include sufficient numbers of women with high levels of prenatal stress only, high levels of postnatal stress only, or both.
4.5 |. Potential mechanism
Although the mechanism through which maternal stress affects infant’s visual attention is unknown, previous literature has suggested potential mechanisms through which prenatal maternal stress could impact neurodevelopment. A possible mechanism is through an impact on the placental enzyme 11-β hydroxysteroid dehydrogenase type 2 (11β-HSD2). Studies suggest that greater production of maternal cortisol may lead to dysregulation of 11β-HSD2 (Beijers et al., 2014; Carpenter et al., 2017; Glover & Hill, 2012). This enzyme limits the amount of cortisol that crosses the placenta, and reaches the developing fetus, by converting cortisol to cortisone. Hence, dysregulation may lead to greater exposure of the fetus to cortisol and this in turn may affect neurodevelopment (Glover & Hill, 2012). Studies have shown that the brain has high levels of glucocorticoid receptors with the highest being the limbic system, the hypothalamus and the cortex (Moisiadis & Matthews, 2014). These are areas that have been shown to regulate the hypothalamic-pituitary-adrenal axis, behavior, and memory acquisition. During development, glucocorticoids have been shown to also affect neurogenesis and gliogenesis (Moisiadis & Matthews, 2014).
4.6 |. Strengths and limitations
There are several strengths and some limitations of this study that should be taken into account when considering its findings and their implications. A major strength is that the VRM task took advantage of state-of-the-art eye-tracking technology that allowed the automated collection of precise looking behavior data. In addition, this task assessed basic components of cognitive function that tend to be stable within individuals across time (Aslin, 2007) and have been shown to be predictive of cognitive abilities later in childhood (Rose & Feldman, 1995, 1997; Rose et al., 2001). Thus, the outcomes measured here may serve as early indicators of long-term neurodevelopment. Another strength was the use of multiple measures of stress including the PSS, a SLE scale, and MTL. A potential limitation of this study is that shortened TL is not specific to stress. The shortening of TL has been associated with other factors like living environment, physical activity, sleep duration (Starkweather et al., 2014), and air pollution (Miri et al., 2019). Another limitation may be the fact that multiple measures of stress were investigated in relation to multiple measures of cognitive outcomes increases the likelihood of spurious findings. Despite this problem, consistent patterns of results such as the tendency for increases in time to reach familiarization and decreases in average run duration to be associated with higher stress across multiple measures of stress suggest that these are not spurious findings.
In conclusion, the findings presented here suggest that maternal stress, assessed in multiple ways, is associated with differences in the performance of infants on a VRM task. In particular, our most consistent findings suggest that visual attention is particularly affected by maternal stress. Our findings provide additional evidence to existing literature that maternal stress can impact early neurodevelopment.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by the Children's Environmental Health & Disease Prevention Research Centers, National Institute of Environmental Health Sciences under grant ES022848; U.S. Environmental Health Protection Agency under grant RD83543401; the Environmental Influences on Child Health Outcomes (ECHO) under grant OD023272; and the NIH Predoctoral Traineeship in Endocrine, Developmental & Reproductive Toxicology under grant T32 ES007326.
Funding information
NIH Predoctoral Traineeship in Endocrine, Developmental & Reproductive Toxicology, Grant/Award Number: T32 ES007326; National Institute of Environmental Health Sciences, Grant/Award Number: ES022848; NIH Office of the Director-Environmental Influences on Child Health Outcomes, Grant/Award Number: OD023272; U.S. Environmental Health Protection Agency, Grant/Award Number: RD83543401
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- Anderson PJ, & Burnett A (2017). Assessing developmental delay in early childhood—Concerns with the Bayley-III scales. Clinical Neuropsychology, 31(2), 371–381. 10.1080/13854046.2016.1216518 [DOI] [PubMed] [Google Scholar]
- Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW; Victorian Infant Collaborative Group. (2010). Underestimation of developmental delay by the new Bayley-III scale. Archives of Pediatrics & Adolescent Medicine, 164(4), 352–356. 10.1001/archpediatrics.2010.20 [DOI] [PubMed] [Google Scholar]
- Aslin RN (2007). What’s in a look? Developmental Science, 10(1), 48–53. 10.1111/j.1467-7687.2007.00563.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Parlett LE, Sathyanarayana S, Liu F, Redmon JB, Wang C, & Swan SH (2013). Prenatal exposure to stressful life events is associated with masculinized anogenital distance (AGD) in female infants. Physiology & Behavior, 114–115, 14–20. 10.1016/j.physbeh.2013.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett ES, Redmon JB, Wang C, Sparks A, & Swan SH (2014). Exposure to prenatal life events stress is associated with masculinized play behavior in girls. NeuroToxicology, 41, 20–27. 10.1016/j.neuro.2013.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beijers R, Buitelaar JK, & de Weerth C (2014). Mechanisms underlying the effects of prenatal psychosocial stress on child outcomes: Beyond the HPA axis. European Child and Adolescent Psychiatry, 23, 943–956. 10.1007/s00787-014-0566-3 [DOI] [PubMed] [Google Scholar]
- Breeman LD, Jaekel J, Baumann N, Bartmann P, & Wolke D (2015). Preterm cognitive function into adulthood. Pediatrics, 136(3), 415–423. 10.1542/peds.2015-0608 [DOI] [PubMed] [Google Scholar]
- Carpenter T, Grecian SM, & Reynolds RM (2017). Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: A systematic review. Journal of Developmental Origins of Health and Disease, 8(2), 244–255. 10.1017/S204017441600074X [DOI] [PubMed] [Google Scholar]
- Cawthon RM (2002). Telomere measurements by quantitative PCR. Nucleic Acids Research, 30(10), e47. 10.1093/nar/30.10.e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, & Williamson G (1988). Perceived stress in a probability sample of the United States. In Spacapan S & Oskamp S (Eds.) The social psychology of health (pp. 31–67). Sage. [Google Scholar]
- Colombo J, Mitchell DW, Coldren JT, & Freeseman LJ (1991). Individual differences in infant visual attention: Are short lookers faster processors or feature processors? Child Development, 62(6), 1247–1257. 10.1111/j.1467-8624.1991.tb01603.x [DOI] [PubMed] [Google Scholar]
- DiPietro JA (2012). Maternal stress in pregnancy: Considerations for fetal development. Journal of Adolescent Health, 51(2 Suppl.), S3–S8. 10.1016/j.jadohealth.2012.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, & Dunn DM (2007). Peabody Picture Vocabulary Test (4th ed.). NCS Pearson Inc. [Google Scholar]
- Dzwilewski KLC, Merced-Nieves FM, Aguiar A, Korrick SA, & Schantz SL (2020). Characterization of performance on an automated visual recognition memory task in 7.5- month-old infants. Neurotoxicology and Teratology, 81(2020), 106904. 10.1016/j.ntt.2020.106904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan JF, Holland CR, & Wheeler K (2007). The prediction, from infancy, of adult IQ and achievement. Intelligence, 35(3), 225–231. 10.1016/j.intell.2006.07.007 [DOI] [Google Scholar]
- Feiler MO, Patel D, Li H, Meacham PJ, Watson GE, Shamlaye C, Yeates A, Broberg K, & van Wijngaarden E (2018). The association between early-life relative telomere length and childhood neurodevelopment. NeuroToxicology, 65, 22–27. 10.1016/j.neuro.2018.01.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover V, & Hill J (2012). Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiology & Behavior, 106, 736–740. 10.1016/j.physbeh.2012.02.011 [DOI] [PubMed] [Google Scholar]
- Hernan MA, Hernandez-Diaz S, Werler MM, Robins JM, & Mitchell AA (2000). Causal knowledge as a prerequisite for confounding evaluation. Examples from birth defects epidemiology. American Journal of Epidemiology, 151(11), S43. 10.1093/aje/155.2.176 [DOI] [PubMed] [Google Scholar]
- Kingston D, McDonald S, Austin MP, & Tough S (2015). Association between prenatal and postnatal psychological distress and toddler cognitive development: A systematic review. PLoS One, 10(5), e0126929. 10.1371/journal.pone.0126929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante DP, Barr RG, Brunet A, Du Fort GG, Meaney ML, Saucier J, Zelazo PR, & King S (2004). Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatric Research, 56(3), 400–410. 10.1203/01.PDR.0000136281.34035.44 [DOI] [PubMed] [Google Scholar]
- Lin J, Epel E, Cheon J, Kroenke C, Sinclair E, Bigos M, Wolkowitzm O, Mellon S, & Blackburn E (2010). Analyses and comparisons of telomerase activity and telomere length in human T and B cells: Insights for epidemiology of telomere maintenance. Journal of Immunological Methods, 352(1–2), 71–80. 10.1016/j.jim.2009.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsell L, Johnson S, Wolke D, O’Reilly H, Morris JK, Kurinczuk JJ, & Marlow N (2018). Cognitive trajectories from infancy to early adulthood following birth before 26 weeks of gestation: A prospective, population-based cohort study. Archives of Disease in Childhood, 103(4), 363–370. 10.1136/archdischild-2017-313414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangin KS, Horwood LJ, & Woodward LJ (2017). Cognitive development trajectories of very preterm and typically developing children. Child Development, 88(1), 282–298. 10.1111/cdev.12585 [DOI] [PubMed] [Google Scholar]
- Marino C, & Gervain J (2019). The novelty effect as a predictor of language outcome. Frontiers in Psychology: Developmental Psychology, 10, 258. 10.3389/fpsycg.2019.00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur MB, Epel E, Kind S, Desai M, Parks CG, Sandler DP, & Khazeni N (2016). Perceived stress and telomere length: A systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain, Behavior, and Immunity, 54, 158–169. 10.1016/j.bbi.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall RB, & Carriger MS (1993). A meta-analysis of infant habituation and recognition memory performance as predictors of later IQ. Child Development, 64(1), 57–79. 10.1111/j.1467-8624.1993.tb02895.x [DOI] [PubMed] [Google Scholar]
- Merced-Nieves FM, Aguiar A, Dzwilewski KLC, Musaad S, Korrick SA, & Schantz SL (2020). Association of prenatal maternal perceived stress with a sexually dimorphic measure of cognition in 4.5-month-old infants. Neurotoxicology and Teratology, 77, 106850. 10.1016/j.ntt.2019.106850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miri M, Nazarzadeh M, Alahabadi A, Ehrampoush MH, Rad A, Lotfi MH, Sheikhha MH, Sakhvidi MJZ, Nawrot TS, & Dadvand P (2019). Air pollution and telomere length in adults: A systematic review and meta-analysis of observational studies. Environmental Pollution, 244, 636–647. 10.1016/j.envpol.2018.09.130 [DOI] [PubMed] [Google Scholar]
- Moisiadis VG, & Matthews SG (2014). Glucocorticoids and fetal programming part 2: Mechanisms. Nature, 10, 403–411. 10.1038/nrendo.2014.74 [DOI] [PubMed] [Google Scholar]
- Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, & Oliveira Guerra R (2016). Systematic review of the association between chronic social stress and telomere length: A life course perspective. Ageing Research Reviews, 26, 37–52. 10.1016/j.arr.2015.12.006 [DOI] [PubMed] [Google Scholar]
- Pascalis O, & de Haan M (2003). Recognition memory and novelty preference: What model? In Hayne H & Fagen J (Eds.) Progress in infancy research (pp. 95–119). Psychology Press. [Google Scholar]
- Rose SA, & Feldman JF (1995). Prediction of IQ and specific cognitive abilities at 11 years from infancy measures. Developmental Psychology, 31(4), 685–696. 10.1037/0012-1649.31.4.685 [DOI] [Google Scholar]
- Rose SA, & Feldman JF (1997). Memory and speed: Their role in the relation of infant information processing to later IQ. Child Development, 68(4), 630–641. 10.1111/j.1467-8624.1997.tb04226.x [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2001). Attention and recognition memory in the 1st year of life: A longitudinal study of preterm and full-term infants. Developmental Psychology, 37(1), 135–151. 10.1037/0012-1649.37.1.135 [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Jankowski JJ (2009). A cognitive approach to the development of early language. Child Development, 80(1), 134–150. 10.1111/j.1467-8624.2008.01250.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, & Wallace IF (1992). Infant information processing in relation to six-year cognitive outcomes. Child Development, 63(5), 1126–1141. 10.1111/j.1467-8624.1992.tb01684.x [DOI] [PubMed] [Google Scholar]
- Starkweather AR, Alhaeeri AA, Montpetit A, Brumelle J, Filler K, Montpetit M, Mohanraj L, Lyon DE, & Jackson-Cook CK (2014). An integrative review of factors associated with telomere length and implications for behavioral research. Nursing Research, 63(1), 36–50. 10.1097/NNR.0000000000000009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vehmeijer FOL, Guxens M, Duijts L, & El Marroun H (2018). Maternal psychological distress during pregnancy and childhood health outcomes: A narrative review. Journal of Developmental Origins of Health and Disease, 10(3), 274–285. 10.1017/S2040174418000557 [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Heyman MB, Elwan D, Shiboski S, Lin J, Blackburn E, & Epel E (2015). Telomere length is associated with oppositional defiant behavior and maternal clinical depression in Latino preschool children. Translational Psychiatry, 5, 1–6. 10.1038/tp.2015.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu P, Sun M, Hao J, Chen Y, Jiang X, Tao R, Huang K, & Tao F (2014). Does prenatal maternal stress impair cognitive development and alter temperament characteristics in toddlers with healthy birth outcomes? Developmental Medicine & Child Neurology, 56, 283–289. 10.1111/dmcn.12378 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.