Abstract
Background
Gastro‐oesophageal reflux disease (GORD) is a common condition with 3% to 33% of people from different parts of the world suffering from GORD. There is considerable uncertainty about whether people with GORD should receive an operation or medical treatment for controlling the condition.
Objectives
To assess the benefits and harms of laparoscopic fundoplication versus medical treatment for people with gastro‐oesophageal reflux disease.
Search methods
We searched the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (UGPD) Trials Register (June 2015), Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 6, 2015), Ovid MEDLINE (1966 to June 2015), and EMBASE (1980 to June 2015) to identify randomised controlled trials. We also searched the references of included trials to identify further trials.
Selection criteria
We considered only randomised controlled trials (RCT) comparing laparoscopic fundoplication with medical treatment in people with GORD irrespective of language, blinding, or publication status for inclusion in the review.
Data collection and analysis
Two review authors independently identified trials and independently extracted data. We calculated the risk ratio (RR) or standardised mean difference (SMD) with 95% confidence intervals (CI) using both fixed‐effect and random‐effects models with RevMan 5 based on available case analysis.
Main results
Four studies met the inclusion criteria for the review, and provided information on one or more outcomes for the review. A total of 1160 participants in the four RCTs were either randomly assigned to laparoscopic fundoplication (589 participants) or medical treatment with proton pump inhibitors (571 participants). All the trials included participants who had had reflux symptoms for at least six months and had received long‐term acid suppressive therapy. All the trials included only participants who could undergo surgery if randomised to the surgery arm. All of the trials were at high risk of bias. The overall quality of evidence was low or very low. None of the trials reported long‐term health‐related quality of life (HRQoL) or GORD‐specific quality of life (QoL).
The difference between laparoscopic fundoplication and medical treatment was imprecise for overall short‐term HRQOL (SMD 0.14, 95% CI ‐0.02 to 0.30; participants = 605; studies = 3), medium‐term HRQOL (SMD 0.03, 95% CI ‐0.19 to 0.24; participants = 323; studies = 2), medium‐term GORD‐specific QoL (SMD 0.28, 95% CI ‐0.27 to 0.84; participants = 994; studies = 3), proportion of people with adverse events (surgery: 7/43 (adjusted proportion = 14.0%); medical: 0/40 (0.0%); RR 13.98, 95% CI 0.82 to 237.07; participants = 83; studies = 1), long‐term dysphagia (surgery: 27/118 (adjusted proportion = 22.9%); medical: 28/110 (25.5%); RR 0.90, 95% CI 0.57 to 1.42; participants = 228; studies = 1), and long‐term reflux symptoms (surgery: 29/118 (adjusted proportion = 24.6%); medical: 41/115 (35.7%); RR 0.69, 95% CI 0.46 to 1.03; participants = 233; studies = 1).
The short‐term GORD‐specific QoL was better in the laparoscopic fundoplication group than in the medical treatment group (SMD 0.58, 95% CI 0.46 to 0.70; participants = 1160; studies = 4).
The proportion of people with serious adverse events (surgery: 60/331 (adjusted proportion = 18.1%); medical: 38/306 (12.4%); RR 1.46, 95% CI 1.01 to 2.11; participants = 637; studies = 2), short‐term dysphagia (surgery: 44/331 (adjusted proportion = 12.9%); medical: 11/306 (3.6%); RR 3.58, 95% CI 1.91 to 6.71; participants = 637; studies = 2), and medium‐term dysphagia (surgery: 29/288 (adjusted proportion = 10.2%); medical: 5/266 (1.9%); RR 5.36, 95% CI 2.1 to 13.64; participants = 554; studies = 1) was higher in the laparoscopic fundoplication group than in the medical treatment group.
The proportion of people with heartburn at short term (surgery: 29/288 (adjusted proportion = 10.0%); medical: 59/266 (22.2%); RR 0.45, 95% CI 0.30 to 0.69; participants = 554; studies = 1), medium term (surgery: 12/288 (adjusted proportion = 4.2%); medical: 59/266 (22.2%); RR 0.19, 95% CI 0.10 to 0.34; participants = 554; studies = 1), long term (surgery: 46/111 (adjusted proportion = 41.2%); medical: 78/106 (73.6%); RR 0.56, 95% CI 0.44 to 0.72); participants = 217; studies = 1) and those with reflux symptoms at short‐term (surgery: 6/288 (adjusted proportion = 2.0%); medical: 53/266 (19.9%); RR 0.10, 95% CI 0.05 to 0.24; participants = 554; studies = 1) and medium term (surgery: 6/288 (adjusted proportion = 2.1%); medical: 37/266 (13.9%); RR 0.15, 95% CI 0.06 to 0.35; participants = 554; studies = 1) was less in the laparoscopic fundoplication group than in the medical treatment group.
Authors' conclusions
There is considerable uncertainty in the balance of benefits versus harms of laparoscopic fundoplication compared to long‐term medical treatment with proton pump inhibitors. Further RCTs of laparoscopic fundoplication versus medical management in patients with GORD should be conducted with outcome‐assessor blinding and should include all participants in the analysis. Such trials should include long‐term patient‐orientated outcomes such as treatment‐related adverse events (including severity), quality of life, and also report on the social and economic impact of the adverse events and symptoms.
Plain language summary
Keyhole surgery versus medical treatment for adults with heart burn or acid regurgitation
Review question
Is laparoscopic fundoplication (keyhole surgery which involves wrapping the top end of the stomach around the bottom of the oesophagus (food pipe or gullet) to form a new valve) beneficial or harmful when compared to medical treatment in adults with heartburn or acid regurgitation (gastro‐oesophageal reflux disease (GORD))?
Background
Gastro‐oesophageal reflux disease or GORD is a condition which develops when stomach contents regurgitate into the oesophagus causing troublesome symptoms such as heartburn (burning sensation at the lower end of the breastbone) or regurgitation (perception of flow of stomach content into the throat or mouth). Long‐term complications of GORD include reflux oesophagitis (injury to lining of oesophagus), bleeding from the oesophagus, narrowing of the oesophagus, change in the nature of the lining of the oesophagus which can sometimes give rise to oesophageal cancer. Approximately 3% to 33% of people have GORD around the world. The risk factors for GORD include a family history of reflux disease in immediate relatives, pregnancy, older age, obesity, cigarette smoking, and excessive alcohol drinking. Apart from lifestyle changes (such as cessation of smoking) and diet changes (avoiding food that causes heartburn), the major forms of treatment for GORD are medical and surgical. The medical treatment is usually aimed at decreasing acidity in the stomach. Currently a group of drugs which suppress acid secretion, called proton pump inhibitors, are considered the best in decreasing acid secretion. The main surgical treatment is fundoplication which involves wrapping around the lower part of the food pipe with stomach. This can be performed by traditional open surgery, keyhole surgery or surgery that is performed without making any cut from within the stomach with the help of an endoscope (in this context, a flexible tube introduced through the mouth to give a view of the food pipe and stomach). The benefits and harms of laparoscopic fundoplication compared to medical treatment in people with GORD is not known. We sought to resolve this issue by searching for existing studies on the topic. We included all studies whose results were reported until 1st October 2014.
Study characteristics
Four studies met the inclusion criteria for the review, and provided information for the review. A total of 1160 participants received either laparoscopic fundoplication (589 participants) or medical treatment (571 participants). The decision on whether a participant received surgery or medical treatment was made using methods similar to tossing a coin, ensuring that the participants in the two groups were similar. All the trials included people who had had reflux symptoms for at least six months, had received long‐term acid suppressive therapy, and could undergo laparoscopic fundoplication if necessary.
Key results
None of the trials reported long‐term health‐related quality of life (HRQoL) or GORD‐specific quality of life (QoL). The difference between laparoscopic fundoplication and medical treatment was imprecise for overall short‐term HRQOL, medium‐term HRQOL, medium‐term GORD‐specific QoL, percentage of people with adverse events, long‐term dysphagia (difficulty in swallowing), and long‐term acid regurgitation. The short‐term GORD‐specific quality of life was better in the laparoscopic fundoplication group than in the medical treatment group. However, it was not clear how much this improvement benefited the patient. The proportion of people with serious adverse events, short‐term dysphagia, and medium‐term dysphagia was higher in the laparoscopic fundoplication group than in the medical treatment group. The proportion of people with heartburn at short term, medium term, and long term, and those with acid regurgitation at short term and medium term was less in the laparoscopic fundoplication group than in the medical treatment group. The severity of difficulty in swallowing, heartburn, or acid regurgitation was not reported. There is considerable uncertainty in the balance of benefits versus harms of laparoscopic fundoplication compared to long‐term medical treatment with proton pump inhibitors. Due to the poor quality of the trials, future high‐quality studies are needed in this field.
Quality of the evidence
The quality of evidence was low or very low. As a result, there is a lot of uncertainty regarding the results.
Summary of findings
Summary of findings for the main comparison. Laparoscopic fundoplication versus medical management for gastro‐oesophageal reflux disease (GORD) in adults.
| Laparoscopic fundoplication versus medical management for gastro‐oesophageal reflux disease (GORD) in adults | ||||||
|
Patient or population: Patients with gastro‐oesophageal reflux disease (GORD) in adults
Settings: Secondary care
Intervention: Laparoscopic fundoplication Control: Medical management | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Medical management | Laparoscopic fundoplication | |||||
| Health‐related quality of life | ||||||
| (< 1 year) | The mean health‐related quality of life (< 1 year) in the intervention groups was 0.14 standard deviations higher (0.02 lower to 0.3 higher) | 605 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD 0.14 (‐0.02 to 0.3) | ||
| (1 to 5 years) | The mean health‐related quality of life (1 to 5 years) in the intervention groups was 0.03 standard deviations higher (0.19 lower to 0.24 higher) | 323 (2 studies) | ⊕⊝⊝⊝ very low1,2,4 | SMD 0.03 (‐0.19 to 0.24) | ||
| GORD‐specific quality of life | ||||||
| (< 1 year) | The mean GORD‐specific quality of life (< 1 year) in the intervention groups was 0.58 standard deviations higher (0.46 to 0.7 higher) | 1160 (4 studies) | ⊕⊕⊝⊝ low1 | SMD 0.58 (0.46 to 0.7) | ||
| (1 to 5 years) | The mean GORD‐specific quality of life (1 to 5 years) in the intervention groups was 0.28 standard deviations higher (0.27 lower to 0.84 higher) | 994 (3 studies) | ⊕⊝⊝⊝ very low1,2,3 | SMD 0.28 (‐0.27 to 0.84) | ||
| Adverse events | ||||||
| Serious adverse events | 124 per 1000 | 181 per 1000 (125 to 262) | RR 1.46 (1.01 to 2.11) | 637 (2 studies) | ⊕⊝⊝⊝ very low1,5 | |
| Adverse events | 10 per 1000 | 140 per 1000 (8 to 1000) | RR 13.98 (0.82 to 237.07) | 83 (1 study) | ⊕⊝⊝⊝ very low1,3,5,6 | |
| Dysphagia | ||||||
| (< 1 year) | 36 per 1000 | 129 per 1000 (69 to 241) | RR 3.58 (1.91 to 6.71) | 637 (2 studies) | ⊕⊝⊝⊝ very low1,6 | |
| (1 to 5 years) | 19 per 1000 | 101 per 1000 (39 to 256) | RR 5.36 (2.1 to 13.64) | 554 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| (5 years or more) | 255 per 1000 | 229 per 1000 (145 to 361) | RR 0.9 (0.57 to 1.42) | 228 (1 study) | ⊕⊝⊝⊝ very low1,3,6 | |
| Heartburn | ||||||
| (< 1 year) | 222 per 1000 | 100 per 1000 (67 to 153) | RR 0.45 (0.3 to 0.69) | 554 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| (1 to 5 years) | 222 per 1000 | 42 per 1000 (22 to 75) | RR 0.19 (0.1 to 0.34) | 554 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| (5 years or more) | 736 per 1000 | 412 per 1000 (324 to 530) | RR 0.56 (0.44 to 0.72) | 217 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| Reflux | ||||||
| (< 1 year) | 199 per 1000 | 20 per 1000 (10 to 48) | RR 0.1 (0.05 to 0.24) | 554 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| (1 to 5 years) | 139 per 1000 | 21 per 1000 (8 to 49) | RR 0.15 (0.06 to 0.35) | 554 (1 study) | ⊕⊝⊝⊝ very low1,6 | |
| (5 years or more) | 357 per 1000 | 246 per 1000 (164 to 367) | RR 0.69 (0.46 to 1.03) | 233 (1 study) | ⊕⊝⊝⊝ very low1,3,6 | |
| Long‐term overall health‐related quality of life and long‐term GORD‐specific quality of life were not reported in any of the trials. | ||||||
| *The basis for the assumed risk was the mean control group risk across studies for all outcomes other than adverse events. For control group risk, 1% was used as the control group risk since there were no adverse events in the control group in the only trial that reported this outcome (Anvari 2011). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The trial(s) was/were at high risk of bias. 2 There was substantial heterogeneity. 3 The confidence intervals overlapped no effect and 25% or more increase or 25% or more decrease or both. 4 There were fewer than 400 participants in both groups. 5 Some trials did not report this outcome although this can be expected to be reported in a randomised controlled trial of laparoscopic fundoplication versus medical treatment. 6 There were fewer than 300 events in total in both groups.
Background
Description of the condition
Gastro‐oesophageal reflux disease or GORD (GERD in North America, as oesophagus is spelt 'esophagus') is a condition which develops when the reflux of stomach contents causes troublesome symptoms or complications or both (Vakil 2006). The symptoms related to GORD are retrosternal burning sensation (heartburn) and regurgitation (perception of flow of refluxed gastric content into the mouth or laryngopharynx) (Vakil 2006). Patients have to determine whether the symptoms are troublesome (Vakil 2006). In general, mild symptoms occurring on two or more days in a week and moderate or severe symptoms occurring on one or more days in a week are considered troublesome by patients (Vakil 2006). The complications include reflux oesophagitis (oesophageal mucosal injury), upper gastrointestinal bleeding, reflux stricture (persistent narrowing of the oesophagus), and Barrett's oesophagus (replacement of the normal stratified squamous epithelium lining of the lower end of oesophagus with simple columnar epithelial lining which is similar to the lining of the stomach) (Vakil 2006), which in turn is a risk factor for oesophageal cancer (Solaymani‐Dodaran 2004). Other researchers have hypothesised that there is a direct link between symptomatic GORD and oesophageal cancer based on their observation of increased oesophageal cancer irrespective of the presence of Barrett's oesophagus (Cook 2014; Lagergren 1999). While there has been significant controversy over the definition of GORD, the international consensus definition of GORD includes asymptomatic patients with complications, does not stipulate the method of diagnosis, and includes reflux which may be weakly acidic or gaseous (Vakil 2006).
There is global variation in the prevalence of GORD. The prevalence is higher in Europe, North and South America, and the Middle East, with a prevalence ranging between 9% and 33% of adults compared to a prevalence ranging between 3% and 8% in East Asia (Dent 2005; El‐Serag 2014). The incidence of GORD is available from the UK and the USA and is about five cases of GORD per 1000 person‐years (Dent 2005; El‐Serag 2014). The risk factors for GORD include genetic factors such as a family history of reflux disease in immediate relatives; demographic factors such as pregnancy, older age and obesity; behavioural factors such as cigarette smoking, excessive alcohol consumption, drug treatments such as non‐steroidal anti‐inflammatory drugs (NSAIDs), oral steroids; and co‐morbidities such as abdominal pain, irritable bowel syndrome, gallstone disease, asthma, chronic obstructive pulmonary disease, chest pain, and angina. Coffee consumption, oral contraceptive consumption, and hormonal replacement therapy are associated with lower prevalence of GORD (Dent 2005). It should be noted that these are associations and no causal association can be shown for many of these factors.
Gastric contents are prevented from entering into the oesophagus by the lower oesophageal sphincter (LOS). However, LOS relaxes transiently resulting in the reflux of gastric contents into the oesophagus, even in normal individuals (Boeckxstaens 2014b). However, increased reflux of gastric contents into the oesophagus and prolonged exposure of the lower oesophagus to gastric contents are believed to contribute to the symptoms and complications of GORD (Boeckxstaens 2014b). Hiatus hernia lowers the competence and pressure of the LOS, reduces the length of the gastro‐oesophageal junction, and alters the opening characteristics of the gastro‐oesophageal junction. This results in an increase in the exposure of the oesophagus to acid (Boeckxstaens 2014b; Gordon 2004). Hiatus hernia is associated with severe GORD symptoms and complications (Boeckxstaens 2014b; Gordon 2004; Vakil 2006).
Based on the international consensus conference definition, GORD may be diagnosed based on typical symptoms alone (heartburn and regurgitation) without any additional diagnostic tests (Vakil 2006). Diagnostic tests that may be performed to confirm the diagnosis include pH testing, impedence monitoring, upper gastrointestinal endoscopy with or without histological examination, or electron microscopy to confirm the presence of one or more of the complications such as reflux oesophagitis, Barrett's oesophagus, and oesophageal cancer (Vakil 2006). Although a number of classification systems based on endoscopic findings are still commonly used (Dent 2008), the international consensus conference classifies GORD into oesophageal syndrome (presence of heartburn, regurgitation, upper abdominal pain, chest pain, which may be sometimes be similar to that of anginal chest pain, reflux oesophagitis, upper gastrointestinal bleeding, stricture, Barrett’s oesophagus, and oesophageal cancer) and extraoesophageal syndromes (chronic cough, chronic laryngitis, asthma, and dental erosions).
Description of the intervention
Apart from lifestyle and diet modifications, the major forms of treatment for GORD are surgical and medical treatments (Boeckxstaens 2014a).
Surgical treatment for GORD is in the form of fundoplication. There are various modifications to original complete fundoplication suggested by Nissen, mainly with regards to the amount and area of the gastric fundus which is plicated and where the wrap is fixed (Watson 1998). Fundoplication can be performed by open or laparoscopic surgery (Watson 1998). Recently endoscopic endoluminal fundoplication, which involves fundoplication without any incision, has been proposed (Zacherl 2014). The complications related to fundoplication include injury to nearby structures such as the liver, spleen, or oesophagus; dysphagia (troublesome difficulty in swallowing) (5%); wrap migration (1%); recurrent regurgitation (1%); recurrent heartburn (1% to 10%); and chest complications (3%) (Singhal 2009; Wileman 2010). Overall, 1% to 14% of patients undergoing fundoplication develop complications (Wileman 2010).
The medical treatment is usually aimed at decreasing acidity in the stomach (which may be in the form of proton pump inhibitors, H2 (histamine H2 receptor) blockers or H2 receptor antagonists, antacids, prokinetic drugs) and surgical treatment (usually in the form of fundoplication, which can be performed by open surgery, laparoscopic surgery, or by endoscopic endoluminal fundoplication) (Boeckxstaens 2014a; Lundell 2001; Moayyedi 2008; Van Meer 2013; Wileman 2010; Zacherl 2014).
For GORD, the medical treatment is usually given orally. Proton pump inhibitors are currently considered the main treatment of GORD (Katz 2010a). Various proton pump inhibitors include omeprazole, lansoprazole, pantoprazole, rabeprazole, and esomeprazole (Katz 2010a). Proton pump inhibitors are generally well tolerated, and adverse effects are relatively infrequent. The adverse effects reported most often with proton pump inhibitors are headache, gastrointestinal disturbances, and rash. Occasionally severe allergic reactions, anaphylactic reactions, muscle weakness, reversible confusional states, mental disturbances, liver failure, kidney damage, and angina have been reported (Martindale 2011).
The proton pump inhibitors may be supplemented with H2 blockers such as ranitidine, antacids such as magnesium hydroxide, and prokinetic agents such as metoclopramide or domperidone (Achem 1998; Boeckxstaens 2014a). Adverse reactions to H2 blockers are generally infrequent. The most common adverse effects reported are diarrhoea and other gastrointestinal disturbances, dizziness, tiredness, headache, muscle and joint pain, and rash (Martindale 2011). The antacids are generally safe. Adverse effects depend upon the antacid taken and the additional ingredients that the antacid preparation contains. The reported adverse effects include constipation, diarrhoea, and flatulence (Martindale 2011). Adverse effects related to prokinetic agents include extrapyramidal symptoms (usually acute dystonic reactions). Parkinsonism and tardive dyskinesia have occasionally been reported, usually during prolonged treatment in elderly patients. Metoclopramide may also cause galactorrhoea or related disorders (Martindale 2011).
How the intervention might work
Fundoplication compresses the lower oesophagus, increasing the LOS pressure and decreasing the intermittent relaxations of the LOS (Watson 1998). This decreases the amount of gastric acid which reaches the oesophagus. Medical treatments such as proton pump inhibitors and H2 (histamine H2 receptor) blockers work by decreasing the acid secretion (Achem 1998; Katz 2010a). Antacids neutralise the gastric acid (Achem 1998). Prokinetics improve the gastric motility and hence acid clearance by the oesophagus (Achem 1998).
Why it is important to do this review
GORD is the most common gastrointestinal diagnosis in outpatient clinical visits in the USA resulting in more than nine million outpatient visits annually (Peery 2012). The estimated direct and indirect costs of treatment and lost productivity due to GORD is USD 10 billion annually in US, EUR 9.3 billion annually in Germany, EUR 2.9 billion annually in Italy, and EUR 1.9 billion annually in Spain (Darba 2011; Sandler 2002). There is currently no consensus in the treatment of GORD. The previous Cochrane Review on Medical versus surgical management for gastro‐oesophageal reflux disease (GORD) in adults included medical management versus laparoscopic fundoplication surgery and concluded that laparoscopic fundoplication surgery is more effective than medical management for the treatment of GORD in the short term to medium term (Wileman 2010). This review is an update of the above review with revised methodology that reflects the current Cochrane methodology (Higgins 2011a). This review will provide the best level of evidence on the comparative benefits and harms of laparoscopic versus medical management for GORD, and so allow patients and the healthcare providers involved in their care to make informed decisions.
Objectives
To assess the benefits and harms of laparoscopic fundoplication versus medical treatment for people with gastro‐oesophageal reflux disease.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs). We included studies reported as full text, those published as abstract only, and unpublished data. We excluded quasi‐randomised studies and observational studies because of the high risk of bias in such study designs.
Types of participants
We included adults (> 16 years of age) with GORD irrespective of the presence of symptoms, complications, or hiatus hernia and judged to be suitable for either surgical or medical management. We excluded patients who have symptomatic oesophageal stricture due to GORD or those who have oesophageal dysplasia or cancer.
Types of interventions
We included trials comparing laparoscopic fundoplication versus medical treatment for gastro‐oesophageal disease irrespective of the nature of the laparoscopic fundoplication or medical treatment. We excluded trials in which the comparisons solely involve comparison of different forms of medical treatment or different forms of laparoscopic fundoplications. We accepted co‐interventions, for example, the use of lifestyle modification advice, provided that they were used equally in both the groups.
Types of outcome measures
Primary outcomes
-
Health‐related quality of life (HRQoL) (using any validated scale).
Short‐term (four weeks to 12 months).
Medium‐term (one year to five years).
Long‐term (five years or more).
-
GORD‐specific quality of life (QoL)
Short‐term (four weeks to 12 months).
Medium‐term (one year to five years).
Long‐term (five years or more).
-
Serious adverse events (within three months of cessation of treatment ‐ for surgery this period refers to three months after index surgery). We will accept the following definitions of serious adverse events.
ICH‐GCP International Conference on Harmonisation ‐ Good Clinical Practice guideline (ICH‐GCP 1996): serious adverse events defined as any untoward medical occurrence that results in death, is life‐threatening, requires inpatient hospitalisation or prolongation of existing hospitalisation, results in persistent or significant disability/incapacity.
Other variations of ICH‐GCP classifications such as Food and Drug Administration (FDA) classification (FDA 2006), Medicines and Healthcare products Regulatory Agency (MHRA) classification (MHRA 2013).
Secondary outcomes
Adverse events (within three months of cessation of treatment ‐ for surgery this period refers to three months after index surgery). We will accept all adverse events reported by the study author irrespective of the severity of the adverse event.
-
Dysphagia.
Short‐term (four weeks to 12 months).
Medium‐term (one year to five years).
Long‐term (five years or more).
-
Heartburn.
Short‐term (four weeks to 12 months).
Medium‐term (one year to five years).
Long‐term (five years or more).
-
Reflux.
Short‐term (four weeks to 12 months).
Medium‐term (one year to five years).
Long‐term (five years or more).
The choice of the above clinical outcomes was to assess the comparative safety and clinical improvement, in terms of reduced symptoms and complications, resulting in an improvement in the health‐related quality of life between surgical and medical treatment in patients with GORD.
Reporting of the outcomes listed here were not an inclusion criteria for the review.
Search methods for identification of studies
Electronic searches
We conducted a literature search to identify all published and unpublished randomised controlled trials (RCTs). The literature search identified potential studies in all languages. We planned to translate the non‐English language papers and fully assess them for potential inclusion in the review as necessary.
We searched the following electronic databases (via the OVID platform) to identify potential studies:
Cochrane Central Register of Controlled Trials (CENTRAL), (The Cochrane Library Issue 6, 2015 (Appendix 1);
Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (UGPD) Trials Register (June 2015);
Ovid MEDLINE (1966 to June 2015) (Appendix 2); and
EMBASE (1980 to June 2015) (Appendix 3).
Details of the previous search strategies are provided in Appendix 4
Searching other resources
We checked reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published and unpublished studies.
We searched for errata or retractions from eligible trials on http://www.ncbi.nlm.nih.gov/pubmed and report the date this was done within the review.
Data collection and analysis
Selection of studies
Two review authors (SG and KG) independently screened titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. We retrieved the full‐text study reports and two review authors (SG and KG) independently screened the full text and identified studies for inclusion. We identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreement through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and characteristics of excluded studies table.
Data extraction and management
We used a standard data collection form for study characteristics and outcome data which has been piloted on at least one study in the review. Two review authors (SG and KG) extracted the following study characteristics from the included studies.
Methods: study design, total duration of the study and run in, number of study centres and location, study setting, withdrawals, date of study.
Participants: number (n), mean age, age range, gender, hiatal hernia status, Barrett's oesophagus status, body mass index, inclusion criteria, exclusion criteria.
Interventions: intervention, comparison, concomitant interventions.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, notable conflicts of interest of trial authors.
Two review authors (SG and KG) independently extracted outcome data from the included studies. If outcomes were reported multiple times for the same time point, for example, short‐term health‐related quality of life was reported at three months and 12 months, the later time point (i.e. 12 months) was chosen for data extraction. For time‐to‐event outcomes, we planned to extract data to calculate the natural logarithm of the hazard ratio and its standard error using the methods suggested by Parmar et al (Parmar 1998).
We included all randomised participants for medium outcomes (for example, dysphagia) and this was not conditional upon the short‐term outcomes (for example, having or not having a dysphagia at 12 months).
We planned to note in the characteristics of included studies table if outcome data were reported in an unusable way. We resolved disagreements by consensus. One review author (KG) copied across the data from the data collection form into the Review Manager file (Review Manager 2014). We double checked that the data were entered correctly by comparing the study reports with how the data are presented in the systematic review.
Assessment of risk of bias in included studies
Two review authors (SG and KG) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). Any disagreement was resolved by discussion. We assessed the risk of bias according to the following domains;
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective outcome reporting;
other bias.
We graded each potential source of bias as high, low or unclear and provide a quote from the study report together with a justification for our judgment in the risk of bias table. We have summarised the risk of bias judgements across different studies for each of the domains listed. Where information on risk of bias relates to unpublished data or correspondence with a trialist, we have noted this in the risk of bias table.
When considering treatment effects, we have taken into account the risk of bias for the studies that contributed to that outcome.
Assesment of bias in conducting the systematic review
We have conducted the review according to the current guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We have reported any deviations from the previous review in the 'Differences between current review and previous review' section of the systematic review.
Measures of treatment effect
We have analysed dichotomous data as risk ratio (RR) and continuous data as standardised mean difference (SMD) since different scales were used for measuring the quality of life. We have ensured that higher scores for continuous outcomes have the same meaning for the particular outcome, explained the direction to the reader and reported where the directions were reversed if this was necessary. We have calculated the rate ratio for outcomes such as adverse events and serious adverse events, where it is possible for the same person to develop more than one adverse event (or serious adverse event). If the authors had calculated the rate ratio of adverse events (or serious adverse events) in the intervention versus control based on Poisson regression, we planned to obtain the rate ratio by the Poisson regression method in preference to rate ratio calculated based on the number of adverse events (or serious adverse events) during a certain period. We planned to calculate the hazard ratio (HR) for time‐to‐event outcomes such as time‐to‐first adverse event (or serious adverse event).
We have undertaken meta‐analyses if this was meaningful, that is if the treatments, participants and the underlying clinical question were similar enough for pooling to make sense.
A common way that trialists indicate when they have skewed data is by reporting medians and interquartile ranges. When we encountered this we planned to note that the data is skewed and consider the implication of this.
Where multiple trial arms are reported in a single trial, we planned to include only the relevant arms. If two comparisons (e.g. omeprazole versus laparoscopic fundoplication and lansoprazole versus laparoscopic fundoplication) must be entered into the same meta‐analysis, we planned to halve the control group to avoid double counting. The alternative way of including such trials with multiple arms is to pool the results of the omeprazole and lansoprazole and compare it with laparoscopic fundoplication. We planned to perform a sensitivity analysis to determine if the results of the two methods of dealing with multi‐arm trials lead to different conclusions.
Unit of analysis issues
The units of analyses were individual patients with GORD. If cluster‐randomised trials were identified, we planned to obtain the effect estimate adjusted for the clustering effect. If this was not available, we planned to perform a sensitivity analysis excluding the trial from the meta‐analysis as the variance of the effect estimate unadjusted for cluster effect is less than the actual variance which is adjusted for cluster‐effect, giving inappropriately more weight to the cluster RCT in the meta‐analysis.
Dealing with missing data
We attempted to contact investigators or study sponsors in order to verify key study characteristics and obtain missing numerical outcome data where possible (e.g. when a study is identified as abstract only). If we were unable to obtain the information from the investigators or study sponsors, we planned to impute mean from median (i.e. consider median as the mean) and standard deviation from standard error, inter‐quartile range, or P values according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011c) but planned to assess the impact of including such studies as indicated in a sensitivity analysis. If we were unable to calculate the standard deviation from standard error, inter‐quartile range, or P values, we planned to impute standard deviation as the highest standard deviation in the remaining trials included in the outcome, fully aware that this method of imputation would decrease the weight of the studies in the meta‐analysis of mean difference and shift the effect towards no effect for standardised mean difference.
Assessment of heterogeneity
We used the I² statistic to measure heterogeneity among the trials in each analysis (Higgins 2003). If we identified substantial heterogeneity as per the Cochrane Handbook for Systematic Reviews of Interventions (> 50% to 60%), we planned to explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
We attempted to contact study authors to ask them to provide missing outcome data. When this was not possible, and the missing data were thought to introduce serious bias, we planned to assess the impact of including such studies in the overall assessment of results by a sensitivity analysis.
Since there were fewer than 10 trials for all the outcomes, we did not create and examine a funnel plot to explore possible publication biases. We planned to use Egger's test to determine the statistical significance of the reporting bias (Egger 1997). We planned to use a P value less than 0.05 to show statistically significant reporting bias.
Data synthesis
We performed analyses using RevMan 5.3 (Review Manager 2014). We used the Mantel Haenszel method for dichotomous data, inverse variance method for continuous data, and generic inverse variance for count data. We planned to use generic inverse variance for time‐to‐event data. We used both the fixed‐effect model and random‐effects model for the analysis. We have used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (Demets 1987). In case of discrepancy between the two models, we have reported both results; otherwise we have reported only the results from the fixed‐effect model.
'Summary of findings' table
We created a 'Summary of findings' table using all outcomes. We used the five GRADE (Grades of Recommendation, Assessment, Development and Evaluation) considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of a body of evidence as it related to the studies that contributed data to the meta‐analyses for prespecified outcomes.
We used methods and recommendations as described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and used GRADEpro software (GRADEpro GDT). We justified all decisions to downgrade or upgrade the quality of studies by using footnotes and making comments to aid the reader's understanding of the review whenever necessary. We planned to consider whether any additional outcome information could not be incorporated into the meta‐analyses and noted this in the comments, stating whether it supported or contradicted the information derived from the meta‐analyses.
Subgroup analysis and investigation of heterogeneity
We planned to carry out the following subgroup analyses.
Presence versus absence of hiatus hernia.
Includes proton pump inhibitor as part of treatment versus those that do not include proton pump inhibitors as part of treatment.
Standard recommended dose versus high dose medical treatment.
Different methods of fundoplication.
We planned to use all the primary outcomes in subgroup analysis. We planned to use the formal chi2 test for subgroup differences to test for subgroup interactions.
Sensitivity analysis
We planned to perform sensitivity analysis to assess the robustness of our conclusions. This would have involved:
Excluding trials at unclear or high risk of bias (one of more of the risk of bias domains (other than blinding of surgeon) classified as unclear or high).
Excluding trials in which either mean or standard deviation or both were imputed.
Excluding cluster RCTs in which the adjusted effect estimates were not reported.
Different methods of dealing with multi‐arm trials (please see Measures of treatment effect).
Reaching conclusions
We have based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We have avoided making recommendations for practice and our implications for research explain any remaining uncertainties to the reader, giving a clear sense of what the focus of any future research in the area should be.
Results
Description of studies
Results of the search
We identified 222 references through electronic searches of the different databases. After removing duplicate references there were 173 references. We excluded 147 clearly irrelevant references through reading abstracts. A total of 26 references were retrieved for further assessment in detail, from the full publication. We excluded nine references for the reasons listed in the 'Characteristics of excluded studies'. Seventeen references of four randomised controlled trials (RCTs) fulfilled the inclusion criteria (Characteristics of included studies). The reference flow is shown in Figure 1.
1.
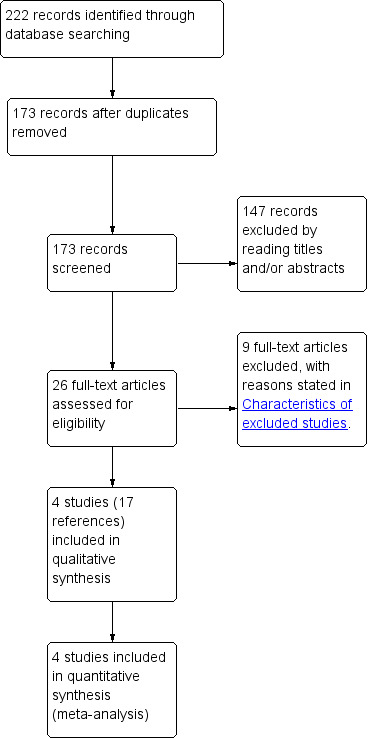
Study flow diagram.
Included studies
The four studies were published between 2005 and 2011. Two were performed in multiple centres in the UK (Grant 2008; Mahon 2005), one was conducted in 11 European countries (Lundell 2008) and the remaining study was conducted at a single centre in Canada (Anvari 2011). The characteristics of the included studies are described in the 'Characteristics of included studies' table. Sample sizes in the trials ranged from 104 to 554 participants, with a total of 1232 randomised participants. Seventy two of these participants were excluded in two trials (Anvari 2011; Mahon 2005). Outcomes were reported on the remaining 1160 participants in the four trials.
Participants
The mean age of participants in the included trials ranged from 43 to 48 years (Anvari 2011; Grant 2008; Lundell 2008; Mahon 2005). The proportion of women in the trials ranged from 28% to 59% in the three trials that provided this information (Anvari 2011; Grant 2008; Lundell 2008). In two trials, the participants had had reflux symptoms for at least 12 months (Anvari 2011; Grant 2008). In the remaining two trials, the participants had had reflux symptoms for at least six months (Lundell 2008; Mahon 2005). In two trials, the participants had received acid suppressive therapy for at least 12 months (Anvari 2011; Grant 2008). In one trial, the participants had received acid suppressive therapy for at least three months (Mahon 2005). In the remaining trial, the participants had received long‐term acid suppressive therapy but the minimum duration of this treatment was not reported (Lundell 2008). None of the trials reported the proportion of participants who had hiatus hernia or Barrett's oesophagus. All the trials included participants who could undergo surgery if randomised to the surgery arm.
Intervention and control
Surgery was by laparoscopic Nissen fundoplication in three trials (Anvari 2011; Lundell 2008; Mahon 2005). In the remaining trial, laparoscopic fundoplication was performed according to the surgeon's preference (Grant 2008). In all the four trials, laparoscopic fundoplications were performed by experienced surgeons (Anvari 2011; Grant 2008; Lundell 2008; Mahon 2005). In all four trials, the medical treatment was proton pump inhibitor. In one trial, the proton pump inhibitor used was esomeprazole 20 mg daily that could be increased step‐wise up to 40 mg daily and could be adjusted to 20 mg twice daily (Lundell 2008). In the remaining trials, proton pump inhibitors were administered according to local protocol (Anvari 2011; Grant 2008; Mahon 2005).
Excluded studies
Two references were non‐randomised studies (Gutschow 2009; Rantanen 2013). Four references included open anti‐reflux surgery (Lundell 2000; Lundell 2007; Lundell 2009; Spechler 2001) and three references included endoscopic anti‐reflux surgery (Trad 2013; Trad 2014; Witteman 2013) as the method of anti‐reflux surgery.
Risk of bias in included studies
The risk of bias is summarised in Figure 2 and Figure 3.
2.
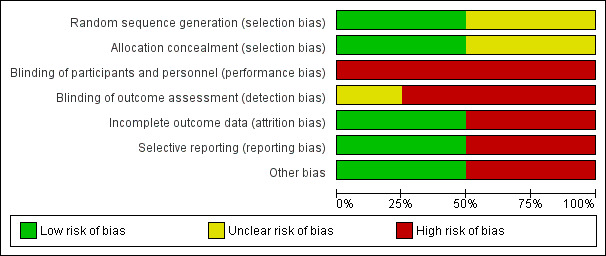
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
3.
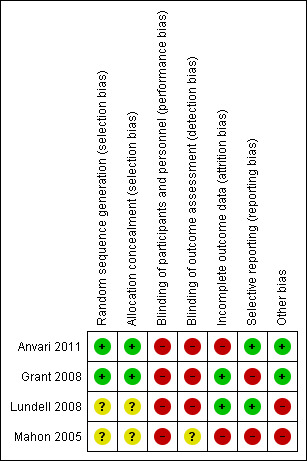
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Two trials described the method of random sequence generation and allocation concealment and can be considered to be at low risk of selection bias (Anvari 2011; Grant 2008). The random sequence generation and allocation concealment were not reported in the remaining two trials (Lundell 2008; Mahon 2005).
Blinding
It is impossible to blind participants and the healthcare providers who perform the intervention in trials comparing medical and surgical treatments. So, all the trials were at high risk of performance bias. It is possible to blind the outcome assessors. However, none of the trials reported blinding of outcome assessors. So, all the trials were at unclear or high risk of detection bias.
Incomplete outcome data
In two trials, 20% and 24% of participants were excluded from analysis (Anvari 2011; Mahon 2005). These trials were at high risk of attrition bias. In the remaining two trials, an intention‐to‐treat analysis was performed and so these two trials were at low risk of attrition bias (Grant 2008; Lundell 2008).
Selective reporting
We were unable to identify a published protocol of any of the trials. Two trials reported the important clinical outcomes adequately and are considered to be at low risk of selective reporting bias (Anvari 2011; Lundell 2008). The remaining two trials did not report the treatment‐related complications adequately and are at high risk of selective reporting bias (Grant 2008; Mahon 2005).
Other potential sources of bias
Two trials were funded by organisations with vested interests in the results and are subject to bias due to their source of funding (Lundell 2008; Mahon 2005). The remaining two trials were funded by organisations without vested interests in the results and were at low risk of source‐of‐funding bias. The surgeons in all the trials were experienced in laparoscopic fundoplication and are free from differential expertise bias.
Effects of interventions
See: Table 1
The effect of intervention on various outcomes is summarised in the 'Summary of findings' Table. Long‐term overall health‐related quality of life and long‐term GORD‐specific quality of life were not reported in any of the trials.
Health related quality of life (HRQoL)
Short‐term (four weeks to 12 months)
Three trials reported short‐term HRQoL (Anvari 2011; Grant 2008; Mahon 2005). One trial used SF‐36 to measure HRQoL (Anvari 2011). One trial used EQ‐5D to measure HRQoL (Grant 2008). The last trial used a general well‐being score to measure HRQoL (Mahon 2005). There was no statistically significant difference in HRQoL between the two groups (SMD 0.14, 95% CI ‐0.02 to 0.30; participants = 605; studies = 3; I2 = 64%). There was substantial heterogeneity in the results. Using the random‐effects model did not alter the conclusions.
Medium‐term (one year to five years)
Two trials reported medium‐term HRQoL (Anvari 2011; Grant 2008). One trial used SF‐36 to measure HRQoL (Anvari 2011). The other trial used EQ‐5D to measure HRQoL (Grant 2008). There was no statistically significant difference in HRQoL between the two groups (SMD 0.03, 95% CI ‐0.19 to 0.24; participants = 323; studies = 2; I2 = 65%). There was substantial heterogeneity in the results. Using the random‐effects model did not alter the conclusions.
GORD‐specific quality of life (QoL)
Short‐term (four weeks to 12 months)
All four trials reported short‐term GORD‐specific QoL (Anvari 2011; Grant 2008; Lundell 2008; Mahon 2005). One trial used the Gastroesophageal Reflux Symptom Score (GERSS) to measure GORD‐specific QoL (Anvari 2011). One trial used REFLUX QoL to measure GORD‐specific QoL (Grant 2008). One trial used the Gastrointestinal Symptom Rating Scale (GSRS) to measure GORD‐specific QoL (Lundell 2008). The last trial used a gastrointestinal well‐being score to measure GORD‐specific QoL (Mahon 2005). The GORD‐specific QoL was significantly better with laparoscopic fundoplication than medical treatment (SMD 0.58, 95% CI 0.46 to 0.70; participants = 1160; studies = 4; I2 = 40%). The heterogeneity was not considered important since all the studies were suggesting better GORD‐specific QoL in the surgical groups than in the medical treatment groups. Using the random‐effects model did not alter the conclusions.
Medium‐term (one year to five years)
Three trials reported medium‐term GORD‐specific QoL (Anvari 2011; Grant 2008; Lundell 2008). One trial used the Gastroesophageal Reflux Symptom Score (GERSS) to measure GORD‐specific QoL (Anvari 2011). One trial used REFLUX QoL to measure GORD‐specific QoL (Grant 2008). The last trial used Gastrointestinal Symptom Rating Scale (GSRS) to measure GORD‐specific QoL (Lundell 2008). The GORD‐specific QoL was significantly better with laparoscopic fundoplication than medical treatment (SMD 0.34, 95% CI 0.22 to 0.47; participants = 994; studies = 3; I2 = 94%) when the fixed‐effect model was used, but on using the random‐effects model, there was no statistically significant difference in GORD‐specific QoL between the two groups (SMD 0.28, 95% CI ‐0.27 to 0.84; participants = 994; studies = 3; I2 = 94%). There was considerable heterogeneity in the results between the studies.
Serious adverse events
Two trials reported the severity of complications (Anvari 2011; Lundell 2008). There were no serious adverse events in either group in one trial (Anvari 2011). In the other trial, the proportion of people with serious adverse events was statistically significantly higher in the laparoscopic fundoplication group than in the medical treatment group (RR 1.46, 95% CI 1.01 to 2.11; participants = 637; studies = 2). Since only one trial contributed to the meta‐analysis (the other trial being a zero‐event trial), we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Adverse events
One trial reported all adverse events (Anvari 2011). There was no statistically significant difference in the proportion of people with adverse events between the groups (RR 13.98, 95% CI 0.82 to 237.07; participants = 83; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Dysphagia
Short‐term (four weeks to 12 months)
Two trials reported short‐term dysphagia (Anvari 2011; Lundell 2008). The proportion of people with dysphagia was statistically significantly higher after laparoscopic fundoplication than medical treatment (RR 3.58, 95% CI 1.91 to 6.71; participants = 637; studies = 2; I2 = 0%). There was no heterogeneity in the results between the studies. Using the random‐effects model did not alter the conclusions.
Medium‐term (one year to five years)
One trial reported medium‐term dysphagia (Lundell 2008). The proportion of people with dysphagia was statistically significantly higher after laparoscopic fundoplication than medical treatment (RR 5.36, 95% CI 2.10 to 13.64; participants = 554; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Long‐term (five years or more)
One trial reported long‐term dysphagia (Grant 2008). There was no statistically significant difference in the proportion of people with dysphagia between the two groups (RR 0.90, 95% CI 0.57 to 1.42; participants = 228; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Heartburn
Short‐term (four weeks to 12 months)
One trial reported short‐term heartburn (Lundell 2008). The proportion of people with heartburn was statistically significantly less after laparoscopic fundoplication than medical treatment (RR 0.45, 95% CI 0.30 to 0.69; participants = 554; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Medium‐term (one year to five years)
One trial reported medium‐term heartburn (Lundell 2008). The proportion of people with heartburn was statistically significantly less after laparoscopic fundoplication than medical treatment (RR 0.19, 95% CI 0.10 to 0.34; participants = 554; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Long‐term (five years or more)
One trial reported long‐term heartburn (Grant 2008). The proportion of people with heartburn was statistically significantly less after laparoscopic fundoplication than medical treatment (RR 0.56, 95% CI 0.44 to 0.72; participants = 217; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Reflux
Short‐term (four weeks to 12 months)
One trial reported short‐term reflux (Lundell 2008). The proportion of people with reflux was statistically significantly less after laparoscopic fundoplication than medical treatment (RR 0.10, 95% CI 0.05 to 0.24; participants = 554; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Medium‐term (one year to five years)
One trial reported medium‐term reflux (Lundell 2008). The proportion of people with reflux was statistically significantly less after laparoscopic fundoplication than medical treatment (RR 0.15, 95% CI 0.06 to 0.35; participants = 554; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Long‐term (five years or more)
One trial reported long‐term reflux (Grant 2008). There was no statistically significant difference in the proportion of people with reflux between the two groups (RR 0.69, 95% CI 0.46 to 1.03; participants = 233; studies = 1). Since only one trial was included in this outcome, we did not assess heterogeneity and the issue of fixed‐effect model versus random‐effects model does not arise.
Subgroup analysis
We did not perform any subgroup analysis. None of the trials reported the results separately for participants with hiatus hernia, so a subgroup analysis of participants with hiatus hernia could not be performed. All the trials included proton pump inhibitors as part of the medical treatment, so a subgroup analysis of trials that included proton pump inhibitors as part of treatment versus those that did not include proton pump inhibitors as part of treatment could not be performed. Only one of the trials specified the dose of proton pump inhibitor, so a subgroup analysis of standard recommended dose versus high dose medical treatment could not be performed. Three trials used laparoscopic Nissen fundoplication and one trial used laparoscopic fundoplication according to the surgeon's preference, so a subgroup analysis of different methods of fundoplication could not be performed.
Sensitivity analysis
We did not perform any sensitivity analysis. All the trials were at high risk of bias, so a sensitivity analysis excluding trials at unclear or high risk of bias could not be performed. We did not impute the mean or standard deviation for any of the comparisons, so a sensitivity analysis excluding trials in which either mean or standard deviation or both were imputed could not be performed. There were no cluster RCTs, so a sensitivity analysis excluding cluster RCTs in which the adjusted effect estimates were not reported could not be performed. All the trials were two‐armed trials, so a sensitivity analysis excluding different methods of dealing with multi‐arm trials could not be performed.
Reporting bias
Two trials did not report the treatment‐related complications adequately. Hence the outcomes 'serious adverse events' and 'adverse events' are subject to reporting bias. Since there were fewer than 10 trials for all the outcomes, we did not create and examine a funnel plot to explore possible publication biases.
Discussion
Summary of main results
In this systematic review of laparoscopic fundoplication versus medical treatment, we identified four RCTs. There was no evidence of any difference in the overall short‐term or medium‐term health‐related quality of life between the groups. The short‐term GORD‐specific quality of life was better in the laparoscopic fundoplication group than in the medical treatment group. However, there was no evidence of any difference in the medium‐term GORD‐specific quality of life. None of the trials reported long‐term overall or GORD‐specific quality of life. The proportion of people with serious adverse events was higher in the laparoscopic fundoplication group than in the medical treatment group. There was no evidence of any difference in the proportion of people with overall adverse events between the groups. The proportion of people with dysphagia was greater in the laparoscopic fundoplication group than in the medical treatment group in the short and medium terms. There was no evidence of any difference in the proportion of people with long‐term dysphagia between the groups. The proportion of people with heartburn was less in the laparoscopic fundoplication group than in the medical treatment group at short term, medium term and long term, and the proportion of people with reflux symptoms was also less in the laparoscopic fundoplication group than in the medical treatment group at short term and medium term. There was no evidence of any difference in the proportion of people with long‐term reflux symptoms between the groups.
All the studies indicated better GORD‐specific quality of life in the short term in the laparoscopic fundoplication group compared to the medical treatment group. Despite using valid scales to measure the GORD‐specific quality of life, it is not clear how the difference translates into clinical importance to the patient, such as fewer work days lost or improved social activity. There was no evidence of a difference in the GORD‐specific quality of life beyond the short term. There was no evidence of a difference in the health‐related quality of life between the groups at any time‐point. It is not reasonable to prefer one treatment over another because of the short‐lived benefits of laparoscopic fundoplication over medical treatment.
Dysphagia was higher in the laparoscopic fundoplication group than in the medical treatment group in the short and medium terms. Heartburn and reflux symptoms were less in the laparoscopic fundoplication group than in the medical treatment group in the short and medium terms. Long‐term heartburn symptoms were less in the laparoscopic fundoplication group than in the medical treatment group but there was no evidence of a difference in the long‐term dysphagia or reflux symptoms. Severity and impact (social and economic) were not reported, so it is difficult to recommend treatments based on these outcomes.
Adverse events and their severity were not reported adequately in two trials (Grant 2008; Mahon 2005). It is important to know the complications related to the two treatment groups and the severity of these complications before the treatment can be recommended. It was not possible to assess this from this systematic review. A review of a US database including 13,050 patients who had undergone laparoscopic fundoplication reported a reoperation rate of approximately 5% at 5 years and 7% at 10 years (Zhou 2015). The majority of these reoperations were 'redo' fundoplications (87% of reoperations) while the remaining 13% of reoperations were reversal of fundoplication (Zhou 2015). Redo fundoplications are generally carried out for failure of improvement of existing symptoms or new symptoms of GORD (Smith 2005). Reversal of fundoplication is usually performed because of symptoms such as bloating and dysphagia. A review of a Danish database including 2465 patients who had undergone an open or laparoscopic fundoplication reported a 30‐day mortality of 0.46% and a reoperation rate of 5% (Funch‐Jensen 2008). Thus, the complications of laparoscopic fundoplication cannot be taken lightly. The major adverse events associated with long‐term proton pump inhibitor treatment are a modest increase in the risk of osteoporosis‐related fractures; an increase in the risk of Clostridium difficile infections in people who are admitted to hospital, from 0.3% to 0.9%; an increase in community‐acquired pneumonias; and an increase of approximately 30% in hospital‐acquired pneumonias from the baseline rate of 2% (Gray 2010; Herzig 2009; Howell 2010; Katz 2010b; Lambert 2015).
The choice between surgical and medical treatment is therefore likely to be based on whether people prefer to undergo a procedure that has a risk of mortality of around 0.5% and a reoperation rate of around 5%, or take long‐term medical treatment which carries a modest increased risk of fractures, Clostridium difficile infections if admitted to hospital, and pneumonia.
Overall completeness and applicability of evidence
All four studies included in this review compared laparoscopic fundoplication with proton pump inhibitor treatment. The results of this systematic review are therefore only applicable when the choice has to be made between laparoscopic fundoplication and proton pump inhibitor treatment in people for whom laparoscopic fundoplication or long‐term proton pump inhibitor treatment is a viable option.
Quality of the evidence
All the trials were considered to be at high risk of bias. While it is impossible to blind participants and healthcare providers who perform the treatment, it is possible to blind outcome assessors. Given that most of the outcomes are subjective outcomes, lack of outcome assessor blinding introduces bias to the effect estimates. Another major source of bias is the post‐randomisation drop‐out of 20% and 24% in two trials (Anvari 2011; Mahon 2005). In another trial, only 60% to 65% of participants were included in the long‐term outcomes (Grant 2008) reflecting the difficulty in long‐term follow‐up of trial participants. Exclusion of the participants is likely to overestimate the treatment benefits. The proportion of people who underwent surgery and who started taking medical treatment (hence exposing the patients to the risk of long‐term medical treatment) was not clear from the trials. There was substantial heterogeneity in some of the outcomes (short‐term and medium‐term overall health‐related quality of life and medium‐term GORD‐specific quality of life). In the absence of significant heterogeneity in participants and treatment (three trials used laparoscopic Nissen fundoplication and one trial used laparoscopic fundoplication according to the surgeon's preference; three trials used a pragmatic approach of medical treatment according to local protocol), the heterogeneity could be because of the instruments used to measure the quality of life. This also introduces additional uncertainty since different validated instruments result in different results. Many of the outcomes had only been reported by one of the included trials. This introduces uncertainty since we can consider that these results were never reproduced. Overall, the quality of evidence was low or very low for all the outcomes.
Potential biases in the review process
We took a systematic approach to each stage in the review process, including the search for literature, selection of titles and abstracts for full critical appraisal and data extraction. Two authors independently assessed all studies and disagreements were resolved by discussion.
Agreements and disagreements with other studies or reviews
The previous version of this review suggested that laparoscopic fundoplication surgery was more effective than medical management for the treatment of GORD at least in the short to medium term, that surgery carries some risk, and whether the benefits of surgery are sustained in the long term remains uncertain (Wileman 2010). Our conclusions have changed with use of current Cochrane Handbook guidelines to interpret the evidence.
Authors' conclusions
Implications for practice.
There is considerable uncertainty in the balance of benefits versus harms of laparoscopic fundoplication compared to long‐term medical treatment with proton pump inhibitors.
Implications for research.
Further RCTs of laparoscopic fundoplication versus medical management in patients with GORD should be conducted with outcome‐assessor blinding, and should include all participants in the analysis. Such trials should include long‐term patient‐orientated outcomes such as treatment‐related adverse events (including severity), quality of life, and also report on the social and economic impact of the adverse events and symptoms.
What's new
| Date | Event | Description |
|---|---|---|
| 16 June 2015 | New citation required and conclusions have changed | The previous version of this review suggested that laparoscopic fundoplication surgery was more effective than medical management for the treatment of GORD at least in the short to medium term and that surgery carries some risk and whether the benefits of surgery are sustained in the long term remains uncertain (Wileman 2010). The conclusions have changed with use of current Cochrane Handbook guidelines to interpret the evidence. |
| 16 June 2015 | New search has been performed | Literature searches were rerun. No new studies were identified. New review methods incorporated. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2010
| Date | Event | Description |
|---|---|---|
| 16 March 2010 | Amended | Citation corrected in Discussion section. |
Acknowledgements
We would like to thank Karin Dearness, Jan Lilleyman, and Cathy Bennett for their help and encouragement with this review. We gratefully acknowledge the work of the review team who were responsible for the original review concept, and wrote earlier published versions of the review. In these earlier versions, Samantha Wileman and Julie Bruce entered the data and wrote the text of the full review. Zygmunt Krukowski provided clinical expertise and Adrian Grant provided review expertise. All authors commented on the review.
Appendices
Appendix 1. CENTRAL search strategy
Date Run: 16 June 2015. Via OVID platform
106 trials
CENTRAL (Cochrane Central Register of Controlled Trials) (The Cochrane Library Issue 8 of 12, August 2014)
ID Search
#1 MeSH descriptor: [Esophagus] explode all trees
#2 (esophag* or oesophag*)
#3 MeSH descriptor: [Gastroesophageal Reflux] explode all trees
#4 gastroesophageal reflux
#5 gastro oesophageal reflux
#6 gord
#7 gerd
#8 MeSH descriptor: [Duodenogastric Reflux] explode all trees
#9 bile reflux
#10 acid reflux
#11 gastric acid secret*
#12 stomach acid secret*
#13 gastric eros*
#14 stomach eros*
#15 MeSH descriptor: [Heartburn] this term only
#16 heartburn
#17 indigestion
#18 MeSH descriptor: [Esophagitis] explode all trees
#19 esophagitis
#20 oesophagitis
#21 low* sphincter* pressur*
#22 MeSH descriptor: [Gastric Emptying] explode all trees
#23 MeSH descriptor: [Gastroparesis] explode all trees
#24 MeSH descriptor: [Gastritis] explode all trees
#25 gastr* empt* disorder*
#26 stomach empt* disorder*
#27 MeSH descriptor: [Dyspepsia] this term only
#28 dyspep*
#29 MeSH descriptor: [Eructation] this term only
#30 eructation
#31 regurgitat*
#32 MeSH descriptor: [Hernia, Hiatal] this term only
#33 {OR #1 ‐ #32}
#34 MeSH descriptor: [Proton Pump Inhibitors] 1 tree(s) exploded
#35 proton pump inhibitor*
#36 ppi
#37 omeprazole
#38 esomeprazole
#39 lansoprazole
#40 pantoprazole
#41 rabeprazole
#42 MeSH descriptor: [Histamine H2 Antagonists] explode all trees
#43 cimetidine
#44 famotidine
#45 nizatidine
#46 ranitidine
#47 burimamide
#48 {OR #34 ‐ #47}
#49 MeSH descriptor: [Fundoplication] this term only
#50 fundoplication*
#51 nissen or rossetti
#52 toupet or lind or watson or besley
#53 {OR #49 ‐ #52}
#54 #33 and #48 and #53
Appendix 2. MEDLINE search strategy
Date of Search: Ovid MEDLINE 16 June 2015
Database: Ovid MEDLINE(R) <1946 to 16 June 2015>
1. exp Esophagus/
2. (esophag$ or oesophag$).mp.
3. exp gastroesophageal reflux/
4. (gastroesophageal adj3 reflux).mp.
5. (gastro adj3 oesophageal adj3 reflux).mp.
6. (gastro adj3 esophageal adj3 reflux).mp.
7. (gerd or gord).mp.
8. exp Duodenogastric Reflux/
9. bile reflux.mp.
10. (acid adj3 reflux).mp.
11. (gastric adj3 acid adj3 secret$).mp.
12. (stomach adj3 acid adj3 secret$).mp.
13. (gastric adj3 eros$).mp.
14. (stomach adj3 eros$).mp.
15. Heartburn/
16. (heartburn or indigestion).mp.
17. exp Esophagitis/
18. (esophagitis or oesophagitis).mp.
19. (low$ adj6 sphincter$ adj3 pressur$).mp.
20. Gastric Emptying/
21. Gastroparesis/
22. exp Gastritis/
23. (gastr$ adj3 empt$ adj3 disorder$).mp.
24. (stomach adj3 empt$ adj3 disorder$).mp.
25. Dyspepsia/
26. dyspep$.mp.
27. Eructation/
28. eructation.mp.
29. regurgitat$.mp.
30. Hernia, Hiatal/
31. hernia$ hiat$.mp.
32. or/1 ‐31
33. exp Proton Pump Inhibitors/
34. (proton adj3 pump adj3 inhibitor$).mp.
35. ppi.mp.
36. omeprazole.mp.
37. esomeprazole.mp.
38. lansoprazole.mp.
39. pantoprazole.mp.
40. rabeprazole.mp.
41. exp proton pumps/ai [Antagonists & Inhibitors]
42. exp Histamine H2 Antagonists/
43. (histamine H2 adj3 antagonist$).mp.
44. H2RA$.mp.
45. cimetidine.mp.
46. famotidine.mp.
47. nizatidine.mp.
48. ranitidine.mp.
49. burimamide.mp.
50. or/33 ‐ 49
51. Fundoplication/
52. fundoplication$.mp.
53. (nissen or rossetti).mp.
54. (toupet or lind or watson or besley).mp.
55. 51 or 52 or 53 or 54
56. 32 and 50 and 55
57. randomized controlled trial.pt.
58. controlled clinical trial.pt.
59. randomized.ab.
60. randomly.ab.
61. placebo.ab.
62. drug therapy.fs.
63. trial.ab.
64. groups.ab.
65. or/57 ‐ 64
66. 56 and 65
Lines 57‐64 were modified from Box 6.4.c: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐maximizing version (2008 revision); Ovid format in Higgins 2011a.
Appendix 3. EMBASE search strategy
Date run in Embase (OVID): 16 June 2015
Database: Embase Classic+Embase 1947 to 16 June 2015
1. exp esophagus/
2. (esophag$ or oesophag$).mp.
3. exp gastroesophageal reflux/
4. (gastroesophageal adj3 reflux).mp.
5. (gastro adj3 oesophageal adj3 reflux).mp.
6. (gastro adj3 esophageal adj3 reflux).mp.
7. (gerd or gord).mp.
8. exp duodenogastric reflux/
9. bile reflux.mp.
10. (acid adj3 reflux).mp.
11. (gastric adj3 acid adj3 secret$).mp.
12. (stomach adj3 acid adj3 secret$).mp.
13. (gastric adj3 eros$).mp.
14. (stomach adj3 eros$).mp.
15. heartburn/
16. (heartburn or indigestion).mp.
17. exp esophagitis/
18. (esophagitis or oesophagitis).mp.
19. (low$ adj6 sphincter$ adj3 pressur$).mp.
20. stomach emptying/
21. stomach paresis/
22. exp gastritis/
23. (gastr$ adj3 empt$ adj3 disorder$).mp.
24. (stomach adj3 empt$ adj3 disorder$).mp.
25. dyspepsia/
26. dyspep$.mp.
27. eructation/
28. eructation.mp.
29. regurgitat$.mp.
30. hiatus hernia/
31. hernia$ hiat$.mp.
32. or/1‐ 31
33. proton pump inhibitor/
34. (proton adj3 pump adj3 inhibitor$).mp.
35. ppi.mp.
36. omeprazole/
37. omeprazole.mp.
38. esomeprazole/
39. esomeprazole.mp.
40. lansoprazole/
41. lansoprazole.mp.
42. pantoprazole/
43. pantoprazole.mp.
44. rabeprazole/
45. rabeprazole.mp.
46. histamine H2 receptor antagonist/
47. (histamine H2 adj3 antagonist$).mp.
48. H2RA$.mp.
49. cimetidine/
50. cimetidine.mp.
51. famotidine/
52. famotidine.mp.
53. nizatidine/
54. nizatidine.mp.
55. ranitidine/
56. ranitidine.mp.
57. burimamide/
58. burimamide.mp.
59. or/33 ‐ 58
60. stomach fundoplication/
61. fundoplication$.mp.
62. (nissen or rossetti).mp.
63. (toupet or lind or watson or besley).mp.
64. 60 or 61 or 62 or 63
65. 32 and 59 and 64
66. crossover procedure/
67. double blind procedure/
68. randomized controlled trial/
69. single‐blind procedure/
70. random$.ab.
71. factorial$.ab.
72. crossover$.ab.
73. cross over$.ab.
74. cross‐over$.ab.
75. placebo$.ab.
76. (doubl$ adj blind$).ab.
77. (singl$ adj blind$).ab.
78. assign$.mp.
79. allocat$.ab.
80. volunteer$.ab.
81. or/66 ‐ 80
82. 65 and 81
Results: 199
Lines 66‐80 from sections 6.4.11.2 Search filters for identifying randomized trials in EMBASE and section 6.3.2.2 What is in The Cochrane Central Register of Controlled Trials (CENTRAL) from EMBASE? in Higgins 2011a.
Appendix 4. Previous search strategies
CENTRAL search strategy
#1 esophagus/ #2 (esophag* or oesophag*) #3 exp gastroesophageal reflux/ #4 (gastroesophageal reflux) #5 gastro oesophageal reflux #6 gord #7 gerd #8 duodenogastric reflux/ #9 bile reflux/ #10 acid reflux #11 gastric acid secret* #12 stomach acid secret* #13 gastric eros* #14 (stomach eros*) #15 heartburn/ #16 (heartburn or indigestion) #17 esophagitis/ #18 esophagitis #19 oesophagitis #20 (low* sphincter* pressur*) #21 les #22 gastric emptying/ #23 gastroparesis/ #24 gastritis/ #25 gastr* empt* disorder* #26 stomach empt* disorder #27 dyspepsia/ #28 dyspep* #29 eructation/ #30 eructat* #31 regurgitat* #32 Hiatal hernia #33 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR ( # AND 20 ) OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30 OR #31 OR #32) #34 proton pump/ #35 proton pump inhibitor* #36 ppi #37 Omeprazole/ #38 omeprazole #39 esomeprazole #40 lansoprazole #41 pantoprazole #42 rabeprazole #43 Histamine H2 Antagonists/ #44 cimetidine #45 Cimetidine/ #46 Famotidine #47 Nizatidine #48 Ranitidine #49 (#34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42 OR #43 OR #44 OR #45 OR #46 OR #47 OR #48) #50 Fundoplication/ #51 nissen or rossetti #52 toupet or lind or watson or besley #53 (partial* fundoplication*) #54 (laparoscop* fundoplication*) #55 (#50 OR #51 OR #52 OR #53 OR #54) #56 (#33 AND #49 AND #55), from 2006 to 2009
EMBASE search strategy
(random$ or placebo$).ti,ab.
((single$ or double$ or triple$ or treble$) and (blind$ or mask$)).ti,ab.
controlled clinical trial$.ti,ab.
RETRACTED ARTICLE/
or/1‐4
(animal$ not human$).sh,hw.
5 not 6
exp esophagus/
(esophag$ or oesophag$).tw.
exp gastroesophageal reflux/
(gastroesophageal adj3 reflux).tw.
(gastro adj3 oesophageal adj3 reflux).tw.
(gastro adj3 esophageal adj3 reflux).tw.
gord.tw.
gerd.tw.
exp duodenogastric reflux/
exp bile reflux/
(acid adj3 reflux).tw.
(gastric adj3 acid adj3 secret$).tw.
(stomach adj3 acid secret$).tw.
(gastric adj3 eros$).tw.
(stomach adj3 eros$).tw.
exp heartburn/
(heartburn or indigestion).tw.
exp esophagitis/
esophagitis.tw.
oesophagitis.tw.
(low$ adj6 sphincter$ adj3 pressur$).tw.
les.tw.
exp gastric emptying/
exp gastroparesis/
exp gastritis/
(gastr$ adj3 empt$ adj3 disorder$).tw.
(stomach adj3 empt$ adj3 disorder).tw.
exp dyspepsia/
dyspep$.tw.
exp eructation/
eructat$.tw.
regurgitat$.tw.
Hernia, Hiatal/
or/8‐40
exp proton pump/
esomeprazole.mp.
(proton adj3 pump adj3 inhibitor$).tw.
ppi.tw.
exp Omeprazole/
omeprazole.tw.
lansoprazole.tw.
pantoprazole.tw.
rabeprazole.tw.
exp Histamine H2 Antagonists/
cimetidine.tw.
exp Cimetidine/
famotidine.tw. or exp Famotidine/
nizatidine.tw. or exp Nizatidine/
ranitidine.tw. or exp Ranitidine/
or/42‐56
exp Fundoplication/
(nissen or rossetti).tw.
(toupet or lind or watson or besley).tw.
(partial$ adj5 fundoplication$).tw.
(laparoscop$ adj5 fundoplication$).tw.
or/58‐62
41 and 57 and 63
7 and 64
limit 65 to yr="2006 ‐Current"
MEDLINE search strategy
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
clinical trials as topic.sh.
randomly.ab.
trial.ti.
or/1‐7
(animals not (humans and animals)).sh.
8 not 9
exp esophagus/
(esophag$ or oesophag$).tw.
exp gastroesophageal reflux/
(gastroesophageal adj3 reflux).tw.
(gastro adj3 oesophageal adj3 reflux).tw.
(gastro adj3 esophageal adj3 reflux).tw.
gord.tw.
gerd.tw.
exp duodenogastric reflux/
exp bile reflux/
(acid adj3 reflux).tw.
(gastric adj3 acid adj3 secret$).tw.
(stomach adj3 acid secret$).tw.
(gastric adj3 eros$).tw.
(stomach adj3 eros$).tw.
exp heartburn/
(heartburn or indigestion).tw.
exp esophagitis/
esophagitis.tw.
oesophagitis.tw.
(low$ adj6 sphincter$ adj3 pressur$).tw.
les.tw.
exp gastric emptying/
exp gastroparesis/
exp gastritis/
(gastr$ adj3 empt$ adj3 disorder$).tw.
(stomach adj3 empt$ adj3 disorder).tw.
exp dyspepsia/
dyspep$.tw.
exp eructation/
eructat$.tw.
regurgitat$.tw.
Hernia, Hiatal/
or/11‐43
exp proton pump/
esomeprazole.mp.
(proton adj3 pump adj3 inhibitor$).tw.
ppi.tw.
exp Omeprazole/
omeprazole.tw.
lansoprazole.tw.
pantoprazole.tw.
rabeprazole.tw.
exp Histamine H2 Antagonists/
cimetidine.tw.
exp Cimetidine/
famotidine.tw. or exp Famotidine/
nizatidine.tw. or exp Nizatidine/
ranitidine.tw. or exp Ranitidine/
or/45‐59
exp Fundoplication/
(nissen or rossetti).tw.
(toupet or lind or watson or besley).tw.
(partial$ adj5 fundoplication$).tw.
(laparoscop$ adj5 fundoplication$).tw.
or/61‐65
44 and 60 and 66
10 and 67
limit 68 to yr="2006 ‐Current"
Data and analyses
Comparison 1. Laparoscopic fundoplication versus medical management.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Health‐related quality of life (< 1 year) | 3 | 605 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.02, 0.30] |
| 2 Health‐related quality of life (1 to 5 years) | 2 | 323 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.03 [‐0.19, 0.24] |
| 3 GORD‐specific quality of life (< 1 year) | 4 | 1160 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.58 [0.46, 0.70] |
| 4 GORD‐specific quality of life (1 to 5 years) | 3 | 994 | Std. Mean Difference (IV, Random, 95% CI) | 0.28 [‐0.27, 0.84] |
| 5 Serious adverse events | 2 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [1.01, 2.11] |
| 6 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Dysphagia (< 1 year) | 2 | 637 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.58 [1.91, 6.71] |
| 8 Dysphagia (1 to 5 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Dysphagia (5 years or more) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 Heartburn (< 1 year) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11 Heartburn (1 to 5 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12 Heartburn (5 years or more) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 13 Reflux (< 1 year) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14 Reflux (1 to 5 years) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Reflux (5 years or more) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.1. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 1 Health‐related quality of life (< 1 year).
1.2. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 2 Health‐related quality of life (1 to 5 years).
1.3. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 3 GORD‐specific quality of life (< 1 year).
1.4. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 4 GORD‐specific quality of life (1 to 5 years).
1.5. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 5 Serious adverse events.
1.6. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 6 Adverse events.
1.7. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 7 Dysphagia (< 1 year).
1.8. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 8 Dysphagia (1 to 5 years).
1.9. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 9 Dysphagia (5 years or more).
1.10. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 10 Heartburn (< 1 year).
1.11. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 11 Heartburn (1 to 5 years).
1.12. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 12 Heartburn (5 years or more).
1.13. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 13 Reflux (< 1 year).
1.14. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 14 Reflux (1 to 5 years).
1.15. Analysis.
Comparison 1 Laparoscopic fundoplication versus medical management, Outcome 15 Reflux (5 years or more).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anvari 2011.
| Methods | Randomised clinical trial | |
| Participants | Country: Canada. Number randomised: 104 Post‐randomisation drop‐outs: 21 (20.2%) Revised sample size: 83 Average age: 43 years Females: 49 (59%) Barretts oesophagus: not stated Hiatus hernia: not stated Body mass index: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: surgery (n = 43) Further details: Laparoscopic Nissen fundoplication Group 2: medical treatment (n = 40) Further details: Same PPI dose as previous treatment. | |
| Outcomes | The outcomes reported were health‐related quality of life, GERD‐specific quality of life, and adverse events. | |
| Notes | Reasons for post‐randomisation drop‐outs: nine: surgical treatment (one did not follow received allocation; eight lost to follow‐up) and 12: medical treatment (two did not follow received allocation; 10 lost to follow‐up). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The uniform random integers, 0 through 9, were generated in Minitab 12 (Minitab Inc, State College, PA) for each stratum….. The groups of 16 for blocks of 2 or 4 were generated at random, using single‐digit random numbers, odd being SC (surgery, control) and even being CS". |
| Allocation concealment (selection bias) | Low risk | Quote: "To this extent the research assistants checking the eligibility were not exposed to the continuing randomization list". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Consequently, this study did not have blinded implementation of the treatment arms". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Consequently, this study did not have blinded implementation of the treatment arms". |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes were reported. |
| Other bias | Low risk | Quote: "Supported by grants from Canadian Institute of Health Research and Ontario Ministry of Health and Long‐term Care". |
Grant 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: United Kingdom Number randomised: 357 Post‐randomisation drop‐outs: not stated Revised sample size: 357 Average age: 46 years Females: 121 (33.9%) Barretts oesophagus: not stated Hiatus hernia: not stated Body mass index: 29 Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: surgery (n = 178) Further details: Laparoscopic fundoplication as per surgeon preference. Group 2: medical treatment (n = 179) Further details: Medical treatment as per local protocol. | |
| Outcomes | The outcomes reported were health‐related quality of life, GORD‐specific quality of life, proportion with heartburn, reflux, and dysphagia. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation was organised centrally by a secure system, using a computer‐generated sequence, stratified by clinical site, with balance in respect of age (18 ‐ 49 or ≥ 50), sex (men or women), and BMI (≤ 28 or > 29) secured by minimisation". |
| Allocation concealment (selection bias) | Low risk | Quote: "Staff in the central trial office entered details of participants on the secure database, then notified participants and respective clinical sites of their allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "There was no subsequent blinding". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "There was no subsequent blinding". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | High risk | Comment: Treatment‐related complications were not reported adequately. |
| Other bias | Low risk | Quote: "This study was funded by the NIHR Health Technology Assessment Programme (as part of project No 97/10/99)". |
Lundell 2008.
| Methods | Randomised clinical trial | |
| Participants | Country: Europe Number randomised: 554 Post‐randomisation drop‐outs: not stated Revised sample size: 554 Average age: 45 years Females: 156 (28.2%) Barretts oesophagus: 60 (10.8%) Hiatus hernia: not stated Body mass index: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: surgery (n = 288) Further details: Laparoscopic Nissen fundoplication Group 2: medical treatment (n = 266) Further details: Esomeprezole 20 mg once daily increased to maximum of 40 mg once daily or 20 mg twice daily. | |
| Outcomes | The outcomes reported were GORD‐specific quality of life, proportion with heartburn, reflux, dysphagia and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Patients undergoing medical treatment did not have sham surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: An intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Low risk | Comment: Important outcomes were reported. |
| Other bias | High risk | Quote: "This study was funded by Astrazeneca, Mölndal, Sweden". |
Mahon 2005.
| Methods | Randomised clinical trial | |
| Participants | Country: United Kingdom Number randomised: 217 Post‐randomisation drop‐outs: 51 (23.5%) Revised sample size: 166 Average age: 48 years Females: not stated Barretts oesophagus: not stated Hiatus hernia: not stated Body mass index: not stated Inclusion criteria
Exclusion criteria
|
|
| Interventions | Participants were randomly assigned to two groups. Group 1: surgery (n = 80) Further details: Laparoscopic Nissen fundoplication Group 2: medical treatment (n = 86) Further details: Proton pump inhibitor adjusted to symptom control. | |
| Outcomes | The outcomes reported were health‐related quality of life and gastrointestinal quality of life. | |
| Notes | Reasons for post‐randomisation drop‐outs: Lost‐to follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: Patients undergoing medical treatment did not have sham surgery. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs. |
| Selective reporting (reporting bias) | High risk | Comment: Treatment‐related complications were not reported adequately. |
| Other bias | High risk | Quote: "This study was funded partly by Janssen Pharmaceuticals relating to physiology studies at the Norfolk Physiology Laboratory in conjunction with their GI Partnership Scheme. Yvette Sharpe performed most of these studies under the supervision of R.L. B.D., D.M. and B. K. were funded partly by Ethicon Endosurgery, UK". |
GERD or GORD = gastro‐oesophageal reflux disease PPI = proton pump inhibitor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gutschow 2009 | Not a randomised controlled trial |
| Lundell 2000 | Open anti‐reflux surgery |
| Lundell 2007 | Open anti‐reflux surgery |
| Lundell 2009 | Open anti‐reflux surgery |
| Rantanen 2013 | Not a randomised controlled trial |
| Spechler 2001 | Open anti‐reflux surgery |
| Trad 2013 | Transoral anti‐reflux surgery |
| Trad 2014 | Transoral anti‐reflux surgery |
| Witteman 2013 | Transoral anti‐reflux surgery |
Differences between protocol and review
The protocol for this review included the comparison of alternative surgical laparoscopic approaches (total versus partial fundoplication) as a secondary objective. A comparison of the alternative surgical approaches for the management of GORD is being addressed by a separate systematic review (MacKay 2010). The risk of bias section has been updated from the protocol and uses the assessment criteria recommended in the updated version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Differences between previous version and current version
The review has been updated according to the updated version of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
The following outcomes were moved to secondary outcomes: heartburn, reflux, and dysphagia.
Serious adverse events have been included as primary outcome.
Adverse events have been include as secondary outcome.
Contributions of authors
Conceiving the review: this is an update of the previous review (Wileman 2010).
Designing the review: this is an update of the previous review (Wileman 2010).
Coordinating the review: SG, KG
Designing search strategies: UGPD
Writing the review: SG, KG
Providing general advice on the review: Not applicable
Securing funding for the review: KG
Performing previous work that was the foundation of the current study: this is an update of the previous review (Wileman 2010).
Sources of support
Internal sources
University of Aberdeen. Health Services Research Unit, UK.
Department of Public Health, University of Aberdeen, UK.
External sources
Chief Scientist Office of the Scottish Government Health Directorates, UK.
-
NHS R&D HTA Programme, UK.
This project was supported by the National Institute for Health Research via Cochrane Programme Grant funding to the CHBG and UGPD Groups. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
SG: none known
KG: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anvari 2011 {published data only}
- Anvari M, Allen C, Goldsmith C. A randomized controlled trial of laparoscopic Nissen fundoplication (LNF) versus proton pump inhibitors for treatment of patients with chronic gastro‐esophageal reflux disease (GERD) who complained of cough. Gastroenterology 2010;138(5 SUPPL):S885. [Google Scholar]
- Anvari M, Allen C, Marshall J, Armstrong D, Goeree R, Ungar W, et al. A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for the treatment of patients with chronic gastroesophageal reflux disease (GERD): 3‐year outcomes. Surgical Endoscopy 2011;25(8):2547‐54. [DOI] [PubMed] [Google Scholar]
- Anvari M, Allen C, Marshall J, Armstrong D, Goeree R, Ungar W, et al. A randomized controlled trial of laparoscopic Nissen fundoplication versus proton pump inhibitors for treatment of patients with chronic gastroesophageal reflux disease: one‐year follow‐up. Surgical Innovation 2006;13(4):238‐49. [DOI] [PubMed] [Google Scholar]
- Goeree R, Hopkins R, Marshall JK, Armstrong D, Ungar WJ, Goldsmith C, et al. Cost‐utility of laparoscopic Nissen fundoplication versus proton pump inhibitors for chronic and controlled gastroesophageal reflux disease: a 3‐year prospective randomized controlled trial and economic evaluation. Value in Health 2011;14(2):263‐73. [DOI] [PubMed] [Google Scholar]
Grant 2008 {published data only}
- Epstein D, Bojke L, Sculpher MJ, The REFLUX trial group. Laparoscopic fundoplication compared with medical management for gastro‐oesophageal reflux disease: cost effectiveness study. BMJ 2009;339:b2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant A, Wileman S, Ramsay C, Bojke L, Epstein D, Sculpher M, et al. The effectiveness and cost‐effectiveness of minimal access surgery amongst people with gastro‐oesophageal reflux disease ‐ a UK collaborative study. The REFLUX trial. Health Technology Assessment 2008;12(31):1‐181. [DOI] [PubMed] [Google Scholar]
- Grant AM, Boachie C, Cotton SC, Faria R, Bojke L, Epstein DM, et al. Clinical and economic evaluation of laparoscopic surgery compared with medical management for gastro‐oesophageal reflux disease: 5‐year follow‐up of multicentre randomised trial (the REFLUX trial). Health Technology Assessment 2013;17(22):1‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AM, Cotton SC, Boachie C, Ramsay CR, Krukowski ZH, Heading RC, et al. Minimal access surgery compared with medical management for gastro‐oesophageal reflux disease: five year follow‐up of a randomised controlled trial (REFLUX). BMJ 2013;346:f1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AM, Wileman SM, Ramsay CR, Mowat NA, Krukowski ZH, Heading RC, et al. Minimal access surgery compared with medical management for chronic gastro‐oesophageal reflux disease: UK collaborative randomised trial. BMJ (Clinical Research edition) 2008;337:a2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant AM, Wileman SM, Ramsay CR, Mowat NA, Krukowski ZH, Heading RC, et al. Minimal access surgery compared with medical management for chronic gastro‐oesophageal reflux disease: UK collaborative randomised trial. BMJ (Online) 2009;338(7686):81‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundell 2008 {published data only}
- Attwood SE, Lundell L, Hatlebakk JG, Eklund S, Junghard O, Galmiche JP, et al. Medical or surgical management of GERD patients with Barrett's Esophagus: the LOTUS trial 3‐year experience. Journal of Gastrointestinal Surgery 2008;12(10):1646‐54. [DOI] [PubMed] [Google Scholar]
- Attwood SEA, Lundell L, Ell C, Galmiche JP, Hatlebakk J, Fiocca R, et al. Standardization of surgical technique in antireflux surgery: the LOTUS trial experience. World Journal of Surgery 2008;32(6):995‐8. [DOI] [PubMed] [Google Scholar]
- Fiocca R, Mastracci L, Engstrom C, Attwood S, Ell C, Galmiche JP, et al. Long‐term outcome of microscopic esophagitis in chronic GERD patients treated with esomeprazole or laparoscopic antireflux surgery in the LOTUS trial. American Journal of Gastroenterology 2010;105(5):1015‐23. [DOI] [PubMed] [Google Scholar]
- Galmiche JP, Hatlebakk JG, Attwood SE, Ell C, Fiocca R, Eklund S, et al. Laparoscopic antireflux surgery vs long‐term esomeprazole treatment for chronic GERD. Final results after 5 yrs of follow up in the LOTUS study. Gastroenterology 2010;138(5 SUPPL):S53. [Google Scholar]
- Lundell L, Attwood S, Ell C, Fiocca R, Galmiche JP, Hatlebakk J, et al. Comparing laparoscopic antireflux surgery with esomeprazole in the management of patients with chronic gastro‐oesophageal reflux disease: a 3‐year interim analysis of the LOTUS trial. Gut 2008;57(9):1207‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahon 2005 {published data only}
- Mahon D, Rhodes M, Decadt B, Hindmarsh A, Lowndes R, Beckingham I, et al. Randomized clinical trial of laparoscopic Nissen fundoplication compared with proton‐pump inhibitors for treatment of chronic gastro‐oesophageal reflux. British Journal of Surgery 2005;92(6):695‐9. [DOI] [PubMed] [Google Scholar]
- Mehta S, Bennett J, Mahon D, Rhodes M. Prospective trial of laparoscopic Nissen fundoplication versus proton pump inhibitor therapy for gastroesophageal reflux disease: seven‐year follow‐up. Journal of Gastrointestinal Surgery 2006;10(9):1312‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gutschow 2009 {published data only}
- Gutschow CA, Schroder W, Bludau M, Leers J, Bollschweiler E, Holscher AH. Azide and non‐azide intraoesophageal bolus movements after Nissen fundoplication and under high‐dose ppi: Evaluation using combined 24‐hour impedance‐ph‐monitoring of the oesophagus. Zeitschrift für Gastroenterologie 2009;47(9):867. [Google Scholar]
Lundell 2000 {published data only}
- Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Thor K, Lamm M, et al. Long‐term management of gastro‐oesophageal reflux disease with omeprazole or open antireflux surgery: results of a prospective, randomized clinical trial. European Journal of Gastroenterology and Hepatology 2000;12(8):879‐87. [DOI] [PubMed] [Google Scholar]
Lundell 2007 {published data only}
- Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Malm A, et al. Seven‐year follow‐up of a randomized clinical trial comparing proton‐pump inhibition with surgical therapy for reflux oesophagitis. British Journal of Surgery 2007;94(2):198‐203. [DOI] [PubMed] [Google Scholar]
Lundell 2009 {published data only}
- Lundell L, Miettinen P, Myrvold HE, Hatlebakk JG, Wallin L, Engstrom C, et al. Comparison of outcomes twelve years after antireflux surgery or omeprazole maintenance therapy for reflux esophagitis. Clinical Gastroenterology and Hepatology 2009;7(12):1292‐8. [DOI] [PubMed] [Google Scholar]
Rantanen 2013 {published data only}
- Rantanen T, Kiljander T, Salminen P, Ranta A, Oksala N, Kellokumpu I. Reflux symptoms and side effects among patients with gastroesophageal reflux disease at baseline, during treatment with PPIs, and after Nissen fundoplication. World Journal of Surgery 2013;37(6):1291‐6. [DOI] [PubMed] [Google Scholar]
Spechler 2001 {published data only}
- Spechler SJ, Lee E, Ahnen D, Goyal RK, Hirano I, Ramirez F, et al. Long‐term outcome of medical and surgical therapies for gastroesophageal reflux disease: follow‐up of a randomized controlled trial. Journal of the American Medical Association 2001;285(18):2331‐8. [DOI] [PubMed] [Google Scholar]
Trad 2013 {published data only}
- Trad KS, Barnes WE, Simoni G, Shughoury AB, Raza M, Heise JA, et al. Transoral fundoplication vs. proton pump inhibitor therapy for treatment of chronic gastroesophageal reflux: short‐term results from a multicenter, randomized, open label clinical trial. Gastroenterology 2013;144(5 SUPPL):S163‐S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trad 2014 {published data only}
- Trad KS, Fox MA, Simoni G, Shughoury AB, Mavrelis PG, Raza M, et al. Efficacy of transoral fundoplication for treatment of chronic gastroesophageal reflux disease incompletely controlled with high‐dose PPI therapy: a randomized, multicenter, open label, crossover study. Gastroenterology 2014;146(5 SUPPL):S127‐S8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Witteman 2013 {published data only}
- Witteman BP, Conchillo JM, Koek GH, Peeters A, Stassen LP, Bouvy ND. Randomized controlled trial of transoral incisionless fundoplication versus proton pump inhibitors for treatment of GERD; an interim analysis. Gastrointestinal Endoscopy 2013;77(5 SUPPL):AB459. [Google Scholar]
Additional references
Achem 1998
- Achem SR, Robinson M. A prokinetic approach to treatment of gastroesophageal reflux disease. Digestive Diseases 1998;16(1):38‐46. [DOI] [PubMed] [Google Scholar]
Boeckxstaens 2014a
- Boeckxstaens G, El‐Serag HB, Smout AJPM, Kahrilas PJ. Symptomatic reflux disease: the present, the past and the future. Gut 2014;63(7):1185‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boeckxstaens 2014b
- Boeckxstaens GE, Rohof WO. Pathophysiology of gastroesophageal reflux disease. Gastroenterology Clinics of North America 2014;43(1):15‐25. [DOI] [PubMed] [Google Scholar]
Cook 2014
- Cook MB, Corley DA, Murray LJ, Liao LM, Kamangar F, Ye W, et al. Gastroesophageal reflux in relation to adenocarcinomas of the esophagus: a pooled analysis from the Barrett's and Esophageal Adenocarcinoma Consortium (BEACON). PloS One 2014;9(7):e103508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Darba 2011
- Darba J, Kaskens L, Plans P, Elizalde JI, Coma M, Cuomo R, et al. Epidemiology and societal costs of gastroesophageal reflux disease and Barrett's syndrome in Germany, Italy and Spain. Expert Review of Pharmacoeconomics & Outcomes Research 2011;11(2):225‐32. [DOI] [PubMed] [Google Scholar]
Demets 1987
- Demets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6:341‐8. [DOI: ] [DOI] [PubMed] [Google Scholar]
Dent 2005
- Dent J, El‐Serag HB, Wallander MA, Johansson S. Epidemiology of gastroesophageal reflux disease: a systematic review. Gut 2005;54:710‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dent 2008
- Dent J. Endoscopic grading of reflux oesophagitis: the past, present and future. Best Practice & Research Clinical Gastroenterology 2008;22(4):585‐99. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
El‐Serag 2014
- El‐Serag HB, Sweet S, Winchester CC, Dent J. Update on the epidemiology of gastro‐oesophageal reflux disease: a systematic review. Gut 2014;63(6):871‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
FDA 2006
- Center for Biologics Evaluation and Research, U.S. Food, Drug Administration. Guidance for industry adverse reactions section of labeling for human prescription drug and biological products — Content and format. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm075057.pdf 2006 (accessed on 4 July 2014).
Funch‐Jensen 2008
- Funch‐Jensen P, Bendixen A, Iversen MG, Kehlet H. Complications and frequency of redo antireflux surgery in Denmark: a nationwide study, 1997‐2005. Surgical Endoscopy 2008;22(3):627‐30. [DOI] [PubMed] [Google Scholar]
Gordon 2004
- Gordon C, Kang JY, Neild PJ, Maxwell JD. The role of the hiatus hernia in gastro‐oesophageal reflux disease. Alimentary Pharmacology and Therapeutics 2004;20(7):719‐32. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime, Inc). GRADEpro GDT: GRADEpro Guideline Development Tool. Available from www.gradepro.org, 2015.
Gray 2010
- Gray SL, LaCroix AZ, Larson J, Robbins J, Cauley JA, Manson JE, et al. Proton pump inhibitor use, hip fracture, and change in bone mineral density in postmenopausal women: Results from the women's health initiative. Archives of Internal Medicine 2010;170(9):765‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herzig 2009
- Herzig SJ, Howell MD, Ngo LH, Marcantonio ER. Acid‐suppressive medication use and the risk for hospital‐acquired pneumonia. JAMA 2009;301(20):2120‐8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. The Cochrane Collaboration, Available from www.cochrane‐handbook.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Higgins 2011c
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 7: Selecting studies and collecting data . In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Howell 2010
- Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, et al. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Archives of Internal Medicine 2010;170(9):784‐90. [DOI] [PubMed] [Google Scholar]
ICH‐GCP 1996
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. Code of Federal Regulation & ICH Guidelines. Media: Parexel Barnett, 1996. [Google Scholar]
Katz 2010a
- Katz PO, Zavala S. Proton pump inhibitors in the management of GERD. Journal of Gastrointestinal Surgery 2010;14 Suppl 1:S62‐6. [DOI] [PubMed] [Google Scholar]
Katz 2010b
- Katz MH. Failing the acid test: benefits of proton pump inhibitors may not justify the risks for many users. Archives of Internal Medicine 2010;170(9):747‐8. [DOI] [PubMed] [Google Scholar]
Lagergren 1999
- Lagergren J, Bergstrom R, Lindgren A, Nyren O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. New England Journal of Medicine 1999;340(11):825‐31. [DOI] [PubMed] [Google Scholar]
Lambert 2015
- Lambert AA, Lam JO, Paik JJ, Ugarte‐Gil C, Drummond MB, Crowell TA. Risk of community‐acquired pneumonia with outpatient proton‐pump inhibitor therapy: a systematic review and meta‐analysis. PloS One 2015;10(6):e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lundell 2001
- Lundell L, Miettinen P, Myrvold HE, Pedersen SA, Liedman B, Hatlebakk JG, et al. Continued (5‐year) followup of a randomized clinical study comparing antireflux surgery and omeprazole in gastroesophageal reflux disease. Journal of the American College of Surgeons 2001;192(2):172‐9. [DOI] [PubMed] [Google Scholar]
MacKay 2010
- MacKay C, Wileman SM, Krukowski ZH, Bruce J. Laparoscopic fundoplication for gastro‐oesophageal reflux disease (GORD) in adults. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008719] [DOI] [PubMed] [Google Scholar]
Martindale 2011
- Sweetman S (editor). Martindale: the complete drug reference (online version), 37th edition. www.pharmpress.com/product/MC_MART/martindale‐the‐complete‐drug‐reference 2011 (accessed 23 September 2014).
MHRA 2013
- Medicines and Healthcare products Regulatory Agency (MHRA). Clinical trials for medicines: Safety reporting ‐ SUSARs and DSURs. http://www.mhra.gov.uk/Howweregulate/Medicines/Licensingofmedicines/Clinicaltrials/Safetyreporting‐SUSARsandASRs/ 2013 (accessed on 4 July 2014).
Moayyedi 2008
- Moayyedi P, Delaney B. GORD in adults. Clinical Evidence (Online) 2008;2008:0403. [PMC free article] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Peery 2012
- Peery AF, Dellon ES, Lund J, Crockett SD, McGowan CE, Bulsiewicz WJ, et al. Burden of gastrointestinal disease in the United States: 2012 update. Gastroenterology 2012;143(5):1179‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Sandler 2002
- Sandler RS, Everhart JE, Donowitz M, Adams E, Cronin K, Goodman C, et al. The burden of selected digestive diseases in the United States. Gastroenterology 2002;122(5):1500‐11. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Singhal 2009
- Singhal T, Balakrishnan S, Hussain A, Grandy‐Smith S, Paix A, El‐Hasani S. Management of complications after laparoscopic Nissen's fundoplication: a surgeon's perspective. Annals of Surgical Innovation and Research 2009;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2005
- Smith CD, McClusky DA, Rajad MA, Lederman AB, Hunter JG. When fundoplication fails: redo?. Annals of Surgery 2005;241(6):861‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Solaymani‐Dodaran 2004
- Solaymani‐Dodaran M, Logan RF, West J, Card T, Coupland C. Risk of oesophageal cancer in Barrett's oesophagus and gastro‐oesophageal reflux. Gut 2004;53(8):1070‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vakil 2006
- Vakil N, Zanten SV, Kahrilas P, Dent J, Jones R, Global Consensus G. The Montreal definition and classification of gastroesophageal reflux disease: a global evidence‐based consensus. American Journal of Gastroenterology 2006;101(8):1900‐20. [DOI] [PubMed] [Google Scholar]
Van Meer 2013
- Meer S, Bogte A, Siersema PD. Long‐term follow up in patients with gastroesophageal reflux disease with specific emphasis on reflux symptoms, use of anti‐reflux medication and anti‐reflux surgery outcome: a retrospective study. Scandinavian Journal of Gastroenterology 2013;48(11):1242‐8. [DOI] [PubMed] [Google Scholar]
Watson 1998
- Watson A. Update: total versus partial laparoscopic fundoplication. Digestive Surgery 1998;15(2):172‐80. [DOI] [PubMed] [Google Scholar]
Zacherl 2014
- Zacherl J, Roy‐Shapira A, Bonavina L, Bapaye A, Kiesslich R, Schoppmann SF, et al. Endoscopic anterior fundoplication with the Medigus Ultrasonic Surgical Endostapler (MUSE) for gastroesophageal reflux disease: 6‐month results from a multi‐center prospective trial. Surgical Endoscopy 2015;29(1):220‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhou 2015
- Zhou T, Harnsberger C, Broderick R, Fuchs H, Talamini M, Jacobsen G, et al. Reoperation rates after laparoscopic fundoplication. Surgical Endoscopy 2015;29(3):510‐4. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Wileman 2010
- Wileman SM, McCann S, Grant AM, Krukowski ZH, Bruce J. Medical versus surgical management for gastro‐oesophageal reflux disease (GORD) in adults. Cochrane Database of Systematic Reviews 2010, Issue 3. [DOI: 10.1002/14651858.CD003243.pub2] [DOI] [PubMed] [Google Scholar]


