Abstract
Background
The growing use of Electronic Health Records (EHRs) is promoting the application of data mining in health-care. A promising use of big data in this field is to develop models to support early diagnosis and to establish natural history. Dravet Syndrome (DS) is a rare developmental and epileptic encephalopathy that commonly initiates in the first year of life with febrile seizures (FS). Age at diagnosis is often delayed after 2 years, as it is difficult to differentiate DS at onset from FS. We aimed to explore if some clinical terms (concepts) are significantly more used in the electronic narrative medical reports of individuals with DS before the age of 2 years compared to those of individuals with FS. These concepts would allow an earlier detection of patients with DS resulting in an earlier orientation toward expert centers that can provide early diagnosis and care.
Methods
Data were collected from the Necker Enfants Malades Hospital using a document-based data warehouse, Dr Warehouse, which employs Natural Language Processing, a computer technology consisting in processing written information. Using Unified Medical Language System Meta-thesaurus, phenotype concepts can be recognized in medical reports. We selected individuals with DS (DS Cohort) and individuals with FS (FS Cohort) with confirmed diagnosis after the age of 4 years. A phenome-wide analysis was performed evaluating the statistical associations between the phenotypes of DS and FS, based on concepts found in the reports produced before 2 years and using a series of logistic regressions.
Results
We found significative higher representation of concepts related to seizures’ phenotypes distinguishing DS from FS in the first phases, namely the major recurrence of complex febrile convulsions (long-lasting and/or with focal signs) and other seizure-types. Some typical early onset non-seizure concepts also emerged, in relation to neurodevelopment and gait disorders.
Conclusions
Narrative medical reports of individuals younger than 2 years with FS contain specific concepts linked to DS diagnosis, which can be automatically detected by software exploiting NLP. This approach could represent an innovative and sustainable methodology to decrease time of diagnosis of DS and could be transposed to other rare diseases.
Keywords: Data mining, Natural Language Processing, Dravet syndrome, Rare Diseases, Early diagnosis
Objectives
Electronic health records (EHRs) contain healthcare data of individuals and population electronically-stored in a digital format [1]. In the last decade, the use of EHRs has become part of routine care across the majority of developed countries [2].
Through data mining techniques, this growing use of EHRs is allowing the development of predictive models aimed to individuate high risk patients and support prevention initiatives [3, 4]. As well, models to support diagnosis and treatment of rare diseases are emerging [5, 6].
EHRs consist of structured and unstructured data. Structured data are produced through constrained choices (drop-down menus, check boxes and pre-filled templates as in registries), whereas unstructured clinical data exist in the form of free text narratives and are often used in clinical care for medical reports [7]. Combining Natural Language Processing (NLP) technology and UMLS (Unified Medical Language System), providers’ notes and narratives can be converted into structured, standardized formats, usable for data mining [8–10].
Dravet Syndrome (DS) is a rare disorder, with a worldwide incidence between 1/40,000 and 1/15,700 [11]. DS is a genetic developmental and epileptic encephalopathy with onset in first year of life, characterized at onset by febrile seizures and convulsive status epilepticus in otherwise healthy infants [12]. Starting by the second year, individuals present multiple seizure types (clonic, tonic–clonic, motor and non-motor onset focal seizures, myoclonic, atypical absences), that are often drug resistant, with developmental slowing leading to definite cognitive impairment [13]. Diagnosis is easier after the age of two as more pathognomonic seizure types and other symptoms are present from this age. Genetic testing shows a pathogenic variant in SCN1A in over 85% of cases reinforcing the diagnosis suspicion, but this testing might take months and is not available for all individuals with suspected DS [14]. However there is a need for early diagnosis in order to avoid worsening therapies and to establish best therapy protocol as seizure control might be partly related to cognitive improvement and a better quality of life [15].
Early diagnosis of individuals with DS is often delayed as it is difficult to differentiate at onset from Febrile Seizures (FS) [16]. These two conditions present substantial clinical differences, leading to exclude one on other diagnosis but might be overlapping at onset. Even if physician awareness of Dravet syndrome has markedly improved in last decades [17], time to diagnosis is still over 2 years [18], and it remains underdiagnosed in adult population and in developing countries [19, 20].
Using data mining, we analysed clinical reports produced before the age of 2 years for individuals with confirmed DS and FS with the aim of identifying specific terms (concepts) allowing early DS suspicion and reducing diagnosis delay. We then explored the differences between the concepts in the reports of two subgroups of individuals with DS: patients with suspected diagnosis before the age of 2 years and patients for whom diagnosis was suspected after the age of two.
Materials and methods
Data were collected from Necker Enfants Malades Hospital, a paediatric University hospital belonging to the Assistance Publique Hopitaux de Paris group (400 paediatric beds, 200 adult beds), which is a national and European reference center for rare and undiagnosed diseases, including the reference a centre for rare epilepsies.
DrWarehouse® [21] (DrWH) is a document-based open-source data warehouse oriented toward narrative clinical reports from the Electronic health records (EHRs). It contains more than 4.5 million clinical free-text documents produced at Necker Hospital from 2009, for more than 465,000 individuals and more than 20 departments. DrWarehouse® uses UMLS Metathesaurus to recognize phenotype concepts inside narrative medical reports. In this manuscript, the word “concepts” will refer to phenotypes extracted automatically from hospital reports, without a priori, by using a UMLS subset of 20,000 phenotypic words or expressions.
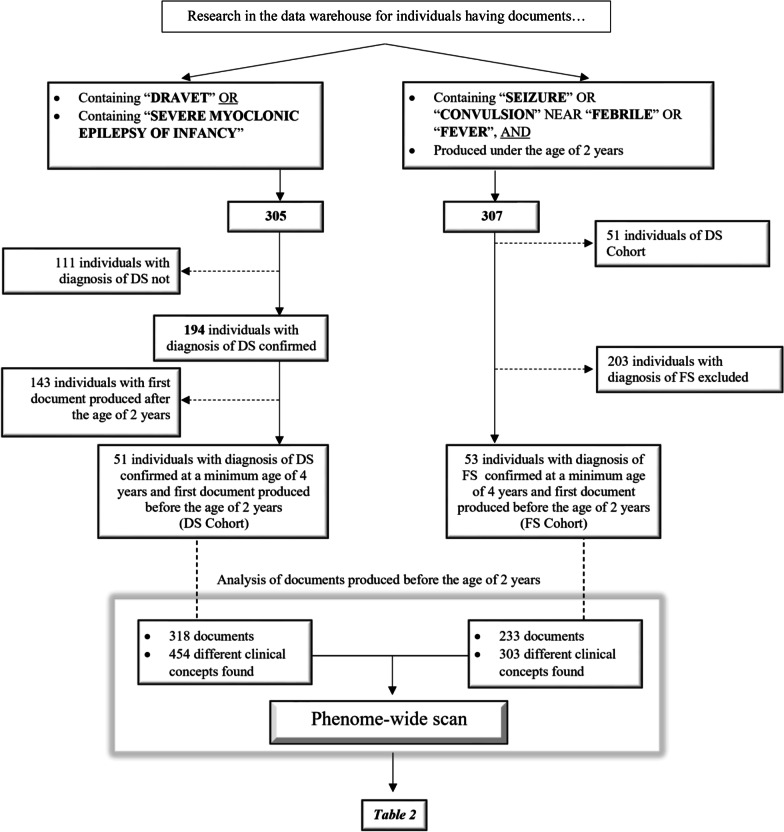
By using the appropriate research field in DrWarehouse®, we searched all individuals who presented in their medical reports the word “Dravet” or “Severe Myoclonic Epilepsy of Infancy” at least in one clinical document. We then selected from this group all individuals that had a definite diagnosis of DS based on clinical and genetic criteria, and evaluated after the age of four where the full blown syndrome can be confirmed. We finally included from this group individuals with at least one clinical report before the age of 2 years and this final selection constituted the “Dravet Syndrome Cohort” (DS Cohort).
Subsequently, we searched in the data of DrWarehouse all individuals whose medical reports produced before the age of two presented the words “seizure”/“seizures” or “convulsion”/“convulsions” in proximity (max 5 words away) to “fever” or “febrile”. From this group, we excluded the individuals of the DS Cohort and individuals in which febrile seizures was a symptom of a more complex condition (infections involving the central nervous system, other encephalopathies, structural brain injury, detected genetic or metabolic pathologies, or epilepsies). The “Febrile Seizures’cohort” (FS cohort) included the individuals from this group aged over year where we confirmed the diagnosis of febrile seizures based on EHRs or by telephone interviewing of the family (FS Cohort) (Fig. 1).
Fig. 1.
Flowchart of the selection procedures and constitution of the cohorts
The phenome-wide scan consists in comparing the distribution of phenotypes between two groups (cases and controls) and estimates the association between the phenotypes and the groups. These associations are assessed sequentially [22, 23]. We evaluated the statistical associations between the phenotypes and the cohorts DS and FS, using a series of multivariate logistic regressions adjusted on gender and age. For the analysis, we used concepts found in clinical reports with a minimum number of occurrences of three individuals, excluding negations and those associated to family members. The p-values were corrected for multiple testing using a false discovery rate (FDR) methodology.
We also compared the phenotype differences in the DS Cohort between the subgroup where diagnosis of DS was confirmed or suspected before the age of 2 years, and the subgroup where DS diagnosis was not reported.
Results
“Dravet Syndrome Cohort” (DS Cohort)
The term “Dravet” and/or “severe myoclonic epilepsy of infancy” appeared in 305 individuals present in the warehouse: 194 of them had a final diagnosis of DS in the last document on the database, 51 had at least one document produced under the age of 2 years. All had a clinical and genetic diagnosis of DS. These individuals constituted the DS Cohort.
DS cohort included 28 males and 23 females. The mean age at first seizure was 5.5 months (min 2–max 12). The average age of the first produced document was 1.05 years, median is 1.15 (min 0.25–max 1.98). The average length of the follow-up of these individuals was 5.68 years, median 4.98 (min 3.75–max 13.42).
In order to compare early characteristics of this population with a population with FS at the same age, documents produced exclusively before 2 years were selected, for a total of 318 documents (mean: 6.24; median: 3 for each individual). 3484 concepts were extracted from the abovementioned documents (mean: 10.9 per document), 454 of which were unique concepts. Concepts present in almost 10% of the population are listed in a decreasing order in the Table 1. The most prevalent concepts were “Seizures” (found in 48 individuals – 94%), “Fever” (43 individuals – 84%), “Epilepsy” (42 individuals – 82%), “Dravet Syndrome” (37 individuals – 73%), “Convulsions” (31 individuals – 61%).
Table 1.
Comparison between concepts found in more than 10% of individuals of DS Cohort (left) and FS Cohort (right)
| UMLS CUI code | Concept | DS cohort | FS cohort | ||
|---|---|---|---|---|---|
| Number of individuals | Frequency (%) | Number of individuals | Frequency (%) | ||
| C0036572 | Seizures | 48 | 94 | 44 | 83 |
| C0015967 | Fever | 43 | 84 | 48 | 91 |
| C0014544 | Epilepsy | 42 | 82 | 35 | 66 |
| C0751122 | Dravet syndrome | 37 | 73 | ||
| C0234972 | Convulsions | 31 | 61 | 40 | 75 |
| C1419856 | SCN1A | 28 | 55 | ||
| C0009952 | Febrile seizures | 21 | 41 | 37 | 70 |
| C0026827 | Hypotonia | 21 | 41 | 19 | 36 |
| C2825055 | Recurrence | 21 | 41 | 30 | 57 |
| C0234535 | Clonic | 17 | 33 | 9 | 17 |
| C1705236 | Deviation | 17 | 33 | ||
| C3809175 | Prolonged seizure | 16 | 31 | 8 | 15 |
| C2830004 | Drowsiness | 16 | 31 | 17 | 32 |
| C0038220 | Status epilepticus | 15 | 29 | 8 | 15 |
| C0259972 | Ketogenic diet | 13 | 25 | ||
| C0235195 | Sedation | 13 | 25 | ||
| C3887612 | Psychomotor agitation | 12 | 24 | 7 | 13 |
| C0009450 | Infection | 12 | 24 | 9 | 17 |
| C0027066 | Myoclonia | 11 | 22 | ||
| C1698630 | Virosis | 11 | 22 | 11 | 21 |
| C0030193 | Pain | 10 | 20 | 6 | 11 |
| C0013144 | Falling asleep | 10 | 20 | 12 | 23 |
| C0026837 | Hypertonus | 10 | 20 | 8 | 15 |
| C0029877 | Otitis | 10 | 20 | 13 | 25 |
| C0522336 | Rolling of eyes | 10 | 20 | 18 | 34 |
| C0010200 | Cough | 10 | 20 | 9 | 17 |
| C0004134 | Ataxia | 9 | 18 | ||
| C0006271 | Bronchiolitis | 9 | 18 | 11 | 21 |
| C0009443 | Rhinitis | 9 | 18 | 14 | 26 |
| C0085639 | Fall | 8 | 16 | ||
| C0424927 | Education | 8 | 16 | ||
| C0024115 | Pneumonia | 8 | 16 | ||
| C0684320 | Regression | 8 | 16 | ||
| C0020517 | Allergies | 7 | 14 | ||
| C0751495 | Focal Seizures | 7 | 14 | ||
| C0015672 | Fatigue | 7 | 14 | 7 | 13 |
| C0026205 | Myosis | 7 | 14 | ||
| C0036973 | Shiverings | 7 | 14 | ||
| C0035561 | Side | 6 | 12 | ||
| C0031350 | Pharingitis | 6 | 12 | 8 | 15 |
| C0424230 | Psychomotor DELAY | 6 | 12 | ||
| C0027441 | Nasopharyngitis | 6 | 12 | ||
| C0549209 | Startle | 6 | 12 | ||
| C0003123 | Anorexia | 5 | 10 | ||
| C0034642 | Rales | 5 | 10 | ||
| C0270844 | Tonic Seizures | 5 | 10 | 6 | 11 |
| C0010520 | Cyanosis | 5 | 10 | 9 | 17 |
| C0011991 | Diarrhea | 5 | 10 | ||
| C0017160 | Gastroenteritis | 5 | 10 | ||
| C0019209 | Hepatomegaly | 5 | 10 | ||
| C0013384 | Movement disorder | 5 | 10 | ||
| C0013604 | Edema | 5 | 10 | ||
| C0232483 | Reflux | 5 | 10 | ||
| C0035203 | Respiration | 5 | 10 | ||
| C0037763 | Spasms | 5 | 10 | ||
| C1504405 | Pyramidal syndrome | 5 | 10 | ||
| C0008049 | Chickenpox | 5 | 10 | 7 | 13 |
| C0596002 | Reflex | 19 | 36 | ||
| C0271429 | Acute otitis media | 9 | 17 | ||
| C0855324 | Normal pulse pressure | 8 | 15 | ||
| C0042963 | Vomiting | 8 | 15 | ||
| C0034150 | Purpura | 7 | 13 | ||
| C0494475 | Tonic–clonic seizures | 6 | 11 | ||
CUI concept unique identifiers
“Febrile Seizure Cohort” (FS Cohort)
The research of the words “seizure” or “convulsion” in individuals’ reports close to the words “febrile” or “fever”, limited to documents produced by the first 2 years of life and excluding individuals of DS Cohort, led to 256 subjects. After exclusion of other aetiologies, we included all 53 subjects with a diagnosis of febrile seizures. Diagnosis was confirmed after age four by reviewing child's medical history, neurological and developmental outcome in the available medical files in addition to a telephone interview with the family.
This cohort was constituted of 17 females and 36 males. The mean age of the first document produced was 1.18 years, while median was 1.3 (min 0.30–max 1.96). The mean duration of follow-up was 4.20 years, median 4.02 (min 3.70–max 5.57). The mean age at first seizure was 12.4 months (min 4–max 21) with 1 individual having an onset before 6 months and 23 before 12 months.
In order to compare phenotypes of FS Cohort with DS Cohort at the same age (before the age of 2 years), documents produced exclusively before 2 years were selected, for a total of 233 documents (mean 4.4; median 3 for each individual). From these, 2053 concepts have been extrapolated (mean 8.8 concepts per document), 303 of which were unique concepts.
The concepts present in more than 10% of individuals are shown in Table 1. The most prevalent concepts were “Fever” (found in 48 individuals—91%), “Seizures” (44 individuals—83%), “Convulsions” (40 individuals—75%), “Febrile Seizures” (37 individuals—70%), “Epilepsy” (35 individuals—66%).
Comparison of DS and FS cohorts
DS cohort was constituted of 54% of males and 46% of females while in FS cohort, gender comparison showed significant difference with 68% of males and 32% of females (p = 0.009).
The different length of follow-up at our centre among the two cohorts shows the higher medical needs for individuals with DS (mean 3.99 years, median 3.11) compared to individuals with FS (mean 1.82 years, median 1.37 years). Indeed, the follow-up at our centre often stops when the diagnosis of FS is confirmed, and children are usually referred back to their paediatrician or general practitioner.
The mean number of documents per individual produced during the same period (0–2 years), was higher in the population with DS (6.2 vs 4.4), as well as the mean number of concepts extrapolated per document (10.9 vs 8.8).
The phenome-wide comparison of both cohorts showed a different representation of a series of concepts (Table 2). Some of these concepts were related to seizures. Concept “Deviation” (p < 0.01), which is found within sentences describing focal seizures, point out to a significant higher occurrence of focal seizures in DS cohort compared to FS cohort. The frequency of “prolonged seizures” concept was also significantly higher in DS cohort (31% compared to 15% in FS cohort, p = 0.05. Another concept, “sedation”, which was used in the medical reports with reference to the post-ictal phase or to the need of rescue medication showed a significant difference (25% in the DS Cohort, 0% in the FS Cohort; p = 0.02). The concept “myoclonia” was not found in the FS Cohort, while was reported in 22% of individuals of DS Cohort (p = 0.02), and the concept “clonic” was reported two folds in the DS Cohort compared to the FS one (33% versus 17%, p = 0.05). The concept “febrile seizures” was significantly higher in the FS Cohort and was found in 70% of individuals compared to 41% of individuals of DS Cohort (p = 0.01). Other non seizures concepts were found only in the DS Cohort, namely “ataxia” (18%; p = 0.02), “regression” (16%; p = 0.03) and “pneumonia” (16%; p = 0.03).
Table 2.
Phenome-wide comparison of DS Cohort and FS Cohort
| UMLS CUI code | Concept | DS individuals with the concept(%) | FS individuals with the concept (%) | DS individuals without the concept (%) | FS individuals without the concept (%) | OR | p value |
|---|---|---|---|---|---|---|---|
| C0751122 | Dravet syndrome | 36 (70.6) | 0 (0) | 15 (29.4) | 53 (100) | 129.60 | 0.00 |
| C1419856 | SCN1A | 28 (54.9) | 0 (0) | 23 (45.1) | 53 (100) | 65.74 | 0.00 |
| C1705236 | Deviation | 17 (33.3) | 4 (7.5) | 34 (66.7) | 49 (92.5) | 6.37 | 0.00 |
| C0259972 | Ketogenic diet | 13 (25.5) | 0 (0) | 38 (74.5) | 53 (100) | 18.47 | 0.01 |
| C0009952 | Febrile seizures | 21 (41.2) | 37 (69.8) | 30 (58.8) | 16 (30.2) | 0.34 | 0.01 |
| C0235195 | Sedation | 13 (25.5) | 4 (7.5) | 38 (74.5) | 49 (92.5) | 4.36 | 0.02 |
| C0027066 | Myoclonia | 11 (21.6) | 3 (5.7) | 40 (78.4) | 50 (94.3) | 4.77 | 0.02 |
| C0004134 | Ataxia | 9 (17.6) | 1 (1.9) | 42 (82.4) | 52 (98.1) | 11.57 | 0.02 |
| C0024115 | Pneumonia | 8 (15.7) | 0 (0) | 43 (84.3) | 53 (100) | 10.05 | 0.03 |
| C0684320 | Regression | 8 (15.7) | 0 (0) | 43 (84.3) | 53 (100) | 10.05 | 0.03 |
| C0234535 | Clonic | 17 (33.3) | 9 (17) | 34 (66.7) | 44 (83) | 2.56 | 0.05 |
| C0085639 | Fall | 8 (15.7) | 2 (3.8) | 43 (84.3) | 51 (96.2) | 4.93 | 0.05 |
| C3809175 | Prolonged seizure | 16 (31.4) | 8 (15.1) | 35 (29.6) | 45 (84.9) | 2.87 | 0.05 |
| C0014544 | Epilepsy | 41 (80.4) | 35 (66) | 10 (19.6) | 18 (34) | 2.34 | 0.06 |
| C0038220 | Status epilepticus | 15 (29.4) | 8 (15.1) | 36 (70.6) | 45 (84.9) | 2.45 | 0.07 |
| C0424230 | Psychomotor delay | 6 (11.8) | 0 (0) | 45 (88.2) | 53 (100) | 7.20 | 0.07 |
| C0549209 | Startle | 6 (11.8) | 0 (0) | 45 (88.2) | 53 (100) | 7.20 | 0.07 |
| C0855324 | Normal pulse pressure | 2 (3.9) | 8 (15.1) | 49 (96.1) | 52 (98.1) | 0.24 | 0.08 |
| C0026205 | Myosis | 7 (13.7) | 2 (3.8) | 44 (86.3) | 51 (96.2) | 4.22 | 0.08 |
| C0036572 | Seizures | 47 (92.9) | 44 (83) | 4 (7.8) | 9 (17) | 2.94 | 0.08 |
| C0271429 | Acute otitis media | 3 (5.9) | 9 (17) | 48 (94.1) | 44 (83) | 0.32 | 0.10 |
| C0003123 | Anorexia | 5 (9.8) | 0 (0) | 46 (90.2) | 53 (100) | 5.87 | 0.11 |
| C0013604 | Edema | 5 (9.8) | 1 (1.9) | 46 (90.2) | 52 (98.1) | 5.87 | 0.11 |
| C0035203 | Respiration | 5 (9.8) | 1 (1.9) | 46 (90.2) | 52 (98.1) | 5.87 | 0.11 |
| C0037763 | Spasms | 5 (9.8) | 1 (1.9) | 46 (90.2) | 52 (98.1) | 5.87 | 0.11 |
| C0034150 | Purpura | 2 (3.9) | 7 (13.2) | 49 (96.1) | 46 (86.8) | 0.28 | 0.12 |
| C0522336 | Rolling of eyes | 10 (19.6) | 18 (34) | 41 (80.4) | 35 (66) | 0.50 | 0.13 |
| C0231218 | Malaise | 1 (2) | 5 (9.4) | 50 (98) | 48 (90.6) | 0.20 | 0.15 |
| C0427008 | Stiffness | 1 (2) | 5 (9.4) | 50 (98) | 48 (90.6) | 0.20 | 0.15 |
| C1336751 | Flat | 1 (2) | 5 (9.4) | 50 (98) | 48 (90.6) | 0.20 | 0.15 |
| C3887612 | Pyschomotor agitation | 12 (23.5) | 7 (13.2) | 39 (76.5) | 46 (86.8) | 2.11 | 0.15 |
| C0042963 | Vomiting | 3 (5.9) | 8 (15.1) | 48 (94.1) | 45 (84.9) | 0.37 | 0.16 |
| C0036973 | Shiverings | 7 (13.7) | 3 (5.7) | 44 (86.3) | 50 (94.3) | 2.76 | 0.16 |
| C0751495 | Focal seizures | 7 (13.7) | 3 (5.7) | 44 (86.3) | 50 (94.3) | 2.76 | 0.16 |
| C2825055 | Recurrence | 21 (41.2) | 30 (56.6) | 30 (58.8) | 23 (43.4) | 0.58 | 0.17 |
| C0013473 | Eating disorders | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0018989 | Hemiparesis | 4 (7.8) | 1 (1.9) | 47 (92.9) | 52 (98.1) | 4.60 | 0.18 |
| C0020649 | Hypotension | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0032290 | Aspiration Pneumonia | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0037036 | Hypersalivation | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0038450 | Stridor | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0205721 | Nosocomial infection | 4 (7.8) | 1 (1.9) | 47 (92.9) | 52 (98.1) | 4.60 | 0.18 |
| C0333641 | Atrophy | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0349506 | Photosensitivity | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C0428167 | FiO2 | 4 (7.8) | 1 (1.9) | 47 (92.9) | 52 (98.1) | 4.60 | 0.18 |
| C0865850 | Acute respiratory insufficiency | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
| C1504405 | Pyramidal syndrome | 4 (7.8) | 0 (0) | 47 (92.9) | 53 (100) | 4.60 | 0.18 |
CUI concept unique identifiers
In addition, a series of concepts were consistently more represented in the DS Cohort than in FS Cohort, without reaching a statistical significance as “status epilepticus” (29% versus 15%; p = 0.07, OR = 2.4), “startle” (12 versus 0%; p = 0.07, OR = 7.2), “psychomotor delay” (12 versus 0%; p = 0.07, OR = 7.2), “pyramidal syndrome” (10 versus 0%; p = 0.18, OR = 4.6) “hemiparesis” (8 versus 2%; p = 0.18, OR = 4.6) and “photosensitivity” (8 versus 0%; p = 0.18, OR = 4.6).
Analysis of the DS cohort in regard to the early diagnosis
In the DS cohort, we compared the subgroup of individuals who had DS diagnosis confirmed or suspected before the age of 2 years of age (n = 36) versus the subgroup where the diagnosis of DS was not suspected (n = 15). In the first, the term (concept) Dravet syndrome was reported in the clinical reports before the age of 2 years while none of the individuals of the second group had any use of this term suggesting that DS diagnosis was not suspected before the age of 2 years. The mean age at first seizure was 5.3 months (min 2–max 12) in the subpopulation that received a diagnosis or a suspected diagnosis before age 2 and 6.1 months (min 2 – max 9) in the group without an early diagnosis (p = 0.2). Individuals who received diagnosis within 2 years showed a higher rate of concepts as “seizures” (p < 0.01), “fever” (p < 0.01), “epilepsy” (p < 0.01), “prolonged seizures” (p < 0.01), “convulsions” (p = 0.01), “myoclonia” (p = 0.02) and “ataxia” (p = 0.04) compared to the second group (Table 3).
Table 3.
Comparison between concepts found in more than 10% individuals of DS Cohort who received the diagnosis/suspicion of DS before (left) and after (right) the age of 2 years
| UMLS CUI code | Concept | DS cohort diag < 2 years | DS cohort diag > 2 years | ||
|---|---|---|---|---|---|
| Number of individuals | Frequency (%) | Number of individuals | Frequency (%) | ||
| C0751122 | Dravet syndrome | 36 | 100 | ||
| C0036572 | Seizures | 36 | 100 | 11 | 79 |
| C0015967 | Fever | 34 | 94 | 8 | 57 |
| C0014544 | Epilepsy | 33 | 92 | 8 | 57 |
| C0234972 | Convulsions | 26 | 72 | 5 | 36 |
| C1419856 | SCN1A | 22 | 61 | 6 | 43 |
| C0026827 | Hypotonia | 17 | 47 | 4 | 29 |
| C2825055 | Recurrence | 17 | 47 | 4 | 29 |
| C0009952 | Febrile seizures | 15 | 42 | 5 | 36 |
| C3809175 | Prolonged seizure | 15 | 42 | ||
| C0234535 | Clonic | 14 | 39 | 3 | 21 |
| C0038220 | Status epilepticus | 13 | 36 | 2 | 14 |
| C0596002 | Reflex | 13 | 36 | 2 | 14 |
| C1705236 | Deviation | 12 | 33 | 5 | 36 |
| C2830004 | Drowsiness | 12 | 33 | 4 | 29 |
| C0259972 | Ketogenic diet | 11 | 31 | 2 | 14 |
| C0235195 | Sedation | 11 | 31 | 2 | 14 |
| C1698630 | Virosis | 11 | 31 | ||
| C3887612 | Psychomotor Agitation | 10 | 28 | 2 | 14 |
| C0009450 | Infection | 10 | 28 | ||
| C0027066 | Myoclonia | 10 | 28 | ||
| C0004134 | Ataxia | 9 | 25 | ||
| C0030193 | Pain | 9 | 25 | ||
| C0013144 | Falling asleep | 9 | 25 | ||
| C0029877 | Otitis | 9 | 25 | ||
| C0009443 | Rhinitis | 9 | 25 | ||
| C0010200 | Cough | 9 | 25 | ||
| C0424927 | Education | 8 | 22 | ||
| C0522336 | Rolling of eyes | 8 | 22 | 2 | 14 |
| C0020517 | Allergies | 7 | 19 | ||
| C0006271 | Bronchiolitis | 7 | 19 | 2 | 14 |
| C0015672 | Fatigue | 7 | 19 | ||
| C0026837 | Hypertonus | 7 | 19 | 3 | 21 |
| C0026205 | Myosis | 7 | 19 | ||
| C0024115 | Pneumonia | 7 | 19 | ||
| C0085639 | Fall | 6 | 17 | ||
| C0494475 | Tonic–clonic seizures | 6 | 17 | ||
| C0751495 | Focal seizures | 6 | 17 | ||
| C0031350 | Pharingitis | 6 | 17 | ||
| C0424230 | Psychomotor delay | 6 | 17 | ||
| C0684320 | Regression | 6 | 17 | 2 | 14 |
| C0549209 | Startle | 6 | 17 | ||
| C0036973 | Shiverings | 6 | 17 | ||
| C0003123 | Anorexia | 5 | 14 | ||
| C0035561 | Side | 5 | 14 | ||
| C0034642 | Rales | 5 | 14 | ||
| C0013604 | Edema | 5 | 14 | ||
| C0035203 | Respiration | 5 | 14 | ||
| C0424230 | Psychomotor delay | 5 | 14 | ||
| C0008049 | Chickenpox | 5 | 14 | ||
| C0004093 | Asthenia | 4 | 11 | ||
| C0270844 | Tonic seizures | 4 | 11 | ||
| C0010520 | Cyanosis | 4 | 11 | ||
| C0011991 | Diarrhea | 4 | 11 | ||
| C0221725 | Bronchial obstruction | 4 | 11 | ||
| C0017160 | Gastroenteritis | 4 | 11 | ||
| C0021400 | Flu | 4 | 11 | ||
| C0037036 | Hypersalivation | 4 | 11 | ||
| C0042769 | Viral infection | 4 | 11 | ||
| C0349506 | Photosensitivity | 4 | 11 | ||
| C0032290 | Aspiration pneumonia | 4 | 11 | ||
| C1272641 | Arterial blood pressure | 4 | 11 | ||
| C0027441 | Nasopharyngitis | 4 | 11 | 2 | 14 |
| C0037763 | Spasms | 4 | 11 | ||
| C0038450 | Stridor | 4 | 11 | ||
| C1504405 | Pyramidal syndrome | 4 | 11 | ||
| C0018916 | Angiome | 2 | 14 | ||
| C0085584 | Encephalopathy | 2 | 14 | ||
| C0018989 | Hemiparesis | 2 | 14 | ||
| C0018991 | Hemiplegia | 2 | 14 | ||
| C0019209 | Hepatomegaly | 2 | 14 | ||
| C0232483 | Reflux | 2 | 14 | ||
CUI concept unique identifiers
Discussion
This study shows that narrative medical reports produced before 2 years include several clinical concepts which are significantly associated with individuals with DS compared to FS, this latter condition representing the main differential diagnosis at the onset. These concepts are consistent with the main clinical findings constituting the criteria for differentiating DS from FS in first 2 years of life.
FS are usually reported after the first year with some cases initiating before 12 months. They are usually brief and generalized [24]. In our study, concepts referred to prolonged (“status epilepticus”, “prolonged seizures”, “sedation”) and focal seizures (“deviation”) are prominent in the DS cohort, emphasizing the higher tendency of individuals with DS to present at onset long lasting and focal febrile seizures compared to individuals with FS [16, 25, 26]. Importantly, individuals with DS develop different types of seizures as myoclonic or atypical absences in addition to the first seizures mimicking FS. We observed in our DS cohort concepts referring to seizures other than febrile convulsions, including “Myoclonia” and “startle”, which is mostly used in narrative reports to depict myoclonic seizure semiology [16, 27, 28]. The concept “hemiparesis” was more frequent in the DS Cohort compared to FS one. This is consistent with the higher occurrence of transitory hemiplegia after long-lasting hemiclonic seizures, a type of seizure being quite suggestive of DS [16, 27, 29].
Some important non-seizure concepts also emerged, differentiating the two cohorts. Subjects with DS and FS show a normal neurodevelopment at the seizure onset, but then psychomotor trajectories deviate [26, 30]. In accordance, concepts related to psychomotor delay were found only in the DS Cohort (“Regression”, “Psychomotor delay”). In addition, “Ataxia” was significantly more reported DS Cohort, reflecting the peculiar gait disorder commonly observed in individuals with DS, and representing an early motor-marker of this condition [28, 31].
Interestingly, the concept “febrile seizures” was found with significant higher frequency in the FS Cohort probably because it was used for a “diagnostic” purpose in the clinical reports.
The study was carried out in a tertiary epilepsy center, so it is plausible that some words have been chosen as a consequence of the clinical suspicion of Dravet Syndrome by highly experienced specialist in epileptology (e.g. “myoclonia”, “ataxia”). However, many of the medical reports were done by physicians without a specific expertise in epilepsy or DS (e.g. emergency care or intensive care physicians), emphasizing the uniformity of expressions used for reporting disease and individuals description, and suggesting that most of key-concepts may have also been found into non-specialists medical reports (e.g. “deviation”, “prolonged seizures”, “startle”).
Several studies show a substantial worldwide issue of diagnostic delay of DS, with a mean age at diagnosis that is usually over 2 years, resulting in “unnecessary, costly, and, at times, invasive testing, and use of ineffective therapies, which can exacerbate seizures, increase the risk of status epilepticus, and worsen cognitive outcome” [17, 32–34]. Moreover, DS is certainly less recognized in adult population and in developing countries [19, 20].
Computer-based models using EHRs able to suggest diagnosis and to avoid misdiagnosis are gaining ground [3, 35]. These models are mostly based on structured data, as image-based or laboratory data [36, 37]. Recently, more complex models of artificial intelligence are emerging, which are able to elaborate diagnosis by extracting clinically relevant information from unstructured data in EHRs [38, 39].
On the basis of our findings, further extensive studies might focus on elaborating a specific computer algorithm which combines significative concepts and their age of appearance within narrative specialists and non-specialists reports, in order to automatically produce an alert signal suggesting possible diagnosis of DS.
Some results of our analysis set out some additional insights. For example, the major incidence of concept “pneumonia” in DS Cohort compared to FS Cohort appears to be relevant, since it can represent both a facilitator of the seizure onset or a complication of an inhalation during a long lasting convulsive seizure or a status epilepticus [40]. In addition, a number of concepts related to peri-ictal nosocomial and respiratory complications were found with higher frequency in reports of individuals with DS (“nosocomial infections”, “acute respiratory insufficiency”, “aspiration pneumonia”, “FiO2”, “stridor”) underlying that convulsive status epilepticus might be a life-threatening condition in this population [40, 41].
Furthermore, in this study the concept “Dravet Syndrome” was found in 72% of individuals of DS Cohort before the age of 2 years. This is concordant with the literature showing the early recognition of DS in France [34].
Some clinical concepts were found with higher frequency in the reports of individuals who received the diagnosis/suspicion of DS before the age of 2 years: the “long-lasting seizure” concepts (“Status epilepticus”, “Prolonged seizures”, “Sedation”), the “myoclonic” concepts (“Myoclonia”, “Startles”), the “drug resistance” concepts (“Ketogenic diet”), as well as “Ataxia”, and “Photosensitivity”. Although statistical significance was not reached for all these concepts as sample was small, these findings may support that these clinical concepts are the most DS diagnosis orienting. We can hypothesise that individuals belonging to the sub-group who did not receive a diagnosis within 2 years presented a less “typical” phenotype. The diagnosis was made later than 2 years of age when the full blown syndrome is often complete with pharmacoresistant seizures and developmental plateauing. However, in this subgroup without early diagnosis with individuals presenting “intermediate” features between only FS and the “complete” DS clinical picture, the median age at first seizure was significantly lower than in FS cohort (6.1 months vs 12.4 months). This finding confirms that age at first seizure might be the strongest predictor of DS in infants who experience febrile seizures [25].
Study limitations
Word sense disambiguation poses a challenge in extracting meaningful data from unstructured text. Clinical notes often contain terms or phrases that have more than one meaning [8], or that need for a contextualisation to understand the real clinical meaning. For example, concept “deviation” apparently do not link to a specific clinical feature, but in the narrative reports of individuals of both cohorts it was mostly used within the description of the seizure semeiology, thus referring to a focal seizure.
The presence of a clinical concept in a medical report does not necessary implies that the individual presents this clinical feature. For instance, the concept “spasms” that we found in five individuals of the DS Cohort, was used within the clinical description of paroxysmal motor events that could suggest epileptic spasms, but was not confirmed in any of them. Similarly, concept “Dravet Syndrome” could be found in reports of subjects who received the diagnosis, or in which a suspicion was made (i.e.: “We see today patient X for the suspicion of Dravet Syndrome”). The method used by Dr Warehouse automatically classifies concepts according to polarity (negation/affirmation) and the experiencer (patient/family). But there may still be errors in the classification. In addition, the classification does not take into account the notion of hypothesis.
In this study, the FS population presents some “atypical” features; for instance, the frequency of the concept “status epilepticus” in these subjects is higher than expected in terms of incidence in individuals with febrile seizures [42, 43]. This might be due to a preferential referral to university hospital of individuals with febrile long lasting seizures or febrile status epilepticus, as they might need further admission to ICU.
Conclusion
Narrative medical reports of individuals younger than 2 years with febrile seizures, contain different words depending if they have or will develop clinical phenotype of DS, or not. The elaboration of algorithm exploiting NLP on the basis of our work, could be useful to early individualize these individuals, in order to establish early diagnosis and adequate therapy that in some instances need to address them to expert epilepsy centres.
This methodology would represent an innovative, “cheap”, transposable and sustainable methodology to reduce time of diagnosis for individuals with Dravet Syndrome and other rare conditions.
Some “key early symptoms” often identified by the patients/care givers and the non-expert physicians are merely linked to a given known disease causing diagnosis delay. Using these symptoms and signs as alerts and warning signs can help to address patients earlier to expert centres for a definite diagnosis. The future step is to validate the impact of the implementing of these “warnings” in the electronic health records on shortening the patient’s odyssey to diagnosis and therapies.
Acknowledgements
Not applicable.
Authors' contributions
TLB collected and interpreted data and drafted the manuscript. MK collected and interpreted data. NG created the software used in the work and revised the manuscript. AN interpreted data and revised the manuscript. RN concepted and designed the work, supervised data collection and interpretation, revised the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by State funding from the Agence Nationale de la Recherche under “Investissements d’Avenir” program (ANR-10-IAHU-01) and the “Fondation Bettencourt Schueller” (RN).
Availability of data and materials
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study had the approval of Necker Hospital ethic committee.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gunter TD, Terry NP. The emergence of national electronic health record architectures in the United States and Australia: models, costs, and questions. J Med Internet Res. 2005;7(1):e3. doi: 10.2196/jmir.7.1.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landi I, Glicksberg BS, Lee HC, Cherng S, Landi G, Danieletto M, et al. Deep representation learning of electronic health records to unlock patient stratification at scale. npj Digit Med. 2020;3:1–11. doi: 10.1038/s41746-020-0301-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olivera P, Danese S, Jay N, Natoli G, Peyrin-Biroulet L. Big data in IBD: a look into the future. Nat Rev Gastroenterol Hepatol. 2019;16(5):312–321. doi: 10.1038/s41575-019-0102-5. [DOI] [PubMed] [Google Scholar]
- 4.Bates DW, Saria S, Ohno-Machado L, Shah A, Escobar G. Big data in health care: Using analytics to identify and manage high-risk and high-cost patients. Health Aff. 2014;33(7):1123–1131. doi: 10.1377/hlthaff.2014.0041. [DOI] [PubMed] [Google Scholar]
- 5.Shen F, Liu S, Wang Y, Wen A, Wang L, Liu H. Utilization of electronic medical records and biomedical literature to support the diagnosis of rare diseases using data fusion and collaborative filtering approaches. J Med Internet Res. 2018;20(10):e11301. doi: 10.2196/11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Southall NT, Natarajan M, Lau LPL, Jonker AH, Deprez B, Guilliams T, et al. The use or generation of biomedical data and existing medicines to discover and establish new treatments for patients with rare diseases-recommendations of the IRDiRC Data Mining and Repurposing Task Force. Orphanet J Rare Dis. 2019;14(1):225. doi: 10.1186/s13023-019-1193-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcelon N, Neuraz A, Salomon R, Bahi-Buisson N, Amiel J, Picard C, et al. Next generation phenotyping using narrative reports in a rare disease clinical data warehouse. Orphanet J Rare Dis. 2018;13:85. doi: 10.1186/s13023-018-0830-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Townsend H. Natural language processing and clinical outcomes: the promise and progress of NLP for improved care. J AHIMA. 2013;84:44–45. [PubMed] [Google Scholar]
- 9.Bodenreider O. The Unified Medical Language System (UMLS): integrating biomedical terminology. Nucleic Acids Res. 2004;32:D267–D270. doi: 10.1093/nar/gkh061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rindflesch TC, Fiszman M. The interaction of domain knowledge and linguistic structure in natural language processing: interpreting hypernymic propositions in biomedical text. J Biomed Inform. 2003;36(6):462–477. doi: 10.1016/j.jbi.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 11.Wu YW, Sullivan J, McDaniel SS, Meisler MH, Walsh EM, Li SX, et al. Incidence of dravet syndrome in a US population. Pediatrics. 2015;136(5):e1310–e1315. doi: 10.1542/peds.2015-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, Guilhoto L, Hirsch E, et al. ILAE classification of the epilepsies position paper of the ILAE: commission for classification and terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dravet C. The core Dravet syndrome phenotype. Epilepsia. 2011;52(SUPPL. 2):3–9. doi: 10.1111/j.1528-1167.2011.02994.x. [DOI] [PubMed] [Google Scholar]
- 14.Hirose S, Scheffer IE, Marini C, De Jonghe P, Andermann E, Goldman AM, et al. SCN1A testing for epilepsy: application in clinical practice. Epilepsia. 2013;54:946–952. doi: 10.1111/epi.12168. [DOI] [PubMed] [Google Scholar]
- 15.Catarino CB, Liu JYW, Liagkouras I, Gibbons VS, Labrum RW, Ellis R, et al. Dravet syndrome as epileptic encephalopathy: evidence from long-term course and neuropathology. Brain. 2011;134(10):2982–3010. doi: 10.1093/brain/awr129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hattori J, Ouchida M, Ono J, Miyake S, Maniwa S, Mimaki N, et al. A Screening test for the prediction of Dravet syndrome before one year of age. Epilepsia. 2008;49(4):626–633. doi: 10.1111/j.1528-1167.2007.01475.x. [DOI] [PubMed] [Google Scholar]
- 17.Lagae L, Brambilla I, Mingorance A, Gibson E, Battersby A. Quality of life and comorbidities associated with Dravet syndrome severity: a multinational cohort survey. Dev Med Child Neurol. 2018;60(1):63–72. doi: 10.1111/dmcn.13591. [DOI] [PubMed] [Google Scholar]
- 18.Bremer A, Lossius MI, Nakken KO. Dravet syndrome—considerable delay in making the diagnosis. Acta Neurol Scand. 2012;125(5):359–362. doi: 10.1111/j.1600-0404.2011.01609.x. [DOI] [PubMed] [Google Scholar]
- 19.Jansen FE, Sadleir LG, Harkin LA, Vadlamudi L, McMahon JM, Mulley JC, et al. Severe myoclonic epilepsy of infancy (Dravet syndrome): recognition and diagnosis in adults. Neurology. 2006;67(12):2224–2226. doi: 10.1212/01.wnl.0000249312.73155.7d. [DOI] [PubMed] [Google Scholar]
- 20.Connolly MB. Dravet syndrome: diagnosis and long-term course. Can J Neurol Sci. 2016;43:S3–8. doi: 10.1017/cjn.2016.243. [DOI] [PubMed] [Google Scholar]
- 21.Garcelon N, Neuraz A, Salomon R, Faour H, Benoit V, Delapalme A, et al. A clinician friendly data warehouse oriented toward narrative reports: Dr. Warehouse. J Biomed Inform. 2018;80:52–63. doi: 10.1016/j.jbi.2018.02.019. [DOI] [PubMed] [Google Scholar]
- 22.Neuraz A, Chouchana L, Malamut G, Le Beller C, Roche D, Beaune P, et al. Phenome-wide association studies on a quantitative trait: application to TPMT enzyme activity and thiopurine therapy in pharmacogenomics. PLoS Comput Biol. 2013;9(12):e1003405. doi: 10.1371/journal.pcbi.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Denny JC, Ritchie MD, Basford MA, Pulley JM, Bastarache L, Brown-Gentry K, et al. PheWAS: Demonstrating the feasibility of a phenome-wide scan to discover gene-disease associations. Bioinformatics. 2010;26(9):1205–1210. doi: 10.1093/bioinformatics/btq126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumann RJ. Technical report: treatment of the child with simple febrile seizures. Pediatrics. 1999;103(6 I):1278–1279. doi: 10.1542/peds.103.6.e86. [DOI] [PubMed] [Google Scholar]
- 25.Cetica V, Chiari S, Mei D, Parrini E, Grisotto L, Marini C, et al. Clinical and genetic factors predicting Dravet syndrome in infants with SCN1A mutations. Neurology. 2017;88(11):1037–1044. doi: 10.1212/WNL.0000000000003716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dravet C, Guerrini R. Dravet syndrome. Arcueil: John Libbey Eurotext; 2011. [Google Scholar]
- 27.Ohki T, Watanabe K, Negoro T, Aso K, Haga Y, Kasai K, et al. Severe myoclonic epilepsy in infancy: evolution of seizures. Seizure. 1997;6(3):219–224. doi: 10.1016/S1059-1311(97)80009-X. [DOI] [PubMed] [Google Scholar]
- 28.Gataullina S, Dulac O. From genotype to phenotype in Dravet disease. Seizure. 2017;44:58–64. doi: 10.1016/j.seizure.2016.10.014. [DOI] [PubMed] [Google Scholar]
- 29.Ragona F, Brazzo D, De Giorgi I, Morbi M, Freri E, Teutonico F, et al. Dravet syndrome: early clinical manifestations and cognitive outcome in 37 Italian patients. Brain Dev. 2010;32:71–77. doi: 10.1016/j.braindev.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Nabbout R, Chemaly N, Chipaux M, Barcia G, Bouis C, Dubouch C, et al. Encephalopathy in children with Dravet syndrome is not a pure consequence of epilepsy. Orphanet J Rare Dis. 2013;8(1):1–8. doi: 10.1186/1750-1172-8-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verheyen K. Motor Development in children with Dravet syndrome. Dev Med Child Neurol. 2019;61:950–956. doi: 10.1111/dmcn.14147. [DOI] [PubMed] [Google Scholar]
- 32.Wirrell EC, Laux L, Donner E, Jette N, Knupp K, Meskis MA, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American Consensus Panel. Pediatr Neurol. 2017;68:18–34. doi: 10.1016/j.pediatrneurol.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 33.Wirrell EC, Laux L, Franz DN, Sullivan J, Saneto RP, Morse RP, et al. Stiripentol in Dravet syndrome: results of a retrospective U.S. study. Epilepsia. 2013;54(9):1595–1604. doi: 10.1111/epi.12303. [DOI] [PubMed] [Google Scholar]
- 34.Nabbout R, Auvin S, Chiron C, Thiele E, Cross H, Scheffer IE, et al. Perception of impact of Dravet syndrome on children and caregivers in multiple countries: looking beyond seizures. Dev Med Child Neurol. 2019;61:1229–1236. doi: 10.1111/dmcn.14186. [DOI] [PubMed] [Google Scholar]
- 35.Shilo S, Rossman H, Segal E. Axes of a revolution: challenges and promises of big data in healthcare. Nat Med. 2020;26(1):29–38. doi: 10.1038/s41591-019-0727-5. [DOI] [PubMed] [Google Scholar]
- 36.Castaneda C, Nalley K, Mannion C, Bhattacharyya P, Blake P, Pecora A, et al. Clinical decision support systems for improving diagnostic accuracy and achieving precision medicine. J Clin Bioinform. 2015;5(1):4. doi: 10.1186/s13336-015-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fitipaldi H, McCarthy MI, Florez JC, Franks PW. A global overview of precision medicine in type 2 diabetes. Diabetes. 2018;67:1911–1922. doi: 10.2337/dbi17-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang H, Tsui BY, Ni H, Valentim CCS, Baxter SL, Liu G, et al. Evaluation and accurate diagnoses of pediatric diseases using artificial intelligence. Nat Med. 2019;25(3):433–438. doi: 10.1038/s41591-018-0335-9. [DOI] [PubMed] [Google Scholar]
- 39.Hully M, Lo Barco T, Kaminska A, Barcia G, Cances C, Mignot C, et al. Deep phenotyping unstructured data mining in an extensive pediatric database to unravel a common KCNA2 variant in neurodevelopmental syndromes. Genet Med. 2021;23:968–971. doi: 10.1038/s41436-020-01039-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD. Mortality in Dravet syndrome: a review. Epilepsy Behav. 2016;64:69–74. doi: 10.1016/j.yebeh.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 41.Kim Y, Bravo E, Thirnbeck CK, Smith-Mellecker LA, Kim SH, Gehlbach BK, et al. Severe peri-ictal respiratory dysfunction is common in Dravet syndrome. J Clin Invest. 2018;128(3):1141–1153. doi: 10.1172/JCI94999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hesdorffer DC, Shinnar S, Lewis DV, Moshé SL, Nordli DR, Pellock JM, et al. Design and phenomenology of the FEBSTAT study. Epilepsia. 2012;53(9):1471–1480. doi: 10.1111/j.1528-1167.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vitaliti G, Castagno E, Ricceri F, Urbino A, Di Pianella AV, Lubrano R, et al. Epidemiology and diagnostic and therapeutic management of febrile seizures in the Italian pediatric emergency departments: a prospective observational study. Epilepsy Res. 2017;129:79–85. doi: 10.1016/j.eplepsyres.2016.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed during the current study are available from the corresponding author on reasonable request.