Abstract
Objective:
Smoking re-exposure after a quit attempt (i.e., lapse) increases relapse risk, but lapse recovery is possible. Using a six-day analogue model of smoking cessation and lapse, this study tested the effect of a single lapse cigarette on the risk of subsequent smoking. Abstinence self-efficacy (ASE) and depressive symptoms (using the Center for Epidemiological Studies Depression Scale, CES-D) were also examined as hypothesized moderators of lapse recovery.
Method:
After receiving cessation counseling and achieving two days of incentivized abstinence, 54 daily smokers (mean age: 41 years, 61% African American, 63% male) were randomly assigned to smoke one cigarette or to a no lapse control condition. Participants were then offered monetary incentives to abstain for three more days and smoking was monitored.
Results:
Compared to the control condition, participants who experienced a lapse had a 2.5 times greater risk of smoking in the first 24 hours. Furthermore, a lapse resulted in much greater risk of subsequent smoking compared to the control condition among individuals with lower post-quit ASE scores (p = .044) and greater CES-D scores (p = .040).
Conclusions:
These findings provide preliminary evidence that a single lapse cigarette after quitting plays a causal role in subsequent smoking and suggest that individuals with lower post-quit ASE and greater depressive symptoms are less likely to recover from a lapse. Future research should investigate factors associated with lapse recovery and failure so that effective lapse-responsive strategies can be developed. Laboratory models provide an efficient and controlled method to examine such processes.
Keywords: smoking relapse, lapse, depressive symptoms, abstinence self-efficacy, treatment analogue model
Smoking is the leading preventable cause of morbidity and mortality in the world (World Health Organization, 2015). While many smokers attempt to quit, relapse rates remain high (Eisenberg et al., 2008). The relapse process has been conceptualized as involving three stages: initial abstinence, lapse, (i.e., smoking exposure after quitting), and relapse (i.e., a return to regular smoking) (Marlatt & Gordon, 1985; Shiffman, 2006). Each stage may have different determinants and research that disentangles these abstinence outcomes may better inform treatment development. Clinical and experimental observations indicate that smoking lapses very often progress to relapse (Juliano, Donny, Houtsmuller, & Stitzer, 2006; Shadel et al., 2011). Furthermore, preclinical research has reliably demonstrated that re-exposure to a drug or drug-related cues after extinction produces reinstatement of drug-seeking behavior (Reiner, Fredriksson, Lofaro, Bossert, & Shaham, 2019). Despite the apparent detrimental effects of a smoking lapse, it is important to recognize that progression to relapse is not inevitable (Conklin et al., 2005; Kirchner, Shiffman, & Wileyto, 2012; Wileyto et al., 2004). Smoking lapses are a potential point of treatment intervention (Bouton, 2000; Juliano, Donny, et al., 2006; Juliano, Houtsmuller, & Stitzer, 2006; Marlatt & Gordon, 1985; Shiffman, 2006); however, the development of lapse-responsive interventions is hindered by our incomplete understanding of causal determinants of lapse recovery or failure.
Treatment analogue models that experimentally control a smoking lapse provide an efficient, controlled, and cost-effective way to investigate factors associated with lapse outcomes. Such models can span multiple days or weeks, and include elements, such as smoking cessation counseling, a quit attempt, and/or abstinence incentives to simulate the quitting process. At least five prior multi-day studies have manipulated a smoking lapse. These studies have demonstrated that lapses with cigarettes containing nicotine (Chornock, Stitzer, Gross, & Leischow, 1991) or no nicotine (Juliano, Donny, et al., 2006) increased relapse risk, that post-lapse increases in craving partially mediated lapse effects (Shadel et al., 2011), and that the smoking medication varenicline decreased the subjective rewarding effects of the lapse, as well as relapse risk (McClure, Vandrey, Johnson, & Stitzer, 2013; Patterson et al., 2009).
The present study is the first to test the effect of a single lapse cigarette on subsequent smoking risk. A lapse is typically defined as the first exposure to smoking (even a single puff) after abstinence (Shiffman, Paty, Gnys, Kassel, & Hickcox, 1996). It has been observed that most naturalistic lapses involve smoking just one cigarette or less (Kirchner et al., 2012), however, prior research has only manipulated lapse episodes consisting of two (McClure et al., 2013; Shadel et al., 2011) or five cigarettes (Chornock et al., 1991; Juliano, Donny, et al., 2006; Patterson et al., 2009).
An additional aim of this study was to test hypothesized moderators of the lapse effect. Abstinence self-efficacy (ASE) and depressive symptoms were targeted as these constructs have been posited to play a role in cessation and relapse processes and are potential treatment targets. Various models of behavior change include ASE as a central determinant of behavior, including lapse recovery (Bandura, 1977; Marlatt & Gordon, 1985; Niaura et al., 1988). In the reformulated Relapse Prevention Model (Witkiewitz & Marlatt, 2004) and in Shiffman’s (2005) conceptualization of relapse risk factors, ASE is proposed as both a tonic/stable and phasic/dynamic determinant of relapse. Prior studies using ecological momentary assessment (EMA) have reported that baseline ASE (tonic) predicts initial lapse risk, while post-lapse tonic daily (but not phasic) variations or repeated drops in ASE after lapses (other than the first lapse) predict relapse (Gwaltney, Shiffman, Balabanis, & Paty, 2005; Kirchner et al., 2012). The strength of the relationship between ASE and smoking outcomes appears to vary based on the timing of the assessment, if smoking is controlled, and the specific smoking outcome being assessed (Gwaltney et al., 2005). The present study is the first controlled lapse study to examine the role of ASE in lapse recovery and offers a high level of control over smoking behavior as ASE was assessed at baseline and after 48 hours of biologically validated smoking abstinence.
Numerous links between depressive symptoms and smoking behavior, including poorer cessation outcomes, have been observed (Bakhshaie, Zvolensky, & Goodwin, 2015; Fluharty, Taylor, Grabski, & Munafo, 2017). Negative affect, a central feature of depression, is a key component of various models of smoking relapse (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Marlatt & Gordon, 1985; Shiffman, 2005). However, very little research has investigated the role of depressive symptoms in lapse recovery. One clinical trial of bupropion found that scores on the Center for Epidemiological Studies Depression Scale (CES-D) predicted slower lapse recovery (Wileyto et al., 2005). We examined the CES-D to follow up on this finding.
The present study employed a six-day laboratory-based analogue model of smoking cessation and lapse. Participants received brief smoking cessation counseling and were instructed to abstain for 48 hours with a $40 incentive. On Day 3, abstinent participants were randomly assigned to smoke a single lapse cigarette (own brand) or to a no lapse control condition. Afterwards participants were incentivized to abstain for three more days. It was hypothesized that those exposed to a lapse cigarette would have greater risk of self-initiated smoking relative to the no lapse control condition. It was further hypothesized that the lapse manipulation would be particularly detrimental for individuals with lower ASE and greater depressive symptoms.
Method
Participants
Eighty-one adult daily cigarette smokers (63% male, 66% African American) were recruited from the Washington D.C. area (Table 1). Participants were not eligible if they smoked fewer than 8 cigarettes per day, had a psychiatric/medical condition that would interfere with study participation, were seeking cessation treatment, or were using smoking pharmacotherapy.
Table 1.
Baseline Characteristics
| Characteristic | Lapse Condition (n = 27) | No Lapse Condition (n = 27) |
|---|---|---|
| Sex | ||
| % Female | 33.33 | 40.74 |
| Race | ||
| % African American | 55.56 | 66.67 |
| % Caucasian | 29.63 | 25.93 |
| % Asian | 3.70 | 3.70 |
| % Other | 11.11 | 3.70 |
| Age (years) | 40.63 (16.65) | 41.19 (13.72) |
| Cigarettes per day | 16.48 (6.35) | 15.39 (6.59) |
| Years smoked | 20.18 (15.77) | 19.52(11.43) |
| FTND (0–10) | 4.67 (1.73) | 4.30 (2.13) |
| % Menthol | 67 | 67 |
| CO (ppm) | 16.96 (6.9) | 20.52 (17.15) |
| Contemplation Ladder (0–10) | 6.41 (2.58) | 6.41 (2.82) |
| CES-D (0 – 60) | 16.33 (11.09) | 14.85 (10.81) |
| Baseline ASE (1–7) | 5.19 (1.80) | 4.85 (1.98) |
| URS (0–10) | 4.57 (2.93) | 4.75 (2.57) |
Note. Values are means and standard deviations (in parentheses) unless otherwise noted. FTND = Fagerstrӧm Test for Nicotine Dependence; CO = carbon monoxide; CES-D = Center for Epidemiologic Studies Depression Scale; ASE = Abstinence Self-efficacy; URS = Urge Rating Scale. There were no significance baseline differences between the groups.
Measures
Participants provided demographic information and smoking history, including the Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991) and completed the Contemplation Ladder to indicate their readiness to quit smoking (Biener & Abrams, 1991). Depressive symptoms were assessed with the CES-D (Radloff, 1977) (Cronbach’s alpha = .90). To assess ASE at baseline and after 48 hours of confirmed abstinence, participants rated their confidence that they could abstain from smoking for the next 24 hours from 1 (not at all confident) to 7 (extremely confident). Single item measures of ASE have been shown to be comparable to multi-item measures of ASE (Gwaltney, Metrik, Kahler, & Shiffman, 2009). Participants rated their cravings, wants, and desires to smoke on a scale from 1 (none at all) to 10 (greatest ever experienced) (Kozlowski, Pillitteri, Sweeney, Whitfield, & Graham, 1996) (Mean score Cronbach’s alpha = .95). Additional measures were administered during the experiment that are not presented herein.
Procedure
This study was approved by the Institutional Review Board at American University. After phone screenings, participants visited the laboratory on six consecutive days. On Day 1, participants completed informed consent and baseline measures, provided an expired air carbon monoxide (CO) sample, and smoked one of their own cigarettes in the lab to equate recent smoke exposure. To simulate a cessation attempt, participants were given a 20-minute smoking cessation counseling session and take-home materials. There was a $40 incentive for 48 hours of confirmed abstinence based on self-report and a CO sample ≤ 7 ppm. Twenty-seven participants (33%) were discharged (see Muench & Juliano, 2017). On Day 3, remaining participants were randomly assigned to a lapse (n = 27) or no lapse control condition (n = 27) (Chornock et al., 1991; Juliano, Donny, et al., 2006; Shadel et al., 2011). In the lapse condition, latency to smoke, number of puffs, and smoking duration were recorded. Those in the no lapse condition sat quietly. Participants then completed measures and attempted abstinence for three more days with incentives of $12, $9, and $6 respectively (Juliano et al., 2006). Participants visited the lab daily and were debriefed during the final session.
Statistical methods.
Analyses were conducted using SPSS v24.0 and R v3.6.3. The primary outcome was latency to self-initiated smoking. Participants who did not report smoking but had CO levels above 7ppm (n = 6) were coded as having smoked as a conservative strategy (Juliano, Donny, et al., 2006; Sweitzer, Denlinger, & Donny, 2013). Cox proportional hazards (PH) models were used to (1) test the effects of the experimental condition on smoking risk 24, 48, and 72 hours post-manipulation; and (2) investigate hypothesized moderators. For moderator analyses, the experimental condition and mean-centered scores of the hypothesized moderator variable were entered on Step 1. The experimental condition by moderator variable cross-product term was entered on Step 2, with a significant effect providing support for moderation (Baron & Kenny, 1986). All observations were censored at 72 hours. The assumptions for Cox PH models were met. One participant had missing data for post-abstinence ASE, which was imputed by carrying the last value forward (i.e., baseline ASE rating, which was the highest possible value). Due to a strong directional hypothesis for the lapse effects, alpha was set at p < .05, one-tailed (Juliano et al., 2006). For all other analyses, alpha was set at p < .05, two-tailed.
Results
Lapse manipulation check.
All participants in the lapse group had adequate smoking exposure. They had a mean latency to smoke of 6.22 seconds (2–23 seconds, SD = 4.41), took a mean of 13.54 puffs (5–35, SD = 7.14) and smoked for a mean of 5.43 minutes (1.41–10.55, SD = 2.07). Mean urge to smoke decreased by a mean of 3.71 (SD = 3.33) in the lapse group compared to 0.19 (SD = 1.60) in the no lapse group, F(1,52) = 24.37, p < .0001, ƞ2 =.323.
Effect of programmed lapse on self-initiated smoking.
Fifty percent of participants reported smoking during the three-day follow-up period with no significant difference between males and females (56% male vs. 40% female). In the first 24 hours post-manipulation, the risk of smoking was 2.5 times greater among those who had a lapse cigarette (Wald = 2.90, HR = 2.508, p = .044, confidence interval (CI) = .871 –7.224) (Figure 1). The risk of smoking in the lapse compared to the control condition was 1.7 times greater by 48 hours (Wald = 1.51, HR = 1.703, CI = .728 – 3.988, p = .110) and 1.5 times greater by 72 hours (Wald = 1.10, HR = 1.505, CI = .704 – 3.217, p = .146).
Figure 1.
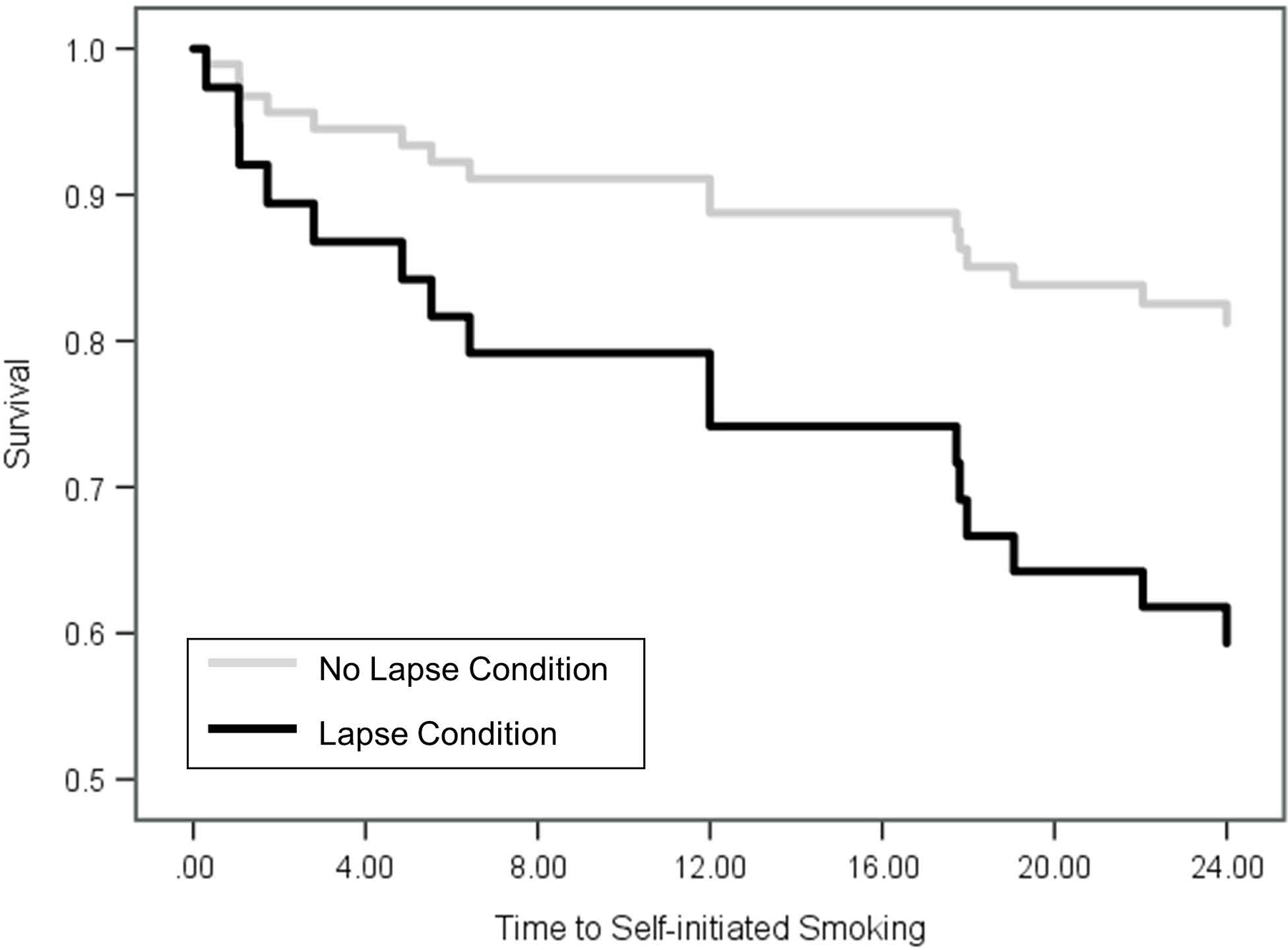
Estimated Survival Function for Self-initiated Smoking in the First 24 Hours after the Experimental Manipulation.
Moderator Variable Analyses
Abstinence self-efficacy.
A significant ASE-by-condition interaction effect indicated that ASE significantly moderated the effect of the experimental manipulation on progression to self-initiated smoking (Wald = 4.07, HR = .579, CI = .340 – .985, p = .044). Participants with lower Day 3 ASE had a much greater risk of smoking when they were in the lapse compared to the no lapse condition (Figure 2). Baseline ASE did not show a significant moderating effect.
Figure 2.
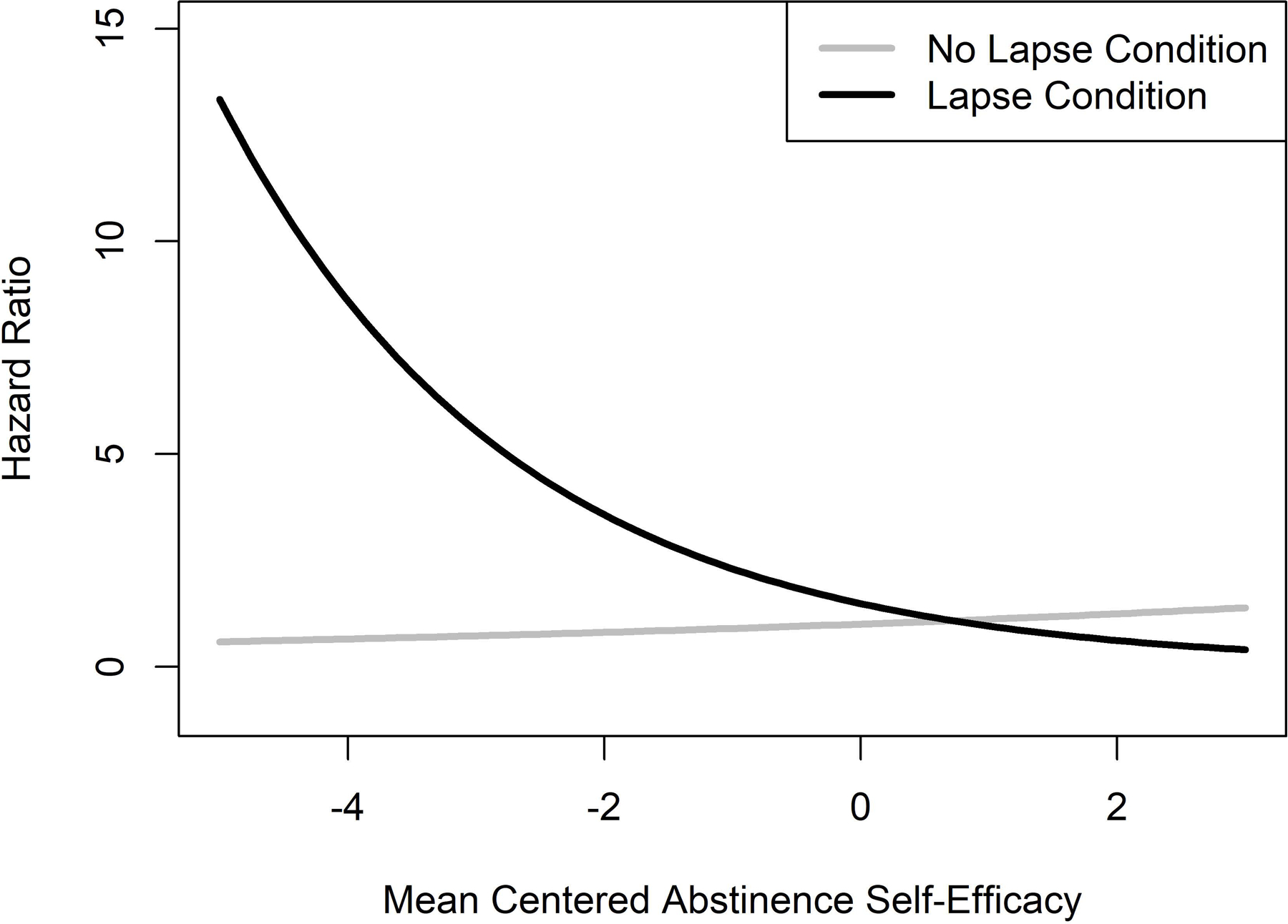
Interaction Effect of Post-Quit Abstinence Self-efficacy and the Experimental Manipulation on Risk of Self-initiated Smoking.
Depressive symptoms.
A significant CES-D-by-condition interaction effect indicated that CES-D scores significantly moderated the effect of the experimental lapse on progression to self-initiated smoking (Wald = 4.25, HR = 1.082, CI = 1.004 – 1.167, p = .040). Individuals with greater CES-D scores had a much greater risk of self-initiated smoking when they were in the lapse compared to the no lapse condition (Figure 3).
Figure 3.
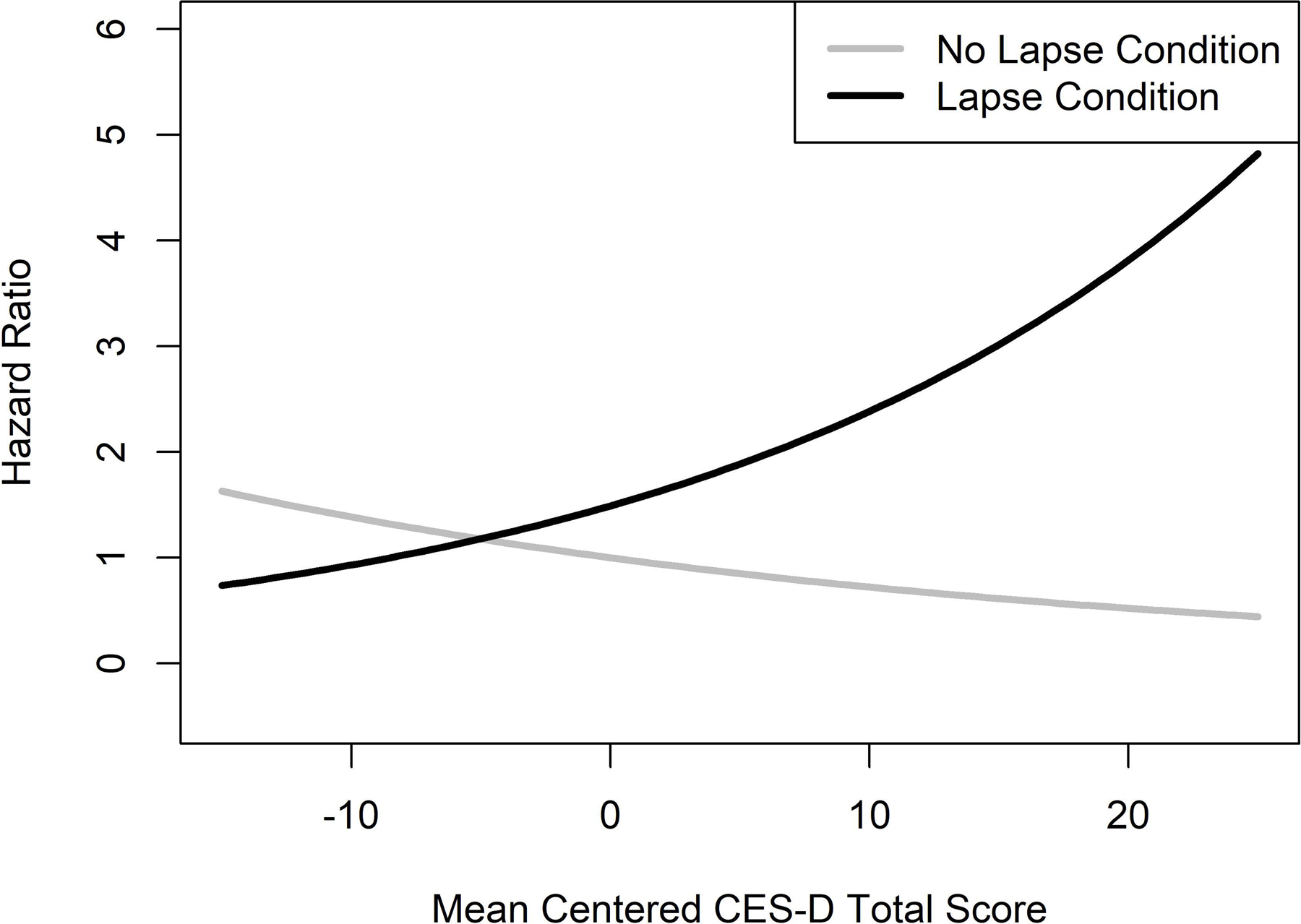
Interaction Effect of Baseline Depressive Symptoms (CES-D Scores) and the Experimental Manipulation on Risk of Self-initiated Smoking.
Discussion
Using a six-day laboratory model, the present study tested the effects of a single lapse cigarette on subsequent smoking risk. These findings provide evidence of a causal link between lapse and subsequent smoking, consistent with prior research (Chornock et al., 1991; Juliano, Donny, et al., 2006; Shadel et al., 2011). Forty-one percent of participants in the lapse condition smoked within the first 24 hours of the manipulation compared to just 18% of controls. Lapse exposure was associated with a 2.5 times greater risk of smoking in the first 24 hours, 1.7 times greater risk by 48 hours, and 1.5 times greater risk by 72 hours. This is the first experimental study to show that just one lapse cigarette increases relapse risk, which adds to prior research showing that 2 or 5 lapse cigarettes increase relapse risk. Moreover, the lapse cigarettes were the participants’ preferred brand, which is more ecologically valid than the use of unfamiliar and often less palatable experimental cigarettes (Juliano et al., 2006). Follow-up research should investigate the dose-response relationship between lapse exposure and lapse outcomes.
The present study is also the first controlled study to examine if specific individual difference variables moderate reactions to a lapse cigarette. We hypothesized that the lapse cigarette would produce greater relapse risk among those with lower post-abstinence ASE and greater baseline depressive symptoms. As Day 3 ASE scores decreased, the risk of smoking increased for those who had a lapse cigarette compared to controls (Figure 1). These findings suggest that ASE may play a crucial role in reactions to high-risk situations, such as a lapse, and support predictions from behavior change theories (Bandura, 1977; Marlatt & Gordon, 1985). Consistent with prior research, we found that post-abstinence ASE was a significant predictor of smoking outcomes, but baseline ASE was not (Gwaltney et al., 2009). Prior research has shown that post-lapse daily decreases in ASE, but not phasic moment to moment changes, predicted relapse (Gwaltney et al., 2005; Van Zundert, Ferguson, Shiffman, & Engels, 2010). Future research should test if treatments that target ASE can prevent lapses (Shadel, Martino, Setodji, Cervone, & Witkiewitz, 2017), as well as facilitate lapse recovery.
As predicted, we found that greater depressive symptoms moderated the effect of the lapse manipulation on subsequent smoking risk. This is consistent with a randomized clinical trial of bupropion versus placebo, in which greater CES-D baseline scores predicted slower lapse recovery, but not initial lapse risk (Wileyto et al., 2005). Our findings also complement prior research demonstrating that higher pre-treatment depressive symptoms reduce patients’ odds of quitting successfully (Cinciripini et al., 2003; Niaura et al., 2001) and that lapses preceded by negative affect are more likely to result in relapse (Brandon, Tiffany, Obremski, & Baker, 1990). Based on the Limited Strength Model, those higher in depressive symptoms may not have enough self-control resources remaining to cope with lapses (Muraven & Baumeister, 2000). Smokers with depressive symptoms may benefit from additional support or pharmacological treatment approaches. Future research should continue to explore cessation and lapse dynamics among individuals with depressive symptoms.
The findings in this study should be considered preliminary pending replication. Limitations include use of a non-treatment-seeking sample of smokers and an experimentally controlled lapse that may not generalize to a self-initiated lapse. The small sample size limited power and the lapse manipulation produced significant effects only in the first 24 hours (albeit, the moderating effects were significant for the full 72 hours). Moreover, there was possible missed detection of smoking based on a CO cutoff of ≤ 7ppm, which would be especially problematic if it differed among the groups. While some have challenged the usefulness of individual differences in predicting cessation outcomes in favor of dynamic changes in smoking-related constructs, the present study indicates that tonic levels of ASE and depressive symptoms appear to have important predictive utility. Individual difference variables, such as ASE and depressive symptoms, not only allow us to identify those who may be at the greatest risk of lapse recovery failure, but also suggest potential psychological targets for lapse responsive interventions. Analogue models strive to simulate the quitting and relapse process while maximizing efficiency. Previous studies have used models ranging from 7 to 35 days, with post-lapse follow-up periods ranging from 4 to 27 days. We utilized a six-day model as smoking risk is greatest in the first hours and days after the manipulation. A criticism of models with brief follow-up is that relapse, often defined as seven days of continuous smoking, cannot be assessed. However, Shadel et al. (2011) found that 95% of first self-initiated smoking episodes were followed by at least seven days of smoking, indicating that this serves as an excellent marker of relapse.
The development of lapse-responsive treatments is still in its infancy (Ferguson, Gitchell, & Shiffman, 2012; Juliano, Houtsmuller, et al., 2006; Vidrine et al., 2016). We hope this research will stimulate future controlled research aimed at identifying factors associated with lapse recovery and failure, as well as the development of effective lapse-responsive strategies.
Public Health Significance Statement.
Understanding factors that increase the risk of smoking relapse will inform the development of more effective smoking cessation treatments. This study provides causal evidence that a single lapse cigarette increases the risk of subsequent smoking. Findings also suggest that individuals who have lower post abstinence self-efficacy and greater depressive symptoms are less likely to recover from a lapse and may need additional support.
Acknowledgments
This research was supported by a grant from the National Institute on Drug Abuse R01DA033235 and an award from the College of Arts and Sciences at American University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A
Data Transparency Statement
The results of the experimental lapse manipulation described in the present manuscript are entirely original and have not been presented or published elsewhere. The findings are based on the dissertation of the first author. Of the 81 participants who began the study 54 achieved the first 48 hours of required abstinence and were randomly assigned to the experimental lapse manipulation. The present manuscript describes the outcomes of the experimental lapse manipulation for these 54 participants, which was the main aim of this NIH-funded study.
A prior publication described characteristics of individuals who did not achieve the first 48 hours of required abstinence and thus were discharged prior to randomization to the experimental lapse manipulation (Muench and Juliano, 2017).
Footnotes
The authors have no conflicts of interest to report.
Contributor Information
Christine Muench, Department of Psychology, American University.
Elizabeth J. Malloy, Department of Math and Statistics, American University
Laura M. Juliano, Department of Psychology, American University
References
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, & Fiore MC (2004). Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychological Review, 111(1), 33–51. 10.1037/0033-295X.111.1.33 [DOI] [PubMed] [Google Scholar]
- Bakhshaie J, Zvolensky MJ, & Goodwin RD (2015). Cigarette smoking and the onset and persistence of depression among adults in the United States: 1994–2005. Comprehensive Psychiatry, 60, 142–148. 10.1016/j.comppsych.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A (1977). Self-efficacy: toward a unifying theory of behavioral change. Psychological Review, 84(2), 191–215. 10.1037/0033-295X.84.2.191 [DOI] [PubMed] [Google Scholar]
- Baron RM, & Kenny DA (1986). The moderator–mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology, 51(6), 1173. [DOI] [PubMed] [Google Scholar]
- Biener L, & Abrams DB (1991). The Contemplation Ladder: validation of a measure of readiness to consider smoking cessation. Health Psychology, 10(5), 360–365. [DOI] [PubMed] [Google Scholar]
- Bouton ME (2000). A learning theory perspective on lapse, relapse, and the maintenance of behavior change. Health Psychology, 19(1S), 57. [DOI] [PubMed] [Google Scholar]
- Brandon TH, Tiffany ST, Obremski KM, & Baker TB (1990). Postcessation cigarette use: the process of relapse. Addictive Behaviors, 15(2), 105–114. [DOI] [PubMed] [Google Scholar]
- Chornock WM, Stitzer ML, Gross J, & Leischow S (1991). Experimental Model of Smokine Re-Exposure: Effects on Relapse. Psychopharmacology (Berl), 108, 495–500. [DOI] [PubMed] [Google Scholar]
- Cinciripini PM, Wetter DW, Fouladi RT, Blalock JA, Carter BL, Cinciripini LG, & Baile WF (2003). The effects of depressed mood on smoking cessation: Mediation by postcessation self-efficacy. Journal of Consulting and Clinical Psychology, 71(2), 292–300. 10.1037/0022-006X.71.2.292 [DOI] [PubMed] [Google Scholar]
- Conklin CA, Perkins KA, Sheidow AJ, Jones BL, Levine MD, & Marcus MD (2005). The return to smoking: 1-year relapse trajectories among female smokers. Nicotine & Tobacco Research, 7(4), 533–540. 10.1080/14622200500185371 [DOI] [PubMed] [Google Scholar]
- Eisenberg MJ, Filion KB, Yavin D, Bélisle P, Mottillo S, Joseph L, … Rinfret S (2008). Pharmacotherapies for smoking cessation: a meta-analysis of randomized controlled trials. Canadian Medical Association Journal, 179(2), 135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SG, Gitchell JG, & Shiffman S (2012). Continuing to wear nicotine patches after smoking lapses promotes recovery of abstinence. Addiction, 107(7), 1349–1353. 10.1111/j.1360-0443.2012.03801.x [DOI] [PubMed] [Google Scholar]
- Fluharty M, Taylor AE, Grabski M, & Munafo MR (2017). The Association of Cigarette Smoking With Depression and Anxiety: A Systematic Review. Nicotine & Tobacco Research, 19(1), 3–13. 10.1093/ntr/ntw140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Metrik J, Kahler CW, & Shiffman S (2009). Self-efficacy and smoking cessation: a meta-analysis. Psychology of Addictive Behaviors, 23(1), 56–66. 10.1037/a0013529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Shiffman S, Balabanis MH, & Paty JA (2005). Dynamic self-efficacy and outcome expectancies: prediction of smoking lapse and relapse. Journal of Abnormal Psychology, 114(4), 661–675. 10.1037/0021-843X.114.4.661 [DOI] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, & Fagerstrom KO (1991). The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. British Journal of Addiction, 86(9), 1119–1127. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Donny EC, Houtsmuller EJ, & Stitzer ML (2006). Experimental evidence for a causal relationship between smoking lapse and relapse. Journal of Abnormal Psychology, 115(1), 166–173. 10.1037/0021-843X.115.1.166 [DOI] [PubMed] [Google Scholar]
- Juliano LM, Houtsmuller EJ, & Stitzer ML (2006). A preliminary investigation of rapid smoking as a lapse-responsive treatment for tobacco dependence. Experimental and Clinical Psychopharmacology, 14(4), 429. [DOI] [PubMed] [Google Scholar]
- Kirchner TR, Shiffman S, & Wileyto EP (2012). Relapse dynamics during smoking cessation: Recurrent abstinence violation effects and lapse-relapse progression. Journal of Abnormal Psychology, 121(1), 187–197. 10.1037/a0024451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski LT, Pillitteri JL, Sweeney CT, Whitfield KE, & Graham JW (1996). Asking questions about urges or cravings for cigarettes. Psychology of Addictive Behaviors, 10(4), 248–260. 10.1037/0893-164X.10.4.248 [DOI] [Google Scholar]
- Marlatt GA, & Gordon JR (1985). Relapse prevention: Maintenance strategies in addictive behavior change. New York, NY: Guilford. [Google Scholar]
- McClure EA, Vandrey RG, Johnson MW, & Stitzer ML (2013). Effects of varenicline on abstinence and smoking reward following a programmed lapse. Nicotine & Tobacco Research, 15(1), 139–148. 10.1093/ntr/nts101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muench C & Juliano LM (2017). Predictors of smoking lapse during a 48-hour laboratory analogue smoking cessation attempt. Psychology of Addictive Behaviors, 31(4), 415. [DOI] [PubMed] [Google Scholar]
- Muraven M, & Baumeister RF (2000). Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin, 126(2), 247–259. [DOI] [PubMed] [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, & Brown R (2001). Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors, 15(1), 13–17. [DOI] [PubMed] [Google Scholar]
- Niaura RS, Rohsenow DJ, Binkoff JA, Monti PM, Pedraza M, & Abrams DB (1988). Relevance of cue reactivity to understanding alcohol and smoking relapse. Journal of Abnormal Psychology, 97(2), 133. [DOI] [PubMed] [Google Scholar]
- Patterson F, Jepson C, Strasser AA, Loughead J, Perkins KA, Gur RC, … Lerman C (2009). Varenicline improves mood and cognition during smoking abstinence. Biological Psychiatry, 65(2), 144–149. 10.1016/j.biopsych.2008.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS (1977). The CES-D scale A self-report depression scale for research in the general population. Applied Psychological Measurement, 1(3), 385–401. [Google Scholar]
- Reiner DJ, Fredriksson I, Lofaro OM, Bossert JM, & Shaham Y (2019). Relapse to opioid seeking in rat models: behavior, pharmacology and circuits. Neuropsychopharmacology, 44(3), 465–477. 10.1038/s41386-018-0234-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Martino SC, Setodji C, Cervone D, & Witkiewitz K (2017). Does self-efficacy causally influence initial smoking cessation? An experimental study. Addictive Behaviors, 73, 199–203. 10.1016/j.addbeh.2017.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadel WG, Martino SC, Setodji C, Cervone D, Witkiewitz K, Beckjord EB, … Shih R (2011). Lapse-induced surges in craving influence relapse in adult smokers: An experimental investigation. Health Psychology, 30(5), 588–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffman S (2005). Dynamic influences on smoking relapse process. Journal of Personality, 73(6), 1715–1748. [DOI] [PubMed] [Google Scholar]
- Shiffman S (2006). Reflections on smoking relapse research. Drug and Alcohol Review, 25(1), 15–20. [DOI] [PubMed] [Google Scholar]
- Shiffman S, Paty JA, Gnys M, Kassel JA, & Hickcox M (1996). First lapses to smoking: within-subjects analysis of real-time reports. Journal of Consulting and Clinical Psychology, 64(2), 366. [DOI] [PubMed] [Google Scholar]
- Sweitzer MM, Denlinger RL, & Donny EC (2013). Dependence and Withdrawal-Induced Craving Predict Abstinence in an Incentive-Based Model of Smoking Relapse. Nicotine & Tobacco Research, 15(1), 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Zundert RM, Ferguson SG, Shiffman S, & Engels RC (2010). Dynamic effects of self-efficacy on smoking lapses and relapse among adolescents. Health Psychology, 29(3), 246–254. [DOI] [PubMed] [Google Scholar]
- Vidrine JI, Spears CA, Heppner WL, Reitzel LR, Marcus MT, Cinciripini PM, … Wetter DW (2016). Efficacy of mindfulness-based addiction treatment (MBAT) for smoking cessation and lapse recovery: A randomized clinical trial. Journal of Consulting and Clinical Psychology, 84(9), 824–838. 10.1037/ccp0000117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wileyto EP, Patterson F, Niaura R, Epstein LH, Brown RA, Audrain-McGovern J, … Lerman C (2005). Recurrent event analysis of lapse and recovery in a smoking cessation clinical trial using bupropion. Nicotine & Tobacco Research, 7(2), 257–268. 10.1080/14622200500055673 [DOI] [PubMed] [Google Scholar]
- Wileyto P, Patterson F, Niaura R, Epstein L, Brown R, Audrain-McGovern J, … Patterson F (2004). Do small lapses predict relapse to smoking behavior under bupropion treatment? Nicotine & Tobacco Research, 6(2), 357–366. 10.1080/1462220042000202463 [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, & Marlatt GA (2004). Relapse prevention for alcohol and drug problems: that was Zen, this is Tao. American Psychologist, 59(4), 224. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2015). WHO report on the global tobacco epidemic, 2015: Raising taxes on tobacco.


