Abstract
Background
Canavalia rosea (Sw.) DC. (bay bean) is an extremophile halophyte that is widely distributed in coastal areas of the tropics and subtropics. Seawater and drought tolerance in this species may be facilitated by aquaporins (AQPs), channel proteins that transport water and small molecules across cell membranes and thereby maintain cellular water homeostasis in the face of abiotic stress. In C. rosea, AQP diversity, protein features, and their biological functions are still largely unknown.
Results
We describe the action of AQPs in C. rosea using evolutionary analyses coupled with promoter and expression analyses. A total of 37 AQPs were identified in the C. rosea genome and classified into five subgroups: 11 plasma membrane intrinsic proteins, 10 tonoplast intrinsic proteins, 11 Nod26-like intrinsic proteins, 4 small and basic intrinsic proteins, and 1 X-intrinsic protein. Analysis of RNA-Seq data and targeted qPCR revealed organ-specific expression of aquaporin genes and the involvement of some AQP members in adaptation of C. rosea to extreme coral reef environments. We also analyzed C. rosea sequences for phylogeny reconstruction, protein modeling, cellular localizations, and promoter analysis. Furthermore, one of PIP1 gene, CrPIP1;5, was identified as functional using a yeast expression system and transgenic overexpression in Arabidopsis.
Conclusions
Our results indicate that AQPs play an important role in C. rosea responses to saline-alkaline soils and drought stress. These findings not only increase our understanding of the role AQPs play in mediating C. rosea adaptation to extreme environments, but also improve our knowledge of plant aquaporin evolution more generally.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-021-03034-1.
Keywords: Aquaporin, Drought, Saline-alkaline soil, Water deficit, Canavalia rosea (Sw.) DC.
Background
Canavalia rosea (Sw.) DC. (also called bay bean) is an extremophile halophyte and nitrogen-fixing legume species that is widely distributed in coastal areas of tropical and subtropical regions and is highly adapted to seawater and drought conditions [1]. The seeds of C. rosea have nutritional and medicinal value and this species constitutes an important wild plant resource. Notably, C. rosea presents better growth potential than most of native species, thereby plays basic and pioneering roles in island greening, sand fixation, and ecological restoration of tropical and subtropical coral islands and coastal zones [2]. Sandy soils, salinization, and seasonal drought are factors that limit growth for many plants in coastal areas or coral reefs. Canavalia rosea belongs to the “mangrove associates” group, in which some elaborate mechanisms for adapting to highly saline and alkaline soils and drought stress have evolved at both the morphological and physiological-molecular levels. Understanding the molecular and evolutionary mechanisms of C. rosea’s adaptation to the special habitats would help to illuminate extremophile adaptations to adverse conditions. Saline-alkaline soils and drought stress both cause plant cellular water deficits [3] and result in water imbalances, from root water uptake to leaf transpiration [4]. Identification of genes involved in responding to water-deficit stress in C. rosea may be valuable for molecular breeding improvement of saline-alkaline and drought-related traits through genetic engineering.
Water is an essential component of any biological system and plants exhibit elaborate adaptations to maintain survival in the presence of water stress. Aquaporins (AQPs) are transmembrane proteins that play critical roles in controlling transmembrane water transport in and out of plant cells by forming water channels [5]. In addition to water transport, AQPs facilitate the transport of small molecules such as urea, H2O2, and NO, and elements such as boron and silicon across cell membranes [6]. Aquaporins are found in a wide variety of taxa, including microbes, animals, and plants, and are the oldest family of major intrinsic proteins (MIPs). Aquaporins have been traditionally classified into four major subfamilies: plasma membrane intrinsic proteins (PIPs), tonoplast intrinsic proteins (TIPs), Nod26-like intrinsic proteins (NIPs), and small and basic intrinsic proteins (SIPs) [7]. Additionally, in some plant genomes, a small number of AQPs have been identified as a fifth subfamily called X-intrinsic proteins (XIPs), which are absent from monocots and Brassicaceae [8]. Furthermore, GlpF-like intrinsic proteins (GIPs) isolated from a moss (Physcomitrella patens) and hybrid intrinsic proteins (HIPs) found in a fern (Selaginella moellendorffii) and a moss (P. patens), which are rare in most plants, are both classified into the AQP family [9, 10].
Structurally, almost all AQPs consist of six transmembrane domains (α-helices, H1 to H6) with N and C termini facing the cytosol [11]. The six transmembrane domains are joined by five interhelical loops (A–E). The conserved loops (B and E) show extremely hydrophobic characteristic, often containing internal repeats of asparagine-proline-alanine residues (NPA motifs). These conserved, hydrophobic loops seem to be the most important features maintaining AQP function by forming short helices [12, 13]. Aromatic/Arginine regions (ar/R) and Froger’s positions are also conserved in most of AQPs [14]. Generally, AQPs are inserted into membranes in a tetrameric structure comprising four independent pores created by AQP monomers [15]. Besides being water channel proteins, some AQPs are also involved in facilitating the transport of CO2 [16], NO [17], glycerol [18], H2O2 [19], some trivalent elements [20], and a wide range of small uncharged solutes [21]. It is clear that AQPs show versatile functions in water uptake, nutrient balancing, long-distance signal transfer, nutrient/heavy metal acquisition in plant development, and stress responses [22].
Unlike the AQP members in yeast (only two genes, AQY1 and AQY2) [23] or animals (only 13 AQPs in mammals) [24], plants AQPs comprised large, highly diverse gene families that may be linked to plants’ greater adaptability to local conditions given their sessile nature [11, 25]. Many AQP gene families have been identified using cDNA and whole-genome analyses in a wide variety of plant species, including Arabidopsis (35 members) [26], maize (31 members) [27], and rice (34 members) [28]. Given advances in whole genome sequencing, AQP-related research has recently gained traction in studies of plant adaptation, especially for halophyte and drought-tolerant plants. As a close relative of Arabidopsis, Eutrema salsugineum has been considered a model extremophile used to identify mechanisms of salt tolerance. The AQP family in E. salsugineum has been characterized by assessing differential gene expression patterns, with research mostly focused on assessing responses to salt, cold, and drought stress [29, 30]. Chickpea (Cicer arietinum) has better drought tolerance than most of leguminous species and its AQP gene family has been characterized to further investigate its adaptability to water deficit [31, 32]. Furthermore the AQP gene family of cassava (Manihot esculenta), a drought-tolerant tuber that is an important food resource in many African countries, has been characterized in terms of its evolution, structure, and expression patterns [33]. Canavalia rosea is more tolerant to drought, high salinity, heat, and low nitrogen and phosphorous than most of leguminous plants. It is therefore of particular interest to identify the complete set of AQPs within C. rosea (CrAQPs) and to perform comparative analyses to understand their evolutionary relationships, particularly regarding the adaptability of this species to coastal and coral reef habitats.
In our study, the availability of whole genome sequence data for C. rosea facilitated genome-wide analysis to identify the evolutionary relationships between C. rosea AQPs and those of related leguminous species. We characterized the structure of CrAQPs and their chromosomal locations. We also investigated the expression profiles of CrAQP genes in various tissues, in response to different abiotic stressors, and in different habitats, along with promoter analyses. Additionally, a single plasma membrane intrinsic protein gene, CrPIP1;5, was functionally identified using heterogeneous transgenic assays.
Results
Identification of the C. rosea AQP family
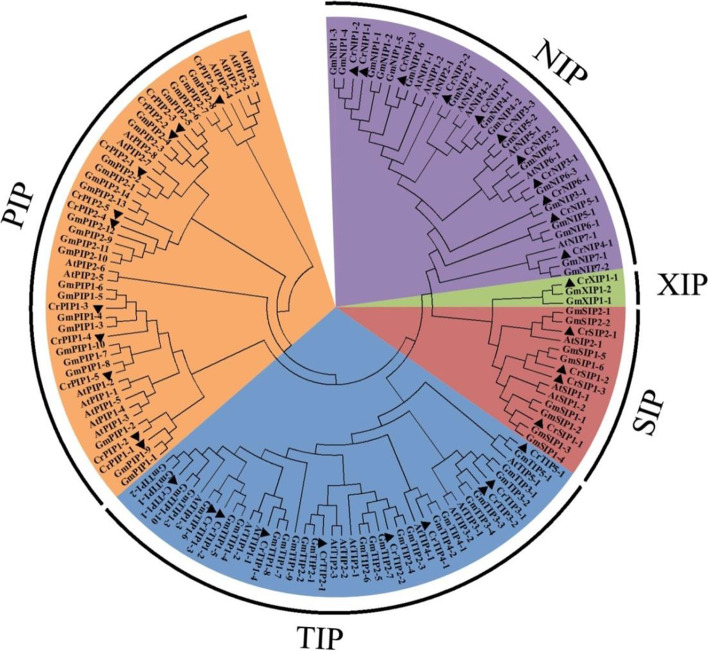
Base on the protein BLAST research and Hidden Markov model profile (Pfam ID: PF00230) search, a total of 37 CrAQP members were identified and annotated in the C. rosea genome database. The set of CrAQPs includes 11 NIPs, 11 PIPs, 10 TIPs, 4 SIPs, and 1 XIP (Table 1), which were named according to their phylogenetic and sequence identity relationships with AtAQP and GmAQP proteins (Table 1). Based on multiple alignments, a neighbor-joining phylogenetic tree was constructed with the amino acid sequences of AQPs from C. rosea, Arabidopsis, and soybean (Fig. 1). The clustering results clearly showed that there was only one sequence encoding for the XIP protein in C. rosea. In addition, the SIP subfamily in C. rosea (CrSIP) had a smaller but more conserved cluster than other three subfamilies in C. rosea (CrNIP, CrPIP, and CrTIP). We also compared the number of AQP genes in C. rosea with other plant genomes (Table 2), including four Leguminosae species (bean [Phaseolus vulgaris, 41 genes], chickpea [Cicer arietinum, 40], wild peanut A [Arachis duranensis, 32], and wild peanut B [Arachis ipaensis, 36]), two Brassicaceae species (Arabidopsis [Arabidopsis thaliana, 35] and salt cress [Eutrema salsugineum, 35]), and three Gramineae species (rice [Oryza sativa, 33], maize [Zea mays, 31], and foxtail millet [Setaria italica, 39]). The numbers of AQP genes in all of these typical diploid species were similar. The soybean (G. max) genome contains 72 AQP genes, which might be due to a whole-genome duplication event in the distant past [34].
Table 1.
Nomenclature and subcellular localization of AQPs identified from C. rosea genome
| Name | Locus | Mw (kD) | PI | GRAVY | No. of Phosphor Sites | Prediction for cellular-localization | |
|---|---|---|---|---|---|---|---|
| WoLF_PSORTa | Plant-mPLoc | ||||||
| CrPIP1;1 | 02T006326 | 31.00 | 9.24 | 0.304 | Ser: 9 Thr: 8 Tyr: 4 | plas | plas |
| CrPIP1;2 | 03T010068 | 30.96 | 9.24 | 0.265 | Ser: 9 Thr: 8 Tyr: 5 | plas | plas |
| CrPIP1;3 | 01T000438 | 30.71 | 8.83 | 0.405 | Ser: 10 Thr: 6 Tyr: 3 | plas | plas |
| CrPIP1;4 | 10T026210 | 34.33 | 7.56 | 0.286 | Ser: 14 Thr: 8 Tyr: 3 | plas | plas |
| CrPIP1;5 | 06T017675 | 30.89 | 8.90 | 0.361 | Ser: 12 Thr: 6 Tyr: 3 | plas | plas |
| CrPIP2;1 | 02T003848 | 26.63 | 8.84 | 0.429 | Ser: 12 Thr: 5 Tyr: 3 | plas/vacu | plas |
| CrPIP2;2 | 08T022691 | 30.62 | 8.24 | 0.348 | Ser: 11 Thr: 9 Tyr: 4 | plas | plas |
| CrPIP2;3 | 09T024941 | 31.07 | 7.66 | 0.383 | Ser: 9 Thr: 10 Tyr: 3 | plas | plas |
| CrPIP2;4 | 07T020364 | 30.69 | 8.28 | 0.497 | Ser: 11 Thr: 7 Tyr: 4 | plas | plas |
| CrPIP2;5 | 04T013368 | 30.77 | 8.28 | 0.462 | Ser: 12 Thr: 7 Tyr: 5 | plas | plas |
| CrPIP2;6 | 03T008319 | 30.53 | 8.82 | 0.401 | Ser: 11 Thr: 6 Tyr: 5 | plas | plas |
| CrTIP1;1 | 03T010879 | 26.00 | 5.32 | 0.744 | Ser: 15 Thr: 2 Tyr: 1 | plas/vacu | vacu |
| CrTIP1;2 | 08T022781 | 25.84 | 5.41 | 0.864 | Ser: 11 Thr: 3 Tyr: 1 | vacu/plas | vacu |
| CrTIP1;3 | 09T025003 | 26.06 | 5.70 | 0.769 | Ser: 7 Thr: 5 Tyr: 1 | plas/vacu | vacu |
| CrTIP1;4 | 03T008366 | 25.65 | 5.78 | 0.863 | Ser: 8 Thr: 5 Tyr: 4 | plas/vacu | vacu |
| CrTIP2;1 | 10T026350 | 25.25 | 4.80 | 1.005 | Ser: 8 Thr: 4 Tyr: 3 | vacu | vacu |
| CrTIP2;2 | 02T007260 | 25.16 | 5.76 | 0.933 | Ser: 11 Thr: 4 Tyr: 3 | plas/vacu | vacu |
| CrTIP3;1 | 07T020862 | 27.08 | 6.34 | 0.661 | Ser: 8 Thr: 4 Tyr: 2 | plas/vacu/ | vacu |
| CrTIP3;2 | 04T012996 | 27.28 | 6.74 | 0.586 | Ser: 9 Thr: 6 Tyr: 2 | plas/cyto_plas | vacu |
| CrTIP4;1 | 02T004418 | 28.53 | 5.77 | 0.798 | Ser: 9 Thr: 4 Tyr: 1 | plas/vacu | vacu |
| CrTIP5;1 | 08T023301 | 26.44 | 7.74 | 0.760 | Ser: 13 Thr: 6 Tyr: 2 | plas | plas |
| CrNIP1;1 | 11T029123 | 28.33 | 6.96 | 0.528 | Ser: 11 Thr: 10 Tyr: 1 | plas/vacu | plas/vacu |
| CrNIP1;2 | 11T029124 | 28.32 | 7.01 | 0.414 | Ser: 12 Thr: 7 Tyr: 2 | plas/vacu | plas/vacu |
| CrNIP1;3 | 01T000942 | 32.85 | 9.18 | 0.460 | Ser: 14 Thr: 10 Tyr: 3 | plas/vacu/E.R | plas |
| CrNIP2;1 | 06T018871 | 27.82 | 6.41 | 0.709 | Ser: 15 Thr: 6 Tyr: 5 | plas/vacu | plas |
| CrNIP2;2 | 01T002720 | 27.48 | 5.11 | 0.650 | Ser: 14 Thr: 4 Tyr: 4 | plas | plas |
| CrNIP3;1 | 09T025384 | 30.90 | 7.67 | 0.465 | Ser: 8 Thr: 10 Tyr: 1 | plas | plas |
| CrNIP3;2 | 02T007404 | 31.72 | 8.83 | 0.460 | Ser: 13 Thr: 14 Tyr: 1 | plas | plas |
| CrNIP3;3 | 04T013473 | 31.04 | 8.95 | 0.459 | Ser: 11 Thr: 8 Tyr: 2 | plas | plas |
| CrNIP4;1 | 04T011155 | 78.97 | 9.08 | 0.092 | Ser: 32 Thr: 17 Tyr: 5 | plas | plas/chlo |
| CrNIP5;1 | 03T010636 | 30.41 | 8.57 | 0.387 | Ser: 16 Thr: 11 Tyr: 1 | plas | plas |
| CrNIP6;1 | 06T018390 | 30.47 | 6.83 | 0.569 | Ser: 16 Thr: 11 Tyr: 1 | plas/vacu | plas |
| CrSIP1;1 | 03T007625 | 26.53 | 8.53 | 0.721 | Ser: 4 Thr: 7 Tyr: 2 | plas | plas |
| CrSIP1;2 | 08T022477 | 25.88 | 9.92 | 0.621 | Ser: 4 Thr: 10 Tyr: 2 | plas | plas |
| CrSIP1;3 | 08T022478 | 17.13 | 10.22 | 0.434 | Ser: 6 Thr: 3 Tyr: 3 | plas/vacu | plas |
| CrSIP2;1 | 03T007754 | 26.21 | 9.80 | 0.547 | Ser: 14 Thr: 4 Tyr: 1 | vacu/plas/E.R | plas |
| CrXIP1;1 | 08T023183 | 31.59 | 5.55 | 0.621 | Ser: 11 Thr: 8 Tyr: 0 | plas | plas |
a The scores predicted by WoLF PSORT equal to or below 3 are ignored
Fig. 1.
Phylogenetic relationships of the 37 CrAQPs from C. rosea, the 75 GmAQPs from soybean, and the 35 AtAQPs from Arabidopsis. The phylogenetic tree is constructed using MEGA 6.0 software, with ClustalW alignment, neighbor-joining (NJ) method, the bootstrap method, and 1000 repetitions. All five subfamilies of AQP gene family are well separated in different clades and represented by different color background
Table 2.
The numbers of AQP genes in different plant species
| Family | Species | Total No | The No. of AQP members in sub-families | ||||
|---|---|---|---|---|---|---|---|
| PIPs | TIPs | NIPs | SIPs | XIPs | |||
| Leguminosae | Canavalia rosea | 37 | 11 | 10 | 11 | 4 | 1 |
| Phaseolus vulgaris | 41 | 12 | 13 | 10 | 4 | 2 | |
| Cicer arietinum | 40 | 9 | 12 | 16 | 3 | 0 | |
| Arachis duranensis | 32 | 9 | 11 | 8 | 3 | 1 | |
| Arachis ipaensis | 36 | 9 | 10 | 10 | 3 | 4 | |
| Glycine max | 72 | 24 | 24 | 17 | 8 | 2 | |
| Brassicaceae | Arabidopsis thaliana | 35 | 13 | 10 | 9 | 3 | 0 |
| Eutrema salsugineum | 35 | 12 | 11 | 9 | 3 | 0 | |
| Gramineae | Oryza sativa | 33 | 11 | 10 | 10 | 2 | 0 |
| Zea mays | 31 | 13 | 11 | 4 | 3 | 0 | |
| Setaria italica | 39 | 12 | 11 | 13 | 3 | 0 | |
The length of CrAQP proteins ranged from 155 aa (CrSIP1;3) to 709 aa (CrNIP4;1), while most were between 230 and 320 aa. The predicted molecular weight and isoelectric points of the CrAQPs ranged from 17.13 kDa to 78.97 kDa and 4.8 to 9.92, respectively (Tables 1 and 3). Thirty four of the 37 CrAQPs included six transmembrane domains and the remaining three members (CrNIP4;1, CrSIP1;3, and CrXIP1;1) possessed seven, three, and five transmembrane domains, respectively (Table 3). The identification of transmembrane regions of CrAQPs is shown in Figure S1.
Table 3.
Conserved amino acid residues (Asn-Pro-Ala, NPA) motifs, aromatic/arginine (ar/R) filters and Froger’s positions (FPs) and trans-membrane (TM) domains of AQPs in C. rosea
| Name | AA | TMD | NPA (LB) | NPA (LE) | NPA space | Ar/Rfilters | Froger’s residues | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | H5 | LE1 | LE2 | P1 | P2 | P3 | P4 | P5 | ||||||
| CrPIP1;1 | 288 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrPIP1;2 | 287 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrPIP1;3 | 286 | 6 | NPA | NPA | 119 | F | H | T | R | E | S | A | F | W |
| CrPIP1;4 | 317 | 6 | NPA | NPA | 119 | F | H | T | R | E | S | A | D | F |
| CrPIP1;5 | 288 | 6 | NPA | NPA | 119 | F | H | T | R | E | S | S | F | W |
| CrPIP2;1 | 250 | 6 | \ | NPA | \ | F | H | T | R | M | S | A | F | W |
| CrPIP2;2 | 285 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrPIP2;3 | 289 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | Y | W |
| CrPIP2;4 | 288 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrPIP2;5 | 287 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrPIP2;6 | 284 | 6 | NPA | NPA | 118 | F | H | T | R | Q | S | A | F | W |
| CrTIP1;1 | 252 | 6 | NPA | NPA | 111 | H | I | A | V | T | S | A | C | W |
| CrTIP1;2 | 252 | 6 | NPA | NPA | 111 | H | I | A | V | T | S | A | Y | W |
| CrTIP1;3 | 252 | 6 | NPA | NPA | 111 | H | I | A | V | T | S | A | Y | W |
| CrTIP1;4 | 250 | 6 | NPA | NPA | 111 | H | I | A | V | T | S | S | Y | W |
| CrTIP2;1 | 249 | 6 | NPA | NPA | 111 | H | I | G | R | T | S | A | Y | W |
| CrTIP2;2 | 248 | 6 | NPA | NPA | 111 | H | I | G | R | T | S | A | Y | W |
| CrTIP3;1 | 255 | 6 | NPA | NPA | 111 | H | I | A | L | T | A | S | F | W |
| CrTIP3;2 | 257 | 6 | NPA | NPA | 111 | H | I | A | R | T | A | A | F | W |
| CrTIP4;1 | 272 | 6 | NPS | NPA | 111 | H | I | A | R | S | S | A | Y | W |
| CrTIP5;1 | 253 | 6 | NPA | NPA | 111 | N | V | G | C | V | A | A | Y | W |
| CrNIP1;1 | 268 | 6 | NPA | NPA | 109 | W | V | A | R | F | S | T | Y | L |
| CrNIP1;2 | 269 | 6 | NPA | NPA | 109 | W | V | A | R | F | S | A | Y | L |
| CrNIP1;3 | 306 | 6 | NPA | NPV | 109 | W | V | A | R | F | S | A | Y | L |
| CrNIP2;1 | 261 | 6 | NPA | NPA | 109 | W | V | A | R | L | S | A | Y | I |
| CrNIP2;2 | 256 | 6 | NPA | NPA | 109 | W | L | A | R | F | S | A | Y | I |
| CrNIP3;1 | 299 | 6 | NPA | NPV | 108 | T | I | G | R | F | T | A | Y | L |
| CrNIP3;2 | 307 | 6 | NPA | NPV | 108 | N | I | S | R | F | T | A | Y | L |
| CrNIP3;3 | 299 | 6 | NPA | NPV | 108 | A | I | G | R | Y | T | A | Y | L |
| CrNIP4;1 | 709 | 7 | NPA | NPA | 108 | A | V | G | R | Y | S | A | Y | I |
| CrNIP5;1 | 290 | 6 | NPA | NPA | 108 | A | S | G | R | F | T | A | Y | F |
| CrNIP6;1 | 287 | 6 | NPA | NPA | 104 | S | I | A | R | Y | S | A | Y | I |
| CrSIP1;1 | 247 | 6 | NPT | NPA | 112 | V | V | P | N | M | A | A | Y | W |
| CrSIP1;2 | 239 | 6 | NPS | NPA | 113 | A | V | P | N | L | A | A | Y | W |
| CrSIP1;3 | 155 | 3 | NPS | NLG | 79 | \ | V | P | F | L | A | A | Y | W |
| CrSIP2;1 | 236 | 6 | NPL | NPA | 108 | S | H | G | S | I | V | A | Y | W |
| CrXIP1;1 | 296 | 5 | SPV | NPA | 127 | V | V | V | R | D | C | A | \ | \ |
Features of AQP proteins
The phosphorylation state of AQP proteins is a key factor regulating the transport of water and other small molecules, or affecting of the protein subcellular localization [14, 22]. In this study, we predicted the possible phosphorylation sites of CrAQPs. In brief, all CrAQPs except for CrXIP1;1 contained all three phosphorylation sites (Ser, Thr, and Tyr; Table 1). We also predicted the subcellular localization of CrAQPs. The two programs used (WoLF_PSORT and Plant-mPLoc) had similar results and most CrAQPs were located in the plasma membrane, although some were located in vacuoles, plastids, and the endoplasmic reticulum (Table 1). The subcellular localizations of CrAQPs showed diverse and broad patterns, indicating that the in vivo compartmentation of CrAQPs is highly variable for each member to regulate transport of water and/or solutes across the plasma membrane and intracellular membrane systems, thereby exercising unique biological functions.
The NPA motifs, ar/R filter, and Froger’s positions of AQPs are critical for their substrate selectivity. A multiple alignment between CrAQPs and other plant AQPs was performed and the conserved NPA motifs and amino acids in ar/R filter and Froger’s positions are characterized in Table 3 and Figure S1. Except for CrPIP2;1, the other 36 CrAQPs all contained two NPA motifs, one in loop B and one in loop E, and most of them were conserved. However, some CrAQPs, such as in CrTIP4;1 and four CrSIPs, displayed a variable third residue in the LB NPA motif, in which the A residue was replaced by S/T/L. In addition, the CrXIP1;1 protein had variable first and third residues in the LB NPA motif (SPV). In loop E, this NPA motif was more conserved and only CrNIP1;3, CrNIP3;1, CrNIP3;2, and CrNIP3;3 showed substitutions of A by V. In CrSIP1;3, the LE NPA motif degenerated into NLG and showed a greater divergence in residues of the two NPA motifs than the other CrAQPs (Table 3). The space between the two conserved NPA motifs varied from 79 to 127 aa and most were between 108 and 119 aa (Table 3). At the ar/R selectivity filters and Froger’s positions, the CrAQPs displayed more differences than in NPA motifs (Table 3). These variabilities determined the substrate specificity of CrAQPs.
Chromosomal locations and evolutionary characterization of CrAQPs
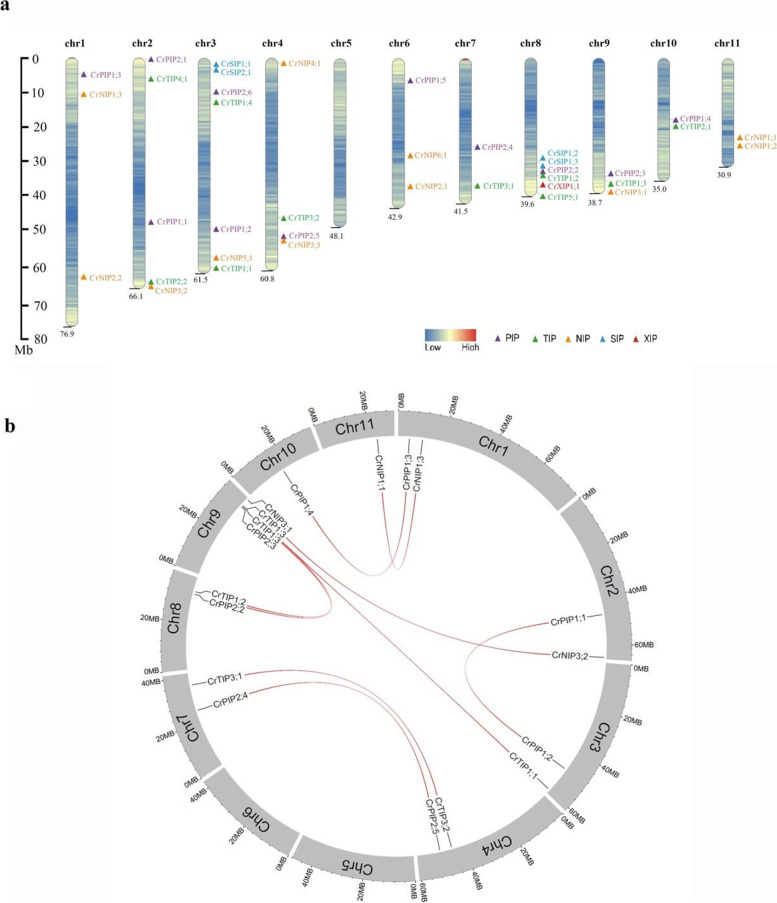
To investigate the evolutionary relationship among CrAQP genes, chromosome maps were constructed (Fig. 2a). There are eleven chromosomes in the C. rosea genome and CrAQP genes were found on all except chromosome 5. On the other ten chromosomes, the CrAQPs were unevenly distributed. Among them, chromosome 3 had seven CrAQP genes, chromosome 8 had six, chromosome 2 had five, chromosome 4 had four, chromosomes 1, 6, and 9 had three, and chromosomes 7, 10, and 11 had two.
Fig. 2.
a Locations of the 37 CrAQPs on 11 chromosomes of C. rosea. b The distribution of segmental duplication of CrAQPs in C. rosea chromosomes
Gene duplication events of CrAQPs were also investigated. A total of eighteen and four CrAQP genes were found to be segmentally and tandemly duplicated, respectively (Table 4). The distribution of segmental duplication of CrAQPs in C. rosea chromosomes was simply showed in Fig. 2b.The selection pressure acting on CrAQP genes was inferred from the ratio of non-synonymous (Ka) to synonymous (Ks) substitution values. Our data indicate that all CrAQP genes were under evolutionary pressure, with an average Ka/Ks ratio of 0.1523. All Ka/Ks ratios were well below one (range: 0.0989–0.2738) (Table 4). These results suggest that CrAQPs experienced strong purifying selection pressure with limited functional divergence after duplication.
Table 4.
Ka/Ks analysis and duplication events for CrAQP genes
| Duplicated pair | Duplicate type | Ka | Ks | Ka/Ks | P-value(Fisher) | Positive selection |
|---|---|---|---|---|---|---|
| CrPIP1;1/CrPIP1;2 | Segmental | 0.073547 | 0.622897 | 0.118072 | 1.87439E-30 | No |
| CrPIP1;3/CrPIP1;4 | Segmental | 0.111758 | 0.842367 | 0.132671 | 1.34665E-32 | No |
| CrPIP2;2/CrPIP2;3 | Segmental | 0.06824 | 0.675207 | 0.101065 | 6.37277E-34 | No |
| CrPIP2;4/CrPIP2;5 | Segmental | 0.048577 | 0.491175 | 0.0989 | 7.70278E-29 | No |
| CrTIP1;1/CrTIP1;3 | Segmental | 0.140454 | 1.19439 | 0.117595 | 9.9E-36 | No |
| CrTIP1;2/CrTIP1;3 | Segmental | 0.065171 | 0.651205 | 0.100078 | 2.28E-30 | No |
| CrTIP3;1/CrTIP3;2 | Segmental | 0.141034 | 0.515046 | 0.273827 | 1.48E-12 | No |
| CrNIP1;1/CrNIP1;3 | Segmental | 0.170454 | 0.652004 | 0.26143 | 7.81E-16 | No |
| CrNIP3;1/CrNIP3;2 | Segmental | 0.13405 | 0.803634 | 0.166804 | 1.27E-28 | No |
| CrNIP1;1/CrNIP1;2 | Tandem | \ | \ | \ | \ | \ |
| CrSIP1;2/CrSIP1;3 | Tandem | \ | \ | \ | \ |
Gene structures and protein motif compositions
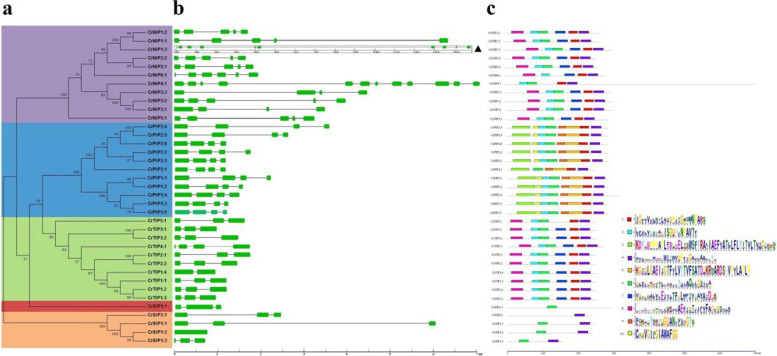
Gene structure analyses performed using the GSDS tool revealed relatively large variation in the number and length of introns/exons that resulted in CrAQPs length variation (720–14,816 bp) across five different CrAQP subfamilies (Fig. 3a and b). The number of introns ranged from zero (CrSIP1;2) to eleven (CrNIP4;1). Most CrNIPs and CrPIPs possessed three to four introns and most CrTIPs had two introns, except for CrTIP4;1, which had three introns. Three of four CrSIPs had two introns, except for CrSIP1;2, which was intronless. The only CrXIP1;1 also had two introns.
Fig. 3.
Phylogenetic relationships, genes’ structure, and motif compositions of the AQP genes in C. rosea. a The phylogenetic tree on the left side is constructed using MEGA 6.0. The five major groups are marked with different color backgrounds. b The exon–intron organization of the CrAQPs is constructed using GSDS 2.0 (in the middle). c The conserved motifs of each group on the right side are identified by the MEME web server. Different motifs are represented by different colored boxes, and the motif sequences are provided in Table S4
To further investigate the function of AQPs in C. rosea, MEME was used to identify the conserved domains of CrAQP proteins. Among the ten motifs identified, motifs 1, 2, 4, 6, 7, and 8 were widely found in CrNIP and CrTIP subfamilies. Most of the CrPIP members shared conserved motifs 1, 2, 3, 4, 5, 6, 9, and 10. Three of the four CrSIPs shared conserved motifs 4 and 6, all except CrSIP2;1. The CrXIP1;1 protein possessed only motif 6. In general, the motif compositions were similar within each CrAQP protein subfamily (Fig. 3c).
Cis-acting regulatory elements
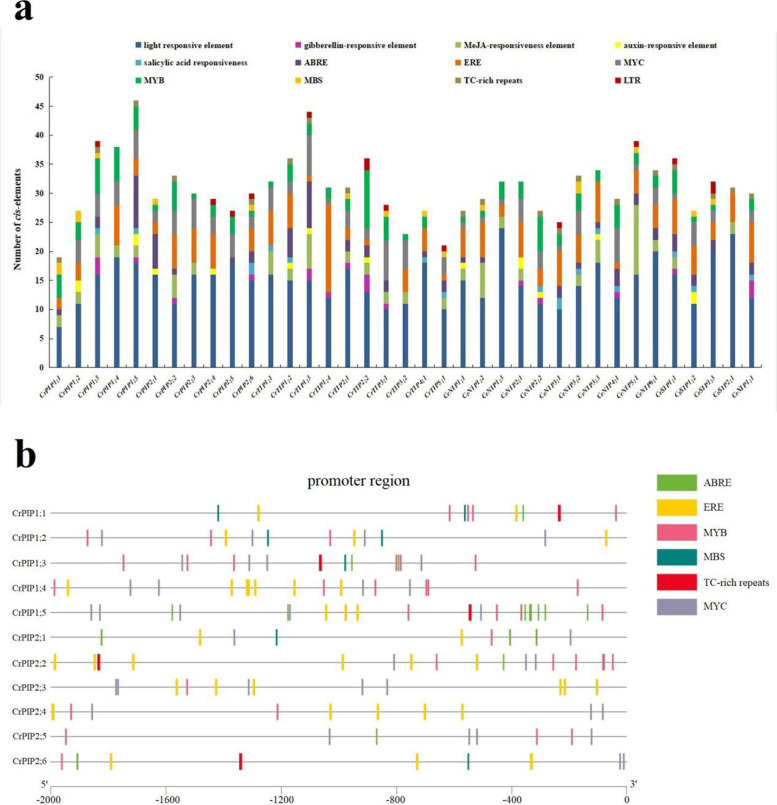
The regulation of CrAQP expression remains a key mediator of CrAQP function, especially in response to stress and plant growth and development. The cis-acting regulatory elements are a series of nucleotide motifs that bind to specific transcription factors, thereby regulating transcription in plants. In this study, we identified putative cis-acting elements in the promoter regions of all of CrAQPs by scanning the online PlantCARE program.
The promoter analyses of all 37 CrAQPs identified 68 putative cis-acting elements, including 25 light responsive elements, 4 ABA responsive elements, 3 gibberellin-responsive elements, 2 MeJA-responsive elements, 2 auxin responsive elements, 1 ethylene-responsive element, 22 abiotic or biotic stress-related responsive elements, and 18 development-related responsive elements (Table S3). We characterized these elements into 12 categories: light responsive elements, gibberellin-responsive elements, MeJA-responsive elements, auxin-responsive elements, salicylic acid responsive elements, ABRE-, ERE-, MYC-, MYB-, MBS-, and TC-rich repeats, and LTR. The numbers of these elements in each CrAQP promoter region are summarized in Fig. 4a. In addition, because PIPs play an important roles in maintaining water balance in plant cells, we summarized the abiotic stress-related cis-acting elements (including ABRE, ERE, MYB, MBS, TC-rich repeats, and MYC) within 11 CrPIP promoter regions (Fig. 4b). The categories and numbers of these elements suggest that mechanisms regulating CrPIP expression are involved in stress responses. However, further functional studies are still necessary to confirm the functions of these cis-acting CrAQP elements.
Fig. 4.
Numbers and distribution of the cis-acting elements in the 37 candidate CrAQP promoter regions. A Summaries of the twelve cis-acting elements in the 37 candidate CrAQPs promoter regions; B Distribution of the six cis-acting elements (ABRE, ERE, MYB, MBS, TC-rich repeat, and MYC) in the eleven CrPIP promoter regions. The elements are represented by different symbols. The scale bar represents 500 bp
Expression profiles of CrAQPs in different tissues and plants residing in different habitats
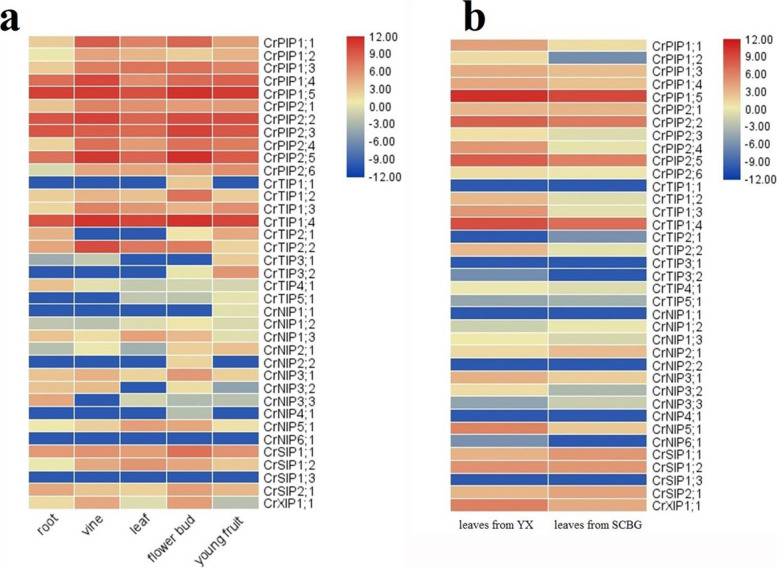
Tissue- and habitat-specific expression profiles of CrAQPs were assessed by examining their Illumina RNA-Seq data representing seven tissue types: roots, vines, young leaves, flowering buds, and young fruits gathered from SCBG, and two mature leaf samples gathered from SCBG and YX Island respectively. Expression of all CrAQPs was detected in at least one of the examined tissues, though the transcript level was diverse. Overall, the CrPIP members had relatively higher expression in all tissues. The subfamilies CrPIP and CrTIP also produced abundant transcripts in most examined tissues (Fig. 5).Young flowering buds and young fruits tended to have high levels of CrAQP expression across the whole family (Fig. 5a). We also focused on the comparison of CrAQPs’ expression levels in adult C. rosea leaves collected from various habitats (YX Island and SCBG), and the results indicated that the most of CrAQPs expressed higher in the YX sample than in the SCBG sample, particularly the CrPIP members (Fig. 5b). These results suggest that CrAQPs might play diverse roles in the growth and development of C. rosea, and in this extremophile halophyte’s adaptation to coral reef habitats.
Fig. 5.
Heatmaps showing (A) the expression levels of the 37 CrAQPs in the root, vine, leaf, flower bud, and young fruit of C. rosea plant and (B) expression differences of the 37 CrAQPs in mature C. rosea leaves planting in South China Botanical Garden (SCBG) and in Yongxing Island (YX)
Expression profiles of CrPIPs in response to different stressors and the ABA treatment
We performed a gene expression analysis on different C. rosea tissues to examine the expression patterns of CrPIP genes under various abiotic stress conditions and an ABA hormone treatment. The purpose of these treatments were to mimic reef and coast adversity as much as possible. We performed qRT-PCR to detect the transcript levels of these subfamily genes. As shown in Fig. 6, expression of all CrPIPs was affected by the stressors and hormone application. We also found several CrPIP members that showed relatively stable expression patterns, even under the various stressors. These genes included CrPIP1;5, CrPIP2;2, CrPIP2;3, and CrPIP2;5 (Fig. 6). Combining these results with the RNA-Seq data (Fig. 5), it is evident that these genes maintained higher expression levels than the other CrAQP genes across different tissues and habitats, suggesting that they may be involved in maintaining basic and primary water homeostasis during C. rosea growth and development. Under high salt stress, CrPIP1;2 showed all induced expression patterns in roots, vines, and leaves, while CrPIP1;1, CrPIP1;3, CrPIP1;4, CrPIP2;1, and CrPIP2;6 showed elevated expression in vine and leaf, and their expression was downregulated in roots. In general, alkaline stress had a smaller effect on the expression of CrPIPs. The genes CrPIP1;2, CrPIP1;3, CrPIP1;4, CrPIP1;5, CrPIP2;1, CrPIP2;4, CrPIP2;5, and CrPIP2;6 were downregulated in root, while CrPIP1;1, CrPIP1;2, CrPIP2;4, and CrPIP2;5 were slightly upregulated in aerial tissues. High osmotic stress increased the expression of CrPIP1;1, CrPIP2;4, and CrPIP2;6, and the ABA treatment increased the expression of CrPIP1;2, CrPIP1;4, CrPIP2;1, and CrPIP2;6 (Fig. 6). These results indicate the role these genes play in multiple abiotic stress responses and ABA signaling response in C. rosea.
Fig. 6.
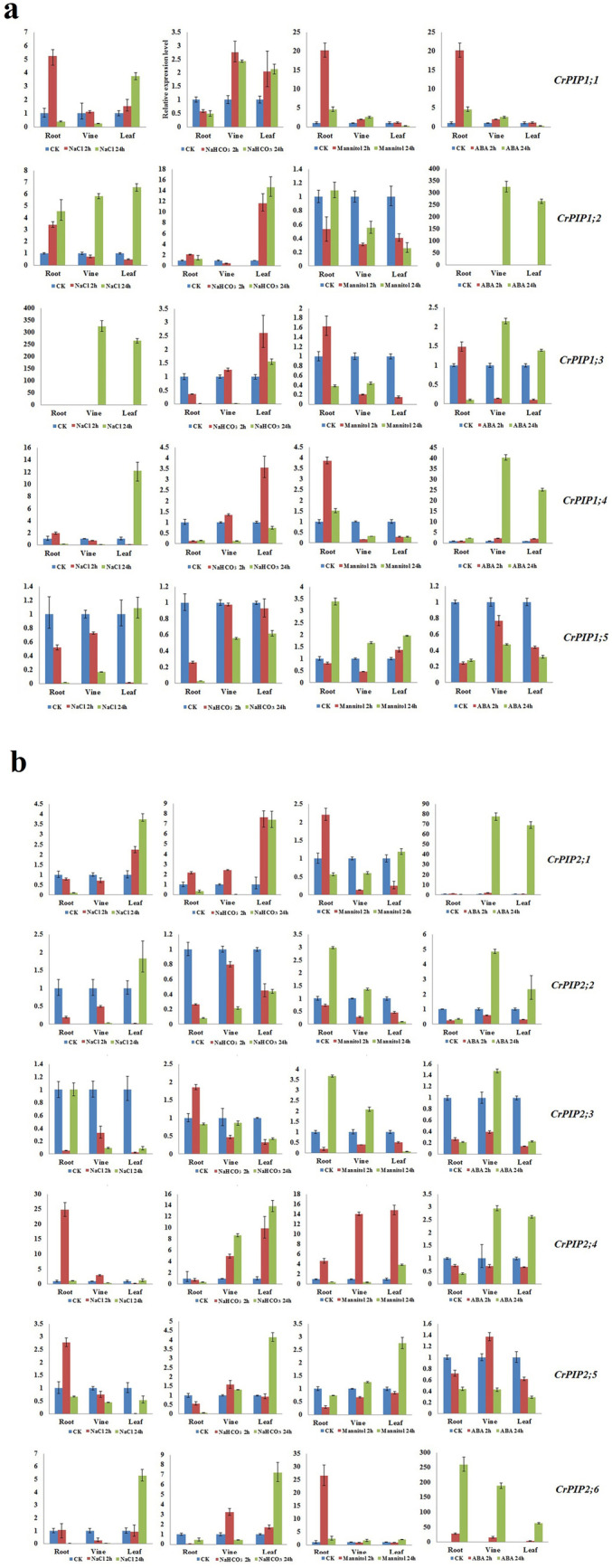
Quantitative RT-PCR detection of the expression levels of the 11 CrPIPs responding to different stresses (600 mM NaCl, 150 mM NaHCO3, 300 mM mannitol, and 100 mM ABA) in C. rosea seedling plants. a 5 CrPIP1s. b 6 CrPIP2s. Relative expression values were calculated using the 2 − ΔCt method with housekeeping gene CrEF-α as reference gene. Bars show mean values ± SD of n = 3–4 technical replicates
Interactions between CrPIP1;5 and CrPIP2;3
A previous study indicated that plant PIP1 and PIP2 members can associate together in heterodimers and tetramers [35]. In our previous study, we have functional identified a C. rosea PIP2 gene, CrPIP2;3, being involved in drought tolerance in transgenic plant [36]. CrPIP1;5 and CrPIP2;3 were both initially isolated from C. rosea cDNA library, and these two members showed much higher expression level in different tissues of C. rosea than other CrAQPs (Fig. 5a), which might indicate their relative importance of maintaining water balance in vivo. In this study we analyzed two PIP members, CrPIP1;5 and CrPIP2;3, to confirm that CrAQPs could form homodimers or heterodimers. To explore CrPIP1;5-CrPIP2;3 interactions, a series of DNA constructs were prepared for a yeast two-hybrid assay (Fig. 7a). BD and AD vectors were co-transformed into yeast AH109. Both CrPIP1;5 and CrPIP2;3 did not self-activate, but both can form homodimers through direct interactions with themselves(Fig. 7b). Furthermore, CrPIP1;5 and CrPIP2;3 can interact with each other (Fig. 7c). Together, these results indicate that, at least in yeast cells, these two CrPIP members can interact with themselves and each other to form active pores for water and small molecule transport across membranes.
Fig. 7.
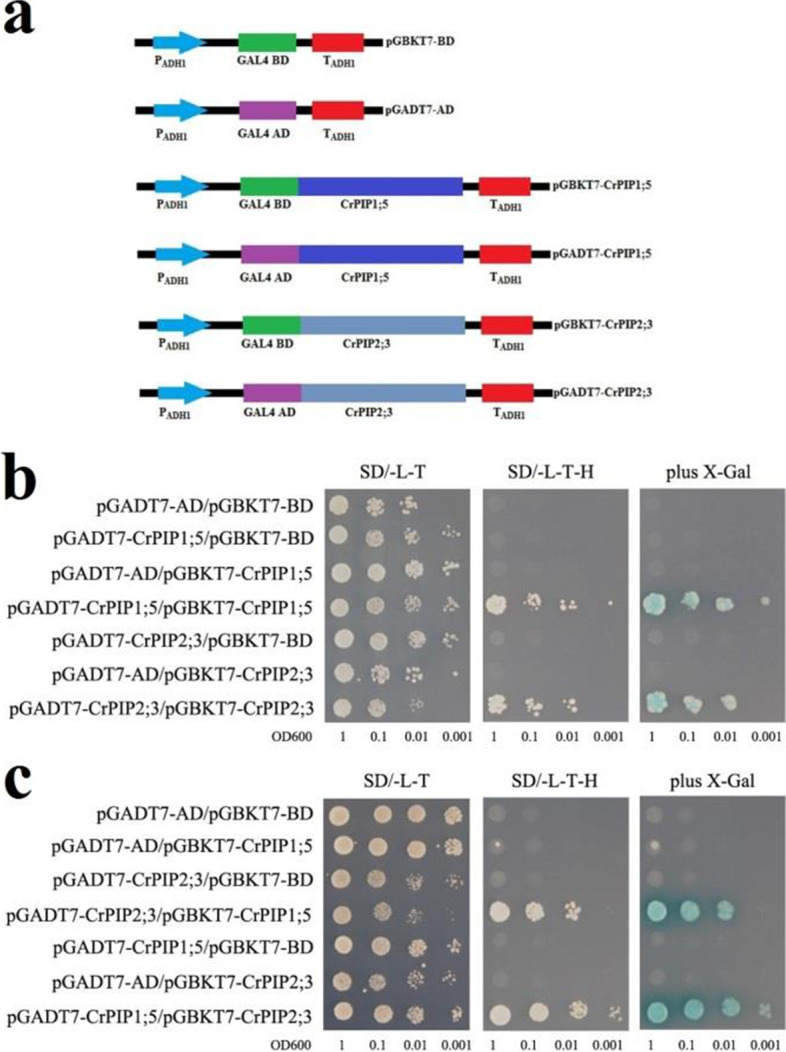
Homodimer or Heterodimer of the CrPIP1; 5 and the CrPIP2;3 detection by yeast two-hybrid assay. a Maps of different constructs. b Both the CrPIP1;5 and the CrPIP2;3 showed self-interacting. c The CrPIP1;5 and the CrPIP2;3 could interact each other
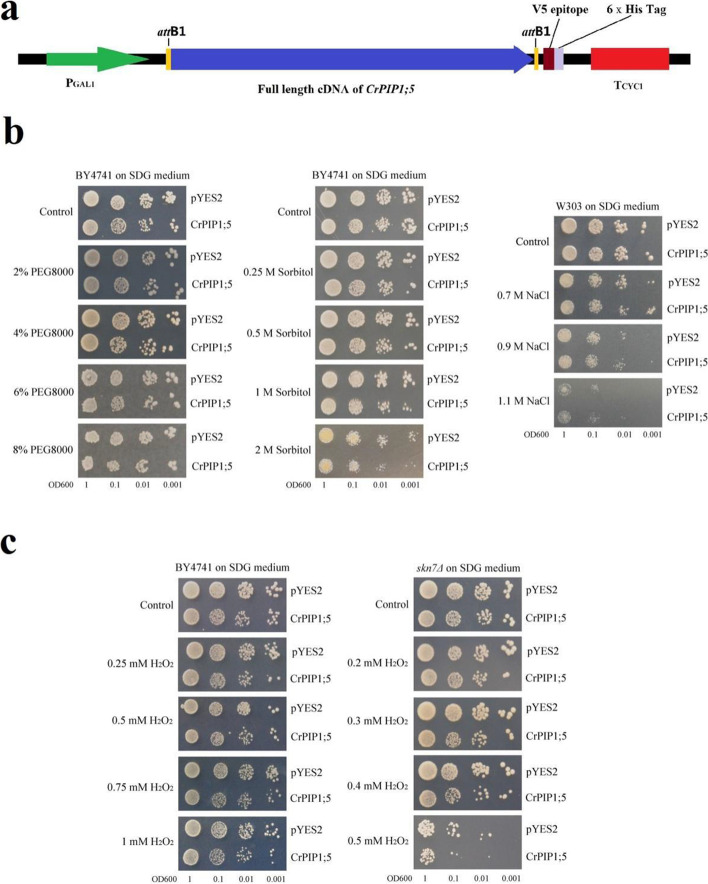
Abiotic stress tolerance of yeast and Arabidopsis heterologously expressing CrPIP1;5
We performed functional identification of CrPIP1;5 using a yeast expression system, constructing with a CrPIP1;5-pYES-DEST52 recombinant vector (Fig. 8a). As seen in Fig. 8b, W303 transformed with either CrPIP1;5 or pYES2 developed normally and did not differ in growth rate from the SDG control plate. However, with the addition of PEG8000 or sorbitol, W303 transformed with CrPIP1;5 showed an obvious growth lag compared to yeast containing the pYES2 control. When NaCl was added to the SDG medium, the W303 yeast containing CrPIP1;5 showed better growth than the control (Fig. 8b). We also checked H2O2 transport activity using the yeast expression system. CrPIP1;5 resulted in increased H2O2 sensitivity of yeast and lower growth rates, while both the BY4741 strain and the H2O2-sensitive mutant strain skn7Δ showed similar growth performance to the SDG control plate (Fig. 8c). These results indicate that, at least in yeast cells, CrPIP1;5 is an active H2O and H2O2 transporter.
Fig. 8.
The spot assays for stress tolerance confirmation of the CrPIP1;5 expression in yeast. a Map of the CrPIP1;5-pYES-DEST52 construct. b High osmotic stress and salt tolerance confirmation in yeast. The growth state of yeast cells (WT, BY4741) transformed with CrPIP1;5-pYES-DEST52 and empty vector pYES2 with or without PEG8000, sorbitol, or NaCl on SDG-Ura plates. The concentration of these stress factors for each treatment was labeled on the left. c H2O2 oxidative stress tolerance confirmation in yeast. The growth state of yeast cells (WT and H2O2-sensitive mutant strain skn7Δ) transformed with CrPIP1;5-pYES-DEST52 and empty vector pYES2 with or without H2O2 on SDG-Ura plates. The concentration of H2O2 for each treatment was labeled on the left
To further assess the effects of CrPIP1;5, we generated transgenic Arabidopsis plants that ectopically expressed CrPIP1;5 under the control of 35S promoters. The plants were confirmed as transgenic using genomic PCR, RT-PCR, and qRT-PCR (Figure S2). Plants from three homozygous T3 lines (OX 1#, OX 5#, and OX 10#) were subjected to the salt, salt-alkaline, high osmotic, and drought tolerance tests. Although our seed germinating assays indicated that CrPIP1;5 OX lines showed no significant difference on germination rates under salt, salt-alkaline, or high osmotic challenges compared with WT seeds (Figure S3), while in the seedling growth assays, CrPIP1;5 OX line seedlings showed slightly growth retardation on the salt and salt-alkaline MS plates compared with WT control (Figure S4).
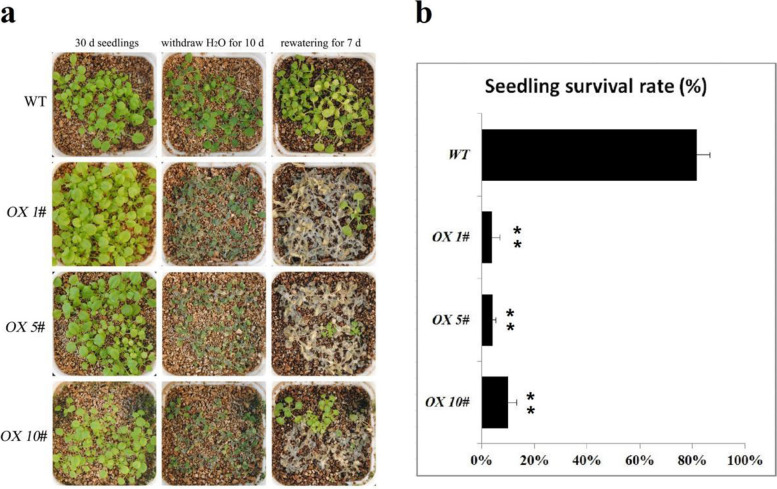
The seeds of WT and CrPIP1;5 OX lines were grown in well-watered conditions for 30 days, and prior to the salt, drought, and alkaline stress treatments, the growth rates of adult plants (WT and three CrPIP1;5 OX lines) were relatively consistent. There was no difference in tolerance between WT and transgenic plants (OX 1#, OX 5#, and OX 10#) under salt (200 mM NaCl) and salt-alkaline (100 mM NaHCO3, pH 8.2) stressors (Figure S5). Apparently, CrPIP1;5 resulted in weak sensitivity to drought (Fig. 9a). After 10 days of water withdrawal, all plants wilted to some degree in both WT and the three CrPIP1;5 OX lines. After re-watering and growing for another 7 days, most of the CrPIP1;5 OX plants did not recover, while most of the WT plants did recover and had a higher survival rate than CrPIP1;5 OX plants (Fig. 9b). This result indicates that overexpression of CrPIP1;5 decreased plant resistance to drought.
Fig. 9.
Drought stress treatment on the growth of the transgenic CrPIP1;5 overexpression lines (OX 1#, OX 5#, and OX 10#) and wild-type (WT) plants under natural growth conditions in vermiculite. a Leaf phenotypes of the transgenic line and WT plants under drought treatment. b The survival rates of the OX lines and WT plants. Bars show mean values ± SD of n = 3–4 biological replicates. The significance level was defined as * (P < 0.05) and ** (P < 0.01)
We found that GFP-fused CrPIP1;5 was constitutively expressed (under the control of CaMV 35S) in transgenic Arabidopsis plants. Root tip fluorescence of roughly three- to four-day-old transgenic Arabidopsis seedlings was easily discerned by confocal microscopy; the GFP-CrPIP1;5 protein was visible in the plasma membranes of transgenic plants, while in control plant roots, the GFP signal was distributed evenly in the whole cytoplasm (Figure S6). These results suggest that subcellular localization of CrPIP1;5 was consistent across the PIP1 subfamily and was predominantly localized to the plasma membrane, as well as partly in endomembrane system. Within the plasma membrane, CrPIP1;5 was folded into a specific transmembrane channel and functioned as a water transporter.
Discussion
Water deficit—caused by drought, high salinity/alkaline, high temperature, cold/freezing conditions or other abiotic stressors—can negatively affect plant growth and survival. However, plants have developed intricate mechanisms to cope with this type stress, including alterations to signal perception and transduction and differential expression of stress responsive genes through complex networks. Aquaporins are a class of integral membrane proteins that facilitate the diffusion of water and other small solutes. Plants often maintain large and diverse AQP families compared to animals and microorganisms. Aquaporins have been reported to play crucial roles in plant water balance and homeostasis under adverse growing conditions [5, 21] and in response to specific biotic challenges [37, 38]. In this study, we performed genome-wide identification and characterization of AQPs in C. rosea to understand the evolution of this family and its molecular roles. We were particularly interested in resolving the molecular mechanisms underlying this extremophile halophyte’s adaptation to coral reef habitats and its responses to acute salt, alkaline, and drought stressors.
The AQP protein family within the C. rosea genome was characterized and 37 putatively functional CrAQP isoforms (based on Pfam domain sequences) were identified, belonging to the PIP (11 isoforms), TIP (10), NIP (11), SIP (4), and XIP (1) subfamilies (Table 1). We performed whole genome sequencing of C. rosea, and our result indicates that this species is diploid, with a 534.94 Mbp genome size (data not published). The number of AQPs was similar to other diploid plant species (Table 2) and their protein sequences were highly similar. This indicates that the number of AQPs and sequence specificity may not be directly related to the adaptation of C. rosea to extreme environments. The roles that CrAQPs play in stress tolerance needs to be further studied from other perspectives, such as transcriptional regulation, protein modification, and the regulation of AQP transmembrane transport activities.
Although numerous studied have identified AQPs in model plant species, research on this gene family has increasingly focused on plants that inhabit novel environments. This is largely because AQP genes are seen as candidates for use in genetic modification of crops to increase agricultural productivity [39, 40]. The saltbush Atriplex canescens is highly tolerant of saline-alkaline soils, drought, heavy metals, and cold, and the AQP genes AcPIP2 and AcNIP5;1 have been shown to be involved in abiotic stress tolerance in this species, and their overexpression in transgenic Arabidopsis caused altered tolerance to drought and salt [41, 42]. Compared with cultivated soybean, the wild Glycine soja is relatively salt-alkaline tolerant. Two AQP genes from G. soja, GsTIP2;1 and GsPIP2;1, minimized tolerance to salt and dehydration stress when overexpressed in Arabidopsis, implying they have negative impacts on stress tolerance by regulating water potential [43, 44]. In most functional analyses conducted in transgenic plants, the overexpression of AQP genes caused elevated tolerance to salt and drought, such as in Malus zumi (gene MzPIP2;1) [45], Sesuvium portulacastrum (SpAQP1) [46], Stipa purpurea (SpPIP1) [47], Simmondsia chinensis (ScPIP1) [48], Thellungiella salsuginea (TsPIP1;1) [49], and Phoenix dactylifera (PdPIP1;2) [50]. The elevated expression of AQP genes in plants can lead to cellular changes in water potential, which cause alterations in water uptake and transpiration, and ultimately modify tolerance to water deficit stress. In this respect, understanding the distribution, expansion, regulation, phylogenetic diversity, and evolutionary selection of AQP genes in extremophile plants like C. rosea is an important step toward potentially improving the water utilization abilities and drought adaptations of other plant species, including agricultural crops.
Plant AQPs play versatile physiological roles in combatting abiotic stress, not only by regulating water content and potential, but also by transporting certain signaling molecules and nutrients. Generally, AQPs consist of six transmembrane helices connected by five loops (A–E) and cytosolic N- and C-termini. Loops B (cytosolic) and E (non-cytosolic) both contain the highly conserved NPA (asparagine-proline-alanine) motifs that form part of the core of these proteins. The aromatic/arginine (ar/R) constriction is located at the non-cytosolic end of the pore. The substrate specificity of AQPs is closely related to several different signature sequences, including NPA motifs, the ar/R filter, and Froger’s positions (FPs) [51]. In all CrAQP NPA motifs, the first two residues were the most conserved, except for CrSIP1;3 and CrXIP1;1, in which the loop B and loop E NPA motifs degenerated into NLG and SPV. The third residue of NPA motifs was more variable, in which A was frequently replaced by either L, S, T, or V. However compared to the NPA motifs, the 10 amino acid residues at the ar/R filter and Froger’s positions were more variable in all CrAQPs (Table 3). In some subfamilies, the ar/R selectivity filter sequences were similar, such as in CrPIPs (F–H-T-R), CrTIP1s (H-I-A-V), and CrNIP1s (W-V-A-R). We also analyzed Froger’s positions (P1–P5), five conserved amino acid residues that are related to glycerol transport in water-conducting AQPs. The P2, P3, P4, and P5 Froger’s positions in CrPIPs were relatively conserved (S-A-F-W), and in CrTIPs, they were less conserved (S-A-Y/F-W). In CrNIPs and CrSIPs, the P3 and P4 positions mostly stayed A and Y. It is supposed that plant TIPs may transport various small solutes, including H2O2, NH4+, and urea, in addition to water [40]. As with other plant TIPs, CrTIPs are mainly located in vacuolar membranes and may be involved in the regulation of water flow across subcellular compartments of organelles [52]. The variation of CrTIPs in ar/R selectivity filter sequences may contribute to their multiple transport functions, and their NPA spacing varies from 79 to 127 amino acid residues, which indicates that CrTIPs might also be involved in the transmembrane transport of multiple small molecules.
Gene structure organization, gene expansion, and gene diversity are critical indicators of the evolution of gene families. The CrPIP and CrTIP subfamilies exhibit relatively stable gene structure in comparison with other subfamilies (Fig. 3). Most of them possess three (CrPIPs) or two (CrTIPs) introns, suggesting that they might share a common ancestral origin. Similar to previous reports showing very few or no intronless AQP genes in other plant species [30, 31, 53], only one intronless AQP was identified in C. rosea. The intronless gene, CrSIP1;2, might have evolved recently through a retrotransposon process. The CrAQP family has undergone a number of duplication events consistent with the highly duplicated nature of plant genomes (Fig. 2; Table 4). The duplication events concerning segmental and tandem duplications identified in this study have also been reported in other plant species [33, 34]. In the present study, some duplicated CrAQPs have distinct patterns of expression in different tissues and habitats, and under different stressors and hormone exposure (Figs. 5 and 6). It is likely that these duplicated gene pairs have similar protein functions yet function in different biological processes, probably mediated by transcriptional regulation or posttranscriptional modification.
Canavalia rosea is a salt- and alkaline-tolerant and drought-adapted halophyte, and abiotic stressors, such as saline-alkaline soil, seasonal drought, strong solar irradiance, and high temperatures, are the main limiting factors that induce osmotic stress and disturb water balance for this species and other tropical seaside plants. For regulating the water transport under different abiotic stresses efficiently, plant often changes the expression of aquaporin genes through a series of complex steps to control the water uptake or management. However, the water management effectiveness and patterns could always depending on the plant growth conditions or tissue type, as well as the different types or degrees of water stress and the specificity of AQP members [54]. In plant, the PIP isoforms were supposed to playing major roles in maintaining plant water homeostasis and responses to abiotic stress [11, 55]. Gene transcript levels are dependent upon the structures of their promoters. Therefore, the cis-acting elements in promoter regions might provide the key to understanding genetic factors influencing the responses of signal molecules and environmental elicitors. We summarized the abiotic stress-related cis-acting elements in CrAQP promoters (Fig. 4) and our findings suggest diversity in CrAQP expression patterns, which could be also considered as a part of the adaptation mechanisms to stress conditions. Chickpea (Cicer arietinum L.) is an important food legume crop with good drought and salinity tolerance than most of other crops [31, 32]. The promoter analyses of CaAQPs also identified a number of cis-regulatory elements, including defense and stress responsive elements. This promoter analysis was also partly support by the experimental expression analyses some of selected CaAQPs, while was not absolutely consistent with the expression patterns of CaAQPs [32]. Furthermore, the expression profiles of CrAQPs in different tissues revealed by RNA-Seq indicate that some of the CrPIP and CrTIP subfamilies had higher expression levels than other subfamilies (Fig. 5a), and habitat-specific RNA-Seq data acquired from leaves further indicated the most of the CrPIP members had greater expression levels in coastal C. rosea (YX) than in inland C. rosea (SCBG; Fig. 5b). Similar results were also observed in leguminous plant, chickpea (Cicer arietinum L.) [32]. In the drought-tolerant genotype chickpea, two subfamilies (CaPIPs and CaTIPs) showed high expression in all tissues as compared to other subfamilies, which indicated these two subfamilies were essential for water transportation and carried drought tolerant. The qRT-PCR results also showed some of PIP members showed much higher expression level in drought-tolerant genotype chickpea than in drought-tolerant genotype chickpea [32]. Our results suggest that differential expression of CrPIPs might be associated with different water use strategies in different habitats, and the higher expression level of CrAQPs in coastal C. rosea plants might be an adaptive mechanism to deal with intracellular and extracellular water-deficit signals. Due to the material limitation, and we cannot collect enough and intact root tissues from YX Island for RNA-seq analysis, so we mimicked the stresses in lab, and analyzed the gene expression with qRT-PCR. The expression patterns of CrPIPs under salt, alkaline, and drought stress and the ABA hormone treatment were further investigated (Fig. 6). The results showed that CrPIP expression was most affected under the saline-alkaline, high osmotic stress, and ABA treatments. Furthermore, some CrPIPs showed clearly different and even opposite expression patterns in roots, vines, and leaves. This can be attributed to the fact that in roots the PIP proteins mainly facilitate water absorption from external environments, while in vines and leaves, the PIPs may play a larger role in transpiration. Broadly, our results suggest a role for PIPs in regulating C. rosea hydraulics and probably adaptation to the challenging environmental conditions found on tropical coral reefs and islands.
In our previous research, we have characterized CrPIP2;3 as a salt/drought stress related gene [36]. Here we performed protein–protein interaction studies using yeast two-hybrid assays and found that two CrPIP members, encoded by CrPIP1;5 and CrPIP2;3, that were highly expressed in all tested tissues and almost constitutively expressed under the abiotic stress challenges and ABA treatment (Figs. 5a and 6). These two CrPIP members could bind to themselves and each other to form homodimers and heterodimers (Fig. 7). This is consistent with previous findings that some PIP1 and PIP2 members could assemble as homotetramers and heterotetramers, thereby triggering channel activities, influencing substrate specificity, and regulating PIP trafficking [56]. Here, our results on the expression patterns of CrPIP1;5 and CrPIP2;3 provide a detailed understanding of their regulatory modes and help to illuminate CrAQP functions. These data are especially helpful for characterizing AQP-interacting protein complexes involved in C. rosea’s adaptations to harsh environmental conditions such as low water availability and saline-alkaline soils.
Our results from the yeast overexpression system indicate that CrPIP1;5 is an active transmembrane H2O and H2O2 transporter (Fig. 8). We assessed the overexpression of CrPIP1;5 in transgenic Arabidopsis, and CrPIP1;5 lead to slightly reduced saline-alkaline and drought tolerance, which showed the exact opposite phenotype compared with CrPIP2;3’s overexpression in Arabidopsis [36]. This also suggests that CrPIP1;5 could play a key role in water transport. Here we speculated that as a foreign AQP gene, CrPIP1;5 might be involved in modifying the function of endogenous PIPs of Arabidopsis, or function more in water flowing out than water absorption in transgenic Arabidopsis roots, thereby resulted in sensitivity to salt and drought stresses. We also found that high levels of salt, alkaline, and ABA slightly decreased the expression of CrPIP1;5 in C. rosea, this further suggests that this gene is highly important for water movement between cells and tissues, and is indeed involved in a stress response pathway that protects yeast cells or plants from water loss under high salinity conditions and promotes water release under high osmotic stress or drought (Figs. 8b and 9). The overexpression of CrPIP1;5 in transgenic Arabidopsis is in contrast to most previous findings [40, 54], suggesting that overexpression of plant PIPs results in specificities of abiotic stress tolerance or sensitivity, which also might be attributed to the protein interaction, post-translational modifications (PTM), protein trafficking of the foreign AQP. While in C. rosea, our results about the expression pattern of CrPIP1;5 regarding to different tissues or habitats (Fig. 5), as well as the transcriptional changes responding to salinity/alkaline, high osmotic stress, and ABA treatment (Fig. 6a), indicated that CrPIP1;5 might be boiled down to a “housekeeping gene” for basic water homeostasis, to some degree. Even the CrPIP1;5 showed higher expression level in YX sample than in SCBG sample (leaves) (Fig. 5b), which might be probably due to the long-term adaptive mechanism that C. rosea plants on YX island have to deal with much tougher water-deficit adversities than plants in SCBG, including water absorption from the external environment and water transportation in vivo. The slightly decreased expression of CrPIP1;5 in C. rosea seedlings caused by stress factors or ABA (Fig. 6a), which might be an emergency protection for alleviating the damages caused by water disturbances, since we only checked the CrPIP1;5’s expression changes in 24 h challenged by different factors. Many studies also proved that the overexpression of plant PIPs could increase sensitivity to drought stress. For example, tobacco PIP1 member, NtAQP1, caused a decline of root hydraulic conductivity and decreased resistance of plants to water stress [57]. Transgenic tobacco (Nicotiana tabacum) plants overexpressing AtPIP1;4 and AtPIP2;5 displayed rapid water loss under dehydration stress and showed enhanced water flow under drought stress [58]. The Glycine soja gene GsPIP2;1 negatively impact salt and drought stress tolerance by regulating water potential when overexpressed in transgenic Arabidopsis [44]. In addition, Arabidopsis plants overexpressing AcPIP2 (a PIP gene from saltbush A. canescens) exhibited drought-sensitive phenotypes [41]. This can be explained by the nature and intensity of stresses, combining with the cooperation between the over-expressed foreign AQPs and the endogenous AQPs. When the water-deficit stress signals were perceived, the foreign AQPs’ over-expression might affect the expression patterns and distribution of endogenous AQPs, and as a result the plant stress-responsive effects might vary among plant species. In our study, the over-expression of CrPIP1;5 showed some sensitivity both in yeast and in Arabidopsis, which only reflect CrPIP1;5 did involve in water stress responses in vivo. That, combined with our previous related research about CrPIP2;3 [36], suggested that overexpression of these two PIP genes in transgenic Arabidopsis promoted plant responses to abiotic stressors by maintaining water homeostasis, as well as decreasing the damage caused by water-deficit stress. Overall, as an active water channel protein with high expression levels in C. rosea different tissues or challenged by different factors, the CrPIP1;5, as well as the CrPIP2;3, might be basic sustainers for water homeostasis during the development of C. rosea plants.
Conclusions
The leguminous nitrogen-fixing plant, C. rosea, presents extreme saline-alkaline and drought resistance and is used as pioneer species on islands and reefs for artificial vegetation construction. In the present study, we conducted a genome-wide analysis and characterization of AQPs in C. rosea. Our results will be helpful for understanding the involvement of this gene family in adaptation to stressful abiotic conditions, particularly through its impact on water balance. We determined that the CrAQP family consists of 37 members distributed across five subfamilies. Each member had subtle variations in gene and protein structures, transcriptional regulation, subcellular localization, substrate-specificity, and post-translational regulatory mechanisms. Expression profiling of CrAQPs revealed higher expression of PIP-associated genes in almost every tissue of C. rosea plants, suggesting that this subfamily likely plays important roles in developmental processes and abiotic stress responses. As predicted, the two PIP1 and PIP2 members, CrPIP1;5 and CrPIP2;3, formed homodimers and heterodimers through protein interactions. We also functionally identified one of the CrPIP1 members, CrPIP1;5, given its highest expression levels in different tissues of C. rosea. Although our results showed that overexpression of CrPIP1;5 could increase sensitivity to saline-alkaline and drought conditions in yeast and plants, combining the high and regulatable expression of CrPIP1;5 in C. rosea, it can be inferred that CrPIP1;5 is one of basic and important functional genes for facilitating water transportation in vivo, and might be involved in the C. rosea ecological adaptation to tropical coral reef. The identification of CrAQPs in this study will be useful for further investigation of the roles that AQPs play in the various developmental stages and physiological processes of C. rosea, as well as elucidating the possible ecological adaptation mechanisms of C. rosea to extreme environments, and identification candidate genes for potential introduction into transgenic agricultural crops.
Methods
Plant materials
Canavalia rosea plants growing on Yongxing Island (YX, 16˚83′93′′ N, 112˚34′00′′ E) and in the South China Botanical Garden (SCBG, 23˚18′76′′ N, 113˚37′02′′ E) were used in this study. The C. rosea plants growing in SCBG were introduced gradually from coastal area in Hainan Province since 2012, and grew steadily for one to seven years with regular water and fertilizer supply in Guangzhou, China. To analyze tissue-specific transcriptional patterns of the identified CrAQPs, roots, stems, leaves, flowers, and fruits were gathered from C. rosea plants grown in SCBG. In addition, to investigate the involvement of the CrAQPs in adaptation to different habitats, adult leaves were gathered from C. rosea plants growing in both YX and SCBG. In brief, the C. rosea tissues were collected outdoors and immediately frozen in liquid nitrogen, then the samples were stored at − 80 °C for subsequent RNA-seq analysis. Three independent biological replicates were used.
Identification of CrAQP genes and gene duplication analysis of the CrAQP family
To identify all putative CrAQP genes, the genome database of C. rosea (data not published) was used to obtain DNA and protein sequences (Table S1). In brief, DIAMOND [59] and InterProscan (https://www.ebi.ac.uk/interpro/search/sequence/) were used to identify all C. rosea proteins with conserved domains and motifs (e < 1e−5), and all proteins were annotated using InterPro and Pfam databases (http://pfam.xfam.org/). The Pfam ID (MIP, PF00230) was used to search the CrAQP protein family, and putative sequences of CrAQP proteins were identified and submitted to SMART (http://smart.embl-heidelberg.de/) and the NCBI Conserved Domain Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) to confirm presence of the AQP domain. Next, the selected CrAQPs were named based on their sequence homology with known AQPs and C. rosea genome annotation.
Gene segmental and tandem duplications were assessed using MCScanX software (http://chibba.pgml.uga.edu/mcscan2/), and tandem duplications were also checked manually according their gene loci. The number of synonymous substitutions per synonymous site (Ka), the number of non-synonymous substitutions per non-synonymous site (Ks), and the P-value from a Fisher's exact test of neutrality were calculated using the Nei-Gojobori model with 1,000-bootstrap replicates [60]. A Ka/Ks ratio < 1 indicates purifying selection, a Ka/Ks ratio = 1 indicates neutral selection, and a Ka/Ks ratio > 1 indicates positive selection.
Multiple sequence alignment and phylogenetic analysis of CrAQP family proteins
A bootstrap neighbor-joining phylogenetic tree was constructed based on multiple alignments of the identified AQPs from C. rosea with AQPs from Arabidopsis and soybean using MEGA 6.0 with 1,000 bootstraps. The sequences of GmAQPs (from soybean; Glycine max) and AtAQPs (from Arabidopsis; Arabidopsis thaliana) were downloaded from the phytozome database (https://phytozome-next.jgi.doe.gov/). Aquaporins were mapped on C. rosea chromosomes according to positional information of the CrAQP genes in the C. rosea genome database and displayed using MapInspect software (http://mapinspect.apponic.com/).
The gene structure for each CrAQP was illustrated using the Gene Structure Display Server 2.0 (http://gsds.cbi.pku.edu.cn/). To identify the biochemical features of all CrAQPs, the ProtParam (http://web.expasy.org/protparam/) was used to predict molecular weights (MW) and isoelectric points (pI) of the candidate CrAQP proteins. The transmembrane domains (TMDs), NPA motifs, and other conserved amino acid residues were recognized by the sequence alignment of CrAQPs with AtAQPs [26] and GmAQPs [34]. The numbers of phosphorylation sites within CrAQPs were predicted using NetPhos 3.1 (http://www.cbs.dtu.dk/services/NetPhos/). Subcellular localization was predicted using the WoLF PSORT server (https://wolfpsort.hgc.jp/) and Plant-mPLoc (http://www.csbio.sjtu.edu.cn/bioinf/plant-multi/). Conserved CrAQP motifs were analyzed using MEME suite (http://meme-suite.org/), with the maximum number of motifs being 10 and the optimum width of motifs ranging from 11 to 50.
Promoter sequence profiling of CrAQPs
Putative CrAQP promoter sequences (2,000 bp upstream of ATG) were retrieved from the C. rosea genomes database (Table S1). Sequences were then uploaded into the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for cis-acting regulatory element analysis. The cis-acting elements were classified as either hormone-specific (gibberellin-responsive elements, MeJA-responsive elements, auxin-responsive elements, salicylic acid-responsive elements, EREs, and ABREs) or abiotic stress-responsive (light responsive elements, MYCs, MYBs, MBSs, TC-rich repeats, and LTREs). The different elements were summarized and several selected CrPIP promoters were visualized using TBtools [61].
Expression analysis of CrAQPs
A transcriptome database of C. rosea was constructed using Illumina HiSeq X sequencing technology. The quality of the RNA-Seq datasets created from seven different tissues (roots, vines, young leaves, flowers, and young siliques collected from C. rosea growing in SCBG; mature leaves from C. rosea growing in SCBG and on YX Island) was examined using FastQC (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/), which produced 40 Gb clean reads. In brief, five different tissue samples were collected from similar young C. rosea plants (two-year-old with flowers and young siliques planted in SCBG), then these samples were cleaned and frozen quickly with liquid nitrogen for organ-specific RNA-Seq analysis. As to the habitat-specific RNA-Seq analysis, the mature leaves gathered separately from C. rosea plants growing in SCBG (seven-year-old plants) or native plants in YX Island, then the mature leaf samples were frozen with liquid nitrogen for future use. The samples mentioned above were collected with 3 independent replicates for each group. Clean reads were mapped to the C. rosea reference genome using Tophat v.2.0.10 (http://tophat.cbcb.umd.edu/). Gene expression levels were calculated as fragments per kilobase of transcript per million mapped reads (FPKM) according to the length of the gene and the read counts mapped to the gene: FPKM = total exon fragments/[mapped reads (millions) × exon length (kb)]. Expression levels (log2) of CrAQPs were visualised as clustered heatmaps using TBtools.
To investigate the involvement of the CrAQP genes in abscisic acid (ABA) and in various stress responses, C. rosea was germinated from seed and 30-day-old seedlings were exposed to stressors. In brief, for the high osmotic stress treatment, seedlings were removed from their pots and carefully washed with distilled water to remove soil from the roots, and then transferred into a 300 mM mannitol solution. For high salt stress, seedlings were soaked in a 600 mM NaCl solution. For alkaline stress, seedlings were soaked in a 150 mM NaHCO3 (pH 8.2) solution. For ABA treatment, a freshly prepared working solution of 100 μM exogenous ABA was sprayed on the leaves of seedlings. The second and/or third mature leaves from the seedling apexes were collected at 0, 2, and 24 h during the previously described stress treatments, with the 0-hourtime point used as the control. All samples were immediately frozen in liquid nitrogen and stored at − 80 °C for subsequent gene expression analysis. Three independent biological replicates were used. Transcript abundance of several CrAQPs' transcript was investigated using a qRT-PCR assay. The extraction and isolation of RNA and the synthesis of first strand cDNAs was performed as the previous report [36], with 3 replicates for each group. In brief, total RNA was extracted from C. rosea seedling tissues under the stress/ABA treatments and reverse transcribed to cDNA. Quantitative RT-PCR was conducted using the LightCycler480 system (Roche, Basel, Switzerland) and TransStart Tip Green qPCR SuperMix (TransGen Biotech, Beijing, China). All of the gene expression data obtained via qRT-PCR was normalized to the expression of CrEF-α (Table S2). The primers used for qRT-PCR (CrEF-αRTF/CrEF-αRTR for the reference gene and other CrAQP-specific primer pairs) are listed in Table S2.
Detection of CrPIP1;5 and CrPIP2;3 homodimers and heterodimers using a yeast two-hybrid assay
The full-length cDNAs of CrPIP1;5 (GenBank accession number MT787665) and CrPIP2;3 (GenBank accession number MT787666) were isolated from the cDNA library of C. rosea seedlings, in which all cDNAs were inserted into a yeast expression vector (pYES-DEST52) using Gateway® techniques (Life Technologies). The recombinant plasmids containing CrPIP1;5 and CrPIP2;3 cDNAs were designated as CrPIP1;5-pYES-DEST52 and CrPIP2;3-pYES-DEST52 and used as template DNA in the following PCR assays. The open reading frame (ORF) regions of CrPIP1;5 and CrPIP2;3 were PCR-amplified using the primer pairs PIP1-5BDF/PIP1-5BDR and PIP2-3BDF/PIP2-3BDR, respectively (Table S2), and then inserted into a pGBKT7 vector using InFusion® techniques (In-Fusion HD® Cloning System, Clontech) to construct the pGBKT7-CrPIP1;5 and pGBKT7-CrPIP2;3 bait plasmids. The prey plasmid, pGADT7-CrPIP1;5, and pGADT7-CrPIP2;3, were generated by cloning the CrPIP1;5 and CrPIP2;3 ORFs into the pGADT7 vector after amplification (using the primers PIP1-5ADF/PIP1-5ADR, PIP2-3ADF/PIP2-3ADR as above; Table S2). Constructed vectors including activation domain (AD) and binding domain (BD) were co-transformed into AH109-competent yeast cells in pairs, and transformants were plated on SD/-Leu-Trp and SD/-Leu-Trp-His mediums to test protein interactions. Transformant spots on SD/-Leu-Trp-His medium were also supplemented with 40 µg/mL 5-bromo-4-chloro-3-indoxyl-α-d-galactopyranoside (X-α-Gal, 2 µL per spot) to further confirm interactions of the different co-transformants. Each experiment was independently repeated three times.
In vivo stress tolerance assay for CrPIP1;5 overexpression in yeast
The recombinant plasmids CrPIP1;5-pYES-DEST52 and the empty vector pYES2 (a negative control) were transformed into the Saccharomyces cerevisiae wild type strains W303 (MATa; his3-11_15; leu2-3_112; ura3-1; trp1Δ2; ade2-1; can1-100), WT (BY47471; MATa; his3Δ1; leu2Δ0; met15Δ0; ura3Δ0), and H2O2-sensitive mutant skn7Δ (BY4741; MATa; ura3Δ0; leu2Δ0; his3Δ1; met15Δ0; YHR206w::kanMX4). The yeast strain W303 was provided by Zhou et al. [62]. The WT (Y00000) and skn7Δ (Y02900) strains were obtained from Euroscarf (http://www.euroscarf.de/index.php?name=News). Plasmids were introduced into yeast strains using a standard polyethylene glycol (PEG)-lithium acetate-based transformation protocol. The yeast spot assay for NaCl, PEG, sorbitol, and H2O2 tolerance were performed as previously described [63].
Functional identification of CrPIP1;5 in transgenic Arabidopsis plants
The coding sequence (CDS) of the CrPIP1;5 cDNA was PCR-amplified using the primer pair PIP1-5OXGF/PIP1-5OXGR (Table S2) and then inserted into plant expression vector pEGAD to generate CrPIP1;5-pEGAD. Thus, transgenic Arabidopsis plants (col-0 genotype, three overexpression lines, OX 1#, OX 5#, and OX 10#) were generated. After confirmation with genomic PCR and quantitative RT-PCR, these T3 homozygous transgenic lines were tested for their stress tolerance according to their seed germination rate as well as seedling and adult plant growth rate. These tests were thereby meant to evaluate the biological functions of CrPIP1;5.
In brief, seed germination rate of the CrPIP1;5 transgenic Arabidopsis (OX 1#, OX 5#, OX 10#, and WT) was measured under the following stress treatments: NaCl (175 mM, 200 mM, and 225 mM; salt stress); 5 mmol/L NaHCO3 plus 95 mmol/L NaCl (pH 8.2), 7.5 mmol/L NaHCO3 plus 92.5 mmol/L NaCl (pH 8.2), and 10 mmol/L NaHCO3 plus 90 mmol/L NaCl (pH 8.2; alkaline stress); mannitol (200 mM, 300 mM, and 400 mM) stress. The goal of these treatments was to detect the effect of the overexpression of CrPIP1;5 on affecting the salt/alkaline/osmotic tolerance of transgenic Arabidopsis seeds during germination. Additionally, root length was calculated to evaluate the influence of the overexpression of CrPIP1;5 on transgenic Arabidopsis seedlings under abiotic stress (100 mM, 150 mM, and 200 mM NaCl for salt stress; 0.5 mmol/L NaHCO3 plus 99.5 mmol/L NaCl, 0.75 mmol/L NaHCO3 plus 99.25 mmol/L NaCl, 1 mmol/L NaHCO3 plus 99 mmol/L NaCl, pH 8.2 for alkaline stress; 200 mM, 300 mM, and 400 mM mannitol for osmotic stress. Wild-type Arabidopsis and Murashige&Skoog medium (MS) or MS plus 100 mM NaCl (pH 8.2) medium were used as controls. The seed germination and seedling growth experiments were both performed on MS plates with or without stress factors, in the same greenhouse environment used to grow the Arabidopsis plants. Drought and salt/alkaline tolerance assays were also performed on transgenic adult Arabidopsis plants. Both WT and transgenic seeds (OX 1#, OX 5#, and OX 10#) were grown on MS medium. Ten-day-old seedlings were transplanted into square pots filled with nutrient solution soaked vermiculite, with identical soil moisture. For drought tolerance assay, thirty to forty plants of each OX lines and WT control were cultured in growth chamber as described above without watering for another 20 days, since the original vermiculite humidity could ensure regular growth of Arabidopsis plants. The water content of vermiculite in the pots was reduced over this timeframe but did not induce drought stress. The plants were then subjected to a drought tolerance assay by continuous withdrawing water, whereby WT and transgenic plants (OX 1#, OX 5#, and OX 10#) turned to continuous drought conditions for 10 days and then rewatered adequately and recovered for another 7 days. Survival rates were then calculated according to the number of living plants at the end of the experiment. For salt tolerance assay, the ten-day-old seedlings of WT and transgenic Arabidopsis (OX 1#, OX 5#, and OX 10#) growing in square pots were irrigated with 200 mM NaCl solution (50 mL each pot) and the phenotype was recorded after 7 days. For alkaline tolerance assay, the same seedlings were irrigated with 100 mM NaHCO3 solution (50 mL each pot) and the phenotype was recorded after 4 days.
Subcellular localization of CrPIP1;5 in Arabidopsis was also detected using GFP fusion protein in seedling roots. The OX homozygous lines of CrPIP1;5-pEGAD and the control (pEGAD) transgenic plants were sterilized and spotted in MS plates to generate seedlings. Then, three- to four-day-old seedlings were detected using a camera fitted to a confocal laser scanning microscope to record the GFP fluorescence of different tissues. To confirm it was the cell membrane that was fluorescing, seedling roots were stained by using a propidium iodide solution (1 mg/mL in phosphate buffer solution).
Statistical analysis
All the experiments in this study were repeated three times independently and results are shown as mean ± SD (n ≥ 3). Pairwise differences between means were analyzed using Student's t-tests in Microsoft Excel 2010.
Supplementary Information
Additional file 1: Figure S1. The structural features of the CrAQPs. a CrNIPs. b CrPIPs. c CrTIPs. d CrXIPs. e CrSIPs.
Additional file 2: Figure S2. The overexpression analyses of CrPIP1;5 in the three transgenic Arabidopsis lines (OX 1#, OX 5#, and OX 10#). a RT-PCR and genomic DNA PCR analysis of CrPIP1;5 in the transgenic Arabidopsis lines and WT plants. b Quantitative RT-PCR analysis of CrPIP1;5 in the transgenic Arabidopsis lines and WT plants.
Additional file 3: Figure S3. Overexpression analyses of CrPIP1;5 in the transgenic Arabidopsis lines (OX 1#, OX 5#, and OX 10#) and stress tolerance analyses of transgenic plants with regards to seed germination rates. a Photographs of the transgenic lines and WT seeds germinated on MS medium or MS medium with NaCl, NaCl plus NaHCO3 (pH 8.2), or mannitol for 7 d. b‒d The seed germination rates in WT and transgenic lines under NaCl (b), NaCl plus NaHCO3 (pH 8.2) (c), and mannitol (d) stresses after 7 d.
Additional file 4: Figure S4. Salt, salt-alkaline, and high osmotic stress analyses of the transgenic plants with CrPIP1;5’s overexpression based on seedling root lengths. Four-day-old seedlings were transplanted into MS medium containing NaCl, NaCl plus NaHCO3 (pH 8.2) or mannitol and then grown for 7 d before measuring the root length. a Photographs of the transgenic lines (CrPIP1;5OX 1#, OX 5#, and OX 10#) and WT seedlings on MS medium or MS medium with NaCl, NaCl plus NaHCO3 (pH 8.2), or mannitol; b‒d The seedling root lengths (mm) in WT and the transgenic lines under NaCl (b), NaCl plus NaHCO3 (pH 8.2) (c), or mannitol (d) stresses after 7 d. Error bars indicate the SD based on over three replicates (n ≥ 3). Asterisks indicate significant differences from the control (Student’ s t-test P values, * p < 0.05 and ** p < 0.01).
Additional file 5: Figure S5. Salt and alkaline stress analyses of the transgenic plants with CrPIP1;5’s overexpression based on the growth of adult Arabidopsis. a Leaf phenotypes of the transgenic Arabidopsis OX lines and WT plants under 200 mM NaCl stress for 7 days. b Leaf phenotypes of the transgenic Arabidopsis OX lines and WT plants under 100 mM NaHCO3 (pH 8.2) stress for 4 days.
Additional file 6: Figure S6. Subcellular localization of the CrPIP1;5 protein. Arabidopsis roots expressing 35S:GFP-CrPIP1;5 fusion proteins (upper two lines) and 35S:GFP (lower line) were observed under a laser scanning confocal microscope.
Additional file 7: Table S1. The obtained CrAQPs’ nucleotide and protein sequences information in this study.
Additional file 8: Table S2. Primers’ information used in this study.
Additional file 9: Table S3. The categories of cis-acting elements identified in the CrAQPs’ promoter regions.
Additional file 10: Table S4. The conserved motif sequences of CrAQPs identified by the MEME web server.
Acknowledgements
We sincerely thank several unknown workers who provided us Canavalia rosea plants and seeds gathered from Hainan province, China.
Abbreviations
- AQP
Aquaporin
- RNA-Seq
RNA sequencing
- PIP
Plasma membrane intrinsic protein
- NIP
Nod26-like intrinsic protein
- TIP
Tonoplast intrinsic protein
- SIP
Small and basic intrinsic protein
- XIP
X-intrinsic protein
- GIP
GlpF-like intrinsic protein
- HIP
Hybrid intrinsic protein
- MIP
Major intrinsic protein
- NPA
Asparagine-proline-alanine
- ar/R
Aromatic/Arginine region
- FPs
Froger’s positions
- GSDS
Gene Structure Dispaly Server
- ABA
Abscisic acid
- SDG
Synthetic dropout medium plus galactose
- qRT-PCR
Quantitative Reverse Transcription Polymerase Chain Reaction
- OX
Over-expression
- WT
Wild type
- GRAVY
Grand average of hydropathicity
- MS
Murashige and Skoog medium
- CK
Control check
- SCBG
South China Botanical Garden
- YX
Yongxing Island
- MW
Molecular weight
- pI
Isoelectric point
- TMD
Transmembrane domain
- FPKM
Fragments per kilobase of transcript per million mapped reads
- AD
Activation domain
- BD
Binding domain
- CDS
Coding sequence
Authors’ contributions
S.J. and M.Z. conceived and designed the experiments; R.L. and J.Z. conducted most of the experiment, collected and analyzed the data; L.P. helped with measurements of physiological parameters; Z.W. and Q.M. helped in sequence analyses; R.L. and M.Z. drafted the manuscript; S.J. and M.Z. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by grants from the National Natural Sciences Foundation of China (No. U1701246 and No. 31570257), the Guangdong Science and Technology Program (No. 2019B121201005), the ‘Strategic Priority Research Program’ of the Chinese Academy of Sciences (No. XDA13020500), and the Key Special Project for Introduced Talents Team of Southern Marine Science and Engineering Guangdong Laboratory (Guangzhou) (No. GML2019ZD0408). The funders had no roles in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Availability of data and materials
All data generated or analyzed during this study are included in this article and its supplementary information files. However, the sequence data in this study can also be accessed at http://doi.org/10.13140/RG.2.2.21213.74727.
Declarations
Ethics approval and consent to participate
No applicable.
Consent for publication
No applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Ruoyi Lin and Jiexuan Zheng contributed equally to this work.
Contributor Information
Mei Zhang, Email: zhangmei@scbg.ac.cn.
Shuguang Jian, Email: jiansg@scbg.ac.cn.
References
- 1.Supriya P, Sridhar KR. Impact of electron beam irradiation on the bioactive principles of seeds of coastal sand dune wild legumes (Canavalia Spp.) Recent Pat Food Nutr Agric. 2019;10(1):57–61. doi: 10.2174/2212798410666180709144200. [DOI] [PubMed] [Google Scholar]
- 2.Huang J, Liu N, Ren H, Jian SG. Physiology and biochemical characteristics of Canavalia rosea under stress. J Trop&Subtrop Bot. 2019;27(02):157–163. [Google Scholar]
- 3.Verslues PE, Agarwal M, Katiyar-Agarwal S, Zhu J, Zhu JK. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 2006;45:523–539. doi: 10.1111/j.1365-313X.2005.02593.x. [DOI] [PubMed] [Google Scholar]
- 4.Munns R, Tester M. Mechanisms of salinity tolerance. Annu Rev Plant Biol. 2008;59:651–681. doi: 10.1146/annurev.arplant.59.032607.092911. [DOI] [PubMed] [Google Scholar]
- 5.Kaldenhoff R, Ribas-Carbo M, Sans JF, Lovisolo C, Heckwolf M, Uehlein N. Aquaporins and plant water balance. Plant Cell Environ. 2008;31(5):658–666. doi: 10.1111/j.1365-3040.2008.01792.x. [DOI] [PubMed] [Google Scholar]
- 6.Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science. 1992;256(5055):385–387. doi: 10.1126/science.256.5055.385. [DOI] [PubMed] [Google Scholar]
- 7.Johanson U, Gustavsson S. A new subfamily of major intrinsic proteins in plants. Mol Biol Evol. 2002;19(4):456–461. doi: 10.1093/oxfordjournals.molbev.a004101. [DOI] [PubMed] [Google Scholar]
- 8.Lopez D, Bronner G, Brunel N, Auguin D, Bourgerie S, Brignolas F, Carpin S, Tournaire-Roux C, Maurel C, Fumanal B, Martin F, Sakr S, Label P, Julien JL, Gousset-Dupont A, Venisse JS. Insights into populus XIP aquaporins: evolutionary expansion, protein functionality, and environmental regulation. J Exp Bot. 2012;63(5):2217–2230. doi: 10.1093/jxb/err404. [DOI] [PubMed] [Google Scholar]
- 9.Anderberg HI, Kjellbom P, Johanson U. Annotation of Selaginella moellendorffii major intrinsic proteins and the evolution of the protein family in terrestrial plants. Front Plant Sci. 2012;3:33. doi: 10.3389/fpls.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gustavsson S, Lebrun AS, Kristina N, Francois C, Johanson U. A novel plant major intrinsic protein in Physcomitrella patens most similar to bacterial glycerol channels. Plant Physiol. 2005;139(1):287–295. doi: 10.1104/pp.105.063198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurel C, Boursiac Y, Luu DT, Santoni V, Shahzad Z, Verdoucq L. Aquaporins in plants. Physiol Rev. 2015;95(4):1321–1358. doi: 10.1152/physrev.00008.2015. [DOI] [PubMed] [Google Scholar]
- 12.Murata K, Mitsuoka K, Hirai T, Walz T, Agre P, Heymann JB, Engel A, Fujiyoshi Y. Structural determinants of water permeation through aquaporin-1. Nature. 2000;407(6804):599–605. doi: 10.1038/35036519. [DOI] [PubMed] [Google Scholar]
- 13.Chaumont F, Moshelion M, Daniels MJ. Regulation of plant aquaporin activity. Biol Cell. 2005;97(10):749–764. doi: 10.1042/BC20040133. [DOI] [PubMed] [Google Scholar]
- 14.Fox AR, Maistriaux LC, Chaumont F. Toward understanding of the high number of plant aquaporin isoforms and multiple regulation mechanisms. Plant Sci. 2017;264:179–187. doi: 10.1016/j.plantsci.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 15.de Groot BL, Grubmüller H. Water permeation across biological membranes: mechanism and dynamics of aquaporin-1 and GlpF. Science. 2001;294(5550):2353–2357. doi: 10.1126/science.1062459. [DOI] [PubMed] [Google Scholar]
- 16.Cooper GJ, Occhipinti R, Boron WF. CrossTalk proposal: physiological CO2 exchange can depend on membrane channels. J Physiol. 2015;593(23):5025–5028. doi: 10.1113/JP270059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Tajkhorshid E. Nitric oxide conduction by the brain aquaporin AQP4. Proteins. 2010;78(3):661–670. doi: 10.1002/prot.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siefritz F, Otto B, Bienert GP, van der Krol A, Kaldenhoff R. The plasma membrane aquaporin NtAQP1 is a key component of the leaf unfolding mechanism in tobacco. Plant J. 2004;37(2):147–155. doi: 10.1046/j.1365-313X.2003.01947.x. [DOI] [PubMed] [Google Scholar]
- 19.Smirnoff N, Arnaud D. Hydrogen peroxide metabolism and functions in plants. New Phytol. 2019;221(3):1197–1214. doi: 10.1111/nph.15488. [DOI] [PubMed] [Google Scholar]
- 20.Poschenrieder C, Busoms S, Barceló J. How plants handle trivalent (+3) elements. Int J Mol Sci. 2019;20(16):3984. doi: 10.3390/ijms20163984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Srivastava AK, Penna S, Nguyen DV, Tran LS. Multifaceted roles of aquaporins as molecular conduits in plant responses to abiotic stresses. Crit Rev Biotechnol. 2016;36(3):389–398. doi: 10.3109/07388551.2014.973367. [DOI] [PubMed] [Google Scholar]
- 22.Verdoucq L, Rodrigues O, Martinière A, Luu DT, Maurel C. Plant aquaporins on the move: reversible phosphorylation, lateral motion and cycling. Curr Opin Plant Biol. 2014;22:101–107. doi: 10.1016/j.pbi.2014.09.011. [DOI] [PubMed] [Google Scholar]
- 23.Soveral G, Prista C, Moura TF, Loureiro-Dias MC. Yeast water channels: an overview of orthodox aquaporins. Biol Cell. 2010;103(1):35–54. doi: 10.1042/BC20100102. [DOI] [PubMed] [Google Scholar]
- 24.Verkman AS, Anderson MO, Papadopoulos MC. Aquaporins: important but elusive drug targets. Nat Rev Drug Discov. 2014;13(4):259–277. doi: 10.1038/nrd4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sutka M, Amodeo G, Ozu M. Plant and animal aquaporins crosstalk: what can be revealed from distinct perspectives. Biophys Rev. 2017;9(5):545–562. doi: 10.1007/s12551-017-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johanson U, Karlsson M, Johansson I, Gustavsson S, Sjövall S, Fraysse L, Weig AR, Kjellbom P. The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. Plant Physiol. 2001;126(4):1358–1369. doi: 10.1104/pp.126.4.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaumont F, Barrieu F, Wojcik E, Chrispeels MJ, Jung R. Aquaporins constitute a large and highly divergent protein family in maize. Plant Physiol. 2001;125(3):1206–1215. doi: 10.1104/pp.125.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen MX, Moon S, Jung KH. Genome-wide expression analysis of rice aquaporin genes and development of a functional gene network mediated by aquaporin expression in roots. Planta. 2013;238(4):669–681. doi: 10.1007/s00425-013-1918-9. [DOI] [PubMed] [Google Scholar]
- 29.Qin S, Liu Y, Han Y, Xu G, Wan S, Cui F, Li G. Aquaporins and their function in root water transport under salt stress conditions in Eutrema salsugineum. Plant Sci. 2019;287:110–199. doi: 10.1016/j.plantsci.2019.110199. [DOI] [PubMed] [Google Scholar]
- 30.Qian W, Yang X, Li J, Luo R, Yan X, Pang Q. Genome-wide characterization and expression analysis of aquaporins in salt cress (Eutrema salsugineum) PeerJ. 2019;7:e7664. doi: 10.7717/peerj.7664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deokar AA, Tar'an B. Genome-wide analysis of the aquaporin gene family in chickpea (Cicer arietinum L.) Front Plant Sci. 2016;7:1802. doi: 10.3389/fpls.2016.01802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hussain A, Tanveer R, Mustafa G, Farooq M, Amin I, Mansoor S. Comparative phylogenetic analysis of aquaporins provides insight into the gene family expansion and evolution in plants and their role in drought tolerant and susceptible chickpea cultivars. Genomics. 2020;112(1):263–275. doi: 10.1016/j.ygeno.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Zou Z, Yang JH. Genome-wide comparison reveals divergence of cassava and rubber aquaporin family genes after the recent whole-genome duplication. BMC Genomics. 2019;20(1):380. doi: 10.1186/s12864-019-5780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feng ZJ, Liu N, Zhang GW, Niu FG, Xu SC, Gong YM. Investigation of the AQP family in soybean and the promoter activity of TIP2;6 in heat stress and hormone responses. Int J Mol Sci. 2019;20(2):262. doi: 10.3390/ijms20020262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bienert MD, Diehn TA, Richet N, Chaumont F, Bienert GP. Heterotetramerization of plant PIP1 and PIP2 aquaporins is an evolutionary ancient feature to guide PIP1 plasma membrane localization and function. Front Plant Sci. 2018;9:382. doi: 10.3389/fpls.2018.00382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng J, Lin R, Pu L, Wang Z, Mei Q, Zhang M, Jian S. Ectopic expression of CrPIP2;3, a plasma membrane intrinsic protein gene from the halophyte Canavalia rosea, enhances drought and salt-alkali stress tolerance in Arabidopsis. Int J Mol Sci. 2021;22(2):565. doi: 10.3390/ijms22020565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang R, Wang M, Chen K, Wang S, Mur LAJ, Guo S. Exploring the roles of aquaporins in plant-microbe interactions. Cells. 2018;7(12):267. doi: 10.3390/cells7120267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Chen L, Dong H. Plant aquaporins in infection by and immunity against pathogens-a critical review. Front Plant Sci. 2019;10:632. doi: 10.3389/fpls.2019.00632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Martinez-Ballesta Mdel C, Carvajal M. New challenges in plant aquaporin biotechnology. Plant Sci. 2014;217–18:71–77. doi: 10.1016/j.plantsci.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 40.Singh RK, Deshmukh R, Muthamilarasan M, Rani R, Prasad M. Versatile roles of aquaporin in physiological processes and stress tolerance in plants. Plant Physiol Biochem. 2020;149:178–189. doi: 10.1016/j.plaphy.2020.02.009. [DOI] [PubMed] [Google Scholar]
- 41.Li J, Yu G, Sun X, Liu Y, Liu J, Zhang X, Jia C, Pan H. AcPIP2, a plasma membrane intrinsic protein from halophyte Atriplex canescens, enhances plant growth rate and abiotic stress tolerance when overexpressed in Arabidopsis thaliana. Plant Cell Rep. 2015;34(8):1401–1415. doi: 10.1007/s00299-015-1796-7. [DOI] [PubMed] [Google Scholar]
- 42.Yu G, Li JT, Sun XH, Zhang XH, Liu JL, Pan HY. Overexpression of AcNIP5;1, a novel nodulin-like intrinsic protein from halophyte Atriplex canescens, enhances sensitivity to salinity and improves drought tolerance in Arabidopsis. Plant Mol Biol Rep. 2015;33:1864–1875. doi: 10.1007/s11105-015-0881-y. [DOI] [Google Scholar]
- 43.Wang X, Li Y, Ji W, Bai X, Cai H, Zhu D, Sun XL, Chen LJ, Zhu YM. A novel Glycine soja tonoplast intrinsic protein gene responds to abiotic stress and depresses salt and dehydration tolerance in transgenic Arabidopsis thaliana. J Plant Physiol. 2011;168(11):1241–1248. doi: 10.1016/j.jplph.2011.01.016. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Cai H, Li Y, Zhu Y, Ji W, Bai X, Zhu D, Sun X. Ectopic overexpression of a novel Glycine soja stress-induced plasma membrane intrinsic protein increases sensitivity to salt and dehydration in transgenic Arabidopsis thaliana plants. J Plant Res. 2015;128(1):103–113. doi: 10.1007/s10265-014-0674-7. [DOI] [PubMed] [Google Scholar]
- 45.Wang L, Li Q, Lei Q, Feng C, Gao Y, Zheng X, Zhao Y, Wang Z, Kong J. MzPIP2;1:an aquaporin involved in radial water movement in both water uptake and transportation, altered the drought and salt tolerance of transgenic Arabidopsis. PLoS One. 2015;10(11):e0142446. doi: 10.1371/journal.pone.0142446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang W, Liu X, Zhu J, Fan W, Zhang Z. An aquaporin gene from halophyte Sesuvium portulacastrum, SpAQP1, increases salt tolerance in transgenic tobacco. Plant Cell Rep. 2016;35(2):385–395. doi: 10.1007/s00299-015-1891-9. [DOI] [PubMed] [Google Scholar]
- 47.Chen Q, Yang S, Kong X, Wang C, Xiang N, Yang Y, Yang Y. Molecular cloning of a plasma membrane aquaporin in Stipa purpurea, and exploration of its role in drought stress tolerance. Gene. 2018;665:41–48. doi: 10.1016/j.gene.2018.04.056. [DOI] [PubMed] [Google Scholar]
- 48.Wang X, Gao F, Bing J, Sun W, Feng X, Ma X, Zhou Y, Zhang GF. Overexpression of the jojoba aquaporin gene, ScPIP1, enhances drought and salt tolerance in transgenic Arabidopsis. Int J Mol Sci. 2019;20(1):153. doi: 10.3390/ijms20010153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li W, Qiang X, Han X, Jiang L, Zhang S, Han J, He R, Cheng X. Ectopic expression of a Thellungiella salsuginea aquaporin gene, TsPIP1;1, increased the salt tolerance of rice. Int J Mol Sci. 2018;19(8):2229. doi: 10.3390/ijms19082229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Patankar HV, Alharrasi I, Alyahyai R, Yaish MW. Functional characterization of date palm aquaporin gene PdPIP1;2 confers drought and salinity tolerance to yeast and Arabidopsis. Genes. 2019;10(5):390. doi: 10.3390/genes10050390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Törnroth-Horsefield S, Wang Y, Hedfalk K, Johanson U, Karlsson M, Tajkhorshid E, Neutze R, Kjellbom P. Structural mechanism of plant aquaporin gating. Nature. 2006;439:688–694. doi: 10.1038/nature04316. [DOI] [PubMed] [Google Scholar]
- 52.Hu W, Hou X, Huang C, Yan Y, Tie W, Ding Z, Wei Y, Liu J, Miao H, Lu Z, Li M, Xu B, Jin Z. Genome-wide identification and expression analyses of aquaporin gene family during development and abiotic stress in banana. Int J Mol Sci. 2015;16(8):19728–19751. doi: 10.3390/ijms160819728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar N, Kumawat S, Khatri P, Singla P, Tandon G, Bhatt V, Shinde S, Patil GB, Sonah H, Deshmukh R. Understanding aquaporin transport system in highly stress-tolerant and medicinal plant species Jujube (Ziziphus jujuba Mill.) J Biotechnol. 2020;324:103–11. doi: 10.1016/j.jbiotec.2020.09.026. [DOI] [PubMed] [Google Scholar]
- 54.Kapilan R, Vaziri M, Zwiazek JJ. Regulation of aquaporins in plants under stress. Biol Res. 2018;51(1):4. doi: 10.1186/s40659-018-0152-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaneff A, Vitali V, Amodeo G. PIP1 aquaporins: Intrinsic water channels or PIP2 aquaporin modulators? FEBS Lett. 2015;589(23):3508–3515. doi: 10.1016/j.febslet.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 56.Roche JV, Törnroth-Horsefield S. Aquaporin protein-protein interactions. Int J Mol Sci. 2017;18(11):2255. doi: 10.3390/ijms18112255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aharon R, Shahak Y, Wininger S, Bendov R, Kapulnik Y, Galili G. Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. Plant Cell. 2003;15(2):439–447. doi: 10.1105/tpc.009225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jang JY, Lee SH, Rhee JY, Chung GC, Ahn SJ, Kang H. Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. Plant Mol Biol. 2007;64(6):621–632. doi: 10.1007/s11103-007-9181-8. [DOI] [PubMed] [Google Scholar]
- 59.Buchfink B, Xie C, Huson DH. Fast and sensitive protein alignment using DIAMOND. Nat Methods. 2015;12(1):59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 60.Nei M, Gojobori T. Simple methods for estimating the numbers of synonymous and nonsynonymous nucleotide substitutions. Mol Biol Evol. 1986;3(5):418–426. doi: 10.1093/oxfordjournals.molbev.a040410. [DOI] [PubMed] [Google Scholar]
- 61.Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R. TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant. 2020;3(8):1194–1202. doi: 10.1016/j.molp.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 62.Zhou Y, Yin X, Duan R, Hao G, Guo J, Jiang X. SpAHA1 and SpSOS1 coordinate in transgenic yeast to improve salt tolerance. PLoS ONE. 2015;10:e0137447. doi: 10.1371/journal.pone.0137447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Su H, Zou T, Lin R, Zheng J, Jian S, Zhang M. Characterization of a phytochelatin synthase gene from Ipomoea pes-caprae involved in cadmium tolerance and accumulation in yeast and plants. Plant Physiol Biochem. 2020;155:743–755. doi: 10.1016/j.plaphy.2020.08.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. The structural features of the CrAQPs. a CrNIPs. b CrPIPs. c CrTIPs. d CrXIPs. e CrSIPs.
Additional file 2: Figure S2. The overexpression analyses of CrPIP1;5 in the three transgenic Arabidopsis lines (OX 1#, OX 5#, and OX 10#). a RT-PCR and genomic DNA PCR analysis of CrPIP1;5 in the transgenic Arabidopsis lines and WT plants. b Quantitative RT-PCR analysis of CrPIP1;5 in the transgenic Arabidopsis lines and WT plants.
Additional file 3: Figure S3. Overexpression analyses of CrPIP1;5 in the transgenic Arabidopsis lines (OX 1#, OX 5#, and OX 10#) and stress tolerance analyses of transgenic plants with regards to seed germination rates. a Photographs of the transgenic lines and WT seeds germinated on MS medium or MS medium with NaCl, NaCl plus NaHCO3 (pH 8.2), or mannitol for 7 d. b‒d The seed germination rates in WT and transgenic lines under NaCl (b), NaCl plus NaHCO3 (pH 8.2) (c), and mannitol (d) stresses after 7 d.
Additional file 4: Figure S4. Salt, salt-alkaline, and high osmotic stress analyses of the transgenic plants with CrPIP1;5’s overexpression based on seedling root lengths. Four-day-old seedlings were transplanted into MS medium containing NaCl, NaCl plus NaHCO3 (pH 8.2) or mannitol and then grown for 7 d before measuring the root length. a Photographs of the transgenic lines (CrPIP1;5OX 1#, OX 5#, and OX 10#) and WT seedlings on MS medium or MS medium with NaCl, NaCl plus NaHCO3 (pH 8.2), or mannitol; b‒d The seedling root lengths (mm) in WT and the transgenic lines under NaCl (b), NaCl plus NaHCO3 (pH 8.2) (c), or mannitol (d) stresses after 7 d. Error bars indicate the SD based on over three replicates (n ≥ 3). Asterisks indicate significant differences from the control (Student’ s t-test P values, * p < 0.05 and ** p < 0.01).
Additional file 5: Figure S5. Salt and alkaline stress analyses of the transgenic plants with CrPIP1;5’s overexpression based on the growth of adult Arabidopsis. a Leaf phenotypes of the transgenic Arabidopsis OX lines and WT plants under 200 mM NaCl stress for 7 days. b Leaf phenotypes of the transgenic Arabidopsis OX lines and WT plants under 100 mM NaHCO3 (pH 8.2) stress for 4 days.
Additional file 6: Figure S6. Subcellular localization of the CrPIP1;5 protein. Arabidopsis roots expressing 35S:GFP-CrPIP1;5 fusion proteins (upper two lines) and 35S:GFP (lower line) were observed under a laser scanning confocal microscope.
Additional file 7: Table S1. The obtained CrAQPs’ nucleotide and protein sequences information in this study.
Additional file 8: Table S2. Primers’ information used in this study.
Additional file 9: Table S3. The categories of cis-acting elements identified in the CrAQPs’ promoter regions.
Additional file 10: Table S4. The conserved motif sequences of CrAQPs identified by the MEME web server.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary information files. However, the sequence data in this study can also be accessed at http://doi.org/10.13140/RG.2.2.21213.74727.