Abstract
Background
Accumulating evidence has established a role for the orexigenic hormone ghrelin in alcohol-seeking behaviors. Accordingly, the ghrelin system may represent a potential pharmacotherapeutic target for alcohol use disorder. Ghrelin modulates several neuroendocrine pathways, such as appetitive, metabolic, and stress-related hormones, which are particularly relevant in the context of alcohol use. The goal of the present study was to provide a comprehensive assessment of neuroendocrine response to exogenous ghrelin administration, combined with alcohol, in heavy-drinking individuals.
Methods
This was a randomized, crossover, double-blind, placebo-controlled human laboratory study, which included 2 experimental alcohol administration paradigms: i.v. alcohol self-administration and i.v. alcohol clamp. Each paradigm consisted of 2 counterbalanced sessions of i.v. ghrelin or placebo administration. Repeated blood samples were collected during each session, and peripheral concentrations of the following hormones were measured: leptin, glucagon-like peptide-1, pancreatic polypeptide, gastric inhibitory peptide, insulin, insulin-like growth factor-1, cortisol, prolactin, and aldosterone.
Results
Despite some statistical differences, findings were consistent across the 2 alcohol administration paradigms: i.v. ghrelin, compared to placebo, increased blood concentrations of glucagon-like peptide-1, pancreatic polypeptide, cortisol, and prolactin, both acutely and during the whole session. Lower levels of leptin and higher levels of aldosterone were also found during the ghrelin vs placebo session.
Conclusion
These findings, gathered from a clinically relevant sample of heavy-drinking individuals with alcohol use disorder, provide a deeper insight into the complex interplay between ghrelin and appetitive, metabolic, and stress-related neuroendocrine pathways in the context of alcohol use.
Keywords: Ghrelin, alcohol, neuroendocrine, metabolism, stress
Significance Statement.
Administration of the hormone ghrelin has been shown to increase alcohol craving and drinking. Therefore, the ghrelin system is being studied as a potential target to develop novel medications to treat alcohol use disorder (AUD). Both ghrelin and alcohol interact with a variety of endocrine pathways, especially those related to appetite, metabolism, and stress. To better understand the complex interplay between ghrelin and other hormones in the context of alcohol use, the present study examined neuroendocrine response to a supraphysiological challenge with exogenous ghrelin, combined with alcohol, in a clinically relevant sample of heavy-drinking individuals with AUD. Results were consistent across 2 experimental alcohol administration paradigms and found a number of endocrine changes in response to exogenous ghrelin administration. This study provides a comprehensive picture of neuroendocrine response to ghrelin plus alcohol and provides a deeper insight into the interplay between ghrelin and appetitive, metabolic, and stress-related hormones in the context of alcohol use.
Introduction
Alcohol is the most commonly used addictive drug worldwide, and alcohol use disorder (AUD) represents a major global public health concern (Rehm et al., 2018). Despite the high prevalence of AUD and considerable medical, psychosocial, and economic burden associated with the disease (Grant et al., 2016), treatment options, including medications, are limited in number and efficacy (Jonas et al., 2014). Therefore, there is a critical need to increase the armamentarium of pharmacotherapies for AUD (Farokhnia et al., 2019a). While most of the research in this regard has focused on central neurobiological mechanisms involved in addictive behaviors, there is growing interest in understanding the role of peripheral/modulatory pathways (e.g., endocrine system, immune factors, gut microbiome) and their potential as novel therapeutic targets for AUD (Engel and Jerlhag, 2014; Ray et al., 2014; Temko et al., 2017).
Among drugs with addictive properties, alcohol has some unique characteristics, as it not only exerts pharmacological actions in the central nervous system (CNS) and the periphery but also has palatable properties and is a direct source of calories. In fact, previous research indicates considerable overlap between biological processes involved in food craving, intake, and metabolism and those that regulate alcohol-seeking and consummatory behaviors (Volkow et al., 2012; Blanco-Gandía et al., 2020). Notably, appetitive/metabolic hormones such as ghrelin, leptin, glucagon-like peptide-1 (GLP-1), and insulin that control homeostatic feeding and metabolism have also been found to regulate hedonic and addictive properties of food and alcohol, mainly through interactions with pathways related to reward processing (van Zessen et al., 2012). In addition, several lines of evidence suggest that stress-related pathways modulate both food and alcohol-seeking behaviors, primarily through negative reinforcement mechanisms (Koob et al., 2014). The hypothalamic-pituitary-adrenal (HPA) axis, a key neuroendocrine pathway involved in stress response, is directly activated by alcohol at the pharmacological level (Zhou and Kreek, 2014). Moreover, previous studies indicate that alterations in the HPA axis may facilitate the transition from mild-to-moderate alcohol drinking to AUD and may contribute to the risk of relapse (Koob, 2010; Blaine and Sinha, 2017). Based on the aforementioned evidence, feeding- and stress-related endocrine pathways may represent novel pharmacotherapeutic targets for AUD. One such pathway is the ghrelin system, with growing evidence from preclinical and clinical studies supporting its role in biobehavioral mechanisms underlying alcohol seeking and consumption (Farokhnia et al., 2019b).
Ghrelin is a 28-amino acid peptide hormone primarily produced by enteroendocrine cells located in the oxyntic glands of the stomach. A portion of the produced proghrelin undergoes acylation via the ghrelin-O-acyltransferase enzyme; the acylated peptide is subsequently cleaved to form acyl-ghrelin. Acyl-ghrelin has been termed the “active” form of ghrelin for its ability to bind to and activate the growth hormone secretagogue receptor 1a (GHSR1a), also known as the ghrelin receptor (Gahete et al., 2014). GHSR1a is a G protein-coupled receptor widely expressed in both central (e.g., hypothalamus, pituitary, ventral tegmental area, amygdala, hippocampus) and peripheral (e.g., gut, pancreas, adipose tissue, adrenal gland, vagus nerve terminals) tissues (Gnanapavan et al., 2002), mediating ghrelin’s functions in the CNS and the periphery.
Ghrelin was first discovered to stimulate the release of growth hormone (GH) from the pituitary through GHSR1a activation (Kojima et al., 1999). Subsequent research has identified a wide range of other key physiological functions, indicating that the ghrelin system is critical for survival (Mani and Zigman, 2017). Chiefly, ghrelin regulates both homeostatic and hedonic food intake (Perelló and Zigman, 2012; Yanagi et al., 2018). Ghrelin also plays a major role in energy balance, as it regulates calorie intake and expenditure and modulates key metabolic processes, such as those involved in lipid and glucose homeostasis (Pradhan et al., 2013; Lv et al., 2018; Gray et al., 2019). Growing evidence suggests that ghrelin may also be considered a stress hormone, as it closely interacts with biobehavioral pathways that regulate stress response, for example, the HPA axis (Morris et al., 2018; Stone et al., 2020). Accordingly, the ghrelin system has been extensively studied in relation to alcohol-related behaviors and is currently under investigation as a potential therapeutic target for AUD (Zallar et al., 2017; Farokhnia et al., 2019b; Lee et al., 2020).
Ghrelin’s effects on alcohol-related outcomes are thought to be primarily driven through brain regions and neural circuits involved in reward processing, stress regulation, and cognition (Jerlhag et al., 2009; Meyer et al., 2014; Koob and Volkow, 2016). Peripherally secreted ghrelin is able to cross the blood-brain barrier and binds to the GHSR1a in the CNS (Banks, 2012). Another route through which ghrelin interacts with the brain is vagal stimulation via activation of the GHSR1a expressed on vagal afferent neurons (Dockray, 2013; Date, 2014; Tamboli et al., 2017; Godlewski et al., 2019). Given the widespread presence of ghrelin receptors in both central and peripheral tissues, ghrelin signaling modulates a myriad of other neuroendocrine pathways, such as appetitive/metabolic and stress-related hormones. These effects are particularly relevant in the context of alcohol use, as neuroendocrine mechanisms may also be implicated in the pathophysiology of AUD. In addition to a large body of preclinical evidence on the interaction between ghrelin, alcohol, and neuroendocrine pathways, secondary analyses from a human laboratory study found reduced levels of leptin and insulin (Haass-Koffler et al., 2015; Haass-Koffler et al., 2016) and increased levels of cortisol and aldosterone (Haass-Koffler et al., 2019) following i.v. administration of ghrelin in heavy-drinking individuals with alcohol dependence. Of note, participants did not receive alcohol in the aforementioned study (Leggio et al., 2014).
The goal of the present study was to provide a comprehensive assessment of neuroendocrine response to a supraphysiological pharmacological challenge with exogenous ghrelin by examining the effects on appetitive/metabolic and stress-related endocrine outcomes in heavy-drinking individuals who also received i.v. alcohol under a controlled experimental setting.
Methods
Setting and Participants
Data were collected under a human laboratory study conducted at the National Institutes of Health (NIH) Clinical Center in Bethesda, Maryland. The protocol was approved by the NIH Addictions Institutional Review Board (13-AA-0043), registered at ClinicalTrials.gov (NCT01779024), and conducted under an Investigational New Drug application (IND #117,778) following review by the Food and Drug Administration. The primary goal of the parent study was to examine the effects exogenous ghrelin on i.v. alcohol self-administration and brain functional activity in heavy alcohol drinkers (Farokhnia et al., 2018). Potential candidates were first screened through a phone interview, followed by an in-person screening visit. Inclusion/exclusion criteria were assessed, and eligible individuals were enrolled after providing written, informed consent. Enrolled participants were non-treatment-seeking, heavy-drinking (>15 and >20 standard drinks per week for females and males, respectively), alcohol-dependent (DSM-IV-TR) individuals with no significant health problems. For the complete list of eligibility criteria, see supplementary Appendix 1.
Design and Procedures
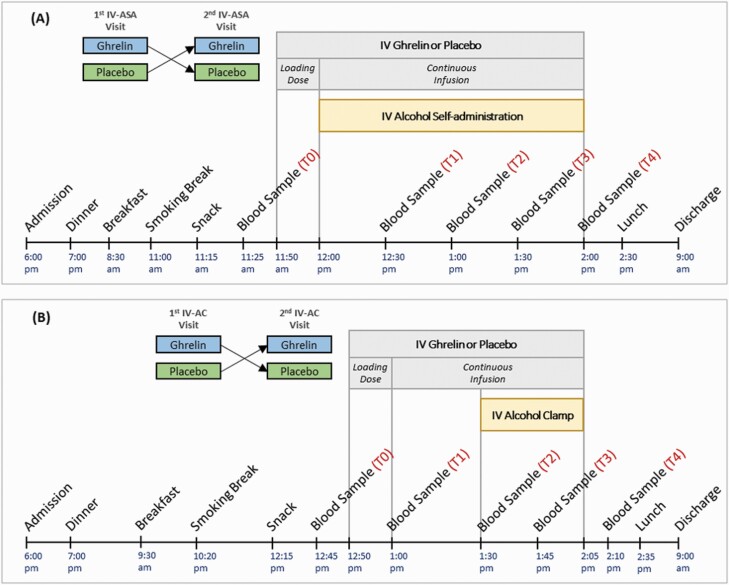
A detailed description of the parent study was previously reported (Farokhnia et al., 2018). Briefly, each participant underwent up to 4 experimental sessions (Figure 1): 2 i.v. alcohol self-administration (IV-ASA) and 2 brain functional magnetic resonance imaging (fMRI) sessions. The fMRI experiment included an i.v. alcohol clamp (IV-AC) designed to achieve a target blood alcohol concentration. Alcohol administration procedures were performed in accordance with the National Institute on Alcohol Abuse and Alcoholism Council Guidelines on Alcohol Administration (https://niaaa.nih.gov/Resources/ResearchResources/job22.htm). Each experiment (IV-ASA and IV-AC) had a crossover, randomized, double-blind, placebo-controlled design during which participants received a 10-minute loading dose of i.v. acyl-ghrelin (3 mcg/kg) or placebo, followed by a continuous acyl-ghrelin (16.9 ng/kg/min) or placebo infusion, until the end of each session. For more details about the experimental compounds, see supplementary Appendix 2.
Figure 1.
Schematic outline of the events and blood sampling time-points during (A) i.v. alcohol self-administration (IV-ASA) experiment (2 visits), and (B) i.v. alcohol clamp (IV-AC) experiment (2 visits). Each participant underwent up to 4 experimental sessions. During each session, a 10-minute loading dose of i.v. acyl-ghrelin (3 mcg/kg) or placebo was administered, followed by a continuous infusion of acyl-ghrelin (16.9 ng/kg/min) or placebo until the end of the session.
Intravenous Alcohol Self-Administration
During each 120-minute IV-ASA session, participants were given the opportunity to self-administer alcohol via the Computer-Alcohol Infusion System by pressing a button in a progressive ratio manner. Each alcohol infusion was designed to increase the breath alcohol concentration (BrAC) by 7.5 mg% over 2.5 minutes, with a subsequent decrease of 0.5 mg%/min, until the following infusion. Participants were not allowed to administer alcohol beyond a maximum BrAC of 120 mg%. Primary results of this paradigm showed that exogenous ghrelin administration, compared to placebo, significantly increased the total amount of alcohol self-administered (Farokhnia et al., 2018).
Intravenous Alcohol Clamp
A predetermined dose of i.v. alcohol was administered as part of a brain fMRI experiment. This alcohol infusion was designed to increase the BrAC linearly to 80 mg% within 20 minutes and maintain (clamp) the BrAC at this level for 15 minutes (therefore, 35 minutes in total). Primary results of this paradigm showed that exogenous ghrelin, compared to placebo, differentially modulated brain functional activity while anticipating alcohol vs food reward (Farokhnia et al., 2018).
Participants were admitted to the NIH Clinical Center the evening before each experimental session and were discharged the morning after; therefore, each experimental session was conducted under a controlled inpatient setting, where parameters such as alcohol intake, smoking breaks, and diet were closely monitored and standardized. Three standardized meals and 1 standardized snack were served during each visit. A washout period of at least 3 days was applied between study visits. For additional details, see Figure 1 and supplementary Appendix 3.
Blood Collection, Processing, and Assays
Repeated blood samples (5 time-points; see Figure 1) were obtained during each experimental visit via a saline lock i.v. catheter that was fixed in the antecubital fossa of the nondominant arm. Blood concentrations of the following hormones were measured: total ghrelin, acyl-ghrelin, GH, leptin, GLP-1, pancreatic polypeptide (PP), gastric inhibitory peptide, insulin, insulin-like growth factor-1 (IGF-1), cortisol, prolactin, and aldosterone. Details regarding blood collection, processing, and assays are presented in supplementary Appendix 4. Values below the lower limit of quantitation (LLOQ) for each assay were set to 1/2 of the LLOQ (Keizer et al., 2015).
Statistical Methods
Demographic characteristics of the study sample were summarized with descriptive statistics (mean and standard error for continuous variables, number and percent for categorical variables). Data from the ghrelin sessions of the IV-ASA and IV-AC were used to characterize kinetic parameters of peripheral acyl-ghrelin and total ghrelin concentrations in this study. Noncompartmental analyses were run, and the linear trapezoidal rule was applied for estimations (Phoenix WinNonlin, Pharsight Corp., version 6.3; Mountain View, CA). Calculated parameters included area under the plasma concentration-time curve (AUC0-last), maximum plasma concentration (Cmax), time of Cmax (Tmax), half-life, mean residence time (MRT), clearance (Cl), and volume of distribution (Vd). Baseline correction was performed using pre-dose concentrations of the placebo session. This correction was done to minimize the within-day variability of each hormone and to provide a more accurate estimation of acyl-ghrelin and total ghrelin kinetic parameters (Scheff et al., 2011). Neuroendocrine outcomes were first tested for statistical outliers and normal distribution. Leptin, GLP-1, and GH data for the IV-ASA experiment and acyl-ghrelin and total ghrelin data for the IV-AC experiment were not normally distributed and therefore were log10 transformed, which led to significant improvement in normality. Baseline (T0) concentrations of the endocrine outcomes were compared between the 2 sessions (i.v. ghrelin vs placebo) of each experiment using independent samples t test. To examine i.v. ghrelin’s acute effect, we first compared the change (Δ) in blood concentrations of each neuroendocrine outcome from baseline (T0) to post-drug (T1) in response to i.v. ghrelin vs placebo using independent samples t test. Next, we examined the pattern/time course of these hormones over a longer timeframe, that is, during the i.v. ghrelin vs placebo sessions. For this purpose, repeated measurements of each neuroendocrine outcome were analyzed via linear mixed-effects models with drug condition (i.v. ghrelin, placebo), blood sampling time-point (T1, T2, T3, T4), and drug × time-point interaction as fixed effects, individual participants as a random effect, and blood concentrations of each hormone as the outcome. Age, gender, body weight, session order (i.v. ghrelin or placebo first), total number of alcohol infusions self-administered (IV-ASA experiment only), and baseline (T0) concentration of each hormone (first time-point) were also included as covariates. Partial eta squared (η 2p) values were also calculated to indicate effect sizes. Analyses were performed using the Statistical Package for the Social Sciences (SPSS) software (IBM Corp., version 25; Armonk, NY), and significance level was set at P < .05 (2-tailed).
Results
Sample Characteristics and Ghrelin Concentrations
Of 77 individuals screened, 18 signed the informed consent and were enrolled. A final sample of 11 and 8 participants completed both IV-ASA sessions and both IV-AC sessions, respectively, and were included in the analyses (supplementary Figure 1). Table 1 summarizes the demographic characteristics of the study sample. Enrolled participants were predominantly male and African American. Although not a required eligibility criterion, all participants had a current diagnosis of alcohol dependence based on DSM-IV-TR.
Table 1.
Demographic Characteristics of the Study Sample
| IV-ASA experiment (n = 11) | IV-AC experiment (n = 8) | |
|---|---|---|
| Age, years, M (SEM) | 39.86 (3.54) | 42.50 (3.12) |
| Gender, males, n (%) | 8 (72.72) | 6 (75) |
| Race, African Americans, n (%) | 9 (81.81) | 7 (87.5) |
| Education, years, M (SEM) | 13.36 (0.49) | 13.75 (0.70) |
| Annual incomea, n (%) | ||
| Below average | 7 (63.63) | 5 (62.5) |
| Average | 3 (27.27) | 2 (25) |
| Above average | 1 (9.09) | 1 (12.5) |
| Body weight, kg, M (SEM) | 77.44 (3.29) | 77.90 (3.50) |
| BMI, kg/m2, M (SEM) | 25.42 (0.87) | 25.88 (0.98) |
| Family history density of problem drinkingb, M (SEM) | 0.10 (0.03) | 0.14 (0.05) |
| Current cigarette smoker, n (%) | 8 (72.72) | 5 (62.5) |
Abbreviations: BMI, body mass index; IV-AC, intravenous alcohol clamp; IV-ASA, intravenous alcohol self-administration; M, mean; SEM, standard error of the mean.
a Below average: <$30 000; average: $30 000-$49 000; above average: ≥$50 000.
b Based on Family Tree Questionnaire: density of relatives (siblings, parents, grandparents) with definite problem drinking (self-reported).
As expected, i.v. ghrelin administration significantly increased blood concentrations of acyl-ghrelin, total ghrelin, and GH (see supplementary Figures 2 and 3), confirming that the supraphysiological challenge with exogenous acyl-ghrelin was successful (i.e., ghrelin levels increased) and pharmacologically effective (i.e., GH levels increased). Ten individuals from the IV-ASA experiment and 6 individuals from the IV-AC experiment had complete data for calculation of acyl-ghrelin and total ghrelin kinetic parameters (supplementary Tables 1 and 2). Respective spaghetti plots are also depicted in supplementary Figures 4 and 5.
Neuroendocrine Outcomes
Intravenous Alcohol Self-Administration Experiment
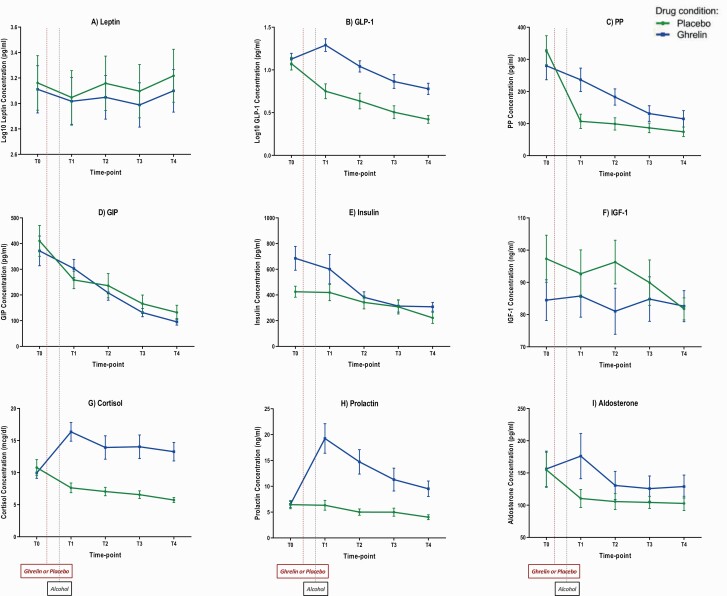
Figure 2 outlines the IV-ASA experiment neuroendocrine outcomes.
Figure 2.
Blood concentrations of neuroendocrine outcomes during ghrelin and placebo sessions of the i.v. alcohol self-administration (IV-ASA) experiment. A 10-minute loading dose of i.v. acyl-ghrelin (3 mcg/kg) or placebo was administered, followed by a continuous infusion of acyl-ghrelin (16.9 ng/kg/min) or placebo until the end of each session. Alcohol self-administration started simultaneously with the ghrelin/placebo continuous infusion (between T0 and T1) and continued until the end of the session (T4). T0: 25 minutes before the start of the ghrelin/placebo loading dose; T1: 30 minutes after the start of the ghrelin/placebo continuous infusion; T2: 60 minutes after the start of the ghrelin/placebo continuous infusion; T3: 90 minutes after the start of the ghrelin/placebo continuous infusion; T4: 120 minutes after the start of the ghrelin/placebo continuous infusion. Blood concentrations of each hormone per time-point are expressed as mean (M) and standard error of the mean (SEM). For statistical results, see Table 2. Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; PP, pancreatic polypeptide.
Baseline (T0) concentrations of the measured hormones did not significantly differ between the 2 IV-ASA sessions, except for significantly higher baseline insulin levels prior to i.v. ghrelin than placebo administration (supplementary Table 3).
Comparison of change (Δ) from T0 to T1 showed that IV ghrelin, compared to placebo, significantly increased blood concentrations of GLP-1 (t [20] = −4.91, P < .001), PP (t [20] = −5.27, P < .001), cortisol (t [19] = −4.65, P < .001), and prolactin (t [18] = −4.56, P < .001) (Figure 2; Table 2).
Table 2.
Comparison of Change in Blood Concentrations of Neuroendocrine Outcomes, From Baseline to Post-Drug Time-Pointa, Between Placebo and Ghrelin Sessions of the IV-ASA Experiment
| Placebo session, M (SEM) | Ghrelin session, M (SEM) | Independent samples t test | |
|---|---|---|---|
| Δ Leptin (Log10) concentration (pg/mL) | −0.11 (0.02) | −0.09 (0.02) | t (18) = −0.62, P = .54 |
| Δ GLP-1 (Log10) concentration (pg/mL) | −0.32 (0.04) | 0.16 (0.08) | t (20) = −4.91,P < .001 |
| Δ PP concentration (pg/mL) | −221.46 (28.44) | −43.87 (18.01) | t (20) = −5.27,P < .001 |
| Δ GIP concentration (pg/mL) | −151.94 (51.00) | −68.62 (79.23) | t (20) = −0.88,P = .38 |
| Δ Insulin concentration (pg/mL) | −81.95 (39.32) | −84.42 (144.79) | t (18) = 0.01,P = .98 |
| Δ IGF-1 concentration (ng/mL) | −4.63 (4.94) | 1.28 (4.19) | t (20) = −0.91,P = .37 |
| Δ Cortisol concentration (mcg/dL) | −3.15 (1.14) | 6.24 (1.70) | t (19) = −4.65,P < .001 |
| Δ Prolactin concentration (ng/mL) | −0.13 (0.78) | 12.74 (2.70) | t (18) = −4.56,P < .001 |
| Δ Aldosterone concentration (pg/mL) | −44.41 (18.31) | 18.07 (33.49) | t (19) = −1.67,P = .11 |
Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; IV-ASA, i.v. alcohol self-administration; M, mean; PP, pancreatic polypeptide; SEM, standard error of the mean.
a Baseline (T0): 25 minutes before the start of the ghrelin/placebo loading dose, post-drug (T1): 30 minutes after the start of the ghrelin/placebo continuous infusion. See Figure 1 for more details.
Analysis of repeated measures during the IV-ASA experiment (T1, T2, T3, and T4) after controlling for baseline (T0) found lower leptin (F [1, 57.78] = 6.28, P = .01) and higher GLP-1 (F [1, 55.28] = 75.73, P < .001), PP (F [1, 61.11] = 55.56, P < .001), insulin (F [1, 50.75] = 21.85, P < .001), cortisol (F [1, 63.34] = 186.75, P < .001), prolactin (F [1, 59.93] = 87.45, P < .001), and aldosterone (F [1, 65.63] = 15.62, P < .001) concentrations under i.v. ghrelin compared to placebo, as indicated by significant drug main effects. Significant drug × time-point interaction effects were also found for PP (F [3, 61.65] = 5.71, P = .002) and prolactin (F [3, 53.39] = 5.65, P = .002) (Figure 2; Table 3). Of note, the significant drug main effect on insulin appears to be a carryover effect of significantly higher levels of insulin at baseline, that is, prior to ghrelin vs placebo administration (Figure 2E).
Table 3.
Drug, Time-Point, and Drug × Time-Point Effects on Neuroendocrine Outcomes During the IV-ASA Experiment
| Druga main effect | Time-pointb main effect | Drug × time-point interaction effect | |
|---|---|---|---|
| Leptin | F (1, 57.78) = 6.28,P = .01 | F (3, 55.38) = 5.26,P = .003 | F (3, 55.38) = 0.03,P = .99 |
| η 2p = 0.09 | η 2p = 0.22 | η 2p = 0.002 | |
| GLP-1 | F (1, 55.28) = 75.73,P < .001 | F (3, 59.24) = 20.43,P < .001 | F (3, 59.38) = 1.75,P = .16 |
| η 2p = 0.57 | η 2p = 0.50 | η 2p = 0.08 | |
| PP | F (1, 61.11) = 55.56,P < .001 | F (3, 61.65) = 14.45,P < .001 | F (3, 61.65) = 5.71,P = .002 |
| η 2p = 0.47 | η 2p = 0.41 | η 2p = 0.21 | |
| GIP | F (1, 66.97) = 3.66,P = .06 | F (3, 60.30) = 25.61,P < .001 | F (3, 60.30) = 1.63,P = .19 |
| η 2p = 0.05 | η 2p = 0.56 | η 2p = 0.07 | |
| Insulin | F (1, 50.75) = 21.85,P < .001 | F (3, 46.58) = 12.36,P < .001 | F (3, 46.58) = 2.15,P = .10 |
| η 2p = 0.30 | η 2p = 0.44 | η 2p = 0.12 | |
| IGF-1 | F (1, 60.68) = 0.11,P = .73 | F (3, 60.18) = 0.29,P = .82 | F (3, 60.21) = 1.05,P = .37 |
| η 2p = 0.001 | η 2p = 0.01 | η 2p = 0.05 | |
| Cortisol | F (1, 63.34) = 186.75,P < .001 | F (3, 57.40) = 3.60,P = .01 | F (3, 57.40) = 0.70,P = .55 |
| η 2p = 0.74 | η 2p = 0.15 | η 2p = 0.03 | |
| Prolactin | F (1, 59.93) = 87.45,P < .001 | F (3, 53.40) = 15.06,P < .001 | F (3, 53.39) = 5.65,P = .002 |
| η 2p = 0.59 | η 2p = 0.45 | η 2p = 0.24 | |
| Aldosterone | F (1, 65.63) = 15.62,P < .001 | F (3, 59.20) = 2.61,P = .05 | F (3, 59.20) = 1.73,P = .17 |
| η 2p = 0.19 | η 2p = 0.11 | η 2p = 0.08 |
Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; IV-ASA, i.v. alcohol self-administration; PP, pancreatic polypeptide.
a Intravenous ghrelin or placebo.
b T1 (30 minutes after the start of the ghrelin/placebo continuous infusion), T2 (60 minutes after the start of the ghrelin/placebo continuous infusion), T3 (90 minutes after the start of the ghrelin/placebo continuous infusion), and T4 (120 minutes after the start of the ghrelin/placebo continuous infusion). T0 (baseline) was included as a covariate in each model, along with age, gender, body weight, session order, and total number of alcohol infusions self-administered. See Figure 1 for more details.
Intravenous Alcohol Clamp Experiment
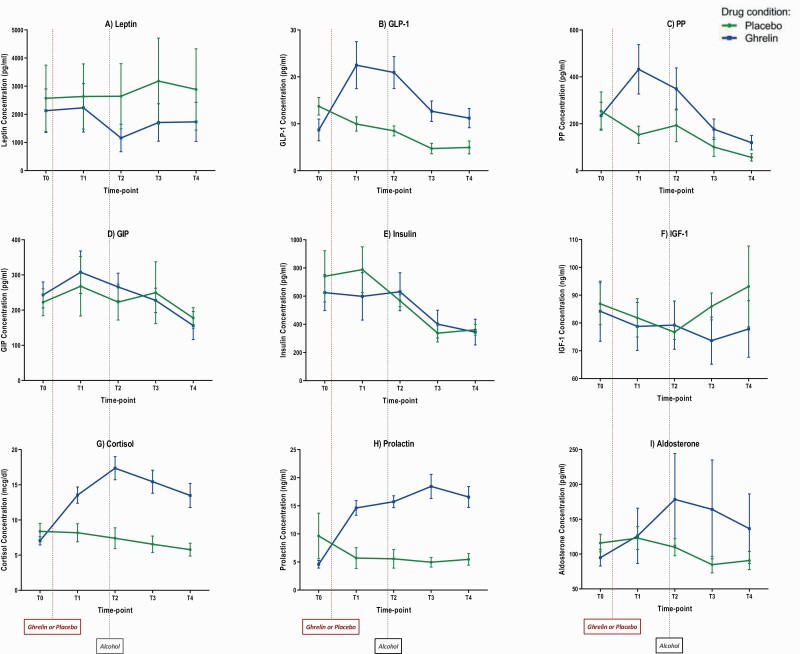
Figure 3 outlines the IV-AC experiment neuroendocrine outcomes. Baseline (T0) concentrations of the measured hormones did not significantly differ between the 2 IV-AC sessions (supplementary Table 4).
Figure 3.
Blood concentrations of neuroendocrine outcomes during ghrelin and placebo sessions of the i.v. alcohol clamp (IV-AC) experiment. A 10-minute loading dose of i.v. acyl-ghrelin (3 mcg/kg) or placebo was administered, followed by a continuous infusion of acyl-ghrelin (16.9 ng/kg/min) or placebo until the end of each session. Alcohol clamp started at T2 and continued until the end of the session (5 minutes before T4). T0: 5 minutes before the start of the ghrelin/placebo loading dose; T1: at the end of the ghrelin/placebo loading dose and the start of the ghrelin/placebo continuous infusion; T2: 30 minutes after the start of the ghrelin/placebo continuous infusion; T3: 45 minutes after the start of the ghrelin/placebo continuous infusion; T4: 70 minutes after the start of the ghrelin/placebo continuous infusion. Blood concentrations of each hormone per time-point are expressed as mean (M) and standard error of the mean (SEM). For statistical results, see Table 3. Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; PP, pancreatic polypeptide.
Comparison of change (Δ) from T0 to T1 showed that i.v. ghrelin, compared to placebo, significantly increased blood concentrations GLP-1 (t [11] = −4.67, P = .001), cortisol (t [11] = −4.76, P = .001), and prolactin (t [10] = −4.96, P = .001); a trend-level increase in PP (t [10] = −1.85, P = .06) was also found (Figure 3; Table 4).
Table 4.
Comparison of Change in Blood Concentrations of Neuroendocrine Outcomes, From Baseline to Post-Drug Time-Pointa, Between Placebo and Ghrelin Sessions of the IV-AC Experiment
| Placebo session, M (SEM) | Ghrelin session, M (SEM) | Independent samples t Test | |
|---|---|---|---|
| Δ Leptin Concentration (pg/mL) | 65.79 (145.13) | 102.95 (175.28) | t (11) = −0.16,P = 87 |
| Δ GLP-1 Concentration (pg/mL) | −3.77 (1.64) | 13.81 (3.16) | t (11) = −4.67,P = 001 |
| Δ PP Concentration (pg/mL) | −36.07 (30.36) | 197.86 (102.96) | t (10) = −1.85,P = 06 |
| Δ GIP Concentration (pg/mL) | 45.58 (47.83) | 64.36 (38.40) | t (11) = −0.31,P = 76 |
| Δ Insulin Concentration (pg/mL) | 47.67 (57.69) | −26.45 (87.58) | t (11) = 0.68, P = 51 |
| Δ IGF-1 Concentration (ng/mL) | −5.06 (3.77) | −5.47 (8.43) | t (11) = 0.04,P = 96 |
| Δ Cortisol Concentration (mcg/dL) | −0.26 (0.72) | 6.91 (1.24) | t (11) = −4.76,P = 001 |
| Δ Prolactin Concentration (ng/mL) | −0.23 (0.24) | 9.73 (1.99) | t (10) = −4.96,P = 001 |
| Δ Aldosterone Concentration (pg/mL) | 2.43 (6.97) | 35.51 (30.35) | t (9) = −0.96,P = 35 |
Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; IV-AC, i.v. alcohol clamp; M, mean; PP, pancreatic polypeptide; SEM, standard error of the mean.
a Baseline (T0): 5 minutes before the start of the ghrelin/placebo loading dose, post-drug (T1): at the end of the ghrelin/placebo loading dose and the start of the ghrelin/placebo continuous infusion. See Figure 1 for more details.
Analysis of repeated measures during the IV-ASA experiment (T1, T2, T3, and T4) after controlling for baseline (T0) found lower IGF-1 (F [1, 19.35] = 5.29, P = .03) and higher GLP-1 (F [1, 31.53] = 30.40, P < .001), PP (F [1, 36.00] = 17.47, P < .001), cortisol (F [1, 22.66] = 42.53, P < .001), prolactin (F [1, 33.37] = 134.14, P < .001), and aldosterone (F [1, 30.15] = 36.44, P < .001) concentrations under i.v. ghrelin, compared to placebo, as indicated by significant drug main effects. A significant drug × time-point interaction effect was also found for cortisol (F [3, 33.55] = 3.34, P = .03) (Figure 3; Table 5). Of note, the significant drug main effect on IGF-1 appears to be driven by an unexpected increase in IGF-1 concentrations at T3 and T4 under the placebo condition (Figure 3F).
Table 5.
Drug, Time-Point, and Drug × Time-Point Effects on Neuroendocrine Outcomes During the IV-AC Experiment
| Druga main effect | Time-pointb main effect | Drug × time-point interaction effect | |
|---|---|---|---|
| Leptin | F (1, 8.58) = 0.19,P = .67 | F (3, 35.04) = 0.44,P = .72 | F (3, 35.06) = 0.97,P = .41 |
| η 2p = 0.02 | η 2p = 0.03 | η 2p = 0.07 | |
| GLP-1 | F (1, 31.53) = 30.40,P < .001 | F (3, 35.07) = 6.36,P = .001 | F (3, 35.07) = 0.85,P = .47 |
| η 2p = 0.49 | η 2p = 0.35 | η 2p = 0.06 | |
| PP | F (1, 36.00) = 17.47,P < .001 | F (3, 36.00) = 7.42,P = .001 | F (3. 36.00) = 1.59,P = .20 |
| η 2p = 0.32 | η 2p = 0.38 | η 2p = 0.11 | |
| GIP | F (1, 37.00) = 0.01,P = .90 | F (3, 37.00) = 2.44,P = .08 | F (3, 37.00) = 0.57,P = .63 |
| η 2p = 0.0004 | η 2p = 0.16 | η 2p = 0.04 | |
| Insulin | F (1, 19.75) = 0.67,P = .42 | F (3, 31.45) = 13.36,P < .001 | F (3, 31.45) = 0.41,P = .74 |
| η 2p = 0.03 | η 2p = 0.56 | η 2p = 0.03 | |
| IGF-1 | F (1, 19.35) = 5.29,P = .03 | F (3, 34.24) = 0.31,P = .81 | F (3, 34.24) = 0.88,P = .46 |
| η 2p = 0.21 | η 2p = 0.02 | η 2p = 0.07 | |
| Cortisol | F (1, 22.66) = 42.53,P < .001 | F (3, 33.55) = 4.32,P = .01 | F (3, 33.55) = 3.34,P = .03 |
| η 2p = 0.65 | η 2p = 0.27 | η 2p = 0.23 | |
| Prolactin | F (1, 33.37) = 134.14,P < .001 | F (3, 32.66) = 1.02,P = 0.39 | F (3, 32.62) = 0.50,P = .68 |
| η 2p = 0.80 | η 2p = 0.08 | η 2p = 0.04 | |
| Aldosterone | F (1, 30.15) = 36.44,P < .001 | F (3, 28.88) = 0.68,P = .56 | F (3, 28.88) = 1.20,P = .32 |
| η 2p = 0.54 | η 2p = 0.06 | η 2p = 0.11 |
Abbreviations: GIP, gastric inhibitory peptide; GLP-1, glucagon-like peptide-1; IGF-1, insulin-like growth factor-1; IV-AC, i.v. alcohol clamp; PP, pancreatic polypeptide.
a Intravenous ghrelin and placebo
b T1 (at the end of the ghrelin/placebo loading dose and the start of the ghrelin/placebo continuous infusion), T2 (30 minutes after the start of the ghrelin/placebo continuous infusion), T3 (45 minutes after the start of the ghrelin/placebo continuous infusion), and T4 (70 minutes after the start of the ghrelin/placebo continuous infusion). T0 (baseline) was included as a covariate in each model, along with age, gender, body weight, and session order. See Figure 1 for more details.
Discussion
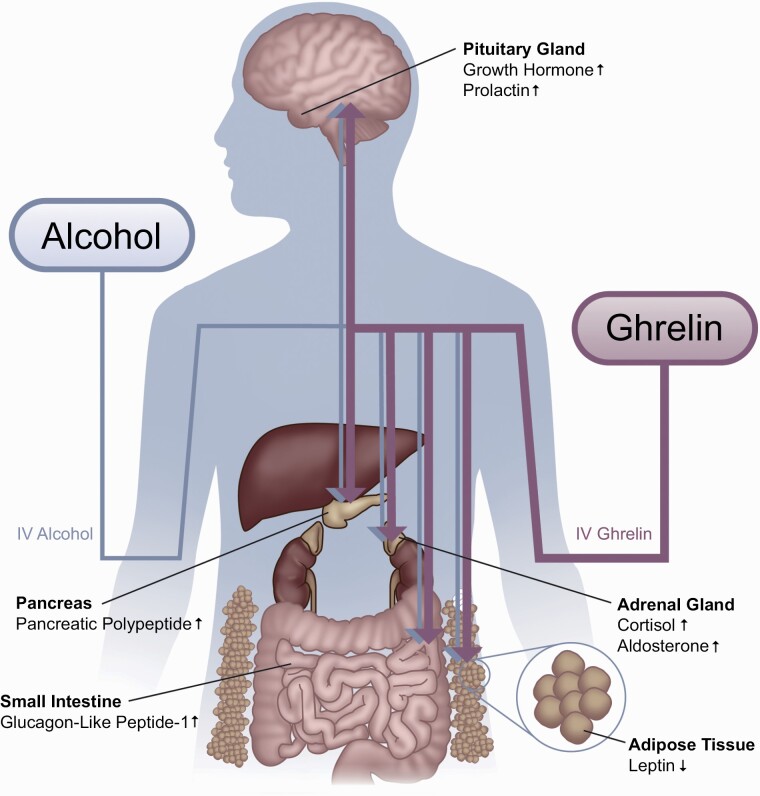
The present study investigated the effects of a supraphysiological challenge with exogenous ghrelin on peripheral concentrations of a range of hormones in heavy-drinking, alcohol-dependent individuals concurrently receiving i.v. alcohol. Despite methodological differences in terms of alcohol dosage, blood sampling time-points, etc., the results were in overall agreement and internally replicated across the 2 alcohol administration paradigms (i.e., IV-ASA and IV-AC). i.v. ghrelin, compared to placebo, significantly increased blood concentrations of GLP-1, PP, cortisol, and prolactin both acutely (Δ T0-T1) and during the session (T1, T2, T3, and T4, while controlling for T0). Lower levels of leptin and higher levels of aldosterone were also found during the ghrelin vs placebo session (Figure 4).
Figure 4.
A graphical summary of the main hormonal changes, found in this study, in response to i.v. ghrelin, compared to placebo. Findings were in overall agreement across the 2 alcohol administration paradigms (i.e., IV-ASA and IV-AC). Increased levels of growth hormone, prolactin, cortisol, aldosterone, pancreatic polypeptide, and glucagon-like peptide-1, and decreased levels of leptin were observed under i.v. ghrelin administration, compared to the placebo condition. Abbreviations: AC, alcohol clamp; ASA, alcohol self-administration.
In addition to the supraphysiological challenge with exogenous ghrelin, the experiments in this study included i.v. administration of alcohol using a well-established computer-based method that limited the variability in alcohol levels/exposure typically observed after oral alcohol consumption (Cyders et al., 2020). The 2 experimental paradigms (IV-ASA and IV-AC) could be considered complementary, as they included different levels and durations of exposure to alcohol as well as different blood sampling time-points in relation to both i.v. ghrelin and alcohol administration (see Figure 1). Specifically, the IV-ASA experiment had a longer duration, included a variable dose of alcohol (proportionate to the amount that each participant decided to self-administer), and all sampling time-points occurred under i.v. ghrelin (or i.v. placebo) plus alcohol. On the other hand, the IV-AC had a shorter duration, included a predetermined dose of alcohol (designed to achieve a target blood alcohol concentration), and had 1 sampling time-point (T1) that occurred under only i.v. ghrelin (or i.v. placebo) infusion, before alcohol administration began, hence providing an opportunity to relatively tease out the effects of “ghrelin” per se vs “ghrelin plus alcohol.” Nonetheless, our observations are consistent with previous work on endocrine response to exogenous ghrelin administration (for review, see Garin et al., 2013b) and provide novel information among a clinically relevant population (i.e., heavy-drinking individuals with alcohol dependence) and in the context of alcohol use, which is also a major modulator of peripheral and central endocrine pathways.
A large body of preclinical and clinical evidence indicates that peripheral leptin levels are associated with biobehavioral correlates of alcohol craving, use, and withdrawal (Bach et al., 2020). Results of the present study are comparable with our previous finding of exogenous ghrelin-induced reduction in circulating leptin levels in heavy-drinking individuals with alcohol dependence in a cue-reactivity study without actual alcohol administration (Haass-Koffler et al., 2015). Ghrelin and leptin have inverse functions in relation to appetite, food intake, metabolism, and alcohol use, and it has been suggested that leptin negatively mediates peripheral ghrelin levels (Klok et al., 2007; Nogueiras et al., 2008). It is important to note that although leptin levels were lower under ghrelin, compared to placebo, during both IV-ASA and IV-AC experiments (Figures 2A and 3A), the difference did not reach statistical significance for the IV-AC experiment. In general, due to inherent limitations of the present study (e.g., small sample size, inter-individual variability, secondary nature of the analyses), we encourage the readers to focus more on the pattern and direction of hormonal changes (depicted in Figures 2–4) rather than pure statistical results (presented in Tables 2–5). This approach is consistent with the overall goal of the present study, that is, to evaluate how these hormones “behave” in response to and under i.v. ghrelin, compared to placebo, in the context of alcohol use.
Akin to the data presented here (Figures 2B and 3B), studies in both rodents and humans have observed an increase in GLP-1 concentrations following ghrelin administration (Tong et al., 2016; Lindqvist et al., 2017). GLP-1 is an incretin produced mainly by L-cells of the intestinal mucosa and plays a key role in regulating food intake and glucose homeostasis (Müller et al., 2019). While some studies suggest that ghrelin’s effect on GLP-1 production and secretion might be driven by direct actions on intestinal L-cells, others do not confirm such a direct interaction (Jepsen et al., 2019). It appears that the crosstalk between insulinostatic ghrelin and insulinotropic GLP-1 is largely mediated through indirect glucose-dependent mechanisms (Djurhuus et al., 2002; Damdindorj et al., 2012; Page et al., 2018), although the exact molecular mechanism remains unknown. Preclinical studies have suggested that ghrelin and GLP-1 signaling may overlap in the nucleus accumbens to regulate alcohol intake and reward (Abtahi et al., 2018). Therefore, targeting these endocrine pathways offers potential novel treatment options for individuals with AUD (Farokhnia et al., 2019b; Jerlhag, 2020). In a previous cue-reactivity study with i.v. ghrelin, we found different results for insulin and GLP-1. Specifically, i.v. ghrelin reduced blood insulin levels without significantly affecting GLP-1 levels (Haass-Koffler et al., 2016). Together, these findings suggest that unlike other hormones investigated here (e.g., leptin, cortisol, and aldosterone, for which we did replicate our previous findings), the crosstalk between ghrelin and hormones such as insulin and GLP-1 may be more subject to variability and sensitive to different experimental conditions and settings. For example, in the previous study, there was no alcohol coadministration, fasting conditions differed, and the overall design was quite different.
Exogenous ghrelin administration, compared to placebo, had a robust effect on PP levels in this study. PP is a 36-amino acid polypeptide produced by PP cells of pancreatic islets (islets of Langerhans). PP is involved in self-regulation of endocrine and exocrine functions of the pancreas and acts primarily as an anorexigenic hormone by suppressing food intake, delaying gastric emptying, and increasing energy expenditure (Lonovics et al., 1981). PP has been suggested to be a biomarker of vagal activity (Schwartz, 1983b). While alcohol has been shown to decrease PP (Sehested et al., 1998), ghrelin has been found to increase PP levels (Arosio et al., 2003). In this study, it is likely that i.v. ghrelin administration initially increased serum PP levels, but this stimulatory effect was blunted by administration of alcohol. This interpretation is supported by comparing the PP results during the IV-ASA vs IV-AC experiments, as the variation in timing of i.v. ghrelin and alcohol administration between the studies shows a distinct stimulatory effect of ghrelin and inhibitory effect of alcohol on PP. In other words, the interval between T0 and T1 of the IV-AC experiment, when only i.v. ghrelin (and no i.v. alcohol) is on board, revealed a clear separation where i.v. ghrelin increased PP concentrations (Figure 3C). Consistent with this observation, during the IV-ASA experiment, which did not include an alcohol-free blood sampling time-point, exogenous ghrelin administration, compared to placebo, clearly blunted the alcohol-induced reduction in PP concentrations, which even resulted in a significant drug × time-point interaction (Figure 2C). Given that the secretion of PP is tightly regulated by vagal cholinergic mechanisms (Schwartz, 1983a) and ghrelin receptors are widely expressed in the vagal afferent neurons (Date, 2012), changes in PP concentrations in response to exogenous ghrelin administration can be interpreted as a proxy of GHS-R1a activity on vagal dendrites in the periphery. Accordingly, peripheral activation of vagal GHS-R1a has been proposed as a main route carrying the ghrelin signal to the CNS (Dockray, 2013; Date, 2014; Tamboli et al., 2017). Of note, we previously reported that exogenous ghrelin administration significantly reduced systolic and diastolic blood pressure in this (Farokhnia et al., 2018) and previous (Leggio et al., 2014) work, which is consistent with other reports on GHSR1a-dependent autonomic activity of ghrelin (Garin et al., 2013a; Zhang et al., 2017).
Consistent with our findings, several preclinical and clinical studies have found that ghrelin stimulates the production and/or secretion of cortisol, aldosterone, and prolactin, leading to increased concentrations of these hormones in the periphery (Arvat et al., 2001; Vestergaard et al., 2007; Messini et al., 2011; Zhang et al., 2017; Haass-Koffler et al., 2019; Akalu et al., 2020). Ghrelin-knockout models in rodents have identified the centrally projecting Edinger-Wesphal nucleus, specifically urocortin 1 neurons, as a link between GHS-R1a activation and corticosterone response (Spencer et al., 2012). Furthermore, GHS-R1a is expressed throughout the CNS, most notably in the hypothalamus pituitary unit, and ghrelin has been found to increase corticotropin-releasing factor mRNA in hypothalamic 4b cells in vitro (Gnanapavan et al., 2002; Shuto et al., 2002; Kageyama et al., 2012). The higher concentrations of aldosterone under i.v. ghrelin, compared to placebo, observed in this study (Figures 2I and 3I) are consistent with previous clinical (Zhang et al., 2017; Haass-Koffler et al., 2019) and preclinical (Andreis et al., 2003; Milosević et al., 2010; Rucinski et al., 2012) reports that ghrelin-induced activation of the HPA axis extends to both glucocorticoids and mineralocorticoids and that manipulation of the ghrelin system has a global stimulatory effect on the adrenal cortex. The precise mechanism of ghrelin-induced increases in prolactin levels remains unclear, although some evidence suggests a mechanistic pairing of GHS-R1a activity and prolactin secretion (Rubinfeld et al., 2004). Together, the established GH response, adrenocorticotropic hormone secretion, and evidence of prolactin release point to ghrelin as a strong pituitary releasing agent. Of note, prolactin has been suggested to be a neuromodulator of extrahypothalamic dopaminergic activity (Hernández et al., 1994), and alcohol has also been found to modulate peripheral prolactin levels (Frias et al., 2000).
The present study had several strengths and limitations. The sample had a relatively small size and was limited to heavy-drinking individuals with alcohol dependence. The strict inclusionary and exclusionary criteria for enrollment (supplementary Appendix 1) resulted in a homogenous sample of participants, thus reducing random variability in our measures and analyses. However, this factor limits the generalizability of our findings. As a human laboratory study, the experimental settings (e.g., drug dosage, meals, blood sampling time-points) were strictly controlled before and during each experiment. Although such a design provides a rigorous research platform, it may not reflect a real-world condition and the results may not be generalizable to other settings. Two different, yet complementary, i.v. alcohol administration paradigms (IV-ASA and IV-AC) were employed, and the results were internally replicated with some statistical differences across the 2 paradigms. Application of i.v. alcohol minimizes variation in alcohol pharmacokinetics and is therefore suitable for such studies, but this route bypasses the gastrointestinal tract and the hepatic first pass metabolism, factors that may influence at least some of the neuroendocrine outcomes investigated here. Given the secondary nature of this study, inter-individual variability, and small sample size, we were not able to analyze possible clinical/behavioral correlates of the observed neuroendocrine effects in response to exogenous ghrelin administration—a relevant question that should be investigated in future studies with a larger sample and an a priori design for this purpose. Another limitation is that glucose levels were not measured during the experiments. Given that ghrelin plays an important role in glucose regulation, whether the effects of exogenous ghrelin on glucose-regulating hormones (e.g., GLP-1) are dependent on, or independent of, glucose levels remains unanswered. Finally, we had a placebo session to be compared to the i.v. ghrelin condition, but i.v. alcohol was not matched with a control and participants received alcohol during all sessions, because of the design of the parent study. The effects of the supraphysiological challenge with ghrelin on neuroendocrine outcomes appeared to surpass the effects of alcohol, at least with the doses used in this study. That said, disentangling the effects of ghrelin vs alcohol would require a fully factorial 2 (i.v. ghrelin vs placebo) × 2 (i.v. alcohol vs control) design.
In conclusion, the findings of the present study, gathered from a clinically relevant sample of heavy-drinking individuals with alcohol dependence, provide a deeper insight into the complex interplay between ghrelin and appetitive, metabolic, and stress-related endocrine pathways in the context of alcohol use. More studies are required to understand the mechanisms underlying these effects and their potential direct and/or indirect relevance to alcohol-related behaviors.
Supplementary Material
Acknowledgments
We thank the clinical and research staff involved in patient care, data collection/analysis, and technical support in the joint National Institute on Drug Abuse (NIDA)/National Institute on Alcohol Abuse and Alcoholism (NIAAA) Clinical Psychoneuroendocrinology and Neuropsychopharmacology Section, in the NIAAA clinical program of the Division of Intramural Clinical and Biological Research (DICBR), at the National Institutes of Health (NIH) Clinical Center (Departments of Nursing, Nutrition, and Pharmacy), and in the Clinical Pharmacokinetics Research Laboratory at the University of Rhode Island. We also thank Dr Vijay Ramchandani (Section on Human Psychopharmacology, NIAAA DICBR) and Dr Reza Momenan (Clinical NeuroImaging Research Core, NIAAA DICBR) for their support in the execution of the parent study, Dr Xiaobai Li (Biostatistics and Clinical Epidemiology Service, NIH Clinical Center) for statistical support and consultation, and Ms Lauren Brick (Visual Media, Administrative Management Branch, NIDA Intramural Research Program [IRP]) for graphical design. The authors also express their gratitude to the participants who took part in this study. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
This work was supported by NIH intramural funding ZIA-DA000635 and ZIA-AA000218 (PI: Lorenzo Leggio), jointly funded by the IRP of the NIDA and the DICBR of the NIAAA. The development of the Computerized Alcohol Infusion System (CAIS) software was supported by Dr Vijay Ramchandani’s Section on Human Psychopharmacology in the NIAAA DICBR and by the NIAAA-funded Indiana Alcohol Research Center (AA007611). Dr Carolina Haass-Koffler is supported by the NIAAA (K01 AA023867; R01 AA026589; R01 AA027760; R21 AA027614) and by the National Institute of General Medical Sciences (NIGMS), Center of Biomedical Research Excellence (COBRE, P20 GM130414).
Statement of Interest
The authors report no biomedical financial interests or potential conflicts of interest. Dr Leggio is a federal employee; outside his federal work, he receives royalties from Rutledge for a textbook and an honorarium from the UK Medical Council on Alcohol for serving as editor-in-chief of Alcohol and Alcoholism.
References
- Abtahi S, Howell E, Currie PJ (2018) Accumbal ghrelin and glucagon-like peptide 1 signaling in alcohol reward in female rats. Neuroreport 29:1046–1053. [DOI] [PubMed] [Google Scholar]
- Akalu Y, Molla MD, Dessie G, Ayelign B (2020) Physiological effect of ghrelin on body systems. Int J Endocrinol 2020:1385138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreis PG, Malendowicz LK, Trejter M, Neri G, Spinazzi R, Rossi GP, Nussdorfer GG (2003) Ghrelin and growth hormone secretagogue receptor are expressed in the rat adrenal cortex: evidence that ghrelin stimulates the growth, but not the secretory activity of adrenal cells. FEBS Lett 536:173–179. [DOI] [PubMed] [Google Scholar]
- Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M (2003) Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab 88:701–704. [DOI] [PubMed] [Google Scholar]
- Arvat E, Maccario M, Di Vito L, Broglio F, Benso A, Gottero C, Papotti M, Muccioli G, Dieguez C, Casanueva FF, Deghenghi R, Camanni F, Ghigo E (2001) Endocrine activities of ghrelin, a natural growth hormone secretagogue (GHS), in humans: comparison and interactions with hexarelin, a nonnatural peptidyl GHS, and GH-releasing hormone. J Clin Endocrinol Metab 86:1169–1174. [DOI] [PubMed] [Google Scholar]
- Bach P, Koopmann A, Kiefer F (2020) The impact of appetite-regulating neuropeptide leptin on alcohol use, alcohol craving and addictive behavior: a systematic review of preclinical and clinical data. Alcohol:agaa044. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- Banks WA (2012) Role of the blood-brain barrier in the evolution of feeding and cognition. Ann N Y Acad Sci 1264:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaine SK, Sinha R (2017) Alcohol, stress, and glucocorticoids: from risk to dependence and relapse in alcohol use disorders. Neuropharmacology 122:136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco-Gandía MC, Miñarro J, Rodríguez-Arias M (2020) Common neural mechanisms of palatable food intake and drug abuse: knowledge obtained with animal models. Curr Pharm Des 26:2372–2384. [DOI] [PubMed] [Google Scholar]
- Cyders MA, Plawecki MH, Corbin W, King A, McCarthy DM, Ramchandani VA, Weafer J, O’Connor SJ (2020) To infuse or ingest in human laboratory alcohol research. Alcohol Clin Exp Res 44:764–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damdindorj B, Dezaki K, Kurashina T, Sone H, Rita R, Kakei M, Yada T (2012) Exogenous and endogenous ghrelin counteracts GLP-1 action to stimulate cAMP signaling and insulin secretion in islet β-cells. FEBS Lett 586:2555–2562. [DOI] [PubMed] [Google Scholar]
- Date Y (2012) Ghrelin and the vagus nerve. Methods Enzymol 514:261–269. [DOI] [PubMed] [Google Scholar]
- Date Y (2014) The vagus nerve and ghrelin function. In: Central functions of the ghrelin receptor (Portelli J, Smolders I, eds), pp 53–61. New York, NY: Springer New York. [Google Scholar]
- Djurhuus CB, Hansen TK, Gravholt C, Ørskov L, Hosoda H, Kangawa K, Jørgensen JO, Holst JJ, Schmitz O (2002) Circulating levels of ghrelin and GLP-1 are inversely related during glucose ingestion. Horm Metab Res 34:411–413. [DOI] [PubMed] [Google Scholar]
- Dockray GJ (2013) Enteroendocrine cell signalling via the vagus nerve. Curr Opin Pharmacol 13:954–958. [DOI] [PubMed] [Google Scholar]
- Engel JA, Jerlhag E (2014) Role of appetite-regulating peptides in the pathophysiology of addiction: implications for pharmacotherapy. CNS Drugs 28:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Grodin EN, Lee MR, Oot EN, Blackburn AN, Stangl BL, Schwandt ML, Farinelli LA, Momenan R, Ramchandani VA, Leggio L (2018) Exogenous ghrelin administration increases alcohol self-administration and modulates brain functional activity in heavy-drinking alcohol-dependent individuals. Mol Psychiatry 23:2029–2038. [DOI] [PubMed] [Google Scholar]
- Farokhnia M, Browning BD, Leggio L (2019a) Prospects for pharmacotherapies to treat alcohol use disorder: an update on recent human studies. Curr Opin Psychiatry 32:255–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhnia M, Faulkner ML, Piacentino D, Lee MR, Leggio L (2019b) Ghrelin: from a gut hormone to a potential therapeutic target for alcohol use disorder. Physiol Behav 204:49–57. [DOI] [PubMed] [Google Scholar]
- Frias J, Rodriguez R, Torres JM, Ruiz E, Ortega E (2000) Effects of acute alcohol intoxication on pituitary-gonadal axis hormones, pituitary-adrenal axis hormones, beta-endorphin and prolactin in human adolescents of both sexes. Life Sci 67:1081–1086. [DOI] [PubMed] [Google Scholar]
- Gahete MD, Rincón-Fernández D, Villa-Osaba A, Hormaechea-Agulla D, Ibáñez-Costa A, Martínez-Fuentes AJ, Gracia-Navarro F, Castaño JP, Luque RM (2014) Ghrelin gene products, receptors, and GOAT enzyme: biological and pathophysiological insight. J Endocrinol 220:R1–24. [DOI] [PubMed] [Google Scholar]
- Garin MC, Burns CM, Kaul S, Cappola AR (2013a) Clinical review: the human experience with ghrelin administration. J Clin Endocrinol Metab 98:1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garin MC, Burns CM, Kaul S, Cappola AR (2013b) The human experience with ghrelin administration. J Clin Endocrinol Metab 98:1826–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, Bhattacharya S, Carpenter R, Grossman AB, Korbonits M (2002) The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab 87:2988. [DOI] [PubMed] [Google Scholar]
- Godlewski G, Cinar R, Coffey NJ, Liu J, Jourdan T, Mukhopadhyay B, Chedester L, Liu Z, Osei-Hyiaman D, Iyer MR, Park JK, Smith RG, Iwakura H, Kunos G (2019) Targeting peripheral CB1 receptors reduces ethanol intake via a gut-brain axis. Cell Metab 29:1320–1333.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Saha TD, Ruan WJ, Goldstein RB, Chou SP, Jung J, Zhang H, Smith SM, Pickering RP, Huang B, Hasin DS (2016) Epidemiology of DSM-5 drug use disorder: results from the national epidemiologic survey on alcohol and related conditions-III. JAMA Psychiatry 73:39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SM, Page LC, Tong J (2019) Ghrelin regulation of glucose metabolism. J Neuroendocrinol 31:e12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Aoun EG, Swift RM, de la Monte SM, Kenna GA, Leggio L (2015) Leptin levels are reduced by intravenous ghrelin administration and correlated with cue-induced alcohol craving. Transl Psychiatry 5:e646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Giovenco DE, Lee MR, Zywiak WH, de la Monte SM, Kenna GA, Swift RM, Leggio L (2016) Serum insulin levels are reduced by intravenous ghrelin administration but do not correlate with alcohol craving in alcohol-dependent individuals. Int J Neuropsychopharmacol 19:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass-Koffler CL, Long VM, Farokhnia M, Magill M, Kenna GA, Swift RM, Leggio L (2019) Intravenous administration of ghrelin increases serum cortisol and aldosterone concentrations in heavy-drinking alcohol-dependent individuals: results from a double-blind, placebo-controlled human laboratory study. Neuropharmacology 158:107711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández ML, Fernández-Ruiz JJ, Navarro M, de Miguel R, Cebeira M, Vaticón L, Ramos JA (1994) Modifications of mesolimbic and nigrostriatal dopaminergic activities after intracerebroventricular administration of prolactin. J Neural Transm Gen Sect 96:63–79. [DOI] [PubMed] [Google Scholar]
- Jepsen SL, Vestergaard ET, Larraufie P, Gribble FM, Reimann F, Jørgensen JOL, Holst JJ, Kuhre RE (2019) Ghrelin does not directly stimulate secretion of glucagon-like peptide-1. J Clin Endocrinol Metab 105:266–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerlhag E (2020) Alcohol-mediated behaviours and the gut-brain axis; with focus on glucagon-like peptide-1. Brain Res 1727:146562. [DOI] [PubMed] [Google Scholar]
- Jerlhag E, Egecioglu E, Landgren S, Salomé N, Heilig M, Moechars D, Datta R, Perrissoud D, Dickson SL, Engel JA (2009) Requirement of central ghrelin signaling for alcohol reward. Proc Natl Acad Sci U S A 106:11318–11323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas DE, Amick HR, Feltner C, Bobashev G, Thomas K, Wines R, Kim MM, Shanahan E, Gass CE, Rowe CJ, Garbutt JC (2014) Pharmacotherapy for adults with alcohol use disorders in outpatient settings: a systematic review and meta-analysis. JAMA 311:1889–1900. [DOI] [PubMed] [Google Scholar]
- Kageyama K, Akimoto K, Yamagata S, Sugiyama A, Murasawa S, Watanuki Y, Tamasawa N, Suda T (2012) Dexamethasone stimulates the expression of ghrelin and its receptor in rat hypothalamic 4B cells. Regul Pept 174:12–17. [DOI] [PubMed] [Google Scholar]
- Keizer RJ, Jansen RS, Rosing H, Thijssen B, Beijnen JH, Schellens JH, Huitema AD (2015) Incorporation of concentration data below the limit of quantification in population pharmacokinetic analyses. Pharmacol Res Perspect 3:e00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klok MD, Jakobsdottir S, Drent ML (2007) The role of leptin and ghrelin in the regulation of food intake and body weight in humans: a review. Obes Rev 8:21–34. [DOI] [PubMed] [Google Scholar]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402:656–660. [DOI] [PubMed] [Google Scholar]
- Koob GF (2010) The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res 1314:3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Buck CL, Cohen A, Edwards S, Park PE, Schlosburg JE, Schmeichel B, Vendruscolo LF, Wade CL, Whitfield TW Jr, George O (2014) Addiction as a stress surfeit disorder. Neuropharmacology 76 Pt B:370–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND (2016) Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 3:760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MR, Tapocik JD, Ghareeb M, Schwandt ML, Dias AA, Le AN, Cobbina E, Farinelli LA, Bouhlal S, Farokhnia M, Heilig M, Akhlaghi F, Leggio L (2020) The novel ghrelin receptor inverse agonist PF-5190457 administered with alcohol: preclinical safety experiments and a phase 1b human laboratory study. Mol Psychiatry 25:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leggio L, Zywiak WH, Fricchione SR, Edwards SM, de la Monte SM, Swift RM, Kenna GA (2014) Intravenous ghrelin administration increases alcohol craving in alcohol-dependent heavy drinkers: a preliminary investigation. Biol Psychiatry 76:734–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Shcherbina L, Fischer AT, Wierup N (2017) Ghrelin is a regulator of glucagon-like peptide 1 secretion and transcription in mice. Front Endocrinol 8:135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonovics J, Devitt P, Watson LC, Rayford PL, Thompson JC (1981) Pancreatic polypeptide. A review. Arch Surg 116:1256–1264. [DOI] [PubMed] [Google Scholar]
- Lv Y, Liang T, Wang G, Li Z (2018) Ghrelin, a gastrointestinal hormone, regulates energy balance and lipid metabolism. Biosci Rep 38: BSR20181061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani BK, Zigman JM (2017) Ghrelin as a survival hormone. Trends Endocrinol Metab 28:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messini CI, Dafopoulos K, Chalvatzas N, Georgoulias P, Anifandis G, Messinis IE (2011) Effect of ghrelin and metoclopramide on prolactin secretion in normal women. J Endocrinol Invest 34:276–279. [DOI] [PubMed] [Google Scholar]
- Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA (2014) A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Mol Psychiatry 19:1284–1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milosević VLj, Stevanović DM, Nesić DM, Sosić-Jurjević BT, Ajdzanović VZ, Starcević VP, Severs WB (2010) Central effects of ghrelin on the adrenal cortex: a morphological and hormonal study. Gen Physiol Biophys 29:194–202. [PubMed] [Google Scholar]
- Morris LS, Voon V, Leggio L (2018) Stress, motivation, and the gut-brain axis: a focus on the ghrelin system and alcohol use disorder. Alcohol Clin Exp Res 24:1378–1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller TD, et al. (2019) Glucagon-like peptide 1 (GLP-1). Mol Metab 30:72–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueiras R, Tschöp MH, Zigman JM (2008) Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann N Y Acad Sci 1126:14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page LC, Gastaldelli A, Gray SM, D’Alessio DA, Tong J (2018) Interaction of GLP-1 and ghrelin on glucose tolerance in healthy humans. Diabetes 67:1976–1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perelló M, Zigman JM (2012) The role of ghrelin in reward-based eating. Biol Psychiatry 72:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan G, Samson SL, Sun Y (2013) Ghrelin: much more than a hunger hormone. Curr Opin Clin Nutr Metab Care 16:619–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Roche DJ, Heinzerling K, Shoptaw S (2014) Opportunities for the development of neuroimmune therapies in addiction. Int Rev Neurobiol 118:381–401. [DOI] [PubMed] [Google Scholar]
- Rehm J, Guiraud J, Poulnais R, Shield KD (2018) Alcohol dependence and very high risk level of alcohol consumption: a life-threatening and debilitating disease. Addict Biol 23:961–968. [DOI] [PubMed] [Google Scholar]
- Rubinfeld H, Hadani M, Taylor JE, Dong JZ, Comstock J, Shen Y, DeOliveira D, Datta R, Culler MD, Shimon I (2004) Novel ghrelin analogs with improved affinity for the GH secretagogue receptor stimulate GH and prolactin release from human pituitary cells. Eur J Endocrinol 151:787–795. [DOI] [PubMed] [Google Scholar]
- Rucinski M, Ziolkowska A, Szyszka M, Hochol A, Malendowicz LK (2012) Evidence suggesting that ghrelin O-acyl transferase inhibitor acts at the hypothalamus to inhibit hypothalamo-pituitary-adrenocortical axis function in the rat. Peptides 35:149–159. [DOI] [PubMed] [Google Scholar]
- Scheff JD, Almon RR, Dubois DC, Jusko WJ, Androulakis IP (2011) Assessment of pharmacologic area under the curve when baselines are variable. Pharm Res 28:1081–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz TW (1983a) Pancreatic polypeptide: a unique model for vagal control of endocrine systems. J Auton Nerv Syst 9:99–111. [DOI] [PubMed] [Google Scholar]
- Schwartz TW (1983b) Pancreatic polypeptide: a hormone under vagal control. Gastroenterology 85:1411–1425. [PubMed] [Google Scholar]
- Sehested J, Heringlake M, Schmidt V (1998) Neurohumoral cardiovascular responses to alcohol and their modulation by peroral fluid. Am J Cardiol 81:761–765. [DOI] [PubMed] [Google Scholar]
- Shuto Y, Shibasaki T, Otagiri A, Kuriyama H, Ohata H, Tamura H, Kamegai J, Sugihara H, Oikawa S, Wakabayashi I (2002) Hypothalamic growth hormone secretagogue receptor regulates growth hormone secretion, feeding, and adiposity. J Clin Invest 109:1429–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Xu L, Clarke MA, Lemus M, Reichenbach A, Geenen B, Kozicz T, Andrews ZB (2012) Ghrelin regulates the hypothalamic-pituitary-adrenal axis and restricts anxiety after acute stress. Biol Psychiatry 72:457–465. [DOI] [PubMed] [Google Scholar]
- Stone LA, Harmatz ES, Goosens KA (2020) Ghrelin as a stress hormone: implications for psychiatric illness. Biol Psychiatry 88:531–540. [DOI] [PubMed] [Google Scholar]
- Tamboli RA, Antoun J, Sidani RM, Clements A, Harmata EE, Marks-Shulman P, Gaylinn BD, Williams B, Clements RH, Albaugh VL, Abumrad NN (2017) Metabolic responses to exogenous ghrelin in obesity and early after Roux-en-Y gastric bypass in humans. Diabetes Obes Metab 19:1267–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temko JE, Bouhlal S, Farokhnia M, Lee MR, Cryan JF, Leggio L (2017) The microbiota, the gut and the brain in eating and alcohol use disorders: a ‘Ménage à Trois’? Alcohol 52:403–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Davis HW, Gastaldelli A, D’Alessio D (2016) Ghrelin impairs prandial glucose tolerance and insulin secretion in healthy humans despite increasing GLP-1. J Clin Endocrinol Metab 101:2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zessen R, van der Plasse G, Adan RA (2012) Contribution of the mesolimbic dopamine system in mediating the effects of leptin and ghrelin on feeding. Proc Nutr Soc 71:435–445. [DOI] [PubMed] [Google Scholar]
- Vestergaard ET, Hansen TK, Gormsen LC, Jakobsen P, Moller N, Christiansen JS, Jorgensen JO (2007) Constant intravenous ghrelin infusion in healthy young men: clinical pharmacokinetics and metabolic effects. Am J Physiol Endocrinol Metab 292:E1829–E1836. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Tomasi D, Baler R (2012) Food and drug reward: overlapping circuits in human obesity and addiction. Curr Top Behav Neurosci 11:1–24. [DOI] [PubMed] [Google Scholar]
- Yanagi S, Sato T, Kangawa K, Nakazato M (2018) The homeostatic force of ghrelin. Cell Metab 27:786–804. [DOI] [PubMed] [Google Scholar]
- Zallar LJ, Farokhnia M, Tunstall BJ, Vendruscolo LF, Leggio L (2017) The role of the ghrelin system in drug addiction. Int Rev Neurobiol 136:89–119. [DOI] [PubMed] [Google Scholar]
- Zhang CJ, Bidlingmaier M, Altaye M, Page LC, D’Alessio D, Tschöp MH, Tong J (2017) Acute administration of acyl, but not desacyl ghrelin, decreases blood pressure in healthy humans. Eur J Endocrinol 176:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kreek MJ (2014) Alcohol: a stimulant activating brain stress responsive systems with persistent neuroadaptation. Neuropharmacology 87:51–58. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.