Abstract
Accumulating evidence shows that certain populations of depressed patients have impaired hypothalamus-pituitary-adrenal (HPA) axis function. Arginine-vasopressin (AVP) is one of the primary factors in HPA axis regulation under stress situations, and AVP and its receptor subtype (V1B receptor) play a pivotal role in HPA axis abnormalities observed in depression. Based on this hypothesis, several non-peptide V1B receptor antagonists have been synthesized, and the efficacies of some V1B receptor antagonists have been investigated in both animals and humans. V1B receptor antagonists exert antidepressant-like effects in several animal models at doses that attenuate the hyperactivity of the HPA axis, and some of their detailed mechanisms have been delineated. These results obtained in animal models were, at least partly, reproduced in clinical trials. At least 2 V1B receptor antagonists (TS-121 and ABT-436) showed tendencies to reduce the depression scores of patients with major depressive disorder at doses that attenuate HPA axis hyperactivity or block the pituitary V1B receptor. Importantly, TS-121 showed a clearer efficacy for patients with higher basal cortisol levels than for those with lower basal cortisol levels, which was consistent with the hypothesis that V1B receptor antagonists may be more effective for patients with HPA axis hyperactivity. Therefore, V1B receptor antagonists are promising approaches for the treatment of depression involving HPA axis impairment such as depression.
Keywords: ABT-436, hypothalamus-pituitary-adrenal axis, SSR149415, TS-121, V1B receptor antagonist
Significance Statement.
Given that dysfunction of the hypothalamus-pituitary-adrenal (HPA) axis is observed in certain populations of depressed patients, the V1B receptor, a receptor subtype of arginine-vasopressin (AVP) that is deeply involved in the regulation of HPA axis, has gained attention as a promising target for the development of novel antidepressants. However, despite encouraging results for V1B receptor antagonists in rodents, the outcomes of clinical studies for the first V1B receptor antagonist, SSR149415, were not necessarily encouraging. However, 2 recent trials with new V1B receptor antagonists (ABT-436 and TS-121) have suggested that these antagonists are effective for the treatment of depressed patients with a highly active HPA axis at dose(s) that block the pituitary V1B receptor or attenuate HPA axis activity. Therefore, revisiting the HPA hypothesis of depression and reconsidering the utility of V1B receptor antagonists as a novel treatment for depression, particularly in patients with impaired HPA axis function, are appropriate.
Introduction
Major depressive disorder (MDD) is among the most disabling medical conditions, with a lifetime prevalence of approximately 20% of the US population (Hasin et al., 2018). All current antidepressant medications have stemmed from the study of mechanisms of serendipitously discovered agents that act on monoamine neurotransmissions. While the large majority of individuals (approximately 70%) with depression exhibit at least some improvement with antidepressant medication, approximately 30% of patients remain resistant to series of treatments (Rush et al., 2006; Trivedi et al., 2006). Moreover, for currently available antidepressants, about 3–6 weeks is required before the manifestation of a significant therapeutic effect. Therefore, the focus of drug discovery research has recently shifted from the currently prescribed monoamine-based antidepressants to non-monoamine-based agents. In March 2019, the US Food and Drug Administration approved 2 novel antidepressants (esketamine for treatment-resistant depression and brexanolone for postpartum depression) with mechanisms that differ from monoamine systems (Cristea and Naudet, 2019). Although these drugs represent breakthroughs for depression therapy, activities to find newer antidepressants with improved safety and compliance are ongoing (Chaki et al., 2006; Chaki, 2017).
Depression is a clinically heterogenous condition defined by several subtypes, the features of which may change over time within the same individual. These different symptom clusters can respond selectively to different treatments. Therefore, different pathophysiological processes are required to be operating in the different subtypes of depression. Based on this nature of depression, it is important to focus on pathophysiological events that differ from the ones targeted by current pharmacotherapies to discover and develop novel antidepressants.
Chronic dysfunction of the hypothalamus-pituitary-adrenal (HPA) axis is generally acknowledged to occur in a subset of MDD patients (Dinan and Scott, 2005; Stetler and Miller, 2011), and patients with treatment-resistant depression or severe depression tend to show HPA axis dysfunction (Juruena et al., 2009; Rosenblat et al., 2015; Nikkheslat et al., 2020). A sustained elevation of HPA activity is considered to be a causal factor in human affective disorders (Dinan, 1994), and irregularities include elevated serum and 24-hour urinary free cortisol, dexamethasone non-suppression (Carroll et al., 1981), a blunted release of adrenocorticotropic hormone (ACTH) to corticotropin-releasing hormone (CRH) challenge (von Bardeleben et al., 1988), and exaggerated ACTH and cortisol responses in a dexamethasone/CRH test (Ising et al., 2007). Moreover, successful pharmacological therapy has been linked to the normalization of HPA activity (Ising et al., 2005; Schüle, 2007). Therefore, dampening HPA axis hyperactivity has been hypothesized to be a potential avenue for the treatment of depression. However, clinical studies with compounds regulating the HPA axis have not been successful to date (Griebel and Holsboer, 2012; Menke, 2019). Among the components that regulate the HPA axis, arginine-vasopressin (AVP) and its receptor subtype have attracted attention. In this review, the role of the AVP system in the HPA axis dysfunction observed in depressed patients will first be summarized, followed by a summary of representative preclinical studies of antagonists of the V1B receptor, an AVP receptor subtype that is deeply involved in the regulation of the HPA axis by AVP. Finally, the potential of V1B receptor antagonists for the treatment of patients with MDD will be presented through a discussion of the clinical studies on V1B receptor antagonists conducted to date. In addition, the potential of V1B receptor antagonists for the treatment of anxiety disorders, which are commonly encountered in patients with MDD, will be briefly mentioned.
Role of AVP and V1B Receptor in the Regulation of the HPA Axis
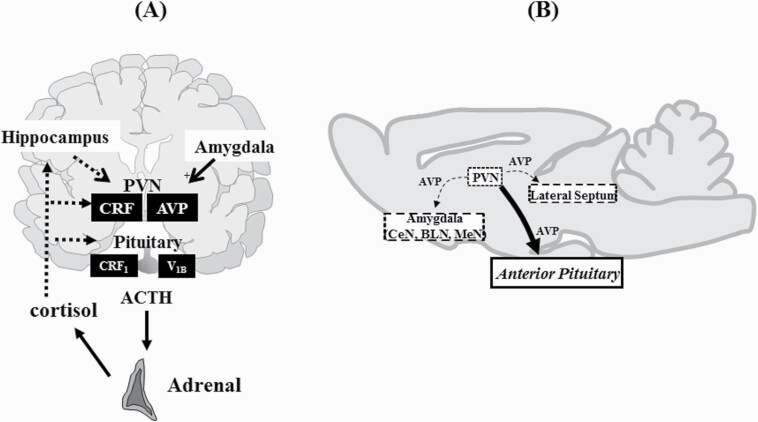
HPA activity is driven by the secretion of CRH from the hypothalamic paraventricular nucleus (PVN), which in turn stimulates the secretion of ACTH. AVP, in conjunction with CRH, is also a primary factor in the regulation of HPA axis activity (Aguilera and Rabadan-Diehl, 2000). AVP is a cyclic nonapeptide produced in the PVN and supraoptic nucleus of the hypothalamus and is released from the median eminence into the pituitary portal circulation, where it strongly potentiates the effects of CRH on ACTH release. AVP exerts its effects through 3 receptor subtypes (V1A, V1B, and V2 receptors), all of which are G-protein coupled receptors (Peter et al., 1995). Of these receptor subtypes, V1B receptor mRNA is expressed in the majority of anterior pituitary corticotrophs that secrete ACTH; therefore, it mediates the regulation of HPA axis activity by AVP. The V1B receptor is also expressed within the brain, particularly within the hypothalamus and limbic brain regions, which have been implicated in stress and emotions (Lolait et al., 1995; Vaccari et al., 1998; Hernando et al., 2001; Corbani et al., 2018). The regulation of the HPA axis by AVP and the extrahypothalamic V1B receptor are illustrated in Figure 1.
Figure 1.
Regulation of hypothalamus-pituitary-adrenal (HPA) axis by arginine-vasopressin (AVP) (A) and extrahypothalamic AVP system (B). (A) Both AVP and corticotropin-releasing factor (CRF) produced in the paraventricular nucleus (PVN) of the hypothalamus act on V1B and CRF1 receptors in the pituitary, respectively, to stimulate adrenocorticotropic hormone (ACTH) release from the anterior pituitary. In response to ACTH, the cortex of the adrenal glands produces cortisol (corticosterone in rodents), which penetrates the blood–brain barrier and exerts its actions in the brain through the high-affinity mineralocorticoid receptors and the low-affinity glucocorticoid receptors. Cortisol provides negative feedback at the limbic, hypothalamic, and pituitary level. In contrast, the amygdala enhances CRF production in the PVN. The AVP and V1B receptor systems are rather resistant to feedback mechanisms via cortisol. Under chronic stress, the proportion of AVP-containing neurons among the CRF neurons in the PVN increases. In addition, the pituitary V1B receptor is upregulated, while the pituitary CRF1 receptor is downregulated by chronic stress. (B) The V1B receptor is expressed in the brain, including the lateral septum and amygdaloid nuclei, and V1B receptors in these nuclei are postulated to play some roles in the regulation of emotion. BLN, basolateral nucleus; CeN, central nucleus; MeN, medial nucleus.
There is a hypothesis that AVP may play a more important role in the regulation of the HPA axis than CRH in chronic stress situations. Repeated stress markedly increases the proportion of AVP-containing neurons among the CRH neurons in the PVN (de Goeij et al., 1992) and increases V1B receptor expression in the pituitary (Rabadan-Diehl et al., 1995). The sensitivity of CRH and AVP to glucocorticoid feedback is markedly different; the mRNA expression of CRH and its receptor (CRH1) is reduced by elevated glucocorticoid levels (Zhou et al., 1996), whereas the mRNA levels of the V1B receptor and the coupling of the receptor to phospholipase C are stimulated by glucocorticoids (Aguilera and Rabadan-Diehl, 2000). These effects may contribute to the refractoriness of AVP-stimulated ACTH secretion to glucocorticoid feedback. Therefore, vasopressinergic regulation of the HPA axis is presumed to be critical for sustaining corticotroph responsiveness in the presence of high levels of circulating glucocorticoids during chronic stress. The predominant roles of the AVP-V1B receptor system in the regulation of the HPA axis in chronic stress are supported by the finding that the correlation between AVP and ACTH is stronger than between CRH and ACTH (Scott and Dinan, 1998). All these findings indicate a switch from a CRH system to an AVP system in the regulation of ACTH release during chronic stress, and V1B receptor antagonists might be an adequate approach for the treatment of psychiatric disorders involving chronically stressful conditions. On the other hand, it should be noted that more recent reports contradict the hypothesis that the AVP-V1B receptor system has a more important role in the regulation of HPA axis under chronic stress. For example, lower ACTH and corticosterone responses to noise stress were noted after repeated restrained stress in rats while vasopressinergic activity was increased, and a V1 receptor antagonist did not block increased ACTH and corticosterone induced by hypertonic saline in repeated chronic stress conditions (Chen et al., 2008). Moreover, AVP-deficient Brattleboro rats show normal HPA axis responses to stress (Makara et al., 2012). Although differences in experimental conditions need to be taken into account, role of the AVP-V1B receptor system in chronic stress remains to be thoroughly investigated.
Role of the AVP-V1B Receptor System in MDD
Several lines of evidence have implicated the AVP-V1B receptor system in depression. First, AVP levels are increased not only in plasma but also in brain nuclei (the PVN and supraoptic nucleus of the hypothalamus and the suprachiasmatic nucleus) in MDD patients (Purba et al., 1996; van Londen et al., 1997; Zhou et al., 2001; Meynen et al., 2006), indicating that AVP is overly activated in depressed patients, although a previous report showed no change in AVP levels in cerebrospinal fluid (CSF) (Heuser et al., 1998). Importantly, the increase in AVP is more pronounced in patients with melancholic-type depression or anxious-retarded depression (van Londen et al., 1997; De Winter et al., 2003; Meynen et al., 2006), which is in line with the reported HPA axis dysfunctions in these patients. In addition, treatment with fluoxetine decreases the AVP levels in the CSF of depressed patients, which is accompanied by a decrease in the depression scores (De Bellis et al., 1993). Moreover, the plasma AVP level is reportedly correlated with cortisol levels during depression in a positive manner, particularly in suicide victims (Inder et al., 1997). There are reports indicating the hyperactivity of the V1B receptor in MDD patients. In patients with MDD, HPA responsiveness to AVP is increased while responsiveness to CRH is decreased (O’Keane et al., 2012), and the administration of the AVP analog desmopressin to patients with MDD induces an augmented ACTH and cortisol secretion compared with that in non-depressed patients (Dinan et al., 2004). These findings suggest that the anterior vasopressin receptors (V1B receptors) are more sensitive to AVP in depressed patients than in healthy individuals. Moreover, a single nucleotide polymorphism of the V1B receptor has been found to protect against major depression (van West et al., 2004). These above-mentioned lines of evidence indicate that hyperactivity of the AVP-V1B receptor system is mainly responsible for the dysfunction of the HPA axis observed in patients with MDD.
In addition to patients with MDD, increased AVP levels have been observed in patients with several other psychiatric disorders. The plasma AVP levels of patients with post-traumatic stress disorder (PTSD) are reportedly higher than those in both healthy controls and traumatic controls (de Kloet et al., 2008); therefore, elevated plasma AVP levels are specifically related to PTSD and not to exposure to traumatic stress. Moreover, the AVP levels in the CSF are increased in patients with either obsessive-compulsive disorder (Altemus et al., 1992) or bulimia nervosa (Demitrack et al., 1992). Although AVP release is reportedly correlated with anxiety symptom responses in healthy individuals challenged with an anxiogenic CCK-B agonist (Abelson et al., 2001), the role of AVP in panic symptoms remains to be clarified, since increased AVP levels were observed by inducers of panic symptoms in both patients with panic disorder and healthy volunteers who did not experience panic symptoms (Peskind et al., 1998).
In addition to clinical studies, animal studies have also shown a correlation between depressive- and anxiety-like behaviors and elevated AVP levels. The Brattleboro rat strain, which displays a spontaneous AVP deficiency stemming from a single nucleotide deletion in the AVP gene, exhibits reduced depressive- and anxiety-like behaviors (Mlynarik et al., 2007; Varga et al., 2015). In contrast, among Wistar rats that have been selectively bred for high or low anxiety-like behavior, the high anxiety-like behavior lines exhibit higher AVP expressions in the PVN of the hypothalamus than the low anxiety-like behavior lines, accompanied by elevated HPA activations in response to stress and a dexamethasone/CRH challenge (Keck et al., 2002). Therefore, the relationship between increased AVP levels and depressive- and anxiety-like behaviors is underpinned by the behaviors of genetic animal models.
Pharmacology of V1B Receptor Antagonists
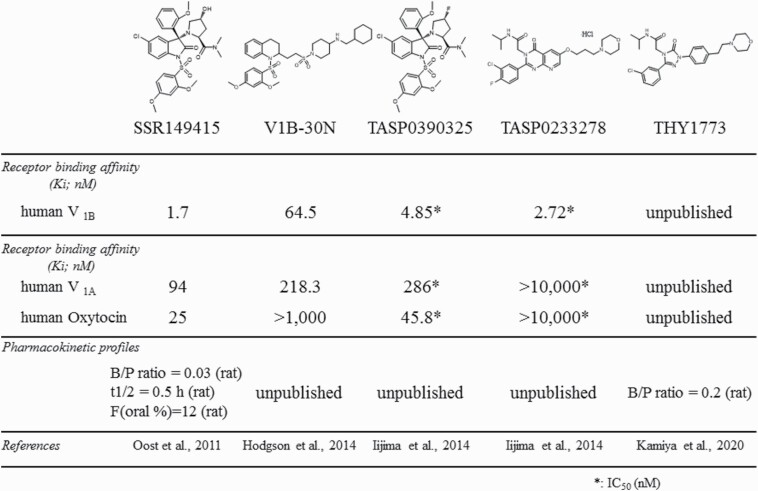
To date, several selective and potent non-peptide V1B receptor antagonists have been synthesized, and their antidepressant potential has been tested in animal models. The chemical structures and some of their profiles of representative V1B receptor antagonists are illustrated in Figure 2.
Figure 2.
Chemical structures and profiles of representative V1B receptor antagonists.
SSR149415 was the first V1B receptor antagonist that could be administered systemically and was used for pharmacological studies in animals (Serradeil-Le Gal et al., 2002). However, SSR149415 had some drawbacks, including receptor selectivity (active against V1A and oxytocin receptors as well; Griffante et al., 2005) and a suboptimal pharmacokinetic profile in rats (extensive first-pass metabolism and low brain penetration [brain/plasma ratio = 0.03], etc.) (Oost et al., 2011). To overcome the drawbacks of SSR149415, 2 approaches were taken: further optimization of the scaffold of SSR149415 and searches for a different scaffold. Using the former approach, compounds with improved pharmacokinetic profiles (oral bioavailability, half-life, and brain penetration) have been successfully synthesized, representatives of which exerted antidepressant-like effects in the forced swimming test (Oost et al., 2011; Geneste et al., 2018). The other approach was to identify a novel scaffold using a high-throughput screening and hit-to-lead (Letourneau et al., 2010), followed by optimization of the lead. These efforts successfully produced several compounds with improved pharmacokinetic profiles, including a lack of CYP enzymes inhibition (Napier et al., 2011a, 2011b). In addition, the compounds produced using this approach had improved selectivity with negligible activity at V1A, V2, and oxytocin receptors as well as a broad panel of unrelated targets (Napier et al., 2011a, 2011b). Moreover, the representative compounds were demonstrated to attenuate increases in ACTH secretion induced by CRH/desmopressin.
Antidepressant-like Effects and underlying Mechanisms of V1B Receptor Antagonists in Rodents
The antidepressant-like effects of representative V1B receptor antagonists are summarized in Table 1. The antidepressant-like effects of V1B receptor antagonists in animal models were first demonstrated using SSR149415, a prototype V1B receptor antagonist (Serradeil-Le Gal et al., 2002). SSR149415 exerted antidepressant-like effects in several animal models, and these studies have been replicated using other V1B receptor antagonists (Table 1). Importantly, the antidepressant-like effects of V1B receptor antagonists occurred at doses capable of attenuating the increase in plasma ACTH induced by stress or CRH/desmopressin, suggesting that V1B receptor antagonists exerted their antidepressant-like effects by inhibiting HPA axis activated by acute stress. V1B receptor antagonists exerted antidepressant-like effects not only in animal models used to evaluate conventional antidepressants but also in an animal model resistant to conventional antidepressants (Iijima et al., 2014; Kamiya et al., 2020), as has been observed for ketamine treatment (Koike et al., 2013). On the other hand, V1B receptor antagonists might not have a rapid onset of action. In an olfactory bulbectomy model, SSR149415 exerted antidepressant-like effects after repeated administrations (7, 14, or 28 days), but not after acute administration (Iijima and Chaki, 2007; Breuer et al., 2009; Poretti et al., 2016). In addition, in a chronic mild stress model, SSR149415 exerted antidepressant-like effects after 7 or 14 days of treatment, similar to conventional antidepressants (Griebel et al., 2002; Alonso et al., 2004; Surget et al., 2008; Bessa et al., 2009). The time course for the antidepressant-like effects is consistent with another V1B receptor antagonist, TASP039025, which required 2 weeks of treatment before noticeable effects in an olfactory bulbectomy model (Iijima et al., 2014). Nonetheless, it is important to note that the antidepressant-like effects of SSR149415 lasted for at least a week after the cessation of treatment (Breuer et al., 2009), suggesting that changes in neuroplasticity may play a role in the actions of V1B receptor antagonists.
Table 1.
Effect of V1B receptor antagonists in animals (antidepressant-like effects)
| Compound | Model | Test | Species/strain | Dose (route) | Results | Reference |
|---|---|---|---|---|---|---|
| SSR149415 | – | FST | Wistar rat | 3, 10, 30 mg/kg (PO) | Decrease immobility | Griebel et al., 2002 |
| UCMS | Physical state, EPM, FST, etc. | CD-1 mouse | 10, 30 mg/kg (IP) | Reverse decreased physical state scale after 2 wk dosing (reverse anxiety- and depressive-like behaviors) | ||
| – | FST | Flinders Sensitive Line rat | 3, 10, 30 mg/kg (IP, dosing for 14 d) | Reverse increased immobility | Overstreet and Griebel, 2005 | |
| – | DRL-72s | Wistar rat | 3, 10, 30 mg/kg (IP) | Increase percentage of lever presses emitted in the IRT bin (inter-response time) | Louis et al., 2006 | |
| Olfactory bulbectomy | Hyperemotionality | Wistar rat | 10, 30 mg/kg (PO, dosing for 14 d) | Reverse increased hyperemotionality (no effect after a single dosing) | Iijima and Chaki, 2007 | |
| Olfactory bulbectomy | Hyperemotionality | Sprague-Dawley rat | 10, 30 mg/kg (IP, dosing for 7 or 14 d) | Reverse increased hyperactivity (no effect after a single dosing) | Breuer et al., 2009 | |
| Olfactory bulbectomy | TST | N:NIH mouse | 30 mg/kg (PO, dosing for 28 d) | Reverse increased immobility | Poretti et al., 2016 | |
| – | FST | CD rat | 3, 10, 30 mg/kg (IP) | No effect | Hodgson et al., 2007 | |
| – | FST | CD-1 mouse | 3, 10, 30 mg/kg (IP) | No effect | ||
| – | TST | CD-1 mouse | 3, 10, 30 mg/kg (IP) | Increase immobility | ||
| UCMS | Physical state | BALB/c mouse | 30 mg/kg (IP, dosing for 4 wk) | Reverse decreased physical state scale after 1 week dosing | Alonso et al., 2004 | |
| UCMS | Physical state, splash test, NSFT | BALB/c mouse | 30 mg/kg (IP, dosing for 3 wk) | Reverse decreased physical state scale after 2 wk dosing (reverse decreased grooming frequency and increased latency to feed) | Surget et al., 2008 | |
| UCMS | FST, SPT, NSFT | Wistar rat | 30 mg/kg (IP, dosing for 14 d) | Reverse increased immobility, reduced sucrose preference, and increased latency to feed | Bessa et al., 2009 | |
| – | FST | Sprague-Dawley rat | 1, 10, 100 ng (intra-septum) | Decrease immobility | Stemmelin et al., 2005 | |
| – | FST | Sprague-Dawley rat | 0.1, 1, 10 ng (intra-BIA) 0.1, 1, 10, 100 ng (intra-CeA) 10, 100 ng (intra-MeA) | Decrease immobility | Salomé et al., 2006 | |
| TASP0233278 | – | FST | Sprague-Dawley rat | 0.3, 1, 3 mg/kg (PO) | Decrease immobility | Iijima et al., 2014 |
| Corticosterone treated | FST | Sprague-Dawley rat | 3 mg/kg (PO) | Reverse increased immobility | ||
| TASP0390325 | – | FST | Sprague-Dawley rat | 0.1, 0.3, 1 mg/kg (PO) | Decrease immobility | |
| Olfactory bulbectomy | Hyperemotionality | Wistar rat | 0.1, 0.3 mg/kg (PO, dosing for 14 days) | Reverse increased hyperemotionality (no effect after a single dosing) | ||
| V1B-30N | – | FST | CD rat | 1, 3, 10, 30 mg/kg (IP) | No effect | Hodgson et al., 2014 |
| – | FST | CD-1 mouse | 3, 10, 30 mg/kg (IP) | No effect | ||
| THY1773 | Corticosterone treated | FST | Sprague-Dawley rat | 0.1, 0.3, 1 mg/kg (PO) | Reverse increased immobility | Kamiya et al., 2020 |
Abbreviations: –, naïve; BLA, basolateral nucleus of amygdala; CeA, central nucleus of amygdala; DRL-72s, differential reinforcement of low-rate 72s; FST, forced swimming test; IP, intraperitoneal; MeA, medial nucleus of amygdala; NSFT, novelty-suppressed feeding test; PO, per os; SPT, sucrose preference test; TST, tail suspension test; UCMS, unpredictable chronic mild stress.
Blue: effective dose(s); black: ineffective dose(s).
Nonetheless, opposing reports on V1B receptor antagonists should be mentioned. Hodgson et al. (2007) reported that V1B receptor antagonists, including SSR149415, did not exert antidepressant-like effects in rodents in the rat forced swimming test, while CP-154 526 (a CRH1 receptor antagonist) exerted effects in the same paradigm. They additionally reported that a newly synthesized V1B receptor antagonist V1B-30N did not have antidepressant-like effects in rodents (Hodgson et al., 2014). Although the reason for this discrepancy is not known, the majority of studies conducted to date have favored antidepressant-like effects of V1B receptor antagonists in several animal models.
The site of action for V1B receptor antagonists remains controversial. Although the V1B receptor in the pituitary has been postulated to play a critical role, a role of central V1B receptors in exerting the antidepressant effects has also been proposed based on the following evidence. SSR149415 reportedly exerted the antidepressant-like effects in hypophysectomized rats, although the effects were weaker than those in normal rats (Griebel et al., 2002). Moreover, the injection of SSR149414 into certain brain nuclei, such as the lateral septum and amygdaloid nuclei (central nucleus, basolateral nucleus, medial nucleus), exerted antidepressant-like effects (Stemmelin et al., 2005; Salomé et al., 2006). In contrast, V1B receptor antagonists such as TASP0390325 and THY1773 exerted antidepressant-like effects at doses resulting in a pituitary V1B receptor occupancy of nearly 50% (Iijima et al., 2014; Koga et al., 2016; Kamiya et al., 2020). Given the rather low brain penetration of these compounds in rodents, it is unlikely that these V1B receptor antagonists exert their effects by directly acting on V1B receptors in the brain. Moreover, studies using central injections were performed using only SSR149415. Because SSR149415 has some activity against the V1A receptor, which is highly expressed in the brain (Allaman-Exertier et al., 2007), the role of the V1A receptor cannot be ruled out. Therefore, further investigation is needed to clarify the roles of central V1B receptors in the antidepressant-like effects of V1B receptor antagonists. For reference, as described in clinical studies, TS-121 (in which the active ingredient is THY1773) tended to reduce the depressive scores at doses resulting in a pituitary V1B receptor occupancy of nearly 50% in a Phase 2a study (Kamiya et al., 2020), providing further evidence that the pituitary V1B receptor plays a critical role in the effects of V1B receptor antagonists.
Hippocampal neurogenesis has been presumed to play an important role in the actions of antidepressants (Duman et al., 2001), and it has been reported that AVP is involved in neuroplasticity (Yang et al., 2017; Sipos et al., 2020). In addition, SSR149415 reversed a decrease in the hippocampal neurogenesis induced by chronic mild stress, which coincided with a reversal of depressive-like behavior (Alonso et al., 2004). Interestingly, the loss of hippocampal neurogenesis as a result of focal hippocampal irradiation did not affect the antidepressant-like effects of SSR149415, while the same manipulation completely blocked the effects of conventional antidepressants (imipramine and fluoxetine) (Surget et al., 2008). Moreover, the antidepressant-like effects of SSR149415 were still observed when neurogenesis was arrested by simultaneous treatment with methylazoxymethanol (Bessa et al., 2009). Therefore, more works need to clarify role of hippocampal neurogenesis in the antidepressant-like effects of V1B receptor antagonists.
Anxiolytic-like effects of V1B Receptor Antagonists in Rodents
Although clinical evidence linking the AVP-V1B receptor system and anxiety disorders is not as plentiful as that for depressive disorders, anxiolytic-like effects of V1B receptor antagonists have been reported in numerous rodent models, the representative results of which are summarized in Table 2. V1B receptor antagonists including SSR149415, TASP0233287, TASP0390325, and V1B-30N exerted anxiolytic-like effects in classical animal models in which benzodiazepine anxiolytics have been shown to be effective. Notably, Hodgson et al. (2007) reported that SSR149425 was more effective in anxiety models than in depression models. In addition, TASP0233287 attenuated sodium lactate–induced panic-like responses in panic-prone rats (Iijima et al., 2014). Interestingly, a CRH1 receptor antagonist also blocked lactate-induced behavior and cardiovascular responses (Shekhar et al., 2011), suggesting that compounds that act on the HPA axis may be useful for the treatment of panic disorder. In contrast, SSR149415 was not effective in a marble burying test in which both selective serotonin reuptake inhibitors and benzodiazepine anxiolytics have been shown to be effective (Hodgson et al., 2007).
Table 2.
Effect of V1B receptor antagonists in animals (anxiolytic-like effects)
| Compound | Model | Test | Species/strain | Dose (route) | Results | Reference |
|---|---|---|---|---|---|---|
| SSR149415 | – | Four-plate | NMRI mouse | 1, 3, 10 mg/kg (PO, IP) | Increase number of punished crossing | Serradeil-Le Gal et al., 2002 |
| – | Vogel | Sprague-Dawley rat | 1, 3, 10 mg/kg (IP) | Increase number of shocks | Griebel et al., 2002 | |
| – | Light/dark | BALB/c mouse | 1, 3, 10, 30 mg/kg (IP) | Increase time in lit box | ||
| – | EPM | Sprague-Dawley rat | 3, 10, 30 mg/kg (PO) | Increase % open arm entries | ||
| Social defeat stress | EPM | Swiss mouse | 3 mg/kg (PO) | Reverse decreased % time open arms | ||
| – | Social interaction | Flinders Sensitive Line rat | 3, 10, 30 mg/kg (IP, dosing for 14 days) | Increase social interaction | Overstreet and Griebel, 2005 | |
| – | Social interaction | Sprague-Dawley rat | 0.1, 0.3 mg/kg (PO) | Increase social interaction | Shimazaki et al., 2006 | |
| – | Separation-induced ultrasonic vocalization | Sprague-Dawley rat pup | 3, 10, 30 mg/kg (IP) | Tendency to decrease number of calls | Iijima and Chaki, 2005 | |
| – | Separation-induced ultrasonic vocalization | Brattleboro rat pup | 10 mg/kg (IP) | Decrease USV duration/frequency | Varga et al., 2015 | |
| – | EPM | CD rat | 3, 10, 30 mg/kg (IP) | Increase % open arm entries | Hodgson et al., 2007 | |
| – | Conditioned lick suppression | CD rat | 3, 10, 30 mg/kg (IP) | Increase number of punished licks | ||
| – | Separation-induced ultrasonic vocalization | CD rat pup | 3, 10, 30 mg/kg (IP) | Decrease number of calls | ||
| – | Separation-induced vocalization | Hartley guinea pig pup | 3, 10, 30 mg/kg (IP) | Decrease number of calls | ||
| – | Marble burying | CD-1 mouse | 3, 10, 30 mg/kg (IP) | No effect | ||
| Social defeat stress | Anxiety-like behaviors | Swiss-Webster mouse | 30 mg/kg (IP) | Reverse anxiety-like behaviors | Litvin et al., 2011 | |
| – | Vogel | Sprague-Dawley rat | 1, 10, 100 ng (intra-septal) | No effect | Stemmelin et al., 2005 | |
| – | EPM | Sprague-Dawley rat | 1, 10, 100 ng (intra-septal) | No effect | ||
| – | EPM | Sprague-Dawley rat | 0.1, 1, 10 ng (intra-BIA) 1, 10, 100 ng (intra-CeA) 10, 100 ng (intra-MeA) | Increase % time spent in open arms no effect no effect | Salomé et al., 2006 | |
| – | EPM | Wistar rat (female) | 100 ng/side (intra-PVN) | No effect | Bayerl et al., 2016 | |
| – | Shock-probe burying | Wistar rat | 1, 10 ng/side (intra-CeA) | Attenuate AVP-increased burying behavior (no effect by itself) | Hernández-Pérez et al., 2018 | |
| TASP0233287 | – | Social interaction | Sprague-Dawley rat | 0.1, 0.3, 1, 3 mg/kg (PO) | Increase social interaction | Iijima et al., 2014 |
| – | Stress-induced hyperthermia | ICR mouse | 3, 10, 30 mg/kg (PO) | Decrease stress-induced hyperthermia | ||
| – | Separation-induced ultrasonic vocalization | Sprague-Dawley rat pup | 3, 10, 30 mg/kg (IP) | Tendency to decrease number of calls | ||
| Forced swim stress | EPM | Sprague-Dawley rat | 0.3, 1, 3 mg/kg (PO) | Reverse decreased time in open arms | ||
| Panic model | Social interaction | Sprague-Dawley rat | 1, 3 mg/kg (PO) | Reverse decreased social interaction | ||
| TASP0390325 | – | Stress-induced hyperthermia | ICR mouse | 3, 10, 30 mg/kg (PO) | Decrease stress-induced hyperthermia | |
| V1B-30N | – | Vogel | CD rat | 3, 10, 30 mg/kg (IP) | Increase number of punished licks | Hodgson et al., 2014 |
| – | Separation-induced ultrasonic vocalization | CD rat pup | 1, 3, 10, 30 mg/kg (IP) | Decrease number of calls | ||
| – | Separation-induced vocalization | Hartley guinea pig pup | 3, 10, 30 mg/kg (IP) | Decrease number of calls |
Abbreviations: –, naive; BIA, basolateral nucleus of amygdala; CeA, central nucleus of amygdala; EPM, elevated plus maze; IP, intraperitoneal; MeA, medial nucleus of amygdala; PO, per os; PVN, paraventricular nucleus.
Blue: effective dose(s); Black: Ineffective dose(s).
There is some debate about the primary site of action for the anxiolytic-like effects of V1B receptor antagonists (central vs pituitary). The injection of SSR149415 into certain brain nuclei (lateral septum, amygdaloid nuclei [central nucleus, medial nucleus], PVN of the hypothalamus) did not exert anxiolytic-like effects, unlike the results for antidepressant-like effects (Stemmelin et al., 2005; Salomé et al., 2006; Bayerl et al., 2016), indicating that central V1B receptors have a minimal role in exerting the anxiolytic-like effects of V1B receptor antagonists. This finding is consistent with our previous report that the anxiolytic-like effect of SSR149415 in a social interaction test was no longer observed in hypophysectomized rats, while the anxiolytic-like effect of chlordiazepoxide was preserved (Shimazaki et al., 2006). Therefore, the pituitary V1B receptor is the premier site in the exertion of anxiolytic-like effects. However, the role of the V1B receptor in certain brain nuclei cannot be fully ruled out, because the injection of SSR149415 into the basolateral nucleus of the amygdala has been reported (Stemmelin et al., 2005), and SSR149145 also attenuated AVP-induced anxiety-like behavior when injected into the central nucleus of amygdala (Hernández-Pérez et al., 2018). Moreover, chronic social defeat increased V1B receptor expression in the medial nucleus of the amygdala and lateral septum (Litvin et al., 2011). However, as described above, SSR149415 has some affinity for the V1A receptor, and V1A receptor antagonists have been shown to exert anxiolytic-like effects (Bleickardt et al., 2009), raising the possibility that the effects of SSR149415 in the brain nuclei may be mediated through the blockade of the V1A receptor. Moreover, an earlier study suggested that the central role of the AVP system on anxiety-like behavior is anxiolytic rather than anxiogenic (Appenrodt et al., 1998). Therefore, not enough evidence exists to support the role of the V1B receptor in the brain in the anxiolytic-like effects of V1B receptor antagonists and anxiety-like behavior.
Clinical Studies of V1B Receptor Antagonists for Patients with MDD
To date, clinical trials of 3 V1B receptor antagonists for the treatment of patients with MDD have been conducted, as summarized in Table 3. SSR149415 was the first V1B receptor antagonist to be tested in clinical studies. In 2 trials, SSR149415 failed to clearly demonstrate efficacy in the treatment of depressive symptoms (Griebel et al., 2012). In 1 of the 2 trials, a high dose of SSR149415 (250 mg, BID) resulted in a greater improvement in the Hamilton Depression Rating Scale (HDRS) score relative to the baseline score compared with a placebo at 8 weeks after the start of administration; however, the administration of escitalopram, which was used as a positive control, did not produce a significant reduction in the HDRS score, causing the trial to fail. Moreover, SSR149415 was not superior in efficacy to the placebo for the treatment of MDD in another trial in which a robust signal detection was obtained with paroxetine. Of note, 2 different doses (100 mg and 250 mg) of SSR149415 did not reduce the cortisol response to CRH challenge, while doses higher than 250 mg of SSR149415 were shown to attenuate this response significantly in a Phase I study. Therefore, the doses used in these trials might have been insufficient to block HPA activity and achieve therapeutic effects. ABT-436, another V1B receptor antagonist, has been demonstrated to reduce HPA parameters (urine total glucocorticoids, plasma ACTH, and serum/urine cortisol) at a dose of 800 mg QD after 7 days of treatment in both healthy adults (Katz et al., 2016) and patients with MDD (Katz et al., 2017). ABT-436 not only reduced the basal HPA parameters but also attenuated the plasma ACTH and serum cortisol responses to a CRH challenge in patients with MDD (Katz et al., 2017), indicating that a dose of 800 mg of ABT-436 attenuated the increased HPA activity in patients with MDD. Importantly, ABT-436 showed favorable symptom responses on 2 (General Distress-Depressive Symptoms and General Distress-Mixed Symptoms) of the 5 subscales of the Mood and Anxiety Symptom Questionnaire (MASQ) compared with the results for a placebo, although favorable symptom changes in the HDRS score were not observed. Given that a cross-sectional analysis of symptom severity measured using MASQ showed a correlation with cortisol levels (Veen et al., 2011) and that a difference in symptom change using the HDRS at 7 days is not typically observed for most antidepressants, the favorable symptom changes obtained using ABT-436 at a dose that attenuates HPA activation supports the potential of V1B receptor antagonists as effective antidepressants. The potential of V1B receptor antagonists for the treatment of depression is underpinned by a trial examining TS-121 (Kamiya et al., 2020). In this trial, the doses that were used were determined based on V1B receptor occupancy in the pituitary as observed using a positron emission tomography (PET) study (ClinicalTrials.gov Identifier: NCT02448212) and a PET tracer that we developed (Koga et al., 2017). Thus, 2 doses (10 mg and 50 mg), which were expected to occupy the V1B receptor by 55.3% (10 mg) and 75.1% (50 mg), were selected. Of note, to our knowledge, this is the first and only clinical study of a V1B receptor antagonist in which the optimal doses were determined based on receptor occupancy. In our previous non-clinical studies, doses producing more than 50% occupancy of the pituitary V1B receptor were shown to exert antidepressant-like effects and to attenuate HPA activation in animal models (Iijima et al., 2014; Koga et al., 2016; Kamiya et al., 2020). TS-121 produced a greater reduction in not only the Montgomery-Åsberg Depression Rating Scale (MADRS) score but also across secondary measures (Clinical Global Impression-Severity, Hamilton Anxiety Rating Scale, Symptoms of Depression Questionnaire) compared with a placebo, although these changes were not statistically significant because of the small sample size. Importantly, higher baseline urinary and hair cortisol levels were associated with a greater separation between the TS-121 and placebo results, indicating that MDD patients with higher HPA activity may respond better to TS-121. This assumption is supported by the results of another trial in which the magnitude of the attenuation in basal HPA activity produced by ABT-436 was larger in MDD patients with higher HPA activity levels than in those with lower HPA activity levels (Katz et al., 2017). These clinical trials support the concept that V1B receptor antagonists are effective at doses that attenuate HPA axis activity and are most efficacious in MDD patients who exhibit HPA axis hyperactivity (Figure 3). Therefore, further clinical studies of V1B receptor antagonists should take these factors into consideration to clarify their potential as antidepressants fully.
Table 3.
Effect of V1B receptor antagonists in clinical trials (depression)
| Compound | Trial | Dose regimen | Patients | Primary endpoint | Results | ClinicalTrial.gov identifier | Reference |
|---|---|---|---|---|---|---|---|
| SSR149415 | Randomized, double-blinded, placebo-controlled study (DFI5878) | SSR149415 (100 mg, 250 mg) or placebo, BID for 8 wk (active control: escitalopram, 10 mg QD) | MDD patients SSR149415 100 mg (n = 80) SSR149415 250 mg (n = 79) placebo n = 75 escitalopram n = 84 | Changes from baseline HDRS total score at wk 8 | • SSR149415 (250 but not 100 mg) had significantly greater reductions in HDRS changes from baseline compared with placebo, while escitalopram had nonsignificant reduction in HDRS • Similar trends of greater improvements in SSR149415 observed in secondary endpoints (CGI-S, MADRS), while these effects did not reach statistical significance |
NCT00358631 | Griebel et al., 2012 |
| Randomized, double-blinded, placebo-controlled study (DFI5879) | SSR149415 (100 mg, 250 mg) or placebo, BID for 8 wk (active control: paroxetine, 20 mg QD) | MDD patients SSR149415 100 mg (n = 82) SSR149415 250 mg (n = 81) placebo n = 77 escitalopram n = 80 |
Changes from baseline HDRS total score at wk 8 | • Differences between placebo and each SSR149415 dose on HDRS score not statistically significant, while paroxetine significantly reduced HDRS score • No statistical difference between placebo and each SSR149414 dose on secondary endpoints (CGI-S, MADRS). |
NCT00361491 | ||
| Randomized, double-blinded, placebo-controlled study (PDY5467) | SSR149415 (100 mg, 250 mg) or placebo, BID for 4 wk | MDD patients SSR149415 100 mg (n = 25) SSR149415 250 mg (n = 24) placebo n = 24 | Plasma cortisol concentration response to CRF administration before and after 27 d dosing | • Differences between placebo and each SSR149415 dose on primary endpoint not statistically significant • SSR149415 group showed greater mean improvements from baseline HDRS score and CGI-S than placebo group, although differences not statistically significant |
NCT01606384 | ||
| ABT-436 | Randomized, double-blinded, placebo-controlled study | ABT-436 (800 mg) or placebo, QD for 7 d | MDD patients ABT-436 800 mg (n = 31) placebo n = 20 | Basal HPA parameters at d 7 | • Basal HPA parameters (urine total glucocorticoids, plasma ACTH, urine/serum/ saliva cortisol, etc.) lower in ABT-436 group than in placebo group • Dynamic HPA parameters (plasma ACTH and serum cortisol response to CRF) lower in ABT- 436 group than in placebo group • ABT-436 had favorable symptom responses on 2 of 5 MASQ subscale compared with placebo but no differences on HDRS score |
NCT01380704 | Katz et al., 2017 |
| TS-121 | Randomized, double-blinded, placebo-controlled study | TS-121 (10 mg, 50 mg) or placebo, QD for 6 wk (adjunctive treatment) | MDD patients with inadequate response to current antidepressant treatment TS-121 10 mg (n = 16) TS-121 50 mg (n = 17) placebo n = 18 | Changes from baseline MADRS score at wk 6 | • TS-121 had greater reductions in MADRS change from baseline compared with placebo but changes not statistically significant • Similar trends of non-significantly greater improvements in TS-121 observed in secondary endpoints (CGI-S, HAM-A, SDQ, etc.), but effects not statistically significant • Higher baseline urinary and hair cortisol associated with greater separation between TS-121 and placebo in MADRS score |
NCT03093025 | Kamiya et al., 2020 |
Abbreviations: ACTH, adrenocorticotropic hormone; CGI-S, Clinical Global Impressions-Severity of Illness Score; CRF, corticotropin-releasing factor; HAM-A, Hamilton Anxiety Rating Scale; HDRS, Hamilton Depression Rating Scale; HPA, hypothalamus-pituitary-adrenal; MADRS, Montgomery-Åsberg Depression Rating Scale; MASQ, Mood and Anxiety Symptom Questionnaire; MDD, major depressive disorder; SDQ, Symptoms of Depression Questionnaire.
Figure 3.
Conceptualized scheme for patient segmentation for V1B receptor antagonists treatment. Among patients with major depressive disorder (MDD), there are certain populations with impaired hypothalamus-pituitary-adrenal (HPA) axis who might show higher cortisol levels in blood, urine, or saliva. Moreover, even among MDD patients with impaired HPA activity, there are certain populations of patients with V1B receptor hyperactivation. It is important to appropriately stratify patients who could respond better to V1B receptor antagonists.
Clinical Studies of V1B Receptor Antagonists for other Psychiatric Disorders
Given that V1B receptor antagonists show anxiolytic-like effects (see Table 2) and reduce alcohol intake in animal models (Zhou et al., 2011; Edwards et al., 2012), clinical studies of V1B receptor antagonists for generalized anxiety disorder (GAD) and alcohol use disorder have been conducted, as summarized in Table 4. Contrary to the convincing results in animal models, SSR149415 (100 or 250 mg, BID for 8 weeks) did not produce statistically significant improvements in both primary and secondary measures of anxiety symptoms (Griebel et al., 2012). However, concluding that V1B receptor antagonists are not efficacious for GAD might be premature because the doses might not have been sufficient to exert the desired effects. In addition, other disorders for which increased AVP levels have been reported, such as PTSD or obsessive-compulsive disorder, are worth considering (Altemus et al., 1992; de Kloet et al., 2008), since there is no evidence of changes in AVP in patients with GAD to date. Notably, TS-121 reduced Hamilton Anxiety Rating Scale of depressed patients compared with placebo (Kamiya et al., 2020). On the other hand, ABT-436 reduced the percentage of heavy drinking days compared with a placebo, although this effect was not statistically significant (Ryan et al., 2017). Therefore, the potential of V1B receptor antagonists for the treatment of alcohol dependence has not been fully determined. Interestingly, patients with relatively greater baseline stress levels responded better to ABT-436 in terms of a reduction in both the frequency of drinking and the number of heavy drinking days, which is consistent with the concept that V1B receptor antagonists may reduce alcohol drinking by ameliorating HPA axis abnormalities. Future trials should be conducted under conditions that maximize the treatment effect of ABT-436 by enriching the treatment population with participants who have clinically elevated anxiety levels (and/or hyper-reactivity to stress).
Table 4.
Effect of V1B Receptor Antagonists in Clinical Trials (Other Stress-related Disorders)
| Compound | Trial | Dose regimen | Patients | Primary endpoint | Results | ClinicalTrial. gov identifier | Reference |
|---|---|---|---|---|---|---|---|
| SSR149415 | Randomized, double-blinded, placebo-controlled study (DFI5880) | SSR149415 (100 mg, 250 mg) or placebo, BID for 8 wk (active control: paroxetine, 20 mg QD) | GAD patients SSR149415 100 mg (n = 79) SSR149415 250 mg (n = 82) placebo n = 81 paroxetine n = 82 | Changes from baseline in HAM-A total score at wk 8 | • Differences between placebo and each SSR149415 dose on HAM-A score were not statistically significant, while paroxetine significantly reduced HAM-A score • No statistical difference between placebo and each SSR149414 dose on secondary endpoints (CGI-S, MADRS) |
NCT00374166 | Griebel et al., 2012 |
| ABT-436 | Randomized, double-blinded, placebo-controlled study | ABT-436 (titrated from 200 to 800 mg) or placebo for 12 wk (wk 2–12, 400 mg BID used; 400 mg BID selected to reduce risk of drop-outs due to gastrointestinal effects) | Alcohol dependence ABT-436 n = 73 placebo n = 71 | Weekly percentage of heavy drinking days | • ABT-436 group showed lower adjusted levels of percentage of heavy drinking days than placebo group, although effect was not statistically significant • ABT-436 group had significantly greater percentage of days abstinent than placebo group • No statistical difference between placebo and ABT-436 on alcohol-related craving and consequences |
NCT01613014 | Ryan et al., 2017 |
Abbreviations: CGI-S, Clinical Global Impressions-Severity of Illness Score; GAD, generalized anxiety disorder; HAM-A, Hamilton Anxiety Rating Scale; MADRS, Montgomery-Åsberg Depression Rating Scale.
Conclusions
Accumulating evidence suggests that certain populations of depressed patients have impaired HPA axis function, and agents ameliorating HPA axis dysfunction have gained attention. Nonetheless, the outcomes of several agents acting on the HPA axis, such as a cortisol synthesis inhibitor and a glucocorticoid receptor antagonist, were inconsistent in clinical trials (Schüle et al., 2009), raising skepticism about the role of HPA axis in the pathophysiology of depression. Although precise reasons for these inconsistent results have not been clarified yet, several factors including differences in clinical designs (dose regimens and patient populations) should be considered.
Given that the HPA axis is regulated by numerous factors and processes and that V1B receptor signaling is overly activated in certain populations of depressed patients, blockade of the V1B receptor may be a more appropriate approach to normalize HPA axis dysfunction in depression among regulators of HPA axis. The results that both ABT-436 and TS-121 tended to improve depressive symptoms when administered at doses that attenuated HPA axis hyperactivity or occupied the pituitary V1B receptor may underpin this hypothesis. Importantly, the efficacy of TS-121 was more apparent in patients with higher basal cortisol levels. These results suggest that V1B receptor antagonists are more effective in patients with impaired HPA axis activities when they are administered at doses that suppress the over-activation of HPA axis activity.
Given that depression is a clinically heterogenous condition defined by several subtypes and that different symptom clusters may respond selectively to different treatments, the development of novel antidepressants that focus on HPA axis dysfunction, which is a pathophysiological event in depression, is important. V1B receptor antagonists could be the best candidate in this domain. Still, several issues, including (1) evaluation of the full potential of V1B receptor antagonists in an adequately powered study, (2) precise mechanisms underlying the antidepressant effects of V1B receptor antagonists, and (3) comparisons with other mechanisms relating HPA axis regulation remain to be investigated.
Acknowledgments
The manuscript was edited by a professional language editor. The author is entirely responsible for the scientific content of the manuscript.
Interest Statement
Shigeyuki Chaki is an employee of Taisho Pharmaceutical Co., Ltd. This article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
References
- Abelson JL, Le Mellédo J, Bichet DG (2001) Dose response of arginine vasopressin to the CCK-B agonist pentagastrin. Neuropsychopharmacology 24:161–169. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Rabadan-Diehl C (2000) Vasopressinergic regulation of the hypothalamic-pituitary-adrenal axis: implications for stress adaptation. Regul Pept 96:23–29. [DOI] [PubMed] [Google Scholar]
- Allaman-Exertier G, Reymond-Marron I, Tribollet E, Raggenbass M (2007) Vasopressin modulates lateral septal network activity via two distinct electrophysiological mechanisms. Eur J Neurosci 26:2633–2642. [DOI] [PubMed] [Google Scholar]
- Alonso R, Griebel G, Pavone G, Stemmelin J, Le Fur G, Soubrié P (2004) Blockade of CRF(1) or V(1b) receptors reverses stress-induced suppression of neurogenesis in a mouse model of depression. Mol Psychiatry 9:278–286; 224. [DOI] [PubMed] [Google Scholar]
- Altemus M, Pigott T, Kalogeras KT, Demitrack M, Dubbert B, Murphy DL, Gold PW (1992) Abnormalities in the regulation of vasopressin and corticotropin releasing factor secretion in obsessive-compulsive disorder. Arch Gen Psychiatry 49:9–20. [DOI] [PubMed] [Google Scholar]
- Appenrodt E, Schnabel R, Schwarzberg H (1998) Vasopressin administration modulates anxiety-related behavior in rats. Physiol Behav 64:543–547. [DOI] [PubMed] [Google Scholar]
- Bayerl DS, Hönig JN, Bosch OJ (2016) Vasopressin V1a, but not V1b, receptors within the PVN of lactating rats mediate maternal care and anxiety-related behaviour. Behav Brain Res 305:18–22. [DOI] [PubMed] [Google Scholar]
- Bessa JM, Ferreira D, Melo I, Marques F, Cerqueira JJ, Palha JA, Almeida OF, Sousa N (2009) The mood-improving actions of antidepressants do not depend on neurogenesis but are associated with neuronal remodeling. Mol Psychiatry 14:764–773; 739. [DOI] [PubMed] [Google Scholar]
- Bleickardt CJ, Mullins DE, Macsweeney CP, Werner BJ, Pond AJ, Guzzi MF, Martin FD, Varty GB, Hodgson RA (2009) Characterization of the V1a antagonist, JNJ-17308616, in rodent models of anxiety-like behavior. Psychopharmacology (Berl) 202:711–718. [DOI] [PubMed] [Google Scholar]
- Breuer ME, van Gaalen MM, Wernet W, Claessens SE, Oosting RS, Behl B, Korte SM, Schoemaker H, Gross G, Olivier B, Groenink L (2009) SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn Schmiedebergs Arch Pharmacol 379:101–106. [DOI] [PubMed] [Google Scholar]
- Carroll BJ, Feinberg M, Greden JF, Tarika J, Albala AA, Haskett RF, James NM, Kronfol Z, Lohr N, Steiner M, de Vigne JP, Young E (1981) A specific laboratory test for the diagnosis of melancholia. Standardization, validation, and clinical utility. Arch Gen Psychiatry 38:15–22. [DOI] [PubMed] [Google Scholar]
- Chaki S (2017) Beyond ketamine: new approaches to the development of safer antidepressants. Curr Neuropharmacol 15:963–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaki S, Okubo T, Sekiguchi Y (2006) Non-monoamine-based approach for the treatment of depression and anxiety disorders. Recent Pat CNS Drug Discov 1:1–27. [DOI] [PubMed] [Google Scholar]
- Chen J, Young S, Subburaju S, Sheppard J, Kiss A, Atkinson H, Wood S, Lightman S, Serradeil-Le Gal C, Aguilera G (2008) Vasopressin does not mediate hypersensitivity of the hypothalamic pituitary adrenal axis during chronic stress. Ann N Y Acad Sci 1148:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbani M, Marir R, Trueba M, Chafai M, Vincent A, Borie AM, Desarménien MG, Ueta Y, Tomboly C, Olma A, Manning M, Guillon G (2018) Neuroanatomical distribution and function of the vasopressin V1B receptor in the rat brain deciphered using specific fluorescent ligands. Gen Comp Endocrinol 258:15–32. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Naudet F (2019) US Food and Drug Administration approval of esketamine and brexanolone. Lancet Psychiatry 6:975–977. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Gold PW, Geracioti TD Jr, Listwak SJ, Kling MA (1993) Association of fluoxetine treatment with reductions in CSF concentrations of corticotropin-releasing hormone and arginine vasopressin in patients with major depression. Am J Psychiatry 150:656–657. [DOI] [PubMed] [Google Scholar]
- de Goeij DC, Jezova D, Tilders FJ (1992) Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res 577:165–168. [DOI] [PubMed] [Google Scholar]
- de Kloet CS, Vermetten E, Geuze E, Wiegant VM, Westenberg HG (2008) Elevated plasma arginine vasopressin levels in veterans with posttraumatic stress disorder. J Psychiatr Res 42:192–198. [DOI] [PubMed] [Google Scholar]
- Demitrack MA, Kalogeras KT, Altemus M, Pigott TA, Listwak SJ, Gold PW (1992) Plasma and cerebrospinal fluid measures of arginine vasopressin secretion in patients with bulimia nervosa and in healthy subjects. J Clin Endocrinol Metab 74:1277–1283. [DOI] [PubMed] [Google Scholar]
- de Winter RF, van Hemert AM, DeRijk RH, Zwinderman KH, Frankhuijzen-Sierevogel AC, Wiegant VM, Goekoop JG (2003) Anxious-retarded depression: relation with plasma vasopressin and cortisol. Neuropsychopharmacology 28:140–147. [DOI] [PubMed] [Google Scholar]
- Dinan TG (1994) Glucocorticoids and the genesis of depressive illness. A psychobiological model. Br J Psychiatry 164:365–371. [DOI] [PubMed] [Google Scholar]
- Dinan TG, O’Brien S, Lavelle E, Scott LV (2004) Further neuroendocrine evidence of enhanced vasopressin V3 receptor responses in melancholic depression. Psychol Med 34:169–172. [DOI] [PubMed] [Google Scholar]
- Dinan TG, Scott LV (2005) Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J Anat 207:259–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Nakagawa S, Malberg J (2001) Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 25:836–844. [DOI] [PubMed] [Google Scholar]
- Edwards S, Guerrero M, Ghoneim OM, Roberts E, Koob GF (2012) Evidence that vasopressin V1b receptors mediate the transition to excessive drinking in ethanol-dependent rats. Addict Biol 17:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geneste H, Bhowmik S, van Gaalen MM, Hornberger W, Hutchins CW, Netz A, Oost T, Unger L (2018) Novel, potent, selective and brain penetrant vasopressin 1b receptor antagonists. Bioorg Med Chem Lett 28:3260–3264. [DOI] [PubMed] [Google Scholar]
- Griebel G, Beeské S, Stahl SM (2012) The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry 73:1403–1411. [DOI] [PubMed] [Google Scholar]
- Griebel G, Holsboer F (2012) Neuropeptide receptor ligands as drugs for psychiatric diseases: the end of the beginning? Nat Rev Drug Discov 11:462–478. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, Maffrand JP, Soubrie P (2002) Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A 99:6370–6375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffante C, Green A, Curcuruto O, Haslam CP, Dickinson BA, Arban R (2005) Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. Br J Pharmacol 146:744–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF (2018) Epidemiology of adult DSM-5 major depressive disorder and its specifiers in the United States. JAMA Psychiatry 75:336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Pérez OR, Crespo-Ramírez M, Cuza-Ferrer Y, Anias-Calderón J, Zhang L, Roldan-Roldan G, Aguilar-Roblero R, Borroto-Escuela DO, Fuxe K, Perez de la Mora M (2018) Differential activation of arginine-vasopressin receptor subtypes in the amygdaloid modulation of anxiety in the rat by arginine-vasopressin. Psychopharmacology (Berl) 235:1015–1027. [DOI] [PubMed] [Google Scholar]
- Hernando F, Schoots O, Lolait SJ, Burbach JP (2001) Immunohistochemical localization of the vasopressin V1b receptor in the rat brain and pituitary gland: anatomical support for its involvement in the central effects of vasopressin. Endocrinology 142:1659–1668. [DOI] [PubMed] [Google Scholar]
- Heuser I, Bissette G, Dettling M, Schweiger U, Gotthardt U, Schmider J, Lammers CH, Nemeroff CB, Holsboer F (1998) Cerebrospinal fluid concentrations of corticotropin-releasing hormone, vasopressin, and somatostatin in depressed patients and healthy controls: response to amitriptyline treatment. Depress Anxiety 8:71–79. [PubMed] [Google Scholar]
- Hodgson RA, Higgins GA, Guthrie DH, Lu SX, Pond AJ, Mullins DE, Guzzi MF, Parker EM, Varty GB (2007) Comparison of the V1b antagonist, SSR149415, and the CRF1 antagonist, CP-154,526, in rodent models of anxiety and depression. Pharmacol Biochem Behav 86:431–440. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Mullins D, Lu SX, Guzzi M, Zhang X, Bleickardt CJ, Scott JD, Miller MW, Stamford AW, Parker EM, Varty GB (2014) Characterization of a novel vasopressin V1b receptor antagonist, V1B-30N, in animal models of anxiety-like and depression-like behavior. Eur J Pharmacol 730:157–163. [DOI] [PubMed] [Google Scholar]
- Iijima M, Chaki S (2005) Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav 82:652–657. [DOI] [PubMed] [Google Scholar]
- Iijima M, Chaki S (2007) An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Prog Neuropsychopharmacol Biol Psychiatry 31:622–627. [DOI] [PubMed] [Google Scholar]
- Iijima M, Yoshimizu T, Shimazaki T, Tokugawa K, Fukumoto K, Kurosu S, Kuwada T, Sekiguchi Y, Chaki S (2014) Antidepressant and anxiolytic profiles of newly synthesized arginine vasopressin V1B receptor antagonists: TASP0233278 and TASP0390325. Br J Pharmacol 171:3511–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inder WJ, Donald RA, Prickett TC, Frampton CM, Sullivan PF, Mulder RT, Joyce PR (1997) Arginine vasopressin is associated with hypercortisolemia and suicide attempts in depression. Biol Psychiatry 42:744–747. [DOI] [PubMed] [Google Scholar]
- Ising M, Künzel HE, Binder EB, Nickel T, Modell S, Holsboer F (2005) The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry 29:1085–1093. [DOI] [PubMed] [Google Scholar]
- Ising M, Horstmann S, Kloiber S, Lucae S, Binder EB, Kern N, Künzel HE, Pfennig A, Uhr M, Holsboer F (2007) Combined dexamethasone/corticotropin releasing hormone test predicts treatment response in major depression - a potential biomarker? Biol Psychiatry 62:47–54. [DOI] [PubMed] [Google Scholar]
- Juruena MF, Pariante CM, Papadopoulos AS, Poon L, Lightman S, Cleare AJ (2009) Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br J Psychiatry 194:342–349. [DOI] [PubMed] [Google Scholar]
- Kamiya M, Sabia HD, Marella J, Fava M, Nemeroff CB, Umeuchi H, Iijima M, Chaki S, Nishino I (2020) Efficacy and safety of TS-121, a novel vasopressin V1B receptor antagonist, as adjunctive treatment for patients with major depressive disorder: a randomized, double-blind, placebo-controlled study. J Psychiatr Res 128:43–51. [DOI] [PubMed] [Google Scholar]
- Katz DA, Liu W, Locke C, Dutta S, Tracy KA (2016) Clinical safety and hypothalamic-pituitary-adrenal axis effects of the arginine vasopressin type 1B receptor antagonist ABT-436. Psychopharmacology (Berl) 233:71–81. [DOI] [PubMed] [Google Scholar]
- Katz DA, Locke C, Greco N, Liu W, Tracy KA (2017) Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized Phase 1b trial. Brain Behav 7:e00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck ME, Wigger A, Welt T, Müller MB, Gesing A, Reul JM, Holsboer F, Landgraf R, Neumann ID (2002) Vasopressin mediates the response of the combined dexamethasone/CRH test in hyper-anxious rats: implications for pathogenesis of affective disorders. Neuropsychopharmacology 26:94–105. [DOI] [PubMed] [Google Scholar]
- Koga K, Yoshinaga M, Uematsu Y, Nagai Y, Miyakoshi N, Shimoda Y, Fujinaga M, Minamimoto T, Zhang MR, Higuchi M, Ohtake N, Suhara T, Chaki S (2016) TASP0434299: a novel pyridopyrimidin-4-one derivative as a radioligand for vasopressin V1B receptor. J Pharmacol Exp Ther 357:495–508. [DOI] [PubMed] [Google Scholar]
- Koga K, Nagai Y, Hanyu M, Yoshinaga M, Chaki S, Ohtake N, Ozaki S, Zhang MR, Suhara T, Higuchi M (2017) High-contrast PET imaging of vasopressin V1B receptors with a novel radioligand, 11C-TASP699. J Nucl Med 58:1652–1658. [DOI] [PubMed] [Google Scholar]
- Koike H, Iijima M, Chaki S (2013) Effects of ketamine and LY341495 on the depressive-like behavior of repeated corticosterone-injected rats. Pharmacol Biochem Behav 107:20–23. [DOI] [PubMed] [Google Scholar]
- Letourneau JJ, Riviello CM, Li H, Cole AG, Ho KK, Zanetakos HA, Desai H, Zhao J, Auld DS, Napier SE, Thomson FJ, Goan KA, Morphy JR, Ohlmeyer MH, Webb ML (2010) Identification and optimization of novel 2-(4-oxo-2-aryl-quinazolin-3(4H)-yl)acetamide vasopressin V3 (V1b) receptor antagonists. Bioorg Med Chem Lett 20:5394–5397. [DOI] [PubMed] [Google Scholar]
- Litvin Y, Murakami G, Pfaff DW (2011) Effects of chronic social defeat on behavioral and neural correlates of sociality: vasopressin, oxytocin and the vasopressinergic V1b receptor. Physiol Behav 103:393–403. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O’Carroll AM, Mahan LC, Felder CC, Button DC, Young WS 3rd, Mezey E, Brownstein MJ (1995) Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A 92:6783–6787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis C, Cohen C, Depoortère R, Griebel G (2006) Antidepressant-like effects of the corticotropin-releasing factor 1 receptor antagonist, SSR125543, and the vasopressin 1b receptor antagonist, SSR149415, in a DRL-72 s schedule in the rat. Neuropsychopharmacology 31:2180–2187. [DOI] [PubMed] [Google Scholar]
- Makara GB, Varga J, Barna I, Pintér O, Klausz B, Zelena D (2012) The vasopressin-deficient Brattleboro rat: lessons for the hypothalamo-pituitary-adrenal axis regulation. Cell Mol Neurobiol 32:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A (2019) Is the HPA axis as target for depression outdated, or is there a new hope? Front Psychiatry 10:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meynen G, Unmehopa UA, van Heerikhuize JJ, Hofman MA, Swaab DF, Hoogendijk WJ (2006) Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: a preliminary report. Biol Psychiatry 60:892–895. [DOI] [PubMed] [Google Scholar]
- Mlynarik M, Zelena D, Bagdy G, Makara GB, Jezova D (2007) Signs of attenuated depression-like behavior in vasopressin deficient Brattleboro rats. Horm Behav 51:395–405. [DOI] [PubMed] [Google Scholar]
- Napier SE, et al. (2011a) Synthesis and SAR studies of novel 2-(4-oxo-2-aryl-quinazolin-3(4H)-yl)acetamide vasopressin V1b receptor antagonists. Bioorg Med Chem Lett 21:1871–1875. [DOI] [PubMed] [Google Scholar]
- Napier SE, et al. (2011b) Synthesis and SAR studies of novel 2-(6-aminomethylaryl-2-aryl-4-oxo-quinazolin-3(4H)-yl)acetamide vasopressin V1b receptor antagonists. Bioorg Med Chem Lett 21:3813–3817. [DOI] [PubMed] [Google Scholar]
- Nikkheslat N, McLaughlin AP, Hastings C, Zajkowska Z, Nettis MA, Mariani N, Enache D, Lombardo G, Pointon L, Cowen PJ, Cavanagh J, Harrison NA, Bullmore ET, Pariante CM, Mondelli V; NIMA Consortium (2020) Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav Immun 87:229–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keane V, Frodl T, Dinan TG (2012) A review of atypical depression in relation to the course of depression and changes in HPA axis organization. Psychoneuroendocrinology 37:1589–1599. [DOI] [PubMed] [Google Scholar]
- Oost T, Backfisch G, Bhowmik S, van Gaalen MM, Geneste H, Hornberger W, Lubisch W, Netz A, Unger L, Wernet W (2011) Potent and selective oxindole-based vasopressin 1b receptor antagonists with improved pharmacokinetic properties. Bioorg Med Chem Lett 21:3828–3831. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G (2005) Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacol Biochem Behav 82:223–227. [DOI] [PubMed] [Google Scholar]
- Peskind ER, Jensen CF, Pascualy M, Tsuang D, Cowley D, Martin DC, Wilkinson CW, Raskind MA (1998) Sodium lactate and hypertonic sodium chloride induce equivalent panic incidence, panic symptoms, and hypernatremia in panic disorder. Biol Psychiatry 44:1007–1016. [DOI] [PubMed] [Google Scholar]
- Peter J, Burbach H, Adan RA, Lolait SJ, van Leeuwen FW, Mezey E, Palkovits M, Barberis C (1995) Molecular neurobiology and pharmacology of the vasopressin/oxytocin receptor family. Cell Mol Neurobiol 15:573–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poretti MB, Sawant RS, Rask-Andersen M, de Cuneo MF, Schiöth HB, Perez MF, Carlini VP (2016) Reduced vasopressin receptors activation mediates the anti-depressant effects of fluoxetine and venlafaxine in bulbectomy model of depression. Psychopharmacology (Berl) 233:1077–1086. [DOI] [PubMed] [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF (1996) Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry 53:137–143. [DOI] [PubMed] [Google Scholar]
- Rabadan-Diehl C, Lolait SJ, Aguilera G (1995) Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. J Neuroendocrinol 7:903–910. [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, McIntyre RS, Alves GS, Fountoulakis KN, Carvalho AF (2015) Beyond monoamines-novel targets for treatment-resistant depression: a comprehensive review. Curr Neuropharmacol 13:636–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rush AJ, Trivedi MH, Wisniewski SR, Nierenberg AA, Stewart JW, Warden D, Niederehe G, Thase ME, Lavori PW, Lebowitz BD, McGrath PJ, Rosenbaum JF, Sackeim HA, Kupfer DJ, Luther J, Fava M (2006) Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 163:1905–1917. [DOI] [PubMed] [Google Scholar]
- Ryan ML, Falk DE, Fertig JB, Rendenbach-Mueller B, Katz DA, Tracy KA, Strain EC, Dunn KE, Kampman K, Mahoney E, Ciraulo DA, Sickles-Colaneri L, Ait-Daoud N, Johnson BA, Ransom J, Scott C, Koob GF, Litten RZ (2017) A phase 2, double-blind, placebo-controlled randomized trial assessing the efficacy of ABT-436, a novel V1b receptor antagonist, for alcohol dependence. Neuropsychopharmacology 42:1012–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomé N, Stemmelin J, Cohen C, Griebel G (2006) Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 187:237–244. [DOI] [PubMed] [Google Scholar]
- Schüle C (2007) Neuroendocrinological mechanisms of actions of antidepressant drugs. J Neuroendocrinol 19:213–226. [DOI] [PubMed] [Google Scholar]
- Schüle C, Baghai TC, Eser D, Rupprecht R (2009) Hypothalamic-pituitary-adrenocortical system dysregulation and new treatment strategies in depression. Expert Rev Neurother 9:1005–1019. [DOI] [PubMed] [Google Scholar]
- Scott LV, Dinan TG (1998) Vasopressin and the regulation of hypothalamic-pituitary-adrenal axis function: implications for the pathophysiology of depression. Life Sci 62:1985–1998. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, Barberis C, Brossard G, Soubrié P, Nisato D, Pascal M, Pruss R, Scatton B, Maffrand JP, Le Fur G (2002) Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine Carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther 300:1122–1130. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Fitz SD, Nakazato A, Chaki S, Steckler T, Schmidt M (2011) A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int J Neuropsychopharmacol 14:355–365. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Iijima M, Chaki S (2006) The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol 543:63–67. [DOI] [PubMed] [Google Scholar]
- Sipos E, Török B, Barna I, Engelmann M, Zelena D (2020) Vasopressin and post-traumatic stress disorder. Stress 23:732–745. [DOI] [PubMed] [Google Scholar]
- Stemmelin J, Lukovic L, Salome N, Griebel G (2005) Evidence that the lateral septum is involved in the antidepressant-like effects of the vasopressin V1b receptor antagonist, SSR149415. Neuropsychopharmacology 30:35–42. [DOI] [PubMed] [Google Scholar]
- Stetler C, Miller GE (2011) Depression and hypothalamic-pituitary-adrenal activation: a quantitative summary of four decades of research. Psychosom Med 73:114–126. [DOI] [PubMed] [Google Scholar]
- Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C (2008) Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry 64:293–301. [DOI] [PubMed] [Google Scholar]
- Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L, Norquist G, Howland RH, Lebowitz B, McGrath PJ, Shores-Wilson K, Biggs MM, Balasubramani GK, Fava M; STAR*D Study Team (2006) Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am J Psychiatry 163:28–40. [DOI] [PubMed] [Google Scholar]
- Vaccari C, Lolait SJ, Ostrowski NL (1998) Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology 139:5015–5033. [DOI] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, De Wied D (1997) Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 17:284–292. [DOI] [PubMed] [Google Scholar]
- van West D, Del-Favero J, Aulchenko Y, Oswald P, Souery D, Forsgren T, Sluijs S, Bel-Kacem S, Adolfsson R, Mendlewicz J, Van Duijn C, Deboutte D, Van Broeckhoven C, Claes S (2004) A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol Psychiatry 9:287–292. [DOI] [PubMed] [Google Scholar]
- Varga J, Fodor A, Klausz B, Zelena D (2015) Anxiogenic role of vasopressin during the early postnatal period: maternal separation-induced ultrasound vocalization in vasopressin-deficient Brattleboro rats. Amino Acids 47:2409–2418. [DOI] [PubMed] [Google Scholar]
- Veen G, van Vliet IM, DeRijk RH, Giltay EJ, van Pelt J, Zitman FG (2011) Basal cortisol levels in relation to dimensions and DSM-IV categories of depression and anxiety. Psychiatry Res 185:121–128. [DOI] [PubMed] [Google Scholar]
- von Bardeleben U, Stalla GK, Müller OA, Holsboer F (1988) Blunting of ACTH response to human CRH in depressed patients is avoided by metyrapone pretreatment. Biol Psychiatry 24:782–786. [DOI] [PubMed] [Google Scholar]
- Yang C, Zhang X, Gao J, Wang M, Yang Z (2017) Arginine vasopressin ameliorates spatial learning impairments in chronic cerebral hypoperfusion via V1a receptor and autophagy signaling partially. Transl Psychiatry 7:e1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou JN, Riemersma RF, Unmehopa UA, Hoogendijk WJ, van Heerikhuize JJ, Hofman MA, Swaab DF (2001) Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch Gen Psychiatry 58:655–662. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ (1996) Modulation of CRF-R1 mRNA in rat anterior pituitary by dexamethasone: correlation with POMC mRNA. Peptides 17:435–441. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Colombo G, Carai MA, Ho A, Gessa GL, Kreek MJ (2011) Involvement of arginine vasopressin and V1b receptor in alcohol drinking in Sardinian alcohol-preferring rats. Alcohol Clin Exp Res 35:1876–1883. [DOI] [PMC free article] [PubMed] [Google Scholar]