Abstract
Background
Hyponatremia associated with antipsychotic drugs is a rare but potentially life-threatening adverse drug reaction; the underlying pharmacological mechanism has not yet been explained.
Methods
We investigated the relationship between pharmacological targets of antipsychotic drugs and the occurrence of hyponatremia by conducting a nested case-control study using the Food and Drug Administration Adverse Event Reporting System database. Multiple logistic regression was used to determine the associations between antipsychotics receptor occupancy and hyponatremia. We also performed a systematic review of clinical studies on this association.
Results
Of 139 816 reports involving at least 1 antipsychotic, 1.1% reported hyponatremia. Olanzapine was the most frequently suspected drug (27%). A significant positive association was found between dopamine D3, D4, and hyponatremia, while adrenergic α 1, serotonin 5-HT1A, and 5-HT2A receptor occupancies were negatively associated. A multivariable stepwise regression model showed that dopamine D3 (adj. odds ratio = 1.21; 95% CI = 1.09–1.34; P < .05) predicted the risk for hyponatremia (P < .05), while serotonin 5-HT2A occupancy (Adj. odds ratio = 0.78; 95% CI = 0.68–0.90; P < .01) exhibited a protective effect against hyponatremia. Among the 11 studies included in the systematic review, incidence rates of hyponatremia diverged between 0.003% and 86%, whereas the odds of developing hyponatremia from effect studies ranged between 0.83 and 3.47.
Conclusions
Antipsychotic drugs having a combined modest occupancy for D3 and 5-HT2A receptors and higher levels of D3 receptor occupancy correspond to different degrees of risk for hyponatremia. Based on the few, relatively large-scale available studies, atypical antipsychotics have a more attenuated risk profile for hyponatremia.
Keywords: Antipsychotics, pharmacodynamics, pharmacovigilance
Significance Statement.
Hyponatremia is known to occur as a rare but clinically important adverse event to treatment with various antipsychotic drugs; however, the mechanisms of this adverse reaction are unknown. Several hypotheses have been described regarding the pathophysiological mechanisms, such as a prolonged blockade of dopamine D2 and a stimulation of serotonin 5-HT1C receptors by antipsychotic drugs. Taking advantage of one of the largest spontaneous reporting systems, we studied the association between the receptor binding profile of antipsychotics and the occurrence of hyponatremia in the Food and Drug Administration Adverse Event Reporting System. Results showed a disruption of the fine balance between dopaminergic (D3) and serotonergic (5-HT2A) transmission induced by antipsychotic drugs. Higher levels of D3 receptor occupancy were associated with increased risk for hyponatremia. This report also summarizes the current evidence from clinical studies on hyponatremia after the antipsychotic therapy. Given the considerable variations and inconsistencies in available evidence, prospective data should be generated more systematically in well-defined and larger-scale populations.
Introduction
The syndrome of inappropriate antidiuretic hormone (SIADH) occurs when there is persistent stimulation of antidiuretic hormone (ADH) resulting in hyponatremia. SIADH commonly presents as euvolemic hyponatremia, and it should be suspected in any patient with hyponatremia, hypoosmolality, and a urine osmolality >100 mOsmol/kg (Verbalis et al., 2016). Hyponatremia (serum sodium concentration < 136 mEq/L) is a prevalent and potentially dangerous medical comorbidity in psychiatric patients (Siegel, 2008). It has been reported to be associated with an increased risk of mortality of 55% and substantial costs for health systems (Wald et al., 2010; Hoorn and Zietse, 2013).
The cause of hyponatremia/SIADH among psychiatric patients is still unclear, and there are 2 conflicting possibilities. Because any CNS abnormality, including mental illness and psychosis, can enhance ADH-release from the pituitary gland, one possibility is that hyponatremia is associated with the exacerbation of the underlying psychiatric conditions such as psychosis-intermittent hyponatremia-polydipsia syndrome and compulsive water drinking/psychogenic polydipsia (Yasir and Mechanic, 2020; Ahmadi and Goldman, 2020). A second possibility is that hyponatremia/SIADH has an iatrogenic cause; a number of drugs are indeed associated with SIADH by enhancing or affecting the release of ADH. The list of the most common drugs includes carbamazepine, oxcarbazepine, chlorpropamide, cyclophosphamide, and selective serotonin reuptake inhibitors (Yasir and Mechanic, 2020); however, hyponatremia/SIADH has also been reported with the use of both typical and atypical antipsychotics (Mannesse et al, 2010; Ali and Bazzano, 2018). The clear association between antipsychotic drug use in a clinical setting and the occurrence of these abnormalities has not been established.
Early observational studies that examined the relationship between hyponatremia and antipsychotic drugs reported higher incidence with typical antipsychotics than atypical antipsychotics, particularly phenothiazines (Kimelman and Albert, 1984; Canuso and Goldman, 1996; Spigset and Hedenmalm, 1996; Mannesse et al, 2010). To date, there has been only 1 comprehensive systematic review addressing this topic, which was conducted in 2010 (Meulendijks et al., 2010). More recently, a systematic review of case reports on hyponatremia induced only by atypical antipsychotics in patients with schizophrenia was published (Ali and Bazzano, 2018).
Hyponatremia/SIADH in atypical antipsychotics may be mediated by the action of serotonin, both by the release of ADH induced by the stimulation of central serotonin 5-HT2 and 5-HT1c receptors and by the increase in the effects of ADH at the renal medullary level (Anderson et al., 1992; Jørgensen et al., 2003). Prolonged blockade of dopamine D2 receptors and subsequent stimulation of the release of ADH and increase in its peripheral response (Hirayama et al., 2001; Milella et al., 2010; Fabrazzo et al., 2019) have also been proposed, limited to typical antipsychotic drugs. Yet, stratified disproportionality analyses of antipsychotics based on chemical structure and receptor affinity profiles of the dopamine D2 receptor and serotonin 5-HT2A have not shown a variation regarding the risk of hyponatremia in VigiBase (Mannesse et al., 2010), leaving unanswered the question of how antipsychotic drugs may lead to hyponatremia.
Given the widespread use of antipsychotics in different neuropsychiatric disorders and in light of the poorly understood pharmacological mechanism of action, it is of clinical relevance to elucidate the plausible pharmacodynamic relationship between hyponatremia and antipsychotics. Moreover, in the past decade, several studies have been carried out that describe the development of hyponatremia in association with antipsychotic drug treatment (Bun et al., 2011; Manu et al., 2012; Yang and Cheng, 2017; Falhammar et al., 2019; Yamamoto et al., 2019); however, to our knowledge, no review has been conducted to summarize findings.
We thus conducted a case-control study by using the US Food and Drug Administration Adverse Event Reporting System (FAERS) database aimed at quantifying the association between antipsychotics and the occurrence of hyponatremia/SIADH. To then clarify whether hyponatremia induction by antipsychotic drugs is driven by their receptor occupancy characteristics (dopaminergic D1, D2, D3, D4, histamine H1, muscarinic M1, M2, M3, central adrenergic α 1a, α 2a, serotonin 5-HT1A, 5-HT2A, 5-HT2C), we carried out a combined pharmacovigilance–pharmacodynamic (PV–PD) analysis. This method has been previously used to study the pharmacological mechanisms of adverse reactions to a variety of drug classes (Montastruc et al., 2015; Carnovale et al., 2019; Mazhar et al., 2019). It can be used to establish an association between pharmacological targets of drugs and their corresponding reporting risks for adverse drug reactions (ADRs) of interest observed in a large pharmacovigilance database. Finally, we performed an updated systematic review of the current literature to include all additional data on antipsychotic-associated hyponatremia.
Experimental Procedures
In Silico Pharmacodynamic Analysis
Setting and Study Design—
Data were obtained from the FAERS, one of the largest spontaneous reporting system databases. The FAERS receives approximately 1.5 million adverse events (AEs), product complaints, and user error reports from healthcare practitioners, consumers, companies, and other sources concerning drugs, vaccines, and medical devices for human use (Harpaz et al., 2016). The database is updated quarterly and designed in accordance with the international safety reporting guidance issued by the International Conference on Harmonization (ICH 2000). AEs are recorded in the FAERS using the Medical Dictionary for Regulatory Activities (MedDRA) preferred terms. The number of safety reports sent to the FDA annually is continuously expanding due to increases in the type and number of products the agency regulates, awareness of the importance of these reports, ease of submitting reports (i.e., digitally), and population size (Duggirala et al., 2016).
This study was designed as a nested case-control study. The base cohort consisted of all ADRs involving any antipsychotic drug (Anatomical Therapeutic Chemical [ATC] code N05A, excluding lithium [ATC N05AN]) as suspected, interacting, or concomitant drug and for which information on binding affinities were available. The study period covered the first quarter of 2004 (representing the beginning of freely available FAERS data) through to the third quarter of 2019.
Data Acquisition and Data Processing—
AEs data recorded in the FAERS were downloaded from the FDA website (http://www.fda.gov/Drugs/InformationOnDrugs/ucm135151.htm). The database consists of 7 datasets, namely patient demographic and administrative information (file descriptor DEMO), drug and biologic information (DRUG), adverse events (REAC), patient outcomes (OUTC), report sources (RPSR), start and end dates of drug therapy (THER), and indications for use/diagnosis (INDI). These 7 datasets were joined by unique identification numbers for each FAERS report and a relational database was built. Data extraction was restricted to reports without missing values for age and sex. We analyzed only reports concerning adults (≥18 years).
Because FAERS may sporadically contain duplicate reports, in case of reports submitted by both the consumer and the sponsor or intentional multiple reporting, data were scrutinized further manually based on similarities in patients, ADRs, and medicinal product data. Duplicate records were detected and deleted accordingly. We further standardized our dataset for possible misspelt or variants of drug names. Drug name text-mapping was accomplished by normalizing multiple drug names into a single generic name by automated matching processes through SQL-database schema. Subsequently, an open-source program, OpenRefine (Ham, 2013), was used to standardize drug name variants in the dataset to make them consistent with the international nonproprietary nomenclature defined by the World Health Organization ATC classification.
Definition of Cases and Controls—
Cases were defined as all Individual Case Safety Reports (ICSRs) where at least 1 MedDRA lower-level term from the standardized MedDRA query for “hyponatremia /SIADH (narrow)” (released in March 2014 with MedDRA Version 15.0) has been coded in the adverse reaction section (outcome of interest) in relation to antipsychotic drug(s). Noncases (controls) were all other ADRs reported in the database during the same period of time (i.e., all ADRs reports without the outcome of interest).
Potential Confounding Factors—
Potential confounding factors retrieved from the case reports included age and sex of the patient, exposure to concomitant medication associated with hyponatremia, reporting year, year since marketing, characteristics of the reporter (physician; pharmacist; other caregivers; pharmaceutical company, indirectly obtained from a healthcare professional; and patient/consumer). Concomitant use of medication associated with hyponatremia/SIADH was defined as one of the drugs summarized in Supplementary Table 1 reported as a concomitant or interacting drug for an ADR.
Pharmacodynamic Data Sources and Methods for Calculation of Antipsychotics Receptor Occupancy—
For each antipsychotic drug studied, we quantified degrees of occupancy at dopaminergic D1, D2, D3, and D4; histamine H1; muscarinic M1, M2, and M3; central adrenergic α 1a and α 2a; and serotonin 5-HT1A, 5-HT2A, and 5-HT2C receptors (Supplementary Table 2). Degree of receptor occupancy was calculated according to an equation derived from the pharmacological receptor theory’s model (Yamada et al., 2002; Kenakin, 2004). By using this approach, receptor-mediated pharmacological actions of drugs can be estimated quantitatively with reasonable accuracy (Matsui-Sakata et al., 2005). The receptor occupancy is expressed by the following equation:
where [Cr] represents the concentration of unbound antipsychotic (nmol/L) and constant Ki (nM) is the equilibrium dissociation constant of a ligand determined in inhibition studies. [Cr] were estimated according the “therapeutic reference ranges” reported in the AGNP Consensus Guidelines for Therapeutic Drug Monitoring in Psychiatry (Hiemke et al., 2018) and data on plasma protein binding reported in DrugBank, Integrated Database of ADMET, and Adverse effects of Predictive Modeling (Legehar et al., 2016) databases and individual monographs or regulatory documents.
For a given drug-receptor pair, the constant Ki is reflective of the binding affinity of the drug to a receptor. For each studied antipsychotic drug, values of binding affinities (Ki) at 12 different receptors potentially involved in iatrogenic hyponatremia were searched in the European Bioinformatics Institute-ChEMBL (ChEMBL, 2020), International Union of Basic and Clinical Pharmacology (IUPHAR, 2020), Psychoactive Drug Screening Program (PDSP, 2020), and BindingDB (Gilson et al., 2016). Only Ki obtained from human species and subsets on data for ligand-receptor combinations studied in CNS tissue or cloned receptors were selected. In case of multiple Ki values for the same receptor-ligand pair, we computed the dispersion, discarded values at the tails of the distribution, and reported the resulting average.
Statistical Analyses—
We first developed descriptive statistics for cases and noncases. The normality of data was also verified by means of the Kolmogorov test, and appropriate parametric and nonparametric analyses were conducted. Descriptive analysis was performed for cases and noncases, in terms of age, female sex, and use of concomitant medication associated with hyponatremia. The Student’s t test was used to assess whether age was distributed differently between cases and noncases, whereas Pearson’s chi-square test was used to assess whether categorical variables (sex and the presence of concomitant medications associated with hyponatremia) were differently distributed between cases and noncases. Tests were 2-tailed, with significance set at a P value of .05.
We then performed univariate and multivariate logistic regression to study the association between receptors’ occupancy and the occurrence of hyponatremia with antipsychotic drugs. Quantitative values of occupancy were converted into a categorical variable by grouping values into 2 categories (low level of occupancy [<50%], and high level of occupancy [≥50%]) and were included in the regression models. In case of several antipsychotics with different receptor occupancies on the same receptor, the highest degree of occupancy was selected.
Logistic regressions used hyponatremia cases as the dependent variable. Potential explanatory variables included categorized values of occupancies ([“high level of occupancy” vs “low level of occupancy” [reference group]) of involved antipsychotic drugs at 12 different receptors investigated along with the confounding variables discussed in section 2.1.4.
First, univariate analyses (model 1) were carried out to calculate the unadjusted association between receptor occupancy and outcome separately for each receptor. Levels of receptor occupancies that were significantly associated with the outcome in the univariate analyses were simultaneously entered in the multivariate model (model 2). A stepwise descending procedure (model 3) was conducted to select the main variables related to hyponatremia. In this modelling, the P value to enter the model was ≤.05, and the P value to leave the model was >.10. The validity of the models was checked using the Hosmer and Lemeshow Goodness-of-Fit Test. The association between receptor occupancy and hyponatremia was estimated as crude and adjusted odds ratios with their corresponding 95% confidence interval (95% CI).
To identify antipsychotics that were reported more frequently than expected in FAERS, we performed a case/noncase comparison among the 19 drugs to measure the disproportionality of drug-associated hyponatremia reporting for each antipsychotic. This method allows the comparison of hyponatremia with other cases of ADRs regarding the exposure to one antipsychotic compared with other antipsychotics (Montastruc et al., 2011), quantified as reporting odds ratio (ROR) and its corresponding 95% CI (Rothman et al., 2004). The ROR for each antipsychotic–ADR combination was defined as the ratio between proportions of cases containing the suspected antipsychotic in the “case” (number of cases with 1 antipsychotic as the suspected drug divided by the number of cases with other antipsychotics) and in the “noncase” (number of noncases with the same antipsychotic as the suspected drug divided by the number of noncases with other antipsychotics) group. We only included antipsychotic drugs that had been reported 3 or more times to be a suspected cause of hyponatremia. A signal of disproportionate reporting was defined when the lower limit of the 95% 2-sided CI for the ROR exceeded the threshold value of 1.
All analyses were performed using counts of unique cases. Data reading, filtering, and processing were conducted through RStudio. All statistical analyses were using STATA (StataCorp, College Station, TX).
Systematic Review
We conducted a systematic review of the published literature in accordance with Preferred Reporting Items for Systemic Reviews and Meta-Analyses guidelines (Liberati et al., 2009) for the evidence of hyponatremia following antipsychotic (excluding lithium) treatment as reported in clinical trials and cohort and case-control studies. Outcome measures were incidence rates and the odds or hazard ratios (OR/HR) of hyponatremia in both inpatients and outpatients treated with antipsychotic drugs.
Search Strategy—
A search was conducted in the MEDLINE, PsychINFO, and EMBASE databases between April 2009 and December 6, 2020, to be current with the most recent literature since the last most comprehensive review by Meulendijks et al. (2010) on antipsychotic-associated hyponatremia studies available in the literature. For the search, which was based on keywords from the systematic catalog or alphabetic index, the following terms were used: “antipsychotic agents,” “neuroleptic agent,” “hyponatremia,” “inappropriate ADH syndrome,” “sodium blood level,” “sodium deficiency,” “sodium depletion,” and “water-electrolyte balance.” The only limit on the search was time. The complete search strategy is available in Supplementary Material 1.
Study Selection, Data Abstraction, and Quality Assessment—
After duplicate removal, the search results were then screened by title and abstract. All potentially relevant publications were retrieved in full text and evaluated in detail. Bibliographies of retrieved articles were examined for further relevant publications. Studies were eligible if they included descriptions of study design and population(s) and case definitions of hyponatremia and if the results on the outcome for participants exposed to antipsychotics were separately identified. We excluded studies that reported polydipsia but not hyponatremia/SIADH or explicitly reported polydipsia. Our study eligibility criteria also excluded lithium. Any disagreements about study selection were resolved by consensus with 2 researchers (F.M. and V.B.).
For each included study, we extracted the following information: study design (study type, study duration, sample size, serum sodium cutoff values used for definition of hyponatremia); patient characteristics (in/out patients and source, age, sex, and number exposed to antipsychotics); and outcomes on antipsychotic-associated hyponatremia. Because of our broad inclusion criteria, we anticipated considerable heterogeneity, which is why we assessed and compared effect studies for study design, sample size, patient characteristics, cutoff values, and definition of hyponatremia.
The quality of the individual studies was assessed by 1 reviewer (F.M.) and independently checked by a second (V.B.); disagreements were resolved by consensus. Cohort studies and case-control studies were assessed with the designated Newcastle-Ottawa Scale (NOS) (Wells et al., 2016). The NOS for cohort studies was adapted for use with cross-sectional studies in a similar manner to previous research (Hermont et al., 2014). The NOS, which was adapted for cross-sectional studies, uses the same star system in the main scale only. The difference is that on this scale, there are 5 stars for the selection dimension, 2 stars for the comparability dimension, and 3 stars for the outcomes dimension, which indicates the quality of the study. Each item is scored 1 or 2 and summed for a total indicating overall study quality as either high (7–9), moderate (5–6), or low (0–4).
Results
In Silico Pharmacodynamic Analysis
Study Population—
From the FAERS we identified 138 194 ICSRs involving at least 1 of the 19 antipsychotics of interest with information on age and sex. Of 138 194 ICSRs, 1520 (1.1%) were related to hyponatremia (cases). Univariate analyses of demographics and characteristics of nested cases and noncases population are presented in Table 1. Compared with noncases, prevalence of hyponatremia cases was higher in female individuals (56% vs 53%; P < .05) and most frequently reported by physicians and patients. The mean age of cases was significantly higher than that of noncases (55.88 vs 47.05 years; P < .0001). Concomitant medication associated with hyponatremia was used in 69% of the cases and in 66% of the noncases (P < .05).
Table 1.
Characteristics of the Nested Cases/Noncases Population in the FAERS (n = 138 192)
| Characteristic | Cases (n = 1520) |
Noncases (n = 136 674) |
P value |
|---|---|---|---|
| Patient age, mean (SD), y | 55.88 ± 16.65 | 47.05 ± 17.14 | <.0001a |
| Sex, females | 849 (56) | 72 366 (53) | .024b |
| Concomitant use of medication associated with hyponatremia | 1049 (69) | 90 205 (66) | .0460b |
| Reporter type | |||
| Physician | 502 (33) | 50 569 (37) | .024b |
| Pharmacist | 304 (20) | 17 768 (13) | |
| Other caregivers | 182 (12) | 13 667 (10) | |
| Patient/consumer | 426 (28) | 41 002 (30) | |
| Unknown | 106 (7) | 13 667 (10) | |
| Reporting year | |||
| 2004–2012 | 684 (45) | 43 736 (32) | .074b |
| 2013–2019 | 836 (55) | 92 938 (68) | |
| Years since marketing | |||
| <10 | 532 (35) | 56 036 (41) | .023b |
| 10–15 | 289 (19) | 38 269 (28) | |
| 15–20 | 426 (28) | 9567 (7) | |
| >20 | 274 (18) | 32 802 (24) |
All data except patient age are shown as number (%).
a t test.
b Chi-square test.
Pharmacovigilance–Pharmacodynamic Analysis for the Association Between Receptor Occupancies and Antipsychotic-Associated Hyponatremia—
Univariate logistic regression analysis showed a significant and positive association between hyponatremia reports and dopamine D3 (OR = 1.20; 95% CI = 1.09–1.31) and D4 (OR = 1.17; 95% CI = 1.06–1.28) receptor occupancies, whereas significant negative association was found with histamine H1 (OR = 0.72; 95% CI = 0.65–0.79) and serotonin 5HT1A (OR = 0.52; 95% CI = 0.47–0.58) and 5HT2A (OR = 0.53; 95% CI = 0.48–0.60) receptor occupancies (Table 2).
Table 2.
The Association Between Antipsychotic Receptor Occupancies and the Occurrence of Hyponatremia, Using Logistic Regression Analyses
| Cases (hyponatremia) |
Noncases (other AEs) |
Crude OR (95% CI) |
AOR (95% CI)a |
AOR (95% CI)a | |||
|---|---|---|---|---|---|---|---|
| Receptor | n | % | n | % | Model 1 (univariate) | Model 2 (multivariate) | Model 3 (stepwise) |
| D2 | 1255 | 66.0 | 113 892 | 66.8 | 0.96 (0.87–1.06) |
0.94 (0.85–1.03) |
— |
| D3 | 765 | 40.2 | 61 177 | 35.9 | 1.20 (1.09–1.31) |
1.43
(1.13–1.56) |
1.21 (1.09–1.34) |
| D4 | 706 | 37.1 | 57 128 | 33.5 | 1.17 (1.06–1.28) |
1.12
(1.03–1.23) |
— |
| H1 | 979 | 51.5 | 101 463 | 59.5 | 0.72 (0.65–0.79) |
0.81 (0.65–1.08) |
— |
| M1 | 909 | 47.8 | 93 675 | 55.0 | 0.75 (0.68–0.82) |
1.02 (0.86–1.29) |
— |
| M2 | 143 | 7.5 | 22 732 | 13.3 | 0.93 (0.44–1.43) |
1.21 (0.95–1.31) |
— |
| α 1 | 937 | 49.3 | 97 469 | 57.2 | 0.72 (0.66–0.79) |
0.70
(0.64–0.77) |
— |
| α 2 | 88 | 4.6 | 8672 | 5.1 | 0.90 (0.73–1.12) |
1.05 (0.84–1.30) |
— |
| 5-HT1A | 488 | 25.7 | 67 774 | 39.8 | 0.52 (0.47–0.58) |
0.53
(0.47–0.58) |
— |
| 5-HT2A | 1563 | 82.2 | 152 651 | 89.6 | 0.53 (0.48–0.60) |
0.59
(0.53–0.67) |
0.78 (0.68–0.90) |
| 5-HT2C | 1416 | 74.5 | 135 657 | 79.6 | 0.84 (0.67–1.03) |
0.95 (0.81–1.18) |
— |
Abbreviations: AOR, adjusted odds ratio; CI, confidence interval; OR, odds ratio.
For each receptor: high receptor occupancy (≥50%) vs low receptor occupancy (<50%) set as the baseline level. D1 receptor not included as none of the antipsychotic possess high occupancy.
Significant values are reported in bold: lower bound of 95% CI >1 meaning an increase of the risk of ADR reporting; upper bound of 95% CI <1 meaning a decrease of the risk of ADR reporting.
a Adjusted for age, gender, concomitant use of medication associated with hyponatremia, type of reporter, and years since marketing.
Using multivariate logistic regression analysis, higher receptor occupancies for dopamine D3 (adjusted [adj]. OR = 1.43; 95% CI = 1.13–1.56), D4 (adj. OR = 1.12; 95% CI = 1.02–1.23), α 1 adrenergic (adj. OR = 0.70; 95% CI = 0.64–0.77), and serotonin 5HT1A (adj. OR = 0.53; 95% CI = 0.47–0.58) and 5HT2A (adj. OR = 0.59; 95% CI = 0.53–0.67) were significant. No other significant association were found for the remaining 6 targets.
Receptor targets significantly associated (D3, D4, α 1, 5HT1A, and 5HT2A) with hyponatremia occurrence were then entered into a stepwise regression model while adjusting for all potential confounders. In the stepwise multivariable analysis, only dopamine D3 (adj. OR = 1.21; 95% CI = 1.09–1.34) receptor occupancy was found to be associated with significantly increased risk for hyponatremia. In contrast, the occurrence of hyponatremia decreased substantially with higher serotonin 5HT2A receptor (adj. OR = 0.78; 95% CI = 0.68–0.90) occupancy.
Case/Noncase Analysis—
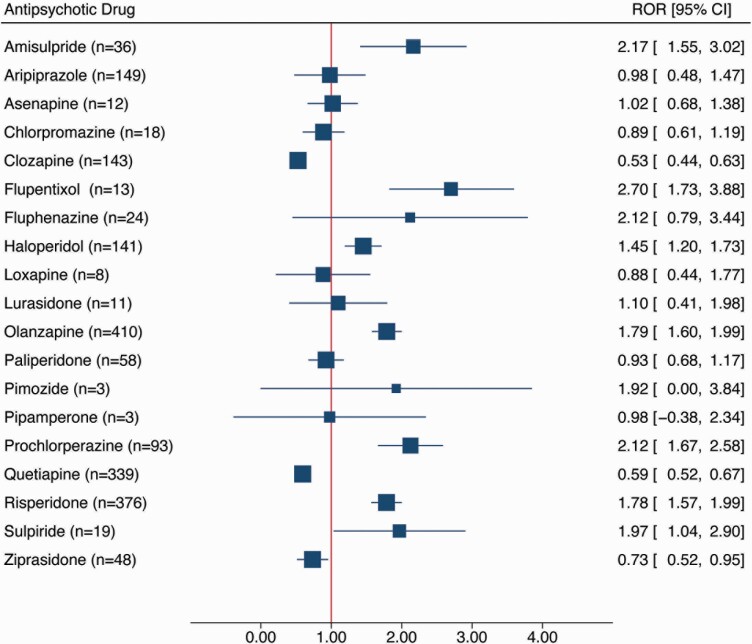
Results of disproportionality analysis for each antipsychotic drug are shown in Figure 1. The highest RORs were found for flupentixol (n = 13; ROR = 2.70; 95% CI = 1.73–3.88), amisulpride (n = 36; ROR = 2.17; 95% CI = 1.55–3.02), prochlorperazine (n = 93; ROR = 2.12; 95% CI = 1.67–2.58), and fluphenazine (n = 24; ROR = 2.12; 95% CI = 0.79–3.44). In contrast, no signal of disproportionate reporting was found for lurasidone, asenapine, clozapine, quetiapine, ziprasidone, loxapine, chlorpromazine, aripiprazole, pipamperone, and paliperidone.
Figure 1.
Case/noncase analysis for the association between antipsychotic exposure and the occurrence of hyponatremia in the US Food and Drug Administration Adverse Event Reporting System. Antipsychotics with less than 3 reports of diabetes are not presented (i.e., promazine, melperone, zuclopenthixol). Abbreviations: 95% CI, 95% confidence interval; ROR, reporting odds ratio.
Supplementary Figures 1 and 2 show the degrees of 5-HT2A and D3 receptor occupancies by antipsychotic drugs plotted against ROR, together with a line describing the logistic model.
Systematic Review of the Literature
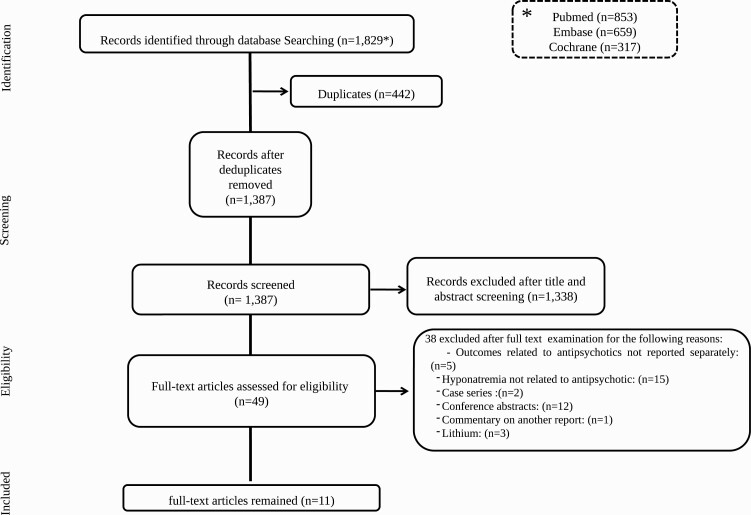
Figure 2 shows the flow of the systematic literature search. The search resulted in 1343 unique titles. All titles were screened, after which 48 full-text articles were assessed for eligibility. Ultimately, 11 studies were identified which satisfied the eligibility criteria.
Figure 2.
Preferred Reporting Items for Systematic Reviews and Meta-analyses flow diagram of process of study selection.
Characteristics of the Reviewed Studies—
No randomized controlled trials reporting hyponatremia following antipsychotic drugs use were found. Eleven observational studies were eligible for the inclusion in our systematic review (Table 3); of these, 6 were case-control studies comparing hyponatremia cases with controls (normonatremia) for their relative exposure to antipsychotic drugs (Bun et al., 2011; Manu et al., 2012; Yang and Cheng, 2017; Falhammar et al., 2019; Yamamoto et al., 2019; Jun et al., 2020), 2 were retrospective cohort studies that included control group as patients not exposed to antipsychotic drugs (Lange-Asschenfeldt et al., 2013; Gandhi et al., 2016), 2 were cross-sectional (Serrano et al., 2014; Shepshelovich et al., 2017), and 1 report from drug-surveillance programs relying on a system of enhanced monitoring of drug-related adverse events (Letmaier et al., 2012). Shepshelovich et al. (2017) exclusively looked for the medication-induced SIADH.
Table 3.
Studies on the Risk or Incidence of Hyponatremia With the Use of Antipsychotics
| Study design | Patient characteristics | Outcome | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, y | Duration (y) | Sample size (n) | SNa+ active monitoring | Cutoff SNa+ (mEq/L) | In/Out | Disease | Age (mean± SD) | F (%) | Exposed to AP (n) | Cases of hyponatremia / no. exposed or no. of controls | Type of AP used | Type of measure | Overall | TAs | ATy APs |
| Case-control studies (hyponatremia vs normonatraemia) | |||||||||||||||
| a Jun et al., 2020 | 5 (with ≥30 d follow- up for recurrent hyponatremia) | 19 173 | No | <135 | Out | Recurrent hyponatremia | >65 (range: 65–75) | 52.1 | 1535 | 79/1,456b | Any | OR [95% CI] |
0.83 [0.64–1.09] | — | — |
| Falhammar et al., 2019 | 9 | 71 741 | No | <135 | In | Ad | Median 76 (range: 18–103) | 72 | 317 (newly initiated) |
148/169b | Any | OR [95% CI] |
1.80 [1.38–2.34] | 2.94 [2.09–4.13] | 1.05 [0.75–1.47] |
| Yamamoto et al., 2019 | 12 | 7442 | Yes | <130 | In/ out |
Epilepsy | 36.2 ± 14.4 | 46.7 | 504 | 17/487b | Any + anticonvulsant (except CBZ) | Incidence (%) |
3.40 | — | — |
| OR [95% CI] |
3.47 [2.03–5.95] | — | — | ||||||||||||
| Yang and Cheng, 2017 | 15 | 2051 | No | <135 | In/ out |
Psychiatric illness | 54.7± 13.9 | 43.8 | 1069 | 92/977b | Any | Incidence (%) |
8.61 | 10.66 | 10.05 |
| HR [95% CI] |
— | 3.13 [1.83–5.34] | 2.09 [1.36–3.23] | ||||||||||||
| Manu et al., 2012 | 1 | 924 | No | <136 | In | Ad | 45.15 ± 19.6 |
46.8 | 642 | 37/605b | Any | Incidence (%) |
6.11 | 11.84 | 5.29 |
| Bun et al., 2011 | 1 | 248 | Yes | <130 | In | Psychiatric illness | 46.45 ± 17.0 | 38.3 | 248 | 91/157b | Any | OR [95% CI] |
1.79 [1.04–3.10] | — | — |
| Cohort Studies (antipsychotic users vs nonantipsychotic users) | |||||||||||||||
| Gandhi et al., 2016 | 9 | 116 016 | No | ≤132 | Out | Ad | 81 ± 7.7 | 66.8 | 58 008 | 86/58 008 | ATyAPs | Incidence (%) |
— | — | 0.15 |
| RR [95% CI] |
— | — | 1.62 [1.15–2.29] | ||||||||||||
| Lange- Asschenfeldt et al., 2013 | 3 | 7113 | Yes | <135 | In | Psychiatric illness | Median 67 (range: 21–101)c | NR | 4976 | 199/4976 | Any | Incidence (%) |
3.99 | 6.00 | 3.40 |
| Cross-sectional | |||||||||||||||
| Shepshelovich et al., 2017 | 6 | 198 | No | <135 | In | SIADH | 66.6 ± 17.3 | 55.5 | 22 | 19/22 | Any | Incidence (%) |
86 | — | — |
| Serrano et al., 2014 | 4 | 219 | No | <135 | In/Out | Psychiatric illness | 44.2 ± 15.7 | 52.5 | 183 | 13/183 | Any | Incidence (%) | 7 | 26 | Clozapine = 3; others = 5 |
| Clozapine vs any other AP | OR [95% CI] |
— | 31.3 [3.9–247.0] | 2.9 [0.5–18.2] | |||||||||||
| Observational multidrug surveillance program/adverse drug reaction monitoring | |||||||||||||||
| Letmaier et al., 2012 | 14 | 263 864 | No | <130 | In | Psychiatric illness | 60.7 ± 15.9 | 55.7 | 189 462 | 5/189 462 | Any | Incidence (%) | 0.003 | Perazine = 0.015; haloperidol = 0.007 | Risperidone = 0.004 |
Abbreviations (patient characteristics and outcome): Ad, hospital admission for hyponatremia; AP, antipsychotic; AtyAPs, atypical antipsychotics; CBZ, carbamazepine; F, females; HR, hazard ratio; In, inpatient; NR, not reported; OR, odds ratio (where provided we report only adjusted ratio); Out, outpatient; RR, relative risk ratio; SIADH, syndrome of inappropriate antidiuretic hormone secretion; TAs, typical antipsychotics.
a Cases and control were drawn from the population in a fully enumerated cohort
b Number of patients without hyponatremia (controls)
c Values related only to patients who experienced hyponatremia .
All included studies were retrospective, of which 3 (Bun et al., 2011; Lange-Asschenfeldt et al., 2013; Yamamoto et al., 2019) explicitly mentioned a systematic monitoring of serum sodium levels, defined as “active monitoring” in this review.
Four large-scale studies reporting hyponatremia following antipsychotic treatment were identified: 1 population-based cohort study (Gandhi et al., 2016) and 2 population-based case-control studies ((Yang and Cheng, 2017; Falhammar et al., 2019) using health administrative databases and 1 in a drug-surveillance program of psychiatric inpatients (Letmaier et al., 2012). The RR, HR, or OR of hyponatremia associated with antipsychotic drugs was calculated in 7 studies: 5 case-control studies (Bun et al., 2011; Yang and Cheng, 2017; Falhammar et al., 2019; Yamamoto et al., 2019; Jun et al., 2020), 1 cohort study (Gandhi et al., 2016), and 1 cross-sectional study (Serrano et al., 2014).
The quality of the included studies was moderate (mean NOS 6.9, SD = 2.3) (Supplementary Table 3). Four studies (1 for cross-sectional and cohort and 3 for case-control studies) were classified as high quality, and the 3 case-control studies were categorized as moderate quality. Three studies (2 for cross-sectional and 1 for cohort studies) were rated as low quality. In general, most studies with low to moderate quality had no score for the comparability section and outcome assessment was unsatisfactory.
Risk or Incidence Rate of Hyponatremia—
Due to considerable heterogeneity in study designs and characteristics of the studied populations and serum sodium (SNa+) threshold values (SNa+ ≤ 130 vs ≤135 mmol/L), incidence rates varied greatly, with the larger-scale studies or without active monitoring of serum sodium levels resulting in more modest rates. Studies with a SNa+ cutoff ≤135 or ≤136 mmol/L resulted in incidence figures between 3.99% and 86%, whereas in studies using stringent a case definition of SNa+ ≤130 or ≤132 mmol/L or severe hyponatremia, incidences between 0.003 % and 3.40% were reported.
The HR, OR, or RR of hyponatremia for pooled antipsychotic drugs was determined in 4 studies. The lowest risk was found by Bun et al. (2011) (adj. OR = 1.79; 95% CI = 1.04–3.40), whereas the higher risk was reported in adult epilepsy patients treated with antipsychotics in addition to antiepileptic drugs (carbamazepine excluded) (adj. OR = 3.47; 95% CI = 2.03–5.95) (Yamamoto et al., 2019). One population-based cohort study reported atypical antipsychotic use compared with nonuse was associated with an increased risk of hospitalization with hyponatremia within 30 days (RR = 1.62; 95% CI = 1.15–2.29) (Gandhi et al., 2016). Jun et al. (2020) found no association between the use of antipsychotics and recurrence of symptomatic or severe hyponatremia in older patients (adj. OR = 0.83; 95% CI = 0.64–1.09).
Several studies exclusively reported the occurrence of hyponatremia in users of atypical or typical antipsychotic users. Use of typical antipsychotic was consistently reported for increased risk of hyponatremia compared with atypical antipsychotic. The Swedish register–based case-control study reported users of typical antipsychotics were more likely to experience severe hyponatremia (adj. OR = 2.94; 95% CI = 2.09–4.13) than those on atypical antipsychotics (adj. OR = 1.05; 95% CI = 0.75–1.47) (Falhammar et al., 2019). Similarly, a population-level case-control study using Taiwan’s claim database reported an elevated risk of hyponatremia with typical antipsychotics (adj. HR = 3.13 [1.83–5.34]) vs atypical (adj. HR = 2.09 [1.36–3.23]) (Yang and Cheng, 2017).
The same distribution was found in the Arzneimittelsicherheit in der Psychiatrie study, with a higher incidence of hyponatremia with the typical antipsychotic perazine (0.015%) and haloperidol (0.007%), and lower with risperidone (0.004%) (Letmaier et al., 2012). In a case-control study by Manu et al. (2012), hyponatremia was reported to occur less with atypical antipsychotics (5.29%; n = 9/76) than with typical antipsychotics (11.84%; n = 28/529); however, no statistically significant difference was found.
Discussion
To date, the mechanism by which antipsychotics induce hyponatremia is not well understood. This is the first study, to our knowledge, aimed at assessing whether the degrees of antipsychotics receptor occupancy explain the occurrence of hyponatremia by using one of the largest spontaneous reporting system databases, that is, FAERS. We found a statistically significant positive association with dopamine D3 receptor degrees of occupancy and hyponatremia occurrences and a negative one with serotonin 5-HT2A degree. These associations persisted after adjustment for potential confounding factors such as sex, age, concomitant medications, and type of reporter.
The evidence for the relationship between neuroendocrine abnormalities and D3 receptors in the literature is scarce. Dopamine D3 is a member of the D2-like receptor family that can couple to effector mechanisms similar to the D2 receptor subtype (Ilani et al., 2001; Le Foll et al., 2009). Dopamine D3 receptors are unique among the D2-like receptors, exhibiting sustained high affinity for dopamine (>20-fold higher than D2 receptors), suggesting that D3 receptors, in vivo, are occupied by endogenous dopamine for extended periods, leading to high spontaneous activation of D3 receptors (Vanhauwe et al., 2000; Richtand et al., 2001). Accordingly, small changes in the number or function of D3 receptors may lead to dramatic effects on synaptic transmission, suggesting that D3 receptors could be critical modulators of normal dopaminergic function. Due to the lack of selective D3 receptor antagonists available on the market, direct evidence of the effects of selective dopamine D3 receptor antagonists on neuroendocrine abnormalities is lacking.
Many atypical antipsychotic drugs and some typical antipsychotic ones have high affinities for both D2 and D3 receptors (Girgis et al., 2011); the high affinity of endogenous dopamine for D3 receptors has been postulated to result in only minimal or no D3 receptor occupancy by antipsychotic drugs in dopamine-rich areas (Graff-Guerrero et al., 2009; Mizrahi et al., 2011; Gross and Drescher, 2012; Mugnaini et al., 2013).
The involvement of D2 receptors in the regulation of neuroendocrine abnormalities has been described in several animal models (Meltzer and Stahl, 1976; Hirayama et al., 2001; Milella et al., 2010). It is generally thought that antipsychotics may stimulate ADH release in the brain by supersensitivity of D2 receptors. Therefore, neuroendocrine dysfunction might be explained, at least in part, by inhibition of the dopamine D3 receptor. Our FAERS analysis does not support this possibility as we did not retrieve any case where cariprazine, a D3-preferring antipsychotic, was suspected in the occurrence of hyponatremia, consistent with the fact that only 1 case of hyponatremia associated with the use of cariprazine leading to drug discontinuation has been reported to date (Kane et al., 2015). It must be acknowledged, however, that cariprazine has only been recently commercialized, and we need to wait for additional observational studies using real-world data on larger-scale samples to reach a definite conclusion.
Some early animal studies showed secretion of ADH through serotonin-mediated effects on central 5-HT2 and 5-HT1C receptors (Brownfield et al., 1988; Anderson et al., 1992; Jørgensen et al., 2003). It has been speculated that the effect of various psychotropic drugs on serotoninergic transmission contribute to excess ADH secretion. However, a study we recently performed using the FAERS database (Mazhar et al., 2019) showed that serotonin-mediated neurotransmission may not be involved in the hyponatremia associated with antidepressant drugs. The results of the present analysis, and our previous results, suggest that the emergence of hyponatremia with antipsychotic is linked when D3 receptor occupancy exceeds a certain threshold, whereas high 5-HT2A occupancy provides relative protection from hyponatremia.
Our multivariable model showed that both blockades of dopamine D3 and serotonin 5-HT2A receptors independently explain the risk of hyponatremia. The case/noncase study we conducted thus supports the hypothesis that hyponatremia induced by antipsychotic drugs results from a disruption of the fine balance between dopaminergic- and serotonergic-mediated transmission. The unbalanced inhibition of dopamine D3 and serotonin 5-HT2A receptors can explain why antipsychotics that have high D3 and low 5-HT2A occupancies, such as amisulpride, sulpiride, and prochlorperazine, were the ones we found most associated with hyponatremia. Consistently, antipsychotics with nearly balanced antagonistic activities at D3 and 5-HT2A (pipamperone, risperidone, chlorpromazine, asenapine, paliperidone, aripiprazole, and quetiapine) or a high 5-HT2A antagonist property were the ones least associated with hyponatremia.
In the systematic review we conducted, we observed a considerable heterogeneity across studies, with incidence rates of hyponatremia following any antipsychotic use diverging between 0.003% and 86%, whereas the odds developing hyponatremia from effect studies range between 0.83 and 3.47. Regarding the classes of antipsychotic drugs, ORs for typical antipsychotics (2.9–31.3) were consistently higher than for atypical antipsychotics (1.1–2.9). The risks associated with individual antipsychotics drugs between 2 classes could not be established due to insufficient information.
Despite this limitation, our review clearly shows that the risk of hyponatremia is higher with typical antipsychotics but not confined to these agents several novel aspects. It also reveals that study methodologies greatly affected outcomes, particularly, the choice (or lack) of nonexposed groups or the presence/absence of active monitoring for hyponatremia. Timing and clinical indication for sodium level checks varied and often were unclearly reported. The commonly accepted definition of hyponatremia is a serum sodium level lower than the arbitrarily defined threshold of 135 mEq/L; however, some studies selected a 130-mEq/L threshold, which may, in fact, be more relevant to the severity or clinical practice, considering that symptoms seldom occur at higher values. Additionally, inconsistencies in hyponatremia case definitions compromised comparison. Studies also differed with respect to confounding factors possibly causing or contributing to hyponatremia; some studies chose to exclude such cases (Yamamoto et al., 2019), whereas others included them but performed multivariate regressions for statistical correction (Gandhi et al., 2016; Yang and Cheng, 2017).
Limitations and Strengths
The use of a spontaneous reporting system database has some important implicit limitations, because reporting is influenced by factors such as the notoriety bias, selection bias, and underreporting (Wiggins et al., 2015). The PV–PD analysis based on pharmacological receptors theory has its own limitations (Pariente et al., 2007); for instance, receptor affinity does not directly reflect the intrinsic activity of a drug. Also, receptor occupancy does not necessarily mean antagonism, and the interaction at the receptor level of antipsychotics is complex and only partially characterized. Moreover, some antipsychotics have high occupancy but fast dissociation, which is sufficient to elicit a biological therapeutic response. Nevertheless, PV–PD analysis provides reliable estimates of putative relationship and in recent past years, studies validated pharmacological preclinical studies in humans by combining real-word data with pharmacodynamic data. We have used unbound therapeutic plasma concentrations to estimate receptor occupancies, whereas a brain concentration would be the ideal approach. However, monitoring of the human brain concentration of unbound drug is experimentally not feasible.
Unlike typical case–control studies, cases and noncases are drawn from different populations; thus, this method cannot be a substitute for the classical case–control but gives relatively reliable results if the database contains several thousand different drug−event combinations. RORs are influenced by potential confounders such as selective underreporting, follow-up period bias, and exposure misclassification bias. Neither the prevalence nor the incidence rate of ADRs can be computed from the analysis of the FAERS dataset because the primary goal of such system is to signal the existence of a possible relationship between a drug or drug class and an ADR, and it does not prove causality.
As a primary limitation, our systematic review included a small number of studies, which may influence the quality and the strength of the results. Moreover, heterogeneity in study design (case–control, cohort, cross-sectional) precluded us from pooling the estimates.
Despite these limitations, the present work has several strengths. The study was conducted using one of the largest pharmacovigilance databases, which includes more than 10 million reports. Furthermore, our base cohort contains a considerably higher number of reports, strengthening the statistical power of the analyses. Furthermore, to contribute in closing the knowledge gap on this issue, we used a relatively innovative approach in the field of pharmacovigilance, and, unlike previous studies that applied a similar approach to investigate pharmacological mechanisms of ADR using 1 pharmacological target in the linear regression model, we considered all relevant targets in our multivariable logistic regression analyses.
Conclusion
This is the first study, to our knowledge, aimed at evaluating the potential association between degrees of antipsychotics receptor occupancy and the occurrence of hyponatremia by using one of the largest spontaneous reporting system databases: FAERS. Of importance, our PV–PD analysis showed that both blockades of dopamine D3 and serotonin 5-HT2A receptors independently explain the risk of hyponatremia. The unbalanced inhibition of dopamine D3 and serotonin 5-HT2A receptors can explain why antipsychotics that have high D3 and low 5-HT2A occupancies (amisulpride, sulpiride, and prochlorperazine) were the ones we found most associated with hyponatremia. Consistently, antipsychotics with nearly balanced antagonistic activities at D3 and 5-HT2A (pipamperone, risperidone, chlorpromazine, asenapine, paliperidone, aripiprazole, and quetiapine) or high 5-HT2A antagonist property were the ones least associated with hyponatremia. Given the fact that antipsychotics have high affinities for multiple receptors and receptor subtypes, the potential for specific adverse events varies for each different antipsychotic. Therefore, drug-specific side-effect profiles should be considered together with patient-specific risk factors when deciding on the optimal treatment for individual patients.
Our review of the current literature, based on the few, nonetheless relatively large-scale studies published to date shows that typical antipsychotics have a more attenuated risk profile for hyponatremia. However, hyponatremia can also occur with these agents, albeit less frequently. We were unable to draw conclusions regarding the risks associated with individual antipsychotic owing to insufficient information. Prospective controlled studies are needed that assess the risk of hyponatremia more systematically in well-defined, larger-scale populations.
Supplementary Materials
Supplementary data are available at International Journal of Neuropsychopharmacology (IJNPPY) online.
Supplementary Table S1. Drugs associated with the occurrence of hyponatremia.
Supplementary Table S2. Receptor occupancies for the 19 antipsychotics of interest.
Supplementary Table S3. The Newcastle Ottawa scale for the studies included in the review.
Supplementary Material S1. The search string.
Supplementary Figure S1. The relationship between 5-HT2A receptor occupancy by antipsychotics and the occurrence of hyponatremia in a nested case/noncase population studied in the FAERS.
Supplementary Figure S2. The relationship between the D3 receptor occupancy by antipsychotics and the occurrence of hyponatremia in a nested case/noncase population studied in the FAERS.
Acknowledgments
F. Mazhar is enrolled in the PhD in Experimental and Clinical Pharmacological Sciences, University of Milano, which supports his fellowship.
This work was supported by the Regional Centre of Pharmacovigilance of Lombardy (to E.C.), the Italian Medicines Agency, Agenzia Italiana del Farmaco (to E.C.), and by the Italian Ministry of Health (Ricerca Corrente 2019–2020 to M.P. and M.N.; and Progetto Finalizzata RF-2016-02363761 to E.C. and A.C.), which are gratefully acknowledged. The funding public institutions had no role in any part of the work.
Statement of Interest
All authors have no conflict of interest to declare.
References
- Ahmadi L, Goldman MB (2020) Primary polydipsia: update. Best Pract Res Clin Endocrinol Metab 34:101469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali SN, Bazzano LA (2018) Hyponatremia in association with second-generation antipsychotics: a systematic review of case reports. Ochsner J 18:230–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson IK, Martin GR, Ramage AG (1992) Central administration of 5-HT activates 5-HT1A receptors to cause sympathoexcitation and 5-HT2/5-HT1C receptors to release vasopressin in anaesthetized rats. Br J Pharmacol 107:1020–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownfield MS, Greathouse J, Lorens SA, Armstrong J, Urban JH, Van de Kar LD (1988) Neuropharmacological characterization of serotoninergic stimulation of vasopressin secretion in conscious rats. Neuroendocrinology 47:277–283. [DOI] [PubMed] [Google Scholar]
- Bun S, Serby MJ, Friedmann P (2011) Psychotropic medication use, hyponatremia, and falls in an inpatient population: a retrospective study. J Clin Psychopharmacol 31:395–397. [DOI] [PubMed] [Google Scholar]
- Canuso CM, Goldman MB (1996) Does minimizing neuroleptic dosage influence hyponatremia? Psychiatry Res 63:227–229. [DOI] [PubMed] [Google Scholar]
- Carnovale C, Mazhar F, Arzenton E, Moretti U, Pozzi M, Mosini G, Leoni O, Scatigna M, Clementi E, Radice S (2019) Bullous pemphigoid induced by dipeptidyl peptidase-4 (DPP-4) inhibitors: a pharmacovigilance-pharmacodynamic/pharmacokinetic assessment through an analysis of the vigibase®. Expert Opin Drug Saf 18:1099–1108. [DOI] [PubMed] [Google Scholar]
- Duggirala HJ, et al. (2016) Use of data mining at the Food and Drug Administration. J Am Med Inform Assoc 23:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrazzo M, Fuschillo A, Perris F, Catapano F (2019) The unmasking of hidden severe hyponatremia after long-term combination therapy in exacerbated bipolar patients: a case series. Int Clin Psychopharmacol 34:206–210. [DOI] [PubMed] [Google Scholar]
- Falhammar H, Lindh JD, Calissendorff J, Skov J, Nathanson D, Mannheimer B (2019) Antipsychotics and severe hyponatremia: a Swedish population-based case-control study. Eur J Intern Med 60:71–77. [DOI] [PubMed] [Google Scholar]
- Gandhi S, McArthur E, Reiss JP, Mamdani MM, Hackam DG, Weir MA, Garg AX (2016) Atypical antipsychotic medications and hyponatremia in older adults: a population-based cohort study. Can J Kidney Health Dis 3:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilson MK, Liu T, Baitaluk M, Nicola G, Hwang L, Chong J (2016) BindingDB in 2015: a public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res 44:D1045–D1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgis RR, Xu X, Miyake N, Easwaramoorthy B, Gunn RN, Rabiner EA, Abi-Dargham A, Slifstein M (2011) In vivo binding of antipsychotics to D3 and D2 receptors: a PET study in baboons with [11C]-(+)-PHNO. Neuropsychopharmacology 36:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Guerrero A, Mizrahi R, Agid O, Marcon H, Barsoum P, Rusjan P, Wilson AA, Zipursky R, Kapur S (2009) The dopamine D2 receptors in high-affinity state and D3 receptors in schizophrenia: a clinical [11C]-(+)-PHNO PET study. Neuropsychopharmacology 34:1078–1086. [DOI] [PubMed] [Google Scholar]
- Gross G, Drescher K (2012) The role of dopamine D(3) receptors in antipsychotic activity and cognitive functions. Handb Exp Pharmacol 213:167–210. [DOI] [PubMed] [Google Scholar]
- Ham K (2013) OpenRefine (version 2.5). http://openrefine.org. Free, open-source tool for cleaning and transforming data. J Med Libr Assoc 101:233–234. [Google Scholar]
- Harpaz R, DuMochel W, Shah NH (2016) Big data and adverse drug reaction detection. Clin Pharmacol Ther 99:268–270. [DOI] [PubMed] [Google Scholar]
- Hermont AP, Oliveira PA, Martins CC, Paiva SM, Pordeus IA, Auad SM (2014) Tooth erosion and eating disorders: a systematic review and meta-analysis. Plos One 9:e111123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiemke C, et al. (2018) Consensus guidelines for therapeutic drug monitoring in neuropsychopharmacology: update 2017. Pharmacopsychiatry 51:9–62. [DOI] [PubMed] [Google Scholar]
- Hirayama T, Kita T, Ogawa Y, Ohsawa H, Yamashita M, Nakashima T, Kishimoto T (2001) Effect of chronic treatment with haloperidol on vasopressin release and behavioral changes by osmotic stimulation of the supraoptic nucleus. Life Sci 69:2147–2156. [DOI] [PubMed] [Google Scholar]
- Hoorn EJ, Zietse R (2013) Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis 62:139–149. [DOI] [PubMed] [Google Scholar]
- Ilani T, Ben-Shachar D, Strous RD, Mazor M, Sheinkman A, Kotler M, Fuchs S (2001) A peripheral marker for schizophrenia: increased levels of D3 dopamine receptor mRNA in blood lymphocytes. Proc Natl Acad Sci U S A 98:625–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) (2000) ICH M2 EWG electronic transmission of individual case safety reports message specification. Version 2.3. Geneva ICH Secretariat. http://estri.ich.org/e2br22/ICH_ICSR_Specification_V2-3.pdf. Accessed March 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Council for Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) . Medical dictionary for regulatoryactivities (MedDRA®). Standardised MedDRA® Queries. https://www.ich.org/page/meddra. Accessed March 13, 2020.
- IUPHAR – International Union of Basic and Clinical Pharmacology (2015) http://www.iuphar.org/. Accessed March 13, 2020.
- Jørgensen H, Riis M, Knigge U, Kjaer A, Warberg J (2003) Serotonin receptors involved in vasopressin and oxytocin secretion. J Neuroendocrinol 15:242–249. [DOI] [PubMed] [Google Scholar]
- Jun K, Kim Y, Ah Y, Lee J (2020) Awareness of the use of hyponatraemia-inducing medications in older adults with hyponatraemia: a study of their prevalent use and association with recurrent symptomatic or severe hyponatraemia. Age Ageing 18:afaa195. [DOI] [PubMed] [Google Scholar]
- Kane JM, Zukin S, Wang Y, Lu K, Ruth A, Nagy K, Laszlovszky I, Durgam S (2015) Efficacy and safety of cariprazine in acute exacerbation of schizophrenia: results from an international, phase III clinical trial. J Clin Psychopharmacol 35:367–373. [DOI] [PubMed] [Google Scholar]
- Kenakin T (2004) Principles: receptor theory in pharmacology. Trends Pharmacol Sci 25:186–192. [DOI] [PubMed] [Google Scholar]
- Kimelman N, Albert SG (1984) Phenothiazine-induced hyponatremia in the elderly. Gerontology 30:132–136. [DOI] [PubMed] [Google Scholar]
- Lange-Asschenfeldt C, Kojda G, Cordes J, Hellen F, Gillmann A, Grohmann R, Supprian T (2013) Epidemiology, symptoms, and treatment characteristics of hyponatremic psychiatric inpatients. J Clin Psychopharmacol 33:799–805. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Gallo A, Le Strat Y, Lu L, Gorwood P (2009) Genetics of dopamine receptors and drug addiction: a comprehensive review. Behav Pharmacol 20:1–17. [DOI] [PubMed] [Google Scholar]
- Legehar A, Xhaard H, Ghemtio L (2016) IDAAPM: integrated database of ADMET and adverse effects of predictive modeling based on FDA approved drug data. J Cheminform 8:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letmaier M, Painold A, Holl AK, Vergin H, Engel R, Konstantinidis A, Kasper S, Grohmann R (2012) Hyponatraemia during psychopharmacological treatment: results of a drug surveillance programme. Int J Neuropsychopharmacol 15:739–748. [DOI] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannesse CK, van Puijenbroek EP, Jansen PA, van Marum RJ, Souverein PC, Egberts TC (2010) Hyponatraemia as an adverse drug reaction of antipsychotic drugs: a case-control study in VigiBase. Drug Saf 33:569–578. [DOI] [PubMed] [Google Scholar]
- Manu P, Ray K, Rein JL, De Hert M, Kane JM, Correll CU (2012) Medical outcome of psychiatric inpatients with admission hyponatremia. Psychiatry Res 198:24–27. [DOI] [PubMed] [Google Scholar]
- Matsui-Sakata A, Ohtani H, Sawada Y (2005) Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet 20:368–378. [DOI] [PubMed] [Google Scholar]
- Mazhar F, Pozzi M, Gentili M, Scatigna M, Clementi E, Radice S, Carnovale C (2019) Association of hyponatraemia and antidepressant drugs: a pharmacovigilance-pharmacodynamic assessment through an analysis of the US Food and Drug Administration Adverse Event Reporting System (FAERS) database. CNS Drugs 33:581–592. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM (1976) The dopamine hypothesis of schizophrenia: a review. Schizophr Bull 2:19–76. [DOI] [PubMed] [Google Scholar]
- Meulendijks D, Mannesse CK, Jansen PA, van Marum RJ, Egberts TC (2010) Antipsychotic-induced hyponatraemia: a systematic review of the published evidence. Drug Saf 33:101–114. [DOI] [PubMed] [Google Scholar]
- Milella MS, Passarelli F, De Carolis L, Schepisi C, Nativio P, Scaccianoce S, Nencini P (2010) Opposite roles of dopamine and orexin in quinpirole-induced excessive drinking: a rat model of psychotic polydipsia. Psychopharmacology (Berl) 211:355–366. [DOI] [PubMed] [Google Scholar]
- Mizrahi R, Agid O, Borlido C, Suridjan I, Rusjan P, Houle S, Remington G, Wilson AA, Kapur S (2011) Effects of antipsychotics on D3 receptors: a clinical PET study in first episode antipsychotic naive patients with schizophrenia using [11C]-(+)-PHNO. Schizophr Res 131:63–68. [DOI] [PubMed] [Google Scholar]
- Montastruc JL, Sommet A, Bagheri H, Lapeyre-Mestre M (2011) Benefits and strengths of the disproportionality analysis for identification of adverse drug reactions in a pharmacovigilance database. Br J Clin Pharmacol 72:905–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montastruc F, Palmaro A, Bagheri H, Schmitt L, Montastruc JL, Lapeyre-Mestre M (2015) Role of serotonin 5-HT2C and histamine H1 receptors in antipsychotic-induced diabetes: a pharmacoepidemiological-pharmacodynamic study in VigiBase. Eur Neuropsychopharmacol 25:1556–1565. [DOI] [PubMed] [Google Scholar]
- Mugnaini M, Iavarone L, Cavallini P, Griffante C, Oliosi B, Savoia C, Beaver J, Rabiner EA, Micheli F, Heidbreder C, Andorn A, Merlo Pich E, Bani M (2013) Occupancy of brain dopamine D3 receptors and drug craving: a translational approach. Neuropsychopharmacology 38:302–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pariente A, Gregoire F, Fourrier-Reglat A, Haramburu F, Moore N (2007) Impact of safety alerts on measures of disproportionality in spontaneous reporting databases: the notoriety bias. Drug Saf 30:891–898. [DOI] [PubMed] [Google Scholar]
- PDSP - Psychoactive Drug Screening Program-Ki Database (2020) https://pdsp.unc.edu/pdspweb/Accessed January 20, 2020.
- Richtand NM, Woods SC, Berger SP, Strakowski SM (2001) D3 dopamine receptor, behavioral sensitization, and psychosis. Neurosci Biobehav Rev 25:427–443. [DOI] [PubMed] [Google Scholar]
- Rothman KJ, Lanes S, Sacks ST (2004) The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf 13:519–523. [DOI] [PubMed] [Google Scholar]
- Serrano A, Rangel N, Carrizo E, Uzcátegui E, Sandia I, Zabala A, Fernández E, Tálamo E, Servigna M, Prieto D, Connell L, Baptista T (2014) Safety of long-term clozapine administration. Frequency of cardiomyopathy and hyponatraemia: two cross-sectional, naturalistic studies. Aust N Z J Psychiatry 48:183–192. [DOI] [PubMed] [Google Scholar]
- Shepshelovich D, Schechter A, Calvarysky B, Diker-Cohen T, Rozen-Zvi B, Gafter-Gvili A (2017) Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol 83:1801–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel AJ (2008) Hyponatremia in psychiatric patients: update on evaluation and management. Harv Rev Psychiatry 16:13–24. [DOI] [PubMed] [Google Scholar]
- Spigset O, Hedenmalm K (1996) Hyponatremia during treatment with clomipramine, perphenazine, or clozapine: study of therapeutic drug monitoring samples. J Clin Psychopharmacol 16:412–414. [DOI] [PubMed] [Google Scholar]
- The European Bioinformatics Institute ChEMBL database (2020) https://www.ebi.ac.uk/chembl/. Accessed January 21, 2020.
- The Metabolomics Innovation Centre . DrugBank. https://www.drugbank.ca/. Accessed January 21, 2020. [Google Scholar]
- Vanhauwe JF, Josson K, Luyten WH, Driessen AJ, Leysen JE (2000) G-protein sensitivity of ligand binding to human dopamine D(2) and D(3) receptors expressed in Escherichia coli: clues for a constrained D(3) receptor structure. J Pharmacol Exp Ther 295:274–283. [PubMed] [Google Scholar]
- Verbalis JG, Greenberg A, Burst V, Haymann JP, Johannsson G, Peri A, Poch E, Chiodo JA 3rd, Dave J (2016) Diagnosing and treating the syndrome of inappropriate antidiuretic hormone secretion. Am J Med 129:537.e9–537.e23. [DOI] [PubMed] [Google Scholar]
- Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE (2010) Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med 170:294–302. [DOI] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’connell D, Peterson J, Welch V, Losos M, Tugwell P (2016) The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed February 23, 2020).
- Wiggins A, Balasubramanian T, Ferraro A (2015) Hyponatraemia and confusion in a 70-year-old female when bupropion was added to dothiepin and escitalopram. Australas Psychiatry 23:507–509. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Ohno Y, Nakashima Y, Fukuda M, Takayanagi R, Sato H, Tsuchiya F, Sawada Y, Iga T (2002) Prediction and assessment of extrapyramidal side effects induced by risperidone based on dopamine D(2) receptor occupancy. Synapse 46:32–37. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Takahashi Y, Imai K, Ohta A, Kagawa Y, Inoue Y (2019) Prevalence and risk factors for hyponatremia in adult epilepsy patients: large-scale cross-sectional cohort study. Seizure 73:26–30. [DOI] [PubMed] [Google Scholar]
- Yang HJ, Cheng WJ (2017) Antipsychotic use is a risk factor for hyponatremia in patients with schizophrenia: a 15-year follow-up study. Psychopharmacology (Berl) 234:869–876. [DOI] [PubMed] [Google Scholar]
- Yasir M, Mechanic OJ (2020) Syndrome of Inappropriate Antidiuretic Hormone Secretion (SIADH). In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.