Abstract
Introduction
Deficits in somatostatin-positive gamma-aminobutyric acid interneurons (SST+ GABA cells) are commonly reported in human studies of mood and anxiety disorder patients. A causal link between SST+ cell dysfunction and symptom-related behaviors has been proposed based on rodent studies showing that chronic stress, a major risk factor for mood and anxiety disorders, induces a low SST+ GABA cellular phenotype across corticolimbic brain regions; that lowering Sst, SST+ cell, or GABA functions induces depressive-/anxiety-like behaviors (a rodent behavioral construct collectively defined as “behavioral emotionality”); and that disinhibiting SST+ cells has antidepressant-like effects. Recent studies found that compounds preferentially potentiating receptors mediating SST+ cell functions, α5-GABAA receptor positive allosteric modulators (α5-PAMs), achieved antidepressant-like effects. Together, the evidence suggests that SST+ cells regulate mood and cognitive functions that are disrupted in mood disorders and that rescuing SST+ cell function via α5-PAM may represent a targeted therapeutic strategy.
Methods
We developed a mouse model allowing chemogenetic manipulation of brain-wide SST+ cells and employed behavioral characterization 30 minutes after repeated acute silencing to identify contributions to symptom-related behaviors. We then assessed whether an α5-PAM, GL-II-73, could rescue behavioral deficits.
Results
Brain-wide SST+ cell silencing induced features of stress-related illnesses, including elevated neuronal activity and plasma corticosterone levels, increased anxiety- and anhedonia-like behaviors, and impaired short-term memory. GL-II-73 led to antidepressant- and anxiolytic-like improvements among behavioral deficits induced by brain-wide SST+ cell silencing.
Conclusion
Our data validate SST+ cells as regulators of mood and cognitive functions and demonstrate that bypassing low SST+ cell function via α5-PAM represents a targeted therapeutic strategy.
Keywords: Somatostatin, GABA, depression, Gabra5, antidepressant
Significance Statement.
Human studies demonstrate somatostatin-positive GABAergic interneuron (SST+ cell) deficits as pathological features of major depressive disorder and anxiety disorders. Studies indicate reduced SST and GABAergic markers across corticolimbic brain regions. Past animal studies identified that SST+ cells regulate mood and cognitive functions related to symptoms of mood disorders but employed SST+ cell ablation or region-specific silencing not representative of disease-related processes. We developed a chemogenetic mouse model of brain-wide low SST+ cell function and performed behavioral characterization to demonstrate a role for these cells in regulating anxiety- and anhedonia-like behaviors, behavioral emotionality, and impaired memory. We next found that the α5-GABAA receptor positive allosteric modulator (α5-PAM), GL-II-73, rescued deficits induced by low SST+ cell function. These findings support SST+ cells as central regulators of symptom-related behaviors and validate α5-GABAA receptors as a therapeutic target to reverse deficits related to low SST+ cell function.
Introduction
Major depressive disorder (MDD) is a severe psychiatric illness affecting 322 million people (Friedrich, 2017; Vos et al., 2017; World Health Organization, 2017; Rehm and Shield, 2019) and is more common among females (5.1%) than males (3.6%) (World Health Organization, 2017). The diagnosis and treatment of MDD is limited by heterogeneity in pathological and clinical presentation, with low mood, anhedonia, physiological, and cognitive impairments and high comorbidity (approximately 40%–70%) with anxiety disorders. First-line antidepressants target monoaminergic imbalances that occur downstream from primary mood disorder pathologies and remain ineffective in 50% of treated patients (Gaynes et al., 2009; Holtzheimer and Mayberg, 2011). Therefore, a need exists to characterize how cellular changes contribute to specific symptoms to develop targeted drug therapies. Four decades of human and animal studies demonstrate reduced level, synthesis, and function of neurons expressing the inhibitory neurotransmitter gamma-aminobutyric acid (GABA) among primary pathological changes across mood disorders (Luscher et al., 2011; Newton et al., 2019). Among these, GABA neurons coexpressing the neuropeptide somatostatin (SST+ GABA cells, or SST+ cells) appear to be selectively vulnerable, wherein deficits were identified downstream of causal factors such as chronic stress and reduced neurotrophic factor signaling (Guilloux et al., 2012; Tripp et al., 2012).
GABA neurons play a critical role in information processing by cortical microcircuits to regulate mood and cognitive functions. These microcircuits encode neuronal information through activity patterns of excitatory glutamatergic pyramidal neuron (PN) ensembles, whereas GABAergic neurons shape this activity differently depending on nonoverlapping cellular identities coexpressing SST, the calcium-binding protein parvalbumin (PV+), or the ionotropic serotonin receptor 5HT3aR with or without vasoactive intestinal peptide (Rudy et al., 2011). SST+ cells gate excitatory PN input via dendritic inhibition and modulate output via perisomatic PN disinhibition through SST-PV+ cell afferents (Fee et al., 2017; Hu et al., 2019). Dendritic SST+ cell inhibition is partially mediated by GABA acting on GABA-A and -B receptors (GABAA-R/GABAB-R) (Urban-Ciecko et al., 2015; Schulz et al., 2018), whereas the SST peptide is released under distinct firing conditions (Katona et al., 2014; Yavorska and Wehr, 2016). SST has shared roles alongside GABA in pre- and postsynaptic PN inhibition (Tallent and Siggins, 1997; Schweitzer et al., 1998), plus distinct roles in negative regulation of stress-related hypothalamic-pituitary adrenal axis activation (Stengel and Taché, 2017).
Histological postmortem MDD studies identified SST and GABA marker reductions at mRNA and protein levels in the prefrontal cortex (PFC) (Sibille et al., 2011), anterior cingulate cortex (Tripp et al., 2011), and amygdala (Guilloux et al., 2012) that were more severe in females (Seney et al., 2013, 2015). Follow-up studies indicated reduced SST mRNA per cell spanning cortical layers (Tripp et al., 2011) and reduced detectable SST+ cell density without overall cell density changes in the amygdala (Douillard-Guilloux et al., 2017). In vivo studies revealed MDD-related reductions in PFC (Hasler et al., 2007) and ACC GABA levels (Price et al., 2009), with reduced GABAA-R and GABAB-R neurotransmission (Bajbouj et al., 2006; Levinson et al., 2010; Radhu et al., 2013). The regional and functional differentiation of GABAA-Rs depends on heteropentameric subunit composition, commonly containing 2 α, 2 β, and 1 γ-subunit (Rudolph et al., 2001; Sieghart and Sperk, 2002). SST+ cell functions are partially mediated by α5 subunit-containing GABAA-Rs given their localization to PN dendrites and PV+ cells in the PFC, hippocampus (HPC), and ventral striatum (Ali and Thomson, 2008; Schulz et al., 2018; Hu et al., 2019). GABRA5 transcripts (encoding α5GABAA-Rs) were also reduced in the PFC of MDD and aged patients (Oh et al., 2019), together suggesting that a low SST+ GABA cellular phenotype, affecting pre- and postsynaptic functions and arising from intrinsic brain-wide cellular vulnerabilities, contributes to common symptoms of mood disorders and age-related processes. Indeed, low SST+ markers are also commonly found in schizophrenia and bipolar disorder (Lin and Sibille, 2013).
Preclinical findings support that disrupted SST+ cell function may contribute to deficits reflecting mood disorder symptoms. In mice, chronic stress (a major risk factor for MDD and anxiety disorders) induced reduced mRNA levels of Sst and the GABA-synthesizing enzyme Gad67 in the cingulate cortex, plus altered SST+ cell but not PN transcripts (Lin and Sibille, 2015; Girgenti et al., 2019). Mice with genetic Sst ablation also recapitulated elevated corticosterone, reduced growth factor and Gad67 expression, and elevated anxiety-/depressive-like behaviors (collectively defined as “behavioral emotionality”; Guilloux et al., 2011). Furthermore, acute chemogenetic or optogenetic PFC SST+ cell silencing elevated behavioral emotionality (Soumier and Sibille, 2014) and working memory impairment (Abbas et al., 2018), whereas genetic disinhibition had antidepressant-like effects (Fuchs et al., 2017). Additionally, corticolimbic infusion of exogenous SST peptide had anxiolytic- and antidepressant-like effects (Engin et al., 2008; Engin and Treit, 2009; Yeung et al., 2011; Prévôt et al., 2017). Therefore, SST+ cells appear to regulate mood-related behaviors, but it is important to mention that genetic ablation and region-specific silencing do not recapitulate the brain-wide low (but intact) SST+ cell phenotype observed in humans (Fee et al., 2017).
Neuroimaging studies demonstrated that GABA levels normalized after pharmacological, cognitive-behavioral, and neuromodulatory antidepressant treatment (Fee et al., 2017). Although first-line antidepressants do not target GABA/SST deficits directly, mixed efficacy has been observed from monotherapy or combination therapy with benzodiazepines (BZDs) that act as nonselective positive allosteric modulators (PAMs) at GABAA-Rs between ɣ2 and α1–3, 5 subunits (Benasi et al., 2018; Gomez et al., 2018; Ogawa et al., 2019). However, BZDs confer side effects and abuse liability attributed to pan-α-subunit selectivity (Vgontzas et al., 1995). Recent preclinical studies assessing BZD derivatives with selectivity for α5-GABAA-Rs (α5-PAMs), which have restricted corticolimbic distribution, found anxiolytic-, antidepressant-like, and procognitive effects in young, aged, and chronic stress–exposed rodents (Koh et al., 2013; Piantadosi et al., 2016; Prevot et al., 2019a). Further, an exogenous formulation of the neurosteroid allopregnanolone was recently approved for postpartum depression and is believed to act partially as a PAM at extrasynaptic GABAA-R compartments where α5-GABAA-Rs are preferentially located (Farrant and Nusser, 2005; Lüscher and Möhler, 2019). However, studies to date have not assessed the potential of α5-PAMs to reverse deficits induced by specific pathologies, and even minor differences in GABAA-R subunit selectivity can have divergent outcomes (Prevot et al., 2019a), thus warranting further investigation.
Given evidence that a brain-wide low SST+ cellular phenotype contributes to mood disorder symptoms, we aimed to identify how acute silencing of brain-wide SST+ cells in mice led to changes along symptom-related behavioral dimensions, including anxiety- and anhedonia-like behavior, behavioral emotionality, and impaired memory. To achieve this, we combined multiple behavioral tests with a novel chemogenetic SST+ cell-silencing model. We then assessed whether the α5-PAM GL-II-73 could rescue behavioral deficits. We predicted that brain-wide SST+ cell silencing induces symptom-related behavioral deficits that can be rescued by α5-PAM.
Materials and Methods
A complete description of methods/study designs can be found in supplemental Information.
Animals
Viral transduction was validated in C57BL/6J SstGfp/+ mice (Taniguchi et al., 2011; Lin and Sibille, 2015). Behavioral and molecular experiments were performed using C57BL/6J SstCre/+ mice (Ssttm2.1(cre)Zjh/J, Jackson Laboratories, Bar Harbour, ME; #913944), postnatal day zero at surgery and 9-16 weeks at testing. Mice were maintained under a 12-hour-light/-dark cycle (7 am-7 pm) with ad libitum food and water and were group housed (4/cage) except during behavioral testing (1/cage). Tests were performed according to Institutional and Canadian Council on Animal Care guidelines.
AAV Vectors and Neonatal Injection
Enhanced serotype inhibitory Designer Receptors Exclusively Activated by Designer Drugs (DREADD) vectors used were AAV-PHP.eB-hSyn-DIO-hM4D(Gi)-mCherry (Addgene #44362, Orono, ME) (Chan et al., 2017). Control vectors used were AAV-PHP.eB-hSyn-DIO-mCherry (Addgene #50459).
Brain-wide SST+ cell-specific hM4Di expression was achieved by low-dose bilateral intracerebroventricular (i.c.v.) infusion of PHP.eB serotype Flip-Excision AAVs in postnatal day zero SstCre/+ mice (Kim et al., 2013) (Supplementary Methods). For validation experiments, SstGfp/+ neonates received DREADD (hM4Di) vectors. For characterization experiments, SstCre/+ neonates received hM4Di (SsthSyn-hM4Di-mCherry mice) or control (SsthSyn-mCherry mice) vectors.
Chemogenetic Inhibition of Brain-Wide SST+ cells and α5-PAM Coadministration
For the first characterization experiment, SsthSyn-hM4Di-mCherry mice received clozapine-N-oxide (CNO = 3.5 mg/kg) or vehicle (Veh = 0.9% saline) i.p. 30 minutes before testing (n = 16/group; 50% female; Fig. 2a). We confirmed that locomotor activity and anxiety-like behavior were not affected by CNO in separate SstCre/+ cohorts not expressing hM4Di (supplemental Fig. 1).
Figure 2.
Validation of brain-wide somatostatin-positive (SST+) cell manipulation. (A) Experimental design for behavioral and biological characterization following chemogenetic SST+ cell silencing in SsthSyn-hM4Di-mCherry mice. (B) Representative image of c-Fos+ cells 110 minutes after treatment with vehicle (Veh, top) or 3.5 mg/kg clozapine-N-oxide (CNO, bottom). CNO-hM4Di activation increased neuronal activity (c-Fos+ cell counts) in the mPFC, HPC, and amygdala (Amy) (n = 12 mice/group, x = males, o = females). (C) From the same cohort, CNO induced a trend elevation in plasma corticosterone (n = 16 mice/group, x = males, o = females). ***P < .001, **P < .01, #P < .1.
For the second characterization experiment, we assessed whether deficits induced by SST+ cell silencing could be rescued by coadministering CNO and GL-II-73 (Prevot et al., 2019a). SsthSyn-mCherry and SsthSyn-hM4Di-mCherry mice were generated, and all groups received 3.5 mg/kg CNO, achieving SST+ cell silencing only in hM4Di-expressing mice (SST-silenced = SsthSyn-hM4Di-mCherry, SST-control = SsthSyn-mCherry). Mice were randomly assigned to receive Veh (vehicle+CNO) or 10 mg/kg GL-II-73 (+vehicle+CNO) totaling 4 groups (n = 10–12/group; 50% female; Fig. 4a).
Figure 4.
GL-II-73 rescues behavioral deficits induced by brain-wide somatostatin-positive (SST+) cell silencing. (A-J) Behavioral tests administered 30 minutes following i.p. injection of vehicle + CNO (Veh) or vehicle + CNO + GL-II-73 (GL-II-73) in SST-control (SsthSyn-mCherry) or SST-silenced (SsthSyn-hM4Di-mCherry) mice (except in PhenoTyper as indicated). (B) Shelter zone time before, during, and after light challenge and (C) derived shelter zone area under the curve (AUC) from light challenge initiation until PhenoTyper test (PT) completion. (D) Time spent in open arms of the elevated plus maze (EPM). (E) Time spent in center zone of the open field test (OFT). (F) Novel environment latency to feed for the novelty suppressed feeding (NSF) test. (G) Novel environment latency to drink milk reward for the novelty-induced hypophagia test (NIH). (H) Sucrose consumed in the sucrose consumption test (SCT). (I) Immobility time in the forced swim test (FST). (J) Percent correct alternations in Y-maze (YM). (K) Pretrial left/right familiar object recall (left) and trial familiar/novel object recall (right) in the novel object recognition test (NORT). (L-O) Z-scores integrating test parameters assessing anxiety-like behavior (L), anhedonia-like behavior (M), overall behavioral emotionality (N), and memory impairment (O). Abbreviations: n, 16 mice/group; x, males, o, females. ***P < .001, **P < .01, *P < .05, #P < .01, † = P < .05 SST-Control+Veh vs SST-Silenced+Veh; $ = P < .05 SST-Silenced+GL-II-73 vs SST-Silenced+Veh; § = significant main effect of treatment; ¥ = significant main effect of group. Homecage parameters in supplemental Table 2.
Behavioral Analyses
In adulthood, anxiety-like behavior was assessed with the PhenoTyper test (PT), elevated plus-maze (EPM), open field test (OFT), and novelty-suppressed feeding test (NSF); anhedonia-like behavior with sucrose consumption test (SCT); mixed anxiety-/anhedonia-like behavior with novelty-induced hypophagia test (NIH); antidepressant-predictive behavior by the forced-swim test (FST); and memory impairment by the Y-maze (YM) and novel object recognition test (NORT) following past study designs (Fee et al., 2020). CNO was administered 30 minutes prior to test initiation, except for the PT where CNO was administered 30 minutes prior to the dark cycle (90 minutes prior to light challenge) to allow habituation.
Z-score normalization captured changes along behavioral dimensions, directionally, relative to controls (i.e., increase = deficit, decrease = improvement) for anxiety- (PT, EPM, OFT, NSF, NIH) and anhedonia-like behavior (NIH, SCT), behavioral emotionality (all previous + FST), and working and short-term memory impairment (YM, NORT) (Guilloux et al., 2011) (supplemental Methods). Viral transduction efficiency and SST+ cell specificity were visually inspected via immunohistochemistry (IHC) in all mice following behavioral experiments.
IHC and Microscopy
The transduction efficiency and SST+ cell specificity of neonatally delivered i.c.v. AAV-PHP.eB-hSyn-DIO-hM4D(Gi)-mCherry was assessed via IHC in sections from paraformaldehyde-perfused SstGfp/+ mice fluorescently labeled for GFP and mCherry (n = 8, 2 fields/region/mouse; Fig. 1a).
Figure 1.
Validation of brain-wide somatostatin-positive (SST+) cell targeting. (A) Experimental design for validation of SST+ cell targeting by 2 × 1010vg AAV-PHP.eB-DIO-hM4Di-mCherry i.c.v. in postnatal day zero SstGfp/+ mice, validated by immunohistochemistry (IHC). (B) Representative images of brain-wide viral transduction in adult mice (red = conditional mCherry expression; scale = 500 μm). (C) Representative images of hM4Di-mCherry (red) and SST-GFP (green) coexpression (white arrow) in the mPFC. (D) Efficiency (mCherry/GFP) and specificity (GFP/mCherry) of viral transduction in mPFC, dHPC, and Amy (n = 8 mice; x = males, o = females).
Forty-eight hours after the last test, SsthSyn-hM4Di-mCherry mice from behavioral experiments were injected with Veh or CNO and perfused 110 minutes later, approximating CNO + c-Fos peaks, to quantify neuronal activation following SST+ cell silencing (indexed by c-Fos+ cell counts) (Dragunow and Faull, 1989; Ferguson et al., 2011) (Fig. 2a). Cell counts were collected for every third section (N = 4–5 sections/region/mouse) in medial PFC (mPFC), dorsal HPC (dHPC), and basolateral amygdala (Amy). Image processing and counting was performed under blinded conditions for Veh/CNO groups (n = 12/group; 50% female) using Fiji (Schindelin et al., 2012).
Corticosterone Measurement
In SsthSyn-hM4Di-mCherry mice from behavior and c-Fos experiments, plasma corticosterone was assessed by ELISA (Arbor Assays, Ann Arbor, MI) 110 minutes after Veh/CNO administration from blood collected via cardiac puncture prior to perfusions (n = 16 mice/group; 50% female).
Data Analysis
Data were analyzed using SPSS (IBM, NY) and expressed as mean ± SEM. For Veh vs CNO, data were analyzed using 2-way ANOVA with treatment and sex as independent variables. For SST-control vs SST-silenced, data were analyzed using 3-way ANOVA, with group, treatment (Veh vs GL-II-73), and sex as independent variables, using Bonferroni-adjusted post hoc where appropriate. Timecourse parameters were assessed by repeated-measures ANOVA using Greenhouse-Geisser correction. Object recall in the NORT was assessed using paired-samples t tests. Given limitations on sample size, sexes were pooled when significant main effects or interactions with sex were not observed.
Results
Intracerebroventricular AAV-PHP.eB DREADD Infusion in Neonatal SstCre Mice Enables Brain-Wide Manipulation of SST+ Cells in Adulthood
Validation of SST+ cell viral targeting was performed in SstGfp/+ mice administered low-dose (2 × 1010vg) AAV-PHP.eB-DIO-hM4Di-mCherry via neonatal i.c.v. infusion (Soumier and Sibille, 2014; Lin and Sibille, 2015) (Fig. 1a–d). In adults, IHC quantification revealed high viral transduction efficiency (mPFC = 95 ± 2%, dHPC = 96 ± 2%, Amy = 56 ± 9%, overall = 82 ± 5%) and SST+ cell specificity (mPFC = 99 ± 1%, dHPC = 98 ± 1%, Amy = 99 ± 1%, overall = 99 ± 1%) that was consistent with Sst expression patterns (NCBI, 2020) (Fig. 1b–d).
Based on established roles for GABA and SST in inhibitory regulation of local neuronal activity and endocrine signaling (Yavorska and Wehr, 2016), chemogenetic SST+ cell silencing was next validated in a separate cohort of SsthSyn-hM4Di-mCherry mice by quantifying neuronal activity via c-Fos+ cell counts (Kovács, 1998) and plasma corticosterone levels 110 minutes following SST+ cell inactivation by CNO (Fig. 2a). Compared with Veh, CNO increased the number of c-Fos+ cells in the mPFC (F1,23 = 30.62; P < .001), HPC (F1,23 = 11.33; P = .003), and Amy (F1,23 = 21.97; P < .001; Fig. 2b). CNO induced a trend-level elevation in corticosterone as assessed by ELISA (F1,28 = 3.51; P = .071; Fig. 2c). No main effect or interactions with sex were found (P > .3).
Brain-Wide SST+ Cell Silencing Increases Anxiety-Like Behaviors, Overall Behavioral Emotionality, and Memory Impairment
In the characterization experiment 1 cohort, we sought to determine whether SST+ cell silencing induced anxiety-like behavior (PT, EPM, OFT, NSF), anhedonia-like behavior (NIH, SCT), antidepressant-predictive behavior (FST), and memory impairment (YM, NORT).
Analysis of PT shelter zone activity before, during, and after a 1-hour light challenge in the dark cycle revealed significant main effects of time (F7.51,217.82 = 2.42; P < .05), group (F1,29 = 6.75; P < .05), and group*time interaction (F7.51,217.82 = 2.18; P < .05; Fig. 3a). CNO significantly increased shelter zone time after injection and persisting for 6 hours after light challenge (P < .05). Overall anxiogenic response, indexed by shelter zone time area under the curve (AUC) from light challenge initiation until test completion, confirmed that CNO significantly increased shelter zone time (F1,29 = 11.97; P < .01; Fig. 3b).
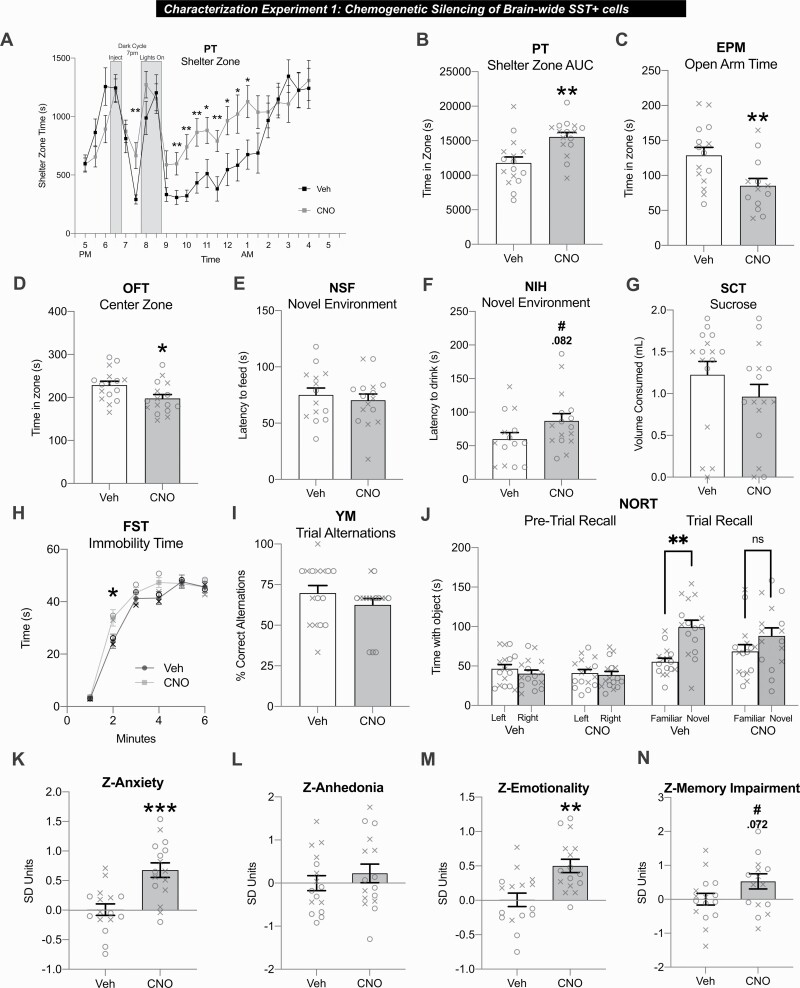
Figure 3.
Brain-wide somatostatin-positive (SST+) cell silencing in SsthSyn-hM4Di-mCherry mice elevates anxiety-like behavior, overall behavioral emotionality, and memory impairment. (A–J) Behavioral tests administered 30 minutes after injection of vehicle (Veh) or 3.5 mg/kg clozapine-N-oxide (CNO) i.p. except for the PhenoTyper Test (PT) (administered 90 minutes prior to light challenge). (A) Shelter zone time before, during, and after light challenge in the PT. (B) Summary area under the curve (AUC) of shelter zone time from light challenge initiation until the test completion. (C) Time spent in open arms of the elevated plus maze (EPM). (D) Time spent in center zone of the open field test (OFT). (E) Novel environment latency to feed in the novelty suppressed feeding (NSF) test. (F) Novel environment latency to drink milk reward in the novelty-induced hypophagia test (NIH). (G) Sucrose consumed in the sucrose consumption test (SCT). (H) Immobility time in the forced swim test (FST). (I) Percent correct alternations in Y-maze (YM). (J) Pretrial left/right familiar object recall (left) and trial familiar/novel object recall (right). (K-N) Z-scores integrating test parameters assessing anxiety-like behavior (K), anhedonia-like behavior (L), behavioral emotionality (M), and memory impairment (N). n = 16 mice/group; male = x/female = o. ***P < .001, **P < .01, *P < .05, #P < .01. Homecage measures in supplemental Table 1.
Anxiogenic CNO effects were also detected from decreased EPM open arm time (F1,25 = 7.06; P < .05; Fig. 3c) and OFT center zone time (F1,29 = 5.86; P < .05; Fig. 3d). Distance travelled was unchanged between groups in the OFT and EPM (supplemental Table 1). In the NSF, no group differences were detected for novel environment latency to feed (Fig. 3e). In the NIH, CNO induced a trend-level increase in novel environment latency to drink (F1,27 = 3.26; P = .082; Fig. 3f). Locomotor activity (PT, EPM, OFT) and home cage latencies to feed or drink (NSF/NIH) did not differ between groups (supplemental Table 1). No main effect or interaction with sex was found for PT, EPM, OFT, NSF, or NIH parameters (P > .1).
In the SCT, CNO did not influence sucrose consumed (Fig. 3g) or sucrose relative to total fluid consumed (supplemental Table 1). There was a main effect of sex as females consumed significantly less sucrose (F2,28 = 7.7; P < .01; supplemental Table 1).
FST analysis revealed a significant effect of time (F3.46, 100.39 = 15.15; P < .001) and trend-level time*treatment interaction (F3.46,100.39 = 2.32; P = .071) as CNO increased immobility in minute 2 (P < .05; Fig. 3h).
In the YM, group differences were not detected for pretrial (supplemental Table 1) or trial alternation rates (Fig. 3i). In the NORT pretrial, left/right familiar object times were equivalent for both groups (Fig. 3j). In the NORT trial, Veh-treated mice spent significantly more time with the novel vs familiar object (t = 4.44, df = 15; P < .001) while CNO-treated mice did not (t = 1.56; df = 15; P = .14), indicating impaired short-term recall. There was no main effect or interaction with sex for FST, YM, or NORT parameters (P > .2).
Given the well-characterized variability associated with preclinical behavioral tests (Willner, 2017; Prevot et al., 2019b), we next used Z-scores to assess the consistency of behavioral responses across tests assessing a priori-related dimensions (Guilloux et al., 2011). CNO significantly increased Z-anxiety scores, reflecting PT, EPM, OFT, NSF, and NIH parameters (F1,29 = 17.53; P < .001; Fig. 3k). Z-anhedonia scores did not differ between groups, reflecting SCT and NIH (Fig. 3l). CNO significantly elevated Z-emotionality scores, reflecting all previous tests plus FST (F1,29 = 13.96; P < .01; Fig. 3m). CNO induced a trend-level increase in Z-memory impairment scores, reflecting YM and NORT (F1,29 = 3.49; P = .072; Fig. 3n).
α5-PAM (GL-II-73) Rescues Behavioral Deficits Induced by Brain-Wide SST+ Cell Silencing
SST-control (SsthSyn-mCherry) and SST-silenced (SsthSyn-hM4Di-mCherry) mice were generated by neonatal infusion of control or DREADD viruses in SstCre/+ mice, and in adulthood administered Veh (vehicle+CNO) or GL-II-73 (vehicle+CNO+GL-II-73) prior to behavioral testing (Fig. 4a).
Analysis of PT shelter zone time revealed a significant main effect of sex (F1,41 = 22.83; P < .001; supplemental Table 2), time (F9.5,389.4 = 6.971; P < .001), and a time*group*treatment interaction (F9.5,389.4 = 2.1; P < .05). SST-silenced+Veh mice had significantly increased shelter zone time after the light challenge (P < .05 vs SST-control+Veh; Fig. 1b), and males overall spent more time in the shelter (supplemental Table 2). This effect was reversed by GL-II-73 in SST-silenced mice (P < .05). For shelter zone AUC, ANOVA revealed a group*treatment interaction (F1,39 = 8.45; P < .01), wherein SST-silenced+Veh mice had increased AUC (P < .01 vs SST-control+Veh) that was similarly reversed by GL-II-73 treatment (P < .05; Fig. 4c). Increased shelter zone AUC was also detected in SST-control+GL-II-73 vs SST-control+Veh mice (P < .05). Locomotor activity did not differ between groups (F1,39 = .65; P > .59; supplemental Table 2).
Analysis of EPM open arm time revealed a significant main effect of group (F1,35 = 11.63; P = .002) and group*treatment interaction (F1,35 = 8.83; P = .005) (Fig. 4d). GL-II-73 increased open arm time in SST-silenced mice (P < .05 vs SST-silenced+Veh). OFT center zone time was unchanged between groups (Fig. 4e). However, CNO+GL-II-73 significantly increased distance travelled in EPM (P = .044) and OFT (P < .001) (supplemental Table 2). In the NSF, no group differences were detected for novel environment latency to feed (Fig. 4f). Analyses of NIH novel environment latency to drink revealed significant main effects of sex (F1,39 = 6.57; P < .05), group (F1,39 = 5.31; P < .05), treatment (F1,40 = 16.66; P < .001), and a trend-level group*treatment interaction (F1,40 = 2.93; P = .095), wherein SST-silenced+Veh mice had increased latency (P < .01 vs SST-control+Veh) that was rescued by GL-II-73 (P < .05) (Fig. 4g) and females overall had increased latency to drink (P < .05; supplemental Table 2). Home cage latency to feed (NSF) or drink (NIH) did not differ between groups (supplemental Table 2).
In the SCT, a significant main effect of treatment was detected; GL-II-73 increased sucrose consumption (F1,38 = 6.25; P < .05; Fig. 4h), but not sucrose ratio (supplemental Table 2), indicating increased overall fluid consumption rather than preference.
In the FST, a significant main effect of time (F3.19,102 = 6.74; P < .001), time*group interaction (F3.19,102 = 3.2; P < .05), and group*treatment interaction were detected (F1,32 = 6.87; P < .05; Fig. 4i). GL-II-73 decreased immobility in SST-silenced mice (P < .05 vs SST-silenced+Veh, SST-control+GL-II-73). However, this may have been confounded by the previously identified hyperlocomotor effect of GL-II-73.
In the YM, pretrial (supplemental Table 2) and trial (Fig. 4j) alternation rates did not differ between groups. In the NORT pretrial, only SST-silenced+GL-II-73 mice spent significantly more time with the right-located object, indicating side preference that was balanced by rotating novel object position in the trial (t = 2.84, df = 10; P = .018; Fig. 4k). In the NORT trial, significant short-term recall was detected for SST-control+Veh (t = 1.62, df = 11; P < .001) and SST-control+GL-II-73 (t = 3.89, df = 10; P = .003). SST+ cell silencing impaired familiar object recall (t = .695, df = 10; P = .503) that was partially rescued by GL-II-73 (t = 1.85, df = 10; P = .09).
For Z-score analysis, EPM, OFT (Z-anxiety), and FST parameters (Z-emotionality) were excluded due to potential locomotor bias, whereas this effect was not detected in other tests. Analysis of Z-anxiety scores (PT, NSF, NIH) revealed a significant main effect of treatment (F1,41 = 11.18; P < .01), a trend-level group effect (F1,41 = 3.94; P = .054), and a significant group*treatment interaction (F1,41 = 4.32; P < .05) (Fig. 4l). SST-silenced+Veh mice had elevated anxiety-like behavior (P < .05 vs SST-control+Veh). GL-II-73 had anxiolytic effects in SST-control+GL-II-73 mice and reversed elevated anxiety-like behavior in SST-silenced+GL-II-73 mice (Ps < .01 relative to SST-silenced+Veh; Fig. 4l). For Z-anhedonia scores (NIH, SCT), ANOVA detected significant treatment effects (F1,41 = 20.13; P < .001), and trend-level group (F1,41 = 3.34; P < .075) and group*treatment effects (F1,41 = 3.8; P = .056). Z-anhedonia was significantly elevated in SST-silenced+Veh mice (P < .05 vs SST-control+Veh) and reduced or rescued in SST-Control+GL-II-73 and SST-Silenced+GL-II-73 mice, respectively (Ps < .01 relative to SST-silenced + Veh; Fig. 4m). Z-emotionality scores (all previous tests) were significantly affected by treatment (F1,41 = 17.5; P < .001), trend-level group effects (F1,41 = 4.1; P = .05), and a significant group*treatment interaction (F1,41 = 4.77; P < .05). Z-emotionality scores were increased in SST-silenced+Veh mice (P < .05 vs SST-control+Veh) and reduced or rescued in SST-Control+GL-II-73 and SST-Silenced+GL-II-73 mice (P < .01; Fig. 4n). Analysis of Z-memory impairment (YM, NORT) revealed a main effect of group (F1,41 = 15.18; P < .001) and trend-level group*treatment interaction (F1,41 = 3.29; P = .07), wherein SST+ cell silencing increased scores (P < .05 vs SST-control + Veh) and GL-II-73 partially rescued these (P = .059; Fig. 4o). There was no main effect or interaction with sex for Z-scores.
Discussion
In this report, we validated a novel approach to silence brain-wide SST+ cell function, finding increased neuronal activity in the PFC, HPC, and amygdala, elevated plasma corticosterone, and elevated anxiety- and anhedonia-like behaviors, behavioral emotionality, and memory impairment. Treatment with GL-II-73, an α5-GABAA-R-selective PAM, rescued behavioral changes. These results reveal that brain-wide low SST+ cell function contributes to behavioral changes related to symptoms of mood disorders, and that this low functioning cellular phenotype and its behavioral consequences can be rescued by α5-GABAA-R–selective PAM.
Silencing Brain-Wide SST+ Cell Function
To selectively silence brain-wide SST+ cell function, we employed neonatal i.c.v. infusion of Flip-Excision AAV-PhP.eB hM4Di vectors in SstCre/+ mice. AAV-PHP.eB capsids have enhanced GABAergic interneuron transduction (Gholizadeh et al., 2013) but previously relied on high-dose systemic administration (Deverman et al., 2016; Chan et al., 2017). We achieved high SST+ cell transduction via low-dose i.c.v. infusion in neonates (Kim et al., 2013) as a cost-effective technique to overcome genetic leakage that is sometimes reported in lox-stop-lox models (Madisen et al., 2012). In SstGfp/+ mice, cell specificity (approximately 98%–99%) and efficiency (56%–95%) were high, but not complete, consistent with systemic AAV-PHP.eB in SstCre/+ mice (Allen et al., 2017). This may be due to lower IHC detectability in SstCre mice that have reduced Sst levels and contain small numbers of non-SST Cre- or GFP-expressing cells (Ma et al., 2006; Taniguchi et al., 2011; Hu et al., 2013; Viollet et al., 2017).
Whereas past approaches used region-specific cell knockdown or Sst deletion (Soumier and Sibille, 2014; Lin and Sibille, 2015), we employed brain-wide inhibitory DREADDs that inhibit presynaptic firing and neurotransmitter release (Zhu and Roth, 2014), thus aiming to silence both GABA and SST functions. These changes may be more closely related to mood and neurodegenerative disorders where an intrinsic vulnerability causes low SST and GABA markers per cell across corticolimbic brain regions (Fee et al., 2017). Indeed, although not measured directly, putative suppression of SST/GABA functions was validated by corticolimbic hyperactivation in the PFC, HPC, and Amy, implying inhibitory neuron silencing and consistent with past approaches (Soumier and Sibille, 2014; Allen et al., 2017). Although we selected 3 regions for c-Fos analyses, SST+ cell targeting was confirmed via qualitative inspection throughout the neocortex in all mice. As a limitation, our approach did not distinguish SST vs GABA roles as both markers are reduced in MDD (Fee et al., 2017). However, corticolimbic hyperactivation is an expected outcome reflecting silencing both factors given their shared roles in PN inhibition (Tallent and Siggins, 1997; Schweitzer et al., 1998; Stengel and Taché, 2017) and consistent with past chemogenetic SST+ cell-silencing studies (Soumier and Sibille, 2014; Allen et al., 2017) and chronic stress studies wherein GABA and SST markers are selectively reduced (Lin and Sibille, 2015; Girgenti et al., 2019; Fee et al., 2020). Plasma corticosterone elevation also validated reduced SST function, given SST roles in inhibitory regulation of corticosteroid release (Engin and Treit, 2009; Prévôt et al., 2017; Prévôt et al., 2018). That corticosterone effects only reached trend level may reflect the long measurement window chosen (designed also for c-Fos half-life). Indeed, Sst-ablated mice had elevated corticosterone at baseline, but not during or after stress (Lin and Sibille, 2015). Behavioral findings were also consistent with low GABAA-R signaling (Ren et al., 2016) or SST knockdown mice (Lin and Sibille, 2015). Finally, given that GABA-AR–acting BZDs and SST receptor-acting analogs confer anxiolytic- and antidepressant-like effects in rodents (Sanders and Shekhar, 1995; Engin et al., 2008; Yeung et al., 2011; Prévôt et al., 2017), evidence suggests that both systems regulate mood and cognitive functions.
Behavioral deficits were induced by repeated acute CNO administration across 9 tests and were relatively lasting (i.e., persisting 6 hours after injection in PT), consistent with past chemogenetic studies (Guettier et al., 2009; Alexander et al., 2009). The amount of 3.5 mg/kg CNO was selected to achieve brain penetrant levels >EC50 for hM4Di for approximately 15–30+ minutes after administration and clearing from plasma within 2 hours (Jendryka et al., 2019). Therefore, consistent with past studies (Smith et al., 2016), we do not expect lingering subchronic effects from repeated hM4Di activation and consider behavioral deficits observed to reflect direct consequences of reduced upstream SST+ cell regulation of corticolimbic functions.
The DREADD approach has several technical caveats, including CNO to clozapine back-metabolism that may confer off-target effects (Gomez et al., 2017; Jendryka et al., 2019). However, CNO-derived clozapine did not exceed hM4Di EC50 at the selected dose (Gomez et al., 2017), and CNO alone did not alter behavior in SstCre/+ control mice (supplemental Fig. 1) or locomotion in SST-control mice (Fig. 3). Furthermore, when all groups received CNO, behavioral changes were detected only in hM4Di-expressing mice (Fig. 4). As human pathologies reflect chronic conditions, we tested chronic SST+ cell silencing via frequent repeated i.p. injections or oral CNO delivery but found no behavioral changes (Unpublished data, Fee, 2020). Rather than this being a consequence of chronic SST+ cell silencing, we attributed these effects to technical limitations of DREADDs, including that CNO has a rapid half-life and is challenging to administer at levels exceeding hM4Di EC50 orally over long time periods without back metabolism and accumulation of lipophilic clozapine (Gomez et al., 2017; Jendryka et al., 2019). Indeed, evidence of chronic chemogenetic hM4Di activation is sparse, including null or opposite effects compared with acute hM4Di activation (Soumier and Sibille, 2014; Poyraz et al., 2016; Urban et al., 2016), whereas chronic excitatory DREADD activation is more common due to the unique pharmacokinetic properties of hM3Dq (Jain et al., 2013). These challenges justify the ongoing development of improved DREADD actuators or delivery systems (Bonaventura et al., 2019).
SST+ Cells and Depressive-Like Behavior
Low SST and GABA markers in postmortem MDD patients and elevated behavioral emotionality in mice with altered SST+ cell function suggest a contributing role in mood disorders (Fee et al., 2017). We replicated past findings, including anxiety-like behaviors observed in Sst knockout (Lin and Sibille, 2015) and acute chemogenetic PFC SST+ cell-silenced mice (Soumier and Sibille, 2014). Our findings extended roles for SST+ cell regulation of behavioral deficits to antidepressant-predictive (e.g., FST) and short-term memory dimensions (e.g., NORT). However, we did not find working memory impairment as with optogenetic PFC SST+ cell silencing, possibly due to distinct inhibition timing, that is, initiated before vs during the test (Abbas et al., 2018).
Notably, past studies found that acute and chronic blockade of PFC SST+ cell function had opposite physiological and anxiogenic outcomes (Seybold et al., 2012; Soumier and Sibille, 2014). However, region-specific manipulations may confer compensatory neural circuit adaptations that are not reflective of human pathology, especially on regional cellular ablation rather than cell silencing (Fee et al., 2017). Indeed, we found behavioral changes closely paralleling rodent chronic stress studies (Nikolova et al., 2018; Prevot et al., 2019b; Fee et al., 2020) that demonstrated selective SST+ cell functional deficits via dysregulated cell integrity pathways (Lin and Sibille, 2015; Girgenti et al., 2019; Oh et al., 2019). These findings suggest that SST+ cell deficits are an intermediary causal factor between upstream risk factors (e.g., chronic stress, altered proteostasis and neurotrophic factor signaling) and symptom emergence (Fee et al., 2017; Prévot and Sibille, 2021).
In SST-control vs SST-silenced mice (using control vs hM4Di viruses), we replicated anxiogenic findings from the first characterization experiment (using Veh vs CNO) but found a stronger loading on anhedonia-like deficits. The lack of reproducibility on individual tests of anxiety-like behavior is a limitation of the current findings. However, the well-noted replicability challenges of individual behavioral tests (Willner, 2017) justified development of the PhenoTyper Test (a fully automated test with higher reproducibility than EPM and OFT; Prevot et al., 2019b) and Z-scoring methods that increase the power and reliability of preclinical phenotyping (Guilloux et al., 2011). To this extent, we found that SST+ cell silencing induced consistent anxiogenic changes detected by the PT and Z-scoring across experiments.
Given that SST+ cell deficits are more severe in females (Seney et al., 2013, 2015), sex was investigated as a main factor. However, differences were sparse as, for example, anhedonia-like behavior was increased in female mice but unrelated to SST+ cell silencing.
Antidepressant Potential of α5-PAM
α5-GABAA-R potentiation via GL-II-73 had antidepressant-, anxiolytic-like, and pro-memory effects in mice with brain-wide low SST+ cell function. GL-II-73 rescued deficits in the EPM and FST, consistent with effects in wild-type mice (Prevot et al., 2019a) and with phenotypes of mice having disinhibited SST+ cell function (Fuchs et al., 2017). However, CNO+GL-II-73 conferred hyperlocomotor effects in the EPM, OFT (but not the PT), and presumably the mobility-dependent FST (supplemental Table 2), so these results were excluded from Z-score analysis. These changes did not appear in past studies of GL-II-73 alone (Prevot et al., 2019b), so we cannot preclude potential drug-drug interactions. However, corrected Z-scoring revealed that GL-II-73 reduced anxiety- and anhedonia-like behavior, overall emotionality, and (trend-level) memory impairment in both SST-control and SST-silenced mice, consistent with past studies (Prevot et al., 2019a). Contrasting findings in chronic stress-exposed mice, GL-II-73 did not improve YM performance, potentially due to the reactive phenotype of SstCre mice and the handling necessary for this test (Viollet et al., 2017). Indeed, SST-controls had lower YM alternation rates compared with wild-type mice in past studies (Prevot et al., 2019a). Finally, GL-II-73 induced trend-level rescue of NORT deficits (a low-handling test), consistent with HPC α5-GABA-AR mediation of short-term memory (Möhler and Rudolph, 2017).
Others reported antidepressant-like and pro-cognitive properties from α5-knockdown or negative allosteric modulators (NAMs) (Martin et al., 2010; Fischell et al., 2015; Zanos et al., 2017; Bugay et al., 2020). Although these findings appear to conflict with α5-PAMs, it is proposed that SST+ cell regulation of microcircuit activity benefits from “tightening” in contexts characterized by underinhibition (i.e., poor information encoding) and “loosening” in contexts characterized by overinhibition (i.e., poor information transfer), supporting the utility of α5-PAM/NAM for different contexts, behaviors, or disorders (Prévot and Sibille, 2021). Indeed, we found that α5-PAM had therapeutic effects in SST-silenced mice with corticolimbic hyperactivity. Another possibility is that α5-PAMs and NAMs could increase the signal-to-noise ratio of corticolimbic microcircuits through different mechanisms, for example, by promoting phasic vs tonic PN activity with α5-PAMs or by normalizing PN signaling through ketamine-like synaptic plasticity with α5-NAMs (Bugay et al., 2020).
Future Directions
Despite marked improvement in sedative and amnesic side-effect profiles of BZDs, α5-selective derivatives such as GL-II-73 have mild α1–3 GABAA-R potentiation ( Prevot et al., 2019a) that may worsen or improve therapeutic efficacy. For example, more selective α5-PAMs (Stamenić et al., 2016) had weaker antidepressant-like and pro-cognitive effects only in chronic stress-exposed female (Piantadosi et al., 2016) and nonstressed male mice ( Prevot et al., 2019a). Given that regional localization impacts α1-5-subunit functional differentiation (Sieghart and Sperk, 2002), characterization studies using gene profiling (Lin and Sibille, 2015) or RNA labeling (Hu et al., 2019) may inform therapeutic strategies.
In conclusion, we demonstrated that brain-wide SST+ cell function regulates mood and cognitive functions and found support that acute disruptions contributing to anxiety- and anhedonia-like behaviors, overall behavioral emotionality, and impaired memory may reflect similar processes in psychiatric diseases over a longer scale. Deficits arising from brain-wide low SST+ cell function were rescued by α5-PAM, representing a promising new avenue for the development of targeted antidepressants.
Supplementary Material
Acknowledgments
We acknowledge the hard work of CAMH institutional animal facility staff for their assistance in breeding, genotyping, and maintaining colonies. Special thanks to K.F., G.F., and K.D. Special thanks to Dr Bryan Roth for providing DREADD vectors (Addgene #44362) and Drs Gradinaru and Deverman for the AAV-PHP.eB serotype technology. Thanks also to the Penn Vector Core for packaging and production of AAV-PHP.eB serotype control viruses (Addgene #50459).
C.F. and T.P. were supported by CAMH Discovery Fund fellowships. C.F. also received an Ontario Graduate Scholarship during the studies. M.B. is supported by a NARSAD young investigator award from the Brain and Behavior Research Foundation (#24034) and the CAMH Discovery Seed Fund and the Canadian Institutes of Health Research (PJT-165852). E.S. was supported by the Brain and Behavior Research Foundation (#25637) and Canadian Institutes of Health Research (PJT-153175). The project was also supported by the Campbell Family Mental Health Research Institute.
Interest Statement
D.K., G.L., P.M., J.C., E.S., M.B., and T.P. are co-inventors or listed on US patent applications that cover GABAergic ligands and/or their use in brain disorders. E.S. is co-founder of Alpha Cog, a biotech company developing ligands, including GL-II-73, as procognitive therapeutics. C.F. and K.M. have no conflicts of interest to disclose.
Author Contributions
C.F., M.B., and E.S. conceived of the study design. C.F. performed all experiments, data acquisition, and analysis with assistance from T.P., K.M., and M.B. C.F. wrote the manuscript with critical input from T.P., M.B., and E.S. D.K., G.L., P.M., and J.C. contributed to distinct steps for the synthesis and development of GL-II-73.
References
- Abbas AI, Sundiang MJM, Henoch B, Morton MP, Bolkan SS, Park AJ, Harris AZ, Kellendonk C, Gordon JA (2018) Somatostatin interneurons facilitate hippocampal-prefrontal synchrony and prefrontal spatial encoding. Neuron 100:926–939.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GM, Rogan SC, Abbas AI, Armbruster BN, Pei Y, Allen JA, Nonneman RJ, Hartmann J, Moy SS, Nicolelis MA, McNamara JO, Roth BL (2009) Remote control of neuronal activity in transgenic mice expressing evolved G protein-coupled receptors. Neuron 63:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM (2008) Synaptic alpha 5 subunit-containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex 18:1260–1271. [DOI] [PubMed] [Google Scholar]
- Allen WE, Kauvar IV, Chen MZ, Richman EB, Yang SJ, Chan K, Gradinaru V, Deverman BE, Luo L, Deisseroth K (2017) Global representations of goal-directed behavior in distinct cell types of mouse neocortex. Neuron 94:891–907.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajbouj M, Lisanby SH, Lang UE, Danker-Hopfe H, Heuser I, Neu P (2006) Evidence for impaired cortical inhibition in patients with unipolar major depression. Biol Psychiatry 59:395–400. [DOI] [PubMed] [Google Scholar]
- Benasi G, Guidi J, Offidani E, Balon R, Rickels K, Fava GA (2018) Benzodiazepines as a monotherapy in depressive disorders: a systematic review. Psychother Psychosom 87:65–74. [DOI] [PubMed] [Google Scholar]
- Bonaventura J, et al. (2019) High-potency ligands for DREADD imaging and activation in rodents and monkeys. Nat Commun 10:4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bugay V, McCoy AM, Lodge DJ, Brenner R, Frazer A, Carreno FR (2020) Mechanisms associated with the antidepressant-like effects of L-655,708. Neuropsychopharmacology 45:2289–2298. https://doi.org/10.1038/s41386-020-0772-2. Accessed August 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan KY, Jang MJ, Yoo BB, Greenbaum A, Ravi N, Wu WL, Sánchez-Guardado L, Lois C, Mazmanian SK, Deverman BE, Gradinaru V (2017) Engineered AAVs for efficient noninvasive gene delivery to the central and peripheral nervous systems. Nat Neurosci 20:1172–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deverman BE, Pravdo PL, Simpson BP, Kumar SR, Chan KY, Banerjee A, Wu WL, Yang B, Huber N, Pasca SP, Gradinaru V (2016) Cre-dependent selection yields AAV variants for widespread gene transfer to the adult brain. Nat Biotechnol 34:204–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douillard-Guilloux G, Lewis D, Seney ML, Sibille E (2017) Decrease in somatostatin-positive cell density in the amygdala of females with major depression. Depress Anxiety 34:68–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R (1989) The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods 29:261–265. [DOI] [PubMed] [Google Scholar]
- Engin E, Stellbrink J, Treit D, Dickson CT (2008) Anxiolytic and antidepressant effects of intracerebroventricularly administered somatostatin: behavioral and neurophysiological evidence. Neuroscience 157:666–676. [DOI] [PubMed] [Google Scholar]
- Engin E, Treit D (2009) Anxiolytic and antidepressant actions of somatostatin: the role of sst2 and sst3 receptors. Psychopharmacology (Berl) 206:281–289. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Fee C, Banasr M, Sibille E (2017) Somatostatin-positive gamma-aminobutyric acid interneuron deficits in depression: cortical microcircuit and therapeutic perspectives. Biol Psychiatry 82:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fee C, Prevot T, Misquitta K, Banasr M, Sibille E (2020) Chronic stress-induced behaviors correlate with exacerbated acute stress-induced cingulate cortex and ventral hippocampus activation. Neuroscience 440:113–129. [DOI] [PubMed] [Google Scholar]
- Ferguson SM, Eskenazi D, Ishikawa M, Wanat MJ, Phillips PE, Dong Y, Roth BL, Neumaier JF (2011) Transient neuronal inhibition reveals opposing roles of indirect and direct pathways in sensitization. Nat Neurosci 14:22–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischell J, Van Dyke AM, Kvarta MD, LeGates TA, Thompson SM (2015) Rapid antidepressant action and restoration of excitatory synaptic strength after chronic stress by negative modulators of Alpha5-containing GABAA receptors. Neuropsychopharmacology 40:2499–2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich MJ (2017) Depression is the leading cause of disability around the world. JAMA 317:1517. [DOI] [PubMed] [Google Scholar]
- Fuchs T, Jefferson SJ, Hooper A, Yee PH, Maguire J, Luscher B (2017) Disinhibition of somatostatin-positive GABAergic interneurons results in an anxiolytic and antidepressant-like brain state. Mol Psychiatry 22:920–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Warden D, Trivedi MH, Wisniewski SR, Fava M, Rush AJ (2009) What did STAR*D teach us? Results from a large-scale, practical, clinical trial for patients with depression. Psychiatr Serv 60:1439–1445. [DOI] [PubMed] [Google Scholar]
- Gholizadeh S, Tharmalingam S, Macaldaz ME, Hampson DR (2013) Transduction of the central nervous system after intracerebroventricular injection of adeno-associated viral vectors in neonatal and juvenile mice. Hum Gene Ther Methods 24:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girgenti MJ, Wohleb ES, Mehta S, Ghosal S, Fogaca MV, Duman RS (2019) Prefrontal cortex interneurons display dynamic sex-specific stress-induced transcriptomes. Transl Psychiatry 9:292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AF, Barthel AL, Hofmann SG (2018) Comparing the efficacy of benzodiazepines and serotonergic anti-depressants for adults with generalized anxiety disorder: a meta-analytic review. Expert Opin Pharmacother 19:883–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez JL, Bonaventura J, Lesniak W, Mathews WB, Sysa-Shah P, Rodriguez LA, Ellis RJ, Richie CT, Harvey BK, Dannals RF, Pomper MG, Bonci A, Michaelides M (2017) Chemogenetics revealed: DREADD occupancy and activation via converted clozapine. Science 357:503–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guettier JM, Gautam D, Scarselli M, Ruiz de Azua I, Li JH, Rosemond E, Ma X, Gonzalez FJ, Armbruster BN, Lu H, Roth BL, Wess J (2009) A chemical-genetic approach to study G protein regulation of beta cell function in vivo. Proc Natl Acad Sci U S A 106:19197–19202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Seney M, Edgar N, Sibille E (2011) Integrated behavioral z-scoring increases the sensitivity and reliability of behavioral phenotyping in mice: relevance to emotionality and sex. J Neurosci Methods 197:21–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilloux JP, Douillard-Guilloux G, Kota R, Wang X, Gardier AM, Martinowich K, Tseng GC, Lewis DA, Sibille E (2012) Molecular evidence for BDNF- and GABA-related dysfunctions in the amygdala of female subjects with major depression. Mol Psychiatry 17:1130–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC (2007) Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry 64:193–200. [DOI] [PubMed] [Google Scholar]
- Holtzheimer PE, Mayberg HS (2011) Stuck in a rut: rethinking depression and its treatment. Trends Neurosci 34:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Cavendish JZ, Agmon A (2013) Not all that glitters is gold: off-target recombination in the somatostatin-IRES-Cre mouse line labels a subset of fast-spiking interneurons. Front Neural Circuits 7:195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Rocco BR, Fee C, Sibille E (2019) Cell type-specific gene expression of Alpha 5 subunit-containing Gamma-aminobutyric acid subtype A receptors in human and mouse frontal cortex. Mol Neuropsychiatry 4:204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, Ruiz de Azua I, Lu H, White MF, Guettier JM, Wess J (2013) Chronic activation of a designer G(q)-coupled receptor improves β cell function. J Clin Invest 123:1750–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendryka M, Palchaudhuri M, Ursu D, van der Veen B, Liss B, Kätzel D, Nissen W, Pekcec A (2019) Pharmacokinetic and pharmacodynamic actions of clozapine-N-oxide, clozapine, and compound 21 in DREADD-based chemogenetics in mice. Sci Rep 9:4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katona L, Lapray D, Viney TJ, Oulhaj A, Borhegyi Z, Micklem BR, Klausberger T, Somogyi P (2014) Sleep and movement differentiates actions of two types of somatostatin-expressing GABAergic interneuron in rat hippocampus. Neuron 82:872–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JY, Ash RT, Ceballos-Diaz C, Levites Y, Golde TE, Smirnakis SM, Jankowsky JL (2013) Viral transduction of the neonatal brain delivers controllable genetic mosaicism for visualising and manipulating neuronal circuits in vivo. Eur J Neurosci 37:1203–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M (2013) Selective GABA(A) α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology 64:145–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovács KJ (1998) c-Fos as a transcription factor: a stressful (re)view from a functional map. Neurochem Int 33:287–297. [DOI] [PubMed] [Google Scholar]
- Levinson AJ, Fitzgerald PB, Favalli G, Blumberger DM, Daigle M, Daskalakis ZJ (2010) Evidence of cortical inhibitory deficits in major depressive disorder. Biol Psychiatry 67:458–464. [DOI] [PubMed] [Google Scholar]
- Lin LC, Sibille E (2013) Reduced brain somatostatin in mood disorders: a common pathophysiological substrate and drug target? Front Pharmacol 4:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin LC, Sibille E (2015) Somatostatin, neuronal vulnerability and behavioral emotionality. Mol Psychiatry 20:377–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüscher B, Möhler H (2019) Brexanolone, a neurosteroid antidepressant, vindicates the gabaergic deficit hypothesis of depression and may foster resilience. F1000Research 8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A (2006) Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci 26:5069–5082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madisen L, et al. (2012) A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci 15:793–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Zurek AA, MacDonald JF, Roder JC, Jackson MF, Orser BA (2010) Alpha5GABAA receptor activity sets the threshold for long-term potentiation and constrains hippocampus-dependent memory. J Neurosci 30:5269–5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möhler H, Rudolph U (2017) Disinhibition, an emerging pharmacology of learning and memory. F1000Res 6:F1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCBI (2020) Sst somatostatin [Mus musculus (house mouse)] - gene - NCBI. https://www.ncbi.nlm.nih.gov/gene/20604. Accessed February 8, 2020.
- Newton DF, Fee C, Nikolova YS, Sibille E (2019) Altered GABAergic function, cortical microcircuitry, and information processing in depression. In: Neurobiology of depression (Joao Q, Carvalho AF, Zarate CA, eds), pp 315–329. Cambridge, MA: Academic Press. [Google Scholar]
- Nikolova YS, Misquitta KA, Rocco BR, Prevot TD, Knodt AR, Ellegood J, Voineskos AN, Lerch JP, Hariri AR, Sibille E, Banasr M (2018) Shifting priorities: highly conserved behavioral and brain network adaptations to chronic stress across species. Transl Psychiatry 8:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Takeshima N, Hayasaka Y, Tajika A, Watanabe N, Streiner D, Furukawa TA (2019) Antidepressants plus benzodiazepines for adults with major depression. Cochrane Database Syst Rev 6:CD001026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Piantadosi SC, Rocco BR, Lewis DA, Watkins SC, Sibille E (2019) The role of dendritic brain-derived neurotrophic factor transcripts on altered inhibitory circuitry in depression. Biol Psychiatry 85:517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piantadosi SC, French BJ, Poe MM, Timić T, Marković BD, Pabba M, Seney ML, Oh H, Orser BA, Savić MM, Cook JM, Sibille E (2016) Sex-dependent anti-stress effect of an α5 subunit containing GABAA receptor positive allosteric modulator. Front Pharmacol 7:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyraz FC, Holzner E, Bailey MR, Meszaros J, Kenney L, Kheirbek MA, Balsam PD, Kellendonk C (2016) Decreasing striatopallidal pathway function enhances motivation by energizing the initiation of goal-directed action. J Neurosci 36:5988–6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévot T, Sibille E (2021) Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol Psychiatry 26:151–167. https://pubmed-ncbi-nlm-nih-gov.myaccess.library.utoronto.ca/32346158/. Accessed August 6, 2020. [DOI] [PubMed] [Google Scholar]
- Prévôt TD, Gastambide F, Viollet C, Henkous N, Martel G, Epelbaum J, Béracochéa D, Guillou JL (2017) Roles of hippocampal somatostatin receptor subtypes in stress response and emotionality. Neuropsychopharmacology 42:1647–1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prévôt TD, Viollet C, Epelbaum J, Dominguez G, Béracochéa D, Guillou JL (2018) sst 2 -receptor gene deletion exacerbates chronic stress-induced deficits: consequences for emotional and cognitive ageing. Prog Neuro-Psychopharmacology Biol Psychiatry 86:390–400. [DOI] [PubMed] [Google Scholar]
- Prevot TD, Li G, Vidojevic A, Misquitta KA, Fee C, Santrac A, Knutson DE, Stephen MR, Kodali R, Zahn NM, Arnold LA, Scholze P, Fisher JL, Marković BD, Banasr M, Cook JM, Savic M, Sibille E (2019a) Novel benzodiazepine-like ligands with various anxiolytic, antidepressant, or pro-cognitive profiles. Mol Neuropsychiatry 5:84–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevot TD, Misquitta KA, Fee C, Newton DF, Chatterjee D, Nikolova YS, Sibille E, Banasr M (2019b) Residual avoidance: a new, consistent and repeatable readout of chronic stress-induced conflict anxiety reversible by antidepressant treatment. Neuropharmacology 153:98–110. [DOI] [PubMed] [Google Scholar]
- Price RB, Shungu DC, Mao X, Nestadt P, Kelly C, Collins KA, Murrough JW, Charney DS, Mathew SJ (2009) Amino acid neurotransmitters assessed by proton magnetic resonance spectroscopy: relationship to treatment resistance in major depressive disorder. Biol Psychiatry 65:792–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhu N, de Jesus DR, Ravindran LN, Zanjani A, Fitzgerald PB, Daskalakis ZJ (2013) A meta-analysis of cortical inhibition and excitability using transcranial magnetic stimulation in psychiatric disorders. Clin Neurophysiol 124:1309–1320. [DOI] [PubMed] [Google Scholar]
- Rehm J, Shield KD (2019) Global burden of disease and the impact of mental and addictive disorders. Curr Psychiatry Rep 21:10. [DOI] [PubMed] [Google Scholar]
- Ren Z, Pribiag H, Jefferson SJ, Shorey M, Fuchs T, Stellwagen D, Luscher B (2016) Bidirectional homeostatic regulation of a depression-related brain state by gamma-aminobutyric acidergic deficits and ketamine treatment. Biol Psychiatry 80:457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph U, Crestani F, Möhler H (2001) GABA(A) receptor subtypes: dissecting their pharmacological functions. Trends Pharmacol Sci 22:188–194. [DOI] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J (2011) Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol 71:45–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders SK, Shekhar A (1995) Anxiolytic effects of chlordiazepoxide blocked by injection of GABAA and benzodiazepine receptor antagonists in the region of the anterior basolateral amygdala of rats. Biol Psychiatry 37:473–476. [DOI] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz JM, Knoflach F, Hernandez MC, Bischofberger J (2018) Dendrite-targeting interneurons control synaptic NMDA-receptor activation via nonlinear α5-GABAA receptors. Nat Commun 9:3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweitzer P, Madamba SG, Siggins GR (1998) Somatostatin increases a voltage-insensitive K+ conductance in rat CA1 hippocampal neurons. J Neurophysiol 79:1230–1238. [DOI] [PubMed] [Google Scholar]
- Seney ML, Chang LC, Oh H, Wang X, Tseng GC, Lewis DA, Sibille E (2013) The role of genetic sex in affect regulation and expression of GABA-related genes across species. Front Psychiatry 4:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seney ML, Tripp A, McCune S, Lewis DA, Sibille E (2015) Laminar and cellular analyses of reduced somatostatin gene expression in the subgenual anterior cingulate cortex in major depression. Neurobiol Dis 73:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold BA, Stanco A, Cho KK, Potter GB, Kim C, Sohal VS, Rubenstein JL, Schreiner CE (2012) Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex. Proc Natl Acad Sci U S A 109:13829–13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibille E, Morris HM, Kota RS, Lewis DA (2011) GABA-related transcripts in the dorsolateral prefrontal cortex in mood disorders. Int J Neuropsychopharmacol 14:721–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieghart W, Sperk G(2002) Subunit composition, distribution and function of GABAA receptor subtypes. Curr Top Mol Chem 2:795–816. [DOI] [PubMed] [Google Scholar]
- Smith KS, Bucci DJ, Luikart BW, Mahler SV (2016) DREADDS: use and application in behavioral neuroscience. Behav Neurosci 130:137–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumier A, Sibille E (2014) Opposing effects of acute versus chronic blockade of frontal cortex somatostatin-positive inhibitory neurons on behavioral emotionality in mice. Neuropsychopharmacology 39:2252–2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamenić TT, Poe MM, Rehman S, Santrač A, Divović B, Scholze P, Ernst M, Cook JM, Savić MM (2016) Ester to amide substitution improves selectivity, efficacy and kinetic behavior of a benzodiazepine positive modulator of GABAA receptors containing the α5 subunit. Eur J Pharmacol 791:433–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stengel A, Taché YF (2017) Activation of brain somatostatin signaling suppresses CRF receptor-mediated stress response. Front Neurosci 11:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallent MK, Siggins GR (1997) Somatostatin depresses excitatory but not inhibitory neurotransmission in rat CA1 hippocampus. J Neurophysiol 78:3008–3018. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Kvitsani D, Fu Y, Lu J, Lin Y, Miyoshi G, Shima Y, Fishell G, Nelson SB, Huang ZJ (2011) A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron 71:995–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Kota RS, Lewis DA, Sibille E (2011) Reduced somatostatin in subgenual anterior cingulate cortex in major depression. Neurobiol Dis 42:116–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp A, Oh H, Guilloux JP, Martinowich K, Lewis DA, Sibille E (2012) Brain-derived neurotrophic factor signaling and subgenual anterior cingulate cortex dysfunction in major depressive disorder. Am J Psychiatry 169:1194–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban DJ, Zhu H, Marcinkiewcz CA, Michaelides M, Oshibuchi H, Rhea D, Aryal DK, Farrell MS, Lowery-Gionta E, Olsen RH, Wetsel WC, Kash TL, Hurd YL, Tecott LH, Roth BL (2016) Elucidation of the behavioral program and neuronal network encoded by dorsal raphe serotonergic neurons. Neuropsychopharmacology 41:1404–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban-Ciecko J, Fanselow EE, Barth AL (2015) Neocortical somatostatin neurons reversibly silence excitatory transmission via GABAb receptors. Curr Biol 25:722–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Kales A, Bixler EO (1995) Benzodiazepine side effects: role of pharmacokinetics and pharmacodynamics. Pharmacology 51:205–223. [DOI] [PubMed] [Google Scholar]
- Viollet C, Simon A, Tolle V, Labarthe A, Grouselle D, Loe-Mie Y, Simonneau M, Martel G, Epelbaum J (2017) Somatostatin-IRES-Cre mice: between knockout and wild-type? Front Endocrinol (Lausanne) 8:131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, et al. (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P (2017) Reliability of the chronic mild stress model of depression: a user survey. Neurobiol Stress 6:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization (2017) Depression and other common mental health disorders: global health estimates. Geneva, Switzerland; World Health organization. [Google Scholar]
- Yavorska I, Wehr M (2016) Somatostatin-expressing inhibitory interneurons in cortical circuits. Front Neural Circuits 10:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung M, Engin E, Treit D (2011) Anxiolytic-like effects of somatostatin isoforms SST 14 and SST 28 in two animal models (Rattus norvegicus) after intra-amygdalar and intra-septal microinfusions. Psychopharmacology (Berl) 216:557–567. [DOI] [PubMed] [Google Scholar]
- Zanos P, Nelson ME, Highland JN, Krimmel SR, Georgiou P, Gould TD, Thompson SM (2017) A negative allosteric modulator for α5 subunit- containing GABA receptors exerts a rapid and persistent antidepressant-like action without the side effects of the NMDA receptor antagonist ketamine in mice. eNeuro 4:ENEURO.0285-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Roth BL (2014) Silencing synapses with DREADDs. Neuron 82:723–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.