Abstract
The aim of this study was to identify and validate a sensitive, high-throughput, and cost-effective SARS-CoV-2 real-time RT-PCR assay to be used as a surveillance and diagnostic tool for SARS-CoV-2 in a university surveillance program. We conducted a side-by-side clinical evaluation of a newly developed SARS-CoV-2 multiplex assay (EZ-SARS-CoV-2 Real-Time RT-PCR) with the commercial TaqPath COVID-19 Combo Kit, which has an Emergency Use Authorization from the FDA. The EZ-SARS-CoV-2 RT-PCR incorporates two assays targeting the SARS-CoV-2 N gene, an internal control targeting the human RNase P gene, and a PCR inhibition control in a single reaction. Nasopharyngeal (NP) and anterior nares (AN) swabs were tested as individuals and pools with both assays and in the ABI 7500 Fast and the QuantStudio 5 detection platforms. The analytical sensitivity of the EZ-SARS-CoV-2 RT-PCR assay was 250 copies/ml or approximately 1.75 genome copy equivalents per reaction. The clinical performance of the EZ-SARS-CoV-2 assay was evaluated using NP and AN samples tested in other laboratories. The diagnostic sensitivity of the assay ranged between 94 and 96% across the detection platforms, and the diagnostic specificity was 94.06%. The positive predictive value was 94%, and the negative predictive value ranged from 94 to 96%. Pooling five NP or AN specimens yielded 93% diagnostic sensitivity. The overall agreement between these SARS-CoV-2 RT-PCR assays was high, supported by a Cohen’s kappa value of 0.93. The EZ-SARS-CoV-2 RT-PCR assay performance attributes of high sensitivity and specificity with AN sample matrix and pooled upper respiratory samples support its use in a high-throughput surveillance testing program.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00705-021-05148-1.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) became a public health emergency due to the rapid and widespread dissemination of the virus at a global scale [1–3]. Fast and accurate diagnosis of SARS-CoV-2 infection is critical for preventing virus transmission through isolation of positive patients, and quarantine of contact persons, and for providing adequate treatment to clinically affected patients [4]. Given that SARS-CoV-2 causes asymptomatic infections [5–7] and that it can be effectively transmitted prior to the development of symptoms [8–11], widespread testing of symptomatic and asymptomatic populations is essential for effective disease control. To achieve widespread testing of large populations, highly sensitive, accurate and efficient tests and testing workflows are needed. Efficiency is especially important, given the high demand for SARS-CoV-2 testing and the resultant supply shortages that limit the number of SARS-CoV-2 and other diagnostic assays that can be performed, as reported by the American Society for Microbiology [12].
SARS-CoV-2 is a newly emerging member of the subgenus Sarbecovirus within the family Coronaviridae [13]. The SARS-CoV-2 genome consists of a single-stranded RNA molecule of approximately 30 kb in length [14]. Assays for detection of SARS-CoV-2 are often based on real-time reverse transcription polymerase chain reaction (RT-PCR) [15, 16], and many RT-PCR assays have been given Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) [17]. The high analytical sensitivity of RT-PCR allows its use for pooled testing, in which multiple samples are mixed and tested in a single reaction, thus increasing testing efficiency and significantly lowering costs [18]. The feasibility of pooled testing for detection of SARS-CoV-2 has been demonstrated in multiple studies [19–22].
We were tasked with identifying a sensitive SARS-CoV-2 assay that could be performed in an efficient manner and provide sensitive and specific surveillance and diagnostic testing capability to support Cornell University’s surveillance program and campus re-opening in the fall semester of 2020. The present study was undertaken to identify and validate a SARS-CoV-2 multiplex RT-PCR assay that could support high-throughput and cost-effective testing with a rapid turnaround time. We evaluated the clinical performance of a newly developed SARS-CoV-2 multiplex RT-PCR assay (targeting two regions of the SARS-CoV-2 N gene, in addition to an internal control targeting the human RNase P gene and a PCR inhibition control) and compared it to the performance of a commercial assay with an EUA from the FDA. To facilitate efficient testing, increase testing capacity, and decrease the overall testing costs of the university surveillance program, assay performance was also evaluated on pooled samples.
Materials and methods
Clinical specimens
De-identified frozen specimens were shared with the Cornell COVID-19 Testing Laboratory (CCTL) by three other COVID-19 testing laboratories in the United States. A total of 201 nasopharyngeal (NP) swabs and 24 anterior nares (AN) swabs were included in this study. Sixty NP swabs were tested in originating laboratory 1 using the Xpert® Xpress SARS-CoV-2 assay (Cepheid, Sunnyvale, CA), and of these, 30 were positive and 30 were negative for SARS-CoV-2. The other 141 NP swabs were tested in originating laboratory 2 using the TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay (Thermo Fisher Scientific Inc., Waltham, MA); 70 were positive and 71 were negative SARS-CoV-2. The AN swabs were originally tested using the New York State Department of Health (NYSDOH) (laboratory 3) SARS-CoV-2 RT-PCR assay; 12 were positive and 12 were negative for SARS-CoV-2.
Sample pooling
Pools of five or 10 samples were made by combining one SARS-CoV-2-positive sample with four or nine negative samples, respectively. Two hundred µl of each sample was included in the pools. Twenty individual positive NP swab samples with original Ct values of 28 or greater were selected to generate the NP pools, and twelve individual positive AN swab samples with original Ct values between 19 and 34 were selected to generate the AN pools.
Nucleic acid extraction
Prior to extraction, each sample was vortexed at 100 × g for 10 s. Nucleic acid was extracted from 200 µl of each individual swab supernatant or pooled samples using a MagMAX Viral/Pathogen II Nucleic Acid Isolation Kit (Applied Biosystems, Foster City, CA) and a KingFisher Flex Magnetic Particle Processor, following the manufacturer’s procedures (Thermo Fisher Scientific Inc.). Nucleic acid was eluted in 50 µl of elution buffer and used for SARS-CoV-2 detection as described below.
SARS-CoV-2 RT-PCR assays
Two assays for SARS-CoV-2 real-time RT-PCR were used in this study: the newly developed EZ-SARS-CoV-2 Real Time RT-PCR assay (Tetracore, Inc., Rockville, MD) and the TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay (Thermo Fisher Scientific Inc.). The EZ-SARS-CoV-2 assay was evaluated and compared to the TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay, which served as a reference assay with an EUA from FDA. Negative extraction, negative amplification, and positive amplification controls were included on each assay run. RT-PCR assays were performed in two detection systems: the ABI 7500 Fast Real-Time PCR System (with SDS v1.5.1 software, Applied Biosystems, Waltham, MA) and the QuantStudio 5 Real-Time PCR System (with QuantStudio Design and Analysis Desktop Software v1.5.1, Applied Biosystems).
The EZ-SARS-CoV-2 Real-Time RT-PCR (Tetracore, Inc.) is a multiplex assay targeting the SARS-CoV-2 N gene (two target regions, with both probes labeled with FAM reporter dye), the human RNase P gene (CY5 reporter dye), and an inhibition control (IC, TAMRA reporter dye). The amplification cycling conditions were 48°C for 15 minutes; 95°C for 2 minutes; 45 cycles of 95°C for 10 seconds and 60°C for 40 seconds, using 18 µl of combined reagents and 7 µl of nucleic acid template. RT-PCR data were analyzed for SARS-CoV-2 and IC targets by setting the threshold at 3% of the final normalized fluorescence of the positive amplification control. For the human RNase P gene, the threshold was set at 3% of the approximate average of the final normalized fluorescence of all samples in a single plate run. The baseline was set automatically by the software for all reporter dyes.
The TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay (Thermo Fisher Scientific Inc.) targets three regions of the SARS-CoV-2 genome (ORF1ab, N, and S genes with FAM, VIC, and ABY reporter dyes, respectively) and an inhibition control target (MS2 bacteriophage, JUN reporter dye). The amplification cycling conditions were 25°C for 2 minutes, 53°C for 10 minutes, 95°C for 2 minutes, and 40 cycles of 95°C for 3 seconds and 60°C for 30 seconds, using 15 µl of combined reagents and 10 µl of template. RT-PCR data were analyzed for SARS-CoV-2 targets by setting the threshold at 10% of the final normalized fluorescence of the positive amplification control. For MS2, the threshold was set at 10% of the approximate average of the final normalized fluorescence of all samples in a single plate run. The baseline was set automatically by the software for all reporter dyes. For interpretation of results, TaqPath RT-PCR data were analyzed with auto settings and imported into Applied Biosystems COVID-19 Interpretive Software version 1.2.
Analytical sensitivity
The limit of detection (LoD) of the EZ-SARS-CoV-2 RT-PCR was determined by preparing twofold serial dilutions in viral transport medium (VTM, Corning product number 25-500-CM, Corning, NY) (medium only) and AN sample matrix (SARS-CoV-2-negative patient samples) ranging from 1,000 to 62.5 copies/ml of a commercial standard containing the full-length SARS-CoV-2 genomic RNA (AccuPlex SARS-CoV-2 Verification Panel – Full Genome, Seracare Life Sciences, Inc., Milford, MA, catalog number 0505-0168). Each serial dilution was tested three times independently using the ABI 7500 Fast and the QuantStudio 5 detection systems. A preliminary LoD was defined as the lowest concentration in which 100% of the replicates were positive. The final LoD of the assay was determined by testing 20 replicates containing 250 and 500 viral genome copies/ml, and the LoD was defined as the lowest concentration in which at least 19/20 (95%) of the replicates were detected.
Analytical specificity
Initial cross-reactivity analyses of the EZ-SARS-CoV-2 RT-PCR primers and probes were performed in silico. Oligonucleotide sequences were compared to a non-redundant database comprised of GenBank NT and RefSeq entries as of 02/11/2020 using nucleotide BLAST. Sequences identified as EST, STS, GSS, WGS, TSA, or patent were excluded. The BLAST search parameters were set for short sequences. The following values were fixed: threshold = 1000, match score = 1, mismatch score = -3. A set of human respiratory pathogen sequences (Supplementary Table S1) were compared to the oligonucleotide sequences by Needleman–Wunsch alignment [23]. Gaps were not allowed. Matches were assigned a score of 1; zero was assigned otherwise. The number of matches was summed to determine the alignment score, and the score was divided by the length of the oligonucleotide to determine the frequency.
To exclude cross-reactivity of the EZ-SARS-CoV-2 RT-PCR assay against other human pathogens and confirm its specificity in the wet test condition, the assay was tested against 40 non-target organisms known to infect humans (Supplementary Table S2). Nucleic acid from each organism (at a concentration of > 106 CFU/ml or > 104 TCID50/ml, when available from the vendor) was extracted using a QIAamp Viral RNA Mini Kit (QIAGEN) and subjected to real-time PCR using the EZ-SARS-CoV-2 assay in a ABI 7500 Fast Real-Time PCR System. Each pathogen was tested in triplicate. SARS-CoV-2-positive and negative controls were included in each assay run.
Reproducibility
The intra- and inter-run reproducibility of the EZ-SARS-CoV-2 PCR assay was evaluated. For this, tenfold serial dilutions containing between 100 and 10,000 genome copies of SARS-CoV-2 per ml (AccuPlex SARS-CoV-2 Verification Panel – Full Genome, Seracare Life Sciences, Inc., catalog number 0505-0168) were prepared in VTM and tested in triplicate and in three independent runs.
Data analysis
Microsoft Excel was used to calculate the mean and variation of replicate Ct values, including standard deviation [SD (Ct)], coefficient of variation [%CV (Ct)], and coefficient of variation based on linearized Ct values [%Ct (2-Ct)]. Contingency (2 × 2) tables were analyzed in Microsoft Excel to determine the diagnostic sensitivity, diagnostic specificity, positive predictive value, and negative predictive value of the assays evaluated herein, as well as the corresponding 95% confidence intervals. Results provided by the originating laboratory were considered to be the reference standard for these calculations and comparisons of the assays evaluated here.
The degree of agreement between assay results was measured by Cohen’s kappa using formulas from Watson and Petrie, 2010 [24]. The plot was generated using GraphPad Prism (version 9.0.0 for Windows, GraphPad Software, San Diego, California USA, www.graphpad.com).
Results
Analytical specificity
The specificity of the primers and probes (N gene assay 1 and 2) of the EZ-SARS-CoV-2 RT-PCR was evaluated in silico and tested against a panel of 40 known human pathogens. In silico BLAST searches and alignment of the N gene assay 1 probe sequences revealed 92% sequence identity to SARS-CoV (AY345986.1), while the forward and reverse primers presented lower sequence identity (70% and 88%, respectively) to SARS-CoV. The forward primer sequence of N gene assay 2 presented 100% sequence identity to SARS-CoV (AY345986.1), whereas the N gene assay 2 reverse primer and probe sequences presented lower sequence identity to SARS-CoV (89% and 78%, respectively). No significant sequence identity was observed between the primers and probes of N gene assays 1 and 2 and the human genome, other coronaviruses, or other common agents of the human microflora (Supplementary Table S1). Most importantly, testing of the panel of 40 known human pathogens did not result in cross-amplification (Supplementary Table S2), confirming the high specificity of the assay.
Analytical sensitivity
The estimated LoD for the EZ-SARS-CoV-2 PCR assay was determined to be 250 copies per ml in VTM and AN sample matrix (Table 1). One replicate at 125 copies per ml was not detected with the AN matrix diluent. The LoD for the TaqPath COVID-19 Real-Time RT-PCR assay was estimated to be 500 copies per ml (equivalent to 5 copies per reaction) (Table 1). The final LoD of the EZ-SARS-CoV-2 assay was determined testing 20 replicates containing 250 and 500 copies per ml. Nineteen of 20 replicates were positive at 250 copies per ml (Table 2), and the final LoD of the assay was determined to be 250 copies per ml, which is equivalent to 1.75 genome copies per reaction.
Table 1.
Percent positive replicates of twofold dilution series detected on the ABI 7500 platform
| Assay | Copies per mla | ||||
|---|---|---|---|---|---|
| Diluent | 1000 | 500 | 250 | 125 | 62.5 |
| EZ-SARS-CoV-2 PCR assay | |||||
| VTM | 100 | 100 | 100 | 100 | 77.78 |
| AN | 100 | 100 | 100 | 88.89 | 88.89 |
| TaqPath COVID-19 real-time RT-PCR assay S gene | |||||
| VTM | 100 | 100 | 100 | 77.78 | 77.78 |
| AN | 100 | 100 | 100 | 55.56 | 33.33 |
| TaqPath COVID-19 real-time RT-PCR assay ORF1ab | |||||
| VTM | 100 | 100 | 77.78 | 100 | 55.56 |
| AN | 100 | 100 | 100 | 100 | 22.22 |
| TaqPath COVID-19 real-time RT-PCR assay N gene | |||||
| VTM | 100 | 100 | 100 | 100 | 44.44 |
| AN | 100 | 100 | 77.78 | 77.78 | 22.22 |
aResults represent percent of replicates detected in three independent runs
Table 2.
Final limit of detection (LoD) of EZ-SARS-CoV-2 Real-Time RT-PCR based on the analysis of twenty replicates at 250 copies per ml in AN matrix on the ABI 7500 platform
| Replicate | Ct value | Replicate | Ct value | Replicate | Ct value | Replicate | Ct value |
|---|---|---|---|---|---|---|---|
| 1 | 35.27 | 6 | 35.16 | 11 | 36.69 | 16 | 36.20 |
| 2 | 36.20 | 7 | 35.48 | 12 | Undetermined | 17 | 37.29 |
| 3 | 34.15 | 8 | 34.90 | 13 | 36.09 | 18 | 35.57 |
| 4 | 34.54 | 9 | 35.13 | 14 | 35.71 | 19 | 37.60 |
| 5 | 36.92 | 10 | 35.30 | 15 | 34.14 | 20 | 35.94 |
Assay reproducibility
The reproducibility of the EZ-SARS-CoV-2 assay was determined within a single test run (intra-run) and between independent runs (inter-run). Serial dilutions with known concentrations of the SARS-CoV-2 genome in VTM were tested in triplicate and in three independent PCR runs. As shown in Tables 3 and 4, the assay demonstrated high intra- and inter-run reproducibility. The standard deviation [SD (Ct)] of Ct values for replicate samples was less than 1.0 when tested on the ABI 7500 detection system. Only one set of replicates had a standard deviation over 1 (100 copies per ml on run 1) when tested using the QuantStudio 5 detection system. None of the coefficient of variation values based on Ct values [%CV (Ct)] were greater than 3%. Additionally, only two of the coefficient of variation values based on linearized Ct values [%Ct (2-Ct)] exceeded 50% when tested on the ABI 7500 system and none exceeded 50% when tested in the QuantStudio 5 detection system.
Table 3.
Intra-assay variation of EZ-SARS-CoV-2 real-time RT-PCR cycle threshold (Ct) values
| Plate | Copies per ml | Mean (Ct) | SD (Ct) | % CV (Ct) | % CV (2−Ct) |
|---|---|---|---|---|---|
| ABI 7500 platform | |||||
| 1 | 104 | 29.91 | 0.09 | 0.30 | 6.18 |
| 1 | 103 | 33.56 | 0.05 | 0.16 | 3.78 |
| 1 | 102 | 36.74 | 0.72 | 1.96 | 53.52 |
| 2 | 104 | 29.86 | 0.03 | 0.10 | 2.09 |
| 2 | 103 | 33.92 | 0.26 | 0.76 | 16.96 |
| 2 | 102 | 38.07 | 0.83 | 2.19 | 62.83 |
| 3 | 104 | 29.45 | 0.12 | 0.41 | 8.47 |
| 3 | 103 | 32.94 | 0.37 | 1.14 | 26.08 |
| 3 | 102 | 35.78 | 0.54 | 1.5 | 36.76 |
| QuantStudio 5 platform | |||||
| 1 | 104 | 29.99 | 0.39 | 1.30 | 26.64 |
| 1 | 103 | 33.02 | 0.08 | 0.24 | 5.67 |
| 1 | 102 | 36.49 | 1.14 | 3.14 | 84.4 |
| 2 | 104 | 29.88 | 0.28 | 0.94 | 19.55 |
| 2 | 103 | 34.13 | 0.32 | 0.93 | 22.28 |
| 2 | 102 | 36.13 | 0.49 | 1.36 | 31.15 |
| 3 | 104 | 29.99 | 0.15 | 0.51 | 10.37 |
| 3 | 103 | 33.28 | 0.13 | 0.38 | 8.60 |
| 3 | 102 | 37.05 | 0.45 | 1.21 | 30.91 |
Table 4.
Inter-assay variation of EZ-SARS-CoV-2 RT-PCR cycle threshold (Ct) values
| Copies per ml | Mean (Ct) | SD (Ct) | %CV (Ct) | %CV (2−Ct) |
|---|---|---|---|---|
| ABI 7500 platform | ||||
| 104 | 29.74 | 0.25 | 0.85 | 18.30 |
| 103 | 33.47 | 0.49 | 1.48 | 36.33 |
| 102 | 36.86 | 1.15 | 3.11 | 66.82 |
| QuantStudio 5 platform | ||||
| 104 | 29.95 | 0.06 | 0.21 | 4.38 |
| 103 | 33.48 | 0.58 | 1.72 | 35.04 |
| 102 | 36.56 | 0.46 | 1.27 | 31.32 |
Additionally, intra-run repeatability of the EZ-SARS-CoV-2 RT-PCR assay was within expected parameters when the number of SARS-CoV-2 genome copies was within the assay LoD; however, at the lower concentration tested (100 copies per ml), greater variability was observed on the ABI 7500 platform with [SD (Ct)] greater than 1, [%CV (Ct)] greater than 3, and [%Ct (2-Ct)] greater than 50%. Importantly, in both inter- and intra-run comparisons, the replicates that demonstrated the greatest variability (>50%) contained the lowest concentration of SARS-CoV-2 genomes/ml (100 copies per ml), which is below the LoD of the assay (250 copies per ml, Table 2).
Clinical performance in individual upper respiratory samples
Clinical evaluation of the EZ-SARS-CoV-2 RT-PCR and comparison of its performance with the reference TaqPath™ COVID-19 Real-Time RT-PCR assay or the Xpert® Xpress SARS-CoV-2 RT-PCR assays were performed on 201 NP samples. Of these, 60 samples were originally tested on the Cepheid GeneXpert System using the Xpert® Xpress SARS-CoV-2 RT-PCR assay (30 had SARS-CoV-2-positive test results and 30 had SARS-CoV-2-negative test results). The remaining 141 samples were originally tested using the TaqPath™ Real-Time RT-PCR assay (70 positive and 71 negative for SARS-CoV-2).
Among the 60 NP samples previously tested using the Xpert® Xpress SARS-CoV-2 RT-PCR assay, the EZ-SARS-CoV-2 RT-PCR detected all 30 positive NP specimens on both the ABI 7500 and QuantStudio 5 platforms (Supplementary Table S3). Of the samples that were expected to be negative, two (n = 2) tested positive on the ABI 7500 platform and three (n = 3) were detected on the QuantStudio 5 platform (Supplementary Table S3). Two of the discrepant samples were detected by the EZ-SARS-CoV-2 RT-PCR assay on both platforms (ABI 7500 and QuantStudio 5).
When the set of 141 NP samples originally tested using the TaqPath™ COVID-19 Real-Time RT-PCR assay were tested using the EZ-SARS-CoV-2 RT-PCR, 66 and 64 of 70 positive NP specimens were detected on the ABI 7500 and the QuantStudio 5 detection system, respectively (Supplementary Table S4). Three of the six discrepant samples were not detected on either platform. Of the NP specimens that were expected to be negative, the EZ-SARS-CoV-2 RT-PCR assay detected four of these as positive on the ABI 7500 platform and three as positive on the QuantStudio 5 platform. One of the discrepant samples was positive on both platforms, and the other five were only detected on a single platform.
The diagnostic sensitivity and specificity of the EZ-SARS-CoV-2 RT-PCR was estimated and compared to those of the TaqPath™ COVID-19 Real-Time RT-PCR using test results from the 201 NP samples included in our study (Table 5). Additionally, comparison of this dataset to the provided results was used to estimate the positive and negative predictive values of the EZ-SARS-CoV-2 RT-PCR assay. As shown in Table 6, the diagnostic sensitivity of the assay ranged between 94 and 96% when using the ABI 7500 and QuantStudio5 platforms, while the diagnostic specificity was 94.06%. The positive predictive value (PPV) for the EZ-SARS-CoV-2 RT-PCR assay was 94%, and the negative predictive value (NPV) ranged from 94 to 96%.
Table 5.
Comparison of EZ-SARS-CoV-2 RT-PCR assay (EZ) results with TaqPath™ COVID-19 Combo Kit Multiplex Real-Time RT-PCR results for 201 NP samples performed on the same elution in the Cornell COVID-19 Testing Laboratory
| ABI 7500 | TaqPath positive | TaqPath negative | QuantStudio 5 | TaqPath positive | TaqPath negative |
|---|---|---|---|---|---|
| EZ positive | 92 | 10 | EZ positive | 91 | 9 |
| EZ negative | 0 | 99 | EZ negative | 2 | 99 |
| Diagnostic sensitivity | 100.00 (100.00-100.00) | Diagnostic sensitivity | 97.85 (94.90–100.80) | ||
| Diagnostic specificity | 90.83 (85.41–96.24) | Diagnostic specificity | 91.67 (86.45–96.88) | ||
| Positive predictive value | 90.20 (84.43–95.97) | Positive predictive value | 91.00 (85.39–96.61) | ||
| Negative predictive value | 100.00 (100.00–100.00) | Negative predictive value | 98.02 (95.30–100.74) | ||
| Kappa | 0.94 (0.90–0.97) | Kappa | 0.93 (0.89–0.97) | ||
Results determined on the ABI 7500 platform are on the left and results determined by the QuantStudio 5 platform are on the right. Estimates are followed by 95% confidence interval in parentheses
Table 6.
Comparison of EZ-SARS-CoV-2 RT-PCR assay (EZ) results with results for 201 NP samples provided by other testing laboratories
| ABI 7500 | Originating lab positive | Originating lab negative | QuantStudio 5 | Originating lab positive | Originating lab negative |
|---|---|---|---|---|---|
| EZ positive | 96 | 6 | EZ positive | 94 | 6 |
| EZ negative | 4 | 95 | EZ negative | 6 | 95 |
| Diagnostic sensitivity | 96.00 (92.16–99.84) | Diagnostic sensitivity | 94.00 (89.35–98.65) | ||
| Diagnostic specificity | 94.06 (89.45–98.67) | Diagnostic specificity | 94.06 (89.45–98.67) | ||
| Positive predictive value | 94.12 (89.55–98.68) | Positive predictive value | 94.00 (89.35–98.65) | ||
| Negative predictive value | 95.96 (92.08–99.84) | Negative predictive value | 94.06 (89.45–98.67) | ||
| Kappa | 0.93 (0.89–0.97) | Kappa | 0.92 (0.88–0.96) | ||
Results determined on the ABI 7500 platform are on the left and results determined by the QuantStudio 5 platform are on the right. Estimates are followed by 95% confidence interval in parentheses
Clinical performance in pooled upper respiratory samples
The performance of the EZ-SARS-CoV-2 assay and its feasibility for testing pooled respiratory samples was also evaluated. Ten individual positive NP swab samples with original Ct values greater than 28 were selected to create 10 pools. Three independent extractions and amplification runs were performed for each pool. Pools were assayed with both the EZ-SARS-CoV-2 RT-PCR assay and the TaqPath COVID-19 Real-Time RT-PCR assay.
To assess a pool size of 10 samples, each of 10 pools comprised one SARS-CoV-2-positive NP swab sample and nine negative NP swabs samples. The average diagnostic sensitivity on pools of 10 samples after three extraction and amplification runs was approximately 70% with the EZ-SARS-CoV-2 RT-PCR assay and approximately 50% with the TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay (Table 7).
Table 7.
Diagnostic sensitivity with a pool size of 10 NP swabs; 10 pools tested
| Assay | Platform | Diagnostic sensitivity |
|---|---|---|
| EZ-SARS-CoV-2 RT-PCR | ABI 7500 | 70 |
| QuantStudio 5 | 73 | |
| TaqPath COVID-19 RT-PCR | ABI 7500 | 50 |
| QuantStudio 5 | 47 |
When a pool size of 5 samples was assessed, using the same 10 individual positive samples now combined with four negative NP samples, the average diagnostic sensitivity increased to 90% with the EZ-SARS-CoV-2 RT-PCR assay and approximately 75% with the TaqPath COVID-19 Combo Kit Multiplex Real-Time RT-PCR assay (Table 8).
Table 8.
Diagnostic sensitivity with a pool size of 5 NP swabs; 10 pools tested
| Assay | Platform | Diagnostic sensitivity |
|---|---|---|
| EZ-SARS-CoV-2 RT-PCR | ABI 7500 | 90 |
| QuantStudio 5 | 90 | |
| TaqPath COVID-19 RT-PCR | ABI 7500 | 77 |
| QuantStudio 5 | 73 |
Given the better performance of the assays on pools of five samples, additional pools of five were created with one positive and four negative samples each, including 10 more NP swab pools and 12 AN swab pools. These pools were extracted and amplified three times using the EZ-SARS-CoV-2 RT-PCR assay. The difference in Ct values between the individual positive sample and the corresponding pool of five is shown in Table 9 for the ABI 7500 platform and in Table 10 for the QuantStudio 5 platform.
Table 9.
Results of EZ-SARS-CoV-2 RT-PCR with pool size of 5 on the ABI 7500 platform
| Pool | Specimen | Individual Ct value | Pooled Ct valuea | Ct change |
|---|---|---|---|---|
| 1 | NP | 33.55 | 34.58 | 1.03 |
| 2 | NP | 35.30 | 36.86 | 1.55 |
| 3 | NP | 33.39 | 34.34 | 0.95 |
| 4 | NP | 36.20 | 36.77 | 0.58 |
| 5 | NP | 33.85 | 36.55 | 2.71 |
| 6 | NP | 33.33 | 34.69 | 1.36 |
| 7 | NP | 35.75 | 36.62 | 0.87 |
| 8 | NP | 40.25 | Not detected | |
| 9 | NP | 35.19 | 37.75 | 2.56 |
| 10 | NP | 35.93 | 37.51 | 1.58 |
| Average (standard deviation) of Ct change | 1.47 (0.74) | |||
| 11 | NP | 30.83 | 33.94 | 3.10 |
| 12 | NP | 28.97 | 31.79 | 2.81 |
| 13 | NP | 33.41 | 32.40 | − 1.01 |
| 14 | NP | 32.86 | 36.19 | 3.33 |
| 15 | NP | 33.95 | 36.49 | 2.54 |
| 16 | NP | 31.77 | 33.50 | 1.72 |
| 17 | NP | 32.00 | 31.16 | − 0.85 |
| 18 | NP | 28.24 | 30.28 | 2.03 |
| 19 | NP | 30.16 | 33.28 | 3.11 |
| 20 | NP | 32.25 | 35.17 | 2.91 |
| Average (standard deviation) of Ct change | 1.97 (1.61) | |||
| 21 | ANb | 19.02 | 18.15 | − 0.87 |
| 22 | AN | 19.86 | 18.90 | − 0.96 |
| 23 | AN | 28.50 | 27.09 | − 1.41 |
| 24 | AN | 31.64 | 38.62 | 6.98 |
| 25 | AN | 31.75 | 34.71 | 2.96 |
| 26 | AN | 32.96 | 37.70 | 4.74 |
| 27 | AN | 33.33 | 37.22 | 3.89 |
| 28 | AN | 25.50 | 29.87 | 4.37 |
| 29 | AN | 34.00 | 39.12 | 5.12 |
| 30 | AN | 22.00 | 24.93 | 2.93 |
| 31 | AN | 23.75 | 25.93 | 2.18 |
| 32 | AN | 32.25 | 34.76 | 2.51 |
| Average (standard deviation) of Ct change | 2.70 (2.63) | |||
aAverage Ct value from three independent extraction and RT-PCR runs
bAN specimens and individual Ct values were kindly provided by the Wadsworth Center, NYS Department of Health. There was not enough volume for both individual and pooled extractions
Table 10.
Results of EZ-SARS-CoV-2 RT-PCR with pool size of 5 on the QuantStudio 5 platform for validation
| Pool | Specimen | Individual Ct value | Pooled Ct valuea | Ct change |
|---|---|---|---|---|
| 1 | NP | 32.96 | 35.04 | 2.08 |
| 2 | NP | 35.42 | 36.96 | 1.53 |
| 3 | NP | 32.65 | 35.04 | 2.39 |
| 4 | NP | 34.67 | 36.62 | 1.95 |
| 5 | NP | 33.11 | 36.97 | 3.86 |
| 6 | NP | 33.41 | 35.46 | 2.05 |
| 7 | NP | 34.74 | 36.84 | 2.11 |
| 8 | NP | 37.15 | 38.41 | 1.26 |
| 9 | NP | 35.13 | 37.04 | 1.91 |
| 10 | NP | 35.72 | 38.29 | 2.57 |
| Average (standard deviation) of Ct change | 2.17 (0.70) | |||
| 11 | NP | 33.00 | 33.88 | 0.88 |
| 12 | NP | 31.60 | 31.67 | 0.08 |
| 13 | NP | 35.53 | 32.24 | − 3.29 |
| 14 | NP | 34.79 | 36.05 | 1.26 |
| 15 | NP | 34.12 | 36.24 | 2.12 |
| 16 | NP | 32.28 | 33.53 | 1.25 |
| 17 | NP | 32.24 | 31.48 | − 0.76 |
| 18 | NP | 28.76 | 30.35 | 1.59 |
| 19 | NP | 30.87 | 33.00 | 2.12 |
| 20 | NP | 32.36 | 34.82 | 2.46 |
| Average (standard deviation) of Ct change | 0.77 (1.73) | |||
| 21 | ANb | 19.02 | 17.98 | − 1.04 |
| 22 | AN | 19.86 | 18.73 | − 1.13 |
| 23 | AN | 28.50 | 26.97 | − 1.53 |
| 24 | AN | 31.64 | 38.44 | 6.80 |
| 25 | AN | 31.75 | 35.01 | 3.26 |
| 26 | AN | 32.96 | 37.95 | 4.99 |
| 27 | AN | 33.33 | Not detected | |
| 28 | AN | 25.50 | 29.92 | 4.42 |
| 29 | AN | 34.00 | 36.82 | 2.82 |
| 30 | AN | 22.00 | 24.89 | 2.89 |
| 31 | AN | 23.75 | 25.98 | 2.23 |
| 32 | AN | 32.25 | 34.53 | 2.28 |
| Average (standard deviation) of Ct change: | 2.36 (2.67) | |||
aAverage Ct value from three independent extraction and RT-PCR runs
bAN specimens and individual Ct values were kindly provided by the Wadsworth Center, NYS Department of Health. There was not enough volume for both individual and pooled extractions.
Twelve pools of five SARS-CoV-2 negative AN samples were generated at the same time as the SARS-CoV-2-positive AN pools. No pools were detected after performing three extractions and RT-PCR assays on the ABI 7500 platform. On the QuantStudio 5 platform, only one pool (pool 4) was detected in one out of three runs, with a Ct value of 41.79.
For pools of five consisting of one positive and four negative samples, the average Ct change observed for the entire study was 2.44, with a standard deviation of 2.90. The expected Ct change for a fivefold dilution is approximately 2.3 cycles. The relationship between EZ-SARS-CoV-2 RT-PCR Ct values of the individual SARS-CoV-2-positive samples and in the corresponding pools of five is shown in Figure 1.
Fig. 1.
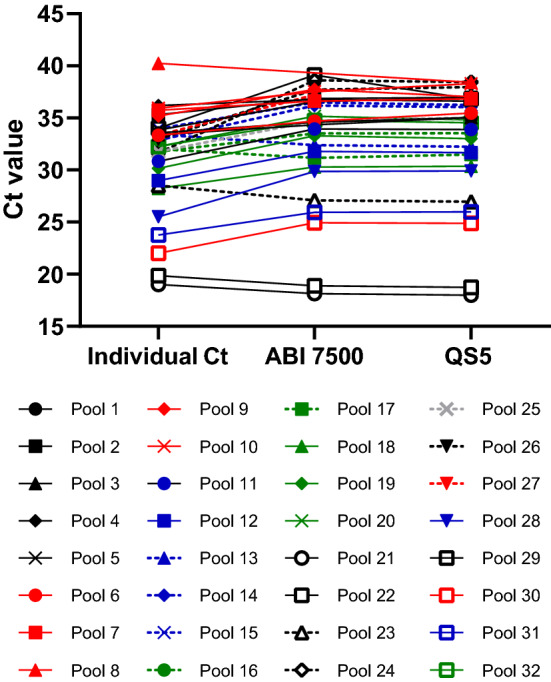
EZ-SARS-CoV-2 RT-PCR Ct values of individual and pooled samples. The Ct value of each individual SARS-CoV-2-positive sample is plotted and connected to the average pooled Ct value (Ct values from each of the three independent extraction and amplification runs averaged together) determined on either the ABI 7500 or QuantStudio 5 platform.
The change in Ct value between positive pools and the corresponding individual positive specimens tested with the EZ-SARS-CoV-2 RT-PCR assay was also evaluated in additional pools. From 54 positive pools with Ct values ranging from 15.66 to 39.53, the average Ct change was 2.84 cycles, with a standard deviation of 1.94.
Positive pooled samples were not detected in seven out of 96 amplifications (32 pools extracted and assayed three times each), resulting in approximately 93% diagnostic sensitivity for pools of five containing one SARS-CoV-2-positive sample.
Discussion
In this study, we evaluated the clinical performance of the EZ-SARS-CoV-2 RT-PCR assay on individual and pooled human upper respiratory specimens. The analytical sensitivity of the EZ-SARS-CoV-2 RT-PCR assay was determined to be 250 copies/ml, which corresponds to approximately 1.75 genome copy equivalents per reaction. In comparison, the analytical sensitivity of the TaqPath COVID-19 RT-PCR assay was determined in this study to be 500 copies/ml (5 genomic copy equivalents per reaction), and it is reported to be 10 genomic copy equivalents per reaction by the manufacturer [25]. The sensitivity of these RT-PCR assays is comparable to that of the US CDC RT-PCR panel for SARS-CoV-2, which is reported to be 5 RNA transcript copies/reaction [26]. In contrast, the sensitivity of the cartridge-based Xpert Xpress SARS-CoV-2 Assay LoD was reported to be 250 copies/ml by the manufacturer in April 2020, but when the required 300-µl sample input volume is considered, the LoD equates to 75 copies per reaction [27]. The specificity of the EZ-SARS-CoV-2 RT-PCR assay was assessed in silico and in vitro with a panel 40 bacterial, fungal, and viral human respiratory pathogens, including human coronavirus strains 229E and OC43, SARS-CoV, and MERS-CoV. The EZ-SARS-CoV-2 RT-PCR assay did not amplify any pathogens other than SARS-CoV-2, confirming the specificity of the assay.
Both the intra-assay and inter-assay variation of the EZ-SARS-CoV-2 RT-PCR assay were low. Importantly, in both inter- and intra-assay comparisons, the replicates that demonstrated the greatest variability (CV (linearized Ct) >50%) contained the lowest concentration of SARS-CoV-2 genomes/ml (100 copies per ml), which is below the assay LoD (250 copies per ml, Table 2).
The diagnostic sensitivity of the EZ-SARS-CoV-2 RT-PCR assay was compared to that of the Cepheid GeneXpert Xpress system using 60 NP samples. All 30 positive samples were correctly identified by both assays; however, the EZ-SARS-CoV-2 RT-PCR assay identified two of the 30 negative samples as positive on both amplification platforms tested, with late positive Ct values. One of these was also identified as positive by the TaqPath COVID-19 RT-PCR assay on both amplification platforms. Given the differences in LoD, these samples may be true positives with low viral load. When compared to a larger set of 70 positives that were initially tested elsewhere using the TaqPath COVID-19 RT-PCR assay, four were not detected with the EZ-SARS-CoV-2 RT-PCR assay on the ABI 7500 Fast platform, and three of these were not detected on the QuantStudio 5 platform either. Given that these were archived samples, the possibility that shipping and/or freeze-thawing may have compromised the integrity of the viral RNA present in some of these samples cannot be formally excluded. Original false positive results in the initial test may also have contributed to these discrepancies. Considering all 201 samples used in the present study, the diagnostic sensitivity of the EZ-SARS-CoV-2 RT-PCR assay was 94-96%, with diagnostic specificity of 94%. The positive predictive value was 94%, and the negative predictive value ranged from 94 to 96% in this comparison. The high level of agreement is also indicated by Cohen’s kappa values of 0.92-0.93.
Testing the same set of 201 diagnostic samples allowed us to compare the performance of the EZ-SARS-CoV-2 RT-PCR assay directly with the TaqPath COVID-19 RT-PCR assay, as they were performed using the same nucleic acid elutions and on the same PCR instruments. The diagnostic sensitivity of the EZ-SARS-CoV-2 RT-PCR assay was higher than the 90% determined for the TaqPath COVID-19 RT-PCR assay on both platforms. However, the diagnostic specificity of the TaqPath COVID-19 RT-PCR assay was slightly higher than the EZ-SARS-CoV-2 RT-PCR assay (97-98% versus 94%). The positive predictive value for the EZ-SARS-CoV-2 RT-PCR assay was approximately 94%, whereas the negative predictive value was approximately 95%. Overall, agreement between the EZ-SARS-CoV-2 and the TaqPath COVID-19 RT-PCR assays was high, supported by Cohen’s kappa values of 0.93-0.94.
To increase our testing capacity and meet the high demand for surveillance testing at Cornell University, the feasibility of testing pooled samples was also investigated. Pooling one SARS-CoV-2-positive NP sample with nine negative NP samples reduced the diagnostic sensitivity to 70% with the EZ-SARS-CoV-2 assay and to 50% with the TaqPath COVID-19 kit. Pools of five samples yielded 93% diagnostic sensitivity, and acceptable performance was found for both NP and AN swab specimens. Pooling is an important strategy to both increase the sample throughput and reduce the costs of supplies.
One limitation of this assay is that the use of 45 amplification cycles creates the possibility of detecting samples with late cycle threshold values that have low reproducibility due to low viral load or yield false positive results. A second limitation is the dependence on a single gene in the SARS-CoV-2 genome. Furthermore, a mutation in the viral strain could impact detection if it occurs in the target region. Although many SARS-CoV-2 mutations occur elsewhere in the genome (mainly S gene), three of the 11 most frequent mutations in the United States occur in the N gene, which encodes the nucleocapsid [28]. It is important to note, however, that none of the N mutations affected the sensitivity of the EZ RT-PCR assay. Additionally, as this study was under review it became evident that some of the S gene mutations that occurred in the B.1.1.7 lineage of SARS-CoV-2 (Alpha variant) affected detection of the S gene target by the TaqPath COVID-19 RT-PCR TaqPath COVID-19 RT-PCR assay, leading to S gene drop out (lack of detection) when this variant is present in the clinical sample.
In summary, the EZ-SARS-CoV-2 RT-PCR assay has excellent performance when using the automated extraction and amplification workflow with human upper respiratory samples. The results of this study support the use of the EZ-SARS-CoV-2 RT-PCR for pooled testing in upper respiratory human samples. Combining the high sensitivity of the EZ-SARS-CoV-2 assay with the ease of AN swab collection and the ability to pool samples will facilitate a high-throughput surveillance testing program. The slightly reduced sensitivity in pooled specimens can be compensated by high-frequency sample collection, especially from those populations at higher risk of SARS-CoV-2 infection and spread.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank the Office of the Vice Provost for Academic Integration for providing financial support for the validation studies described here. We would like to thank Drs. Lars Westblade and Melissa Cushing from Weill Cornell Medicine, Dr. Kiril Dimitrov from Texas Medical Veterinary Diagnostic Laboratory, and Dr. Kirsten St. George from Wadsworth Center, NYS Department of Health for providing archived clinical samples used for the validation study described here.
Author contributions
The EZ-SARS-CoV-2 RT-PCR assay was designed by RR, AS, and WMN. Samples were acquired by EP and DGD. Validation plan was devised by ML, RT and DGD. Samples were processed and validation experiments were performed by ML, RV, BC, and XZ. Specificity experiments were performed by RR and AS. Data were analyzed and the first manuscript was drafted by RLT and DGD. All authors read and approved the final manuscript.
Funding
This study was funded by the Cornell University Office of the Vice Provost for Academic Integration, Ithaca, New York, USA.
Availability of data
Raw data are available upon request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The validation study was discussed with Cornell’s IRB, and given that the specimens used were residual de-identified specimens collected for diagnostic purposes, no formal review of the protocol was deemed necessary. The Cayuga Medical Center’s (CMC) IRB has reviewed and approved the diagnostic assay development and validation protocol (Protocol no. 0420EP).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bchetnia M, Girard C, Duchaine C, Laprise C. The outbreak of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): a review of the current global status. J Infect Public Health. 2020;13:1601–1610. doi: 10.1016/j.jiph.2020.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohrabi C, Alsafi Z, O’Neill N, et al. World Health Organization declares global emergency: a review of the 2019 novel coronavirus (COVID-19) Int J Surg. 2020;76:71–76. doi: 10.1016/j.ijsu.2020.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Emergency Committee (2021) Statement on the sixth meeting of the International Health Regulations (2005) Emergency Committee regarding the coronavirus disease (COVID-19) pandemic. https://www.who.int/news/item/15-01-2021-statement-on-the-sixth-meeting-of-the-international-health-regulations-(2005)-emergency-committee-regarding-the-coronavirus-disease-(covid-19)-pandemic. Accessed 2 Feb 2021
- 4.World Health Organization (2020) Considerations in the investigation of cases and clusters of COVID-19: interim guidance. https://www.who.int/publications/i/item/considerations-in-the-investigation-of-cases-and-clusters-of-covid-19. Accessed 2 Feb 2021
- 5.Buitrago-Garcia D, Egli-Gany D, Counotte MJ, et al. Occurrence and transmission potential of asymptomatic and presymptomatic SARSCoV-2 infections: a living systematic review and meta-analysis. PLoS Med. 2020;17:e100334. doi: 10.1371/journal.pmed.1003346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han D, Li R, Han Y, et al. Covid-19: Insight into the asymptomatic sars-cov-2 infection and transmission. Int J Biol Sci. 2020;16:2803–2811. doi: 10.7150/ijbs.48991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection : a narrative review. Ann Intern Med. 2020;173:362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arons MM, Hatfield KM, Reddy SC, et al. Presymptomatic SARS-CoV-2 infections and transmission in a skilled nursing facility. N Engl J Med. 2020;382:2081–2090. doi: 10.1056/nejmoa2008457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng HY, Jian SW, Liu DP, et al. Contact Tracing Assessment of COVID-19 transmission dynamics in taiwan and risk at different exposure periods before and after symptom onset. JAMA Intern Med. 2020;180:1156–1163. doi: 10.1001/jamainternmed.2020.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kimball A, Hatfield KM, Arons M, et al. Asymptomatic and presymptomatic SARS-CoV-2 infections in residents of a long-term care skilled nursing facility—King County, Washington, March 2020. MMWR Morb Mortal Wkly Rep. 2020;69:377–381. doi: 10.15585/mmwr.mm6913e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei WE, Li Z, Chiew CJ, et al. Presymptomatic transmission of SARS-CoV-2—Singapore, January 23–March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:411–415. doi: 10.15585/mmwr.mm6914e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.(2021) Supply shortages impacting COVID-19 and non-COVID testing. In: Am Soc Microbiol. https://asm.org/Articles/2020/September/Clinical-Microbiology-Supply-Shortage-Collecti-1. Accessed 3 Feb 2021
- 13.Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus taxonomy. New York: Elsevier; 2012. Order—Nidovirales; pp. 784–794. [Google Scholar]
- 15.Shabani E, Dowlatshahi S, Abdekhodaie MJ. Laboratory detection methods for the human coronaviruses. Eur J Clin Microbiol Infect Dis. 2021;40:225–246. doi: 10.1007/s10096-020-04001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cheng MP, Papenburg J, Desjardins M, et al. Diagnostic testing for severe acute respiratory syndrome-related coronavirus 2: a narrative review. Ann Intern Med. 2020;172:726–734. doi: 10.7326/M20-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(2021) In Vitro Diagnostics EUAs | FDA. In: US Food Drug Adm. website. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas. Accessed 8 Feb 2021
- 18.Dorfman R. The detection of defective members of large populations. Ann Math Stat. 1943;14:436–440. doi: 10.1214/aoms/1177731363. [DOI] [Google Scholar]
- 19.Abdalhamid B, Bilder CR, McCutchen EL, et al. Assessment of specimen pooling to conserve SARS CoV-2 testing resources. Am J Clin Pathol. 2020;153:715–718. doi: 10.1093/ajcp/aqaa064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ben-Ami R, Klochendler A, Seidel M, et al. Large-scale implementation of pooled RNA extraction and RT-PCR for SARS-CoV-2 detection. Clin Microbiol Infect. 2020;26:1248–1253. doi: 10.1016/j.cmi.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirotsu Y, Maejima M, Shibusawa M, et al. Pooling RT-qPCR testing for SARS-CoV-2 in 1000 individuals of healthy and infection-suspected patients. Sci Rep. 2020;10:18899. doi: 10.1038/s41598-020-76043-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deka S, Kalita D. Effectiveness of sample pooling strategies for SARS-CoV-2 mass screening by RT-PCR: a scoping review. J Lab Physicians. 2020;12:212–218. doi: 10.1055/s-0040-1721159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Needleman SB, Wunsch CD. A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol. 1970;48:443–453. doi: 10.1016/0022-2836(70)90057-4. [DOI] [PubMed] [Google Scholar]
- 24.Watson PF, Petrie A. Method agreement analysis: a review of correct methodology. Theriogenology. 2010;73:1167–1179. doi: 10.1016/j.theriogenology.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Thermo Fisher Scientific Inc. TaqPath COVID-19 Combo Kit and TaqPath COVID-19 Combo Kit advanced instructions for use, publication number MAN0019181, revision H.0. Thermo Fisher Scientific Inc; 2020. [Google Scholar]
- 26.Lu X, Wang L, Sakthivel SK, et al. US CDC real-time reverse transcription PCR panel for detection of severe acute respiratory syndrome Coronavirus 2. Emerg Infect Dis. 2020;26:1654–1665. doi: 10.3201/eid2608.201246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cepheid . Xpert Xpress SARS-CoV-2 Assay ENGLISH package insert 302–3750 revision C. Cepheid; 2020. [Google Scholar]
- 28.Wang R, Chen J, Gao K, et al. Analysis of SARS-CoV-2 mutations in the United States suggests presence of four substrains and novel variants. Commun Biol. 2021;4:1–14. doi: 10.1038/s42003-021-01754-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available upon request.


