Abstract
Objectives
Various symptoms and considerable organ dysfunction persist following infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Uncertainty remains about the potential mid- and long-term health sequelae. This prospective study of patients hospitalized with coronavirus disease 2019 (COVID-19) in Liège University Hospital, Belgium aimed to determine the persistent consequences of COVID-19.
Methods
Patients admitted to the University Hospital of Liège with moderate-to-severe confirmed COVID-19, discharged between 2 March and 1 October 2020, were recruited prospectively. Follow-up at 3 and 6 months after hospital discharge included demographic and clinical data, biological data, pulmonary function tests (PFTs) and high-resolution computed tomography (CT) scans of the chest.
Results
In total, 199 individuals were included in the analysis. Most patients received oxygen supplementation (80.4%). Six months after discharge, 47% and 32% of patients still had exertional dyspnoea and fatigue. PFTs at 3-month follow-up revealed a reduced diffusion capacity of carbon monoxide (mean 71.6 ± 18.6%), and this increased significantly at 6-month follow-up (P<0.0001). Chest CT scans showed a high prevalence (68.9% of the cohort) of persistent abnormalities, mainly ground glass opacities. Duration of hospitalization, intensive care unit admission and mechanical ventilation were not associated with the persistence of symptoms 3 months after discharge.
Conclusion
The prevalence of persistent symptoms following hospitalization with COVID-19 is high and stable for up to 6 months after discharge. However, biological, functional and iconographic abnormalities improved significantly over time.
Keywords: long COVID, post-COVID, COVID-19, sequelae, post-acute COVID-19
Introduction
In December 2019, a novel coronavirus – severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) – was detected in Wuhan, China, and identified as the aetiological agent of coronavirus disease 2019 (COVID-19). Clinical manifestations range from the absence of symptoms to acute respiratory distress syndrome and multi-organ failure (Frix et al., 2020; Guiot et al., 2020a; Radermecker et al., 2020; Schoneveld et al., 2021). For most patients, the infection is mild with low-grade fever and cough, but 15% of patients experience respiratory impairment combined with diffuse alveolar damage, pulmonary (hyper)inflammatory infiltrates and microvascular thrombosis associated with elevated levels of inflammatory markers, and require hospital care (Radermecker et al., 2020; Fang et al., 2021). In addition to respiratory symptoms, multiple organs can be affected. Disorders include thrombotic complications, myocardial dysfunction and arrhythmia, acute coronary syndromes, acute kidney injury (AKI), gastrointestinal symptoms, hepatocellular injury, hyperglycaemia and ketosis, neurological illnesses, ocular symptoms and dermatological complications (Gabarre et al., 2020; Gupta et al., 2020; Iadecola et al., 2020; Li et al., 2020; Ronco et al., 2020). AKI in hospitalized patients is high, with prevalence ranging from approximately 20% to approximately 80% according to the definition used for AKI and the type of hospitalization [usual vs intensive care unit (ICU)]. AKI has been associated with death (Cheng et al., 2020; Wu et al., 2020; Chan et al., 2021; Chatterjee et al., 2021). To date, few and discrepant results are available regarding the evolution of patients with AKI and/or proteinuria (Bouquegneau et al., 2021; Delanaye et al, 2021; Gupta et al., 2021).
More recently, data have emerged that symptoms related to COVID-19 persist after the acute phase of infection. Terminology found in the literature mainly refers to ‘post-COVID conditions’, ‘long COVID’, ‘post-COVID syndrome’ or ‘post-acute COVID-19’ (Amenta et al., 2020; National Institute for Health and Care Excellence, 2020; Ayoubkhani et al., 2021). The National Institute for Health and Care Excellence (2020) has defined long COVID as ‘signs and symptoms that develop during or after an infection consistent with COVID-19 which continue for more than 12 weeks and are not explained by an alternative diagnosis’.
The incidence, natural history and aetiology of these symptoms have not been determined to date, and may vary depending on numerous factors including viral, individual or treatment features. A growing number of studies have highlighted that a noteworthy proportion of patients who have suffered from SARS-CoV-2 infection experience ‘post-COVID syndrome’ (Salamanna et al., 2021). Learning more about the whole range of short- and long-term health effects associated with COVID-19 has become a key priority. Tools constructed from patients’ lived experiences would provide validated and reliable instruments to monitor the symptoms and impact of ‘post-COVID syndrome’ (Tran et al., 2021). Chopra et al. (2021) reported a high death rate within 60 days of discharge. For most patients who survived, ongoing physical and psychological morbidities were common. Carvalho-Schneider et al. (2021) confirmed that two-thirds of adults with non-critical COVID-19 had complaints 2 months after symptom onset, mainly anosmia/ageusia, dyspnoea or fatigue. Garrigues et al. (2020) found that patients with COVID-19 discharged from hospital experienced persistent symptoms, most commonly fatigue and dyspnoea, for up to 3 months after diagnosis. Huang et al. (2021) presented the results of a large study with long-term follow-up, and reported that fatigue or muscle weakness, sleep difficulties, and anxiety or depression were common, even 6 months after symptom onset. More severely ill patients had higher risk of pulmonary diffusion abnormality, fatigue or muscle weakness, and anxiety or depression. In addition, lung function was altered, with reduced diffusion capacity and long-term lung abnormalities shown on imaging, mainly represented by ground glass opacities (GGOs) and interstitial lung abnormalities (Guiot et al., 2020b,c). More recently, Ayoubkhani et al. (2021) quantified rates of organ-specific dysfunction in individuals with COVID-19 after discharge from hospital compared with a matched control group from the general population. Mean follow-up in the study by Amenta et al. (2020) was 140 days. Admission to hospital for COVID-19 was associated with increased risk of re-admission and death after discharge. Rates of multi-organ dysfunction after discharge were higher in individuals with COVID-19 compared with those in the matched control group, suggesting extrapulmonary pathophysiology. Long-term complications after COVID-19 are thus associated with severity but not limited to hospitalized patients (Hirschtick et al., 2021). A Danish population-based cohort study found that the absolute risk of severe post-acute complications after SARS-CoV-2 infection not requiring hospital admission was low (Lund et al., 2021). Blomberg et al. (2021) found that young, home-isolated adults with mild COVID-19 were at risk of long-lasting dyspnoea and cognitive symptoms. Kashif et al. (2021) also reported various post-viral sequelae, the most common being fatigue, in patients following recovery from mild COVID-19.
These data indicate that the toll of COVID-19 extends well beyond hospitalization. Studies aimed at determining long-term pathophysiology across organ systems are needed urgently. As such, this prospective study investigated patients hospitalized with COVID-19 to determine the persistent consequences of COVID-19, and to characterize risk factors associated with the symptoms of long-term COVID-19.
Methods
Study design and participants
Patients admitted to the University Hospital of Liège with moderate-to-severe confirmed COVID-19, discharged between 2 March and 1 October 2020, were recruited prospectively. Inclusion criteria were: (1) age ≥16 years; (2) confirmed SARS-CoV-2 infection; and (3) hospitalization at the University Hospital of Liège, Belgium. Recruitment was performed on a voluntary basis.
All patients who died before the first follow-up visit, those for whom no recommended follow-up was available, and those who declined to participate were excluded from the study.
All discharged patients met uniform discharge criteria according to standard clinical care.
The protocol was approved by the Ethics Committee of CHU Liège (No. B707201422832; ref: 2021-131).
Clinical and biological parameters
The following variables were considered at patient admission: age; weight; height; body mass index (BMI); history of hypertension (based on medical records and/or the presence of antihypertensive medications at admission); history of diabetes (based on medical records and/or the presence of specific therapy at admission); active smoking; and history of chronic kidney disease (CKD) (based on medical records, not biological data). Biological data of interest were considered at the closest time to discharge (maximum 72 h before discharge). All biological data were generated from a single laboratory (Unilab, CHU de Liège) accredited for ISO 15189 Guideline (regulatory standard for medical labs). The following variables were measured: C-reactive protein (CRP), procalcitonin, serum creatinine, lactate dehydrogenase (LDH), albumin, sodium, potassium, total calcium and bicarbonate (Abbott Alinity instrument); leukocytes, lymphocytes, platelet counts and haemoglobin (Sysmex SE-9000 Hematology analyzer); and D-dimer (Innovance D-Dimer kit on the Siemens CS5100 automate).
AKI was defined by the Kidney Disease Improving Global Outcomes (KDIGO) AKI criteria (Kellum et al., 2013). The KDIGO criteria for AKI applied serum creatinine variations alone. The baseline serum creatinine value was defined using the most recent value found in the centralized electronic medical records of CHU Liège preceding the current admission. Whenever data were not available, the serum creatinine level measured at the time of admission was used as the baseline value. Estimated glomerular filtration rate (eGFR) was calculated using the Chronic Kidney Disease Epidemiology Collaboration formula (Levey et al., 2009). Renal data on 30 patients from this cohort have been published previously (Bouquegneau et al., 2021).
Pulmonary function tests
Pulmonary function tests (PFTs) were performed in the routine respiratory laboratory at Liège University Hospital. Forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) were measured in accordance with the recommendations of the European Respiratory Society (ERS) (Laszlo, 2006). The results were expressed in millilitres and % predicted. The Tiffeneau index or FEV1/FVC was expressed as a percentage. Total lung capacity (TLC) was measured by body plethysmography according to the ERS recommendations. The diffusion capacity of carbon monoxide (DLCO) and DLCO/AV were measured using the single-breath carbon monoxide gas transfer method, and expressed as % predicted (Sensor Medics 2400 He/CO Analyzer System, Bilthoven, The Netherlands). In addition, differences between pre- and post-COVID-19 PFTs were evaluated when available.
CT scanner description
All CT images used in the study were acquired on one of five multi-detector CT scanners (Siemens Edge Plus: Siemens Healthineers; Erlangen, Germany, GE Revolution CT, GE Brightspeed: GE Healthcare Chicago, Illinois, USA) available at the University Hospital of Liège. As CT images were collected prospectively as part of standard care, no standardized scan protocol was available over the complete dataset. Two thoracic radiologists performed CT scan analysis (PC: 3 years of experience, CD: 6 years of experience).
Statistical analysis
Results were summarized using mean and standard deviation (SD) or median and interquartile range (IQR) for quantitative variables. Qualitative variables were presented as frequency tables (number and percentage).
Chi-squared test and Fisher's exact test, as appropriate, were used to compare proportions, and non-parametric Kruskal–Wallis test was used to compare quantitative variables. Linear or logistic regression models were used to analyse the impact of patient characteristics and the severity of hospital stay on clinical and pulmonary consequences. Student's t-test for paired observations was used to analyse changes in pulmonary data pre- vs post COVID. Changes in CT scans and biological parameters were studied with McNemar's test and signed rank test for paired observations.
Results were considered significant at the 5% level (P<0.05). Missing data were not replaced and calculations were always performed using the maximum number of data available. Data analysis was performed using SAS Version 9.4 for Windows. R Version 3.6.1 was used for the figures.
Results
Patients’ characteristics
In total, 199 individuals were included in the analysis (Table S1, see online supplementary material). The demographic, clinical and treatment characteristics of the patients are given in Tables S1 and S2 (see online supplementary material). The mean age was 60.5 ± 13.9 years, and 63.3% of subjects were male. Mean BMI was 28.6 kg/m2 (Table S1, see online supplementary material). The percentages of comorbidities were high: 44.4% for hypertension, 36.2% for diabetes and 36.4% for obesity. In addition, 20.6% of subjects had a chronic pulmonary disease.
The median duration of hospitalization was 9 days (Table S2, see online supplementay material). Most patients received oxygen supplementation (80.4%; Table S2, see online supplementary material). Approximately one-quarter (26.1%) of patients were hospitalized in an ICU, 16.6% had endotracheal intubation, >80% were treated with hydroxychloroquine and the huge majority (90.4%) also received antibiotics (Table S2, see online supplementary material).
Evolution of symptomatology
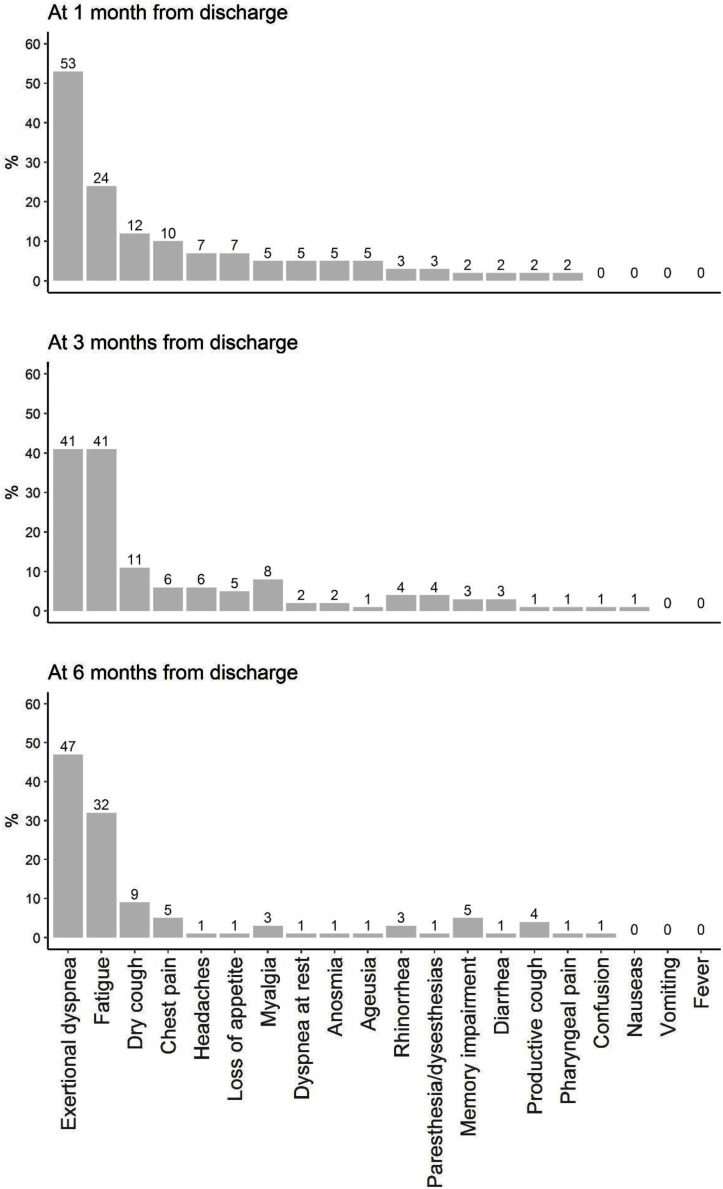
Persistence of symptoms was analysed systematically. Figure 1 represents the symptoms at 1 month, 3 months and 6 months after discharge. The most common symptoms were exertional dyspnoea and fatigue, followed by cough and chest pain (Figure 1 and Table S3, see online supplementary material). Shortness of breath was present in 53% of individuals at 1 month after discharge, and remained highly prevalent at 3 (41%) and 6 (47%) months after discharge. Fatigue was common 1 month after discharge. Surprisingly, the prevalence of fatigue increased at 3 months after discharge (41%). A high proportion of individuals still complained about fatigue 6 months after discharge (32%). Dry cough was common at 1 month after discharge (12%). The proportion of individuals presenting with a dry cough remained high at 3 and 6 months after discharge (11% and 9%, respectively). The prevalence of chest pain decreased from 10% at 1 month after discharge to 5% at 6 months after discharge. In contrast, there was a slight increase in the proportion of patients presenting with memory impairment over time. The proportion of individuals presenting with symptoms after discharge was high and remained elevated at 6 months after discharge. The most common symptoms at 1, 3 and 6 months after discharge were shortness of breath and fatigue.
Figure 1.
Changes in symptoms.
Changes in biological data
Changes in biological data are presented in Table 1 . The level of haemoglobin increased significantly from discharge to 1 month after discharge, and from 1 month after discharge to 3 months after discharge (P<0.0001). At discharge, 37.5% had anaemia, and this number decreased to 15.5% at 3 months after discharge.
Table 1.
Evolution of biological data
| At discharge(± 72 h) | Follow-upafter 1 month | Follow-upafter 3 months | |
|---|---|---|---|
| Days since hospital discharge, median (IQR) | 34 (28–46) | 93 (84–106) | |
| WBC (10³/mm³) | |||
| N | 178 | 57 | 71 |
| Median (IQR) | 6.0 (4.8–7.4) | 6.2 (5.3–7.8) | 6.9 (5.5–8.4) |
| n (%) out of rangea | 52 (29.2) | 9 (15.8) | 12 (16.9) |
| P-value evolutionb | 0.69 (n=53) | 0.57 (n=37) | |
| Haemoglobin (g/dL) | |||
| N | 176 | 55 | 71 |
| Median (IQR) | 12.3 (10.8–13.5) | 12.9 (11.9–14.0) | 13.9 (12.4–15.2) |
| n (%) <lower rangea | 66 (37.5) | 12 (21.8) | 11 (15.5) |
| P-value evolutionb | <0.0001 (n=50) | <0.0001 (n=35) | |
| Lymphocytes (%) | |||
| N | 176 | 55 | 71 |
| Median (IQR) | 24.5 (18.1–30.3) | 29.6 (24.8–37.3) | 31.4 (24.4–37.6) |
| n (%) out of rangea | 45 (25.6) | 9 (16.4) | 10 (14.1) |
| P-value evolutionb | <0.0001 (n=50) | 0.90 (n=35) | |
| Lymphocytes (10³/mm³) | |||
| N | 176 | 54 | 68 |
| Median (IQR) | 1.5 (1.1–1.8) | 1.5 (1.2–2.3) | 2.0 (1.7–2.5) |
| n (%) out of rangea | 42 (23.9) | 4 (7.4) | 4 (5.9) |
| P-value evolutionb | <0.0001 (n=49) | 0.86 (n=34) | |
| D-dimer (µg/L) | |||
| N | 75 | 46 | 72 |
| Median (IQR) | 841 (501–1534) | 446 (258–737) | 322 (190–599) |
| n (%) out of rangea | 57 (76.0) | 20 (43.5) | 24 (33.3) |
| P-value evolutionb | 0.0010 (n=16) | <0.0001 (n=28) | |
| TGO (ASAT) aspartate aminotransferase (U/L) | |||
| N | 168 | 54 | 72 |
| Median (IQR) | 35 (24–58) | 26 (22–31) | 26 (24–32) |
| n (%) out of rangea | 87 (51.8) | 10 (18.5) | 16 (22.2) |
| P-value evolutionb | 0.0078 (n=44) | 0.0030 (n=34) | |
| TGP (ALAT) alanine aminotransferase (U/L) | |||
| N | 167 | 55 | 72 |
| Median (IQR) | 47 (23–89) | 28 (17–45) | 26 (20–36) |
| n (%) out of rangea | 15 (9.0) | 0 (0.0) | 0 (0.0) |
| P-value evolutionb | 0.0002 (n=45) | 0.90 (n=34) | |
| Creatinine | |||
| N | 179 | 58 | 73 |
| Median (IQR) | 0.84 (0.70–1.0) | 0.83 (0.76–0.99) | 0.94 (0.77–1.1) |
| n (%) >upper rangea | 41 (22.9) | 12 (20.7) | 24 (32.9) |
| P-value evolutionb | 0.90 (n=54) | <0.0001 (n=38) | |
| GFR estimation (CKD-EPI) | |||
| N | 179 | 58 | 73 |
| Median (IQR) | 90.7 (76.8–101.2) | 90.7 (72.2–100.1) | 83.7 (63.2–93.1) |
| n (%) <60 | 26(14.5) | 8 (13.8) | 17 (23.3) |
| P-value evolutionb | 0.28 (n=38) | <0.0001 (n=38) | |
| Creatinine (without IRA patients) | |||
| N | 161 | 51 | 59 |
| Median (IQR) | 0.80 (0.70–0.95) | 0.83 (0.73–0.95) | 0.90 (0.74–1.0) |
| n (%) >upper rangea | 27 (16.8) | 8 (15.7) | 13 (22.0) |
| P-value evolutionb | 0.37 (n=48) | 0.0001 (n=31) | |
| GFR estimation (without IRA patients) | |||
| N | 161 | 51 | 59 |
| Median (IQR) | 92.8 (80.9–101.8) | 92.0 (74.1–101.6) | 86.5 (70.9–95.2) |
| n (%) <60 | 14 (8.7) | 5 (9.8) | 8 (13.6) |
| P-value evolutionb | 0.41 (n=48) | 0.0001 (n=31) | |
| Creatinine kinase (UI/L) | |||
| N | 94 | 59 | 61 |
| Median (IQR) | 58.5 (35.0–118) | 54.0 (22.0–99.0) | 112.0 (53.0–150.0) |
| n (%)>upper rangea | 13 (13.8) | 7 (11.9) | 11 (18.0) |
| P-value evolutionb | 0.66 (n=22) | <0.0001 (n=33) | |
| CRP (mg/L) | |||
| N | 180 | 53 | 70 |
| Median (IQR) | 20.4 (9.0–48.1) | 5.6 (1.3–6.7) | 2.7 (1.4–4.9) |
| n (%) >upper rangea | 158 (87.8) | 18 (34.0) | 17 (24.3) |
| P-value evolutionb | <0.0001 (n=49) | 0.28 (n=36) | |
| Ferritin (µg/L) | |||
| N | 31 | 50 | 63 |
| Median (IQR) | 671 (386–1012) | 179 (127–332) | 95 (46–171) |
| n (%) >upper rangea | 27 (87.1) | 21 (42.0) | 13 (20.6) |
| P-value evolutionb | - | <0.0001 (n=28) | |
IQR, interquartile range ; WBC, white blood cells ; GFR, glomerular filtration rate; CRP, C-reactive protein.
Reference ranges: WBC (10³/mm³): 4.6–10.1; haemoglobin (g/dL): 11.7–15.0; lymphocyte (%): 17.5–45.5; lymphocyte (10³/mm³): 1.1–3.7; D-dimer (µg/L): <500; TGO (ASAT) (U/L): 5–34, TGP (ALAT) (U/L): <150; creatinine (mg/dL): 0.55–1.02; GFR (MDRD) (mL/min/1.73 m²): <60; creatinine kinase (UI/L): 29–168; CRP (mg/L): 0.0–5.0; ferritin (µg/L): 5–204.
Signed rank test for paired observations, at discharge vs 1 month after discharge, or at 1 month after discharge vs 3 months after discharge (n=number of paired observations available).
The percentage of lymphocytes increased significantly from discharge to 1 month after discharge (P<0.0001). The level of D-dimer decreased significantly from discharge to 1 month after discharge (P=0.001), and further diminished from 1 month after discharge to 3 months after discharge (P<0.0001). The median CRP value decreased significantly after discharge, and returned to normal at 1 month after discharge (P<0.0001). eGFR did not differ at discharge and at 1 month after discharge (Table 1), but decreased significantly at 3 months after discharge (P<0.0001) in 38 patients with data available for 1 and 3 months after discharge. Excluding the patient with AKI during hospitalization, median eGFR remained significantly different at 1 and 3 months after discharge [92.0 mL/min/1.73 m2 (IQR 74.1–101.6) and 86.5 mL/min/1.73 m2 (IQR 70.9–95.2), respectively; n=31]. Mean eGFR remained in the normal range for followed patients. From the 73 patients with eGFR data available at 3 months after discharge, 17 patients had eGFR <60 mL/min/1.73 m2; of these, 14 (82.4%) patients had eGFR <60 mL/min/1.73 m2 at hospital admission.
Twenty-one patients developed AKI during hospitalization, with eight patients needing renal replacement therapy, excluding the three patients on chronic haemodialysis before admission; all five patients were weaned off dialysis at discharge. Median proteinuria (n=145) was 301 (IQR 174–583) at discharge and 80 (IQR 59–125) mg/g (n=40) at 1 month after discharge (P<0.0001) (data not shown). No further decrease was observed at 3 months after discharge [76 (IQR 56–138) mg/g (n=31); not significant].
Pulmonary function tests
Of the overall population, 156 patients were evaluated; of these, 128 patients did not present any chronic lung disease based on medical history. Of the 28 patients of the population with chronic lung disease, 36% were suffering from chronic obstructive pulmonary disease.
Interestingly, the whole patient population exhibited low DLCO (71.6 ± 18.6%) (Table 2 ). Both FEV1 and FVC were in normal ranges (90.8 ± 20.8% and 88.6% ± 18.2, respectively).
Table 2.
Pulmonary function tests of patients at follow-up (3 months).
| n | n (%) | Mean ± SD | Median (IQR) | |
|---|---|---|---|---|
| Delay between discharge and follow-up | 156 | 101 ± 34 | 94 (77–119) | |
| FEV1 (L) | 156 | 2.73 ± 0.913 | ||
| FEV1 (% predicted) | 156 | 90.8 ± 20.8 | ||
| FEV1 <80% predicted | 156 | 34 (21.8) | ||
| FVC | 156 | 3.41 ± 1.08 | ||
| FVC (% predicted) | 156 | 88.6 ± 18.2 | ||
| FVC <80% predicted | 156 | 43 (27.6) | ||
| Tiffeneau index % | 156 | 79.7 ± 9.07 | ||
| Tiffeneau index <70% | 156 | 17 (10.9) | ||
| DLCO | 132 | 6.04 ± 2.04 | ||
| DLCO (% predicted) | 132 | 71.6 ± 18.6 | ||
| DLCO <75% predicted | 132 | 72 (54.6) | ||
| DLCO_AV | 132 | 1.25 ± 0.320 | ||
| DLCO_AV (% predicted) | 132 | 87.8 ± 20.6 | ||
| DLCO_AV <80% predicted | 132 | 46 (34.9) | ||
| TLC | 85 | 5.36 ± 1.45 | ||
| TLC (% predicted) | 87 | 87.7 ± 21.9 | ||
| TLC <80% predicted | 87 | 31 (35.6) | ||
| FRC | 73 | 3.32 ± 1.03 | ||
| FRC (% predicted) | 73 | 102.2 ± 29.2 | ||
| FRC <80% predicted | 73 | 14 (19.2) | ||
| RV | 79 | 2.00 ± 0.742 | ||
| RV (% predicted) | 79 | 89.6 ± 30.0 | ||
| RV <80% predicted | 79 | 29 (36.7) | ||
| sGaw | 72 | 0.940 ± 0.332 | ||
| sGaw (% predicted) | 58 | 87.3 ± 34.1 | ||
| sGAw <80% predicted | 58 | 24 (41.4) |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; DLCO, diffusion lung capacity for carbon monoxide; AV, alveolar ventilation; TLC, total lung capacity; FRC, functional residual capacity; RV, residual volume; sGaw, specific conductance; SD, standard deviation; IQR, interquartile range.
Table 3.
Changes in chest computed tomography scan at 3 months.
| Baseline | Follow-up | Evolution | P-value | |
|---|---|---|---|---|
| Delay between baseline evaluation and follow-up visit, median (IQR) | - | 94 (76–114) | ||
| n (%) Ground glass opacities | 145 (98.0) | 102 (68.9) | <0.0001a | |
| n (%) Bronchiectasis | 5 (3.4) | 10 (6.8) | 0.059a | |
| n (%) Reticular pattern | 5 (3.4) | 7 (4.7) | 0.32a | |
| n (%) Honeycombing | 3 (2.0) | 1 (0.7) | 0.16a | |
| n (%) Pleural effusion | 15 (10.1) | 6 (4.0) | 0.020a | |
| n (%) Crazy paving | 91 (61.5) | 8 (5.4) | <0.0001a | |
| n (%) Thin condensations | 128 (86.5) | 101 (68.2) | <0.0001a | |
| n (%) Organizing pneumonia | 9 (6.1) | 1 (0.7) | 0.011a | |
| n (%) Condensation | 69 (46.6) | 20 (13.5) | <0.0001a | |
| Lung involvement (%) | ||||
| Mean ± SD | 32.4 ± 21.4 | 10.4 ± 14.9 | -22.0 ± 23.1 | <0.0001b |
IQR, interquartile range; SD, standard deviation.
McNemar test.
Student's t-test.
Some patients were evaluated with PFTs at 3 months and 6 months after discharge. Reassuringly, they exhibited a significant improvement in several parameters including FEV1, FVC and DLCO (Table S4, see online supplementary material).
Changes between pre- and post-COVID-19 PFTs were evaluated. The median re-assessment between two PFTs (first before COVID-19 infection, second at follow-up) was at 22 ± 51 months. Data are shown in Table S5 (see online supplementary material). Patients presented a significant decrease in FVC and DLCO after COVID-19. Median FVC decreased from 87.3 ± 18.9% to 81.6 ± 19.7% of predicted values (P<0.05). DLCO also decreased significantly from 65.4 ± 26.0 to 58.0 ± 21.1% predicted.
Chest CT scan evaluation
Changes in CT scans over time were evaluated at 3 months [median 94 days (IQR 76–114)] for 149 patients. Compared with CT scans performed at hospital admission, the main persistent pattern was GGOs (68.9% of the cohort) with thin condensations (68.2%), condensations (46.6%) and crazy paving (6.8%). Re-evaluation of CT scans showed that most images were improved with a significant reduction in GGOs (P<0.0001), pleural effusion (P<0.05), organizing pneumonia (P<0.05), condensations (P<0.0001), crazy paving (P<0.0001) and condensations (P<0.0001). No significant differences were found between the two CT scans in terms of honeycombing, reticular pattern and bronchiectasis. As expected, quantification of lung involvement identified a substantial decline in COVID-19 lesions from a mean of 32.4 ± 21.4% to 10.4 ± 14.9% at 3 months after discharge.
Characterization of patients with persistent symptoms
The persistence of symptomatology was correlated with clinical and demographic features (Table S6, see online supplementary material) and with selected biological data (haemoglobin, D-dimer), PFTs (DLCO) and chest CT scan (GGOs) (Table S7, see online supplementary material). Unfortunately, it was not possible to determine a typical clinical or biological profile of patients at risk of presenting long-term symptoms. In particular, duration of hospitalization, ICU admission and mechanical ventilation were not associated with the persistence of symptoms at 3 months after discharge.
More patients (56.6%) with decreased DLCO had persistent symptoms compared with patients with normal DLCO (31.8%), although the P-value was at the limit of statistical relevance (P=0.051) (Table S7, see online supplementary material).
Discussion
For some individuals, COVID-19 can cause symptoms that last for weeks or months after the infection has gone. Post-COVID conditions are being seen in a growing number of patients reporting a constellation of symptoms after SARS-CoV-2 infection that are persistent, debilitating, and have yet to be fully explained by known or measurable mechanisms (Burke and Rio, 2021). Pioneering studies have shown that COVID-19 survivors often suffer from fatigue or muscle weakness, sleep difficulties, and anxiety or depression for up to 6 months after infection (Bellan et al., 2021; Huang et al., 2021). In addition, individuals discharged from hospital after COVID-19 have increased rates of multi-organ dysfunction compared with the expected risk in the general population (Ayoubkhani et al., 2021). The prevalence of post-COVID conditions seems to be associated with severity (Lund et al., 2021), although a recent report suggested that this is also common in young home-isolated adults with mild COVID-19 (Blomberg et al., 2021). Augustin et al. (2021) observed the ongoing presence of shortness of breath, anosmia, ageusia or fatigue as long-lasting symptoms even in non-hospitalized patients at 4 and 7 months post-infection.
This study found a very high prevalence of extreme tiredness and shortness of breath for up to 6 months after discharge in patients hospitalized for COVID-19. The prevalence of anosmia/ageusia was low in this study compared with other recent reports (Augustin et al., 2021).
Surprisingly, the frequency of these symptoms remained high and stable over time, indicating that longer follow-up is needed to determine their duration. A better understanding of the causes of symptom persistence is needed to provide solutions and to design prevention strategies.
The severity of the persistent symptoms was not correlated with PFTs or CT scan images. Interestingly, PFT re-assessment did not find any significant decrease in lung volume, but an isolated reduction in DLCO. Of note, patients who had benefited from previous PFT evaluation were suffering from a significant reduction in FVC and DLCO at 3 months after discharge. Contrary to what was seen in PFTs, a clear improvement in CT scan images was noted over time, with significant residual lesions at 3 months after discharge in up to 68.9% of the cohort (mainly GGOs and thin condensations).
Based on these observations, the alveolar and vascular lung lesions seen in patients with COVID-19 may explain the reduction in DLCO as well as the persistent lung abnormalities at 3 months. The high prevalence of thromboembolic events is also a potential parameter to consider in the reduction of DLCO over time without any restrictive or obstructive lung disease based on the PFT assessment. Reinforcing this hypothesis, the median level of D-dimer remained elevated over time. Other studies have demonstrated a higher rate of thromboembolic events in survivors of COVID-19 at 6 months of follow-up, raising the question of the number of undiagnosed cases of segmental or subsegmental pulmonary embolism (Al-Aly et al., 2021)). The endothelial inflammation classically described in COVID-19 remains a good hypothesis for the increased incidence of pulmonary embolism, and potentially the leading cause of the persistent reduction in DLCO over time with a specific increase in D-dimer level (Radermecker et al., 2020). Together with direct cellular damage and the robust innate immune response with inflammatory cytokine production, the pro-coagulant state and endothelial damage induced by SARS-CoV-2 infection may contribute to various organ-specific sequelae of post-acute COVID-19 (Nalbandian et al., 2021; Nauen et al., 2021).
The mid- and long-term renal consequences of SARS-CoV-2 infection remain unknown. In this study, no discharged survivors required prolonged renal replacement therapy (n=5). Although COVID-19 was associated with a high prevalence of AKI (10.9%), few follow-up data are available to evaluate the long-term impact and risk of CKD. Large cohort studies have demonstrated that >70% of patients recovered from AKI at discharge (Charytan et al., 2021). In the present study cohort, patients with eGFR <60 mL/min/1.73 m2 at 3 months are in majority patients who have eGFR <60 mL/min/1.73 m2 at hospital admission and probably are patients with CKD prior to admission. The significant, but probably not clinically relevant, difference between eGFR at 1 month and 3 months after discharge remains in the normal range (eGFR >60 mL/min/1.73 m2). Loss of lean mass during hospitalization may explain the lower creatinine observed at 1 month after discharge, with the likely increase in lean mass at 3 months after discharge explaining the increase in creatinine.
This study had several limitations, including the limited number of participants and the absence of a control group of non-COVID-19 patients. Loss to follow-up may also have been non-random, so the proportion of patients with adverse outcomes may be biased.
This study confirmed the very high prevalence of persistent symptoms following hospitalization for COVID-19, and revealed worrying stability of post-COVID symptoms over time. High percentages of biological, functional and iconographic abnormalities were observed several months after discharge. In contrast with symptomatology, these objective parameters improved over time.
In conclusion, the long-term consequences of COVID-19 should not be neglected and will likely represent an additional burden on the healthcare system in terms of human and economic resources. Understanding the natural history of COVID-19 sequelae and the elements involved is a key priority to mitigate the long-term effects of COVID-19 on multiple organs and tissues.
Acknowledgments
Acknowledgements
The authors wish to thank the study participants.
Conflict of interest statement
None declared.
Funding
This study was funded by the Leon Fredericq Foundation.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.07.016.
Appendix. Supplementary materials
References
- Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequalae of COVID-19. Nature. 2021;594:259–264. doi: 10.1038/s41586-021-03553-9. [DOI] [PubMed] [Google Scholar]
- Amenta EM, Spallone A, Rodriguez-Barradas MC, El Sahly HM, Atmar RL, Kulkarni PA. Postacute COVID-19: an overview and approach to classification. Open Forum Infect Dis. 2020;7:ofaa509. doi: 10.1093/ofid/ofaa509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin M, Schommers P, Stecher M, Dewald F, Gieselmann L, Gruell H, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health – Eur. 2021;6 doi: 10.1016/j.lanepe.2021.100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I, et al. Post-COVID syndrome in individuals admitted to hospital with COVID-19: retrospective cohort study. BMJ. 2021;372:n693. doi: 10.1136/bmj.n693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellan M, Soddu D, Balbo PE, Baricich A, Zeppegno P, Avanzi GC, et al. Respiratory and psychophysical sequelae among patients with COVID-19 four months after hospital discharge. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2020.36142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blomberg B, Mohn KG-I, Brokstad KA, Zhou F, Linchausen DW, Hansen B-A, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021 doi: 10.1038/s41591-021-01433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouquegneau A, Huart J, Lutteri L, Erpicum P, Grosch S, Résimont G, et al. Survivors of COVID-19 mostly recover from tubular proteinuria and acute kidney injury after hospital discharge. J Nephrol. 2021:1–3. doi: 10.1007/s40620-021-01075-1. Epub ahead of print. PMID: 34089518; PMCID: PMC8179082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke MJ, Rio C del. Long COVID has exposed medicine's blind-spot. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00333-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho-Schneider C, Laurent E, Lemaignen A, Beaufils E, Bourbao-Tournois C, Laribi S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. 2021;27:258–263. doi: 10.1016/j.cmi.2020.09.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32:151–160. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charytan DM, Parnia S, Khatri M, Petrilli CM, Jones S, Benstein J, et al. Decreasing incidence of acute kidney injury in patients with COVID-19 critical illness in New York City. Kidney Int Rep. 2021;6:916–927. doi: 10.1016/j.ekir.2021.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A, Wu G, Primakov S, Oberije C, Woodruff H, Kubben P, et al. Can predicting COVID-19 mortality in a European cohort using only demographic and comorbidity data surpass age-based prediction: an externally validated study. PloS One. 2021;16 doi: 10.1371/journal.pone.0249920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Luo R, Wang K, Zhang M, Wang Z, Dong L, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97:829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra V, Flanders SA, O'Malley M, Malani AN, Prescott HC. Sixty-day outcomes among patients hospitalized with COVID-19. Ann Intern Med. 2021;174:576–578. doi: 10.7326/M20-5661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanaye P, Huart J, Bouquegneau A, Jouret F. Long-term effects of COVID-19 on kidney function. Lancet. 2021;397:1807. doi: 10.1016/S0140-6736(21)00881-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang FC, Benson CA, del Rio C, Edwards KM, Fowler VG, Jr, Fredricks DN, et al. COVID-19 – lessons learned and questions remaining. Clin Infect Dis. 2021;72:2225–2240. doi: 10.1093/cid/ciaa1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frix AN, Schoneveld L, Ladang A, Henket M, Duysinx B, Vaillant F, et al. Could KL-6 levels in COVID-19 help to predict lung disease? Respir Res. 2020;21:309. doi: 10.1186/s12931-020-01560-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L. Acute kidney injury in critically ill patients with COVID-19. Intensive Care Med. 2020;46:1339–1348. doi: 10.1007/s00134-020-06153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrigues E, Janvier P, Kherabi Y, Bot AL, Hamon A, Gouze H, et al. Post-discharge persistent symptoms and health-related quality of life after hospitalization for COVID-19. J Infect. 2020;81:e4–e6. doi: 10.1016/j.jinf.2020.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot J, Vaidyanathan A, Deprez L, Zerka F, Danthine D, Frix A-N, et al. Development and validation of an automated radiomic CT signature for detecting COVID-19. Diagnostics (Basel) 2020;11(1):41. doi: 10.3390/diagnostics11010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiot J, Danthine D, Deprez L, Louis R, Lovinfosse P, Meunier P. Chest radiological lesions in COVID-19 : from classical imaging to artificial intelligence. Rev Med Liege Sup. 2020;75(Suppl. 1):81–85. [PubMed] [Google Scholar]
- Guiot J, Henket M, Frix AN, Delvaux M, Denis A, Giltay L, et al. Single-center experience of patients with interstitial lung diseases during the early days of the COVID-19 pandemic. Respir Investig. 2020;58(6):437–439. doi: 10.1016/j.resinv.2020.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Coca SG, Chan L, Melamed ML, Brenner SK, Hayek SS, et al. AKI treated with renal replacement therapy in critically ill patients with COVID-19. J Am Soc Nephrol. 2021;32:161–176. doi: 10.1681/ASN.2020060897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschtick JL, Titus AR, Slocum E, Power LE, Hirschtick RE, Elliott MR, et al. Population-based estimates of post-acute sequelae of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection (PASC) prevalence and characteristics. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iadecola C, Anrather J, Kamel H. Effects of COVID-19 on the nervous system. Cell. 2020;183:16–27. doi: 10.1016/j.cell.2020.08.028. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashif A, Chaudhry M, Fayyaz T, Abdullah M, Malik A, Anwer JMA, et al. Follow-up of COVID-19 recovered patients with mild disease. Sci Rep. 2021;11:13414. doi: 10.1038/s41598-021-92717-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellum JA, Lameire N, KDIGO AKI Guideline Work Group Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1) Crit Care. 2013;17:204. doi: 10.1186/cc11454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laszlo G. Standardisation of lung function testing: helpful guidance from the ATS/ERS Task Force. Thorax. 2006;61:744–746. doi: 10.1136/thx.2006.061648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu T, Yang N, Han D, Mi X, Li Y, et al. Neurological manifestations of patients with COVID-19: potential routes of SARS-CoV-2 neuroinvasion from the periphery to the brain. Front Med. 2020;14:533–541. doi: 10.1007/s11684-020-0786-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund LC, Hallas J, Nielsen H, Koch A, Mogensen SH, Brun NC, et al. Post-acute effects of SARS-CoV-2 infection in individuals not requiring hospital admission: a Danish population-based cohort study. Lancet Infect Dis. 2021 doi: 10.1016/S1473-3099(21)00211-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian A, Sehgal K, Gupta A, Madhavan MV, McGroder C, Stevens JS, et al. Post-acute COVID-19 syndrome. Nat Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute for Health and Care Excellence . NICE; London: 2020. Overview. COVID-19 rapid guideline: managing the long-term effects of COVID-19.https://www.nice.org.uk/guidance/ng188 Available at. accessed 2 April 2021. [PubMed] [Google Scholar]
- Nauen DW, Hooper JE, Stewart CM, Solomon IH. Assessing brain capillaries in coronavirus disease 2019. JAMA Neurol. 2021;78:760–762. doi: 10.1001/jamaneurol.2021.0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radermecker C, Detrembleur N, Guiot J, Cavalier E, Henket M, d'Emal C, et al. Neutrophil extracellular traps infiltrate the lung airway, interstitial, and vascular compartments in severe COVID-19. J Exp Med. 2020;217(12):e20201012. doi: 10.1084/jem.20201012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronco C, Reis T, Husain-Syed F. Management of acute kidney injury in patients with COVID-19. Lancet Respir Med. 2020;8:738–742. doi: 10.1016/S2213-2600(20)30229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamanna F, Veronesi F, Martini L, Landini MP, Fini M. Post-COVID-19 syndrome: the persistent symptoms at the post-viral stage of the disease. A systematic review of the current data. Front Med (Lausanne) 2021;8:653516. doi: 10.3389/fmed.2021.653516. https://www.frontiersin.org/articles/10.3389/fmed.2021.653516/full Available at. accessed 1 July 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoneveld L, Ladang A, Henket M, Frix A-N, Cavalier E, Guiot J, et al. YKL-40 as a new promising prognostic marker of severity in COVID infection. Crit Care. 2021;25:66. doi: 10.1186/s13054-020-03383-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran V-T, Riveros C, Clepier B, Desvarieux M, Collet C, Yordanov Y, et al. Development and validation of the long COVID symptom and impact tools, a set of patient-reported instruments constructed from patients’ lived experience. Clin Infect Dis. 2021 doi: 10.1093/cid/ciab352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Yang P, Xie Y, Woodruff HC, Rao X, Guiot J, et al. Development of a clinical decision support system for severity risk prediction and triage of COVID-19 patients at hospital admission: an international multicentre study. Eur Respir J. 2020;56(2):2001104. doi: 10.1183/13993003.01104-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.