Abstract
Objectives
To examine the relationship between antibody status and cycle threshold (Ct) values, the prognostic value of the latter for COVID-19 patients, and the inter-assay comparability of SARS-CoV-2 Ct values.
Methods
In 347 COVID-19 inpatients, SARS-CoV-2 Ct values (via reverse transcription-quantitative polymerase chain reaction) on admission were compared between 2 assays and correlated with the antibody response (in the course of the disease), the clinical course and the time since onset of symptoms.
Results
Ct values for 2 of 3 target genes showed significant differences between the 2 assays used (P=0.012 and P<0.0001). Ct values were significantly higher for antibody positive patients (P<0.0001) and positively correlated with the amount of time since onset of symptoms (R: 0.332–0.363; P<0.001). Patients with fatal outcomes showed higher viral loads than survivors (P<0.0001).
Conclusions
Ct values depend strongly on assay used and target gene examined and should not be used as quantitative values to guide therapeutic or diagnostic decisions. The inverse association between antibody status and viral load suggests that antibodies contribute to the elimination of the virus, independent of the outcome, which is influenced by the viral load on admission and might depend more strongly on other parts of the immune response.
Keywords: SARS-CoV-2, COVID-19, RT-qPCR, Ct value, anti-SARS-CoV-2 antibodies
Introduction
We previously reported data concerning anti-SARS-CoV-2 antibodies in polymerase chain reaction (PCR)-confirmed COVID-19 patients from a high-incidence region in northern Spain (Markewitz et al., 2021). In brief, we were able to show that neither the presence nor the level of anti-SARS-CoV-2 specific antibodies served as prognostic markers in our cohort but that they correlated with the amount of time since onset of symptoms. Recently, there has been much debate on the diagnostic value of the naïve (i.e., without reference to a standard curve) cycle threshold (Ct) results of the reverse transcription-quantitative PCR (RT-qPCR) that is the gold standard in the laboratory diagnosis of acute cases of COVID-19. In short, the Ct describes the number of PCR cycles that have been performed when the fluorescent signal of the amplification products of the PCR crosses a defined threshold. While the Ct value certainly correlates with the viral load of the examined sample, it is not to be regarded as a quantitative unit. In order for PCR results to be quantified, a standard curve has to be calculated for every PCR using controls that contain a known quantity of the nucleic acid that is to be detected (Han et al., 2020; Markewitz et al., 2021). As research into SARS-CoV-2 intensifies the naïve Ct values of qualitative RT-qPCR are increasingly reported and the results interpreted as if they were quantitative results (Bullard et al., 2020; Marot et al., 2021; Seeni et al., 2021; Shen et al., 2020; Zou et al., 2020). In some cases, researchers have generated their own standard curves in order to gain quantitative results (Wyllie et al., 2020), while some commercial distributors have made quantified biologic reference materials available. Nonetheless, one has to add that swabs of any kind remain a suboptimal material for quantitative PCR, as the contained viral load is to a large degree dependent on pre-analytic processes, mainly the quality of sample collection. Therefore, the reporting of naïve Ct values as a quantitative value without reference to a standard curve is to be viewed with the utmost caution. Treating Ct values as a semi-quantitative correlate of the viral load of a sample, reporting general trends or relative differences between Ct values obtained using the same assays may, however, serve as an approximation to quantitative PCR results for SARS-CoV-2.
The aims of our current study were:
-
1)
To examine the relationship between antibody status and Ct values for SARS-CoV-2 measured via RT-qPCR
-
2)
To assess the prognostic value of Ct values for COVID-19 patients
-
3)
To examine the inter-assay comparability of SARS-CoV-2 Ct values
Methods
Study location and population
Participants of the current study were recruited from PCR-confirmed COVID-19 inpatients treated at the University Hospital of Donostia/San Sebastián (Basque country, Spain) during March and April of 2020. The study was approved by the Ethics Committee for Clinical Research of Euskadi (CEIC-E) (PI2020064). All participants gave written informed consent. For minors under the age of 18 years and patients who were too ill to consent, this consent was obtained from legally authorized representatives as mandated by the local ethics committee. The study was conducted in accordance with the Declaration of Helsinki (World Medical Association, 2013).
Collection of swabs and RT-qPCR
Oropharyngeal swabs were collected and tested immediately after collection via RT-qPCR between March 26 and April 11, 2020. For each patient, 1 swab was collected on admission (at a median of 6.5 days since onset of symptoms (interquartile range (IQR): ±6 days)). All samples were tested for 3 different target genes: the E gene (which is common to all members of the subgenus Sarbecovirus, to which SARS-CoV-2 belongs), the N gene and the RdRp gene (the latter 2 being specific for SARS-CoV-2). To that end, 2 different assays were used: the Allplex 2019-nCoV Assay (by Seegene Inc., Seoul, South Korea), containing all 3 genes (henceforth referred to as Assay 1); and the Viasure SARS-CoV-2 assay (Certest Biotec, Zaragoza, Spain; containing the N gene) in combination with LightMix Modular SARS-CoV-2 primers from TibMolBiol (Berlin, Germany) that contained the E gene and RdRP gene (this combination being henceforth referred to as Assay 2). This mixture of different assays was necessary during the first wave of the COVID-19 crisis due to shortages of reagents from any single manufacturer. The interpretation of each assay was performed according to the manufacturer's instructions: for all Allplex assays, a Ct ≤40 was considered positive for all target genes. For the Viasure assay, this cut-off was <38 and for the TibMolBiol assays it was 36 (±2 cycles). If at least 2 target genes yielded Ct values in a very high range of 35–40, the sample was reported as “indeterminate” and retesting with a new sample was recommended.
Collection of sera and antibody testing
The sample collection and test protocols employed in examining the anti-SARS-CoV-2 antibody status has been described previously, as has the collection of the clinical data of the patients (Markewitz et al., 2021). In brief, 1 serum sample per patient was collected after confirmation of COVID-19 via RT-qPCR, at a median of 10 days since onset of symptoms (IQR: ±7 days; therefore, at a median of 3.5 days after the collection of the oropharyngeal swabs). The serologic tests (antibodies of the classes immunoglobulin (Ig)A and IgG against the S1-subunit of the Spike-protein of SARS-CoV-2) were performed at the Institute of Clinical Chemistry, at the University Hospital Schleswig-Holstein (Kiel/Lübeck, Germany) using the Anti-SARS-CoV-2 IgA and IgG ELISA kits from EUROIMMUN (Lübeck, Germany) according to the manufacturer's instructions. At the time, no quantitative assay was commercially available and the results of the test were reported as a (dimensionless) ratio of the optical density (OD) of the sample at 450 nm compared to the OD of a calibrator. The OD, or extinction, of the examined samples is determined by the concentration of anti-S1 antibodies, therefore the OD ratio is a semi-quantitative correlate of a patient's antibody levels, allowing comparisons with other sera measured with the same technique. Consequently, the OD ratios were used for all analyses of the patients’ antibody levels. Ratios of ≥0.8 to <1.1 were considered borderline, ratios of ≥1.1 positive.
Statistical analysis
As a measure for average values, the median (± IQR) is reported throughout the Results section. For associations between continuous variables, correlation coefficients were calculated according to Pearson and Spearman. For the statistical significance of differences between groups, analyses of variance (ANOVA) were performed, unless otherwise specified. Differences were treated as statistically significant for P-values ≤0.05. Levels of significance were indicated in all relevant figures as follows: ns: P>0.05; *: P≤0.05; **: P≤0.01; ***: P≤0.001; ****: P≤0.0001. All data files were processed in the free software for statistical computing and graphics R (version 4.0.3) with the integrated development environment RStudio (version 1.3.1093) (R Core Team, 2020).
Results
Study population
As previously reported (Markewitz et al., 2021), the cohort comprised 347 patients (144 (41.5%) female; 203 (58.5%) male) with a median age of 66 years (±18; range: 10–95 years). Of these 347 patients, 54 (15.6%) were treated in the ICU and 38 (11%) died.
Comparison of the assays
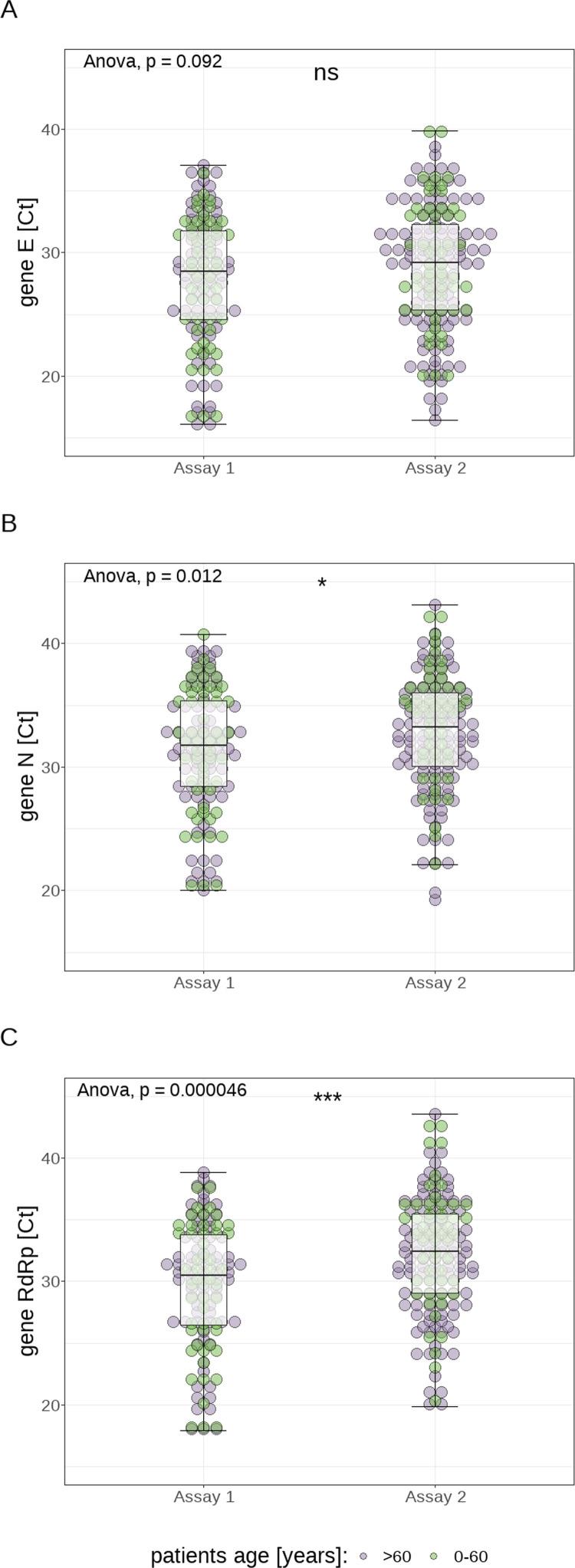
The median Ct values of the examined samples (cumulatively for both assays used) were 28.5 (E gene; range: 16.1–39.9; ±6.9), 32.3 (N gene; range: 19.3–43.1; ±6.3) and 31.2 (RdRp gene; range: 17.9–43.6; ±6.5). Comparing the 2 different methods used for the detection of SARS-CoV-2 RNA, statistically higher Ct values were measured for the detection of the N gene (33.2±6 vs 31.7±7; P=0.012) and the RdRp gene (32.4±6.5 vs 30.5±7.3; p<0.0001) using Assay 2 compared with Assay 1, whereas a similar trend could be observed for the E gene (29.2±6.9 vs. 28.5±7.2; p=0.092) (see Figure 1 ).
Fig. 1.
Visualization of the distribution of cycle threshold (Ct) values for the different genes as measured by the 2 different assays: gene E (panel A), gene N (panel B), and gene RdRp (panel C)
Ct values and antibody detection
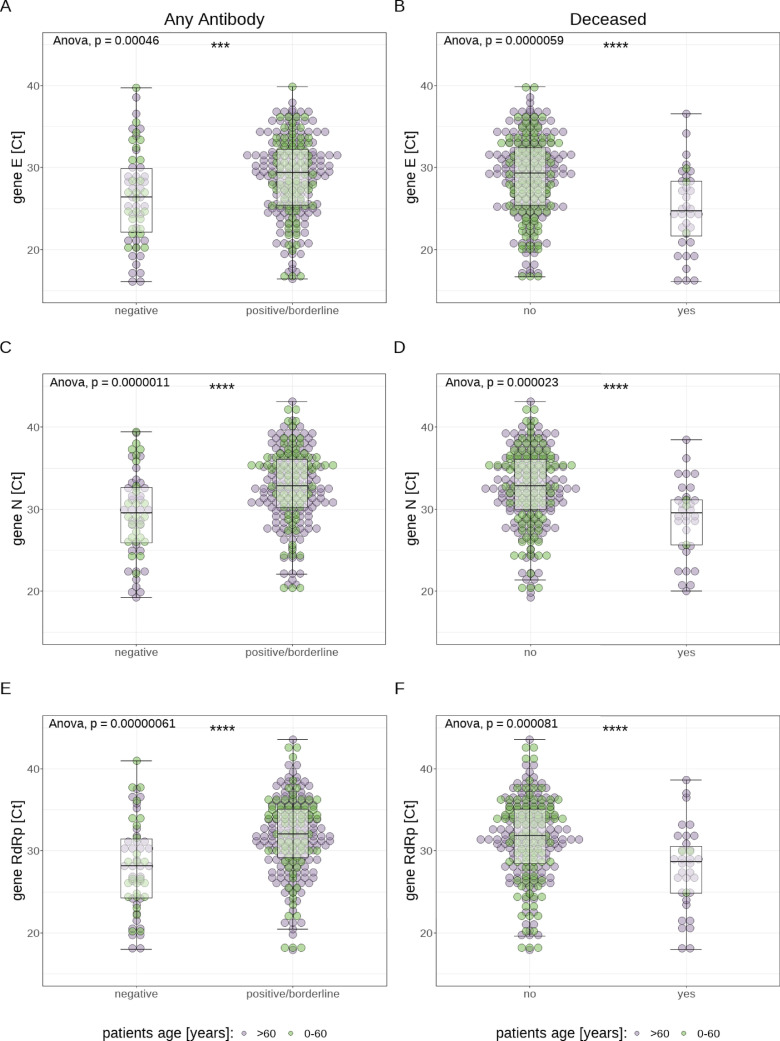
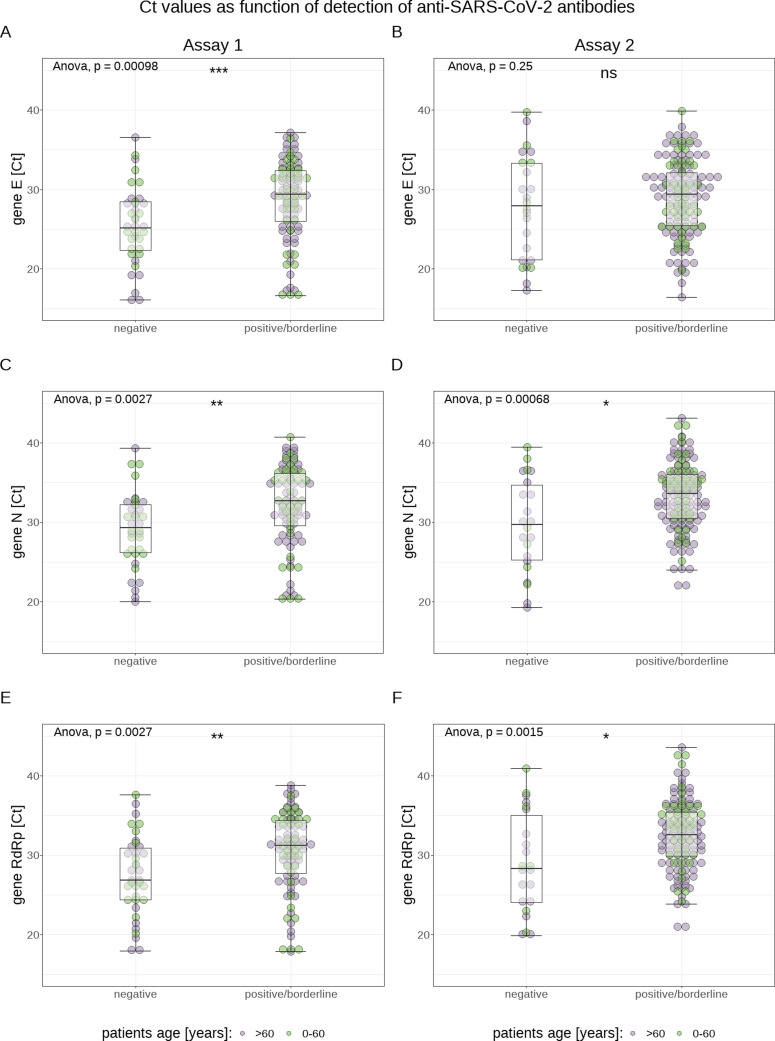
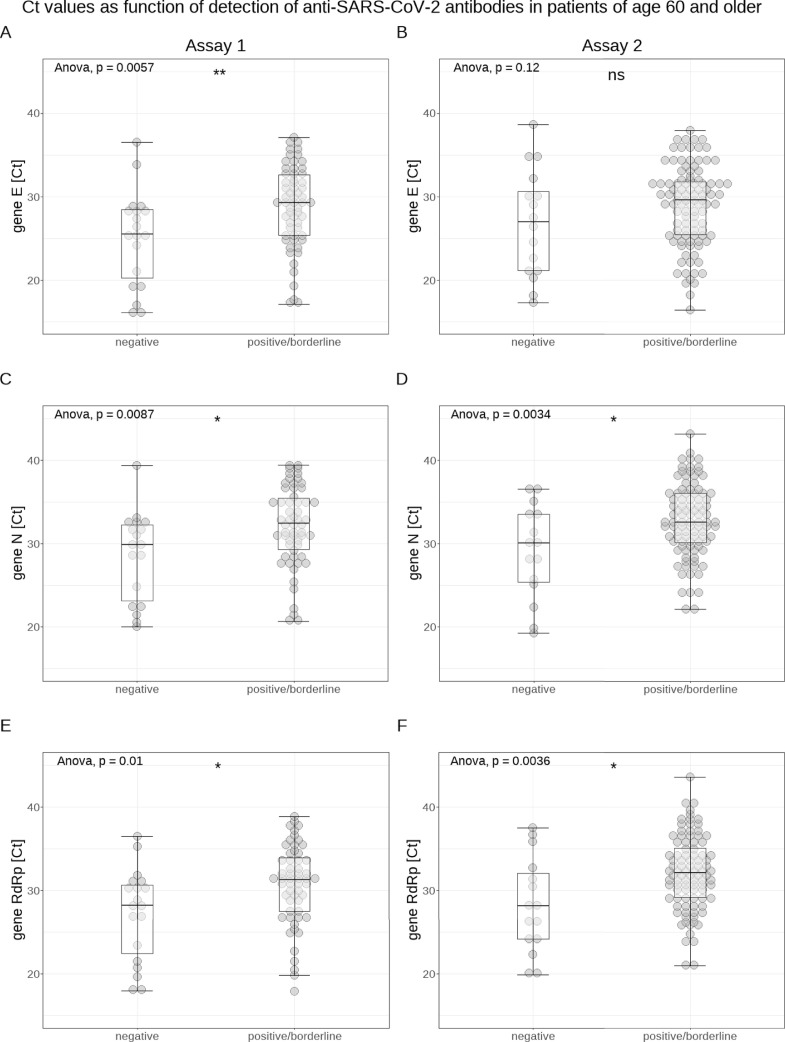
Analyzing the data of both methods cumulatively, Ct values of antibody-positive (IgG and/or IgA) patients were significantly higher (E gene: 29.4±6.8 vs 26.4±7.8; N gene: 32.9±5.8 vs 29.5±6.7; RdRp gene: 32.0±5.9 vs 28.2±7.2; each P<0.0001) for all 3 genes than those of antibody negative patients (for a visualization of the association between antibody positivity and Ct value, see Figure 2 ). In addition, Ct values were significantly lower (E gene: 24.7±6.6 vs 29.3±7; N gene: 29.5±5.5 vs 32.8±6.1; RdRp gene: 28.7±5.7 vs 31.8±6.6; each P<0.0001) for all 3 genes in samples from patients who died later on than in samples from surviving patients (see Figure 2). Patients who were treated in the intensive care unit (ICU) had significantly lower Ct values only for the E gene (25.5±6 vs 29.1±6.8; P<0.001) compared with non-ICU patients. When both assays were analyzed separately, the association between higher Ct values and antibody positivity remained significant for both methods and all 3 genes, except for gene E measured with Assay 2 (see Figure 3 ). In order to test whether the increased case fatality rate (CFR) in older patients was due to their antibodies possibly having a lower impact on the viral load, we also analyzed the association between Ct values and antibody positivity for patients over 60 years only. We found statistically significantly higher Ct values (for both PCR assays and all 3 genes, except for the E gene measured with Assay 2) for antibody (IgA and/or IgG) positive patients compared with antibody negative patients (see Figure 4 ).
Fig. 2.
Visualization of the association between presence of antibodies of any class and cycle threshold (Ct) values (panels A, C, and E, shown cumulatively for both assays, but for each examined target gene separately), as well as of the association between fatal outcome and Ct values (panels B, D, and F, again shown separately for each target gene)
Fig. 3.
Visualization of the association between presence of antibodies of any class and cycle threshold (Ct) values for both assays separately: Assay 1 (panels A, C, and E, shown for each examined target gene separately) and Assay 2 (panels B, D, and F)
Fig. 4.
Visualization of the association between presence of antibodies of any class and cycle threshold (Ct) values in all patients 60 years of age or older (shown for each assay and target gene separately: Assay 1: panels A, C, and E; Assay 2: panels B, D, and F)
Ct values and interval between onset of symptoms and sample collection
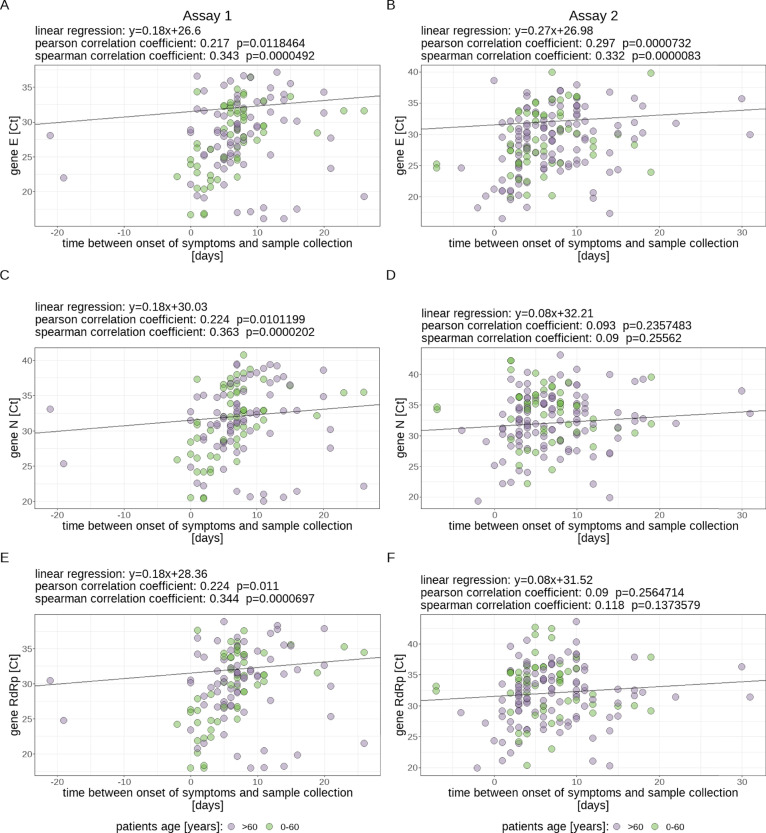
Statistically highly significant (P<0.001) correlations of only low to medium effect size could be shown between the Ct value of all 3 genes measured with Assay 1 and the amount of days since onset of symptoms (Spearman correlation coefficients: E gene 0.343; N gene 0.363; RdRp gene: 0.344) whereas for Assay 2 the same effect could be seen only for the E gene (Spearman correlation coefficient: 0.332; see Figure 5 ).
Fig. 5.
Visualization of the correlation between cycle threshold (Ct) values (for each assay and examined target gene separately) and amount of days since onset of symptoms after which the oropharyngeal swab was collected. Assay 1: panels A, C, and E; Assay 2: panels B, D, and F
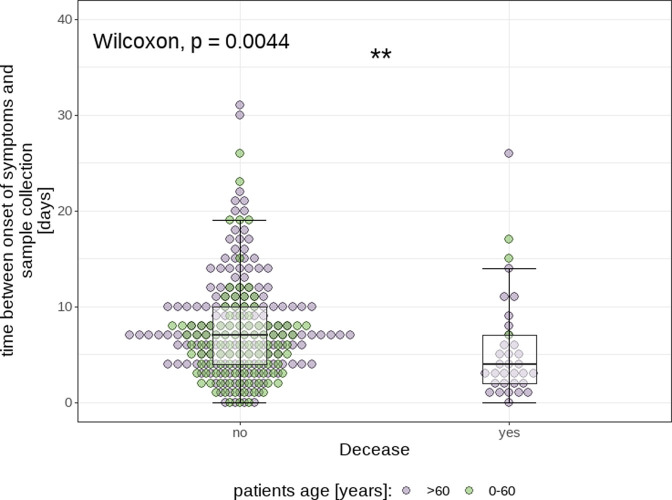
Finally, the time between symptom onset and acquisition of the oropharyngeal swab was statistically significantly (Wilcoxon signed-rank test, P<0.005) shorter for patients who died in the course of their disease (median: 3.5±5 days) than for those who survived (median: 7.0±6 days; see Figure 6 ). The Wilcoxon signed-rank test was used for this comparison, as the data range of both group is cut off at y=0 and can thus not be assumed to be normally distributed.
Fig. 6.
Visualization of the association between fatal outcome and the amount of time since onset of symptoms at which the oropharyngeal swab was collected
Association between age and outcome
As previously reported (Markewitz et al., 2021) there was a statistically significant association between old age and an adverse outcome, the mean age of deceased patients (75.2 years; ±10.9) being significantly higher than the mean age of surviving patients (63 years; ±13•8; p<0.001). While the CFR was 3.2% for patients in the age bracket from 40–49 years, it is 32.6% for patients above 80 years. Overall, 89.5% of all patients who died were 60 years of age or older.
When interpreting these results, it is important to keep in mind that the conclusions drawn may only be applied to inpatients, as all patients included in this study had been admitted to the hospital at some point in their disease (COVID-19).
Discussion
Our results show that Ct values from different assays, even if measured in the same laboratory, are not readily comparable, as there can be significant and systematic differences in the levels of Ct values measured, both between different assays and between different target genes of the same assay. Therefore, it is our opinion that Ct values should not be treated as quantitative values that can be compared, independent of the assay used or target gene examined, or used to guide therapeutic decisions (Carroll and McNamara, 2021; Han et al., 2020; Markewitz et al., 2021). Findings from studies that draw conclusions from the Ct value concerning infectivity are therefore to be interpreted with utmost caution. In our opinion, the comparison of relative differences in Ct values of distinct target genes measured with the same assay (as we have done) is nevertheless valid.
Further, our results show that higher Ct values as a correlate of lower viral loads on admission are associated with the detection of antibodies in the patient's serum. An obvious explanation for this finding would be that the antibodies contributed to the higher Ct values which imply a reduced viral load. This is further supported by our finding that the viral load (via Ct values) measured in the swabs was negatively correlated with the amount of days since onset of symptoms, while we could show the opposite to be true for anti-SARS-CoV-2 IgG in the previous study (Markewitz et al., 2021). Both findings on their own are not surprising as they corroborate findings of earlier studies. It has been shown that the sensitivity of tests for antibodies against SARS-CoV-2 depends on the time since symptom onset and is especially low in the first week (Deeks et al., 2020; Long et al., 2020). Similarly, the decline of the viral load in oro-/nasopharyngeal swabs over time has been described before (Kim et al., 2021; Se et al., 2020; Singanayagam et al., 2020). Our data suggests that there is a connection between the 2, the most obvious explanation being, as mentioned, that the antibodies we measured contribute to the reduction of the patient's viral load.
Lastly, our results show that patients who have a higher viral load on admission (as measured by a lower Ct value in the PCR) are at a greater risk of dying later in the course of the disease. Interestingly, this effect does not appear to be mediated by the antibody response, as neither the general presence of antibodies against SARS-CoV-2, nor their measured levels could be used as a predictor of disease severity as measured by the duration of hospitalization, treatment in the ICU or death in our cohort (Markewitz et al., 2021). This higher viral load on admission that is associated with a fatal outcome is probably, to at least some degree, mediated by the finding that patients with a fatal outcome are admitted earlier in the disease in our cohort. The latter finding might be explained by patients with a fatal outcome developing symptoms that justify hospitalization earlier than surviving patients. This, in turn, is most likely caused by a failure to control the disease during the first 5–7 days in the course of the infection through mechanisms other than the antibody response (which cannot be expected to be present as early (R. Markewitz et al., 2021)). Which mechanisms these are can only be speculated from our data. Possible candidates might be the innate immune response, which plays an important part in the host's early response to an infection with SARS-CoV-2 (Bernardes et al., 2020; Birra et al., 2020), or the T-cell response to SARS-CoV-2. The theory of the outcome of COVID-19 being shaped early (i.e., within the first 5 days since onset of symptoms) in the course of the disease is further corroborated by the finding that the early administration of convalescent plasma prevents severe cases of COVID-19 (Libster et al., 2021). In a synopsis where the CFR increases with age (a correlation that has been found by others as well (Gudbjartsson et al., 2020; Verity et al., 2020)), a possible explanation for the described association between viral load (or the timing of admission) and death might be the senescence of the immune system that is part of the general aging of the immune system that can be observed in elderly patients (Ak et al., 2015; Bernardes et al., 2020; Nikolich-Žugich, 2018).
In conclusion, our findings imply that anti-SARS-CoV-2 antibodies against the Spike-protein contribute to the reduction of the viral load (as measured by higher Ct values), while also showing that a fatal outcome is associated with a failure to reduce the viral load early (within the first 5-7 days) in the disease through parts of the immune response other than antibodies.
Limitations of this study are: only inpatients were included. For further research it might be interesting to include outpatients as well in order to gain a clearer picture of the relationship between viral load and clinical course of COVID-19. Another limitation is that our sample size did not permit us to compare the Ct values on admission between survivors and deceased patients adjusted for the time since onset of symptoms at which time the sample was taken. In a larger cohort, it might be possible to examine whether the Ct values on admission of patients with a fatal outcome are higher than those of survivors, independently of the amount of time since onset of symptoms. In addition, we did not perform neutralization assays, therefore, we were not able to make observations on the neutralizing properties of the antibodies we measured. Lastly, since they have become available in the meantime, quantitative anti-SARS-CoV-2 IgG assays should be used for further studies, instead of reporting the semiquantitative OD ratios, as we have done.
Acknowledgments
Conflict of Interest
Dr. Banales reports grants from INCYTE, personal fees for lecturer from BAYER and INTERCEPT, and consulting for OWL METABOLOMICS, outside the submitted work. All other authors have no conflict of interest to declare.
Author Contributions
R.M. wrote the article. L.B., J.M.B. and R.M. were responsible for study design, data collection, data analysis and data interpretation. K.-P.W., D.P., J.D. and R.J. contributed to data collection and analysis. A.F. helped design the study and provided logistical support. B.N. contributed to data and sample collection as well as data analysis J.M.M, R.M. and A.T analyzed and interpreted the data. M.A.G.-S. was responsible for patient recruitment contributed to data analysis. All authors approved the final version of the manuscript.
Funding
No funding sources to disclose.
References
- Ak S., Ga H., A M. Evolution of the immune system in humans from infancy to old age [WWW Document] Proc. Biol. Sci. 2015 doi: 10.1098/rspb.2014.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardes J.P., Mishra N., Tran F., Bahmer T., Best L., Blase J.I., Bordoni D., Franzenburg J., Geisen U., Josephs-Spaulding J., Köhler P., Künstner A., Rosati E., Aschenbrenner A.C., Bacher P., Baran N., Boysen T., Brandt B., Bruse N., Dörr J., Dräger A., Elke G., Ellinghaus D., Fischer J., Forster M., Franke A., Franzenburg S., Frey N., Friedrichs A., Fuß J., Glück A., Hamm J., Hinrichsen F., Hoeppner M.P., Imm S., Junker R., Kaiser S., Kan Y.H., Knoll R., Lange C., Laue G., Lier C., Lindner M., Marinos G., Markewitz R., Nattermann J., Noth R., Pickkers P., Rabe K.F., Renz A., Röcken C., Rupp J., Schaffarzyk A., Scheffold A., Schulte-Schrepping J., Schunk D., Skowasch D., Ulas T., Wandinger K.-P., Wittig M., Zimmermann J., Busch H., Hoyer B.F., Kaleta C., Heyckendorf J., Kox M., Rybniker J., Schreiber S., Schultze J.L., Rosenstiel P., HCA Lung Biological Network, Deutsche COVID-19 Omics Initiative (DeCOI) Longitudinal Multi-omics Analyses Identify Responses of Megakaryocytes, Erythroid Cells, and Plasmablasts as Hallmarks of Severe COVID-19. Immunity. 2020;53 doi: 10.1016/j.immuni.2020.11.017. 1296-1314.e9https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birra D., Benucci M., Landolfi L., Merchionda A., Loi G., Amato P., Licata G., Quartuccio L., Triggiani M., Moscato P. COVID 19: a clue from innate immunity. Immunol. Res. 2020:1–8. doi: 10.1007/s12026-020-09137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard J., Dust K., Funk D., Strong J.E., Alexander D., Garnett L., Boodman C., Bello A., Hedley A., Schiffman Z., Doan K., Bastien N., Li Y., Van Caeseele P.G., Poliquin G. Predicting infectious SARS-CoV-2 from diagnostic samples. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2020 doi: 10.1093/cid/ciaa638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll A., McNamara E. Comparison and correlation of commercial SARS-CoV-2 real-time-PCR assays, Ireland, June 2020. Eurosurveillance. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.6.2002079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deeks J.J., Dinnes J., Takwoingi Y., Davenport C., Spijker R., Taylor-Phillips S., Adriano A., Beese S., Dretzke J., Ferrante di Ruffano L., Harris I.M., Price M.J., Dittrich S., Emperador D., Hooft L., Leeflang M.M., Van den Bruel A. Cochrane COVID-19 Diagnostic Test Accuracy Group, 2020. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020;6 doi: 10.1002/14651858.CD013652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E., Arnthorsson A.O., Helgason D., Bjarnadottir K., Ingvarsson R.F., Thorsteinsdottir B., Kristjansdottir S., Birgisdottir K., Kristinsdottir A.M., Sigurdsson M.I., Arnadottir G.A., Ivarsdottir E.V., Andresdottir M., Jonsson F., Agustsdottir A.B., Berglund J., Eiriksdottir B., Fridriksdottir R., Gardarsdottir E.E., Gottfredsson M., Gretarsdottir O.S., Gudmundsdottir S., Gudmundsson K.R., Gunnarsdottir T.R., Gylfason A., Helgason A., Jensson B.O., Jonasdottir A., Jonsson H., Kristjansson T., Kristinsson K.G., Magnusdottir D.N., Magnusson O.T., Olafsdottir L.B., Rognvaldsson S., le Roux L., Sigmundsdottir G., Sigurdsson A., Sveinbjornsson G., Sveinsdottir K.E., Sveinsdottir M., Thorarensen E.A., Thorbjornsson B., Thordardottir M., Saemundsdottir J., Kristjansson S.H., Josefsdottir K.S., Masson G., Georgsson G., Kristjansson M., Moller A., Palsson R., Gudnason T., Thorsteinsdottir U., Jonsdottir I., Sulem P., Stefansson K. Humoral Immune Response to SARS-CoV-2 in Iceland. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2026116. 0, null. https://doi.org/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.S., Byun J.-H., Cho Y., Rim J.H. RT-PCR for SARS-CoV-2: quantitative versus qualitative. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M.-C., Cui C., Shin K.-R., Bae J.-Y., Kweon O.-J., Lee M.-K., Choi S.-H., Jung S.-Y., Park M.-S., Chung J.-W. Duration of Culturable SARS-CoV-2 in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021;384:671–673. doi: 10.1056/NEJMc2027040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libster R., Pérez Marc G., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., Rondan A., Lescano G., Cruz P., Ritou Y., Fernández Viña V., Álvarez Paggi D., Esperante S., Ferreti A., Ofman G., Ciganda Á., Rodriguez R., Lantos J., Valentini R., Itcovici N., Hintze A., Oyarvide M.L., Etchegaray C., Neira A., Name I., Alfonso J., López Castelo R., Caruso G., Rapelius S., Alvez F., Etchenique F., Dimase F., Alvarez D., Aranda S.S., Sánchez Yanotti C., De Luca J., Jares Baglivo S., Laudanno S., Nowogrodzki F., Larrea R., Silveyra M., Leberzstein G., Debonis A., Molinos J., González M., Perez E., Kreplak N., Pastor Argüello S., Gibbons L., Althabe F., Bergel E., Polack F.P. Early High-Titer Plasma Therapy to Prevent Severe Covid-19 in Older Adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long Q.-X., Liu B.-Z., Deng H.-J., Wu G.-C., Deng K., Chen Y.-K., Liao P., Qiu J.-F., Lin Y., Cai X.-F., Wang D.-Q., Hu Y., Ren J.-H., Tang N., Xu Y.-Y., Yu L.-H., Mo Z., Gong F., Zhang X.-L., Tian W.-G., Hu L., Zhang X.-X., Xiang J.-L., Du H.-X., Liu H.-W., Lang C.-H., Luo X.-H., Wu S.-B., Cui X.-P., Zhou Z., Zhu M.-M., Wang J., Xue C.-J., Li X.-F., Wang L., Li Z.-J., Wang K., Niu C.-C., Yang Q.-J., Tang X.-J., Zhang Y., Liu X.-M., Li J.-J., Zhang D.-C., Zhang F., Liu P., Yuan J., Li Q., Hu J.-L., Chen J., Huang A.-L. Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat. Med. 2020;26:845–848. doi: 10.1038/s41591-020-0897-1. [DOI] [PubMed] [Google Scholar]
- Markewitz R., Torge A., Wandinger K.-P., Pauli D., Franke A., Bujanda L., Marimón J.M., Banales J.M., Gutierrez-Stampa M.A., Nafría B., Junker R. Clinical correlates of anti-SARS-CoV-2 antibody profiles in Spanish COVID-19 patients from a high incidence region. Sci. Rep. 2021;11:4363. doi: 10.1038/s41598-021-83969-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markewitz R.D.H., Wandinger K.-P., Junker R. Saliva for Detection of SARS-CoV-2. N. Engl. J. Med. 2021;384 doi: 10.1056/NEJMc2032165. [DOI] [PubMed] [Google Scholar]
- Marot S., Calvez V., Louet M., Marcelin A.-G., Burrel S. Interpretation of SARS-CoV-2 replication according to RT-PCR crossing threshold value. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2021 doi: 10.1016/j.cmi.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolich-Žugich J. The twilight of immunity: emerging concepts in aging of the immune system. Nat. Immunol. 2018;19:10–19. doi: 10.1038/s41590-017-0006-x. [DOI] [PubMed] [Google Scholar]
- R Core Team R: A Language and Environment for Statistical Computing; Vienna, Austria; 2020. [Google Scholar]
- Se K., Hs J., Y Y., Su S., S K., Th O., Uj K., Sj K., Hc J., Si J., Kh P. Viral kinetics of SARS-CoV-2 in asymptomatic carriers and presymptomatic patients [WWW Document] Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020 doi: 10.1016/j.ijid.2020.04.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeni R., Firzli T., Riddle M.S., Krasner C., Ashraf S., Siddiqui F. Using COVID-19 Cycle Threshold and Other Lab Values as Predictors of Hospitalization Need. J. Med. Virol. 2021 doi: 10.1002/jmv.26835. [DOI] [PubMed] [Google Scholar]
- Shen C., Wang Z., Zhao F., Yang Yang, Li J., Yuan J., Wang F., Li D., Yang M., Xing L., Wei J., Xiao H., Yang Yan, Qu J., Qing L., Chen L., Xu Z., Peng L., Li Y., Zheng H., Chen F., Huang K., Jiang Y., Liu D., Zhang Z., Liu Y., Liu L. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singanayagam A., Patel M., Charlett A., Bernal J.L., Saliba V., Ellis J., Ladhani S., Zambon M., Gopal R. Duration of infectiousness and correlation with RT-PCR cycle threshold values in cases of COVID-19, England, January to May 2020. Eurosurveillance. 2020;25 doi: 10.2807/1560-7917.ES.2020.25.32.2001483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verity R., Okell L.C., Dorigatti I., Winskill P., Whittaker C., Imai N., Cuomo-Dannenburg G., Thompson H., Walker P.G.T., Fu H., Dighe A., Griffin J.T., Baguelin M., Bhatia S., Boonyasiri A., Cori A., Cucunubá Z., FitzJohn R., Gaythorpe K., Green W., Hamlet A., Hinsley W., Laydon D., Nedjati-Gilani G., Riley S., van Elsland S., Volz E., Wang H., Wang Y., Xi X., Donnelly C.A., Ghani A.C., Ferguson N.M. Estimates of the severity of coronavirus disease 2019: a model-based analysis. Lancet Infect. Dis. 2020;20:669–677. doi: 10.1016/S1473-3099(20)30243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–2194. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Wyllie A.L., Fournier J., Casanovas-Massana A., Campbell M., Tokuyama M., Vijayakumar P., Warren J.L., Geng B., Muenker M.C., Moore A.J., Vogels C.B.F., Petrone M.E., Ott I.M., Lu P., Venkataraman A., Lu-Culligan A., Klein J., Earnest R., Simonov M., Datta R., Handoko R., Naushad N., Sewanan L.R., Valdez J., White E.B., Lapidus S., Kalinich C.C., Jiang X., Kim D.J., Kudo E., Linehan M., Mao T., Moriyama M., Oh J.E., Park A., Silva J., Song E., Takahashi T., Taura M., Weizman O.-E., Wong P., Yang Y., Bermejo S., Odio C.D., Omer S.B., Dela Cruz C.S., Farhadian S., Martinello R.A., Iwasaki A., Grubaugh N.D., Ko A.I. Saliva or Nasopharyngeal Swab Specimens for Detection of SARS-CoV-2. N. Engl. J. Med. 2020;383:1283–1286. doi: 10.1056/NEJMc2016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L., Ruan F., Huang M., Liang L., Huang H., Hong Z., Yu J., Kang M., Song Y., Xia J., Guo Q., Song T., He J., Yen H.-L., Peiris M., Wu J. SARS-CoV-2 Viral Load in Upper Respiratory Specimens of Infected Patients. N. Engl. J. Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]