Abstract
Background
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is commonly associated with myocardial injury and heart failure. The pathophysiology behind this phenomenon remains unclear, with many diverse and multifaceted hypotheses. To contribute to this understanding, we describe the underlying cardiac findings in fifty patients who died with coronavirus disease 2019 (COVID-19).
Methods
Included were autopsies performed on patients with a positive SARS-CoV-2 reverse-transcriptase-polymerase-chain reaction test from the index hospitalization. In the case of out-of-hospital death, patients were included if post-mortem testing was positive. Complete autopsies were performed according to a COVID-19 safety protocol, and all patients underwent both macroscopic and microscopic examination. If available, laboratory findings and echocardiograms were reported.
Results
The median age of the decedents was 63.5 years. The most common comorbidities included hypertension (90.0%), diabetes (56.0%) and obesity (50.0%). Lymphocytic inflammatory infiltrates in the heart were present in eight (16.0%) patients, with focal myocarditis present in two (4.0%) patients. Acute myocardial ischemia was observed in eight (16.0%) patients. The most common findings were myocardial fibrosis (80.0%), hypertrophy (72.0%), and microthrombi (66.0%). The most common causes of death were COVID-19 pneumonia in 18 (36.0%), COVID-19 pneumonia with bacterial superinfection in 12 (24.0%), and COVID-19 pneumonia with pulmonary embolism in 10 (20.0%) patients.
Conclusions
Cardiovascular comorbidities were prevalent, and pathologic changes associated with hypertensive and atherosclerotic cardiovascular disease were the most common findings. Despite markedly elevated inflammatory markers and cardiac enzymes, few patients exhibited inflammatory infiltrates or necrosis within cardiac myocytes. A unifying pathophysiologic mechanism behind myocardial injury in COVID-19 remains elusive, and additional autopsy studies are needed.
Keywords: Severe acute respiratory syndrome coronavirus 2, Coronavirus disease 2019, Cardiac pathology, Cardiovascular disease
Abbreviations: COVID-19, Coronavirus Disease 2019; SARS-CoV-2, Severe Acute Respiratory Syndrome Coronavirus 2
1. Introduction
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), was officially recognized in December 2019. This virus rapidly spread throughout the world causing significant morbidity and mortality. Though originally characterized by varying degrees of respiratory failure,[1] extrapulmonary involvement has been well documented.[2] Of particular concern are the effects COVID-19 exhibits on the cardiovascular system. More than half of COVID-19 patients undergoing echocardiography exhibit left or right ventricular abnormalities, often unsuspected,[3] and myocardial injury (signified by elevated cardiac biomarkers) is associated with excess morbidity and mortality in patients with COVID-19.[4], [5], [6]
Autopsy reports have described a varied prevalence of cardiac pathologies associated with COVID-19, including acute ischemia, myocarditis, pericarditis, cytokine storm and non-myocarditis inflammation, and microvascular injury with resultant thrombosis.[7], [8], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21] The discrepancy in these findings may be due to the absence of a predefined standard COVID-19 autopsy protocol.[7] In particular, while persistent myocardial inflammation has been of concern in COVID-19 survivors (with cardiac magnetic resonance imaging showing persistent myocardial inflammation in up to 60.0% of patients),[22] the prevalence of pathologically confirmed inflammatory infiltrates and/or myocarditis is much lower.[7,8]
Our understanding of the cardiac pathologies caused by COVID-19 remains limited, in part due to limited number of autopsy studies available. In this report, we describe the cardiac pathology found on autopsy in 50 patients who died with COVID-19.
2. Methods
Deceased patients were included from the index hospitalization in which they tested positive for SARS-CoV-2 by reverse-transcriptase-polymerase-chain-reaction. In the case of out-of-hospital death, patients were included if post-mortem testing was positive. Consent for autopsy, and use for research, was obtained from the appropriate next of kin for each patient. Complete autopsies were performed at the University of Alabama at Birmingham, Birmingham, AL, USA and were performed according to an institutional COVID-19 safety protocol (Supplementary Methods). Hearts were examined in the usual fashion, with sections obtained from the anterior, lateral, and posterior walls of the left ventricle, interventricular septum, posterior right ventricle, coronary and pulmonary arteries. Blocks were processed for light microscopy and slides stained with hematoxylin and eosin for microscopic evaluation. All finalized autopsy reports were included in this analysis.
Review of the electronic medical record was performed to obtain clinical data from patients hospitalized at the University of Alabama at Birmingham. Complete medical records were not available for patients who died at outside hospitals, we were therefore unable to include laboratory and imaging data for those patients. However, demographic and comorbid conditions were available and included. We assessed the prevalence of the following comorbid conditions: obesity, hypertension, diabetes mellitus, hyperlipidemia, coronary artery disease, congestive heart failure (defined as either preserved or reduced ejection fraction), chronic atrial fibrillation and/or atrial flutter, chronic lung disease (defined as chronic obstructive pulmonary disease, asthma, or pulmonary fibrosis), and chronic kidney disease. Median laboratory values were obtained for the following variables: brain natriuretic peptide, troponin I, D-dimer, ferritin, C-reactive protein, erythrocyte sedimentation rate, and Interleukin-6. Peak measurements, rather than initial measurements, were recorded to compare peak systemic inflammation and myocardial injury with pathologic findings. Transthoracic echocardiogram reports were reviewed for the following variables: left ventricular ejection fraction, left ventricular wall motion abnormalities, right ventricular ejection fraction, and right ventricular size. If available in our electronic medical record, these variables were compared to the most recent echocardiogram prior to hospitalization. Cause of death was extracted from the final autopsy report.
Myocardial injury was defined as a troponin I greater than the 99th percentile for the upper reference limit.[5,23] Ventricular hypertrophy was defined as left ventricular free wall thickness greater than 1.5 cm and/or right ventricular free wall thickness greater than 0.5 cm.[24,25] Significant coronary artery disease was defined as ≥75% stenosis in any coronary vessel.[26] Microscopic changes of acute ischemia were characterized by reversible hydropic degeneration, irreversible contraction band necrosis, hyper-eosinophilic myocytes with nuclear loss, and myocyte necrosis with interstitial neutrophils.[27] Myocarditis was defined as an inflammatory infiltrate consisting of ≥14 lymphocytes/mm2 including ≤ four monocytes/mm2 with the presence of CD3-positive T lymphocytes ≥ seven cells/mm2 in the setting of myocyte degeneration and necrosis.[28,29] COVID-19 pneumonia was defined histologically as diffuse alveolar damage and/or organizing pneumonia.[30] This study was approved by the University of Alabama at Birmingham, Birmingham, AL, USA institutional review board.
3. Results
3.1. Demographics and Comorbidities
Patient demographic, laboratory, and echocardiographic variables are shown in Table 1 . Age, race, and comorbidities were available for all 50 patients. Complete records were available for 29 of the 50 patients. The median age of the descendants was 63.5 years (range 31-94 years). Black individuals represented 31 (62.0%) of the patients, with White and Hispanic individuals representing 18 (36.0%) and 1 (2.0%), respectively. Hypertension was observed in 45 (90.0%) patients. Diabetes was present in 28 (56.0%), obesity in 25 (50.0%), and hyperlipidemia in 16 (32.0%) patients. A known diagnosis of coronary artery disease was present in seven (14.0%) and congestive heart failure in five (10.0%) patients. History of atrial fibrillation or flutter was present in four (8.0%) patients. Chronic lung or kidney disease were each present in 15 (30.0%) patients.
Table 1.
Patient demographic, laboratory, and echocardiographic variables
| Patient Characteristics | Number (Proportion) | n = 50 |
|---|---|---|
| Baseline Demographics | ||
| Age, median (range) | 63.5 (31-94) | 50 |
| Male | 36 (72%) | 50 |
| Race or Ethnicity | ||
| White | 18 (36%) | 50 |
| Black | 31 (62%) | 50 |
| Hispanic | 1 (2%) | 50 |
| Clinical comorbidities | ||
| Obesity | 25 (50%) | 50 |
| Hypertension | 45 (90%) | 50 |
| Diabetes | 28 (56%) | 50 |
| Hyperlipidemia | 16 (32%) | 50 |
| Coronary artery disease | 7 (14%) | 50 |
| Congestive heart failure | 5 (10%) | 50 |
| Atrial arrhythmia | 4 (8%) | 50 |
| Chronic lung disease | 15 (30%) | 50 |
| Chronic kidney disease | 15 (30%) | 50 |
| Labs (reference) | Median (range) | |
| BNP (0-100 pg/mL) | 278 (17-3794) | 15 |
| Troponin-I (3-20 ng/L) | 85.5 (8-9394) | 28 |
| D-dimer (0-240 ng/mL) | 1753 (525-20000) | 23 |
| Ferritin (23.9-336.2 ng/mL) | 8228.5 (139-104841) | 20 |
| CRP (0-10.9 mg/L) | 161.9 (6.1-475.7) | 21 |
| ESR (0-10 mm/hr) | 60 (2-119) | 20 |
| Interleukin-6 (<2 pg/mL) | 25.7 (13.0-131.2) | 5 |
| Echocardiographic findings | ||
| New left ventricular dysfunction | 3 (14%) | 21 |
| New right ventricular dysfunction | 14 (67%) | 21 |
BNP: Brain natriuretic peptide; CRP: C-reactive protein; ESR: Erythrocyte sedimentation rate
3.2. Laboratory Findings
Brain natriuretic peptide was available for 15 patients, of which it was elevated in 10 (66.7%), with median value 278 pg/mL (range 17-3794; reference 0-100 pg/mL). Troponin-I was elevated in 24 of the 28 patients (85.7%), with median value of 85.5 ng/L (range 8-9394; reference 3-20 ng/mL). D-dimer was elevated in 23 of 23 patients (100.0%), with median value 1753 ng/mL ([range 525-20000; with 20000 being the upper limit of detection in our assay]; reference 0-240 ng/mL). Ferritin was elevated in 16 of 20 patients (80.0%), with median value of 8228.5 ng/mL (range 139-104841; reference 23.9-336.2 ng/mL). C-reactive protein was elevated in 20 of 21 patients (95.2%), with median value of 161.9 mg/L (range 6.1-475.7; reference 0-10.9 mg/L). Erythrocyte sedimentation rate was elevated in 18 of 20 patients (90.0%), with median value of 60 mm/hr (range 2-119; reference 0-10 mm/hr). Interleukin-6 levels were elevated in five of five patients (100.0%), with median value of 25.7 pg/mL (range 13-131.2; reference <2 pg/mL).
3.3. Echocardiogram Findings
Transthoracic echocardiograms were available for 21 of 50 patients, with abnormalities present in 15 (71.4%) studies. Of the 21 patients, six had received a prior echocardiogram in our electronic medical record. New onset reduced left ventricular ejection fraction was observed in three of the 21 (14.3%) patients, all exhibiting an ejection fraction ≤ 25%. Of these patients, one echocardiogram exhibited wall motion abnormalities consistent with stress – induced (Takotsubo) cardiomyopathy, while the others revealed severe global hypokinesis of the left ventricle. Right ventricular dilation and/or reduced right ventricular systolic function were present in 14 (66.7%) patients – of the six patients with prior echocardiograms, this was a new finding in three and a markedly worse finding in one patient.
3.4. Macroscopic Pathologic Findings
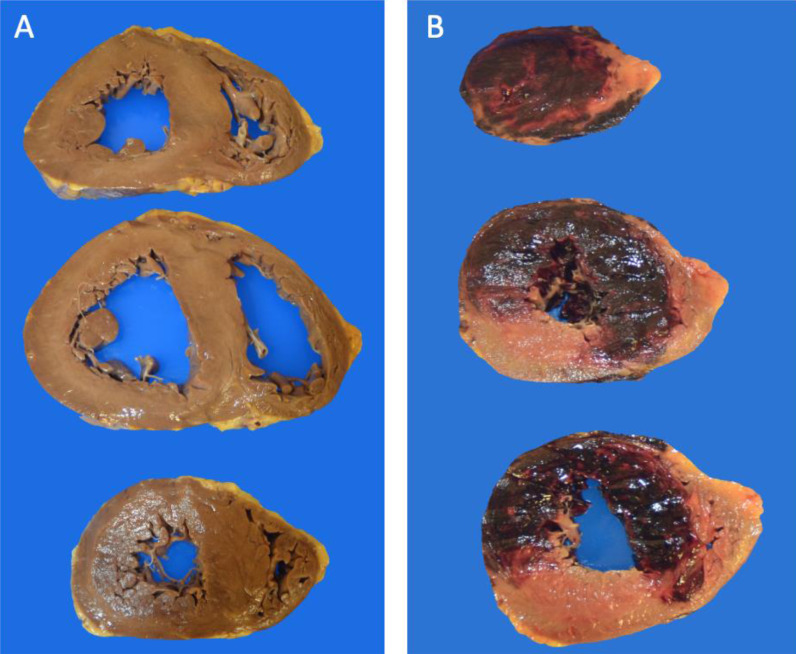
Pathologic findings and cause of death are listed in Table 2 . Median heart weight was 500 grams (range 280-1020 gm). Significant coronary artery obstruction was present in 14 (28.0%) patients. Left and/or right ventricular hypertrophy was present in 36 (72.0%) patients, with the presence of left ventricular dilation and right ventricular dilation in 26 (52.0%) and 28 (56.0%) patients, respectively (Fig. 1 A). Gross evidence of acute infarction was seen in four (8.0%) patients (Fig. 1B), with evidence of remote infarction seen in six (12.0%) patients. Overt pulmonary thromboemboli were observed in 25 (50.0%) patients.
Table 2.
Macroscopic and microscopic findings at autopsy and cause of death
| Autopsy Characteristics | Count (proportion) | n = 50 |
|---|---|---|
| Gross Pathology | ||
| Weight (g), median (range) | 500 (280-1020) | 50 |
| Coronary stenosis ≥75% | 14 (28%) | 50 |
| Hypertrophy | 36 (72%) | 50 |
| Left ventricular dilation | 26 (52%) | 50 |
| Right ventricular dilation | 28 (56%) | 50 |
| Acute myocardial infarction | 4 (8%) | 50 |
| Remote myocardial infarction | 6 (12%) | 50 |
| Pulmonary thromboemboli | 25 (50%) | 50 |
| Microscopic Pathology | ||
| Myocardial Fibrosis | 40 (80%) | 50 |
| Interstitial Fibrosis | 30 (60%) | 50 |
| Perivascular Fibrosis | 23 (46%) | 50 |
| Subendocardial Fibrosis | 10 (20%) | 50 |
| Acute Ischemia | 8 (16%) | 50 |
| Lymphocytic Inflammation | 8 (16%) | 50 |
| Microthrombi | 33 (66%) | 50 |
| Myocarditis | 2 (4%) | 50 |
| Pericarditis | 1 (2%) | 50 |
| Pulmonary Vasculitis | 2 (4%) | 50 |
| Cardiac Sarcoidosis | 1 (2%) | 50 |
| Cause of Death | ||
| COVID-19 pneumonia | 18 (36%) | 50 |
| COVID-19 pneumonia with bacterial coinfection | 12 (24%) | 50 |
| COVID-19 pneumonia and pulmonary embolism | 10 (20%) | 50 |
| COVID-19 pneumonia with myocardial infarction | 1 (2%) | 50 |
| Myocardial infarction | 3 (6%) | 50 |
| Dilated cardiomyopathy | 2 (4%) | 50 |
| Cardiac sarcoidosis | 1 (2%) | 50 |
| Other a | 3 (6%) | 50 |
Included are cerebrovascular accident, glioblastoma multiforme, and acute on chronic decompensated cirrhosis.
Fig. 1.
Gross cardiac pathology. (A) Specimen with biventricular dilation and straightening of the interventricular septum as a result of increased pulmonary pressure. (B) Acute transmural hemorrhagic infarction extending from the apex to base with involvement of the anterior and lateral walls and interventricular septum.
3.5. Microscopic Findings
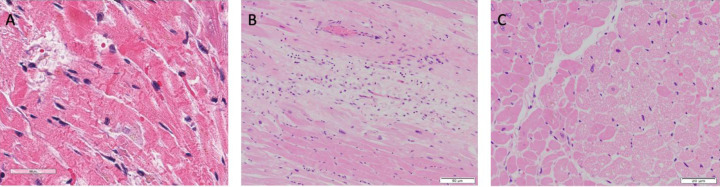
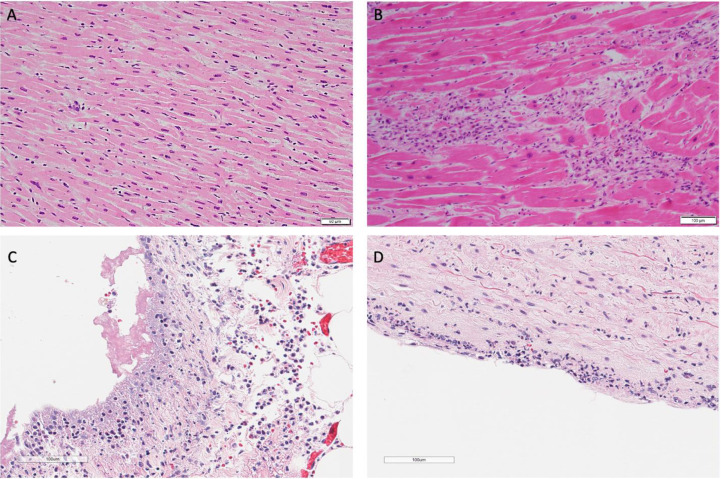
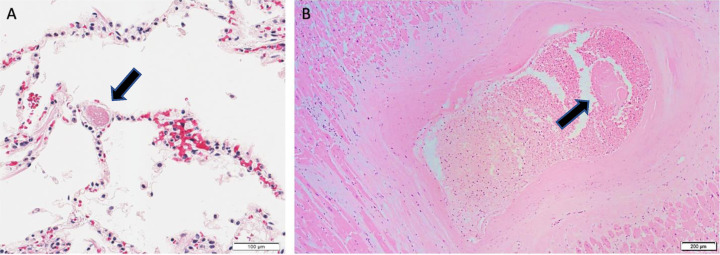
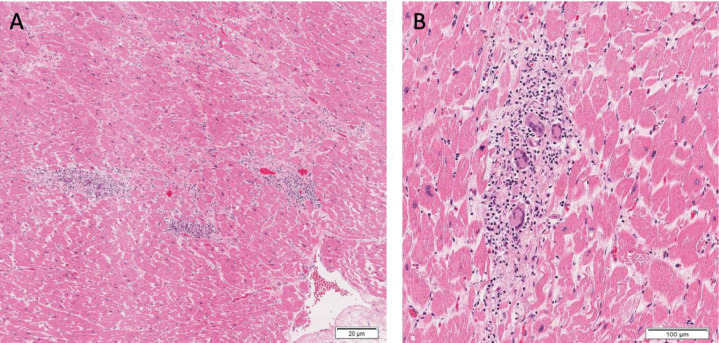
On microscopic exam, myocardial fibrosis was present in 40 (80.0%) patients. This was further subdivided into interstitial, perivascular, and subendocardial fibrosis, and these findings were observed in 30 (60%), 23 (46.0%), and 10 (20.0%) patients. Microscopic changes of acute ischemia were seen in eight (16.0%) patients (Fig. 2 A-C). Presence of lymphocytic inflammation was seen in eight (16.0%) patients (Fig. 3 A), with two (4.0%) patients meeting criteria for a focal myocarditis (Fig. 3B). [28,29] Pericarditis was observed in one (2.0%) patient (Fig. 3C). A focal vasculitis of the pulmonary vasculature was observed in two (4.0%) patients (Fig. 3D). Microthrombi were present in 33 (66.0%) patients. In patients who exhibited evidence of microthrombi, the pulmonary vasculature was universally involved. Additional organs exhibiting microthrombi included the heart (intramural vessel) in two (4.0%) patients, (Fig. 4 A-B), the kidneys in three (6.0%) patients, the liver in one (2.0 %) patient, the colon in one (2.0%) patient, the prostate in one (2.0%) patient, and the brain in one (2.0%) patient. Immunohistochemical staining was performed on one patient exhibiting giant cell granulomas in the heart, lungs, and lymph nodes. In this patient, lymphocytes in the intramyocardial areas of the giant cells were positive for CD3, CD4, and CD68 – these findings were consistent with an incidental diagnosis of cardiac sarcoidosis (Fig. 5 A-B).
Fig. 2.
(A) Acute ischemic injury with irreversible contraction band necrosis (H&E 20x) in a patient with moderate atherosclerotic stenosis of the left anterior descending coronary artery. (B) Hypereosinophilic myocytes with contraction bands and nuclear loss surrounding an area of granulation tissue and myocardial fibrosis (H&E 20x). (C) Reversible vacuolar degeneration of myocytes typical of myocardial ischemia (H&E 40x).
Fig. 3.
(A) Mild interstitial lymphocytic infiltration without myocyte damage (H&E 20x). (B) Focal myocarditis characterized by mononuclear cell infiltrate with myocyte damage (H&E 10x). (C) Fibrinous pericarditis (H&E 20x). (D) Pulmonary artery with subendothelial inflammatory infiltrate (H&E 20x).
Fig. 4.
(A) Microthrombus within pulmonary vasculature (H&E 20x). (B) Non-occlusive organizing thrombus of an intramural vessel of the lateral left ventricle (H&E 4x).
Fig. 5.
Patchy and multifocal lymphocytes and intramyocardial giant cells compatible with a diagnosis of cardiac sarcoidosis. (H&E 4x (A); 10x (B)). The findings of sarcoidosis were incidental in the setting of known heart failure with reduced ejection fraction requiring ICD placement, atrial fibrillation requiring multiple cardioversions, and severe mitral regurgitation.
3.6. Underlying cause of death
In this cohort, cause of death in 18 (36.0%) patients was attributed to COVID-19 pneumonia and/or acute respiratory distress syndrome. COVID-19 pneumonia with the presence of bacterial coinfection was reported as the cause of death in 12 (24.0%) patients. Pulmonary embolism in the setting of COVID-19 was reported in 10 (20.0%) patients. COVID-19 pneumonia with myocardial infarction was reported in one (2.0%) patient. Myocardial infarction without evidence of COVID-19 pneumonia was reported as the cause of death in three (6.0%) patients. Fulminant dilated cardiomyopathy was reported as the cause of death in two (4.0%) patients. Cardiac sarcoidosis was reported as the cause of death in one (2.0%) patient. No evidence of cardiopulmonary COVID-19 sequalae were found in three (6.0%) patients – death in these patients were attributed to cerebrovascular accident, glioblastoma multiforme, and acute on chronic decompensated cirrhosis.
4. Discussion
In the present study we discovered high rates of myocardial fibrosis and hypertrophy, sequelae of chronic cardiovascular disease. Increased frequency of pulmonary thromboembolism and microthrombi formation were also observed. Despite the markedly elevated inflammatory markers in many patients, there was a relative low frequency of myocardial inflammatory cell infiltration or myocyte necrosis.
Of the limited echocardiograms available, acute left ventricular dysfunction was uncommon. Of the three patients with new onset left ventricular dysfunction, the autopsy findings varied. The patient with stress – induced (Tokatsubo) cardiomyopathy revealed a focal area of mononuclear cells composed of lymphocytes and plasma cells. One was found to have acute ischemia in the setting of myocardial infarction, while the other did not reveal any inflammatory infiltrates. Right sided ventricular dysfunction was more prevalent. Of these patients, 13 (92.8%) were associated with significant COVID-19 pneumonia and eight (57.1%) were associated with pulmonary thromboembolism. This data may further support the use of echocardiography for risk stratification of COVID-19 patients suspected of having thromboembolism in order to expedite treatment for this comorbid condition.[31,32]
Myocardial injury in COVID-19 has been attributed to multiple etiologies and include direct viral infection of myocytes, hyperinflammation or “cytokine storm”, endothelial dysfunction, and microthrombosis.[33] Despite most patients exhibiting markedly elevated acute phase reactants, inflammatory infiltrates were relatively uncommon in cardiac tissue, with focal lymphocytic infiltrates found in only eight (16.0%) patients. Focal areas of vasculitis in the pulmonary vasculature were seen in only two (4.0%) patients. While “cytokine storm” storm has received much attention in the literature, it is unclear how this contributes to cardiac dysfunction given these findings. These results appear to be consistent across prior studies.[8]
An extant issue is whether there is an association between COVID-19 and myocarditis with resultant heart failure. Despite concern for myocarditis in both non-critical and critically ill patients, biopsy proven myocarditis remains rare and inconsistent across autopsy reports.[[7], [8], [9], [10], [11], [12], [13], [14],[17], [18], [19], [20], [21],34] In this cohort, only two (4.0%) patients met criteria for a focal myocarditis – with the cause of death in these patients attributed to COVID-19 pneumonia with bacterial coinfection and COVID-19 pneumonia with pulmonary embolism. In the two patients in which the cause of death was listed as a dilated cardiomyopathy, neither patient exhibited a lymphocytic inflammatory infiltrate within the heart, and findings were most consistent with chronic hypertensive and atherosclerotic cardiovascular disease. Our report supports prior studies’ findings that fulminant myocarditis is an uncommon cause of myocardial injury and/or cardiac dysfunction in COVID-19.
The coagulopathy associated with COVID-19 is increased when compared to other etiologies of diffuse alveolar damage,[35,36] and is believed to be related to significant microvascular dysfunction.[16,31,[37], [38], [39], [40]] The etiology of microthrombi formation is multifactorial, and may be related to direct infection of endothelial cells leading to cell death with resultant exposure to the basement membrane, acute immobilization with venous stasis, increases in clotting factors, and increased blood viscosity.[33] In our cohort of patients, microthrombi formation was common, occurring in 66.0% of patients. However, its role in myocardial injury is unclear as the majority of microthrombi identified in this series were pulmonary microthrombi. Current theories suggest endothelial dysfunction and microthrombi may lead to increased myocardial ischemia.[33] However, despite the overall high rates of microthrombi formation, acute ischemia along with myocyte necrosis was found in only eight (16.0%) patients within this cohort, of which only one case showed evidence of cardiac microthrombi. Of the four patients in this cohort whose listed cause of death was myocardial infarction, only one patient showed evidence of COVID-19 pneumonia. In the remaining three cases, there were no characteristic pulmonary features, cardiac microthrombi or microthrombi elsewhere to attribute to COVID-19. While COVID-19 pneumonia has been associated with myocardial infarction,[41], [42], [43] the question remains as to whether SARS-CoV-2 infection may cause myocardial infarction in the absence of the COVID-19 syndrome. These results call into question the prevailing view that microthrombi play a predominant role in myocyte injury.[15]
This cohort of patients exhibited high frequencies of cardiovascular risk factors, including hypertension, diabetes, and obesity. Pathologically, this correlated with a high prevalence of coronary artery disease, myocyte hypertrophy and fibrosis, and ventricular dilation, all of which are known chronic sequalae of cardiovascular co-morbidities. While many questions remain, it is possible that myocardial damage and resultant dysfunction are sequalae of reduced cardiac reserve in the setting of long-term exposure to cardiovascular risk factors.
4.1. Limitations
As with many autopsy reports, this study is limited due to the small sample size and population distribution (Southeastern United States); therefore, generalizability is unclear. Additionally, complete medical records for every patient were not available, leading to difficulties in correlating the clinical course with pathological findings. Lastly, we did not regularly perform immunostaining nor electron microscopy on the cardiac specimens. Nonetheless, we believe this large autopsy study provides important information regarding the cardiac pathophysiology related to COVID-19.
5. Conclusion
In this autopsy cohort, cardiovascular risk factors were prevalent, and correlated with chronic cardiac pathological changes observed at autopsy. There was a predominance of micro and macro-thrombi, with little evidence of inflammatory infiltrate or necrosis within cardiac myocytes. Ultimately, a unifying pathologic etiology underlying COVID-19 myocardial injury remains elusive. Continued autopsy studies are needed to further understand this disease process and to guide future therapeutic interventions.
Ethical Standards
The authors confirm they have obtained approval from the University of Alabama at Birmingham, Birmingham, AL, USA Institutional Review Board for the creation and publication of this manuscript and are in compliance with national and institutional ethical standards.
Acknowledgments
None
Footnotes
There were no grants, contracts, or other forms of financial support used in the making of this report.
The authors have nothing to disclose.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.carpath.2021.107370.
Appendix. Supplementary materials
References
- 1.Guan W-j, Ni Z-y, Hu Y, Liang W-h, Ou C-q, He J-x. Clinical characteristics of coronavirus disease 2019 in China. New England Journal of Medicine. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS. Extrapulmonary manifestations of COVID-19. Nature Medicine. 2020;26:1017–1032. doi: 10.1038/s41591-020-0968-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR. Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging. 2020;21:949–958. doi: 10.1093/ehjci/jeaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clerkin KJ, Fried JA, Raikhelkar J, Sayer G, Griffin JM, Masoumi A. COVID-19 and Cardiovascular Disease. Circulation. 2020;141:1648–1655. doi: 10.1161/CIRCULATIONAHA.120.046941. [DOI] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiology. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonow RO, Fonarow GC, O’Gara PT, Yancy CW. Association of coronavirus disease 2019 (COVID-19) with myocardial injury and mortality. JAMA Cardiology. 2020;5:751–753. doi: 10.1001/jamacardio.2020.1105. [DOI] [PubMed] [Google Scholar]
- 7.Halushka MK, Vander Heide RS. Myocarditis is rare in COVID-19 autopsies: cardiovascular findings across 277 postmortem examinations. Cardiovasc Pathol. 2021;50 doi: 10.1016/j.carpath.2020.107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hooper JE, Padera RF, Dolhnikoff M, da Silva LFF, Duarte-Neto AN, Kapp ME. A postmortem portrait of the coronavirus disease 2019 (COVID-19) pandemic: a large multi institutional autopsy survey study. Arch Pathol Lab Med. 2021;145:529–535. doi: 10.5858/arpa.2020-0786-SA. [DOI] [PubMed] [Google Scholar]
- 9.Basso C, Leone O, Rizzo S, De Gaspari M, van der Wal AC, Aubry MC. Pathological features of COVID-19-associated myocardial injury: a multi center cardiovascular pathology study. Eur Heart J. 2020;41:3827–3835. doi: 10.1093/eurheartj/ehaa664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bradley BT, Maioli H, Johnston R, Chaudhry I, Fink SL, Xu H. Histopathology and ultrastructural findings of fatal COVID-19 infections in Washington State: a case series. Lancet. 2020;396:320–332. doi: 10.1016/S0140-6736(20)31305-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindner D, Fitzek A, Bräuninger H, Aleshcheva G, Edler C, Meissner K. Association of cardiac infection with SARS-CoV-2 in confirmed COVID-19 autopsy cases. JAMA Cardiol. 2020;5:1281–1285. doi: 10.1001/jamacardio.2020.3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiese A, Manetti AC, La Russa R, Di Paolo M, Turillazzi E, Frati P. Autopsy findings in COVID-19-related deaths: a literature review. Forensic Sci Med Pathol. 2021;17:279–296. doi: 10.1007/s12024-020-00310-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edler C, Schröder AS, Aepfelbacher M, Fitzek A, Heinemann A, Heinrich F. Dying with SARS-CoV-2 infection-an autopsy study of the first consecutive 80 cases in Hamburg, Germany. Int J Legal Med. 2020;134:1275–1284. doi: 10.1007/s00414-020-02317-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menter T, Haslbauer JD, Nienhold R, Savic S, Hopfer H, Deigendesch N. Postmortem examination of COVID-19 patients reveals diffuse alveolar damage with severe capillary congestion and variegated findings in lungs and other organs suggesting vascular dysfunction. Histopathology. 2020;77:198–209. doi: 10.1111/his.14134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pellegrini D, Kawakami R, Guagliumi G, Sakamoto A, Kawai K, Gianatti A. Microthrombi as a major cause of cardiac injury in COVID-19. Circulation. 2021;143:1031–1042. doi: 10.1161/CIRCULATIONAHA.120.051828. [DOI] [PubMed] [Google Scholar]
- 16.Wichmann D, Sperhake JP, Lütgehetmann M, Steurer S, Edler C, Heinemann A. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Michele S, Sun Y, Yilmaz MM, Katsyv I, Salvatore M, Dzierba AL. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Falasca L, Nardacci R, Colombo D, Lalle E, Di Caro A, Nicastri E. Postmortem findings in Italian patients with COVID-19: a descriptive full autopsy study of cases with and without comorbidities. J Infect Dis. 2020;222:1807–1815. doi: 10.1093/infdis/jiaa578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsoukkary SS, Mostyka M, Dillard A, Berman DR, Ma LX, Chadburn A. Autopsy findings in 32 patients with COVID-19: a single-institution experience. Pathobiology. 2021;88:56–68. doi: 10.1159/000511325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fox SE, Li G, Akmatbekov A, Harbert JL, Lameira FS, Brown JQ. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 21.Hanley B, Naresh KN, Roufosse C, Nicholson AG, Weir J, Cooke GS. Histopathological findings and viral tropism in UK patients with severe fatal COVID-19: a post-mortem study. Lancet Microbe. 2020;1:e245–e253. doi: 10.1016/S2666-5247(20)30115-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Puntmann VO, Carerj ML, Wieters I, Fahim M, Arendt C, Hoffmann J. Outcomes of cardiovascular magnetic resonance imaging in patients recently recovered from coronavirus disease 2019 (COVID-19) JAMA Cardiology. 2020;5:1265–1273. doi: 10.1001/jamacardio.2020.3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thygesen K, Alpert Joseph S, Jaffe Allan S, Chaitman Bernard R, Bax Jeroen J, Morrow David A. Fourth universal definition of myocardial infarction. Circulation. 2018;138:e618–e651. doi: 10.1161/CIR.0000000000000617. 2018. [DOI] [PubMed] [Google Scholar]
- 24.Westaby JD, Miles C, Chis Ster I, Cooper STE, Antonios TF, Meijles D. Characterisation of hypertensive heart disease: pathological insights from a sudden cardiac death cohort to inform clinical practice. Journal of Human Hypertension. 2021 doi: 10.1038/s41371-021-00507-6. [DOI] [PubMed] [Google Scholar]
- 25.Walker Isom C, Helm Robert A, Scott Ralph C. Right ventricular hypertrophy. Circulation. 1955;11:215–222. doi: 10.1161/01.cir.11.2.215. [DOI] [PubMed] [Google Scholar]
- 26.Basso C, Aguilera B, Banner J, Cohle S, d’Amati G, de Gouveia RH. Guidelines for autopsy investigation of sudden cardiac death: 2017 update from the Association for European Cardiovascular Pathology. Virchows Arch. 2017;471:691–705. doi: 10.1007/s00428-017-2221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duncanson ER, Mackey-Bojack SM. Histologic examination of the heart in the forensic autopsy. Acad Forensic Pathol. 2018;8:565–615. doi: 10.1177/1925362118797736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fung G, Luo H, Qiu Y, Yang D, McManus B. Myocarditis. Circulation Research. 2016;118:496–514. doi: 10.1161/CIRCRESAHA.115.306573. [DOI] [PubMed] [Google Scholar]
- 29.Caforio AL, Pankuweit S, Arbustini E, Basso C, Gimeno-Blanes J, Felix SB. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2013;34:2636–2648. doi: 10.1093/eurheartj/eht210. 2648a-2648d. [DOI] [PubMed] [Google Scholar]
- 30.Borczuk AC. Pulmonary pathology of COVID-19: a review of autopsy studies. Curr Opin Pulm Med. 2021;27:184–192. doi: 10.1097/MCP.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 31.Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. Journal of the American College of Cardiology. 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bikdeli B, Lobo JL, Jiménez D, Green P, Fernández-Capitán C, Bura-Riviere A. Early use of echocardiography in patients with acute pulmonary embolism: findings from the RIETE registry. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.118.009042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Unudurthi SD, Luthra P, Bose RJC, McCarthy JR, Kontaridis MI. Cardiac inflammation in COVID-19: Lessons from heart failure. Life Sci. 2020;260 doi: 10.1016/j.lfs.2020.118482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bearse M, Hung YP, Krauson AJ, Bonanno L, Boyraz B, Harris CK. Factors associated with myocardial SARS-CoV-2 infection, myocarditis, and cardiac inflammation in patients with COVID-19. Mod Pathol. 2021;34:1345–1357. doi: 10.1038/s41379-021-00790-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thille AW, Esteban A, Fernández-Segoviano P, Rodriguez JM, Aramburu JA, Vargas-Errázuriz P. Chronology of histological lesions in acute respiratory distress syndrome with diffuse alveolar damage: a prospective cohort study of clinical autopsies. Lancet Respir Med. 2013;1:395–401. doi: 10.1016/S2213-2600(13)70053-5. [DOI] [PubMed] [Google Scholar]
- 36.Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA. Lung Histopathology in coronavirus disease 2019 as compared with severe acute respiratory sydrome and H1N1 Influenza: a systematic review. Chest. 2021;159:73–84. doi: 10.1016/j.chest.2020.09.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18:1421–1424. doi: 10.1111/jth.14830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llitjos JF, Leclerc M, Chochois C, Monsallier JM, Ramakers M, Auvray M. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rovas A, Osiaevi I, Buscher K, Sackarnd J, Tepasse PR, Fobker M. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis. 2021;24:145–157. doi: 10.1007/s10456-020-09753-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piazza G, Campia U, Hurwitz S, Snyder JE, Rizzo SM, Pfeferman MB. Registry of arterial and venous thromboembolic complications in patients with COVID-19. J Am Coll Cardiol. 2020;76:2060–2072. doi: 10.1016/j.jacc.2020.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Modin D, Claggett B, Sindet-Pedersen C, Lassen MCH, Skaarup KG, Jensen JUS. Acute COVID-19 and the Incidence of Ischemic Stroke and Acute Myocardial Infarction. Circulation. 2020;142:2080–2082. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in hospitalized patients with COVID-19 in a New York city health system. Jama. 2020;324:799–801. doi: 10.1001/jama.2020.13372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bangalore S, Sharma A, Slotwiner A, Yatskar L, Harari R, Shah B. ST-Segment elevation in patients with Covid-19 - a case series. N Engl J Med. 2020;382:2478–2480. doi: 10.1056/NEJMc2009020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.