Abstract
Aims
To create and compare survival models from admission laboratory indices in people hospitalized with coronavirus disease 2019 (COVID-19) with and without diabetes.
Methods
Retrospective observational study of patients with COVID-19 with or without diabetes admitted to Sheffield Teaching Hospitals from 29 February to 01 May 2020. Predictive variables for in-hospital mortality from COVID-19 were explored using Cox proportional hazard models.
Results
Out of 505 patients, 156 (30.8%) had diabetes mellitus (DM) of which 143 (91.7%) had type 2 diabetes. There were significantly higher in-hospital COVID-19 deaths in those with DM [DM COVID-19 deaths 54 (34.6%) vs. non-DM COVID-19 deaths 88 (25.2%): P < 0.05]. Activated partial thromboplastin time (APPT) > 24 s without anticoagulants (HR 6.38, 95% CI: 1.07–37.87: P = 0.04), APTT > 24 s with anticoagulants (HR 24.01, 95% CI: 3.63–159.01: P < 0.001), neutrophil–lymphocyte ratio > 8 (HR 6.18, 95% CI: 2.36–16.16: P < 0.001), and sodium > 136 mmol/L (HR 3.27, 95% CI: 1.12–9.56: P = 0.03) at admission, were only associated with in-hospital COVID-19 mortality for those with diabetes.
Conclusions
At admission, elevated APTT with or without anticoagulants, neutrophil–lymphocyte ratio and serum sodium are unique factors that predict in-hospital COVID-19 mortality in patients with diabetes compared to those without. This novel finding may lead to research into haematological and biochemical mechanisms to understand why those with diabetes are more susceptible to poor outcomes when infected with Covid-19, and contribute to identification of those most at risk when admitted to hospital.
Keywords: COVID-19, Mortality, Diabetes mellitus, Anticoagulants, Activated partial thromboplastin time (APTT), Neutrophil-lymphocyte ratio
1. 1: Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and is an ongoing pandemic. COVID-19 deaths in the U.K. are now in excess of 100,000 [1]. There are 3.9 million people with confirmed diabetes in the U.K. [2] and nationally 18.1% of hospital inpatients have diabetes, making it one of the most commonly encountered conditions [3]. Type 1 and type 2 diabetes mellitus have been shown to confer an increased risk of developing severe COVID-19 with poor early outcomes and an attendant increase in mortality [4], [5], [6]. This is consistent with diabetes being a well-recognised risk factor in several other infectious respiratory diseases [7], [8], [9].
Data describing the clinical risk-phenotypes for COVID-19 severity in the context of diabetes are rapidly emerging [10], [11], [12], [13], [14]. These data could inform clinical risk stratification and direct future research into the pathogenesis and treatment of severe COVID-19.
Current evidence suggests that individuals with diabetes are at an increased risk of severe COVID-19 infection and consequently poor hospital outcomes, and that this risk is influenced by a number of potentially modifiable and non-modifiable factors. However, there is a lack of precise data on clinical characteristics including admission laboratory parameters that predict outcomes in a cohort of hospitalized patients with diabetes and COVID-19 compared to patients with COVID-19 who do not have diabetes. We hypothesized that routine admission observations and laboratory tests could predict outcomes on in-hospital stay with COVID-19. The aim of this study was to compare admission characteristics and mortality between subjects with COVID-19 with and without diabetes mellitus, at two large University hospitals in England.
2. Subjects, materials and methods
We conducted an exploratory, retrospective, observational, cohort study at Sheffield Teaching Hospitals (comprising the Northern General and Royal Hallamshire Hospitals) between 29 February 2020 and 01 May 2020 coinciding with the first COVID-19 wave in the U.K. Patients were included if they had a documented past medical history of diabetes and were PCR positive for SARS-CoV-2 with samples obtained from a nasopharyngeal swab. We included a comparator group of all patients who were consecutively admitted to our hospitals and had SARS-CoV-2 PCR confirmed COVID-19 during the study period but without diabetes. Absence of diabetes was determined by checking documented past medical history and an HbA1c < 6.5% (48 mmol/mol) on all available laboratory data (historical electronic records extend until 2011). Given the novel nature of COVID-19 and thus our exploratory study design, we aimed to be comprehensive in collection of admission clinical and routine laboratory data. However, we specifically collected detailed data on vital signs including respiratory rate, albumin status, serum sodium, potassium and renal function, total leukocyte counts and subsets including neutrophils and lymphocytes, and coagulation profiles. This was in keeping with our hypothesis and aims and based on emerging literature linking these characteristics with morbidity and mortality in COVID-19 [15], [16], [17], [18], [19], [20]. Demographic, clinical and laboratory data were extracted using computer search algorithms from electronic medical records and by the investigators from paper case notes.
Socioeconomic deprivation was assessed using the English index of multiple deprivation [21]. These are widely used datasets in the U.K. to classify deprivation in small areas (based on residential post code). There are seven domains of deprivation which combine to create the index in a given locality including: income, employment, health deprivation and disability, education, crime, barriers to housing and services, and living environment. Cause-specific mortality (COVID-19 death versus non-COVID-19 death) was ascertained from death certificates for all patients. Deaths due to COVID-19 were defined as those where COVID-19 was a documented cause of death on the death certificate. In addition, raw data were rigorously assessed for accuracy and quality by a member of the research team not involved in data collection and efforts made to minimize missing data by manual reinterrogation of case records. The study was approved by the East-Midlands-Leicester South Research Ethics Committee (20/EM/0145).
2.1. Statistical analysis
We analyzed clinical characteristics of patients with and without diabetes who had COVID-19 to determine which characteristics predict in-hospital mortality from COVID-19. Given the retrospective study design there were missing variables that are indicated in the results tables (Table 1, Table 3 and Supplementary Table 1). Continuous variables are represented as mean (M) and standard deviation (SD). Categorical variables are represented by the number of cases (N) and the percentage (%) of the total. Comparisons between the diabetes and non-diabetes group were performed using students t test for continuous variables and χ2 test for categorical variables. Although t tests are relatively robust to violations of assumptions of normality and homogeneity of variance, Q-Q plots were used to determine normality and Levene's test was used to test for homogeneity of variance and subsequent adjustment of P values if required. Possible predictive variables for mortality were explored using Cox proportional hazards models. The data set were split to generate a diabetes-COVID-19 and a non-diabetes-COVID-19 model. The models incorporated admission characteristics including: demographic variables, vital signs, and laboratory values. The covariates were selected according to significance within the bivariate analysis. The final model incorporated: age, BMI, index of multiple deprivation, on admission respiratory rate, albumin, sodium, potassium, urea, creatinine, neutrophil–lymphocyte ratio, and activated partial thromboplastin time (APPT) with or without use of anticoagulants on admission. As this was an exploratory analysis, if the variable was linked to decreased survival then the optimal cut-off was determined to establish maximum separation between survivors and non-survivors. As a more prolonged APTT was determined to be a strong predictor of early mortality, but it was predicted that clinicians would want to understand if this was related to therapeutic anticoagulation, an extra category was added for longer APTT with therapeutic anticoagulation. The diabetes-COVID-19 model also included admission HbA1c and additional glucose categories on admission. These were hypoglycaemia [<4 mmol/L (72 mg/dL)], euglycemia [4.1–11 mmol/L (74–198 mg/dL) or hyperglycaemia on admission stratified as: glucose 11.1–14 mmol/L (200–252 mg/dL), 14.1–20 mmol/L (254–360 mg/dL), 20.1–27 mmol/L (262–486 mg/dL) or >27.1 mmol/L (488 mg/dL) (see Table 3 for frequency of events in final model variables). Kaplan-Meier curves were generated to represent COVID-19 specific mortality in the diabetes group by admission neutrophil–lymphocyte ratio and admission APTT with or without use of anticoagulants. Time to COVID-19 specific death was the primary outcome for analyses, with data from those patients that were discharged alive or were still admitted as hospital inpatients at the end of the data collection period counted as a censored observation. Analyses were conducted in SPSS version 26 and in all analyses a P value of <0.05 was considered statistically significant. The values reported are for a two-tailed P value.
Table 1.
Baseline clinical characteristics of 349 patients without diabetes and 156 patients with diabetes prior to hospitalization with COVID-19.
| Clinical Features | Number of patients with available data non-DM/ DM | Non-DM | DM | P |
|---|---|---|---|---|
| Age (years)* | 348/154 | 68.6 ± 18.1 | 71.8 ± 14.9 | <0.05 |
| BMI (kg/m2)* | 294/148 | 26.9 ± 7.1 | 29.2 ± 8.5 | <0.05 |
| Length of stay (days*) | 349/156 | 11.2 ± 12.9 | 13.0 ± 12.0 | NS |
| HbA1c % (mmol/mol)* | 147 | – | 7.8 ± 3.7 (61 ± 17) | – |
| Type of Diabetes N (%) |
156 | – | Type 1: 12 (7.7) Type 2: 143 (91.7) Steroid: 1 (0.6) |
– |
| Sex, N (%) | 349/156 | Male 199 (57) Female 150 (43) |
Male 96 (61) Female 60 (39) |
NS |
| Smoker, N (%) | 175/144 | 26 (14.9) | 4 (2.7) | <0.05 |
| Frailty scoreA, N (%) | 229/80 | Mild 107 (46.7) | Mild 18 (22.5) | <0.05 |
| Moderate 74 (32.3) | Moderate 41 (51.2) | <0.05 | ||
| Severe 48 (21) | Severe 21 (26.3) | NS | ||
| Index of multiple deprivationB, N (%) | 347/154 | Most 184 (53.2) | Most 83 (53.8) | NS |
| Intermediate 98 (28.2) | Intermediate 47 (30.5) | NS | ||
| Least 65 (18.7) | Least 24 (15.5) | NS | ||
| Comorbidities, N (%) | 349/156 | IHD 60 (17.2) | IHD 52 (33.3) | <0.05 |
| Stroke/TIA 53 (15.2) | Stroke/TIA 39 (25.0) | <0.05 | ||
| RRTC7 (2.0) | RRTc 19 (12.1) | <0.05 | ||
| Asthma 38 (10.9) | Asthma 16 (10.3) | NS | ||
| COPD 51 (14.6) | COPD 23 (14.7) | NS | ||
| HTN 140 (40.1) | HTN 97 (62.2) | <0.05 | ||
| HF 41 (12.0) | HF 44 (28.2) | <0.05 | ||
| Dementia 52 (15.2) | Dementia 25 (16.0) | NS | ||
| Malignancy 75 (21.5) | Malignancy 22 (14.1) | <0.05 |
A: Frailty measured by Rockwood score and grouped: mild (1–3), moderate (4–6), and severe (7–9). B: Index of multiple deprivation post code centiles grouped: most deprivation (1–3), intermediate deprivation (4–7), and least deprivation (8–10). C: RRT established prior to COVID-19 infection. *M = mean and SD = standard deviation, t tests were used to compare continuous and χ2 for categorical data. Abbreviations: DM, diabetes mellitus: Non-DM, no diabetes mellitus; BMI, body mass index; IHD, ischemic heart disease; TIA, transient ischemic attack; RRT, renal replacement therapy; COPD, chronic obstructive pulmonary disease; HF, heart failure; NS, non-significant.
Table 3.
Frequency of events for variables included in the final non-DM-COVID-19 and DM-COVID-19 Cox proportional models (Table 2).
| Variable | Non-DM (Total N = 349) |
DM (Total N = 156) |
||
|---|---|---|---|---|
| Survived N (% of total) | Died N (% of total) | Survived N (% of total) | Died N (% of total) | |
| Age categories (years) Available data N = 348 (99.7%) | Available data N = 154 (98.7%) | |||
| 16–60 | 109 (31.3) | 4 (1.1) | 26 (16.9) | 4 (2.6) |
| 60.1–70 | 37 (10.6) | 9 (2.6) | 20 (13.0) | 9 (5.8) |
| >70.1 | 114 (32.8) | 75 (21.6) | 54 (35.1) | 41 (26.6) |
| BMI categories (kg/m2) Available data N = 294 (84.2%) | Available data N = 148 (94.9%) | |||
| 18.5–24.9 | 71 (24.1) | 28 (9.5) | 27 (18.2) | 16 (10.8) |
| 25–29.9 | 70 (23.8) | 16 (5.4) | 31 (20.9) | 13 (8.8) |
| 30–34.9 | 39 (13.3) | 9 (3.1) | 18 (12.2) | 9 (6.1) |
| 35–39.9 | 17 (5.8) | 7 (2.4) | 12 (8.1) | 2 (1.4) |
| >40 | 9 (3.1) | 2 (0.7) | 9 (6.1) | 6 (4.1) |
| 0–18.5 | 20 (6.8) | 6 (2.0) | 1 (0.7) | 4 (2.7) |
| Index of multiple deprivation Available data N = 347 (99.4%) (centiles) | Available data N = 154 (98.7%) | |||
| 1–3 (most deprivation) | 134 (38.6) | 50 (14.4) | 53 (34.4) | 30 (19.5) |
| 4–6 (intermediate deprivation) | 69 (19.9) | 29 (8.4) | 30 (19.5) | 17 (11.0) |
| 7–10 (least deprivation) | 56 (16.1) | 9 (2.6) | 17 (11.0) | 7 (4.5) |
| Admission respiratory rate Available data N = 341 (97.7%) (breaths per minutes) | Available data N = 151 (96.8%) | |||
| >22 | 57 (16.7) | 26 (7.6) | 26 (17.2) | 16 (10.6) |
| <22 | 202 (59.0) | 56 (16.4) | 74 (49.0) | 35 (23.2) |
| Admission albumin (g/L) Available data N = 337 (96.5%) | Available data N = 147 (94.2%) | |||
| >37 | 173 (51.3) | 32 (9.4) | 45 (30.6) | 17 (11.6) |
| <37 | 76 (22.5) | 56 (16.6) | 52 (35.3) | 33 (22.4) |
| Admission sodium (mmol/L) Available data N = 346 (99.1%) | Available data N = 152 (97.4%) | |||
| >136 | 140 (40.5) | 50 (14.5) | 32 (21.1) | 25 (16.4) |
| <136 | 119 (34.4) | 37 (10.7) | 68 (44.7) | 27 (17.8) |
| Admission potassium (mmol/L) Available data N = 346 (99.1%) | Available data N = 152 (97.4%) | |||
| >3.5 | 211 (61.0) | 67 (19.4) | 80 (52.6) | 35 (23.0) |
| <3.5 | 48 (13.9) | 20 (5.8) | 20 (13.2) | 17 (11.2) |
| Admission urea (mmol/L) Available data N = 346 (99.1%) | Available data N = 152 (97.4%) | |||
| >7 | 81 (23.4) | 63 (18.2) | 63 (41.4) | 41 (27.0) |
| <7 | 178 (51.4) | 24 (6.9) | 37 (24.3) | 11 (7.2) |
| Admission creatinine (μmol/L) Available data N = 346 (99.1%) Available data N = 152 (97.4%) | ||||
| >100 | 59 (17.1) | 47 (13.6) | 56 (36.8) | 35 (23.0) |
| <100 | 200 (57.8) | 40 (11.6) | 44 (28.9) | 17 (11.2) |
| Admission neutrophil–lymphocyte Available data N = 346 (99.1%) ratio* | Available data N = 152 (97.4%) | |||
| >8 | 65 (18.8) | 36 (10.4) | 27 (17.8) | 26 (17.1) |
| <8 | 193 (55.8) | 52 (15.0) | 73 (48.0) | 26 (17.1) |
| Admission APTT (seconds) Available data N = 309 (88.5%) | Available data N = 126 (80.8%) | |||
| >24 | 118 (38.2) | 43 (13.9) | 38 (30.2) | 17 (13.5) |
| >24 and on anticoagulant | 32 (10.4) | 19 (6.1) | 23 (18.3) | 20 (15.9) |
| <24 | 79 (25.6) | 18 (5.8) | 24 (19.0) | 4 (3.2) |
| Admission glucose categories Available data N = 322 (92.2%) mmol/L (mg/dL) | Available data N = 148 (94.8%) | |||
| <4 (72) | 3 (0.9) | 1 (0.3) | 4 (2.7) | 4 (2.7) |
| 4.1–11 (74–198) | 230 (71.4) | 82 (25.4) | 57 (38.5) | 29 (19.5) |
| 11.1–14 (200–252) | 2 (0.6) | 3 (0.9) | 16 (10.8) | 7 (4.7) |
| 14.1–20 (254–360) | 0 | 1 (0.3) | 11 (7.4) | 7 (4.7) |
| 20.1–27 (362–486) | 0 | 0 | 5 (3.3) | 3 (2.0) |
| >27.1 (488) | 0 | 0 | 3 (2.0) | 2 (1.3) |
Neutrophil-lymphocyte ratio calculated as total neutrophil count × 109/L divided by total lymphocyte count × 109/L. Abbreviations: DM, diabetes mellitus: Non-DM, no diabetes mellitus; APTT, activated partial thromboplastin time.
3. Results
3.1. Baseline characteristics
There were 505 patients admitted with COVID-19 who were eligible for inclusion. Of the 505 patients, 156 patients had diabetes (DM) and 349 patients did not have diabetes (non-DM). Patients in the DM group were older than non-DM (mean age 71.8 ± 14.9 years DM vs. 68.6 ± 18.1 years non-DM: P < 0.05) and had a higher BMI (mean BMI 29.2 ± 8.5 kg/m2 DM vs. 26.9 ± 7.1 kg/m2 non-DM: P < 0.05). The mean HbA1c in the DM group was 7.8% (61 mmol/mol) and 143 (91.7%) patients had type 2 diabetes. Comorbidities were more common in the DM group, likewise they had higher frailty scores. A higher proportion of patients had a diagnosis of malignancy in the non-DM group. Table 1 summarizes baseline characteristics for the study population.
3.2. Laboratory characteristics on admission
On admission, patients in the DM group compared to non-DM had higher total white cell count (mean white cell count 9.2 ± 5.1 × 109/L DM vs. 8.1 ± 4.5 × 109/L non-DM: P < 0.05), higher neutrophil count (mean neutrophil count 7.1 ± 4.6 × 109/L DM vs. 6.2 ± 4.1 × 109/L non-DM: P < 0.05), and CRP levels (mean CRP 100.3 ± 99.6 mg/L DM vs. 82.2 ± 89.2 mg/L non-DM: P < 0.05). Admission lymphocyte counts (mean lymphocyte count 1.3 ± 1.9 × 109/L DM vs. 1.2 ± 1.0 × 109/L non-DM: P = 0.39) and neutrophil–lymphocyte ratios (mean neutrophil–lymphocyte ratio 9.0 ± 10.8 DM vs. 8.0 ± 9.2 non-DM: P = 0.39) did not differ significantly between groups. Admission glucose values were significantly higher in the DM group (mean glucose 11.0 ± 6.4 mmol/L DM vs. 6.4 ± 1.6 mmol/L non-DM: P < 0.001). Estimated glomerular filtration rate (eGFR) was lower in the DM group (mean eGFR 43.2 ± 23.6 mL/min/1.73 m2 DM vs. 57.3 ± 22.7 mL/min/1.73 m2 non-DM: P < 0.001) and admission urea higher (mean urea 11.6 ± 7.4 mmol/L DM vs. 8.2 ± 5.9 mmol/L non-DM: P < 0.001). Admission prothrombin time (mean prothrombin time 13.2 ± 6.9 s DM vs. 11.8 ± 2.3 s non-DM: P < 0.05) and APTT (mean APTT 29.5 ± 14.5 s DM vs. 25.9 ± 4.7 s non-DM: P < 0.01) were both higher in the DM group. Fibrinogen levels were significantly higher in the DM group (mean fibrinogen 5.7 ± 1.2 g/L DM vs. 5.2 ± 1.4 g/L non-DM: P < 0.001) and D-dimer levels elevated in both groups but with no statistically significant between group difference (Supplementary Table 1).
3.3. COVID-19 mortality and intensive care admission
There were significantly higher COVID-19 deaths in the DM group compared to non-DM [DM COVID-19 deaths 54 (34.6%) vs. non-DM COVID-19 deaths 88 (25.2%): P < 0.05] (Supplementary Table 2). There were a total of 34 (6.7%) admissions to the Intensive Care Unit (ICU) and 12 (2.3%) patients required mechanical ventilation. There was no statistically significant difference in the proportion of patients admitted to ICU or mechanically ventilated when comparing the DM to the non-DM group (Supplementary Fig. 1). A significantly higher proportion of DM patients were established on renal replacement at baseline (Table 1) and continued on this through admission. There was, however, no de novo initiation of renal replacement therapy in those with diabetes.
3.4. Admission factors associated with in-hospital COVID-19 mortality
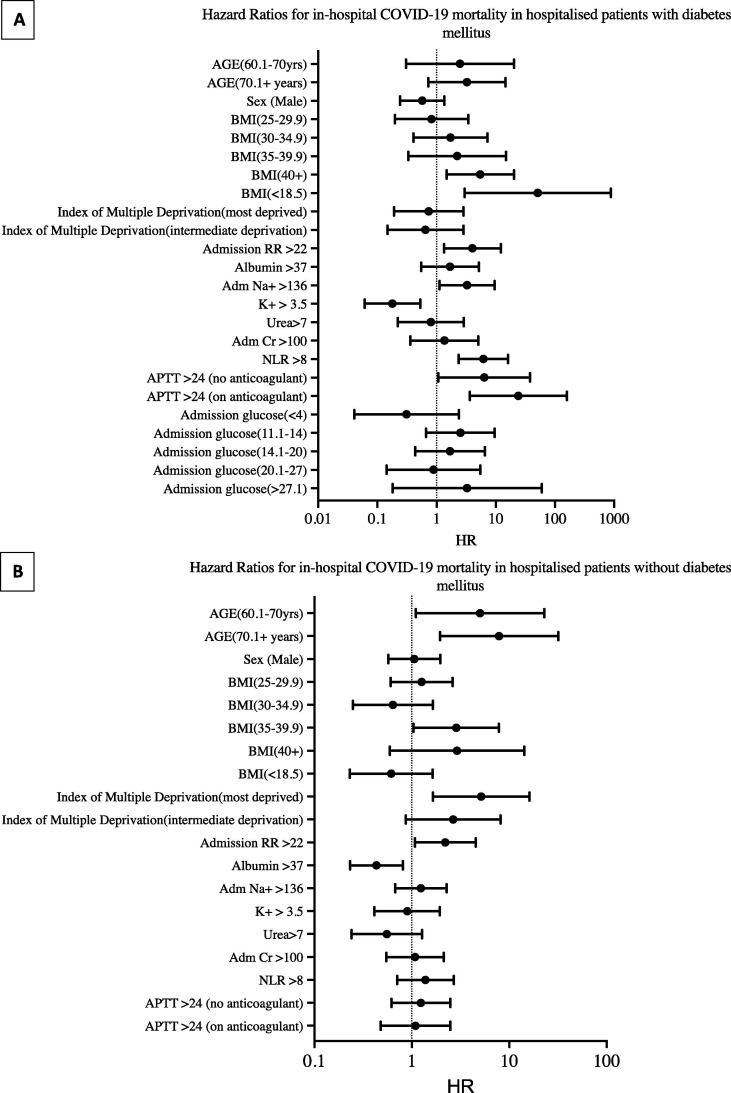
In the DM Cox proportional hazard model (Table 2 and Fig. 1 ), compared to a BMI of 18.5–24.9 kg/m2, a BMI of >40 kg/m2 (HR 5.45, 95% CI: 1.47–20.23: P = 0.01) or <18.5 kg/m2 (HR 51.21 , 95% CI: 2.97–882.31: P = 0.007), serum sodium >136 mmol/L (HR 3.27, 95% CI: 1.12–9.56: P = 0.03), a neutrophil–lymphocyte ratio > 8 (HR 6.18, 95% CI: 2.36–16.16: P < 0.001), an APPT > 24 s without anticoagulants (HR 6.38, 95% CI: 1.07–37.87: P = 0.04), and an APTT > 24 s with anticoagulant use on admission (HR 24.01, 95% CI: 3.63–159.01: P < 0.001) were associated with in-hospital COVID-19 mortality (Fig. 2 ). However, admission hypoglycaemia < 4 mmol/L (HR 0.31, 95% CI: 0.04–2.37: P = 0.26), blood glucose between 11.1 and 14 mmol/L (HR 2.52, 95% CI: 0.66–9.59: P = 0.17), blood glucose between 14.1 mmol/L and 20 mmol/L (HR 1.68, 95% CI: 0.43–6.56: P = 0.45), 20.1 and 27 mmol/L (HR 0.89, 95% CI: 0.14–5.48: P = 0.90), or >27 mmol/L (HR 3.28, 95% CI: 0.18–59.63: P = 0.42) were not associated with time to in-hospital COVID-19 death. Admission potassium levels > 3.5 mmol/L (HR 0.18, 95% CI: 0.06–0.53: P = 0.002) conferred a decreased risk of mortality in those with DM.
Table 2.
Cox proportional models* reporting hazard ratios for in-hospital COVID-19 mortality in 505 hospitalised patients (overall study population) stratified by diabetes status.
| Variable | Non-DM-COVID-19 model HR (95% CI) | P | DM-COVID-19 model HR (95% CI) | P |
|---|---|---|---|---|
| Age categories (years) | 0.01 | – | 0.28 | |
| 16–60 | Reference | – | Reference | – |
| 60.1–70 | 6.48 (1.40–29.93) | 0.02 | 2.49 (0.30–20.33) | 0.39 |
| >70 | 8.61 (2.15–34.52) | 0.002 | 3.36 (0.73–14.65) | 0.12 |
| Sex | ||||
| Female | Reference | – | Reference | – |
| Male | 1.09 (0.58–2.06) | 0.79 | 0.57 (0.24–1.36) | 0.21 |
| BMI categories (kg/m2) | 0.07 | 0.02 | ||
| 18.5–24.9 | Reference | – | Reference | – |
| 25–29.9 | 1.25 (0.60–2.61) | 0.55 | 0.82 (0.20–3.43) | 0.79 |
| 30–34.9 | 0.64 (0.23–1.79) | 0.39 | 1.71 (0.41–7.22) | 0.46 |
| 35–39.9 | 3.11 (1.12–8.60) | 0.03 | 2.23 (0.33–14.93) | 0.41 |
| > 40 | 2.88 (0.59–14.09) | 0.19 | 5.45 (1.47–20.23) | 0.01 |
| 0–18.5 | 0.62 (0.23–1.65) | 0.34 | 51.21 (2.97–882.31) | 0.007 |
| Index of multiple deprivation (centiles) | 0.01 | 0.85 | ||
| 7–10 (least deprivation) | Reference | – | Reference | – |
| 1–3 (most deprivation) | 5.40 (1.72–16.96) | 0.004 | 0.73 (0.19–2.84) | 0.65 |
| 4–6 (intermediate deprivation) | 2.91 (0.95–8.98) | 0.06 | 0.65 (0.15–2.83) | 0.56 |
| Admission respiratory rate > 22 (breaths per minutes) | 2.14 (1.02–4.48) | 0.04 | 4.03 (1.33–12.21) | 0.014 |
| Admission albumin > 37 (g/L) | 0.43 (0.23–0.80) | 0.01 | 1.69 (0.55–5.16) | 0.36 |
| Admission sodium > 136 (mmol/L) | 1.41 (0.75–2.63) | 0.28 | 3.27 (1.12–9.56) | 0.03 |
| Admission potassium > 3.5 (mmol/L) | 0.90 (0.41–1.96) | 0.79 | 0.18 (0.06–0.53) | 0.002 |
| Admission urea > 7 (mmol/L) | 0.67 (0.29–1.55) | 0.35 | 1.35 (0.36–5.06) | 0.65 |
| Admission creatinine > 100 (μmol/L) | 1.09 (0.54–2.18) | 0.81 | 0.80 (0.22–2.88) | 0.73 |
| Admission neutrophil–lymphocyte ratio > 8 | 1.36 (0.70–2.64) | 0.37 | 6.18 (2.36–16.6) | <0.001 |
| Admission APTT | 0.79 | 0.01 | ||
| Admission APTT > 24 (seconds) | 1.28 (0.64–2.58) | 0.49 | 6.38 (1.07–37.07) | 0.04 |
| Admission APTT > 24 s and on anticoagulant | 1.15 (0.50–2.63) | 0.75 | 24.01 (3.63–159.01) | <0.001 |
| Admission glucose categories mmol/L (mg/dL) | 0.54 | |||
| Glucose 4.1–11 (74–198) | Reference | Reference | ||
| Hypoglycaemia < 4 (72) | – | – | 0.31 (0.04–2.37) | 0.26 |
| Hyperglycaemia 11.1–14 (200–252) | – | – | 2.52 (0.66–9.59) | 0.17 |
| Hyperglycaemia 14.1–20 (254–360) | – | – | 1.68 (0.43–6.56) | 0.45 |
| Hyperglycaemia 20.1–27 (362–486) | – | – | 0.89 (0.14–5.48) | 0.90 |
| Hyperglycaemia > 27.1 (488) | – | – | 3.28 (0.18–59.63) | 0.42 |
| Admission HbA1c (mmol/mol) | – | – | 1.01 (0.98–1.03) | 0.52 |
Age and sex were essential values, additional covariates selected based on significance on bivariate analysis for the non-DM-COVID-19 model were: BMI, index of multiple deprivation, admission respiratory rate, and albumin. The DM-COVID-19 model included: BMI, admission respiratory rate, sodium, potassium, neutrophil–lymphocyte ratio, and admission APTT with or without use of anticoagulants. Additional variables in the DM model that were non-significant were: on HbA1c on admission, hypoglycaemia and stratified hyperglycaemia, and serum creatinine > 100 (μmol/L) on admission. Reference categories for analyses were as follows: age 16–60 years, female sex, BMI 18.5–24.9 kg/m2, and index of multiple deprivation 7–10 (least deprivation) centiles. Abbreviations: DM, diabetes mellitus: Non-DM, no diabetes mellitus; BMI, body mass index; APTT, activated partial thromboplastin time.
Fig. 1.
Forest plots showing hazard ratios for in-hospital COVID-19 mortality generated using Cox proportional hazards in those with diabetes mellitus (panel A, N = 156) and without diabetes mellitus (panel B, N = 349). See Table 2 for corresponding 95% CI and P values. Abbreviations: BMI, body mass index; RR, respiratory rate; Na+, sodium; K+, potassium; Cr, creatinine; NLR, neutrophil–lymphocyte ratio; APTT, activated partial thromboplastin time. Units: BMI (kg/m2), RR (breaths per minute), albumin (g/L), Na+ (mmol/L), K+ (mmol/L), urea (mmol/L), creatinine (μmol/L), NLR, calculated as total neutrophil count × 109/L divided by total lymphocyte count × 109/L, APTT (seconds), glucose (mmol/L).
Fig. 2.
Kaplan-Meier analyses illustrating survival in hospital inpatients with diabetes and COVID-19. Data are presented as cumulative survival on the y-axis and length of stay in days on the x-axis. A: Survival by admission APTT (seconds) with or without use of anticoagulants. B: Survival by admission neutrophil–lymphocyte ratio (calculated as total neutrophil count × 109/L divided by total lymphocyte count × 109/L). Abbreviations: NLR, neutrophil–lymphocyte ratio; APTT, activated partial thromboplastin time.
In the non-DM Cox proportional hazard model (Table 2 and Fig. 1), compared to a BMI of 18.5–24.9 kg/m2, a BMI of 35–39.9 kg/m2 (HR 3.11, 95% CI: 1.12–8.60: P = 0.03) was associated with higher in-hospital mortality. Compared to an age between 16 and 60 years, age between 60.1 and 70 years (HR 6.48, 95% CI: 1.40–29.93: P = 0.02), and age > 70.1 years (HR 8.61, 95% CI 2.15–34.52: P = 0.002) was associated with in-hospital COVID-19 mortality. The lowest index of multiple deprivation (most deprivation centiles 1–3) predicted poorer survival than the highest indices (least deprivation centiles 7–10) in the non-DM group (HR 5.40, 95% CI 1.72–16.96: P = 0.004). Admission albumin levels > 37 g/L (HR 0.43, 95% CI: 0.23–0.80: P = 0.01) were associated with reduced in-hospital COVID-19 mortality in the non-DM group (Table 2). The only common predictive factor between the diabetes and non-diabetes groups was the admission respiratory rate. An admission respiratory rate > 22 breaths per minutes (HR 4.03, 95% CI: 1.33–12.21: P = 0.014) predicted mortality in the DM group and the non-DM group (HR 2.14, 95% CI: 1.02–4.48: P = 0.04)
4. Discussion
In this exploratory, retrospective, observational study of 505 hospital inpatients with or without diabetes admitted with COVID-19 in the U.K., we aimed to compare between groups: (1) baseline clinical features; (2) the proportion of people that died in-hospital with COVID-19; and (3) analyze admission laboratory characteristics associated with in-hospital COVID-19 mortality.
We present the novel findings that in those with diabetes, an elevated APTT with or without use of anticoagulants, elevated admission neutrophil–lymphocyte ratio, and admission serum sodium predicted in-hospital COVID-19 mortality. We also found that on admission those with diabetes compared to those without were older, had a higher BMI, were more moderately frail, and had a higher burden of cardiovascular comorbidities, and chronic kidney disease. A higher proportion of patients with diabetes died in-hospital from COVID-19. However, there was no significant difference in rates of ICU admission and need for invasive ventilation between groups. In those with and without diabetes, an elevated admission respiratory rate was associated with COVID-19 deaths.
To our knowledge, we report for the first time an association between an elevated admission APTT (>24 s with or without anticoagulants) and neutrophil–lymphocyte ratio (>8) with increased in-hospital COVID-19 mortality in those with diabetes but not in those without diabetes. A high neutrophil–lymphocyte ratio reflects elevated neutrophils and relative lymphopenia and has been associated with COVID-19 severity and death [4], [19], [22]. Diabetes is associated with a dysregulated immune status [23] and our findings are mechanistically consistent with downstream inflammation driving multi-organ failure and death. COVID-19 is associated with increased thromboembolic risk and disseminated intravascular coagulation is a recognised lethal sequela [20], [24]. Diabetes in the absence of COVID-19 is a prothrombotic state [25]. Our findings of an elevated admission APTT increasing COVID-19 mortality in those with diabetes may represent an aggregated increased risk of death from subsequent intra-vessel coagulation across tissue beds with consumption of clotting factors. It is intriguing, however, that the association between an elevated APTT and in-hospital COVID-19 mortality was also observed in those in the diabetes group that were established on anticoagulants. This is counterintuitive as one would hypothesize that those established on anticoagulants at admission would be protected from COVID-19 related thromboembolic events. Indeed, in observational cohorts, anticoagulant therapy has reduced mortality in patients hospitalized with COVID-19 [26], [27]. Admission anticoagulant use may be a marker for cardiovascular and cerebrovascular comorbidities that increase susceptibility to adverse in-hospital outcomes from COVID-19 in patients with diabetes. However, a direct adverse causal effect from admission anticoagulants on COVID-19 mortality cannot be excluded. It is also notable that at the time of writing, the Antithrombotics Inpatient and Antithrombotic Therapy to Ameliorate Complications of COVID-19 (ATTACC) trial has paused randomization to therapeutic anticoagulation among critically ill patients with COVID-19 [28]. This was on the recommendation of the Data Safety and Monitoring Board recognizing potential for harm with full dose anticoagulation in this group.
Our finding of an admission serum sodium > 136 mmol/L predicting mortality in diabetes is of note. Hypernatremic dehydration has been noted in COVID-19 and is thought to be secondary to fever and insensible water losses [29]. In those with diabetes, admission hyperglycaemia resulting in osmotic diuresis, and a low renal physiological reserve due to a higher prevalence of chronic kidney disease, may have increased vulnerability. We demonstrate that at admission tachypnea (respiratory rate > 22 breaths per minute) is associated with in-hospital COVID-19 mortality in those with and without diabetes. We therefore extend previous findings from the CORONADO study [10] by demonstrating an association between admission tachypnea and in-hospital COVID-19 specific mortality as a distinct end-point in those with or without diabetes over the full length of hospital stay.
A U shaped relationship between BMI and COVID-19 mortality in diabetes has been well established at a population level in England [11]. This was also observed in our diabetes cohort, where increased mortality was seen at a very low BMI (<18.5 kg/m2) and at the highest BMI (>40 kg/ m2). This suggests that frailty and susceptibility to COVID-19 is seen at the extremes of weight in those with diabetes. In the non-DM cohort, there was no significant overall effect of BMI.
One notable finding was a lack of association between admission hyperglycaemia (>11 mmol/L or higher) and in-hospital COVID-19 mortality in the diabetes group as seen in published cohort studies [12], [13]. However, in both studies, patients did not have a confirmed previous diagnosis of diabetes and hyperglycaemia was somewhat arbitrarily defined as a plasma glucose ≥ 7 mmol/L. The most likely explanation for our findings is that there were very small numbers of individuals who presented to the hospital whose first recorded blood glucose was out of range. Another possible explanation for our findings is that those with higher levels of admission hyperglycaemia may have been treated more intensively including use of intravenous insulin which has strong anti-inflammatory properties [30]. Optimal treatment of admission hyperglycaemia (>7.77 mmol/L) with insulin infusion in COVID-19 with or without diabetes has been reported to reduce IL-6 and D-dimer levels as well as reducing overall risk of severe disease (ICU admission, mechanical ventilation, death) [14].
We confirm that patients with diabetes admitted with COVID-19 are older, have a higher BMI compared to those without diabetes, are more likely to be male, and the vast majority (91.7%) have type 2 diabetes [6], [10], [12], [31]. In keeping with a comparably sized (178 patients with diabetes and 272 without) cohort of patients admitted with COVID-19 to a single-centre in Boston [6] and a multicentre French cohort (1317 patients) in the CORONADO study [10], we report a higher prevalence of ischaemic heart disease, chronic kidney disease, hypertension, and heart failure in those with diabetes. Further, we demonstrate for the first time, using a validated Clinical Frailty Score [32], that patients with diabetes and COVID-19 are more moderately frail at admission compared to those without. This may have important implications for COVID-19 severity and medical decision making including escalation to ICU in this cohort, as a recent observational study of 1564 patients from the U.K. has demonstrated that COVID-19 outcomes are better predicted by frailty than either age or comorbidities [33].
In line with original diabetes cohorts from Wuhan, China [31], [34], our data show higher total admission white cell and neutrophil counts in those with diabetes and COVID-19 compared to those without. However, admission lymphocyte counts were not significantly different between groups as previously noted in an Italian population [12]. Patients with diabetes also had a higher admission CRP compared to those without as reported from Italy [12] and China, in those with and without diabetes and COVID-19 [35]. These findings are noteworthy as higher admission leukocytes and CRP have been associated with COVID-19 severity and need for critical care [10], [22], [36]. We report that patients with diabetes compared to those without had significantly elevated prothrombin time (PT) on admission as previously shown [34] but extend previous data by demonstrating a significantly elevated APTT in our diabetes cohort. Prolonged PT and APPT have both been associated with COVID-19 mortality [20].
We found that compared to patients without diabetes, a significantly higher proportion of patients with diabetes died from COVID-19 in-hospital. This is consistent with a rich body of literature demonstrating an association between diabetes and increased COVID-19 mortality [5], [37], [38], [39]. However, we did not find a statistically significant difference between the number of patients with diabetes admitted to ICU or mechanically ventilated compared to those without diabetes, as has been reported previously in cohorts from China [40], the U.S [6] and meta-analyses [39], [41]. One possible explanation for our findings is that only 34 out of 505 (6.7%) of patients in our study were admitted to ICU, of which only 12 (2.3%) were mechanically ventilated, whereas in other cohorts from the U.S and France mechanical ventilation rates were 20–30% [6], [10]. These relatively small numbers may mean that we were statistically underpowered to detect between group differences in ICU admission and mechanical ventilation. In addition, differing ICU capacities and more frailty as evidenced by a higher burden of comorbidities in our diabetes group compared to the U.S and French cohorts may have also resulted in reduced ICU admissions and mechanical ventilation.
One strength of our study is that, to our knowledge, it is the first to comprehensively assess in two major U.K. University hospitals the relationship between admission clinical characteristics and in-hospital COVID-19 specific mortality in a cohort of patients with and without diabetes. In addition, we did not limit our cohort to those only in critical care and we examined survival over the entire inpatient admission. However, this study has some limitations. First, the retrospective observational design means that we have identified potentially important associations that can be viewed as being suggestive and useful for hypothesis generation but not causal. Second, our data are from a single city in the U.K. with a predominantly white population. However, the effects of non-white ethnicity with diabetes increasing COVID-19 mortality in England have already been reported at a population level [11]. Third, whilst we adopted a standardized approach to data extraction from electronic records and paper notes to ensure validity, there were, however, missing data not recorded or measured at source. Nonetheless, we had data on > 80% of all variables included in the final models. Finally, our new findings need confirmation in large studies across multiple sites. Our study was exploratory and numbers in both the diabetes and non-diabetes groups were constrained by hospital admissions in the first COVID-19 wave. Further, the number of COVID-19 deaths in both groups were low for some variable categories including very high or low BMI giving wide confidence intervals that require cautious interpretation.
In conclusion, we identified novel indicators of poor prognosis that were unique to patients with diabetes: (1) an APTT on admission>24 s with or without use of anticoagulants increased the risk of death from COVID-19 between 6 and 24 times; (2) a neutrophil–lymphocyte ratio greater than 8 on admission increased the risk of death from COVID-19 by 6 times; and (3) sodium greater than 136 mmol/L on admission increased the risk of death from COVID-19 by 3 times. Future mechanistic work is needed to dissect if these variables are diabetes specific risk factors for COVID-19 mortality as opposed to risk markers. In addition, these variables need to be evaluated in large, multicentre, prospective studies and if confirmed could serve as important components of risk stratification models allowing COVID-19 prognostication at hospital admission in those with diabetes.
Author contribution
A.I., M.F.A., M.G., and J.E. co-conceived the study. A.I. and M.F.A. developed the protocol and study data collection tools with contributions from M.G. and J.E. Data collection was performed by A.I., M.F.A., T.H.J., S.E.T., and J.E. Analysis was performed by M.G. The first draft of the manuscript was prepared by A.I. with critical input on multiple versions of the manuscript from all authors. T.H.J. and M.G. provided specific input on literature review and methodological sections respectively. All authors approved the final submitted version. J.E. is the guarantor of this work, and as such had full access to all study data and thus takes responsibility for data integrity and accuracy of data analysis.
Funding
No specific funding support was required for this study. A.I. is supported by a National Institute for Health Research (NIHR) Academic Clinical Lectureship.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.diabres.2021.108955.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Government U. Coronavirus (COVID-19) in the UK; 2020.
- 2.UK D. Diabetes Prevalence; 2019.
- 3.Digital N. National Diabetes Inpatient Audit England; 2019.
- 4.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barron E., Bakhai C., Kar P., Weaver A., Bradley D., Ismail H., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8(10):813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seiglie J., Platt J., Cromer S.J., Bunda B., Foulkes A.S., Bassett I.V., et al. Diabetes as a risk factor for poor early outcomes in patients hospitalized with COVID-19. Diabetes Care. 2020;43(12):2938–2944. doi: 10.2337/dc20-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li S., Wang J., Zhang B., Li X., Liu Y. Diabetes mellitus and cause-specific mortality: a population-based study. Diabetes Metabol J. 2019;43(3):319. doi: 10.4093/dmj.2018.0060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang J.K., Feng Y., Yuan M.Y., Yuan S.Y., Fu H.J., Wu B.Y., et al. Plasma glucose levels and diabetes are independent predictors for mortality and morbidity in patients with SARS. Diabetic Med: J Brit Diabetic Assoc. 2006;23(6):623–628. doi: 10.1111/j.1464-5491.2006.01861.x. [DOI] [PubMed] [Google Scholar]
- 9.Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med 2011;364:829–41. [DOI] [PMC free article] [PubMed]
- 10.Cariou B., Hadjadj S., Wargny M., Pichelin M., Al-Salameh A., Allix I., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63(8):1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holman N., Knighton P., Kar P., O'Keefe J., Curley M., Weaver A., et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8(10):823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppelli A., Giannarelli R., Aragona M., Penno G., Falcone M., Tiseo G., et al. Hyperglycemia at hospital admission is associated with severity of the prognosis in patients hospitalized for COVID-19: the Pisa COVID-19 study. Diabetes Care. 2020;43(10):2345–2348. doi: 10.2337/dc20-1380. [DOI] [PubMed] [Google Scholar]
- 13.Wang S., Ma P., Zhang S., Song S., Wang Z., Ma Y., et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63(10):2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sardu C., D’Onofrio N., Balestrieri M.L., Barbieri M., Rizzo M.R., Messina V., et al. Outcomes in patients with hyperglycemia affected by COVID-19: can we do more on glycemic control? Diabetes Care. 2020;43(7):1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knight S.R., Ho A., Pius R., Buchan I., Carson G., Drake T.M., et al. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ (Clin Res Ed) 2020;370 doi: 10.1136/bmj.m3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Yang Y., Zhang C., Huang F., Wang F., Yuan J., et al. Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci. 2020;63(3):364–374. doi: 10.1007/s11427-020-1643-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasomsong W., Ungthammakhun C., Phiboonbanakit D., Prapaso S., Luvira V., Dhitiwat C. Low serum potassium among patients with COVID-19 in Bangkok, Thailand: Coincidence or clinically relevant? Trop Doct. 2021;51(2):212–215. doi: 10.1177/0049475520978174. [DOI] [PubMed] [Google Scholar]
- 18.Ruiz-Sánchez JG, Núñez-Gil IJ, Cuesta M, Rubio MA, Maroun-Eid C, Arroyo-Espliguero R, et al. Prognostic impact of hyponatremia and hypernatremia in COVID-19 pneumonia. A HOPE-COVID-19 (Health Outcome Predictive Evaluation for COVID-19) Registry Analysis. Front Endocrinol (Lausanne). 2020;11:599255. [DOI] [PMC free article] [PubMed]
- 19.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med (CCLM) 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 20.Tang N., Li D., Wang X., Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thrombosis Haemostasis: JTH. 2020;18(4):844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Housing CaLGEIoD; 2019.
- 22.Petrilli C.M., Jones S.A., Yang J., Rajagopalan H., O'Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ (Clin Res Ed) 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drucker D.J. Coronavirus infections and type 2 diabetes-shared pathways with therapeutic implications. Endocrine Rev. 2020;41 doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung W.-S., Lin C.-L., Kao C.-H. Diabetes increases the risk of deep-vein thrombosis and pulmonary embolism. A population-based cohort study. Thromb Haemost. 2015;114(10):812–818. doi: 10.1160/TH14-10-0868. [DOI] [PubMed] [Google Scholar]
- 26.Tang N., Bai H., Chen X., Gong J., Li D., Sun Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thrombosis Haemostasis: JTH. 2020;18(5):1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nadkarni G.N., Lala A., Bagiella E., Chang H.L., Moreno P.R., Pujadas E., et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol. 2020;76(16):1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Research NIfH. Randomised, embedded, multi-factorial, adaptive platform trial for community-acquired pneumonia; 2020.
- 29.Christ-Crain M., Hoorn E.J., Sherlock M., Thompson C.J., Wass J.A.H. Endocrinology in the time of COVID-19: management of diabetes insipidus and hyponatraemia. Eur J Endocrinol. 2020;183:G9. doi: 10.1530/EJE-20-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dandona P., Ghanim H., Bandyopadhyay A., Korzeniewski K., Ling Sia C., Dhindsa S., et al. Insulin suppresses endotoxin-induced oxidative, nitrosative, and inflammatory stress in humans. Diabetes Care. 2010;33:2416–2423. doi: 10.2337/dc10-0929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen Y., Yang D., Cheng B., Chen J., Peng A., Yang C., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43(7):1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 32.Rockwood K., Song X., MacKnight C., Bergman H., Hogan D.B., McDowell I., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hewitt J., Carter B., Vilches-Moraga A., Quinn T.J., Braude P., Verduri A., et al. The effect of frailty on survival in patients with COVID-19 (COPE): a multicentre, European, observational cohort study. Lancet Public Health. 2020;5:e444–e451. doi: 10.1016/S2468-2667(20)30146-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan Y., Yang Y., Wang F., Ren H., Zhang S., Shi X., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res Care. 2020;8(1):e001343. doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu L., She Z.-G., Cheng X.u., Qin J.-J., Zhang X.-J., Cai J., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31(6):1068–1077.e3. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang J.-J., Dong X., Cao Y.-Y., Yuan Y.-D., Yang Y.-B., Yan Y.-Q., et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy. 2020;75(7):1730–1741. doi: 10.1111/all.14238. [DOI] [PubMed] [Google Scholar]
- 37.Kumar A., Arora A., Sharma P., Anikhindi S.A., Bansal N., Singla V., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab Syndr. 2020;14(4):535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fadini G.P., Morieri M.L., Longato E., Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest. 2020;43(6):867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roncon L., Zuin M., Rigatelli G., Zuliani G. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J Clin Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guan W.-J., Liang W.-H., Zhao Y.i., Liang H.-R., Chen Z.-S., Li Y.-M., et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55(5):2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li B.o., Yang J., Zhao F., Zhi L., Wang X., Liu L., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin Res Cardiol. 2020;109(5):531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.