Abstract
Background
To date, there is no effective treatment for the new coronavirus disease (COVID-19). We aimed to systematically review the literature on the association between the combination of tocilizumab (TCZ) and systemic corticosteroid therapy (SCT) on outcomes of COVID-19 patients.
Methods
We searched MEDLINE, Cochrane Central, and preprints, for studies in which health outcomes were compared between adults with severe COVID-19 who received TCZ and SCT and those who received standard of care without TCZ. Record screening, data extraction, and risk of bias assessment were performed in duplicate. Random effect models were used when pooling crude numbers and adjusted effect estimates of study outcomes.
Results
Our search identified seventeen studies. The pooled crude mortality rate was lower in the combination arm (relative risk, RR=0.62, 95% confidence interval [CI]=0.42 – 0.91; I2=60%). The adjusted mortality rates were also lower in the combination arm (RR=0.58, 95% CI=0.42 – 0.81; I2=71%). The rate of superinfections did not differ between the two interventions.
Conclusions
The findings of this study show that combination of TCZ and SCT compared to SOC has lower mortality rates. There is an urgent need for well-designed randomized trials to assess the safety and efficacy of this combination in subjects with severe COVID-19.
Keywords: COVID-19, tocilizumab, systemic corticosteroid therapy, coronavirus, severe COVID-19
Introduction
A severe pneumonia-associated respiratory syndrome caused by a new strain coronavirus was identified in December 2019 (COVID-19) and found to be caused by a novel enveloped beta-corona virus (SARS-CoV-2) (Fu et al., 2020; Guan et al., 2020). The disease severity ranged from asymptomatic to severe (Mehta et al., 2020). Presently, there are no effective therapies for COVID-19 supported by high-level evidence (Hossen et al., 2020; Sanders et al., 2020). Current treatment options include 1) RNA-dependent RNA polymerase inhibitors (e.g., remdesivir), 2) protease inhibitors (e.g., lopinavir/ritonavir), 3) blockade of virus-cell membrane fusion with a recombinant human angiotensin-converting enzyme (e.g., ivermectin), and 4) the modulation of the human immune system (e.g., interleukin-6 blockers), 5) Janus kinase inhibitor (e.g., ruxitinib), 6) anti-tumour necrosis factor, 7) convalescent plasma, and finally 8) corticosteroids (Hossen et al., 2020; Sanders et al., 2020).
One of the promising options for severe COVID-19 is tocilizumab (TCZ), which is currently approved in to treat adults with moderately to severely active rheumatoid arthritis who have not responded to or tolerated previous therapy (Burmester et al., 2017; Mehta et al., 2020). It is also proven to be effective in cytokine release syndrome (CRS) (Burmester et al., 2017; Mehta et al., 2020). An IgG1 subclass of humanized monoclonal antibody (mAb), TCZ acts by blocking IL-6 from binding to the membrane-bound and soluble IL-6R. This action results in inhibiting IL-6 activity (Burmester et al., 2017; Yazici et al., 2012). Patients with severe COVID-19 infection may present with CRS, causing hypotension, fever, and high levels of C-reactive protein (CRP) and abnormal coagulation parameters (Shimabukuro-Vornhagen et al., 2018; Xiong et al., 2020). That in some cases may develop to uncontrolled inflammatory response causing shock and multiorgan system failure (Shimabukuro-Vornhagen et al., 2018). Core cytokines founded to be elevated in the serum of patients with CRS were IL-6, IL-10 and IFN-γ (Shimabukuro-Vornhagen et al., 2018). It has been noticed that SARS-CoV-2 infection is associated with a noticeable elevation in IL-6 (Xiong et al., 2020). Therefore, targeting IL-6 is of crucial role in the management of COVID-19 related CRS and organ failure. As an anti-IL-6 agent, TCZ is used to improve symptoms of severe CRS caused by chimeric antigen receptor (CAR) T cells (Brudno and Kochenderfer, 2016). Since changes in inflammatory cytokines were related to the severity of COVID-19, suppression of the cytokine storm is rationally acceptable (Choy et al., 2020).
In several studies of severe COVID-19, TCZ was used for the management of elevated IL-6 level and CRS (Alattar et al., 2020; Klopfenstein et al., 2020; Luo et al., 2020; Sise et al., 2020). These observational studies generally show a favorable effect of TCZ in reduction of CRP and IL-6 (Alattar et al., 2020; Luo et al., 2020). A retrospective analysis of patients with severe COVID‐19 who received TCZ revealed a rapid decline in oral temperature and CRP levels (Alattar et al., 2020). Besides, TCZ use was related to the dramatic reduction in inflammatory markers, radiological improvement and reduced ventilator support requirements (Alattar et al., 2020). The use of systemic corticosteroid therapy (SCT) in the treatment of infectious diseases has long been controversial. Corticosteroids have received worldwide attention as a potentially effective treatment for COVID-19 infection (Sanders et al., 2020). Throughout the pandemic, SCT has been used in both non-severe and severe COVID-19 patients. The use of SCT has prompted several studies to either corroborate or refute positive findings (RECOVERY Collaborative Group, 2020).
There are currently a few cohort studies that assessed the effectiveness and potential adverse effects of combining TCZ and STC. To address the current gaps in our knowledge in the management of COVID-19, we aimed to meta-analyze the results of observational studies to test the hypothesis that TCZ combined with STC is associated with improved survival and lower rates of intubation in severs COVID-19 pneumonia. Meanwhile, we aimed to explore potential sources of between-studies heterogeneity by performing subgroup and sensitivity analyses.
Methods
Search strategy and study selection
This systematic review and meta-analysis adhered to the PRISMA guidelines (Liberati et al., 2009). This study was registered in PROSPERO 2020 CRD42020216024. MEDLINE and Cochrane CENTRAL were searched using the Ovid platform from inception to 18th May 2021. We used no restrictions by language or study design. A search strategy was developed with the combination of terms and keywords related to the disease of interest (e.g., COVID*) and the interventions of interest (e.g., tocilizumab, and corticosteroids*). We also searched for unpublished manuscripts using the medRxiv services operated by Cold Spring Harbor Laboratory and Research Square preprints. The full search strategy is provided in the Supplementary material.
Two investigators (BA and SA) independently screened all citations by title and abstract using Abstrackr (Wallace et al., 2012), and a third investigator (HA) resolved any disagreements. Full texts of these studies were retrieved, and two investigators (BA and SA) independently screened them for inclusion. A third investigator (HA) resolved any disagreements. Here, the disagreement was almost entirely due to oversight, not a difference in opinion. Two reviewers (BA and SA) manually screened the bibliographies of included articles to identify additional eligible studies.
We followed a priori study eligibility criteria for study selection. We included observational studies that compared the combination of TCZ and SCT with no TCZ treatment for the management of SARS-CoV-2. We also included subgroup analyses from randomized controlled trials (RCTs), and they were treated as observational in nature, since trials were not randomized based on subgroups. Studies were included regardless of dose, duration, and type of steroid agent used. We determined a priori to report on the following outcomes because they were judged to be essential or critical for decision making, including death, intubation, and superinfection. The primary outcome, which is death, was defined as hospital mortality or all-cause mortality, as reported in the included studies. The secondary outcome was a composite of intubation, or death, and superinfection. For studies that did not report a composite of intubation and death as one of their outcomes, we considered the mortality outcome to be the composite.
Data extraction and quality assessment
Two investigators (BA and SA) extracted data independently from the included studies. A third investigator (HA) was consulted to resolve any discrepancies. The information was recorded using a standardized data collection form for study characteristics and outcome findings. We extracted information about the study region, design, sample size, inclusion criteria, disease severity, intervention (including drug type, dose, duration of use, start time, and cointerventions), study funding, and outcomes.
Two independent investigators (KA and AA) used the Newcastle-Ottawa Scale as a tool to assess the quality of non-randomized studies (Wells et al., 2012). For any conflicts, a third investigator (HA) was consulted. The scale awards a maximum of nine stars to each study, four for selection of participants and measurement of exposure, two for comparability of cohorts based on the design or analysis, and three for assessment of outcomes and adequacy of follow-up. Studies assigned zero starts for at least four elements in the scale were of low quality.
To assess the quality of the RCTs included we used the Cochrane Risk of Bias tool–version 2 (RoB 2) (Higgins et al., 2019). This was done by two independent investigators. Bias was assessed with the following domains: bias arising from the randomization process, bias due to deviations from the intended interventions, bias due to missing outcome data, bias in measurement of outcomes, and bias arising from selective reporting of results. The overall risk of bias for each trial was categorized as low if the risk of bias was low in all domains, medium if the risk of bias was unclear in at least one domain, or high if the risk of bias was high in at least one domain per the risk of bias tool. Any disagreements were resolved disagreements by discussion and consensus.
Data synthesis
Studies that reported on at least one of the study outcomes were included in the meta-analysis. We performed meta-analyses of associations by either using crude numbers or adjusted analysis depending on the availability of these data, using DerSimonian and Laird random-effects models (DerSimonian and Laird, 1986). For crude analysis, we pooled the data by using relative risk or risk ratios (RRs) when the number of events was available. The analysis was first performed by combining only observational studies, and then repeated by adding the subgroup analyses from RCTs. Since the trials did not randomize subjects based on TCZ and SCT combination, this intervention was rather reported as subgroup only, these analyses were considered observational in nature. Analyses were performed separately for studies that provided adjusted as opposed to crude numbers. We used adjusted hazard ratios (HRs), and odds ratios (ORs), and their 95% confidence intervals, of associations, reported. However, we first converted the ORs to RRs before combining the calculated RRs and HRs using the method of Zhang and Yu (Zhang and Yu, 1998). We included the most adjusted HRs or ORs reported; if only unadjusted ORs were reported, we included them as well. All analyses were conducted using R statistical programming version 4.0.3.
Heterogeneity was assessed using the chi-square test and quantified by using the I2 statistic. If results would have shown substantial heterogeneity (I2 > 75%), we planned to explore it using subgroup analysis of the following variables: type of SCT used, the dose of SCT, the dose of TCZ, and severity of the disease. However, the available data did not permit such analyses to be performed.
Sensitivity analysis was conducted for all outcomes using the leave-one-out analysis. We also repeated the analysis by including only studies that used SCT as a comparison arm instead of the standard of care (SOC). Also, sensitivity analyses were performed by excluding low-quality studies and unpublished data (i.e., available on MedRxiv only). For analyses using adjusted ORs or HRs, we repeated the analysis separately for studies reporting ORs and for those using HRs. For the treatment failure outcome (composite of intubation or death), we repeated the analysis by restricting the definition of the outcome to those studies which report a composite outcome of either intubation or death.
Results
Study characteristics and quality assessment
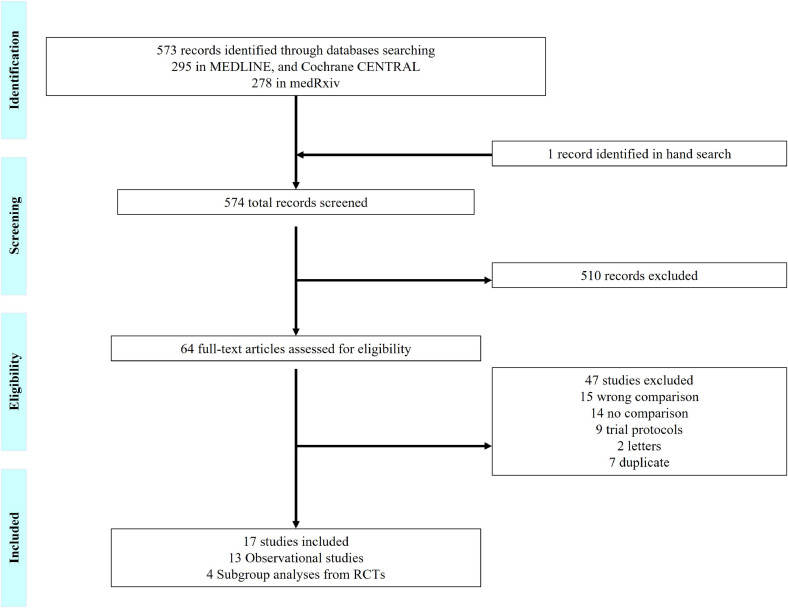
A total of 574 articles were identified and screened; one additional study was identified when hand-searching relevant review articles. In total, seventeen studies met our inclusion criteria (figure 1 ) (Aomar-Millán et al., 2021; Callejas Rubio et al., 2020; Colaneri et al 2020; Hermine et al., 2020 [CORIMUNO-TOCI 1 Trial]; Rosas et al., 2021 [COVACTA Trial]; Salama et al., 2021 [EMPACTA Trial]; Giacobbe et al., 2020; González-Castro et al., 2021; López-Medrano et al., 2021; Mahale et al., 2020; Mikulska et al., 2020; Narain et al., 2021; Ramiro et al., 2020; Horby and Lim, 2021, RECOVERY Collaborative Group 2021 [RECOVERY Trial]; Rodríguez-Baño et al., 2021; Ruiz-Antorán et al., 2021; Van den Eynde et al., 2021). Their sample size ranged between 92 and 5,776 participants, and thirteen of them were observational studies, while four were subgroup analyses from RCTs. One study, however, did not state the design clearly and was assumed to be a cohort investigation. All studies reported on the combination of TCZ and SCT as the intervention arm. There were variations in the definition of the SOC (i.e., controlled) arm across studies, with most having intravenous (IV) corticosteroids as the comparison arm, while few used SOC without SCT (Table 1 ). Participants mean age (in years) ranged from 56 to 74 in the combination arm and from 56 to 78 in the control arm. The most common comorbidity was hypertension, which was reported in only twelve studies, followed by diabetes mellitus and cardiovascular disease. Characteristics of study subjects are summarized in Table 2 .
Figure 1.
Study Selection
RCT: randomized controlled trials.
Table 1.
Characteristics of included studies
| Authors | Region | Study design | N | Severity | TCZ and SCT | No TCZ | Risk of Bias⁎⁎ |
|---|---|---|---|---|---|---|---|
| Aomar-Millán et al | Spain | Retrospective cohort study | 143 | Severe COVID-19 pneumonia and hyperinflammation | TCZ and Methylprednisolone | Methylprednisolone | Low |
| Callejas Rubio et al | Spain | Retrospective observational study | 92 | NR | TCZ and SCT | SCT pulses alone | High |
| Colaneri et al | Italy | Retrospective cohort study | 112 | Hospitalized COVID-19 pneumonia | TCZ and Methylprednisolone | Methylprednisolone | Low |
| CORIMUNO-TOCI 1 Trial | France | Open label, randomized clinical trial | 131 | Moderate or severe pneumonia | TCZ and SCT | Usual care and SCT | Low |
| COVACTA Trial | Europe and North America | Randomized, double-blind, placebo-controlled, phase 3 trial | 438 | Hospitalized with severe COVID-19 pneumonia | TCZ and SCT | SCT | Low |
| EMPACTA Trial | Global | Randomized, double-blind, placebo-controlled, phase 3 trial | 389 | Hospitalized with COVID-19 pneumonia | TCZ and SCT | SCT | Low |
| Giacobbe et al | Italy | Retrospective observational study | 78 | Admitted to intensive care units | TCZ and Methylprednisolone | Methylprednisolone alone | Low |
| González-Castro et al | Spain | Retrospective study | 208 | Severe COVID-19 disease | TCZ and SCT | SCT | Low |
| López-Medrano et al | Spain | Retrospective cohort study | 275 | Severe COVID- 19 pneumonia | TCZ and SCT | SCT alone | Low |
| Mahale et al | India | Retrospective cohort study | 134 | Hypoxic COVID-19 patients | TCZ and Methylprednisolone | Methylprednisolone | Low |
| Mikulska et al | Italy | Observational study | 215 | Severe COVID-19 pneumonia and systemic inflammation | TCZ and Methylprednisolone | Methylprednisolone alone | Low |
| Narain et al | United States | Retrospective observational study | 5,776 | COVID-19 cytokine storm | TCZ and SCT | SCT/SOC | Low |
| Ramiro et al | The Netherlands | Cohort study* | 172 | COVID-19 associated cytokine storm syndrome | TCZ and SCT | SOC (neither including SCT nor TCZ) | Low |
| RECOVERY Trial | United Kingdom | Randomized, controlled, open-label, trial | 4116 | Severe COVID-19 | TCZ and SCT | Usual care and corticosteroids | Low |
| Rodríguez-Baño et al | Spain | Retrospective cohort study | 1014 | NR | TCZ and SCT | No treatment | Low |
| Ruiz-Antorán et al | Spain | Retrospective, cohort study | 506 | Severe COVID-19 pneumonia | TCZ and SCT | SCT | Low |
| Van den Eynde et al | Spain | Single-center retrospective study | 255 | Severe COVID-19 pneumonia | TCZ and SCT | SOC (non-immunomodulatory) | Low |
COVID-19: coronavirus disease 2019; NR: not reported; SCT: systematic corticosteroids; SOC: stander of care; TCZ: tocilizumab.
: Not clearly stated.
: Using Newcastle Ottawa scale for observational studies and Cochrane Risk of Bias tool for randomized controlled trials.
Table 2.
Demographic characteristics of patients in the included studies
| Authors | Age (years) | Males % | CRP (mg/L) | Hypertension % | Cardiovascular disease % | Diabetes mellitus % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TCZ and SCT | No TCZ | TCZ and SCT | No TCZ | TCZ and SCT | No TCZ | TCZ and SCT | No TCZ | TCZ and SCT | No TCZ | TCZ and SCT | No TCZ | |
| Aomar‑Millán et al | 62.17 | 68.18 | 67.8% | 52.7% | 136.69 | 99.38 | 54.2% | 50% | 6.8% | 5.4% | 20.3% | 16.2% |
| Callejas Rubio et al | 63.9 | 63% | NR | |||||||||
| Colaneri et al | 62.33* | 63.7* | 90% | 69% | 21.38* | 14.88* | 38% | 22% | 9.5% | 7.7% | 9.5% | 8.8% |
| CORIMUNO-TOCI 1 Trial | 64* | 63.3* | 70% | 66% | 119.5* | 127* | NR | 33% | 30% | 33% | 34% | |
| COVACTA Trial | 60.9 | 60.6 | 69.7% | 70.1% | 168.4 | 172.6 | 60.5% | 65.3% | 29.9% | 24.3% | 35.7% | 43.1% |
| EMPACTA Trial | 56 | 55.6 | 60.2% | 57% | 124.5* | 143.4* | 47.6% | 49.6% | NR | 42% | 37.8% | |
| Giacobbe et al | 66* | 77% | 43.7* | 105* | 45% | NR | 18% | |||||
| González-Castro et al | 67 | 65 | 77% | 86% | 22 | 29 | 55% | 57% | NR | 28% | 22% | |
| López-Medrano et al | 74.4 | 78.4 | 56.2% | 56.4% | 148 | 159 | NR | 7.5% | 24.9% | 27.5% | 24.3% | |
| Mahale et al | 55.6* | 67.9% | 118* | 46% | 19% | 44% | ||||||
| Mikulska et al | 61 | 67.5 | 62.5% | 71.1% | 88* | 82* | NR | |||||
| Narain et al | 65* | 65/67* | 72.9% | 61.5/64.6% | > 25* | 49.3% | 47/49% | 13% | 12.8/13% | 33.9% | 31.9/33% | |
| Ramiro et al | 67 | 67 | 79% | 79% | 160 | 167 | 22% | 31% | 20% | 13% | 11% | 27% |
| RECOVERY Trial | 63.3 | 63.9 | 66% | 69% | 143 | 144 | NR | 22% | 24% | 28% | 29% | |
| Rodríguez-Baño et al | 65* | 69* | 71.9% | 69% | 112 | 112 | 48.3% | 50.9% | 11.3% | 18% | 17.2% | 20.9% |
| Ruiz-Antorán et al | 65 | 71.3 | 68.7% | 58.8% | 149.5 | 148 | 48.5% | 60.9% | 24.3% | 31.1% | 29.1% | 28.6% |
| Van den Eynde et al | 73.3* | 73.7* | 66.7% | 65.3% | 71.5* | 132.4* | 60.3% | 68.6% | 29.5% | 40.7% | 29.5% | 37.3% |
CRP: C-reactive protein; NR: not reported; SCT: systematic corticosteroids; TCZ: tocilizumab.
: Median.
The use of SCT had been mostly given as either methylprednisolone 250 mg IV pulse or 0.5-1 mg/Kg daily for five days. For TCZ, seven studies had a prespecified criterion for TCZ use. Thirteen out of the included studies used IV 8 mg/kg as the dose for TCZ while using a subcutaneous route in addition to the intravenous route in one study. There were only four studies reported the time of the first TCZ dose. Hydroxychloroquine is the most used SOC in both treatment arms. Supplementary Tables 2 and 4 describe the medications used in each study.
Fourteen of the seventeen studies reported on death and therefore were also included in the treatment failure analysis. Only six studies reported the exact composite outcome of intubation or death. For the secondary outcome, six out of the seventeen studies reported on superinfection. However, studies used different definition for superinfection. Only two study defined it as superinfection, and two studies as a bacterial infection. One study defined it as bacteremia without a source, and another one as an intensive care unit acquired bloodstream infection. The description of the outcomes included in each study can be found in supplementary Table 3.
In regard to quality assessment, all the studies scored either good or fair quality in the three domains (selection, comparability, outcome). However, one study was of low quality in all the domains. Table 1 summarizes the quality assessment for the included studies.
Death rates
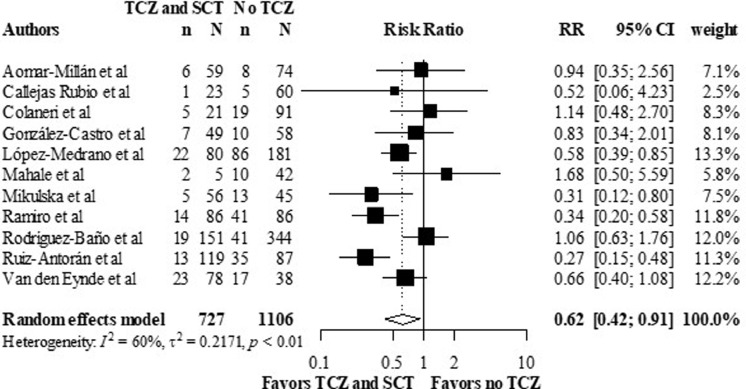
All the thirteen observational studies that reported on death rates, except four, presented crude death numbers. Adjusted HRs were reported in five studies, while adjusted ORs were reported in two, and five reported unadjusted ORs. For crude analysis, overall RR was 0.62, 95% CI (0.42 – 0.91), favoring the TCZ and SCT combination arm, versus the no TCZ arm (figure 2 ). Heterogenicity reported as I2 was 60% (p < 0.01).
Figure 2.
Crude mortality rates in the TCZ and SCT arm versus no TCZ arm, using data from observational studies.
Meta-analysis of the crude mortality outcome.
CI: confidence interval; RR: relative risk; SCT: systematic corticosteroids; TCZ: tocilizumab.
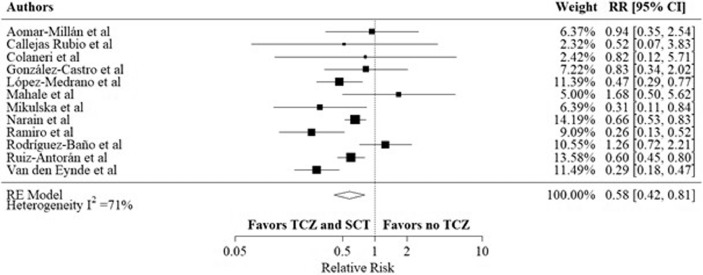
In adjusted effect estimates, most studies controlled for age, gender, comorbidities, previously received medications, and baseline laboratory inflammatory markers. Two studies included propensity score adjustments. Overall, the RR was 0.58, 95% CI (0.42 – 0.81) with I2 was 71% (p < 0.001), favoring the combination arm (figure 3 ).
Figure 3.
Adjusted mortality rates in the TCZ and SCT arm versus no TCZ arm, using data from observational studies.
Meta-analysis of the most adjusted mortality rates using HRs and RRs as reported in each included study.
CI: confidence interval; HR: hazard ratio; RR: relative risk; SCT: systematic corticosteroids; TCZ: tocilizumab.
For analyses of combining observational studies and subgroup analyses from RCTs, the crude death numbers were reported in thirteen studies. The overall RR was 0.69, 95% CI (0.49 – 0.95), I2 was 68%, (p < 0.01) (supplementary figure 1). The adjusted death rates were reported in fourteen studies. The overall RR was 0.64, 95% CI (0.48 – 0.86), I2 was 69%, (p < 0.001) (supplementary figure 4).
Treatment failure
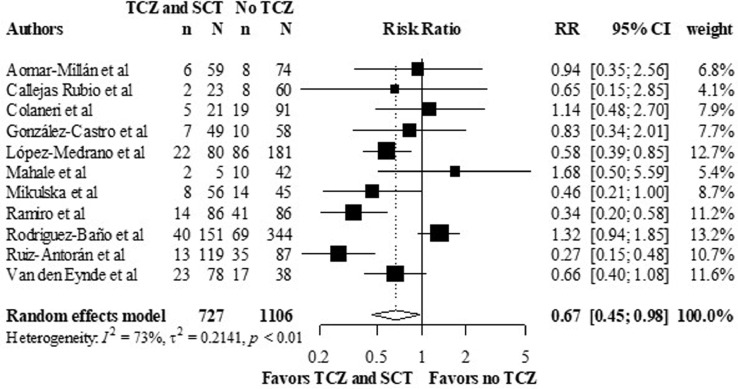
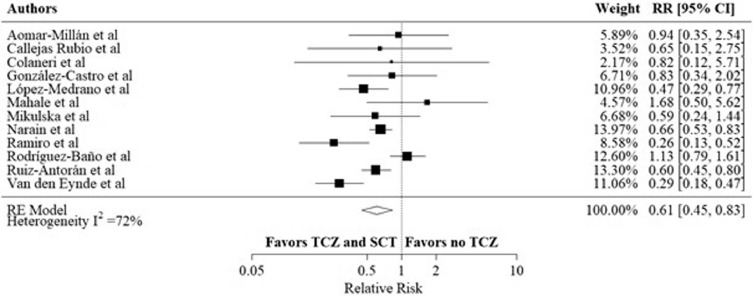
Three studies defined treatment failure to include intubation or death. In the other included studies, death outcome was used as a substitute for treatment failure. Five studies reported treatment failure in HRs, and three studies reported this outcome as OR, which was converted to RR. Eleven out of the observational studies reported the crude number of treatment failure events. The overall RR was 0.67, 95% CI (0.45 – 0.98), I2 was 73% (p < 0.01) (figure 4 ), favoring the combination arm. The adjusted treatment failure rates were reported in twelve observational studies. The overall RR was 0.61, 95% CI (0.45 – 0.83) (figure 5 ), I2 was 72% (p < 0.001).
Figure 4.
Crude composite outcome rates of intubation or death in the TCZ and SCT arm versus no TCZ arm, using data from observational studies.
Meta-analysis of the composite outcome rates of intubation or death.
CI: confidence interval; RR: relative risk; SCT: systematic corticosteroids; TCZ: tocilizumab.
Figure 5.
Adjusted composite outcome rates of intubation and death in the TCZ and SCT arm versus no TCZ arm, using data from observational studies.
Meta-analysis of the most adjusted composite outcome rates of intubation and death using HRs and RRs as reported in each included study.
CI: confidence interval; HR: hazard ratio; RR: relative risk; SCT: systematic corticosteroids; TCZ: tocilizumab.
When combining observational studies and subgroup analyses from RCTs, fifteen studies reported crude numbers of treatment failure events. The overall RR was 0.69, 95% CI (0.52 – 0.93), I2 was 69%, (p < 0.01) (supplementary figure 9). For adjusted treatment failure rates overall RR was 0.59, 95% CI (0.43 – 0.80), I2 was 86%, (p < 0.001) (supplementary figure 13).
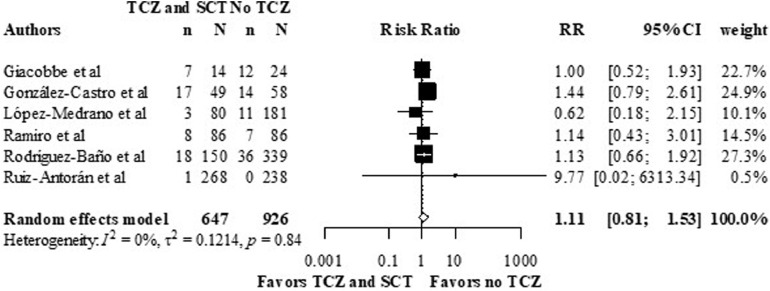
Superinfection
Superinfection rates were described in five studies, in which all of them reported unadjusted analyses. The overall RR was 1.11, 95% CI (0.81 – 1.53), I2 was 0%, (p = 0.84) (figure 6 ).
Figure 6.
Crude superinfection rates in the TCZ and SCT arm versus no TCZ arm from, using data from observational studies.
Meta-analysis of the superinfection outcome.
CI: confidence interval; RR: relative risk; SCT: Systematic Corticosteroids; TCZ: Tocilizumab.
Sensitivity analysis
There was almost no significant influence on the results of crude mortality rates after performing multiple sensitivity tests. However, the association was borderline significant after including only steroids as a comparator and excluding low-quality (supplementary figures 2 and 3).
For adjusted death rates, the sensitivity analysis yielded similar results to crude analysis. The association between the intervention and the adjusted death rates remained significant after omitting each study in the leave-one-out analysis. When including studies that compared TCZ and SCT with SCT instead of SOC in general, the association remained significant (supplementary figure 5). The results remained the same when including only studies of high quality (supplementary figure 6). When separating the analysis by HRs and ORs, there was a significant relationship using HRs but not with ORs (supplementary figure 7 and 8). When running the sensitivity analysis for the treatment failure outcomes, the results were like the death rates in both crude and adjusted analyses (supplementary material figures 10-12 and 14-18). Furthermore, for crude superinfection rates, the analyses remained non-significant using sensitivity analyses (supplementary figures 19).
Discussion
The present systematic review and meta-analysis, to our knowledge, is the first to synthesize available evidence on the effect of the combination of TCZ and SCT in subjects with severe COVID-19. Our findings suggest that the use of both TCZ and SCT, compared to no TCZ use in subjects with severe COVID-19, reduces the risk of mortality. These results were consistent when using both unadjusted and adjusted data obtained from the observational studies included. Even after performing several sensitivity analyses, the results remained relatively unchanged. For the outcome of treatment failure, although crude analysis showed a trend towards lower event rates in the TCZ and SCT combination arm, this effect was more substantial using adjusted models. Furthermore, the rate of superinfections, using only unadjusted analysis, suggests that there are no differences between the two treatment arms in rates of infections.
Generally, there was moderate to high heterogeneity across study outcomes. Many factors may have contributed to the heterogeneity observed, including different inclusion criteria, variability in TCZ and SCT regimen, specifically lower doses of TCZ, co-interventions used, and the different confounders used in the adjusted models. Due to the limited data available from currently available studies, we were unable to perform subgroup analyses to investigate this heterogeneity. However, when we limited our analysis to studies that used SCT rather than SOC as the comparison arm, heterogeneity was decreased substantially, in both the mortality and treatment failure outcomes.
Although no prior review has focused on the effect of TCZ and SCT combination, several studies have looked at the effect of either TCZ or SCT separately. The beneficial effects of TCZ compared to SOC was observed in previous reports that used data from observational studies (Kotak et al., 2020; Tleyjeh et al., 2021). On the other hand, when pooling data from randomized trials, there was no added benefit of TCZ (Tleyjeh et al., 2021). In a meta-analysis assessing safety and efficacy of TCZ in reducing mortality among critically ill COVID-19 patients, a subgroup analysis by corticosteroid use showed lower mortality rates, RR was 0.56, 95% CI (0.34 – 0.92), and post-drug infection RR was 1.29 95% CI (0.41 – 4.04) (Boregowda et al., 2020). Although this is consistent with our results, the studies included in this subgroup analysis differs substantially from our review. Here we have included studies that used both TCZ and SCT in all patients in the combination arm. In contrast, in the previous report, not all subjects in the included studies were using SCT in the combination arm (Boregowda et al., 2020).
For corticosteroid use in COVID-19 patient, the results vary between studies (RECOVERY Collaborative Group, 2020; Sterne et al., 2020; Tlayjeh et al., 2020). In the Randomized Evaluation of COVID-19 Therapy (ROCEVRY) trial, the use of dexamethasone resulted in lower mortality rates in subjects with COVID-19 who received mechanical ventilation or oxygen (RECOVERY Collaborative Group, 2020). These results were supported by data from a meta-analysis which included randomized trials and compared the mortality risk in COVID-19 patient who used SCT versus stander of care or placebo (Sterne et al., 2020). Their findings show that SCT use was associated with lower mortality rates (Sterne et al., 2020). However, other meta-analyses, which included observational studies or randomized control trials, found that corticosteroids use was associated with higher mortality rates Sarkar et al., 2020; Yang et al., 2020. In contrast, others found no association between CST and mortality (Tlayjeh et al., 2020).
In this review, for our main analysis we included observational studies only. However, there were four randomized trials that on the use of TCZ in subjects with severe COVID-19, reported posthoc analyses based on SCT use, which we included in our secondary analyses (Hermine et al., 2020; Rosas et al., 2021; RECOVERY Collaborative Group, 2021; Salama et al., 2021). For instance, in the CORIMUNO-TOC 1 trial, the combination of SCT with TCZ use was associated with a lower treatment failure rate, and adjusted HR was 0.13, 95% CI (0.021 to 0.78) (Hermine et al., 2020). Also, the results of the RECOVERY trial showed a lower rate of both mortality and treatment failure in combination group, and RR was 0.79, 95% CI (0.70-0.89), RR was 0.81, 95% CI (0.74-0.89), respectively (RECOVERY Collaborative Group, 2021). These results are in accordance with our findings. Nevertheless, there is a still need for trials that are designed to assess the efficacy of TCZ and SCT combination, versus SCT alone.
The strengths of this study include a quantitative synthesis and the inclusion of both published and unpublished studies, and performing analyses using both unadjusted and adjusted data. However, there are several limitations. First, the included studies were mostly observational and mostly retrospective, which may suffer from the risk of confounding, selection bias and data loss. Nevertheless, we performed the analysis once using crude numbers and using adjusted analysis, and the results remained consistent. Second, the majority of studies were from Europe, and the United States, which may limit the generalizability of the results to other populations. Third, there were multiple variations between the studies, including the different definitions of the comparison arm, the variability between co-interventions, and the level of disease severity across groups. Fourth, the studies only included subjects with severe COVID-19. Therefore, the results of these analyses may not apply to patients with mild to moderate disease. Furthermore, even though the studies in this review included only those with severe COVID-19, still the characteristics of the population and level of severity differed across studies and might impact the current study outcomes.
In conclusion, this study shows that the combination of TCZ and SCT in subjects with severe COVID-19, compared to no TCZ is associated with lower rates of mortality, and somewhat intubation, with no change in risk of superinfection. However, these results were derived from observational studies; therefore, well-designed randomized trials are urgently warranted to confirm our findings.
Acknowledgments
Role of the Funding Source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributors
All authors contributed to the conception and design of the study and reviewed all documents and materials.
Conflict of Interest
All authors declare no competing interests.
Funding Source
This research project was supported by a grant from the "Research Center of the Center for Female Scientific and Medical Colleges", Deanship of Scientific Research, King Saud University.
Ethical Approval
This study did not require ethical approval because the meta-analysis was based on published research and the original data are anonymous.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2021.07.021.
Appendix. Supplementary materials
References
- Alattar R, Ibrahim TBH, Shaar SH, Abdalla S, Shukri K, Daghfal JN, et al. Tocilizumab for the Treatment of Severe COVID-19. J Med Virol. 2020 doi: 10.1002/jmv.25964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aomar-Millán IF, Salvatierra J, Torres-Parejo Ú, Nuñez-Nuñez M, Hernández-Quero J, Anguita-Santos F. Glucocorticoids alone versus tocilizumab alone or glucocorticoids plus tocilizumab in patients with severe SARS-CoV-2 pneumonia and mild inflammation. Med Clin (Barc) 2021;156 doi: 10.1016/j.medcli.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boregowda U, Perisetti A, Nanjappa A, Gajendran M, Kutti Sridharan G, Goyal H. Addition of Tocilizumab to the Standard of Care Reduces Mortality in Severe COVID-19: A Systematic Review and Meta-Analysis. Front Med. 2020 doi: 10.3389/fmed.2020.586221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brudno JN, Kochenderfer JN. Toxicities of chimeric antigen receptor T cells: Recognition and management. Blood. 2016;127:3321–3330. doi: 10.1182/blood-2016-04-703751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burmester GR, Choy E, Kivitz A, Ogata A, Bao M, Nomura A, et al. Low immunogenicity of tocilizumab in patients with rheumatoid arthritis. Ann Rheum Dis. 2017;76:1078–1085. doi: 10.1136/annrheumdis-2016-210297. [DOI] [PubMed] [Google Scholar]
- Choy EH, De Benedetti F, Takeuchi T, Hashizume M, John MR, Kishimoto T. Translating IL-6 biology into effective treatments. Nat Rev Rheumatol. 2020;16 doi: 10.1038/s41584-020-0419-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaneri M, Bogliolo L, Valsecchi P, Sacchi P, Zuccaro V, Brandolino F, et al. Tocilizumab for treatment of severe covid-19 patients: Preliminary results from smatteo covid19 registry (smacore). Microorganisms 2020;8. https://doi.org/10.3390/microorganisms8050695. [DOI] [PMC free article] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986 doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Fu B, Xu X, Wei H. Why tocilizumab could be an effective treatment for severe COVID-19? J Transl Med. 2020 doi: 10.1186/s12967-020-02339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobbe DR, Battaglini D, Ball L, Brunetti I, Bruzzone B, Codda G, et al. Bloodstream infections in critically ill patients with COVID-19. Eur J Clin Invest. 2020;50 doi: 10.1111/ECI.13319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Castro A, Cuenca Fito E, Fernandez A, Escudero Acha P, Rodríguez Borregán JC, Peñasco Y. Combined therapy of tocilizumab and corticosteroids in severe SARS-CoV-2 disease. Med Intensiva. 2021 doi: 10.1016/J.MEDIN.2021.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermine O, Mariette X, Tharaux P-L, Resche-Rigon M, Porcher R, Ravaud P. Effect of Tocilizumab vs Usual Care in Adults Hospitalized With COVID-19 and Moderate or Severe Pneumonia. JAMA Intern Med. 2020 doi: 10.1001/jamainternmed.2020.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins J, Savović J, Page MJ, Sterne JAC. RoB 2: A revised Cochrane risk-of-bias tool for randomized trials. Br Med J. 2019 [Google Scholar]
- Hossen MS, Barek MA, Jahan N, Safiqul Islam M. A Review on Current Repurposing Drugs for the Treatment of COVID-19: Reality and Challenges. SN Compr Clin Med. 2020;2 doi: 10.1007/s42399-020-00485-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejas Rubio JL, Luna Del Castillo JD, de la Hera Fernández J, Guirao Arrabal E, Colmenero Ruiz M, Ortego Centeno N. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Med Clin (Barc) 2020;155:159–161. doi: 10.1016/J.MEDCLI.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klopfenstein T, Zayet S, Lohse A, Balblanc J-C, Badie J, Royer P-Y, et al. Tocilizumab therapy reduced intensive care unit admissions and/or mortality in COVID-19 patients. Med Mal Infect. 2020:1–4. doi: 10.1016/j.medmal.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotak S, Khatri M, Malik Mehreen, Malik Maria, Hassan W, Amjad A, et al. Use of Tocilizumab in COVID-19: A Systematic Review and Meta-Analysis of Current Evidence. Cureus. 2020 doi: 10.7759/cureus.10869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009 doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Medrano F, Pérez-Jacoiste Asín MA, Fernández-Ruiz M, Carretero O, Lalueza A, Maestro de la Calle G, et al. Combination therapy with tocilizumab and corticosteroids for aged patients with severe COVID-19 pneumonia: A single-center retrospective study. Int J Infect Dis. 2021;105:487–494. doi: 10.1016/j.ijid.2021.02.099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo P, Liu Y, Qiu L, Liu X, Liu D, Li J. Tocilizumab treatment in COVID-19: A single center experience. J Med Virol. 2020:1–5. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahale N, Rajhans P, Godavarthy P, Narasimhan VL, Oak G, Marreddy S, et al. A Retrospective Observational Study of Hypoxic COVID-19 Patients Treated with Immunomodulatory Drugs in a Tertiary Care Hospital. Indian J Crit Care Med. 2020;24:1020–1027. doi: 10.5005/jp-journals-10071-23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikulska M, Nicolini LA, Signori A, Di Biagio A, Sepulcri C, Russo C, et al. Tocilizumab and steroid treatment in patients with COVID-19 pneumonia. PLoS One. 2020;15:1–16. doi: 10.1371/journal.pone.0237831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narain S, Stefanov DG, Chau AS, Weber AG, Marder G, Kaplan B, et al. Comparative Survival Analysis of Immunomodulatory Therapy for Coronavirus Disease 2019 Cytokine Storm. Chest. 2021;159:933–948. doi: 10.1016/j.chest.2020.09.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramiro S, Mostard RLM, Magro-Checa C, Van Dongen CMP, Dormans T, Buijs J, et al. Historically controlled comparison of glucocorticoids with or without tocilizumab versus supportive care only in patients with COVID-19-associated cytokine storm syndrome: Results of the CHIC study. Ann Rheum Dis. 2020;79:1143–1151. doi: 10.1136/annrheumdis-2020-218479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group. Horby P, Lim WS, et al. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RECOVERY Collaborative Group Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet (London, England) 2021;397:1637–1645. doi: 10.1016/S0140-6736(21)00676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Baño J, Pachón J, Carratalà J, Ryan P, Jarrín I, Yllescas M, et al. Treatment with tocilizumab or corticosteroids for COVID-19 patients with hyperinflammatory state: a multicentre cohort study (SAM-COVID-19) Clin Microbiol Infect. 2021;27:244–252. doi: 10.1016/J.CMI.2020.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosas IO, Bräu N, Waters M, Go RC, Hunter BD, Bhagani S, et al. Tocilizumab in Hospitalized Patients with Severe Covid-19 Pneumonia. N Engl J Med. 2021;384 doi: 10.1056/nejmoa2028700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Antorán B, Sancho-López A, Torres F, Moreno-Torres V, de Pablo-López I, García-López P, et al. Combination of Tocilizumab and Steroids to Improve Mortality in Patients with Severe COVID-19 Infection: A Spanish, Multicenter, Cohort Study. Infect Dis Ther. 2021;10:347–362. doi: 10.1007/s40121-020-00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama C, Han J, Yau L, Reiss WG, Kramer B, Neidhart JD, et al. Tocilizumab in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;384:20–30. doi: 10.1056/nejmoa2030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic Treatments for Coronavirus Disease 2019 (COVID-19): A Review. JAMA - J Am Med Assoc. 2020;323 doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sarkar S, Khanna P, Soni KD. Are the steroids a blanket solution for COVID-19? A systematic review and meta-analysis. J Med Virol. 2020 doi: 10.1002/jmv.26483. [DOI] [PubMed] [Google Scholar]
- Shimabukuro-Vornhagen A, Gödel P, Subklewe M, Stemmler HJ, Schlößer HA, Schlaak M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6 doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sise ME, Baggett M V, Shepard J-AO, Stevens JS, Rhee EP. Case 17-2020: A 68-Year-Old Man with Covid-19 and Acute Kidney Injury. N Engl J Med. 2020:1–10. doi: 10.1056/NEJMcpc2002418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterne JAC, Murthy S, Diaz J.V., Slutsky AS, Villar J, Angus DC, et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA - J Am Med Assoc. 2020 doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tleyjeh IM, Kashour Z, Damlaj M, Riaz M, Tlayjeh H, Altannir M, et al. Efficacy and safety of tocilizumab in COVID-19 patients: A living systematic review and meta-analysis. Clin Microbiol Infect. 2021;27(2):215–227. doi: 10.1016/j.cmi.2020.10.036. Feb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlayjeh H, Mhish OH, Enani MA, Alruwaili A, Tleyjeh R, Thalib L, et al. Association of corticosteroids use and outcomes in COVID-19 patients: A systematic review and meta-analysis. J Infect Public Health. 2020 doi: 10.1016/j.jiph.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Eynde E, Gasch O, Oliva JC, Prieto E, Calzado S, Gomila A, et al. Corticosteroids and tocilizumab reduce in-hospital mortality in severe COVID-19 pneumonia: a retrospective study in a Spanish hospital. Infect Dis (Auckl) 2021;53:291–302. doi: 10.1080/23744235.2021.1884286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Abstrackr. IHI’12 - Proc. 2nd ACM SIGHIT Int. Heal. Informatics Symp. 2012. Deploying an interactive machine learning system in an Evidence-based Practice Center. [DOI] [Google Scholar]
- Wells G, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. (Available from URL http//www.Ohri.ca/Programs/Clinical_epidemiology/OxfordAsp) 2012. doi: 10.2307/632432. [DOI]
- Xiong M, Liang X, Wei Y. Changes in Blood Coagulation in Patients with Severe Coronavirus Disease 2019 (COVID-19): a Meta-Analysis. Br J Haematol. 2020:2019–2021. doi: 10.1111/bjh.16725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Liu Jialong, Zhou Y, Zhao X, Zhao Q, Jing Liu. The effect of corticosteroid treatment on patients with coronavirus infection: a systematic review and meta-analysis. J Infect. 2020 doi: 10.1016/j.jinf.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazici Y, Curtis JR, Ince A, Baraf H, Malamet RL, Teng LL, et al. Efficacy of tocilizumab in patients with moderate to severe active rheumatoid arthritis and a previous inadequate response to disease-modifying antirheumatic drugs: The ROSE study. Ann Rheum Dis. 2012;71:198–205. doi: 10.1136/ard.2010.148700. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yu KF. What's the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. J Am Med Assoc. 1998;280 doi: 10.1001/jama.280.19.1690. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.