Abstract
Myocarditis has been described previously as a rare side effect of both influenza and smallpox vaccines. In this report, we present a case of acute perimyocarditis in a young, healthy man after vaccination with the mRNA-1273 severe acute respiratory syndrome coronavirus -2 (SARS-CoV-2; Moderna) vaccine. He presented with chest pain and decompensated heart failure 3 days after administration of his second dose, and his symptoms resolved by 9 days post-inoculation. This case highlights a rare but potentially serious side effect of this mRNA vaccine that primary care physicians and cardiologists should be aware of in order to identify and appropriately manage these patients.
Résumé
La myocardite a auparavant été considérée comme un effet secondaire rare des vaccins antigrippaux et antivarioliques. Dans ce rapport, nous présentons le cas d’un homme, jeune et en bonne santé, atteint d’une périmyocardite aiguë après avoir reçu le vaccin à ARNm-1273 contre le coronavirus du syndrome respiratoire aigu sévère 2 (SARS-CoV-2, de l’anglais severe acute respiratory syndrome coronavirus-2; Moderna). Il a éprouvé une douleur thoracique et présenté une décompensation cardiaque 3 jours après l’administration de la seconde dose. Les symptômes se sont résorbés 9 jours après l’inoculation. Ce cas illustre un effet secondaire rare, mais potentiellement sérieux, de ce vaccin à ARNm, que les médecins de premier recours et les cardiologues doivent connaître pour être en mesure de détecter et prendre en charge adéquatement ces patients.
Case
A 34-year-old previously healthy man presented to the hospital with a 3-day history of fevers and myalgias and a 2-day history of a dull, retrosternal chest pain that was both positional and pleuritic in nature. He had received his second dose of the mRNA-1273 severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) (Moderna, Cambridge, MA) vaccine 1 day prior to his symptom onset. He took no medications, did not use any recreational drugs, and his review of infectious systems was otherwise negative.
Novel Teaching Points.
-
•
Clinicians should be aware that patients presenting with chest pain after vaccination with mRNA vaccines (Moderna or Pfizer-BioNTech) may have myocarditis or perimyocarditis.
-
•
The natural history of mRNA vaccine–associated myocarditis is not known, but case reports suggest a favourable prognosis and rapid recovery.
-
•
Myocarditis after mRNA vaccination appears to be rare, and the goal of this report is not to deter clinicians or patients from vaccination, but rather to raise awareness of this clinical entity.
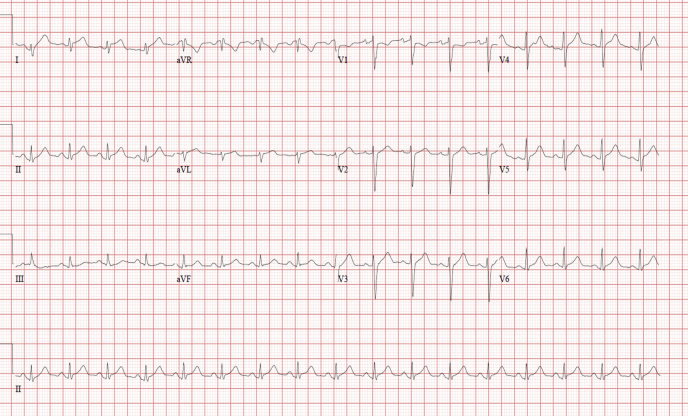
On physical exam, he was febrile (39.1oC), tachycardic with a heart rate of 102, had blood pressure of 103/67 mm Hg, and was tachypneic, with a respiratory rate of 28 and oxygen saturation of 93% on room air. His jugular venous pressure was elevated, he had no murmurs or rubs, and he had mild crackles to the lung bases. Electrocardiography showed lateral PR-segment depression and ST-segment elevation mirrored in the aVR with PR-segment elevation and ST-segment depression (Fig. 1). Laboratory investigation on admission showed a high-sensitivity troponin T concentration of 4026 ng/L (normal < 14 ng/L), which peaked at 5203 ng/L; N-terminal pro-B-type natriuretic peptide [NT-proBNP] concentration of 1551 ng/L (normal < 125 ng/L); a white blood cell count of 8.4 x 109/L; lactate level at 1.1 mmol/L, and a C-reactive protein level of 111 mg/L. Sputum and blood cultures were negative, and nasopharyngeal coronavirus disease 2019 (COVID-19) polymerase chain reaction was nonreactive. Chest radiograph revealed mild pulmonary edema. His symptoms, physical exam, and investigations were suspicious for perimyocarditis.
Figure 1.
12-lead electrocardiogram obtained on presentation shows ST-segment elevation in I, aVL, and V4-V6, with PR-segment elevation and ST-segment depression in aVR.
A transthoracic echocardiogram revealed reduced left ventricular ejection fraction (LVEF) of 43%, without pericardial effusion (see Videos 1-4
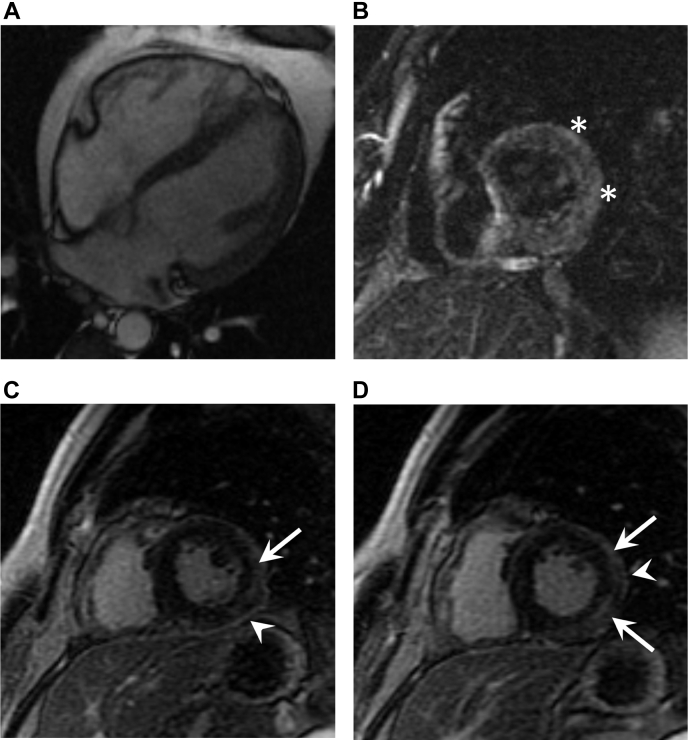
 , view videos online). Cardiac magnetic resonance imaging (MRI) performed on day 4 of admission showed normalization of the LVEF to 54%, with subepicardial late gadolinium enhancement in the anterolateral and inferolateral segments, as well as patchy myocardial edema on T2-weighted images (Fig. 2), meeting the Lake Louise criteria for myocarditis. The MRI also demonstrated pericardial enhancement consistent with inflammation, confirming the clinical suspicion of perimyocarditis. By day 5 of his admission, his symptoms had resolved and his high-sensitivity troponin T and C-reactive protein concentrations improved to 59 ng/L and 20 mg/L, respectively. Given his clinical, biochemical, and LVEF improvement, an endomyocardial biopsy was deferred. He was discharged from the hospital symptom-free on medical therapy with high-dose aspirin, colchicine, bisoprolol, and rampiril, with a plan for close outpatient follow-up.
, view videos online). Cardiac magnetic resonance imaging (MRI) performed on day 4 of admission showed normalization of the LVEF to 54%, with subepicardial late gadolinium enhancement in the anterolateral and inferolateral segments, as well as patchy myocardial edema on T2-weighted images (Fig. 2), meeting the Lake Louise criteria for myocarditis. The MRI also demonstrated pericardial enhancement consistent with inflammation, confirming the clinical suspicion of perimyocarditis. By day 5 of his admission, his symptoms had resolved and his high-sensitivity troponin T and C-reactive protein concentrations improved to 59 ng/L and 20 mg/L, respectively. Given his clinical, biochemical, and LVEF improvement, an endomyocardial biopsy was deferred. He was discharged from the hospital symptom-free on medical therapy with high-dose aspirin, colchicine, bisoprolol, and rampiril, with a plan for close outpatient follow-up.
Figure 2.
(A) Four-chamber FIESTA (fast imaging employing steady-state acquisition) sequence in diastole demonstrating normal indexed cardiac chamber sizes (left ventricular end-diastolic volume: 153 mL; right ventricular end-diastolic volume: 167 mL) and no pericardial effusion. (B) Short-axis oblique triple inversion recovery sequence at the level of the mid–left ventricle demonstrating patchy myocardial edema (asterisks). (C, D) Short-axis oblique late gadolinium enhancement inversion recovery sequence (TI = 260 ms) demonstrating pericardial enhancement (arrowheads) and subepicardial late gadolinium enhancement in the mid–left ventricular anterolateral and inferolateral segments (arrows).
Discussion
Myocarditis is an acute inflammatory disease of the myocardium predominantly associated with infectious agents (often viruses), toxic substances, or systemic immune-mediated disorders. The gold standard for diagnosis is via histopathologic sampling with endomyocardial biopsy, but this is not always feasible or practical. A probable diagnosis can be achieved in the appropriate clinical context based on elevated cardiac enzymes as well as functional and structural abnormalities on cardiac echocardiogram and MRI.1
Vaccine-related myocarditis is rare but has been documented with live-attenuated influenza and smallpox vaccines.2,3 The causality is uncertain, and the mechanism is not fully understood, but there are some hypotheses that post-vaccine myopericarditis may be secondary to lymphocytic infiltration resulting in an immune-mediated myocardial injury.3
COVID-19 vaccine mRNA-1273, developed by ModernaTx, Inc., is a pre-fusion SARS-CoV-2 spike glycoprotein (S) antigen encoded in mRNA and formulated in lipid nanoparticles, representing a novel vaccination technology with ongoing surveillance for potential unrecognized side effects. During the phase-3 study for this vaccine, no cases of myocarditis were documented in any of the 30,420 participants.4
Cardiac involvement in COVID-19 infection is well recognized, with manifestations ranging from myocardial injury to cardiogenic shock. Myocarditis itself is a known complication of coronavirus disease.5 With the global vaccination effort well underway, and millions of mRNA vaccines administered, the potential for myocarditis after vaccination is being increasingly recognized in case series.6,7 As in the case of our patient, most reported cases describe younger male patients presenting within days of their second vaccine dose who have a self-limited course without malignant arrhythmias or need for advanced circulatory support.6,7 Although this patient and others reported thus far have had a favourable course and outcome, recognition of this entity is important in managing these patients appropriately, and may have yet unknown implications. The purpose of this case is to highlight a potential rare side effect of this vaccine for clinicians to be aware of; it is not meant to deter clinicians and patients from receiving the benefit of the proven efficacy and overall safety of this mRNA vaccine.
Acknowledgments
Funding Sources
The authors have no funding sources to declare.
Disclosures
The authors have no conflicts of interest to disclose.
Footnotes
Ethics Statement: This case study presentation is in compliance with all Research Ethics Board policies at the University of British Columbia and Providence Health Care.
See page 1412 for disclosure information.
To access the supplementary material accompanying this article, visit CJC Open at https://www.cjcopen.ca/ and at https://doi.org/10.1016/j.cjco.2021.07.008.
Supplementary Material
Parasternal long axis view showing infero-lateral wall hypokinesis.
Parasternal short axis view showing infero-lateral hypokinesis.
Apical 4-chamber view showing antero-lateral wall hypokinesis.
Apical 2 chamber view showing inferior wall hypokinesis. Ejection fraction 43% by Simpson’s Method.
References
- 1.Turgeon P.Y., Massot M., Beaupré F., et al. Effect of acute immunosuppression on left ventricular recovery and mortality in fulminant viral myocarditis: a case series and review of literature. CJC Open. 2020;3:292–302. doi: 10.1016/j.cjco.2020.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mei R., Raschi E., Forcesi E., et al. Myocarditis and pericarditis after immunization: gaining insights through the Vaccine Adverse Event Reporting System. Int J Cardiol. 2018;273:183–186. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 3.Cassimatis D.C., Atwood J.E., Engler R.M., et al. Smallpox vaccination and myopericarditis: a clinical review. J Am Coll Cardiol. 2004;43:1503–1510. doi: 10.1016/j.jacc.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siripanthong B., Nazarian S., Muser D., et al. Recognizing COVID-19-related myocarditis: the possible pathophysiology and proposed guideline for diagnosis and management. Heart Rhythm. 2020;17:1463–1471. doi: 10.1016/j.hrthm.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larson K.F., Ammirati E., Adler E.D., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosner C.M., Genovese L., Tehrani B.N., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Parasternal long axis view showing infero-lateral wall hypokinesis.
Parasternal short axis view showing infero-lateral hypokinesis.
Apical 4-chamber view showing antero-lateral wall hypokinesis.
Apical 2 chamber view showing inferior wall hypokinesis. Ejection fraction 43% by Simpson’s Method.