Abstract
COVID-19 has been associated with significant risk for cardiac arrhythmias, particularly in patients with underlying cardiac conditions or prior histories of arrhythmia. It has been shown that a Brugada pattern can be unmasked in febrile patients with COVID-19. Herein we report a unique case of an afebrile patient without known prior history of Brugada presenting with Brugada pattern on ECG.
Keywords: arrhythmias, COVID-19
Background
SARS-CoV-2 has shown to incite numerous multi-systemic syndromes since it was first reported in Wuhan, China in late 2019.1 Most notably, COVID-19 displays a predilection for the respiratory system and can precipitate acute respiratory distress syndrome in severe cases. Given the virus’s high degree of transmissibility and the potential for poor outcomes, it has proven to be a serious public health threat and was deemed a global pandemic by the WHO in March 2020. While the pulmonary manifestations are the most characteristic and well described of the organ systems, COVID-19 can also trigger various cardiovascular pathologies, including acute coronary syndromes, myocarditis, arrhythmias, heart failure and certain vasculitides.2 COVID-19 carries arrhythmogenic properties which can unmask or exacerbate cardiac arrhythmias and channelopathies such as long QT syndrome, short QT syndrome and Brugada syndrome (BrS).3–7 Here we report a case of Brugada ECG pattern during an acute COVID-19 in a patient previously without any identifiable risk factors.
Case presentation
A 44-year-old Hispanic man with no significant medical history presented with generalised fatigue, headaches, productive cough of whitish to yellow sputum, myalgias, abdominal pain and non-bloody diarrhoea with fevers (T.max of 39.2°C) for 6 days prior to admission and worsening shortness of breath for 2 days. He reported getting a COVID-19 test 1 week prior to admission at an urgent care centre which was positive. A chest X-ray at that time showed clear lungs without any acute cardiopulmonary abnormalities. He denied any significant family history including sudden cardiac death (SCD), arrhythmias, myocardial infarction or syncope. He did not recall any exposure to coronavirus and had no sick contacts. He lives at home with his wife and children. He works in maintenance, denies any alcohol, tobacco and illicit drug use. He denied any lightheadedness, dizziness, chest pain/palpitations, nausea, vomiting, loss of taste and smell. His last known fever was a day prior to presentation and stated he had been taking acetaminophen at home and over-the-counter cough syrup.
On presentation to the Emergency Department (ED), he was a healthy-looking man who appeared in mild distress. He was afebrile (36.8°C); Blood Pressure (BP), 136/86 mm Hg; heart rate, 110 beats/min; respiratory rate, 24 breaths/min and oxygen saturation 95% on room air. Pupils were equal, round and reactive to light with normal conjunctiva and no scleral icterus. Lungs with decreased breath sounds; no wheezing, rales or rhonchi. Cardiovascular examination revealed increased rate and normal rhythm, no murmurs. The abdomen was diffusely tender to palpation, but not distended and with normal bowel sounds and no hepatosplenomegaly. The bladder was not distended, and there was no costovertebral tenderness. There was full range of motion in all joints without swelling, tenderness or oedema. There were no focal neurologic deficits or meningeal signs.
Investigations
Laboratory data demonstrated mild hyponatremia 134 mmol/L (135–145 mmol/L), and normal potassium 3.9 mmol/L (3.5–5.0 mmol/L), magnesium 2.0 mmol/L (1.5–2.0 mmol/L) and brain natriuretic peptide level 20 pg/mL (0–120 pg/mL). Liver enzyme tests showed aspartate aminotransferase 92 units/L (10–45 units/L), alanine aminotransferase 120 units/L (10–45 units/L), normal alkaline phosphatase 84 units/L (40–125 units/L). His ferritin was normal at 234 ng/mL (20–450 ng/mL); however, his other inflammatory markers were elevated: erythrocyte sedimentation rate (ESR) 49 mm/hour (0–15 mm/hour), lactate dehydrogenase 782 units/L (300–600 units/L), D-dimer 0.48 mcg/mL FEq unit (0.2–0.38 mcg/mL FEq unit), interleukin-6 (IL-6) 53.9 pg/mL (0.0–13.0 pg/mL) and procalcitonin level 0.113 ng/mL (0.00–0.099 ng/mL). The remaining laboratory values were unremarkable, and the troponin level was normal. He tested positive for SARS-CoV-2 via PCR and was placed on airborne isolation precautions in a dedicated COVID-19 unit with telemetry and pulse oximetry monitoring. Portable chest radiograph demonstrated moderate pulmonary interstitial and alveolar oedema with enlarged heart (figure 1). The ECG showed a Brugada-type 1 pattern ‘coved’ ST-segment elevation that concaves down with inverted T waves in V1–V2 leads with no reciprocal changes (figure 2).
Figure 1.
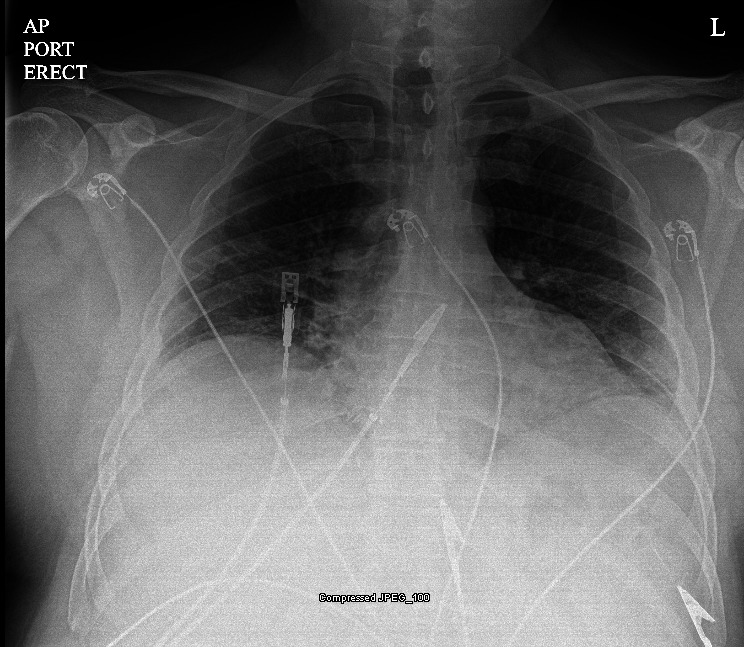
Portable chest radiograph.
Figure 2.
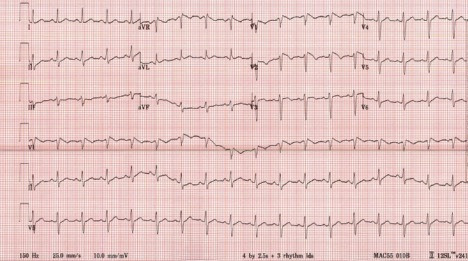
Initial 12 lead ECG.
Treatment
The patient was managed conservatively with supportive care: antipyretics (acetaminophen) and benzonatate for symptomatic relief of his cough. He was monitored overnight, remained afebrile and did not require supplemental oxygen therapy, so there was no need to initiate remdesivir or dexamethasone for treatment of COVID-19. He was discharged home after 48 hours of monitoring with follow-up with cardiology in 3 weeks given his low adverse cardiac risk.
Outcome and follow-up
At his follow-up in the outpatient cardiology clinic 3 weeks later, he reported doing well without fatigue, fevers, shortness of breath at rest and exertion. His vital signs were T, 36.7°C; BP, 136/93 mm Hg; heart rate, 82 beats/min. ECG was done which showed normal sinus rhythm at 75 beats/min, normal PR interval 154 ms (0.12–0.20 ms) without ST–T elevations or signs of ischaemic and QTc of 413 ms (400–440 ms) (figure 3). A Zio patch was placed to monitor for any arrhythmias, which demonstrated sinus rhythm without any significant supraventricular tachycardia, atrial fibrillation, ventricular tachycardia (VT) or pauses.
Figure 3.
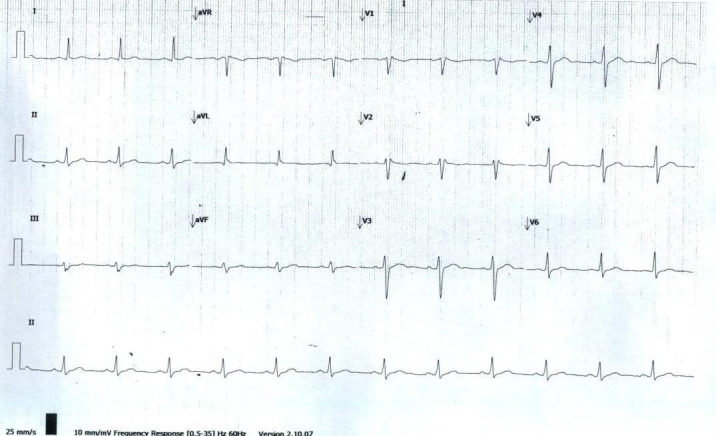
Repeat 12 lead ECG.
Discussion
BrS is an inherited cardiac channelopathy that compromises ion channel integrity and leads to transmembrane current alterations in cardiomyocytes. The clinical significance of BrS is underscored by its association with ventricular fibrillation (VF) with progression to SCD. Its true prevalence is unknown, as a consequence of frequently being asymptomatic and thus underdiagnosed, but is estimated to be between 1:2000 and 1:5000, and is likely more common in Asian populations.8 Although BrS exhibits characteristic ECG patterns, patients are often young, healthy and unaware of their condition until after a syncopal episode or sudden cardiac arrest. In fact, the peak presentation is said to be between 38 and 48 years of age.9 ECG criteria for diagnosis include ST elevation and/or R′ in V1–V3 followed by an inverted T-wave (Coved pattern—type 1) or by a Saddleback pattern (type 2).10 The most commonly identified genetic lesion is a loss-of-function mutation in SCN5A, which encodes cardiac sodium channel alpha subunit NaV1.5.11 Many susceptibility loci have been identified in association with BrS, although SCN5A is the only gene classified as having definitive evidence as a cause for BrS.12
The distinction between BrS and Brugada pattern (asymptomatic but with characteristic ECG findings) has important implications for patient management. Placement of an implantable cardioverter-defibrillator (ICD) is indicated in patients with BrS who have survived sudden cardiac arrest or have sustained VT, but individuals with BrS pattern and no signs, symptoms or family history of BrS do not benefit from a defibrillator.13 Despite being asymptomatic, those who have Brugada pattern on ECG carry a risk of VF up to 12% at 10 years, so the clinical threshold to perform an ECG should remain low if there is any suspicion of BrS.14
BrS is often elicited from various inciting stressors, such as fever, alcohol and certain medications, including those commonly used in critical care scenarios. Fever is a particularly well described variable in the aetiology and prognosis of BrS, with higher temperatures increasing the risk for cardiac arrest in BrS.15–17 It is known that the biochemical capacity of cardiac sodium channels declines at higher temperatures, and, importantly, fever-exacerbated BrS appears to not be mutation-specific.18 19 Our patient’s DNA was not sequenced to determine his mutation status at the SCN5A locus, but it is noteworthy that some authors contend that even heterozygous carriers for the mutation can display meaningful pathology in the context of fever or other unmasking states.19 COVID-19 is typified by the presence of fever, among a constellation of other constitutional and/or pulmonary symptoms, and thus can be an important risk factor in unmasking BrS. The severe inflammatory response frequently seen with COVID-19 can lead to fever-induced arrhythmias and, to some extent, dictate the prognostic outcomes. In our case it is notable that the patient was afebrile during the hospital stay.
A prior case report had demonstrated unmasking of Brugada pattern in a febrile patient with COVID-19.7 One study demonstrated that the prevalence of Brugada in patients with fever is 20 times higher than in afebrile patients, which emphasises the potent unmasking power of systemic fever.20 To our knowledge, our case is unique in that the patient remained afebrile, suggesting an alternate mechanism for unmasking the Brugada pattern perhaps related to the inflammatory response. Given this patient’s elevated inflammatory markers, one could surmise that even if this individual’s fever was suppressed by antipyretics like acetaminophen, the milieu of circulating cytokines such as IL-6 and other mediators (procalcitonin, ESR and so on) may contribute to the transient malfunctioning of the cardiac sodium channels, which precipitates Brugada pattern on ECG. Previous reports have noted that acetaminophen use in this context seems to reduce the risk for fever-induced cardiac arrest, but as is illustrated by our case, it remains unclear if antipyretic use has any effect on the development of Brugada pattern.16 Despite this uncertainty, healthcare providers should be aware of this phenomenon and prioritise treatment of fever and avoidance of drugs that could provoke BrS, such as sodium channel blockers or tricyclic antidepressants, if characteristic ECG changes are recognised in the setting of COVID-19.
Current guidelines for management of BrS is based on symptoms with the exception of patients with a strong family history of sudden cardiac arrest. For these asymptomatic patients with Brugada pattern on ECG without family history of SCD, syncope and ventricular tachyarrhythmias, there is no indication for treatment.21 However, ICD placement may be considered if ventricular arrhythmia is induced during an electrophysiology (EP) study. In symptomatic patients (ie, those with sustained ventricular tachyarrhythmias leading to syncope with type I Brugada on ECG or those with prior cardiac arrest), the recommendation is ICD placement. Certain patients may refuse ICD placement if he or she is not a candidate for the procedure (limited life expectancy or significant comorbidities); these individuals can be managed medically with antiarrhythmic therapy (ie, quinidine or amiodarone). Lastly, catheter ablation is also an option for those who fail antiarrhythmic therapy or continue to receive repeated shocks from ICD due to high burden of ventricular tachyarrhythmias.22 23
Learning points.
This is a classic arrhythmia that has been known to be exacerbated by fever. We have seen Brugada pattern with a number of COVID-19 cases and do not know the long-term cardiac risk factors in these patients.
These patients should be closely monitored on telemetry or in the intensive care unit if there is any respiratory compromise.
Fever should be adequately treated and make sure to rule out other infectious aetiologies.
There are no indications for invasive interventions in asymptomatic individuals with Brugada pattern on ECG, unless there is a family history of channelopathies, syncope or sudden cardiac death, or if a ventricular arrhythmia is induced during an electrophysiology study.
Footnotes
Contributors: OMA and AB thought of the idea to write the case report. We discussed the idea with CMT who was helpful with developing the idea, reviewing the case report including the EKG. We also had the help of ZF, our medical student who was helpful with the chart review, literature review and write-up as well. All authors discussed the results and contributed to the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Lu H, Stratton CW, Tang Y-W. Outbreak of pneumonia of unknown etiology in Wuhan, China: the mystery and the miracle. J Med Virol 2020;92:401–2. 10.1002/jmv.25678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hessami A, Shamshirian A, Heydari K. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis [published online ahead of print, 2020 Oct 16]. Am J Emerg Med 2020;S0735-6757:30908–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu C-I, Postema PG, Arbelo E, et al. SARS-CoV-2, COVID-19, and inherited arrhythmia syndromes. Heart Rhythm 2020;17:1456–62. 10.1016/j.hrthm.2020.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsimploulis A, Rashba EJ, Rahman T, et al. Medication unmasked Brugada syndrome and cardiac arrest in a COVID-19 patient. HeartRhythm Case Rep 2020;6:554–7. 10.1016/j.hrcr.2020.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vidovich MI. Transient Brugada-Like Electrocardiographic Pattern in a Patient With COVID-19. JACC Case Rep 2020;2:1245–9. 10.1016/j.jaccas.2020.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lugenbiel P, Roth L, Seiz M, et al. The arrhythmogenic face of COVID-19: Brugada ECG pattern during acute infection. Eur Heart J Case Rep 2020;4:1–2. 10.1093/ehjcr/ytaa230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chang D, Saleh M, Garcia-Bengo Y, et al. COVID-19 infection unmasking Brugada syndrome. HeartRhythm Case Rep 2020;6:237–40. 10.1016/j.hrcr.2020.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brugada J, Campuzano O, Arbelo E, et al. Present status of Brugada syndrome: JACC state-of-the-art review. J Am Coll Cardiol 2018;72:1046–59. [DOI] [PubMed] [Google Scholar]
- 9.Milman A, Andorin A, Gourraud J-B, et al. Age of first arrhythmic event in Brugada syndrome: data from the SABRUS (survey on arrhythmic events in Brugada syndrome) in 678 patients. Circ Arrhythm Electrophysiol 2017;10:e005222. 10.1161/CIRCEP.117.005222 [DOI] [PubMed] [Google Scholar]
- 10.Bayés de Luna A, Brugada J, Baranchuk A. Current electrocardiographic criteria for diagnosis of Brugada pattern: a consensus report [published correction appears in J Electrocardiol. 2013 Jan-Feb;46(1):76]. J Electrocardiol. 2012;45:433–42. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe H, Koopmann TT, Le Scouarnec S, et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J Clin Invest 2008;118:2260–8. 10.1172/JCI33891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseini SM, Kim R, Udupa S, et al. Reappraisal of reported genes for sudden arrhythmic death: evidence-based evaluation of gene validity for Brugada syndrome. Circulation 2018;138:1195–205. 10.1161/CIRCULATIONAHA.118.035070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Probst V, Veltmann C, Eckardt L, et al. Long-Term prognosis of patients diagnosed with Brugada syndrome: results from the finger Brugada syndrome registry. Circulation 2010;121:635–43. 10.1161/CIRCULATIONAHA.109.887026 [DOI] [PubMed] [Google Scholar]
- 14.Sacher F, Probst V, Maury P, et al. Outcome after implantation of a cardioverter-defibrillator in patients with Brugada syndrome: a multicenter study-part 2. Circulation 2013;128:1739–47. 10.1161/CIRCULATIONAHA.113.001941 [DOI] [PubMed] [Google Scholar]
- 15.Michowitz Y, Milman A, Sarquella-Brugada G, et al. Fever-Related arrhythmic events in the multicenter survey on arrhythmic events in Brugada syndrome. Heart Rhythm 2018;15:1394–401. 10.1016/j.hrthm.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 16.Amin AS, Meregalli PG, Bardai A, et al. Fever increases the risk for cardiac arrest in the Brugada syndrome. Ann Intern Med 2008;149:216–8. 10.7326/0003-4819-149-3-200808050-00020 [DOI] [PubMed] [Google Scholar]
- 17.Mizusawa Y, Morita H, Adler A, et al. Prognostic significance of fever-induced Brugada syndrome. Heart Rhythm 2016;13:1515–20. 10.1016/j.hrthm.2016.03.044 [DOI] [PubMed] [Google Scholar]
- 18.Dumaine R, Towbin JA, Brugada P, et al. Ionic mechanisms responsible for the electrocardiographic phenotype of the Brugada syndrome are temperature dependent. Circ Res 1999;85:803–9. 10.1161/01.RES.85.9.803 [DOI] [PubMed] [Google Scholar]
- 19.Keller DI, Rougier J-S, Kucera JP, et al. Brugada syndrome and fever: genetic and molecular characterization of patients carrying SCN5A mutations. Cardiovasc Res 2005;67:510–9. 10.1016/j.cardiores.2005.03.024 [DOI] [PubMed] [Google Scholar]
- 20.Adler A, Topaz G, Heller K, et al. Fever-induced Brugada pattern: how common is it and what does it mean? Heart Rhythm 2013;10:1375–82. 10.1016/j.hrthm.2013.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Takagi M, Tatsumi H, Yoshiyama M. Approach to the asymptomatic patients with Brugada syndrome. Indian Pacing Electrophysiol J 2007;7:73–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Brugada J, Campuzano O, Arbelo E, et al. Present status of Brugada syndrome. J Am Coll Cardiol 2018;72:1046–59. 10.1016/j.jacc.2018.06.037 [DOI] [PubMed] [Google Scholar]
- 23.Priori SG, Wilde AA, Horie M, et al. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by Hrs, EHRA, and APHRS in may 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm 2013;10:1932–63. 10.1016/j.hrthm.2013.05.014 [DOI] [PubMed] [Google Scholar]


