Abstract
Background:
Inflammatory cytokines have been implicated in organ fibrosis, however their role in the development of arthrofibrosis after total knee arthroplasty (TKA) has not been well explored. The purpose of this study was to assess whether perioperative synovial fluid or blood plasma cytokine levels are associated with reduced early post-TKA range of motion (ROM).
Methods:
179 patients with end-stage idiopathic osteoarthritis undergoing TKA were enrolled in this prospective cohort study. Synovial fluid and blood plasma were collected pre-arthrotomy and plasma was collected longitudinally in the Post-Acute Care Unit (PACU) and on post-operative days (PODs) 1 and 2. Stiffness was defined as ≤ 95° ROM measured with a goniometer at 6 weeks (± 2 weeks).
Results:
Thirty-two out of 162 (19.8%) patients analyzed were stiff at 6 weeks postoperatively. Postoperative plasma levels of 9 cytokines (Eotaxin3, IL5, Il12_23p40, IP10, VEGF, IL7, IL12p70, IL16, IL17a) were significantly different between stiff and non-stiff patients on POD 1 and/or POD2. An association between preoperative plasma and synovial fluid cytokine levels and the development of postoperative stiffness was not detected.
Conclusions:
The results of this study suggest that there is a distinct acute postoperative cytokine response profile in patients who develop stiffness 6 weeks after TKA. This profile was characterized by significant differences in levels of 9 cytokines over the first two postoperative days. These results identify cytokines that are potential biomarkers for risk of early stiffness after TKA and may play a role in the pathophysiology of this outcome.
Keywords: total knee arthroplasty, postoperative stiffness, biological risk factors, cytokines, arthrofibrosis, chronic fibrotic inflammation
Introduction
More than 700,000 total knee arthroplasties (TKAs) are performed in the United States each year and the annual volume is predicted to rise to 1.5 million in 2050 [1]. Post-TKA knee stiffness is one of the most common early post-operative complications and the most common reason for reoperation (when including manipulation under anesthesia) with a reported rate of occurrence between 1.3–12% [2–4]. There is no consensus in literature regarding a universal range of motion definition that qualifies as knee “stiffness”. Yercan et al have defined knee stiffness as a flexion contracture greater than 10 degrees and/or flexion less than 95 degrees during the first 6 postoperative weeks [4]. Similarly, flexion of less than or equal to 95 degrees at 6 weeks was used in this study of “stiffness” post-TKA.
Post-surgical arthrofibrosis, which refers to a pathologic stiffening of a joint, may be triggered by an exaggerated inflammatory response leading to the production of excessive peri-articular fibrous scar tissue [5, 6]. Histopathology typically shows sub-synovial fibrosis with synovial hyperplasia, chronic inflammatory infiltration, and excessive and unregulated proliferation of collagen and fibroblasts [7]. As a common complication following TKA, this benign-appearing connective tissue hyperplasia can cause significant disability among patients because the restricted range of motion and concomitant knee pain may severely hinder postoperative rehabilitation, clinical outcomes, and negatively impact basic activities of daily living [8]. While post-operative knee stiffness is often multifactorial, the pathogenesis of post-operative arthrofibrosis is unclear and no clear biological intrinsic risk factors have been identified [3], it is likely some patients have a biological predisposition for formation of scar tissue leading to stiffness post-TKA [9]. Cytokines and adipokines have been found to play an important role in the development of organ fibrosis (such as in the pulmonary and hepatic systems) [10, 11]. For example, Interleukin (IL)-33 is strongly associated with fibrosis in chronic liver injury [12] and is increased in systemic sclerosis patients, correlating with the extent of skin sclerosis and the severity of pulmonary fibrosis. Furthermore, Demols et al showed that IL-10, a potent anti-inflammatory cytokine, controls the regeneration phase and limits the severity of fibrosis and glandular atrophy induced by repeated episodes of acute pancreatitis in mice [13]. Finally, when IL-13 was inhibited independently, it was identified as the dominant effector cytokine of fibrosis in several experimental models [14, 15].
However, the role of cytokine-mediated inflammation in the development of arthrofibrosis after TKA has not been well studied. For this reason, this study aims to describe the perioperative systemic (plasma) and local (synovial fluid) cytokine profiles of patients who do and do not develop stiffness after TKA. Specifically, our aims are to address the following questions: 1) Are preoperative plasma cytokine levels associated with development of stiffness after TKA? 2) Are preoperative synovial fluid cytokine levels associated with development of early postoperative stiffness after TKA? 3) Are early postoperative plasma cytokine levels (POD 1 and POD 2) associated with development of stiffness after TKA? Our hypothesis is that levels of a subset of plasma and synovial fluid cytokines are associated with rates of postoperative stiffness at 6 weeks after surgery.
Methods
One hundred seventy-nine patients with idiopathic end-stage OA scheduled for TKA were enrolled in this single-institution, prospective cohort study. The study protocol was approved by the Institutional Review Board (IRB#2015–361) and written informed consent was obtained from all participants. The trial was registered before patient enrollment at clinicaltrials.gov (NCT02626533; date of registration: 12/8/2015). Data were collected and hosted electronically through the Clinical and Translational Science Center’s Research Electronic Data Capture (REDCap).
Enrollment included adult patients receiving a unilateral TKA for severe osteoarthritis (OA) by participating surgeons from a single academic institution between May 2016 and February 2018. Severe OA designation was defined as radiologist description of “severe,” “end-stage,” or “bone-on-bone” osteoarthritis.
Exclusion criteria included a contraindication to regional anesthesia (our institutional standard of care), contraindications to the perioperative administration of non-steroidal anti-inflammatory drugs (NSAIDS), dexamethasone, or acetaminophen, a history of daily opioid use of 6 weeks or greater or usage of non-prescribed opioids, preoperative steroid usage within 6 months of surgery, a diagnosis or history of rheumatic or autoimmune disease, post-traumatic arthritis, periarticular injection within three months prior to surgery, American Society of Anesthesiologists (ASA) physical status score >3, pregnant women, and any active infections or current antibiotic use.
17 patients were excluded from analysis after enrollment, leaving 162 patients for analysis of clinical data and biological samples (Figure 1).
Figure 1.
STROBE patient flow diagram detailing the number of patients assessed for eligibility, enrolled in the study and included in the analysis.
We divided our cohort into two groups: 1) Group A included patients who were found to have postoperative stiffness at 6 weeks, and 2) Group B included those without post-TKA stiffness at 6 weeks. Stiffness was defined as ≤ 95° range of motion (ROM) measured by goniometer at 6 weeks (± 2 weeks). Blinded examination and measurement of post-implant radiographs was performed.
Synovial fluid was aspirated pre-arthrotomy and blood plasma was collected at multiple time points including prior to incision (pre-op), on arrival to the recovery room (PACU), and on postoperative days (POD) 1 and POD 2. Cytokines and adipokines that are known to play roles in immunity, inflammation, and angiogenesis were measured using commercially available quantitative antibody-based immunoassays: the V-Plex Human Cytokine 30-Plex Panel (29 cytokines) and individual adiponectin and leptin detection plates (Mesoscale - Rockville, Maryland, USA). These well validated assay plates have lower limits of detection in the picomolar range with details for individual cytokines presented in product inserts available on the Mesoscale company website.
Statistical Analysis
Categorical demographics and preoperative variables are summarized as counts and percentages (%) and were compared between stiffness and non-stiffness groups using Chi-Square or Fisher’s exact test. Continuous demographic and preoperative variables are presented as means and standard deviations (SD), and were compared between groups using t-test.
All continuous cytokine values are presented are means and SDs. All cytokine values were log-transformed for analysis due to skewed distributions. Undetectable cytokine levels with a minimum detection limit are imputed as ½ of the detection limit value. Undetectable cytokine levels with a maximum detection limit are imputed as the detection limit value [16]. Batch effect was identified in synovial fluid samples. A linear model for microarray data package in R was used after normalizing the cytokine values to remove the batch effect [17].
Log transformed plasma cytokine levels were compared between the stiffness (A) and non-stiffness (B) groups via generalized linear modeling using the generalized estimating equation (GEE) approach. GEE was used to account for the correlation between repeated cytokine measurements at pre-op baseline, PACU, POD1, and POD2, for the same patient, adjusting for log transformed preoperative plasma cytokine levels. Effect sizes are presented as exponentiated geometric means with 95% confidence intervals (CIs) for each group, and ratios of geometric means between stiffness and non-stiffness group with 95% CIs.
Log transformed preoperative synovial fluid cytokine levels were compared between stiff and non-stiff groups using t-test. Because of low numbers, cytokine levels of stiff patients who underwent manipulation and those who did not are presented without t-test.
Given the study outcomes are exploratory, no adjustment was performed for multiple testing. All statistical hypothesis tests were 2-sided. Statistical analyses were performed with SAS version 9.4 (SAS Institute) and R version 3.6.0.
Results
Thirty-two out of 162 (19.8%) patients met the criteria for postoperative stiffness at 6 weeks following TKA and 130 patients were in the non-stiff group. Both outcome groups were comparable in their demographic and preoperative clinical characteristics (Table 1). In addition, tobacco use, statin use, exposure to intraoperative tranexamic acid, and postoperative deep vein thrombosis (DVT) prophylaxis did not differ between groups (Table 1).
Table 1:
Patient Characteristics
| Stiff (n=32) | Non-Stiff (n=130) | P value | |
|---|---|---|---|
| Female, Count (%) | 17 (53.10) | 75 (57.7) | 0.641 |
| White, Count (%) | 26 (81.3) | 117 (89.3) | 0.22 |
| Age, mean (SD) | 65.8 (8.9) | 67.3 (7.9) | 0.351 |
| BMI, mean (SD) | 32.5 (7.7) | 31.1 (6.5) | 0.3 |
| Baseline ROM, mean (SD) | 99 (23) | 109 (13) | 0.023 |
| Joint Fluid Volume (cc), mean (SD) | 4.6 (2.4) | 4.9 (2.7) | 0.586 |
| Has Previous Surgery, Count (%) | 13 (40.6) | 54 (41.5) | 0.925 |
| History of Diabetes, Count (%) | 3 (9.4) | 17 (13.1) | 0.571 |
|
History of Hyperlipidemia, Count
(%) |
16 (50) | 57 (43.8) | 0.531 |
| History of Tobacco Use, Count (%) | 12 (37.5) | 48 (36.9) | 0.952 |
| Statin Use, Count (%) | 17 (53.1) | 50 (38.5) | 0.134 |
| Used any Intra-op TXA, Count (%) | 30 (93.8) | 123 (94.6) | 0.848 |
| DVT prophylaxis, Count (%) | |||
| Apixaban | 0 (0) | 1 (0.8) | 0.955 |
| Aspirin | 16 (50) | 66 (50.8) | |
| Coumadin/Warfarin | 10 (31.3) | 41 (31.5) | |
| Xarelto/Rivaroxaban | 6 (18.8) | 21 (16.2) | |
| None | 0 (0) | 1 (0.8) | |
| TKA Design | 0.809 | ||
| BCS (bi-cruciate stabilized) | 4 (12.5) | 12 (9.2) | |
| CR (cruciate retaining) | 1 (3.1) | 5 (3.8) | |
| PS (posterior stabilized) | 27 (84.4) | 114 (87.0) | |
| Polyethylene* | 0.558 | ||
| Conventional | 20 (62.5) | 74 (56.5) | |
| XLPE | 12 (37.5) | 57 (43.5) | |
| Femoral components* | |||
| CoCr (cobalt-chromium) | 17 (53.1) | 59 (45.0) | 0.554 |
| Oxinium | 15 (46.9) | 72 (55.0) |
For implant information not available in the electronic medical record, information was recorded with guidance from the manufacturer.
There were no significant differences between stiff and non-stiff patients for pre-operative levels of any of the 31 cytokines measured in plasma or in synovial fluid (Table 2).
Table 2:
Log transformed baseline synovial fluid cytokine values compared between Stiff and Non-Stiff Groups
| Stiff | Non-Stiff | |||||
|---|---|---|---|---|---|---|
| Cytokine | No. | Mean (SD) | No. | Mean (SD) | Differences in means (95% CI) | p-value |
| Adiponectin | 31 | 1.7 (0.9) | 122 | 1.6 (1.0) | 0.06 (−0.34, 0.46) | 0.7576 |
| Eotaxin | 31 | 5.8 (0.6) | 121 | 5.9 (0.5) | −0.14 (−0.33, 0.09) | 0.239 |
| Eotaxin3 | 29 | 2.7 (1.7) | 120 | 2.5 (1.2) | 0.00 (−0.38, 0.29) | 0.9883 |
| GM_CSF | 31 | −1.6 (0.6) | 121 | −1.5 (0.6) | 0.00 (−0.03, 0.00) | 0.5563 |
| IFNGamma | 31 | 0.6 (1.3) | 122 | 0.6 (1.7) | 0.07 (−0.43, 0.76) | 0.6766 |
| IL_10 | 31 | −2.5 (1.0) | 122 | −2.4 (1.3) | 0.15 (−0.31, 0.57) | 0.466 |
| IL1_alpha | 28 | −2.7 (1.5) | 109 | −2.8 (1.2) | 0.00 (−0.00, 0.00) | 0.5471 |
| IL1_beta | 31 | −2.7 (1.2) | 122 | −2.8 (1.7) | 0.05 (0.00, 0.72) | 0.223 |
| IL2 | 31 | −2.0 (1.5) | 122 | −1.8 (1.4) | −0.11 (−0.71, 0.31) | 0.5542 |
| IL4 | 31 | −4.0 (1.2) | 122 | −3.9 (1.7) | −0.03 (−0.61, 0.48) | 0.9259 |
| IL5 | 31 | −1.5 (0.9) | 121 | −1.4 (1.1) | 0.00 (−0.21, 0.00) | 0.2631 |
| IL6 | 31 | 5.1 (1.6) | 122 | 5.3 (1.9) | 0.16 (−0.58, 0.89) | 0.6746 |
| IL7 | 31 | 2.2 (0.4) | 121 | 2.3 (0.5) | 0.12 (−0.07, 0.31) | 0.2251 |
| IL8 | 31 | 5.0 (1.1) | 120 | 5.3 (1.4) | −0.17 (−0.58, 0.25) | 0.4162 |
| Il12_23p40 | 31 | 6.2 (0.5) | 121 | 6.2 (0.6) | −0.01 (−0.23, 0.21) | 0.9453 |
| IL12p70 | 31 | −2.6 (1.1) | 122 | −2.4 (1.5) | 0.00 (−0.32, 0.20) | 0.7272 |
| IL13 | 31 | −0.1 (0.9) | 122 | 0.1 (1.1) | 0.00 (−0.53, 0.22) | 0.6514 |
| IL15 | 31 | 3.9 (0.4) | 121 | 3.9 (0.4) | −0.01 (−0.15, 0.14) | 0.9236 |
| IL16 | 31 | 9.5 (0.8) | 121 | 9.4 (0.9) | 0.10 (−0.19, 0.39) | 0.463 |
| IL17a | 30 | 0.4 (0.5) | 119 | 0.5 (0.7) | 0.01 (−0.25, 0.28) | 0.9143 |
| IP10 | 30 | 8.5 (0.7) | 119 | 8.9 (1.0) | −0.24 (−0.50, 0.01) | 0.0605 |
| Leptin | 31 | 4.0 (1.8) | 120 | 4.0 (1.9) | 0.04 (−0.80, 0.74) | 0.9028 |
| MCP1 | 31 | 8.6 (0.5) | 121 | 8.6 (0.6) | 0.01 (−0.23, 0.25) | 0.9508 |
| MCP4 | 31 | 3.9 (1.0) | 121 | 3.6 (1.3) | 0.23 (−0.07, 0.53) | 0.1549 |
| MDC | 31 | 8.3 (0.5) | 121 | 8.4 (0.4) | 0.09 (−0.07, 0.26) | 0.271 |
| MIP1alpha | 31 | 4.2 (1.1) | 120 | 4.5 (0.7) | −0.16 (−0.43, 0.10) | 0.2523 |
| MIP1beta | 31 | 5.7 (0.8) | 121 | 5.8 (0.8) | 0.06 (−0.26, 0.37) | 0.7161 |
| TARC | 31 | 4.3 (0.6) | 121 | 4.4 (0.7) | 0.10 (−0.18, 0.38 | 0.4822 |
| TNFalpha | 31 | 0.5 (1.7) | 122 | 0.3 (0.7) | 0.01 (−0.19, 0.20) | 0.9548 |
| TNFbeta | 31 | −0.6 (0.9) | 121 | −0.7 (1.0) | 0.09 (−0.27, 0.50) | 0.6392 |
| VEGF | 31 | 8.7 (0.8) | 121 | 8.9 (0.8) | 0.16 (−0.14, 0.47) | 0.2935 |
Abbreviations- SD: standard deviation, CI: confidence interval, GM_CSF: granulocyte-macrophage colony stimulating factor, IFNgamma: interferon-gamma, IL: interleukin, IP: interferon-gamma induced protein, MCP: monocyte chemoattractant protein, MDC: macrophage-derived chemokine, MIP: macrophage inflammatory protein, TARC: thymus and activation-regulated chemokine, TNF: tumor necrosis factor, VEGF: vascular endothelium growth factor
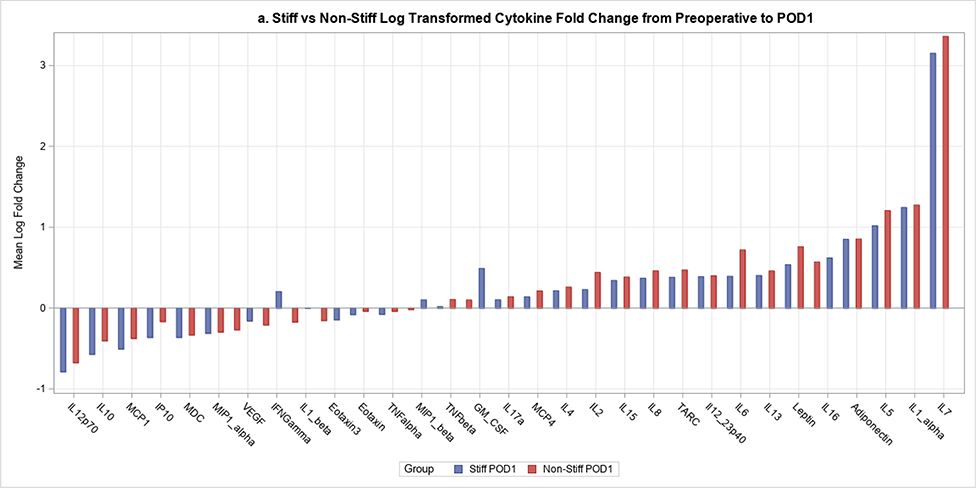
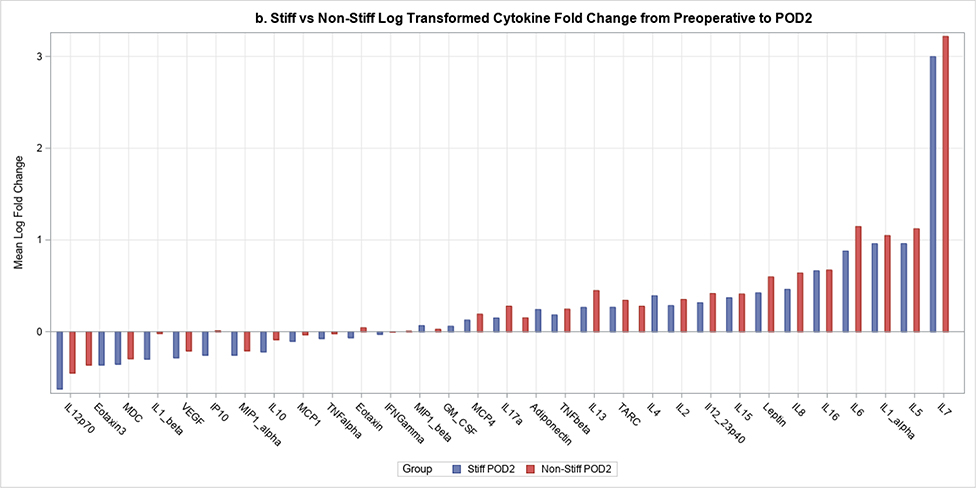
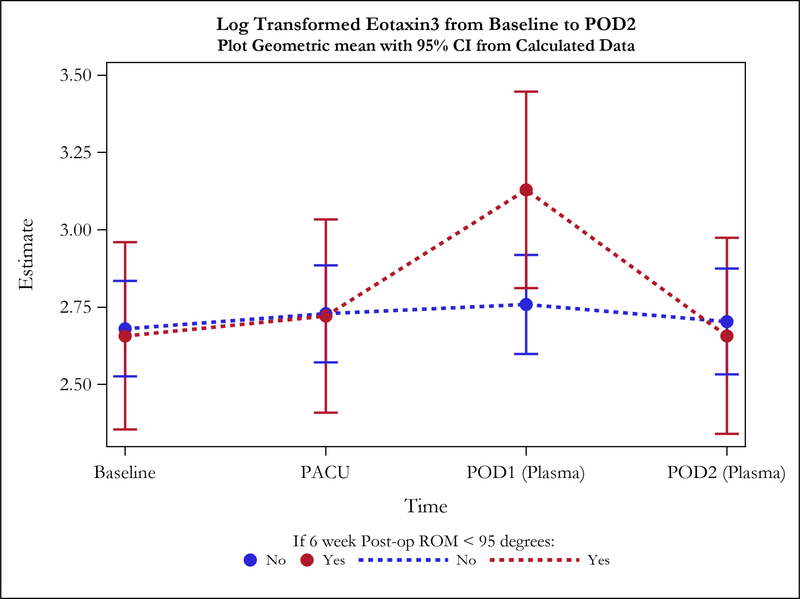
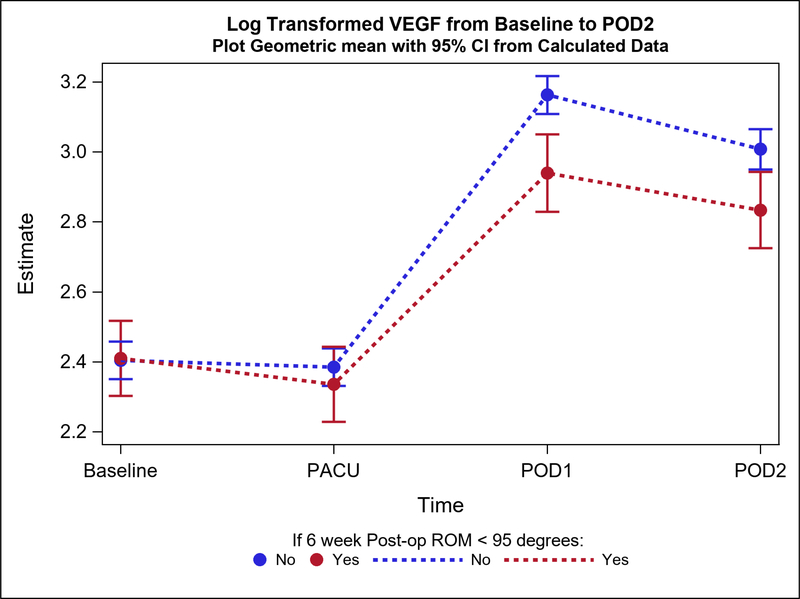
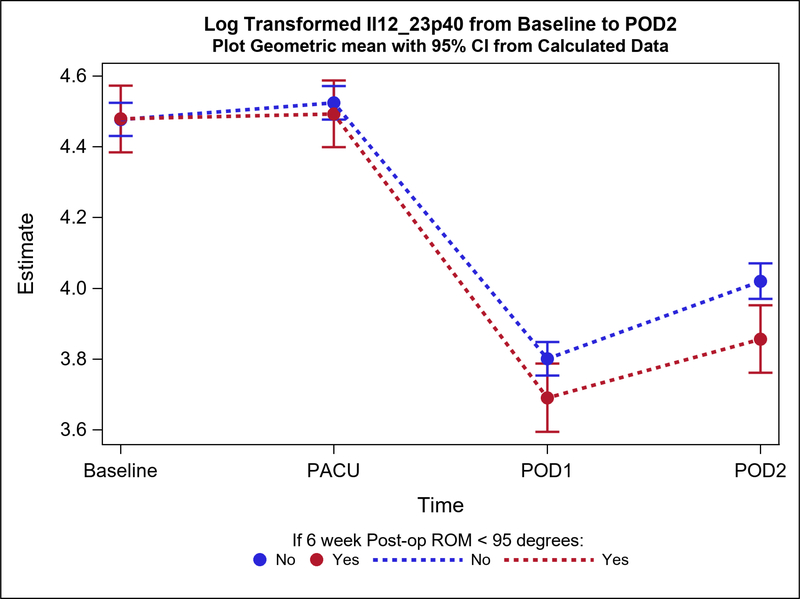
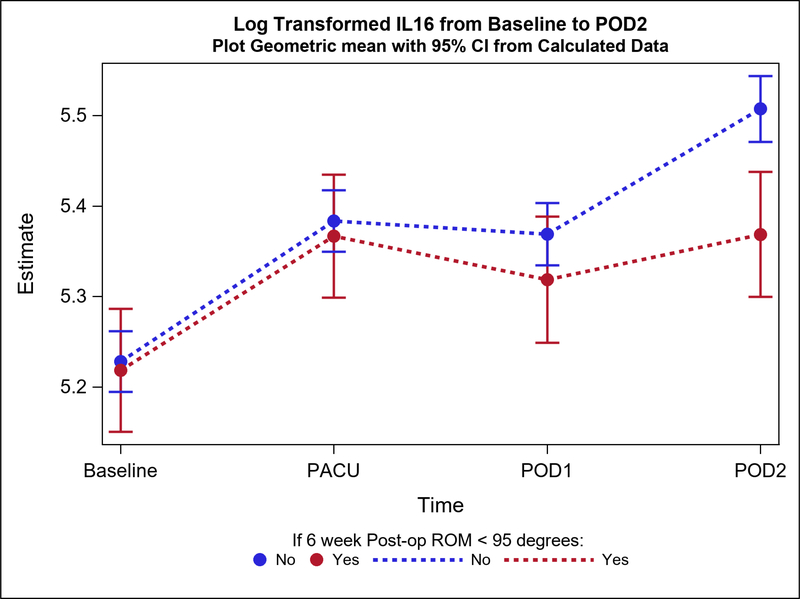
Postoperative plasma levels of 9 out of the 31 cytokines/adiponectins studied were significantly different between stiff and non-stiff patients (Table 3). Particularly, the geometric means of five cytokines of interest were significantly different between groups on POD1 (Figure 2a). These included Eotaxin 3 (p value=0.04) (Figure 3), IL-5 (p=0.04), Interferon Gamma Induced Protein 10 (IP10; p=0.04), IL-12/23p40 (p=0.04), and Vascular Endothelial Growth Factor (VEGF; p=0.0004) (Figure 4). In addition to differences observed between groups by POD1, two of these cytokines showed significant differences on POD2 as well (IL-12/23p40, p=0.003 at POD2; VEGF, p=0.005 at POD2) (Figures 2b, 4 and 5). Finally, the geometric means of four additional cytokines of interest were significantly different between groups only on POD2 (no differences on POD1) (Figure 2b). These included IL-7 (p=0.04), IL-12p70 (p=0.03), IL-16 (p=0.0005) (Figure 6), and IL-17a (p=0.008).
Table 3:
Plasma cytokine levels between stiff and non-stiff groups over study time periods (bold and underlined: cytokines with significant differences between groups).
| Stiff | Non-Stiff | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cytokine | Time | Number | Mean (SD) | Geometric Mean (95% CI) | Number | Mean (SD) | Geometric Mean (95% CI) | Ratio of Geometric Mean (Stiffness/Nonstiffness) (95% CI) | p-value |
| Adiponectin | Baseline | 32 | 16.3 (10.3) | 12.69 (11.63, 13.86) | 130 | 15.2 (9.8) | 12.61 (12.07, 13.17) | 1.01 (0.91, 1.11) | 0.895 |
| PACU | 32 | 16.4 (10.6) | 12.69 (11.63, 13.86) | 129 | 15.3 (9.9) | 12.63 (12.09, 13.20) | 1.01 (0.91, 1.11) | 0.918 | |
| POD1 | 30 | 14.9 (9.2) | 11.70 (10.69, 12.80) | 126 | 14.7 (9.0) | 12.13 (11.61, 12.68) | 0.96 (0.87, 1.07) | 0.476 | |
| POD2 | 31 | 15.7 (9.5) | 11.89 (10.88, 12.99) | 109 | 15.0 (10.2) | 13.12 (12.51, 13.75) | 0.91 (0.82, 1.00) | 0.055 | |
| Eotaxin | Baseline | 31 | 135.7 (53.5) | 129.19 (118.94, 140.33) | 129 | 140.6 (63.6) | 129.82 (124.66, 135.19) | 1.00 (0.91, 1.09) | 0.918 |
| PACU | 31 | 133.1 (52.1) | 124.53 (114.50, 135.44) | 127 | 132.1 (53.3) | 123.15 (118.20, 128.30) | 1.01 (0.92, 1.11) | 0.814 | |
| POD1 | 30 | 122.9 (51.4) | 112.34 (103.15, 122.35) | 123 | 121.8 (51.1) | 111.45 (106.89, 116.21) | 1.01 (0.92, 1.11) | 0.869 | |
| POD2 | 30 | 93.6 (34) | 90.23 (82.85, 98.28) | 103 | 99.3 (38.7) | 90.98 (86.93, 95.23) | 0.99 (0.90, 1.09) | 0.866 | |
| Eotaxin3 | Baseline | 32 | 40.4 (81.8) | 14.25 (10.53, 19.29) | 123 | 25.6 (37.3) | 14.59 (12.50, 17.03) | 0.98 (0.70, 1.37) | 0.892 |
| PACU | 30 | 30.2 (50.2) | 15.20 (11.12, 20.78) | 127 | 27.0 (34.8) | 15.31 (13.09, 17.91) | 0.99 (0.70, 1.41) | 0.969 | |
| POD1 | 29 | 43.3 (66.8) | 22.86 (16.64, 31.40) | 122 | 22.3 (19.3) | 15.78 (13.44, 18.52) | 1.45 (1.02, 2.07) | 0.040 | |
| POD2 | 29 | 31.7 (51.7) | 14.26 (10.38, 19.58) | 104 | 25.2 (29.3) | 14.93 (12.58, 17.71) | 0.96 (0.67, 1.37) | 0.802 | |
| GM_CSF | Baseline | 31 | 0.4 (0.4) | 0.28 (0.21, 0.37) | 130 | 0.5 (0.5) | 0.31 (0.27, 0.36) | 0.88 (0.64, 1.22) | 0.456 |
| PACU | 32 | 0.8 (2.5) | 0.32 (0.24, 0.42) | 129 | 0.5 (0.4) | 0.34 (0.30, 0.40) | 0.92 (0.67, 1.27) | 0.613 | |
| POD1 | 30 | 1.4 (4.1) | 0.33 (0.24, 0.44) | 126 | 0.4 (0.5) | 0.25 (0.22, 0.29) | 1.31 (0.94, 1.83) | 0.108 | |
| POD2 | 31 | 0.5 (1.0) | 0.27 (0.20, 0.36) | 109 | 0.6 (1.0) | 0.31 (0.27, 0.36) | 0.86 (0.62, 1.20) | 0.383 | |
| IFNGamma | Baseline | 32 | 2.9 (1.7) | 2.46 (2.00, 3.02) | 130 | 3.6 (4.7) | 2.42 (2.19, 2.68) | 1.02 (0.81, 1.28) | 0.894 |
| PACU | 32 | 2.6 (1.5) | 2.13 (1.74, 2.62) | 129 | 3.6 (4.7) | 2.42 (2.18, 2.68) | 0.88 (0.70, 1.11) | 0.288 | |
| POD1 | 30 | 1.7 (0.9) | 1.39 (1.12, 1.72) | 126 | 2.5 (2.9) | 1.61 (1.45, 1.78) | 0.86 (0.68, 1.09) | 0.221 | |
| POD2 | 31 | 2.9 (3.4) | 1.98 (1.61, 2.44) | 109 | 3.1 (3.1) | 2.24 (2.00, 2.50) | 0.88 (0.70, 1.12) | 0.303 | |
| IL_10 | Baseline | 32 | 0.2 (0.2) | 0.17 (0.14, 0.22) | 130 | 0.2 (0.2) | 0.17 (0.15, 0.18) | 1.05 (0.83, 1.34) | 0.664 |
| PACU | 32 | 1.2 (0.9) | 0.92 (0.74, 1.14) | 129 | 1.0 (0.9) | 0.76 (0.68, 0.84) | 1.21 (0.95, 1.54) | 0.124 | |
| POD1 | 30 | 0.8 (0.6) | 0.60 (0.48, 0.75) | 126 | 0.8 (0.7) | 0.59 (0.53, 0.66) | 1.02 (0.80, 1.31) | 0.849 | |
| POD2 | 31 | 0.6 (0.4) | 0.46 (0.37, 0.57) | 109 | 0.5 (0.3) | 0.47 (0.42, 0.53) | 0.97 (0.76, 1.24) | 0.816 | |
| IL1_alpha | Baseline | 32 | 0.4 (0.6) | 0.16 (0.10, 0.25) | 127 | 0.5 (0.8) | 0.16 (0.13, 0.20) | 1.03 (0.63, 1.66) | 0.915 |
| PACU | 31 | 0.6 (1.0) | 0.18 (0.11, 0.28) | 127 | 0.5 (1.1) | 0.16 (0.13, 0.19) | 1.14 (0.69, 1.85) | 0.612 | |
| POD1 | 29 | 0.5 (1.1) | 0.15 (0.10, 0.24) | 124 | 0.7 (3.2) | 0.13 (0.10, 0.16) | 1.17 (0.71, 1.93) | 0.546 | |
| POD2 | 29 | 0.3 (0.4) | 0.12 (0.08, 0.19) | 105 | 0.5 (1.3) | 0.16 (0.12, 0.20) | 0.75 (0.45, 1.26) | 0.279 | |
| IL1_beta | Baseline | 32 | 0.1 (0.1) | 0.07 (0.06, 0.10) | 122 | 0.6 (5.4) | 0.08 (0.07, 0.09) | 0.98 (0.73, 1.31) | 0.894 |
| PACU | 32 | 0.1 (0.1) | 0.08 (0.06, 0.10) | 121 | 0.1 (0.1) | 0.08 (0.07, 0.09) | 0.98 (0.73, 1.31) | 0.884 | |
| POD1 | 30 | 0.1 (0.2) | 0.10 (0.07, 0.13) | 122 | 0.4 (2.0) | 0.12 (0.10, 0.14) | 0.82 (0.61, 1.11) | 0.198 | |
| POD2 | 31 | 0.2 (0.2) | 0.10 (0.08, 0.13) | 104 | 0.2 (0.2) | 0.11 (0.10, 0.13) | 0.89 (0.66, 1.21) | 0.469 | |
| IL2 | Baseline | 32 | 0.7 (3.5) | 0.09 (0.08, 0.11) | 125 | 0.1 (0.2) | 0.09 (0.08, 0.10) | 0.99 (0.81, 1.21) | 0.936 |
| PACU | 32 | 0.7 (3.3) | 0.10 (0.08, 0.12) | 129 | 0.1 (0.2) | 0.10 (0.09, 0.11) | 1.00 (0.82, 1.23) | 0.972 | |
| POD1 | 30 | 0.7 (3.1) | 0.11 (0.09, 0.14) | 122 | 0.2 (0.3) | 0.12 (0.11, 0.13) | 0.95 (0.77, 1.17) | 0.626 | |
| POD2 | 31 | 0.6 (2.8) | 0.14 (0.11, 0.16) | 109 | 0.2 (0.2) | 0.12 (0.11, 0.14) | 1.10 (0.89, 1.35) | 0.39 | |
| IL4 | Baseline | 32 | 0.0 (0.0) | 0.01 (0.01, 0.01) | 130 | 0.0 (0.0) | 0.01 (0.01, 0.01) | 1.02 (0.80, 1.30) | 0.873 |
| PACU | 32 | 0.0 (0.0) | 0.01 (0.01, 0.01) | 129 | 0.0 (0.0) | 0.01 (0.01, 0.01) | 0.88 (0.69, 1.11) | 0.275 | |
| POD1 | 30 | 0.0 (0.0) | 0.03 (0.02, 0.03) | 126 | 0.0 (0.1) | 0.03 (0.03, 0.03) | 0.86 (0.68, 1.11) | 0.245 | |
| POD2 | 31 | 0.0 (0.0) | 0.02 (0.02, 0.03) | 109 | 0.0 (0.0) | 0.03 (0.02, 0.03) | 0.86 (0.67, 1.10) | 0.220 | |
| IL5 | Baseline | 32 | 0.3 (0.3) | 0.25 (0.18, 0.35) | 130 | 0.4 (0.5) | 0.27 (0.22, 0.31) | 0.95 (0.65, 1.39) | 0.795 |
| PACU | 32 | 0.4 (0.5) | 0.23 (0.16, 0.32) | 129 | 0.4 (0.5) | 0.25 (0.21, 0.30) | 0.90 (0.62, 1.32) | 0.595 | |
| POD1 | 30 | 0.6 (0.7) | 0.36 (0.26, 0.51) | 126 | 2.5 (7.9) | 0.54 (0.45, 0.64) | 0.68 (0.46, 1.00) | 0.048 | |
| POD2 | 31 | 1.2 (2.1) | 0.62 (0.44, 0.87) | 109 | 2.7 (9.3) | 0.84 (0.70, 1.01) | 0.73 (0.50, 1.08) | 0.115 | |
| IL6 | Baseline | 32 | 1.1 (0.9) | 0.78 (0.62, 0.99) | 130 | 1.1 (1.8) | 0.75 (0.67, 0.84) | 1.04 (0.81, 1.35) | 0.738 |
| PACU | 32 | 1.4 (1.6) | 1.03 (0.82, 1.30) | 129 | 5.5 (48.9) | 1.07 (0.95, 1.20) | 0.97 (0.75, 1.25) | 0.803 | |
| POD1 | 30 | 23 (15.1) | 18.13 (14.29, 23.00) | 126 | 29.3 (27.4) | 21.49 (19.12, 24.14) | 0.84 (0.65, 1.10) | 0.208 | |
| POD2 | 31 | 19.6 (12.6) | 15.79 (12.49, 19.96) | 109 | 23.7 (22.8) | 18.71 (16.52, 21.20) | 0.84 (0.65, 1.10) | 0.210 | |
| IL7 | Baseline | 32 | 1.4 (0.8) | 1.15 (1.01, 1.31) | 130 | 1.3 (0.6) | 1.13 (1.06, 1.20) | 1.02 (0.88, 1.18) | 0.775 |
| PACU | 32 | 1.6 (0.7) | 1.40 (1.23, 1.59) | 129 | 1.7 (1.0) | 1.56 (1.46, 1.66) | 0.90 (0.78, 1.04) | 0.148 | |
| POD1 | 30 | 2.0 (1.0) | 1.66 (1.45, 1.89) | 126 | 2.0 (1.2) | 1.79 (1.68, 1.91) | 0.93 (0.80, 1.08) | 0.319 | |
| POD2 | 31 | 2.1 (0.9) | 1.83 (1.61, 2.09) | 109 | 2.3 (1.3) | 2.13 (1.99, 2.29) | 0.86 (0.74, 1.00) | 0.043 | |
| IL8 | Baseline | 32 | 4.6 (3.5) | 3.96 (3.51, 4.46) | 130 | 4.8 (6.1) | 3.97 (3.74, 4.21) | 1.00 (0.87, 1.14) | 0.959 |
| PACU | 32 | 4.9 (4.2) | 4.03 (3.58, 4.54) | 128 | 4.9 (6.2) | 3.85 (3.63, 4.09) | 1.05 (0.92, 1.19) | 0.508 | |
| POD1 | 30 | 6.9 (5.2) | 5.81 (5.14, 6.56) | 126 | 7.3 (7.7) | 5.96 (5.61, 6.33) | 0.97 (0.85, 1.12) | 0.705 | |
| POD2 | 31 | 6.5 (5.6) | 5.47 (4.85, 6.17) | 109 | 7.8 (9.4) | 6.06 (5.69, 6.46) | 0.90 (0.79, 1.03) | 0.138 | |
| Il12_23p40 | Baseline | 32 | 100.5 (51) | 88.13 (80.23, 96.82) | 130 | 98.7 (46.2) | 87.99 (83.98, 92.19) | 1.00 (0.90, 1.11) | 0.975 |
| PACU | 32 | 101.0 (48.0) | 89.39 (81.37, 98.19) | 129 | 103.7 (48.4) | 92.23 (88.00, 96.67) | 0.97 (0.87, 1.08) | 0.557 | |
| POD1 | 30 | 46.1 (24.4) | 40.07 (36.38, 44.13) | 126 | 50.6 (27.8) | 44.72 (42.65, 46.89) | 0.90 (0.80, 1.00) | 0.045 | |
| POD2 | 31 | 55.4 (33.1) | 47.30 (43.00, 52.02) | 109 | 63.9 (39.4) | 55.70 (52.97, 58.59) | 0.85 (0.76, 0.95) | 0.003 | |
| IL12p70 | Baseline | 32 | 0.1 (0.0) | 0.08 (0.07, 0.09) | 124 | 0.1 (0.3) | 0.08 (0.07, 0.09) | 0.98 (0.82, 1.18) | 0.835 |
| PACU | 32 | 0.1 (0.0) | 0.08 (0.07, 0.09) | 123 | 0.1 (0.3) | 0.09 (0.08, 0.09) | 0.91 (0.76, 1.10) | 0.335 | |
| POD1 | 30 | 0.1 (0.1) | 0.12 (0.10, 0.14) | 125 | 0.2 (0.4) | 0.13 (0.12, 0.14) | 0.93 (0.77, 1.12) | 0.460 | |
| POD2 | 31 | 0.1 (0.1) | 0.10 (0.09, 0.12) | 108 | 0.2 (0.4) | 0.13 (0.11, 0.14) | 0.82 (0.68, 0.99) | 0.037 | |
| IL13 | Baseline | 32 | 0.3 (0.3) | 0.31 (0.26, 0.35) | 123 | 0.3 (0.2) | 0.31 (0.29, 0.33) | 0.99 (0.84, 1.17) | 0.910 |
| PACU | 32 | 0.4 (0.3) | 0.31 (0.27, 0.36) | 123 | 0.4 (0.2) | 0.31 (0.29, 0.33) | 0.99 (0.84, 1.17) | 0.949 | |
| POD1 | 30 | 0.5 (0.3) | 0.44 (0.38, 0.51) | 123 | 0.6 (0.4) | 0.45 (0.42, 0.49) | 0.97 (0.82, 1.14) | 0.691 | |
| POD2 | 31 | 0.5 (0.3) | 0.44 (0.38, 0.51) | 107 | 0.6 (0.4) | 0.47 (0.43, 0.51) | 0.95 (0.80, 1.12) | 0.530 | |
| IL15 | Baseline | 32 | 1.8 (0.5) | 1.68 (1.56, 1.80) | 130 | 1.7 (0.6) | 1.66 (1.60, 1.72) | 1.01 (0.93, 1.09) | 0.827 |
| PACU | 32 | 1.8 (0.8) | 1.65 (1.53, 1.77) | 129 | 1.7 (0.5) | 1.63 (1.57, 1.69) | 1.01 (0.93, 1.09) | 0.831 | |
| POD1 | 30 | 3.5 (1.6) | 3.11 (2.89, 3.35) | 126 | 3.2 (1.5) | 2.94 (2.84, 3.05) | 1.06 (0.98, 1.15) | 0.167 | |
| POD2 | 31 | 3.5 (1.1) | 3.25 (3.02, 3.50) | 109 | 3.4 (1.0) | 3.26 (3.14, 3.39) | 1.00 (0.92, 1.08) | 0.952 | |
| IL16 | Baseline | 32 | 189.8 (66.1) | 184.64 (172.50, 197.65) | 130 | 198.2 (61.3) | 186.46 (180.27, 192.86) | 0.99 (0.92, 1.07) | 0.800 |
| PACU | 32 | 219.1 (70.8) | 214.19 (200.09, 229.27) | 129 | 229.0 (67.6) | 217.80 (210.52, 225.33) | 0.98 (0.91, 1.06) | 0.666 | |
| POD1 | 30 | 209.2 (71.3) | 204.12 (190.34, 218.89) | 126 | 232.5 (97.7) | 214.64 (207.40, 222.14) | 0.95 (0.88, 1.03) | 0.205 | |
| POD2 | 31 | 220.0 (73.9) | 214.60 (200.31, 229.92) | 109 | 262.9 (99.7) | 246.53 (237.70, 255.68) | 0.87 (0.81, 0.94) | 0.000 | |
| IL17a | Baseline | 32 | 2.2 (2.0) | 1.61 (1.36, 1.90) | 128 | 2.2 (2.0) | 1.61 (1.48, 1.75) | 1.00 (0.83, 1.20) | 0.983 |
| PACU | 32 | 1.8 (1.4) | 1.39 (1.18, 1.65) | 125 | 2.2 (2.2) | 1.63 (1.50, 1.77) | 0.86 (0.71, 1.03) | 0.108 | |
| POD1 | 30 | 1.5 (1.1) | 1.13 (0.95, 1.34) | 123 | 2.1 (2.4) | 1.36 (1.25, 1.48) | 0.83 (0.68, 1.00) | 0.055 | |
| POD2 | 31 | 1.5 (1.1) | 1.24 (1.05, 1.47) | 106 | 2.4 (4.6) | 1.61 (1.47, 1.77) | 0.77 (0.64, 0.93) | 0.008 | |
| IP10 | Baseline | 32 | 332.5 (344.3) | 267.28 (240.44, 297.13) | 130 | 304.7 (197.5) | 267.32 (253.65, 281.74) | 1.00 (0.89, 1.13) | 0.998 |
| PACU | 32 | 307.8 (284.4) | 251.71 (226.43, 279.82) | 128 | 331.3 (333.6) | 279.38 (264.93, 294.62) | 0.90 (0.80, 1.01) | 0.084 | |
| POD1 | 30 | 193.3 (124.0) | 161.72 (145.00, 180.36) | 126 | 199.6 (102.8) | 183.30 (173.75, 193.37) | 0.88 (0.78, 1.00) | 0.043 | |
| POD2 | 31 | 316.6 (385.5) | 242.08 (217.42, 269.53) | 109 | 294.4 (172.0) | 258.84 (244.43, 274.09) | 0.94 (0.83, 1.06) | 0.280 | |
| Leptin | Baseline | 32 | 30.6 (34.6) | 14.85 (12.82, 17.19) | 128 | 30.9 (33.2) | 14.71 (13.69, 15.81) | 1.01 (0.86, 1.19) | 0.914 |
| PACU | 32 | 32.4 (35.8) | 14.68 (12.68, 17.00) | 126 | 30.4 933.7) | 14.48 (13.45, 15.59) | 1.01 (0.86, 1.19) | 0.868 | |
| POD1 | 30 | 34.0 (37.8) | 34.54 (29.53, 40.40) | 125 | 57.1 (50.2) | 34.52 (32.06, 37.16) | 1.00 (0.84, 1.19) | 0.994 | |
| POD2 | 31 | 39.8 (39.4) | 19.08 (16.39, 22.19) | 108 | 34.5 (35.5) | 17.23 (15.92, 18.65) | 1.11 (0.93, 1.31) | 0.243 | |
| MCP1 | Baseline | 32 | 67.4 (18.0) | 64.22 (58.44, 70.57) | 130 | 66.3 (19.8) | 63.95 (61.02, 67.01) | 1.00 (0.90, 1.12) | 0.937 |
| PACU | 32 | 56.2 (20.6) | 52.35 (47.64, 57.53) | 128 | 54.5 (19.0) | 51.62 (49.24, 54.13) | 1.01 (0.91, 1.13) | 0.794 | |
| POD1 | 30 | 79.1 (32.1) | 73.63 (66.80, 81.15) | 126 | 85.7 (36.4) | 79.16 (75.48, 83.03) | 0.93 (0.83, 1.04) | 0.189 | |
| POD2 | 31 | 79.6 (30.1) | 73.17 (66.48, 80.52) | 109 | 85.0 (40.2) | 77.64 (73.76, 81.72) | 0.94 (0.85, 1.05) | 0.283 | |
| MCP4 | Baseline | 32 | 54.7 (18.7) | 49.73 (45.47, 54.40) | 130 | 69.6 (210.8) | 49.22 (47.08, 51.46) | 1.01 (0.91, 1.12) | 0.839 |
| PACU | 32 | 48.7 (15.0) | 44.68 (40.85, 48.87) | 129 | 64.5 (181.3) | 46.64 (44.60, 48.78) | 0.96 (0.87, 1.06) | 0.399 | |
| POD1 | 30 | 40.5 (16.9) | 34.95 (31.87, 38.33) | 125 | 37.2 (14.1) | 35.34 (33.77, 36.98) | 0.99 (0.89, 1.10) | 0.833 | |
| POD2 | 31 | 37.3 (11.8) | 34.90 (31.87, 38.23) | 109 | 48.4 (107.5) | 36.87 (35.14, 38.70) | 0.95 (0.85, 1.05) | 0.295 | |
| MDC | Baseline | 32 | 696.3 (220.3) | 645.24 (612.45, 679.78) | 130 | 677.0 (229.8) | 641.43 (625.05, 658.23) | 1.01 (0.95, 1.07) | 0.841 |
| PACU | 32 | 695.9 (220.6) | 643.70 (611.00, 678.16) | 129 | 689.6 (221.7) | 659.39 (642.44, 676.78) | 0.98 (0.92, 1.03) | 0.417 | |
| POD1 | 30 | 494.8 (153.0) | 471.48 (446.91, 497.39) | 126 | 500.0 (156.6) | 477.05 (464.67, 489.76) | 0.99 (0.93, 1.05) | 0.698 | |
| POD2 | 31 | 528.9 (128.2) | 500.18 (474.45, 527.31) | 109 | 529.9 (148.2) | 518.25 (503.98, 532.92) | 0.97 (0.91, 1.02) | 0.243 | |
| MIP1alpha | Baseline | 32 | 26.0 (23.5) | 19.66 (16.16, 23.92) | 127 | 26.4 (24.0) | 20.11 (18.23, 22.19) | 0.98 (0.78, 1.22) | 0.839 |
| PACU | 32 | 23.2 (20.3) | 19.05 (15.61, 23.25) | 128 | 24.2 (21.0) | 18.61 (16.85, 20.55) | 1.02 (0.82, 1.28) | 0.834 | |
| POD1 | 30 | 26.7 (21.4) | 21.16 (17.25, 25.96) | 122 | 26.8 (32.9) | 19.58 (17.70, 21.66) | 1.08 (0.86, 1.36) | 0.504 | |
| POD2 | 31 | 23.9 (18.2) | 20.75 (16.97, 25.39) | 103 | 27.3 (28.2) | 20.32 (18.22, 22.67) | 1.02 (0.81, 1.28) | 0.856 | |
| MIP1beta | Baseline | 32 | 42.9 (17.5) | 37.64 (34.21, 41.42) | 130 | 44.1 (54.9) | 37.25 (35.52, 39.05) | 1.01 (0.91, 1.12) | 0.845 |
| PACU | 32 | 43.9 (18.9) | 38.20 (34.71, 42.04) | 129 | 43.6 (51.1) | 37.30 (35.56, 39.13) | 1.02 (0.92, 1.14) | 0.662 | |
| POD1 | 30 | 58.8 (14.3) | 54.71 (49.58, 60.36) | 126 | 82.0 (225.4) | 59.63 (56.82, 62.58) | 0.92 (0.82, 1.02) | 0.123 | |
| POD2 | 31 | 56.2 (26.1) | 49.19 (44.64, 54.20) | 109 | 69.4 (165.1) | 52.59 (49.95, 55.37) | 0.94 (0.84, 1.04) | 0.232 | |
| TARC | Baseline | 32 | 19.5 (6.7) | 19.21 (16.82, 21.94) | 128 | 22.4 (12.5) | 19.41 (18.16, 20.74) | 0.99 (0.85, 1.15) | 0.890 |
| PACU | 32 | 20.6 (7.4) | 20.46 (17.88, 23.42) | 127 | 25.0 (11.9) | 21.89 (20.47, 23.42) | 0.93 (0.80, 1.09) | 0.379 | |
| POD1 | 30 | 17.9 (6.0) | 18.13 (15.78, 20.84) | 122 | 23.3 (23.9) | 18.65 (17.41, 19.98) | 0.97 (0.83, 1.14) | 0.721 | |
| POD2 | 31 | 20.8 (17.1) | 17.81 (15.57, 20.38) | 106 | 26.0 (24.6) | 19.06 (17.71, 20.50) | 0.93 (0.80, 1.09) | 0.387 | |
| TNFalpha | Baseline | 32 | 1.8 (0.7) | 1.62 (1.50, 1.75) | 130 | 1.7 (0.6) | 1.61 (1.55, 1.67) | 1.01 (0.92, 1.10) | 0.887 |
| PACU | 32 | 1.6 (0.6) | 1.45 (1.34, 1.57) | 129 | 1.6 (0.5) | 1.49 (1.43, 1.55) | 0.97 (0.89, 1.06) | 0.54 | |
| POD1 | 30 | 1.8 (0.8) | 1.65 (1.52, 1.79) | 126 | 1.9 (0.7) | 1.79 (1.72, 1.86) | 0.92 (0.85, 1.01) | 0.080 | |
| POD2 | 31 | 2.1 (0.9) | 1.95 (1.80, 2.11) | 109 | 2.2 (0.7) | 2.07 (1.98, 2.16) | 0.94 (0.86, 1.03) | 0.180 | |
| TNFbeta | Baseline | 32 | 0.4 (0.2) | 0.33 (0.28, 0.38) | 130 | 0.4 (0.2) | 0.34 (0.31, 0.36) | 0.97 (0.82, 1.14) | 0.723 |
| PACU | 32 | 0.4 (0.2) | 0.32 (0.28, 0.37) | 129 | 0.4 (0.2) | 0.33 (0.30, 0.35) | 0.99 (0.84, 1.16) | 0.859 | |
| POD1 | 30 | 0.3 (0.1) | 0.28 (0.24, 0.32) | 126 | 0.3 (0.1) | 0.26 (0.24, 0.28) | 1.09 (0.92, 1.28) | 0.325 | |
| POD2 | 31 | 0.3 (0.2) | 0.25 (0.21, 0.29) | 109 | 0.3 (0.3) | 0.27 (0.25, 0.29) | 0.91 (0.77, 1.07) | 0.256 | |
| VEGF | Baseline | 32 | 12.0 (4.8) | 11.13 (10.00, 12.40) | 130 | 11.7 (4.0) | 11.07 (10.50, 11.68) | 1.01 (0.89, 1.13) | 0.925 |
| PACU | 32 | 11.8 (6.6) | 10.34 (9.29, 11.51) | 129 | 11.8 (5.1) | 10.86 (10.29, 11.46) | 0.95 (0.84, 1.07) | 0.421 | |
| POD1 | 30 | 20.0 (6.9) | 18.92 (16.93, 21.13) | 126 | 25.8 (11.6) | 23.63 (22.38, 24.95) | 0.80 (0.71, 0.91) | 0.000 | |
| POD2 | 31 | 18.9 (8.0) | 17.01 (15.25, 18.96) | 109 | 21.4 (7.7) | 20.23 (19.09, 21.45) | 0.84 (0.74, 0.95) | 0.005 | |
Abbreviations- SD: standard deviation, CI: confidence interval, GM_CSF: granulocyte-macrophage colony stimulating factor, IFNgamma: interferon-gamma, IL: interleukin, IP: interferon-gamma induced protein, MCP: monocyte chemoattractant protein, MDC: macrophage-derived chemokine, MIP: macrophage inflammatory protein, TARC: thymus and activation-regulated chemokine, TNF: tumor necrosis factor, VEGF: vascular endothelium growth factor
Figure 2.
2a. The mean fold change for each cytokine from preoperative levels to POD1 for stiff and non-stiff patient groups.
2b. The mean fold change for each cytokine from preoperative levels to POD2 for stiff and non-stiff patient groups.
Figure 3.
The increase from baseline to POD1 in Eotaxin3 plasma levels of postoperative stiff patients was significantly higher compared to postoperative non-stiff patients, while no differences were observed between groups on POD2.
Figure 4.
The increase from baseline to POD1 and POD2 in VEGF plasma levels of postoperative stiff patients was significantly lower compared to postoperative non-stiff patients.
Figure 5.
The decrease from baseline to POD1 and POD2 in IL12_23p40 plasma levels of group postoperative stiff patients was significantly higher compared to patients who did not develop postoperative stiffness.
Figure 6.
The increase from baseline to POD2 in IL-16 plasma levels of postoperative stiff patients was significantly lower compared to patients who did not develop postoperative stiffness, while no significant differences were observed between groups on POD1.
28% (9/32) of patients who were stiff at 6 weeks post-TKA subsequently underwent manipulation under anesthesia. Patients who underwent manipulation had lower ROM at 6 weeks (69 vs. 89 degrees flexion). The cytokine levels of each group are presented in Table 4.
Table 4:
Cytokine levels of stiff patients who underwent manipulation versus no manipulation
| Cytokine | Time | Stiff and Manipulation (n=9) | Stiff and No Manipulation (n=23) | ||
|---|---|---|---|---|---|
| n | mean (SD) | n | mean (SD) | ||
| Adiponectin | Baseline (JF) | 9 | 4.27 (2.59) | 22 | 3.57 (2.58) |
| Baseline (plasma) | 9 | 21.08 (10.73) | 23 | 14.37 (9.70) | |
| PACU | 9 | 19.45 (11.30) | 23 | 15.19 (10.27) | |
| POD1 | 9 | 17.74 (8.11) | 21 | 13.69 (9.55) | |
| POD2 | 9 | 17.17 (10.21) | 22 | 15.08 (9.41) | |
| Eotaxin | Baseline (JF) | 8 | 52.82 (18.47) | 22 | 70.50 (57.53) |
| Baseline (plasma) | 9 | 139.19 (73.62) | 23 | 134.48 (46.64) | |
| PACU | 9 | 123.19 (37.46) | 22 | 137.15 (57.33) | |
| POD1 | 9 | 113.09 (60.12) | 20 | 127.10 (48.14) | |
| POD2 | 9 | 78.68 (30.81) | 21 | 100.03 (33.90) | |
| Eotaxin3 | Baseline (JF) | 9 | 9.67 (10.74) | 20 | 16.65 (16.68) |
| Baseline (plasma) | 9 | 18.65 (15.05) | 23 | 48.94 (95.26) | |
| PACU | 9 | 14.63 (9.17) | 21 | 36.81 (58.83) | |
| POD1 | 9 | 19.62 (19.42) | 21 | 53.95 (77.65) | |
| POD2 | 9 | 9.86 (8.69) | 21 | 39.97 (58.81) | |
| GM_CSF | Baseline (JF) | 8 | 0.61 (0.76) | 22 | 0.54 (0.63) |
| Baseline (plasma) | 9 | 0.26 (0.19) | 23 | 0.41 (0.42) | |
| PACU | 9 | 0.32 (0.23) | 23 | 0.95 (2.92) | |
| POD1 | 9 | 0.31 (0.31) | 21 | 1.81 (4.83) | |
| POD2 | 9 | 0.24 (0.22) | 22 | 0.59 (1.22) | |
| IFNGamma | Baseline (JF) | 9 | 1.53 (0.80) | 22 | 3.03 (5.81) |
| Baseline (plasma) | 9 | 2.93 (1.70) | 23 | 2.94 (1.76) | |
| PACU | 9 | 2.93 (2.00) | 23 | 2.47 (1.32) | |
| POD1 | 9 | 1.65 (1.22) | 21 | 1.69 (0.73) | |
| POD2 | 9 | 2.50 (1.45) | 22 | 3.03 (3.91) | |
| IL_10 | Baseline (JF) | 9 | 0.20 (0.13) | 22 | 0.22 (0.15) |
| Baseline (plasma) | 9 | 0.17 (0.04) | 23 | 0.23 (0.19) | |
| PACU | 9 | 1.21 (1.12) | 23 | 1.23 (0.90) | |
| POD1 | 9 | 0.69 (0.29) | 21 | 0.84 (0.71) | |
| POD2 | 9 | 0.71 (0.51) | 22 | 0.50 (0.31) | |
| IL1_alpha | Baseline (JF) | 8 | 0.33 (0.65) | 20 | 0.28 (0.36) |
| Baseline (plasma) | 9 | 0.19 (0.21) | 23 | 0.50 (0.63) | |
| PACU | 9 | 0.34 (0.33) | 22 | 0.65 (1.17) | |
| POD1 | 9 | 0.49 (1.07) | 20 | 0.46 (1.11) | |
| POD2 | 9 | 0.31 (0.42) | 20 | 0.28 (0.44) | |
| IL1_beta | Baseline (JF) | 9 | 0.13 (0.11) | 22 | 0.23 (0.21) |
| Baseline (plasma) | 9 | 0.14 (0.13) | 23 | 0.08 (0.05) | |
| PACU | 9 | 0.12 (0.12) | 23 | 0.08 (0.06) | |
| POD1 | 9 | 0.16 (0.08) | 21 | 0.14 (0.23) | |
| POD2 | 9 | 0.12 (0.09) | 22 | 0.17 (0.28) | |
| IL2 | Baseline (JF) | 9 | 0.23 (0.18) | 22 | 0.47 (0.77) |
| Baseline (plasma) | 9 | 0.08 (0.06) | 23 | 0.94 (4.13) | |
| PACU | 9 | 0.08 (0.03) | 23 | 0.91 (3.88) | |
| POD1 | 9 | 0.09 (0.04) | 21 | 0.93 (3.71) | |
| POD2 | 9 | 0.12 (0.07) | 22 | 0.86 (3.27) | |
| IL4 | Baseline (JF) | 9 | 0.10 (0.09) | 22 | 0.08 (0.08) |
| Baseline (plasma) | 9 | 0.01 (0.00) | 23 | 0.01 (0.00) | |
| PACU | 9 | 0.01 (0.00) | 23 | 0.01 (0.00) | |
| POD1 | 9 | 0.03 (0.02) | 21 | 0.04 (0.05) | |
| POD2 | 9 | 0.02 (0.01) | 22 | 0.04 (0.04) | |
| IL5 | Baseline (JF) | 9 | 0.82 (1.27) | 22 | 0.61 (0.73) |
| Baseline (plasma) | 9 | 0.25 (0.19) | 23 | 0.37 (0.36) | |
| PACU | 9 | 0.23 (0.19) | 23 | 0.40 (0.54) | |
| POD1 | 9 | 0.50 (0.50) | 21 | 0.62 (0.84) | |
| POD2 | 9 | 0.81 (0.44) | 22 | 1.43 (2.41) | |
| IL6 | Baseline (JF) | 9 | 92.57 (126.50) | 22 | 47.44 (46.12) |
| Baseline (plasma) | 9 | 0.87 (0.52) | 23 | 1.09 (1.06) | |
| PACU | 9 | 1.05 (0.56) | 23 | 1.56 (1.89) | |
| POD1 | 9 | 23.74 (16.10) | 21 | 22.62 (15.01) | |
| POD2 | 9 | 16.31 (5.75) | 22 | 20.96 (14.44) | |
| IL7 | Baseline (JF) | 9 | 5.24 (1.64) | 22 | 4.61 (1.56) |
| Baseline (plasma) | 9 | 1.20 (0.58) | 23 | 1.47 (0.91) | |
| PACU | 9 | 1.49 (1.02) | 23 | 1.67 (0.62) | |
| POD1 | 9 | 1.83 (0.85) | 21 | 2.00 (1.07) | |
| POD2 | 9 | 1.89 (0.54) | 22 | 2.13 (1.02) | |
| IL8 | Baseline (JF) | 9 | 49.78 (59.52) | 22 | 37.78 (27.45) |
| Baseline (plasma) | 9 | 4.59 (3.48) | 23 | 4.60 (3.63) | |
| PACU | 9 | 5.55 (4.32) | 23 | 4.62 (4.23) | |
| POD1 | 9 | 6.10 (3.04) | 21 | 7.21 (6.41) | |
| POD2 | 9 | 6.06 (3.34) | 22 | 6.73 (6.34) | |
| Il12_23p40 | Baseline (JF) | 9 | 81.14 (47.15) | 22 | 72.63 (23.48) |
| Baseline (plasma) | 9 | 78.71 (28.25) | 23 | 108.96 (55.67) | |
| PACU | 9 | 80.39 (32.80) | 23 | 109.08 (51.18) | |
| POD1 | 9 | 38.07 (12.56) | 21 | 49.51 (27.54) | |
| POD2 | 9 | 48.03 (20.93) | 22 | 58.45 (36.91) | |
| IL12p70 | Baseline (JF) | 9 | 0.60 (1.32) | 22 | 0.23 (0.27) |
| Baseline (plasma) | 9 | 0.06 (0.03) | 23 | 0.09 (0.05) | |
| PACU | 9 | 0.07 (0.03) | 23 | 0.08 (0.05) | |
| POD1 | 9 | 0.12 (0.08) | 21 | 0.14 (0.10) | |
| POD2 | 9 | 0.11 (0.07) | 22 | 0.12 (0.09) | |
| IL13 | Baseline (JF) | 9 | 1.02 (0.78) | 22 | 1.25 (1.02) |
| Baseline (plasma) | 9 | 0.28 (0.08) | 23 | 0.37 (0.33) | |
| PACU | 9 | 0.28 (0.08) | 23 | 0.39 (0.36) | |
| POD1 | 9 | 0.39 (0.25) | 21 | 0.56 (0.37) | |
| POD2 | 9 | 0.35 (0.20) | 22 | 0.57 (0.32) | |
| IL15 | Baseline (JF) | 9 | 16.70 (3.61) | 22 | 15.36 (3.80) |
| Baseline (plasma) | 9 | 1.87 (0.71) | 23 | 1.74 (0.41) | |
| PACU | 9 | 1.74 (0.77) | 23 | 1.80 (0.77) | |
| POD1 | 9 | 3.85 (1.73) | 21 | 3.29 (1.49) | |
| POD2 | 9 | 3.46 (0.95) | 22 | 3.49 (1.16) | |
| IL16 | Baseline (JF) | 9 | 1080.8 (1257.1) | 22 | 762.18 (404.71) |
| Baseline (plasma) | 9 | 193.33 (70.99) | 23 | 188.42 (65.69) | |
| PACU | 9 | 210.89 (59.80) | 23 | 222.37 (75.71) | |
| POD1 | 9 | 200.00 (60.26) | 21 | 213.11 (76.59) | |
| POD2 | 9 | 202.32 (63.89) | 22 | 227.18 (77.90) | |
| IL17a | Baseline (JF) | 9 | 1.69 (0.85) | 21 | 1.32 (0.67) |
| Baseline (plasma) | 9 | 1.66 (1.17) | 22 | 2.42 (2.23) | |
| PACU | 9 | 1.63 (1.01) | 22 | 1.82 (1.58) | |
| POD1 | 9 | 1.24 (1.07) | 20 | 1.59 (1.12) | |
| POD2 | 9 | 1.27 (0.62) | 21 | 1.53 (1.20) | |
| IP10 | Baseline (JF) | 9 | 353.50 (158.26) | 21 | 447.62 (241.99) |
| Baseline (plasma) | 9 | 257.28 (115.01) | 23 | 361.87 (398.72) | |
| PACU | 9 | 248.03 (110.87) | 23 | 331.20 (327.83) | |
| POD1 | 9 | 177.36 (101.96) | 21 | 200.20 (134.03) | |
| POD2 | 9 | 238.88 (122.11) | 22 | 348.35 (450.49) | |
| Leptin | Baseline (JF) | 8 | 22.33 (30.09) | 22 | 34.00 (36.44) |
| Baseline (plasma) | 9 | 22.66 (32.20) | 23 | 35.82 (37.00) | |
| PACU | 8 | 21.96 (32.57) | 23 | 38.25 (39.21) | |
| POD1 | 7 | 37.79 (38.34) | 20 | 57.80 (46.54) | |
| POD2 | 8 | 24.74 (29.88) | 21 | 46.30 (41.84) | |
| MCP1 | Baseline (JF) | 9 | 378.22 (99.77) | 22 | 440.32 (184.54) |
| Baseline (plasma) | 9 | 68.80 (19.69) | 23 | 66.86 (17.80) | |
| PACU | 9 | 65.48 (27.42) | 23 | 52.63 (16.58) | |
| POD1 | 9 | 85.10 (34.66) | 21 | 76.47 (31.40) | |
| POD2 | 9 | 78.03 (29.40) | 22 | 80.22 (31.10) | |
| MCP4 | Baseline (JF) | 9 | 14.11 (8.21) | 22 | 18.91 (17.51) |
| Baseline (plasma) | 9 | 60.37 (19.52) | 23 | 52.43 (18.36) | |
| PACU | 9 | 48.33 (13.48) | 23 | 48.82 (15.80) | |
| POD1 | 9 | 38.44 (17.59) | 21 | 41.32 (16.98) | |
| POD2 | 9 | 37.76 (12.86) | 22 | 37.11 (11.59) | |
| MDC | Baseline (JF) | 9 | 317.89 (107.57) | 22 | 348.27 (107.38) |
| Baseline (plasma) | 9 | 596.56 (193.16) | 23 | 735.26 (221.70) | |
| PACU | 9 | 604.89 (214.93) | 23 | 731.48 (216.97) | |
| POD1 | 9 | 477.56 (168.54) | 21 | 502.19 (149.56) | |
| POD2 | 9 | 493.78 (146.82) | 22 | 543.32 (120.54) | |
| MIP1alpha | Baseline (JF) | 9 | 16.37 (10.83) | 22 | 25.75 (16.54) |
| Baseline (plasma) | 9 | 21.67 (16.79) | 23 | 27.67 (25.80) | |
| PACU | 9 | 16.15 (6.27) | 22 | 26.10 (23.29) | |
| POD1 | 9 | 17.51 (9.41) | 20 | 30.77 (24.10) | |
| POD2 | 9 | 15.38 (6.54) | 22 | 27.04 (20.08) | |
| MIP1beta | Baseline (JF) | 9 | 67.88 (50.75) | 22 | 58.21 (29.71) |
| Baseline (plasma) | 9 | 46.96 (25.60) | 23 | 41.27 (13.59) | |
| PACU | 9 | 50.03 (25.21) | 23 | 41.57 (15.80) | |
| POD1 | 9 | 61.23 (17.69) | 21 | 57.80 (13.02) | |
| POD2 | 9 | 67.03 (40.88) | 22 | 51.78 (16.28) | |
| TARC | Baseline (JF) | 9 | 18.19 (8.93) | 22 | 22.01 (9.19) |
| Baseline (plasma) | 9 | 20.47 (8.44) | 23 | 19.12 (6.09) | |
| PACU | 8 | 20.67 (6.76) | 23 | 20.58 (7.70) | |
| POD1 | 8 | 18.09 (4.90) | 21 | 17.86 (6.54) | |
| POD2 | 8 | 16.98 (5.67) | 22 | 22.40 (19.89) | |
| TNFalpha | Baseline (JF) | 9 | 1.99 (2.22) | 22 | 1.20 (0.41) |
| Baseline (plasma) | 9 | 1.54 (0.57) | 23 | 1.86 (0.72) | |
| PACU | 9 | 1.36 (0.49) | 23 | 1.68 (0.69) | |
| POD1 | 9 | 1.67 (0.72) | 21 | 1.91 (0.83) | |
| POD2 | 9 | 2.20 (1.29) | 22 | 2.13 (0.77) | |
| TNFbeta | Baseline (JF) | 9 | 1.06 (1.03) | 22 | 0.85 (0.91) |
| Baseline (plasma) | 9 | 0.35 (0.21) | 23 | 0.36 (0.22) | |
| PACU | 9 | 0.30 (0.23) | 23 | 0.39 (0.25) | |
| POD1 | 9 | 0.32 (0.16) | 21 | 0.28 (0.14) | |
| POD2 | 9 | 0.28 (0.18) | 22 | 0.28 (0.15) | |
| VEGF | Baseline (JF) | 9 | 445.33 (336.82) | 22 | 495.41 (271.86) |
| Baseline (plasma) | 9 | 11.83 (4.04) | 23 | 12.13 (5.15) | |
| PACU | 9 | 13.78 (10.21) | 23 | 11.04 (4.70) | |
| POD1 | 9 | 21.72 (7.44) | 21 | 19.23 (6.75) | |
| POD2 | 9 | 19.40 (7.61) | 22 | 18.68 (8.35) | |
Abbreviations- SD: standard deviation, GM_CSF: granulocyte macrophage colony stimulating factor, IFNgamma: interferon-gamma, IL: interleukin, IP: interferon-gamma induced protein, MCP: monocyte chemoattractant protein, MDC: macrophage-derived chemokine, MIP: macrophage inflammatory protein, TARC: thymus and activation-regulated chemokine, TNF: tumor necrosis factor, VEGF: vascular endothelium growth factor
Discussion
The slow progression of many systemic fibrotic diseases, which often develop over many years, makes clinical trials expensive and often prohibitive [11]. The relatively short time-course for development of post-TKA arthrofibrosis and the quantitative clinical endpoint provide a unique opportunity for the development of serum biomarkers to assess the risk for this negative postoperative outcome [6]. Many patients manifest symptoms of clinically significant stiffness within 2 months of TKA [4], allowing for early and consistent capture of this outcome at the standard 6-week postoperative follow-up appointment as is common in arthroplasty literature [2]. The aim of this study was to identify differences in perioperative cytokine levels that are associated with early postoperative stiffness after TKA. While there are no established threshold values for cytokine levels of clinical significance, we posit that cytokines with statistically significant different levels between patient groups are worthy of: 1. consideration as potentially useful biomarkers of risk and 2. further exploration as factors that may contribute to the pathophysiology of early stiffness.
The key finding of our study was the significant association between specific levels of 9 cytokines in the early post-operative period (POD 1 and POD 2) and the risk of 6-week postoperative knee stiffness (≤95 degrees flexion) following TKA. Specifically, patients suffering from stiffness after TKA (group A) presented significantly different plasma levels of nine cytokines (IL-5, IL-7, IL-16, VEGF, Eotaxin 3, IL-12/23p40, IL-17a, IL-12p70, and IP10), measured during the first two days after surgery, compared to non-stiff patients (group B). Based on these findings, we suggest that the acute inflammatory biologic response to TKA surgery in the first two days postoperatively may correlate with early clinical/functional outcomes, including the development of knee joint stiffness at 6 weeks.
Various clinical risk factors for arthrofibrosis after TKA have been identified, including reduced preoperative knee range of motion, history of prior knee surgery, etiology of arthritis, incorrect positioning, balancing, or oversized components and alternative explanations such as infection, complex regional pain syndrome, and heterotopic ossification [18]. However, there is limited evidence regarding potential biological risk factors. Freeman et al reported an increased infiltration of inflammatory cells in arthrofibrosis, suggesting that the normal resolution of the postoperative inflammatory reaction fails to occur, resulting in a persistent inflammation of the synovial tissue [19]. On a cellular level arthrofibrosis is characterized by upregulated myofibroblast proliferation with reduced apoptosis, adhesions, and aggressive synthesis of extracellular matrix that can fill and contract joint pouches and tissues, often also resulting in peri-articular heterotrophic ossification [20–22]. The process begins when stress signals stimulate immune cells, resulting in a cascade of cytokines and cellular mediators that drive fibroblasts to differentiate into myofibroblasts which secrete fibrillar collagens and transforming growth factor-β (TGF-β) [23]. The components and timing of this inflammatory cascade have not been well characterized.
There are relatively few studies into the pathogenesis and molecular biology of arthrofibrosis. However, there is considerable research being done to characterize other forms of fibrosis and develop pharmacologic interventions to manage these, often life-threatening, conditions. The burden of diseases where inflammation and fibrosis play important roles continues to grow and therefore the need for safe and effective anti-fibrotic therapies is great and likely to increase [11]. In a literature review, Borthwick et al highlighted the wide range of functions a single cytokine can perform on numerous cell types and suggested the possibility that targeting a single cytokine may provide a way of blocking/reversing at least some of the fibrotic process [11]. Current research into the pathogenesis of organ fibrosis now informs the understanding of arthrofibrosis [23]. By identifying common pathogenic pathways, we can potentially fast-track the road to effective interventions for prevention and treatment of arthrofibrosis [24–26].
In this study of patients who develop early post-TKA stiffness, we describe several cytokine profiles that are similar to those described in other models and others that differ. For example, our results describe an association between levels of postoperative plasma IL-7 and the development of early stiffness. In a study designed to investigate the relation between pro-inflammatory and anti-inflammatory cytokines in congenital intra-abdominal adhesions, Junga et al found that moderate to numerous connective tissue cells contained IL-7 [27]. Moreover, Hsieh et al demonstrated that IL-7 has the potential to inhibit high glucose-induced renal proximal tubular fibrosis in a cell culture model, consistent with our finding that patients with higher levels of IL7 are less likely to develop stiffness [28].
IL-5 may play an important role in fibrosis through the recruitment, differentiation and activation of eosinophils [11]. In a murine model of bleomycin-induced pulmonary fibrosis, analysis of lung RNA showed significant increases in lung IL-5 mRNA content between days 3 and 14 after induction of lung injury, suggesting a novel role for this cytokine in driving development of pulmonary fibrosis via its ability to recruit and activate eosinophils [29]. In our study, lower levels of early postoperative plasma IL-5 were associated with the development of arthrofibrosis following TKA, suggesting that it’s role is humans may be protective and/or that the early kinetics differ from those later in the course of the development of fibrosis [30].
IL-17 has been shown to play a role in the development of fibrosis in multiple organ systems [31]. Wilson et al used three models of pulmonary fibrosis to reveal a critical role for IL-17a in fibrosis, illustrating the potential utility of targeting IL-17a in the treatment of inflammation-induced fibrosis [32]. The same group has also found that IL-17a-driven fibrosis is facilitated by IL-12/23p40 [32]. In contrast, in our study we found an association between lower early postoperative plasma IL-17a and IL-12/23p40 levels and the development of arthrofibrosis following TKA.
Tamaki et al found that IL-16 promotes cardiac fibrosis and myocardial stiffening in heart failure, while the showed that blockade of IL-16 could be a possible therapeutic option for cardiac fibrosis [33]. In addition, Kawabata et al found that IL-16 may play a role in the pathogenesis of systematic sclerosis, a chronic fibrotic inflammatory disease, suggesting that inhibition of IL-16 activation may be effective in treating this disease. Finally, Domagalski et al found that IP10 expression is strongly associated with liver fibrosis in patients with chronic hepatitis C [34]. In contrast, as with IL5, IL17, and IL12_23p40, we found early postoperative IL-16, and IP10 plasma cytokine levels to be lower in those who went on to develop arthrofibrosis following TKA.
A pilot clinical study by Brown et al demonstrated that intra-articular injection of Anakinra, an interleukin-1 receptor (IL-1R) antagonist, into patients who had developed arthrofibrosis following TKA, improved range of motion and pain with 75% of patients able to return to prior activity levels [35]. Dixon et al found that fibroblasts isolated from the infra-patellar fat pad and synovial membrane express high levels of IL-1R1 on the cell surface and are induced to adopt a highly inflammatory phenotype in response to stimulation with IL-1α and IL-1β [36]. In our study, IL-1α and IL-1β levels were not significantly different in the first two days after surgery between patients who went on to develop stiffness and those who did not develop stiffness. It is possible that IL-1α, IL-1β and the IL-1R play a greater role in later stages of the development of arthrofibrosis and may not be good targets for early intervention.
Finally, in a level II study, Emami et al. described the successful use of intra-articular injection of bevacizumab, a monoclonal antibody against VEGF, for prevention of arthrofibrosis in rabbits [37]. In contrast, we present evidence that VEGF plasma levels measured on POD1 (p=0.0004) and POD2 (p=0.005) were markedly lower in the group with early stiffness (at 6 weeks) compared to the non-stiff group. Low VEGF levels immediately after surgery may contribute to development of the hypoxemic environment seen in the fibrotic joint, while VEGF levels may rise later in the post-operative period. It is also possible that while serum levels decrease, peri-articular VEGF levels increase as part of the acute local inflammatory reaction in the joint.
Of note, patients in our cohort who later developed stiffness had lower average preoperative ROM (data not shown), however we did not detect a significant difference in preoperative plasma (data not shown) or synovial fluid (Table 3) cytokine levels between groups. Similarly, pre-operative presence of osteophytes on x-ray and volume of pre-arthrotomy synovial fluid were not found to be associated with postoperative stiffness.
Taken together, a comparison of the findings in this study with those from other models of fibrosis suggest that there may be incomplete overlap between fibrotic processes in different models and different organs. Moreover, the early postoperative inflammatory response measured in blood may be predictive of and yet differ from dysregulated local inflammatory conditions in the joint that drive development of fibrosis. It must be noted that the significant differences in cytokine levels described in this paper indicate an association rather than causation. Defining the detailed kinetics and localization of cytokines during development of arthrofibrosis are areas worthy of future clinical and pre-clinical studies. Elucidating these pathways may move us closer to achieving the complimentary goals of 1) identification of clinically useful early biological markers of arthrofibrosis risk and 2) clarification of target pathways and optimal timing for interventions to prevent arthrofibrosis.
This study was not without limitations. In this cohort of patients, surgical implants were not standardized and there were variations in surgical technique, rehabilitation protocol and scheduled length of stay (LOS). However, it can be argued that these limitations strengthen the generalizability of our findings, since they apply across a cohort with different types of implants, incisions, rehabilitation protocols and LOS. Another potential limitation is that implant sizing (overstuffing) and/or malalignment and malrotation can present clinically as stiffness comparable to that caused by arthrofibrosis.
It should be noted that some cytokines that are likely to be associated with arthrofibrosis were not present on the commercially available cytokine array used in this study and were thus not examined, including IL-33, BMP-2 and TGF-β [38]. In addition, while post-operative stiffness was determined at 6 weeks, we did not measure cytokine levels at time points after initial discharge from the hospital. The temporal pattern of cytokine expression between POD2 and week 6 after surgery is an area worthy of further research. Such information may guide potential therapeutic interventions and dosing regimens to prevent the development of early post-TKA stiffness.
Conclusions
A distinct acute postoperative inflammatory response profile was described in patients who developed postoperative stiffness at 6 weeks after TKA, characterized by significant differences in nine cytokines over the first two postoperative days. These results support the theory that the biologic response to surgery in the first two days postoperatively may be predictive of clinically significant early postoperative outcomes. Future research directed towards early modification of the inflammatory response may identify interventions to reduce post-TKA stiffness.
Supplementary Material
Acknowledgments
Funding: This work was supported by the Anesthesiology Department Research & Education Fund at Hospital for Special Surgery, the Adult Reconstruction and Joint Replacement Marmor Research Award and the Marina French Research Grant. Research reported in this publication was supported by the National Center for Advancing Translational Science of the National Institute of Health Under Award Number UL1TR002384 and UL1TR000457. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Inacio MCS, Paxton EW, Graves SE, Namba RS, Nemes S. Projected increase in total knee arthroplasty in the United States – an alternative projection model. Osteoarthritis Cartilage 2017;25(11):1797–1803. [DOI] [PubMed] [Google Scholar]
- 2.Tibbo ME, Limberg AK, Salib CG, Ahmed AT, van Wijnen AJ, Berry DJ, et al. Acquired idiopathic stiffness after total knee arthroplasty: A systematic review and meta-analysis. J Bone Joint Surg Am 2019;101(14):1320–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gonzalez Della Valle A, Leali A, Haas S. Etiology and surgical interventions for stiff total knee replacements. HSS Journal 2007;3(2):182–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yercan HS, Sugun TS, Bussiere C, Ait Si Selmi T, Davies A, Neyret P. Stiffness after total knee arthroplasty: prevalence, managementand outcomes. Knee 2006;13(2):111–117. [DOI] [PubMed] [Google Scholar]
- 5.Sanders T, Kremers H, Bryan A, Kremers W, Stuart M, Krych A. Procedural intervention for arthrofibrosis after ACL reconstruction: trends over two decades. Knee Surg Sports Traumatol Arthrosc 2017; 25(2): 532–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Parvataneni HK, Shah VP, Howard H, Cole N, Ranawat AS, Ranawat CS. Controlling pain after total hip and knee arthroplasty using a multimodal protocol with local periarticular injections: a prospective randomized study. J Arthroplasty 2007;22(6 Suppl 2):33–8. [DOI] [PubMed] [Google Scholar]
- 7.Gollwitzer H, Burgkart R, Diehl P, Gradinger R, Bühren V. Therapy of arthrofibrosis after total knee arthroplasty. Orthopade 2006;35(2):143–52. [DOI] [PubMed] [Google Scholar]
- 8.Thompson R, Novikov D, Cizmic Z, Feng JE, Fideler K, Sayeed Z, et al. Arthrofibrosis after total knee arthroplasty: Pathophysiology, diagnosis, and management. Orthop Clin North Am 2019;50(3):269–279. [DOI] [PubMed] [Google Scholar]
- 9.Su EP, Su SL, Gonzalez Della Valle AG. Stiffness after TKR: how to avoid repeat surgery. Orthopedics 2010;33(9):658. [DOI] [PubMed] [Google Scholar]
- 10.Yang J, Lin SC, Chen G, He L, Hu Z, Chan L, et al. Adiponectin promotes monocyte-to-fibroblast transition in renal fibrosis. J Am Soc Nephrol 2013;24(10):1644–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borthwick LA, Wynn TA, Fisher AJ. Cytokine mediated tissue fibrosis. Biochim Biophys Acta 2013;1832(7):1049–1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marvie P, Lisbonne M, L’Helgoualc’h A, Rauch M, Turlin B, Preisser L, et al. Interleukin-33 overexpression is associated with liver fibrosis in mice and humans. J Cell Mol Med 2010;14:1726–1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Demols A, Van Laethem JL, Quertinmont E, Degraef C, Delhaye M, Geerts A, et al. Endogenous interleukin-10 modulates fibrosis and regeneration in experimental chronic pancreatitis. Am J Physiol Gastrointest Liver Physiol 2002;282(6):G1105–G1112. [DOI] [PubMed] [Google Scholar]
- 14.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest 1999;104(6):777–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolodsick JE, Toews GB, Jakubzick C, Hogaboam C, Moore TA, McKenzie A, et al. Protection from fluorescein isothiocyanate-induced fibrosis in IL-13-deficient, but not IL-4-deficient, mice results from impaired collagen synthesis by fibroblasts. J Immunol 2004;172(7):4068–4076. [DOI] [PubMed] [Google Scholar]
- 16.Hornung RW, Reed LD. Estimation of Average Concentration in the Presence of Nondetectable Values. Applied Occupational and Environmental Hygiene 1990;5(1):46–51. [Google Scholar]
- 17.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Research 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manrique J, Gomez MM, Parvizi J. Stiffness after total knee arthroplasty. J Knee Surg 2015. April;28(2):119–126. [DOI] [PubMed] [Google Scholar]
- 19.Freeman TA, Parvizi J, Della Valle CJ, Steinbeck MJ. Reactive oxygen and nitrogen species induce protein and DNA modifications driving arthrofibrosis following total knee arthroplasty. Fibrogenesis Tissue Repair 2009;2(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watson RS, Gouze E, Levings PP, Bush ML, Kay JD, Jorgensen MS, et al. Gene delivery of TGF-beta1 induces arthrofibrosis and chondrometaplasia of synovium in vivo. Lab. Invest 2010;90:1615–1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freeman TA, Parvizi J, Dela Valle CJ, Steinbeck MJ. Mast cells and hypoxia drive tissue metaplasia and heterotopic ossification in idiopathic arthrofibrosis after total knee arthroplasty. Fibrogenesis Tissue Repair 2010;3:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monument MJ, Hart DA, Salo PT, Befus AD, Hildebrand KA. Posttraumatic elbow contractures: targeting neuroinflammatory fibrogenic mechanisms. J. Orthop. Sci 2013;18(6):869–877. [DOI] [PubMed] [Google Scholar]
- 23.Usher KM, Zhu S, Mavropalias G, Carrino JA, Zhao J, Xu J. Pathological mechanisms and therapeutic outlooks for arthrofibrosis. Bone Res 2019;7:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rockey DC, Bell PD, Hill JA. Fibrosis—a common pathway to organ injury and failure. N. Engl. J. Med 2015;372(12):1138–1149. [DOI] [PubMed] [Google Scholar]
- 25.Nanthakumar CB, Hatley RJ, Lemma S, Gauldie J, Marshall RP, Macdonald SJ. Dissecting fibrosis: therapeutic insights from the small-molecule toolbox. Nat. Rev. Drug. Discov 2015;14(10):693–720. [DOI] [PubMed] [Google Scholar]
- 26.Stone RC, Pastar I, Ojeh N, Chen V, Liu S, Garzon KI, et al. Epithelial–mesenchymal transition in tissue repair and fibrosis. Cell Tissue Res 2016;365(3):495–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Junga A, Pilmane M, Ābola Z, Volrāts O. Interleukin (IL)-1, 4, 6, 7, 8, 10 Appearance in Congenital Intra-Abdominal Adhesions in Children Under 1 Year of Age. Appl Immunohistochem Mol Morphol 2018;26(9):664–669. [DOI] [PubMed] [Google Scholar]
- 28.Hsieh PF, Liu SF, Lee TC, Huang JS, Yin LT, Chang WT, et al. The role of IL-7 in renal proximal tubule epithelial cells fibrosis. Mol Immunol 2012;50(1–2):74–82. [DOI] [PubMed] [Google Scholar]
- 29.Gharaee-Kermani M, Phan SH. Lung interleukin-5 expression in murine bleomycin-induced pulmonary fibrosis. Am J Respir Cell Mol Biol 1997;16(4):438–447. [DOI] [PubMed] [Google Scholar]
- 30.Gieseck RL 3rd, Wilson MS, Wynn TA. Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 2018;18(1):62–76 [DOI] [PubMed] [Google Scholar]
- 31.Ahmed S, Misra DP, Agarwal V. Interleukin-17 pathways in systemic sclerosis-associated fibrosis. Rheumatol Int 2019;39(7):1135–1143. [DOI] [PubMed] [Google Scholar]
- 32.Wilson NJ, Boniface K, Chan JR, McKenzie BS, Blumenschein WM, Mattson JD, et al. Development, cytokine profile and function of human interleukin 17-producing helper T cells. Nat Immunol 2007;8(9):950–957. [DOI] [PubMed] [Google Scholar]
- 33.Tamaki S, Mano T, Sakata Y, Ohtani T, Takeda Y, Kamimura D, et al. Interleukin-16 promotes cardiac fibrosis and myocardial stiffening in heart failure with preserved ejection fraction. PLoS One 2013;8(7):e68893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Domagalski K, Pawłowska M, Kozielewicz D, Dybowska D, Tretyn A, Halota W. The Impact of IL28B Genotype and Liver Fibrosis on the Hepatic Expression of IP10, IFI27, ISG15, and MX1 and Their Association with Treatment Outcomes in Patients with Chronic Hepatitis C. PLoS One 2015;10(6):e0130899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown CA, Toth AP, Magnussen B. Clinical benefits of intra-articular anakinra for arthrofibrosis. Orthopedics 2010;33(12):877. [DOI] [PubMed] [Google Scholar]
- 36.Dixon D, Coates J, del Carpio Pons A, Horabin J, Walker A, Abdul N, et al. A potential mode of action for Anakinra in patients with arthrofibrosis following total knee arthroplasty. Sci Rep 2015;5:16466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Emami MJ, Jaberi FM, Azarpira N, Vosoughi AR, Tanideh N. Prevention of arthrofibrosis by monoclonal antibody against vascular endothelial growth factor: a novel use of bevacizumab in rabbits. Orthop Traumatol Surg Res 2012;98(7):759–64. [DOI] [PubMed] [Google Scholar]
- 38.Pfitzner T, Geissler S, Duda G, Perka C, Matziolis G. Increased BMP expression in arthrofibrosis after TKA. Knee Surg Sports Traumatol Arthrosc. 2012;20(9):1803–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.