Abstract
Acute myocardial infarction (AMI), and the heart failure (HF) that often follows, are leading causes of death and disability worldwide. Crucially, there are currently no effective treatments, other than myocardial reperfusion, for reducing myocardial infarct (MI) size and preventing HF following AMI. Thus, there is an unmet need to discover novel cardioprotective therapies to reduce MI size, and prevent HF in AMI patients. Although a large number of therapies have been shown to reduce MI size in experimental studies, the majority have failed to benefit AMI patients. Failure to deliver cardioprotective therapy to the ischemic heart in sufficient concentrations following AMI is a major factor for the lack of success observed in previous clinical cardioprotection studies. Therefore, new strategies are needed to improve the delivery of cardioprotective therapies to the ischemic heart following AMI. In this regard, nanoparticles have emerged as drug delivery systems for improving the bioavailability, delivery, and release of cardioprotective therapies, and should result in improved efficacy in terms of reducing MI size and preventing HF. In this article, we provide a review of currently available nanoparticles, some of which have been FDA-approved, in terms of their use as drug delivery systems in cardiovascular disease and cardioprotection.
1.0. Introduction
Cardiovascular disease (CVD) is the leading cause of death and disability worldwide, with a major manifestation being acute myocardial infarction (AMI) (Thygesen et al., 2018), a major complication of which is adverse left ventricular (LV) remodelling, and heart failure (HF). Although improvements in AMI treatment have seen a decline in mortality, an increasing number of patients still go on to develop heart failure (Benjamin et al., 2018, 2019). HF impacts significantly on patient morbidity and quality of life, exerting a huge healthcare and economic burden on society. The size of the myocardial infarct (MI), adverse LV remodelling, and the extent of LV dysfunction are the strongest predictors of mortality following AMI. There remains a clinical unmet need, therefore, to discover novel therapies for reducing MI size and preventing adverse LV remodeling, so as to reduce the risk of developing HF and improve survival in AMI patients. Although, a large number of therapies, which have been shown to be effective in the preclinical setting in animal AMI models have failed to improve clinical outcomes in the cmilinical setting in AMI patients (Cung et al., 2015; Bonora et al., 2019; Kleinbongard et al., 2019).
One major reason for this failure to translate cardioprotection into the clinical setting for patient benefit, is the failure to deliver the cardioprotective drug into the ischemic myocardium at sufficient concentrations to be efficacious. In this regard, development of nanoparticles (NPs) drug delivery system (DDS) (Rudramurthy and Swamy, 2018), have the potential to increase the bioavailability and delivery of cardioprotective therapies to the ischemic heart in the setting of AMI, thereby increasing cardioprotective efficacy, and reducing the risk of off-target side effects (Figure 1) (Bhatia, 2016). At present, there are some Food and Drug Administration (FDA)-approved nanomedicines, mostly based on liposomal, polymeric, and nanocrystal formulations. However, trends are changing and nanoparticles composed of other materials have been undergoing clinical trials in recent years (Bobo et al., 2016). The present article provides an update on recent advances in nanoparticle research as drug carriers for therapies, which have the potential to reduce MI size, prevent adverse LV remodeling, and reduce the risk of HF in AMI patients.
Figure 1.

Schematic illustration of nanoparticle delivery in acute myocardial infarction.
2. Nanoparticles and cardioprotection
Nanoparticles can be categorized into organic and inorganic subtypes, among which many have been applied to the treatment of CVD including AMI. These include iron oxide NPs, gold NPs (AuNPs), silica NPs, liposomes (exosomes), polymeric NPs, micelles, hybrid hydrogel NPs, and biomolecule-based NPs (Figure 2). The advantages and disadvantages of these different NPs as DDSs are summarized in Table 1.
Figure 2.

Schematic structure of various nanoparticles that have been applied as drug delivery systems in cardiovascular disease and cardioprotection.
Table 1.
The advantages and disadvantages of different types of nanoparticles as a drug delivery system (DDS)
| ADVANTAGES | DISADVANTAGES | |
|---|---|---|
| IRON OXIDE NPS | Non-radiation exposure for imaging Specificity for tracking in vivo processes Intravenously administered Non-invasive imaging |
Can persist in non-living cells Does not discriminate between endogenous iron content possibly due to a hemorrhage |
| GOLD NPS | Inert, stable, biocompatible, antioxidant, Near infra-red absorbance | Cardiotoxicity with long exposure Interferes with electrical processes of the heart |
| SILICA-BASED NPS | Easy synthesis Chemically and thermally stable Enables oral administration owing to no premature release |
Serious reports of toxicity |
| LIPOSOMES | Easily tailored for targeting Delivers either hydrophobic or hydrophilic cargo Retains size characteristics in plasma Biodegradable Augments aqueous solubility of insoluble drugs In vivo stability Long half-life Increases bioavailability Enhanced permeability through biological barriers Spontaneously forming in aqueous media |
Undergoes endosome recycling, which lowers its efficiency |
| EXOSOMES | Naturally carrying miRNAs and other molecules with cardioprotection | Unpredictable pharmacokinetics and pharmacodynamics Hard to isolate from other extracellular vesicles |
| PLGA NPS | Resorbable, biocompatible Metabolizable Easily upscaled for production |
Encapsulation of hydrophilic agents is challenging |
| PROTEIN-BASED NPS | Metabolizable Easy to functionalize Amphiphilic Naturally having biochemical moieties for ligand binding No cellular toxicity |
Batch-to-batch variation and scaling-up difficulty (Elzoghby et al, 2012) Limited source Potential antigenicity |
| APTAMERS | Low batch-to-batch variability Upscalable production Thermally stable (does not require refrigeration storage) Biodegradable Easy synthesis Low immunogenicity |
Susceptible to nucleases Hard to predict pharmacokinetics Short lifetimes in vivo |
| SELF-ASSEMBLING PEPTIDES | Controllable kinetics Provide biochemical signals Biodegradable Allows for mechanical/electrical integration Allows for regeneration of myocardium and vasculature Supports cell recruitment |
Limited administration routes (Relying on intramyocardial delivery) |
2.1. Inorganic nanoparticles
Magnetic iron oxide nanoparticles
Cardiovascular magnetic resonance (CMR) is the imaging modality of choice for quantifying LV function and MI size, and for assessing the efficacy of novel cardioprotective therapies in AMI patients (Ibanez et al., 2019). NPs offer several possibilities for CMR as contrast agents (Grimaldi et al., 2019), with paramagnetic iron oxide NPs (IONs) leading this technology field. Animal studies have shown that modifications of IONs make it possible to selectively track processes that accompany an AMI, such as cell death, inflammation, angiogenesis, and LV remodeling. Cross-linked IONs coupled to annexin have been used to detect apoptosis in the heart following AMI (Sosnovik et al., 2005). When used in conjunction with a gadolinium chelate, it was possible to differentiate in vivo between necrotic and apoptotic myocardium (Sosnovik et al., 2009). Subsequent studies have shown smaller IONs containing annexin A5 with faster pharmacokinetics, thereby reducing the time between administration and actual imaging (Figge et al., 2014). Iron oxide microparticles tagged with a VCAM antibody have been used to visualize myocardial vasculature in the rat heart following AMI, with areas devoid of signal depicting areas of activated vasculature in the infarcted area (Grieve et al., 2013). As promising as targeted IONs may seem, they have some limitations as they remain in nonliving cells for longer time than expected. Besides, it is impossible to distinguish between IONs and endogenous iron signals coming from intramyocardial hemorrhage (Sosnovik and Caravan, 2019).
A few studies in humans have been conducted to evaluate IONs as CMR contrast agents. An early trial to test ferucarbotran, which is based on superparamagnetic IONs, concluded that no benefits were achieved over gadolinium-based contrast agents in terms of imaging infarction in both ST-segment elevation myocardial infarction (STEMI) and non-ST-segment elevation myocardial infarction patients (Yilmaz et al., 2013b). This could have been due to the dose of iron applied (Yilmaz et al., 2013a). Later, as a proof of concept, ferumoxytol, a formulation of ultra-small superparamagnetic IONs coated with carboxymethyl-dextran was administered intravenously to ten STEMI patients. Ferumoxytol improved the contrast at the infarcted area with no reported adverse effects, although it tended to accumulate in remote myocardium as well as in liver and spleen (Alam et al., 2012). Ferumoxytol was also tested in a clinical trial of fourteen STEMI patients and yielded good results in terms of safety and visualization of the infarcted myocardium (Yilmaz et al., 2013a). Currently, Feraheme (ferumoxytol) is approved by the FDA, but only indicated as an iron deficiency treatment.
Gold nanoparticles
Gold nanoparticles (AuNPs) have demonstrated moderate potential to confer protection in the isoproterenol-induced cardiac injury rat model (Vinodhini et al., 2014; Ahmed et al., 2017). However, there have been reports of cardiotoxicity with strong dependence on the size of the particles and the duration of exposure, when administered via intraperitoneal injection (Abdelhalim, 2011). It has been reported that 40 nm AuNPs can produce significant changes in the normal process of ventricular repolarization, whereas 5 nm particles might affect depolarization. AuNPs (100 nm) were shown to produce left ventricle hypertrophy, and AuNPs were demonstrated to decrease cardiac output and cause apoptosis and fibrosis, irrespective of size (Zhang et al., 2018). Conjugation of AuNPs with polyethylene glycol (PEG) may reduce toxicity, with PEGylated AuNPs shown to not affect LV function and structure after seven days of daily administration (Yang et al., 2013). Under chronic exposure, 10 nm PEGylated AuNPs administration for 2 weeks did increase LV mass but this reverted to basal values after four and twelve weeks of chronic administration (Yang, Tian, & Li, 2016). Remarkably, PEGylated AuNPs tend to accumulate in the fibrotic heart but not in the healthy heart (Yang et al., 2019). Consistent with this finding, the amount of PEGylated gold nano-rods located in the heart of transgenic mice that expressed TNF-α under the α-myosin heavy chain promoter were three times larger than in wild type animals (Higuchi et al., 2019). As a DDS, AuNPs have been shown to prevent progression to doxorubicin-induced heart failure, whether or not conjugated to levosimendan. Intriguingly, it was demonstrated that ultrasound intensified cardiomyocyte uptake of AuNPs, with selective accumulation in the mitochondria (Spivak et al., 2013). Under a regime of short-term dosing of isoproterenol, PEGylated AuNPs downregulated the β1 adrenergic receptor and IL-6 in myocardium and hence, lessened the hypertrophic effects of isoproterenol (Qiao et al., 2017). As a novel strategy, AuNPs loaded with a DNAzyme that specifically cleaves TNF-α mRNA had a pronounced local anti-inflammatory effect. Such an effect correlated with an amelioration in LV function in a rat model of permanent ligation of the left descending coronary artery (Somasuntharam et al., 2016).
Silica nanoparticles
Silica NPs are regarded as attractive DDS because their porous texture permits the loading of significant amounts of drugs (Mehmood et al., 2017). These NPs have been used to deliver cardioprotective therapies to the ischemic heart following AMI. Adenosine adsorbed at the surface of silica NPs have been shown to accumulate passively in the ischemic-region of the heart in a rat model, and confer postconditioning-like protection against acute ischemia/reperfusion injury (IRI) (Galagudza et al., 2012). In another case, curcumin attached to mesoporous silica NPs elevated the serum levels of antioxidant enzymes and conferred protection to myofibril organization in rats with doxorubicin-induced cardiomyopathy (Yadav et al., 2019). Strikingly, in a zebrafish heart failure model, mesoporous silica NPs coupled with a hydrogen peroxide probe allowed the controlled release of captopril under hydrogen peroxide stimulation and improve cardiac function (Tan et al., 2017). A similar approach had been used to control the release of curcumin by means of light or heat-sensitive silica NPs (Yan et al., 2012). As with NPs of other compositions, further functionalization might enable porous silicon NPs to selectively attain heart cells (Ferreira et al., 2016; Ornelas-Soto et al., 2017).
In the area of stem cell therapy, it has been shown that stem cell distribution to the heart following intramyocardial engraftment can be assessed by tracking fluorescently labeled silica NPs. Notably, engulfment of silica NPs by human mesenchymal stem cells does not appear to alter the stemness potential nor the proliferative capacity of the stem cells (Gallina et al., 2015).
However, silica NPs have been reported to be cardiotoxic. When administered to rats via intratracheal applications, silica NPs produced dose-dependent alterations in the myocardium, mainly in mitochondrial structure, which became swollen and had disintegrated cristae (Du et al., 2019). Additionally, in the same preparation, silica NPs increased the serum concentration of pro-inflammatory cytokines and oxidative stress biomarkers as well as heart silicon content (Du et al., 2013). These findings are consistent with in vitro observations in H9c2 cells, where silica NPs induced loss in viability, presumably due to oxidative stress (Ye et al., 2010). In contrast, other studies did not show toxic effects with silica NPs and organomodified silica NPs, although toxicity was only assessed acutely, by measuring heart rate and hemodynamic parameters (Galagudza et al., 2010; Sonin et al., 2015).
2.2. Organic or Natural nanoparticles
Liposomes and exosomes
Liposomes are phospholipidic spherical structures consisting of one or multiple bilayers that, because of their physico-chemical properties, can be loaded with either hydrophilic or hydrophobic materials (Alavi et al., 2017). Liposomes are perhaps the most studied nanocarriers. Numerous animal studies have shown that liposomes are able to deliver to the myocardium a plethora of molecules that preserve cardiac function after an ischemic event, such as adenosine (Takahama et al., 2009), erythropoietin/granulocyte-colony stimulating factor (Yamada et al., 2019), miRNAs (Higashi et al., 2015), oligonucleotides (Liu et al., 2014), and even plant derivatives (Allijn et al., 2017).
One of the crucial advantages of liposomes is that they can be easily tailored for controlled biological interactions, rendering them highly versatile vehicles (Hu et al., 2015). For instance, it has been shown that adding antibodies against cardiac troponin I to liposomes conferred specificity to damaged myocardium (Liu et al., 2014). In this same line, ligands of the angiotensin II type I receptor attached to liposomes provided specificity towards the infarcted heart (Dvir et al., 2011). Interestingly, by using in vivo phage display screening, Dasa et al. (2015) identified a considerable amount of seven-residue peptides that could direct liposomes to various cell types (cardiomyocytes, endothelial cells, myofibroblasts) at the infarct zone and borderline area. Furthermore, liposomes are susceptible to more than one modification. Wang et al. (2017) appended both a peptide that ensured specific delivery and the HIV-derived cell penetrating peptide TAT to the surface of a liposome. This design gave better results than either modification alone in terms of cardiomyocyte uptake.
Circulating leukocytes are another potential target for attenuating cardiac damage following acute IRI. In this respect, liposomes with encapsulated siRNA against the CCR2 receptor significantly reduced inflammatory monocyte infiltration in mice myocardium subjected to AMI. As expected, this correlated with reduced MI size (Leuschner et al., 2011; Dutta and Nahrendorf, 2015). Furthermore, despite claims regarding the poor efficiency of liposomes as siRNA carriers (Sahay et al., 2013), significant improvements in the field have been achieved through the synthesis of novel lipidic compounds. Uemura et al. (2019) synthesized lipid NPs composed of an original cationic lipid. Anti-CD45 siRNA entrapped in these NPs was effectively delivered to phagocytic cells. Knockdown efficiencies were as high as 91% in cultured macrophages and 30% in mice circulating phagocytes. Of note, in the clinical setting, liposomal prostaglandin E1 administered intravenously immediately after percutaneous coronary intervention (PCI) protected coronary microcirculation and reduced major adverse cardiac events within six months of follow-up in STEMI patients (Wei et al., 2015). Likewise, in unstable angina patients that underwent PCI, that same formulation lowered periprocedural myocardial injury within 24 hours after PCI (Fan et al., 2015).
Similar to liposomes in structure, exosomes are naturally occurring extracellular vesicles in nano-size implicated in intercellular communication (Pegtel and Gould, 2019). Exosomes have been demonstrated to play a cardioprotective role in ischemic heart disease and represent potential therapeutic agents (Yellon and Davidson, 2014; Davidson et al., 2017). Indeed, mesenchymal stem cell derived exosomes appear to act via the same pathways as ischemic conditioning, namely through the activation of the reperfusion injury salvage kinase (RISK) pathway (Arslan et al., 2013). Furthermore, remote ischemic preconditioning has been shown to modestly raise the levels of circulating extracellular vesicles in human patients (Frey et al., 2019). Among the many mediators to be found in exosomes, miRNAs have enticed much interest. Exosomes containing miR-21 and miR-146a are thought to be the responsible entities that promote angiogenesis, cardiomyocyte survival, and cardiac regeneration following cardiosphere derived cell transplantation (Ibrahim et al., 2014; Xiao et al., 2016; Gallet et al., 2017). Moreover, various stimuli (oxidative stress, hypoxia, shockwave therapy) have proven to be effective in triggering the release of miRNA-enriched exosomes, both in vitro and in vivo, with beneficial effects on cardiac function (Xiao et al., 2016; Zhu et al., 2018; Gollman-Tepeköylü et al., 2019). These findings suggest the possibility of therapeutically inducing regeneration, circumventing the need for actual cell transplantation. Nevertheless, one limitation of these studies is that delivery of exosomes depends on direct intramyocardial injection. To overcome poor retention, Vandergrieff et al. (2018) tagged exosomes with a cardiac homing peptide, which led to specific accumulation at the heart after intravenous application. If exosomes are to be translated into the clinical setting, a better understanding of their physiological role, pharmacokinetics, pharmacodynamics, and a more reliable way of isolating them from other extracellular vesicles must be sought (Davidson et al., 2017).
Polymeric nanoparticles
Polymeric NPs encompass a large list of natural and synthetic polymeric materials capable of transporting drugs either encapsulated or adsorbed on their surface (El-Say and El-Sawy, 2017). Amid the vast amount of materials, the copolymer Poly (lactic-co-glycolic acid) (PLGA) has several advantages. PLGA exhibits biocompatibility, biodegradability, and offers the chance of sustained release and targeted delivery. Importantly, PLGA usage is currently approved by the FDA and the European Medicines Evaluation Agency (EMEA) (Danhier et al., 2012; Sharma et al., 2016). Here we provide some relevant examples of PLGA NPs as a bioabsorbable DDS in CVD.
PLGA NPs are able to ferry vascular endothelial growth factor (VEGF) to the infarcted heart and once there, release the growth factor in a sustained manner. Regardless of the size, nano and micrometer-sized PLGA particles (115 nm vs. 4.89 μm) have been shown to significantly increase vascular density in acute myocardial IRI rodent models (Simoń-Yarza et al., 2013; Oduk et al., 2018). Moreover, when VEGF-PLGA microparticles were given in combination with orally administered coenzyme Q10-PLGA NPs, a further improvement in the ejection fraction was achieved (Simoń-Yarza et al., 2013). Insulin like growth factor (IGF) incorporated into PLGA NPs distributed mainly at the heart and gave better results when compared to freely infused IGF in terms of cardioprotection (Chang et al., 2013). Other growth factors such as neuregulin and fibroblast growth factor 1 (FGF1) had similar favorable effects on vessel formation and maturation, and enhanced systolic function when embedded in PLGA formulations (Pascual-Gil et al., 2017). PLGA microparticles loaded with antagomir-92a have been used to downregulate the anti-angiogenic miR92a in a swine myocardial IRI model, and resulted in enhanced angiogenesis and less adverse LV remodeling (Bellera et al., 2014). Apart from directly increasing vessel density and cardiomyocyte survival, another feasible cardioprotective strategy is attenuating inflammation. PLGA-pioglitazone has been shown to reduce adverse post-AMI LV remodeling by decreasing the proportion of M1/M2 macrophages in the post-infarcted mouse and pig hearts (Tokutome et al., 2019). In a similar manner, pitavastatin bound to PLGA particles, but not pitavastatin in solution, prevented post-AMI adverse LV remodeling by inhibiting inflammatory monocyte recruitment (Mao et al., 2017). Targeting the opening of the mitochondrial permeability transition pore (MPTP), a critical determinant of cardiomyocyte death following acute IRI, PLGA particles conjugated to cyclosporine A resulted in a greater reduction in MI size when compared to cyclosporine A alone (Ikeda et al., 2016). Furthermore, the addition of the Szeto-Schiller 31 peptide to cyclosporine A increased the accumulation of cyclosporine A at the inner mitochondrial membrane, and increased cardioprotective efficacy (Zhang et al., 2019).
In addition to PLGA-based polymeric NPs, cyclodextrin-based NPs are also suitable systems for drug delivery. Cyclodextrins are carbohydrates composed of several units of glucose. Ingestion of cyclodextrins is considered safe by the FDA. Nonetheless, parenteral administration is restricted to a few cases because of hemolysis, as well as hepatic and renal toxicity (Braga, 2019). Cyclodextrin-based nanosponges are hyper-crosslinked polymeric particles that resemble porous spheres made of interlinked cyclodextrins. Nanosponges are able to encapsulate and adsorb a variety of molecules, which make them appealing as DDS. Moreover, nanosponges partially overcome toxicity issues that restrict cyclodextrins from more widespread use (Allahyari et al., 2019). Recently, Femminò and colleagues (2018) proposed alpha-cyclodextrin nanosponges as a system for delivery of oxygen to infarcted tissue. In a hypoxia/reoxygenation in vitro protocol, preconditioning H9c2 myoblasts with oxygen-loaded alpha-cyclodextrin nanosponges returned cell viability to normoxic control levels (Femminò et al., 2018).
Micelle
Lipid-based micelles can passively accumulate at the infarct area with a lower latency than liposomes, and are more likely to accumulate in cardiomyocytes rather than in immune cells or vessels, even when administered one week after acute IRI (Geelen et al., 2013). In an analogous manner, polymeric micelles constituted of amphiphilic PEG and phosphatidyl-ethanolamine (PE) also concentrate at the damaged myocardium but not at the remote healthy tissue (Lukyanov et al., 2004). Moreover, to take advantage of matrix metalloproteinase (MMP) over activity seen throughout the remodeling of the post-infarcted myocardium, Nguyen et al. (2015a) coupled lipid micelles with a MMP-targeting peptide, ensuring localization of such micelles within the remodeling heart issue. Retention of micelles was achieved by binding MMP recognition sequences to micelles in order to produce aggregates that remained up to 28 days at the remodeling heart (Nguyen et al., 2015b). Micelles can also be targeted to other cell types by changing the surface composition. This is the case of CCR antibodies that when attached to lipid micelles can decrease monocytic infiltration at the injury site (Wang et al., 2018a). Micelles allow for the solubilization of hydrophobic drugs, and as a consequence, increase their bioavailability, reduce adverse off-target effects and potentiate their ability to cross biological barriers (Musacchio and Torchilin, 2013). These characteristics make micelles a suitable DDS in the cardiovascular system. Recently, the clinically approved drug rapamycin, given chronically as a micellar nanoformulation (Rapatar), was able to reduce MI size in diabetic mice (Samidurai et al., 2017). Polymeric micelles used to deliver hydrogen sulfide donors, the release of which depends on endocytosis, produced robust protective effects on hypoxic cardiomyocytes (Takatani-Nakase et al., 2017).
Micelles have been tested for passive delivery of a variety of natural antioxidants that in their unmodified forms lack aqueous solubility. Polymeric Pluronic micelles simultaneously transporting curcumin and resveratrol lowered effector caspase activity and ROS levels in H9c2 cells treated with doxorubicin (Carlson et al., 2014). A study from the same group indicated that co-delivery of quercetin and resveratrol offered similar results in H9c2 cells, while in vivo, this combination conferred cardioprotection (Cote et al., 2015). In line with these observations, ginsenoside administered within a Pluronic micelles formulation reduced cardiac dysfunction produced by doxorubicin, presumably by safeguarding mitochondria integrity (Li et al., 2017). Another plant derivative, tilianin, a natural flavonoid, was loaded into polymeric micelles and was tested in a hypoxia-reoxygenation preparation. Either tilianin alone or entrapped in micelles significantly preserved the viability of cultured cardiomyoblasts after the simulated injury. Nonetheless, water solubility of micelles was much superior than that of the flavonoid alone (Wang et al., 2018b). In the same line, the isoflavone puerarin, embedded in PEG-PE micelles, counteracted the pro-apoptotic effects of isoprenaline in H9c2 cells. In fact, the conjugate presented a better safety profile than puerarin alone, in terms of its natural hemolytic capacity (Li et al., 2018). The addition of triphenylphosphonium cation further improved puerarin antiapoptotic efficacy, and confocal microscopy revealed that the cation drove the micelles specifically to the mitochondria (Li et al., 2019).
Hybrid Hydrogel Nanoparticles
Hydrogels are another kind of hydrophilic polymeric materials, which can work as scaffolds for cells, drugs, and other NPs (Utech and Boccaccini, 2016). For example, the hybrid system of alginate hydrogel and PLGA microspheres carrying HSP27 considerably lowered fibrosis and cell death at the infarct zone (Lee et al., 2013). Also, the composite of hydrogel and polymeric NPs carrying miRNAs represents a feasible means of cardiac restoration therapy with extended lifetime (Yang et al., 2019b). Intriguingly, a system of PLGA nanoparticle-based oxygen supply was developed and showed encouraging results as a treatment of ischemic disease. In brief, the system consisted of PLGA microspheres that released hydrogen peroxide that was rapidly converted into molecular oxygen and water by the catalase enzyme attached to a hydrogel scaffold (Fan et al., 2018). Regarding cell therapy, cardiac progenitor cells hold promise for myocardium regeneration after cardiac injury, since they are able to differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells (Le and Chong, 2016). Nonetheless, retention of such cells remains a challenge. Recently, it was shown that acrylate-based, degradable, thermal and pH-sensitive hydrogels could solve this problem and make it possible for cardiosphere-derived cells to survive and differentiate at the infarct zone (Li et al., 2016).
Biomolecule-based nanoparticles
Protein-based nanoparticles
Typically, protein-based NPs are derived from water-soluble or water-insoluble proteins. Proteins have a list of properties that makes them appealing as materials for nanoparticle synthesis. Proteins are metabolizable, amphiphilic, and possess a variety of biochemical moieties for ligand binding (Lohcharoenkal et al., 2014). A particularly studied case is that of albumin. It is widely known that albumin transports both endogenous and exogenous molecules in the bloodstream because of its multiple ligand binding sites. Beyond binding, albumin guarantees an extended lifetime mostly due to renal reabsorption and endothelial recycling that keeps it circulating for up to nineteen days (Larsen et al., 2016). Associated in a non-covalent manner, a protein or peptide fused to albumin, can covalently bind ligands to one of the chemical groups exposed at its surface.
Decafluorobutane-filled albumin microbubbles infiltrate into myocardium when the heart is exposed to ultrasound, indicating the capability of these particles to be used as drug vehicles. However, ultrasound destruction of albumin microbubbles is accompanied by hemorrhage and transient left ventricle dysfunction. For these reasons, the risk of using this technique should be seriously considered (Vancraeynest et al., 2006). In a series of studies Ji et al. (2011) investigated the feasibility of bovine serum albumin NPs linked to ultrasonic microbubbles as a gene delivery system for in vivo transfection of tissue plasminogen activator (tPA). Applying ultrasound following the injection of the aforementioned formulation resulted in targeted delivery to cardiomyocytes (Ji et al., 2011). This strategy for heart-targeted delivery of tPA based on albumin NPs was later tested in canine models of tricuspid valve replacement and coronary artery bypass grafting. In the first case, treatment with albumin NPs prevented intrachamber thrombosis and improved survival (Ji et al., 2012). In the second case, intravascular thrombosis and intimal thickening of the vein graft were potently reduced in the group treated with albumin NPs, suggesting a potential therapy for preventing coronary conduit restenosis after cardiac surgery (Ji et al., 2014). Likewise, alendronate given in an albumin nanoparticle formulation reduced the neointima/media ratio in a rat restenosis model by targeting macrophages (Markovsky et al., 2007). Another way of achieving tPA local targeting of myocardium consisted of implanting a Dacron slice coated with gelatin, which contained the tPA plasmid directly into the left atrial cavity (Liu et al., 2015).
Besides albumin, gelatin is also a promising protein material for drug delivery (Lohcharoenkal et al., 2014). In the permanent ligated hind limb, blood perfusion increased when VEGF was attached to NPs composed of glycidyl methacrylated dextran and gelatin (Xie et al., 2013). In the infarcted myocardium, co-delivery of 6-Bromoindirubin-3-oxime and IGF-1 encapsulated in gelatin NPs produced an increase in cardiomyocyte proliferation and capillary density. Importantly, this was reflected as ameliorated left ventricle functional parameters measured six weeks after surgery (Fang et al., 2015). Finally, there are some proteins with a natural tropism towards scarred myocardium. An interesting example is that of fibronectin binding domain of the Streptococcus gordonii adhesin. Recently, this motif was fused to supercharged green fluorescent protein. This chimera was bound by electrostatic forces to the plasma membrane of mesenchymal stem cells, and as a consequence these cells acquired the ability to specifically concentrate at the heart and remain there, offering hope for cell therapy (Xiao et al., 2019).
Nucleic acid-based nanoparticles
Aptamers are small single-stranded RNA/DNA oligonucleotides with the ability to recognize and bind to specific biological motifs with high affinity. In this respect, aptamers resemble antibodies, but display clear advantages over them, including lower cost of production, thermal and pH stability, low immunogenicity, and low batch-to-batch variability (Mittal et al., 2018; Tan et al., 2019). Aptamers are currently being investigated for their use as anticoagulants, anti-thrombotics and thrombolytic agents, since they are able to reversibly bind the factors implicated in the clotting cascade (Hu and Zhang, 2015). The DNA-based aptamer ARC1779 that works by binding to the A1 domain of the von Willebrand factor (vWF), demonstrated that it is capable of reducing platelet aggregation in humans (Gilbert et al., 2007). ARC1779 also effectively and safely reduced the number of embolic signals of patients that underwent carotid endarterectomy. In this context, it was suggested that it could be used as an adjunct in the treatment of myocardial ischemia (Markus et al., 2011). Amid the recent advances, the RNA-based DTRI-031 aptamer, which also targets the vWF, was shown to reestablish the blood flow of the occluded carotid arteries in both a murine and a clinically relevant canine model. DTRI-031 aptamer displayed nuclease resistance and, of note, susceptibility to be switched off by an antidote oligonucleotide. This last characteristic would strengthen the safety of aptamers in a possible clinical scenario (Nimjee et al., 2019).
Aptamers have been explored as a DDS too. In a proof of concept study, an aptamer-peptide chimera was developed. The chimera consisted of an anti-PDGFRβ aptamer and a small novel peptide (R7W-MP) with therapeutic effects on the L-type calcium channel. The chimera successfully restored the L-type calcium channel density in serum-starved HL-1 cells (Romanelli et al., 2018). Aptamers can be delivered attached to self-folding RNA origami, with the advantage of possible co-delivery of more than one aptamer. Such an approach was utilized for the delivery of two anti-thrombin aptamers, extending their lifetime and reinforcing, up to seven times, their anticoagulant activity (Krissanaprasit et al., 2019). Other forms of nucleic acid-based carriers are DNA nanotubes. DNA nanotubes tailored with a pH-sensitive motif released dexamethasone at the ischemic-reperfused skeletal muscle, where the nanotubes decreased leukocyte transmigration via downregulation of vascular adhesion molecules (Sellner et al., 2017). Further experiments would unveil the efficacy of DNA nanotubes in a myocardial ischemia context.
Peptide Self-assembly
For the purpose of cardiac regeneration, self-assembling peptides are a promising option as platforms for cell growth as they can act as growth factors reservoirs. Self-assembling peptides are characterized by having predictable gelling kinetics, and biodegradability into natural amino acids. They would allow for cell recruitment, provide the biochemical signals for cell growth, and integrate without interfering with the mechanical and electrical processes of the heart (French et al., 2016). The intramyocardial injection of self-assembling peptide nanofibers along with PDGF immediately following ligation of the left coronary artery reduced MI size and increased vascular density, such that after four months of coronary ligation, regional blood flow approached normality (Hsieh et al., 2006). The fusion of a heparin-binding motif enabled self-assembling peptides for prolonged release of VEGF, such that after one month considerable amounts of VEGF could still be detected. As expected, microvascular density was also higher compared with non-treated and VEGF-only-treated animals, a phenomenon which correlated with a better contractility performance (Guo et al., 2012). Regarding cell therapy, it would be convenient for transplanted cells to encounter an optimum microenvironment for their implantation. In this regard, a self-assembling peptide scaffold with IGF-1 incorporated by means of a biotin-streptavidin bond improved the survival of transplanted cardiomyocytes (Davis et al., 2006).
3. Current Challenges and Future
Although the development of NPs as DDSs has increased in the past decade, NPs with multi-functions have mainly been applied in the field of cancer research. In clinical trials, using therapeutic NPs in a treatment regimen is rarely used as a first line treatment, but are only available to patients as a last resort, when they have stopped responding to other treatments (Satalkar et al., 2016). Also, limited cases have been successfully translated into clinical settings to treat other diseases besides cancer. In this section, we discuss some of the challenges facing NPs delivery systems in CVD and cardioprotection (Ho et al., 2016).
3.1. Biological Challenges
As the main organ and critical pump in the body, the heart is sensitive and very resistant to foreign objects. This makes delivering drugs into the heart muscle more challenging. In most cases, biodistribution in the heart after administration of nanomedicine is minimal. In acute myocardial IRI, the coronary microvasculature becomes leaky, a well-known phenomenon in tumors, termed the enhanced permeability and retention (EPR) effect (Weis, 2008). The EPR effect makes it possible for NPs to deliver therapeutics into the ischemic heart. Another challenge is from the immune system, which is easy to be stimulated by foreign injection with NPs resulting in fast clearance from the blood. To allow the NPs to remain in the body for a longer time and to avoid being removed by immune cells, NPs can be PEGylated or PEG-terminated. Similarly, using hydrophilic sugar like dextran to coat iron-oxide NPs bring about the same effect. Another way to extend the half-life before immune recognition is decorating biological proteins, like albumin. In term of acute myocardial IRI, there is a special need to protect the NPs in the acute phase when most IR injury occurs. This sets a critical requirement for DDSs to accumulate in the heart rapidly and efficiently.
Yet another challenge comes from the intrinsic complexity of IRI pathophysiology. Since multiple independent pathways are known to contribute to the pathophysiology of IRI, it is important to stress the need for a multi-target approach to cardioprotection (Rossello and Yellon, 2018). From a therapeutic standpoint, NPs could be loaded with a combination of multi-target cargoes intended to combat IRI. In this manner, additive cardioprotection can be achieved by targeting two or more signaling pathways or the same pathway at different points, or even several cell types, including cardiomyocytes, endothelial cells, platelets, macrophages, and fibroblasts (Davidson et al., 2019).
3.2. Toxicity Challenges
Concern over patient safety is one of the main issues impeding the translation of nanomedicine into clinical practice. As a new type of therapy, toxicity studies of nanomedicine have been commonly neglected in the past decades. Despite an increasing interest in safety aspects of various nanomaterials, there is currently no standard to evaluate and categorize their toxic levels. As such, a systematic toxicity protocol should be standardized. The application in the heart requires special attention to cellular toxicity and to the biological fate of the materials, especially when salvaging cells is the target in cardioprotection. Using FDA-proven and biodegradable materials to constructing NPs is a strategy for translational nanomedicine. Physical clearance must be investigated in those non-degradable materials such as inorganic NPs for cardiac application.
3.3. Technological Challenges
Technological challenges of NPs in the lab include scale-up synthesis, performance optimization, and performance predictions. In preclinical studies, NPs are synthesized in small batches, and it is not always possible to scale up for large quantity synthesis. For performance optimization, nanoparticle formulations that provide the best results in animal models have the most potential in clinical trials, but these are not systematically optimized. Correlation between animal and human data is essential for predictions, as there are many differences and similarities between them. Thus, this poses a challenge for predicting nanoparticle performance at the preclinical and clinical level. Efforts to correlate the results should be implemented as soon as possible to determine any general trends, if possible, to allow for better results. There are also difficulties in analyzing and determining the effectiveness of the nanomedicine in humans, as most quantitative techniques require organ isolation or tissue harvesting. Non-invasive imaging techniques may be a solution in studying time-dependent biodistribution. However, imaging the beating heart with spatial resolution is still challenging.
3.4. Administration Routes
Intravenous injection has been the main route used for administration of NPs intended as treatment of AMI. To achieve more specific local delivery, in the case of hydrogels and self-assembling peptides, intramyocardial injection is preferred with proper retention. However, this invasive route has limited clinical availability and has a higher risk. Other routes for cardiac delivery of NPs have been explored, including oral (Simoń-Yarza et al., 2013), inhalation (Miragoli et al., 2018) and intraperitoneal injection (Abdelhalim, 2011), as potential alternatives to the intravenous route. Further investigations are needed to verify whether these routes are suitable in other type of NPs in AMI treatment. Overall, the advantages and disadvantages of each route are briefly summarized in Figure 3.
Figure 3.
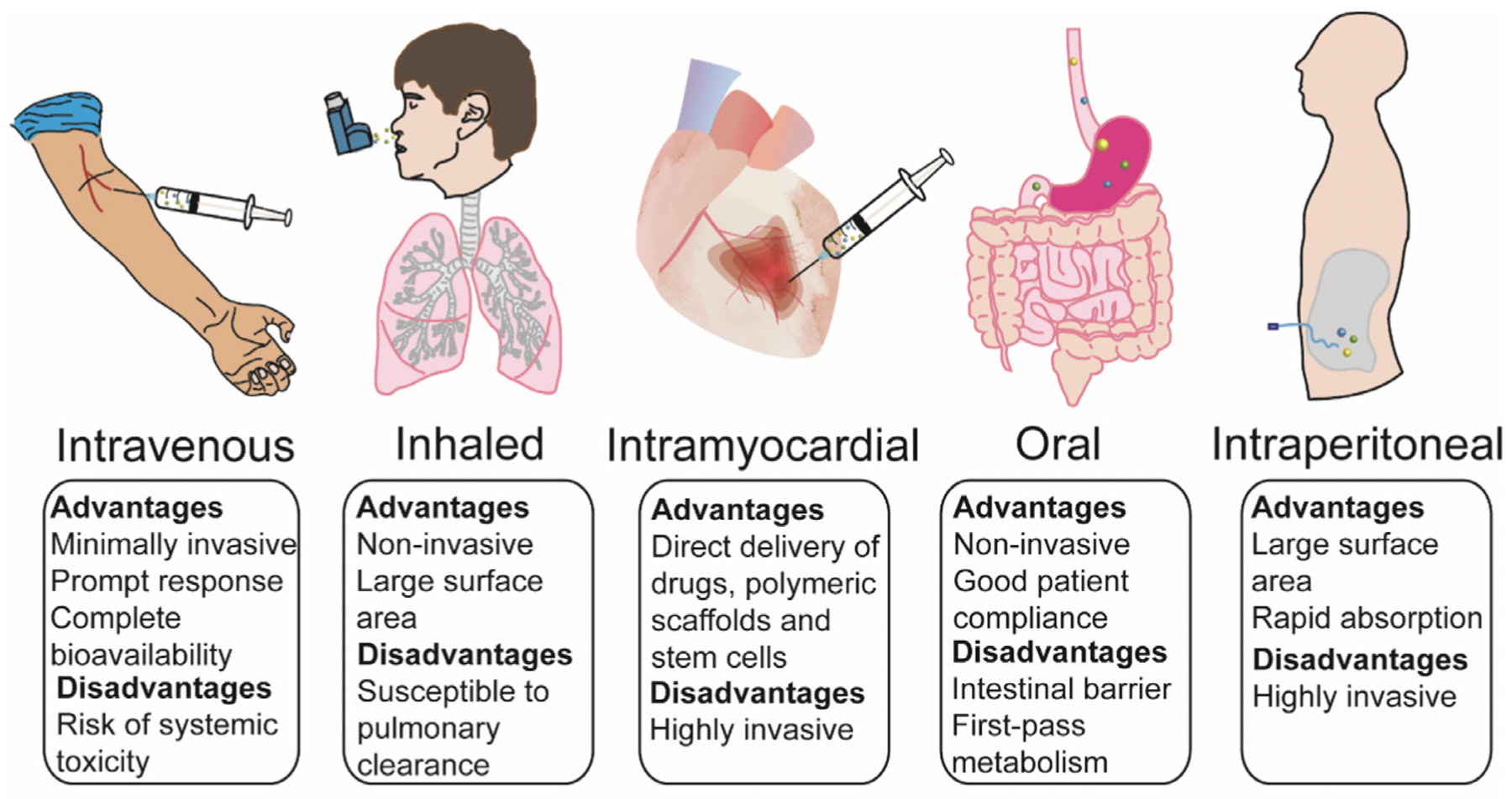
Potential routes of nanoparticle administration applied in cardiovascular disease - their advantages/disadvantages Table 1. The advantages and disadvantages of different types of nanoparticles as a drug delivery system (DDS).
4. Conclusions
Over recent years nanomedicine has been used to increase the bioavailability and delivery of therapies to treat CVD, including acute myocardial IRI. NPs, which hold promise as DDS, are liposomes, polymeric NPs, and micelles, and of these, PLGAs and liposomes are already FDA approved for use in clinical trials. The advances in NPs make their application promising in term of delivering diagnosis and targeting therapeutics to the ischemic heart following AMI. There are challenges in translating nanomedicine into the clinic, and these are primarily concerns related to safety. To overcome these challenges, further investigation of NP design in relation to both safety and efficacy are essential for the future of translational nanomedicine in CVD and cardioprotection.
Acknowledgements:
William Boisvert was supported by grant U54 MD007601 from the NIH. Shengjie Lu was supported by the Singapore Ministry of Health’s National Medical Research Council under Open Fund-Young Individual Research Grant (OF-YIRG)-MOH-000230, Academic Medicine Research Grant- AM/TP014/2018 (SRDUKAMR1811) from SingHealth Duke-NUS Academic Medicine Centre, as well as a Khoo Postdoctoral Fellowship Award (KPFA) - DUKE-NUS-KPFA/2018/0026 from the Estate of Tan Sri Khoo Teck Puat, Singapore. Derek Hausenloy was supported by the British Heart Foundation (CS/14/3/31002), the National Institute for Health Research University College London Hospitals Biomedical Research Centre, Duke-National University Singapore Medical School, Singapore Ministry of Health’s National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). HA Cabrera-Fuentes was supported by the Russian Government Program for competitive growth of Kazan Federal University, Kazan (Russian Federation), by the Singapore Heart Foundation (SHF/FG657P/2017), and by the von Behring-Röntgen-Foundation (Marburg, Germany).
Footnotes
Conflicts of interest statement
The authors declare that they have no conflicts of interest.
References
- Abdelhalim MAK (2011) Exposure to gold nanoparticles produces cardiac tissue damage that depends on the size and duration of exposure. Lipids Health Dis 10:205 Available at: http://www.lipidworld.com/content/10/1/205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed SM, Abdelrahman SA, Salama AE (2017) Efficacy of gold nanoparticles against isoproterenol induced acute myocardial infarction in adult male albino rats. Ultrastruct Pathol 41:168–185 Available at: 10.1080/01913123.2017.1281367. [DOI] [PubMed] [Google Scholar]
- Alam SR, Shah AS V, Richards J, Lang NN, Barnes G, Joshi N, MacGillivray T, McKillop G, Mirsadraee S, Payne J, Fox KAA, Henriksen P, Newby DE, Semple SIK (2012) Ultrasmall superparamagnetic particles of iron oxide in patients with acute myocardial infarction early clinical experience. Circ Cardiovasc Imaging 5:559–565. [DOI] [PubMed] [Google Scholar]
- Alavi M, Karimi N, Safaei M (2017) Application of various types of liposomes in drug delivery systems. Adv Pharm Bull 7:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allijn IE, Czarny BMS, Wang X, Chong SY, Weiler M, da Silva AE, Metselaar JM, Lam CSP, Pastorin G, de Kleijn DP V, Storm G, Wang JW, Schiffelers RM (2017) Liposome encapsulated berberine treatment attenuates cardiac dysfunction after myocardial infarction. J Control Release 247:127–133 Available at: 10.1016/j.jconrel.2016.12.042. [DOI] [PubMed] [Google Scholar]
- Allahyari S, Trotta F, Valizadeh H, Jelvehgari M, Zakeri-Milani P (2019) Cyclodextrin-based nanosponges as promising carriers for active agents. Expert Opinion on Drug Delivery 16:467–479. [DOI] [PubMed] [Google Scholar]
- Arslan F, Lai RC, Smeets MB, Akeroyd L, Choo A, Aguor ENE, Timmers L, van Rijen HV, Doevendans PA, Pasterkamp G, Lim SK, de Kleijn DP (2013) Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res 10:301–312 Available at: 10.1016/j.scr.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Bellera N, Barba I, Rodriguez-Sinovas A, Ferret E, Asín MA, Gonzalez-Alujas MT, Pérez-Rodon J, Esteves M, Fonseca C, Toran N, del Blanco BG, Pérez A, Garcia-Dorado D (2014) Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J Am Heart Assoc 3:8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin EJ et al. (2018) Heart disease and stroke statistics - 2018 update: A report from the American Heart Association. Circulation 137:E67–E492 Available at: https://www.ahajournals.org/doi/10.1161/CIR.0000000000000558 [Accessed January 14, 2020]. [DOI] [PubMed] [Google Scholar]
- Benjamin EJ et al. (2019) Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 139:e56–e528 Available at: http://www.ncbi.nlm.nih.gov/pubmed/30700139 [Accessed January 14, 2020]. [DOI] [PubMed] [Google Scholar]
- Bhatia S (2016) Nanoparticles Types, Classification, Characterization, Fabrication Methods and Drug Delivery Applications. Springer. [Google Scholar]
- Bobo D, Robinson KJ, Islam J, Thurecht KJ, Corrie SR (2016) Nanoparticle-Based Medicines: A Review of FDA-Approved Materials and Clinical Trials to Date. Pharm Res 33:2373–2387. [DOI] [PubMed] [Google Scholar]
- Bonora M, Wieckowski MR, Sinclair DA, Kroemer G, Pinton P, Galluzzi L (2019) Targeting mitochondria for cardiovascular disorders: therapeutic potential and obstacles. Nat Rev Cardiol 16:33–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga SS (2019) Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LJ, Cote B, Alani AW, Rao DA (2014) Polymeric micellar co-delivery of resveratrol and curcumin to mitigate in vitro doxorubicin-induced cardiotoxicity. J Pharm Sci 103:2315–2322. [DOI] [PubMed] [Google Scholar]
- Chang MY, Yang YJ, Chang CH, Tang ACL, Liao WY, Cheng FY, Yeh CS, Lai JJ, Stayton PS, Hsieh PCH (2013) Functionalized nanoparticles provide early cardioprotection after acute myocardial infarction. J Control Release 170:287–294 Available at: 10.1016/j.jconrel.2013.04.022. [DOI] [PubMed] [Google Scholar]
- Cote B, Carlson LJ, Rao DA, Alani AWG (2015) Combinatorial resveratrol and quercetin polymeric micelles mitigate doxorubicin induced cardiotoxicity in vitro and in vivo. J Control Release 213:128–133 Available at: 10.1016/j.jconrel.2015.06.040. [DOI] [PubMed] [Google Scholar]
- Cung TT et al. (2015) Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med 373:1021–1031. [DOI] [PubMed] [Google Scholar]
- Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V (2012) PLGA-based nanoparticles: An overview of biomedical applications. J Control Release 161:505–522 Available at: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- Dasa SSK, Suzuki R, Gutknecht M, Brinton LT, Tian Y, Michaelsson E, Lindfors L, Klibanov AL, French BA, Kelly KA (2015) Development of target-specific liposomes for delivering small molecule drugs after reperfused myocardial infarction. J Control Release 220:556–567 Available at: 10.1016/j.jconrel.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson SM, Ferdinandy P, Andreadou I, Bøtker HE, Heusch G, Ibáñez B, Ovize M, Schulz R, Yellon DM, Hausenloy DJ, Garcia-Dorado D, (CA16225) CCA (2019) Multitarget Strategies to Reduce Myocardial Ischemia/Reperfusion Injury: JACC Review Topic of the Week. Journal of the American College of Cardiology 73:89–99. [DOI] [PubMed] [Google Scholar]
- Davidson SM, Takov K, Yellon DM (2017) Exosomes and Cardiovascular Protection. Cardiovasc Drugs Ther 31:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis ME, Hsieh PCH, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT (2006) Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A 103:8155–8160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Chen S, Cui G, Yang Y, Zhang E, Wang Q, Lavin MF, Yeo AJ, Bo C, Zhang Y, Li C, Liu X, Yang X, Peng C, Shao H (2019) Silica nanoparticles induce cardiomyocyte apoptosis via the mitochondrial pathway in rats following intratracheal instillation. Int J Mol Med 43:1229–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Z, Zhao D, Jing L, Cui G, Jin M, Li Y, Liu X, Liu Y, Du H, Guo C, Zhou X, Sun Z (2013) Cardiovascular toxicity of different sizes amorphous silica nanoparticles in rats after intratracheal instillation. Cardiovasc Toxicol 13:194–207. [DOI] [PubMed] [Google Scholar]
- Dutta P, Nahrendorf M (2015) Monocytes in myocardial infarction. Arterioscler Thromb Vasc Biol 35:1066–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dvir T, Bauer M, Schroeder A, Tsui JH, Anderson DG, Langer R, Liao R, Kohane DS (2011) Nanoparticles targeting the infarcted heart. Nano Lett 11:4411–4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Say KM, El-Sawy HS (2017) Polymeric nanoparticles: Promising platform for drug delivery. Int J Pharm 528:675–691 Available at: 10.1016/j.ijpharm.2017.06.052. [DOI] [PubMed] [Google Scholar]
- Elzoghby AO, Samy WM, Elgindy NA (2012) Albumin-based nanoparticles as potential controlled release drug delivery systems. J Control Release 157:168–182. [DOI] [PubMed] [Google Scholar]
- Fan Y, Jiang Y, Fu X, Cai J, Wang Y, Li W, Gu X, Xing K, Bai S, Bi X (2015) Effects of liposomal prostaglandin E1 on periprocedural myocardial injury in patients with unstable angina undergoing an elective percutaneous coronary intervention. Coron Artery Dis 26:671–677. [DOI] [PubMed] [Google Scholar]
- Fan Z, Xu Z, Niu H, Gao N, Guan Y, Li C, Dang Y, Cui X, Liu XL, Duan Y, Li H, Zhou X, Lin PH, Ma J, Guan J (2018) An Injectable Oxygen Release System to Augment Cell Survival and Promote Cardiac Repair Following Myocardial Infarction. Sci Rep 8:1–22 Available at: 10.1038/s41598-018-19906-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang R, Qiao S, Liu Y, Meng Q, Chen X, Song B, Hou X, Tian W (2015) Sustained co-delivery of BIO and IGF-1 by a novel hybrid hydrogel system to stimulate endogenous cardiac repair in myocardial infarcted rat hearts. Int J Nanomedicine 10:4691–4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Femminò S, Penna C, Bessone F, Caldera F, Dhakar N, Cau D, Pagliaro P, Cavalli R, Trotta F (2018) α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model. Polymers (Basel) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MPA, Ranjan S, Correia AMR, Mäkilä EM, Kinnunen SM, Zhang H, Shahbazi MA, Almeida PV, Salonen JJ, Ruskoaho HJ, Airaksinen AJ, Hirvonen JT, Santos HA (2016) In vitro and in vivo assessment of heart-homing porous silicon nanoparticles. Biomaterials 94:93–104. [DOI] [PubMed] [Google Scholar]
- Figge L, Appler F, Chen HH, Sosnovik DE, Schnorr J, Seitz O, Taupitz M, Hamm B, Schellenberger E (2014) Direct coupling of annexin A5 to VSOP yields small, protein-covered nanoprobes for MR imaging of apoptosis. Contrast Media Mol Imaging 9:291–299. [DOI] [PubMed] [Google Scholar]
- French KM, Somasuntharam I, Davis ME (2016) Self-assembling peptide-based delivery of therapeutics for myocardial infarction. Adv Drug Deliv Rev 96:40–53 Available at: 10.1016/j.addr.2015.04.023. [DOI] [PubMed] [Google Scholar]
- Frey UH, Klaassen M, Ochsenfarth C, Murke F, Thielmann M, Kottenberg E, Kleinbongard P, Klenke S, Engler A, Heusch G, Giebel B, Peters J (2019) Remote ischaemic preconditioning increases serum extracellular vesicle concentrations with altered micro-RNA signature in CABG patients. Acta Anaesthesiol Scand 63:483–492. [DOI] [PubMed] [Google Scholar]
- Galagudza M, Korolev D, Postnov V, Naumisheva E, Grigorova Y, Uskov I, Shlyakhto E (2012) Passive targeting of ischemic-reperfused myocardium with adenosine-loaded silica nanoparticles. Int J Nanomedicine 7:1671–1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagudza MM, Korolev DV, Sonin DL, Postnov VN, Papayan GV, Uskov IS, Belozertseva AV, Shlyakhto EV (2010) Targeted drug delivery into reversibly injured myocardium with silica nanoparticles: Surface functionalization, natural biodistribution, and acute toxicity. Int J Nanomedicine 5:231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallet R, Dawkins J, Valle J, Simsolo E, De Couto G, Middleton R, Tseliou E, Luthringer D, Kreke M, Smith RR, Marbán L, Ghaleh B, Marbán E (2017) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 38:201–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallina C, Capelôa T, Saviozzi S, Accomasso L, Catalano F, Tullio F, Martra G, Penna C, Pagliaro P, Turinetto V, Giachino C (2015) Human mesenchymal stem cells labelled with dye-loaded amorphous silica nanoparticles: long-term biosafety, stemness preservation and traceability in the beating heart. Journal of Nanobiotechnology 13:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen T, Paulis LE, Coolen BF, Nicolay K, Strijkers GJ (2013) Passive targeting of lipid-based nanoparticles to mouse cardiac ischemia-reperfusion injury. Contrast Media Mol Imaging 8:117–126. [DOI] [PubMed] [Google Scholar]
- Gilbert JC, DeFeo-Fraulini T, Hutabarat RM, Horvath CJ, Merlino PG, Marsh HN, Healy JM, BouFakhreddine S, Holohan TV, Schaub RG (2007) First-in-human evaluation of anti-von Willebrand factor therapeutic aptamer ARC1779 in healthy volunteers. Circulation 116:2678–2686. [DOI] [PubMed] [Google Scholar]
- Gollman-Tepeköylü C et al. (2019) miR-19a-3p containing exosomes improve function of ischemic myocardium upon shock wave therapy. Cardiovasc Res. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Lønborg J, Mazhar J, Tan TC, Ho E, Liu CC, Lay W, Gill AJ, Kuchel P, Bhindi R, Figtree GA (2013) Cardiac magnetic resonance imaging of rapid VCAM-1 up-regulation in myocardial ischemia-reperfusion injury. Eur Biophys J 42:61–70. [DOI] [PubMed] [Google Scholar]
- Grimaldi AM, Forte E, Infante T, Cavaliere C, Salvatore M, Cademartiri F (2019) Future perspectives of nanoparticle-based contrast agents for cardiac magnetic resonance in myocardial infarction. Nanomedicine Nanotechnology, Biol Med 17:329–341. [DOI] [PubMed] [Google Scholar]
- Guo HD, Cui GH, Yang JJ, Wang C, Zhu J, Zhang LS, Jiang J, Shao SJ (2012) Sustained delivery of VEGF from designer self-assembling peptides improves cardiac function after myocardial infarction. Biochem Biophys Res Commun 424:105–111 Available at: 10.1016/j.bbrc.2012.06.080. [DOI] [PubMed] [Google Scholar]
- Higashi K, Yamada Y, Minatoguchi S, Baba S, Iwasa M, Kanamori H, Kawasaki M, Nishigaki K, Takemura G, Kumazaki M, Akao Y, Minatoguchi S (2015) MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am J Physiol - Hear Circ Physiol 309:H1813--H1826. [DOI] [PubMed] [Google Scholar]
- Higuchi Y, Niidome T, Miyamoto Y, Komohara Y, Tokunou T, Kubota T, Horiuchi T (2019) Accumulation of gold nano-rods in the failing heart of transgenic mice with the cardiac-specific expression of TNF-α. Heart Vessels 34:538–544 Available at: 10.1007/s00380-018-1241-2. [DOI] [PubMed] [Google Scholar]
- Ho YT, Poinard B, Kah JCY (2016) Nanoparticle drug delivery systems and their use in cardiac tissue therapy. Nanomedicine 11:693–714 Available at: https://www.futuremedicine.com/doi/10.2217/nnm.16.6 [Accessed January 14, 2020]. [DOI] [PubMed] [Google Scholar]
- Hsieh PCH, MacGillivray C, Gannon J, Cruz FU, Lee RT (2006) Local controlled intramyocardial delivery of platelet-derived growth factor improves postinfarction ventricular function without pulmonary toxicity. Circulation 114:637–644. [DOI] [PubMed] [Google Scholar]
- Hu D, Tang S, Peng H, Wang Q (2015) The Bright Future of Liposome Mediated Drug Delivery. Biochem Physiol 04:e133. [Google Scholar]
- Hu PP, Zhang KH (2015) The modulation of coagulation by aptamers: An up-to-date review. Blood Coagul Fibrinolysis 26:1–6. [DOI] [PubMed] [Google Scholar]
- Ibanez B et al. (2019) Cardiac MRI Endpoints in Myocardial Infarction Experimental and Clinical Trials: JACC Scientific Expert Panel. J Am Coll Cardiol 74:238–256 Available at: http://www.ncbi.nlm.nih.gov/pubmed/31296297 [Accessed January 17, 2020]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim AGE, Cheng K, Marbán E (2014) Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Reports 2:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda G, Matoba T, Nakano Y, Nagaoka K, Ishikita A, Nakano K, Funamoto D, Sunagawa K, Egashira K (2016) Nanoparticle-Mediated Targeting of Cyclosporine A Enhances Cardioprotection Against Ischemia-Reperfusion Injury Through Inhibition of Mitochondrial Permeability Transition Pore Opening. Sci Rep 6:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Ji J, He Xia (2011) Preparation of ultrasound microbubbles crosslinked to albumin nanoparticles packaged with tissue-type plasminogen activator gene plasmid and method of in vivo transfection. J Exp Pharmacol:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Ji SY, Yang JA, He X, Yang XH, Ling WP, Chen XL (2012) Ultrasound-targeted transfection of tissue-type plasminogen activator gene carried by albumin nanoparticles to dog myocardium to prevent thrombosis after heart mechanical valve replacement. Int J Nanomedicine 7:2911–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Yang JA, He X, Ling WP, Chen XL (2014) Cardiac-targeting transfection of tissue-type plasminogen activator gene to prevent the graft thrombosis and vascular anastomotic restenosis after coronary bypass. Thromb Res 134:440–448 Available at: 10.1016/j.thromres.2014.04.018. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Bøtker HE, Ovize M, Hausenloy DJ, Heusch G (2019) Co-morbidities and co-medications as confounders of cardioprotection - does it matter in the clinical setting? Br J Pharmacol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissanaprasit A, Key C, Fergione M, Froehlich K, Pontula S, Hart M, Carriel P, Kjems J, Andersen ES, LaBean TH (2019) Genetically Encoded, Functional Single-Strand RNA Origami: Anticoagulant. Adv Mater 31:1–7. [DOI] [PubMed] [Google Scholar]
- Larsen MT, Kuhlmann M, Hvam ML, Howard KA (2016) Albumin-based drug delivery: harnessing nature to cure disease. Mol Cell Ther 4:1–12 Available at: 10.1186/s40591-016-0048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le T, Chong J (2016) Cardiac progenitor cells for heart repair. Cell Death Discov 2:2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Cha MJ, Lim KS, Kim JK, Lee SK, Kim YH, Hwang KC, Lee KY (2013) Injectable microsphere/hydrogel hybrid system containing heat shock protein as therapy in a murine myocardial infarction model. J Drug Target 21:822–829. [DOI] [PubMed] [Google Scholar]
- Leuschner F et al. (2011) Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol 29:1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Ni J, Li M, Chen J, Han L, Zhu Y, Kong D, Mao J, Wang Y, Zhang B, Zhu M, Gao X, Fan G (2017) Ginsenoside Rg3 micelles mitigate doxorubicin-induced cardiotoxicity and enhance its anticancer efficacy. Drug Deliv 24:1617–1630 Available at: 10.1080/10717544.2017.1391893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Wu J, Zhang J, Wang J, Xiang D, Luo S, Li J, Liu X (2018) Puerarin-loaded PEG-PE micelles with enhanced antiapoptotic effect and better pharmacokinetic profile. Drug Deliv 25:827–837 Available at: 10.1080/10717544.2018.1455763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WQ, Wu JY, Xiang DX, Luo SL, Hu X Bin, Tang TT, Sun TL, Liu XY (2019) Micelles loaded with puerarin and modified with triphenylphosphonium cation possess mitochondrial targeting and demonstrate enhanced protective effect against isoprenaline-induced H9c2 cells apoptosis. Int J Nanomedicine 14:8345–8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Fan Z, Xu Y, Lo W, Wang X, Niu H, Li X, Xie X, Khan M, Guan J (2016) PH-Sensitive and Thermosensitive Hydrogels as Stem-Cell Carriers for Cardiac Therapy. ACS Appl Mater Interfaces 8:10752–10760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Li M, Sun S, Li B, Du D, Sun J, Cao F, Li H, Jia F, Wang T, Chang N, Yu H, Wang Q, Peng H (2014) The use of antibody modified liposomes loaded with AMO-1 to deliver oligonucleotides to ischemic myocardium for arrhythmia therapy. Biomaterials 35:3697–3707 Available at: 10.1016/j.biomaterials.2013.12.099. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang K, Jiang X, Xia J, Xiang D (2015) Effects of tissue-type plasminogen site-specific transgene in gelatin-coated dacron on the fibrinolysis activity of rabbit left atrium. Acta Cardiol Sin 31:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohcharoenkal W, Wang L, Chen YC, Rojanasakul Y (2014) Protein nanoparticles as drug delivery carriers for cancer therapy. Biomed Res Int 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanov AN, Hartner WC, Torchilin VP (2004) Increased accumulation of PEG-PE micelles in the area of experimental myocardial infarction in rabbits. J Control Release 94:187–193. [DOI] [PubMed] [Google Scholar]
- Mao Y, Koga JI, Tokutome M, Matoba T, Ikeda G, Nakano K, Egashira K (2017) Nanoparticle-mediated delivery of pitavastatin to monocytes/macrophages inhibits left ventricular remodeling after acute myocardial infarction by inhibiting monocyte-mediated inflammation. Int Heart J 58:615–623. [DOI] [PubMed] [Google Scholar]
- Markovsky E, Koroukhov N, Golomb G (2007) Additive-free albumin nanoparticles of alendronate for attenuating inflammation through monocyte inhibition. Nanomedicine 2:545–553. [DOI] [PubMed] [Google Scholar]
- Markus HS, McCollum C, Imray C, Goulder MA, Gilbert J, King A (2011) The von willebrand inhibitor ARC1779 reduces cerebral embolization after carotid endarterectomy: A randomized trial. Stroke 42:2149–2153. [DOI] [PubMed] [Google Scholar]
- Mehmood A, Ghafar H, Yaqoob S, Gohar UF, Ahmad B (2017) Mesoporous Silica Nanoparticles: A Review. J Dev Drugs 06. [Google Scholar]
- Miragoli M, Ceriotti P, Iafisco M, Vacchiano M, Salvarani N, Alogna A, Carullo P, Ramirez-Rodríguez GB, Patrício T, Esposti LD, Rossi F, Ravanetti F, Pinelli S, Alinovi R, Erreni M, Rossi S, Condorelli G, Post H, Tampieri A, Catalucci D (2018) Inhalation of peptide-loaded nanoparticles improves heart failure. Science Translational Medicine 10:eaan6205. [DOI] [PubMed] [Google Scholar]
- Mittal R, Jhaveri VM, McMurry HS, Kay SIS, Sutherland KJ, Nicole L, Mittal J, Jayant RD (2018) Recent treatment modalities for cardiovascular diseases with a focus on stem cells, aptamers, exosomes and nanomedicine. Artif Cells, Nanomedicine Biotechnol 46:831–840 Available at: 10.1080/21691401.2018.1436555. [DOI] [PubMed] [Google Scholar]
- Musacchio T, Torchilin VP (2013) Advances in polymeric and lipid-core micelles as drug delivery systems. In: Polymeric Biomaterials: Medicinal and Pharmaceutical Applications, Volume 2, pp 65–84. [Google Scholar]
- Nguyen J, Sievers R, Motion JPM, Kivimäe S, Fang Q, Lee RJ (2015a) Delivery of lipid micelles into infarcted myocardium using a lipid-linked matrix metalloproteinase targeting peptide. Mol Pharm 12:1150–1157. [DOI] [PubMed] [Google Scholar]
- Nguyen MM, Carlini AS, Chien MP, Sonnenberg S, Luo C, Braden RL, Osborn KG, Li Y, Gianneschi NC, Christman KL (2015b) Enzyme-Responsive Nanoparticles for Targeted Accumulation and Prolonged Retention in Heart Tissue after Myocardial Infarction. Adv Mater 27:5547–5552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimjee SM et al. (2019) Preclinical Development of a vWF Aptamer to Limit Thrombosis and Engender Arterial Recanalization of Occluded Vessels. Mol Ther 27:1228–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oduk Y, Zhu W, Kannappan R, Zhao M, Borovjagin AV, Oparil S, Jay Zhang J (2018) VEGF nanoparticles repair the heart after myocardial infarction. Am J Physiol - Hear Circ Physiol 314:H278--H284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornelas-Soto N, Rubio-Govea R, Guerrero-Beltrán CE, Vázquez-Garza E, Bernal-Ramírez J, García-García A, Oropeza-Almazán Y, García-Rivas G, Contreras-Torres FF (2017) Enhancing internalization of silica particles in myocardial cells through surface modification. Mater Sci Eng C 79:831–840. [DOI] [PubMed] [Google Scholar]
- Pascual-Gil S, Simón-Yarza T, Garbayo E, Prósper F, Blanco-Prieto MJ (2017) Cytokine-loaded PLGA and PEG-PLGA microparticles showed similar heart regeneration in a rat myocardial infarction model. Int J Pharm 523:531–533. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Gould SJ (2019) Exosomes. Annu Rev Biochem 88:487–514. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Zhu B, Tian A, Li Z (2017) PEG-coated gold nanoparticles attenuate β-adrenergic receptor-mediated cardiac hypertrophy. Int J Nanomedicine 12:4709–4719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanelli A, Affinito A, Avitabile C, Catuogno S, Ceriotti P, Iaboni M, Modica J, Condorelli G, Catalucci D (2018) An anti-PDGFRβ aptamer for selective delivery of small therapeutic peptide to cardiac cells. PLoS One 13:1–9 Available at: 10.1371/journal.pone.0193392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossello X, Yellon DM (2018) The RISK pathway and beyond. Basic Research in Cardiology 113:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudramurthy GR, Swamy MK (2018) Potential applications of engineered nanoparticles in medicine and biology: an update. J Biol Inorg Chem 23:1185–1204. [DOI] [PubMed] [Google Scholar]
- Sahay G, Querbes W, Alabi C, Eltoukhy A, Sarkar S, Zurenko C, Karagiannis E, Love K, Chen D, Zoncu R, Buganim Y, Schroeder A, Langer R, Anderson DG (2013) Efficiency of siRNA delivery by lipid nanoparticles is limited by endocytic recycling. Nat Biotechnol 31:653–658 Available at: 10.1038/nbt.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samidurai A, Salloum FN, Durrant D, Chernova OB, Kukreja RC, Das A (2017) Chronic treatment with novel nanoformulated micelles of rapamycin, Rapatar, protects diabetic heart against ischaemia/reperfusion injury. Br J Pharmacol 174:4771–4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satalkar P, Elger BS, Hunziker P, Shaw D (2016) Challenges of clinical translation in nanomedicine: A qualitative study. Nanomedicine Nanotechnology, Biol Med 12:893–900 Available at: https://linkinghub.elsevier.com/retrieve/pii/S1549963415006218 [Accessed January 14, 2020]. [DOI] [PubMed] [Google Scholar]
- Sellner S, Kocabey S, Zhang T, Nekolla K, Hutten S, Krombach F, Liedl T, Rehberg M (2017) Dexamethasone-conjugated DNA nanotubes as anti-inflammatory agents in vivo. Biomaterials 134:78–90. [DOI] [PubMed] [Google Scholar]
- Sharma S, Parmar A, Kori S, Sandhir R (2016) PLGA-based nanoparticles: A new paradigm in biomedical applications. TrAC - Trends Anal Chem 80:30–40 Available at: 10.1016/j.trac.2015.06.014. [DOI] [Google Scholar]
- Simoń-Yarza T, Tamayo E, Benavides C, Lana H, Formiga FR, Grama CN, Ortiz-de-Solorzano C, Ravi Kumar MN V, Prosper F, Blanco-Prieto MJ (2013) Functional benefits of PLGA particulates carrying VEGF and CoQ10 in an animal of myocardial ischemia. Int J Pharm 454:784–790 Available at: 10.1016/j.ijpharm.2013.04.015. [DOI] [PubMed] [Google Scholar]
- Somasuntharam I, Yehl K, Carroll SL, Maxwell JT, Martinez MD, Che PL, Brown ME, Salaita K, Davis ME (2016) Knockdown of TNF-α by DNAzyme gold nanoparticles as an anti-inflammatory therapy for myocardial infarction. Biomaterials 83:12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonin DL, Korolev DV, Postnov VN, Naumysheva EB, Pochkaeva EI, Vasyutina ML, Galagudza MM (2015) Silicon-containing nanocarriers for targeted drug delivery: Synthesis, physicochemical properties and acute toxicity. Drug Deliv 23:1747–1756. [DOI] [PubMed] [Google Scholar]
- Sosnovik DE, Caravan P (2019) Targeted MR Imaging in Cardiovascular Disease. In: Cardiovascular Magnetic Resonance Imaging, Second. (Kwong RY, Jerosch-Herold M, Heydari B, eds), pp 439–449. New York: Springer. [Google Scholar]
- Sosnovik DE, Garanger E, Aikawa E, Nahrendorf M, Jose-Figuiredo L, Dai G, Reynolds F, Rosenzweig A, Weissleder R, Josephson L (2009) Molecular MRI of cardiomyocyte apoptosis with simultaneous delayed-enhancement MRI distinguishes apoptotic and necrotic myocytes in vivo: Potential for midmyocardial salvage in acute ischemia. Circ Cardiovasc Imaging 2:460–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosnovik DE, Schellenberger EA, Nahrendorf M, Novikov MS, Matsui T, Dai G, Reynolds F, Grazette L, Rosenzweig A, Weissleder R, Josephson L (2005) Magnetic resonance imaging of cardiomyocyte apoptosis with a novel magneto-optical nanoparticle. Magn Reson Med 54:718–724. [DOI] [PubMed] [Google Scholar]
- Spivak MY, Bubnov RV, Yemets IM, Lazarenko LM, Tymoshok NO, Ulberg ZR (2013) Development and testing of gold nanoparticles for drug delivery and treatment of heart failure: A theranostic potential for PPP cardiology. EPMA J 4:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahama H, Minamino T, Asanuma H, Fujita M, Asai T, Wakeno M, Sasaki H, Kikuchi H, Hashimoto K, Oku N, Asakura M, Kim J, Takashima S, Komamura K, Sugimachi M, Mochizuki N, Kitakaze M (2009) Prolonged Targeting of Ischemic/Reperfused Myocardium by Liposomal Adenosine Augments Cardioprotection in Rats. J Am Coll Cardiol 53:709–717 Available at: 10.1016/j.jacc.2008.11.014. [DOI] [PubMed] [Google Scholar]
- Takatani-Nakase T, Katayama M, Matsui C, Hanaoka K, Van Der Vlies AJ, Takahashi K, Nakase I, Hasegawa U (2017) Hydrogen sulfide donor micelles protect cardiomyocytes from ischemic cell death. Mol Biosyst 13:1705–1708 Available at: 10.1039/C7MB00191F. [DOI] [PubMed] [Google Scholar]
- Tan KX, Pan S, Jeevanandam J, Danquah MK (2019) Cardiovascular therapies utilizing targeted delivery of nanomedicines and aptamers. Int J Pharm 558:413–425 Available at: 10.1016/j.ijpharm.2019.01.023. [DOI] [PubMed] [Google Scholar]
- Tan SY, Teh C, Ang CY, Li M, Li P, Korzh V, Zhao Y (2017) Responsive mesoporous silica nanoparticles for sensing of hydrogen peroxide and simultaneous treatment toward heart failure. Nanoscale 9:2253–2261. [DOI] [PubMed] [Google Scholar]
- Thygesen K, Joseph Alpert DS, Allan Jaffe US, Chaitman BR, Bax JJ, Morrow DA, White HD, Zealand N, Bucciarelli-Ducci Hugo Katus Fausto J Pinto Elliott M Antman CA, Hamm CW, Angeles Alonso Garcia Richard Underwood MS, Canty Jr Alexander R Lyon PJ Devereaux Jose Luis Zamorano Bertil Lindahl William S Weintraub JM (2018) 4th Universal Definition of MI. J Am Coll Cardiol 72:2231–2264.30153967 [Google Scholar]
- Tokutome M, Matoba T, Nakano Y, Okahara A, Fujiwara M, Koga J-I, Nakano K, Tsutsui H, Egashira K (2019) Peroxisome proliferator-activated receptor-gamma targeting nanomedicine promotes cardiac healing after acute myocardial infarction by skewing monocyte/macrophage polarization in preclinical animal models. Cardiovasc Res 115:419–431 Available at: https://academic.oup.com/cardiovascres/article/115/2/419/5066356 [Accessed January 17, 2020]. [DOI] [PubMed] [Google Scholar]
- Uemura Y, Naoi T, Kanai Y, Kobayashi K (2019) The efficiency of lipid nanoparticles with an original cationic lipid as a siRNA delivery system for macrophages and dendritic cells. Pharm Dev Technol 24:263–268 Available at: 10.1080/10837450.2018.1469149. [DOI] [PubMed] [Google Scholar]
- Utech S, Boccaccini AR (2016) A review of hydrogel-based composites for biomedical applications: enhancement of hydrogel properties by addition of rigid inorganic fillers. Springer US. [Google Scholar]
- Vancraeynest D, Havaux X, Pouleur AC, Pasquet A, Gerber B, Beauloye C, Rafter P, Bertrand L, Vanoverschelde JLJ (2006) Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur Heart J 27:237–345. [DOI] [PubMed] [Google Scholar]
- Vandergriff A, Huang K, Shen D, Hu S, Hensley MT, Caranasos TG, Qian L, Cheng K (2018) Targeting regenerative exosomes to myocardial infarction using cardiac homing peptide. Theranostics 8:1869–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinodhini A, Govindaraju K, Singaravelu G, Mohamed Sadiq A, Kumar VG (2014) Cardioprotective potential of biobased gold nanoparticles. Colloids Surfaces B Biointerfaces 117:480–486 Available at: 10.1016/j.colsurfb.2014.01.006. [DOI] [PubMed] [Google Scholar]
- Wang J, Seo MJ, Deci MB, Weil BR, Canty JM, Nguyen J (2018a) Effect of CCR2 inhibitor-loaded lipid micelles on inflammatory cell migration and cardiac function after myocardial infarction. Int J Nanomedicine 13:6441–6451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Huang H, Zhang L, Bai Y, Chen H (2017) PCM and TAT co-modified liposome with improved myocardium delivery: In vitro and in vivo evaluations. Drug Deliv 24:339–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wang Y, Wang X, Hu P (2018b) Tilianin-loaded reactive oxygen species-scavenging nano-micelles protect H9c2 cardiomyocyte against hypoxia/reoxygenation-induced injury. [DOI] [PubMed]
- Wei LY, Fu XH, Li W, Bi X Le, Bai SR, Xing K, Wang YB (2015) Effect of intravenous administration of liposomal prostaglandin E1 on microcirculation in patients with ST elevation myocardial infarction undergoing primary percutaneous intervention. Chin Med J (Engl) 128:1147–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weis SM (2008) Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol 15:243–249 Available at: https://insights.ovid.com/crossref?an=00062752-200805000-00016 [Accessed January 14, 2020]. [DOI] [PubMed] [Google Scholar]
- Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY (2016) Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis 7:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao W, Green TIP, Liang X, Delint RC, Perry G, Roberts MS, Le Vay K, Back CR, Ascione R, Wang H, Race PR, Perriman AW (2019) Designer artificial membrane binding proteins to direct stem cells to the myocardium. Chem Sci 10:7610–7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J, Wang H, Wang Y, Ren F, Yi W, Zhao K, Li Z, Zhao Q, Liu Z, Wu H, Gu C, Yi D (2013) Induction of Angiogenesis by Controlled Delivery of Vascular Endothelial Growth Factor Using Nanoparticles. Cardiovasc Ther 31:12–18. [DOI] [PubMed] [Google Scholar]
- Yadav YC, Pattnaik S, Swain K (2019) Curcumin loaded mesoporous silica nanoparticles: assessment of bioavailability and cardioprotective effect. Drug Dev Ind Pharm 0:000 Available at: 10.1080/03639045.2019.1672717. [DOI] [PubMed] [Google Scholar]
- Yamada Y, Minatoguchi S, Endo N, Kanamori H, Kawasaki M, Nishigaki K, Mikami A, Minatoguchi S (2019) Post-MI treatment with G-CSF and EPO-liposome with SLX repairs infarcted myocardium through EPCs mobilization and activation of prosurvival signals in rabbits. Pharmacol Res Perspect 7:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Teh C, Sreejith S, Zhu L, Kwok A, Fang W, Ma X, Nguyen KT, Korzh V, Zhao Y (2012) Functional mesoporous silica nanoparticles for photothermal-controlled drug delivery in vivo. Angew Chemie - Int Ed 51:8373–8377. [DOI] [PubMed] [Google Scholar]
- Yang C, Tian A, Li Z (2016) Reversible cardiac hypertrophy induced by PEG-coated gold nanoparticles in mice. Sci Rep 6:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Tian A, Wu J, Meng Z, Zhang Y, Nie G, Li Z (2019a) Gold Nanoparticles for Targeting the Fibrotic Heart: A Probe Indicating Vascular Permeability. J Nanosci Nanotechnol 19:7546–7550. [DOI] [PubMed] [Google Scholar]
- Yang C, Yang H, Wu J, Meng Z, Xing R, Tian A, Tian X, Guo L, Zhang Y, Nie G, Li Z (2013) No overt structural or functional changes associated with PEG-coated gold nanoparticles accumulation with acute exposure in the mouse heart. Toxicol Lett 222:197–203 Available at: 10.1016/j.toxlet.2013.07.018. [DOI] [PubMed] [Google Scholar]
- Yang H, Qin X, Wang H, Zhao X, Liu Y, Wo HT, Liu C, Nishiga M, Chen H, Ge J, Sayed N, Abilez OJ, Ding D, Heilshorn SC, Li K (2019b) An in Vivo miRNA Delivery System for Restoring Infarcted Myocardium. ACS Nano 13:9880–9894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Y, Liu J, Chen M, Sun L, Lan M (2010) In vitro toxicity of silica nanoparticles in myocardial cells. Environ Toxicol Pharmacol 29:131–137. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Davidson SM (2014) Exosomes nanoparticles involved in cardioprotection? Circ Res 114:325–332. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Dengler MA, Van Der Kuip H, Yildiz H, Rösch S, Klumpp S, Klingel K, Kandolf R, Helluy X, Hiller KH, Jakob PM, Sechtem U (2013a) Imaging of myocardial infarction using ultrasmall superparamagnetic iron oxide nanoparticles: A human study using a multi-parametric cardiovascular magnetic resonance imaging approach. Eur Heart J 34:462–475. [DOI] [PubMed] [Google Scholar]
- Yilmaz A, Rösch S, Klingel K, Kandolf R, Helluy X, Hiller KH, Jakob PM, Sechtem U (2013b) Magnetic resonance imaging (MRI) of inflamed myocardium using iron oxide nanoparticles in patients with acute myocardial infarction - Preliminary results. Int J Cardiol 163:175–182 Available at: 10.1016/j.ijcard.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Zhang CX, Cheng Y, Liu DZ, Liu M, Cui H, Zhang B Le, Mei QB, Zhou SY(2019) Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J Nanobiotechnology 17:1–16 Available at: 10.1186/s12951-019-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Xue Y, Ni Y, Ning F, Shang L, Ma A (2018) Size dependent effects of Gold Nanoparticles in ISO-induced Hyperthyroid Rats. Sci Rep 8:1–13 Available at: 10.1038/s41598-018-27934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lu K, Zhang N, Zhao Y, Ma Q, Shen J, Lin Y, Xiang P, Tang Y, Hu X, Chen J, Zhu W, Webster KA, Wang J, Yu H (2018) Myocardial reparative functions of exosomes from mesenchymal stem cells are enhanced by hypoxia treatment of the cells via transferring microRNA-210 in an nSMase2-dependent way. Artif Cells, Nanomedicine Biotechnol 46:1659–1670 Available at: 10.1080/21691401.2017.1388249. [DOI] [PMC free article] [PubMed] [Google Scholar]


