Abstract
Decline in ovarian reserve with advancing age is associated with reduced fertility and the emergence of metabolic disturbances, osteoporosis, and neurodegeneration. Recent studies have provided insight into connections between ovarian insufficiency and systemic aging, although the basic mechanisms that promote ovarian reserve depletion remain unknown. Here, we sought to determine if chronological age is linked to changes in ovarian cellular senescence, transcriptomic, and epigenetic mechanisms in a mouse model. Histological assessments and transcriptional analyses revealed the accumulation of lipofuscin aggresomes and senescence-related transcripts (Cdkn1a, Cdkn2a, Pai-1 and Hmgb1) significantly increased with advancing age. Transcriptomic profiling and pathway analyses following RNA sequencing, revealed an upregulation of genes related to pro-inflammatory stress and cell-cycle inhibition, whereas genes involved in cell-cycle progression were downregulated; which could be indicative of senescent cell accumulation. The emergence of these senescence-related markers preceded the dramatic decline in primordial follicle reserve observed. Whole Genome Oxidative Bisulfite Sequencing (WGoxBS) found no genome-wide or genomic context-specific DNA methylation and hydroxymethylation changes with advancing age. These findings suggest that cellular senescence may contribute to ovarian aging, and thus, declines in ovarian follicular reserve. Cell-type-specific analyses across the reproductive lifespan are needed to fully elucidate the mechanisms that promote ovarian insufficiency.
Keywords: Aging, Cellular senescence, DNA methylation, Epigenetics, Ovary
1.1. INTRODUCTION
Female mammalian aging is associated with the depletion of the ovarian follicular reserve. The ovarian reserve is composed of primordial follicles and is determined in utero (te Velde et al., 1998b). Primordial follicles consist of an oocyte surrounded by a single layer of granulosa cells (Edson et al., 2009) that remain in a quiescent state until activated by a variety of endocrine factors. The primordial follicles become primary follicles as part of an irreversible process, and may further grow until ovulation or become atretic at any stage of development (Baker, 1963). An active ovary is the primary source of steroid hormones in the female (Baird, 1974). Once the primordial reserve is severely reduced, ovarian activity and hormonal production dramatically declines thereby leading to the menopausal transition. Menopause is causally linked to a variety of morbidities including diabetes, cardiovascular disease, various cancers, and neurodegenerative diseases (Faddy et al., 1992; Kim et al., 2000; La Vecchia et al., 2011; te Velde et al., 1998a; Wellons et al., 2012). Therefore, increased age at menopause is linked to reduced mortality and increased lifespan (Ossewaarde et al., 2005). Preclinical studies have also demonstrated that old female mice receiving ovaries from young donors have increased lifespan (Mason et al., 2009), which further supports the relationship between ovarian function and systemic aging parameters. Collectively, these studies strongly suggest that the ovary is a pivotal player in somatic aging throughout the body, highlighting the importance of understanding the intrinsic mechanisms that promote ovarian aging.
Another important factor related to ovarian aging is the inability to achieve successful fertilization in women past 45 years of age. This is particularly important because the age at which the first child is born in the United States has risen substantially over the past few decades (Eickmeyer et al., 2017), which increases the risk of unsuccessful pregnancies (Chandra and Stephen, 2010). It is well-established that the decreasing number of follicles during aging is accompanied by decreased oocyte quality (Broekmans et al., 2009). Extensive damage occurring throughout oocyte meiosis can have serious consequences if an adequate cellular response is not activated, resulting in infertility or development of defective embryos that are unable to result in full-term pregnancies (Adriaens et al., 2009; Kirk and Lyon, 1982). Several factors can influence oocyte quality during aging. Oocytes enclosed in primordial follicles remain arrested in prophase I of meiosis for a long period (Chiang et al., 2012; Mehlmann, 2005), which increases the chances of oocytes accumulating DNA damage, as shown in aged female mice and humans (Titus et al., 2013). Therefore, better understanding of ovarian aging processes could lead to the development of therapies that curtail oocyte damage and age-related infertility. A recent study in primates demonstrated that the genes most regulated in aged oocytes and granulosa cells are related to antioxidant defenses (Wang et al., 2020), further highlighting the importance of cellular damage in infertility. Despite the encompassing systemic effects of the ovaries, the molecular mechanisms that underlie ovarian aging are largely unexplored. One of the molecular hallmarks of aging is cellular senescence, a tumor suppressor mechanism that results in an irreversible cell cycle arrest response to a variety of biochemical stressors (Hernandez-Segura et al., 2018; Sharpless and Sherr, 2015). We hypothesize that senescence could be occurring in the ovary and contribute to ovarian insufficiency.
Cells are continuously exposed to factors such as DNA damage, mitochondria dysfunction, and telomere attrition which provoke cells to enter a state of senescence characterized by expression of tumor suppressive genes (TP53, CDKN1a, and CDKN2a) (Wang et al., 2009; Wang et al., 2017). Although non-dividing, senescent cells remain metabolically active and exhibit a secretory characteristic termed the senescence-associated secretory phenotype (SASP) (Malaquin et al., 2016). The SASP consists of pro-inflammatory cytokines, chemokines, and matrix metallopeptidase proteins (Hernandez-Segura et al., 2018; Malaquin et al., 2016; Sharpless and Sherr, 2015). These secreted factors act in a paracrine manner to alter the local microenvironment which results in the emergence of additional senescent cells and recruitment of immune cells (Hernandez-Segura et al., 2018). While there are minimal studies to directly implicate cellular senescence in ovarian aging, DNA damage and telomere shortening have been suggested to promote age-associated decline in oocyte quality and follicle number (Wang et al., 2009). Moreover, some studies suggest that the aging ovary can also display several markers associated with senescent cell burden including increased markers of pro-inflammatory stress, DNA double-strand breaks, and lipofuscin aggresome accumulation (Briley et al., 2016; Saccon et al., 2020; Uri-Belapolsky et al., 2014; Urzua et al., 2018b).
Epigenetic processes have also been recently recognized as a potential mechanism by which cellular senescence emerges (Yang and Sen, 2018). Epigenetic mechanisms in the form of DNA methylation, histone, or telomere modifications control DNA accessibility for transcription (Booth and Brunet, 2016) and are considered central regulators of the aging process (López-Otín et al., 2013). DNA methylation involves the addition of a methyl (mC) or hydroxymethyl (hmC) group to the 5C-position of a cytosine ring in a CG or CH context (where H stands for A, T or C) (He and Ecker, 2015; Moore et al., 2013). DNA methyltransferases (DNMTs) maintain methylation patterns during cell division (DNMT1) and de novo methylation are added by DNMT3a and DNMT3b (Jones and Takai, 2001; Lyko, 2018). Tet methylcytosine dioxygenases oxidize mC into hmC and other rare forms of DNA modification (Rasmussen and Helin, 2016). DNA methylation has been reported to play a role in the regulation of oogenesis and oocyte maturation (Menezo et al., 2016). Altered DNA methylation have been proposed to be associated with several reproductive diseases and may underlie ovarian exhaustion that occurs with advancing age (Menezo et al., 2016; Qian et al., 2015). A loss in genomic DNA methylation with ovarian aging has been proposed (Uysal and Ozturk, 2020), but has not been examined with modern sequencing techniques that provide a more quantitative analysis. Interestingly, these modern methods often times produce results that are contrary to early theories suggesting that hypomethylation occurs with advancing age (Hadad et al., 2016; Unnikrishnan et al., 2018). Therefore, it remains unknown if the ovary displays age-related epigenetic and transcriptomic changes from the point of sexual maturation throughout the period of declining reproductive health and in the context of cellular senescence.
A multitude of studies have shown that senescent cells increase with age in adipose, liver, skin and lung tissues (Stout et al., 2014; Wang et al., 2009), although other tissues including heart, skeletal muscle and kidney do not display a similar pattern of accumulation (Wang et al., 2009). This suggests that the accumulation of senescent cells cannot be generalized to all organ systems, thus, it is imperative to explore the role of cellular senescence in ovarian aging (Hernandez-Segura et al., 2018). Despite the aforementioned contradictory reports, senescent cells and their SASP are increasingly being targeted as potential pharmacological approaches to improve lifespan and health by alleviating age-related pathologies in a variety of tissues (Baker et al., 2011; Yousefzadeh et al., 2018; Zhu et al., 2015). Therefore, the removal of senescent cells may be an important therapeutic approach to preserve ovarian function, if cellular senescence is indeed playing a role in ovarian aging and functional declines. In this study follicle numbers, markers of cellular senescence, DNA modification levels, and transcriptome profiles were assessed across mouse ages ranging from sexual maturity to declining fertility.
2. METHODS
2.1. Animals:
Female C57BL/6 mice were obtained from the NIA aging colony at 2 months of age, maintained under specific pathogen-free (SPF) conditions at the University of Oklahoma Health Sciences Center (OUHSC) barrier animal facility. Ovarian samples were collected at 3, 6, 9 and 12 months of age. All experimental procedures were performed according to protocols approved by the OUHSC Institutional Animal Care and Use Committee (IACUC). Mice were euthanized by decapitation and ovaries were excised and stored in 10% buffered formaldehyde for histological analyses or flash-frozen in liquid nitrogen for DNA/RNA analyses.
2.2. Histological analysis:
Ovaries were collected and fixed in 10% buffered formaldehyde, washed in an alcohol gradient, hydrated with water, diaphanized with xylol and embedded in paraplast. The ovaries were sectioned into a thickness of 5 μm with a semi-automatic microtome (Leica RM2245, Leica Biosystems Newcastle Ltd, Newcastle Upon Tyne, United Kingdom). Representative sections of the ovary were selected and placed on standard histological slides. The slides, after drying in the oven at 56°C for 24 hours, were stained with hematoxylin-eosin and mounted with coverslips and synthetic resin (Sigma, St. Louis, MO, USA). Five ovarian sections of each mouse (n=5 per age group) were used for calculation of follicular density. The areas of the sections were measured using a digital camera coupled to a microscope (Nikon Eclipse E200, Nikon Corporation, Japan) and the software TCCapture, the number of follicles were then counted. Measurement of the section was carried out using the 4X objectives and counting of the follicles was performed with 40X objectives. The quantified follicles were those that had a clearly visible oocyte nucleus. Follicles were classified as primordial when surrounded by a single layer of flattened granulosa cells, as primary when surrounded by a single layer of cuboid granulosa cells, as a transition follicle when surrounded by both flattened and cuboid cells, as secondary follicle when surrounded by more than one layer of granulosa cuboid cells, with no visible antrum and when the follicle had a clearly defined antral space and a layer of cumulus granulosa cells around the oocyte, it was classified as a tertiary follicle (Myers et al., 2004). The number of follicles for each of the five ovarian sections was then divided by the area of the section, providing the density of follicles (number of follicles per mm2). A mean of the five sections densities was used as a representative of the follicular density for an individual mouse.
2.3. Lipofuscin staining and analysis:
The lipofuscin staining test was performed with the Sudan black dye on a subset of the histological slides from the ovarian sections. Lipofuscin is a heterogeneous pigmented by-product due to failure of intracellular catabolism, conventionally found in the lysosomes or cytosol of post-mitotic cells in aging (Snyder and Crane, 2020). The protocol used was modified from Urzua et al. (2018a) and Evangelou and Gorgoulis (2017). Briefly, the histological slides were dewaxed with xylol, washed in an alcohol gradient until reaching 70% alcohol and rehydrated with water. After diluting the Sudan black in 70% alcohol, and avoiding its precipitation, a 10 mL syringe with a disc filter was used to drop Sudan black on a clean slide, after which a slide containing the sections was inverted over the Sudan black solution for approximately 2 minutes. After the procedure, the section slide was separated using tweezers and washed with 50% alcohol and distilled water. The slides were then assembled with glycerol and observed under the light microscope in the 4, 10 and 40X objectives. Images obtained at 10X objectives (3 per mice) were quantified for lipofuscin positive area. The number of pixels in the images was calculated using the software Image J and presented relative to the total area of the section evaluated.
2.4. Genomic DNA and RNA extraction:
Genomic DNA and RNA were isolated from ovarian tissues (n=10/age group) using the AllPrep DNA/RNA Mini Kit (Qiagen, Germantown, MD) as previously described(Hadad et al., 2016). Isolated DNA and RNA were quantified by Nanodrop (Thermofisher Scientific, Madison, USA). Quality was assessed by genomic DNA and RNA ScreenTape analyses (Agilent Technologies, Frankfurt, Germany). Only samples with RNA and DNA integrity numbers > 7 were used for subsequent experiments.
2.5. Quantitative PCR (qPCR):
Quantitation of gene expression levels was performed with qPCR as previously described (Chucair-Elliott et al., 2019; Masser et al., 2014; Simpson et al., 2017). cDNA was synthesized using the ABI High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems Inc., Foster City, CA) from 25ng of purified RNA (n=10/age group). qPCR was performed with gene-specific primer probe fluorogenic exonuclease assays (TaqMan, Life Technologies, Waltham, MA) and the QuantStudio 12K Flex Real-Time PCR System (Applied Biosystems). Relative gene expression (RQ) was calculated with Expression Suite v 1.0.3 software using the 2−ΔΔCt analysis method (Livak and Schmittgen, 2001) with GAPDH as an endogenous control. Statistical analysis of the qPCR data was performed using GraphPad Prism 8 (San Diego, CA). One-way ANOVA analyses, with time as the factor, were performed followed by the Student-Newman-Keuls’ (SNK) multiple comparison test (p<0.05). The expression assays used for the analyses of the senescent-related markers Cyclin dependent kinase inhibitor 1a (Cdkn1a) and 2a (Cdkn2a), Plasminogen activator inhibitor-1 (PAI-1) and High mobility group box 1 (HMGB1) were Mm00432448_m1, Mm00494449_m1, Mm00435858_m1 and Mm00849805_gH respectively. Similarly, confirmation of the gene expression levels of C-C Motif Chemokine Ligand 8 (CCL8), Oocyte-specific homeobox 5 (OBOX5), lipocalin-2 (LCN2), newborn ovary homeobox (NOBOX), Cytokine receptor-like factor 1 (CRLF1) and Scavenger Receptor Family Member Expressed on T cells 1 (SCART1) was done using the expression assays Mm01297183_m1, Mm07300216_m1, Mm01324470_m1, Mm00453743_m1, Mm00517026_m1 and Mm00556657_m1 respectively.
2.6. Library preparation and RNA sequencing (RNA-seq):
Strand-specific libraries for RNA sequencing were prepared using the NEBNext Ultra II Directional Library Prep Kit for Illumina (New England Biolabs Inc., Ipswich, MA) according to previously described methods (Chucair-Elliott et al., 2019) with n=5/group samples randomly selected from the whole cohort of mice. Using 25ng of isolated total RNA from each sample, mRNA was purified using Oligo dT-attached magnetic beads. Isolated mRNA was fragmented and primed for first and second strand cDNA synthesis successively. For strand specificity, the incorporation of dUTP instead of dTTP in the second strand cDNA synthesis does not allow amplification past this dUTP with the polymerase. Using Agencourt Ampure XP beads (Beckman Coulter Life Sciences, Indianapolis, IN), the prepared double-stranded cDNA was purified for end repair, the addition of a single ‘A’ base and adapter ligation. The adapter ligated cDNA products were further purified and enriched using PCR to make the final library for subsequent sequencing. The libraries were sized using HS DNA ScreenTape (Agilent Technologies) and quantified using Qubit dsDNA HS assay kit (Thermofisher Scientific). Libraries for each sample were normalized and pooled at 4nM and sequenced using an Illumina NovaSeq 6000 system (SP, PE50bp).
2.7. RNA-seq data analysis:
Following sequencing, reads were trimmed, aligned, differential expression statistics and correlation analyses were performed in Strand NGS software package (Version 3.4, Avadis, Bangalore, India), as previously described (Chucair-Elliott et al., 2019). Reads were aligned against the Mm10 build of the mouse genome (2014.11.26). Alignment and filtering criteria included: adapter trimming, fixed 5bp trim from 5’ and 2bp from 3’ ends, a maximum number of one novel splice allowed per read, a minimum of 90% identity with the reference sequence, a maximum of 5% gap, trimming of 3’ end with Q<30. Alignment was performed directionally with Read 1 aligned in reverse and Read 2 in forward orientation. Normalization was performed with the DESeq algorithm (Anders and Huber, 2010). Transcripts with an average read count value >20 in at least 100% of the samples in at least one time-point were considered expressed at a level sufficient for quantitation and those transcripts below this level were considered not detected/not expressed and excluded, as these low levels of reads are close to background and are highly variable. For statistical analysis of differential expression, a one-way ANOVA across time points with a Benjamini-Hochberg Multiple Testing Correction (FDR<0.1) was used. For those transcripts meeting this statistical criterion, a fold change >|1.25| pairwise cutoff 6M, 9M, or 12M vs 3M) was used to eliminate those genes which were statistically significant but unlikely to be biologically significant and orthogonally confirmable due to their very small magnitude of change (GEO repository with accession code GSE 154890). Visualizations of hierarchical clustering and principal components analysis (PCA) were performed in Strand (NGS). Gene lists were imported into the IPA software Ingenuity Pathway Analysis (IPA) 01.12 (Qiagen Bioinformatics) to assess pathway/biological function enrichment analysis. Gene list comparisons for overlap were performed by a hypergeometric test.
2.8. Library construction and oxidative bisulfite sequencing (OxBS-seq):
Oxidative bisulfite sequencing protocols were carried out as previously described (Chucair-Elliott et al., 2019). For each sample (n=3/age group), 1000ng of isolated gDNA was brought to a volume of 50ul with 1X low-EDTA TE buffer. Each sample was sheared with the Covaris E220 sonicator (Covaris, Inc., Woburn, MA) to an average 200 base pair size using the following settings: intensity of 5, duty cycle of 10%, 200 cycles per burst, 2 cycles of 60 seconds, at 7 °C. The sized products were confirmed by capillary electrophoresis (DNA HS D1000 Agilent). Fragments were then purified using Ampure XP beads (Beckman Coulter Life Sciences, Indianapolis, IN) and quantified using Qubit dsDNA HS assay kit (Thermofisher Scientific). For oxidative bisulfite (oxBS) and bisulfite (BS) workflows, two aliquots of the 200ng DNA in 12ul volumes were prepared with a 1ul spike-in control DNA (0.8ng/ul) of known levels of mC, hmC and fC at individual sites. End repair, adapter ligation (Tecan Genomics, Inc., Redwood City, CA) and final repair were performed according to the manufacturer’s protocol (Ovation Ultralow Methyl-Seq Library System – NuGEN). Products of the oxBS workflow were oxidized and bisulfite converted and those for BS workflow were only bisulfite converted using the True Methyl oxBS module (NuGEN) with desulfonation and purification. Following purification, 22ul of the libraries were eluted from the magnetic beads and qPCR was used to determine the number of cycles (N) required for library amplification. Bisulfite-converted samples were amplified for 9 cycles while oxBS samples were amplified for 15 cycles [95° C- 2 min, N (95°C-15 s, 60°C-1 min, 72° C-30s)]. Amplified libraries were purified with Agencourt beads and eluted in 1X low-EDTA TE buffer, validated and quantified using HS DNA ScreenTape Agilent Technologies. Libraries were normalized to a concentration of 4nM, pooled, denatured and diluted to 12pM for sequencing on MiSeq (Illumina) according to manufacturer’s guidelines with the exception of a custom sequencing primer (MetSeq Primer) that was spiked in with the Illumina Read 1 primer to a final concentration of 0.5 μM.
2.9. OxBS-seq data analysis:
Methylation (mCG and mCH) and hydroxymethylation (hmCG) levels were determined as previously described (Chucair-Elliott et al., 2019). Reads were adaptor-trimmed and filtered using Trimmomatic/0.35 prior to alignment. End-trimming removed leading and trailing bases with Q-score<25, cropped 5 bases off the 5’ end and 3 bases off the 3’ end of reads, dropped reads less than 25 bases long, and dropped reads with average Q-score<25. Trimmed reads were aligned to the mouse reference genome (GRCm38/mm10) using Bismark with Bowtie. BAMs were then de-duplicated using Bismark. Methylation call percentages for each CpG and non-CpG (CH) site within the genome were calculated by dividing the methylated counts over the total counts for that site in the oxidative bisulfite - converted libraries (OXBS). Genome-wide CpG and CH methylation levels were calculated separately. Hydroxymethylation levels in CpG (hmCG) contexts were calculated by subtracting call levels from the oxidative bisulfite-converted (OXBS) libraries from the bisulfite-converted (BS) libraries. BAM files generated by MethylSeq (BaseSpace, Illumina) analysed with MethylKit to generate context-specific (CpG/CH) coverage text files. Bisulfite conversion efficiency for C, mC, and hmC was estimated using CEGX spike-in control sequences (Supplementary figure). Untrimmed fastq files were run through CEGX QC v0.2, which output a fastqc_data.txt file containing the conversion mean for C, mC, and hmC. CG islands and gene body text files were extracted from the UCSC genome Table browser. The context specific CpG/CH MethylKit files were intersected with the CGI and gene body bed files using bedtools and percent methylation was calculated by dividing the average percentage methylation at all common sites by the total number of sites.
3. RESULTS
3.1. Follicle Quantitation:
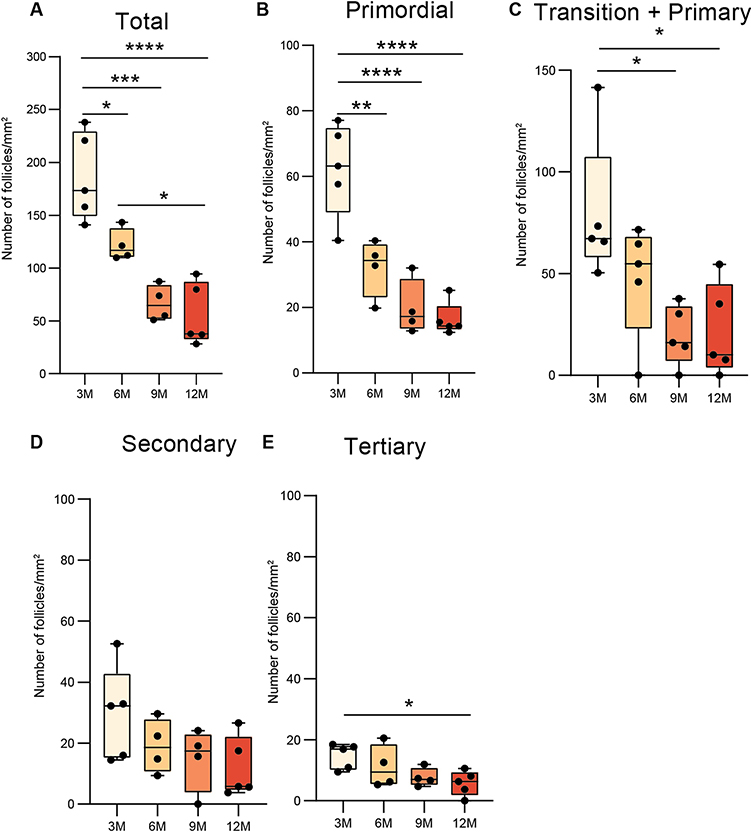
We first determined the number of ovarian follicles in 3M, 6M, 9M and 12M C57BL/6 mice. The age groups were selected to capture important time points ranging from sexual maturation through the period of significant declines in reproductive health (Manzano Nieves et al., 2019; Selesniemi et al., 2008). Oocytes with clearly visible nuclei were counted in five different ovarian sections from each animal (n=5/group). The total number of follicles decreased significantly with advancing age (Figure 1A). A similar decrease was observed in the number of primordial, primary, and tertiary follicles with increasing age, with the greatest reductions occurring at ages 9M and 12M (Figure 1B, C, E). 3.2 Lipofuscin staining: To test the hypothesis that the ovary accumulates senescent cells with age, histological assessment of lipofuscin aggresomes was performed. There was a significant increase in lipofuscin aggresomes at 9M and 12M of age suggesting an increase in senescent cell burden (Figure 2A&B). There was also a strong negative correlation between number of primordial follicles and lipofuscin staining (r=−0.74 and p=0.005) (Figure 2C).
Figure 1: Follicular density decreases with age.
Box plots demonstrates age-related decreases in follicular density represented as the mean of 5 ovarian sections per mouse (n = 5 mice/ group; *p<0.05, **p<0.01, ***p<0.005 and ****p<0.001; one-way ANOVA with Tukey post hoc test). For each section the follicular density was calculated as the number of follicles in the section divided by the area of the section. (A) Total, all follicles with clearly visible oocyte nucleus (B) Primordial follicles representing follicles surrounded by a single layer of flattened granulosa cells (C) Transition + Primary representing follicles surrounded by single layer of cuboidal cells or both flattened and cuboidal cells (D) Secondary follicles are surrounded by more than one layer of granulosa cuboidal cells with no visible antrum (E) Tertiary represents follicles with well-defined antral space and a layer of cumulus granulosa cells around the oocyte.
Figure 2: Lipofuscin aggresomes accumulate with age.
(A) Sudan black staining of ovarian sections for lipofuscin aggresomes with age. Images acquired at 10X magnification with dark blue areas representing lipofuscin (B) Quantification of lipofuscin positive area as a percentage of the total area of section evaluated expressed as mean ± SEM (n=3 mice/group; *p<0.05, **p<0.01; one-way ANOVA with Tukey post hoc test). (C) Correlation of lipofuscin staining to number of primordial follicles.
3.3. Senescence markers:
We also evaluated select transcriptional markers of cellular senescence by qPCR. We found that CDKN1a (p21) and CDKN2a (p16), plasminogen activator inhibitor-1 (PAI-1), and high mobility group 1 (HMG1) were all increased by 9M and/or 12M of age as compared to 3M of age (Figure 3). For some genes a significant increase was also evident between 6M and 9M or 12M mice.
Figure 3 -. Aging causes senescence-related markers in the mouse ovary to increase.
qPCR analysis of ovarian Cdkn1a (p21), Cdkn2a (p16), PAI-1 and HMGB1 reveal age-related increases in all markers by 9 and/or 12 months of age as compared to younger animals (3 and/or 6 months of age) (n=10/group, one-way ANOVA, *p<0.05, **p<0.01, ***p<0.001 SNK post hoc).
3.4. Transcriptomic analysis:
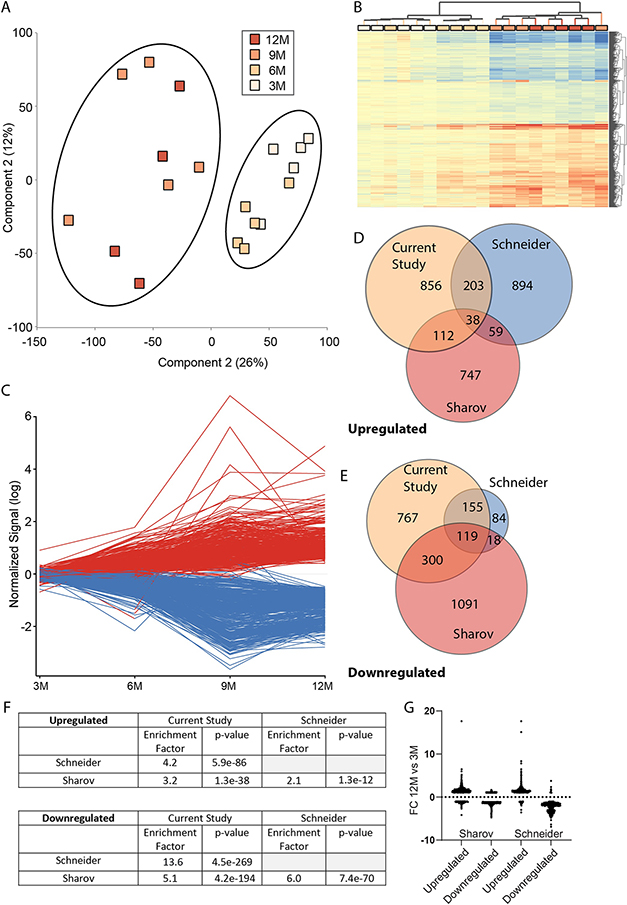
To determine the ovarian transcriptomic profile across the age groups, RNA sequencing was performed (n=5/group). An average of 15.5 million ± 1.1million reads passed filtering and alignment per sample. Using a read count cut-off of >20 reads per gene for all samples in at least one group, a total of 13,869 genes were detected. Principle component analysis of samples using all expressed genes demonstrated a separation of 3 and 6M from 9 and 12M samples (Figure 4A), in agreement with the histological follicle counting and lipofuscin staining. Differentially expressed transcripts between the age groups were identified through a one-way ANOVA with a Benjamini-Hochberg multiple comparison test (FDR<0.1) and SNK post-hoc verses 3M (p<0.05). Transcripts were further filtered to only those with a fold change >|1.25| in at least one pair-wise significant comparison to eliminate very small magnitude changes that are more likely to be by chance and of limited biological importance. A total of 2,550 genes were identified as differentially expressed by age (Supplemental Table 1). Hierarchical clustering of differentially expressed genes showed a separation of 3 and 6M samples from 9 and 12M samples indicating a shift in transcriptomic patterns in older animals with both increases and decreases in expression observed (Figure 4B). These expression differences occurred steadily, growing with age, and replotted as a line graph demonstrated fairly linear increases or decreases with age across the timepoints (Figure 4C).
Figure 4 – Age-related ovarian transcriptomic patterns.
A) Using the full complement of genes detected as expressed in the study a principle component analysis was performed and a segregation of 3 and 6 month from 9 and 12 month was evident. B) 2,550 genes (Up/down) were differentially expressed with age and hierarchical clustering separated 3- and 6 -month from 9- and 12 -month groups. C) When examined for their trajectory over ages differentially expressed genes generally demonstrated a linear change (increase/decrease) across the time points. D&E) Comparison of statistically significant changes to other studies. F) Table of hypergeometric test results revealing an enrichment of commonly differentially expressed gene across studies. G) Differentially expressed genes from other studies were plotted for fold change gene expression between 12 and 3M in this study regardless of statistical significance demonstrate consistent pattern of gene expression change with aging.
To increase rigor, genes differentially expressed with age were compared across this study and two prior studies examining ovarian gene expression with aging. Sharov et al.,(Sharov et al., 2008) studied the transcriptomic profile with age in the ovaries at four timepoints (1M, 6M, 16M, 24M) using a microarray assay while Schneider et al., (Schneider et al., 2017) examined the transcriptomic profile between 5–6M and 21–22M old mice using RNA sequencing. Despite the differences in assay and time points with the current study a number of commonalities in age-related increases and decreases were observed (Figure 4D&E). These commonalities were more than expected by random chance especially between the Schneider study and this study (Figure 4F). Across all three studies 157 common age-regulated genes (38 upregulated and 119 downregulated genes) were observed. Furthermore, when all differentially expressed genes which could be mapped to this dataset were examined for gene expression changes with age (12M vs 3M) regardless of statistical significance demonstrates that most genes demonstrate a positive fold change for upregulated genes and negative fold change for downregulated genes indicating a common pattern of expression change (Figure 4G).
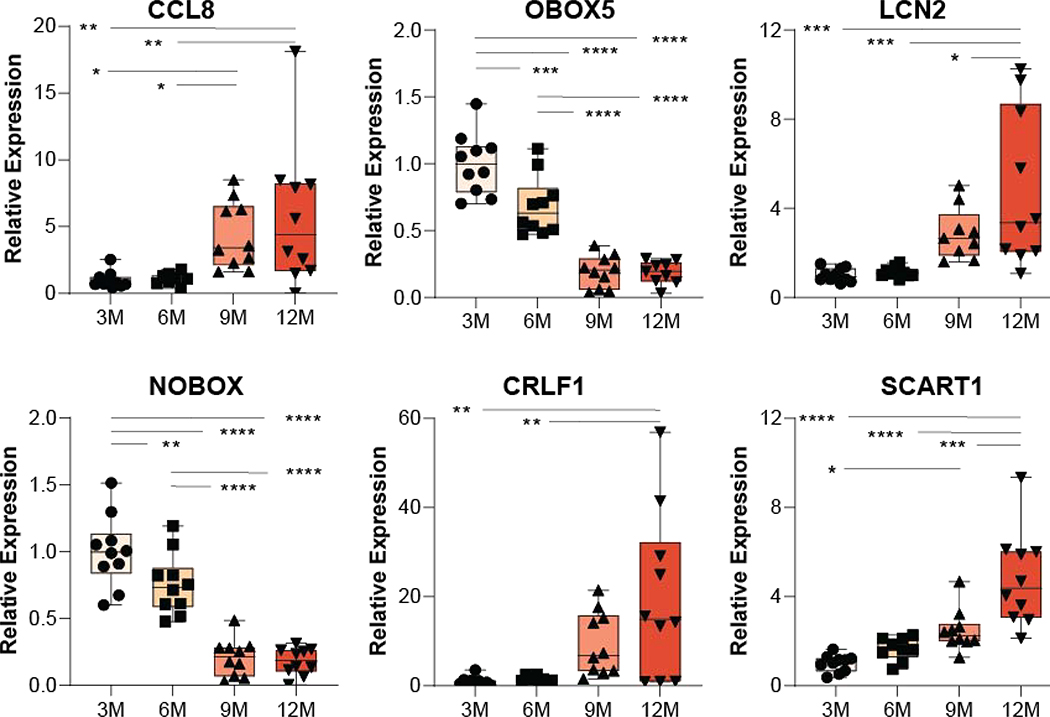
Building on these results six target genes from the RNA-Seq analysis were chosen for RT-qPCR confirmation in the full sample set. All six genes confirmed the RNA-Seq findings with Ccl8, Lcn2, Crlf1, and Scart1 while expression of Obox5 and Nobox decreased with age (Figure 5). This demonstrate the technical reproducibility of the RNA-Seq findings.
Figure 5 – Confirmation of differential expression of select RNA-Seq target genes.
qPCR analysis reveals age-related increases in the expression of the inflammatory-related genes, CCL8, LCN2, CRLF1 and SCART1 by 9 and/or 12 months of age while the expression of the oocyte-specific gene OBOX5 and the folliculogenesis-related gene NOBOX, decreases with age in agreement with the RNA sequencing experiment (n=10/group, one-way ANOVA, *p<0.05, **p<0.01, ***p<0.001 SNK post hoc).
To further contextualize this set of genes, pathway analysis was performed to identify canonical pathways, disease and functions, and upstream regulators affected with aging. Pathways and processes linked to increased inflammation were broadly evident. Multiple pathway and processes related to cell cycle inhibition – either as suppressed cell cycle or as induced checkpoint inhibition (Figure 6A&B). These findings concur with genes regulated by CDKN1a, CDNK2A, and retinoblastoma protein 1, and TP53 (p53) demonstrating patterns of induction, indicative of cell cycle inhibition (Figure 6C).
Figure 6 – Pathway, process and regulator analysis of transcriptomic changes with aging.
Significant differences in gene expression were analyzed by Ingenuity Pathway Analysis. Z-scores, indicating activation or inhibition of pathways (A), processes (B), or regulators (C) are presented.
3.5. DNA Modifications:
Alterations to DNA modification patterns with aging are an area of intense interest but remain largely unexplored in the context of ovarian aging. Levels of methylation in CG and CH dinucleotide contexts (mCG and mCH, respectively) as well as hydroxymethylation in CG and CH contexts (hmCG and hmCH), were determined by oxidative bisulfite sequencing across the four age groups (n=3/group). Spike-in controls confirmed equal conversion efficiencies across samples (Supplemental Figure 1). Low coverage sequencing analysed ~ 350,000 CG and CH sites. Genomic mCG levels (~68%) were consistent across all age groups (Figure 7A). Substantial genomic levels of hmCG (~6%) were also evident and did not change with aging (Figure 6A). Non-CpG (CH) methylation levels have previously received less attention but have been suggested to be abundant and relevant for oocyte maturation (Yu et al., 2017). There was no observed difference in total genomic CH methylation with aging, with an average mCH level < 1% across all age groups (Figure 7A). Consistent with our data from other tissues, non-CG hydroxymethylation (hmCH) was not detected in any samples (Hadad et al., 2016; Lister and Mukamel, 2015).
Figure 7 – Genomic DNA Methylation and hydroxymethylation levels do not change with ovarian aging.
No age-related differences were observed in total genomic (A) or segregated by autosomes (B) and X chromosomes (C) mCG, hmCG, or mCH modification levels. Reproducible levels of hmCG were observed and low levels of mCH were evident.
With the role of epigenetic modifications in X chromosome inactivation and their possible role in “escape” of some genes from X inactivation with age (Berletch et al., 2011; Prothero et al., 2009), we segregated X chromosome and autosomal methylation. There were no age differences in total levels of mCG, mCH and hmCG levels across the age groups when separated into autosomes (Figure 7B) and X chromosome (Figure 7C). Lower X chromosome hmCG levels (~3%) were evident when compared to autosomal hmCG levels (~6%) (p < 0.001 by 2-way ANOVA followed by the Tukey’s multiple comparison test). On the other hand, mCG levels were relatively higher in X chromosomes than in autosomes which may be consistent with genomic imprinting in the X chromosomes (p< 0.001 by 2-way ANOVA followed by the Tukey’s multiple comparison test).
To study the methylation patterns in specific genomic contexts, analysis across gene bodies (−4kb upstream the TSS, within gene body and +4kb downstream the TES) demonstrated no differences in mCG and hmCG levels (Supplemental Figure 2A). CGI units refer to not only the CpG islands, where CG dinucleotides are enriched, but also to the shores (±2kb from CpG islands) as well as shelves (±2kb from shores) of CpG islands (CGIs). Consistent with previous data from other tissues, CpG islands were mainly unmethylated with higher methylation levels in the shores and shelves. There were no observed aging differences in mCG and hmCG levels in all CGI units with age (Supplemental Figure 2B).
Roughly half of mammalian genomes is made up of several million copies of transposable and other repetitive elements including long interspersed nuclear elements (LINEs), short interspersed nuclear elements (SINEs), long terminal repeats (LTRs) and simple repeat elements (Calos and Miller, 1980; de Koning et al., 2011; Smit, 1996). Although the repeat elements make the bulk of the human genome, they are often not examined for DNA modification levels. Thus, we examined the methylation and hydroxymethylation levels in the various repeat elements and found no significant changes across the various age groups (Figure 8A–C). In total, there were no gains or losses in genome-wide methylation or hydroxymethylation levels with aging regardless of the subdivision.
Figure 8 – Methylation and hydroxymethylation of genomic repeat elements is not altered with aging.
No age-related differences were observed in LINEs, SINEs, LTRs, or Simple Repeats by methylation in the CG context (A), hydroxymethylation in the CG context (B), or methylation in the CH context (C). While mCG levels were consistent across repeat types hmCG levels varied by type of repeat. CH methylation was consistent with the exceptions of LTRs which demonstrated a lower level.
4. DISCUSSION
It is well known that the number of primordial follicles gradually decreases as female mammals age. In the current study, ovarian primordial follicle density reduced 74% from 3 to 12 months of age, similar to the reduction observed in the number of total follicles in the ovary. Our observed rate of primordial follicle depletion from 3 to 12 months is similar to the observed previously (Pelosi et al., 2013). Although some have showed a less steep decline in C57BL/6 mice, with the primordial reserve reducing by half by 10 months of age (Kevenaar et al., 2006). A clear menopausal transition is not observed in mice, but there is a severe reduction in the ovarian reserve similar to what is observed in humans (Broekmans et al., 2009).
The occurrence of cellular senescence is well-defined in several tissues outside of the ovary (Muñoz-Espín and Serrano, 2014). Cellular senescence impacts tissue function and increases the secretion of pro-inflammatory cytokines from the SASP (Tchkonia et al., 2013). It has been reported that ovarian aging is followed by increased pro-inflammatory cytokines secretion (Schneider et al., 2017; Uri-Belapolsky et al., 2014), macrophage infiltration (Saccon et al., 2020), fibrosis (Briley et al., 2016), DNA damage (Saccon et al., 2020; Titus et al., 2013; Zhang et al., 2015) and telomere shortening (Krishnamurthy et al., 2004), which are all indicative of increased cellular senescence. Lipofuscin is considered a marker of cellular senescence and consists of an aggregate of oxidized proteins that accumulates with advancing age (Georgakopoulou et al., 2013; Jung et al., 2007). Studies have reported lipofuscin staining as having equal, if not better, sensitivity than β-galactosidase staining, a classical measure of cellular senescence (Georgakopoulou et al., 2013; Kurz et al., 2000). Our current study shows a clear temporal association between decreased ovarian follicular population with aging and accumulation of ovarian lipofuscin. This is mirrored by increases in transcriptional markers of senescence (CDKN1A, CDKN2A, PAI-1, and HMGB1) with advancing age. Senescent cells are typically characterized by a high expression of the CDK inhibitors, CDKN1A and CDKN2A (Hernandez-Segura et al., 2017), known to inhibit the phosphorylation of retinoblastoma protein (RB) resulting in permanent cell cycle arrest (Rodier and Campisi, 2011). Our results demonstrate a consistent increase in CDKN1A and CDKN2A with age during the reproductive lifespan. The senescent secretome includes serine protease inhibitor PAI-1, an important downstream target of the antiproliferative protein p53 and HMGB1, whose secretion have been found to be p53-dependent (Kortlever and Bernards, 2006; Kortlever et al., 2006) was also increased at the mRNA level.
Extending these molecular analyses, transcriptomic data demonstrate linear changes across the ages examined. These data serve as a resource for the field and further highlight areas for further investigation. We observed gene expression profiles demonstrating a repression of the transcription factors NOBOX and FIGLA with age, which are crucial for the maintenance of primordial follicles quiescence. NOBOX and FIGLA null ovaries have rapid loss of the primordial reserve soon after birth (Lim and Choi, 2012). We also observe similar transcriptomic profile demonstrating a decreased expression of amphiregulin (AREG) with aging. AREG is a member of the epidermal growth factor (EGF) family and play a key role in oocyte maturation and ovulation (Huang et al., 2015). In women undergoing IVF, AREG expression in granulosa cell correlates with oocyte retrieval and embryo number (Huang et al., 2015). Advanced age lead to up-regulation of pathways for inflammasome and leukocyte extravasation, indicating effects of SASP and migration of immune cells for clearance of senescent cells. Pathway analysis further confirms the presence of senescent cells as indicated by a gene expression profile indicative of an upregulation of genes encoding CDKN2a, RB1 and p53 and SASP related genes such as IFNG and TNF. These findings also demonstrate a molecular shift between 6M and 9M of age that could be a prime point for investigation of regulatory switches to senescence and inflammation.
There is little available data on the role epigenetic mechanisms play in ovarian aging. Most epigenomic studies have examined the establishment of epigenetic marks necessary for oocyte maturation and development (Gahurova et al., 2017; Sendzikaite and Kelsey, 2019; Smallwood et al., 2011). In this study, we sought to characterize the genome-wide and context-specific DNA methylation changes that occur in the ovaries with age. We found that genome-wide methylation did not change with age. In contrast, a study by Yue et al. examining global DNA methylation levels in oocytes from young (6–8 weeks) and old (35–40 weeks) mice using fluorescence staining reported decreased DNA methylation levels in oocytes with maternal age (Yue et al., 2012). Similar findings, also with antibody based approaches have been recently reported (Uysal and Ozturk, 2020). The likely source of the differences between those findings and the highly consistent genome-wide mCG (as well as hmCG, and mCH levels) observed here are in the methods used. As we have described in detail elsewhere (Unnikrishnan et al., 2019), staining approaches have significant quantitative limitations for measurement of DNA modifications. These differences could also arise from the Yue et al. study examining oocytes while we evaluated whole ovaries. Despite the long-standing hypothesis that genomic hypomethylation occurs with aging in a number of tissues, this has not been confirmed by modern sequencing data across a number of studies (Chen et al., 2012; Chouliaras et al., 2012; Unnikrishnan et al., 2019).
Transposable elements (TEs) and simple repeat elements comprise over half of the genome (Calos and Miller, 1980; de Koning et al., 2011; Smit, 1996). TEs are mobile and capable of insertion into new genomic locations affecting genome structure and integrity, with a reported increased TE mobility with aging (Ryu et al., 2011). As part of the multiple pathways to silence TEs and regulate genomic instability are DNA modifications (Jones and Takai, 2001) thus, we sought to understand its regulation in ovarian aging. However, similar to global methylation, we found no changes in DNA methylation or hydroxymethylation in the ovary with age regards various TEs, LINEs, SINEs and LTRs. Taken together, the epigenomic and transcriptomic results suggest that while genome-wide de-methylation or altered hydroxymethylation levels do not occur with aging. However, it remains to be explored with higher depth sequencing if specific sites/genes are differentially modified with aging and if these relate to altered gene expression. As has proven to be the case with other organ systems where changes in DNA modifications are location specific and not genome-wide.
A related finding from this study is that hydroxymethylation is relatively abundant in the ovary. The discovery that TET enzymes convert methylated cytosines (mC) to hydroxymethylated cytosines (hmC) (Kriaucionis and Heintz, 2009; Tahiliani et al., 2009), has led to the emerging attention on the role hmC plays in genome regulation. Traditional sodium bisulfite sequencing approaches are incapable of differentiating between mC and hmC requiring the need for novel approaches such as oxidative bisulfite sequencing (oxBS) (Booth et al., 2012; Wu and Zhang, 2011). The findings demonstrate the need to differentiate between mCG and hmCG in studies of the ovary, as well as the need to include non-CG methylation (mCH) in data analyses.
The main finding of this study is that declines in ovarian reserve with age occur in combination with increases in markers of cellular senescence. A critical question that remains to be examined is if the time course of senescence in specific ovarian cell populations and if cellular senescence is a driver or symptom of declining ovarian reserve. Future studies should also examine a broad range of senescence markers. Cell-type specific transcriptomic and epigenomic analyses are needed to reveal the importance of senescence and epigenetic changes in oocytes and surrounding somatic cells. Single cell approaches may aid in this approach but are limited to smaller cell sizes and have reduced transcriptomic and epigenomic coverage. An alternative approach could be to use ribosomal profiling and nuclei labeling approaches such as NuTRAP (Chucair-Elliott et al., 2020) with cell-type specific cre lines for different ovarian cells types. Lastly, the effects of interventions, such as senolytics, on reproductive fitness need to examined.
Supplementary Material
Highlights.
Ovarian primordial follicle depletion with aging occurs concomitantly with increased markers of cellular senescence
Age-related ovarian transcriptomic changes demonstrate increased inflammation and suppressed cell cycling
Ovaries have significant levels of hydroxymethylation and non-CG methylation
Neither genomic hypomethylation nor hyperhydroxymethylation is evident with ovarian aging
ACKNOWLEDGMENTS
This work was supported by grants from the NIH [R01AG059430 and P30AG050911-01 (WMF); F31AG064861 (SRO); R00AG051661 and R01AG069742 (MBS)]; VA [I01BX003906 (WMF)]; and AFAR (VAA). Support was also obtained from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Fundação de Amparo à Pesquisa do Estado do Rio Grande do Su (FAPERGS) (AS). Funding agencies has no role in the design, execution, analysis or interpretation of these findings. The authors thank the Oklahoma Nathan Shock Center, Oklahoma Medical Research Foundation Clinical Genomics Center, and Jake Gittes for technical assistance.
Footnotes
DECLARATION OF INTEREST
The authors have no conflicts of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DATA AVAILABILITY
The RNA sequencing data from this study has been deposited in GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE154890) under the accession code GSE154890.
REFERENCES
- Adriaens I, Smitz J, Jacquet P, 2009. The current knowledge on radiosensitivity of ovarian follicle development stages. Human reproduction update 15, 359–377. [DOI] [PubMed] [Google Scholar]
- Anders S, Huber W, 2010. Differential expression analysis for sequence count data. Genome biology 11, R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DT, 1974. The endocrinology of ovarian steroid secretion. Eur J Obstet Gynecol Reprod Biol 4, 31–39. [DOI] [PubMed] [Google Scholar]
- Baker DJ, Wijshake T, Tchkonia T, LeBrasseur NK, Childs BG, Van De Sluis B, Kirkland JL, van Deursen JM, 2011. Clearance of p16 Ink4a-positive senescent cells delays ageing-associated disorders. Nature 479, 232–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TG, 1963. A Quantitative and Cytological Study of Germ Cells in Human Ovaries. Proc R Soc Lond B Biol Sci 158, 417–433. [DOI] [PubMed] [Google Scholar]
- Berletch JB, Yang F, Xu J, Carrel L, Disteche CM, 2011. Genes that escape from X inactivation. Human genetics 130, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth LN, Brunet A, 2016. The aging epigenome. Molecular cell 62, 728–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth MJ, Branco MR, Ficz G, Oxley D, Krueger F, Reik W, Balasubramanian S, 2012. Quantitative sequencing of 5-methylcytosine and 5-hydroxymethylcytosine at single-base resolution. Science 336, 934–937. [DOI] [PubMed] [Google Scholar]
- Briley SM, Jasti S, McCracken JM, Hornick JE, Fegley B, Pritchard MT, Duncan FE, 2016. Reproductive age-associated fibrosis in the stroma of the mammalian ovary. Reproduction (Cambridge, England) 152, 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekmans FJ, Soules MR, Fauser BC, 2009. Ovarian aging: mechanisms and clinical consequences. Endocrine reviews 30, 465–493. [DOI] [PubMed] [Google Scholar]
- Calos MP, Miller JH, 1980. Transposable elements. Cell 20, 579–595. [DOI] [PubMed] [Google Scholar]
- Chandra A, Stephen EH, 2010. Infertility service use among U.S. women: 1995 and 2002. Fertil Steril 93, 725–736. [DOI] [PubMed] [Google Scholar]
- Chen H, Dzitoyeva S, Manev H, 2012. Effect of aging on 5-hydroxymethylcytosine in the mouse hippocampus. Restorative neurology and neuroscience 30, 237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T, Schultz RM, Lampson MA, 2012. Meiotic origins of maternal age-related aneuploidy. Biol Reprod 86, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouliaras L, van den Hove DLA, Kenis G, Keitel S, Hof PR, van Os J, Steinbusch HWM, Schmitz C, Rutten BPF, 2012. Prevention of age-related changes in hippocampal levels of 5-methylcytidine by caloric restriction. Neurobiology of aging 33, 1672–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Ocañas SR, Stanford DR, Ansere VA, Buettner KB, Porter H, Eliason NL, Reid JJ, Sharpe AL, Stout MB, Beckstead MJ, Miller BF, Richardson A, Freeman WM, 2020. Inducible cell-specific mouse models for paired epigenetic and transcriptomic studies of microglia and astroglia. Communications Biology 3, 693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chucair-Elliott AJ, Ocanas SR, Stanford DR, Hadad N, Wronowski B, Otalora L, Stout MB, Freeman WM, 2019. Tamoxifen induction of Cre recombinase does not cause long-lasting or sexually divergent responses in the CNS epigenome or transcriptome: implications for the design of aging studies. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Koning APJ, Gu W, Castoe TA, Batzer MA, Pollock DD, 2011. Repetitive elements may comprise over two-thirds of the human genome. PLoS Genet 7, e1002384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson MA, Nagaraja AK, Matzuk MM, 2009. The mammalian ovary from genesis to revelation. Endocrine reviews 30, 624–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickmeyer KJ, Payne KK, Brown SL, Manning WD, 2017. Crossover in the median age at first marriage and first birth: Thirty-Five years of change. Family Profiles, FP-17–22. National Center for Family & Marriage Research. [Google Scholar]
- Evangelou K, Gorgoulis VG, 2017. Sudan Black B, The Specific Histochemical Stain for Lipofuscin: A Novel Method to Detect Senescent Cells. Methods Mol Biol 1534, 111–119. [DOI] [PubMed] [Google Scholar]
- Faddy MJ, Gosden RG, Gougeon A, Richardson SJ, Nelson JF, 1992. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human reproduction 7, 1342–1346. [DOI] [PubMed] [Google Scholar]
- Gahurova L, Tomizawa S. i., Smallwood SA, Stewart-Morgan KR, Saadeh H, Kim J, Andrews SR, Chen T, Kelsey G, 2017. Transcription and chromatin determinants of de novo DNA methylation timing in oocytes. Epigenetics & chromatin 10, 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgakopoulou EA, Tsimaratou K, Evangelou K, Fernandez M-PJ, Zoumpourlis V, Trougakos IP, Kletsas D, Bartek J, Serrano M, Gorgoulis VG, 2013. Specific lipofuscin staining as a novel biomarker to detect replicative and stress-induced senescence. A method applicable in cryo-preserved and archival tissues. Aging (Albany NY) 5, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadad N, Masser DR, Logan S, Wronowski B, Mangold CA, Clark N, Otalora L, Unnikrishnan A, Ford MM, Giles CB, 2016. Absence of genomic hypomethylation or regulation of cytosine-modifying enzymes with aging in male and female mice. Epigenetics & chromatin 9, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Ecker JR, 2015. Non-CG methylation in the human genome. Annual review of genomics and human genetics 16, 55–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M, 2017. Unmasking transcriptional heterogeneity in senescent cells. Current Biology 27, 2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M, 2018. Hallmarks of cellular senescence. Trends in cell biology 28, 436–453. [DOI] [PubMed] [Google Scholar]
- Huang Y, Zhao Y, Yu Y, Li R, Lin S, Zhang C, Liu P, Qiao J, 2015. Altered amphiregulin expression induced by diverse luteinizing hormone receptor reactivity in granulosa cells affects IVF outcomes. Reprod Biomed Online 30, 593–601. [DOI] [PubMed] [Google Scholar]
- Jones PA, Takai D, 2001. The role of DNA methylation in mammalian epigenetics. Science 293, 1068–1070. [DOI] [PubMed] [Google Scholar]
- Jung T, Bader N, Grune T, 2007. Lipofuscin: formation, distribution, and metabolic consequences. Annals of the New York Academy of Sciences 1119, 97–111. [DOI] [PubMed] [Google Scholar]
- Kevenaar ME, Meerasahib MF, Kramer P, van de Lang-Born BMN, de Jong FH, Groome NP, Themmen APN, Visser JA, 2006. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology 147, 3228–3234. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Kim TH, Ryu WS, Ryoo UH, 2000. Influence of menopause on high density lipoprotein-cholesterol and lipids. Journal of Korean medical science 15, 380–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk M, Lyon MF, 1982. Induction of congenital anomalies in offspring of female mice exposed to varying doses of X-rays. Mutation research 106, 73–83. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Bernards R, 2006. Senescence, wound healing, and cancer: the PAI-1 connection. Cell cycle 5, 2697–2703. [DOI] [PubMed] [Google Scholar]
- Kortlever RM, Higgins PJ, Bernards R, 2006. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nature cell biology 8, 877–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriaucionis S, Heintz N, 2009. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science 324, 929–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE, 2004. Ink4a/Arf expression is a biomarker of aging. The Journal of clinical investigation 114, 1299–1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurz DJ, Decary S, Hong Y, Erusalimsky JD, 2000. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. Journal of cell science 113, 3613–3622. [DOI] [PubMed] [Google Scholar]
- La Vecchia C, Giordano SH, Hortobagyi GN, Chabner B, 2011. Overweight, obesity, diabetes, and risk of breast cancer: interlocking pieces of the puzzle. Oncologist 16, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim EJ, Choi Y, 2012. Transcription factors in the maintenance and survival of primordial follicles. Clin Exp Reprod Med 39, 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lister R, Mukamel EA, 2015. Turning over DNA methylation in the mind. Frontiers in neuroscience 9, 252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD, 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyko F, 2018. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nature Reviews Genetics 19, 81. [DOI] [PubMed] [Google Scholar]
- Malaquin N, Martinez A, Rodier F, 2016. Keeping the senescence secretome under control: molecular reins on the senescence-associated secretory phenotype. Experimental gerontology 82, 39–49. [DOI] [PubMed] [Google Scholar]
- Manzano Nieves G, Schilit Nitenson A, Lee H-I, Gallo M, Aguilar Z, Johnsen A, Bravo M, Bath KG, 2019. Early life stress delays sexual maturation in female mice. Frontiers in molecular neuroscience 12, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason JB, Cargill SL, Anderson GB, Carey JR, 2009. Transplantation of young ovaries to old mice increased life span in transplant recipients. J Gerontol A Biol Sci Med Sci 64, 1207–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masser DR, Bixler GV, Brucklacher RM, Yan H, Giles CB, Wren JD, Sonntag WE, Freeman WM, 2014. Hippocampal subregions exhibit both distinct and shared transcriptomic responses to aging and nonneurodegenerative cognitive decline. J Gerontol A Biol Sci Med Sci 69, 1311–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, 2005. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction 130, 791–799. [DOI] [PubMed] [Google Scholar]
- Menezo YJR, Silvestris E, Dale B, Elder K, 2016. Oxidative stress and alterations in DNA methylation: two sides of the same coin in reproduction. Reproductive biomedicine online 33, 668–683. [DOI] [PubMed] [Google Scholar]
- Moore LD, Le T, Fan G, 2013. DNA methylation and its basic function. Neuropsychopharmacology 38, 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muñoz-Espín D, Serrano M, 2014. Cellular senescence: from physiology to pathology. Nature reviews Molecular cell biology 15, 482–496. [DOI] [PubMed] [Google Scholar]
- Myers M, Britt KL, Wreford NG, Ebling FJ, Kerr JB, 2004. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127, 569–580. [DOI] [PubMed] [Google Scholar]
- Ossewaarde ME, Bots ML, Verbeek AL, Peeters PH, van der Graaf Y, Grobbee DE, van der Schouw YT, 2005. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology 16, 556–562. [DOI] [PubMed] [Google Scholar]
- Pelosi E, Omari S, Michel M, Ding J, Amano T, Forabosco A, Schlessinger D, Ottolenghi C, 2013. Constitutively active Foxo3 in oocytes preserves ovarian reserve in mice. Nature communications 4, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prothero KE, Stahl JM, Carrel L, 2009. Dosage compensation and gene expression on the mammalian X chromosome: one plus one does not always equal two. Chromosome research 17, 637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian Y, Tu J, Tang NLS, Kong GWS, Chung JPW, Chan W-Y, Lee T-L, 2015. Dynamic changes of DNA epigenetic marks in mouse oocytes during natural and accelerated aging. The international journal of biochemistry & cell biology 67, 121–127. [DOI] [PubMed] [Google Scholar]
- Rasmussen KD, Helin K, 2016. Role of TET enzymes in DNA methylation, development, and cancer. Genes & development 30, 733–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodier F, Campisi J, 2011. Four faces of cellular senescenceFour faces of senescence. The Journal of cell biology 192, 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SH, Kang K, Yoo T, Joe CO, Chung JH, 2011. Transcriptional repression of repeat-derived transcripts correlates with histone hypoacetylation at repetitive DNA elements in aged mice brain. Experimental gerontology 46, 811–818. [DOI] [PubMed] [Google Scholar]
- Saccon TD, Rovani MT, Garcia DN, Mondadori RG, Cruz LAX, Barros CC, Bartke A, Masternak MM, Schneider A, 2020. Primordial follicle reserve, DNA damage and macrophage infiltration in the ovaries of the long-living Ames dwarf mice. Experimental Gerontology 132, 110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider A, Matkovich SJ, Saccon T, Victoria B, Spinel L, Lavasani M, Bartke A, Golusinski P, Masternak MM, 2017. Ovarian transcriptome associated with reproductive senescence in the long-living Ames dwarf mice. Mol Cell Endocrinol 439, 328–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selesniemi K, Lee HJ, Tilly JL, 2008. Moderate caloric restriction initiated in rodents during adulthood sustains function of the female reproductive axis into advanced chronological age. Aging cell 7, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sendzikaite G, Kelsey G, 2019. The role and mechanisms of DNA methylation in the oocyte. Essays Biochem 63, 691–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharov AA, Falco G, Piao Y, Poosala S, Becker KG, Zonderman AB, Longo DL, Schlessinger D, Ko MSH, 2008. Effects of aging and calorie restriction on the global gene expression profiles of mouse testis and ovary. BMC Biology 6, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ, 2015. Forging a signature of in vivo senescence. Nature Reviews Cancer 15, 397–408. [DOI] [PubMed] [Google Scholar]
- Simpson JT, Workman RE, Zuzarte PC, David M, Dursi LJ, Timp W, 2017. Detecting DNA cytosine methylation using nanopore sequencing. Nature Methods 14, 407. [DOI] [PubMed] [Google Scholar]
- Smallwood SA, Tomizawa S. i., Krueger F, Ruf N, Carli N, Segonds-Pichon A, Sato S, Hata K, Andrews SR, Kelsey G, 2011. Dynamic CpG island methylation landscape in oocytes and preimplantation embryos. Nature genetics 43, 811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smit AFA, 1996. The origin of interspersed repeats in the human genome. Current opinion in genetics & development 6, 743–748. [DOI] [PubMed] [Google Scholar]
- Snyder AN, Crane JS, 2020. Histology, Lipofuscin, StatPearls, Treasure Island (FL). [PubMed] [Google Scholar]
- Stout MB, Tchkonia T, Pirtskhalava T, Palmer AK, List EO, Berryman DE, Lubbers ER, Escande C, Spong A, Masternak MM, 2014. Growth hormone action predicts age-related white adipose tissue dysfunction and senescent cell burden in mice. Aging (Albany NY) 6, 575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tahiliani M, Koh KP, Shen Y, Pastor WA, Bandukwala H, Brudno Y, Agarwal S, Iyer LM, Liu DR, Aravind L, 2009. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 324, 930–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchkonia T, Zhu Y, Van Deursen J, Campisi J, Kirkland JL, 2013. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. The Journal of clinical investigation 123, 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BC, 1998a. Developmental and endocrine aspects of normal ovarian aging. Mol Cell Endocrinol 145, 67–73. [DOI] [PubMed] [Google Scholar]
- te Velde ER, Scheffer GJ, Dorland M, Broekmans FJ, Fauser BCJM, 1998b. Developmental and endocrine aspects of normal ovarian aging. Molecular and Cellular Endocrinology 145, 67–73. [DOI] [PubMed] [Google Scholar]
- Titus S, Li F, Stobezki R, Akula K, Unsal E, Jeong K, Dickler M, Robson M, Moy F, Goswami S, Oktay K, 2013. Impairment of BRCA1-related DNA double-strand break repair leads to ovarian aging in mice and humans. Science translational medicine 5, 172ra121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Freeman WM, Jackson J, Wren JD, Porter H, Richardson A, 2019. The role of DNA methylation in epigenetics of aging. Pharmacology & therapeutics 195, 172–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unnikrishnan A, Hadad N, Masser DR, Jackson J, Freeman WM, Richardson A, 2018. Revisiting the genomic hypomethylation hypothesis of aging. Annals of the New York Academy of Sciences 1418, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uri-Belapolsky S, Shaish A, Eliyahu E, Grossman H, Levi M, Chuderland D, Ninio-Many L, Hasky N, Shashar D, Almog T, 2014. Interleukin-1 deficiency prolongs ovarian lifespan in mice. Proceedings of the National Academy of Sciences 111, 12492–12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzua U, Chacon C, Espinoza R, Martinez S, Hernandez N, 2018a. Parity-Dependent Hemosiderin and Lipofuscin Accumulation in the Reproductively Aged Mouse Ovary. Anal Cell Pathol (Amst) 2018, 1289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urzua U, Chacon C, Espinoza R, Martínez S, Hernandez N, 2018b. Parity-Dependent Hemosiderin and Lipofuscin Accumulation in the Reproductively Aged Mouse Ovary. Analytical Cellular Pathology 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal F, Ozturk S, 2020. The loss of global DNA methylation due to decreased DNMT expression in the postnatal mouse ovaries may associate with infertility emerging during ovarian aging. Histochemistry and Cell Biology. [DOI] [PubMed] [Google Scholar]
- Wang C, Jurk D, Maddick M, Nelson G, Martin Ruiz C, Von Zglinicki T, 2009. DNA damage response and cellular senescence in tissues of aging mice. Aging cell 8, 311–323. [DOI] [PubMed] [Google Scholar]
- Wang S, Zheng Y, Li J, Yu Y, Zhang W, Song M, Liu Z, Min Z, Hu H, Jing Y, He X, Sun L, Ma L, Esteban CR, Chan P, Qiao J, Zhou Q, Izpisua Belmonte JC, Qu J, Tang F, Liu GH, 2020. Single-Cell Transcriptomic Atlas of Primate Ovarian Aging. Cell. [DOI] [PubMed] [Google Scholar]
- Wang T, Zhang M, Jiang Z, Seli E, 2017. Mitochondrial dysfunction and ovarian aging. American Journal of Reproductive Immunology 77, e12651. [DOI] [PubMed] [Google Scholar]
- Wellons M, Ouyang P, Schreiner PJ, Herrington DM, Vaidya D, 2012. Early menopause predicts future coronary heart disease and stroke: the Multi-Ethnic Study of Atherosclerosis. Menopause 19, 1081–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H, Zhang Y, 2011. Mechanisms and functions of Tet protein-mediated 5-methylcytosine oxidation. Genes & development 25, 2436–2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Sen P, 2018. The senescent cell epigenome. Aging (Albany NY) 10, 3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, 2018. Fisetin is a senotherapeutic that extends health and lifespan. EBioMedicine 36, 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Dong X, Gravina S, Kartal Ö, Schimmel T, Cohen J, Tortoriello D, Zody R, Hawkins RD, Vijg J, 2017. Genome-wide, single-cell DNA methylomics reveals increased non-CpG methylation during human oocyte maturation. Stem cell reports 9, 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue M. x., Fu X. w., Zhou G. b., Hou Y. p., Ming DU, Wang L, Zhu S. e., 2012. Abnormal DNA methylation in oocytes could be associated with a decrease in reproductive potential in old mice. Journal of assisted reproduction and genetics 29, 643–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhang X, Zeng M, Yuan J, Liu M, Yin Y, Wu X, Keefe DL, Liu L, 2015. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. Journal of assisted reproduction and genetics 32, 1069–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, 2015. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging cell 14, 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data from this study has been deposited in GEO repository (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE154890) under the accession code GSE154890.