Abstract
Abstract
Despite advancements in the areas of omics and chemoinformatics, potent novel biotherapeutic molecules with new modes of actions are needed for leishmaniasis. The socioeconomic burden of leishmaniasis remains alarming in endemic regions. Currently, reports from existing endemic areas such as Nepal, Iran, Brazil, India, Sudan and Afghanistan, as well as newly affected countries such as Peru, Bolivia and Somalia indicate concerns of chemoresistance to the classical antimonial treatment. As a result, effective antileishmanial agents which are safe and affordable are urgently needed. Natural products from both flora and fauna have contributed immensely to chemotherapeutics and serve as vital sources of new chemical agents. This review focuses on a systematic cross-sectional view of all characterized anti-leishmanial compounds from natural sources over the last decade. Furthermore, IC50/EC50, cytotoxicity and suggested mechanisms of action of some of these natural products are provided. The natural product classification includes alkaloids, terpenes, terpenoids, and phenolics. The plethora of reported mechanisms involve calcium channel inhibition, immunomodulation and apoptosis. Making available enriched data pertaining to bioactivity and mechanisms of natural products complement current efforts geared towards unraveling potent leishmanicides of therapeutic relevance.
Graphic Abstract

Keywords: Chemotherapeutics, Chemoinformatics, Natural products, Cytotoxicity, Leishmaniasis, Phenotypic screening
Introduction
The debilitating rate of parasitic infections in the tropical and subtropical regions of developing countries has become alarming [1]. Vector-borne neglected tropical diseases and related synergetic co-infections, particularly leishmaniasis are very challenging and sophisticated to treat [2]. This is partly due to the existence of diverse parasitic species with different bionomics and sophisticated overlap between virulent factors. Activated immune response during disease exacerbation coupled with emerging resistance by both parasites and vectors against various treatment regimens have also contributed to this challenge [2, 3].
Leishmania, the etiological agent of leishmaniasis, is transmitted globally by over 90 different female sand-fly species of the Phlebotomus family, spread across 98 countries and four continents, with annual estimates of 1 million new cases and 30,000 deaths as at 2017 [2, 4]. The exact disease burden is unknown, but statistics indicate that over 350 million people are at risk, signifying a prominent public health risk [2, 5, 6].
Leishmaniasis is curable if the disease is diagnosed early and the appropriate medication is administered. Typically, leishmaniasis is initially marked by dermotropic ulcers, which then progress into the visceral tissues, resulting in a late and more debilitating condition that can often lead to death if left untreated. In some cases, the destruction of the mucocutaneous membrane especially the nose, throat, and mouth have also been very common [2]. The degree of clinical outcome and its corresponding immunopathology depends primarily on the type of causative species, age of host, and the balance between the host immune response and how the parasites subvert these defense mechanisms. In cases where the victim’s immune system is strong, Leishmania pathogens behave as opportunists by remaining dormant until the host’s immunity is compromised. Additionally, when the host is immunosuppressed, relapses are usually prevalent resulting in treatment failures.
Some challenges associated with the management of leishmaniasis include systemic toxicity of administrated drugs, high cost of existing therapeutic options, lengthy treatment periods and drug resistance. Furthermore, confounding factors such as parasite diversity has hampered various intervention strategies and halted global efforts, necessitating an immediate search for new drug leads for development as the next generation of antileishmanial agents [7–9]. In lieu of this, the review seeks to bring to the fore the various classes of natural products recently discovered with antileishmanial potentials over the last decade. Even though, the review primarily reported compounds with potent bioactivity, few with low potency were reported since these could be optimized or their scaffolds may serve as skeletons for the development of future leishmanicides.
Trends in leishmanial chemotherapy and current panorama
Protection against leishmaniasis started with mimicking natural immunity through live inoculations [10] until modernized techniques including killed promastigotes and knocked out parasites came into play. Unfortunately, the presence of persistent lesions and the difficulty in estimating their efficacy rendered these approaches less effective [10, 11]. Efforts to alleviate leishmaniasis via chemotherapy include the use of pentavalent antimonial, which was essentially a small tartrate complex of antimony first reported in 1925 by Brahmachari [12, 13]. Although, antimoniate (SbV) is still active after reduction by arsenate reductase to SbIII, Leishmania parasites are also susceptible to SbV via oxidative stress.
Gene amplification studies involving the Adenosine Triphosphate (ATP) binding cassette transporters including the multi-resistance proteins that act as efflux pumps have been shown to contribute to antimony resistance in clinical isolates [14, 15]. Likewise, deletions of aquaporin membrane carrier genes and phenotypic changes of the parasite with subsequent induced effects on the microbicide activity and the efflux rate of antimony reaching the macrophages also contribute to the resistance [16].
In the mid-1960’s pentamidine became the second choice to antimony resistant strains [17]. However, its utility like the antimonial was hampered due to severe vasomotor side effects and complex interactions with the pancreas which leads to the destruction of β-cells causing diabetes mellitus [17].
In the quest to expedite the time it takes for drugs to reach the market, strategies such as deciphering the cellular similarities between disease causing pathogens from phenotypic screening were developed. In the early 1960s, the anti-fungal amphotericin B from Streptomyces nodosus was used for treating leishmaniasis [18, 19]. This choice was widely accepted in most endemic areas due to its efficacy but not so in other areas especially East Africa (L. donovani) and South America (L. infantum) [20].
The anticancer agent alkyl phosphocholine (miltefosine) was the first oral formulation with strong protection against visceral leishmaniasis. Miltefosine works by modulating an apoptosis process induced by mitochondria membrane depolarization and phospholipid biosynthesis inhibition [21]. The main drawback in administering miltefosine for leishmaniasis treatment includes longer elimination time, lengthy treatment course, and miscarriage in pregnant patients after use [22].
A new and simple formulation of an old antibiotic paromomycin which inhibited translation with different modes of application (enteral, parenteral and topical) was also repurposed for leishmaniasis in 1967 [23, 24]. Unlike the other treatment options, paromomycin’s toxic effects are very minimal, but its efficacy is quite poor. New optimum carriers targeting pathogen macrophage using albumin has recently been reported to increase efficiency [25].
Following the failure of miltefosine, a collaboration between the Walter Reed Army Institute of Research (WRAIR, USA) and GlaxoSmithKline (UK) identified sitamaquine as a promising alternative, but its apparent loss of efficacy in tegumentary leishmaniasis limited its use [26]. Subsequently, findings from amphotericin B use and its high curative rate in patients influenced another repurposing strategy using the oral anti-fungal azoles (fluconazole, itraconazole, and ketoconazole) as suitable control and cost-effective therapy [27, 28].
Due to the therapeutic challenges, new chemotypes with high potency in tandem with immunostimulatory activity targeting new proteins applicable to both visceral and cutaneous leishmaniasis cases are desperately needed.
Natural products as possible sources of new drugs against leishmaniasis
The lack of effective vaccines for control and concerted elimination campaign [2], and recent snail paced progress on leishmanial vaccine development does not guarantee any optimism. With the advancements in synthetic organic chemistry, combinatorial chemistry, and computational de novo drug discovery strategies, as well as high throughput screening techniques, only a few synthetically constructed drugs have been useful in combating leishmaniasis. Even with this, few natural product scaffolds represent major pharmacophores responsible for their curative effects. Between 2005 and 2010, about 19 natural products were registered for treatment of infectious diseases [29]. Similarly, over 69% of new small molecules used for the treatment of infectious diseases originated from natural products [30, 31].
Despite the large molecular weights of natural products which renders some of them less druglike, structural diversity, large chemical space and safety are characteristics that overrides synthetic alternatives. Treatments using extracts from plant families from endemic regions include Fabaceae [32], Annonaceae, Euphorbiaceae [31, 33, 34], Rutaceae [35–37], Myrsinaceae [31, 38], Liliaceae [39], Araliaceae [38], Simaroubaceae [40], as well as endophytes genera Alternaria [41], Arthrinium, Penicillium, Cochloibus, Fusarium, Colletotrichum, and Gibberella [42]. Additionally, the exploration of marine natural products has led to the identification of interesting natural products with diverse biomolecular functions [43, 44].
Since the mid-eighties when the search for anti-leishmanial natural products became prominent, numerous metabolites originating from plants to current antileishmanial therapies have been reported. Lately, credible chemical entities from marine sources and endophytic species have also been reviewed [45–51]. This review presents the various classes of natural products from both flora and fauna that have been isolated over the last decade with anti-leishmanial properties. Also, the IC50/EC50 values and suggested mechanisms of action of these natural products are discussed.
Classification of natural products with anti-leishmania properties
Alkaloids
Among the characterized bioactive constituents from nature, alkaloids have provided a broad-spectrum activity against different ailments and demonstrated their suitability as potential drug leads. Phenotypic alterations in ultrastructure form of the infective cells and immunomodulatory investigation studies of isolated alkaloids within the last decade reveal 27 alkaloids (Table 1) with varying efficacies from strong to weak activity. The natural product 3 isolated from Cissampelos sympodialis acts as a calcium channel inhibitor with immunomodulatory effects through the enhancement of nitric oxide (NO) production in macrophages [52]. Studies of 4 from Croton pullei reported significant alterations in organelle membranes of the endoplasmic reticulum, kinoplast and golgi body, depicting an apoptosis-like process [53]. Treatment with spectaline alkaloids, 16 and 17 from dichloromethane fractions of the flower Senna spectabilis of Leishmania promastigotes also portray a similar molecular mechanism like its structurally related piperine amide alkaloid, which either modulates the sterol biosynthetic pathway or acts as an inhibitor of cell proliferation by mitochondrion organelle destruction [54]. Although, the exact mode of action has not been fully elucidated, 21 from Berberine vulgaris like the active alkaloid in Berberine aristate perpetuates a similar activity through respiration incapacitation and apoptosis [55]. However, 21 was identified as a potential cell membrane disruptor via sterol biosynthesis inhibition [56], while 22 induces reactive oxygen species (ROS) generation. Structural activity relation (SAR) studies of high affinity protein kinase inhibitors, staurosporine-based compounds (24-27) revealed the 4th C methyl amine and 7th C hydrogen acceptor as the cause for the reinforced activity observed in L. donovani, which had major morphological changes in the flagella pocket and plasma membrane because of signal blockage via phosphokinase (PK) inhibition.
Table 1.
23 alkaloids isolated from various flora and fauna together with their IC50 and toxicity tested on some Leishmania species
| Natural product source | Chemical structure | Class of natural product | IC50/μg/mL | Organism tested | Toxicology | References |
|---|---|---|---|---|---|---|
|
Paenibaccillus sp. (Marine) |
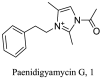 |
Imidazole | 28.1 |
L. donovani (Promastigote) |
Low toxicity profiles to mouse macrophages RAW 264.7 cell lines. > 250 µM | [57] |
|
Paenibaccillus sp. (Marine) |
 |
Imidazole | 0.203 |
L. major (Promastigote) |
MIC = 25 μM | [58] |
| 1.90 |
L. donovani (Promastigote) |
|||||
| Cissampelos sympodialis |  |
Isoquinoline | 80.0 |
L. chasi (Promastigote) |
IC50 = 0.056 μM against human laryngeal cancer cells (HEP-2cells) and 0.067 μM against human mucoepide cells (NCIH-292) | [52] |
| Croton pullei var. glabrior |  |
Piperidine | 6.27 |
L. amazonensis (Amastigote) |
Nontoxic as against murine macrophages after treatment with 79 µM of julocrotine | [53] |
| Aconitum spicatum | 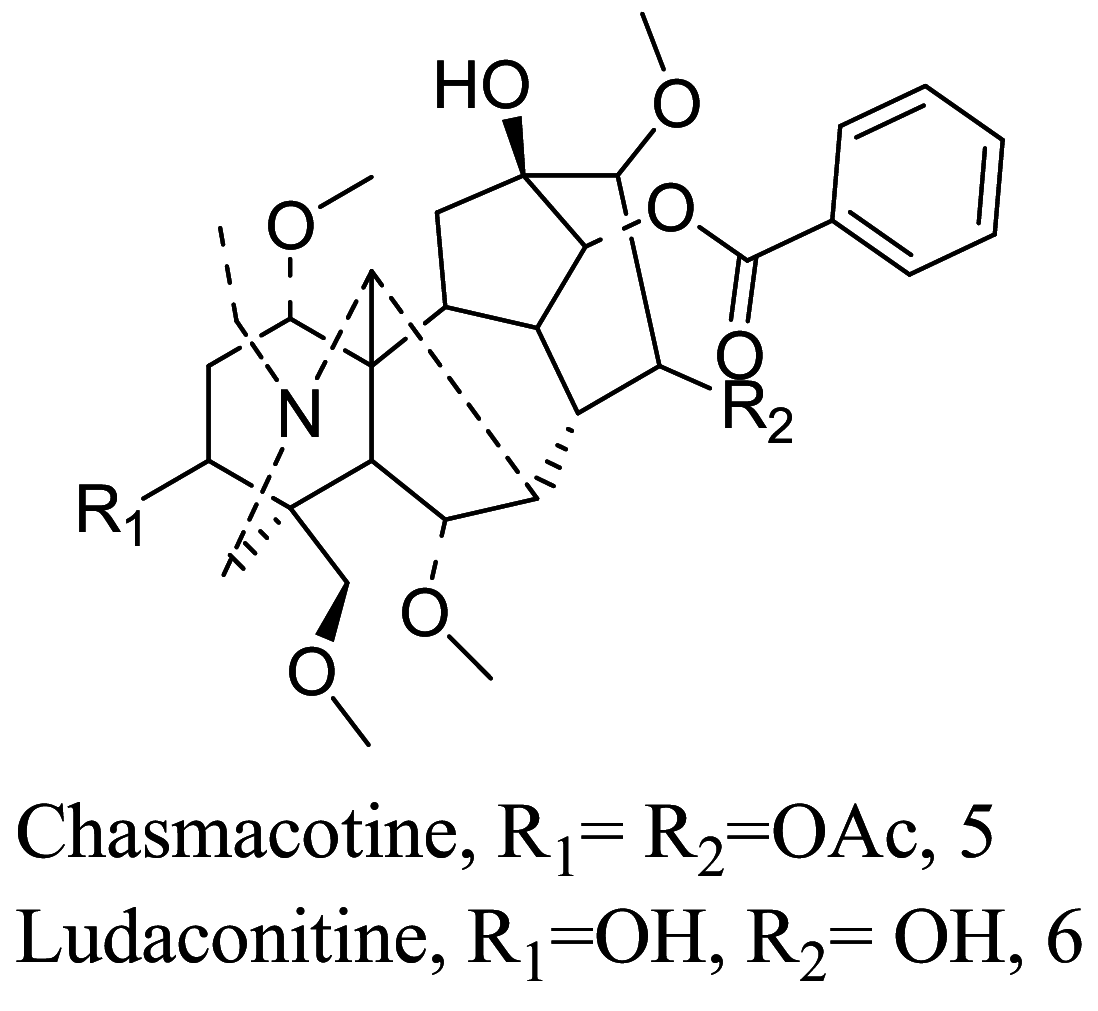 |
Pyrrolidine | 56.0 | L. major | No toxicity against MCF7, HeLa, PC3 cancer cell lines and 3T3 normal fibroblast cell line at 30 µM | [59] |
| 36.1 | ||||||
| Helietta apiculata |  |
Quinoline | 17.3 | L. donovani | [60] | |
| Quinoline | 25.5 | |||||
| Thalictrum alpinum |  |
Isoquinoline | 0.175 | L. donovani | [61] | |
| 0.639 | ||||||
| 6.60 | ||||||
| Trichosprum sp. |  |
Piperazine | 96.3 | L. donovani | [62] | |
| Piperazine | 82.5 | L. donovani | ||||
| Piper choba | 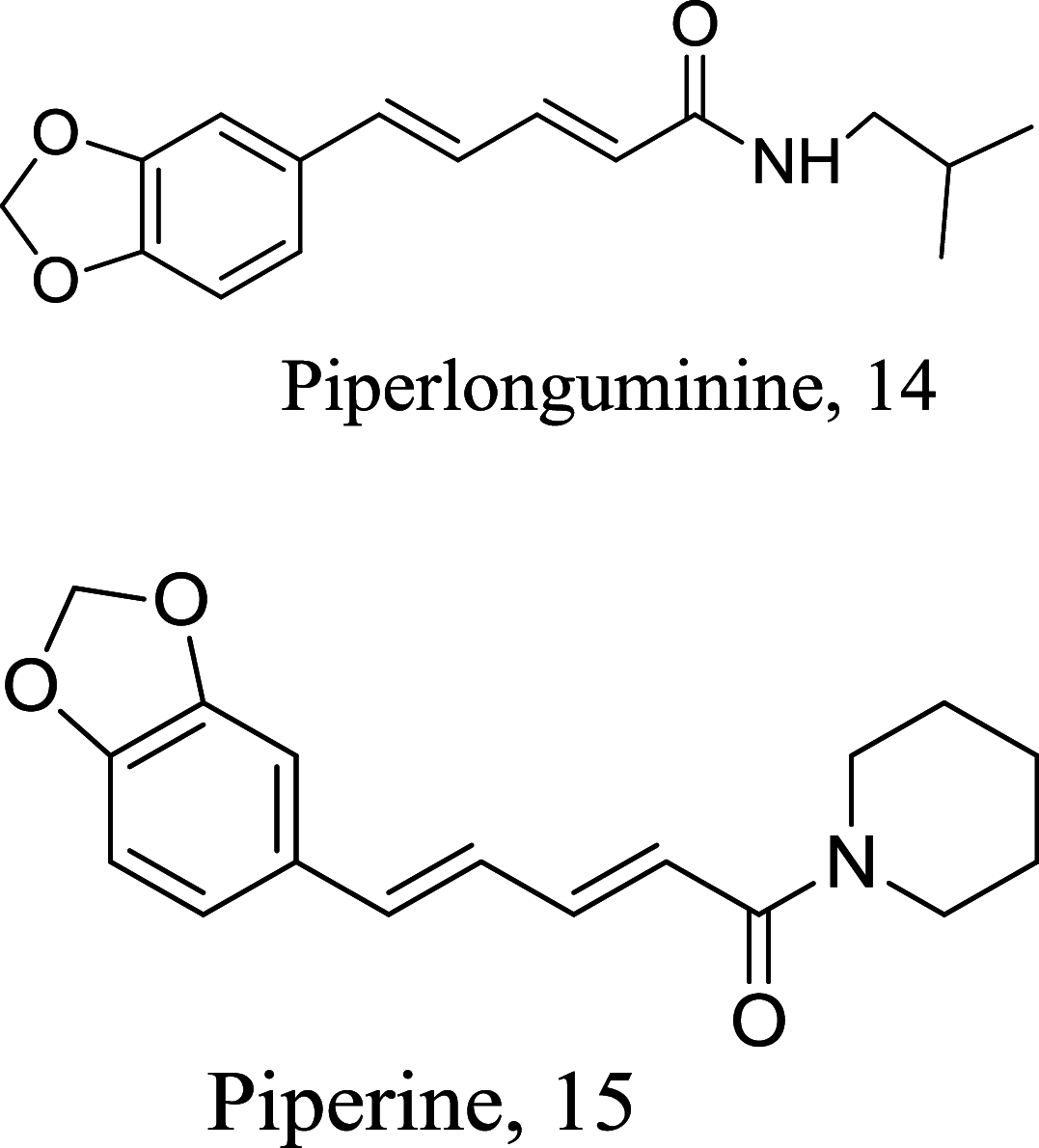 |
Amide | 16.0 |
L. donovani (Promastigotes) |
CC50 = 0.76 μM and 0.83 μM against brine shrimp cells | [63] |
| Amide | 30.0 | |||||
| Senna spectabilis | 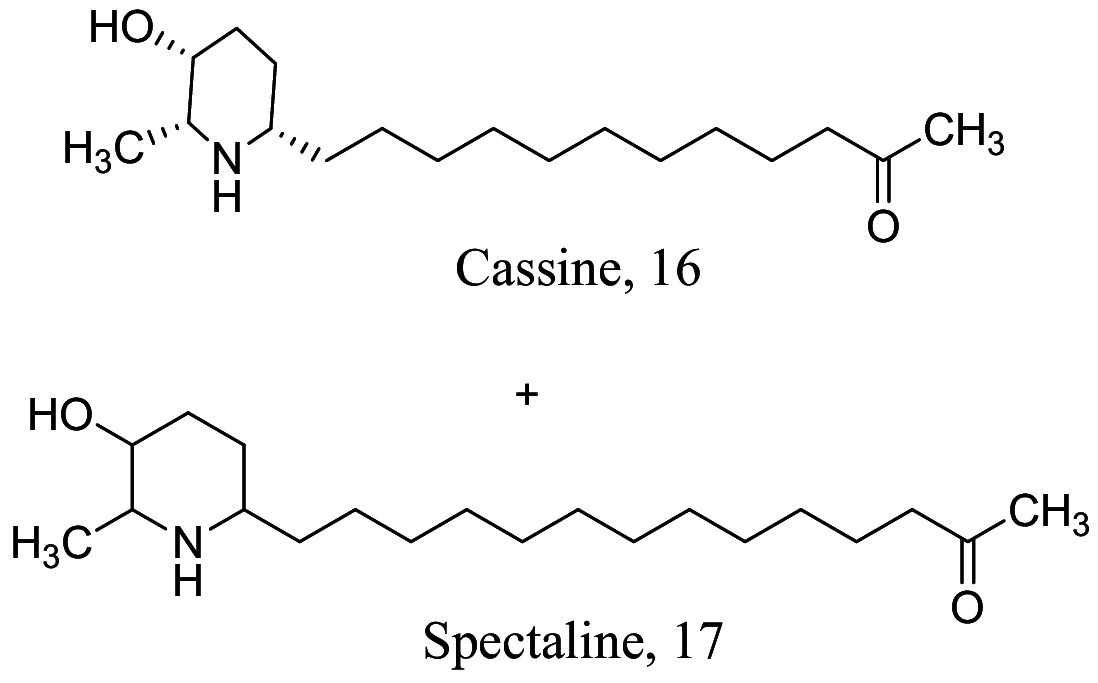 |
Piperidine | 24.9 |
L. major (Promastigotes) |
No observed lethality against J774 murine macrophage | [54] |
| Aspidosperma ramiflorum | 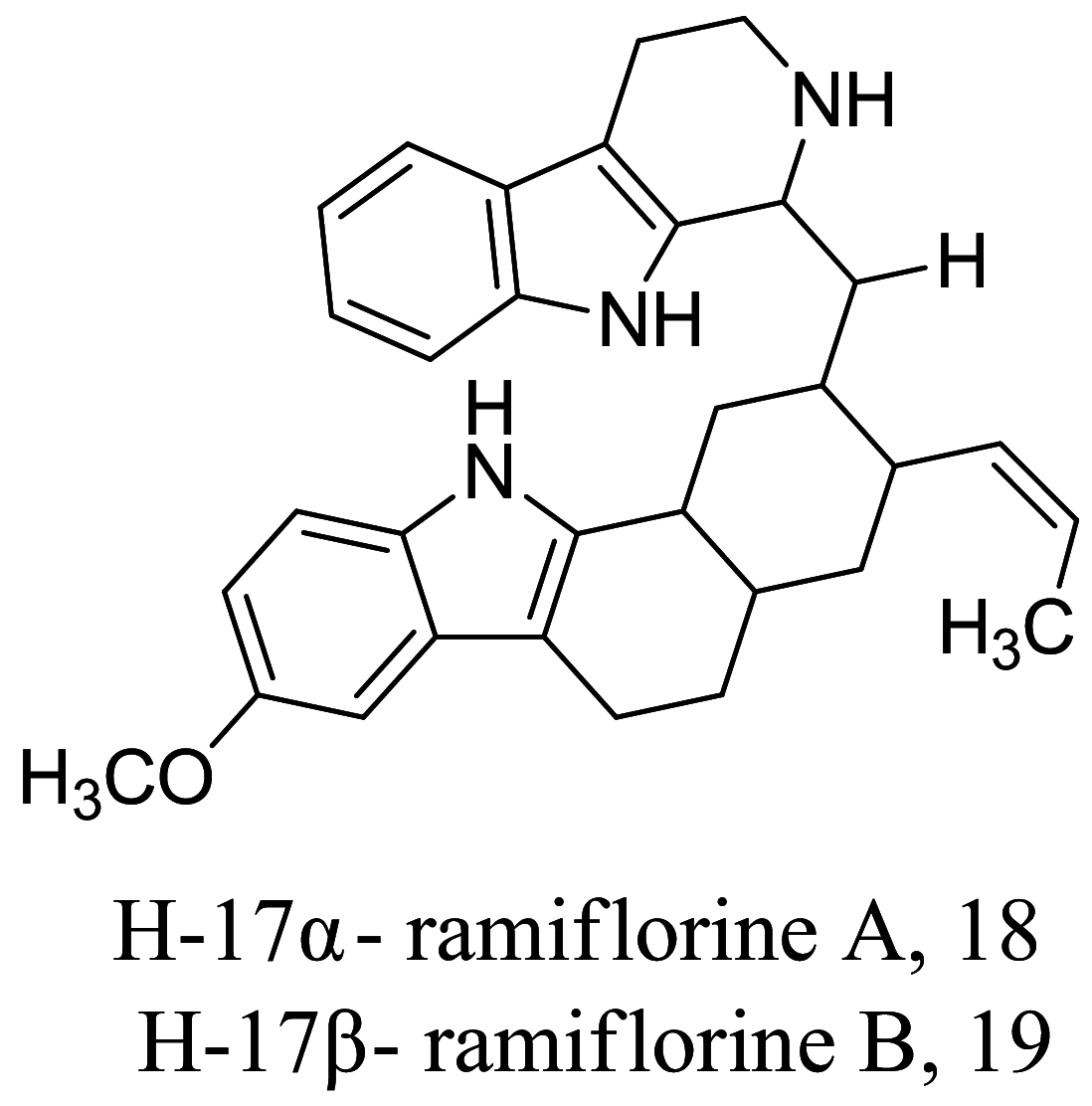 |
Indole | 18.5 |
L. amazonensis (Promastigotes) |
[64] | |
| 12.6 | ||||||
| Beilschmiedia alloiophylla |  |
quinoline | 2.95 | [65] | ||
| Berberis vulgaris | 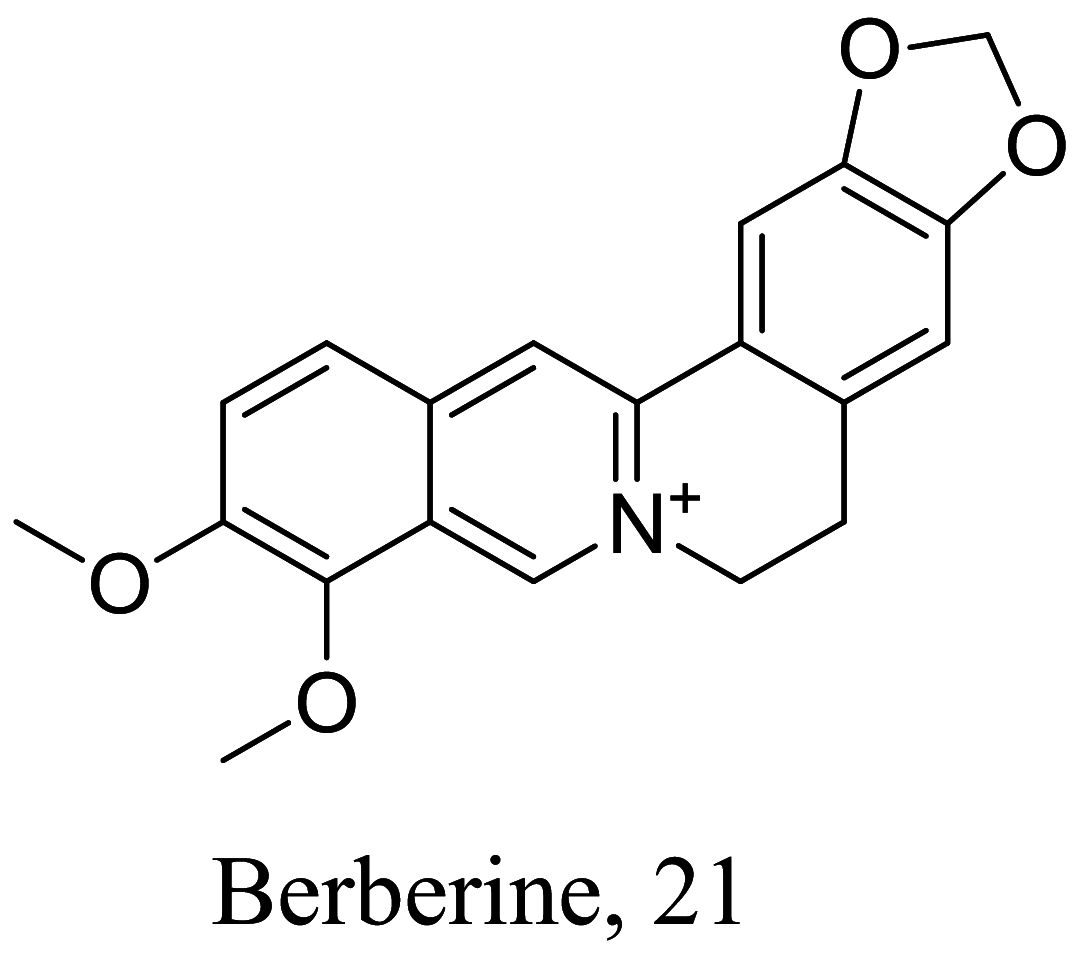 |
Isoquinoline | 2.10 |
L. major L. tropica (Promastigotes) |
Observed toxicity against murine macrophage was at 9.18 μM | [66] |
| 2.90 | ||||||
| Piper longum | 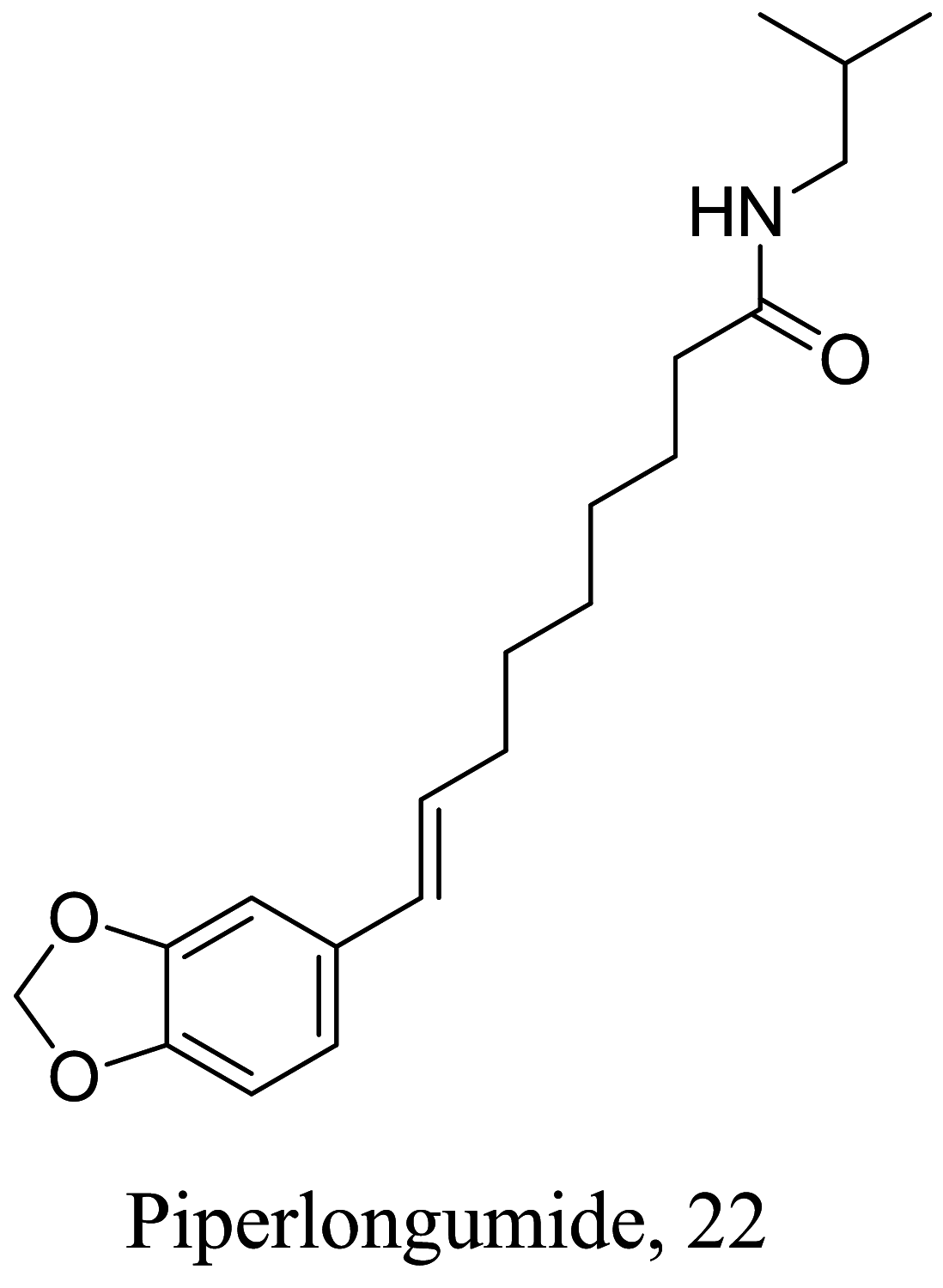 |
Amide | 9.12 |
L. donovani (promastigotes) |
Test against J774A.1 cell line indicated a high cytotoxicity at 5.05 ± 0.64 μg/mL. 393 |
[67] |
| 2.81 |
L. donovani (amastigotes) |
|||||
|
Spongia sp. and Ircinia sp. (Marine) |
 |
Indole | 9.6 | Toxicity profile against mammalian L6 cells was | [68] | |
|
Streptomyces sanyensis (Marine) |
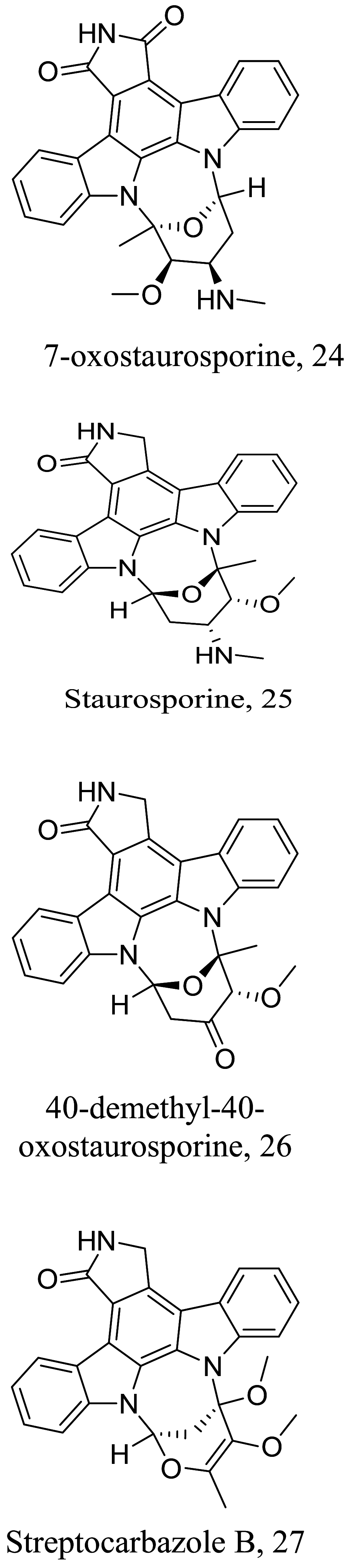 |
Indolocarbazole | 0.0075 |
L. amanzonensis (promastigotes) |
The series showed low selectivity against murine macrophage J774A.1 with CC50 of 5.20 | [69] |
| 0.0012 |
L. donovani (promastigotes) |
|||||
| 0.0002 |
L. amanzonensis (amastigotes) |
|||||
| Indolocarbazole | 0.00017 |
L. amanzonensis (promastigotes) |
8.74 | |||
| 0.0045 |
L. donovani (promastigotes) |
|||||
| 0.0224 |
L. amanzonensis (amastigotes) |
|||||
| Indolocarbazole | 0.037 |
L. amanzonensis (promastigotes) |
> 40 | |||
| > 0.089 |
L. donovani (promastigotes) |
|||||
| 0.005 |
L. amanzonensis (amastigotes) |
|||||
| Indolocarbazole | 0.0224 |
L. amanzonensis (promastigotes) |
> 40 | |||
| > 0.089 |
L. donovani (promastigotes) |
Phenolics
As characterized by hydroxy-phenyl groups, polyphenolics are widely distributed in nature and have been isolated from different plants. In traditional medicine phenolics have received much interest in phyto-therapeutics for the treatment of ailments ranging from non-infectious to infectious diseases. These chemotypes include compounds like coumarins, flavonoids, quinones, lignans, flavone glycosides amongst others (Table 2). Flavonoids from Selaginella sellowi when tested against different forms of Leishmania revealed a pro-drug mechanism for 28 but an activated NO generation for 29 [70]. The difference in the mode of action of these two flavonoids may be due to their conformational orientations. Similar investigations to understand the possible cause of apoptosis induced by 30 and 31 suggested a mitochondrial dysfunction with no influence on ROS [71]. However, evidence from suicidal action of some quercetin analogues have also indicated iron chelation, arginase inhibition, and topoisomerase II intercalation as possible mechanisms [72]. From the same Nectandra genus, inhibitory activity of 34 and 43 have been fully elucidated. Results indicated an inactivation of exacerbatory immunogens with reduced calcium levels and depolarized mitochondria potential [73]. Studies with similar compounds against melanoma cells indicated an apoptosis process confirming the depolarization activity [74]. Deciphering the exact mechanism underpinning the leishmanicidal action of isolated compounds from Connarus seberosus, it was revealed that defects in the mitochondria and plasma membrane structure with the evidence of lipid accumulation were caused by 55 and 56 [75]. Comparing 58 and its 3-O-methyl analog, 59, to rosmarinic acid (based on the shared catechol nucleus), their potential mode of action is suggested as inhibition of reactive oxygen species [76, 77]. 75 as a chemo-preventive agent acts by reducing inflammatory symptoms by suppressing NF-κB expression and other pro-inflammatory factors including iNOS, COX-2, TNF-α, IL-1β, and IL-6 [78]. Compound 74 emulates an apoptosis induced suicidal mechanism which involves DNA fragmentation, inhibition of inflammation cytokines and the activation of caspases with downstream effects on gene transcriptional process [79]. Structural similarities of anti-inflammatory coumarins with 74 precludes a similar mechanism of action [80]. From the isolates of Arrabidaea brachypoda only 67 altered organelle structure and function by attenuating cytoplasm puncturing and golgi apparatus swellings [81].
Table 2.
Various classes of phenolic compounds with their IC50 exhibiting antileishmanial properties
| Natural product source | Chemical structure | Class of phenolic | IC50/μg/mL | Organism tested | Toxicology | References |
|---|---|---|---|---|---|---|
| Selaginella sellowi | 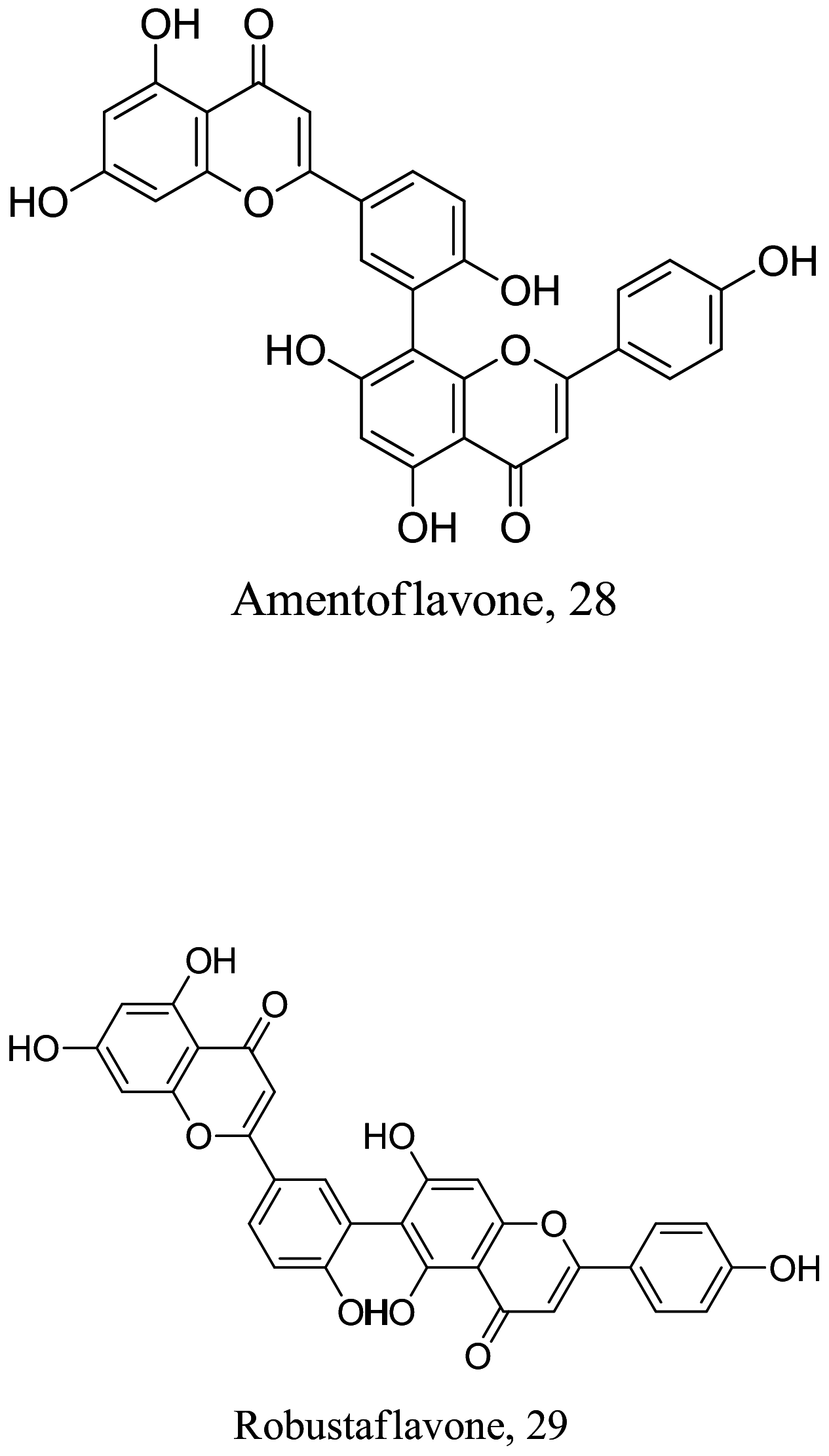 |
Flavonoid | 0.10 | L. amazonensis | IC50 = 5.57 and 4.09 µM against Murine macrophages (J774.A1) and fibroblast cells (NIH/3T3) | [70] |
| Flavonoid | 2.80 | CC50 = 5.75 and 47.4 µM against murine macrophage (J774.A1) and fibroblast cells (NIH/3T3). | ||||
| Strychnos pseudoquina | 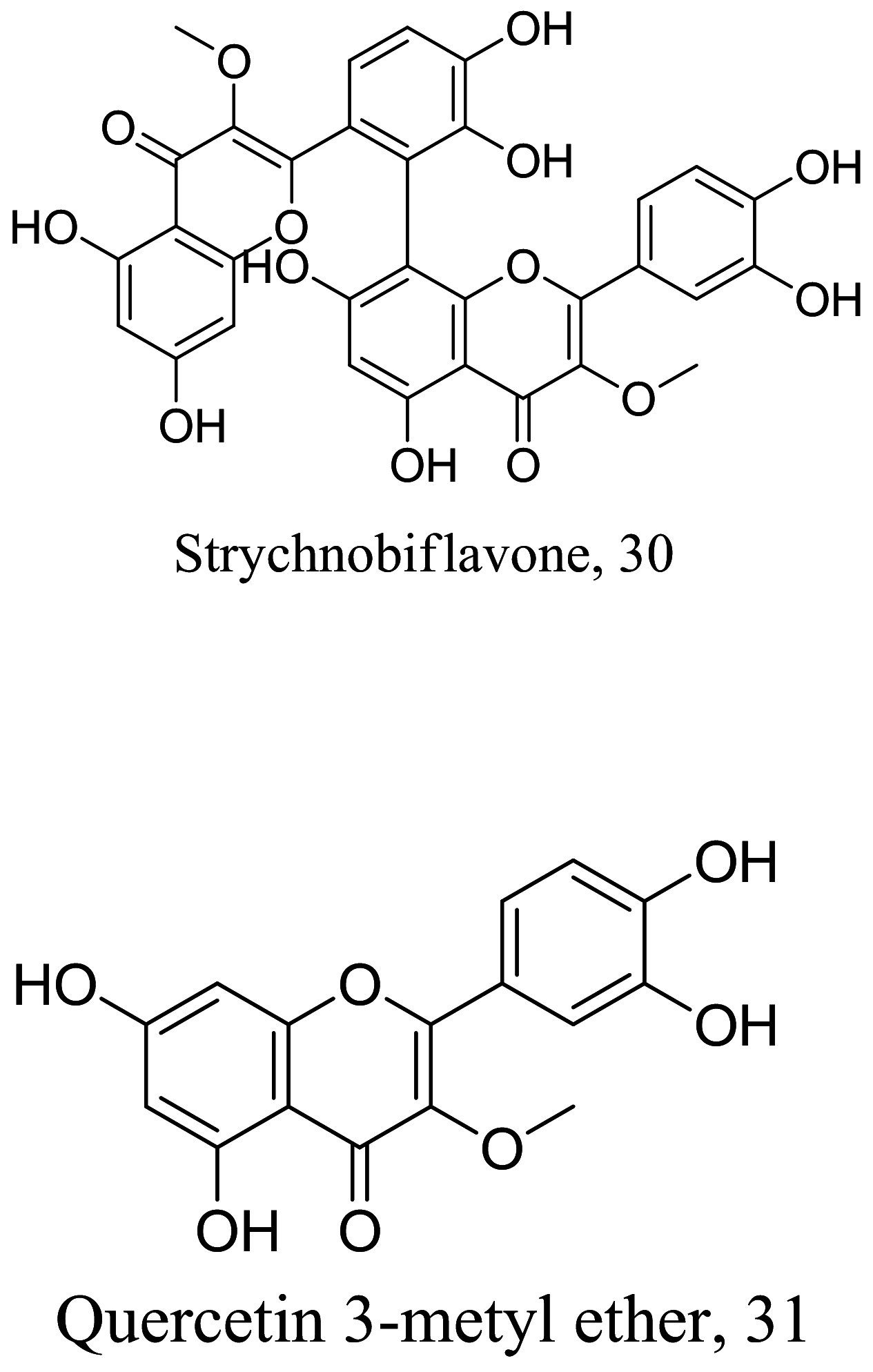 |
Flavonoid |
11.9 2.02 |
L. infantum L. amazonensis |
Low-toxicity to infected murine macrophage up to 125 μM and low hemolytic activity in red blood cells | [71] |
| Flavonoid | 2.56 | L. amazonensis | No significant toxicity > 199 μM | |||
|
Lendenfeldia. dendyi and Sinularia dura (Marine) |
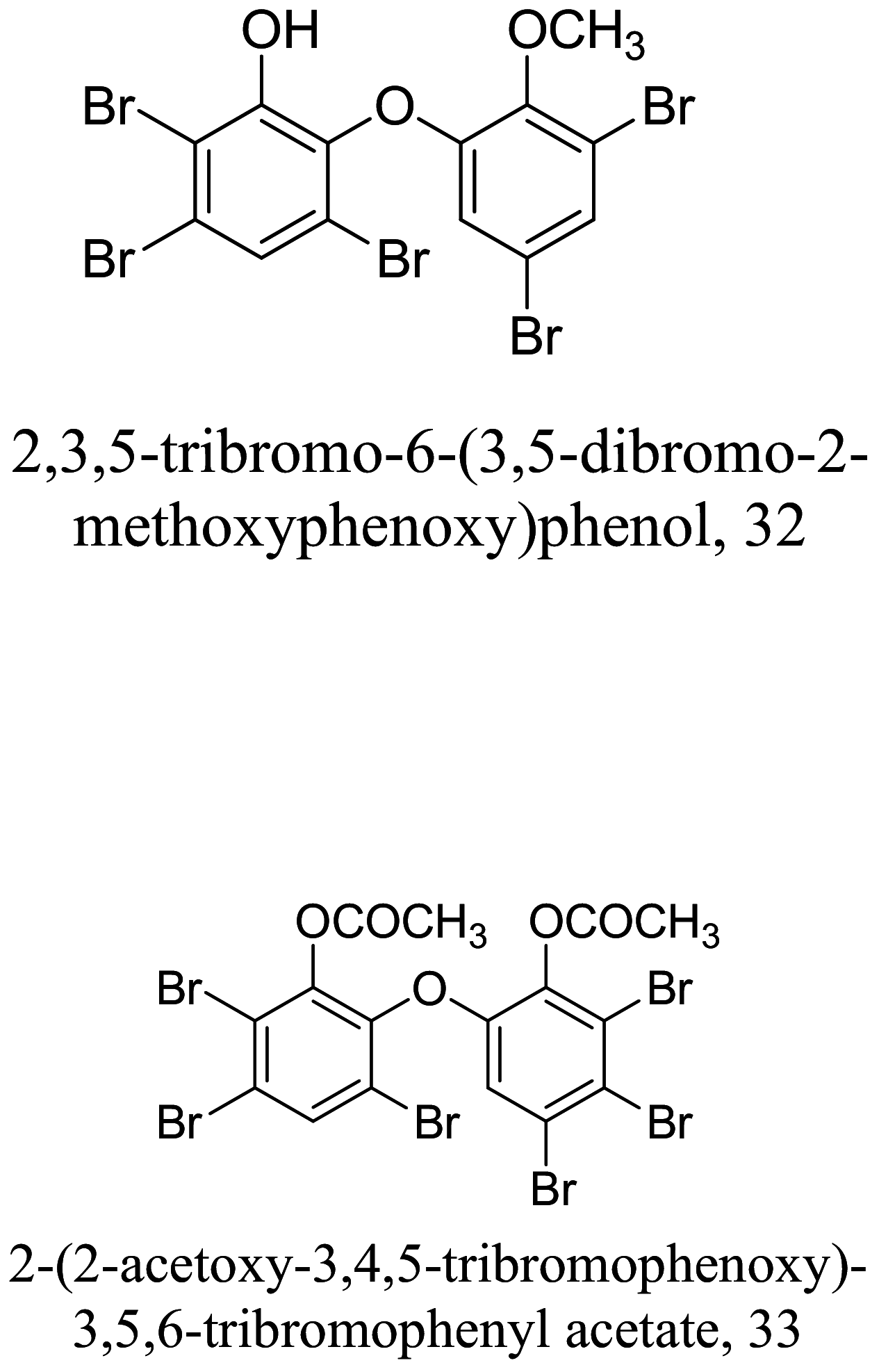 |
Phenyl ether | 18.0 |
L. donovani (Promastigotes) |
Low toxicity profile to VERO cells, pig kidney epithelia, human dermal carcinoma oral | [82] |
| Phenyl ether | 13.6 | L. donovani | Ductile carcinoma breast, human malignant melanoma up to 13 µM | |||
| Nectandra leucantha | 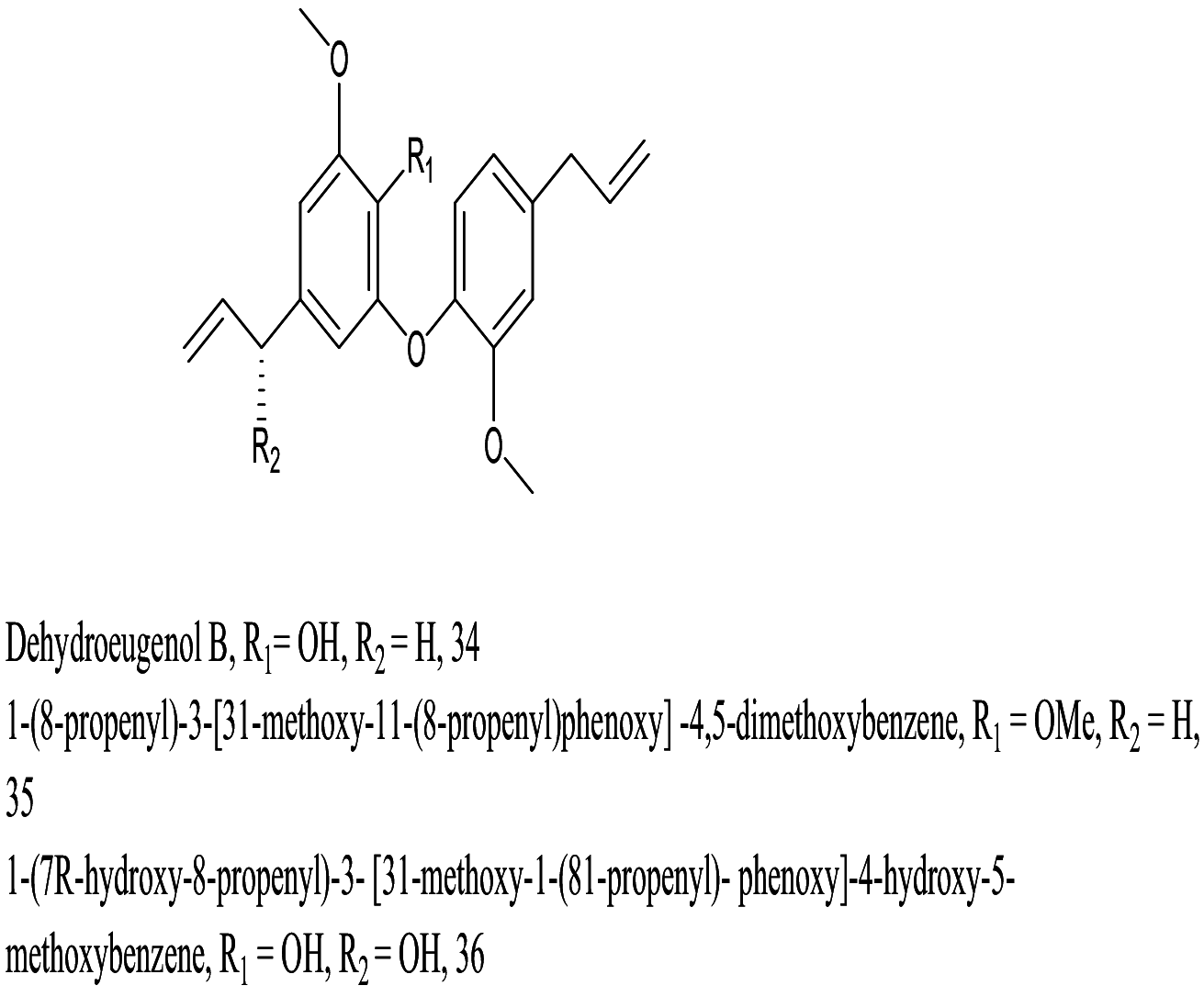 |
Phenyl ether |
8.70 6.00 34.9 |
L. donovani (Intra Amastigotes) |
Nontoxic to mammalian peritoneal macrophages up to >293.8 μM 112.1 μM >292.1 μM |
[83] |
| Alpinia galanga | 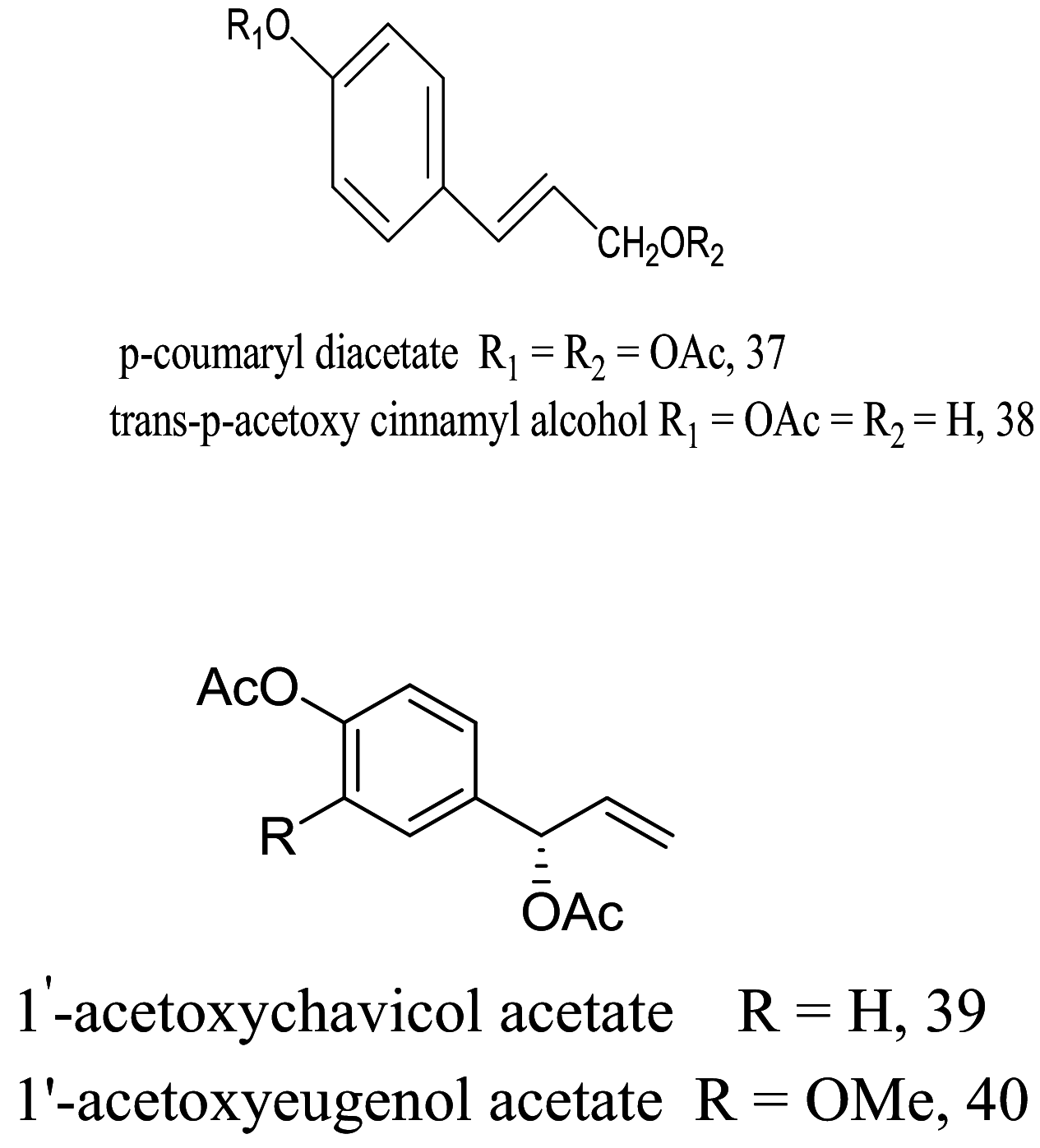 |
Monolignols |
10.5 16.6 |
L. donovani (Promastigotes) |
[84] | |
| Phenol ester |
8.80 5.60 |
|||||
| Hellieta apiculata |  |
Coumarin |
35.8 27.5 32.1 |
L. amazonensis L. infantum L. brazilensis |
[60] | |
| Coumarin |
18.5 27.4 21.5 |
L. amazonensis L. infantum L. brazilensis |
||||
| Nectandra oppositifolia | 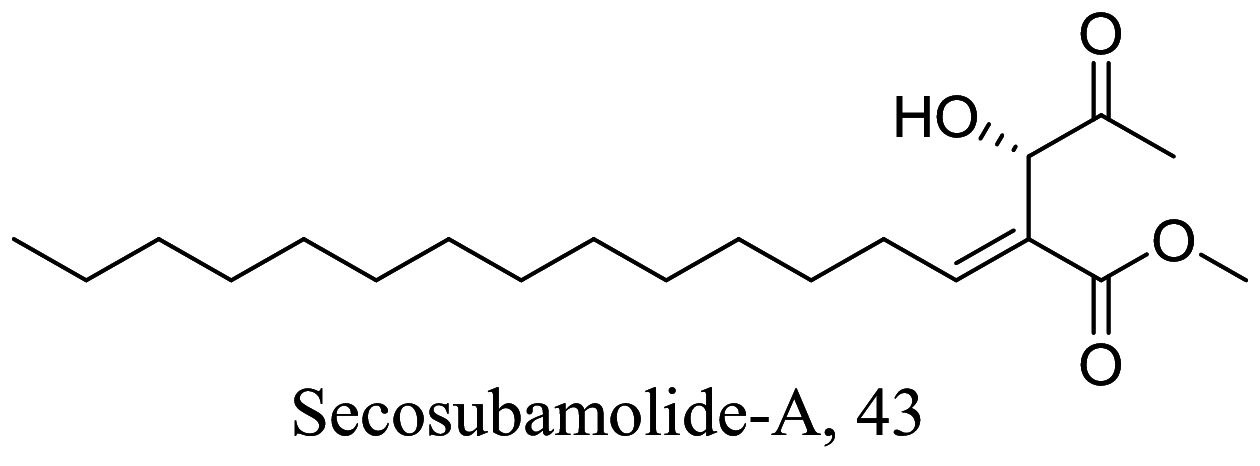 |
Butanolide | 3.58 | Nontoxic against NCTC cell up to 42.3 µM | [85] | |
| Piper regnellii var. pallescens | 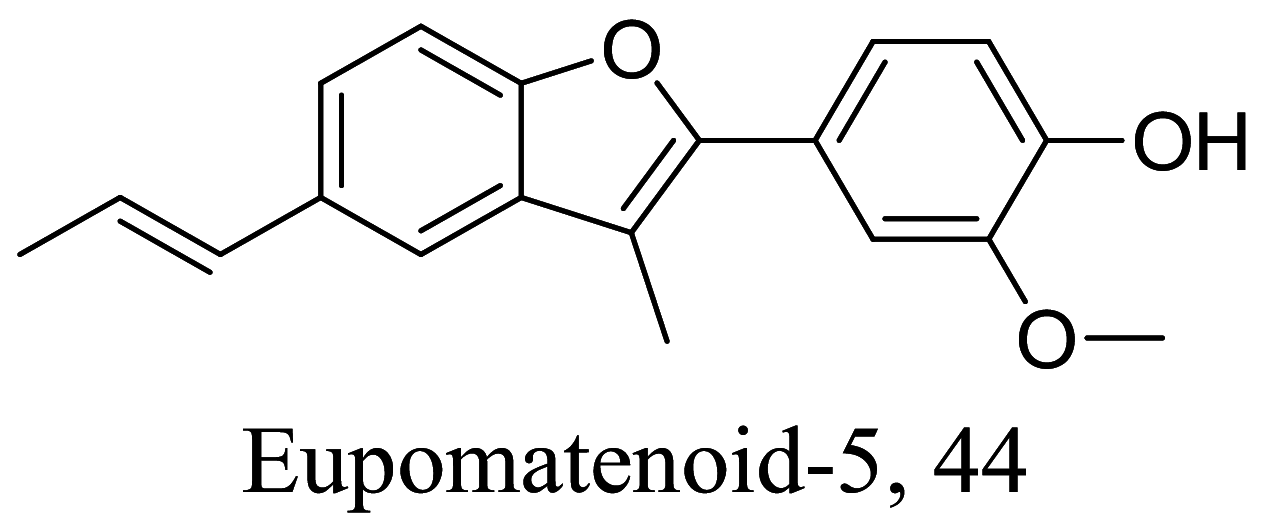 |
Lignan | 5.00 | L. amazonensis | [86] | |
| Nectandra cuspidata | 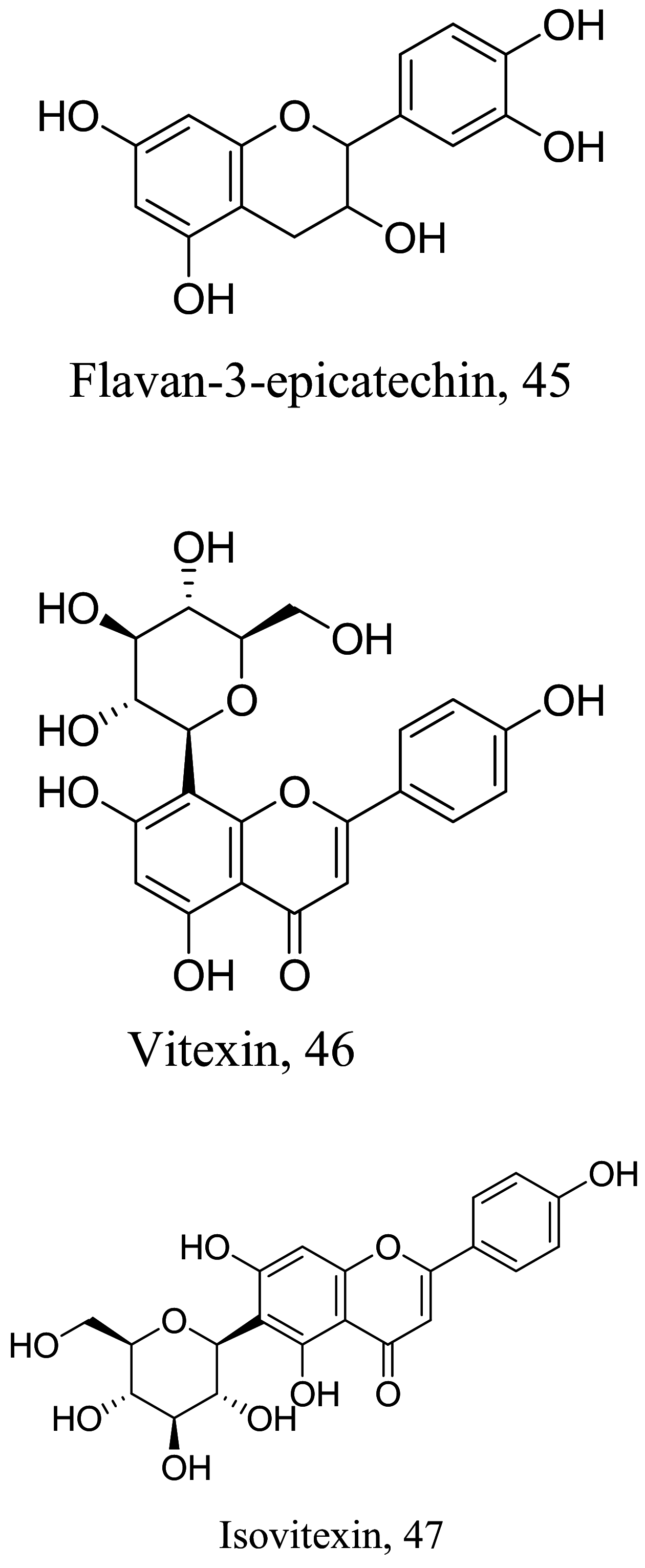 |
Flavonoid | 38.5 |
L. amazonensis (Amastigotes) |
Low cytotoxicity in J774.A1 macrophages | [87] |
| Flavanoid | 71.3 | |||||
| Flavanoid | 34.0 | |||||
| Plumbago zeylanica | 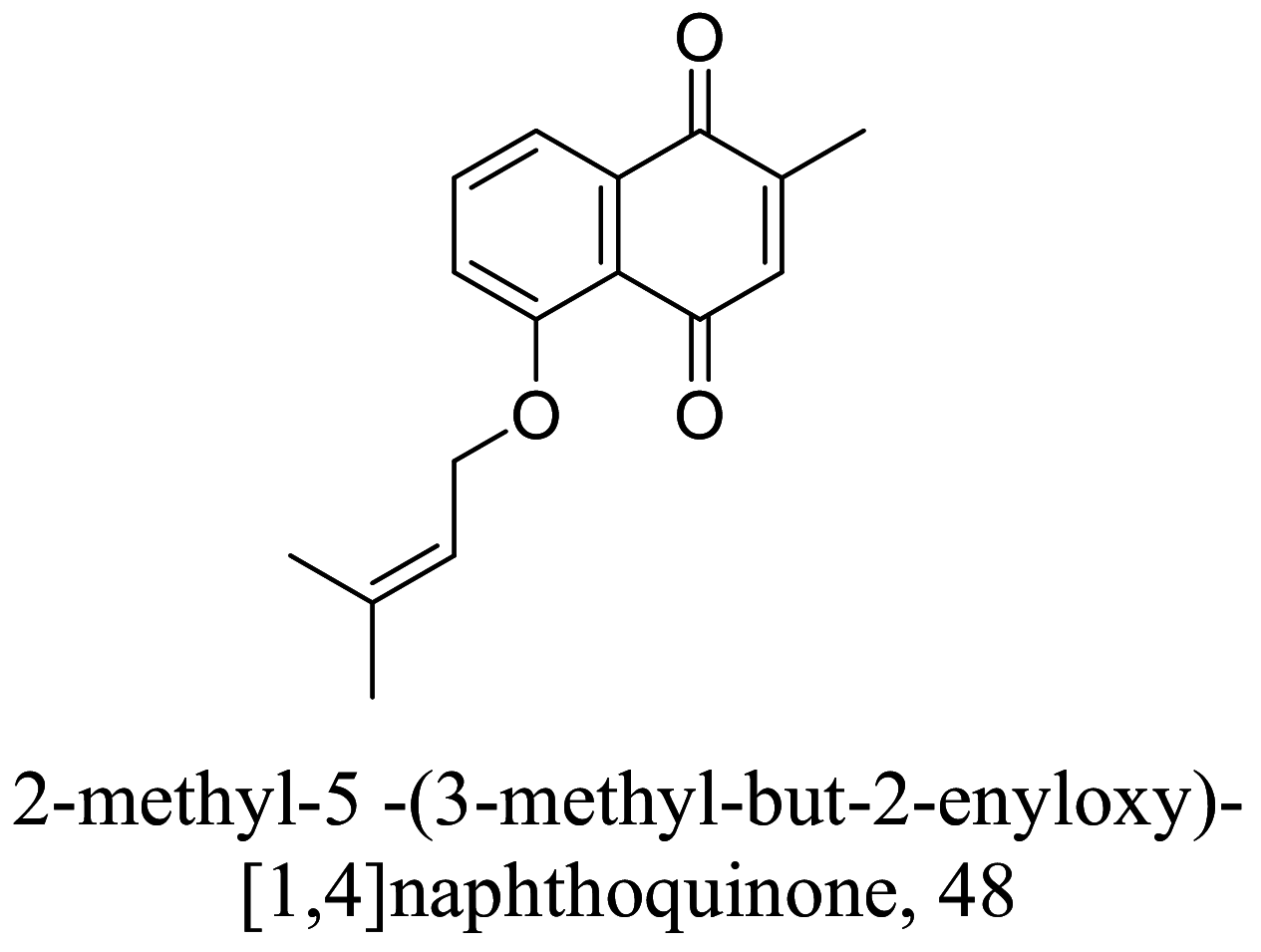 |
Quinone |
1.05 (EC50) |
L. donovani (Amastigotes) |
Very toxic to on RAW 264.7 macrophage cell lines | [88] |
| Ocimum gratissimum | 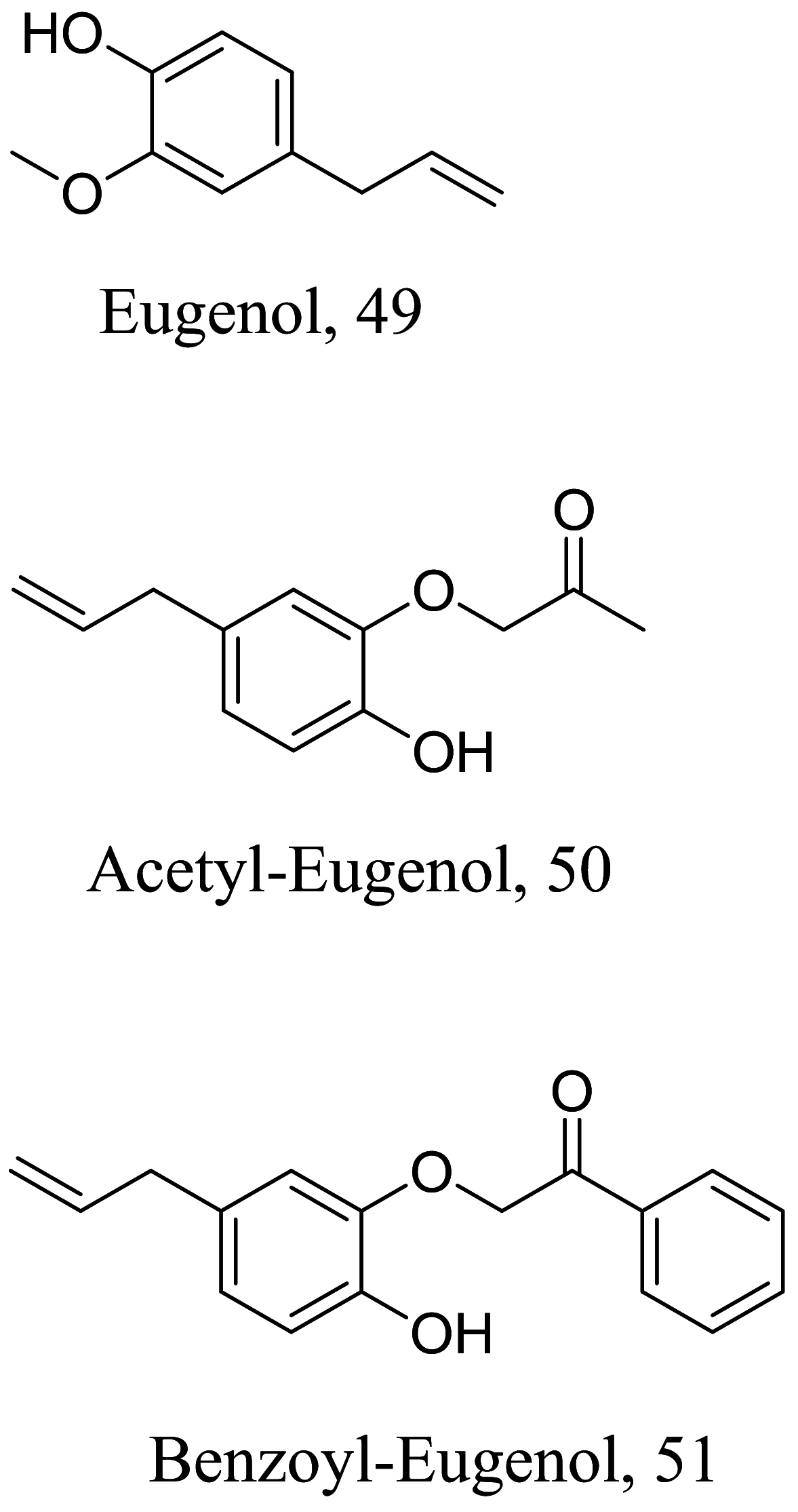 |
Monolignol | 0.81 | L. infantum | Nontoxic in murine macrophages RAW 264.7 cells lines 29.0 µM | [89–91] |
| Monolignol | 18.5 | >100 µM | ||||
| Monolignol | 14.9 | 97.7 µM | ||||
| Vernonia polyanthes | 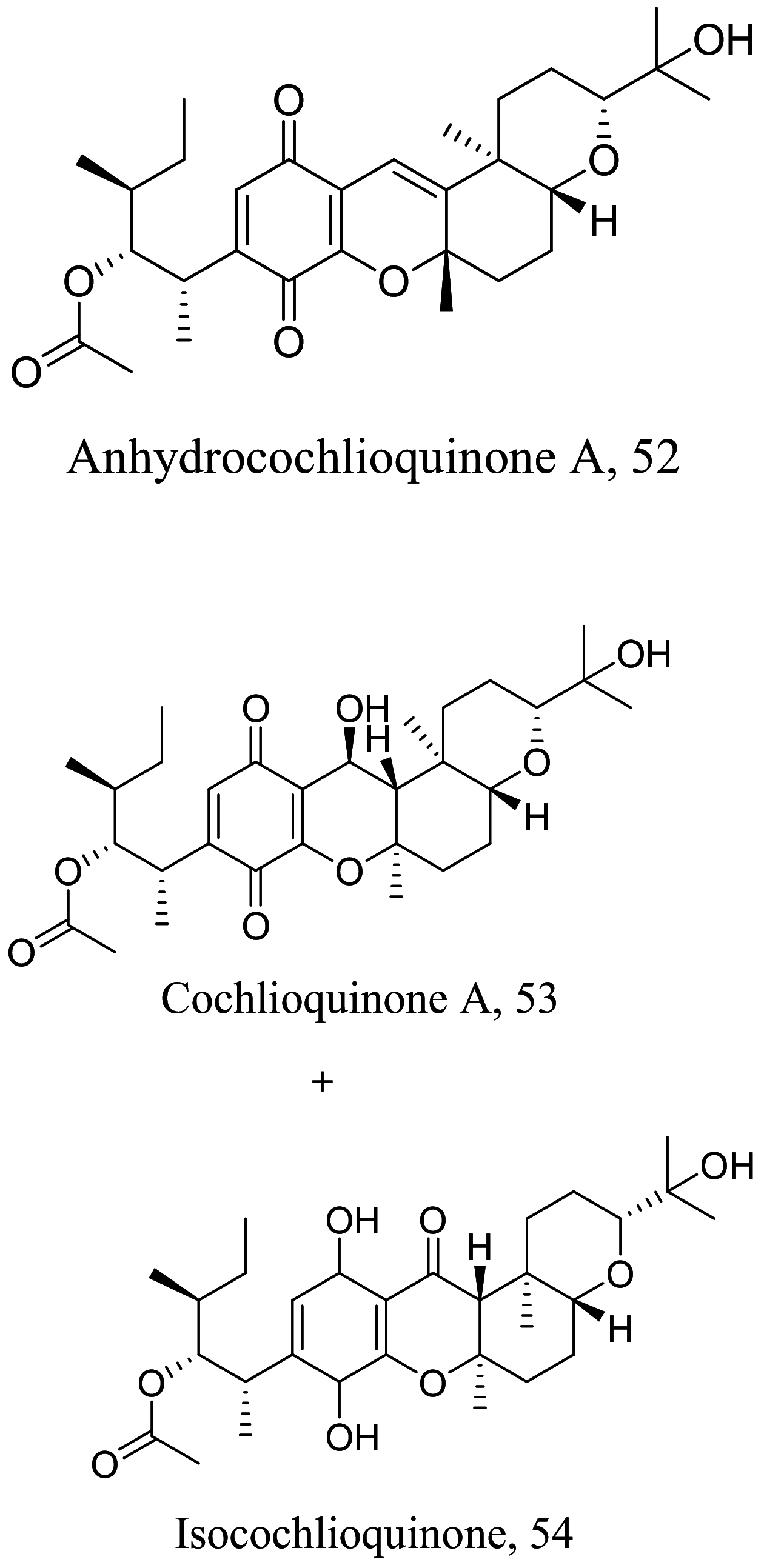 |
Quinone | 50.5 |
L. amazonensis (Promastigotes) |
At conc. > 52.4 µM in infected murine macrophages | [92] |
| Quinone | 10.2 | At conc. > 37.23 µM in infected murine macrophages | ||||
| Connarus Suberosus | 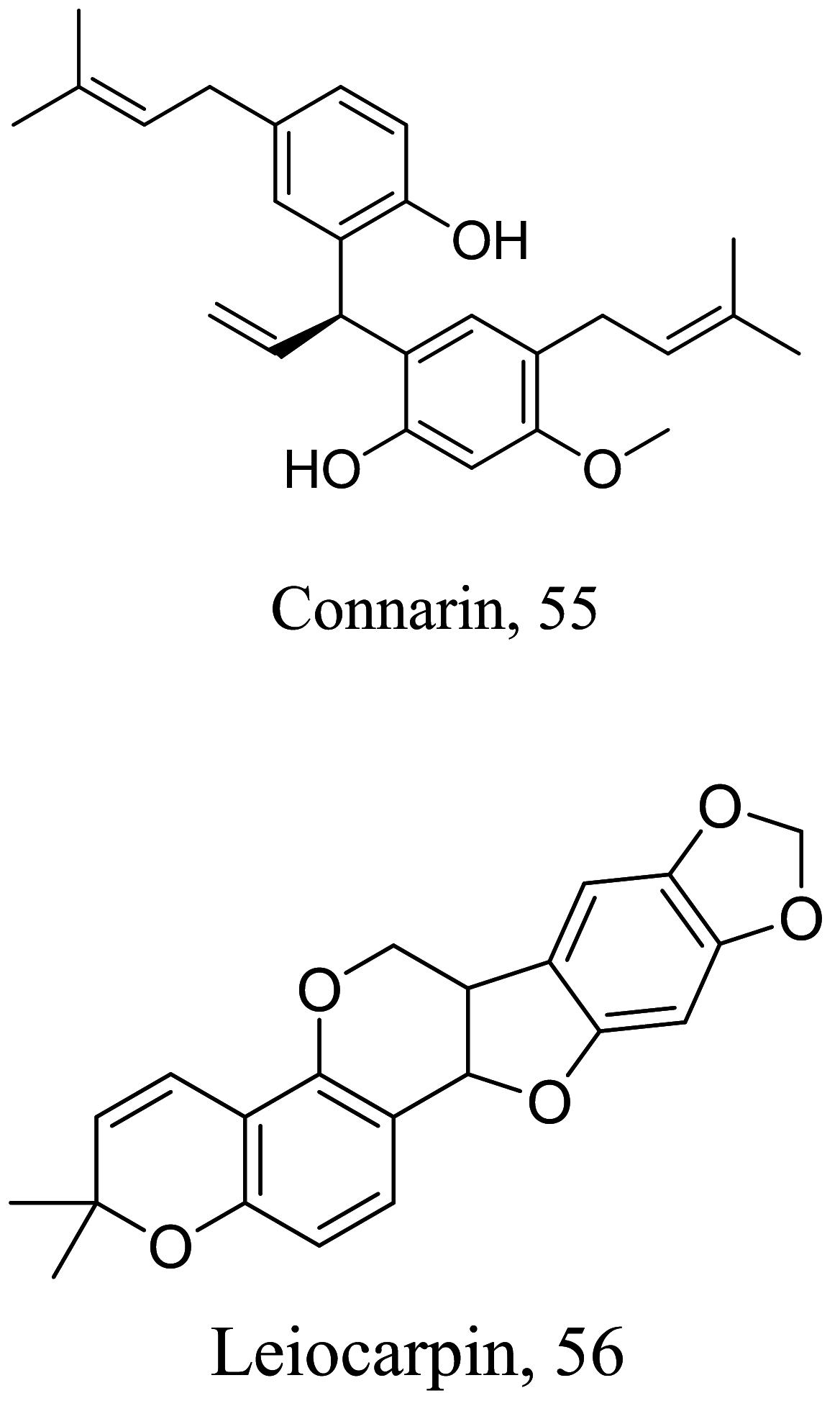 |
Chromanone |
1.13 4.5 5.2 |
L.amazonensis (amastigotes) L. amazonensis (promastigotes) L.infantum (promastigotes) |
Toxic at 18.3 µM against murine macrophages. | [75] |
| Lignan |
11.4 15.5 7.1 |
L. amazonensis (promastigotes) L.infantum (promastigotes) L. amazonensis (promastigotes |
Reduction cell viability was at 116 µM. | |||
| Piper aduncum | 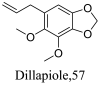 |
Lignan |
0.31 0.28 |
L. amazonensis (promastigotes) L. braziliensis (promastigotes) |
Critical changes in the morphology of 3T3 fibroblast cell lines and its viability was observed at 25 µM and above. | [93] |
| Hyptis pectinata | 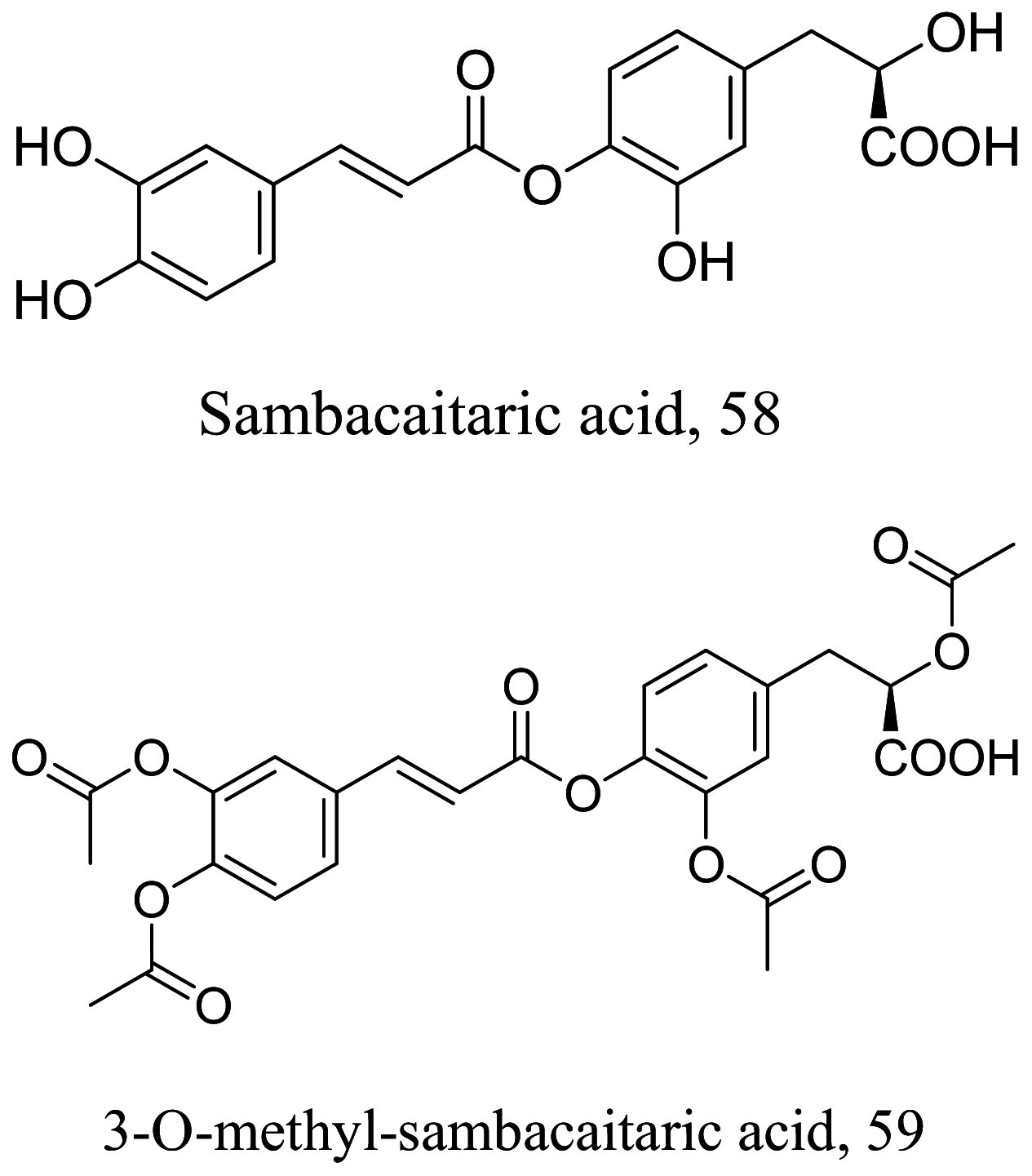 |
Flavonoid | 2.5 |
L. braziliensis (promastigotes) |
N.T | [76] |
| Flavonoid | >36.0 | |||||
| Geosmithia langdonii |  |
Phenyl propene | 0.05 |
L. donovani (promastigotes) |
N.T | [94] |
| Geosmithia langdonii | 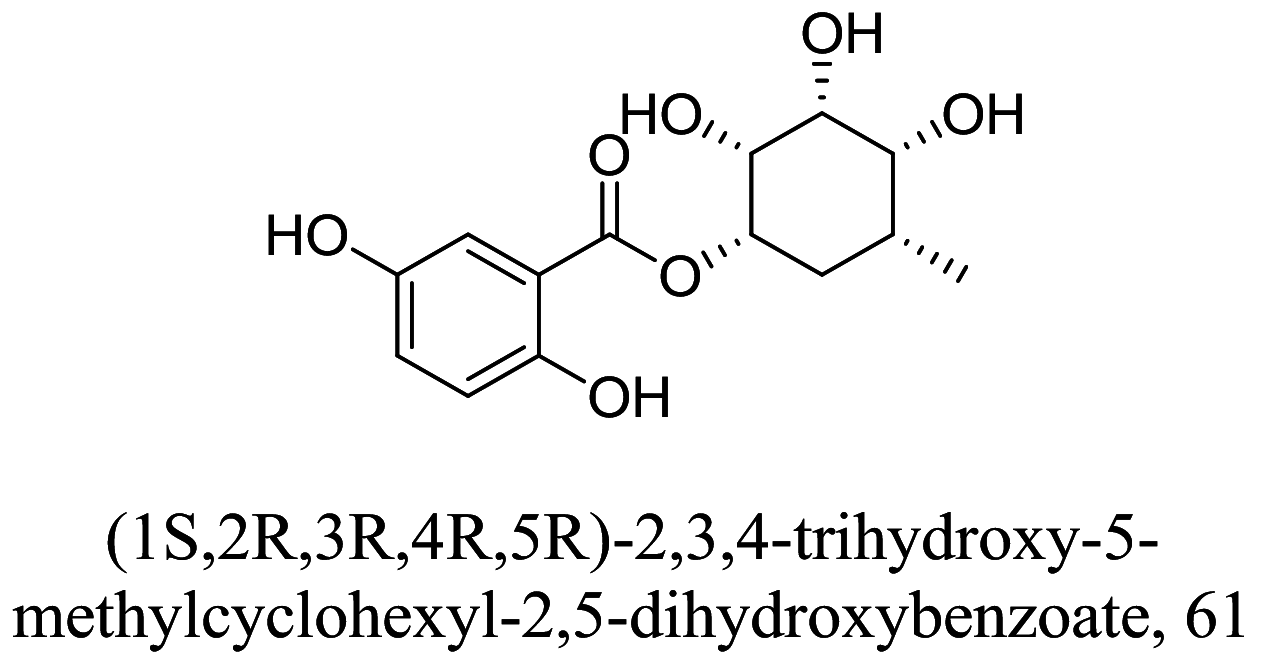 |
Carbasugar Carbasugar |
0.34 0.20 |
L. donovani (promastigotes) |
N.T | [95] |
| Ferula narthex | 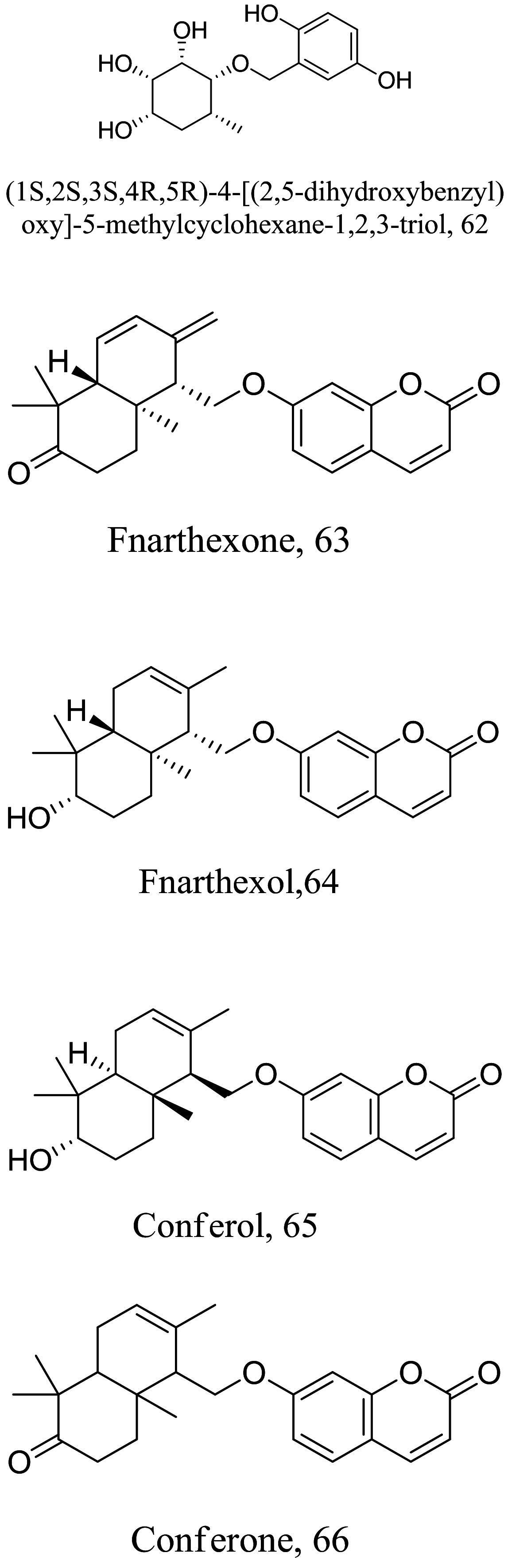 |
Coumarin | 43.77 |
L. amanzonensis (promastigotes) |
N.T | [96] |
| Coumarin | 46.81 | |||||
| Coumarin | 11.51 | |||||
| Coumarin | 46.77 | |||||
| Arrabidaea brachypoda | 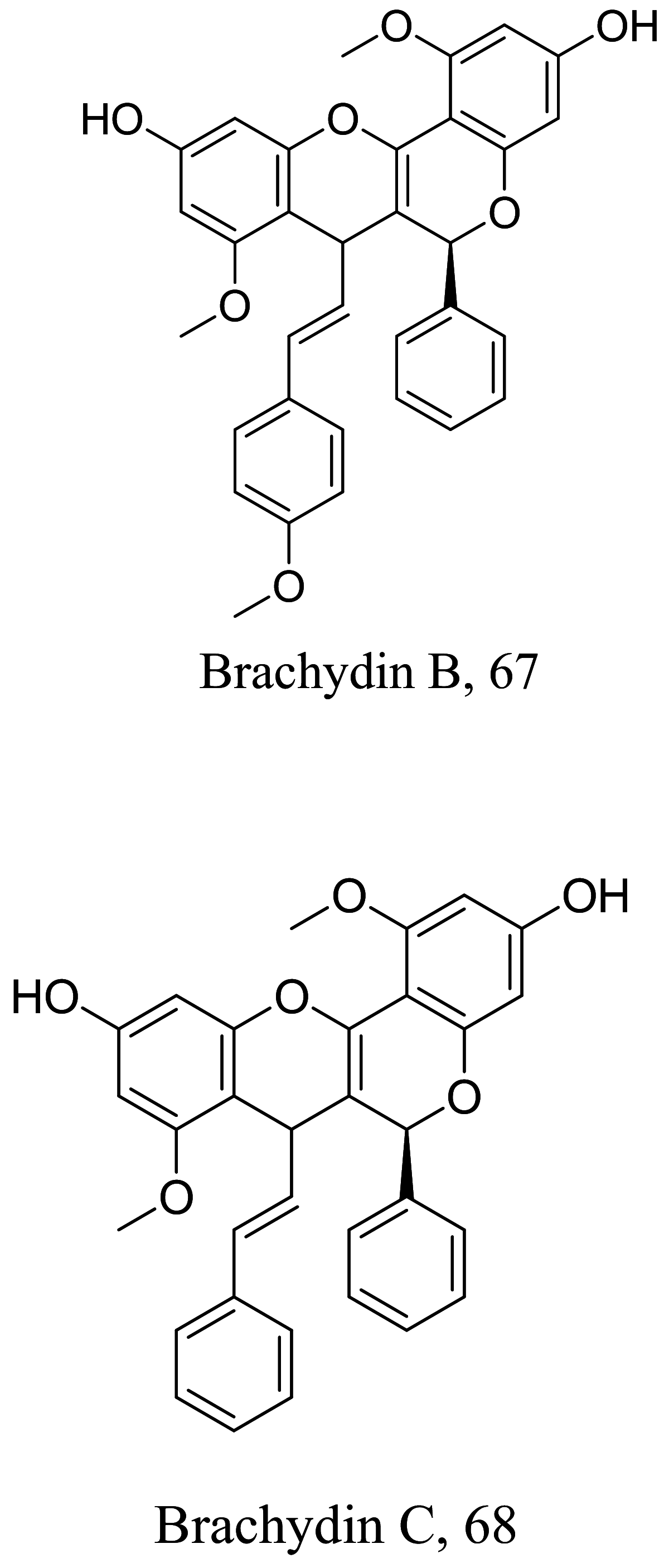 |
Flavonoid |
0.004 0.017 0.013 0.024 |
L. amanzonensis (amastigotes) L. amanzonensis (promastigotes) L. brazilensis (promastigotes) L. infantum (promastigotes) |
High lethality against macrophages at concentration above 20 μM | [81] |
| Flavonoid |
0.02 0.017 0.037 0.012 |
L. amanzonensis (amastigotes) L. amanzonensis (promastigotes) L. brazilensis (promastigotes) L. infantum (promastigotes) |
||||
| Trixis antimenorrhoea |  |
Flavonoid |
78 96 |
L. amazonensis (promastigote) L. brazilensis (promastigote) |
N. T | [97] |
| Flavonoid |
19 5.8 |
|||||
| Anogeissus leiocarpus | 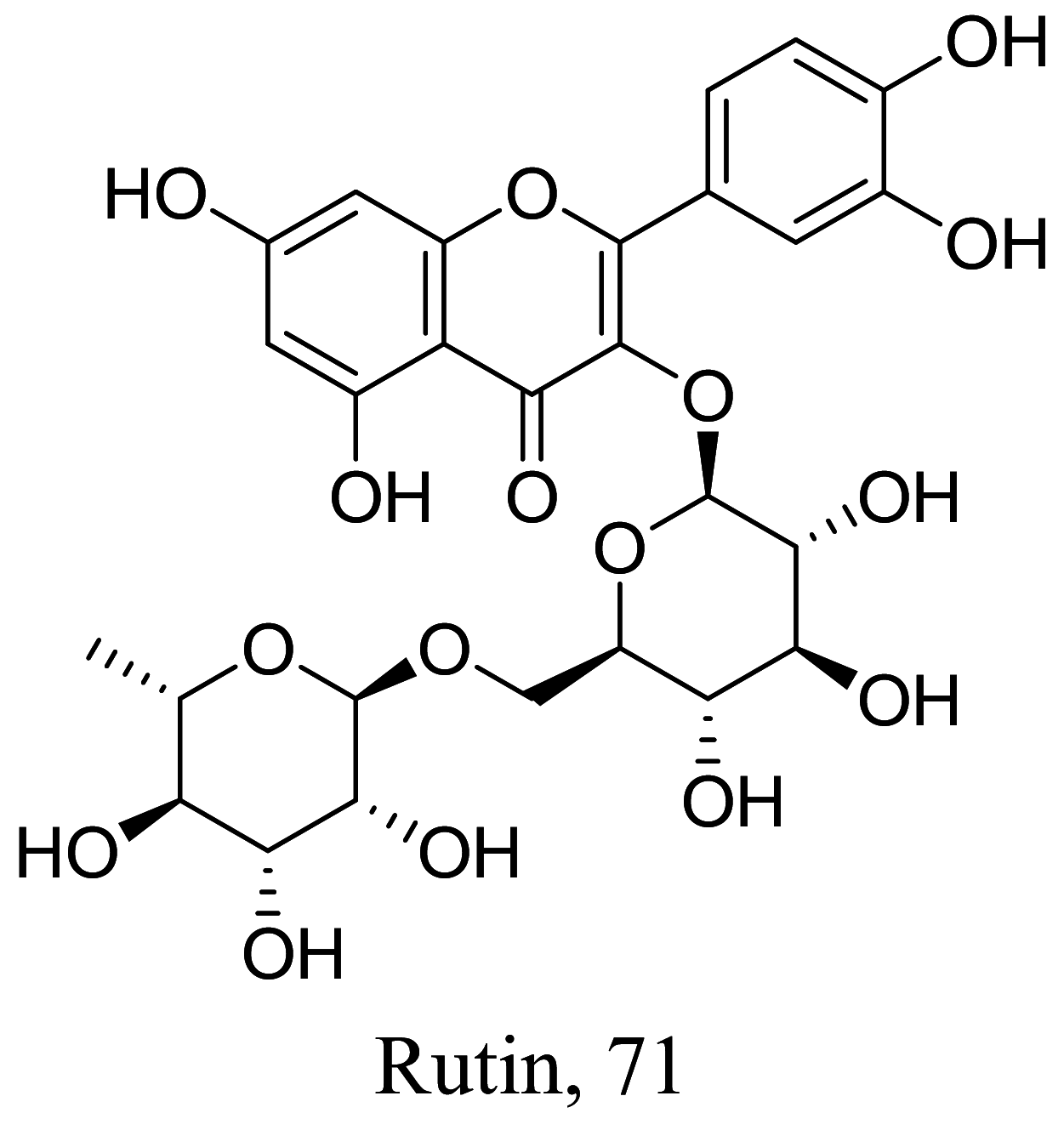 |
Flavonoid | 0.003 |
L. donovani (promastigotes) |
CC50 > 100 µg/ml | [98] |
| Sassafras albidum | 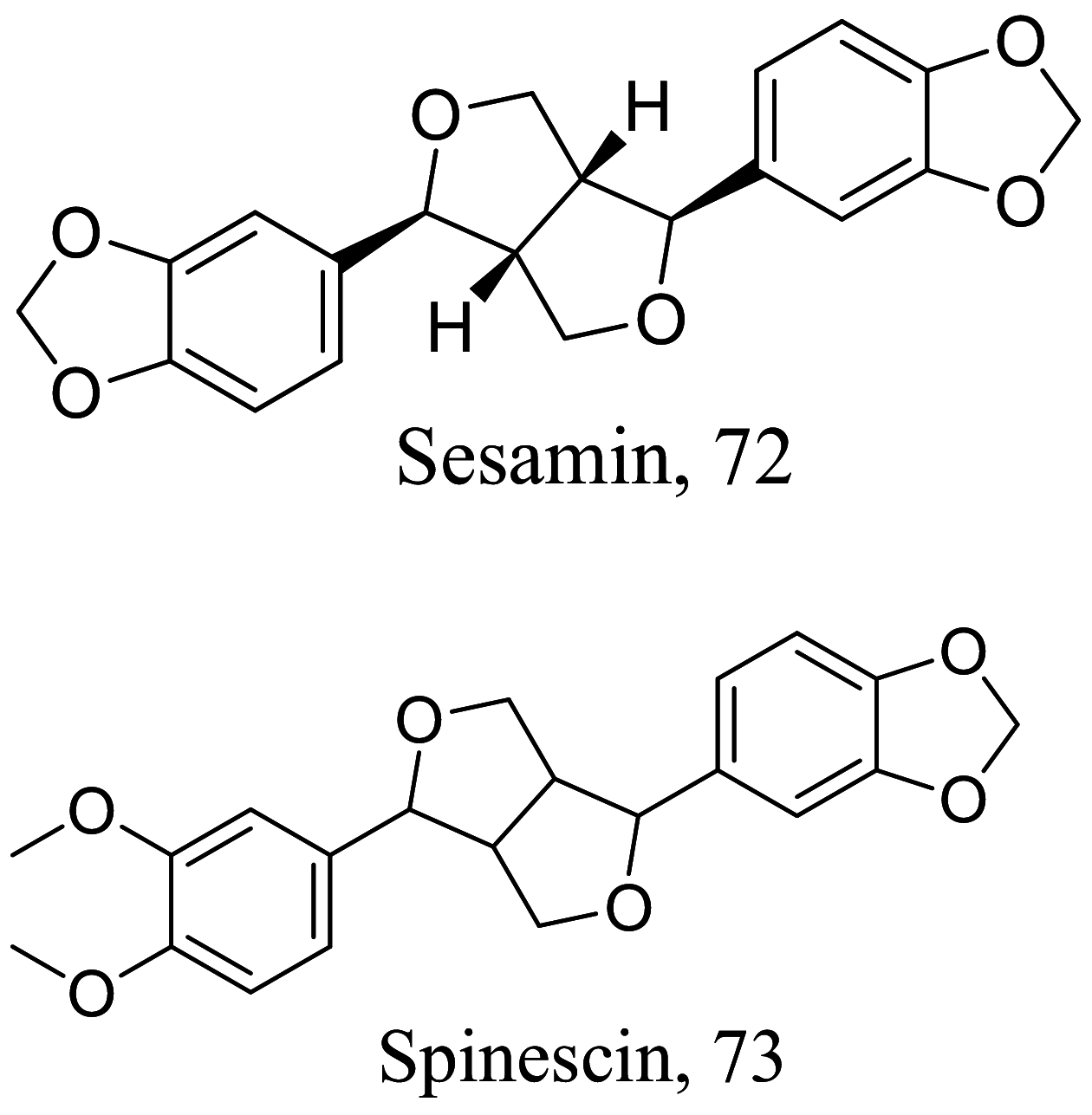 |
Lignan | 15.8 |
L. amazonensis (Promastigote) |
Nontoxic against BALB/c mouse macrophages up to 282 |
[36] |
| Lignan | 45.4 | 190 | ||||
| Zanthoxylum tingoassuiba | 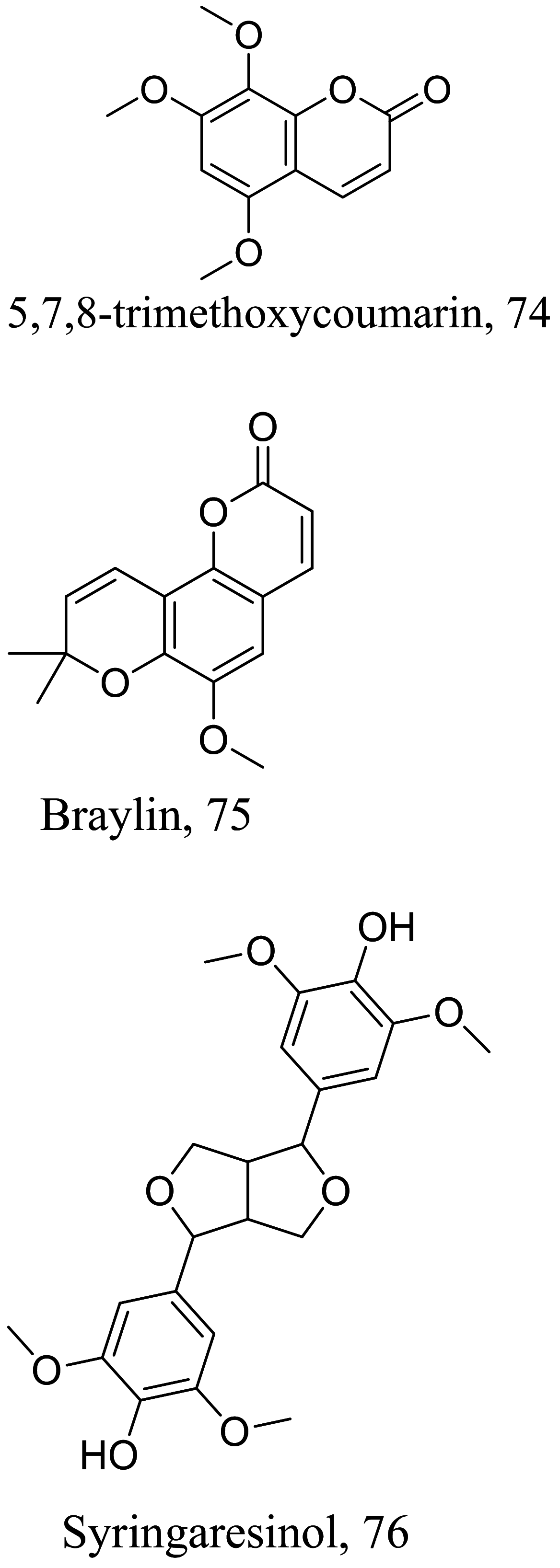 |
Coumarin | 57.7 |
L. amazonensis (Promastigote) |
N. T | [99] |
| Coumarin | 70.0 | |||||
| Lignan | 12.0 |
Terpenes and terpenoids
Another group of secondary metabolites with interesting anti-parasitic activities are terpenes. Ultrastructural changes of 79 in phenotypic screenings indicated mitochondrial blebs and lipid deformities [100, 101]. 80 isolated from essential oils of Tetradenia riparia were found to distort promastigote structure especially the fate of its chromatin followed by an apoptosis process which is suspected to be caused by caspase activation [102, 103] (Tables 3 and 4).
Table 3.
Various classes of terpenes and terpenoids with their IC50 exhibiting antileishmanial properties
| Natural product source | Chemical structure | Class of natural product | IC50/μg/mL | Organism tested | Toxicology | References |
|---|---|---|---|---|---|---|
| Parinari excelsa | 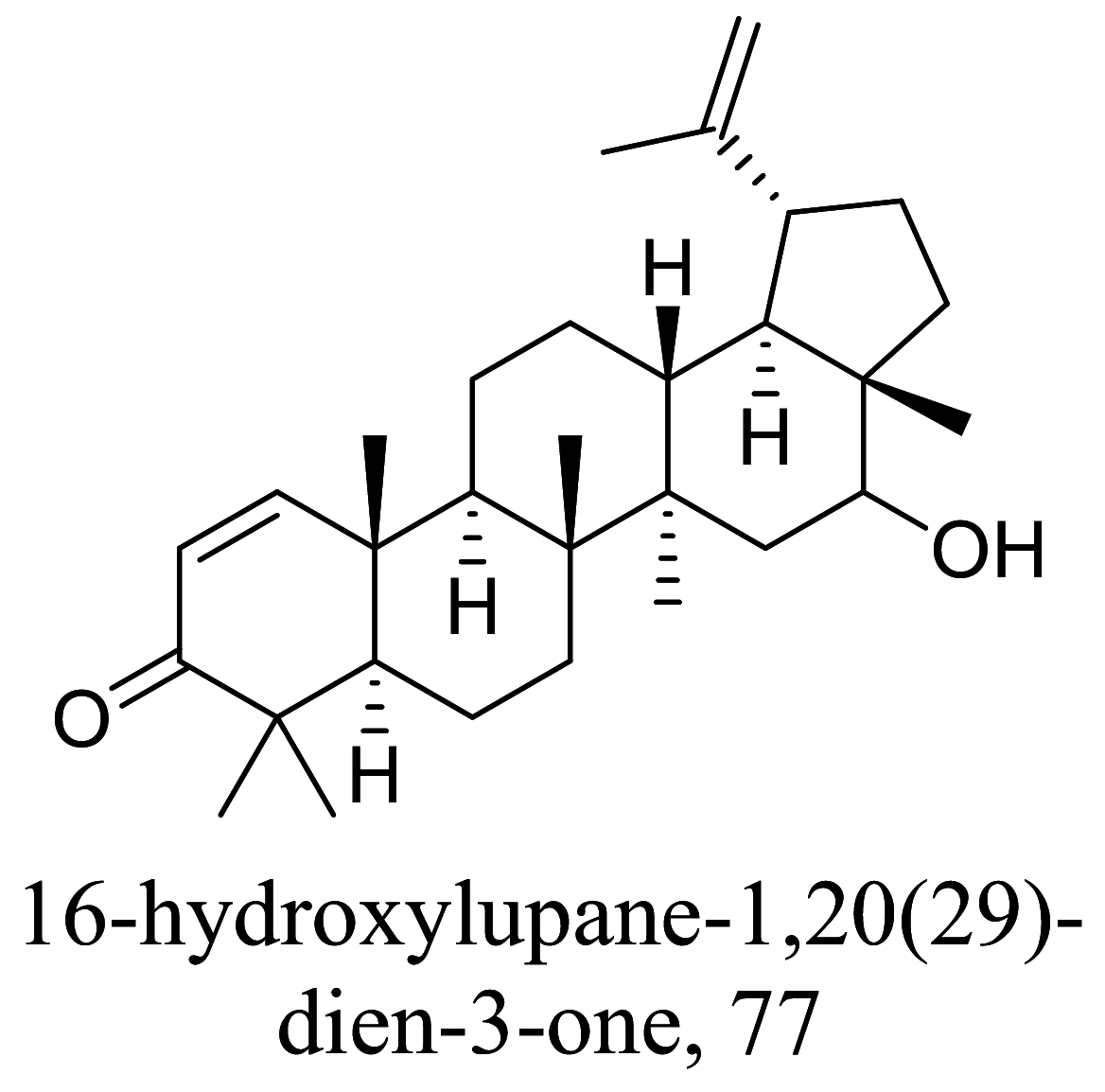 |
Triterpenoid | 0.05 |
L. donovani (amastigotes) |
Cell viability assay with L6 cell lines revealed the lethal concentration at 73.5 μg/mL | [120] |
| Morinda lucida |  |
Monoterpenoid | 1.17 |
L. donovani (promastigotes) |
[121] | |
|
Canistrocarpus cervicornis (Marine) |
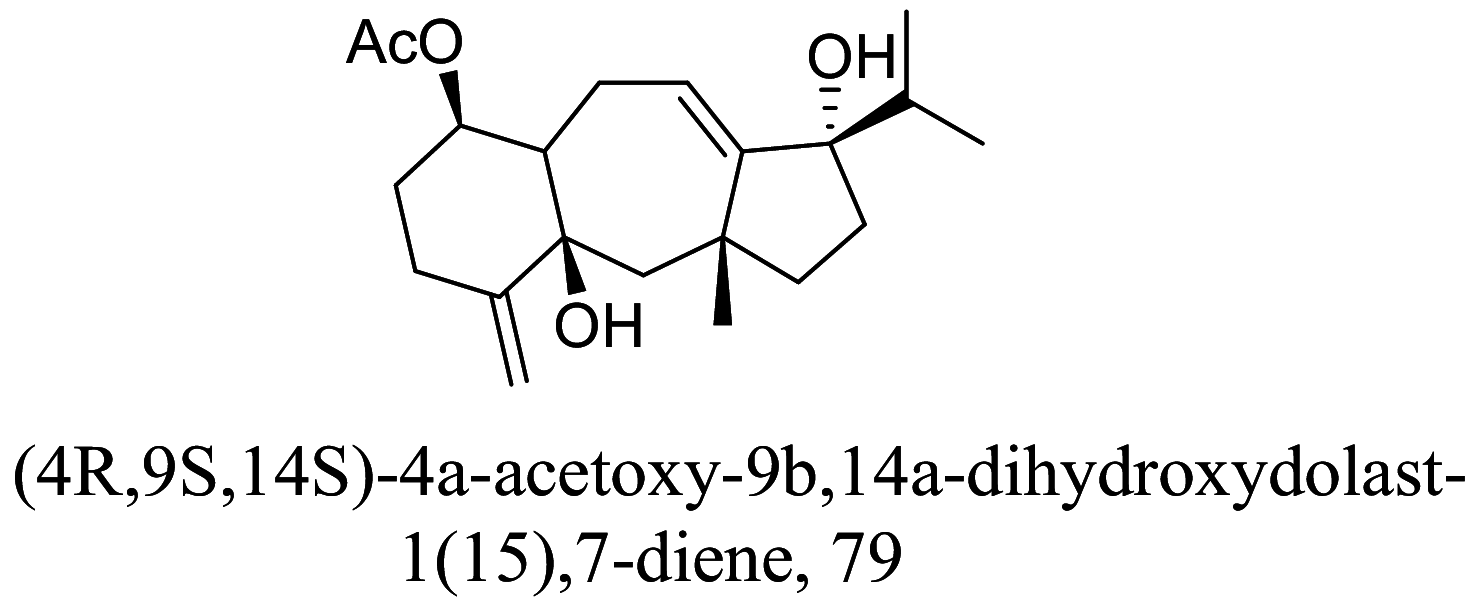 |
Diterpene | 4.00 |
L. amazonensis (Intra Amastigotes) |
Non-toxic up to 515 µM in human macrophage strains J774G8 | [100] |
| Tetradenia riparia | 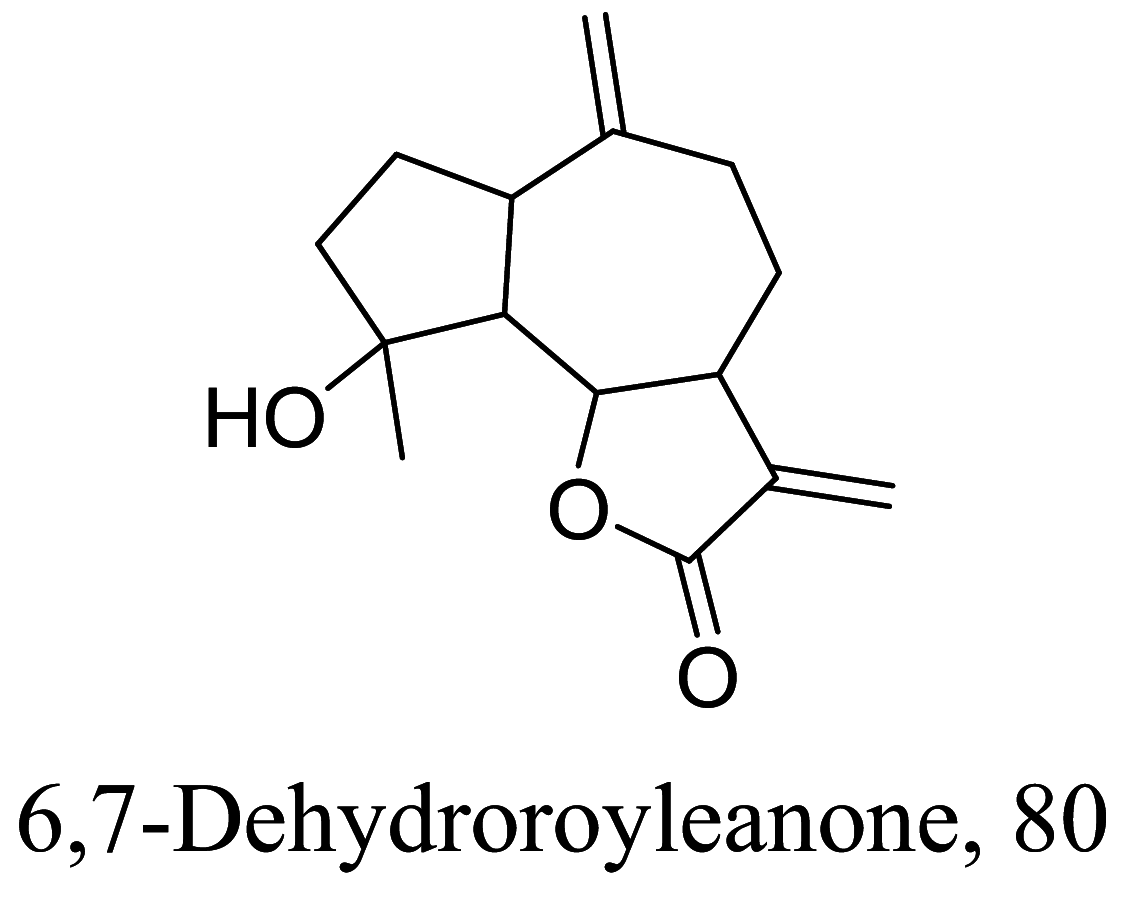 |
Sesquiterpene | 2.45 |
L. amazonensis (Promastigotes) |
high toxicity against mouse peritoneal macrophages = 1.69 µM | [122] |
|
Laurencia dendroidea (Marine) |
 |
Sesquiterpene | 10.8 |
L. amazonensis (Intra Amastigotes) |
CC50 in macrophages and lymph nodes in amastigotes cervical BALB/c mice 160.2 and 172.8 µM |
[123] |
| Sesquiterpene | 1.50 | 112.9 and 120.2 µM | ||||
| Sesquiterpen | 1.62 | 133.5 and 139.3 µM | ||||
| Combretum leprotum |  |
Triterpene | 3.30 |
L. amazonensis (Promastigotes) |
Non-toxic against mouse peritoneal macrophages | [124] |
| Triterpene | 3.48 | |||||
| Triterpene | 5.80 | |||||
| Vanillosmopsis arborea | 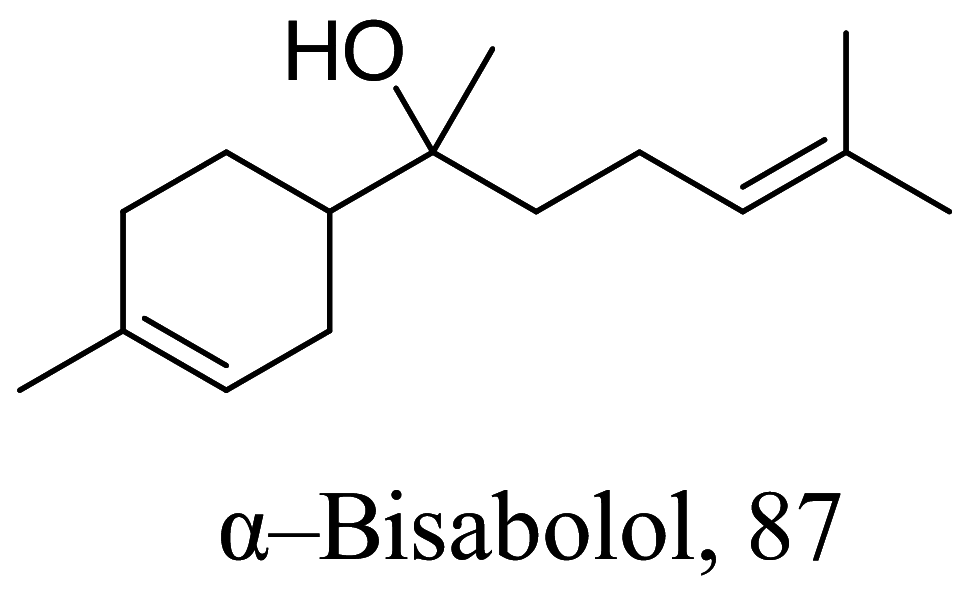 |
Sesquiterpene | 10.7 |
L. amazonensis (Amastigotes) |
Low cytotoxicity to macrophage J774.G8 cell lines 451 µM | [106] |
| Croton cajucara | 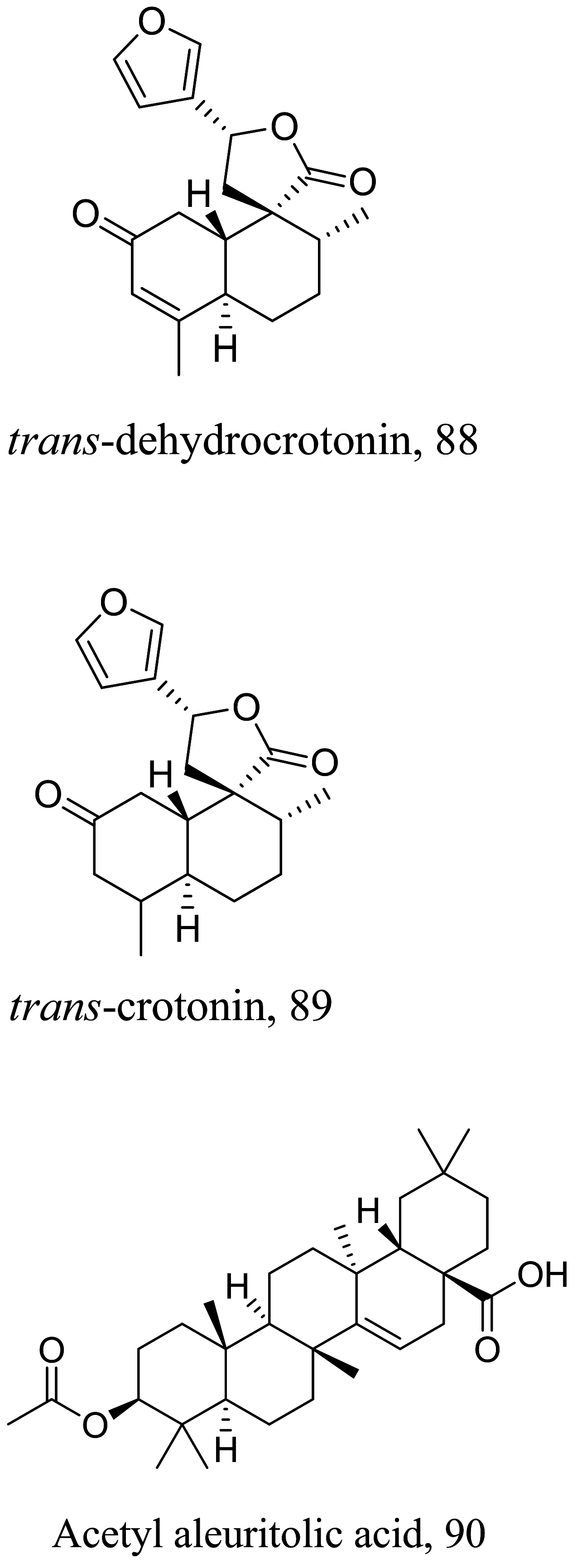 |
Diterpene | 20.0 |
L. amazonensis (Axenic Amastigotes) |
[108] | |
| Diterpene | 41.4 | |||||
| Triterpene | 58.3 | |||||
| Croton sylvaticus | 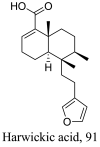 |
Diterpenoid |
10.0 10.0 |
L. major (Promastigotes) L. donovani (Promastigotes) |
Observed toxicity was low at 247.83 µM | [125] |
| Sterculia villosa | 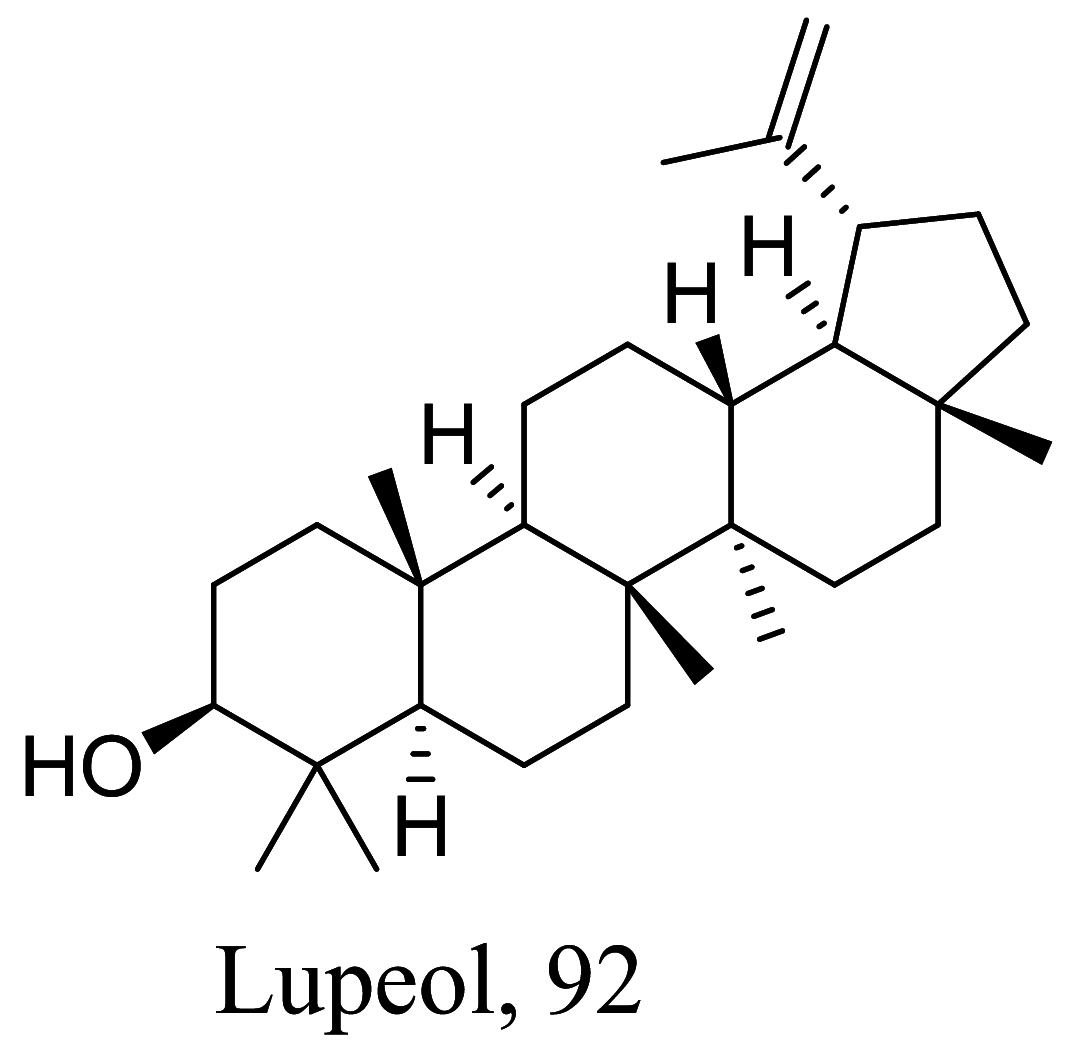 |
Triterpenoid | 15.0 |
L. donovani (Intracellular Amastigotes) |
N.T | [126] |
| Salvia deserta | 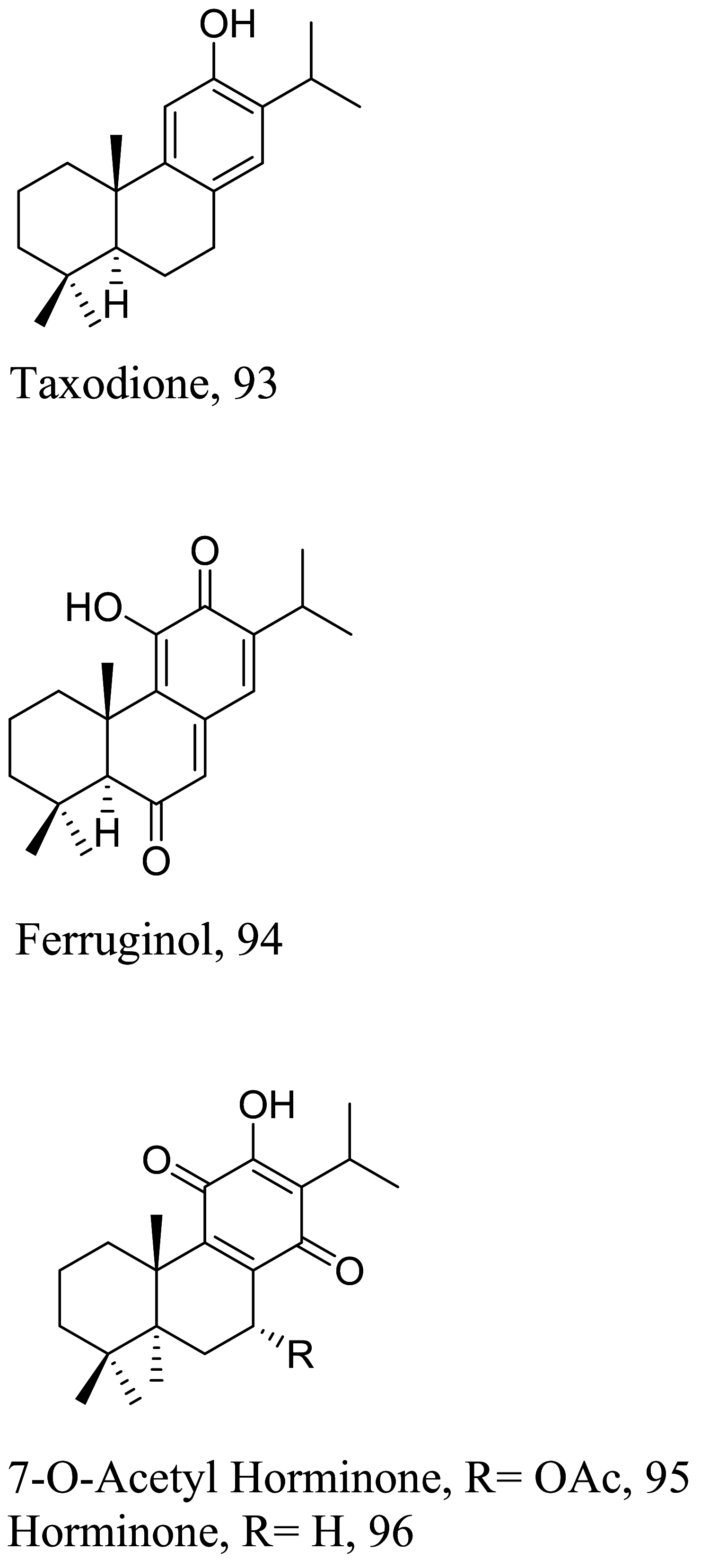 |
Diterpenoid | 0.46 | L. donovani | N. T | [35] |
| Diterpenoid | 3.30 | |||||
| Diterpenoid |
7.40 29.4 |
|||||
| Garcinia achachairu | 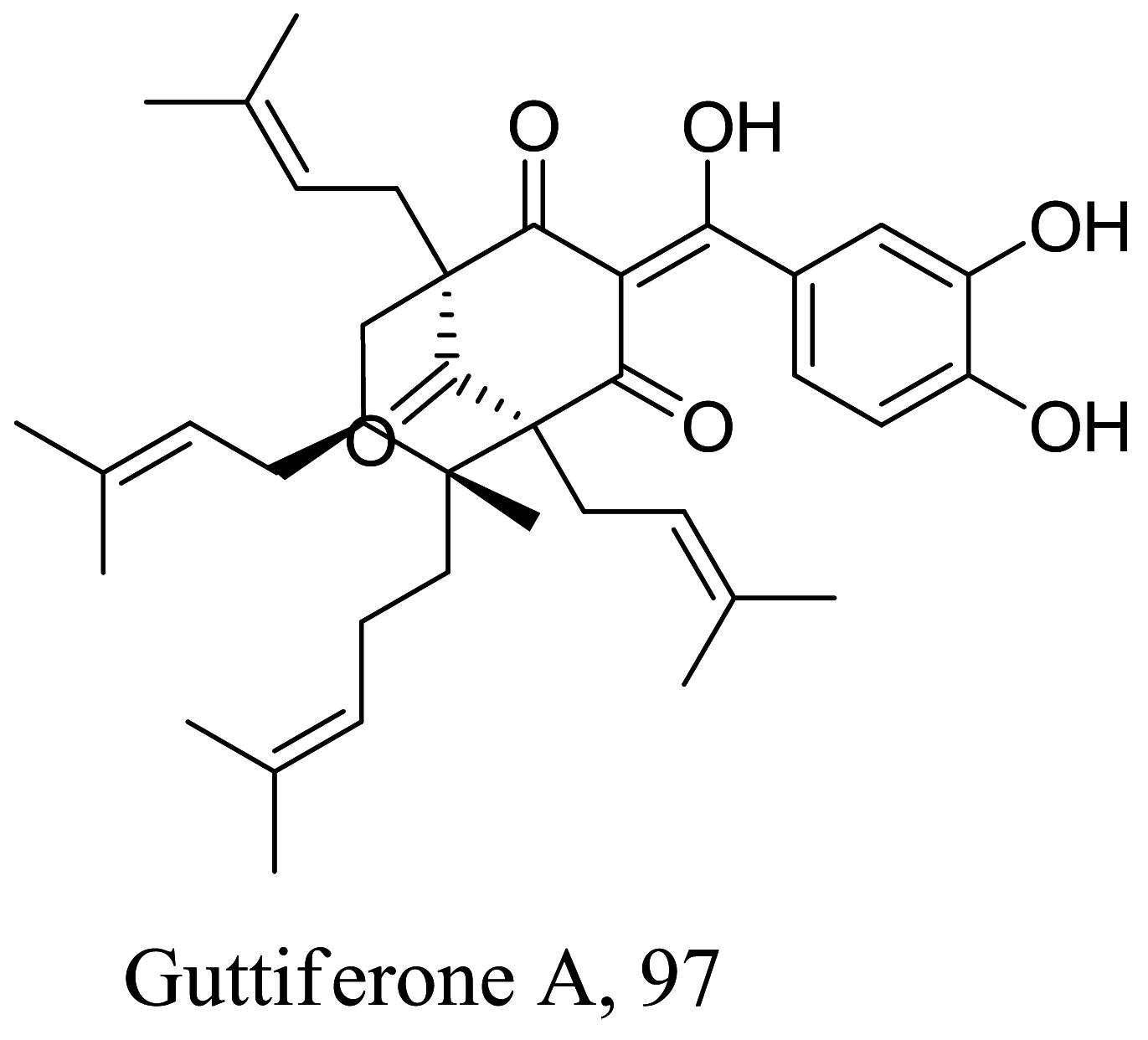 |
Monoterpenoid |
10.4 18.4 |
L. amazonensis L. brazilensis |
N. T | [127] |
| Rapanea ferruginea | 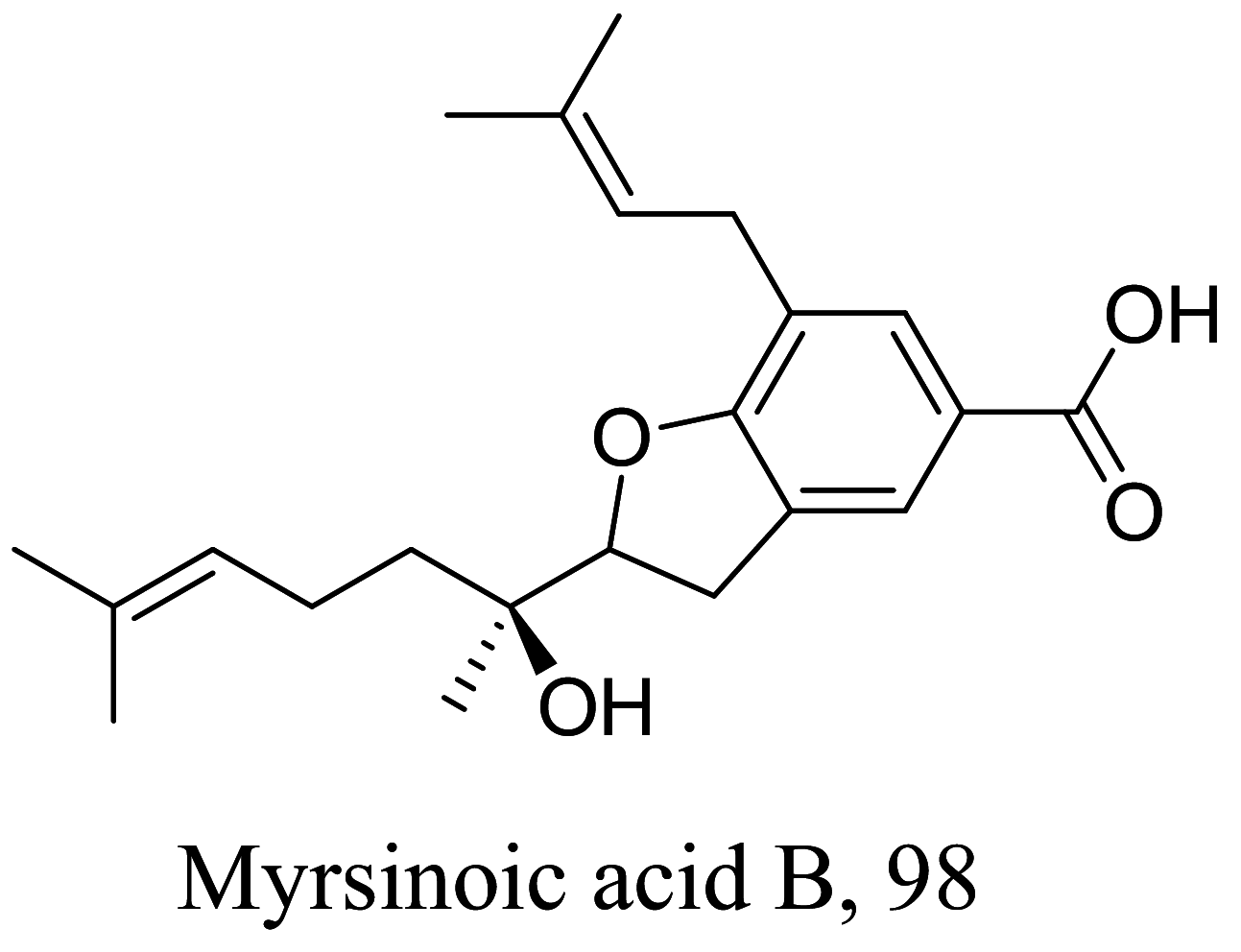 |
24.1 6.10 |
L. amazonensis L. brazilensis |
N. T | [127] | |
| Calea zacatechichi | 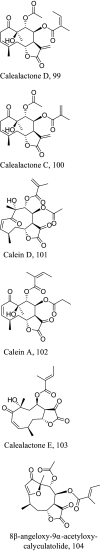 |
Sesquiterpene Lactone |
1.89 | L. donovani | [116] | |
|
Sesquiterpene Lactone |
0.771 | |||||
|
Sesquiterpene Lactone |
0.898 | |||||
|
Sesquiterpene Lactone |
1.74 | |||||
|
Sesquiterpene Lactone |
3.09 | |||||
|
Sesquiterpene Lactone |
1.60 | |||||
| Tanacetum parthenium |  |
Sesquiterpene Lactone |
2.60 |
L. amazonensis (promastigotes) |
Low toxicity towards J774G8 cells | [128] |
| Plumeria bicolor | 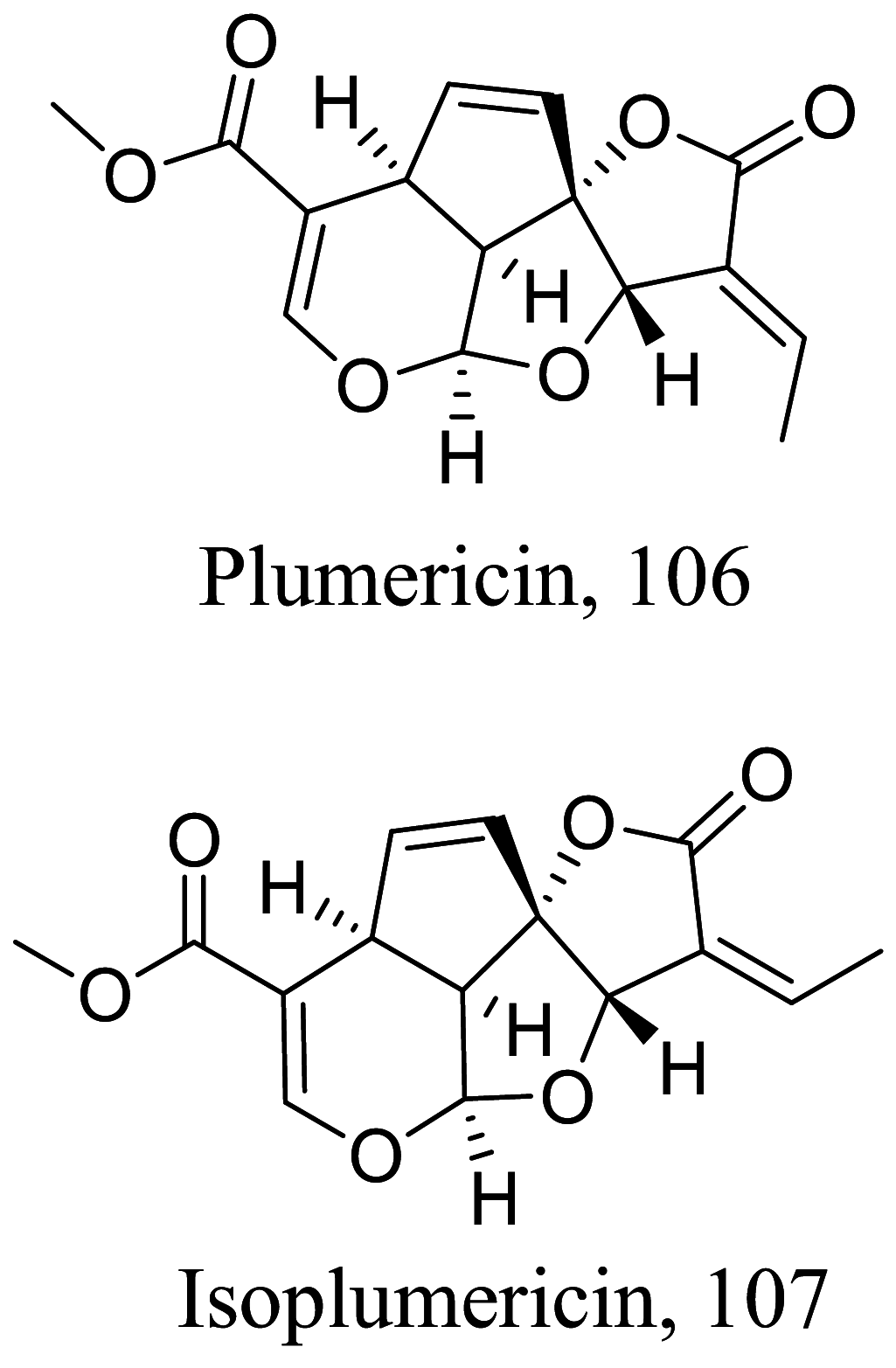 |
Monoterpene lactone |
0.409 |
L. donovani (Amastigotes) |
CC50 = 20.6 µM | [129] |
|
Monoterpene Lactone |
1.19 | CC50 = 24 μM | ||||
| Pseudelephantopus spicatus | 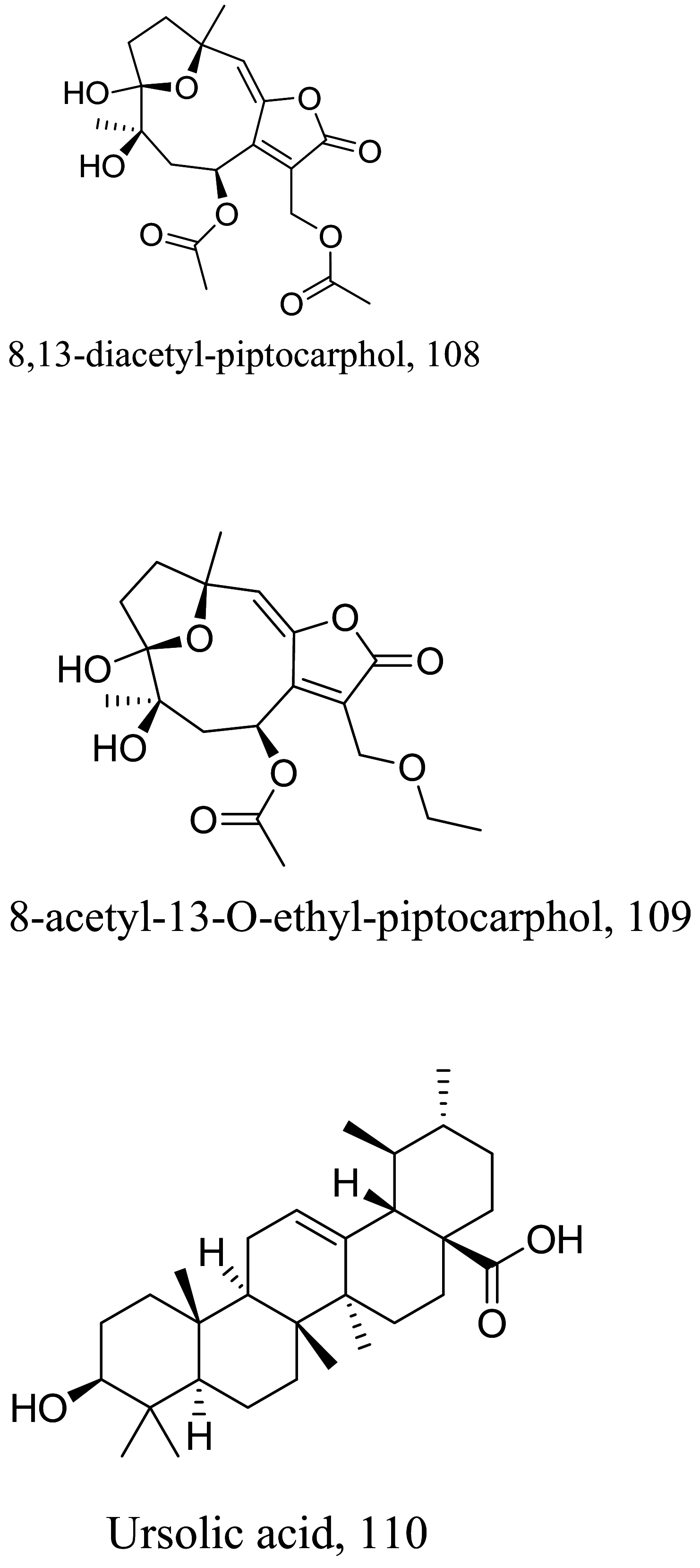 |
Sesquiterpene lactone |
0.0794 | L. amazonensis |
High selectivity towards parasites as compared to mammalian cells with >100, > 100 µM and > 100 µM µM against Hela, L929 and B16F10 cell lines |
[130] |
|
Sesquiterpene lactone |
0.142 | 58.5 µM, > 100 µM and > 100 µM against Hela, L929 and B16F10 cell lines | ||||
| Triterpenoid | 0.451 | Toxic towards RAW264.7, HONE-1, KB and HT 29 cell lines with 15.6 µM, 8.8 µM,8.2 µM and 4.7 µM respectively | ||||
| Calea pinnatifida | 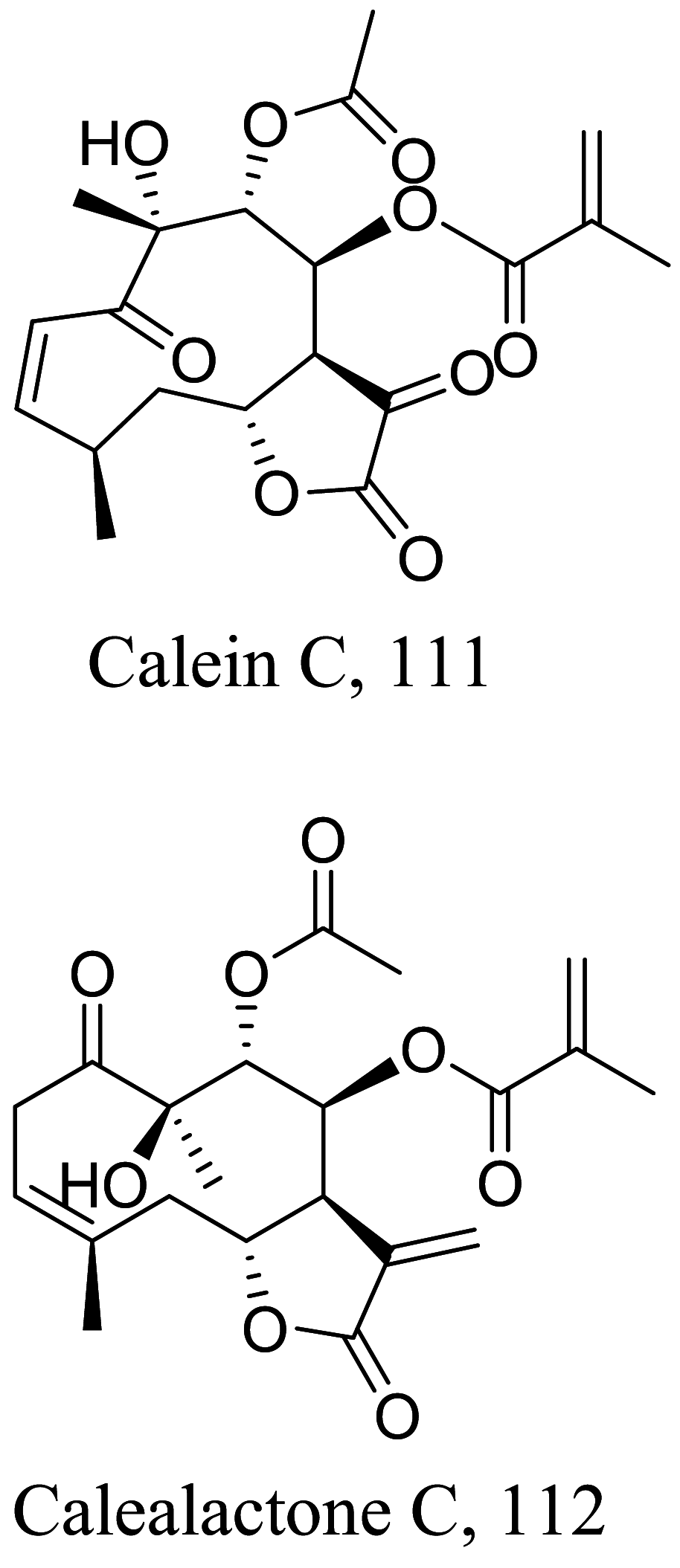 |
Sesquiterpene Lactone |
1.73 |
L. amazonensis (Promastigotes) |
At 4.11 µM, toxic to J774 macrophages | [115] |
|
Sesquiterpene lactone |
4.24 |
L. amazonensis (Amastigotes) |
75.5 µM | |||
|
Spongia sp. and Ircinia sp. (Marine) |
 |
Diterpene | 0.75 | L. donovani |
Toxicity profile against mammalian L6 cells was 9.64 |
[68] |
| Sesterterpene | 5.60 | 127 | ||||
| Sesterterpene | 4.80 | 83.1 | ||||
| Sesterterpene | 10.2 | > 217 | ||||
| Triterpene | 15.9 | >146 | ||||
| Sesterterpene | 14.2 | >254 | ||||
| Tetraterpene | 18.9 | 4.36 | ||||
| Baccharis tola | 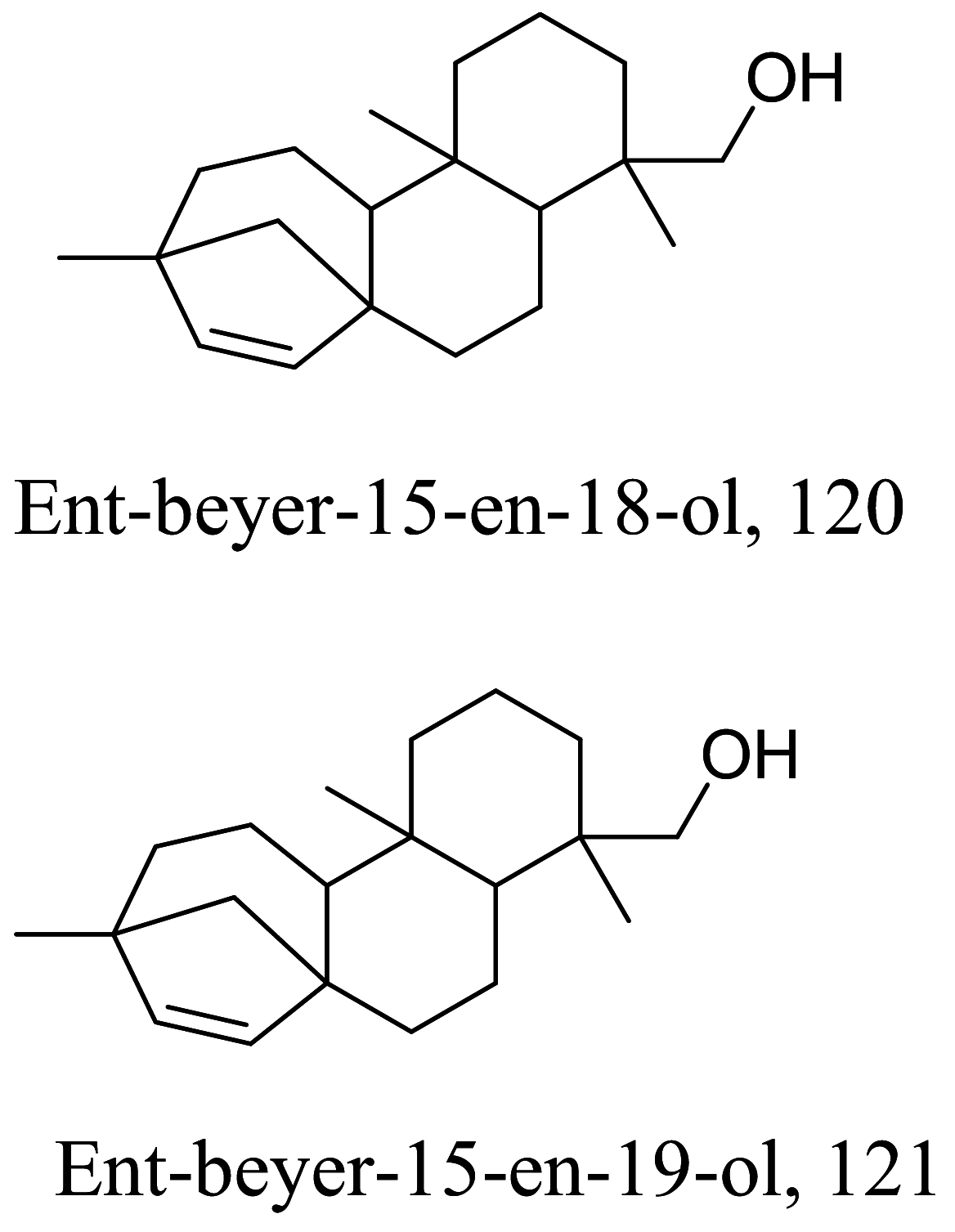 |
Diterpenoid | 4.60 | L. brazilensis | All compounds showed high cytotoxicity in human U937 macro phages with values lower than 347 μM | [131] |
| Diterpenoids | 5.30 | |||||
| Jatropha muitifida | 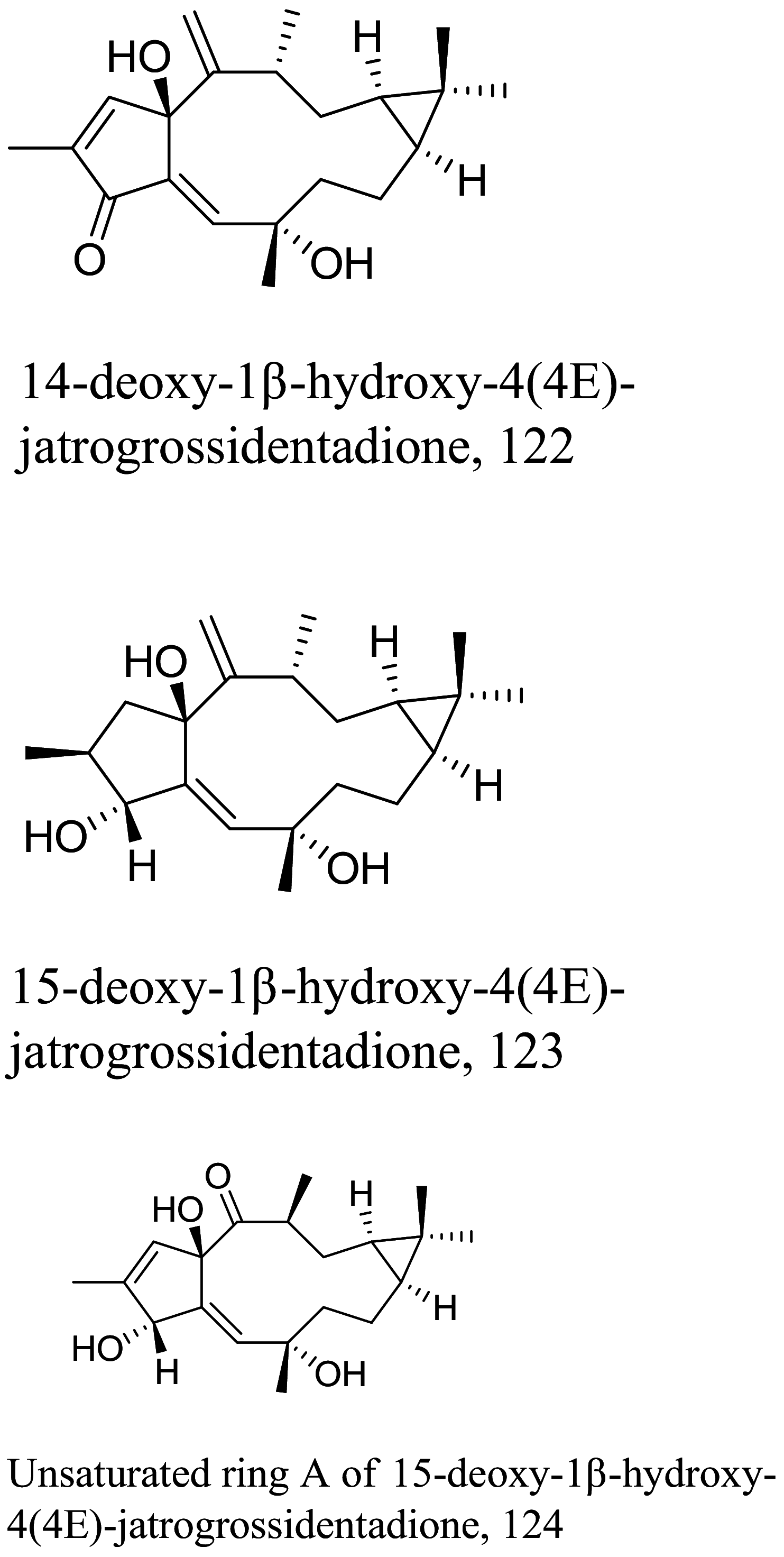 |
Diterpenoid | 11.9 | L. donovani | Low toxicity profile against VERO cells | [132] |
| Diterpenoid | 4.69 | |||||
| Diterpenoid | 4.56 | |||||
| Psidium Guajava | 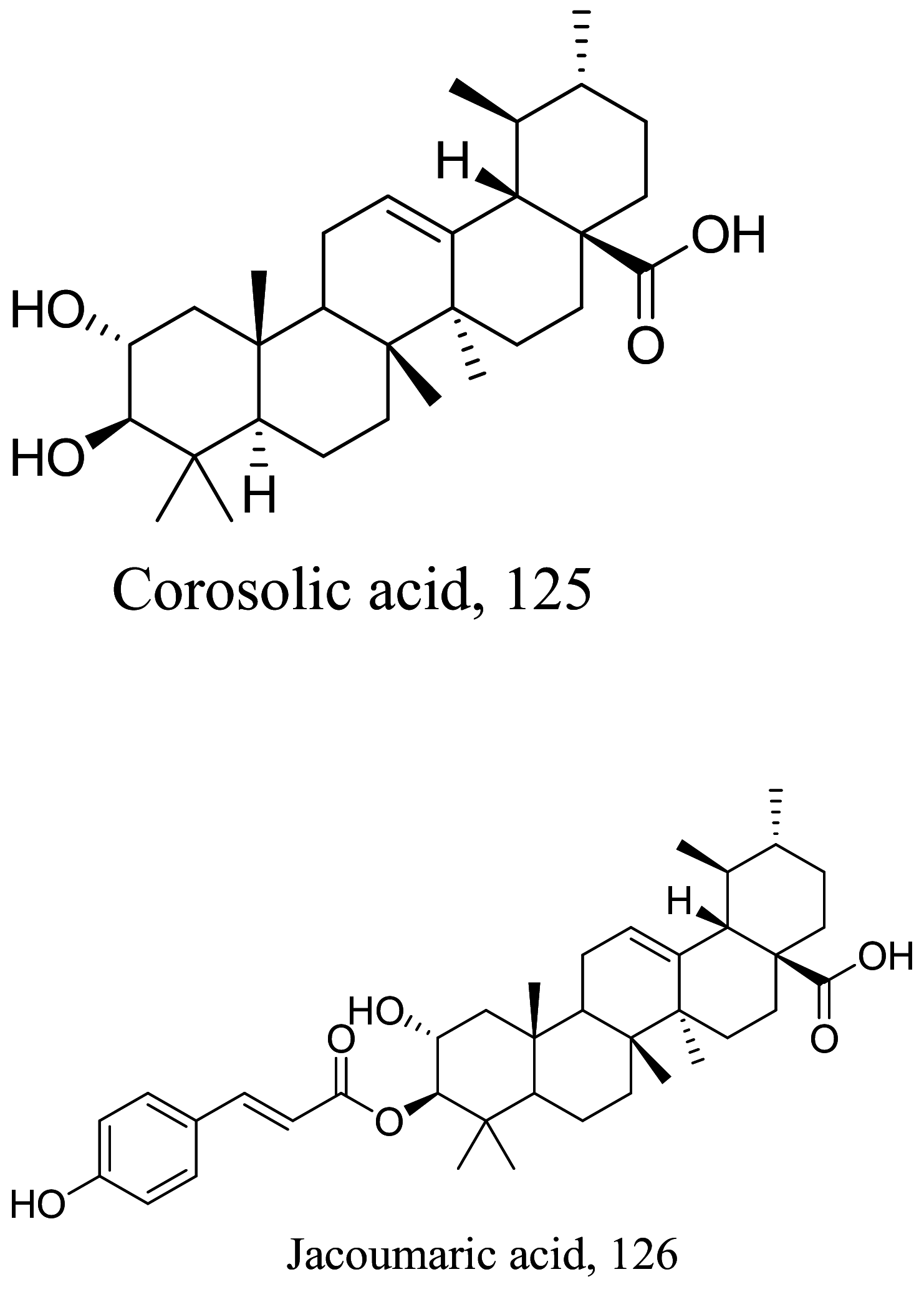 |
Triterpene | 1.01 |
L. infantum (Axenic Amastigotes) |
At conc. = 12.2 µM in mouse macrophage cell lines J774A.1 | [133] |
| Triterpene | 1.32 | At conc. = 20.8 µM against same cell lines | ||||
|
Cystoseira baccata (Marine) |
 |
Diterpenoids | 20.4 |
L. infantum (promastigotes) |
Non-toxic up to 126.6 |
[134] |
| Diterpenoids | 44.5 | 84.5 | ||||
| Pseudelephantopus spiralis | 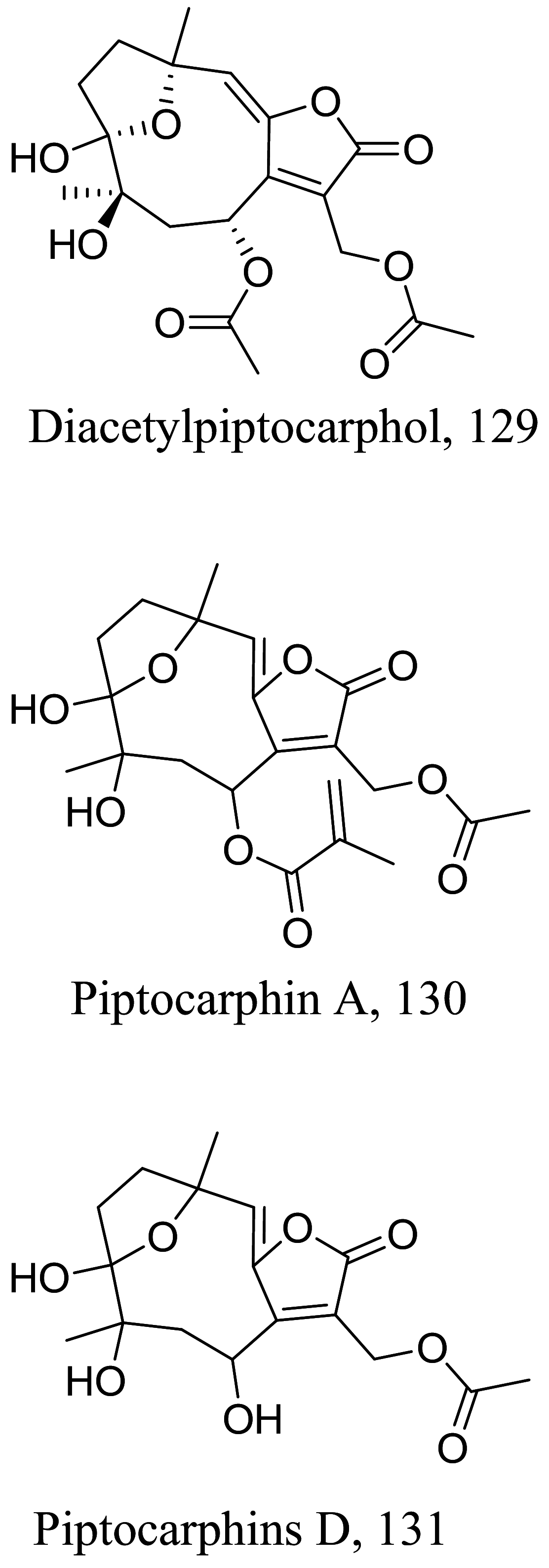 |
Sesquiterpene lactone |
0.06 0.012 |
L. infantum (promastigotes) L. infantum (amastigotes) |
1.47 ± 0.08 | [135] |
|
Sesquiterpene lactone |
0.02 0.005 |
L. infantum (promastigotes) |
0.97 ± 0.07 | |||
|
Sesquiterpene lactone |
0.244 0.048 |
L. infantum (promastigotes) L. infantum (amastigotes) |
5.57 ± 1.9 | |||
| Nectria pseudotrichia | 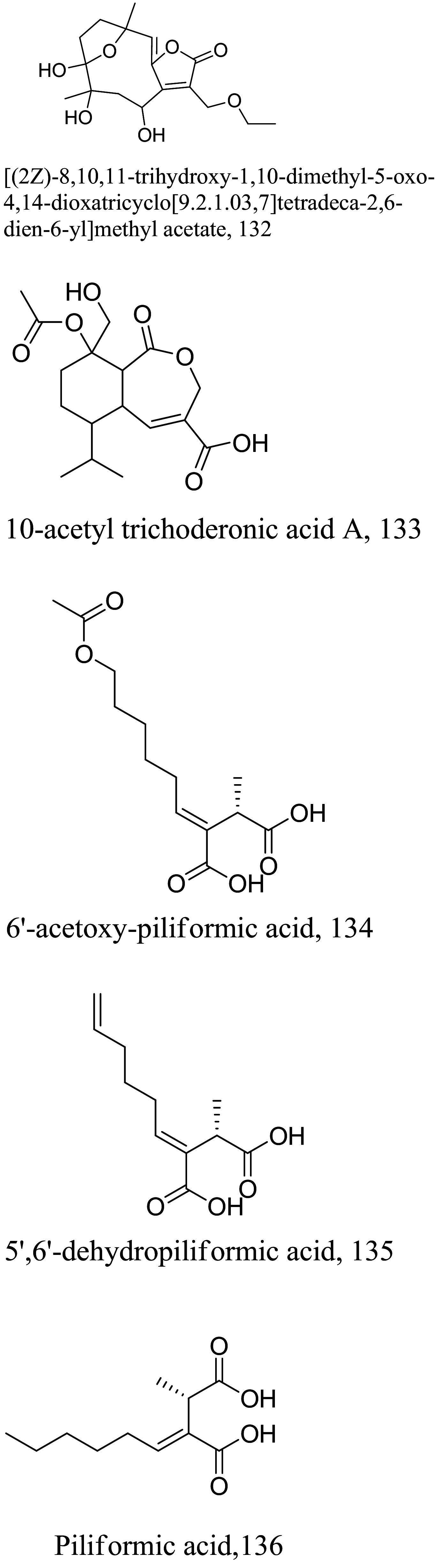 |
Sesquiterpene Lactone |
0.092 0.023 |
L. infantum (promastigotes) L. infantum (amastigotes) |
3.17 ± 1.0 Highly selective to parasites compared to VERO cells and THP-1 (a human leukaemia monocytic cell line). All > 200 µM. |
[136] |
| Sesquiterpenoid | 0.063 |
L. braziliensis (amastigotes) |
||||
| Monoterpene | 0.104 | |||||
| Monoterpene | 0.117 | |||||
| Monoterpene | 0.37 | |||||
| Croton echioides | 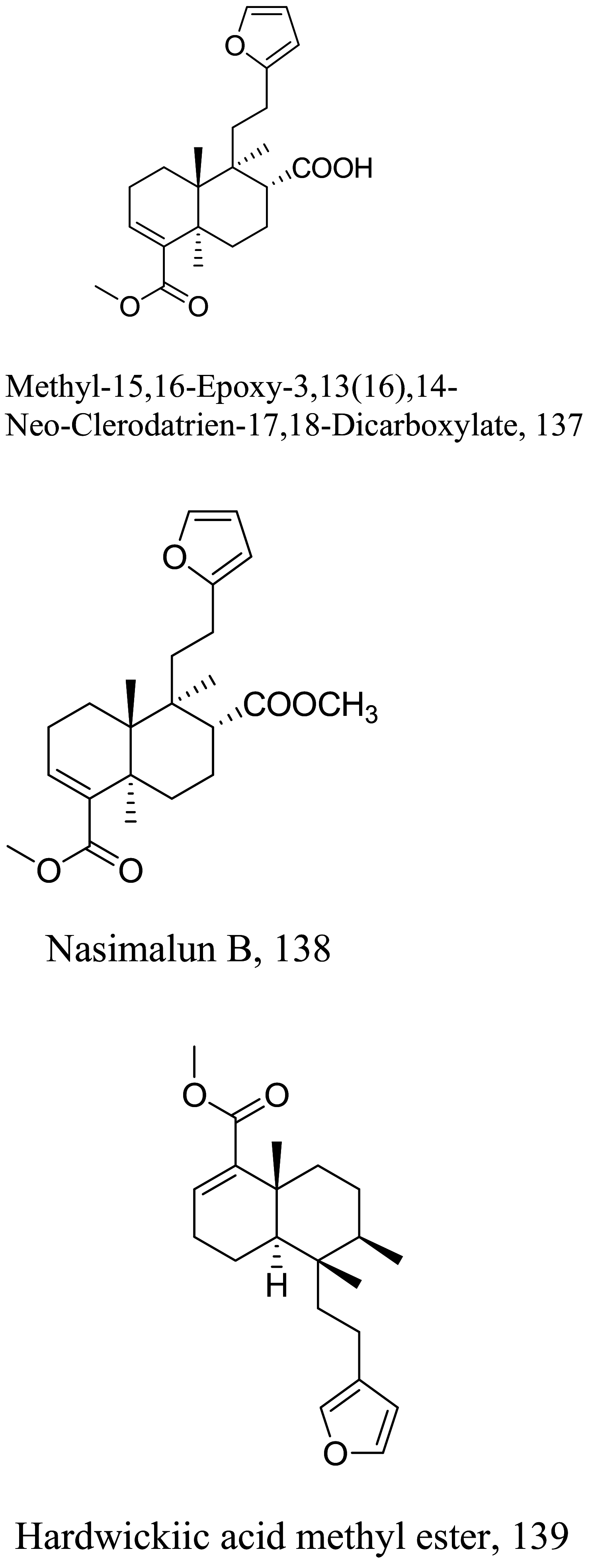 |
Diterpenoid | 0.11 |
L.amansonensis (promastigotes) |
N.T | [137] |
| Diterpenoid | 0.027 | |||||
| Diterpenoid | 0.025 | |||||
| Taxodium distichum | 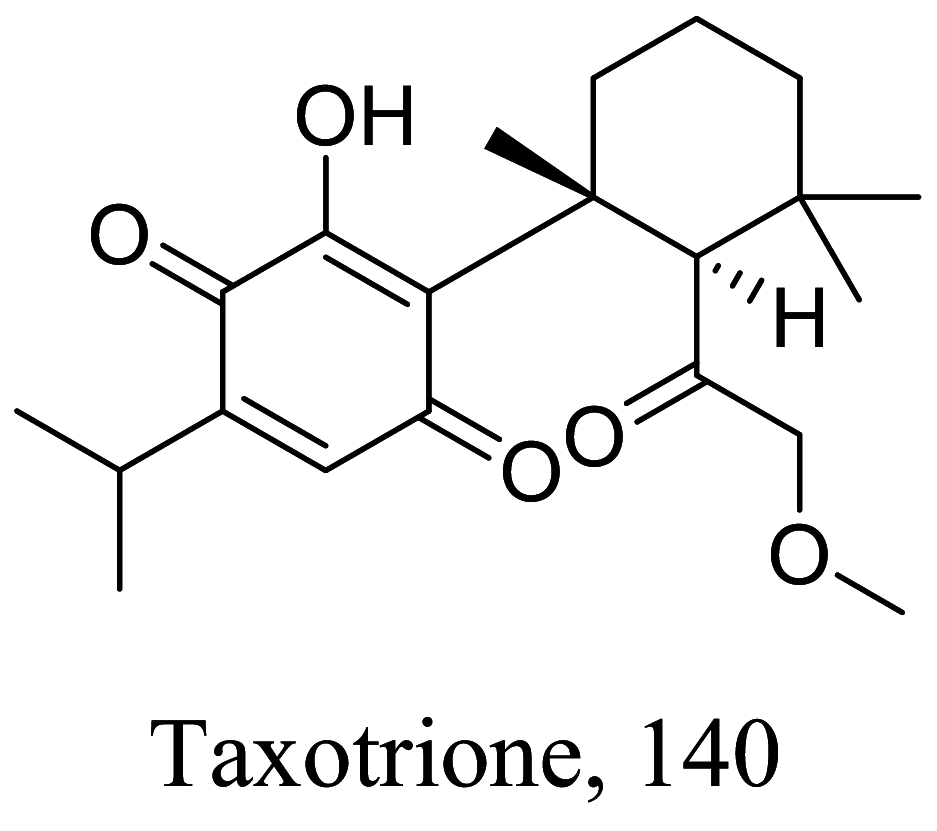 |
Diterpenoid |
2.5 0.52 |
L. donovani (promastigotes) L. amazonensis |
High toxicity against HT-29 colorectal carcinoma cells | [138] |
| Lippia sidoides | 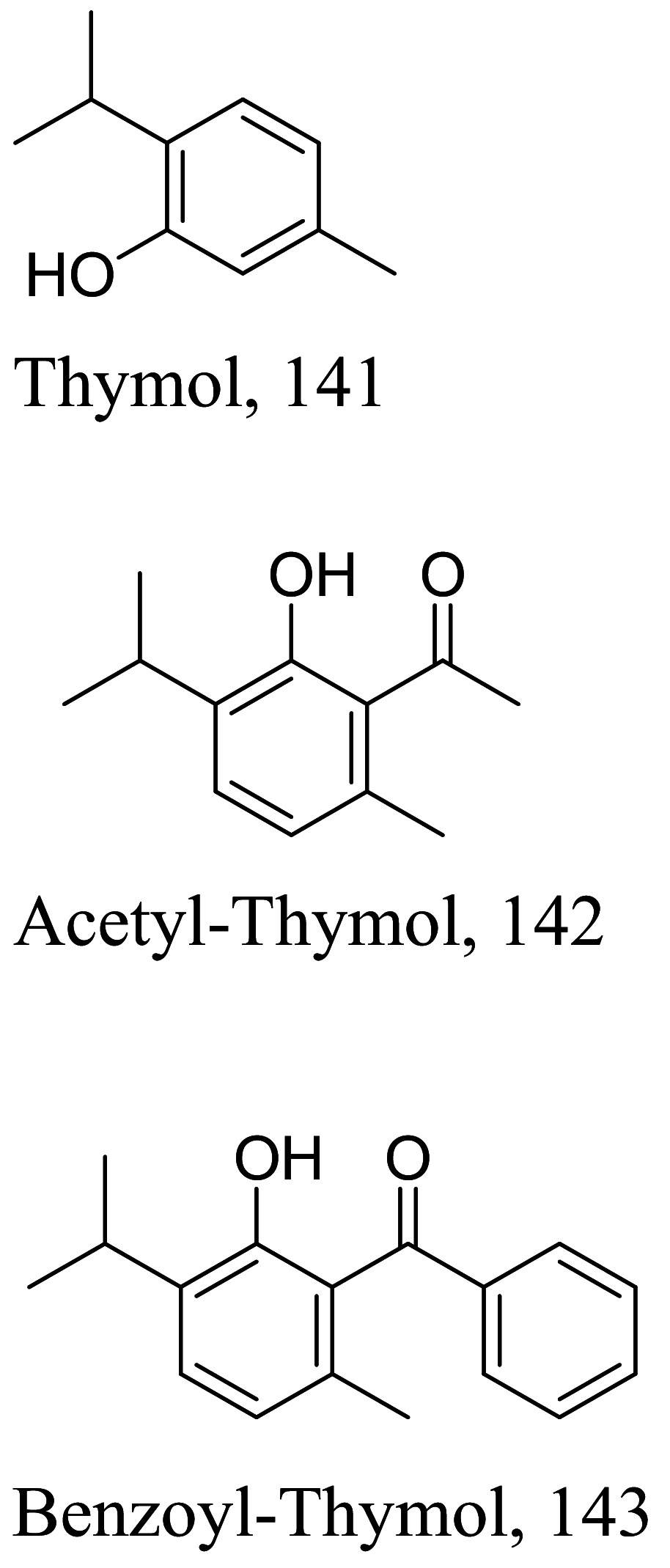 |
Monoterpene | 23.9 |
L. amazonensis (Promastigotes) |
36.5 µM | [139] |
| Monoterpene | 11.0 | >100 µM | ||||
| Monoterpene | 15.1 | 63.6 µM | ||||
| Trixis antimenorrhoea |  |
Sesquiterpene |
0.3 0.96 |
L. amazonensis (promastigote) L. brazilensis (promastigote) |
N. T | [97] |
|
Bifurcaria bifurc-ata (Marine) |
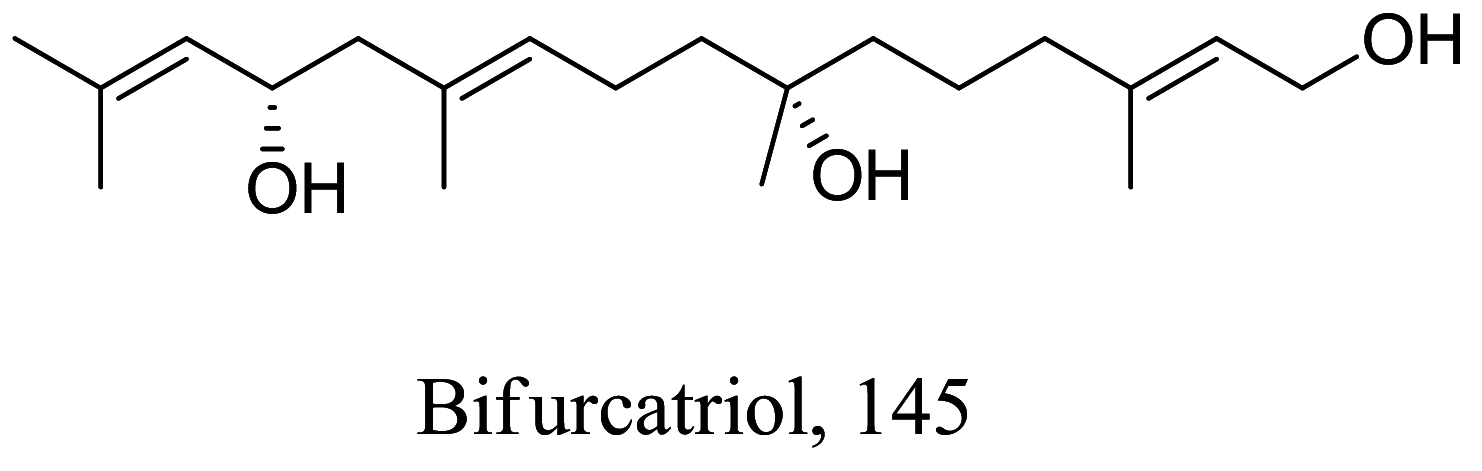 |
Diterpene | 18.8 | L.donovani | Toxicity potential against L6 primary myoblast cell was observed at 56.6 µM | [140] |
|
Dictyota spiralis (Marine) |
 |
Diterpene | 15.47 |
L. amazonensis (promastigote) |
23.4 | [141] |
| Diterpene | 36.81 |
L. amazonensis (promastigote) |
69 | |||
|
Stypopodium zonale (Marine) |
 |
Diterpene | 9 |
L. amazonensis (amastigotes) |
8.4 μM | [142] |
|
Plumarella delicatissima (Marine) |
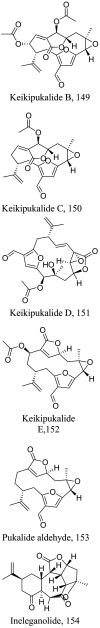 |
Diterpene | 0.025 |
L. donovani (amastigotes) |
Cytotoxicity potential against human lung carcinoma, cells exhibited low toxic potentials which were >50 |
|
| Diterpene | 0.026 | >50 | ||||
| Diterpene | 0.034 | >50 | ||||
| Diterpene | 0.022 | >50 | ||||
| Diterpene | 1.9 | >50 | ||||
| Diterpene | 4.4 | >50 | ||||
|
Laurencia viridis (Marine) |
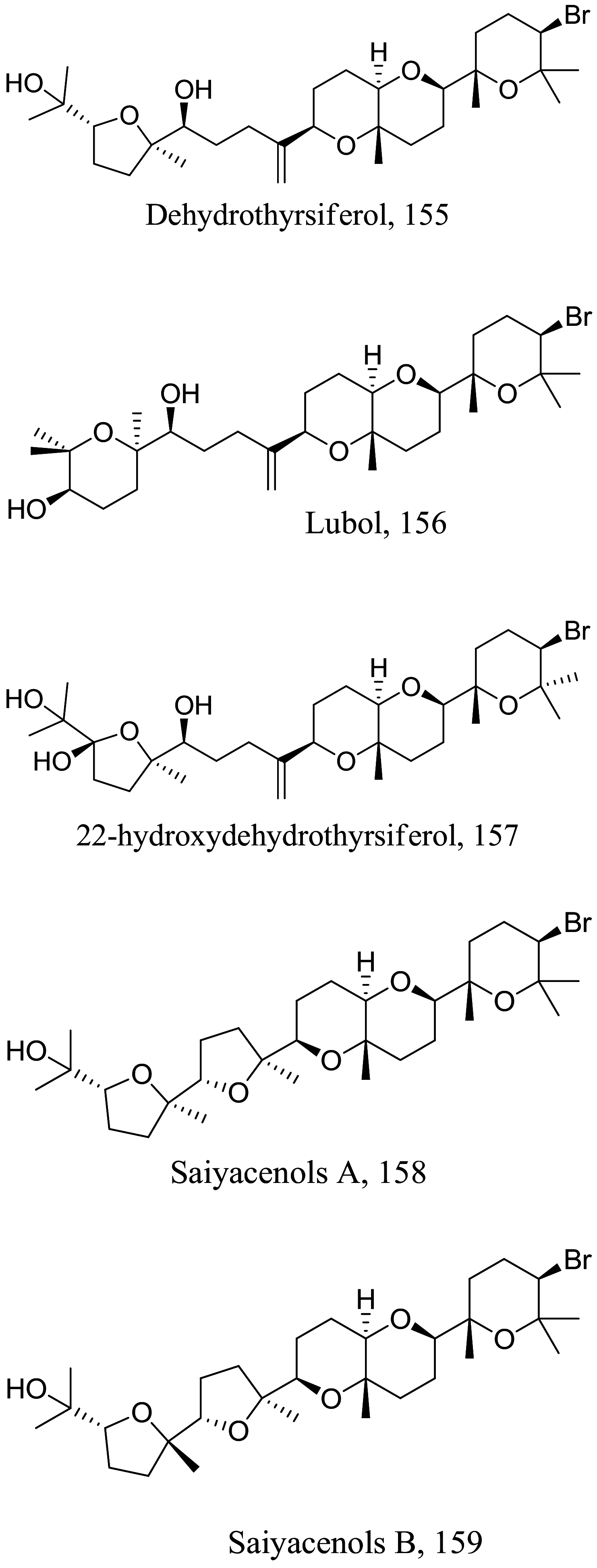 |
Diterpene |
8.36 28.26 |
L. amazonensis (Promastigote) L. donovani (promastigotes) |
0.22 | [143] |
| Diterpene |
7.00 18 |
L. amazonensis (Promastigote) L. donovani (promastigotes) |
4.6 | |||
| Diterpene | 34.65 | 0.6 | ||||
| Diterpene | 12.96 | 1.4 | ||||
| Diterpene | 10.32 | >100 | ||||
|
Dysidea avara (Marine) |
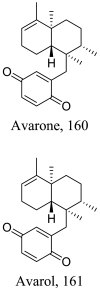 |
Sesquiterpene |
28.21 20.28 7.64 |
L. infantum (Promastigotes) L. tropica (Promastigotes) L. infantum (Amastigote) |
Low toxicity against human microvascular endothelial cells and (human acute monocytic leukemia cells with CC50 62.19 and > 100 respectively. | [144] |
| Sesquiterpene |
7.42 7.08 3.19 |
L. infantum (Promastigotes) L. tropica (Promastigotes) L. infantum (Amastigote) |
36.8 31.75 |
Table 4.
Various classes of steroids, fatty alcohol, lignan, and butanolide with their IC50 exhibiting antileishmanial properties
| Natural product source | Chemical structure | IC50 | Organism tested | Toxicity | References | |
|---|---|---|---|---|---|---|
| Sassafras albidum | 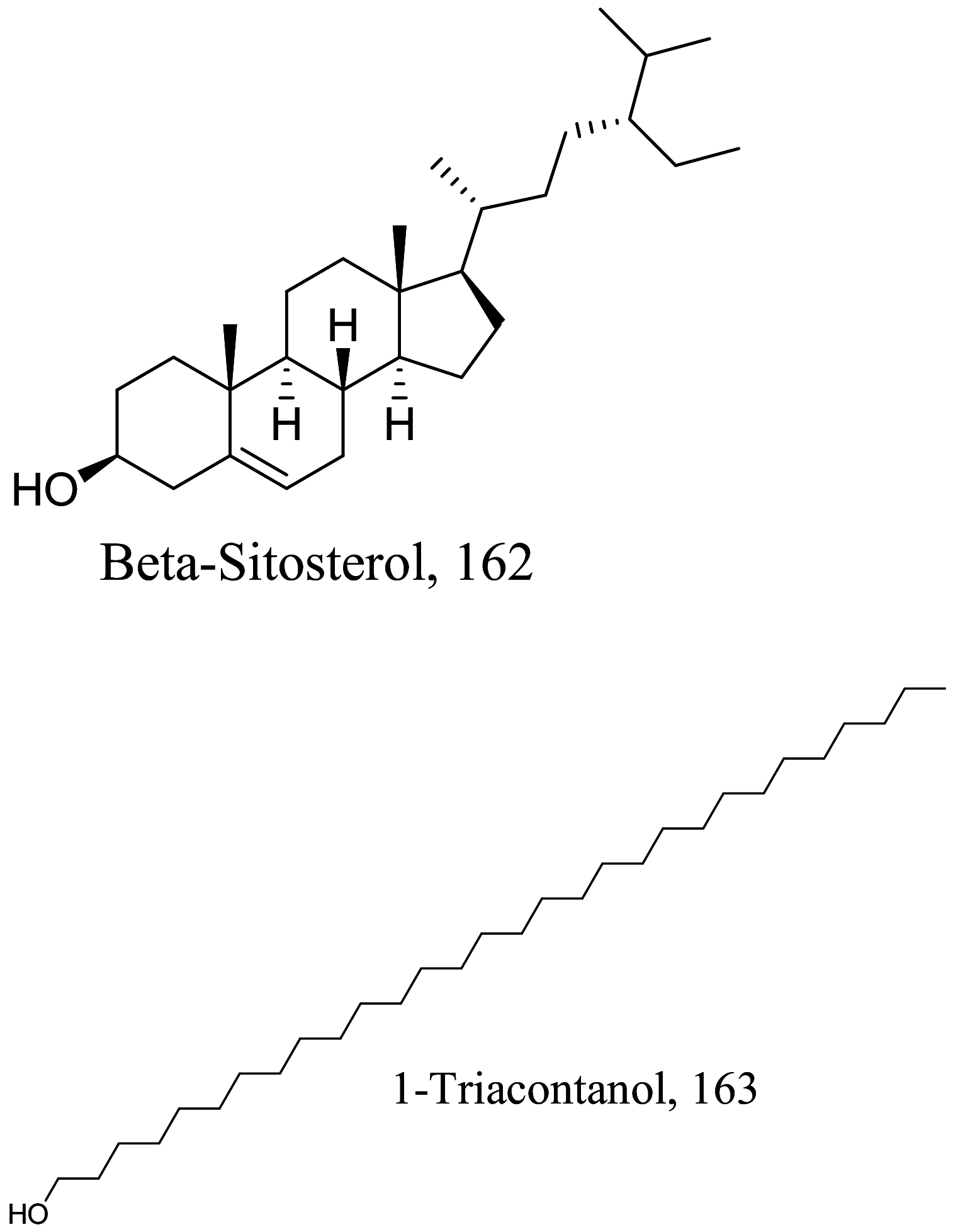 |
Steroid | 54.3 |
L. amazonensis (Promastigote) |
Nontoxic against BALB/c mouse macrophages up to 182 |
[36] |
| Fatty alcohol | 19.9 | 157 | ||||
| Trametes versicolor | 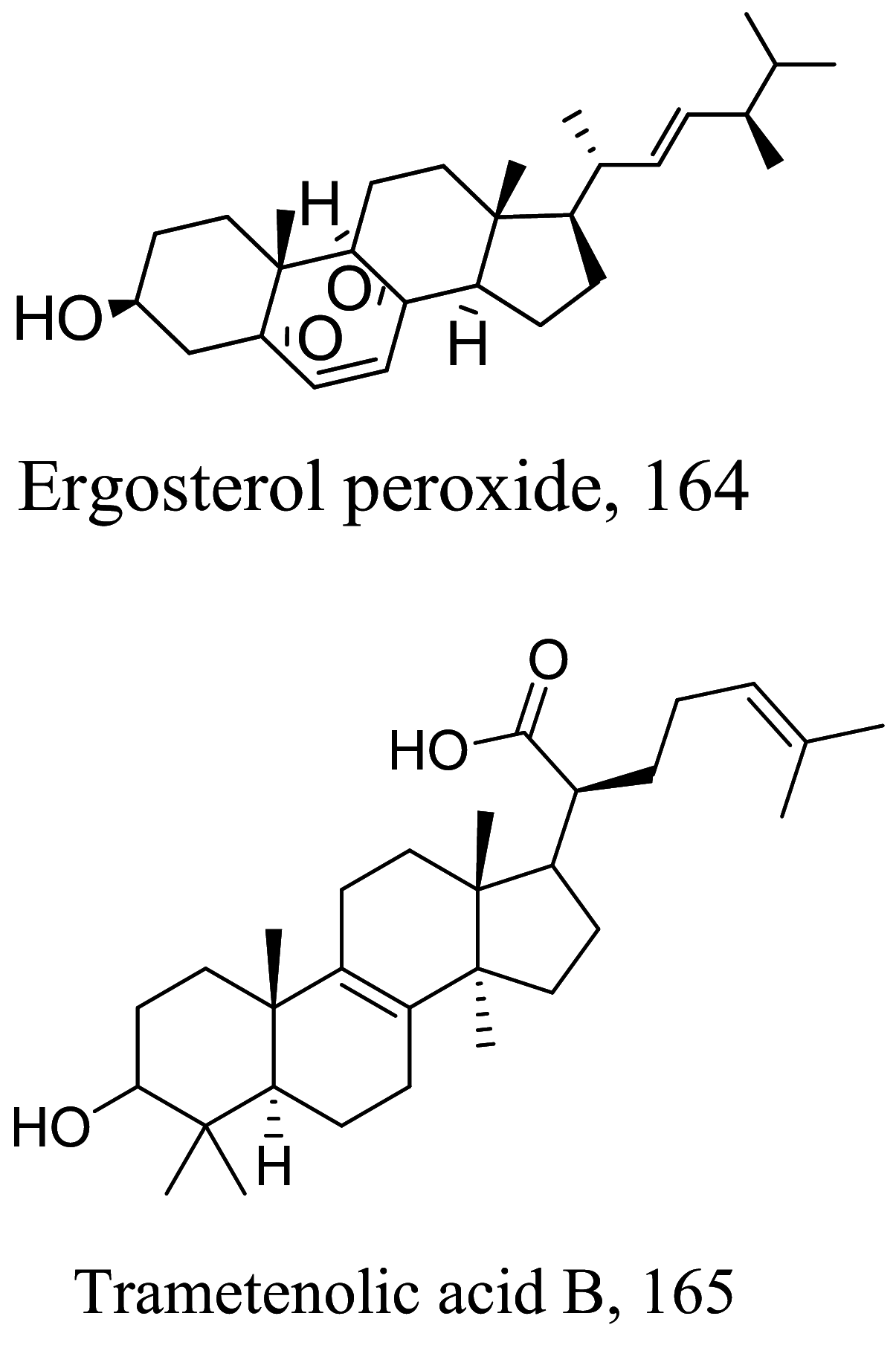 |
Steroid | 1.70 |
L. amazonensis (Amastigote) |
Toxicity profile against peritoneal macrophages 42.9 μM |
[152] |
| Steroid | 0.07 | 39.4 μM | ||||
| Aspergillus terreus | 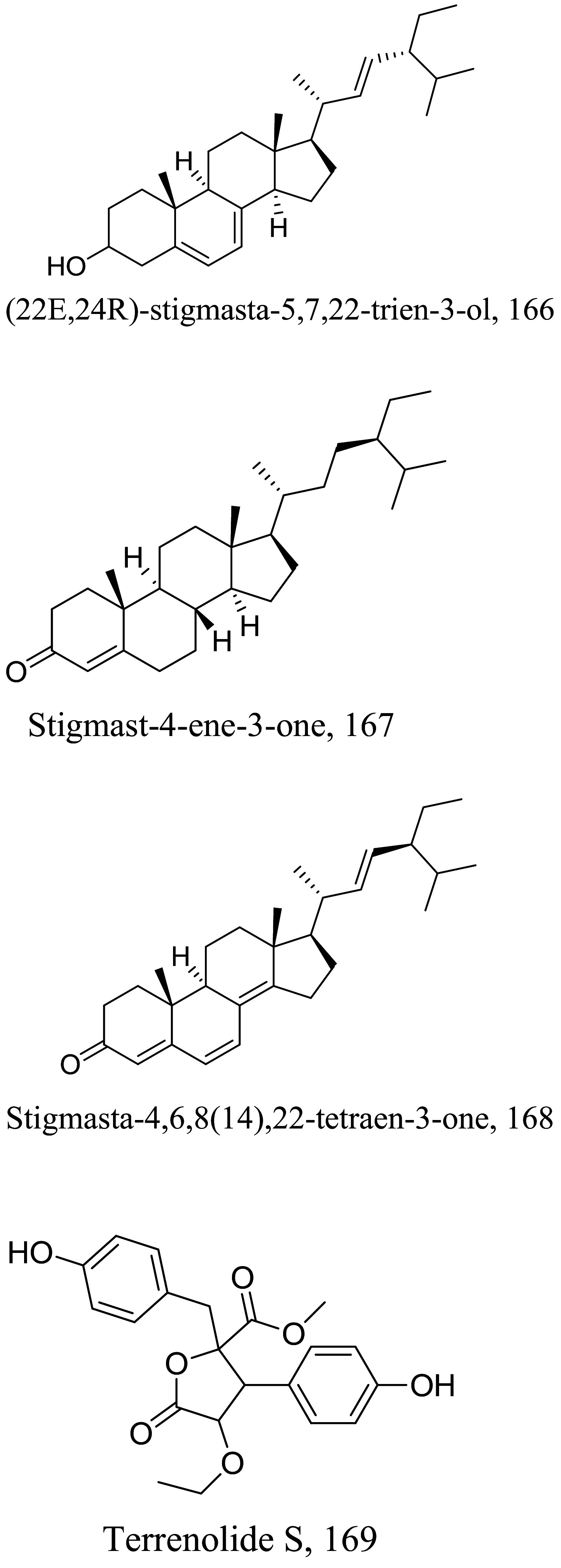 |
Steroid | 11.2 | L. donovani | N. T | [153] |
| Steroid | 15.3 | |||||
| Steroid | 54.3 | |||||
| Butenolide | 7.27 | |||||
| Solanum sisymbriifolium |  |
Steroid |
6.60 3.10 |
L. amazonensis L. brazilensis |
N.T | [127] |
| Steroid |
> 100 59.8 |
L. amazonensis L. brazilensis |
N. T | |||
|
Paecilomyces sp. (Marine) |
 |
18.2 | L. amazonensis (Intra-Amastigote) | Non-toxic up to 183 µM in mouse peritoneal macrophage. | [154] | |
| 7.89 | L. amazonensis | |||||
| Musa paradisiaca |  |
Steroid | 201 |
L. infantum (Amastigote) |
Low toxicity profiles against mammalian raw cell lines 462 µM |
[155] |
| Steroid | 185 | 569 µM | ||||
| Steroid | 127 | 1147 µM | ||||
| Steroid | 98.5 | 150 µM | ||||
| Pentalinon andrieuxi | 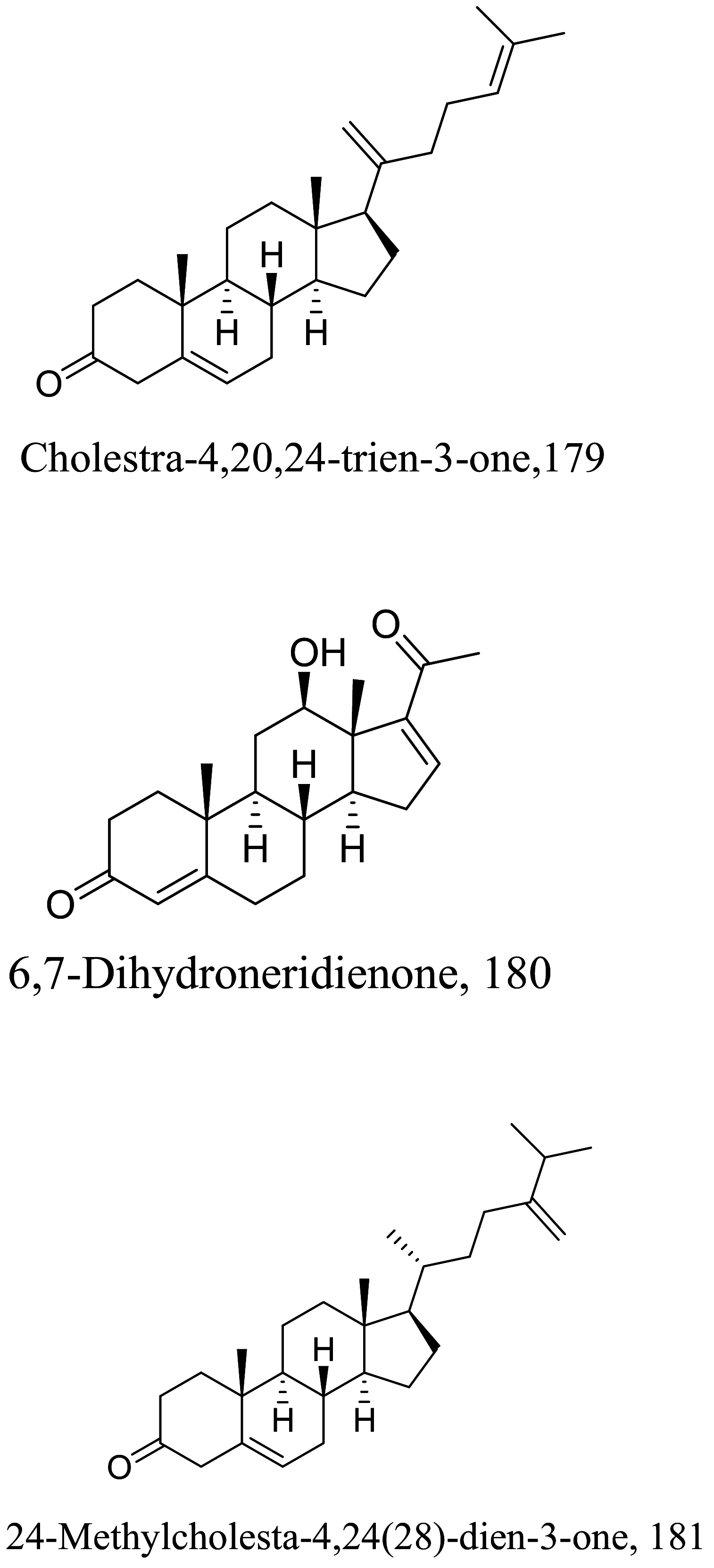 |
Steroid |
0.08 0.009 |
L. mexicana (promastigotes) |
[150] | |
| Steroid |
0.03 0.004 |
L. mexicana (amastigotes) |
||||
| Steroid |
0.06 0.009 |
|||||
| Porophyllum ruderale | 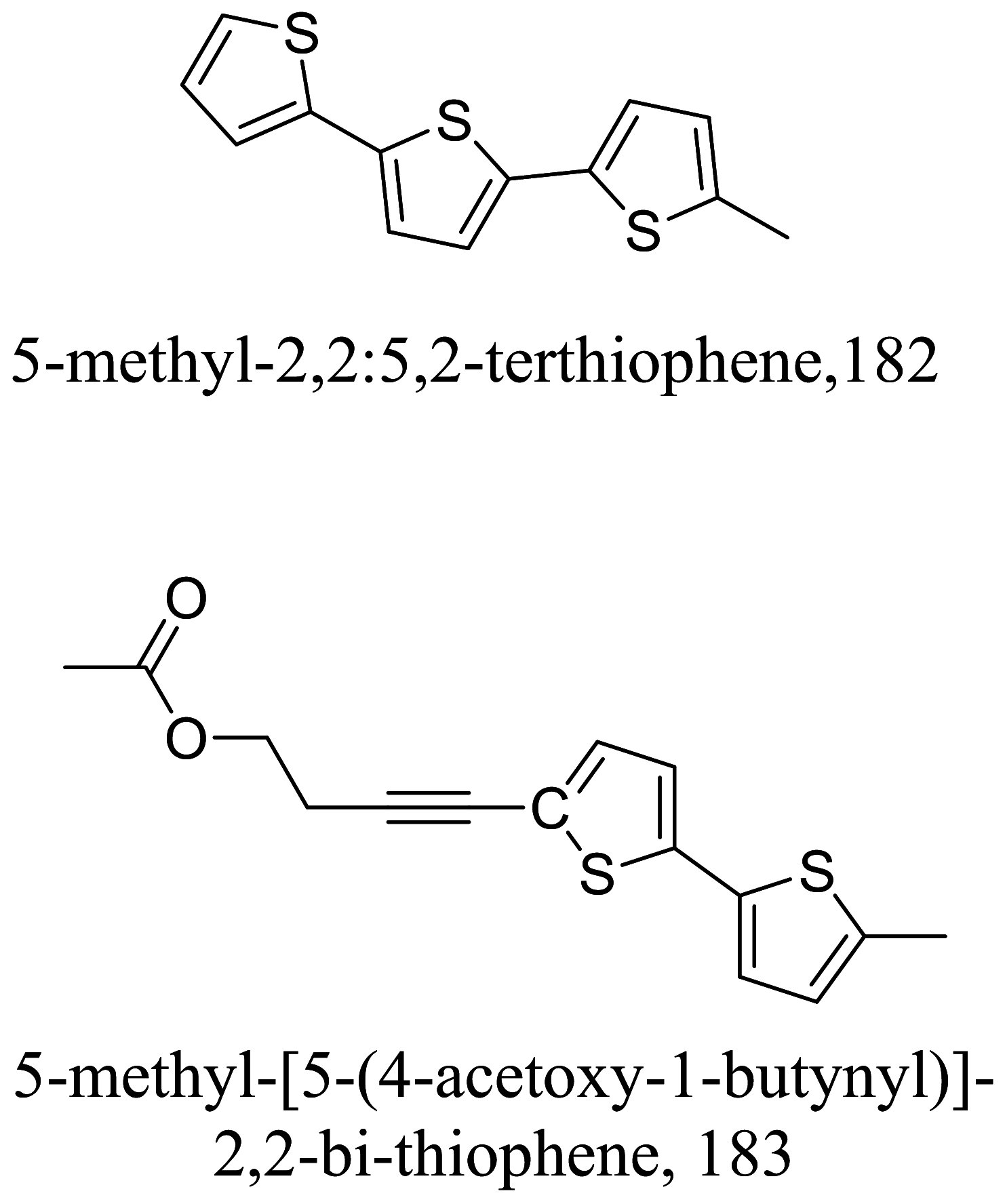 |
Terthiophene |
37 51 |
L. amanzonensis (amastigotes) |
CC50 = 370 μg/mL CC50 = 335 μg/mL |
[151] |
|
Marine Cyanobacteria (Marine) |
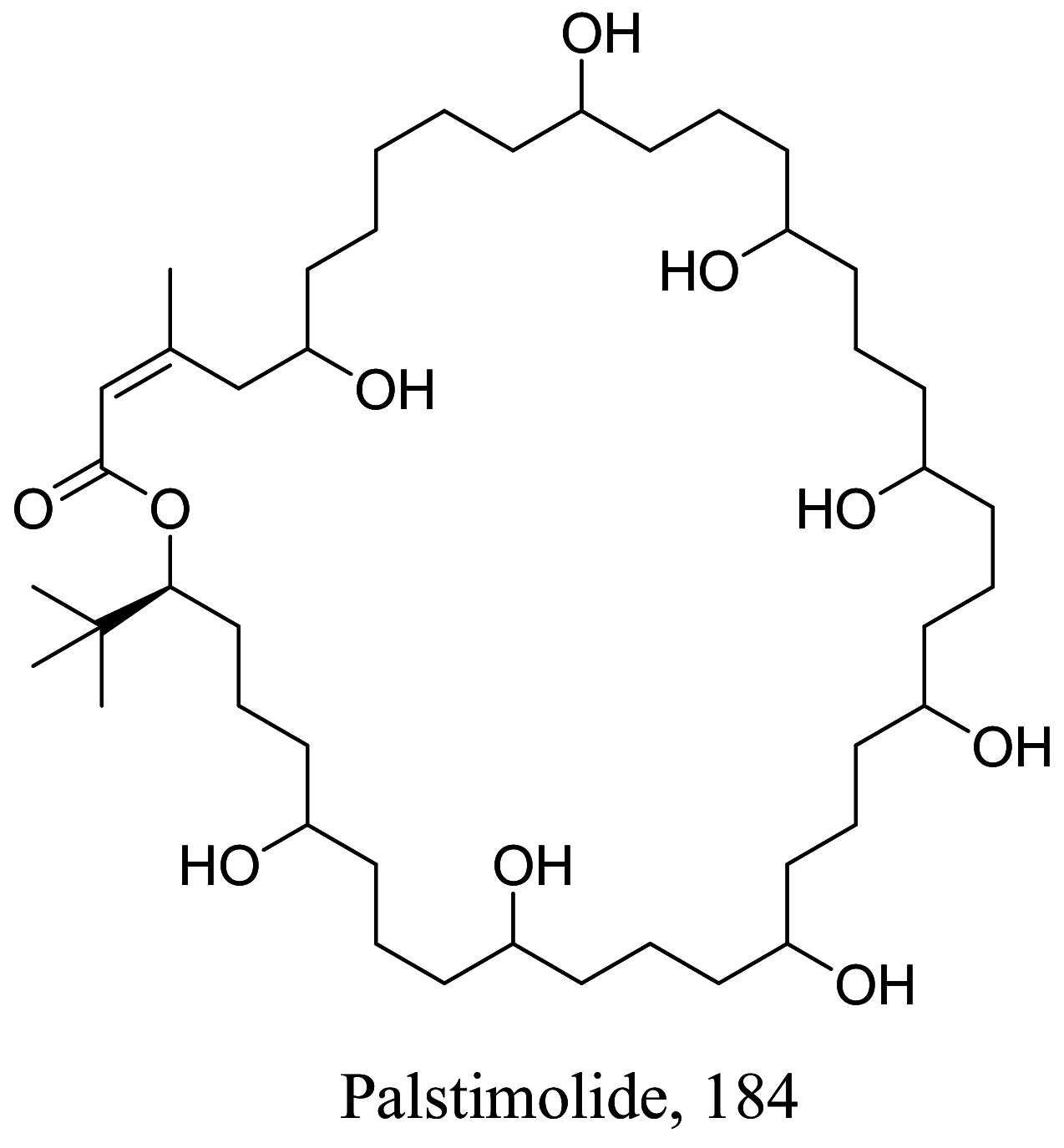 |
Macrolide | 4.67 µM |
L. donovani (amastigotes) |
N.T | [156] |
Halogenated terpenes 72 and 83 from Laurencia dendroidea which only differ primarily in a double bond character also targets the same organelle via redox perturbation [104, 105]. The natural product 87 from Vanillosmopsis arborea show promising activity through apoptosis induction characterized by mitochondrial dysfunction and oxidative stress [106]. Similar mode of action was reported for 87 isolated from Tunisia chamomile essential oil against L. amazonensis and L. infantum [107]. Effects of clerodone terpenes, 88, 89 and 90 from the stem bark of Croton cajucara have been shown to obstruct ROS protection via trypanothione reductase inhibition [108].
Interest in marine natural products which led to the evaluation of marine terpenes like pentacyclic triterpene 92, which exhibited an anti-inflammatory action with enhanced levels of T cells and Th1 cytokines when compared to its control [109].
Elucidation of the exact mechanism of action of four triterpenes from the roots of Salvia deserta showed that despite the strong antioxidant capacity of 93, it also kill parasites by inhibiting isopentenyl diphosphate condensation with the major target being farnesyl diphosphate synthase [110]. Studies to also understand the molecular basics of 94 shows a similar action like 80, but fragmentation of DNA strands has been described for diterpene 95 and 96 [111, 112]. Inhibition of oxidative pathways particularly IFN-γ-related signaling by similar diterpenoid quinones isolated from the roots of Salvia officinalis has also been shown to prevent disease proliferation and further protecting the host specie [113]. Recent studies in estimating the role of the energy production in the form of ATP in Leishmania with acyl phloroglucinol derivatives has revealed 97 as a mitochondria complex II/III inhibitor [114].
Like terpenes which are formed by the head to tail condensation of isoprene units, terpenoids (terpenes with oxygen-containing functional group) also represent a unique group of natural products with high functionalization and promising pharmacological activity. Isolation of six germacranolides from the leaves and stems of the Calea species have shown promising activities against L. donovani and L. amazonensis [115, 116]. Among them morphological assessment studies with 100 and 111 indicated alterations in the nucleus and mitochondria describing an apoptosis like process through the mitosis motor downregulation pathway [115]. Due to the similar core structure shared with germacra-1(10),11(13)-dien-12,6-olide a similar mechanism is envisaged for its counterpart 104 by aiding in generating ROS complementing the elucidated apoptosis process. The natural product 106 shares same structural core therefore may possess similar mode of action in addition to the inhibition of thiol-antioxidant enzymes [117]. Interestingly, 106 and its iso-conformer have also been disclosed to induce a pro-inflammatory inhibition via the NF-KB pathway [118]. On the other hand, 110 and 125 have also exerted multi-spectral activities including suppression of cell proliferation modulators and upregulation of microbicidal NO species [119].
Steroids
Steroids are a class of natural or synthetic organic compounds with three six membered rings fused with a five membered ring. Ergosterol, the main sterol in Leishmania parasite constitute a major component of the cell membrane and mitochondrion of the parasite which when inhibited leads to parasite death. 164 extracted from Trametes versicolor mimics Leishmania ergosterol due to similarities in core structure but a break in oxygen–oxygen bond in ergosterol peroxide unleashes oxidation on lipids, proteins and nucleic acids of the parasite by free radical reaction leading to serious toxicity to the Leishmania parasite [145]. Apart from the biological formation of bridge peroxides, the deleterious effects of other lanostane type steroids on membrane state and integrity causing parasite death has been reported [146, 147]. Also, anti-infective studies of Sassafras albidum and its bioactivity guided fractionation reported a sterol and fatty alcohol, 162 and 163 respectively [36] as promising antileishmanial compounds. 162 which differs from cholesterol at C24 position is believed to kill the parasite via an apoptosis mechanism involving DNA fragmentation, inhibition of inflammation cytokines and the activation of caspases [148, 149]. Evaluating the suicidal action of active isolates from Pentalinon andrieuxii, 181 induced changes in immune responses particularly via necrosis and apoptosis characterized by increase in IL2 and IFN-γ which insinuates the control of pro-inflammatory cytokines by anti-inflammatory counterparts [150]. 182 halted the process of electron transport and ATP generation in the mitochondria [151]. In addition, plasma membrane alterations with the administration of the other isolates depicts a sterol metabolism inhibition as a contributing factor to parasite death [151].
Conclusion
Though humans and natural products did not co-evolve, chemical prototypes from natural origins have numerous targets in both human and animal diseases. Their structural diversity, large chemical space and safety are intriguing characteristics that makes them very attractive. Diverse biomolecular functions including anti-leishmanial potentials are possessed by various plant families including Fabaceae, Annonaceae, Euphorbiaceae, Rutaceae, Myrsinaceae, Liliaceae, Araliaceae and Simaroubaceae, as well as endophytes genera Alternaria, Arthrinium, Penicillium, Cochloibus, Fusarium, Colletotrichum, and Gibberella, and marine natural product possess.
Management of leishmaniasis is plagued with systemic toxicity, high cost of existing drugs, lengthy treatment periods, drug resistance and parasite diversity. Different classes of natural products such as alkaloids, terpenes, terpenoids, and phenolics are examples of compounds evaluated towards the treatment of leishmaniasis. They exert their antileishmanial activities through calcium channel inhibitors, immunomodulatory through the enhancement of NO in macrophages, alterations in organelle membranes of the endoplasmic reticulum, respiration incapacitation and apoptosis. Other antileishmanial related mechanisms include cell membrane disruption via sterol biosynthesis inhibition, reactive oxygen species (ROS) generation, iron chelation, arginase inhibition, topoisomerase II intercalation, suppressing NF-κB expression and other pro-inflammatory, and trypanothione reductase inhibition.
Author contributions
POS, RKA and SKK initiated the work, POS wrote the first draft supervised by SKK and POS All the authors contributed to the writing of the review, read and accepted the final draft article.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Contributor Information
Patrick O. Sakyi, Email: patrick.sakyi@uenr.edu.gh, Email: opsakyi@st.ug.edu.gh
Richard K. Amewu, Email: ramewu@ug.edu.gh
Robert N. O. A. Devine, Email: robert.devine.stu@uenr.edu.gh
Emahi Ismaila, Email: Ismaila.emahi@uenr.edu.gh.
Whelton A. Miller, Email: wmiller6@luc.edu
Samuel K. Kwofie, Email: skkwofie@ug.edu.gh
References
- 1.Tiuman TS, Santos AO, Ueda-Nakamura T, Filho BPD, Nakamura CV. Int. J. Infect. Dis. 2011;15:e525. doi: 10.1016/j.ijid.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 2.WHO (2020). https://www.Who.Int/News-Room/Fact-Sheets/Detail/Leishmaniasis
- 3.Pérez-Cabezas B, Cecílio P, Gaspar TB, Gärtner F, Vasconcellos R, Cordeiro-da-Silva A. Front. Cell. Infect. Microbiol. 2019;9:30. doi: 10.3389/fcimb.2019.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.PAHO/WHO (2017). https://www.paho.org/hq/dmdocuments.
- 5.Okwor I, Uzonna J. Am. J. Trop. Med. Hyg. 2016;94:489. doi: 10.4269/ajtmh.15-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.R. Arenas, E. Torres-Guerrero, M. R. Quintanilla-Cedillo, and J. Ruiz-Esmenjaud, F1000Research 6, (2017). [DOI] [PMC free article] [PubMed]
- 7.N. Tiwari, M. R. Gedda, V. K. Tiwari, S. P. Singh, and R. K. Singh, Mini-Reviews Med. Chem. 18, (2018). [DOI] [PubMed]
- 8.Jain K, Jain NK. J. Immunol. Methods. 2015;422:1. doi: 10.1016/j.jim.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Emami S, Tavangar P, Keighobadi M. Eur. J. Med. Chem. 2017;135:241. doi: 10.1016/j.ejmech.2017.04.044. [DOI] [PubMed] [Google Scholar]
- 10.N. Dunning and S. Ali, Bioscience Horizons 2, (2009)
- 11.S. Srivastava, P. Shankar, J. Mishra, and S. Singh, Parasites and Vectors 9, (2016). [DOI] [PMC free article] [PubMed]
- 12.P. L. Olliaro, P. J. Guerin, S. Gerstl, A. A. Haaskjold, J. A. Rottingen, and S. Sundar, Lancet Infect. Dis. 5, 763 (2005). [DOI] [PubMed]
- 13.Olliaro PL, Guerin PJ, Gerstl S, Haaskjold AA, Rottingen JA, Sundar S. Lancet Infect. Dis. 2005;5:763. doi: 10.1016/S1473-3099(05)70296-6. [DOI] [PubMed] [Google Scholar]
- 14.J. N. Rugani, C. M. F. Gontijo, F. Frézard, R. P. Soares, and R. L. Do Monte-Neto, Mem. Inst. Oswaldo Cruz 114, (2019) [DOI] [PMC free article] [PubMed]
- 15.Frézard F, Monte-Neto R, Reis PG. Biophys. Rev. 2014;6:119. doi: 10.1007/s12551-013-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mandal G, Mandal S, Sharma M, Charret KS, Papadopoulou B, Bhattacharjee H, Mukhopadhyay R. PLoS Negl. Trop. Dis. 2015;9:e0003500. doi: 10.1371/journal.pntd.0003500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundar S, Chakravarty J. Expert Opin. Pharmacother. 2015;16:237. doi: 10.1517/14656566.2015.973850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza ACO, Amaral AC. Front. Microbiol. 2017;8:336. doi: 10.3389/fmicb.2017.00336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nagle AS, Khare S, Kumar AB, Supek F, Buchynskyy A, Mathison CJN, Chennamaneni NK, Pendem N, Buckner FS, Gelb MH, Molteni V. Chem. Rev. 2014;114:11305. doi: 10.1021/cr500365f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tamiru A, Tigabu B, Yifru S, Diro E, Hailu A, Infect BMC. Dis. 2016;16:548. doi: 10.1186/s12879-016-1746-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luque-Ortega JR, Rivas L. Antimicrob. Agents Chemother. 2007;51:1327. doi: 10.1128/AAC.01415-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.J. Pijpers, M. L. Den Boer, D. R. Essink, and K. Ritmeijer, PLoS Negl. Trop. Dis. 13, 1 (2019). [DOI] [PMC free article] [PubMed]
- 23.Sundar S, Chakravarty J. Expert Opin. Investig. Drugs. 2008;17:787. doi: 10.1517/13543784.17.5.787. [DOI] [PubMed] [Google Scholar]
- 24.Wiwanitkit V. Ther. Clin. Risk Manag. 2012;8:323. doi: 10.2147/TCRM.S30139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan W, Kumar R, Singh S, Arora SK, Kumar N. Drug Test. Anal. 2013;5:468. doi: 10.1002/dta.389. [DOI] [PubMed] [Google Scholar]
- 26.Loiseau PM, Cojean S, Schrével J, Parasite J. La Société Française Parasitol. 2011;18:115. doi: 10.1051/parasite/2011182115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gintjee TJ, Donnelley MA, Thompson GR. J. Fungi. 2020;6:28. doi: 10.3390/jof6010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Macedo-Silva ST, Urbina JA, De Souza W, Rodrigues JCF. PLoS ONE. 2013;8:e83247. doi: 10.1371/journal.pone.0083247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra BB, Tiwari VK. Eur. J. Med. Chem. 2011;46:4769. doi: 10.1016/j.ejmech.2011.07.057. [DOI] [PubMed] [Google Scholar]
- 30.Patridge E, Gareiss P, Kinch MS, Hoyer D. Drug Discov. Today. 2016;21:204. doi: 10.1016/j.drudis.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 31.I. V. Ogungbe, M. Singh, and W. N. Setzer, Antileishmanial Natural Products from Plants, 1st ed. (Elsevier B.V., 2012)
- 32.Santana DB, da Costa RC, Araújo RM, de Paula JE, Silveira ER, Braz-Filho R, Espindola LS. Brazilian. J. Pharmacogn. 2015;25:401. [Google Scholar]
- 33.Simoben CV, Ntie-Kang F, Akone SH, Sippl W. Nat. Products Bioprospect. 2018;8:151. doi: 10.1007/s13659-018-0165-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ullah N, Nadhman A, Siddiq S, Mehwish S, Islam A, Jafri L, Hamayun M. Phyther. Res. 2016;30:1905. doi: 10.1002/ptr.5710. [DOI] [PubMed] [Google Scholar]
- 35.Búfalo J, Cantrell CL, Jacob MR, Schrader KK, Tekwani BL, Kustova TS, Ali A, Boaro CSF. Planta Med. 2015;82:131. doi: 10.1055/s-0035-1557875. [DOI] [PubMed] [Google Scholar]
- 36.Pulivarthi D, Steinberg KM, Monzote L, Piñón A, Setzer WN. Nat. Prod. Commun. 2015;10:1229. [PubMed] [Google Scholar]
- 37.Scotti L, Ishiki H, Mendonca FJB, Silva MS, Scotti MT. Mini-Reviews. Med. Chem. 2015;15:253. doi: 10.2174/138955751503150312141854. [DOI] [PubMed] [Google Scholar]
- 38.Sen R, Chatterjee M. Phytomedicine. 2011;18:1056. doi: 10.1016/j.phymed.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 39.Venkatesan SK, Saudagar P, Shukla AK, Dubey VK. Interdiscip. Sci. Comput Life Sci. 2011;3:217. doi: 10.1007/s12539-011-0101-x. [DOI] [PubMed] [Google Scholar]
- 40.Gabriel RS, Amaral ACF, Lima IC, Cruz JD, Garcia AR, Souza HAS, Adade CM, Vermelho AB, Alviano CS, Alviano DS, Rodrigues IA. Rev. Bras. Farmacogn. 2019;29:755. [Google Scholar]
- 41.Cota BB, Rosa LH, Caligiorne RB, Rabello ALT, AlmeidaAlves TM, Rosa CA, Zani CL. FEMS Microbiol. Lett. 2008;285:177. doi: 10.1111/j.1574-6968.2008.01221.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosa LH, Gonçalves VN, Caligiorne RB, Alves TMA, Rabello A, Sales PA, Romanha AJ, Sobral MEG, Rosa CA, Zani CL. Brazilian. J. Microbiol. 2010;41:420. doi: 10.1590/S1517-838220100002000024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pech-Puch D, Pérez-Povedano M, Lenis-Rojas OA, Rodríguez J, Jiménez C. Mar. Drugs. 2020;18:59. doi: 10.3390/md18010059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayibor K, Kwain S, Osei E, Nartey AP, Tetevi GM, Owusu KB-A, Camas M, Camas AS, Kyeremeh K. Int. J. Biol. Chem. Sci. 2019;13:1918. [Google Scholar]
- 45.M. M. Basyoni, Parasitol. United J. 5, (2012).
- 46.R. M. de Oliveira, S. de A. Melo, T. A. da Penha-Silva, F. Almeida-Souza, and A. L. Abreu-Silva, in Leishmaniases as Re-Emerging Dis. (InTech, 2018)
- 47.Yamthe LRT, Appiah-Opong R, Fokou PVT, Tsabang N, Boyom FF, Nyarko AK, Wilson MD. Mar. Drugs. 2017;15:323. doi: 10.3390/md15110323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.I. A. Rodrigues, A. M. Mazotto, V. Cardoso, R. L. Alves, A. C. F. Amaral, J. R. D. A. Silva, A. S. Pinheiro, and A. B. Vermelho, Mediators Inflamm. 2015, (2015) [DOI] [PMC free article] [PubMed]
- 49.Toghueo RMK. Nat. Products Bioprospect. 2019;9:311. doi: 10.1007/s13659-019-00220-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fatima N, Muhammad S, Mumtaz A, Tariq H, Shahzadi I, Said M, Dawood M. Br. J. Pharm. Res. 2016;12:1. [Google Scholar]
- 51.Oliveira M, Barreira L, Gangadhar KN, Rodrigues MJ, Santos T, Varela J, Custódio L. Phytochem. Rev. 2016;15:663. [Google Scholar]
- 52.E. C. Da Silva, C. D. Rayol, P. L. Medeiros, R. C. B. Q. Figueiredo, M. R. Piuvezan, J. M. Brabosa-Filho, A. Fernandes Marinho, T. G. Silva, G. C. G. Militão, A. P. P. Cassilhas, and P. P. De Andrade, Sci. World J. 2012, (2012)
- 53.Guimarães LRC, Rodrigues APD, Marinho PSB, Muller AH, Guilhon GMS, Santos LS, Do Nascimento JLM, Silva EO. Parasitol. Res. 2010;107:1075. doi: 10.1007/s00436-010-1973-0. [DOI] [PubMed] [Google Scholar]
- 54.S. M. M. Amyra Amat Sain, Azimah Amanah, Zuriati Zahari, Roshan Jahn Mohd Salim, Int. J. Pharmacol. Phytochem. Ethnomedicine 3, 1 (2016).
- 55.Zhu N, Cao X, Hao P, Zhang Y, Chen Y, Zhang J, Li J, Gao C, Li L. Cell Stress Chaperones. 2020;25:417. doi: 10.1007/s12192-020-01081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zorić N, Kosalec I, Tomić S, Bobnjarić I, Jug M, Vlainić T, Vlainić J, Complement BMC. Altern. Med. 2017;17:268. doi: 10.1186/s12906-017-1773-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tetevi GM, Kwain S, Mensah T, Camas AS, Camas M, Dofuor AK, Azerigyik FA, Oluwabusola E, Deng H, Jaspars M, Kyeremeh K. Molbank. 2019;2019:M1094. [Google Scholar]
- 58.Osei E, Kwain S, Mawuli GT, Anang AK, Owusu KBA, Camas M, Camas AS, Ohashi M, Alexandru-Crivac CN, Deng H, Jaspars M, Kyeremeh K. Mar. Drugs. 2019;17:9. doi: 10.3390/md17010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shyaula SL, Tamang T, Ghouri N, Adhikari A, Marasini S, Bajracharya GB, Manandhar MD, Choudhary MI. Nat. Prod. Res. 2016;30:2590. doi: 10.1080/14786419.2015.1114941. [DOI] [PubMed] [Google Scholar]
- 60.Ferreira ME, Rojas de Arias A, Yaluff G, de Bilbao NV, Nakayama H, Torres S, Schinini A, Guy I, Heinzen H, Fournet A. Phytomedicine. 2010;17:375. doi: 10.1016/j.phymed.2009.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Naman CB, Gupta G, Varikuti S, Chai H, Doskotch RW, Satoskar AR, Kinghorn AD. J. Nat. Prod. 2015;78:552. doi: 10.1021/np501028u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.A. M. Metwaly, M. M. Ghoneim, and A. Musa, Two new antileishmanial diketopiperazine alkaloids from the endophytic fungus Trichosporum Sp (2015)
- 63.Naz T, Mosaddik A, Rahman MM, Muhammad I, Haque ME, Cho SK. Nat. Prod. Res. 2012;26:979. doi: 10.1080/14786419.2010.535166. [DOI] [PubMed] [Google Scholar]
- 64.Cunha ADC, Chierrito TPC, MacHado GMDC, Leon LLP, Da Silva CC, Tanaka JC, De Souza LM, Gonalves RAC, De Oliveira AJB. Phytomedicine. 2012;19:413. doi: 10.1016/j.phymed.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 65.Mollataghi A, Coudiere E, Hadi AHA, Mukhtar MR, Awang K, Litaudon M, Ata A. Fitoterapia. 2012;83:298. doi: 10.1016/j.fitote.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 66.Mahmoudvand H, Mousavi SAA, Sepahvand A, Sharififar F, Ezatpour B, Gorohi F, Dezaki ES, Jahanbakhsh S. ISRN Pharmacol. 2014;2014:1. doi: 10.1155/2014/602436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghosal S, Deb A, Mishra P, Vishwakarma R. Planta Med. 2012;78:906. doi: 10.1055/s-0031-1298537. [DOI] [PubMed] [Google Scholar]
- 68.Orhan I, Şener B, Kaiser M, Brun R, Tasdemir D. Mar. Drugs. 2010;8:47. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cartuche L, Sifaoui I, López-Arencibia A, Bethencourt-Estrella CJ, Nicolás-Hernández DS, Lorenzo-Morales J, Piñero JE, Díaz-Marrero AR, Fernández JJ. Biomolecules. 2020;10:1. doi: 10.3390/biom10040657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rizk YS, Fischer A, de Cunha MC, Rodrigues PO, Marques MCS, de Matos MFC, Kadri MCT, Carollo CA, de Arruda CCP. Mem. Inst. Oswaldo Cruz. 2014;109:1050. doi: 10.1590/0074-0276140312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lage PS, Chávez-Fumagalli MA, Mesquita JT, Mata LM, Fernandes SOA, Cardoso VN, Soto M, Tavares CAP, Leite JPV, Tempone AG, Coelho EAF. Parasitol. Res. 2015;114:4625. doi: 10.1007/s00436-015-4708-4. [DOI] [PubMed] [Google Scholar]
- 72.Fonseca-Silva F, Inacio JDF, Canto-Cavalheiro MM, Almeida-Amaral EE. PLoS ONE. 2011;6:e14666. doi: 10.1371/journal.pone.0014666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Grecco SS, Costa-Silva TA, Jerz G, de Sousa FS, Conserva GAA, Mesquita JT, Galuppo MK, Tempone AG, Neves BJ, Andrade CH, Cunha RLOR, Uemi M, Sartorelli P, Lago JHG. Phytomedicine. 2017;24:62. doi: 10.1016/j.phymed.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 74.de Sousa FS, Grecco SS, Girola N, Azevedo RA, Figueiredo CR, Lago JHG. Phytochemistry. 2017;140:108. doi: 10.1016/j.phytochem.2017.04.024. [DOI] [PubMed] [Google Scholar]
- 75.Morais LS, Dusi RG, Demarque DP, Silva RL, Albernaz LC, Bao SN, Merten C, Antinarelli LMR, Coimbra ES, Espindola LS. PLoS ONE. 2020;15:1. doi: 10.1371/journal.pone.0241855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.R. A. Falcao, P. L. A. Do Nascimento, S. A. De Souza, T. M. G. Da Silva, A. C. De Queiroz, C. B. B. Da Matta, M. S. A. Moreira, C. A. Camara, and T. M. S. Silva, Evidence-Based Complement. Altern. Med. 2013, (2013) [DOI] [PMC free article] [PubMed]
- 77.Alagawany M, El-Hack MEA, Farag MR, Gopi M, Karthik K, Malik YS, Dhama K. Anim. Health. Res. Rev. 2017;18:167. doi: 10.1017/S1466252317000081. [DOI] [PubMed] [Google Scholar]
- 78.Bajpai VK, Alam MB, Quan KT, Ju MK, Majumder R, Shukla S, Huh YS, Na MK, Lee SH, Han YK. Sci. Rep. 2018;8:9216. doi: 10.1038/s41598-018-27585-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Espírito-Santo RF, Meira CS, DosSantosCosta R, Filho OPS, Evangelista AF, Trossini GHG, Ferreira GM, Da Velozo ES, Villarreal CF, Soares MBP. PLoS ONE. 2017;12:0179174. doi: 10.1371/journal.pone.0179174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gao W, Li Q, Chen J, Wang Z, Hua C. Molecules. 2013;18:15613. doi: 10.3390/molecules181215613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.V. P. C. Rocha, C. Q. Da Rocha, E. F. Queiroz, L. Marcourt, W. Vilegas, G. B. Grimaldi, P. Furrer, E. Allémann, J. L. Wolfender, and M. B. P. Soares, Molecules 24, (2019)
- 82.Radwan MM, Wanas AS, Fronczek FR, Jacob MR, Ross SA. Med. Chem. Res. 2015;24:3398. [Google Scholar]
- 83.Da Costa-Silva TA, Grecco SS, De Sousa FS, Lago JHG, Martins EGA, Terrazas CA, Varikuti S, Owens KL, Beverley SM, Satoskar AR, Tempone AG. J. Nat. Prod. 2015;78:653. doi: 10.1021/np500809a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kaur A, Singh R, Dey CS, Sharma SS, Bhutan KK, Singh IP. Indian J. Exp. Biol. 2010;48:314. [PubMed] [Google Scholar]
- 85.Da Costa-Silva TA, Conserva GAA, Galisteo AJ, Tempone AG, Lago JHG. J. Venom. Anim. Toxins Incl. Trop. Dis. 2019;25:1. doi: 10.1590/1678-9199-JVATITD-2019-0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vendrametto MC, dos Santos AO, Nakamura CV, Filho BPD, Cortez DAG, Ueda-Nakamura T. Parasitol. Int. 2010;59:154. doi: 10.1016/j.parint.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 87.dos Ferreira LAO, de Oliveira MM, Faleiro FL, Scariot DB, Boeing JS, Visentainer JV, Romagnolo MB, Nakamura CV, Truiti MCT. Nat. Prod. Res. 2018;32:2825. doi: 10.1080/14786419.2017.1378214. [DOI] [PubMed] [Google Scholar]
- 88.Mishra BB, Gour JK, Kishore N, Singh RK, Tripathi V, Tiwari VK. Nat. Prod. Res. 2013;27:480. doi: 10.1080/14786419.2012.696254. [DOI] [PubMed] [Google Scholar]
- 89.Dutra FL, Oliveira MM, Santos RS, Silva WS, Alviano DS, Vieira DP, Lopes AH. Acta Trop. 2016;164:69. doi: 10.1016/j.actatropica.2016.08.026. [DOI] [PubMed] [Google Scholar]
- 90.de Morais SM, Vila-Nova NS, Bevilaqua CML, Rondon FC, Lobo CH, De Moura AAAN, Sales AD, Rodrigues APR, De Figuereido JR, Campello CC, Wilson ME, De Andrade HF. Bioorganic. Med. Chem. 2014;22:6250. doi: 10.1016/j.bmc.2014.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ueda-Nakamura T, Mendonça-Filho RR, Morgado-Díaz JA, Maza PK, Filho BPD, Cortez DAG, Alviano DS, do Rosa MSS, Lopes AHCS, Alviano CS, Nakamura CV. Parasitol. Int. 2006;55:99. doi: 10.1016/j.parint.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 92.do Nascimento AM, Soares MG, da Torchelsen FKVS, de Araujo JAV, Lage PS, Duarte MC, Andrade PHR, Ribeiro TG, Coelho EAF, do Nascimento AM. World J. Microbiol. Biotechnol. 2015;31:1793. doi: 10.1007/s11274-015-1932-0. [DOI] [PubMed] [Google Scholar]
- 93.Parise-Filho R, Pasqualoto KFM, Magri FMM, Ferreira AK, Da Silva BAVG, Damião MCFCB, Tavares MT, Azevedo RA, Auada AVV, Polli MC, Brandt CA. Arch. Pharm. (Weinheim). 2012;345:934. doi: 10.1002/ardp.201200212. [DOI] [PubMed] [Google Scholar]
- 94.Malak LG, Ibrahim MA, Bishay DW, Abdel-Baky AM, Moharram AM, Tekwani B, Cutler SJ, Ross SA. J. Nat. Prod. 2014;77:1987. doi: 10.1021/np5000473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Malak LG, Ibrahim MA, Moharram AM, Pandey P, Tekwani B, Doerksen RJ, Ferreira D, Ross SA. J. Nat. Prod. 2018;81:2222. doi: 10.1021/acs.jnatprod.8b00473. [DOI] [PubMed] [Google Scholar]
- 96.Bashir S, Alam M, Adhikari A, Shrestha RL, Yousuf S, Ahmad B, Parveen S, Aman A, Choudhary MI. Phytochem. Lett. 2014;9:46. [Google Scholar]
- 97.Maldonado EM, Salamanca E, Giménez A, Saavedra G, Sterner O. Phytochem. Lett. 2014;10:281. [Google Scholar]
- 98.Attioua B, Lagnika L, Yeo D, Antheaume C, Kaiser M, Weniger B, Lobstein A, Vonthron-Sénécheau C. Int. J. Pharm. Sci. Rev. Res. 2011;11:1. doi: 10.3109/13880209.2011.633270. [DOI] [PubMed] [Google Scholar]
- 99.Costa RS, Filho OPS, Júnior OCSD, Silva JJ, LeHyaric M, Santos MAV, Velozo ES. Braz. J. Pharmacogn. 2018;28:551. [Google Scholar]
- 100.dos Santos AO, Britta EA, Bianco EM, Ueda-Nakamura T, Filho BPD, Pereira RC, Nakamura CV. Mar. Drugs. 2011;9:2369. doi: 10.3390/md9112369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.A. Santos, E. Britta, T. Ueda-Nakamura, B. D. Filho, E. Bianco, V. Teixeira, R. Pereira, and C. Nakamura, Planta Med. 75, (2009)
- 102.Garcia C, Silva CO, Monteiro CM, Nicolai M, Viana A, Andrade JM, Barasoain I, Stankovic T, Quintana J, Hernández I, González I, Estévez F, Díaz-Lanza AM, Reis CP, Afonso CAM, Pesic M, Rijo P. Future. Med. Chem. 2018;10:1177. doi: 10.4155/fmc-2017-0239. [DOI] [PubMed] [Google Scholar]
- 103.Sitarek P, Toma M, Ntungwe E, Kowalczyk T, Skała E, Wieczfinska J, Śliwiński T, Rijo P. Biomolecules. 2020;10:194. doi: 10.3390/biom10020194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Desoti VC, Lazarin-Bidóia D, Sudatti DB, Pereira RC, Alonso A, Ueda-Nakamura T, Filho BPD, Nakamura CV, de Silva SO. Mar. Drugs. 2012;10:1631. doi: 10.3390/md10081631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Freile-Pelegrín Y, Tasdemir D. Bot. Mar. 2019;62:211. [Google Scholar]
- 106.A. V. Colares, F. Almeida-Souza, N. N. Taniwaki, C. D. S. F. Souza, J. G. M. Da Costa, K. D. S. Calabrese, and A. L. Abreu-Silva, Evidence-Based Complement. Altern. Med. 2013, (2013) [DOI] [PMC free article] [PubMed]
- 107.Hajaji S, Sifaoui I, López-Arencibia A, Reyes-Batlle M, Jiménez IA, Bazzocchi IL, Valladares B, Akkari H, Lorenzo-Morales J, Piñero JE. Parasitol. Res. 2018;117:2855. doi: 10.1007/s00436-018-5975-7. [DOI] [PubMed] [Google Scholar]
- 108.Lima GS, Castro-Pinto DB, MacHado GC, Maciel MAM, Echevarria A. Phytomedicine. 2015;22:1133. doi: 10.1016/j.phymed.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 109.Saleem M. Cancer Lett. 2009;285:109. doi: 10.1016/j.canlet.2009.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu YL, Lindert S, Zhu W, Wang K, McCammon JA, Oldfield E. Proc. Natl. Acad. Sci. USA. 2014;111:E2530. doi: 10.1073/pnas.1409061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jia Y, Wu C, Zhang B, Zhang Y, Li J. Hum. Exp. Toxicol. 2019;38:227. doi: 10.1177/0960327118792050. [DOI] [PubMed] [Google Scholar]
- 112.K. Becker, S. Schwaiger, B. Waltenberger, D. Fuchs, C. K. Pezzei, H. Schennach, H. Stuppner, and J. M. Gostner, Oxid. Med. Cell. Longev. (2018). [DOI] [PMC free article] [PubMed]
- 113.Slameňová D, Mašterová I, Lábaj J, Horváthová E, Kubala P, Jakubíková J, Wsólová L. Basic Clin. Pharmacol. Toxicol. 2004;94:282. doi: 10.1111/j.1742-7843.2004.pto940605.x. [DOI] [PubMed] [Google Scholar]
- 114.Monzote L, Lackova A, Staniek K, Cuesta-Rubio O, Gille L. Parasitology. 2015;142:1239. doi: 10.1017/S0031182015000608. [DOI] [PubMed] [Google Scholar]
- 115.Caldas LA, Yoshinaga ML, Ferreira MJP, Lago JHG, de Souza AB, Laurenti MD, Passero LFD, Sartorelli P. Bioorg. Chem. 2019;83:348. doi: 10.1016/j.bioorg.2018.10.059. [DOI] [PubMed] [Google Scholar]
- 116.Wu H, Fronczek FR, Burandt CL, Zjawiony JK. Planta Med. 2011;77:749. doi: 10.1055/s-0030-1250584. [DOI] [PubMed] [Google Scholar]
- 117.Nakagawa Y, Iinuma M, Matsuura N, Yi K, Naoi M, Nakayama T, Nozawa Y, Akao Y. J. Pharmacol. Sci. 2005;97:242. doi: 10.1254/jphs.fp0040456. [DOI] [PubMed] [Google Scholar]
- 118.Fakhrudin N, Waltenberger B, Cabaravdic M, Atanasov AG, Malainer C, Schachner D, Heiss EH, Liu R, Noha SM, Grzywacz AM, Mihaly-Bison J, Awad EM, Schuster D, Breuss JM, Rollinger JM, Bochkov V, Stuppner H, Dirsch VM. Br. J. Pharmacol. 2014;171:1676. doi: 10.1111/bph.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yamamoto ES, Campos BLS, Jesus JA, Laurenti MD, Ribeiro SP, Kallás EG, Rafael-Fernandes M, Santos-Gomes G, Silva MS, Sessa DP, Lago JHG, Levy D, Passero LFD. PLoS ONE. 2015;10:e0144946. doi: 10.1371/journal.pone.0144946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Attioua B, Yeo D, Lagnika L, Harisolo R, Antheaume C, Weniger B, Kaiser M, Lobstein A, Vonthron-Sénécheau C. Pharm. Biol. 2012;50:801. doi: 10.3109/13880209.2011.633270. [DOI] [PubMed] [Google Scholar]
- 121.Azerigyik FA, Amoa-Bosompem M, Tetteh T, Ayertey F, Antwi AN, Owusu KB-A, Dadzie KK, Djameh GI, Tetteh-Tsifoanya M, Iwanaga S, Appiah AA, Ohta T, Uto T, Shoyama Y, Ohta N, Gwira TM, Ohashi M. European. J. Med. Plants. 2018;25:1. [Google Scholar]
- 122.Demarchi IG, Thomazella MV, de Terron MS, Lopes L, Gazim ZC, Cortez DAG, Donatti L, Aristides SMA, Silveira TGV, Lonardoni MVC. Exp. Parasitol. 2015;157:128. doi: 10.1016/j.exppara.2015.06.014. [DOI] [PubMed] [Google Scholar]
- 123.Da MacHado FLS, Pacienza-Lima W, Rossi-Bergmann B, De Gestinari LMS, Fujii MT, Paula JC, Costa SS, Lopes NP, Kaiser CR, Soares AR. Planta Med. 2011;77:733. doi: 10.1055/s-0030-1250526. [DOI] [PubMed] [Google Scholar]
- 124.Teles CBG, Moreira LS, Silva ADAE, Facundo VA, Zuliani JP, Stábeli RG, Silvm I. J. Braz. Chem. Soc. 2011;22:936. [Google Scholar]
- 125.J.A. Crentsil, L.R.T. Yamthe, B.Z. Anibea, E. Broni, S. K. Kwofie, J.K.A. Tetteh, and D. Osei-Safo, Front. Pharmacol. 11, (2020) [DOI] [PMC free article] [PubMed]
- 126.Das A, Jawed JJ, Das MC, Sandhu P, De UC, Dinda B, Akhter Y, Bhattacharjee S. Int. J. Antimicrob. Agents. 2017;50:512. doi: 10.1016/j.ijantimicag.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 127.V.C. Filho, C. Meyre-Silva, R. Niero, L.N. Bolda Mariano, F. Gomes Do Nascimento, I. Vicente Farias, V.F. Gazoni, B. Dos Santos Silva, A. Giménez, D. Gutierrez-Yapu, E. Salamanca, and A. Malheiros, Evidence-Based Complement. Altern. Med. 2013, (2013) [DOI] [PMC free article] [PubMed]
- 128.da Silva BP, Cortez DA, Violin TY, Filho BPD, Nakamura CV, Ueda-Nakamura T, Ferreira ICP. Parasitol. Int. 2010;59:643. doi: 10.1016/j.parint.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 129.Singh S, Sharma U, Kumar P, Singh D, Dobhal M. Indian J. Med. Res. 2011;134:709. doi: 10.4103/0971-5916.91005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Odonne G, Herbette G, Eparvier V, Bourdy G, Rojas R, Sauvain M, Stien D. J. Ethnopharmacol. 2011;137:875. doi: 10.1016/j.jep.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 131.Murillo JA, Gil JF, Upegui YA, Restrepo AM, Robledo SM, Quiñones W, Echeverri F, Martin AS, Olivo HF, Escobar G. Bioorganic. Med. Chem. 2019;27:153. doi: 10.1016/j.bmc.2018.11.030. [DOI] [PubMed] [Google Scholar]
- 132.Falodun A, Imieje V, Erharuyi O, Joy A, Langer P, Jacob M, Khan S, Abaldry M, Hamann M. Doc. Head. Asian Pac J Trop Biomed. 2014;4:374. doi: 10.12980/APJTB.4.2014C1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Phakeovilay C, Bourgeade-Delmas S, Perio P, Valentin A, Chassagne F, Deharo E, Reybier K, Marti G. Molecules. 2019;24:4536. doi: 10.3390/molecules24244536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bruno de Sousa C, Gangadhar KN, Morais TR, Conserva GAA, Vizetto-Duarte C, Pereira H, Laurenti MD, Campino L, Levy D, Uemi M, Barreira L, Custódio L, Passero LFD, Lago JHG, Varela J. Exp. Parasitol. 2017;174:1. doi: 10.1016/j.exppara.2017.01.002. [DOI] [PubMed] [Google Scholar]
- 135.Girardi C, Fabre N, Paloque L, Ramadani AP, Benoit-Vical F, González-Aspajo G, Haddad M, Rengifo E, Jullian V. J. Ethnopharmacol. 2015;170:167. doi: 10.1016/j.jep.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 136.Cota BB, Tunes LG, Maia DNB, Ramos JP, De Oliveira DM, Kohlhoff M, de Alves TMA, Souza-Fagundes EM, Campos FF, Zani CL. Mem. Inst. Oswaldo Cruz. 2018;113:102. doi: 10.1590/0074-02760170217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Novello CR, Düsman E, Balbinot RB, de Paula JC, Nakamura CV, de Mello JCP, Sarragiotto MH. Nat. Prod. Res. 2020;0:1. doi: 10.1080/14786419.2020.1851221. [DOI] [PubMed] [Google Scholar]
- 138.Naman CB, Gromovsky AD, Vela CM, Fletcher JN, Gupta G, Varikuti S, Zhu X, Zywot EM, Chai H, Werbovetz KA, Satoskar AR, Kinghorn AD. J. Nat. Prod. 2016;79:598. doi: 10.1021/acs.jnatprod.5b01131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.de Medeiros MGF, da Silva AC, das Citó AMGL, Borges AR, de Lima SG, Lopes JAD, Figueiredo RCBQ. Parasitol. Int. 2011;60:237. doi: 10.1016/j.parint.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 140.Smyrniotopoulos V, Merten C, Kaiser M, Tasdemir D. Mar. Drugs. 2017;15:1. doi: 10.3390/md15080245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.O. Chiboub, I. Sifaoui, J. Lorenzo-Morales, M. Abderrabba, M. Mejri, J.J. Fernández, J.E. Piñero, A.R. Díaz-Marrero, Mar. Drugs 17, 1 (2019). [DOI] [PMC free article] [PubMed]
- 142.D.C. Soares, M.M. Szlachta, V.L. Teixeira, A.R. Soares, E.M. Saraiva, Mar. Drugs 14, (2016). [DOI] [PMC free article] [PubMed]
- 143.Díaz-marrero AR, López-arencibia A, Bethencout-estrella CJ, Cen-pacheco F, Sifaoui I, Hernández A, Duque-ramírez MC, Universitario I, González DBA, Ag I, De Investigaciones C, De Canarias B, De La U, Ull L. Bioorg. Chem. 2019;92:103276. doi: 10.1016/j.bioorg.2019.103276. [DOI] [PubMed] [Google Scholar]
- 144.C. Imperatore, R. Gimmelli, M. Persico, M. Casertano, A. Guidi, F. Saccoccia, G. Ruberti, P. Luciano, A. Aiello, S. Parapini, S. Avunduk, N. Basilico, C. Fattorusso, Mar. Drugs 18, (2020). [DOI] [PMC free article] [PubMed]
- 145.T. Meza-Menchaca, A. Ramos-Ligonio, A. López-Monteon, A.V. Limón, L.A. Kaluzhskiy, T.V. Shkel, N.V. Strushkevich, L.F. Jiménez-García, L.T.A. Moreno, V. Gallegos-García, J. Suárez-Medellín, Á. Trigos, Biomolecules 9, (2019). [DOI] [PMC free article] [PubMed]
- 146.Mukherjee S, Xu W, Hsu FF, Patel J, Huang J, Zhang K. Mol. Microbiol. 2019;111:65. doi: 10.1111/mmi.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Borba-Santos LP, Visbal G, Gagini T, Rodrigues AM, De Camargo ZP, Lopes-Bezerra LM, Ishida K, De Souza W, Rozental S. Front. Microbiol. 2016;7:1. doi: 10.3389/fmicb.2016.00311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lomenick B, Shi H, Huang J, Chen C. Bioorganic Med. Chem. Lett. 2015;25:4976. doi: 10.1016/j.bmcl.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park C, Moon DO, Ryu CH, Choi BT, Lee WH, Kim GY, Choi YH. Acta Pharmacol. Sin. 2008;29:341. doi: 10.1111/j.1745-7254.2008.00761.x. [DOI] [PubMed] [Google Scholar]
- 150.Pan L, Lezama-Davila CM, Isaac-Marquez AP, Calomeni EP, Fuchs JR, Satoskar AR, Kinghorn AD. Phytochemistry. 2012;82:128. doi: 10.1016/j.phytochem.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Takahashi HT, Britta EA, Longhini R, Ueda-Nakamura T, Palazzo De Mello JC, Nakamura CV. Planta Med. 2013;79:330. doi: 10.1055/s-0032-1328258. [DOI] [PubMed] [Google Scholar]
- 152.Leliebre-Lara V, Fidalgo LM, Pferschy-Wenzig EM, Kunert O, Lima CN, Bauer R. Molecules. 2016;21:1045. doi: 10.3390/molecules21081045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Elkhayat ES, Ibrahim SRM, Mohamed GA, Ross SA. Nat. Prod. Res. 2016;30:814. doi: 10.1080/14786419.2015.1072711. [DOI] [PubMed] [Google Scholar]
- 154.G.H. Braun, H.P. Ramos, A.C.B.B. Candido, R.C.N. Pedroso, K.A. Siqueira, M.A. Soares, G.M. Dias, L.G. Magalhães, S.R. Ambrósio, A.H. Januário, R.C.L.R. Pietro, Nat. Prod. Res. 1 (2019) [DOI] [PubMed]
- 155.Silva AAS, Morais SM, Falcão MJC, Vieira IGP, Ribeiro LM, Viana SM, Teixeira MJ, Barreto FS, Carvalho CA, Cardoso RPA, Andrade-Junior HF. Phytomedicine. 2014;21:1419. doi: 10.1016/j.phymed.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 156.Keller L, Siqueira-neto JL, Souza JM, Eribez K, Lamonte GM, Smith JE, Gerwick WH. Molecules. 2020;25:1604. doi: 10.3390/molecules25071604. [DOI] [PMC free article] [PubMed] [Google Scholar]


