Abstract
Ischemic and traumatic injuries to CNS remain leading causes of death and disability worldwide, despite decades of research into risk factors, therapies, and preventative measures. Recent studies showed that CNS injuries significantly alter the cerebral microRNAome that impact the secondary brain damage as well as plasticity and recovery. Many microRNA based therapies are currently in various clinical trials for different pathologic conditions indicating their therapeutic potential. In the present review, we discuss the role of miR-21 in acute CNS injuries which is currently thought to be a potent neuroprotective microRNA. We emphasize on the potential of miR-21 in promoting cell and tissue survival and preventing inflammation and apoptosis. We also discussed the role of miR-21 in conditioning the brain to promote ischemic tolerance. Finally, we discussed some of the challenges and difficulties to develop miR-21 as a neuroprotective therapy in humans.
Keywords: Stroke, CNS injury, therapy, miR-21, neuroprotection
Various classes of non-coding RNAs (ncRNAs) that represent >98% of the transcriptional output in humans are considered as major controllers of transcription and translation (Cech and Steitz, 2014). The ncRNAs are diverse in size. They can be small like microRNAs (miRNAs) and piwi-interacting RNAs or medium-sized like small nucleolar RNAs and small Cajal body-specific RNAs or big like long noncoding RNAs (lncRNAs) and telomere-associated RNAs (Vemuganti, 2013). The ncRNAs are also very diverse in their function (Chandran et al., 2017). Ribosomal RNAs and transfer RNAs are the most studied ncRNAs that control translation. Whereas, miRNAs which are ~22 nucleotides long also control translation by binding to 6–8 nucleotide complementary seed sequences in the 3’ untranslated regions (3’-UTRs) of mRNAs (Lee et al., 2002; Diederichs and Haber, 2007). The lncRNAs are known to modulate transcription by binding to other RNAs, chromatin modifying proteins, transcription factors and DNA (Kung et al., 2013).
Of the various classes of ncRNAs, miRNAs are the most studied. New miRNAs are continuously being discovered in all species and as of now 2,588 mature miRNAs were discovered in humans and 1,915 in mouse (http://www.mirbase.org/). The miRNAs are transcribed from specific genes by RNA polymerase II as several hundred base pairs of stem-loop structures called primary miRNAs (pri-miRNAs) (Lee et al., 2002). A pri-miRNA will be cleaved in the nucleus by RNase Drosha in association with a protein called DiGeorge Syndrome Critical Region 8 (DGCR8) to release 1 to 6 precursor miRNAs (pre-miRNAs) (Lee et al., 2003). The pre-miRNAs are ~80 nucleotide long hairpin structures that will be transported to cytosol by exportin-5 and cleaved by the RNase III Dicer to release mature miRNAs which are 18–24 nucleotides long (Diederichs and Haber, 2007).
Recent studies have shown that miRNAs play crucial roles in vertebrate development and disease progression including heart failure, diabetes, cancer, and various brain and kidney pathologies (Kantharidis et al., 2011; Jansson and Lund, 2012; Jimenez-Mateos and Henshall, 2013; Olson, 2014). Several miRNA-based therapies also transitioned from preclinical studies to clinical trials. These include antimiR-122 for hepatitis C, antimiR-155 for cutaneous T-cell lymphoma, miR-34 mimic for solid tumors, miR-16 mimic for mesothelioma, miR-29 mimic for scleroderma (Rupaimoole and Slack, 2017). Preclinical studies showed that miR-122 is required for the replication of hepatitis C virus in liver (Baek et al., 2014). Following this lead, miravirsen (miR-122 antagonist) is currently in a phase 2a clinical trial and the initial results showed decreased viral load of hepatitis C for up to 14 weeks after the last treatment (Janssen et al., 2013; van der Ree et al., 2014). MiR-34a is a tumor suppressor miRNA that is often downregulated or lost in solid tumors, including breast, colorectal, liver, lung, and prostate cancer (Saito et al., 2015; Adams et al., 2016). Transfection with a miR-34a mimic decreased invasion/migration in cultured human hepatocellular carcinoma cells (Li et al., 2009) and promoted apoptosis in multiple myeloma cell lines (Di Martino et al., 2012). Furthermore, mouse models of prostate cancer and diffuse B-cell lymphoma showed tumor regression and improved survivorship after intravenous treatment with miR-34a mimic (Liu et al., 2011; Craig et al., 2012). Encouragingly, MRX34 (miR-34a mimic encapsulated in liposomes) recently met acceptable safety standards and showed potential antitumor activity in a phase I clinical trial (Beg et al., 2017). These clinical trials show the potential of miRNA-based therapies for cancers as well as other conditions like stroke with not many available drugs (Mozaffarian et al., 2016).
miRNAs modulate secondary neuronal damage following acute injuries to CNS
Traumatic and ischemic insults to the brain or spinal cord lead to significant neurological deficits that are exacerbated by secondary neuronal damage mediated by many synergistic pathophysiologic mechanisms that include excitotoxicity, inflammation, oxidative stress and apoptosis (Dreier, 2011; Dirnagl, 2012; Li et al., 2017). Recent studies showed that both ischemic and traumatic injuries to CNS are associated with altered cerebral miRNA expression profiles (Vijayan and Reddy, 2016; Di Pietro et al., 2017). Many preclinical studies also showed that secondary brain damage and neurological dysfunction can be curtailed and/or restorative mechanisms like neurogenesis and angiogenesis can be induced by manipulating specific miRNAs (Lou et al., 2012; Liu et al., 2013b; Zeng et al., 2014). In this section, we discuss the role of miRNAs after CNS injury, using stroke as a specific example. Role of miRNAs after traumatic brain injury (TBI) and spinal cord injury (SCI) is discussed at a later stage in the manuscript.
Cerebral miRNA expression profiles were shown to be significantly altered as early as 30 minutes and as late as 3 days of reperfusion following focal or global cerebral ischemia in rodents (Jeyaseelan et al., 2008; Dharap et al., 2009; Yuan et al., 2010). Pathway analysis showed that ischemia leads to upregulation of miRNAs that target pro-survival mRNAs and down-regulation of miRNAs that target pro-apoptotic and/or pro-inflammatory mRNAs (Jeyaseelan et al., 2008; Liu et al., 2010a; Hunsberger et al., 2012). This leads to an unfavorable environment for cellular survival in the ischemic brain.
Many preclinical studies evaluated the significance of modulating specific miRNAs to decrease secondary brain damage and/or to induce plasticity/regeneration after stroke. The miR-145 was reported to be significantly upregulated in rat brain following transient focal ischemia, and treatment with antagomiR-145 was shown to decrease infarction (Dharap et al., 2009). This neuroprotective effect was observed to be mediated by de-repression of miR-145 target superoxide dismutase-2 (SOD2), which is an antioxidant protein that alleviates oxidative stress in the ischemic brain (Dharap et al., 2009). Many other miRNAs were also shown to modulate post-ischemic pathology. Notable examples are let-7f, miR-23a and miR-497. Improved neurological outcome and decreased infarct volume after focal ischemia was shown by inhibiting let-7f (targets insulin-like growth factor 1), miR-23a (targets X-linked inhibitor of apoptosis) and miR-497 (targets pro-apoptotic Bcl2) (Yin et al., 2010; Siegel et al., 2011; Selvamani et al., 2012).
Due to the redundancy of seed sequences and presence of binding sites for multiple miRNAs in the 3’UTRs, miRNAs act in concert to affect specific pathways. For example, several miRNAs modulate post-ischemic inflammation by targeting nuclear factor kappa B (NF-κB) pathway (Buchan et al., 2000; Xu et al., 2012). When rats were transfected with an adenoviral vector overexpressing miR-22, its target nuclear receptor coactivator 1 (NCOA1; a NF-κB coactivator) was repressed leading to neuroprotection after stroke (Yu et al., 2015). MyD88, a protein adaptor for receptors that mediate nuclear translocation of NF-κB is targeted by miR-203 (Yang et al., 2015). Treatment with a miR-203 mimic was shown to silence NF-κB signaling resulting in decreased infarct volume, less edema and improved motor function recovery after stroke in mice (Yang et al., 2015). AntagomiR-181a administered either intravenous or intracerebroventricular was protective against ischemic injury in mice, likely due to degradation of GRP78, which is a NF-κB coactivator (Ouyang et al., 2012; Xu et al., 2015).
miRNAs and ischemic tolerance in the brain
Hibernating animals survive without brain damage for months with very low oxygen and glucose and this physiologic adaptation makes them resistant to stroke-induced brain damage (Zhou et al., 2001; Dave et al., 2006). Several studies have implicated miRNAs in the torpor response in different organs including liver, heart and skeletal muscle of hibernating animals including ground squirrels, bats and Dromiciops (Yuan et al., 2015; Hadj-Moussa et al., 2016; Wu et al., 2016). The brain of torpid hibernating brown bats showed altered miRNA expression when compared to non-torpid littermates (Biggar and Storey, 2014). Notably, they showed altered expression of 10 miRNAs (2 decreased and 8 increased) which target the translation of proteins that modulate focal adhesion and axon guidance (Biggar and Storey, 2014). We will discuss the therapeutic potential of miR-21 which is one of the upregulated miRNAs observed in this study. Ischemic tolerance can be induced in many species by a short duration ischemic insult which prepares the organs for a subsequent long duration ischemic event (Liu et al., 1992; Stetler et al., 2014; Varga et al., 2014). This preconditioning (PC) effect was shown to be associated with significantly altered miRNA expression profiles in rats, mice and gerbils. Following ischemic PC induced by a 10 min middle cerebral artery occlusion (MCAO) in adult rats, several miRNAs that target neuroprotective pathways were shown to be downregulated while those that target cell death pathways were upregulated in cerebral cortex from 6h to 3 days (Dharap and Vemuganti, 2010). Interestingly, miR-21 was the miRNA that showed a consistent induction (from 6h to 3 days) and also the highest fold increase in rat brain after ischemic PC (Dharap and Vemuganti, 2010). A study of in vitro ischemic PC using oxygen-glucose deprivation (OGD) in rat hippocampal neurons also showed miR-21 upregulation at 1 day of re-oxygenation (Keasey et al., 2016). Altered miRNA expression profiles were also shown in the mouse brain from 3h to 3 days following ischemic PC induced by a short duration MCAO (Lee et al., 2010; Lusardi et al., 2010). Suppression of miR-132 and subsequent induction of the transcriptional repressor methyl CpG binding protein 2 (MeCP2) were implicated as the major mediator of PC in mouse brain (Lusardi et al., 2010). These authors showed that MeCP2 knockout mice will not develop ischemic tolerance when subjected to PC.PC induced by a short duration global ischemia in gerbils altered the expression of several miRNAs for up to 6 months, although the consequence of this was not evaluated further (Sun et al., 2015). Whereas, Lee et al. (2010) attributed the PC-induced ischemic tolerance to upregulation of miR-200 leading to degradation of its target prolyl hydroxylase 2, which is a part of the pathway that modulates hypoxia-inducible factor 1α (Ratcliffe et al., 2017).
miR-21 as a therapeutic candidate for CNS injury
The previous sections indicated that stroke alters many miRNAs and several of them can serve as biomarkers to identify a disease state as well as serve as therapeutic targets to protect the brain. Of these, miR-21, one of the first miRNAs isolated from mammalian tissue, stands out as a strong candidate for translation to human stroke therapy (Lagos-Quintana et al., 2002; Krichevsky and Gabriely, 2009; Kumarswamy et al., 2011). Putative functions of miR-21 have been investigated in many pathologies including cardiac ischemia, hepatic fibrosis and different types of cancers (Pan et al., 2010; Zhang et al., 2013b; Gu et al., 2015). Additionally, several predicted gene targets of miR-21 have been validated (Cheng et al., 2010; Buscaglia and Li, 2011). Importantly, miR-21 represses the translation of proteins that promote apoptosis and inflammation and hence helps the post-ischemic outcome (Xu et al., 2014).
Anti-apoptotic and anti-inflammatory functions of miR-21
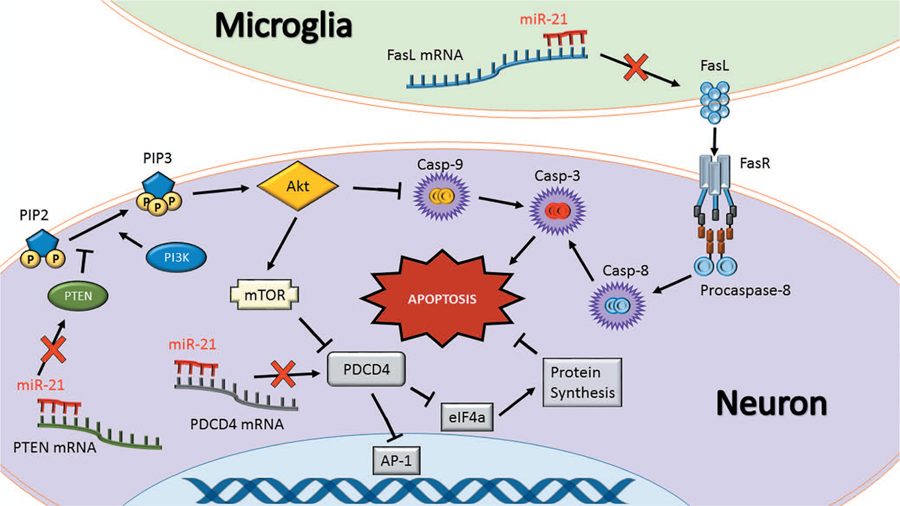
The best studied targets of miR-21 are programmed cell death 4 (PDCD4), Fas ligand (FasL) and phosphatase and tensin homologue (PTEN) (Fig. 1). All of these are strongly implicated in promoting apoptosis and inflammation (Lu et al., 2008; Sayed et al., 2010; Sheedy et al., 2010; Das et al., 2014; Choi et al., 2015; Wu et al., 2015; Yang et al., 2016a). The dual role of these proteins emphasizes that apoptosis and inflammation potentiate each other in a feedback loop. PDCD4 promotes apoptosis by binding to the translational initiation factor eIF4a and arresting translation of activator protein 1 (AP-1) (Yang et al., 2001; Loh et al., 2009). FasL is expressed on the surface of immune cells and binds to Fas death receptor to promote apoptosis via caspase cleavage and/or p53 activation (Benchimol, 2001; Broughton et al., 2009). PTEN dephosphorylates phosphatidylinositol 1,4,5-trisphosphate (PIP3) to form phosphatidylinositol 4,5-bisphosphate (PIP2), preventing PIP3-mediated activation of the kinase Akt, resulting in caspase cleavage (Tu et al., 2013; Yang et al., 2014). Loss of PDCD4, FasL and PTEN contributes to increased survival of cancer cells (Chen et al., 2003; Viard-Leveugle et al., 2003; Peng et al., 2016). Many studies implicated miR-21 as an oncomiR since its enrichment in primary tumors negatively correlates with the expression of these proteins (Chen et al., 2008; Li et al., 2013a; Shang et al., 2015). However, it is key to bear in mind that this anti-apoptotic function which is deleterious for cancer is beneficial in conditions of acute brain injury by promoting cell survival.
Figure 1: miR-21 regulates apoptosis.
Fas ligand (FasL), programmed cell death protein 4 (PDCD4), and phosphatase and tensin homolog (PTEN) are the best-characterized targets of miR-21. These proteins synergistically promote apoptosis. FasL is either membrane-bound or soluble and physically binds to the Fas death receptor (FasR) to initiate a caspase cleavage cascade resulting in apoptosis. PTEN is a tumor suppressor and a negative regulator of PI3K that phosphorylates PIP2 to form PIP3 that activates Akt pathway leading to cell death. As such, prevention of PTEN by miR-21 can lead to better cell survival following conditions like stroke and TBI. PI3K and Akt also modulate caspase-9 cleavage and hence, PTEN inhibition leads to prevention of apoptosis. Furthermore, PI3K/Akt activates mammalian target of rapamycin (mTOR) that inhibits PDCD4 activity. PDCD4 causes a protein expression bottleneck by inhibiting eukaryotic initiation factor 4a (eIF4a) and the transcription factor activator protein 1 (AP-1). Preventing eIF4a activity causes endoplasmic reticulum stress and inhibiting AP-1 prevents expression of beneficial genes such as vascular endothelial growth factor (VEGF). As inhibition of FasL, PTEN or PDCD4 together is a powerful strategy to prevent apoptosis and hence miR-21 is an attractive therapeutic to protect the post-injury brain. Tipped arrows indicate activation and blunted arrows indicate inhibition.
The anti-inflammatory effect of miR-21 has been demonstrated in models of acute inflammation, peritonitis, cardiovascular disease and kidney injury (Feng et al., 2014; Toldo et al., 2014; Jia et al., 2015; Barnett et al., 2016; Zhang and Shu, 2016). The miR-21 target PDCD4 in particular has been shown to activate NF-κB transcription leading to inhibition of the anti-inflammatory cytokine IL-10 (Yang et al., 2001; Young et al., 2010). PTEN/Akt signaling is required for site-directed immune cell migration due to control of its downstream factors mammalian target of rapamycin (mTOR) and FoxO1, which modulate cell growth and metabolism (Li et al., 2000; Hedrick et al., 2012; Zhang et al., 2013a; Xie et al., 2014). Additionally, PTEN knockout mice are resistant to pneumonia infection (Schabbauer et al., 2010). The membrane bound isoform of FasL promotes neutrophil recruitment acting as a chemoattractant for these cells (Hohlbaum et al., 2000; Dupont and Warrens, 2007).
In addition to FasL, PDCD4, and PTEN, miR-21 targets many mRNAs that encode pathologically relevant pro-inflammatory proteins. The transcription factor signal transducer and activator of transcription 3 (STAT3) is a validated target of miR-21 (Wang et al., 2015). Once activated by transmembrane receptor kinases, STAT-3 translocate into the nucleus and transcribes many genes including SOD2, Bcl family and intracellular adhesion molecule 1 (Dauer et al., 2005; Yu et al., 2009). All these are known to modulate oxidative stress, inflammation and apoptosis. Interestingly, colon and skin cancer cells implicate miR-21/PTEN/Akt axis and STAT3 in a feedback regulatory loop such that STAT3 knockdown prevents miR-21 expression, and overexpression of miR-21 increases IL-6 levels which in turn activates STAT3 (Iliopoulos et al., 2010; Lu et al., 2015).
Clinical studies implicated that miR-21 silencing could be therapeutically beneficial in conditions like chronic inflammatory diseases, diabetic nephropathy and renal fibrosis (Zhong et al., 2011; Wang et al., 2014; McClelland et al., 2015). Increased levels of miR-21 in the blood of patients with chronic kidney fibrosis suggest that miR-21 might be a robust biomarker of this disease (Glowacki et al., 2013). MiR-21 expression was also shown to be elevated significantly in blood samples from patients suffering with chronic cardiopulmonary diseases, cardiac fibrosis, idiopathic pulmonary fibrosis and coronary heart disease (Liu et al., 2010b; Li et al., 2015; Lorenzen et al., 2015). A seemingly contradictory finding attributed to miR-21 is silencing its target SMAD7, a signaling molecule that binds to the intracellular region of the transforming growth factor beta receptor (TGFβR), and thus prevents phosphorylation of SMAD2 and SMAD3 (Wang et al., 2014; Choi et al., 2016). When SMAD2/3 are phosphorylated, they translocate into nucleus to promote transcription of extracellular matrix proteins such as collagen I and fibronectin (Meng et al., 2015b). The role of miR-21 in controlling SMAD7 was thought to be important in various conditions like hepatic fibrosis, atrial fibrosis, pulmonary fibrosis and carcinoma-associated fibroblast formation (Li et al., 2013b; He et al., 2016; Kwon et al., 2016; Yang et al., 2016b).
Role of miR-21 in stem cell integrity and angiogenesis
Embryonic and new born mouse brains were shown to be enriched with miR-21 that correlates with SOX2 levels till postnatal day 7 (Polajeva et al., 2012). Furthermore, miR-21 and SOX2 levels showed similar pattern of induction in mouse brain tumor samples, and treatment with antagomiR-21 significantly reduced SOX2 levels in both mouse and human glioma cells (Polajeva et al., 2012). As SOX2 is a transcription factor required for neural stem cell pluripotency, this study indicates the role of miR-21 in stem cell pluripotency, brain development and glioma expansion. A recent study showed increased proliferation of neural progenitor cells cultured in hypoxic conditions that can be attributed to activation of the miR-21/PTEN/Akt axis (Chen et al., 2017).
Increased levels of miR-21 are associated with better integrity of cardiac stem cells (CSC) and their proliferation via its target PTEN. Rat CSCs transfected with miR-21 mimic were robustly protected against lethal H2O2 exposure which was associated with decreased PTEN levels resulting in increased Bcl:Bax ratio, decreased cleaved caspase-3 levels and fewer apoptotic cells (Hori and Nishida, 2009; Deng et al., 2016). CSCs from both mice and rats showed increased proliferation and trans-well migration after transfection with miR-21 mimic (Zhou et al., 2016; Shi et al., 2017). Improved proliferation/migration of stem cells was mimicked and attenuated respectively, by small-molecule inhibitors of PTEN and PI3K, suggesting that PTEN degradation is a key mechanism of miR-21-mediated proliferation/migration of CSCs (Shi et al., 2017).
Exosomes from stem cell are known to contain high levels of pro-survival proteins, RNAs and intact mitochondria (Lai et al., 2011; Phinney et al., 2015). Exosomes derived from human endometrial mesenchymal stem cells and cardiomyocyte progenitor cells were found to be enriched with miR-21. Rat cardiomyocytes cultured in exosome conditioned medium were resistant to oxidative stress-mediated apoptosis in vitro and this protection was attributed to uptake of miR-21 and degradation of its target PDCD4 (Xiao et al., 2016). A subsequent study showed that exosome-conditioned rat cardiomyocytes exhibited improved engraftment and survival after transplantation into the ischemic rat heart, and this improvement was attributed to prevention of PTEN translation by miR-21 (Wang et al., 2017). Together, these studies support a pro-recovery role of miR-21 after an injury to promote plasticity after stroke (Barkho and Zhao, 2011; Goritz and Frisen, 2012).
Role of miR-21 in stroke
Preclinical models show a highly phasic pattern of miR-21 expression after ischemic insults. Rat cortical neurons subjected to OGD showed induction of miR-21 by 1 day (Ziu et al., 2011). However, in microglia subjected to hypoxia, miR-21 was downregulated leading to induction of its target Fas ligand that was thought to promote apoptosis (Zhang et al., 2012). Adult rats subjected to global ischemia or embolic focal ischemia also showed induction of miR-21 at 1 day of reperfusion (Deng et al., 2013; Liu et al., 2013a; Li et al., 2016b). In the rat MCAO model of focal ischemia, miR-21 upregulated significantly in the penumbra from 2 to 7 days post reperfusion (Buller et al., 2010). These studies indicate that miR-21 might be a player in promoting neuroprotection as well as plasticity and recovery after stroke.
MiR-21 was shown to inhibit apoptosis by preventing FasL protein expression after OGD (Buller et al., 2010). In cultured rat cortical neurons subjected to OGD, FasL levels and apoptosis were curtailed by transfection with miR-21 mimic and induced by transfection with antagomiR-21 (Buller et al., 2010). A subsequent study showed that when rat hippocampal neurons were cultured in conditioned medium from rat microglia subject to OGD, there was an induction of FasL and increased apoptosis (Zhang et al., 2012). Furthermore, transfecting the microglia with a miR-21 mimic before the exposure to OGD attenuated FasL expression and prevented neuronal death in hippocampal neurons cultured with the conditioned medium (Zhang et al., 2012). Peripheral blood samples from ischemic and hemorrhagic stroke patients collected at an acute stage (12 to 24h after stroke) showed significantly lower miR-21 levels compared to healthy age-matched controls (Zhou and Zhang, 2014; Wang et al., 2016). Interestingly, elevated miR-21 in the peripheral blood correlated with increased incidence of stroke-associated infections and decreased serum interferon gamma (IFNγ) levels (Lin et al., 2016). This is attributes a putative anti-inflammatory effect to miR-21.
Role of miR-21 in traumatic brain injury
Neuronal death and neurological dysfunction after TBI is also synergistically mediated by apoptosis, inflammation and oxidative stress similar to stroke (Masel and DeWitt, 2010). However, there are certain key differences between TBI and stroke pathologies. For example, post-TBI lesions exhibit significant diffuse axonal injury and increased necrosis unlike post-stroke infarcts (Vieira et al., 2016). Furthermore, TBI is highly associated with hemorrhages that contribute to the secondary brain damage (Nolan, 2005). Despite these differences, preclinical studies show similar patterns of miR-21 expression in TBI and stroke. In rats, fluid percussion injury (FPI) upregulated miR-21 expression in the contused cortex at 1 to 7 days after the injury (Lei et al., 2009; Ge et al., 2014; Ge et al., 2015). Whereas, controlled cortical impact (CCI) injury in rats was shown to down-regulate miR-21 levels at 3h with subsequent upregulation from 1 to 3 days after the injury (Redell et al., 2011). Mice subjected to CCI injury also showed upregulation of miR-21 from 6h to 7 days post injury (Sandhir et al., 2014; Meissner et al., 2016). The miR-21 was reported to be enriched in the extracellular vesicles isolated from the contused hemisphere of mice at 7 days after CCI injury (Harrison et al., 2016). Expression patterns of miR-21 are different in aged and young mice, such that miR-21 levels returned to sham levels by 1 day after injury in aged mice, while they stayed elevated till 7 days post injury in young mice (Sandhir et al., 2014). Compared to young mice, aged mice showed a higher degree of secondary brain damage and increased expression of the miR-21 target proteins PTEN, PDCD4 and tissue inhibitor of metalloproteinase 3 (TIMP3) (Sandhir et al., 2014). Intracerebroventricular injection of miR-21 mimic to rats after FPI was shown to suppress PTEN expression with corresponding increased levels of p-Akt, decreased TUNEL staining, lower Bcl2/Bax ratio and decreased cleaved caspase-3 levels that led to reduced lesion size and curtailed edema and improved behavioral outcome (Ge et al., 2014; Ge et al., 2015). Thus, miR-21 was shown to inhibit apoptosis by promoting the degradation of PTEN. The miR-21-PTEN-Akt axis was also explored in cultured rat cortical neurons subjected to scratch injury (Morrison et al., 2011). Neurons transfected with miR-21 mimic 1 day before scratch injury showed PTEN degradation, increased Bcl2/Bax ratio, decreased TUNEL staining and decreased levels of cleaved caspase-3 and 9 similar to that observed in the in vivo TBI models (Han et al., 2014). Apoptosis after TBI may be modulated by PDCD4, which inversely correlated with mir-21 expression in CCI-contused rats and mice (Redell et al., 2011; Sandhir et al., 2014). All these studies indicate that induction of miR-21 is a neuroprotective adaptation to protect the brain after TBI.
After TBI, miR-21 seems to be playing a neurorestorative role as well. At 3 days post-FPI, rats treated with miR-21 mimic showed induction of claudin-5 and occludin in the injury lesion and boundary (Ge et al., 2015). At 7 days post-FPI, miR-21 treated rats showed increased microvascular density and vascular endothelial growth factor (VEGF) expression (Ge et al., 2014). At both 3 and 7 days post-FPI, edema was decreased, and expression of Ang1 and Tie2 was increased compared to controls; however the mechanism of miR-21-mediated effects on Ang1/Tie2 axis was not evaluated in detail (Ge et al., 2014; Ge et al., 2015). These studies in general indicate that miR-21 promotes blood-brain barrier (BBB) integrity and angiogenesis after TBI. Many targets of miR-21 are pro-inflammatory and pro-apoptotic. When rat brain microvascular endothelial cells transfected with miR-21 mimic were subjected to scratch injury, expression of the pro-inflammatory TNFα, IL-6 and NF-κB decreased and the expression of the anti-inflammatory IL-10 increased (Ge et al., 2016). Furthermore, the scratch injured cells transfected with miR-21 mimic showed decreased apoptosis (as measured by Annexin V and cleaved caspase-9 staining) and elevated levels of claudin-5, occludin, Ang1, and Tie2 that might promote endothelial cell function (Ge et al., 2016).
Role of miR-21 in spinal cord injury
SCI causes extensive sensory and motor deficits that are synergistically mediated by several secondary injury mechanisms that include excitotoxicity, inflammation, oxidative stress and apoptosis (Silva et al., 2014). Despite these mechanistic similarities, SCI pathology is distinct from stroke pathology and poses its own set of challenges (Oyinbo, 2011). The average SCI patient is younger than the average stroke patient and hence the post-injury period of survival is longer. SCI has an extended chronic phase that is characterized by the formation of a perilesional glial scar composed of astrocytes and extracellular matrix that prevents axonal growth (Fawcett and Asher, 1999).
Rats subjected to SCI showed increased miR-21 expression between 3 and 35 days post-injury (Liu et al., 2009; Bhalala et al., 2012; Yunta et al., 2012; Hu et al., 2013). A single bolus of intravenous administration of the omega-3 fatty acid docosahexaenoic acid (DHA) that improves learning, memory, and motor function recovery after SCI (Lim et al., 2013) increased miR-21 levels leading to decreased levels of its target PTEN (Liu et al., 2015). Furthermore, neuronal cultures treated with DHA showed increased neurite growth which was attenuated by antagomiR-21 treatment (Liu et al., 2015). Intrathecal administration of antagomiR-21 after SCI led to increased expression of FasL and PTEN, bigger lesion size and less recovery of the hind limb function (Hu et al., 2013). A recent study showed that in rats subjected to weight drop SCI, treatment with antagomiR-21 increased TIMP3 expression and decreased vascular density, whereas treatment with either miR-21 mimic or TIMP3 siRNA induced MMP2 and MMP9 expression and improved capillary network formation (Hu et al., 2016).
Though the mechanism is unclear, miR-21 was shown to play a role in ameliorating astroglial hypertrophy. Lentivirus-mediated overexpression of miR-21 decreased GFAP expression and cell size in primary mouse astrocytes (Sahni et al., 2010). Transgenic mice that overexpress miR-21 showed decreased GFAP density and thinner astrocytic processes at 14 and 35 days after SCI compared to wild-type controls (Bhalala et al., 2012). This group further demonstrated that transgenic mice that express a miR-21 sponge show persistent astrocytic activation and hypertrophy up to 35 days post-SCI. Mice expressing the miR-21 sponge showed increased axon density across the SCI lesion, which is contradictory to the evidence presented above that miR-21 antagonism is associated with poor outcome after SCI (Hu et al., 2013). The authors justified this discrepancy by hypothesizing that the activated, hypertrophic astrocytes in the miR-21 sponge expressing mice prevented formation of the glial scar (Bhalala et al., 2012). Overall, these studies indicate that overexpression of miR-21 at an acute stage after SCI is potentially therapeutic, but inhibition of miR-21 during the chronic phase after SCI might promote recovery.
Role of miR-21 in other CNS pathologies
MiR-21 is also implicated in several non-ischemic, non-traumatic CNS pathologies. It was shown to be upregulated in the cerebral cortex of autism patients (Mor et al., 2015), in the CSF of patients with viral CNS infection (Goswami et al., 2017) and in peripheral blood mononuclear cells from multiple sclerosis patients (Fenoglio et al., 2011). Studies showed that exposure of murine cortical neurospheres to high doses of alcohol repress miR-21 (Sathyan et al., 2007; Balaraman et al., 2012). However, the functional significance of miR-21 in all these conditions is not known.
Role of miR-21 in epilepsy, HIV/SIV and Parkinson’s disease (PD) was evaluated in detail. In the rat hippocampus, miR-21 was observed to be significantly down-regulated at 1 day, but upregulated from 2 to 30 days following lithium pilocarpine (LiP)-induced epilepsy (Hu et al., 2011; Risbud et al., 2011; Meng et al., 2015a; Roncon et al., 2015; Chak et al., 2016). In both juvenile and adult epileptic patients, miR-21 was reported to be upregulated in the hippocampus (Peng et al., 2013; Roncon et al., 2015). These findings corroborate earlier studies which show that suppression of hippocampal and forebrain neurotrophin 3 (NT3; a miR-21 target gene) is associated with development of status epilepticus in LiP-treated rats (Schmidt-Kastner and Olson, 1995; Mudo et al., 1996). In the brains of SIV-infected rhesus monkeys and HIV-infected humans, miR-21 was reported to be upregulated in neurons, and trafficked to microglia via extracellular vesicles (Yelamanchili et al., 2015). This neuronal upregulation of miR-21 inversely correlated with levels of its target monocyte enhancement factor 2c (MEF2c) (Yelamanchili et al., 2010) which is a transcription factor associated with potassium channel signaling required for neuronal survival and learning (Barbosa et al., 2008). In the context of HIV/SIV, miR-21-mediated decrease in MEF2c resulted in outward potassium neuronal currents, which is a sign of neuronal commitment to apoptosis (Yelamanchili et al., 2010). Cerebral mir-21 levels were observed to be markedly upregulated in the MPTP-induced PD in mice (Su et al., 2016). Mir-21 was shown to target lysosome-associated membrane protein 2 which increases the degradation of α-synuclein by promoting chaperone-mediated autophagy and thus might prevent the PD-like pathology (Xilouri et al., 2013; Su et al., 2016).
Steps to therapeutic translation
The evidence presented previously suggests that miR-21 is already an ideal candidate for translation to the clinic. However, most of these studies were conducted with either cell cultures or rodent models of stroke, TBI and SCI. For therapeutic clinical translation of miR-21, further robust and large-scale preclinical studies are needed. For example, the post-stroke therapies need to be tested following the recommendations of the Stroke Treatment Academic Industry Roundtable (STAIR) criteria that include testing both sexes, different age groups, minimal efficacious dose with no toxicity, half-life, different routes of administration, window of therapeutic opportunity, longer-term multiple functional outcomes, extensive physiological monitoring, multiple disease models and importantly randomization and blinding of the studies (Saver et al., 2009; Savitz et al., 2011). It is also important to develop a proper tissue-specific delivery strategy without the toxicity of a transfection agent and preventing the RNase-mediated degradation are important for translating a miRNA therapy to humans. Studies are underway to test encapsulating the miRNA in liposomes and attaching a targeting moiety like in dendrimers to increase tissue-specific delivery and to chemically modify a miRNA by locked nucleic acid or phosphorothioate modifications to resist the RNases (Rupaimoole and Slack, 2017). Given the pro-survival function of miR-21, the most pressing concern is to confirm that patients don’t develop tumors with miR-21 therapy (Karsy et al., 2012; Pfeffer et al., 2015; Li et al., 2016a; Li et al., 2016c). The path to therapeutic translation is clear, but needs more studies.
Summary and Conclusions
All the above studies suggest that miR-21 is a promising candidate for therapeutic amelioration of secondary neuronal injury following acute and chronic insults to CNS. Importantly, increasing miR-21 levels might precondition the CNS to induce tolerance in case of an insult. The pro-survival effects of miR-21 in the CNS are typically attributed to inhibition of pro-apoptotic pathways controlled by the miR-21 targets PDCD4, PTEN, RECK, and FasL. Dozens of other pro-apoptotic targets of miR-21 have been verified in non-CNS paradigms (Buscaglia and Li, 2011).
Preventing apoptosis often results in decreased inflammatory burden by decreasing the amount of injured tissue requiring repair or clearance. However, miR-21 may directly affect inflammation via its gene targets PELI1, IL-12a, PTEN, FasL, and PDCD4, which regulate the NF-κB pathway. Further evidence for a directly anti-inflammatory role of miR-21 comes from investigations of peritonitis and LPS exposure, in which groups with elevated miR-21 levels survived and recovered better than the control groups (Feng et al., 2014; Barnett et al., 2016).
Encouragingly, the benefits of miR-21 are not limited to preventing damage, but are also tied to its ability to promote cell survival after injury. Neural and cardiac stem cells treated with miR-21 (via either transfection or culturing with miR-21 enriched exosomes) subjected to stressful stimuli such as hypoxia or H2O2 exposure showed decreased apoptosis and improvements in proliferation and migration, which are often considered as signs of stem cell integrity (Chen et al., 2017; Shi et al., 2017). MiR-21 was also shown to promote angiogenic recovery after SCI via its target TIMP3, which inhibits MMP2 and MMP9, and was shown to block VEGF binding to its receptor (Qi et al., 2003; Hu et al., 2016).
In conclusion, these studies implicate miR-21 in improving post-injurious outcomes in both preventative and therapeutic contexts. Overall, mi R-21 shows great promise as a neuroprotective agent against stroke and possibly other acute CNS injuries.
Acknowledgements
This work was supported in part by the U.S. Department of Veterans Affairs Merit Review Grant I01-BX002985, and by National Institute of Health Grants RO1-144AAC1463 and RO1-144AAC1715. Mary Lopez was supported by a pre-doctoral Advanced Opportunity Fellowship through SciMed GRS at the University of Wisconsin-Madison.
References
- Adams BD, Parsons C, Slack FJ (2016) The tumor-suppressive and potential therapeutic functions of miR-34a in epithelial carcinomas. Expert opinion on therapeutic targets 20:737–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek J, Kang S, Min H (2014) MicroRNA-targeting therapeutics for hepatitis C. Archives of pharmacal research 37:299–305. [DOI] [PubMed] [Google Scholar]
- Balaraman S, Winzer-Serhan UH, Miranda RC (2012) Opposing actions of ethanol and nicotine on microRNAs are mediated by nicotinic acetylcholine receptors in fetal cerebral cortical-derived neural progenitor cells. Alcoholism, clinical and experimental research 36:1669–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa AC, Kim MS, Ertunc M, Adachi M, Nelson ED, McAnally J, Richardson JA, Kavalali ET, Monteggia LM, Bassel-Duby R, Olson EN (2008) MEF2C, a transcription factor that facilitates learning and memory by negative regulation of synapse numbers and function. Proceedings of the National Academy of Sciences of the United States of America 105:9391–9396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkho BZ, Zhao X (2011) Adult neural stem cells: response to stroke injury and potential for therapeutic applications. Current stem cell research & therapy 6:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett RE, Conklin DJ, Ryan L, Keskey RC, Ramjee V, Sepulveda EA, Srivastava S, Bhatnagar A, Cheadle WG (2016) Anti-inflammatory effects of miR-21 in the macrophage response to peritonitis. Journal of leukocyte biology 99:361–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg MS, Brenner AJ, Sachdev J, Borad M, Kang YK, Stoudemire J, Smith S, Bader AG, Kim S, Hong DS (2017) Phase I study of MRX34, a liposomal miR-34a mimic, administered twice weekly in patients with advanced solid tumors. Investigational new drugs 35:180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benchimol S (2001) p53-dependent pathways of apoptosis. Cell death and differentiation 8:1049–1051. [DOI] [PubMed] [Google Scholar]
- Bhalala OG, Pan L, Sahni V, McGuire TL, Gruner K, Tourtellotte WG, Kessler JA (2012) microRNA-21 regulates astrocytic response following spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 32:17935–17947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggar KK, Storey KB (2014) Identification and expression of microRNA in the brain of hibernating bats, Myotis lucifugus. Gene 544:67–74. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG (2009) Apoptotic mechanisms after cerebral ischemia. Stroke; a journal of cerebral circulation 40:e331–339. [DOI] [PubMed] [Google Scholar]
- Buchan AM, Li H, Blackburn B (2000) Neuroprotection achieved with a novel proteasome inhibitor which blocks NF-kappaB activation. Neuroreport 11:427–430. [DOI] [PubMed] [Google Scholar]
- Buller B, Liu X, Wang X, Zhang RL, Zhang L, Hozeska-Solgot A, Chopp M, Zhang ZG (2010) MicroRNA-21 protects neurons from ischemic death. The FEBS journal 277:4299–4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buscaglia LE, Li Y (2011) Apoptosis and the target genes of microRNA-21. Chinese journal of cancer 30:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cech TR, Steitz JA (2014) The noncoding RNA revolution-trashing old rules to forge new ones. Cell 157:77–94. [DOI] [PubMed] [Google Scholar]
- Chak K, Roy-Chaudhuri B, Kim HK, Kemp KC, Porter BE, Kay MA (2016) Increased precursor microRNA-21 following status epilepticus can compete with mature microRNA-21 to alter translation. Experimental neurology 286:137–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandran R, Mehta SL, Vemuganti R (2017) Non-coding RNAs and neuroprotection after acute CNS injuries. Neurochemistry international. [DOI] [PMC free article] [PubMed]
- Chen R, Liu Y, Su Q, Yang Y, Wang L, Ma S, Yan J, Xue F, Wang J (2017) Hypoxia stimulates proliferation of rat neural stem/progenitor cells by regulating mir-21: an in vitro study. Neuroscience letters 661:71–76. [DOI] [PubMed] [Google Scholar]
- Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, Ozaki I, Petersen I (2003) Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. The Journal of pathology 200:640–646. [DOI] [PubMed] [Google Scholar]
- Chen Y, Liu W, Chao T, Zhang Y, Yan X, Gong Y, Qiang B, Yuan J, Sun M, Peng X (2008) MicroRNA-21 down-regulates the expression of tumor suppressor PDCD4 in human glioblastoma cell T98G. Cancer letters 272:197–205. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Zhu P, Yang J, Liu X, Dong S, Wang X, Chun B, Zhuang J, Zhang C (2010) Ischaemic preconditioning-regulated miR-21 protects heart against ischaemia/reperfusion injury via anti-apoptosis through its target PDCD4. Cardiovascular research 87:431–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi B, Kim HA, Suh CH, Byun HO, Jung JY, Sohn S (2015) The relevance of miRNA-21 in HSV-induced inflammation in a mouse model. International journal of molecular sciences 16:7413–7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SI, Jin JY, Maeng YS, Kim TI, Kim EK (2016) TGF-beta regulates TGFBIp expression in corneal fibroblasts via miR-21, miR-181a, and Smad signaling. Biochem Biophys Res Commun 472:150–155. [DOI] [PubMed] [Google Scholar]
- Craig VJ, Tzankov A, Flori M, Schmid CA, Bader AG, Muller A (2012) Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 26:2421–2424. [DOI] [PubMed] [Google Scholar]
- Das A, Ganesh K, Khanna S, Sen CK, Roy S (2014) Engulfment of apoptotic cells by macrophages: a role of microRNA-21 in the resolution of wound inflammation. J Immunol 192:1120–1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB (2005) Stat3 regulates genes common to both wound healing and cancer. Oncogene 24:3397–3408. [DOI] [PubMed] [Google Scholar]
- Dave KR, Prado R, Raval AP, Drew KL, Perez-Pinzon MA (2006) The arctic ground squirrel brain is resistant to injury from cardiac arrest during euthermia. Stroke; a journal of cerebral circulation 37:1261–1265. [DOI] [PubMed] [Google Scholar]
- Deng W, Wang Y, Long X, Zhao R, Wang Z, Liu Z, Cao S, Shi B (2016) miR-21 Reduces Hydrogen Peroxide-Induced Apoptosis in c-kit+ Cardiac Stem Cells In Vitro through PTEN/PI3K/Akt Signaling. Oxidative medicine and cellular longevity 2016:5389181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng X, Zhong Y, Gu L, Shen W, Guo J (2013) MiR-21 involve in ERK-mediated upregulation of MMP9 in the rat hippocampus following cerebral ischemia. Brain research bulletin 94:56–62. [DOI] [PubMed] [Google Scholar]
- Dharap A, Vemuganti R (2010) Ischemic pre-conditioning alters cerebral microRNAs that are upstream to neuroprotective signaling pathways. Journal of neurochemistry 113:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharap A, Bowen K, Place R, Li LC, Vemuganti R (2009) Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 29:675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Martino MT et al. (2012) Synthetic miR-34a mimics as a novel therapeutic agent for multiple myeloma: in vitro and in vivo evidence. Clinical cancer research : an official journal of the American Association for Cancer Research 18:6260–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pietro V, Ragusa M, Davies D, Su Z, Hazeldine J, Lazzarino G, Hill LJ, Crombie N, Foster M, Purrello M, Logan A, Belli A (2017) MicroRNAs as Novel Biomarkers for the Diagnosis and Prognosis of Mild and Severe Traumatic Brain Injury. Journal of neurotrauma 34:1948–1956. [DOI] [PubMed] [Google Scholar]
- Diederichs S, Haber DA (2007) Dual role for argonautes in microRNA processing and posttranscriptional regulation of microRNA expression. Cell 131:1097–1108. [DOI] [PubMed] [Google Scholar]
- Dirnagl U (2012) Pathobiology of injury after stroke: the neurovascular unit and beyond. Annals of the New York Academy of Sciences 1268:21–25. [DOI] [PubMed] [Google Scholar]
- Dreier JP (2011) The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nature medicine 17:439–447. [DOI] [PubMed] [Google Scholar]
- Dupont PJ, Warrens AN (2007) Fas ligand exerts its pro-inflammatory effects via neutrophil recruitment but not activation. Immunology 120:133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain research bulletin 49:377–391. [DOI] [PubMed] [Google Scholar]
- Feng J, Li A, Deng J, Yang Y, Dang L, Ye Y, Li Y, Zhang W (2014) miR-21 attenuates lipopolysaccharide-induced lipid accumulation and inflammatory response: potential role in cerebrovascular disease. Lipids in health and disease 13:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenoglio C, Cantoni C, De Riz M, Ridolfi E, Cortini F, Serpente M, Villa C, Comi C, Monaco F, Mellesi L, Valzelli S, Bresolin N, Galimberti D, Scarpini E (2011) Expression and genetic analysis of miRNAs involved in CD4+ cell activation in patients with multiple sclerosis. Neuroscience letters 504:9–12. [DOI] [PubMed] [Google Scholar]
- Ge X, Han Z, Chen F, Wang H, Zhang B, Jiang R, Lei P, Zhang J (2015) MiR-21 alleviates secondary blood-brain barrier damage after traumatic brain injury in rats. Brain research 1603:150–157. [DOI] [PubMed] [Google Scholar]
- Ge X, Huang S, Gao H, Han Z, Chen F, Zhang S, Wang Z, Kang C, Jiang R, Yue S, Lei P, Zhang J (2016) miR-21–5p alleviates leakage of injured brain microvascular endothelial barrier in vitro through suppressing inflammation and apoptosis. Brain research 1650:31–40. [DOI] [PubMed] [Google Scholar]
- Ge XT, Lei P, Wang HC, Zhang AL, Han ZL, Chen X, Li SH, Jiang RC, Kang CS, Zhang JN (2014) miR-21 improves the neurological outcome after traumatic brain injury in rats. Scientific reports 4:6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glowacki F, Savary G, Gnemmi V, Buob D, Van der Hauwaert C, Lo-Guidice JM, Bouye S, Hazzan M, Pottier N, Perrais M, Aubert S, Cauffiez C (2013) Increased circulating miR-21 levels are associated with kidney fibrosis. PloS one 8:e58014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goritz C, Frisen J (2012) Neural stem cells and neurogenesis in the adult. Cell stem cell 10:657–659. [DOI] [PubMed] [Google Scholar]
- Goswami S, Banerjee A, Kumari B, Bandopadhyay B, Bhattacharya N, Basu N, Vrati S (2017) Differential Expression and Significance of Circulating microRNAs in Cerebrospinal Fluid of Acute Encephalitis Patients Infected with Japanese Encephalitis Virus. Molecular neurobiology 54:1541–1551. [DOI] [PubMed] [Google Scholar]
- Gu GL, Xu XL, Sun XT, Zhang J, Guo CF, Wang CS, Sun B, Guo GL, Ma K, Huang YY, Sun LQ, Wang YQ (2015) Cardioprotective Effect of MicroRNA-21 in Murine Myocardial Infarction. Cardiovascular therapeutics 33:109–117. [DOI] [PubMed] [Google Scholar]
- Hadj-Moussa H, Moggridge JA, Luu BE, Quintero-Galvis JF, Gaitan-Espitia JD, Nespolo RF, Storey KB (2016) The hibernating South American marsupial, Dromiciops gliroides, displays torpor-sensitive microRNA expression patterns. Scientific reports 6:24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Chen F, Ge X, Tan J, Lei P, Zhang J (2014) miR-21 alleviated apoptosis of cortical neurons through promoting PTEN-Akt signaling pathway in vitro after experimental traumatic brain injury. Brain research 1582:12–20. [DOI] [PubMed] [Google Scholar]
- Harrison EB, Hochfelder CG, Lamberty BG, Meays BM, Morsey BM, Kelso ML, Fox HS, Yelamanchili SV (2016) Traumatic brain injury increases levels of miR-21 in extracellular vesicles: implications for neuroinflammation. FEBS open bio 6:835–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Zhang K, Gao X, Li L, Tan H, Chen J, Zhou Y (2016) Rapid atrial pacing induces myocardial fibrosis by down-regulating Smad7 via microRNA-21 in rabbit. Heart and vessels 31:1696–1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrick SM, Hess Michelini R, Doedens AL, Goldrath AW, Stone EL (2012) FOXO transcription factors throughout T cell biology. Nature reviews Immunology 12:649–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohlbaum AM, Moe S, Marshak-Rothstein A (2000) Opposing effects of transmembrane and soluble Fas ligand expression on inflammation and tumor cell survival. The Journal of experimental medicine 191:1209–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori M, Nishida K (2009) Oxidative stress and left ventricular remodelling after myocardial infarction. Cardiovascular research 81:457–464. [DOI] [PubMed] [Google Scholar]
- Hu J, Ni S, Cao Y, Zhang T, Wu T, Yin X, Lang Y, Lu H (2016) The Angiogenic Effect of microRNA-21 Targeting TIMP3 through the Regulation of MMP2 and MMP9. PloS one 11:e0149537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu JZ, Huang JH, Zeng L, Wang G, Cao M, Lu HB (2013) Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. Journal of neurotrauma 30:1349–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Zhang C, Long L, Long X, Feng L, Li Y, Xiao B (2011) Expression profile of microRNAs in rat hippocampus following lithium-pilocarpine-induced status epilepticus. Neuroscience letters 488:252–257. [DOI] [PubMed] [Google Scholar]
- Hunsberger JG, Fessler EB, Wang Z, Elkahloun AG, Chuang DM (2012) Post-insult valproic acid-regulated microRNAs: potential targets for cerebral ischemia. American journal of translational research 4:316–332. [PMC free article] [PubMed] [Google Scholar]
- Iliopoulos D, Jaeger SA, Hirsch HA, Bulyk ML, Struhl K (2010) STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Molecular cell 39:493–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR (2013) Treatment of HCV infection by targeting microRNA. The New England journal of medicine 368:1685–1694. [DOI] [PubMed] [Google Scholar]
- Jansson MD, Lund AH (2012) MicroRNA and cancer. Molecular oncology 6:590–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaseelan K, Lim KY, Armugam A (2008) MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke; a journal of cerebral circulation 39:959–966. [DOI] [PubMed] [Google Scholar]
- Jia P, Teng J, Zou J, Fang Y, Wu X, Liang M, Ding X (2015) Xenon Protects Against Septic Acute Kidney Injury via miR-21 Target Signaling Pathway. Critical care medicine 43:e250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Mateos EM, Henshall DC (2013) Epilepsy and microRNA. Neuroscience 238:218–229. [DOI] [PubMed] [Google Scholar]
- Kantharidis P, Wang B, Carew RM, Lan HY (2011) Diabetes complications: the microRNA perspective. Diabetes 60:1832–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsy M, Arslan E, Moy F (2012) Current Progress on Understanding MicroRNAs in Glioblastoma Multiforme. Genes & cancer 3:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keasey MP, Scott HL, Bantounas I, Uney JB, Kelly S (2016) MiR-132 Is Upregulated by Ischemic Preconditioning of Cultured Hippocampal Neurons and Protects them from Subsequent OGD Toxicity. Journal of molecular neuroscience : MN 59:404–410. [DOI] [PubMed] [Google Scholar]
- Krichevsky AM, Gabriely G (2009) miR-21: a small multi-faceted RNA. Journal of cellular and molecular medicine 13:39–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumarswamy R, Volkmann I, Thum T (2011) Regulation and function of miRNA-21 in health and disease. RNA biology 8:706–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kung JT, Colognori D, Lee JT (2013) Long noncoding RNAs: past, present, and future. Genetics 193:651–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon OS, Kim KT, Lee E, Kim M, Choi SH, Li H, Fornace AJ Jr., Cho JH, Lee YS, Lee JS, Lee YJ, Cha HJ (2016) Induction of MiR-21 by Stereotactic Body Radiotherapy Contributes to the Pulmonary Fibrotic Response. PloS one 11:e0154942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T (2002) Identification of tissue-specific microRNAs from mouse. Current biology : CB 12:735–739. [DOI] [PubMed] [Google Scholar]
- Lai RC, Chen TS, Lim SK (2011) Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regenerative medicine 6:481–492. [DOI] [PubMed] [Google Scholar]
- Lee ST, Chu K, Jung KH, Yoon HJ, Jeon D, Kang KM, Park KH, Bae EK, Kim M, Lee SK, Roh JK (2010) MicroRNAs induced during ischemic preconditioning. Stroke; a journal of cerebral circulation 41:1646–1651. [DOI] [PubMed] [Google Scholar]
- Lee Y, Jeon K, Lee JT, Kim S, Kim VN (2002) MicroRNA maturation: stepwise processing and subcellular localization. The EMBO journal 21:4663–4670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, Kim VN (2003) The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419. [DOI] [PubMed] [Google Scholar]
- Lei P, Li Y, Chen X, Yang S, Zhang J (2009) Microarray based analysis of microRNA expression in rat cerebral cortex after traumatic brain injury. Brain research 1284:191–201. [DOI] [PubMed] [Google Scholar]
- Li C, Sun J, Xiang Q, Liang Y, Zhao N, Zhang Z, Liu Q, Cui Y (2016a) Prognostic role of microRNA-21 expression in gliomas: a meta-analysis. Journal of neuro-oncology 130:11–17. [DOI] [PubMed] [Google Scholar]
- Li G, Morris-Blanco KC, Lopez MS, Yang T, Zhao H, Vemuganti R, Luo Y (2017) Impact of microRNAs on ischemic stroke: From pre- to post-disease. Progress in neurobiology. [DOI] [PubMed]
- Li N, Fu H, Tie Y, Hu Z, Kong W, Wu Y, Zheng X (2009) miR-34a inhibits migration and invasion by down-regulation of c-Met expression in human hepatocellular carcinoma cells. Cancer letters 275:44–53. [DOI] [PubMed] [Google Scholar]
- Li P, Mao WM, Zheng ZG, Dong ZM, Ling ZQ (2013a) Down-regulation of PTEN expression modulated by dysregulated miR-21 contributes to the progression of esophageal cancer. Digestive diseases and sciences 58:3483–3493. [DOI] [PubMed] [Google Scholar]
- Li Q, Zhang D, Wang Y, Sun P, Hou X, Larner J, Xiong W, Mi J (2013b) MiR-21/Smad 7 signaling determines TGF-beta1-induced CAF formation. Scientific reports 3:2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yu P, Zeng Q, Luo B, Cai S, Hui K, Yu G, Zhu C, Chen X, Duan M, Sun X (2016b) Neuroprotective Effect of Hydrogen-Rich Saline in Global Cerebral Ischemia/Reperfusion Rats: Up-Regulated Tregs and Down-Regulated miR-21, miR-210 and NF-kappaB Expression. Neurochemical research 41:2655–2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Fan Q, He S, Tang T, Liao Y, Xie J (2015) MicroRNA-21 negatively regulates Treg cells through a TGF-beta1/Smad-independent pathway in patients with coronary heart disease. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology 37:866–878. [DOI] [PubMed] [Google Scholar]
- Li Y, Shang YM, Wang QW (2016c) MicroRNA-21 promotes the proliferation and invasion of neuroblastoma cells through targeting CHL1. Minerva medica 107:287–293. [PubMed] [Google Scholar]
- Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D (2000) Roles of PLC-beta2 and -beta3 and PI3Kgamma in chemoattractant-mediated signal transduction. Science 287:1046–1049. [DOI] [PubMed] [Google Scholar]
- Lim SN, Huang W, Hall JC, Michael-Titus AT, Priestley JV (2013) Improved outcome after spinal cord compression injury in mice treated with docosahexaenoic acid. Experimental neurology 239:13–27. [DOI] [PubMed] [Google Scholar]
- Lin SP, Ye S, Chen XH, Jiang HL, Mao HF, Chen MT, Ma QJ, Long Y, Fan Y, Lin PY (2016) Increased expression of microRNA-21 in peripheral blood mediates the down-regulation of IFN-gamma and increases the prevalence of stroke-associated infection. Journal of the neurological sciences 366:235–239. [DOI] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG (2011) The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nature medicine 17:211–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu DZ, Tian Y, Ander BP, Xu H, Stamova BS, Zhan X, Turner RJ, Jickling G, Sharp FR (2010a) Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FJ, Lim KY, Kaur P, Sepramaniam S, Armugam A, Wong PT, Jeyaseelan K (2013a) microRNAs Involved in Regulating Spontaneous Recovery in Embolic Stroke Model. PloS one 8:e66393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, Kaminski N, Abraham E (2010b) miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. The Journal of experimental medicine 207:1589–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu NK, Wang XF, Lu QB, Xu XM (2009) Altered microRNA expression following traumatic spinal cord injury. Experimental neurology 219:424–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Chopp M, Wang XL, Zhang L, Hozeska-Solgot A, Tang T, Kassis H, Zhang RL, Chen C, Xu J, Zhang ZG (2013b) MicroRNA-17–92 cluster mediates the proliferation and survival of neural progenitor cells after stroke. The Journal of biological chemistry 288:12478–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Kato H, Nakata N, Kogure K (1992) Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain research 586:121–124. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Yip PK, Adams L, Davies M, Lee JW, Michael GJ, Priestley JV, Michael-Titus AT (2015) A Single Bolus of Docosahexaenoic Acid Promotes Neuroplastic Changes in the Innervation of Spinal Cord Interneurons and Motor Neurons and Improves Functional Recovery after Spinal Cord Injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 35:12733–12752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh PG, Yang HS, Walsh MA, Wang Q, Wang X, Cheng Z, Liu D, Song H (2009) Structural basis for translational inhibition by the tumour suppressor Pdcd4. The EMBO journal 28:274–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen JM et al. (2015) Osteopontin is indispensible for AP1-mediated angiotensin II-related miR-21 transcription during cardiac fibrosis. European heart journal 36:2184–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YL, Guo F, Liu F, Gao FL, Zhang PQ, Niu X, Guo SC, Yin JH, Wang Y, Deng ZF (2012) miR-210 activates notch signaling pathway in angiogenesis induced by cerebral ischemia. Molecular and cellular biochemistry 370:45–51. [DOI] [PubMed] [Google Scholar]
- Lu X, Luo F, Liu Y, Zhang A, Li J, Wang B, Xu W, Shi L, Liu X, Lu L, Liu Q (2015) The IL-6/STAT3 pathway via miR-21 is involved in the neoplastic and metastatic properties of arsenite-transformed human keratinocytes. Toxicology letters 237:191–199. [DOI] [PubMed] [Google Scholar]
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y (2008) MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene 27:4373–4379. [DOI] [PubMed] [Google Scholar]
- Lusardi TA, Farr CD, Faulkner CL, Pignataro G, Yang T, Lan J, Simon RP, Saugstad JA (2010) Ischemic preconditioning regulates expression of microRNAs and a predicted target, MeCP2, in mouse cortex. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 30:744–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masel BE, DeWitt DS (2010) Traumatic brain injury: a disease process, not an event. Journal of neurotrauma 27:1529–1540. [DOI] [PubMed] [Google Scholar]
- McClelland AD, Herman-Edelstein M, Komers R, Jha JC, Winbanks CE, Hagiwara S, Gregorevic P, Kantharidis P, Cooper ME (2015) miR-21 promotes renal fibrosis in diabetic nephropathy by targeting PTEN and SMAD7. Clin Sci (Lond) 129:1237–1249. [DOI] [PubMed] [Google Scholar]
- Meissner L, Gallozzi M, Balbi M, Schwarzmaier S, Tiedt S, Terpolilli NA, Plesnila N (2016) Temporal Profile of MicroRNA Expression in Contused Cortex after Traumatic Brain Injury in Mice. Journal of neurotrauma 33:713–720. [DOI] [PubMed] [Google Scholar]
- Meng F, You Y, Liu Z, Liu J, Ding H, Xu R (2015a) Neuronal calcium signaling pathways are associated with the development of epilepsy. Molecular medicine reports 11:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XM, Tang PM, Li J, Lan HY (2015b) TGF-beta/Smad signaling in renal fibrosis. Frontiers in physiology 6:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mor M, Nardone S, Sams DS, Elliott E (2015) Hypomethylation of miR-142 promoter and upregulation of microRNAs that target the oxytocin receptor gene in the autism prefrontal cortex. Molecular autism 6:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison B 3rd, Elkin BS, Dolle JP, Yarmush ML (2011) In vitro models of traumatic brain injury. Annual review of biomedical engineering 13:91–126. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D et al. (2016) Heart Disease and Stroke Statistics-2016 Update: A Report From the American Heart Association. Circulation 133:e38–360. [DOI] [PubMed] [Google Scholar]
- Mudo G, Jiang XH, Timmusk T, Bindoni M, Belluardo N (1996) Change in neurotrophins and their receptor mRNAs in the rat forebrain after status epilepticus induced by pilocarpine. Epilepsia 37:198–207. [DOI] [PubMed] [Google Scholar]
- Nolan S (2005) Traumatic brain injury: a review. Critical care nursing quarterly 28:188–194. [DOI] [PubMed] [Google Scholar]
- Olson EN (2014) MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Science translational medicine 6:239ps233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang YB, Lu Y, Yue S, Xu LJ, Xiong XX, White RE, Sun X, Giffard RG (2012) miR-181 regulates GRP78 and influences outcome from cerebral ischemia in vitro and in vivo. Neurobiology of disease 45:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyinbo CA (2011) Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta neurobiologiae experimentalis 71:281–299. [DOI] [PubMed] [Google Scholar]
- Pan X, Wang ZX, Wang R (2010) MicroRNA-21: a novel therapeutic target in human cancer. Cancer biology & therapy 10:1224–1232. [DOI] [PubMed] [Google Scholar]
- Peng J, Omran A, Ashhab MU, Kong H, Gan N, He F, Yin F (2013) Expression patterns of miR-124, miR-134, miR-132, and miR-21 in an immature rat model and children with mesial temporal lobe epilepsy. Journal of molecular neuroscience : MN 50:291–297. [DOI] [PubMed] [Google Scholar]
- Peng W et al. (2016) Loss of PTEN Promotes Resistance to T Cell-Mediated Immunotherapy. Cancer discovery 6:202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer SR, Yang CH, Pfeffer LM (2015) The Role of miR-21 in Cancer. Drug development research 76:270–277. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Di Giuseppe M, Njah J, Sala E, Shiva S, St Croix CM, Stolz DB, Watkins SC, Di YP, Leikauf GD, Kolls J, Riches DW, Deiuliis G, Kaminski N, Boregowda SV, McKenna DH, Ortiz LA (2015) Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nature communications 6:8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polajeva J, Swartling FJ, Jiang Y, Singh U, Pietras K, Uhrbom L, Westermark B, Roswall P (2012) miRNA-21 is developmentally regulated in mouse brain and is co-expressed with SOX2 in glioma. BMC cancer 12:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B (2003) A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nature medicine 9:407–415. [DOI] [PubMed] [Google Scholar]
- Ratcliffe P, Koivunen P, Myllyharju J, Ragoussis J, Bovee JV, Batinic-Haberle I, Vinatier C, Trichet V, Robriquet F, Oliver L, Gardie B (2017) Update on hypoxia-inducible factors and hydroxylases in oxygen regulatory pathways: from physiology to therapeutics. Hypoxia (Auckland, NZ) 5:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redell JB, Zhao J, Dash PK (2011) Altered expression of miRNA-21 and its targets in the hippocampus after traumatic brain injury. Journal of neuroscience research 89:212–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risbud RM, Lee C, Porter BE (2011) Neurotrophin-3 mRNA a putative target of miR21 following status epilepticus. Brain research 1424:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncon P, Soukupova M, Binaschi A, Falcicchia C, Zucchini S, Ferracin M, Langley SR, Petretto E, Johnson MR, Marucci G, Michelucci R, Rubboli G, Simonato M (2015) MicroRNA profiles in hippocampal granule cells and plasma of rats with pilocarpine-induced epilepsy--comparison with human epileptic samples. Scientific reports 5:14143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nature reviews Drug discovery 16:203–222. [DOI] [PubMed] [Google Scholar]
- Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D, McGuire TL, Stupp SI, Kessler JA (2010) BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. The Journal of neuroscience : the official journal of the Society for Neuroscience 30:1839–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Nakaoka T, Saito H (2015) microRNA-34a as a Therapeutic Agent against Human Cancer. Journal of clinical medicine 4:1951–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandhir R, Gregory E, Berman NE (2014) Differential response of miRNA-21 and its targets after traumatic brain injury in aging mice. Neurochemistry international 78:117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan P, Golden HB, Miranda RC (2007) Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. The Journal of neuroscience : the official journal of the Society for Neuroscience 27:8546–8557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M (2009) Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke; a journal of cerebral circulation 40:2594–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz SI, Chopp M, Deans R, Carmichael T, Phinney D, Wechsler L (2011) Stem Cell Therapy as an Emerging Paradigm for Stroke (STEPS) II. Stroke; a journal of cerebral circulation 42:825–829. [DOI] [PubMed] [Google Scholar]
- Sayed D, He M, Hong C, Gao S, Rane S, Yang Z, Abdellatif M (2010) MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. The Journal of biological chemistry 285:20281–20290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabbauer G, Matt U, Gunzl P, Warszawska J, Furtner T, Hainzl E, Elbau I, Mesteri I, Doninger B, Binder BR, Knapp S (2010) Myeloid PTEN promotes inflammation but impairs bactericidal activities during murine pneumococcal pneumonia. J Immunol 185:468–476. [DOI] [PubMed] [Google Scholar]
- Schmidt-Kastner R, Olson L (1995) Decrease of neurotrophin-3 mRNA in adult rat hippocampus after pilocarpine seizures. Experimental neurology 136:199–204. [DOI] [PubMed] [Google Scholar]
- Selvamani A, Sathyan P, Miranda RC, Sohrabji F (2012) An antagomir to microRNA Let7f promotes neuroprotection in an ischemic stroke model. PloS one 7:e32662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang C, Guo Y, Hong Y, Liu YH, Xue YX (2015) MiR-21 up-regulation mediates glioblastoma cancer stem cells apoptosis and proliferation by targeting FASLG. Molecular biology reports 42:721–727. [DOI] [PubMed] [Google Scholar]
- Sheedy FJ, Palsson-McDermott E, Hennessy EJ, Martin C, O’Leary JJ, Ruan Q, Johnson DS, Chen Y, O’Neill LA (2010) Negative regulation of TLR4 via targeting of the proinflammatory tumor suppressor PDCD4 by the microRNA miR-21. Nature immunology 11:141–147. [DOI] [PubMed] [Google Scholar]
- Shi B, Deng W, Long X, Zhao R, Wang Y, Chen W, Xu G, Sheng J, Wang D, Cao S (2017) miR-21 increases c-kit+ cardiac stem cell proliferation in vitro through PTEN/PI3K/Akt signaling. PeerJ 5:e2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel C, Li J, Liu F, Benashski SE, McCullough LD (2011) miR-23a regulation of X-linked inhibitor of apoptosis (XIAP) contributes to sex differences in the response to cerebral ischemia. Proceedings of the National Academy of Sciences of the United States of America 108:11662–11667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva NA, Sousa N, Reis RL, Salgado AJ (2014) From basics to clinical: a comprehensive review on spinal cord injury. Progress in neurobiology 114:25–57. [DOI] [PubMed] [Google Scholar]
- Stetler RA, Leak RK, Gan Y, Li P, Zhang F, Hu X, Jing Z, Chen J, Zigmond MJ, Gao Y (2014) Preconditioning provides neuroprotection in models of CNS disease: paradigms and clinical significance. Progress in neurobiology 114:58–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su C, Yang X, Lou J (2016) Geniposide reduces alpha-synuclein by blocking microRNA-21/lysosome-associated membrane protein 2A interaction in Parkinson disease models. Brain research 1644:98–106. [DOI] [PubMed] [Google Scholar]
- Sun M, Yamashita T, Shang J, Liu N, Deguchi K, Feng J, Abe K (2015) Time-dependent profiles of microRNA expression induced by ischemic preconditioning in the gerbil hippocampus. Cell transplantation 24:367–376. [DOI] [PubMed] [Google Scholar]
- Toldo S, Das A, Mezzaroma E, Chau VQ, Marchetti C, Durrant D, Samidurai A, Van Tassell BW, Yin C, Ockaili RA, Vigneshwar N, Mukhopadhyay ND, Kukreja RC, Abbate A, Salloum FN (2014) Induction of microRNA-21 with exogenous hydrogen sulfide attenuates myocardial ischemic and inflammatory injury in mice. Circulation Cardiovascular genetics 7:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y, Wan L, Fan Y, Wang K, Bu L, Huang T, Cheng Z, Shen B (2013) Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PloS one 8:e75872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Ree MH, van der Meer AJ, de Bruijne J, Maan R, van Vliet A, Welzel TM, Zeuzem S, Lawitz EJ, Rodriguez-Torres M, Kupcova V, Wiercinska-Drapalo A, Hodges MR, Janssen HL, Reesink HW (2014) Long-term safety and efficacy of microRNA-targeted therapy in chronic hepatitis C patients. Antiviral research 111:53–59. [DOI] [PubMed] [Google Scholar]
- Varga ZV, Zvara A, Farago N, Kocsis GF, Pipicz M, Gaspar R, Bencsik P, Gorbe A, Csonka C, Puskas LG, Thum T, Csont T, Ferdinandy P (2014) MicroRNAs associated with ischemia-reperfusion injury and cardioprotection by ischemic pre- and postconditioning: protectomiRs. American journal of physiology Heart and circulatory physiology 307:H216–227. [DOI] [PubMed] [Google Scholar]
- Vemuganti R (2013) All’s well that transcribes well: non-coding RNAs and post-stroke brain damage. Neurochemistry international 63:438–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viard-Leveugle I, Veyrenc S, French LE, Brambilla C, Brambilla E (2003) Frequent loss of Fas expression and function in human lung tumours with overexpression of FasL in small cell lung carcinoma. The Journal of pathology 201:268–277. [DOI] [PubMed] [Google Scholar]
- Vieira RC, Paiva WS, de Oliveira DV, Teixeira MJ, de Andrade AF, de Sousa RM (2016) Diffuse Axonal Injury: Epidemiology, Outcome and Associated Risk Factors. Frontiers in neurology 7:178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan M, Reddy PH (2016) Peripheral biomarkers of stroke: Focus on circulatory microRNAs. Biochimica et biophysica acta 1862:1984–1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhu Y, Jin F, Tang L, He Z (2016) Differential expression of circulating microRNAs in blood and haematoma samples from patients with intracerebral haemorrhage. J Int Med Res 44:419–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JY, Gao YB, Zhang N, Zou DW, Wang P, Zhu ZY, Li JY, Zhou SN, Wang SC, Wang YY, Yang JK (2014) miR-21 overexpression enhances TGF-beta1-induced epithelial-to-mesenchymal transition by target smad7 and aggravates renal damage in diabetic nephropathy. Molecular and cellular endocrinology 392:163–172. [DOI] [PubMed] [Google Scholar]
- Wang K, Jiang Z, Webster KA, Chen J, Hu H, Zhou Y, Zhao J, Wang L, Wang Y, Zhong Z, Ni C, Li Q, Xiang C, Zhang L, Wu R, Zhu W, Yu H, Hu X, Wang J (2017) Enhanced Cardioprotection by Human Endometrium Mesenchymal Stem Cells Driven by Exosomal MicroRNA-21. Stem cells translational medicine 6:209–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Brandt S, Medeiros A, Wang S, Wu H, Dent A, Serezani CH (2015) MicroRNA 21 is a homeostatic regulator of macrophage polarization and prevents prostaglandin E2-mediated M2 generation. PloS one 10:e0115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CW, Biggar KK, Luu BE, Szereszewski KE, Storey KB (2016) Analysis of microRNA expression during the torpor-arousal cycle of a mammalian hibernator, the 13-lined ground squirrel. Physiological genomics 48:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T, Liu Y, Fan Z, Xu J, Jin L, Gao Z, Wu Z, Hu L, Wang J, Zhang C, Chen W, Wang S (2015) miR-21 Modulates the Immunoregulatory Function of Bone Marrow Mesenchymal Stem Cells Through the PTEN/Akt/TGF-beta1 Pathway. Stem Cells 33:3281–3290. [DOI] [PubMed] [Google Scholar]
- Xiao J, Pan Y, Li XH, Yang XY, Feng YL, Tan HH, Jiang L, Feng J, Yu XY (2016) Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell death & disease 7:e2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie S, Chen M, Yan B, He X, Chen X, Li D (2014) Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PloS one 9:e94496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xilouri M, Brekk OR, Kirik D, Stefanis L (2013) LAMP2A as a therapeutic target in Parkinson disease. Autophagy 9:2166–2168. [DOI] [PubMed] [Google Scholar]
- Xu LJ, Ouyang YB, Xiong X, Stary CM, Giffard RG (2015) Post-stroke treatment with miR-181 antagomir reduces injury and improves long-term behavioral recovery in mice after focal cerebral ischemia. Experimental neurology 264:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Yang L, Hong LZ, Zhao XY, Zhang HL (2012) Direct protection of neurons and astrocytes by matrine via inhibition of the NF-kappaB signaling pathway contributes to neuroprotection against focal cerebral ischemia. Brain research 1454:48–64. [DOI] [PubMed] [Google Scholar]
- Xu X, Kriegel AJ, Jiao X, Liu H, Bai X, Olson J, Liang M, Ding X (2014) miR-21 in ischemia/reperfusion injury: a double-edged sword? Physiological genomics 46:789–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Liu W, Yan X, Zhou H, Zhang H, Liu J, Yu M, Zhu X, Ma K (2016a) Effects of mir-21 on Cardiac Microvascular Endothelial Cells After Acute Myocardial Infarction in Rats: Role of Phosphatase and Tensin Homolog (PTEN)/Vascular Endothelial Growth Factor (VEGF) Signal Pathway. Medical science monitor : international medical journal of experimental and clinical research 22:3562–3575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang F, Wang Y, Xue J, Ma Q, Zhang J, Chen YF, Shang ZZ, Li QQ, Zhang SL, Zhao L (2016b) Effect of Corilagin on the miR-21/smad7/ERK signaling pathway in a schistosomiasis-induced hepatic fibrosis mouse model. Parasitology international 65:308–315. [DOI] [PubMed] [Google Scholar]
- Yang HS, Jansen AP, Nair R, Shibahara K, Verma AK, Cmarik JL, Colburn NH (2001) A novel transformation suppressor, Pdcd4, inhibits AP-1 transactivation but not NF-kappaB or ODC transactivation. Oncogene 20:669–676. [DOI] [PubMed] [Google Scholar]
- Yang Q, Yang K, Li A (2014) microRNA-21 protects against ischemia-reperfusion and hypoxia-reperfusion-induced cardiocyte apoptosis via the phosphatase and tensin homolog/Akt-dependent mechanism. Molecular medicine reports 9:2213–2220. [DOI] [PubMed] [Google Scholar]
- Yang Z, Zhong L, Zhong S, Xian R, Yuan B (2015) miR-203 protects microglia mediated brain injury by regulating inflammatory responses via feedback to MyD88 in ischemia. Molecular immunology 65:293–301. [DOI] [PubMed] [Google Scholar]
- Yelamanchili SV, Chaudhuri AD, Chen LN, Xiong H, Fox HS (2010) MicroRNA-21 dysregulates the expression of MEF2C in neurons in monkey and human SIV/HIV neurological disease. Cell death & disease 1:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelamanchili SV, Lamberty BG, Rennard DA, Morsey BM, Hochfelder CG, Meays BM, Levy E, Fox HS (2015) MiR-21 in Extracellular Vesicles Leads to Neurotoxicity via TLR7 Signaling in SIV Neurological Disease. PLoS pathogens 11:e1005032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin KJ, Deng Z, Huang H, Hamblin M, Xie C, Zhang J, Chen YE (2010) miR-497 regulates neuronal death in mouse brain after transient focal cerebral ischemia. Neurobiology of disease 38:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MR, Santhanam AN, Yoshikawa N, Colburn NH (2010) Have tumor suppressor PDCD4 and its counteragent oncogenic miR-21 gone rogue? Molecular interventions 10:76–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Pardoll D, Jove R (2009) STATs in cancer inflammation and immunity: a leading role for STAT3. Nature reviews Cancer 9:798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Wu M, Zhao P, Huang Y, Wang W, Yin W (2015) Neuroprotective effects of viral overexpression of microRNA-22 in rat and cell models of cerebral ischemia-reperfusion injury. Journal of cellular biochemistry 116:233–241. [DOI] [PubMed] [Google Scholar]
- Yuan L, Geiser F, Lin B, Sun H, Chen J, Zhang S (2015) Down but Not Out: The Role of MicroRNAs in Hibernating Bats. PloS one 10:e0135064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Wang JY, Xu LY, Cai R, Chen Z, Luo BY (2010) MicroRNA expression changes in the hippocampi of rats subjected to global ischemia. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 17:774–778. [DOI] [PubMed] [Google Scholar]
- Yunta M, Nieto-Diaz M, Esteban FJ, Caballero-Lopez M, Navarro-Ruiz R, Reigada D, Pita-Thomas DW, del Aguila A, Munoz-Galdeano T, Maza RM (2012) MicroRNA dysregulation in the spinal cord following traumatic injury. PloS one 7:e34534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, He X, Wang Y, Tang Y, Zheng C, Cai H, Liu J, Fu Y, Yang GY (2014) MicroRNA-210 overexpression induces angiogenesis and neurogenesis in the normal adult mouse brain. Gene therapy 21:37–43. [DOI] [PubMed] [Google Scholar]
- Zhang L, Dong LY, Li YJ, Hong Z, Wei WS (2012) miR-21 represses FasL in microglia and protects against microglia-mediated neuronal cell death following hypoxia/ischemia. Glia 60:1888–1895. [DOI] [PubMed] [Google Scholar]
- Zhang W, Shu L (2016) Upregulation of miR-21 by Ghrelin Ameliorates Ischemia/Reperfusion-Induced Acute Kidney Injury by Inhibiting Inflammation and Cell Apoptosis. DNA and cell biology 35:417–425. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang X, Yang H, Liu H, Lu Y, Han L, Liu G (2013a) Kinase AKT controls innate immune cell development and function. Immunology 140:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zha Y, Hu W, Huang Z, Gao Z, Zang Y, Chen J, Dong L, Zhang J (2013b) The autoregulatory feedback loop of microRNA-21/programmed cell death protein 4/activation protein-1 (MiR-21/PDCD4/AP-1) as a driving force for hepatic fibrosis development. The Journal of biological chemistry 288:37082–37093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Chung AC, Chen HY, Meng XM, Lan HY (2011) Smad3-mediated upregulation of miR-21 promotes renal fibrosis. Journal of the American Society of Nephrology : JASN 22:1668–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou F, Zhu X, Castellani RJ, Stimmelmayr R, Perry G, Smith MA, Drew KL (2001) Hibernation, a model of neuroprotection. The American journal of pathology 158:2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Zhang J (2014) Identification of miRNA-21 and miRNA-24 in plasma as potential early stage markers of acute cerebral infarction. Molecular medicine reports 10:971–976. [DOI] [PubMed] [Google Scholar]
- Zhou Q, Sun Q, Zhang Y, Teng F, Sun J (2016) Up-Regulation of miRNA-21 Expression Promotes Migration and Proliferation of Sca-1+ Cardiac Stem Cells in Mice. Medical science monitor : international medical journal of experimental and clinical research 22:1724–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziu M, Fletcher L, Rana S, Jimenez DF, Digicaylioglu M (2011) Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PloS one 6:e14724. [DOI] [PMC free article] [PubMed] [Google Scholar]