Abstract
Context:
Irisin, a new myokine, has been found to be a biological marker of insulin resistance (IR). There is conflicting evidence on serum irisin level in adult women with polycystic ovarian syndrome (PCOS), and data are lacking in adolescents with PCOS.
Aims:
We aimed to evaluate serum Irisin levels and study its association with indices using blood glucose and insulin levels in adolescents with PCOS.
Settings and Design:
This case–control study was conducted in the gynecology outpatient department.
Materials and Methods:
This study was carried out from August 2015 to June 2017. Eighty-two adolescent girls aged 15–19 years were included in the study. Fasting irisin, insulin,blood glucose; 2nd-h insulin (HOMA2-IR), and Quantitative Insulin Sensitivity Check Index (QUICKI) for all participants with addition of 2nd-h blood glucose for cases.
Statistical Analysis:
A correlation between serum irisin level and various biochemical parameters was done using Pearson's correlation.
Results:
Fasting serum irisin was significantly higher among PCOS cases (8.43 [5.84, 13.11]) when compared to controls 4.90 (2.37, 9.09), median (interquartile range) (P = 0.002). Serum irisin was found to have a significant moderate positive correlation with 2nd-h blood glucose, fasting/2nd-h insulin, and HOMA2-IR. A significant moderate negative correlation was observed between irisin and QUICKI.
Conclusions:
Serum irisin levels were significantly elevated in adolescents with PCOS when compared to the controls. A significant correlation of serum irisin with blood glucose and insulin indices indicates that serum irisin could serve as a marker of IR and may help in its diagnosis in adolescents with PCOS.
KEYWORDS: Adolescents, HOMA2-IR, polycystic ovarian syndrome, QUICKI, serum irisin
INTRODUCTION
Polycystic ovarian syndrome (PCOS) is one of the most prevalent endocrine-metabolic disorders afflicting females of reproductive age and often initially manifests in adolescence. The prevalence of PCOS in Indian adolescents is 9.13% in the community.[1] Insulin resistance (IR) is involved in PCOS pathophysiology.[2] Euglycemic-hyperinsulinemic clamp technique is the gold standard method of assessing IR. Being an expensive and tiresome study requiring continuous insulin infusion with blood sample withdrawal, it is not a practical method to assess IR.[3] Other surrogate markers of IR include indices using blood glucose and insulin levels, adiponectin, sex hormone-binding globulin, etc.,[4,5] but none of these have been found to be very useful. A marker that correlates with the second-best method, namely blood glucose and insulin indices, would therefore be a particularly useful adjunct to assist in the diagnosis of IR in adolescents with PCOS.
A new myokine, irisin, has been found to be a biological marker of IR.[6,7] In adult women, it has been demonstrated that irisin is increased in PCOS and may be a suitable biological marker.[8,9,10,11,12] However, three recent studies have found that adult women with PCOS had either significantly lower mean serum irisin concentrations,[13] or although decreased, there was no difference when compared to controls.[14,15] Thus, there is conflicting evidence on serum irisin level in adult women with PCOS and adequate data are lacking in adolescents with PCOS. Therefore, this study is proposed to evaluate serum irisin levels and study its association with indices using blood glucose and insulin levels in adolescents with PCOS.
MATERIALS AND METHODS
This case–control study was conducted in the department of obstetrics and gynecology of a tertiary care hospital from August 2015 to June 2017. The ethics committee approved the study protocol. Adolescent girls aged 15–19 years, diagnosed with PCOS according to Rotterdam PCOS consensus criteria 200316 which require 2 out of the 3 following characteristics, namely evidence of ovulatory dysfunction (consecutive menstrual intervals >90 days even in the 1st year after menstrual onset, menstrual intervals persistently <21 or >45 days 2 or more years after menarche, and lack of menses by 15 years or 2–3 years after breast budding); androgen excess (moderate to severe hirsutism,persistent acne unresponsive to topical therapy; and persistent elevation of serum total and/or free testosterone level) and polycystic ovaries (12 or more follicles, 2–9 mm diameter and/or ovarian volume >10 ml in at least one ovary).
Girls in the same age group presenting with minor gynecological complaints such as dysmenorrhea, white discharge per vaginum, and lower abdominal pain were included as controls. Since adolescent girls were the participants of this study, they and their parents were given detailed information about this study and its purpose. After obtaining informed consent, namely written assent from participants and parental consent for minors (13–18 years) and written consent from participants >18 years, an interview schedule containing sociodemographic details and detailed history was elicited. After the questionnaire was completed, they were examined in the presence of a female staff nurse, with privacy being maintained throughout. Physical examinations including height, weight, body mass index (BMI), acanthosis nigricans, secondary sexual characteristics, hirsutism score, and abdominal examination were carried out. Ultrasound findings and serum testosterone levels in cases were noted. The procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional or regional) or with the Helsinki Declaration of 1975, as revised in 1983.
Between days 2 and 7 of the menstrual cycle, venous blood was drawn from the antecubital vein of the participants after overnight fasting. Four milliliters of venous blood was taken in the fasting state for estimation of serum irisin, serum insulin, and blood glucose levels from both cases and controls. Then, 75-g glucose was administered, and after 2 h, 4 ml of venous blood was taken for assessment of insulin levels and blood glucose levels in cases and 2 ml of venous blood was collected from controls for analyzing serum insulin levels. The samples collected were stored at −80°C in the Department of Endocrinology, JIPMER, and processed after all cases and controls were recruited. Serum irisin levels were analyzed using enzyme-linked immunoassay (ELISA) kit manufactured by Bioassay Technology Laboratory (catalog no. E3253Hu) having a standard curve range of 0.2–60 ng/mL and sensitivity of 0.095 ng/mL. Serum insulin levels were measured using ELISA kits of the same company (catalog no. E0010Hu) having a standard curve range of 0.2–60 mIU/L and sensitivity of 0.11 mIU/L. Blood glucose levels were estimated in the Department of Biochemistry, JIPMER. Parameters studied in cases were fasting serum irisin, fasting and 2nd-h serum insulin, fasting and 2nd-h blood glucose. In controls, fasting serum irisin, fasting and 2nd-h serum insulin, and fasting blood glucose were assessed. HOMA2-IR and QUICKI using blood glucose and serum insulin levels were calculated using HOMA2-IR calculator version 2.2 and analog calculator in Microsoft Office Excel. Poststudy abnormal tests were communicated to the participants, and appropriate referral and treatment were instituted.
Sample size was calculated to detect a difference of 50 μg/L in irisin levels between adolescents and PCOS patients (standard deviation [SD] – 70 and 90 μg/L, respectively).[9] Sample size was estimated to be 41 in each group. Calculations were done based on 80% power and 95% confidence levels using Open Epi software (Dean AG, Sullivan KM, Sir MM. OpenEpi: Open source Epidemiologic Statistics for Public Health, Version 3.01). Outcome variables were irisin levels and indices using blood glucose and insulin levels. Data were entered in Excel and analyzed using SPSS version 21 (IBM Corp. Armonk, NY, USA). P < 0.05 was set as statistical significance.
Continuous variables such as age, height, weight, and BMI were measured as mean ± (SD). Categorical variables such as education status, socioeconomic status, prevalence of acanthosis nigricans, obesity, chief complaints, presence of impaired glucose tolerance (IGT), and IR were expressed using proportions. Biochemical parameters such as blood glucose levels, serum insulin, serum irisin, HOMA2-IR, and QUICKI were expressed as median interquartile range as the distribution was nonnormal; comparison between the two groups was done using Mann–Whitney test. Pearson's Chi-square test was used to test for significant difference between the two groups with respect to BMI, fasting blood glucose, serum testosterone, serum irisin, fasting insulin, 2nd-h insulin, HOMA2-IR, and QUICKI. A correlation between serum irisin level and various biochemical parameters was done using Pearson's correlation, and scatter plots were constructed. Strength of relationship was expressed as r value with good, moderate, and poor correlation. Receiver operating characteristic (ROC) curve was constructed in order to assess the diagnostic utility of serum irisin as a marker in IR among cases and controls. Area under the curve was obtained to assess the discriminatory power of the marker. The corresponding sensitivity and specificity for a given serum irisin value have been reported as %.
RESULTS
The mean age of girls among cases was 16.4 ± 1.1 years and mean age of girls in the control group was 17.7 ± 1.1 years, with a range of 15–19 years. Three-fourth of the cases (73.2%) were in middle adolescence whereas majority of the controls (85.4%) were in late adolescence. Majority of the cases (63.4%) were in high school whereas maximum controls (70.7%) were graduates. Participants of study population were classified according to income cutoffs from Modified Prasad's Socioeconomic Classification[17] 2016. Out of total 82 participants, majority of the cases belonged to class 1 (48.8%), while among controls, maximum belonged to class 2 (56.1%).
The most common complaint in girls among cases was infrequent cycles (97.5%) followed by primary infertility (2.4%), and among controls, symptoms were pain abdomen (39%), white discharge per vaginum (29%), dysmenorrhea (19.5%), primary amenorrhea (4.8%), heavy menstrual bleeding (2.4%), primary infertility (2.4%), and secondary amenorrhea (2.4%). The mean age of menarche in girls in cases was 13.15 ± 0.98 years with a range of 11–15 years and in controls was 13.05 ± 0.669 years with a range of 11–14 years. According to Rotterdam's criteria for the diagnosis of PCOS, three-fourth (78.0%) of cases had all the three features followed by combination of infrequent cycles and clinical and/or biochemical signs of hyperandrogenism (14.7%) and infrequent cycles and polycystic ovaries (7.3%). Mean body weight and BMI were higher among the cases, and this difference was statistically significant (χ2 = 30.22, P < 0.0001). Twelve percent of the cases had acanthosis nigricans.
Fasting blood glucose was significantly higher in cases when compared to controls, but the level was below the cutoff for the diagnosis of diabetes. Two cases had (4.8%) IGT based on fasting blood sugar. 46.3% had IGT with 2nd-h blood glucose after 75-g oral glucose. Among these, 17 had only raised 2nd-h glucose whereas 2 cases had derangement of both fasting and 2nd-h glucose. Fasting and 2nd-h insulin levels were significantly higher in cases when compared to controls. Seven (17%) of the cases had high fasting insulin levels suggestive of IR in relation to normal fasting blood glucose values. Based on HOMA2-IR, IR was present in 90.2% of the cases and 19.5% of the controls. Both with HOMA2-IR and QUICKI, there was a statistically significant difference between cases and controls. Anthropometric and biochemical parameters are summarized in Table 1.
Table 1.
Anthropometric and biochemical parameters in cases and controls
| Characteristics | Cases | Controls | P |
|---|---|---|---|
| Weight (kg) (mean±SD) | 58.80±10.107 | 50.41±3.464 | 0.001 |
| BMI (kg/m2) (mean±SD) | 25.58±4.353 | 21.67±1.287 | 0.001 |
| Fasting blood glucose (mg/dL), median (IQR) | 84 | 79 | 0.004 |
| Fasting insulin level (mIU/L), median (IQR) | 11.76 (10.35-21.72) | 6.02 (5.26-9.81) | 0.001 |
| Second-hour insulin level (mIU/L), median (IQR) | 11.10 (8.53-18.30) | 6.32 (4.04-11.17) | 0.001 |
| Fasting serum irisin level (ng/ml), median (IQR) | 8.43 (5.84-13.11) | 4.90 (2.37-9.09) | 0.002 |
| HOMA2-IR, median (IQR) | 2.63 (2.03-5.25) | 1.13 (10.0-1.75) | 0.001 |
| QUICKI, median (IQR) | 0.33 (0.30-0.34) | 0.37 (0.34-0.38) | 0.001 |
SD=Standard deviation, IQR=Interquartile range, BMI=Body mass index, HOMA2-IR=homeostatic model assessment 2-Insulin resistance, QUICKI=Quantitative insulin sensitivity check index
Among PCOS cases, serum irisin level ranged from 3.1 to 44.4 ng/ml, and in controls, it ranged from 0.3 to 35.7 ng/ml. Fasting serum irisin was significantly higher among PCOS cases (8.43 ng/ml) when compared to controls (median: 4.9 ng/ml) (P = 0.002) [Figure 1]. The range of serum irisin levels in normal, overweight, and obese girls was 3.34–44.42 ng/mL, 3.1–42.74 ng/dL, and 3.66–41.9 ng/dL, respectively. A correlation of serum irisin with blood glucose, insulin, and indices is depicted in Table 2 and Figure 2.
Figure 1.
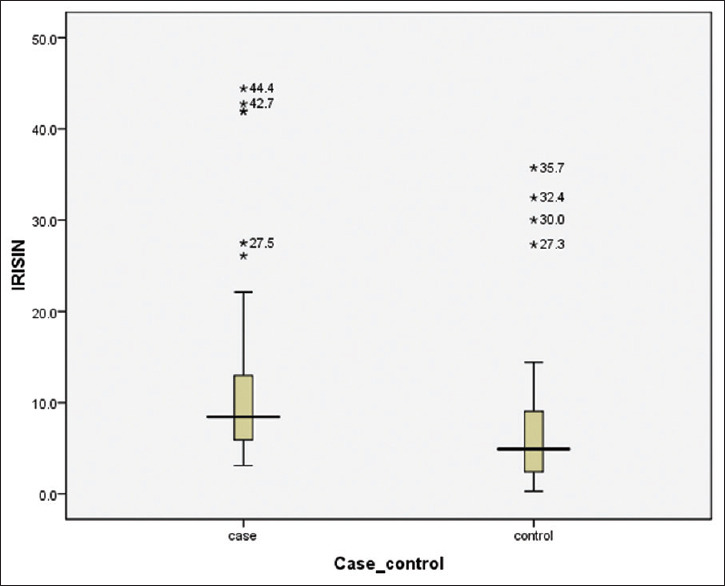
Serum irisin levels in study groups
Table 2.
Correlation of serum irisin with anthropometric, hormonal and biochemical parameters
| Parameters | Correlation coefficient (r) | P |
|---|---|---|
| BMI | −0.14 | 0.36 |
| Serum testosterone | −0.236 | 0.137 |
| Fasting blood glucose | 0.262 | 0.099 |
| Second-hour blood glucose | 0.579 | 0.000 |
| Fasting serum insulin | 0.481 | 0.001 |
| Second-hour serum insulin | 0.579 | 0.000 |
| HOMA2-IR | 0.494 | 0.001 |
| QUICKI | −0.452 | 0.003 |
BMI=Body mass index, HOMA2-IR=homeostatic model assessment 2-Insulin resistance, QUICKI=Quantitative insulin sensitivity check index
Figure 2.
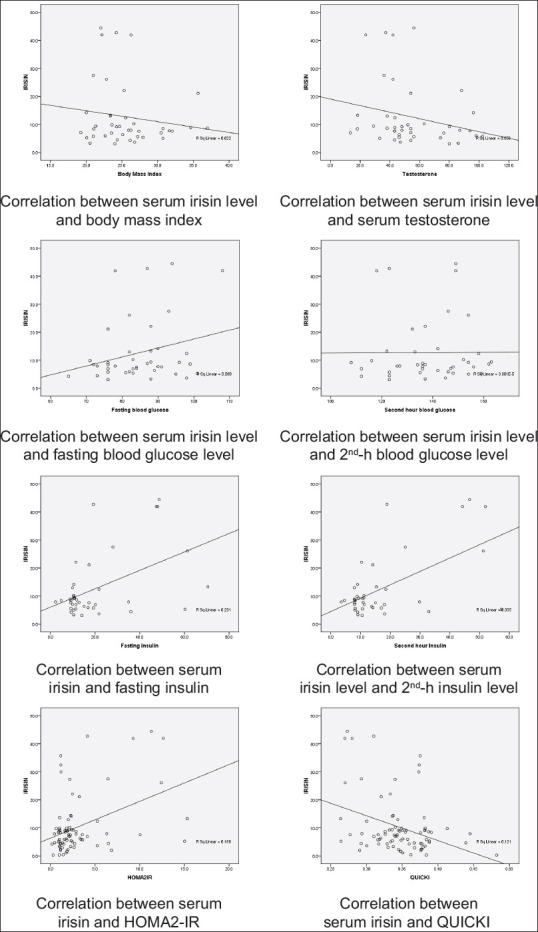
Correlation between serum irisin and various parameters
There was a weak negative correlation of serum irisin with BMI and testosterone however which was not statistically significant. With fasting blood glucose, serum irisin had an insignificant weak positive correlation and a significant moderate correlation with 2nd-h blood glucose. A positive moderate correlation was found between serum irisin level and fasting/2nd-h serum insulin which was statistically significant. There was also a statistically significant moderate positive correlation between serum irisin levels with HOMA2-IR. However, serum irisin level and QUICKI were significantly moderate negatively correlated.
ROC curve was constructed for comparison of irisin values among normal controls and PCOS cases for the diagnosis of IR based on HOMA2-IR [Figure 3]. Area under the curve was 0.626 (P = 0.053). Sensitivity and specificity for a range of irisin level were between 4.5 and 5.5 ng/ml. As the discriminating power of this marker was not very good, a single best cutoff was not possible, but for the given range of serum irisin 4.49–5.50 ng/mL, sensitivity ranges from 73.5% to 79.6% and specificity ranges from 36.4% to 51.5% [Table 3].
Figure 3.
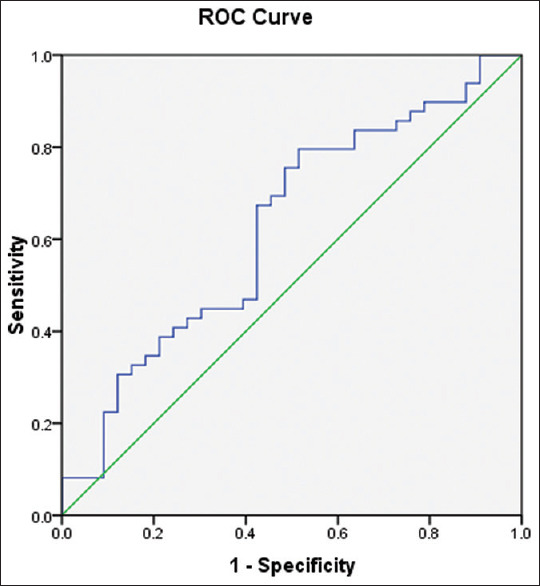
Receiver operating characteristic curve for the diagnosis of insulin resistance
Table 3.
Sensitivity and specificity of serum irisin within minimum and maximum cutoff values
| Serum irisin | Sensitivity (%) | Specificity (%) |
|---|---|---|
| 4.49 | 79.6 | 36.4 |
| 4.575 | 79.6 | 39.4 |
| 4.685 | 79.6 | 42.4 |
| 4.81 | 79.6 | 45.5 |
| 4.975 | 79.6 | 48.5 |
| 5.135 | 77.6 | 48.5 |
| 5.24 | 75.5 | 48.5 |
| 5.33 | 75.5 | 51.5 |
| 5.505 | 73.5 | 51.5 |
DISCUSSION
To the best of our knowledge, this is the first study to evaluate serum irisin levels in adolescents with PCOS and its association with blood glucose and insulin indices. Serum irisin levels were significantly elevated in adolescents with PCOS when compared to the controls, and there was a significant correlation of serum irisin with blood glucose and insulin indices in our study.
There are eight studies in the literature which have evaluated serum irisin in women with PCOS as this is a novel marker of recent interest. The studies available so far have all been in adult women with PCOS in the age group ranging from 20 to 35 years.[8,9,10,11,12,13,14,15] We included girls of 15–19 years in our study because anovulatory cycles are common in the first few years following menarche.
Out of the girls with PCOS, 49% were obese and another 22% were overweight in our study. The difference in BMI between cases and controls was statistically significant (P < 0.0001) similar to other studies in adult women.[8,9,11,12,14] Fasting blood glucose in adolescents with PCOS was significantly higher in cases when compared to controls in this trial comparable to other studies,[8,12,14] but the level was below the cutoff for the diagnosis of diabetes. Serum insulin in the fasting state and 2nd h was significantly higher in cases in concordance with other data.[8,9,11,12,14] Difference in blood glucose indices, namely HOMA2-IR[8,9,11,12,14] and QUICKI,[9] was statistically significant between both the groups in our study similar to other studies in adult women with PCOS. 46.3% had IGT according to fasting and 2nd-h glucose and 17% had IR based on fasting insulin level. Using HOMA2-IR and QUICKI, IR was found in 90% of the cases, thus depicting a high preponderance of IR in PCOS which was missed out on the traditional testing methods, namely fasting and 2nd-h blood glucose estimation.
Serum irisin levels in our study were within the range (5.44–479 ng/ml) reported in eight studies which have assessed serum irisin levels in adult women with PCOS. It is unlikely that storage would have affected the levels. There is no clear explanation for the increase in serum irisin levels in PCOS. It could be due to its association with metabolic syndrome or a protective mechanism in the prediabetic-diabetic state of PCOS before diabetes ensues or irisin resistance state due to failure of its action. Fasting serum irisin was significantly higher among PCOS cases in our study. This is in agreement with five studies so far which evaluated serum irisin in PCOS in adults.[8,9,10,11,12] However few recent studies have found that adult women with PCOS had either significantly lower mean serum irisin concentrations13 or no difference when compared to controls14 or higher levels in obese PCOS15. Thus, there is conflicting evidence on serum irisin level in adult women with PCOS when compared to controls. The range of serum irisin levels in normal, overweight, and obese girls (3.34–44.42 ng/mL, 3.1–42.74 ng/ml, and 3.66–41.9 ng/ml, respectively) was similar on comparison between the three groups.
In our study, serum irisin showed a weak negative correlation with BMI which was not statistically significant. The negative correlation[13] is in contrast to all other data which showed a positive correlation to BMI[11,12,15] or no correlation.[14,15] The absence of correlation could be possible because of lower levels of serum irisin in the two studies described.[14,15] Serum irisin had a statistically insignificant weak negative correlation with serum testosterone level in our study whereas another study[9] in 202 adult women with PCOS found no correlation.[15] There was a weak positive relationship of serum irisin with fasting blood glucose in our study but was statistically not significant. This is similar to a case–control study[13] done to evaluate the relationship between serum irisin level in PCOS to parameters of metabolic disturbances in 179 PCOS women and 122 controls. The reason for this is probably because only 4.8% of the cases had IGT. Furthermore, with 2nd-h blood glucose, serum irisin showed a significant moderate positive correlation in agreement with a case–control study which assessed the association of irisin with the development of PCOS.[9] There was a positive relationship of serum irisin with fasting blood glucose (weak positive) and 2-h blood glucose (significant moderate positive) in our study in agreement with studies on adult PCOS[9,13] Serum irisin had a positive relationship with both fasting and 2nd-h glucose which reiterates the pathophysiology of IR in PCOS. Serum irisin had a significant moderate positive correlation with fasting serum insulin which is similar to another study[10] or weak negative correlation.[11] 46.3% of the girls with PCOS in our study had IGT, and this could be the reason for the positive correlation.
With respect to 2nd-h serum insulin, serum irisin again showed a moderately positive correlation which was statistically significant. However, this association has not been studied in the data available so far, although the increased level of 2nd-h insulin in cases when compared to controls has been reported in few studies.[8,9,12]
Serum irisin showed a statistically significant moderate positive correlation with HOMA2-IR which is consistent with a study[8] evaluating irisin levels and the effect of metformin treatment in women with PCOS. There was a significant decline in levels of serum irisin and HOMA2-IR after metformin therapy. In our study, there was a negative correlation between serum irisin and QUICKI of statistical significance which is in agreement with a study which focused on the development of PCOS.[9] ROC curve was constructed for comparison of irisin values among normal controls and PCOS cases for the diagnosis of IR based on HOMA2-IR. Area under the curve was 0.626 (P = 0.053). As the discriminating power of serum irisin was not very good, a single best cutoff was not possible, but for the given range of serum irisin 4.49–5.50 ng/mL, sensitivity ranges from 73.5 to 79.6 and specificity ranges from 36.4 to 51.5. Analysis of ROC curve derived in a previous study[9] indicated that the optimal cutoff point for fasting irisin was 814.2 ng/ml.
This is the first study to evaluate serum irisin levels and assess its association with indices using blood glucose and insulin levels in adolescents with PCOS. The estimation of serum irisin levels was done in a targeted age group in this study. Restricting the inclusion age to 15–19 years reflects the levels in adolescent girls. Limitations of the study were that the approximated irisin cutoff value for the diagnosis of IR has not yet been elucidated. Since the study population was recruited from a tertiary hospital, the relationship between serum irisin and blood glucose and insulin indices may not reflect all adolescent girls with PCOS.
CONCLUSIONS
Serum irisin levels were significantly elevated in adolescents with PCOS when compared to the controls. A significant correlation of serum irisin with blood glucose and insulin indices indicates that serum irisin could serve as a marker of IR and may help in the diagnosis of IR in adolescents with PCOS. As young girls with PCOS are at high risk of developing IGT, early identification of IR by a panel of HOMA2-IR, QUICKI, and irisin in addition to GTT may play a pivotal role in institution of lifestyle changes that may help in delaying the progression to type 2 DM. Further, follow-up of these participants may yield additional insight into the progression of IR and type 2 DM in young girls with PCOS. Further studies are required with large sample size to arrive at cutoff point of serum irisin for the diagnosis of IR.
Financial support and sponsorship
Intramural fund of Rs. 64,000 for our PG project which was utilized for ELISA kits, reagents, calibrators, etc. (JIP/Res/Intra-MD-MS/phs1/01/2016-17).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nidhi R, Padmalatha V, Nagarathna R, Amritanshu R. Prevalence of polycystic ovarian syndrome in Indian adolescents. J Pediatr Adolesc Gynecol. 2011;24:223–7. doi: 10.1016/j.jpag.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Diamanti-Kandarakis E. Insulin resistance in PCOS. Endocrine. 2006;30:13–7. doi: 10.1385/ENDO:30:1:13. [DOI] [PubMed] [Google Scholar]
- 3.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: A method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–23. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 4.Singh B, Saxena A. Surrogate markers of insulin resistance: A review. World J Diabetes. 2010;1:36–47. doi: 10.4239/wjd.v1.i2.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conway G, Dewailly D, Diamanti-Kandarakis E, Escobar-Morreale HF, Franks S, Gambineri A, et al. The polycystic ovary syndrome: A position statement from the European Society of Endocrinology. Eur J Endocrinol. 2014;171:P1–29. doi: 10.1530/EJE-14-0253. [DOI] [PubMed] [Google Scholar]
- 6.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park KH, Zaichenko L, Brinkoetter M, Thakkar B, Sahin-Efe A, Joung KE, et al. Circulating irisin in relation to insulin resistance and the metabolic syndrome. J Clin Endocrinol Metab. 2013;98:4899–907. doi: 10.1210/jc.2013-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li M, Yang M, Zhou X, Fang X, Hu W, Zhu W, et al. Elevated circulating levels of irisin and the effect of metformin treatment in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2015;100:1485–93. doi: 10.1210/jc.2014-2544. [DOI] [PubMed] [Google Scholar]
- 9.Chang CL, Huang SY, Soong YK, Cheng PJ, Wang CJ, Liang IT. Circulating irisin and glucose-dependent insulinotropic peptide are associated with the development of polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:E2539–48. doi: 10.1210/jc.2014-1180. [DOI] [PubMed] [Google Scholar]
- 10.Adamska A, Karczewska-Kupczewska M, Lebkowska A, Milewski R, Górska M, Otziomek E, et al. Serum irisin and its regulation by hyperinsulinemia in women with polycystic ovary syndrome. Endocr J. 2016;63:1107–12. doi: 10.1507/endocrj.EJ16-0249. [DOI] [PubMed] [Google Scholar]
- 11.Bostancı MS, Akdemir N, Cinemre B, Cevrioglu AS, Özden S, Ünal O. Serum irisin levels in patients with polycystic ovary syndrome. Eur Rev Med Pharmacol Sci. 2015;19:4462–8. [PubMed] [Google Scholar]
- 12.Li H, Xu X, Wang X, Liao X, Li L, Yang G, et al. Free androgen index and Irisin in polycystic ovary syndrome. J Endocrinol Invest. 2016;39:549–56. doi: 10.1007/s40618-015-0403-7. [DOI] [PubMed] [Google Scholar]
- 13.Abali R, Temel Yuksel I, Yuksel MA, Bulut B, Imamoglu M, Emirdar V, et al. Implications of circulating irisin and Fabp4 levels in patients with polycystic ovary syndrome. J Obstet Gynaecol. 2016;36:897–901. doi: 10.3109/01443615.2016.1174200. [DOI] [PubMed] [Google Scholar]
- 14.Gao S, Cheng Y, Zhao L, Chen Y, Liu Y. The relationships of irisin with bone mineral density and body composition in PCOS patients. Diabetes Metab Res Rev. 2016;32:421–8. doi: 10.1002/dmrr.2767. [DOI] [PubMed] [Google Scholar]
- 15.Pukajło K, Łaczmański Ł, Kolackov K, Kuliczkowska-Płaksej J, Bolanowski M, Milewicz A, et al. Irisin plasma concentration in PCOS and healthy subjects is related to body fat content and android fat distribution. Gynecol Endocrinol. 2015;31:907–11. doi: 10.3109/09513590.2015.1065482. [DOI] [PubMed] [Google Scholar]
- 16.Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81:19–25. doi: 10.1016/j.fertnstert.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Raj MS, Shilpa S, Maheswaran R. Revised socio-economic status scale for urban and Rural India Revision for 2015. Socioeconomica. 2015;4:167–74. [Google Scholar]


