To the Editor: The coronavirus disease 2019 (Covid-19) pandemic has uniquely affected prisons and jails across the country. The incidence of Covid-19 among incarcerated persons is nearly six times that among nonincarcerated community members.1 The Centers for Disease Control and Prevention, the National Academy of Medicine, and the American Medical Association have recommended prioritization of prison and jail populations for deployment of Covid-19 vaccines, but vaccine rollout has varied across these settings,2 and few studies have been conducted on the effectiveness of vaccination efforts in congregate housing. Most of such studies have been performed in skilled nursing facilities, where vaccine effectiveness has been measured at 63 to 64%.3,4
Rhode Island is one of six states that have a unified carceral system. The Rhode Island Department of Corrections (RIDOC) maintains six facilities that include a jail-like intake facility, buildings with three levels of security (minimum, medium, and maximum), and a women’s building on the same campus. The RIDOC offered Covid-19 vaccines — the two-dose BNT162b2 (Pfizer–BioNTech) or mRNA-1273 (Moderna) — to all incarcerated persons and staff members. Since November 2020, the standard of care at the RIDOC facilities has included weekly universal polymerase-chain-reaction (PCR) testing for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) among all incarcerated persons and staff members.
We conducted a study to analyze weekly PCR test results that were obtained in the RIDOC system from March 9 to May 6, 2021. RIDOC policy includes a 10-day isolation period for all persons who have symptoms or a positive Covid-19 test. A test-based end-of-isolation strategy was initiated on March 10. According to this protocol, if negative results were obtained on two PCR tests that had been performed 24 hours apart, isolation could end early.
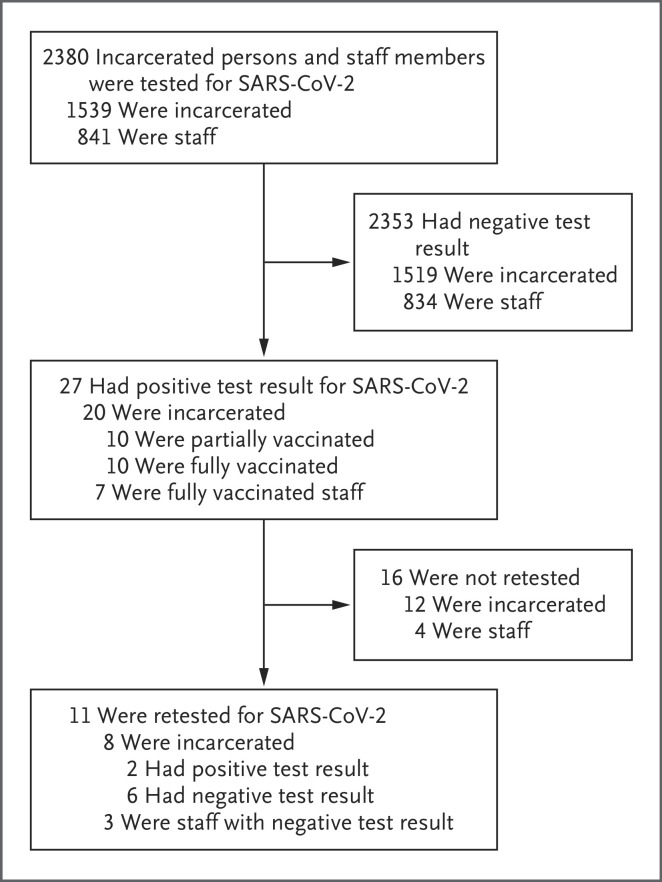
Among the 4638 persons who were tested during the study period, 2380 who had received at least one dose of a SARS-CoV-2 vaccine were included in the analysis (Figure 1). Of these persons, 27 (1.13%) had positive results for SARS-CoV-2. Of the 8847 tests that were administered to incarcerated persons during the study period, 20 (0.22%; 95% confidence interval [CI], 0.14 to 0.36) were positive. Among 4140 tests administered to staff members who had been vaccinated, positive results were obtained on 7 tests (0.17%; 95% CI, 0.16 to 0.18). The incidence of positive tests per person tested was 20 of 1539 (1.3%; 95% CI, 0.8 to 2.0) among incarcerated persons and 7 of 841 (0.8%; 95% CI, 0.3 to 1.7) among staff members. All the cases of Covid-19 were asymptomatic.
Figure 1. Testing and Breakthrough SARS-CoV-2 Infections among Vaccinated Persons in a Prison Complex.
Of the 27 vaccinated staff members and incarcerated persons who had positive results for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, 8 (30%) had also tested positive for SARS-CoV-2 more than 3 months earlier.
Of the 27 vaccinated persons with positive test results, 5 had received one dose of vaccine, 5 had received a second dose within 2 weeks before infection, and 17 had received a second dose at least 2 weeks before infection. Eight persons (30%) had also tested positive for SARS-CoV-2 more than 3 months earlier (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). Repeat PCR testing was performed in 11 of the 27 persons (41%) who had positive test results; 9 persons tested negative, and 2 tested positive. The median interval between the collection of the initial sample and follow-up testing was 2 days (range, 2 to 7 days).
In this analysis, we found that SARS-CoV-2 breakthrough infections were identified only rarely after vaccination in a carceral setting in Rhode Island. Thus, vaccination of staff members and incarcerated persons, along with a policy of expanded decarceration,5 appeared to be effective in preventing the transmission of SARS-CoV-2.
Supplementary Appendix
Disclosure Forms
This letter was published on July 7, 2021, at NEJM.org.
Footnotes
Supported by a grant (UG1DA050072, to Drs. Brinkley-Rubinstein and Martin and Ms. Peterson) from the National Institute on Drug Abuse.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Macmadu A, Berk J, Kaplowitz E, Mercedes M, Rich JD, Brinkley-Rubinstein L. COVID-19 and mass incarceration: a call for urgent action. Lancet Public Health 2020;5(11):e571-e572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Peterson M, Behne F, Denget B, Nowtony K, Brinkley-Rubinstein L. Uneven rollout of COVID-19 vaccinations in United States prisons. Health Affairs Blog. April 15, 2021. (https://www.healthaffairs.org/do/10.1377/hblog20210413.559579/full/).
- 3.Teran RA, Walblay KA, Shane EL, et al. Postvaccination SARS-CoV-2 infections among skilled nursing facility residents and staff members — Chicago, Illinois, December 2020–March 2021. MMWR Morb Mortal Wkly Rep 2021;70:632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton A, Jacobs Slifka KM, Edens C, et al. Effectiveness of the Pfizer-BioNTech COVID-19 vaccine among residents of two skilled nursing facilities experiencing COVID-19 outbreaks — Connecticut, December 2020–February 2021. MMWR Morb Mortal Wkly Rep 2021;70:396-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vest N, Johnson O, Nowotny K, Brinkley-Rubinstein L. Prison population reductions and COVID-19: a latent profile analysis synthesizing recent evidence from the Texas State prison system. J Urban Health 2021;98:53-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.