To the Editor: A second wave of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in India is leading to the emergence of SARS-CoV-2 variants. The B.1.617.1 (or kappa) and B.1.617.2 (or delta) variants were first identified in India and have rapidly spread to several countries throughout the world. These variants contain mutations within the spike protein located in antigenic sites recognized by antibodies with potent neutralizing activity.1-3 We used serum samples obtained from infected and vaccinated persons to assess neutralizing activity against the SARS-CoV-2 variants in a live-virus assay.
For the analyses, we used B.1.617.1 virus that had been isolated from a mid-turbinate swab obtained from a patient in Stanford, California, in March 2021 (hCoV-19/USA/CA-Stanford-15_S02/2021) and B.1.617.2 virus from a nasal swab that had been obtained from a patient in May 2021 (hCoV-19/USA/PHC658/2021). As compared with the WA1/2020 variant (nCoV/USA_WA1/2020; spike 614D), the B.1.617.1 and B.1.617.2 variants contain mutations in key regions within the spike, including the N-terminal antigenic supersite,4 the receptor-binding domain, and the polybasic furin cleavage site (Tables S1 and S2 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). We used an in vitro, live-virus focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus])5 on a Vero E6 cell line (engineered to express TMPRSS2) to compare the neutralizing-antibody responses against WA1/2020 in serum samples from 24 persons who had recovered from coronavirus disease 2019 (Covid-19) (obtained 31 to 91 days after symptom onset),1 from 15 persons who had received the mRNA-1273 (Moderna) vaccine (obtained 35 to 51 days after the second dose), and from 10 persons who had received the BNT162b2 (Pfizer–BioNTech) vaccine (obtained 7 to 27 days after the second dose).
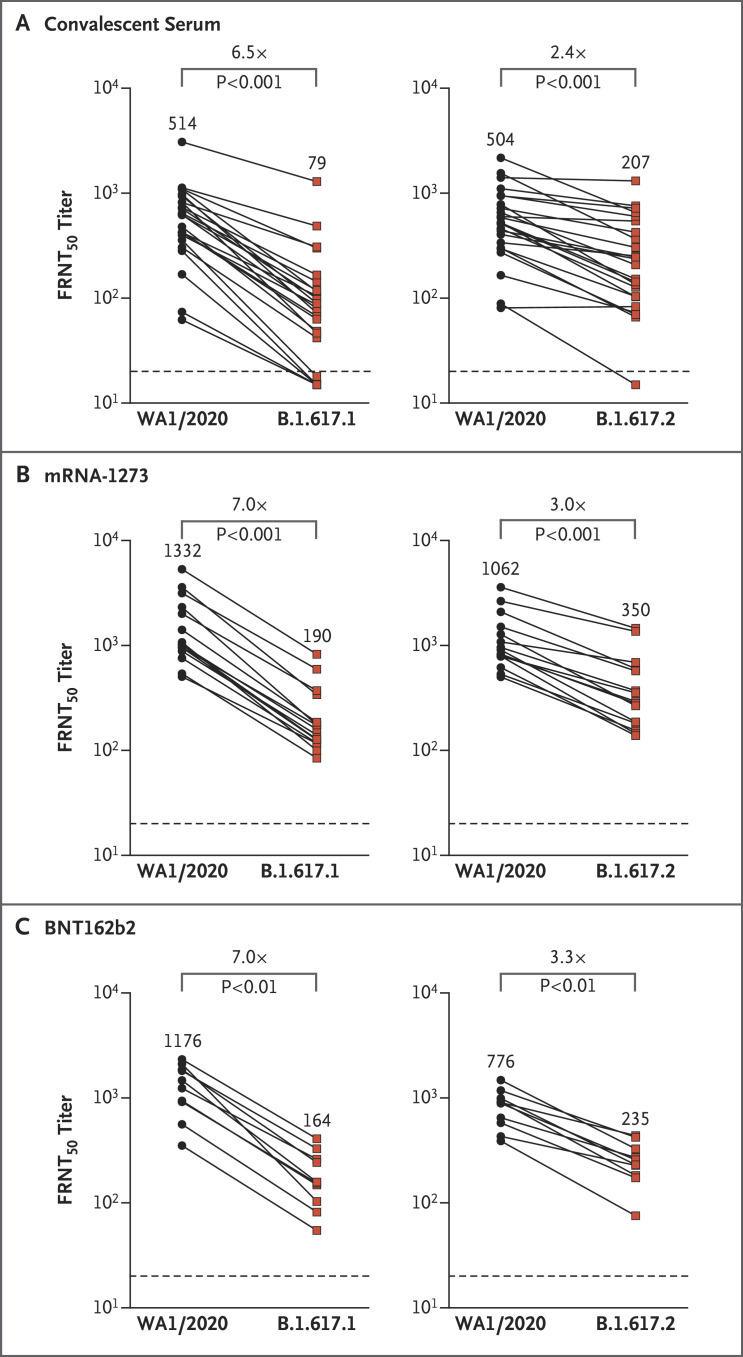
All samples from infected and vaccinated persons showed less neutralizing activity against both the B.1.617.1 and B.1.617.2 variants than against WA1/2020 (Figure 1). Among convalescent serum samples, the FRNT50 geometric mean titer (GMT) against B.1.617.1 was 79 (95% confidence interval [CI], 49 to 128), as compared with 514 (95% CI, 358 to 740) against WA1/2020 (five samples had undetectable activity against the B.1.617.1 variant); the GMT against B.1.617.2 was 207 (95% CI, 135 to 319), as compared with 504 (95% CI, 358 to 709) against WA1/2020 (one sample had undetectable activity against the B.1.617.2 variant). Among the mRNA-1273 samples, the GMT against B.1.617.1 was 190 (95% CI, 131 to 274), as compared with 1332 (95% CI, 905 to 1958) against WA1/2020; the GMT against B.1.617.2 was 350 (95% CI, 229 to 535), as compared with 1062 (95% CI, 773 to 1460) against WA1/2020. Among the BNT162b2 vaccine serum samples, the GMT against B.1.617.1 was 164 (95% CI, 104 to 258), as compared with 1176 (95% CI, 759 to 1824) against WA1/2020; the GMT against B.1.617.2 was 235 (95% CI, 164 to 338), as compared with 776 (95% CI, 571 to 1056) against WA1/2020. Among the three sample groups, the GMTs against the B.1.617.1 and B.1.617.2 variants were significantly lower than those against the WA1/2020 strain.
Figure 1. Neutralizing-Antibody Responses against the WA1/2020, B.1.617.1, and B.1.617.2 Variants.
Shown is the neutralizing activity against natural infection with severe acute respiratory syndrome coronavirus 2 among 24 samples from persons who had recovered from coronavirus disease 2019 (obtained 31 to 91 days after symptom onset) (Panel A), 15 samples from persons who had received the mRNA-1273 (Moderna) vaccine (obtained 35 to 51 days after the second dose) (Panel B), and 10 samples from persons who had received the BNT162b2 (Pfizer–BioNTech) vaccine (obtained 7 to 27 days after the second dose) (Panel C). Two independent neutralization assays were performed: activity against B.1.617.1 was compared with that against WA1/2020, and activity against B.1.617.2 was compared with that against WA1/2020. The focus reduction neutralization test (FRNT50 [the reciprocal dilution of serum that neutralizes 50% of the input virus]) geometric mean titers for WA1/2020, B.1.617.1, and B.1.617.2 are shown in each panel. The connecting lines between WA1/2020 and B.1.617.1 or WA1/2020 and B.1.617.2 represent matched serum samples. The horizontal dashed lines along the x axes indicate the limit of detection (FRNT50 geometric mean titer, 20). Normality of the data was determined with the use of the Shapiro–Wilk normality test. Nonparametric pairwise analyses for neutralization titers were performed with the use of the Wilcoxon matched-pairs signed-rank test.
Our results show that the B.1.617.1 variant was 6.8 times less susceptible, and the B.1.617.2 variant was 2.9 times less susceptible, to neutralization by serum from persons who had recovered from Covid-19 and from vaccinated persons than was the WA1/2020 variant. Despite this finding, a majority of the convalescent serum samples (79% [19 of 24 samples] against B.1.617.1 and 96% [23 of 24 samples] against B.1.617.2) and all serum samples from vaccinated persons still had detectable neutralizing activity above the threshold of detection against both variants through 3 months after infection or after the second dose of vaccine. Thus, protective immunity conferred by the mRNA vaccines is most likely retained against the B.1.617.1 and B.1.617.2 variants.
Supplementary Appendix
Disclosure Forms
This letter was published on July 7, 2021, at NEJM.org.
Footnotes
Supported in part by grants (NIH P51 OD011132, 3U19AI057266-17S1, 1U54CA260563, and HHSN272201400004C [to Emory University] and 75N93021C00016 [to St. Jude Children’s Research Hospital]) from the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health; intramural funding from the NIAID; the Oliver S. and Jennie R. Donaldson Charitable Trust; Emory Executive Vice President for Health Affairs Synergy Fund award; the Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children’s Healthcare of Atlanta; the Emory-UGA Center of Excellence for Influenza Research and Surveillance; COVID-Catalyst-I3 funds from the Woodruff Health Sciences Center and Emory School of Medicine; and Woodruff Health Sciences Center 2020 COVID-19 CURE Award. The funders had no role in the design or conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication.
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Edara VV, Norwood C, Floyd K, et al. Infection- and vaccine-induced antibody binding and neutralization of the B.1.351 SARS-CoV-2 variant. Cell Host Microbe 2021;29(4):516.e3-521.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Z, VanBlargan LA, Bloyet L-M, et al. Identification of SARS-CoV-2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe 2021;29(3):477.e4-488.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plante JA, Mitchell BM, Plante KS, Debbink K, Weaver SC, Menachery VD. The variant gambit: COVID-19’s next move. Cell Host Microbe 2021;29:508-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cerutti G, Guo Y, Zhou T, et al. Potent SARS-CoV-2 neutralizing antibodies directed against spike N-terminal domain target a single supersite. Cell Host Microbe 2021;29(5):819.e7-833.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanderheiden A, Edara VV, Floyd K, et al. Development of a rapid focus reduction neutralization test assay for measuring SARS-CoV-2 neutralizing antibodies. Curr Protoc Immunol 2020;131(1):e116-e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.